Hyperbaric oxygen therapy improves neurocognitive functions of post-stroke patients – a retrospective analysis
Abstract
Background:
Previous studies have shown that hyperbaric oxygen therapy (HBOT) can improve the motor functions and memory of post-stroke patients in the chronic stage.
Objective:
The aim of this study is to evaluate the effects of HBOT on overall cognitive functions of post-stroke patients in the chronic stage. The nature, type and location of the stroke were investigated as possible modifiers.
Methods:
A retrospective analysis was conducted on patients who were treated with HBOT for chronic stroke (>3 months) between 2008-2018. Participants were treated in a multi-place hyperbaric chamber with the following protocols: 40 to 60 daily sessions, 5 days per week, each session included 90 min of 100% oxygen at 2 ATA with 5 min air brakes every 20 minutes. Clinically significant improvements (CSI) were defined as > 0.5 standard deviation (SD).
Results:
The study included 162 patients (75.3% males) with a mean age of 60.75±12.91. Of them, 77(47.53%) had cortical strokes, 87(53.7%) strokes were located in the left hemisphere and 121 suffered ischemic strokes (74.6%).
HBOT induced a significant increase in all the cognitive function domains (p < 0.05), with 86% of the stroke victims achieving CSI. There were no significant differences post-HBOT of cortical strokes compared to sub-cortical strokes (p > 0.05). Hemorrhagic strokes had a significantly higher improvement in information processing speed post-HBOT (p < 0.05). Left hemisphere strokes had a higher increase in the motor domain (p < 0.05). In all cognitive domains, the baseline cognitive function was a significant predictor of CSI (p < 0.05), while stroke type, location and side were not significant predictors.
Conclusions:
HBOT induces significant improvements in all cognitive domains even in the late chronic stage. The selection of post-stroke patients for HBOT should be based on functional analysis and baseline cognitive scores rather than the stroke type, location or side of lesion.
1Introduction
Stroke is the second-most cause of mortality and the third leading cause for disability, worldwide (Langhorne, Bernhardt, & Kwakkel, 2011; Lozano et al., 2012; Ojaghihaghighi, Vahdati, Mikaeilpour, & Ramouz, 2017; Ottenbacher & Jannell, 1993; Powers et al., 2018). When strokes transpire, whether they are ischemic or hemorrhagic, the injured brain region correlates with its related loss of function which may be visual, motor, sensory or cognitive impairments. Most stroke studies focus on motor functions. However, it is estimated that nearly half of the survivors suffer from different degrees of cognitive dysfunction (Kelly-Hayes et al., 2003; Lee, Joshi, Wang, Pashos, & Christensen, 2007; Yoneda et al., 2005).
The two leading subtypes of stroke are ischemic stroke, in 68% of the cases, and the less frequent hemorrhagic stroke, in 32% of the cases (Caplan, 1989; Krishnamurthi et al., 2013; Powers et al., 2018; Zhang, Lo, Mychaskiw, & Colohan, 2005). Even though the two pathophysiological processes are diametrically opposed during the initiation phase, in the subacute chronic phase they culminate in comprised blood supply and subsequent brain ischemia (Caplan, 1989; Krishnamurthi et al., 2013; Powers et al., 2018). When the insult results in cognitive dysfunction, usually more than one cognitive domain is involved such as memory, attention and visual spatial (VS) (Al-Qazzaz, Ali, Ahmad, Islam, & Mohamad, 2014; Cumming, Marshall, & Lazar, 2013). The significant factors that affect the cognitive impairments’ severity are older age, previous history of stroke, and the pre-injury global cognitive function (GCF) (Ballard, Rowan, Stephens, Kalaria, & Kenny, 2003; Mok et al., 2004; Patel, Coshall, Rudd, & Wolfe, 2003; Rasquin, Verhey, van Oostenbrugge, Lousberg, & Lodder, 2004). It has been shown that hemorrhagic strokes cause significantly more cognitive impairments compared to ischemic strokes, and are more associated with cognitive deficits across multiple domains (Cumming et al., 2013). Cortical strokes were found with higher proportions of cognitive impairments in the memory domain than subcortical ones (Lange, Waked, Kirshblum, & DeLuca, 2000; Nys et al., 2007; Schouten, Schiemanck, Brand, & Post, 2009). Yet, higher cortical functions such as expressive aphasia were significantly impaired in subcortical stroke patients as well as lower performances in the information processing speed (IPS) domain compared with cortical stroke patients (Lange et al., 2000; Nys et al., 2007; T. Wagner & A. Cushman, 2017). With respect to dominant vs. non-dominant hemispheric lesion, there is evidence of a more severe cognitive impairments and an overall higher incidence of dementia following an insult in the dominant hemisphere (Censori et al., 1996; de Oliveira, Correia Marin Sde, & Ferreira Bertolucci, 2013; Tatemichi et al., 1993).
Reducing the impact of post-stroke cognitive impairment is an important goal due to the higher mortality and institutionalization rates of those patients (Pasquini, Leys, Rousseaux, Pasquier, & Henon, 2007; Tatemichi et al., 1994). Rehabilitation includes a multidisciplinary approach which includes physiotherapy, speech and language therapy, cognitive rehabilitation therapy, medications and more. However, these programs have limited success (Hebert et al., 2016; Prvu Bettger & Stineman, 2007; Roine, Kajaste, & Kaste, 1993; Williams, Jiang, Matchar, & Samsa, 1999). Cognitive recovery after stroke occurs mainly within the first 30 days, with some post-stroke patients continuing to gain progress up to three months from injury, yet even with domain specific interventions, improvement is minimal (Langhorne et al., 2011; Maulden, Gassaway, Horn, Smout, & DeJong, 2005; Ovbiagele & Nguyen-Huynh, 2011).
Hyperbaric oxygen therapy (HBOT), the application of hyperbaric pressure in conjunction with increased oxygen content, has been shown in several clinical studies to have the capacity to induce neuroplasticity even years after an acute insult (Boussi-Gross et al., 2013; Boussi-Gross et al., 2015; Efrati & Ben-Jacob, 2014; Efrati et al., 2013; Efrati et al., 2015; Hadanny & Efrati, 2016; Hadanny, Fishlev, Bechor, Meir, & Efrati, 2016; Hadanny et al., 2015a, 2015b; Tal et al., 2015a, 2015b; Tal, Hadanny, Sasson, Suzin, & Efrati, 2017; Yildiz et al., 2004). The elevated oxygen concentration in the blood and injured tissue during treatment (Calvert, Cahill, & Zhang, 2007; Niklas, Brock, Schober, Schulz, & Schneider, 2004; Reinert et al., 2003) helps supply the energy needed to regenerate damaged brain tissue. It has been shown that HBOT induced neuroplasticity is mediated by stimulating cell proliferation (Mu et al., 2013), neurogenesis of endogenous neural stem cells (Yang et al., 2008), regeneration of axonal white matter (Chang et al., 2009), improved maturation and myelination of injured neural fibers (Haapaniemi, Nylander, Kanje, & Dahlin, 1998; Vilela, Lazarini, & Da Silva, 2008), and stimulation of axonal growth, thus increasing the ability of neurons to function and communicate with each other (Bradshaw, Nelson, Fanton, Yates, & Kagan-Hallet, 1996; Mukoyama, Iida, & Sobue, 1975). A retrospective analysis of post-stroke patients in the late chronic stage revealed that HBOT can significantly improve the memory domain (Boussi-Gross et al., 2015). However, the overall neurocognitive effects of HBOT and its relation to the different stroke types and anatomical locations were not investigated yet.
The aim of the current study is to investigate the effects of HBOT on the overall cognitive domains of post-stroke patients in the late chronic stage. The nature, type and location of the stroke as possible modifiers of HBOT effects were also investigated.
2Methods
2.1Participants
A retrospective study including post-stroke patients, more than three months post-injury, treated with HBOT between January 2008 and December 2017. The study was approved by our Institutional Review Board (approval number: 0206-17-ASF).
Inclusion criteria: stroke more than three months prior to their first cognitive evaluation, completion of 40 or 60 hyperbaric oxygen sessions and at least two cognitive evaluations, 1–3 weeks prior to the first HBOT session to and 1–3 weeks after last HBOT session.
Exclusion criteria: insufficient details of stroke nature, history of a potential additional brain injury (traumatic brain injury, anoxic brain injury, subarachnoid hemorrhage, etc.), lack of pre or post-HBOT cognitive evaluations.
2.2Study design
The data were collected retrospectively from patients’ medical records and included age, gender, level of education, handedness, stroke details (type, injured hemisphere, location of stroke, time from injury to HBOT, symptoms prior to treatment), number of HBOT sessions, chronic medical conditions (diabetes mellitus type II (DM II), hypertension (HTN), dyslipidemia, ischemic heart disease (IHD), previous stroke, smoking status), and chronic prescribed medications (anti-aggregation (AA)), statins, hypoglycemic medications, HTN medications). Data of the HBOT protocol, and adverse events were also collected.
The main analysis was to compare the stroke nature (hemorrhagic and ischemic) including all stroke locations: cortical, subcortical, atypical locations (i.e. cerebellum or brain stem) and multiple locations. A second analysis (i.e. the location analysis) compared the two main stroke locations, cortical and subcortical. To minimize unknown hemisphere dominance in left handed patients, a third analysis (i.e. the dominance analysis) included only the right-handed patients for evaluating the effect of the injured hemisphere.
2.3Stroke subsets
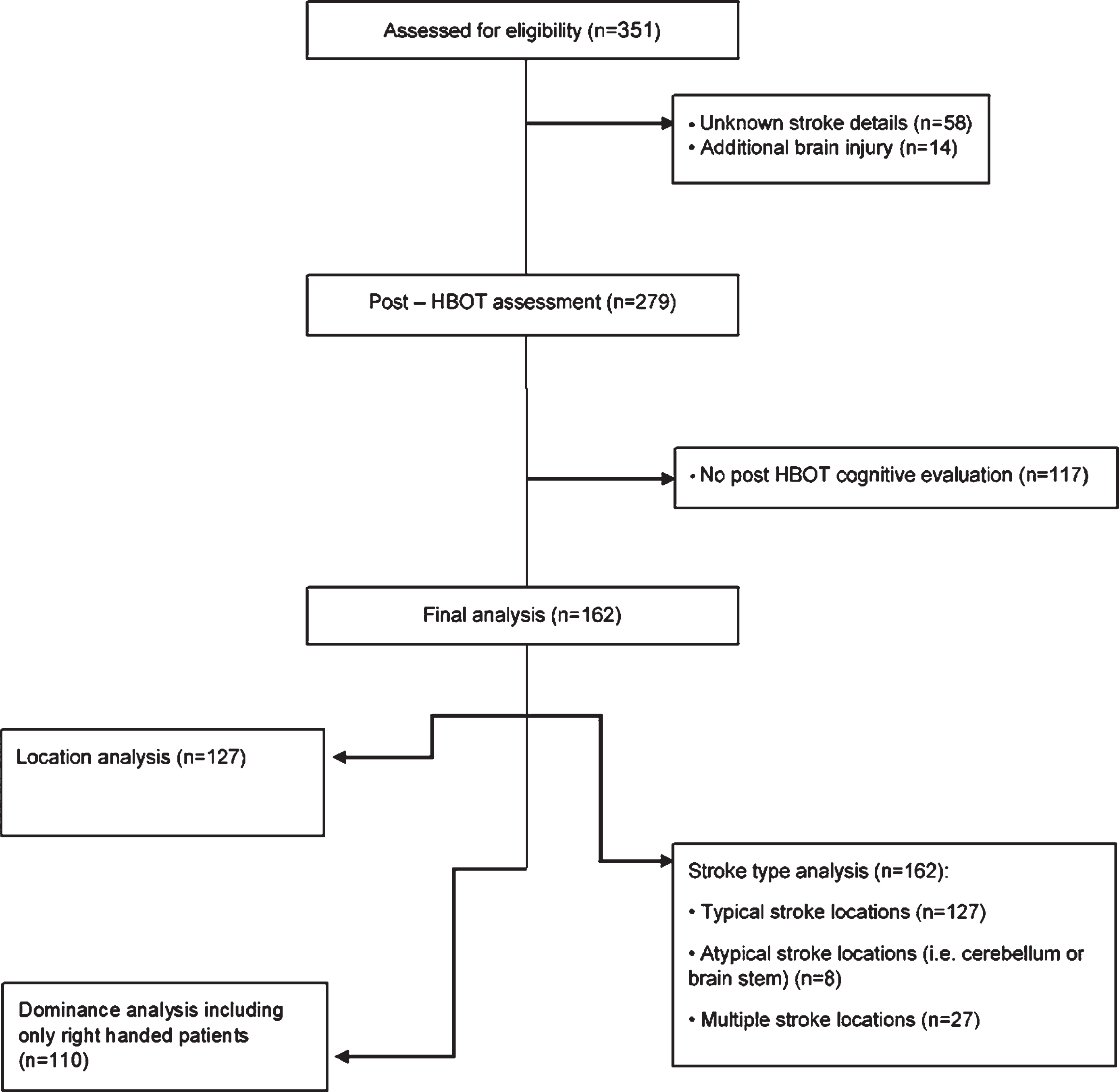
Patients were divided into different groups based on their stroke prerequisites, retrieved from original imaging and medical records: by anatomical location: cortical (i.e. frontal, temporal, parietal and occipital cortex) or subcortical (i.e. basal ganglia (BG), cerebellum, pons, internal capsule and thalamus), by the injured hemisphere: right or left, and by stroke type: ischemic or hemorrhagic (See Fig. 1).
Fig. 1
Flowchart of the patients included in the study.
2.4Hyperbaric oxygen treatment
Participants were treated in a multi-place hyperbaric chamber (Haux-Life-Support GmbH, Germany) with the following protocols: 40 to 60 daily sessions, 5 days per week, each session included 90 min of 100% oxygen at 2 ATA with 5 min air breaks every 20 minutes.
2.5Cognitive evaluation
All the patients were inspected using the NeuroTrax computerized cognitive testing battery (NeuroTrax Corporation, Bellaire, TX). The NeuroTrax system and a detailed description of the tests included were detailed in previous publications(Achiron et al., 2013; Thaler et al., 2012; Zur, Naftaliev, & Kesler, 2014) and are also available on the NeuroTrax website. In brief, NeuroTrax tests evaluate multiple aspects of brain cognitive functions including: memory, executive function (EF), visuospatial skills (VS), verbal function (VF), attention, information processing speed (IPS) and motor skills (MS). Cognitive domain scores were normalized for age, gender and education-specific levels.
The participants completed two validated alternate test forms of the NeuroTrax test battery at baseline and post-HBOT, to allow for iterative administrations with minimal learning effects. Test-retest reliability of the tests were found to be high in both normal and injured populations, without significant learning effects except in the VF & VS domains (Dwolatzky et al., 2003; L. Melton, 2005). Due to the low test-retest reliability of these domains, they were not evaluated in the current study.
2.6Statistical analysis
Data were expressed as mean±SD for parametric variables and frequencies, and percentages for nonparametric variables. Parametric variables were analyzed by paired-sample T tests for intra-group comparison and independent-sample t-tests for inter-group comparison, whereas nonparametric variables were analyzed by Pearson Chi-square test or Fisher’s exact test (where suitable) to identify significant variables. Normal distribution for all continuous variables was tested using the Kolmogorov-Smirnov test.
Clinically significant improvement (CSI) was defined as an absolute increase of 7.5 points of the normalized score (0.5 of one standard deviation) in at least one cognitive domain. The cut-off for CSI was determined by previous studies (Fischer et al., 2000; Schwid, Goodman, Weinstein, McDermott, & Johnson, 2007).
Multiple linear regression models were performed to determine independent predictors for the post-treatment cognitive score. Multivariate logistic regression models were performed to control for potential confounders and to determine independent predictors for CSI. Models included the following covariates: age, sex, stroke type, location of stroke along with side of injured hemisphere, time from injury to HBOT, chronic medical conditions (DM II, HTN, dyslipidemia, IHD, active smoking), number of HBOT sessions and baseline score before HBOT treatment.
The alpha level was set to 0.05 (p-Value < 0.05). The data were statistically analyzed using SPSS version 22 software.
3Results
3.1Participants’ characteristics
Of the 351 patients who were assed for eligibility, a total of 162 met the inclusion criteria and were included in the final analysis (Fig. 1). The patients’ average age was 60.75±12.91 years old (23–83) and 122 (75.3%) were males. The average time from the stroke to HBOT was 2.78±3.3 years. Regarding the stroke type, 121 patients (74.69%) suffered from an ischemic stroke while 41 (25.31%) had a hemorrhagic stroke. In 50 patients (30.86%), the stroke was in the subcortical level, while 77 patients (47.53%) had a stroke in the cortical level and the remaining 35 patients (21.6%) were affected in atypical locations or multiple locations. With respect to the side of injury, 87 strokes (53.7%) were located in the right hemisphere, and 62 strokes (38.3%) were in the left hemisphere. Baseline participants characteristics are summarized in Table 1.
Table 1
Patients’ baseline characteristics
| Analysis | Entire cohort (n = 162) | Location analysis (n = 127) | Dominance analysis (n = 110) | |
| Age (years) | 60.75±12.91 | 60.86±12.57 | 61.23±12.3 | |
| Sex – Males | 122 (75.3%) | 97 (76.4%) | 78 (70.9%) | |
| Dominant hand – Right | 120 (74.1%) | 94 (74%) | 110 (100%) | |
| Time from injury | 2.78±3.3 | 2.53±2.95 | 2.63±3.18 | |
| Num. of HBOT sessions | 40 sessions | 26 (16%) | 22 (17.3%) | 20 (18.2%) |
| 60 sessions | 136 (84%) | 105 (82.7%) | 90 (81.8%) | |
| Type of stroke | Ischemic | 121 (74.69%) | 98 (77.17%) | 85 (77.3%) |
| Hemorrhagic | 41 (25.31%) | 29 (22.8%) | 25 (22.7%) | |
| Location of injury | Subcortical | 54 (33.3%) | 50 (39.4%) | 36 (32.7%) |
| Cortical | 80 (49.4%) | 77 (60.6%) | 58 (52.7%) | |
| Atypical &multiple locations | 28 (17.3) | – | 16 (14.5%)* | |
| Side of injury | Right | 62 (38.3%) | 53 (41.7%) | 56 (50.9%) |
| Left | 87 (53.7%) | 74 (58.3%) | 54 (49.1%) | |
| Bilateral | 13 (8%) | – | – | |
| Symptoms | Cognitive | 77 (47.5%) | 60 (47.2%) | 49 (44.5%) |
| Motor | 132 (81.5%) | 104 (81.9%) | 90(81.8%) | |
| Speech | 65 (40.1%) | 54 (42.5%) | 43 (39.1%) | |
| CN | 67 (41.4%) | 54 (42.5%) | 46 (41.8%) | |
| Ataxia | 57 (35.2%) | 39 (30.7%) | 34 (30.9%) | |
| Comorbidities | DM II | 48 (29.6%) | 37 (29.1%) | 28 (25.5%) |
| HTN | 107 (66%) | 82 (64.6%) | 74 (67.3%) | |
| Dyslipidemia | 107 (66%) | 82 (64.6%) | 75 (68.2%) | |
| IHD | 39 (24.1%) | 30 (23.6%) | 28 (25.5%) | |
| Previous stroke | 18 (11.1%) | 12 (9.4%) | 10 (9.1%) | |
| Smoker | 29 (17.9%) | 23 (18.1%) | 15 (13.6%) | |
| Medications | AA | 105 (64.8%) | 78 (61.4%) | 70 (63.6%) |
| Statins | 104 (64.2%) | 79 (62.2%) | 72 (65.5%) | |
| DM II medications | 37 (22.8%) | 27 (21.3%) | 20 (18.2%) | |
| HTN medications | 107 (66%) | 84 (66.1%) | 74 (67.3%) | |
Data are expressed as means±standard deviation. *Cerebellum insult only. HBOT – hyperbaric oxygen treatment, CN – cranial nerves, DM II – diabetic mellitus type 2, HTN – hypertension, AA – anti-aggregates.
3.2Cognitive function changes
Basic analysis results revealed statistically significant improvements of all the cognitive domains after HBOT by 2.34-20 (p < 0.05, see Table 2). The memory domain had the most prominent improvements of mean absolute change (MAC) (6.19±20, p = 0.0004, see Table 2). CSI was achieved in 86% of the patients in the entire cohort (see Fig. 4). The effects of the HBOT on the cognitive scores is summarized in Table 2.
Table 2
Cognitive domains – mean absolute changes of the entire cohort
| Pre score | Post score | Pre-Post MAC | P-value* | |
| GCF | 87.48 ± 12.26 | 91.14 ± 12.10 | 3.53 ± 7.68 | <0.0001 |
| Memory | 82.09 ± 19.32 | 88.29 ± 19.15 | 6.12 ± 15.46 | <0.0001 |
| EF | 88.61 ± 14.15 | 91.09 ± 12.65 | 2.54 ± 10.37 | 0.003 |
| Attention | 85.19 ± 17.08 | 87.83 ± 15.75 | 2.95 ± 12.63 | 0.04 |
| IPS | 83.54 ± 15.45 | 86.34 ± 17.07 | 2.34 ± 9.28 | 0.005 |
| MS | 91.91 ± 17.13 | 95.21 ± 15.89 | 3.96 ± 14.27 | 0.001 |
Data are expressed as means±standard deviation. *Significant by two-tailed paired t-test. Bold text marks statistical significance (P < 0.05). GCF – global cognitive function, EF – executive function, IPS – information processing speed, MS – motor skills.
3.2.1Ischemic vs. hemorrhagic
At baseline, there were significant differences in baseline characteristics between patients with ischemic compared to patients with hemorrhagic stroke which included age, presence of comorbidities, dyslipidemia and IHD, and medications prescribed (AA and statins) (p < 0.05, see Table 3). In addition, the memory domain mean score of the ischemic stroke patients was significantly higher at baseline, compared to hemorrhagic stroke patients (83.87 vs 76.82, p = 0.043, Table 4).
Table 3
Baseline characteristics comparison of patients with ischemic and hemorrhagic strokes
| Main analysis | Ischemic (n = 121) | Hemorrhagic (n = 41) | P-value* | |
| Age (years) | 62.78±12.3 | 54.77±12.97 | 0.001 | |
| Sex – males | 90 (74.4%) | 32 (78%) | 0.64 | |
| Dominant hand – right | 91 (75.2%) | 29 (70.7%) | 0.575 | |
| Time from injury | 2.82±3.52 | 2.61±2.61 | 0.71 | |
| Location of injury | Subcortical | 38 (31.4%) | 16 (39%) | 0.138 |
| Cortical | 65 (53.7%) | 15 (36.6%) | ||
| Atypical &multiple locations | 18 (14.9) | 10 (24.4) | ||
| Side of injury | Right | 66 (54.5%) | 21 (51.2%) | 0.524 |
| Left | 47 (38.8%) | 15 (36.6%) | ||
| Bilateral | 8 (6.6%) | 5 (12.2%) | ||
| Symptoms | Cognitive | 53 (43.8%) | 24 (58.5%) | 0.104 |
| Motor | 98 (81%) | 34 (82.9%) | 0.784 | |
| Speech | 45 (37.2%) | 20 (48.8%) | 0.193 | |
| CN | 52 (43%) | 15 (36.6%) | 0.476 | |
| Ataxia | 40 (33.1%) | 17 (41.5%) | 0.333 | |
| Comorbidities | DM II | 40 (33.1%) | 8 (19.5%) | 0.078 |
| HTN | 85 (70.2%) | 22 (53.7%) | 0.068 | |
| Dyslipidemia | 90 (74.4%) | 17 (41.5%) | <0.0004 | |
| IHD | 35 (28.9%) | 4 (9.8%) | 0.003 | |
| Previous stroke | 15 (12.4%) | 3 (7.3%) | 0.374 | |
| Smoker | 25 (20.7%) | 4 (9.8%) | 0.071 | |
| Medications | AA | 92 (76%) | 13 (31.7%) | <0.0001 |
| Statins | 86 (71.1%) | 18 (43.9%) | 0.003 | |
| DM II medications | 31 (25.6%) | 6 (14.6%) | 0.113 | |
| HTN medications | 85 (70.2%) | 22 (53.7%) | 0.068 | |
Data are expressed as means±standard deviation. *Significant by two-tailed paired t-test. Bold text marks statistical significance (P < 0.05). CN – cranial nerves, DM II – diabetic mellitus type 2, HTN – hypertension, AA – anti-aggregates.
Table 4
Pre-hyperbaric oxygen treatment cognitive domains scores
| Ischemic/hemorrhagic analysis | Location analysis | Dominance analysis | |||||||
| Ischemic | Hemorrhagic | P -Value* | Subcortical | Cortical | P -Value* | Rt. Injury | Lt. Injury | P -Value* | |
| GCF | 88.18±12.52 | 85.42±11.36 | 0.214 | 88.76±11.26 | 84.95±13.53 | 0.1 | 88.81±11.77 | 87.8±14.28 | 0. 686 |
| Memory | 83.87 ± 18.45 | 76.82 ± 21.06 | 0.043 | 81.68±18.4 | 80.72±20.18 | 0.788 | 87.15±18. 9 | 81.96±18.94 | 0.153 |
| EF | 88.6±14.26 | 88.63±13.98 | 0.992 | 92.37 ± 13.61 | 85.19 ± 15.11 | 0.009 | 89.68±14.69 | 90.29±15.14 | 0.834 |
| Attention | 85.65±17.54 | 83.8±15.73 | 0.56 | 88.44 ± 14.02 | 80.8 ± 19.57 | 0.012 | 84.93±17.57 | 86.33±18.33 | 0.685 |
| IPS | 84.68±15.7 | 79.66±14.09 | 0.106 | 83.55±14.22 | 82.7±16.61 | 0.28 | 85.24±16.99 | 81.21±14.07 | 0.2 |
| MS | 91.56±17.79 | 92.93±15.15 | 0.667 | 92.79±16.25 | 89.69±19.58 | 0.362 | 91.9±15.48 | 90.18±18.59 | 0.608 |
Data are expressed as means±standard deviation. *Significant by two-tailed paired t-test. Bold text marks statistical significance (P < 0.05). GCF – global cognitive function, EF – executive function, VS – visual spatial, IPS – information processing speed, MS – motor skills.
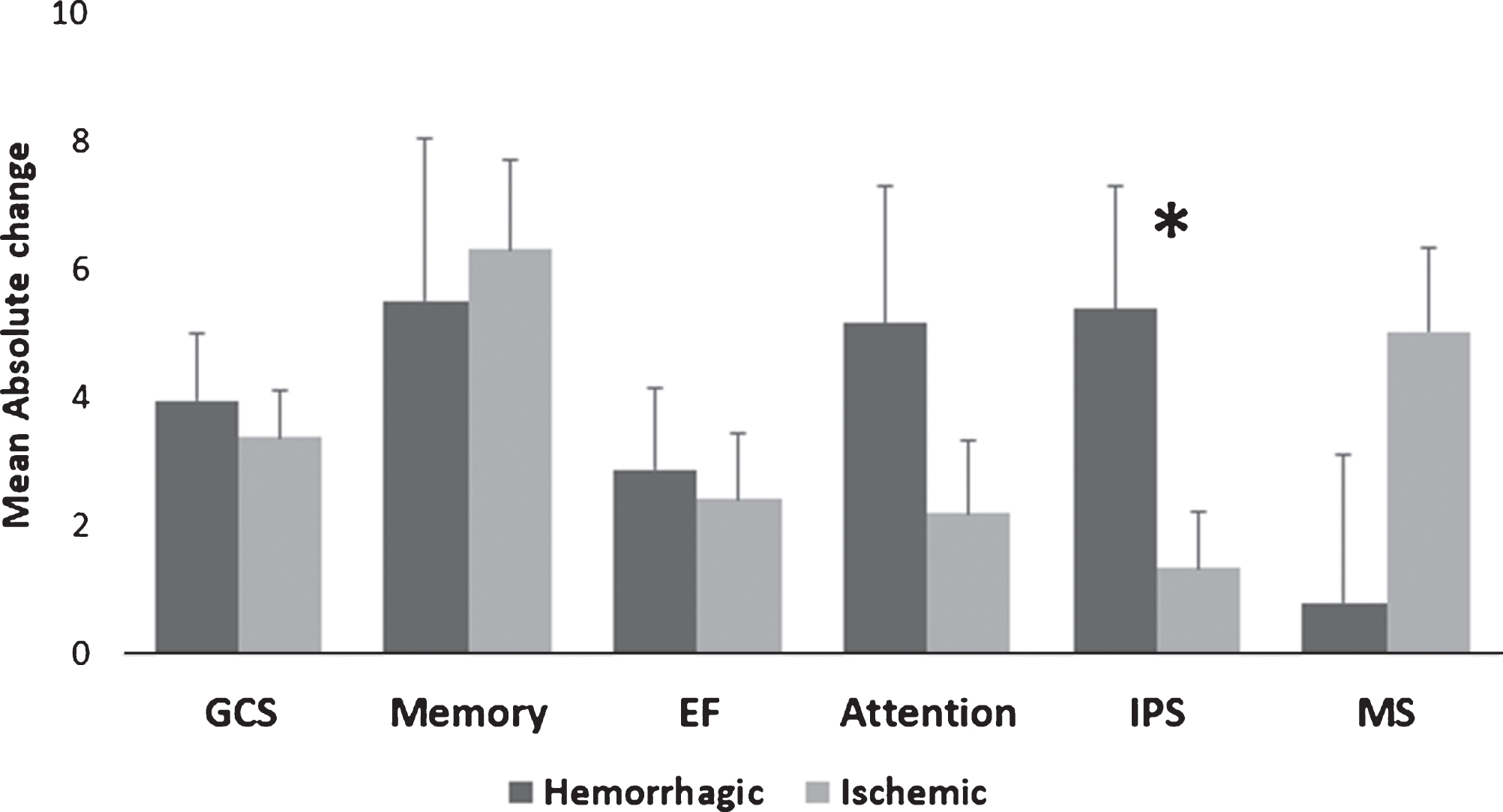
Post-HBOT, the IPS domain had a significantly higher MAC in the hemorrhagic stroke patients compared to the ischemic stroke patients (5.39 vs. 1.36, p = 0.035, see Fig. 2). There were no other significant differences in the surplus of the cognitive domains (p > 0.05, see Fig. 2). In addition, there were no significant changes in the CSI between hemorrhagic stroke patients compared to ischemic stroke patients (94.6% vs. 83.33%, p > 0.05, see Fig. 4).
Fig. 2
Hemorrhagic/ischemic stroke MAC comparison of cognitive scores post-HBOT. Only the IPS domain was significantly increased after HBOT for the hemorrhagic stroke patients compared to ischemic strokes. Statistical significance (p < 0.05) is marked by *. Bars represent means+standard deviation. Abbreviations: MAC – mean absolute change, HBOT – hyperbaric oxygen treatment, GCS – global cognitive scale, EF – executive function, IPS – information processing speed, MS – motor skills.
3.2.2Cortical vs. subcortical
At baseline, there were significant differences in speech symptoms and presence of HTN between patients with subcortical strokes compared to cortical stroke (p < 0.05, see Table 5).
Table 5
Baseline characteristics comparison of patients with cortical and subcortical strokes
| Location analysis | Subcortical (n = 50) | Cortical (n = 77) | P-value* | |
| Age (years) | 62.31±11.28 | 59.91±13.32 | 0.296 | |
| Sex – males | 42 (84%) | 55 (71.4%) | 0.091 | |
| Dominant hand – right | 36 (72%) | 58 (75.3%) | 0.679 | |
| Time from injury | 2.58±2.87 | 2.51±3 | 0.895 | |
| Type of stroke | Ischemic | 35 (70%) | 63 (81.8%) | 0.121 |
| Hemorrhagic | 15 (30%) | 14 (18.2%) | ||
| Side of injury | Right | 22 (44%) | 31 (40.3%) | 0.676 |
| Left | 28 (56%) | 46 (59.7%) | ||
| Symptoms | Cognitive | 23 (46%) | 37 (48.1%) | 0.823 |
| Motor | 43 (86%) | 61 (79.2%) | 0.321 | |
| Speech | 15 (30%) | 39 (50.6%) | 0.019 | |
| CN | 20 (40%) | 34 (44.2%) | 0.647 | |
| Ataxia | 20 (40%) | 19 (24.7%) | 0.077 | |
| Comorbidities | DM II | 19 (38%) | 18 (23.4%) | 0.087 |
| HTN | 38 (76%) | 44 (57.1%) | 0.026 | |
| Dyslipidemia | 34 (68%) | 48 (62.3%) | 0.518 | |
| IHD | 10 (20%) | 20 (26%) | 0.443 | |
| Previous stroke | 7 (14%) | 5 (6.5%) | 0.192 | |
| Smoker | 9 (18%) | 14 (18.2%) | 0.979 | |
| Medications | AA | 27 (54%) | 51 (66.2%) | 0.175 |
| Statins | 31 (62%) | 48 (62.3%) | 0.97 | |
| DM II medications | 13 (26%) | 14 (18.2%) | 0.311 | |
| HTN medications | 36 (72%) | 48 (62.3%) | 0.097 | |
Data are expressed as means±standard deviation. *Significant by two-tailed paired t-test. Bold text marks statistical significance (P < 0.05). CN – cranial nerves, DM II – diabetic mellitus type 2, HTN – hypertension, AA – anti-aggregates.
Compared to cortically located strokes, the EF & attention domains at baseline were significantly higher in the subcortically located strokes (92.37 vs. 85.19, p = 0.009, 88.44 vs. 80.78, p = 0.012, respectively, see Table 4). There were no other significant differences in cognitive domains (p > 0.05, see Table 4).
Post-HBOT, there were no significant differences between patients with subcortical strokes compared to cortical strokes (p > 0.05, see Supplementary Fig. I). Moreover, there were no significant changes in the CSI between subcortical strokes compared to cortical strokes (90% vs. 87.23%, p > 0.05, see Fig. 4).
3.2.3Dominant vs. non-dominant hemisphere
Including only right-handed patients, at baseline, there were significant differences in speech and motor symptoms between patients with left dominant hemisphere strokes compared to right non-dominant hemisphere strokes (p < 0.05, see Table 6). There were no significant differences at baseline cognitive function between the dominant and non-dominant hemisphere strokes (p > 0.05, see Table 4).
Table 6
Baseline characteristics comparison of patients with dominant and non-dominant strokes
| Rt. handed analysis | Non-dominant (n = 56) | Dominant (n = 54) | P-value* | |
| Age (years) | 60.73±13.78 | 61.75±10.65 | 0.668 | |
| Sex – males | 38 (67.9%) | 40 (74.1%) | 0. 477 | |
| Time from injury | 2.57±2.59 | 2.7±3.72 | 0.827 | |
| Type of stroke | Ischemic | 44 (78.6%) | 41 (75.9%) | 0.741 |
| Hemorrhagic | 12 (21.4%) | 13(24.1%) | ||
| Location of injury | Subcortical | 20 (35.7%) | 16 (29.6%) | 0.72 |
| Cortical | 29 (51.8%) | 29 (53.7%) | ||
| Atypical Locations | 7 (12.5%) | 9 (16.7%) | ||
| Symptoms | Cognitive | 21 (37.5%) | 28 (51.9%) | 0.132 |
| Motor | 50 (89.3%) | 40 (74.1%) | 0.04 | |
| Speech | 13 (23.2%) | 30 (55.6%) | 0.0004 | |
| CN | 20 (35.7%) | 26 (48.1%) | 0.19 | |
| Ataxia | 17 (30.4%) | 17 (31.5%) | 0.9 | |
| Comorbidities | DM II | 14 (25%) | 14 (25.9%) | 0.912 |
| HTN | 37 (66.1%) | 37 (68.5%) | 0.787 | |
| Dyslipidemia | 37 (66.1%) | 38 (70.4%) | 0.632 | |
| IHD | 13 (23.2%) | 15 (27.8%) | 0.587 | |
| Previous stroke | 2 (3.6%) | 8 (14.8%) | 0.044 | |
| Smoker | 10 (17.9%) | 5 (9.3%) | 0.19 | |
| Medications | AA | 35 (62.5%) | 35 (64.8%) | 0.803 |
| Statins | 35 (62.5%) | 37 (68.5%) | 0.511 | |
| DM II medications | 12 (22.2%) | 8 (14.3%) | 0.286 | |
| HTN medications | 34 (60.7%) | 40 (74.1%) | 0.137 | |
Data are expressed as means±standard deviation. *Significant by two-tailed paired t-test. Bold text marks statistical significance (P < 0.05). CN – cranial nerves, DM II – diabetic mellitus type 2, HTN – hypertension, AA – anti-aggregates.
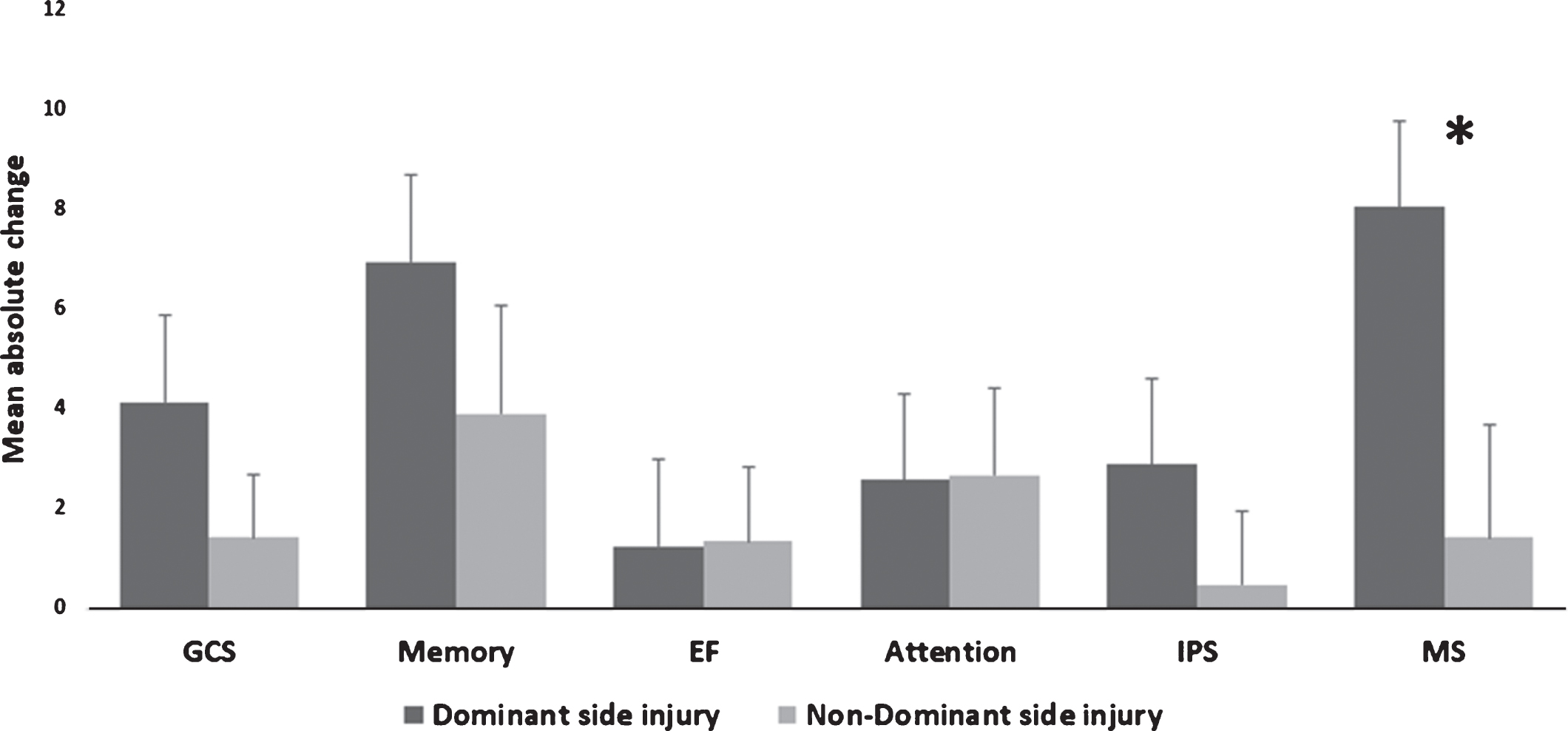
Post-HBOT, there were significantly larger increases in MAC in the motor domains for patients with left hemisphere strokes compared to right hemisphere strokes (8.02 vs. 1.42, p = 0.023, see Fig. 3). There were no other significant differences for the surplus cognitive domains (p > 0.05, see Fig. 3).
Fig. 3
Dominant/non-dominant MAC comparison of cognitive scores post-HBOT. The motor domain was significantly increased after HBOT at the dominant (i.e. left sided) stroke patients compared to non-dominant strokes. Statistical significance (p < 0.05) is marked by *. Bars represent means+standard deviation. Abbreviations: MAC – mean absolute change, HBOT – hyperbaric oxygen treatment, GCS – global cognitive scale, EF – executive function, IPS – information processing speed, MS – motor skills.
There were no significant changes in the CSI between left dominant hemisphere strokes compared to right non-dominant hemisphere stroke patients (90.57% vs. 76.47%, p > 0.05, see Fig. 4).
Fig. 4
Clinically significant improvement comparisons of hemorrhagic vs. ischemic, cortical vs. sub-cortical and dominant vs. non-dominant stroke patients. Scores were not significantly different in all the domains (p > 0.05). Bars represent percentages.
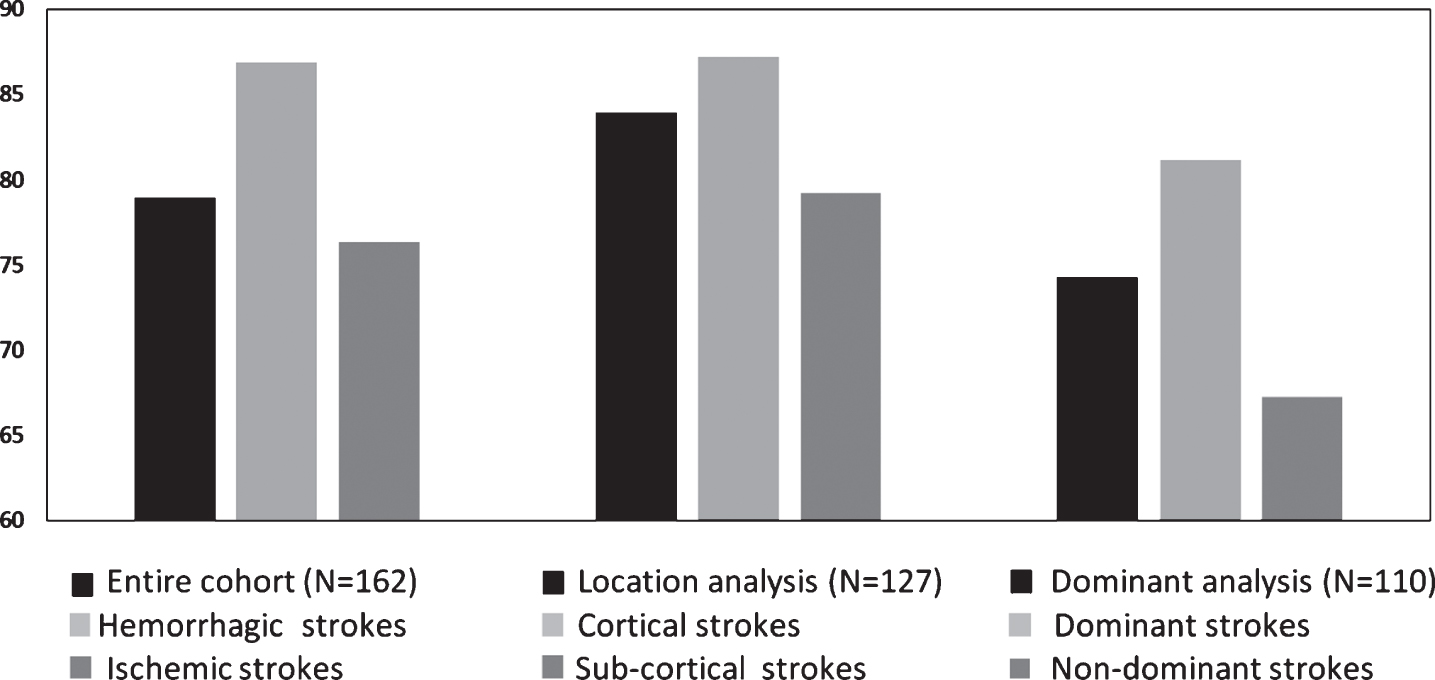
Fig. 5
Cortical/subcortical (i.e. BG) MAC comparison of cognitive scores post HBOT. Scores MAC were not significantly different in all the domains (p > 0.05). Bars represent means+standard deviation. Abbreviations: MAC – mean absolute change, HBOT – hyperbaric oxygen treatment, GCS – global cognitive scale, EF – executive function, IPS – information processing speed, MS – motor skills.
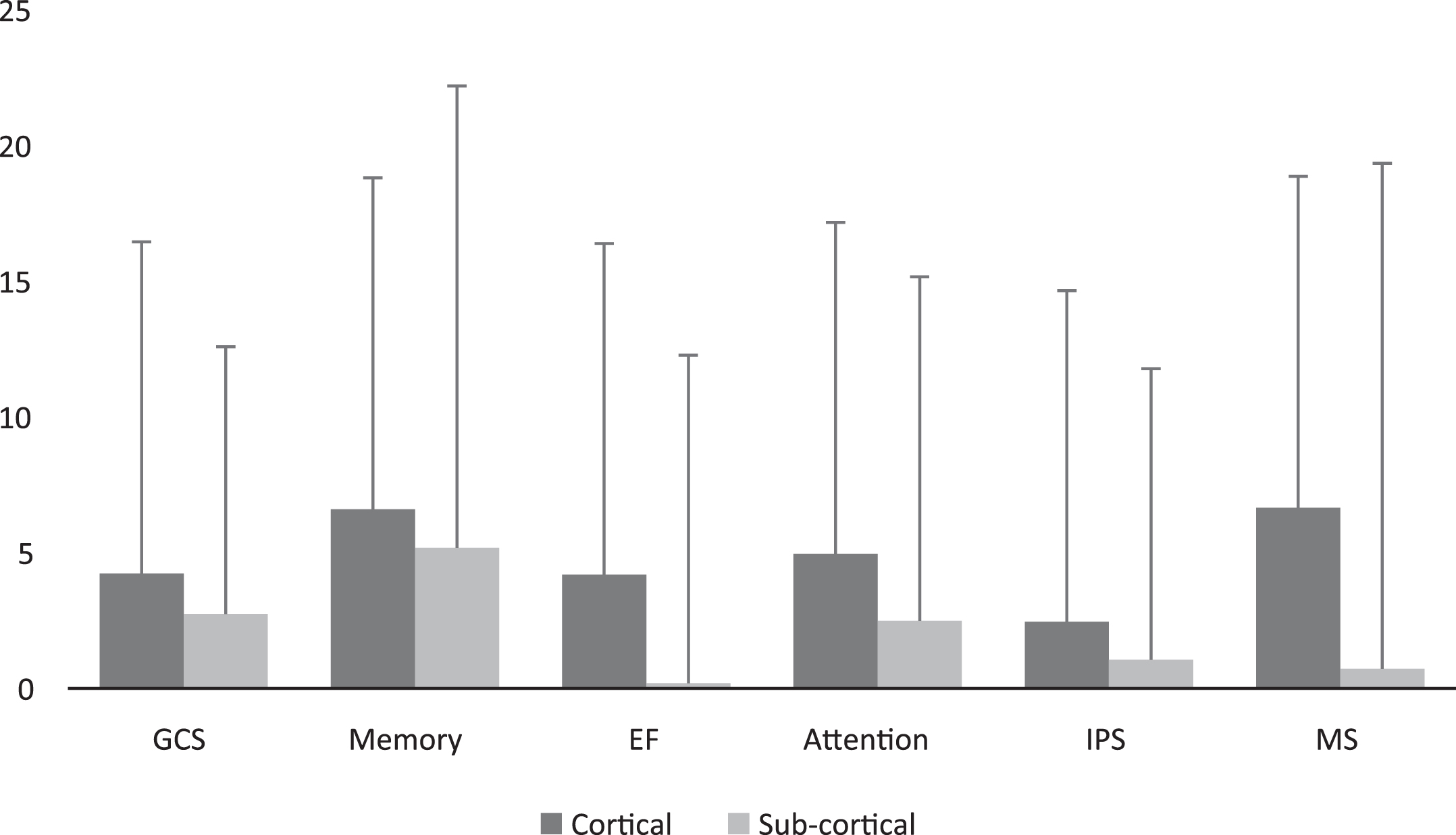
3.3Cognitive scores outcome predictors
Forward stepwise multivariate linear regression models were performed on the entire cohort as well as on the location and dominance cohorts. The only major statistically significant predictor on the post-HBOT score in all of the domains and analyses was the baseline cognitive domain score. Age, gender, handedness, stroke details (type, injured hemisphere, location, time from injury to HBOT), number of HBOT sessions, chronic medical conditions (DM II, HTN, dyslipidemia, IHD, previous stroke), smoking status, chronic prescribed medications (AA, statins, DM II medications, HTN medications) had no effect in most domains.
HTN was a significant predictor on post-HBOT score in the GCS for the dominance analysis only, and the number of HBOT sessions was a significant predictor on post-HBOT in the EF domain for all analyses.
Forward stepwise multivariate logistic regression models were performed on the three different analyses, to evaluate significant predictors for CSI percentage. Low baseline cognitive memory domain score was the only statistically significant predictor on the CSI prevalence in the main and location analyses (OR = 0.94 ([0.909– 0.972], p < 0.0003), OR = 0.948 ([0.912– 0.985], p = 0.007), respectively). In the dominance analysis, the low baseline cognitive memory domain score and shorter times that passed since the event to HBOT were the statistically significant predictors on the post-HBOT score (OR = 0.949 ([0.912– 0.986], p = 0.008), OR = 0.82 ([0.692– 0.972], p = 0.022), respectively).
3.4Safety
There were twelve (7.4%) side effect reports in the entire cohort. Eight experienced barotrauma (8/162, 4.93%). Barotraumas were mild and all patients fully recovered after a few days. In addition, three patients (1.85%) reported minor otalgia without objective barotrauma. One patient (0.06%) reported a mild headache during recompression. In addition, two patients with histories of known seizures prior to HBOT suffered seizures after a few sessions of HBOT. The seizures did not occur while in the hyperbaric chamber and once the patients reported about them, their anti-epileptic drugs were modified, and they resumed HBOT shortly.
4Discussion
In the current study, the effect of HBOT on post-stroke patients in the late chronic stages was analyzed. Even though the patients were treated after a median of 1.5±3.3 years post-stroke, there were significant cognitive improvements in all the cognitive domains which were measured using objective computerized tests. Moreover, clinical significant improvements (CSI) were achieved in 86% of patients, with the most significant measurable improvements gained in the dominant hemisphere stroke patients. Low baseline memory score was the significant predictor for CSI. Hemorrhagic stroke patients had significantly higher improvement in IPS, but no other differences were found compared to ischemic strokes. There were no significant differences in HBOT effects on subcortical compared to cortical strokes. Patients with strokes located in the dominant hemisphere had significantly larger improvement in the MS domain.
In the current study, there were significant improvements in all the cognitive domains which reconfirms previous studies that evaluated the therapeutic effect of HBOT in the chronic late stage of post-stroke patients (Boussi-Gross et al., 2015; Hadanny et al., 2015a; Emily R. Rosario et al., 2018; Vila, Balcarce, Abiusi, Dominguez, & Pisarello, 2005). In a previous study, there were significant improvements in the neurological functions, tested by the National Institutes of Health stroke scale (NIHSS), activities of daily living (ADL) and quality of life (Efrati et al., 2013). However, cognitive domains were not reported. A later retrospective study reported significant improvements in the memory domain after HBOT. Yet, the other cognitive domains were not explored and the stroke nature was not evaluated as a possible confounder (Boussi-Gross et al., 2015). Churchill published a prospective study (Churchill et al., 2013) that included 22 patients at least one year after stroke. HBOT induced improvement in symptoms reports (51% memory, 51% attention/concentration, 48% balance/coordination, 45% endurance, 20% sleep). However, on standardized evaluations of cognition and questionnaires no significant changes were reported. Another small prospective study on seven patients showed verbal memory and executive function improvements in addition to sleep and quality of life changes (E. R. Rosario et al., 2018).
The differences between hemorrhagic and ischemic strokes were mild but evident in the high cognitive function domain (i.e. the IPS) which correlates with the usually more severe outcomes of post-hemorrhagic stroke patients and the cognitive deficits across multiple domains (Cumming et al., 2013). This domain is more sensitive than the other cognitive domains to an insult due to its integrating role on other domains and its influence on downstream processes, which is manifested in the domains score. Nevertheless, hemorrhagic stroke patients showed significant improvements post-HBOT and the low baseline cognitive domain score remained the major predictor for the post-HBOT domain score.
The lack of any significant differences after HBOT between cortical and subcortical strokes is surprising. Similar to our study, previous studies showed subcortical stroke patients have higher post-stroke cognitive scores compared to cortical stroke patients (Gottesman & Hillis, 2010; Kalaria & Ballard, 2001). However, post– HBOT, there were no significant differences between the two types. Even though it is expected that subcortical strokes will have lower proportions of memory impairments (and conversely for the IPS domain), no such differences were seen after HBOT treatment. Our results indicate that the excess oxygen from HBOT treatments functions on all ischemic areas regardless of their anatomical area. As expected, the higher improvements in the MS domain seen in the dominant stroke patients, lies in the basic functionality of the dominant side.
The lack of any significant difference regarding HBOT’s beneficial effects to the stroke’s origin and location could be explained by the common pathophysiological final path of injury, i.e. ischemic/metabolic dysfunctional cells in injured non necrotic brain regions. As seen in previous studies, stroke patients may have chronic penumbra even years after the insult, which can be identified using SPECT imaging (Churchill et al., 2013; Jacobs, Winter, Alvis, & Small, 1969). Oxygenation improves energy metabolism in the border zones of focal cerebral ischemia represented by significant reduction of areas with tissue acidosis and areas with ATP depletion (Sun, Marti, & Veltkamp, 2008; Sun, Strelow, Mies, & Veltkamp, 2011).HBOT can also decrease the post ischemic inflammatory response by reducing blood-brain-barrier damage (Veltkamp et al., 2005), inflammatory cytokines release (Yu, Xue, Liang, Zhang, & Zhang, 2015) and suppresses the aggravated response of astrocytes and microgliosis (Gunther et al., 2005). Recently, it was shown HBOT mitigates the inflammatory response of the neuronal cells through the transfer of mitochondria from astrocytes (Lippert & Borlongan, 2019). HBOT reduces apoptosis which enables to preserve more brain tissues and promote neurologic functional recovery (Yin et al., 2003). Opening of mitochondrial ATP-sensitive potassium channel plays a role in this antiapoptotic effect of early hyperbaric oxygenation (Lou, Chen, Ding, Eschenfelder, & Deuschl, 2006). The intermittent hyperoxic exposure during HBOT can induce hypoxia inducible factor-1 alpha (HIF-1α) by the so called “Hyperoxic-Hypoxic paradox”(Duan, Shao, Yu, & Ren, 2015; Milosevic et al., 2009; Poli & Veltkamp, 2009; Soejima et al., 2013). HIF-1α is transcriptional regulator of genes involved in angiogenesis, energy metabolism, and neuronal cell proliferation induced by HBOT (Duan et al., 2015; Milosevic et al., 2009; Poli & Veltkamp, 2009; Soejima et al., 2013).
In summary, HBOT induces neuroplasticity, by two main physiological effects: increasing tissue oxygenation – the rate limiting factor for all regenerative mechanisms, and the repeated oxygen level fluctuations which increases HIF-1α which in turn triggers the regenerative processes in the metabolically injured brain areas regardless of the stroke origin (Efrati & Ben-Jacob, 2014; Efrati et al., 2013). Therefore, the selection of stroke patients for HBOT should be based on functional imaging and baseline cognitive domain scores rather than stroke type, location or side of lesion.
The current study presents the largest cohort of post-stroke patients treated with HBOT in the late chronic stage. However, it has several limitations, which are mostly related to the fact that data were collected retrospectively. Still, the findings presented here are in agreement with previous prospective RCT’s in which the neuroplasticity effects of HBOT were established [28, 37, 48]. These therapeutic effects were seen in our study in the chronic stage when patients are not expected to improve. Another study limitation is the missing data on the treatment’s long-term effects. Further long-term prospective studies should be performed.
Another important limitation relates to the HBOT protocol which was inconsistent in the cohort, where several patients received 40 sessions compared to 60 sessions in most patients. Although significant neurotherapeutic effects were shown with both these protocols, the optimal protocol, which induces maximal neuroplasticity with minimal side effects, remains unknown.
5Conclusions
HBOT was found, in the largest post-stroke population published, to induce significant improvements in all cognitive function domains even at the late chronic stage. Patients selection for HBOT should be based on functional imaging and baseline cognitive function, regardless of stroke type and location. Further studies are needed to validate these findings for the optimal patient selection.
Acknowledgments
We would like to thank Dr. Mechael Kanovsky for his editing of this manuscript.
Funding: No external funding source was used for this study.
References
1 | Achiron, A. , Chapman, J. , Magalashvili, D. , Dolev, M. , Lavie, M. , Bercovich, E. ,... Barak, Y. ((2013) ). Modeling of cognitive impairment by disease duration in multiple sclerosis: A cross-sectional study. PloS One, 8: (8), e71058. doi: 10.1371/journal.pone.0071058 |
2 | Al-Qazzaz, N.K. , Ali, S.H. , Ahmad, S.A. , Islam, S. , & Mohamad, K. ((2014) ). Cognitive impairment and memory dysfunction after a stroke diagnosis: A post-stroke memory assessment. Neuropsychiatric Disease and Treatment, 10: , 1677–1691. doi: 10.2147/NDT.S67184 |
3 | Ballard, C. , Rowan, E. , Stephens, S. , Kalaria, R. , & Kenny, R.A. ((2003) ). Prospective follow-up study between 3 and 15 months after stroke: Improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke, 34: (10), 2440–2444. doi: 10.1161/01.STR.0000089923.29724.CE |
4 | Boussi-Gross, R. , Golan, H. , Fishlev, G. , Bechor, Y. , Volkov, O. , Bergan, J. ,... Efrati, S. ((2013) ). Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury – randomized prospective trial. PLoS One, 8: (11), e79995. doi: 10.1371/journal.pone.0079995 |
5 | Boussi-Gross, R. , Golan, H. , Volkov, O. , Bechor, Y. , Hoofien, D. , Beeri, M.S. ,... Efrati, S. ((2015) ). Improvement of memory impairments in poststroke patients by hyperbaric oxygen therapy. Neuropsychology, 29: (4), 610–621. doi: 10.1037/neu0000149 |
6 | Bradshaw, P.O. , Nelson, A.G. , Fanton, J.W. , Yates, T. , & Kagan-Hallet, K.S. ((1996) ). Effect of hyperbaric oxygenation on peripheral nerve regeneration in adult male rabbits. Undersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc, 23: (2), 107–113. |
7 | Calvert, J.W. , Cahill, J. , & Zhang, J.H. ((2007) ). Hyperbaric oxygen and cerebral physiology. Neurological Research, 29: (2), 132–141. doi: 10.1179/016164107X174156 |
8 | Caplan, L.R. ((1989) ). Intracranial branch atheromatous disease: A neglected, understudied, and underused concept. Neurology, 39: (9), 1246–1250. |
9 | Censori, B. , Manara, O. , Agostinis, C. , Camerlingo, M. , Casto, L. , Galavotti, B. ,... Mamoli, A. ((1996) ). Dementia after first stroke. Stroke, 27: (7), 1205–1210. |
10 | Chang, C.C. , Lee, Y.C. , Chang, W.N. , Chen, S.S. , Lui, C.C. , Chang, H.W. ,... Wang, Y.L. ((2009) ). Damage of white matter tract correlated with neuropsychological deficits in carbon monoxide intoxication after hyperbaric oxygen therapy. Journal of Neurotrauma, 26: (8), 1263–1270. doi: 10.1089/neu.2008-0619 |
11 | Churchill, S. , Weaver, L.K. , Deru, K. , Russo, A.A. , Handrahan, D. , Orrison, W.W. Jr ,... Elwell, H.A. ((2013) ). A prospective trial of hyperbaric oxygen for chronic sequelae after brain injury (HYBOBI. Undersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc, 40: (2), 165–193. |
12 | Cumming, T.B. , Marshall, R.S. , & Lazar, R.M. ((2013) ). Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. International Journal of Stroke: Official Journal of the International Stroke Society, 8: (1), 38–45. doi: 10.1111/j.1747-4949.2012.00972.x |
13 | de Oliveira, F.F. , Correia Marin Sde, M. , & Ferreira Bertolucci, P.H. ((2013) ). Communicating with the non-dominant hemisphere: Implications for neurological rehabilitation. Neural Regeneration Research, 8: (13), 1236–1246. doi: 10.3969/j.issn.1673-5374.2013.13.009 |
14 | Duan, S. , Shao, G. , Yu, L. , & Ren, C. ((2015) ). Angiogenesis contributes to the neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. The International Journal Neuroscience, 125: (8), 625–634. doi: 10.3109/00207454.2014.956101 |
15 | Dwolatzky, T. , Whitehead, V. , Doniger, G.M. , Simon, E.S. , Schweiger, A. , Jaffe, D. , & Chertkow, H. ((2003) ). Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatrics, 3: , 4. doi: 10.1186/1471-2318-3-4 |
16 | Efrati, S. , & Ben-Jacob, E. ((2014) ). Reflections on the neurotherapeutic effects of hyperbaric oxygen. Expert Review in Neurotherapeutics, 14: (3), 233–236. doi: 10.1586/14737175.2014.884928 |
17 | Efrati, S. , Fishlev, G. , Bechor, Y. , Volkov, O. , Bergan, J. , Kliakhandler, K. ,... Golan, H. ((2013) ). Hyperbaric oxygen induces late neuroplasticity in post stroke patients–randomized, prospective trial. PloS One, 8: (1), e53716. doi: 10.1371/journal.pone.0053716 |
18 | Efrati, S. , Golan, H. , Bechor, Y. , Faran, Y. , Daphna-Tekoah, S. , Sekler, G. ,... Buskila, D. ((2015) ). Hyperbaric oxygen therapy can diminish fibromyalgia syndrome–prospective clinical trial. PLoS One, 10: (5), e0127012. doi: 10.1371/journal.pone.0127012 |
19 | Fischer, J.S. , Priore, R.L. , Jacobs, L.D. , Cookfair, D.L. , Rudick, R.A. , Herndon, R.M. ,... Kooijmans-Coutinho, M. F. ((2000) ). Neuropsychological effects of interferon beta-1a in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Grou. Annals of Neurology, 48: (6), 885–892. |
20 | Gottesman, R.F. , & Hillis, A.E. ((2010) ). Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. The Lancet. Neurology, 9: (9), 895–905. doi: 10.1016/S1474-4422(10)70164-2 |
21 | Gunther, A. , Kuppers-Tiedt, L. , Schneider, P.M. , Kunert, I. , Berrouschot, J. , Schneider, D. , & Rossner, S. ((2005) ). Reduced infarct volume and differential effects on glial cell activation after hyperbaric oxygen treatment in rat permanent focal cerebral ischaemia. The European Journal of Neuroscience, 21: (11), 3189–3194. doi: 10.1111/j.1460-9568.2005.04151.x |
22 | Haapaniemi, T. , Nylander, G. , Kanje, M. , & Dahlin, L. ((1998) ). Hyperbaric oxygen treatment enhances regeneration of the rat sciatic nerve. Experimental Neurology, 149: (2), 433–438. doi: 10.1006/exnr.1997.6745 |
23 | Hadanny, A. , & Efrati, S. ((2016) ). The efficacy and safety of hyperbaric oxygen therapy in traumatic brain injury. Expert Review of Neurotherapeutics, 16: (4), 359–360. doi: 10.1586/14737175.2016.1157018 |
24 | Hadanny, A. , Fishlev, G. , Bechor, Y. , Meir, O. , & Efrati, S. ((2016) ). Nonhealing Wounds Caused by Brown Spider Bites: Application of Hyperbaric Oxygen Therapy. Advances in Skin & Wound Care, 29: (12), 560–566. doi: 10.1097/01.ASW.0000504578.06579.20 |
25 | Hadanny, A. , Golan, H. , Fishlev, G. , Bechor, Y. , Volkov, O. , Suzin, G. ,... Efrati, S. , ((2015) a). Hyperbaric oxygen can induce neuroplasticity and improve cognitive functions of patients suffering from anoxic brain damage. Restorative Neurology and Neuroscience, 33: (4), 471–486. doi: 10.3233/RNN-150517 |
26 | Hadanny, A. , Golan, H. , Fishlev, G. , Bechor, Y. , Volkov, O. , Suzin, G. ,... Efrati, S. , ((2015) b). Hyperbaric oxygen can induce neuroplasticity and improve cognitive functions of patients suffering from anoxic brain damage. Restorative Neurology and Neuroscience, 33: (4), 471–486. doi: 10.3233/RNN-150517 |
27 | Hebert, D. , Lindsay, M.P. , McIntyre, A. , Kirton, A. , Rumney, P.G. , Bagg, S. ,... Teasell, R. ((2016) ). Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. International Journal of Stroke : Official Journal of the International Stroke Society, 11: (4), 459–484. doi: 10.1177/1747493016643553 |
28 | Jacobs, E.A. , Winter, P.M. , Alvis, H.J. , & Small, S.M. ((1969) ). Hyperoxygenation effect on cognitive functioning in the aged. The New England Journal of Medicine, 281: (14), 753–757. doi: 10.1056/NEJM196910022811402 |
29 | Kalaria, R.N. , & Ballard, C. ((2001) ). Stroke and cognition. Current Atherosclerosis Reports, 3: (4), 334–339. |
30 | Kelly-Hayes, M. , Beiser, A. , Kase, C.S. , Scaramucci, A. , D’Agostino, R.B. , & Wolf, P.A. ((2003) ). The influence of gender and age on disability following ischemic stroke: the Framingham study. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 12: (3), 119–126. doi: 10.1016/S1052-3057(03)00042-9 |
31 | Krishnamurthi, R.V. , Feigin, V.L. , Forouzanfar, M.H. , Mensah, G.A. , Connor, M. , Bennett, D.A. ,... Group, G.B.D.S.E. ((2013) ). Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. The Lancet. Global Health, 1: (5), e259–281. doi: 10.1016/S2214-109X(13)70089-5 |
32 | L. Melton, J. ((2005) ). Psychometric Evaluation of the Mindstreams Neuropsychological Screening Tool. |
33 | Lange, G. , Waked, W. , Kirshblum, S. , & DeLuca, J. ((2000) ). Organizational strategy influence on visual memory performance after stroke: cortical/subcortical and left/right hemisphere contrasts. Archives of Physical Medicine and Rehabilitation, 81: (1), 89–94. |
34 | Langhorne, P. , Bernhardt, J. , & Kwakkel, G. ((2011) ). Stroke rehabilitation. Lancet, 377: (9778), 1693–1702. doi: 10.1016/S0140-6736(11)60325-5 |
35 | Lee, W.C. , Joshi, A.V. , Wang, Q. , Pashos, C.L. , & Christensen, M.C. ((2007) ). Morbidity and mortality among elderly Americans with different stroke subtypes. Advances in Therapy, 24: (2), 258–268. |
36 | Lippert, T. , & Borlongan, C.V. ((2019) ). Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer. CNS Neuroscience & Therapeutics, 25: (8), 815–823. doi: 10.1111/cns.13124 |
37 | Lou, M. , Chen, Y. , Ding, M. , Eschenfelder, C.C. , & Deuschl, G. ((2006) ). Involvement of the mitochondrial ATP-sensitive potassium channel in the neuroprotective effect of hyperbaric oxygenation after cerebral ischemia. Brain Research Bulletin, 69: (2), 109–116. doi: 10.1016/j.brainresbull.2005.11.009 |
38 | Lozano, R. , Naghavi, M. , Foreman, K. , Lim, S. , Shibuya, K. , Aboyans, V. ,... Memish, Z.A. ((2012) ). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380: (9859), 2095–2128. doi: 10.1016/S0140-6736(12)61728-0 |
39 | Maulden, S.A. , Gassaway, J. , Horn, S.D. , Smout, R.J. , & DeJong, G. ((2005) ). Timing of initiation of rehabilitation after stroke. Archives of Physical Medicine and Rehabilitation, 86: (12 Suppl 2), S34–S40. doi: 10.1016/j.apmr.2005.08.119 |
40 | Milosevic, J. , Adler, I. , Manaenko, A. , Schwarz, S.C. , Walkinshaw, G. , Arend, M. ,... Schwarz, J. ((2009) ). Non-hypoxic stabilization of hypoxia-inducible factor alpha (HIF-alpha): Relevance in neural progenitor/stem cells. Neurotox Research, 15: (4), 367–380. doi: 10.1007/s12640-009-9043-z |
41 | Mok, V.C. , Wong, A. , Lam, W.W. , Fan, Y.H. , Tang, W.K. , Kwok, T. ,... Wong, K.S. ((2004) ). Cognitive impairment and functional outcome after stroke associated with small vessel disease. Journal of Neurology, Neurosurgery, and Psychiatry, 75: (4), 560–566. |
42 | Mu, J. , Ostrowski, R.P. , Soejima, Y. , Rolland, W.B. , Krafft, P.R. , Tang, J. , & Zhang, J.H. ((2013) ). Delayed hyperbaric oxygen therapy induces cell proliferation through stabilization of cAMP responsive element binding protein in the rat model of MCAo-induced ischemic brain injury. Neurobiology of Disease, 51: , 133–143. doi: 10.1016/j.nbd.2012.11.003 |
43 | Mukoyama, M. , Iida, M. , & Sobue, I. ((1975) ). Hyperbaric oxygen therapy for peripheral nerve damage induced in rabbits with clioquinol. Experimental Neurology, 47: (3), 371–380. |
44 | Niklas, A. , Brock, D. , Schober, R. , Schulz, A. , & Schneider, D. ((2004) ). Continuous measurements of cerebral tissue oxygen pressure during hyperbaric oxygenation–HBO effects on brain edema and necrosis after severe brain trauma in rabbits. Journal of the Neurological Sciences, 219: (1-2), 77–82. doi: 10.1016/j.jns.2003.12.013 |
45 | Nys, G.M. , van Zandvoort, M.J. , de Kort, P.L. , Jansen, B.P. , de Haan, E.H. , & Kappelle, L.J. ((2007) ). Cognitive disorders in acute stroke: Prevalence and clinical determinants. Cerebrovascular Diseases, 23: (5-6), 408–416. doi: 10.1159/000101464 |
46 | Ojaghihaghighi, S. , Vahdati, S.S. , Mikaeilpour, A. , & Ramouz, A. ((2017) ). Comon of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World Journal of Emergency Medicine, 8: (1), 34–38. doi: 10.5847/wjem.j.1920-8642.2017.01.006 paris. |
47 | Ottenbacher, K.J. , & Jannell, S. ((1993) ). The results of clinical trials in stroke rehabilitation research. Archives of Neurology, 50: (1), 37–44. |
48 | Ovbiagele, B. , & Nguyen-Huynh, M.N. ((2011) ). Stroke epidemiology: Advancing our understanding of disease mechanism and therapy. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics, 8: (3), 319–329. doi: 10.1007/s13311-011-0053-1 |
49 | Pasquini, M. , Leys, D. , Rousseaux, M. , Pasquier, F. , & Henon, H. ((2007) ). Influence of cognitive impairment on the institutionalisation rate 3 years after a stroke. Journal of Neurology, Neurosurgery, and Psychiatry, 78: (1), 56–59. doi: 10.1136/jnn2006.102533 |
50 | Patel, M. , Coshall, C. , Rudd, A.G. , & Wolfe, C.D. ((2003) ). Natural history of cognitive impairment after stroke and factors associated with its recovery. Clinical Rehabilitation, 17: (2), 158–166. doi: 10.1191/0269215503cr596oa |
51 | Poli, S. , & Veltkamp, R. ((2009) ). Oxygen therapy in acute ischemic stroke – experimental efficacy and molecular mechanisms. Current Molecular Medicine, 9: (2), 227–241. doi: 10.2174/156652409787581619 |
52 | Powers, W.J. , Rabinstein, A.A. , Ackerson, T. , Adeoye, O.M. , Bambakidis, N.C. , Becker, K. ,... American Heart Association Stroke, C. ((2018) ). 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 49: (3), e46–e110. doi: 10.1161/STR.0000000000000158 |
53 | Prvu Bettger, J.A. , & Stineman, M.G. ((2007) ). Effectiveness of multidisciplinary rehabilitation services in postacute care: state-of-the-science. A review. Archives of Physical Medicine and Rehabilitation, 88: (11), 1526–1534. doi: 10.1016/j.apmr.2007.06.768 |
54 | Rasquin, S.M. , Verhey, F.R. , van Oostenbrugge, R.J. , Lousberg, R. , & Lodder, J. ((2004) ). Demographic and CT scan features related to cognitive impairment in the first year after stroke. Journal of Neurology, Neurosurgery, and Psychiatry, 75: (11), 1562–1567. doi: 10.1136/jnn2003.024190 |
55 | Reinert, M. , Barth, A. , Rothen, H.U. , Schaller, B. , Takala, J. , & Seiler, R.W. ((2003) ). Effects of cerebral perfusion pressure and increased fraction of inspired oxygen on brain tissue oxygen, lte and glucose in patients with severe head injury. Neurochirurgica, 145: (5), 341–349; discussion 349-350. doi: 10.1007/s00701-003-0027-0 Acta. |
56 | Roine, R.O. , Kajaste, S. , & Kaste, M. ((1993) ). Neuropsychological sequelae of cardiac arrest. Jama, 269: (2), 237–242. |
57 | Rosario, E.R. , Kaplan, S.E. , Khonsari, S. , Vazquez, G. , Solanki, N. , Lane, M. ,... Rosenberg, S.S. ((2018) ). The Effect of Hyperbaric Oxygen Therapy on Functional Impairments Caused by Ischemic Stroke. Neurology Research International, 2018: , 12. doi: 10.1155/2018/3172679 |
58 | Rosario, E.R. , Kaplan, S.E. , Khonsari, S. , Vazquez, G. , Solanki, N. , Lane, M. ,... Rosenberg, S.S. ((2018) ). The Effect of Hyperbaric Oxygen Therapy on Functional Impairments Caused by Ischemic Stroke. Neurology Research International, 2018: , 3172679. doi: 10.1155/2018/3172679 |
59 | Schouten, E.A. , Schiemanck, S.K. , Brand, N. , & Post, M.W. ((2009) ). Long-term deficits in episodic memory after ischemic stroke: evaluation and prediction of verbal and visual memory performance based on lesion characteristics. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 18: (2), 128–138. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.017 |
60 | Schwid, S.R. , Goodman, A.D. , Weinstein, A. , McDermott, M.P. , & Johnson, K.P. ((2007) ). Cognitive function in relapsing multiple sclerosis: Minimal changes in a 10-year clinical trial. Journal of the Neurological Sciences, 255: (1), 57–63. doi: 10.1016/j.jns.2007.01.070 |
61 | Soejima, Y. , Hu, Q. , Krafft, P.R. , Fujii, M. , Tang, J. , & Zhang, J.H. ((2013) ). Hyperbaric oxygen preconditioning attenuates hyperglycemia-enhanced hemorrhagic transformation by inhibiting matrix metalloproteinases in focal cerebral ischemia in rats. Experimental Neurology, 247: , 737–743. doi: 10.1016/j.expneurol.2013.03.019 |
62 | Sun, L. , Marti, H.H. , & Veltkamp, R. ((2008) ). Hyperbaric oxygen reduces tissue hypoxia and hypoxia-inducible factor-1 alpha expression in focal cerebral ischemia. Stroke, 39: (3), 1000–1006. doi: 10.1161/STROKEAHA.107.490599 |
63 | Sun, L. , Strelow, H. , Mies, G. , & Veltkamp, R. ((2011) ). Oxygen therapy improves energy metabolism in focal cerebral ischemia. Brain Research, 1415: , 103–108. doi: 10.1016/j.brainres.2011.07.064 |
64 | T. Wagner, M. , & A. Cushman, L. ((2017) ). Intellectual and Memory Functions After Cortical and Subcortical Stroke (Vol. 2). |
65 | Tal, S. , Hadanny, A. , Berkovitz, N. , Sasson, E. , Ben-Jacob, E. , & Efrati, S. , ((2015) a). Hyperbaric oxygen may induce angiogenesis in patients suffering from prolonged post-concussion syndrome due to traumatic brain injury. Restorative Neurology and Neuroscience, 33: (6), 943–951. doi: 10.3233/RNN-150585 |
66 | Tal, S. , Hadanny, A. , Berkovitz, N. , Sasson, E. , Ben-Jacob, E. , & Efrati, S. , ((2015) b). Hyperbaric oxygen may induce angiogenesis in patients suffering from prolonged post-concussion syndrome due to traumatic brain injury. Restorative Neurology and Neuroscience, 33: (6), 943–951. doi: 10.3233/RNN-150585 |
67 | Tal, S. , Hadanny, A. , Sasson, E. , Suzin, G. , & Efrati, S. ((2017) ). Hyperbaric Oxygen Therapy Can Induce Angiogenesis and Regeneration of Nerve Fibers in Traumatic Brain Injury Patients. Frontiers in Human Neuroscience, 11: , 508. doi: 10.3389/fnhum.2017.00508 |
68 | Tatemichi, T.K. , Desmond, D.W. , Paik, M. , Figueroa, M. , Gropen, T.I. , Stern, Y. ,... et al. ((1993) ). Clinical determinants of dementia related to stroke. Annals of Neurology, 33: (6), 568–575. doi: 10.1002/ana.410330603 |
69 | Tatemichi, T.K. , Paik, M. , Bagiella, E. , Desmond, D.W. , Pirro, M. , & Hanzawa, L.K. ((1994) ). Dementia after stroke is a predictor of long-term survival. Stroke, 25: (10), 1915–1919. |
70 | Thaler, A. , Mirelman, A. , Gurevich, T. , Simon, E. , Orr-Urtreger, A. , Marder, K. ,... Consortium, L.A.J. ((2012) ). Lower cognitive performance in healthy G2019S LRRK2 mutation carriers. Neurology, 79: (10), 1027–1032. doi: 10.1212/WNL.0b013e3182684646 |
71 | Veltkamp, R. , Siebing, D.A. , Sun, L. , Heiland, S. , Bieber, K. , Marti, H.H. ,... Schwaninger, M. ((2005) ). Hyperbaric oxygen reduces blood-brain barrier damage and edema after transient focal cerebral ischemia. Stroke; A Journal of Cerebral Circulation, 36: (8), 1679–1683. doi: 10.1161/01.STR.0000173408.94728.79 |
72 | Vila, J.F. , Balcarce, P.E. , Abiusi, G.R. , Dominguez, R.O. , & Pisarello, J.B. ((2005) ). Improvement in motor and cognitive impairment after hyperbaric oxygen therapy in a selected group of patients with cerebrovascular disease: A prospective single-blind controlled trial. Undersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc, 32: (5), 341–349. |
73 | Vilela, D.S. , Lazarini, P.R. , & Da Silva, C.F. ((2008) ). Effects of hyperbaric oxygen therapy on facial nerve regeneration. Acta oto-laryngologica, 128: (9), 1048–1052. doi: 10.1080/00016480701827525. |
74 | Williams, G.R. , Jiang, J.G. , Matchar, D.B. , & Samsa, G.P. ((1999) ). Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke, 30: (12), 2523–2528. |
75 | Yang, Y.J. , Wang, X.L. , Yu, X.H. , Wang, X. , Xie, M. , & Liu, C.T. ((2008) ). Hyperbaric oxygen induces endogenous neural stem cells to proliferate and differentiate in hypoxic-ischemic brain damage in neonatal rats. Undersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc, 35: (2), 113–129. |
76 | Yildiz, S. , Kiralp, M.Z. , Akin, A. , Keskin, I. , Ay, H. , Dursun, H. , & Cimsit, M. ((2004) ). A new treatment modality for fibromyalgia syndrome: Hyperbaric oxygen therapy. The Journal of International Medical Research, 32: (3), 263–267. doi: 10.1177/147323000403200305 |
77 | Yin, D. , Zhou, C. , Kusaka, I. , Calvert, J.W. , Parent, A.D. , Nanda, A. , & Zhang, J.H. ((2003) ). Inhibition of apoptosis by hyperbaric oxygen in a rat focal cerebral ischemic model. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 23: (7), 855–864. doi: 10.1097/01.WCB.0000073946.29308.55 |
78 | Yoneda, Y. , Okuda, S. , Hamada, R. , Toyota, A. , Gotoh, J. , Watanabe, M. ,... Hasegawa, Y. ((2005) ). Hospital cost of ischemic stroke and intracerebral hemorrhage in Japanese stroke centers. Health Policy, 73: (2), 202–211. doi: 10.1016/j.healthpol.2004.11.016 |
79 | Yu, M. , Xue, Y. , Liang, W. , Zhang, Y. , & Zhang, Z. ((2015) ). Protection mechanism of early hyperbaric oxygen therapy in rats with permanent cerebral ischemia. Journal of Physical Therapy Science, 27: (10), 3271–3274. doi: 10.1589/jpts.27.3271 |
80 | Zhang, J.H. , Lo, T. , Mychaskiw, G. , & Colohan, A. ((2005) ). Mechanisms of hyperbaric oxygen and neuroprotection in stroke. Pathophysiology: The Official Journal of the International Society for Pathophysiology, 12: (1), 63–77. doi: 10.1016/j.pathophys.2005.01.003 |
81 | Zur, D. , Naftaliev, E. , & Kesler, A. ((2014) ). Evidence of Multidomain Mild Cognitive Impairment in Idiopathic Intracranial Hypertension. Journal of Neuro-Ophthalmology : The Official Journal of the North American Neuro-Ophthalmology Society. doi: 10.1097/WNO.0000000000000199 |




