A double-blind, randomized, controlled study of two dose strengths of dalfampridine extended release on walking deficits in ischemic stroke
Abstract
Background:
Stroke-induced ischemia affects both cortex and underlying white matter. Dalfampridine extended release tablets (D-ER) enhance action potential conduction in demyelinated axons, which may positively affect post-stroke recovery.
Objective:
Based on promising preliminary data, we compared efficacy of D-ER administered at 7.5 mg or 10 mg with placebo on post-stroke ambulation. Primary study outcome (response) was a ≥20% increase on the 2-minute walk test (2 MinWT) at 12 weeks after first drug administration.
Methods:
This was a multicenter, randomized, placebo-controlled, 3-arm, parallel-group, safety and efficacy trial. After obtaining baseline measures of 2 MinWT, Walk-12, and Timed Up and Go, subjects entered a 2-week, single-blind placebo run-in period and were randomized 1:1:1 to receive 7.5 mg D-ER, 10 mg D-ER, or placebo, dosed twice-daily for 12 weeks. Follow-up evaluations occurred at weeks 14 and 16 when subjects were off study drug.
Results:
The study was terminated early with 377 of planned 540 patients enrolled, due to no treatment effect. At week 12, mean increase in distances walked in 2 minutes were similar among the 3 study groups (14.9±40.0 feet; 19.4±39.6 feet; and 20.4±38.3 feet for placebo, 7.5 mg D-ER, and 10 mg D-ER, respectively). The proportion of subjects who showed ≥20% improvement on 2 MinWT at week 12 was 13.5%, 14.0%, and 19.0%, for placebo, 7.5 mg D-ER, and 10 mg D-ER, respectively; these were nonsignificant changes from baseline for all groups.
Conclusions:
D-ER at either a 7.5-mg or 10-mg dose did not significantly increase performance on the 2 MinWT in stroke survivors with gait impairment, although this study was terminated early before full enrollment. (Clinical Trial # NCT02271217).
1Introduction
Stroke remains a leading cause of long-term disability (Benjamin, Blaha, Chiuve, et al., 2017). The ability to “get out and about” into the community is considered essential or very important by 75% of community-dwelling stroke survivors (Lord, McPherson, McNaughton, Rochester, & Weatherall, 2004). Yet, up to two-thirds of this growing population exhibit ambulation difficulties that affect independence and quality of life. Indeed, many community-dwelling stroke survivors exhibit lower extremity deficits that hinder ambulation outside of their homes or communities (Corr, & Bayer, 1992; Hill, Ellis, Bernhardt, Maggs, & Hull, 1997), limiting their ability to resume valued activities (Bohannan, & Larkin, 1985; Pai, Rogers, Hedman, & Hanke, 1994).
Clinicians target lower extremity impairments affecting post-stroke ambulation using a variety of training strategies (e.g., walking using a treadmill) and technologies (e.g., robotics, electrical stimulation, treadmills with specialized belts). However, many approaches have limited evidence supporting their use (Morone, Bragoni, Iosa, et al., 2011; Teasell, Foley, Bhogal, Speechley, 2003). Likewise, regimens integrating technology are usually prohibitive in community settings due to their cost and requirements for specialized equipment.
In addition to the loss of neurons in affected areas of the brain, ischemic stroke is known to produce long-term neurologic impairment, in part by loss of myelin function in affected white matter areas of the nervous system tissue where axons are otherwise spared (Chida, Kokubo, Sato, et al., 2011; Menon, & Shorvon, 2009; Shi, Hu, Leak, et al., 2015). Based on promising findings from other neurological disorders associated with myelin degradation, it has been hypothesized that administration of dalfampridine may improve post-stroke ambulation by potentiating conduction in demyelinated fibers by blocking voltage-dependent potassium channels (Dunn, & Blight, 2011) and also possibly by increasing synaptic transmission and muscle twitch tension, due to the activation of N- and L-type calcium channels (Wu, Li, Chen, & Pan, 2009). This hypothesis was tested in a proof of concept study of dalfampridine involving 83 chronic stroke patients, which showed evidence of improvement in walking using the timed 25-foot walk test (Simpson, Goldenberg, Kasner, et al., 2015).
We sought to confirm and extend these initial findings in a larger study by investigating the effects of 2 dosage strengths of dalfampridine extended release (D-ER) tablets, taken twice-daily for 12 weeks, on the 2-minute walk test (2 MinWT) (Gershon, Wagster, Hendrie, Fox, Cook, & Nowinski, 2013) in subjects with ischemic stroke. The study was terminated early, with 377 of the planned 540 patients enrolled. Analyses of the data from these patients are presented here.
2Methods
2.1Study design, subject recruitment, and study criteria
This was a prospective, multicenter, double-blind, randomized, placebo-controlled, 3-arm, parallel-group trial to evaluate the safety, efficacy, and tolerability of D-ER among individuals with post-ischemic, stroke-related, chronic walking deficits. This study was initially planned as an adaptive design, with a blinded interim analysis to provide information regarding study futility or estimates for potential sample-size increases. The sponsor elected to stop enrollment after 377 participants since an unblinded analysis did not show sufficient efficacy to support further development of D-ER to improve post-stroke walking deficits. The primary endpoint was a comparison of the proportion of subjects in each treatment arm (7.5 mg D-ER, 10 mg D-ER, and placebo) showing ≥20% improvement in the 2 MinWT between baseline and end of the 12-week treatment period. The study occurred at 81 sites across the United States and Canada (Clinical Trial ID # NCT02271217, October 20, 2014). The total study duration for each subject was up to 20 weeks, which included a single-blind, placebo run-in period, double-blind treatment period, and a 4-week off-drug follow-up period. The active double-blind treatment period was 12 weeks (Fig. 1). Inclusion criteria comprised ischemic stroke ≥6 months prior to enrollment, clinical evidence of a stable walking deficit due to ischemic stroke, and Modified Rankin Scale score of 1–3. Exclusion criteria included previous use of dalfampridine, fampridine, or 4-aminopyridine (4-AP), a history of seizures, and diagnosis of multiple sclerosis. Full criteria are detailed in Appendix A. The protocol and informed consent forms were approved by the Institutional Review Board or Independent Ethics Committee at each site before study initiation.
Fig.1
Study design. DB, double blind; D-ER, dalfampridine extended release.
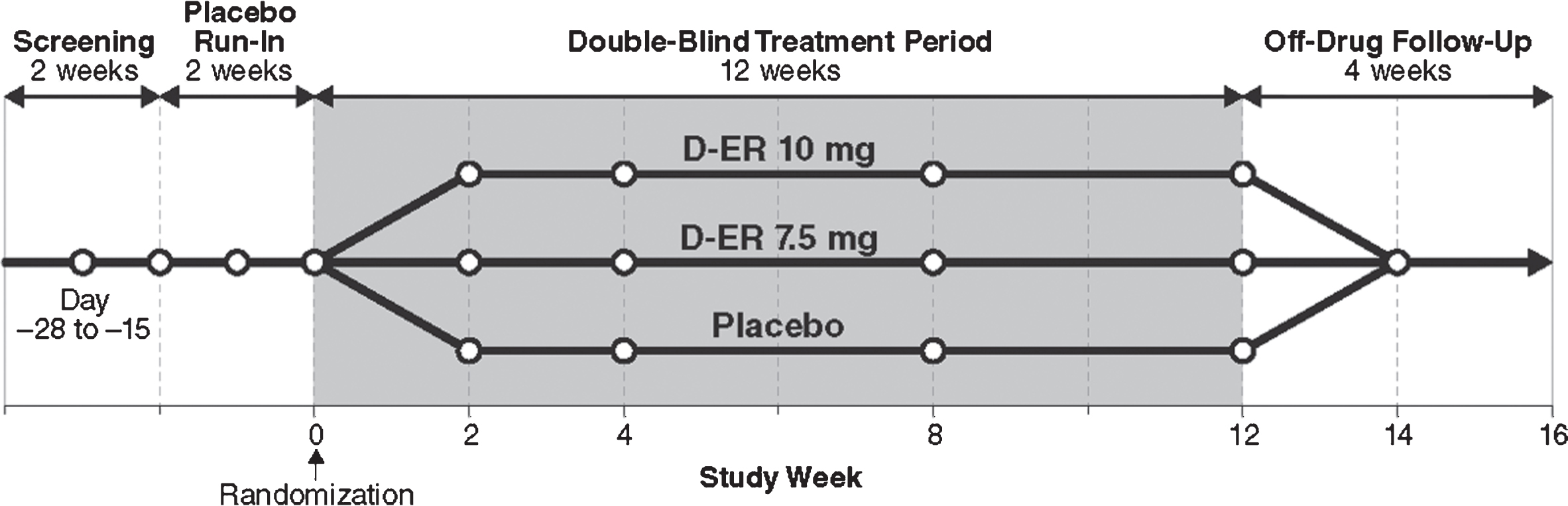
2.2Testing schedule and outcome measures
Week 0 indicates the start of study drug administration, which was preceded by a 2-week placebo run-in. The 2 MinWT was the primary efficacy outcome and was measured at weeks 2, 4, 8, and 12 (Fig. 1). The 2 MinWT measures the distance that subjects can ambulate in 2 minutes and is, thus, considered an endurance measure. The rationale for choosing the 2MinWT as the primary outcome was its excellent psychometric properties, including high inter- and intra-rater reliability, established norms, ease of repeatability across multiple study sites, and clinical relevance to mobility impairment after stroke (Kosak, & Smith, 2005). Subjects were instructed to walk as fast as possible around cones on a marked course, while timed. Distance was measured in feet and inches. Assistive devices (e.g., ankle foot orthoses) were permitted and kept consistent from test to test.
Secondary measures were (a) the Walk-12, administered to ascertain self-perceived limitations in walking and mobility (Hobart, Riazi, Lamping, Fitzpatrick, Thompson, 2003), in which subjects rated limitations in mobility during the preceding 2 weeks using a 5-point scale from 1 (not at all) to 5 (extremely), with a negative change indicative of perceived ambulation improvement, and (b) the Timed Up and Go (TUG), which measures mobility and balance and is predictive of fall risk (Podsiadlo, & Richardson, 1991). In the TUG, subjects were asked to stand up from a chair, walk 10 feet at a comfortable pace, turn around, walk back, and be seated. Timing began from the moment the subject lifted his or her pelvis from the chair until the subject returned pelvis into the chair.
Subjects were also monitored for safety data, collected via adverse event reporting; laboratory tests; vital signs; physical examinations; electrocardiogram; and assessments of suicidality (Columbia-Suicide Severity Rating Scale, C-SSRS) (Posner, Brown, Stanley, et al., 2011).
2.3Randomization and intervention
All tablets were plain (non-debossed) and visually identical among the 3 dosage forms of 7.5 mg and 10 mg D-ER and placebo. Treatment conditions were assigned according to a computer-generated, centralized, randomization scheme created prior to the start of the study. During the study, double-blind treatment assignment was not known by subjects, study personnel at the sites, or to the sponsor staff and monitors.
After giving informed consent and completing screening tests, eligible subjects began a 2-week, single-blind placebo run-in period in which they were instructed to take study drug approximately every 12 hours, until week 0, when subjects were randomized at a ratio of 1:1:1 to receive 7.5 mg D-ER, 10 mg D-ER, or matching placebo tablets, dosed twice-daily for 12 weeks. Subjects took their last dose of study treatment at the end of week 12. Follow-up evaluations occurred at weeks 14 and 16 while subjects were off study drug.
2.4Statistical analysis
Due to early termination of the study, the statistical analysis plan was modified to provide descriptive statistics summarized by treatment for the primary and secondary outcome measures. No formal analyses was performed for this trial; however, a limited post hoc, mixed-model analysis was included for motor-outcome measures in the interest of this report.
3Results
3.1Subject demographics
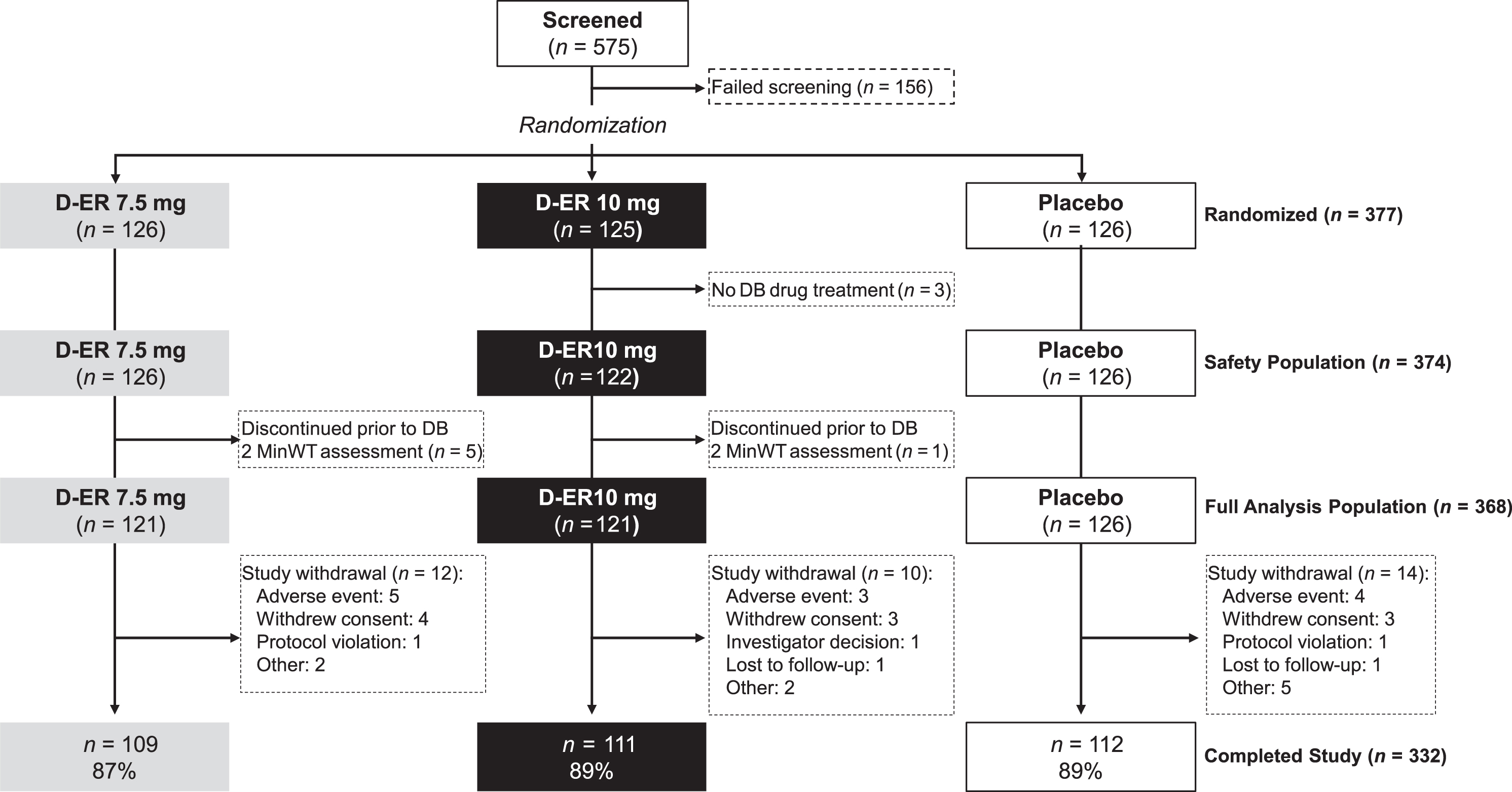
The first patient was enrolled on 23 December 2014, the last subject completed on 16 September 2016, and the study was terminated 21 November 2016. Of the 575 subjects screened for eligibility, 156 were screen failures; 419 were enrolled into the single-blind placebo run-in period, of which 377 were randomized into the double-blind treatment period. The most common reasons for screen failure were not meeting study criteria, disinterest in study participation, and inadequate transportation to make all study visits. Table 1 summarizes the baseline characteristics of the 377 enrolled subjects and Fig. 2 depicts subject flow. Three subjects were randomized but discontinued the study prior to receiving double-blind study treatment, while an additional 6 subjects discontinued the study prior to 2MinWT assessment during the double-blind period. Forty-two subjects started the placebo run-in, but elected not to continue with the second phase of the study and, thus, were not randomized. Of the subjects who withdrew, one was randomized to 10 mg D-ER; all others were randomized to 7.5 mg D-ER. Thus, the full analysis population described below was comprised of 368 subjects.
Table 1
Demographic and Stroke Characteristics of Randomized Sample
| Placebo | D-ER 7.5 mg | D-ER 10 mg | Total | |
| Characteristic at screening | (n = 126) | (n = 126) | (n = 125) | (n = 377) |
| Age, years | ||||
| Mean (SE) | 62.9 (1.04) | 61.7 (0.93) | 64.3 (0.90) | 62.9 (0.55) |
| SD | 11.68 | 10.48 | 10.01 | 10.77 |
| Median (min, max) | 64 (26, 86) | 63 (24, 85) | 65 (34, 86) | 64 (24, 86) |
| Sex, n (%) | ||||
| Male | 87 (69.0%) | 75 (59.5%) | 79 (63.2%) | 241 (63.9%) |
| Female | 39 (31.0%) | 51 (40.5%) | 46 (36.8%) | 136 (36.1%) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 18 (14.3%) | 21 (16.7%) | 20 (16.0%) | 59 (15.6%) |
| Not Hispanic or Latino | 108 (85.7%) | 105 (83.3%) | 105 (84.0%) | 318 (84.4%) |
| Race, n (%) | ||||
| White | 100 (79.4%) | 105 (83.3%) | 94 (75.2%) | 299 (79.3%) |
| Black/African American | 18 (14.3%) | 12 (9.5%) | 21 (16.8%) | 51 (13.5%) |
| Asian | 3 (2.4%) | 4 (3.2%) | 2 (1.6%) | 9 (2.4%) |
| Native Hawaiian or other Pacific Islander | 0 (0.0%) | 0 (0.0%) | 2 (1.6%) | 2 (0.5%) |
| American Indian/Alaskan Native | 3 (2.4%) | 1 (0.8%) | 2 (1.6%) | 6 (1.6%) |
| Other | 2 (1.6%) | 4 (3.2%) | 4 (3.2%) | 10 (2.7%) |
| Weight (kg) | ||||
| Mean (SE) | 84.0 (1.3) | 82.4 (1.3) | 84.5 (1.3) | 83.6 (0.8) |
| SD | 14.9 | 14.5 | 14.8 | 14.7 |
| Median (min, max) | 83.3 (48.8, 117.0) | 80.7 (49.0, 124.3) | 83.5 (55.8, 122.0) | 82.2 (48.8, 124.3) |
| Height (cm) | ||||
| Mean (SE) | 172.4 (0.9) | 170.1 (1.0) | 171.7 (0.8) | 171.4 (0.5) |
| SD | 9.7 | 11.0 | 8.9 | 9.9 |
| Median (min, max) | 171.3 (144.8, 196.1) | 170.2 (127.5, 195.6) | 170.2 (153.0, 193.0) | 170.3 (127.5, 196.1) |
| BMI | ||||
| Mean (SE) | 28.16 (0.340) | 28.42 (0.329) | 28.55 (0.338) | 28.37 (0.194) |
| SD | 3.82 | 3.689 | 3.783 | 3.758 |
| Median (min, max) | 28.3 (18.2, 34.9) | 28.2 (18.0, 38.0) | 28.3 (20.3, 35.0) | 28.3 (18.0, 38.0) |
| Number of strokes | ||||
| Mean (SE) | 1.2 (0.04) | 1.2 (0.04) | 1.2 (0.05) | 1.2 (0.02) |
| SD | 0.46 | 0.43 | 0.54 | 0.48 |
| Median (min, max) | 1 (1, 3) | 1 (1, 3) | 1 (1, 4) | 1 (1, 4) |
| Type of most recent stroke, n (%) | ||||
| Ischemic | 119 (94.4%) | 120 (95.2%) | 120 (96.0%) | 359 (95.2%) |
| Transient ischemic attack | 3 (2.4%) | 3 (2.4%) | 0 (0.0%) | 6 (1.6%) |
| Other | 4 (3.2%) | 3 (2.4%) | 5 (4.0%) | 12 (3.2%) |
| Duration since most recent stroke (months) | ||||
| Mean (SE) | 56.2 (5.15) | 55.5 (5.66) | 54.7 (5.11) | 55.5 (3.06) |
| SD | 57.84 | 63.48 | 57.18 | 59.42 |
| Median (min, max) | 38.2 (6, 382) | 31.2 (6, 328) | 35.5 (6, 402) | 35 (6, 402) |
| Location of most recent stroke, n (%) | ||||
| Internal carotid artery | 2 (1.6%) | 5 (4.0%) | 2 (1.6%) | 9 (2.4%) |
| Middle cerebral artery | 54 (42.9%) | 43 (34.1%) | 46 (36.8%) | 143 (37.9%) |
| Anterior cerebral artery | 1 (0.8%) | 3 (2.4%) | 1 (0.8%) | 5 (1.3%) |
| Posterior cerebral artery | 2 (1.6%) | 5 (4.0%) | 4 (3.2%) | 11 (2.9%) |
| Vertebral | 2 (1.6%) | 3 (2.4%) | 2 (1.6%) | 7 (1.9%) |
| Basilar | 6 (4.8%) | 9 (7.1%) | 9 (7.2%) | 24 (6.4%) |
| Other | 48 (38.1%) | 38 (30.2%) | 49 (39.2%) | 135 (35.8%) |
| Unknown | 11 (8.7%) | 20 (15.9%) | 12 (9.6%) | 43 (11.4%) |
| Primary affected side of body, n (%) | ||||
| Right | 61 (48.4%) | 65 (51.6%) | 57 (45.6%) | 183 (48.5%) |
| Left | 65 (51.6%) | 60 (47.6%) | 66 (52.8%) | 191 (50.7%) |
| Missing | 0 (0.0%) | 1 (0.8%) | 2 (1.6%) | 3 (0.8%) |
| Area of brain affected by most recent stroke, n (%) | ||||
| Cortical | 44 (34.9%) | 40 (31.7%) | 53 (42.4%) | 137 (36.3%) |
| Subcortical | 56 (44.4%) | 65 (51.6%) | 63 (50.4%) | 184 (48.8%) |
| Unknown | 26 (20.6%) | 21 (16.7%) | 7 (5.6%) | 54 (14.3%) |
| Missing | 0 (0.0%) | 0 (0.0%) | 2 (1.6%) | 2 (0.5%) |
D-ER, dalfampridine extended release; SE, standard error.
Fig.2
MILESTONE patient disposition. DB, double blind; D-ER, dalfampridine extended release.
3.2Motor outcomes
Table 2 summarizes the proportion of responders on the 2MinWT by visit. The percentages of responders at week 12 attaining≥20% improvement on the 2MinWT were 13.5% (n = 17 of 126) for placebo, 14.0% (n = 17 of 121) for 7.5 mg D-ER, and 19.0% (n = 23 of 121) for 10 mg D-ER. The mean (SD) increase in 2 MinWT distance from baseline was 14.9 (40.0) feet for placebo, 19.4 (39.6) feet for 7.5 mg D-ER, and 20.4 (38.3) feet for 10 mg D-ER (Table 2). By post hoc analysis of mixed-model least squares means, these were nonsignificant changes (Table 3).
Table 2
Number (%) of Responders with ≥ 20% Increase on 2 MinWT by Visit and Group
| Placebo | D-ER 7.5 mg | D-ER 10 mg | ||
| Visit | (n = 126) | (n = 121) | (n = 121) | |
| Week 2 | Responder, n (%) | 11 (8.7) | 15 (12.4) | 12 (9.9) |
| Nonresponder, n (%) | 115 (91.3) | 106 (87.6) | 109 (90.1) | |
| Week 4 | Responder, n (%) | 19 (15.1) | 16 (13.2) | 18 (14.9) |
| Nonresponder, n (%) | 101 (80.2) | 104 (86.0) | 101 (83.5) | |
| Missing, n (%) | 6 (4.8) | 1 (0.8) | 2 (1.7) | |
| Week 8 | Responder, n (%) | 16 (12.7) | 18 (14.9) | 20 (16.5) |
| Nonresponder, n (%) | 103 (81.7) | 98 (81.0) | 97 (80.2) | |
| Missing, n (%) | 7 (5.6) | 5 (4.1) | 4 (3.3) | |
| Week 12 | Responder, n (%) | 17 (13.5) | 17 (14.0) | 23 (19.0) |
| Nonresponder, n (%) | 97 (77.0) | 96 (79.3) | 90 (74.4) | |
| Missing, n (%) | 12 (9.5) | 8 (6.6) | 8 (6.6) | |
| Week 14 | Responder, n (%) | 16 (12.7) | 15 (12.4) | 15 (12.4) |
| Nonresponder, n (%) | 97 (77.0) | 93 (76.9) | 97 (80.2) | |
| Missing, n (%) | 13 (10.3) | 13 (10.7) | 9 (7.4) | |
| Week 16 | Responder, n (%) | 17 (13.5) | 17 (14.0) | 16 (13.2) |
| Nonresponder, n (%) | 95 (75.4) | 93 (76.9) | 96 (79.3) | |
| Missing, n (%) | 14 (11.1) | 11 (9.1) | 9 (7.4) |
D-ER, dalfampridine extended release; 2 MinWT, 2-Minute Walk Test.
Table 3
Post Hoc Mixed-Model Analysis of Least Squares Mean Change From Baseline in Motor Tests
| Test | Placebo | D-ER 7.5 mg | D-ER 10 mg |
| 2 MinWT | 14.9 | 19.3 (P = 0.39) | 20.5 (P = 0.28) |
| Walk-12 | –5.63 | –2.90 (P = 0.18) | –1.56 (P = 0.05) |
| TUG | –0.45 | –0.43 (P = 1.00) | –0.34 (P = 0.89) |
P-values compared with placebo. 2 MinWT, 2-Minute Walk Test; D-ER, dalfampridine extended release; TUG, Timed Up and Go; Walk-12, 12-Item Multiple Sclerosis Walking Scale.
The secondary study objectives were to evaluate the effects of D-ER at week 12 on patient-reported walking disability using the Walk-12, and mobility and balance as measured by TUG. The mean (SD) Walk-12 scores were 45.4 (23.0) for placebo, 48.3 (25.4) for 7.5 mg D-ER, and 49.3 (26.0) for 10 mg D-ER, representing changes from baseline of –5.78, –3.01, and –1.49, respectively. A post hoc analysis of mixed-model least squares means showed a significant difference between the 10 mg D-ER group and placebo (P < 0.05) (Table 3). Mean (SD) changes in TUG were –0.40 (4.5), –0.48 (6.0), and –0.25 (7.4) (placebo, 7.5 mg D-ER, and 10 mg D-ER, respectively). A post hoc analysis of mixed-model least squares means showed no significant differences.
3.3Safety and tolerability
No new safety signal was observed (Table 4). D-ER was well-tolerated by the 248 subjects who were treated with 7.5 mg or 10 mg twice daily. The most common (≥3%) treatment-emergent adverse events (TEAEs) for D-ER–treated subjects were fall, urinary tract infection, dizziness, nasopharyngitis, and headache. The most common TEAEs for placebo were fatigue, nasopharyngitis, fall, arthralgia, pain in extremity, back pain, headache, and hypertension. These events are consistent with the characteristics of the post-stroke population, or with known adverse events in past clinical studies administering D-ER. Seizure, which is a known risk with D-ER, did not occur in the D-ER group, but did occur in one placebo-treated subject. No hepatic abnormalities were observed, and no deaths were reported.
Table 4
Frequency (≥1% of All D-ER–Treated Subjects) of Treatment-Emergent Adverse Events by System Organ Class and Preferred Term
| System Organ Class/Preferred Terma | Placebo (n = 126) | D-ER 7.5 mg (n = 126) | D-ER 10 mg (n = 122) | Overall D-ER (n = 248) |
| Subjects with any TEAE, n (%) | 75 (59.5%) | 85 (67.5%) | 75 (61.5%) | 160 (64.5%) |
| Gastrointestinal Disorders | ||||
| Diarrhea | 3 (2.4%) | 3 (2.4%) | 3 (2.5%) | 6 (2.4%) |
| Nausea | 2 (1.6%) | 2 (1.6%) | 2 (1.6%) | 4 (1.6%) |
| Vomiting | 1 (0.8%) | 0 (0.0%) | 3 (2.5%) | 3 (1.2%) |
| General Disorders and Administration Site Conditions | ||||
| Fatigue | 8 (6.3%) | 4 (3.2%) | 3 (2.5%) | 7 (2.8%) |
| Gait disturbance | 0 (0.0%) | 6 (4.8%) | 0 (0.0%) | 6 (2.4%) |
| Asthenia | 0 (0.0%) | 4 (3.2%) | 0 (0.0%) | 4 (1.6%) |
| Edema peripheral | 1 (0.8%) | 1 (0.8%) | 2 (1.6%) | 3 (1.2%) |
| Infections and Infestations | ||||
| Urinary tract infection | 3 (2.4%) | 8 (6.3%) | 11 (9.0%) | 19 (7.7%) |
| Nasopharyngitis | 5 (4.0%) | 4 (3.2%) | 5 (4.1%) | 9 (3.6%) |
| Upper respiratory tract infection | 3 (2.4%) | 4 (3.2%) | 1 (0.8%) | 5 (2.0%) |
| Bronchitis | 1 (0.8%) | 1 (0.8%) | 2 (1.6%) | 3 (1.2%) |
| Gastroenteritis viral | 1 (0.8%) | 2 (1.6%) | 1 (0.8%) | 3 (1.2%) |
| Influenza | 1 (0.8%) | 1 (0.8%) | 2 (1.6%) | 3 (1.2%) |
| Injury, Poisoning, and Procedural Complications | ||||
| Fall | 7 (5.6%) | 12 (9.5%) | 13 (10.7%) | 25 (10.1%) |
| Contusion | 1 (0.8%) | 3 (2.4%) | 4 (3.3%) | 7 (2.8%) |
| Excoriation | 3 (2.4%) | 2 (1.6%) | 4 (3.3%) | 6 (2.4%) |
| Ligament sprain | 0 (0.0%) | 0 (0.0%) | 3 (2.5%) | 3 (1.2%) |
| Investigations | ||||
| Blood glucose increased | 0 (0.0%) | 2 (1.6%) | 1 (0.8%) | 3 (1.2%) |
| Musculoskeletal and Connective Tissue Disorders | ||||
| Arthralgia | 4 (3.2%) | 2 (1.6%) | 5 (4.1%) | 7 (2.8%) |
| Pain in extremity | 5 (4.0%) | 2 (1.6%) | 5 (4.1%) | 7 (2.8%) |
| Back pain | 6 (4.8%) | 1 (0.8%) | 4 (3.3%) | 5 (2.0%) |
| Muscular weakness | 3 (2.4%) | 2 (1.6%) | 2 (1.6%) | 4 (1.6%) |
| Neck pain | 0 (0.0%) | 2 (1.6%) | 2 (1.6%) | 4 (1.6%) |
| Muscle spasms | 1 (0.8%) | 0 (0.0%) | 3 (2.5%) | 3 (1.2%) |
| Musculoskeletal pain | 2 (1.6%) | 2 (1.6%) | 1 (0.8%) | 3 (1.2%) |
| Myalgia | 1 (0.8%) | 1 (0.8%) | 2 (1.6%) | 3 (1.2%) |
| Nervous System Disorders | ||||
| Dizziness | 3 (2.4%) | 10 (7.9%) | 4 (3.3%) | 14 (5.6%) |
| Headache | 6 (4.8%) | 6 (4.8%) | 2 (1.6%) | 8 (3.2%) |
| Balance disorder | 0 (0.0%) | 2 (1.6%) | 3 (2.5%) | 5 (2.0%) |
| Psychiatric Disorders | ||||
| Depression | 2 (1.6%) | 2 (1.6%) | 2 (1.6%) | 4 (1.6%) |
| Insomnia | 2 (1.6%) | 1 (0.8%) | 2 (1.6%) | 3 (1.2%) |
| Respiratory, Thoracic, and Mediastinal Disorders | ||||
| Cough | 1 (0.8%) | 2 (1.6%) | 5 (4.1%) | 7 (2.8%) |
| Skin and Subcutaneous Tissue Disorders | ||||
| Rash | 0 (0.0%) | 3 (2.4%) | 2 (1.6%) | 5 (2.0%) |
| Vascular Disorders | 7 (5.6%) | 3 (2.4%) | 5 (4.1%) | 8 (3.2%) |
| Hypertension | 5 (4.0%) | 2 (1.6%) | 4 (3.3%) | 6 (2.4%) |
aSystem organ class expanded to show preferred terms ≥1%. D-ER, dalfampridine extended release; TEAE, treatment-emergent adverse event.
4Discussion
D-ER is a broad-spectrum blocker of voltage-dependent potassium channels, and studies have shown improvement in action potential conduction in demyelinated axons at concentrations as low as 1 Mm (Dunn & Blight, 2011). Concurrently, therapeutic plasma concentrations associated with improved ambulation are observed when mean plasma concentrations are ∼0.25μM. The effects of D-ER at this low concentration appear to vary, and it is presumed that potassium channels on affected, demyelinated axons in stroke are sensitive to blockade at the lower concentrations of D-ER that would correspond to plasma levels achieved clinically in patients (Dunn & Blight, 2011). To provide more information on optimal dosing, the current study examined the primary effect of two doses of D-ER on ambulation in chronic stroke survivors. Our experimental hypothesis was that a greater proportion of subjects in the D-ER group, compared with subjects in the placebo group, would achieve ≥20% increase in the 2 MinWT distance between baseline and end of the 12-week treatment period, and that the higher dose would be more effective than the lower dose.
Despite promising preliminary data and full compliance with the provision of D-ER at the prescribed doses, we found limited effects associated with either D-ER dosage, as compared with placebo, on any measure. There were no treatment-emergent adverse events. It is of note that the study design controlled for the effect of many confounding factors. In particular, subjects were in the chronic phase of stroke, when changes in 2 MinWT distance through spontaneous recovery would be unlikely. Furthermore, subjects received no adjunctive therapies to D-ER that could separately influence outcomes. Within these constraints, results were unlikely to be due to chance, making rejection of our experimental hypothesis justified. However, this study was terminated early, with 377 of the planned 540 patients enrolled.
That said, a question to consider is whether there are subpopulations of stroke survivors who might demonstrate benefit from D-ER administration. For instance, given the hypothesized mechanisms associated with this approach, one may speculate that corticospinal tract integrity (CST) and/or brain white matter tract integrity could, at the minimum, affect response and perhaps even be a study criterion for future efficacy trials testing this approach. Any changes in CST as a result of a novel pharmacological intervention could be readily measured and correlated with motor changes. Indeed, one might expect a relationship between presence (or absence) of sufficient axons in white matter tracts, action potential propagation, and/or motor response that would impact CST integrity and, ultimately, measures of lower extremity motor control and/or ambulation. Accordingly, measures of myelination should be considered for future studies. Concurrently, targeting the therapy toward a particular interval of vulnerability post-stroke, (e.g., the first week, when demyelination and Wallerian degeneration have been shown to occur), may also prove more efficacious than applying D-ER to all chronic stroke survivors, and should be influential in future study designs.
Finally, the proof-of concept study that led to the current investigation applied the Fugl-Meyer (FMA), box and block, grip test, and 25-foot walk test to demonstrate effects of D-ER. In deciding to focus solely on the lower extremity, the current study did not repeat any of these outcome measures. Moreover, considering the hypothesis that D-ER would result in improved conduction along de-myelinated axons, an impairment-based measure such as the lower extremity FMA would be a strong choice as it would discriminate changes in motor control. Future studies should examine multiple domains of impairment, functional limitation, and participation in both the UE and the LE to ascertain changes attributable to the provision of D-ER.
5Conclusions
Despite promising preliminary data, D-ER at either 7.5 mg or 10 mg did not meet the primary endpoint of increased walking ability in chronic stroke patients, compared with placebo control.
Conflict of interest statement
Stephen J. Page received grant support for the conduct of this trial from Acorda Therapeutics.
Scott E. Kasner received grant support for the conduct of this trial from Acorda Therapeutics.
Marcia Bockbrader received grant support for the conduct of this trial from Acorda Therapeutics.
Mark Goldstein has received grant funding for Acorda Therapeutics clinical trials for Eli Lilly, Biogen, Genentech, Roche, Sunovion, Pfizer, and Novartis.
Seth P. Finklestein received grant support for the conduct of this trial from Acorda Therapeutics, andis a Consultant to Constant Pharmaceuticals, NeuroVasc, and AZTherapies, and is a Principal at Stemetix.
MingMing Ning participated in this clinical trial supported by Acorda Therapeutics.
Waleed El-Feky received grant support for the conduct of this trial from Acorda Therapeutics, and is a speaker for Allergan.
Christina A. Wilson has received royalties for stroke-related articles from UpToDate and Medlink Neurology.
Holly Roberts is an employee of, and owns stock in, Acorda Therapeutics, Inc.
Appendices
Appendix A
Inclusion criteria included: men or women aged 18 years or older who experienced an ischemic stroke≥6 months prior to enrollment; clinical evidence of a stable walking deficit due to an ischemic stroke, as judged by the investigator, based on review of medical records and physical exam; Modified Rankin Scale score of 1–3; sufficient ambulatory ability to independently complete the 2 MinWT and the 10 MWT at screening; and body mass index from 18–35 kg/m2 inclusive.
Key exclusion criteria included: previous use of dalfampridine, fampridine, or 4-aminopyridine (4-AP); a history of seizures, except simple febrile seizures; moderate to severe renal impairment; severe depression as determined by the Beck Depression Inventory (BDI) score ≥30; diagnosis of multiple sclerosis or a medical or neurological disorder that would interfere with the assessments during the study.
Acknowledgments
This study was sponsored by Acorda Therapeutics. No remuneration was received for the writing of this manuscript. Initial copyediting and figure preparation were provided by The Curry Rockefeller Group, LLC, Tarrytown, NY, funded by Acorda Therapeutics.
References
1 | Benjamin, E.J. , Blaha, M.J. , Chiuve, S.E. , et al. ((2017) ) Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association, Circulation, 201: , e146–e603. doi: 10.1161/CIR.0000000000000485 |
2 | Bohannan, R. , Larkin, P. ((1985) ) Lower extremity weight bearing under various standing conditions in independently ambulatory patients with hemiplegia, Physical Therapy, 65: , 1323–1325. doi: 10.1093/ptj/65.9.1323 |
3 | Chida, Y. , Kokubo, Y. , Sato, S. , et al. ((2011) ) The alterations of oligodendrocyte, myelin in corpus callosum, and cognitive dysfunction following chronic cerebral ischemia in rats, Brain Research, 1414: , 22–31. doi: 10.1016/j.brainres.2011.07.026 |
4 | Corr, S. , Bayer, A. ((1992) ) Poor functional status of stroke patients after hospital discharge: scope for intervention? British Journal of Occupational Therapy, 55: , 383–385. |
5 | Dunn, J. , Blight, A. ((2011) ) Dalfampridine: a brief review of its mechanism of action and efficacy as a treatment to improve walking in patients with multiple sclerosis, Current Medical Research and Opinion, 27: , 1415–1423. doi: 10.1185/03007995.2011.583229 |
6 | Gershon,R.C., Wagster,M.V., Hendrie,H.C., et al. ((2013) ) NIH toolbox for assessment of neurological and behavioral function, Neurology, 80: ((11 Suppl 3)), S2–6. doi: 10.1212/WNL.0b013e3182872e5f |
7 | Hill, K. , Ellis, P. , Bernhardt, J. , et al. ((1997) ) Balance and mobility outcomes for stroke patients: a comprehensive audit, Australian Journal of Physiotherapy, 43: , 173–180. doi: 10.1016/s0004-9514(14)60408-6 |
8 | Hobart, J.C. , Riazi, A. , Lamping, D.L. , et al. ((2003) ) Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12), Neurology, 60: , 31–36. doi: 10.1212/wnl.60.1.31 |
9 | Kosak, M. , Smith, T. ((2005) ) Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke, Journal of Rehabilitation Research and Development, 42: , 103–107. doi: 10.1682/jrrd.2003.11.0171 |
10 | Lord, S.E. , McPherson, K. , McNaughton, H.K. , et al. ((2004) ) Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Archives of Physical Medicine and Rehabilitation, 85: , 234–239. doi: 10.1016/j.apmr.2003.05.002 |
11 | Menon, B. , Shorvon, S.D. ((2009) ) Ischaemic stroke in adults and epilepsy, Epilepsy Research, 87: , 1–11. doi: 10.1016/j.eplepsyres.2009.08.007 |
12 | Morone, G. , Bragoni, M. , Iosa, M. ((2011) ) Who may benefit from robotic-assisted gait training? A randomized clinical trial in patients with subacute stroke, Neurorehabilitation and Neural Repair, 25: , 636–644. doi: 10.1177/1545968311401034 |
13 | Pai, Y.C. , Rogers, M.W. , Hedman, L.D. , et al. ((1994) ) Alterations in weight-transfer capabilities in adults with hemiparesis, Physical Therapy, 74: , 647–657; discussion 657-659. doi: 10.1093/ptj/74.7.647 |
14 | Podsiadlo, D. , Richardson, S. ((1991) ) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons, Journal of the American Geriatric Society, 39: , 142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x |
15 | Posner, K. , Brown, G.K. , Stanley, B. , et al. ((2011) ) The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults, American Journal of Psychiatry, 168: , 1266–1277. doi: 10.1176/appi.aj2011.10111704 |
16 | Shi, H. , Hu, X. , Leak, R.K. , et al. ((2015) ) Demyelination as a rational therapeutic target for ischemic or traumatic brain injury, Experimental Neurology, 272: , 17–25. doi: 10.1016/j.expneurol.2015.03.017 |
17 | Simpson, D.M. , Goldenberg, J. , Kasner, S. , et al. ((2015) ) Dalfampridine in chronic sensorimotor deficits after ischemic stroke: a proof of concept study, Journal of Rehabilitation Medicine, 47: , 924–931. doi: 10.2340/16501977-2033 |
18 | Teasell, R.W. , Foley, N.C. , Bhogal, S.K. , et al. ((2003) ) An evidence-based review of stroke rehabilitation, Topics in Stroke Rehabilitation, 10: , 29–58. doi: 10.1310/8YNA-1YHK-YMHB-XTE1 |
19 | Thomalla, G. , Glauche, V. , Weiller, C. , et al. ((2005) ) Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging, Journal of Neurology, Neurosurgery, and Psychiatry, 76: , 266–268. doi: 10.1136/jnn2004.046375 |
20 | Wu, Z.Z. , Li, D.P. , Chen, S.R. , Pan, H.L. ((2009) ) Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit, Journal of Biological Chemistry, 284: , 36453–36461. doi: 10.1074/jbc.M109.075523 |




