Evaluation of risk factors for developing COVID-19 in healthcare professionals working at two university hospitals in Turkey
Abstract
BACKGROUND:
Healthcare workers (HCWs) were seriously affected by the coronavirus disease 2019 (COVID-19). It is a priority to protect HCWs against COVID-19 and ensure the continuity of the health care system.
OBJECTIVE:
To evaluate the risk factors for COVID-19 in HCWs and the effectiveness of the measures taken on protection.
METHODS:
A nested case-control study was conducted in two hospitals serving on the same campus which are affiliated with a university from Turkey, between 03.12.2020 and 05.22.2020. We aimed to recruit three controls working in the same unit with the cases diagnosed with COVID-19 by polymerase chain reaction (PCR) and whose SARS-CoV-2 PCR test is negative. Self-reported data were collected from the HCWs by the face-to-face method. Descriptive and analytical methods were used and a logistic regression model was built.
Results:
The study was completed with 271 HCWs, 72 cases, and 199 controls. Household contact with a COVID-19 patient or a patient with symptoms compatible with COVID-19 was found to be significantly higher in the cases than in the controls (p = 0.02, p < 0.001). When the measures for control the COVID-19 were analyzed, using a medical mask (OR = 0.28, 95% confidence interval = 0.11–0.76, p = 0.01) by COVID-19 patient and using the respiratory mask by HCWs (OR = 0.13, 95% CI = 0.03–0.52, p = 0.004) during close contact was found to be protective against COVID-19 transmission.
Conclusion:
This study showed an association with using medical masks by the patients as an important protective precaution for the transmission of COVID-19 to HCWs. Respiratory masks should be used by HCWs while in close contact with COVID-19 patients regardless of aerosol-producing procedures.
1Introduction
Coronavirus disease 2019 (COVID-19) was first discovered with the clustering of pneumonia cases in Wuhan, China at the end of 2019 and rapidly spread all over the world and caused a devastating pandemic. The etiologic agent was identified on 01.07.2020 as a new coronavirus which was later named as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1].
A high number of healthcare workers (HCWs) were infected by SARS-CoV-2 in countries such as China and Italy with a heavy disease burden in the first period of the pandemic and the outcome was tragic in some of them [2]. The lack of certain evidence about some issues on the contagiousness of the disease (such as the infectivity of asymptomatic people or the duration of contagiousness), lack of regular screening, and the shortage of personal protective equipment (PPE) made it difficult to prevent the spread of SARS-CoV-2 in HCWs in the first period of the pandemic [3]. While the number of people who required health care increased rapidly under pandemic conditions, there was a decrease in the health care workforce. It resulted in collapsing the healthcare system from time to time [4, 5]. The high risk of transmission of SARS-CoV-2 from HCWs to their colleagues, home residents, and patients distinguishes them from other risk groups [6, 7]. So, protecting HCWs became a priority.
Possible risk factors were determined through the experiences at the beginning of the pandemic and the effects of the measures against these risk factors need to be carefully analyzed. In this study, we aimed to evaluate the risk factors associated with the development of COVID-19 among HCWs working at Hacettepe University Adult and Oncology Hospitals (HUH). Although several studies evaluated risk factors for the transmission of COVID-19 in HCWs, the usual method was comparing the SARS-CoV-2 infected HCWs without non-infected HCWs. However, the risk of transmission is not the same in every unit of the hospital. So, we planned a case-control study by matching the SARS-CoV-2 infected HCWs with their colleagues who worked at the same unit in the same period.
2Materials and methods
The research was carried out at HUH located in Ankara the capital city of Turkey. The study was conducted in the two separate hospital buildings of the university that serves adult patients within the same campus, namely the oncology hospital and the adult hospitals. The oncology hospital is mainly reserved for in/out-patient care of cancer patients, yet, other patients may use the same setting for some occurrences, such as radiologic imaging, etc. Similarly, the resident physicians, hematology consultants, and oncology consultants of the university hospitals rotate between the two buildings, as necessary. The adult hospital served as a pandemic hospital during the study period. The first patient with laboratory-confirmed COVID-19 at HUH was detected on March 23, 2020 [8].
This research was a case-control study nested in a surveillance system that was focused on HCWs from HUH who were tested for COVID-19 disease. The hospital-based COVID-19 surveillance was initiated by the hospital infection control committee on 03.12.2020. All SARS-CoV-2 polymerase chain reaction (PCR) test results were recorded and followed via the electronic hospital information management system. HCWs who were tested by PCR between 03.12.2020 and 05.22.2020 were defined as the universe of the study.
A naso-oropharyngeal sample from the HCWs was taken at “COVID-19 Initial Evaluation Outpatient Clinic”. SARS-CoV-2 PCR was performed as described previously [9]. The HCWs who had a positive SARS-CoV-2 PCR between 03.12.2020 and 05.22.2020 were the case group. Each case was matched with three controls. The controls were determined among individuals who worked in the same unit at the time of the RT-PCR test of the case, who did not have fever, cough, shortness of breath, myalgia, sore throat, headache, diarrhea, and in whom SARS-CoV-2 PCR test was negative.
Demographic characteristics, occupational or non-occupational risk factors for COVID-19, and PPE usage of the participants were questioned through the standardized 55 question survey specially prepared for the study. The survey was completed by a researcher through face-to-face interviews except for 12 participants who filled out the survey personally due to lack of time for the interview. The surveys were completed fourteen days preceding SARS-CoV-2 PCR.
The body mass indexes of the participants were calculated as body weight (kg) / height squared (m2) based on the personal statement and classified according to the WHO classification [10]. All obesity categories from grades 1 to 3 (due to the insufficient number of participants in the categories) were combined in the analyses. The educational status was classified as secondary school and below (1st–12th grades), or higher since the compulsory education period in Turkey is until the end of secondary school [11]. In the evaluation of smoking status, pack-years were calculated over the number of years smoked and the number of cigarettes consumed per day. The sequence for donning and doffing of the PPEs was accepted as “correct” when expressed as stated in the Hacettepe University Hospitals COVID-19 Personal Protective Equipment Use Guide [12]. The compliance of HCWs with hand hygiene was evaluated over five moments of WHO for hand hygiene [13]. The staff who were directly involved in patient care were defined as healthcare providers. The staff who work at the laundry, pharmacy, administration offices, and medical device offices were defined as supportive service workers. Each case was matched with the controls from the same working unit to avoid the confounding effect of the working unit. As providing direct care to the patient can significantly change the risk of COVID-19, a subgroup analysis was performed among healthcare providers.
2.1Statistical analysis
As descriptive statistics, continuous variables were given as mean ± standard deviation for normally distributed data, and as median and interquartile range (IQR) for data with the non-normal distribution. Categorical variables were reported as numbers and percentage distributions. Categorical variables were compared by the Chi-Square test or Fischer’s exact test and continuous variables were compared by the independent-samples t-test for normally distributed data or Mann Whitney U test for non-normal distributed data. The odds ratios (OR) and their confidence interval (CI) 95% were calculated to give potential association as an effect size value. No data imputation was applied for the missing data. Type 1 error probabilities were accepted as 0.05 for all statistical tests. Statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) version 23 software (IBM Corp., Armonk, NY, USA).
The conditional logistic regression analysis was conducted to examine the effect of measures taken to prevent the development of COVID-19 in 234 healthcare providers. The model included gender, presence of any comorbidity, and age as possible confounders. The variables such as COVID-19 patients wearing a medical mask during the close contact with the HCWs, the HCW wearing a respiratory mask during the close contact with the confirmed patient, and performing the aerosol-generating procedure on a COVID-19 patient were included as covariants. An attempt was made to find the most explanatory model by using conditional logistic regression analysis with the “Enter” method and it was completed in 172 participants due to missing data (26.4%).
2.2Power analysis
Due to the lack of a pioneering study at the research planning stage, the minimum sample size required could not be estimated in the planning period. Power analysis was conducted retrospectively for the variables included in the modelling phase, using “epi.sscc” command in the “epiR” package, R ver. 3.6.1. Accordingly, the power of the study for testing the association between COVID-19 was found to be 81.7%, 51.8%, and 4.1% for wearing a respiratory mask during close contact, wearing a medical mask during close contact, and “performing the aerosol-generating procedure on a COVID-19 patient respectively.
2.3Ethical considerations
Ethical approval was obtained from Hacettepe University Non-interventional Clinical Research Ethics Committee (Approval date: 05.22.2020, number 2020/10-40), and further administrative approval was obtained from Hacettepe University Hospitals Administration (Approval date: 06.05.2020, number: 27043162-000). All participants had informed consent. The study was conducted in accordance with the Declaration of Helsinki (2008). The results of the research were presented to the hospital administration as an executive summary.
3Results
SARS-CoV-2 PCR test was performed at least once on 1383 (23.3%) of 5947 personnel working at HUH between 03.12.2020 and 05.22.2020. The SARS-CoV-2 PCR test was positive in 75. Although we aimed to select three controls for each case, there were not enough control candidates who were suitable for the selection criteria. So, four cases were matched with one control, and nine cases were matched with two controls. The study included a total of 271 HCWs, 72 cases, and 199 controls (Fig. 1). Sixty-two (86.1%) out of 72 cases and 172 (86.4%) out of 199 were working as healthcare providers and others were from the supportive services. Since there was no difference between the general group and healthcare providers through the subgroup analysis, the results that concerned all HCWs were presented over the general group, such as the use of medical masks. There was no statistical difference between the demographic characteristics of the cases and controls (Table 1). No HCW died in the study group during the study period.
Fig. 1
Flowchart of the study.
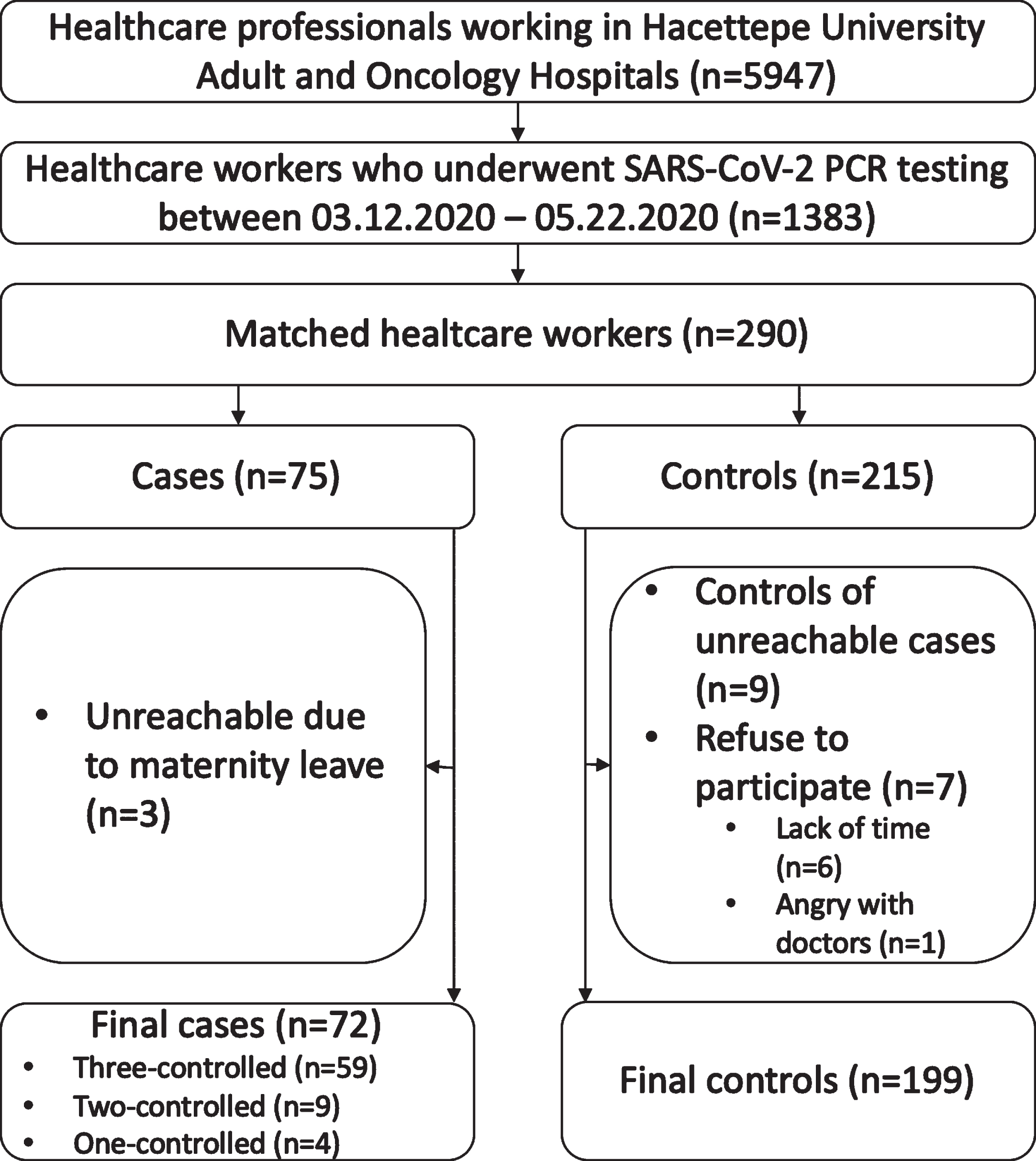
Table 1
Sociodemographic features of the cases and the controls
| Sociodemographic features | Cases | Controls | OR (95% CI) | p-value |
| n (%a) | n (%a) | |||
| Male gender | 32 (44.4) | 83 (41.7) | 1.12 (0.65–1.93) | 0.69 |
| Body mass index | 0.44 | |||
| Underweight | 4 (5.6) | 5 (2.6) | 2.17 (0.55–8.50) | |
| Normal (reference) | 38 (53.5) | 103 (52.8) | 1.00 | |
| Overweight | 23 (32.4) | 60 (30.8) | 1.4 (0.57–1.91) | |
| Obesity | 6 (8.5) | 27 (13.8) | 0.60 (0.23–1.57) | |
| Educational status | 0.52 | |||
| Compulsatory (1st–12th) (ref.) | 22 (30.6) | 48 (24.1) | 1.00 | |
| Associate or Bachelor degree | 33 (45.8) | 95 (47.7) | 0.76 (0.40–1.44) | |
| Postgraduate | 17 (23.6) | 56 (28.1) | 0.66 (0.32–1.38) | |
| Occupation | 0.95 | |||
| Nurse (reference) | 25 (34.7) | 70 (35.2) | 1.00 | |
| Doctor | 17 (23.6) | 49 (24.6) | 0.97 (0.47–1.99) | |
| Housekeeping staff | 9 (12.5) | 27 (13.6) | 0.93 (0.39–2.25) | |
| Cleaning staff | 6 (8.3) | 11 (5.5) | 1.53 (0.51–4.56) | |
| Others | 15 (20.8) | 42 (21.1) | 1.00 (0.47–2.11) | |
| Presence of any comorbidity | 23 (31.9) | 63 (31.7) | 1.01 (0.57–1.81) | 0.96 |
| Routine use of any medication | 19 (26.4) | 42 (21.1) | 1.34 (0.72–2.50) | 0.36 |
| Immunosuppressive therapy | 3 (4.2) | 3 (1.5) | 2.84(0.56–14.41) | 0.19b |
| Smoking status | ||||
| Active | 21 (29.2) | 62 (31.2) | 0.90 (0.49–1.64) | 0.92 |
| Ex-smoker | 6 (8.3) | 18 (9.0) | 0.88 (0.33–2.36) | |
| Never smoked (reference) | 45 (62.5) | 119 (59.8) | 1.00 | |
| Having a child | 43 (59.7) | 102 (51.3) | 1.41 (0.82–2.44) | 0.22 |
| Having home care patients | 5 (6.9) | 15 (7.5) | 0.92 (0.32–2.62) | 0.87 |
| Median | Median | z-value | p-value | |
| (IQR) | (IQR) | |||
| Age (years) | 34.0 (13.8) | 33.0(10.0) | 1.35 | 0.18 |
| Body mass index (kg/m2) | 24.0 (5.5) | 24.4 (5.0) | 0.85 | 0.39 |
| Cumulative smoking exposure (pack-years) | 7.5 (11.7) | 9.5 (14.0) | 0.10 | 0.92 |
| Total working time (years) | 10.0 (11.0) | 7.0 (12.0) | 1.11 | 0.27 |
| Total working time in the current unit (years) | 2.3 (4.8) | 3.0 (4.0) | 0.25 | 0.81 |
CI = Confidence interval, IQR = Interquartile range, OR = Odds ratio. aColumn percentages. bFischer’s exact test.
Among the analyzed HCWs no statistically significant occupational risk factors were identified. Having a household contact either with COVID-19 patient (OR = 21.32; 95% CI = 2.60–176.57; p < 0.001) or a person with symptoms compatible with COVID-19 (OR = 31.94; 95% CI = 4.01–254.43; p < 0.001) increased the risk for COVID-19 in HCWs (Table 2).
Table 2
Distribution of possible risk factors for developing COVID-19 of cases and controls
| Non -occupational possible risk | Cases | Controls | OR (95% CI) | p-value |
| factors | n (%a) | n (%a) | ||
| Presence of a SARS-CoV-2 positive person in the household | 7 (9.7) | 1 (0.5) | 21.32 (2.6–176.57) | <0.001b |
| Presence of symptomatic person in the household | 10 (13.9) | 1 (0.5) | 31.94(4.01–254.43) | <0.001b |
| Take part in a community of five people except for the hospital | 5 (6.9) | 12 (6.0) | 1.16 (0.40–3.42) | 0.78 |
| Transportation routec | ||||
| Public transport | 29 (40.3) | 82 (41.2) | 0.96 (0.56–1.67) | 0.89 |
| Work bus | 1 (1.4) | 3 (1.5) | 0.92 (0.09–8.99) | 1.00b |
| Taxi | 3 (4.2) | 6 (3.0) | 1.40 (0.34–5.76) | 0.70b |
| Private car | 38 (52.8) | 93 (46.7) | 1.27 (0.74–2.19) | 0.38 |
| Pedestrian | 11 (15.3) | 34 (17.1) | 0.88 (0.42–1.84) | 0.72 |
| Median (IQR) | Median (IQR) | z-value | p-value | |
| Household size | 3 (2) | 3 (2) | 1.79 | 0.073 |
| Occupational possible risk factors | Cases n (%a) | Controls n (%a) | OR (95% CI) | p-value |
| Social distance adherence while eating and drinking | ||||
| Always | 20 (27.8) | 64 (32.2) | 0.37 (0.11–1.29) | 0.41 |
| Often | 24 (33.3) | 74 (37.2) | 0.38 (0.12–1.24) | |
| Sometimes | 13 (18,1) | 37 (18.6) | 0.41 (0.12–1.45) | |
| Rarely | 9 (12.5) | 17 (8.5) | 0.62 (0.16–2.40) | |
| Never (reference) | 6 (8.3) | 7 (3.5) | 1.00 | |
| Contact with a COVID-19 patient | 65 (90.3) | 190(95.5) | 0.44 (0.16–1.22) | 0.14b |
| Contact closer than one meter with a COVID-19 patient | 57 (79.2) | 172(86.4) | 0.60 (0.30–1.20) | 0.14 |
| Caregiving to a a COVID-19 patient | 50 (69.4) | 144(72.4) | 0.87 (0.48–1.57) | 0.64 |
| Aerosol generating processc,d | 22 (44.0) | 66 (45.8) | 0.93 (0.49–1.77) | 0.82 |
| Endotracheal aspiration | 8 (36.4) | 36 (54.5) | 0.48 (0.18–1.29) | 0.14 |
| Nebulizer therapy | 10 (45.5) | 30 (45.5) | 1.00 (0.38–2.64) | 1.00 |
| Nasooropharyngeal sampling | 5 (22.7) | 24 (36.4) | 0.52 (0.17–1.57) | 0.24 |
| Contact with a COVID-19 patient’s surroundingsd | 49 (98.0) | 140(97.2) | 1.40 (0.15–12.83) | 1.00b |
| Median | Median | z-value | p-value | |
| (IQR) | (IQR) | |||
| Longest daily working time (hour) | 12 (8) | 12 (8) | 0.77 | 0.44 |
| Average daily working time (hour) | 6.9 (2.9) | 6.9 (2.3) | 0.29 | 0.78 |
CI = Confidence interval, IQR = Interquartile range, OR = Odds ratio. aColumn percentages. bFischer’s exact test. cParticipants could select one more answer. dAmong healthcare providers.
Wearing a medical mask of a COVID-19 patient during close contact with a HCW protected the HCW 1.92 (95% CI = 1.04–3.57) times from developing COVID-19 (p = 0.034). The statistical difference in protecting from SARS-CoV-2 infection was only significant for the respiratory mask (OR = 2.56; 95% CI = 1.39–4.76; p = 0.003) amongst for PPEs during close contact with a COVID-19 patient (Table 3).
Table 3
Distribution of personal protective equipment usage during the close contacta with a COVID-19 patient among cases and controls
| Personal protective equipment | Cases | Controls | OR (95% CI) | p-value |
| n (%b) | n (%b) | |||
| COVID-19 patients wearing a medical mask during close contact | 31 (54.4) | 120 (69.8) | 0.52 (0.28–0.96) | 0.034 |
| Participant wearing a medical mask during close contact | 53 (93.0) | 156 (90.7) | 1.36 (0.44–4.25) | 0.79c |
| Participant wearing a respirator mask during close contact | 19 (33.3) | 97 (56.4) | 0.39 (0.21–0.72) | 0.003 |
| Participant wearing a face shield during close contact | 25 (43.9) | 100 (58.1) | 0.56 (0.31–1.03) | 0.061 |
| Participant wearing a gown during close contact | 32 (56.1) | 106 (61.6) | 0.80 (0.43–1.46) | 0.46 |
| Participant wearing gloves during close contact | 43 (75.4) | 123 (71.5) | 1.22 (0.62–2.44) | 0.57 |
CI = Confidence interval, OR = Odds ratio. aAmong healthcare workers contact closer than one meter with a COVID-19 case (57 cases, 172 controls). bColumn percentages. cFischer’s exact test. Participants could select one more answer.
Wearing face shields and gowns at all times of HCWs protected themselves from catching COVID-19 by 2.78 (95% CI = 1.49–5.26) and 1.96 (95% CI = 1.03–3.70) times, respectively, compared with not wearing them all the time. Accessing respiratory masks, face shields, and gowns at all times for HCWs was negatively associated with catching COVID-19 by OR = 0.37 (95% CI = 0.20–0.70), OR = 0.60 (95% CI = 0.29–1.23), and OR = 0.58 (95% CI = 0.27–1.27), respectively. However, the only statistically significant association was detected for always accessing the respiratory mask. When the frequency of accessing and using PPEs was evaluated together, only 72.2% of the HCWs who can always access the face shield and 78.8% of the HCWs who can always access the gown stated that they always use it.
Respiratory masks were not able to be worn in almost all conditions due to their accessibility. In line with the recommendation of the hospital infection control committee, we accept wearing a respiratory mask while entering the rooms of patients with COVID-19 as a reference. There was a statistically significant difference when this approach was compared with never wearing a respiratory mask in any condition (OR = 2.82; 95% CI = 1.49–5.35). There was a trend in favoring to use a respiratory mask during all hospital stays (OR = 0.47; 95% CI = 0.10–2.22) or only during close contact with a COVID-19 patient / aerosol-generating procedures (OR = 1.41; 95% CI = 0.59–3.37) without reaching statistical significance which requires a further evaluation with a higher number of HCWs.
Donning the PPEs in the order determined by the hospital PPE usage guideline was 27.3% in the cases, 35.1% in the controls, and doffing the PPEs in the order determined by the hospital PPE usage guideline was 54.5% in the cases, and 56.5% in the controls, and there was no statistically significant difference between the groups (p = 0.29, p = 0.80, respectively).
HCWs in the case and control groups made similar statements regarding compliance with each of the five indications for hand hygiene recommended by the World Health Organization (p = 0.86–1.00).
During the pandemic, the statement of official training for the use of PPE was 79.2% in the case group and 80.4% in the control group. There was no statistically significant difference between the groups (p = 0.82).
According to the conditional logistic regression analysis result, the probability of the healthcare providers contracting COVID-19 was 3.22 (95% CI = 1.25–8.33) times lower if the confirmed patient wore a medical mask during close contact, and 5.88 (95% CI = 2.00–16.67) times lower if the HCW wore a respiratory mask during close contact (Table 4).
Table 4
Odds ratios associated with contracting COVID-19 among healthcare providers
| Crude ORa | 95% CI | p-value | adjORb | 95% CI | p-value | |
| Male gender | 1.16 | 0.56–2.43 | 0.69 | 1.39 | 0.51–3.77 | 0.52 |
| Age (years) | 1.07 | 1.02–1.12 | 0.011 | 1.01 | 0.94–1.08 | 0.77 |
| Presence any comorbidity (ref. = none) | 1.08 | 0.57–2.05 | 0.81 | 0.80 | 0.33–1.94 | 0.62 |
| COVID-19 patients wearing a medical mask during close contact (ref. = not wearing) | 0.39 | 1.19–0.80 | 0.010 | 0.31 | 0.12–0.80 | 0.015 |
| Healthcare providers wearing a respiratory mask during close contact (ref. = not wearing) | 0.18 | 0.07–0.44 | <0.001 | 0.17 | 0.06–0.50 | 0.001 |
| Performing aerosol-generating procedure on a COVID-19 patient (ref. = not performing) | 0.89 | 0.39–2.02 | 0.78 | 0.50 | 0.18–1.37 | 0.18 |
CI = Confidence interval, OR = Odds ratio, adj = adjusted, ref = reference. aUnivariate logistic regression. bMultivariate conditional logistic regression with all six variables.
4Discussion
In this study, it was shown that using a respiratory mask by HCWs, wearing a medical mask by COVID-19 patient protected from the transmission of COVID-19 when the HCWs were in close contact with a COVID-19 patient. Household contact with a COVID-19 patient or a person with symptoms suggesting COVID-19 were the risk factors for COVID-19 in HCWs. Epidemiological data quality due to its nested case-control design, and higher sample size compared to previous studies in Turkey makes this study interesting.
The rate of seropositivity was higher in nurses and residents than in other groups in our hospital who were screened between March 24, 2020, and September 10, 2020. We did not find any difference regarding occupation but our results can be influenced by using PCR as the screening test and a shorter period [7]. In a study conducted at a university hospital in Italy, the risk of developing COVID-19 was found to be 2.03 (95% CI = 1.18 –3.49) times in physicians compared to non-physician HCWs [14]. In two studies from Turkey, higher rates of seropositivity were reported in nurses, cleaning personnel, and physicians [15, 16]. Moreover, the majority of the restrictions that limited crowding and social events were over from June 01, 2020. So, the studies that covered a different period can result in different findings [17].
In the case-control study that was conducted by Celebi et al., the rate of COVID-19 was higher in HCWs who were working at wards where COVID-19 patients received care [18]. When we planned this study, such a result was predicted, and the HCWs were matched according to their working units. Thus, the effect of the working unit as a confounder was controlled. For this reason, it is expected that the case and control groups are similar to each other for contact with a patient, close contact with a patient, and contact with a patient’s surroundings, which were examined as possible occupational risk factors, and the similarity between the groups indicated that the control expected from matching provided. On the other hand, no comments should be made in terms of the effect of the possible occupational risk factors indicated by the results of this study on the development of the disease.
Household contact with a laboratory-confirmed COVID-19 patient or a patient with symptoms consistent with COVID-19 was one of the most important risk factors for COVID-19 for HCWs in our study that was reported previously from Turkey [19]. In the early stages of the pandemic in China, 131 HCWs were followed, and it was found that a family history of the disease increased the risk 2.76 times (95% CI = 2.02–3.77) [20]. In a case-control study that evaluated 1130 HCWs in 67 countries between April and May 2020, the rate of developing COVID-19 was 3.8 (95% CI = 1.5–9.3) times higher than the case of sharing the same house with a confirmed COVID-19 patient and the risk increased 3.0 (95% CI = 1.6–5.8) times in case of contact with a person who had symptoms consistent with COVID-19, respectively. In the same study, attending events with more than 10 people outside the hospital and home, meeting in restaurants and bars, and using public transportation were found to be associated with an increased risk of COVID-19 [21]. The effect of activities outside the home and hospital as well as international travels could not be determined in this study, since a general closure was implemented in Turkey for a significant part of the period when this research was conducted [22, 23]. In a case-control study involving 967 HCWs from 25 healthcare centers in the USA between May and December 2020, the OR of developing COVID-19 was 6.2 (95% CI = 4.1–9.4) among those who had contact with a COVID-19 patient outside of work. As a consequence, COVID-19 contacts outside the hospital had an important role in contracting COVID-19 in HCWs [24]. A recently published study from our center showed that SARS-CoV-2 seropositivity rates were higher in HCWs who had SARS-CoV-2 infected family member at home and the seropositivity rate in HCWS did not differ according to the working departments [19].
In this study, there was no statistical difference between the case and control groups when the HCWs contacted each other more than one meter during actions such as eating, drinking, and smoking when they must remove their masks. A case-control study conducted in Turkey found this situation with an increased risk of COVID-19 [18] and the kitchen workers had the highest seropositivity rate for COVID-19 at our hospital. (17) In our study, we thought that there might be a bias related to information gathering in the direction of “giving the desired answer”, since HCWs may have been reluctant to meet socially negatively due to data collection by face-to-face interview method.
We were not able to define the protective effect of wearing medical masks by hospital staff. Wearing a medical mask has been mandatory in patient-care settings from the beginning of the research, and in all settings since April 01, 2020. Due to the small number of HCWs who did not wear medical masks, the impact of enforcement of medical masks on HCWs could not be revealed statistically. However, our finding was clear that when patients wear a mask, it protected the HCWs. As both sides wore masks due to local regulations in patient-care settings, the role of the medical mask should not be ignored. In a cross-sectional study conducted in Iran on 192 HCWs, the majority of whom were physicians and nurses, the participants stated that they usually wore N95 or medical masks (92.4%) as a PPE, and almost all of those who wore them either always or mostly, similar to this study [25]. Lentz et al. found that using a medical mask in the hospital was protective against COVID-19, according to the results of multivariate analysis, except for aerosol-generating procedures [21]. In a cluster-randomized trial of 342,183 adults in Bangladesh, the symptomatic seroprevalence ratio was determined as 0.889 (95% CI = 0.780–0.997) when universal medical masks were used appropriately at the community level [26]. The efficacy of universal medical mask application at the community level suggested that its use in the hospital environment might be more important.
As in the whole world, access to respiratory (FFP2/FFP3) masks was limited by the stocks in our hospitals. Especially in the early stages of the pandemic, their use could be quite limited [27]. In the period of shortage, it was only possible to use it during aerosol-generating procedures for COVID-19 patients as recommended by the World Health Organization and Turkish Ministry of Health Scientific Committee guidelines. According to this study, HCWs who stated that they used a respiratory mask when in close contact with COVID-19 patients provided a level of protection close to 3 times that of those who did not. HCWs who stated that they never used a respiratory mask were found to be 2.82 times increased risk for COVID-19 transmission when compared with HCWs who wore a respiratory mask when entering the COVID-19 patient’s room. Lentz et al. [21] reported that using a respiratory mask was protective in all HCWs regardless of performing aerosol-generating procedures.
One of the recommended PPEs to prevent the transmission of the COVID-19 is the use of face shield/eye protectors [28]. In this study, it was observed that the use of face shield/eye protectors nearly provides 2 times higher protection rate from infection. Studies with a higher number of participants are required to prove a statistically significant effect.
It is recommended to use gloves and gowns to prevent contamination by contact. Another precaution in this respect is performing hand hygiene appropriately [28]. We detected a high rate of compliance with hand hygiene in our study, but this finding was limited with statements in a face-to-face questionnaire. There was a statistically significant difference between those who stated that they always used only the gown and those who state that they did not always use it. This situation posed the risk of contaminating the clothes of HCWs, especially when a gown was not worn, and then transferred to the mucous membranes by hand. However, even if the HCW was in contact with the patient and his/her surroundings, the risk could be reduced with the practice of hand hygiene [21]. It was thought that the use of gowns may have a more critical role because of this indirect contamination risk.
Finding out whether the frequency of using PPE is related to being able to access the equipment is important for estimating whether it would be worthwhile to refine equipment logistics. Approximately 80% of HCWs stated that they always had access to a face shield, while 62% stated that they always wore it. Similarly, 84% of HCWs stated that they always had access to the gown, while 70% stated that they always used it. It was observed that HCWs used gloves and medical masks as much as they could reach, but they did not use face shields and gowns even if they had access. It would be important to plan to investigate the reasons for not using the face shield and gown when contacting a COVID-19 patient although they are accessible.
Proper use of PPEs is as important as the presence of PPEs [29]. The order of donning and doffing PPEs was questioned to investigate this issue in this study. About 30% of the study group described donning and about 50% of doffing in accordance with the guideline released by the infection control committee. Although the difference between groups was not statistically significant, it was less accurately described in the case group. With direct observation, it will be possible to have more clear information about the subject [29]. In a study evaluating compliance with direct observation in a university hospital in Germany, full compliance for donning was determined as 73% in non-COVID-19 wards, 79% in COVID-19 wards, the compliance for doffing was 76% and 85%, respectively [30]. One hundred and seven direct observations in a hospital from Israel documented the compliance rate as 50% for donning and 37% for doffing [31]. However, the effect of compliance status on protection from COVID-19 was not investigated in these studies.
In a study that used self-reporting for hand hygiene compliance investigation, the rate was above 90% although there were slight differences according to the indications when compared with our study [32]. The report from Tanzania showed extremely lower rates of compliance to hand hygiene as 5% during direct observation shows the impact of direct observation [33].
Although we think that there was a relatively high level of compliance in our hospital at the time this study was conducted, it might not have been as high as the statements of the HCWs. Although there was no statistical difference between hand hygiene compliance and developing COVID-19 as a result of this study, in a study conducted in the first period of the epidemic in China, it was reported that unqualified handwashing increased the risk of disease by 2.64 (95% CI = 1.04–6.71) times [20].
There are some limitations of our study. Only a limited number (23.3%) of the staff in the hospital were tested for COVID-19 during our study period. Some asymptomatic cases might have been missed. There is a possibility of selection bias since 3 (4%) HCWs from the case group could not be included because they had taken maternity leave, and 7 (3.4%) HCWs from the control group did not accept to participate in the study. However, due to the low percentage of these people in the total study group, it was thought that the effect on the results would be limited.
The analytical sensitivity and specificity of SARS-CoV-2 PCR was reported as 99.4%, and 99.0%, respectively [34]. However, sensitivity, and specifcity can be influenced by several factors such as stage of the disease, sampling methodology, PCR kit, etc. Especially in daily practice, there is a possibility that some of the COVID-19 might be missed [35]. At the time of the study, repeating the PCR test was used to deal with false negativity. In our case group 24 HCWs were diagnosed after PCR tests were repeated and 108 controls had repeated PCR tests which remained negative.
To avoid information bias, data collection was done by face-to-face interview method as much as possible. A questionnaire form was given to 12 (4.4%) HCWs who could not answer the questionnaire due to their workload, and the questionnaires were collected at the end of the day. In addition, data collection was done by a researcher. Thus, we aimed to avoid inter-observer differences. The data collection process was completed in a short time (minimum-largest; 16–168 days) to cope with the “recall bias”.
Although the face-to-face interview method helps to avoid missing data, there is a possibility of erroneous statements, especially in terms of some criticizable situations such as hand hygiene, smoking status, etc. To prevent this situation, attention was paid to being alone during the interviews and the participants were informed that the information would never be shared with anyone.
5Conclusion
It was found that the risk of developing COVID-19 in HCWs significantly increases in household contact with COVID-19 patients. During the patient care, wearing a medical mask by COVID-19 patient and wearing a respiratory mask by the HCW protected the HCW from COVID-19. The impact of using a face shield and gown during close contact with COVID-19 patients should be underlined.
Ethical approval
Ethical approval was obtained from Hacettepe University Non-interventional Clinical Research Ethics Committee (Approval date: 05.22.2020, number: 2020/10-40), and further administrative approval was obtained from Hacettepe University Hospitals Administration (Approval date: 06.05.2020, number: 27043162-000).
Informed consent
All participants provided informed consent prior to enrollment. No identifying information is presented in this work.
Conflict of interest
GM provided consultancy to the United Nations Office of Turkey in the past 36 months. GM received an honorarium from 3M and Pfizer for Congress Lecture and lecture, respectively in the past 36 months. GM has no current declaration of conflict of interest. The other authors report no conflicts of interest relevant to this study.
Acknowledgments
The authors would like to thank all healthcare workers for their devoted work during the pandemic. The results of this study were presented orally at the 9th International Academic Platform of Infectious Diseases and Clinical Microbiology Specialty Society of Turkey, which was held online (20–23 May 2021).
Funding
The authors report no funding.
References
[1] | Novel Coronavirus (2019-nCoV) Situation Report - 1 World Health Organisation: World Health Organisation; (2020) . Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 |
[2] | Chirico F , Sacco A , Nucera G , Ferrari G , Vinci MR , Zaffina S , et al. Lockdown measures and COVID-19 related deaths during the first and second COVID-19 waves in Italy: a descriptive study. J Health Soc Sci. (2021) ;6: (3):379–90. |
[3] | The L . COVID-19: protecting health-care workers. Lancet. (2020) ;395: (10228):922. |
[4] | Barranco R , Ventura F . Covid-19 and infection in health-care workers: An emerging problem. Med Leg J. (2020) ;88: (2):65–6. |
[5] | Chirico F , Leiter M . Tackling stress, burnout, suicide and preventing the “Great resignation” phenomenon among healthcare workers (during and after the COVID-19 pandemic) for maintaining the sustainability of healthcare systems and reaching the Sustainable Development Goals. Journal of Health and Social Sciences. (2022) :9–13. |
[6] | Yapici G , Kurt AO , Solmaz ET , Yeniocak Tunc A , Bozdag F , Bugdayci Yalcin BN , et al. Assessment of COVID-19 Risky Contact of Healthcare Workers in an University Hospital. Mikrobiyol Bul. (2021) ;55: (2):161–79. |
[7] | Ozdemir A , Demir Cuha M , Telli Dizman G , Alp A , Metan G , Sener B . SARS-CoV-2 Seroprevalence Among Healthcare Workers: Retrospective Analysis of the Data From A University Hospital in Turkey. Mikrobiyol Bul. (2021) ;55: (2):223–32. |
[8] | Uyaroğlu OA , Başaran NÇ , Özışık L , Taş Z , Dizman GT , İnkaya AÇ , et al. First Confirmed Cases of novel coronavirus in a university hospital in Turkey: housemate internists. Acta Medica. (2021) ;52: (1):75–81. |
[9] | Telli Dizman G , Metan G , Ayaz Ceylan CM , Altunay H , Uzun M , Gursoy G , et al. A COVID-19 First Evaluation Clinic at a University Hospital in Turkey. Turk J Med Sci. (2021) . |
[10] | Body Mass Index - BMI: World Health Organisation Regional Office for Europe. Available from: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi |
[11] | Turkish Higher Education System: the Council of the Higher Education. Available from: https://wwwstudyinturkey.gov.tr/StudyinTurkey/ShowDetail?rID=Ec/rgHEN8Zg=&&cId=PE4Nr0mMoY4= |
[12] | COVID-19 Kişisel Koruyucu Malzeme Kullanım Rehberi: Hacettepe Üniversitesi Hastaneleri; (2020) . Available from: https://www.hastane.hacettepe.edu.tr/pdf/covid19_bilgilendirme_temmuz_2020.pdf |
[13] | SAVE LIVES - Clean Your Hands: The World Health Organisation; (2021) . Available from: https://www.who.int/campaigns/world-hand-hygiene-day. |
[14] | Garzaro G , Clari M , Ciocan C , Grillo E , Mansour I , Godono A , et al. COVID-19 infection and diffusion among the healthcare workforce in a large university-hospital in northwest Italy. Med Lav. (2020) ;111: (3):184–94. |
[15] | Ogutlu A , Karabay O , Erkorkmaz U , Guclu E , Sen S , Aydin A , et al. Novel coronavirus seropositivity and related factors among healthcare workers at a university hospital during the prevaccination period: a cross-sectional study. Ann Clin Microbiol Antimicrob. (2021) ;20: (1):31. |
[16] | Alkurt G , Murt A , Aydin Z , Tatli O , Agaoglu NB , Irvem A , et al. Seroprevalence of coronavirus disease (COVID-19) among health care workers from three pandemic hospitals of Turkey. PLoS One. (2021) ;16: (3):e0247865. |
[17] | Açıl S . ‘Kontrollü sosyal hayat’a geçişin ilk büyük adımları bugün atılacak: Anadolu Ajansı; (2020) . Available from: ‘Kontrollü sosyal hayat’a geçişin ilk büyük adımları bugün atılacak. |
[18] | Celebi G , Piskin N , Celik Beklevic A , Altunay Y , Salci Keles A , Tuz MA , et al. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control. (2020) ;48: (10):1225–30. |
[19] | Sonmezer MC , Erul E , Sahin TK , Rudvan Al I , Cosgun Y , Korukluoglu G , et al. Seroprevalence of SARS-CoV-2 Antibodies and Associated Factors in Healthcare Workers before the Era of Vaccination at a Tertiary Care Hospital in Turkey. Vaccines (Basel). (2022) :10: (2). |
[20] | Ran L , Chen X , Wang Y , Wu W , Zhang L , Tan X . Risk Factors of Healthcare Workers With Coronavirus Disease A Retrospective Cohort Study in a Designated Hospital of Wuhan in China. Clin Infect Dis. (2020) ;71: (16):2218–21. |
[21] | Lentz RJ , Colt H , Chen H , Cordovilla R , Popevic S , Tahura S , et al. Assessing coronavirus disease (COVID-19) transmission to healthcare personnel: The global ACT-HCP case-control study. Infect Control Hosp Epidemiol. (2021) ;42: (4):381–7. |
[22] | Aytekin E . Türkiye, Kovid-19 salgınına karşı kitlesel tedbirlerde Avrupa’dan erken davrandı: Anadolu Ajansı; (2020) . Available from: https://www.aa.com.tr/tr/koronavirus/turkiye-kovid-19-salginina-karsi-kitlesel-tedbirlerde-avrupa-dan-erken-davrandi/1792042 |
[23] | Irtak K , Parlak T . İstanbul havalimanlarında yurt dışı uçuşları yeniden başladı: Anadolu Ajansı; (2020) . Available from: https://www.aa.com.tr/tr/turkiye/istanbul-havalimanlarinda-yurt-disi-ucuslari-yeniden-basladi/1872857 |
[24] | Chea N , Brown CJ , Eure T , Ramirez RA , Blazek G , Penna AR , et al. Risk Factors for SARS-CoV-2 Infection Among US Healthcare Personnel, May-December 2020. Emerg Infect Dis. (2022) ;28: (1):95–103. |
[25] | Zangoue M , Safari H , Royce SG , Zangooie A , Rezapour H , Zangouei A , et al. The high level of adherence to personal protective equipment in health care workers efficiently protects them from COVID-19 infection. Work. (2021) ;69: (4):1191–6. |
[26] | Abaluck J , Kwong LH , Styczynski A , Haque A , Kabir MA , Bates-Jefferys E , et al. Impact of community masking on COVID-19: A cluster-randomized trial in Bangladesh. Science. (2021) :eabi9069. |
[27] | Unoki T , Tamoto M , Ouchi A , Sakuramoto H , Nakayama A , Katayama Y , et al. Personal Protective Equipment Use by Healthcare Workers in Intensive Care Unit During the COVID-19 Pandemic in Japan: Comparative Analysis With the PPE-SAFE Survey. Acute Med Surg (2020) . |
[28] | 2019-nCoV Hastalığı Sağlık Çalışanları Rehberi 1ed: T.C. Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü; (2020) . |
[29] | Dehghani F , Omidi F , Yousefinejad S , Taheri E . The hierarchy of preventive measures to protect workers against the COVID-19 pandemic: A review. Work. (2020) ;67: (4):771–7. |
[30] | Neuwirth MM , Mattner F , Otchwemah R . Adherence to personal protective equipment use among healthcare workers caring for confirmed COVID-19 and alleged non-COVID-19 patients. Antimicrob Resist Infect Control. (2020) ;9: (1):199. |
[31] | Lamhoot T , Ben Shoshan N , Eisenberg H , Fainberg G , Mhiliya M , Cohen N , et al. Emergency department impaired adherence to personal protective equipment donning and doffing protocols during the COVID-19 pandemic. Isr J Health Policy Res. (2021) ;10: (1):41. |
[32] | Ashinyo ME , Dubik SD , Duti V , Amegah KE , Ashinyo A , Asare BA , et al. Infection prevention and control compliance among exposed healthcare workers in COVID-19 treatment centers in Ghana: A descriptive cross-sectional study. PLoS One. (2021) ;16: (3):e0248282. |
[33] | Powell-Jackson T , King JJC , Makungu C , Spieker N , Woodd S , Risha P , et al. Infection prevention and control compliance in Tanzanian outpatient facilities: a cross-sectional study with implications for the control of COVID-19. Lancet Glob Health. (2020) ;8: (6):e780–e9. |
[34] | Calik Basaran N , Uyaroglu OA , Telli Dizman G , Ozisik L , Sahin TK , Tas Z , et al. Outcome of noncritical COVID-19 patients with early hospitalization and early antiviral treatment outside the ICU. Turk J Med Sci. (2021) ;51: (2):411–20. |
[35] | Nucera G , Chirico F , Raffaelli V , Marino P . Current challenges in COVID-19 diagnosis: a narrative review and implications for clinical practice. Italian Journal of Medicine. (2021) ;15: (3). |



