Hyperbaric oxygen can induce neuroplasticity and improve cognitive functions of patients suffering from anoxic brain damage
Abstract
Purpose: Cognitive impairment may occur in 42–50% of cardiac arrest survivors. Hyperbaric oxygen therapy (HBO2) has recently been shown to have neurotherapeutic effects in patients suffering from chronic cognitive impairments (CCI) consequent to stroke and mild traumatic brain injury.
The objective of this study was to assess the neurotherapeutic effect of HBO2 in patients suffering from CCI due to cardiac arrest.
Methods: Retrospective analysis of patients with CCI caused by cardiac arrest, treated with 60 daily sessions of HBO2. Evaluation included objective computerized cognitive tests (NeuroTrax), Activity of Daily Living (ADL) and Quality of life questionnaires. The results of these tests were compared with changes in brain activity as assessed by single photon emission computed tomography (SPECT) brain imaging.
Results: The study included 11 cases of CCI patients. Patients were treated with HBO2, 0.5–7.5 years (mean 2.6 ± 0.6 years) after the cardiac arrest. HBO2 was found to induce modest, but statistically significant improvement in memory, attention and executive function (mean scores) of 12% , 20% and 24% respectively. The clinical improvements were found to be well correlated with increased brain activity in relevant brain areas as assessed by computerized analysis of the SPECT imaging.
Conclusions: Although further research is needed, the results demonstrate the beneficial effects of HBO2 on CCI in patients after cardiac arrest, even months to years after the acute event.
1Introduction
Despite advanced cardiac life support (ACLS), the mortality rate from sudden death after cardiac arrest is 85–95% (Nichol et al., 2008). Moreover, 33–50% of ACLS survivors develop significant neurological dysfunction with variable extent, and these percentages rise to nearly 100% in the rare survivors of non-witnessed arrests (Allen & Buckberg, 2012).
The neuropathology of anoxic brain injury (ABI) includes neural toxicity, intracellular calcium accumulation, oxygen free radicals, neurotransmitters and excitatory amino acid release, reperfusion injury and endothelial injury (Guo, Yu, & Ma, 2011). The hippocampus, basal ganglia (especially the globus pallidum, thalamus) and deep central white matter regions are the first to suffer damage due to their high metabolic requirements (Hopkins & Bigler, 2012). As the duration of anoxia increases, global nonspecific damage in other parts of the brain develops. Accordingly, the clinical presentation includes impairments in visual perception, expression, cognition and motor coordination (Fitzgerald, Aditya, Prior, McNeill, & Pentland, 2010; Hopkins & Bigler, 2012). Cognitive impairment is common and may occur in 42–50% of the survivors (Moulaert, Verbunt, van Heugten, & Wade, 2009; van Alem, de Vos, Schmand, & Koster, 2004). Memory, attention and executive function impairments are the most common (van Alem et al., 2004).
Unfortunately, to date, there is no effective therapy that can induce neuroplasticity with significant cognitive improvement in these survivors. While neurorehabilitation starting in the first 3 months may result in modest improvement (Fertl, Vass, Sterz, Gabriel, & Auff, 2000), significant cognitive improvement later than 3 months is considered to be very rare (Lim, Verfaellie, Schnyer, Lafleche, & Alexander, 2014). In general, in comparison to the recovery from traumatic brain injury (TBI), ABI patients are left with more severe cognitive impairments and worse functional debilitation (Shah, Al-Adawi, Dorvlo, &Burke, 2004).
The neuroplasticity effects of hyperbaric oxygen therapy (HBO2) on the chronic damaged neuronal tissue have recently been evaluated in patients with chronic neurological deficiencies due to stroke or TBI (Boussi-Gross et al., 2013, 2014; Efrati et al., 2013). HBO2 includes the inhalation of 100% oxygen at pressures exceeding 1 atmosphere absolute (ATA) in order to enhance the amount of oxygen dissolved in the body tissues. As explained in detail by Efrati and Ben-Jacob (2014), the diverse and powerful innate repair mechanisms activated by HBO2 are associated both with the elevated level of dissolved oxygen and with the elevated pressure. The beneficial physiological effects of HBO2 on the injured brain and cognitive function have been demonstrated in several animal models (Boussi-Gross et al., 2014; Palzur et al., 2004; Palzur, Zaaroor, Vlodavsky, Milman, & Soustiel, 2008).
There is previous evidence for cognitive improvements after HBO2 in various neurological conditions (Barrett et al., 1998; Boussi-Gross et al., 2013, 2014; Jacobs, Winter, Alvis, & Small, 1969; Mukherjee et al., 2014; Rossignol, Rossignol, James, Melnyk, & Mumper, 2007; Tapeantong & Poungvarin, 2009); however, the neuroplasticity effects of HBO2 on patients suffering from ABI have not yet been investigated.
The purpose of the current study was to present the first series of cases of ABI patients suffering fromchronic cognitive impairments after cardiac arrest treated by HBO2.
2Methods
2.1Participants
Retrospective analysis of patients with CCI caused by cardiac arrest, treated at the institute of Hyperbaric Medicine, Assaf Harofeh Medical Center, Israel, between January 2008 and December 2014. The study was approved by the institutional review board of the hospital.
Inclusion criteria: patients who have completed two cognitive evaluations and assessments of SPECT brain imaging, before and after HBO2.
All patients applied for HBO2 on their own interest and funded the sessions. Imaging and functional tests were funded by Assaf Harofe Hyperbaric institute. This were not informed on a research trial before or after their sessions.
2.2Hyperbaric oxygen treatment
Patients were treated in a multiplace hypberbaric chamber (HAUX-Life-Support GmbH.) for 60 daily sessions, 5 days per week. Each session consisted of 60 minutes exposure to 100% oxygen at 1.5 ATA with 5 minutes air breaks every 30 minute. Acceptable compression and decompression rates of0.8 meter per minute were used. Oxygen was supplied by tight masks.
2.3Cognitive assessment
Patients’ cognitive functions were assessed by NeuroTrax computerized cognitive tests (NeuroTrax Corp., TX) (Dwolatzky et al., 2003). NeuroTrax tests evaluate various aspects of brain functions and include: Verbal Memory (immediate and delayed recognition), Non-Verbal Memory (immediate and delayed recognition), Go-No-Go Response Inhibition, Problem Solving, Stroop Interference, Finger Tapping, Catch Game, Staged Information Processing Speed (single digit, two-digit and three-digit arithmetic), Verbal Function and Visual Spatial Processing. Cognitive index scores were computed from normalized outcome parameters for these tests for memory, executive function, attention, information processing speed, visual spatial, verbal function and motor skills domains (Achiron et al., 2013; Thaler et al., 2012; Zur, Naftaliev, & Kesler, 2014). A global cognitive score was computed as the average of all index scores for eachindividual.
After administration, NeuroTrax data were uploaded to the NeuroTrax central server, and outcome parameters were automatically calculated using software blind to diagnosis or testing site. To account for the well known effects of age and education on cognitive performance, each outcome parameter was normalized and fit to an IQ-like scale (mean = 100, S.D. = 15) according to the patient’s age and education. The normative data used by NeuroTrax consists of test data from cognitively healthy individuals in controlled research studies at more than 10 sites (Doniger, 2014).
Notably, the patients were given two different test versions of the NeuroTrax test battery before and after HBO2, to allow repeated administrations with minimal learning effect. Test-retest reliability for those versions was evaluated and found high, with no significant learning effect (Schweiger, 2003; Melton, 2005).
2.4Activities of Daily Living assessment
The Activities of Daily Living (ADL) were evaluated by a questionnaire covering the following functions: bathing, dressing, grooming, oral care, toileting, walking, climbing stairs, eating, shopping, cooking, managing medications, using phone, housework, doing laundry, driving and managing finances (Katz, 1983). For each function, the patient made a dependency rating ranging from independent, needs help, dependent or does not do at all, with a total score ranging from 0 (worst) to 51 (best).
2.5Quality of Life assessment
Quality of Life (QOL) was evaluated by the EQ-5D questionnaire (Rabin & de Charro, 2001). EQ-5D essentially consists of 2 pages: the EQ-5D descriptive system and the EQ visual analogue scale (EQ-VAS). The EQ-5D descriptive system covers mobility, self-care, usual activities, pain/discomfort and anxiety/depression [range: 5 (best) – 15 (worst)]. The EQ-VAS records the respondent’s self-rated health on a vertical, visual analogue scale [range: 0 (worst) −100 (best)].
2.6Brain functional imaging
Brain activity was assessed using single photon emission computed tomography (SPECT) 1-2 weeks prior to and after the HBO2 period. The imaging was conducted using 925–1,110 MBq (25–30 mCi) of technetium-99m-methyl-cysteinate-dimmer (Tc-99m-ECD) at 40–60 min post injection using a dual detector gamma camera (ECAM or Symbia T, Siemens Medical Systems) equipped with high resolution collimators. Data were acquired in 3-degree steps and reconstructed iteratively with Chang method (μ= 0.12/cm) attenuation correction (Jaszczak, Chang, Stein, & Moore, 1979).
Both SPECT studies were normalized to the median brain activity in the entire brain and were then reoriented into Talairach space using NeuroGam (Segami Corporation) to identify Brodmann cortical areas and in order to compute the mean perfusion in each Brodmann area (BA). In addition, volume rendered brain perfusion images were reconstructed and normalized to entire brain median activity. All SPECT analyses were done by study team members who were blinded to the laboratory and clinical data. SPECT scans were performed late morning to midday. On the day of the SPECT scan, patients were treated with only their chronic medications and were instructed not to smoke. Changes in perfusion in all Brodmann areas for each subject were determined by calculating the percentage of the difference of the normalized activity value between post-treatment and pre-treatment divided by the pre-treatment value.
2.7Statistical analysis
Continuous data were expressed as means ± standard errors. Relative changes were calculated by subtracting the pre-treatment score from the post-treatment score, and dividing by the pre-treatment score. Normal distribution for all continuous variables was tested using Kolmogorov-Smirnov test. The cognitive index scores and ADL mean differences before and after HBO2 were analyzed using two tailed paired t-test. EQ-VAS mean difference before and after HBO2 was analyzed using Wilcoxon signed ranks test. Alpha level was set to 0.05. The calculated sample size for 25% change in means was 10 patients. Data were statistically analyzed using SPSS software (version 22.0).
3Results
3.1Patients’ profile
Of 28 patients suffering from ABI treated at the institute of Hyperbaric Medicine, Assaf Harofeh Medical Center, Israel between January 2008 and December 2014, eleven patients had two neuro-cognitive evaluations before and after HBO2.
Among these patients, average age was 45.6 ± 4.4 years (29–70), and 9 out of 11 patients (81%) were males. All patients had chronic stable neuro-cognitive impairment, and they began HBO2 5 months to 7.5 years (mean 2.6 ± 0.6 years) after cardiac arrest. Baseline patients’ characteristics are summarizedin Table 1.
3.2Neuro-cognitive evaluation
High inter-patient variability was present in the baseline cognitive indices. The magnitude of the change in a cognitive score has different implications for patients at low or high baseline levels. Hence, we inspect the effect of HBO2 on the relative changes, i.e. the change relative to the baseline value. Calculating mean ofrelative changes is more informative than calculating the changes in mean values, especially in small groups with high patient to patient variability.
One of the patients that had prominent improvement from non-measureable scores, due to insufficient responses prior to treatment, to measureable scores in all cognitive domains after treatment was not included in the means’ analysis.
The effect of the hyperbaric oxygen treatment on the patients’ cognitive functions, as assessed by the eight cognitive summary scores, is summarized in Table 2. As can be seen, HBO2 induced significant improvement in the global cognitive scores with a mean relative change of 8% (p = 0.006). The most prominent improvement was in executive functions indices, with 24% mean relative change (p = 0.011). Attention indices improved by mean relative change of 20% (p = 0.06). Memory indices by mean relative change of 12% (p = 0.08) (Table 2, Fig. 1).
3.3Activity of daily living
Two of the patients could not complete the ADL questionnaire before HBO2. In the nine patients who completed the pre and post HBO2 questionnaire, ADL score was significantly improved after HBO2 by an average of 5 points (p = 0.002) (Table 3, Fig. 1).
3.4Quality of life evaluation
Nine patients completed pretreatment quality of life questionnaires. Compared to the pre-treatment score, EQ-VAS and EQ-5D scores after HBO2 improved significantly (p = 0.027 and p = 0.015, respectively) (Table 3, Fig. 1).
3.5Functional imaging of the brain-SPECT
Five patients had brain SPECT evaluations before and after HBO2. Six patients refused brain-SPECT imaging due to radiation exposure. The mean relative changes of all patients revealed significant improvement (higher than 10%) in brain activity (reflected by perfusion) following HBO2 in the cingulate gyrus (BA 31,32,23,24), inferior frontal gyrus (BA 47,44,45), perirhinal cortex (BA 36), primary visual cortex (BA 17) and parietal lobe (BA 7)(Figs. 2 and 3).
3.6Representative clinical cases
3.6.1Case 1: 52-year-old male, one year post ventricular fibrillation, suffering from ataxia and significant cognitive decline
Baseline cognitive evaluation showed a very low memory index score and low executive function, verbal function, information processing speed, attention, and motor skills index scores. After 60 HBO2 sessions, the patient had improvements in verbal function, executive function and attention from scores of 71, 73 and 68 to 106, 90 and 81, respectively. Improvement was also seen in memory from 25 to 43, yet the score was still low. Clinically, with regards to his ADL after HBO2, the patient was able to participate in housework, managing finances and shopping.
Brain SPECT evaluation showed the that the largest post HBO2 increase in brain activity was in the perirhinal cortex (BA 36) with over 50% increase, in the primary visual cortex (BA 17,18) with over 30% increase and in the cingulate gyrus (BA 23, 31) with over 20% increase (Fig. 4).
3.6.2Case 2: 48-year-old male, 2 years post resuscitation, suffering from motor dysphasia, left-right disorientation and significant cognitive dysfunction
Cognitive evaluation at baseline showed low memory, visual spatial and executive function scores in addition to low attention and motor scores. After 60 HBO2 sessions, the patient had improvement in memory, visual spatial and executive function from scores of 58, 70 and 74 to 68, 76 and 79, respectively. Clinically, he had complete resolution of dysphasia. Improvements were also noticed in ADL abilities such as ability to do laundry and manage finances. Additionally, he was able to return to his previous work.
Brain SPECT evaluation showed the largest post HBO2 increase in the parietal lobes (BA 5,7), temporal lobe with over 25% increase, in the inferior orbital gyrus (BA 45,47), cingulate gyrus (BA 23,24), in Wernike’s area (BA 39) and in the primary visual cortex (BA 17,18) with over 15% increase (Fig. 5). See Appendix for three additional case reports.
3.7Safety
The treatment was well tolerated and all patients completed the treatment protocol. No significant adverse reactions were recorded in any of the patients.
4Discussion
The neurotherapeutic effects of HBO2 in patients suffering from CCI caused by cardiac arrest mediated anoxic brain injury, were evaluated by both clinical and brain imaging measures. Even though the acute injury was 5 months to 7.5 years (mean 31.3 ± 7.7 months) prior to treatment, HBO2 was associated with significant cognitive improvement in all patients. The clinical improvements were well documented by neurocognitive tests and correlated with improved ability to perform the activities of daily living and quality of life. The most significant measurable improvements were in executive function, attention and memory.
Important validation and clues for future, larger scale studies were provided by the SPECT brain imaging. We found the clinical improvement to be well correlated with increased activity in the relevant brain area. More specifically, the brain areas that had the most significant increase in metabolic activation were in the perirhinal cortex (BA36), the pre-frontal cortex (BA 8,9,10,11), inferior frontal gyrus (BA 45,47), the anterior cingulate gyrus (BA 23,24) and the parietal lobes (BA 5,7). A good correlation was found between the improved neurocognitive functions and the brain areas corresponding to these functions:
- The perirhinal cortex activation after HBO2 was most prominent in patients that had significant memory improvement. The perirhinal cortex has a critical role in object recognition memory while interacting with the hippocampus (Brown & Aggleton, 2001). Because the memoryassessments in the cognitive tests were indeed recognition tasks, this area might be expected to be involved.
- The pre-frontal cortex (BA 10, 11) and, more specifically, the inferior frontal gyrus (BA 45, 47) activation after HBO2 were prominent in all patients with significant executive function improvements. The right frontal gyrus is known to mediate a go/no go task (Aron, Robbins, & Poldrack, 2004), which was among the executive function tests used in the present study. The prefrontal gyrus is presumed to act as a filtering system that enhances goal directed activities and inhibits irrelevantactivations. This filtering mechanism enables executive control (Miller & Cohen, 2001).
- The anterior cingulate gyrus (BA 23, 24) activation after HBO2 was seen in the subjects with attention improvement. The anterior cingulate gyrus is presumed to be involved in error detection, especially in a Stroop task (Bush, Luu, & Posner, 2000), which was used in the attention tests. Lesions in this area can cause inattention to akinetic mutism (Bush et al., 2000).
- The posterior parietal lobes are involved in visual-spatial processing. Lesions in the right parietal lobe are known to cause visual spatial construction deficits (Mishkin & Ungerleider, 1982). Activations of these areas were seen in the patients with visual-spatial index improvement.
The changes revealed by inspection of the pre and post SPECT images indicate that HBO2 can induce reactivation of neuronal activity in stunned areas in agreement with earlier studies (Barrett, 1998; Churchill et al., 2013; Jacobs et al., 1969). This implies that increasing the plasma dissolved oxygen with hyperbaric oxygenation is a potent mean of delivering sufficient oxygen to the brain for repair processes and induction of neuroplasticity.
In the past 30 years, there have been several studies on HBO2 effects in the delayed phase (>1 month) of anoxic brain injury caused by different triggers and in varying ages (Collet et al., 2001; Jain, 2009; Montgomery et al., 1999; van Bever Donker, 1999, 2001). Most studies were focused on children with cerebral palsy (Hardy et al., 2002). Neubauer et al reported on 8 adults with non-cardiac related anoxic enceophalopathy who showed clinical improvement after more than 100 sessions of HBO2 using different hyperbaric protocols (Neubauer & James, 1998). Golden et al. showed improved cognitive functions in 21 adults with chronic brain injury (Golden, Golden, & Neubauer, 2006); however, the causes and extent of the injuries were not reported. As in the current study, the Neubauer and Golden studies showed good correlations with brain activity as evaluated by brain SPECT. In Churchill et al. prospective study, 13 patients with anoxic brain injury had subjective cognitive improvement after HBO2however no change was observed in standardizedtesting (Churchill et al., 2013).
The NeuroTrax cognitive tests, used for the cognitive evaluation, are validated for test-retest evaluations. Data from healthy young adults retested withNeuroTrax test battery on the same day, (Doniger, Simon, & Zivotofsky, 2006) demonstrated small mean relative changes (<5%), and intra class correlations provided support for good reliability. Middle age adults retested with an alternate form of the same battery, (A. Schweiger, 2003) showed stable cognitive scores with small mean relative changes (<5%) except in verbal function (16%) and information processing speed (8%). In the current study, after HBO2, the patients showed improvement in global cognitive function memory and executive function indices of 14–42% , with retesting more than 3 months after the baseline evaluation.
Our findings are in agreement with the previous studies evaluating the effects of HBO2 in the chronic phase of ABI (Hardy et al., 2002; Golden et al., 2006). To our knowledge, this is the first case series of HBO2 application in the chronic phase of brain anoxia focused on patients after cardiac arrest. The use of objective cognitive tests in tandem with objective metabolic brain imaging in the current study, strengthens the conclusion that HBO2 can induce neuroplasticity even at the late chronic stage of ABI.
This study has several limitations. The major one relates to the retrospective methodology and the relatively small number of patients. Still, the findings presented here are in agreement with and reinforce similar findings from previous prospective controlled trials in which the neuroplasticity effects of HBO2 were demonstrated in chronic stages of other brain related injuries (Boussi-Gross et al., 2013). These studies provided convincing evidence that HBO2 can induce neuroplasticity at chronic stages in areas with metabolic dysfunction. Moreover, the natural history of anoxic brain injury implies that maximal recovery is expected during the first 3 months, and no further significant improvement is expected after 3 months (Lim et al., 2014). Accordingly, any significant improvement after 5 months may be attributed to the intervention used. Although significant clinical improvement at a relatively late chronic stage and high correlation with the improved metabolic activity support the validity of the findings, larger randomized prospective controlled trials are needed.
Another important limitation relates to the HBO2 protocol. Although significant neurotherapeutic effects were achieved with 60 sessions of 1.5 ATA for 60 minutes, the exact protocol needed to induce maximal neuroplasticity with minimal side effects remains unknown. There is cumulative data that even smaller increase in pressure might be as effective (Efrati & Ben-Jacob, 2014).
As stated above, further randomized controlledtrials with large patient cohorts are needed to betterunderstand who the best candidates are and what the optimal HBO2 protocol is for patients suffering from ABI.
5Conclusions
Hyperbaric oxygen therapy may improve cognitive functions even during the late chronic phase after ABI resulting from cardiac arrest. Due to the growing numbers of cardiac arrest survivors, the application of HBO2 in this population should be considered. Further prospective randomized clinical studies should be carried out in order to evaluate the patients who can benefit the most from the treatment and the optimal HBO2 protocol (dose and duration) for thispopulation.
Declaration of interest
The authors have no financial or personal relationships to disclose regarding this research and data.
Appendix
Case 3: 70 years old male, 5 years post cardiac arrest, suffering from motor dysphasia, 4 limbs spasticity with no ability to walk, swallowing difficulties and incontinence.
At baseline, the patient could not complete the cognitive evaluation due to very low communication and cognitive skills. After 60 HBO2 sessions, clinically, he had considerable improvement in speech, controlled continence, swallowing and walking capabilities. In addition, in the neuro-cognitive test he had significant improvement in memory, attention, verbal and visual spatial indices from unmeasurables to scores of 98, 70, 102, 89 and 89.7 respectively.
In brain SPECT evaluation, the greatest improvement in brain perfusion (higher than 20%) following HBO2 was seen in the frontal lobes – pre frontal gyrus (BA 10) orbito-frontal gyrus (BA 11) inferior frontal gyrus (BA 45,47), pre motor cortex (BA 6). Improvement (higher than 10%) was also seen in the parietal lobes (BA 7), temporal lobes (BA 20,38) and cingulate gyrus (BA 24,32) (Fig. 6).
Case 4: 57 years old male, 2.5 years post ventricular fibrillation, suffered from dysarthria and significant cognitive dysfunction.
Cognitive evaluation showed low memory, verbal and visual spatial, executive function, information processing speed and attention index scores. Motor skills were high at baseline. After 60 HBO2 daily sessions, cognitive evaluation revealed improvement to in executive function, verbal, visual spatial and memory indices from scores of 98, 88, 84, 69 to 111, 104, 104, 79 respectively.
Clinically, in addition to cognitive improvement, the patient had considerable improvement in speech and daily function – he returned to work and drive by himself.
SPECT evaluation demonstrated the greatest improvement in brain perfusion after HBO2 in the inferior frontal gyrus (BA 45,47), posterior cingulate gyrus (BA 31,32) with over 20% change. Over 10% changes in prefrontal gyrus (BA 8,9), Broca’s and Wernike’s areas (BA 22,39,44) (Fig. 7).
Case 5: 30 years old male, 5 months post ventricular fibrillation suffered from ataxia and significant cognitive decline.
Cognitive evaluation at baseline showed very low memory, attention and executive function scores, and low information processing speed, motor skills, visual spatial and verbal scores. After 60 HBO2 sessions, cognitive evaluation showed improvement in executive function and attention from scores of 44, 38 to 62 and 59 respectively.
SPECT evaluation revealed the greatest improvement in brain perfusion after HBO2 in the perirhinal cortex (BA 36) with over 20% change, inferior frontal gyrus (BA 47), cingulate gyrus (BA 24), primary visual gyrus (BA 17), prefrontal gyrus (BA 10), and inferior temporal gyrus (BA 20) with over 15% change(Fig. 8).
Acknowledgments
The authors would like to thank Dr. Glen Doniger (Neurotrax Corp.) for his contribution. NeuroTrax Corp. did not have a role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation or approval of the manuscript, or decision to submit the manuscript for publication.
The study was funded by the Assaf HaRofeh Medical Center research fund.
REFERENCE
2 | Achiron A, Chapman J, Magalashvili D, Dolev M, LavieM , Bercovich E(2013) Modeling of cognitiveimpair-ment by disease duration in multiple sclerosis: Across-sectional studyPloS One8: 8e71058doi: 10.1371/journal.pone.0071058 |
3 | Allen BS, Buckberg GD(2012) Studies of isolatedglobal brain ischaemia: I. Overview of irreversible brain injuryand evolution of a new concept – redefining the timeof brain deathEuropean Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery41: 511321137doi: 10.1093/ejcts/ezr315 |
4 | Aron AR, Robbins TW, Poldrack RA(2004) Inhibition and the right inferior frontal cortexTrends in Cognitive Sciences8: 4170177doi: 10.1016/j.tics.2004.02.010 |
5 | Barrett KF, Masel BE, Harch PG, Ingram F, Corson KP, Mader JT(1998) Cerebral blood flow changes and cognitiveimprovement in chronic stable traumatic brain injuries treatedwith hyperbaric oxygen therapyNeurology50: 178179 |
6 | Barrett KFMB, Harch PG, Ingram F, Corson KP(1998) Cerebral blood flow changes and cognitive improvement inchronic stable traumatic brain injuries treated with hyperbaricoxygen therapy. ANeurology50: 178179 |
7 | Boussi-Gross R, Golan H, Fishlev G, Bechor Y, Volkov O, Bergan J(2013) Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury - randomized prospective trialPloS One8: 11e79995doi: 10.1371/journal.pone.0079995 |
8 | Boussi-Gross R, Golan H, Volkov O, Bechor Y, Hoofien D, Schnaider Beeri M(2014) Improvement of MemoryImpairments in Poststroke Patients by Hyperbaric Oxygen TherapyNeuropsychologydoi: 10.1037/neu0000149 [Epub ahead ofprint] |
9 | Brown MW, Aggleton JP(2001) Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature reviewsNeuroscience2: 15161doi: 10.1038/35049064 |
10 | Bush G, Luu P, Posner MI(2000) Cognitive and emotional influences in anterior cingulate cortexTrends in Cognitive Sciences4: 6215222 |
11 | Churchill S, Weaver LK, Deru K, Russo AA, Handrahan D, Orrison WWJr(2013) A prospective trial of hyperbaric oxygen for chronic sequelae after brain injury (HYBOBIUndersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc40: 2165193 |
12 | Collet JP, Vanasse M, Marois P, Amar M, Goldberg J, Lambert J(2001) Hyperbaric oxygen for children with cerebral palsy: A randomised multicentre trial. HBO-CP Research GrouLancet357: 9256582586 |
13 | Doniger, G.M. (2014). Guide to Normative Data. Retrieved July, 16th, 2014, from http://www.neurotrax.com/docs/norms_guide.pdf |
14 | Doniger GM, Simon ES, Zivotofsky AZ(2006) Comprehensive computerized assessment of cognitive sequelae of a complete hour fastBehavioral Neuroscience120: 4804816doi: 10.1037/0735-7044.120.4.804 |
15 | Dwolatzky T, Whitehead V, Doniger GM, Simon ES, Schweiger A, Jaffe D(2003) Validity of a novel computerized cognitive battery for mild cognitive impairmentBMC Geriatrics3: 4doi: 10.1186/1471-2318-3-4 |
16 | Efrati S, Ben-Jacob E(2014) How and why hyperbaric oxygen therapy can bring new hope for children suffering from cerebral palsy–an editorial perspectiveUndersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc41: 27176 |
17 | Efrati S, Ben-Jacob E(2014) Reflections on theneurotherapeutic effects of hyperbaric oxygenExpert Rev Neurother14: 3233236doi: 10.1586/14737175.2014.884928 |
18 | Efrati S, Fishlev G, Bechor Y, Volkov O, Bergan J, Kliakhandler K(2013) Hyperbaric oxygen induces lateneuroplasticity in post stroke patients–randomized, prospectivetrialPloS One8: 1e53716doi: 10.1371/journal.pone.0053716 |
19 | Fertl E, Vass K, Sterz F, Gabriel H, Auff E(2000) Neurological rehabilitation of severely disabled cardiac arrest survivors. Part I. Course of post-acute inpatient treatmentResuscitation47: 3231239 |
20 | Fitzgerald A, Aditya H, Prior A, McNeill E, Pentland B(2010) Anoxic brain injury: Clinical patterns and functionaloutcomes. A study of casesBrain injury: [BI]24: 1113111323doi: 10.3109/02699052.2010.506864 |
21 | Golden Z, Golden CJ, Neubauer RA(2006) Improving neuropsychological function after chronic brain injury with hyperbaric oxygenDisability and Rehabilitation28: 2213791386doi: 10.1080/09638280600638364 |
22 | Guo MF, Yu JZ, Ma CG(2011) Mechanisms related to neuron injury and death in cerebral hypoxic ischaemiaFolia Neuropathologica/Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences49: 27887 |
23 | Hardy P, Collet JP, Goldberg J, Ducruet T, Vanasse M, Lambert J(2002) Neuropsychological effects of hyperbaric oxygen therapy in cerebral palsyDevelopmental Medicine and Child Neurology44: 7436446 |
24 | Hopkins RO, Bigler ED(2012) Neuroimaging of anoxic injury: Implications for neurorehabilitationNeuroRehabilitation31: 3319329doi: 10.3233/NRE-2012-0799 |
25 | Jacobs EA, Winter PM, Alvis HJ, Small SM(1969) Hyperoxygenation effect on cognitive functioning in the agedThe New England Journal of Medicine281: 14753757doi: 10.1056/NEJM196910022811402 |
26 | Jain KK(2009) Textbook of Hyperbaric Medicine |
27 | Jaszczak RJ, Chang LT, Stein NA, Moore FE(1979) Whole-body single-photon emission computed tomography using dual,large-field-of-view scintillation camerasPhysics inMedicine and Biology24: 611231143 |
28 | Katz S(1983) Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily livingJournal of the American Geriatrics Society31: 12721727 |
29 | Lim C, Verfaellie M, Schnyer D, Lafleche G, Alexander MP(2014) Recovery, long-term cognitive outcome and quality oflife following out-of-hospital cardiac arrestJournal ofrehabilitation medicine: Official Journal of the UEMS EuropeanBoard of Physical and Rehabilitation Medicine46: 691697doi: 10.2340/16501977-1816 |
30 | Melton JL(2005) Psychometric evaluation of the Mindstreams neuropsychological screening tool0610Navy Experimental Diving Unit (US)Panama City (FL) |
31 | Miller EK, Cohen JD(2001) An integrative theory of prefrontal cortex functionAnnual Review of Neuroscience24: 167202doi: 10.1146/annurev.neuro.24.1.167 |
32 | Mishkin M, Ungerleider LG(1982) Contribution of striateinputs to the visuospatial functions of parieto-preoccipitalcortex in monkeysBehavioural Brain Research6: 15777 |
33 | Montgomery D, Goldberg J, Amar M, Lacroix V, Lecomte J, Lambert J(1999) Effects of hyperbaric oxygen therapy on children with spastic diplegic cerebral palsy: A pilot projectUndersea & Hyperbaric Medicine: Journal of the Undersea and Hyperbaric Medical Society, Inc26: 4235242 |
34 | Moulaert VR, Verbunt JA, van Heugten CM, Wade DT(2009) Cognitive impairments in survivors of out-of-hospitalcardiac arrest: A systematic reviewResuscitation80: 3297305doi: 10.1016/j.resuscitation.2008.10.034 |
35 | Mukherjee A, Raison M, Sahni T, Arya A, Lambert J, Marois P(2014) Intensive rehabilitation combined withHBO therapy in children with cerebral palsy: A controlledlongitudinal studyUndersea & Hyperbaric Medicine: Journalof the Undersea and Hyperbaric Medical Society, Inc41: 27785 |
36 | Neubauer RA, James P(1998) Cerebral oxygenation and the recoverable brain. S-SNeurological Research20: Suppl 136 |
37 | Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP(2008) Regional variation in out-of-hospital cardiac arrest incidence and outcomeJama300: 1214231431doi: 10.1001/jama.300.12.1423 |
38 | Palzur E, Vlodavsky E, Mulla H, Arieli R, Feinsod M, Soustiel JF(2004) Hyperbaric oxygen therapy for reduction of secondary brain damage in head injury: An animal model of brain contusionJournal of Neurotrauma21: 14148doi: 10.1089/089771504772695931 |
39 | Palzur E, Zaaroor M, Vlodavsky E, Milman F, Soustiel JF(2008) Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane propertiesBrain Research1221: 126133doi: 10.1016/j.brainres.2008.04.078 |
40 | Rabin R, de Charro F(2001) EQ-D: A measure of health status from the EuroQol GrouAnnals of Medicine33: 5337343 |
41 | Rossignol DA, Rossignol LW, James SJ, Melnyk S, Mumper E(2007) The effects of hyperbaric oxygen therapy on oxidative stress, inflammation, and symptoms in children with autism: An open-label pilot studyBMC Pediatrics7: 36doi: 10.1186/1471-2431-7-36 |
1 | Schweiger A, G MD, Dwolatzky T, Jaffe D, Simon ES(2003) Reliability of a novel computerized neuropsychological battery for mild cognitive impairmentActa Neuropsychologica1: 4407413 |
42 | Shah MK, Al-Adawi S, Dorvlo AS, Burke DT(2004) Functional outcomes following anoxic brain injury: A comparison with traumatic brain injuryBrain Injury: [BI]18: 2111117doi: 10.1080/0269905031000149551 |
43 | Tapeantong T, Poungvarin N(2009) Delayed encephalopathy and cognitive sequelae after acute carbon monoxide poisoning: Report of a case and review of the literatureJ Med Assoc Thai92: 1013741379 |
44 | Thaler A, Mirelman A, Gurevich T, Simon E, Orr-Urtreger A, Marder K(2012) Lower cognitiveperformance in healthy GS LRRK mutation carriersNeurology79: 1010271032doi: 10.1212/WNL.0b013e3182684646 |
45 | van Alem AP, de Vos R, Schmand B, Koster RW(2004) Cognitive impairment in survivors of out-of-hospital cardiac arrestAmerican Heart Journal148: 3416421doi: 10.1016/j.ahj.2004.01.031 |
46 | van Bever Donker SC(1999) Hyperbaric oxygen therapy for children with cerebral palsySouth African Medical Journal=Suid-Afrikaanse Tydskrif vir Geneeskunde89: 4360361 |
47 | van Bever Donker SC(2001) Hyperbaric oxygen therapy for children with cerebral palsySouth African Medical Journal=Suid-Afrikaanse Tydskrif vir Geneeskunde91: 11909 |
48 | Zur D, Naftaliev E, Kesler A(2014) Evidence ofmultidomain mild cognitive impairment in idiopathic intracranialhypertensionJournal of Neuro-Ophthalmology: The OfficialJournal of the North American Neuro-Ophthalmology Society35: 12630doi: 10.1097/WNO.0000000000000199 |
Figures and Tables
Fig.1
Improvements in cognitive functions ADL and quality of life (EQ-5D, EQ-VAS). A. Mean changes of the corresponding cognitive indices at baseline and after HBO2. Statistical significance (p < 0.05) is marked by *. Note lower EQ-5D score represents higher quality of life. Bars represent means + standard errors. B. The mean percentage of the relative changes (Post-Pre)/Pre.
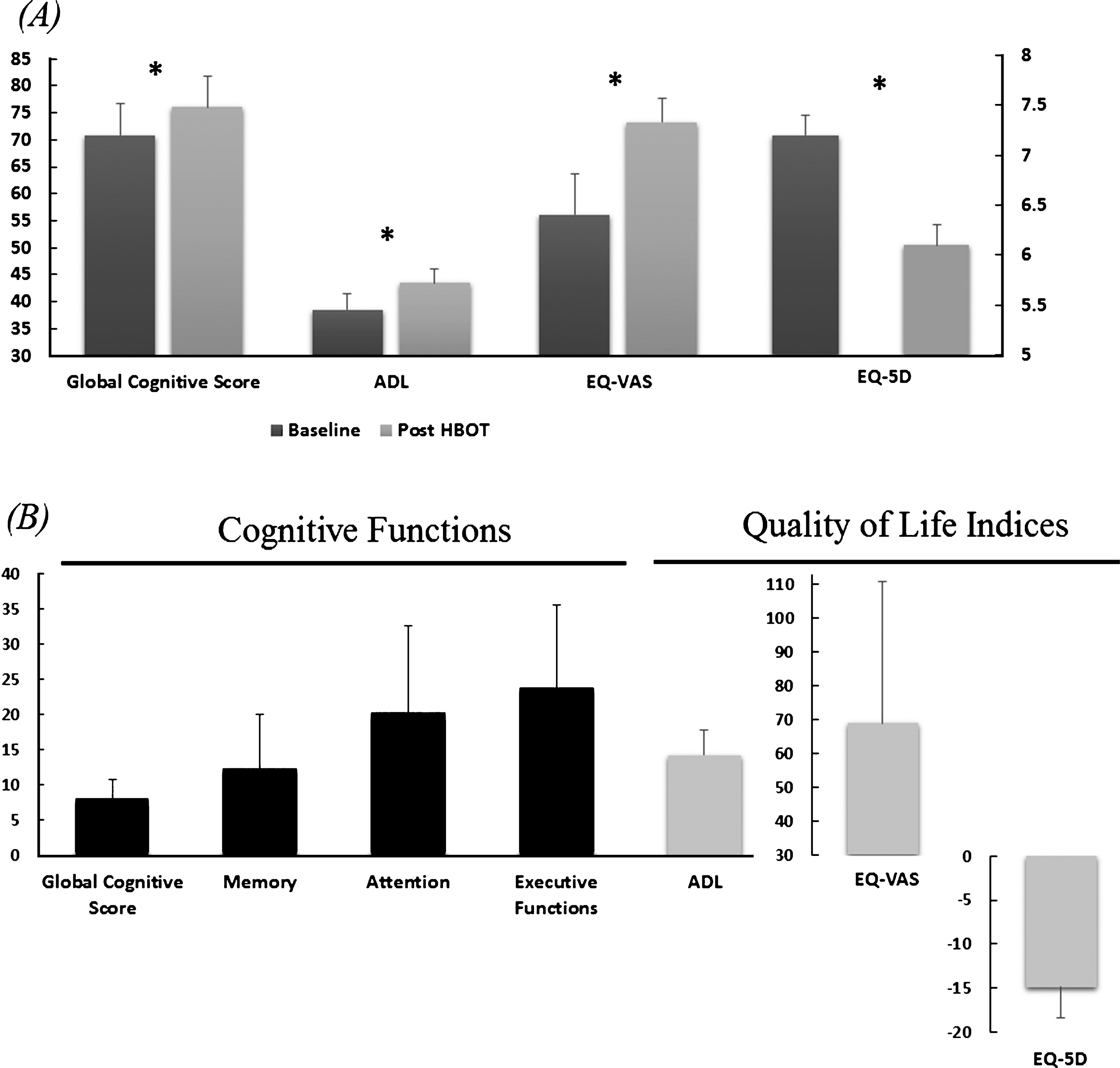
Fig.2
The mean CBF relative change after hyperbaric oxygen therapy calculated per Brodmann area.
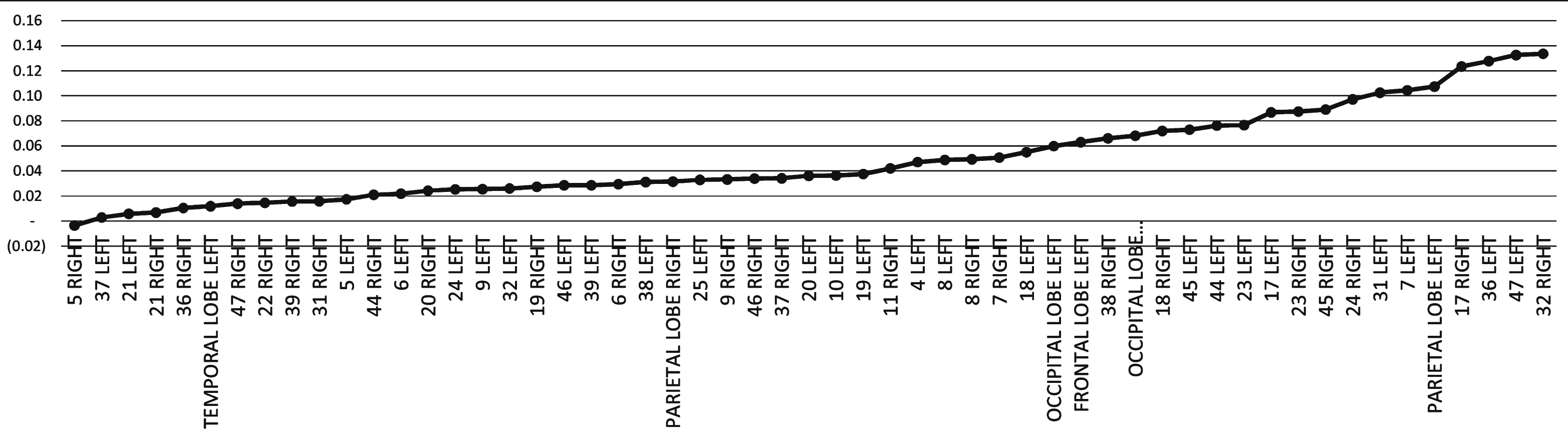
Fig.3
The Brodmann areas that were most improved after HBO2; perirhinal cortex (BA36) in red, the pre-frontal cortex (BA 8,9,10,11) in blue, inferior frontal gyrus (BA 45,47) in yellow, the anterior cingulate gyrus (BA 23,24) in green, primary visual cortex (BA 17) in purple and the parietal lobes (BA 5,7) in orange.
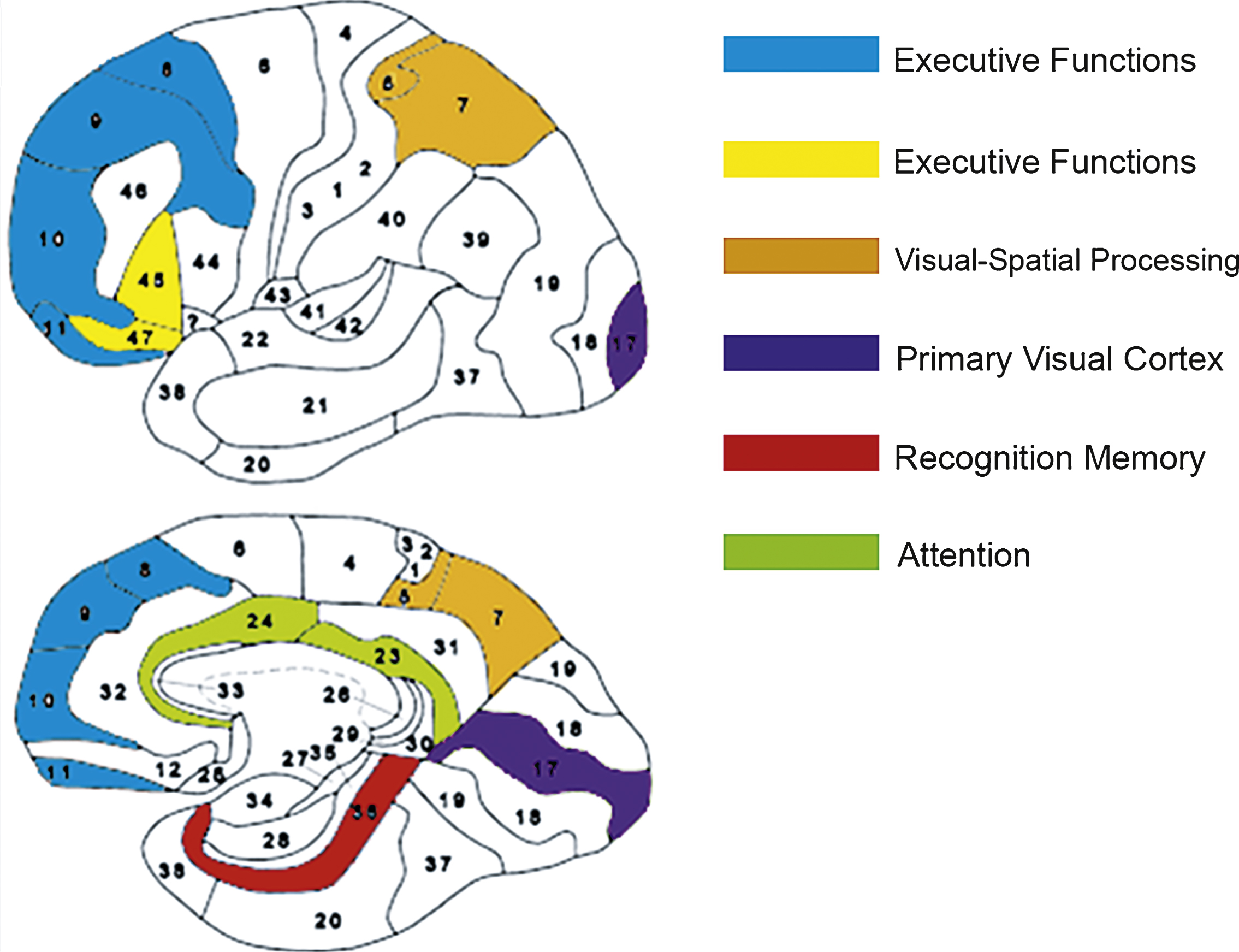
Fig.4
Case 1 SPECT calculated change after HBO2 compared to baseline. In the first two rows colors represent maximal functional brain activity relative to brain median activity, where in the bottom row colors represent the regional change in functional brain activity. White and red areas show the highest changes in CBF.
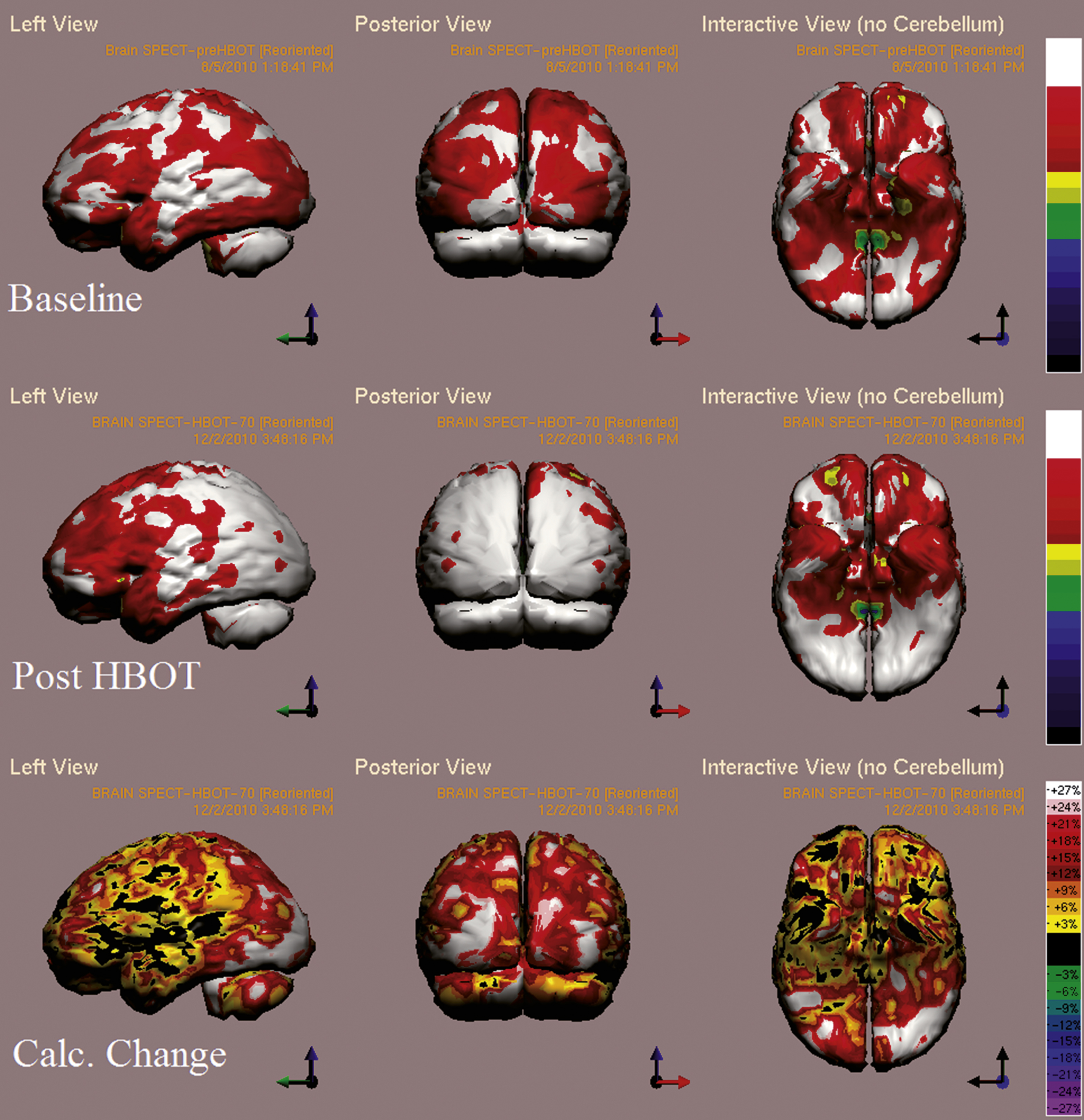
Fig.5
Case 2 SPECT calculated change after HBO2 compared to baseline. In the first two rows colors represent maximal functional brain activity relative to brain median activity, where in the bottom row colors represent the regional change in functional brain activity. White and red areas show the highest changes in CBF.
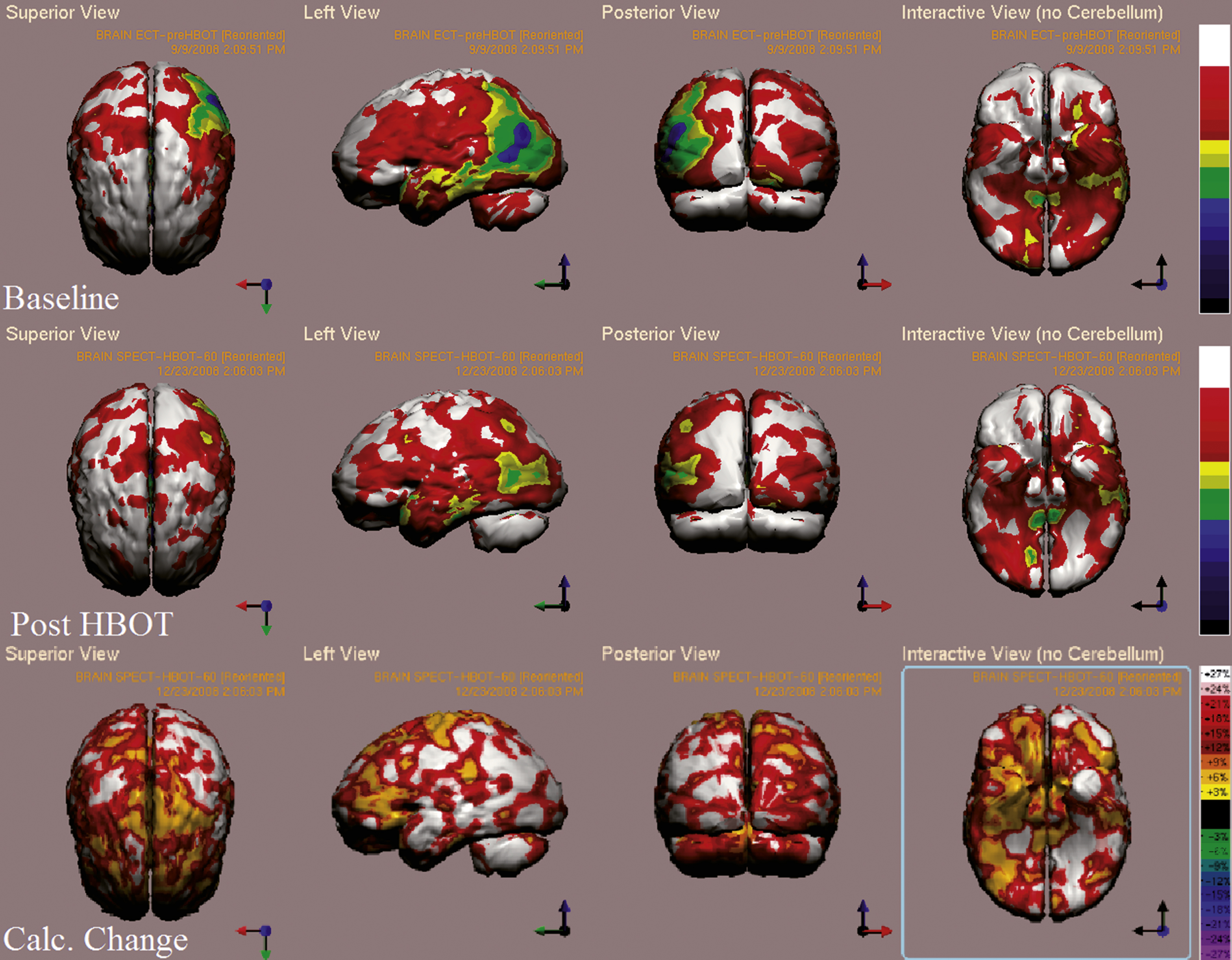
Fig.6
Case 3 SPECT calculated change after HBO2 compared to baseline. White and red areas show the highest changes in CBF. In the first two rows color represent metabolic activity, where in third row colors represent the subtraction of the two previous rows, i.e. the change in metabolic activity. Memory, attention, verbal and visual spatial indices improved from non measureable scores to 98, 70, 102, 89 and 89.7 respectively.
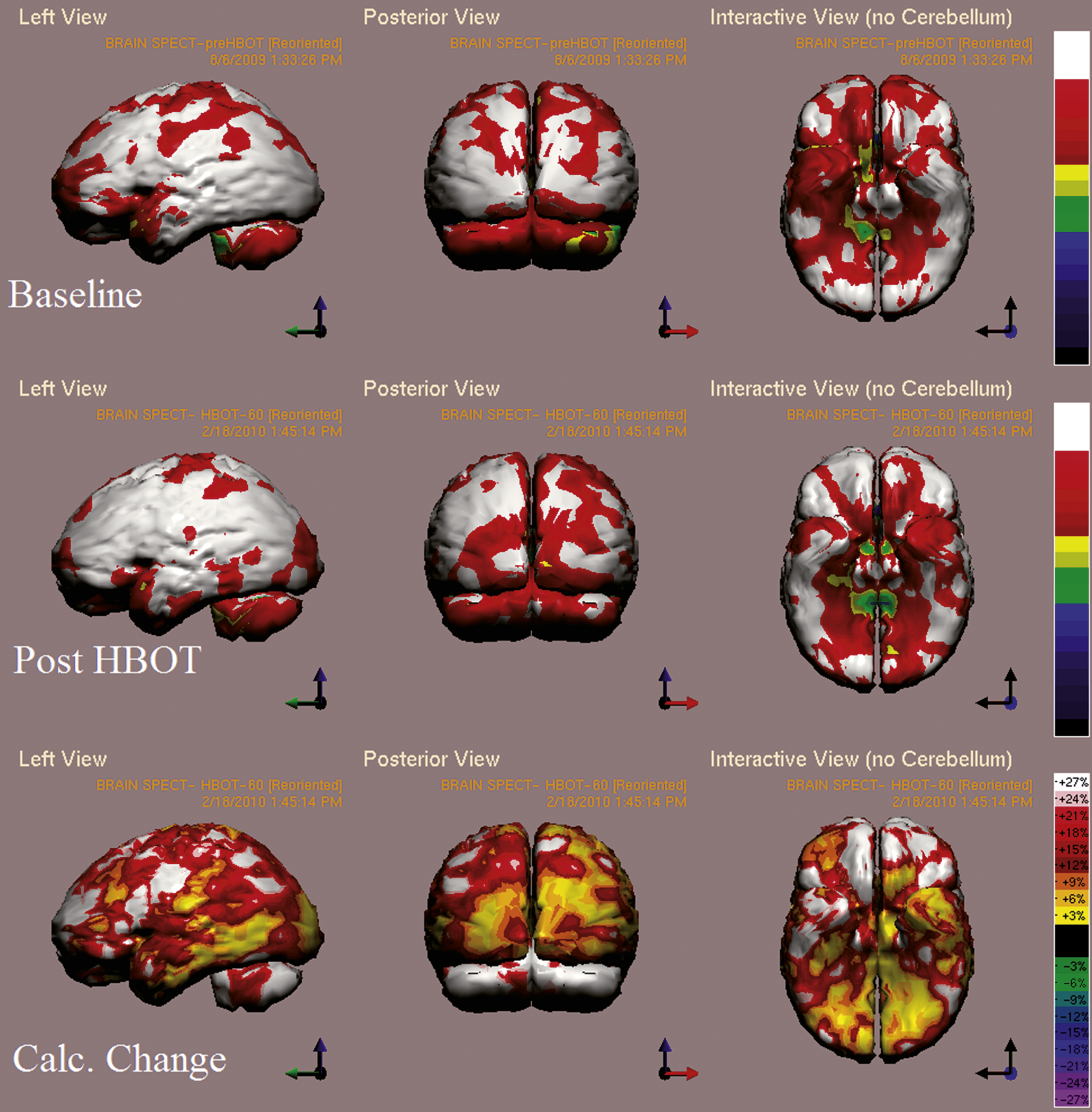
Fig.7
Case 4 SPECT calculated change after HBO2 compared to baseline. In the first two rows color represent maximal functional activity relative to brain median activity, where in bottom row colors represent the change in regional brain activity. White and red areas show the highest changes in CBF. Executive function, verbal, visual spatial and memory indices improved from scores of 98, 88, 84, 69 to 111, 104, 104, 79 respectively.
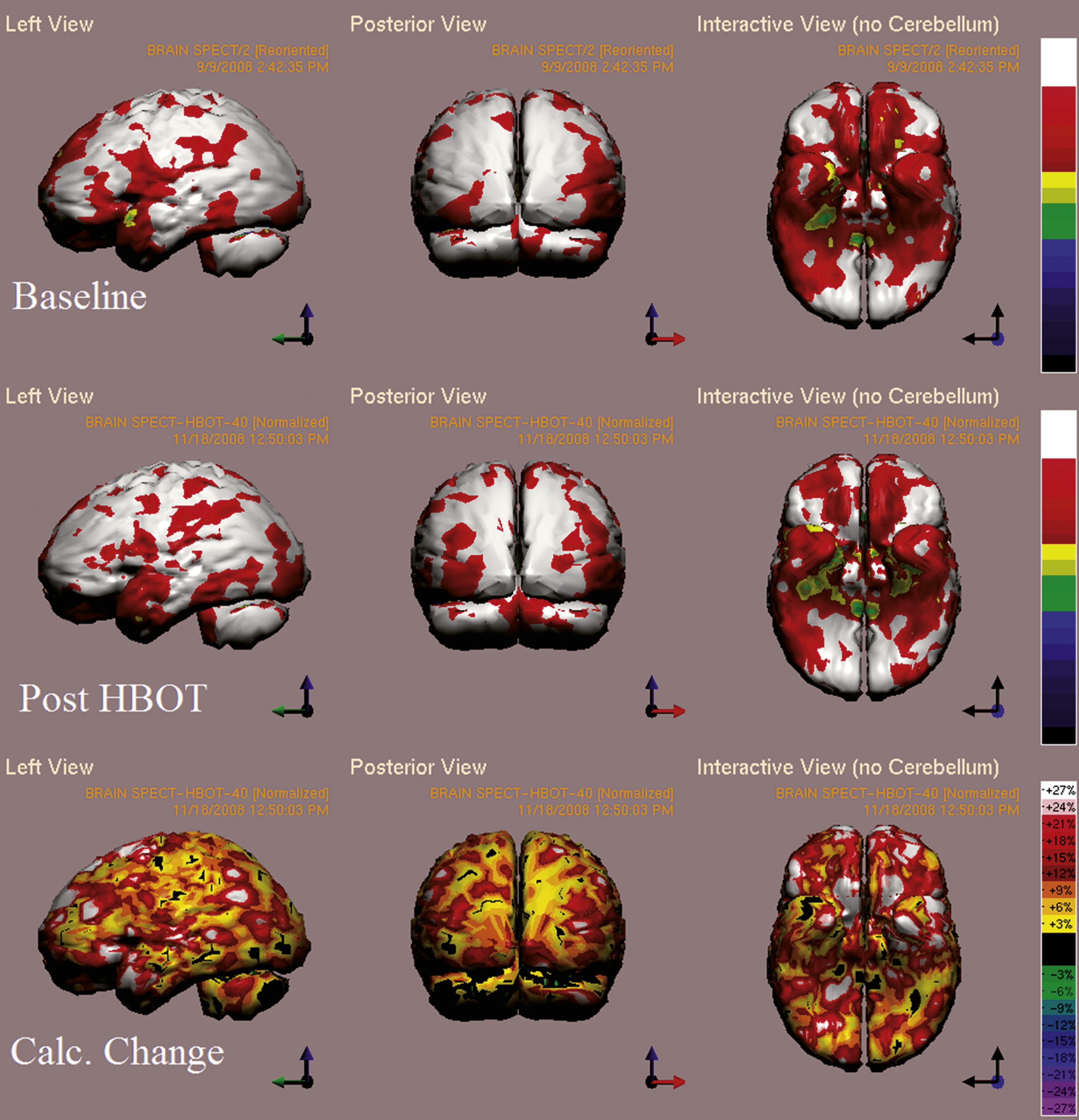
Fig.8
Case 5 SPECT calculated change after HBO2 compared to baseline. In the first two rows color represent maximal brain activity relative to brain median activity, where in the bottom row colors represent the change in regional brain function i.e. the change in metabolic activity. A global improvement of brain activity is demonstrated more accentuated in both occipital cortices. White and red areas show the highest changes in CBF. Executive function and attention indices improved from 44 and 38 to 62 and 59, respectively.
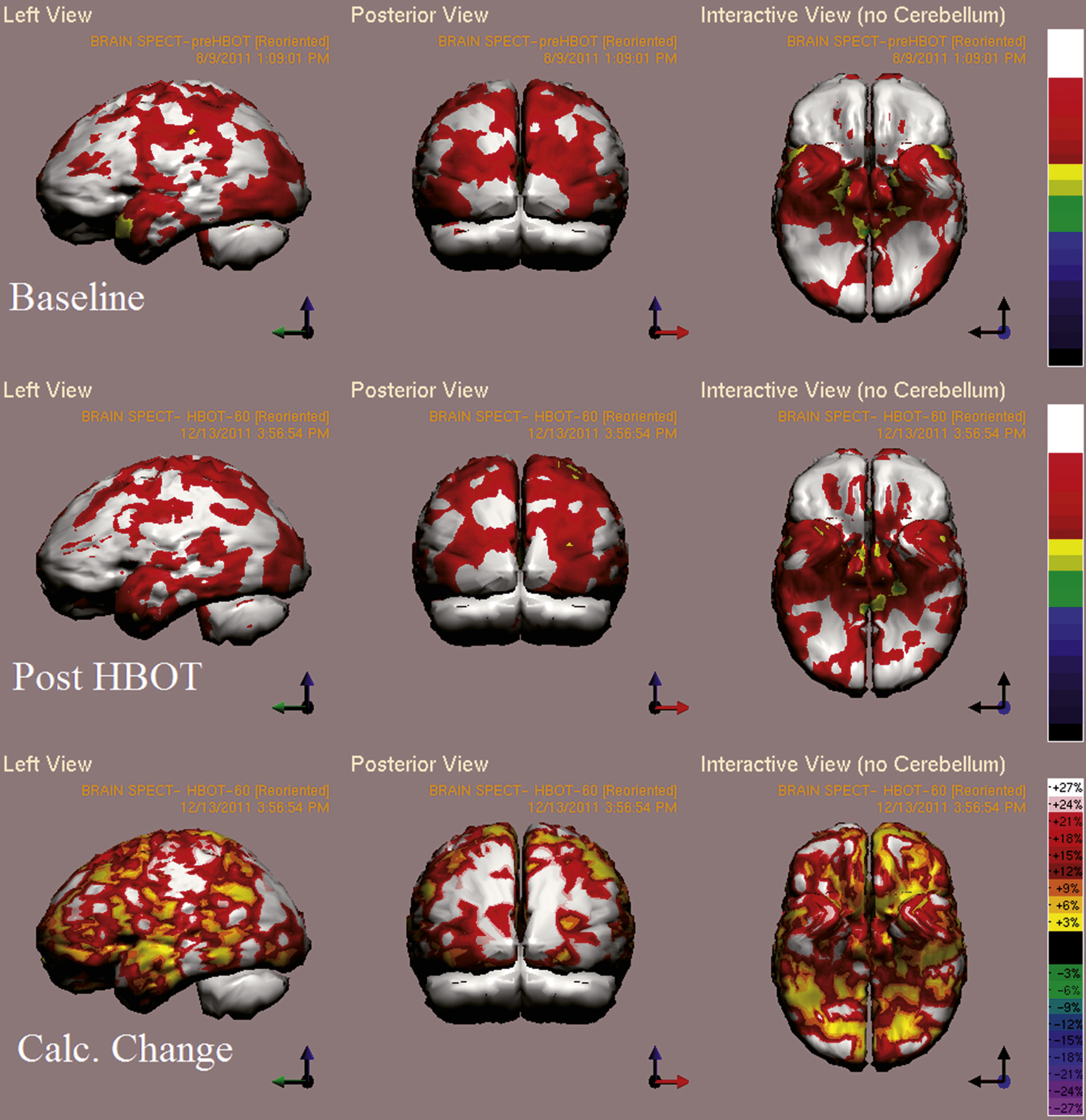
Table 1
Basic characteristics of patients at baseline
| Characteristic | Total patients (11) |
| Age (years) | 45.6 ± 4.4 |
| Males | 81% |
| Time from injury (months) | 31.3 ± 7.7 |
| Cardiac ejection fraction | 52.2 ± 2.3% |
| Years of education | 13.4 ± 0.5 |
| Chronic Diseases | |
| Known Ischemic Heart Disease | 1/11 (9%) |
| Known Arrhythmia (Including Long-QT) | 2/11 (18%) |
| Diabetes Mellitus Type II | 2/11 (18%) |
| Hypertension | 3/11 (27%) |
| Dyslipidemia | 5/11 (45%) |
| Smoking history | 3/11 (27%) |
| Medications | |
| Beta-Blockers | 8/11 (72%) |
| Statins | 5/11 (45%) |
| Anti-Platelets | 5/11 (45%) |
| ACE-Inhibitors | 3/11 (27%) |
| Anti-coagulants | 1/11 (9%) |
Note the average time of 31 months from cardiac arrest. Data are expressed as means ± standard errors.
Table 2
Cognitive indices at baseline, after Hyperbaric Oxygen Therapy (HBO2). Mean relative change is calculated by mean change divided by the pre HBO2 mean score. Data are expressed as means ± standard errors
| Cognitive function | Pre HBO2 | Post HBO2 | Mean Change | Mean Relative change % | T, df | P-value |
| Global Cognitive Score | 70.9 ± 5.9 | 76.0 ± 5.8 | 5.0 ± 1.4 | 8.2 ± 2.7% | 3.5, 9 | 0.006 * |
| Memory | 54.9 ± 8.5 | 59.9 ± 8.3 | 4.9 ± 2.5 | 12.3 ± 7.8% | 1.9, 9 | 0.080 |
| Executive | 71.3 ± 6.7 | 82.9 ± 5.0 | 11.5 ± 3.6 | 23.8 ± 11.8% | 3.5,9 | 0.011 * |
| Attention | 66.13 ± 8.3 | 72.9 ± 5.7 | 6.8 ± 3.1 | 20.3 ± 9.1% | 2.1,9 | 0.060 |
| Information processing speed | 67.5 ± 6.4 | 70.9 ± 7.2 | 3.4 ± 3.0 | 5.1 ± .4.8% | 1.1,7 | 0.310 |
| Visual spatial | 79.4 ± 8.8 | 83.8 ± 9.1 | 4.4 ± 3.3 | 6.4 ± 4.3% | 1.3,9 | 0.221 |
| Verbal skills | 75.4 ± 10.4 | 78.1 ± 11.1 | 2,7 ± 4.8 | 4.1 ± 6.4% | 0.563,8 | 0.589 |
| Motor skills | 87.6 ± 6.0 | 88.0 ± 4.7 | 0.4 ± 2.7 | 2.1 ± 3.6% | 0.155,9 | 0.880 |
One of the patients that had prominent improvement from non-measureable scores due to insufficient responses prior to treatment to measurable scores in all cognitive domains after treatment was not included in this analysis. *significant (p < 0.05) by two-tailed paired t-test.
Table 3
Activity of Daily Living (ADL) and quality of life (EQ-5D and EQ-VAS) scores before and after Hyperbaric Oxygen Therapy. Mean relative change is calculated by mean change divided by the pre HBO2 mean score. Data are expressed as means ± standard errors
| Score | Pre Treatment ADL | Post HBO2 ADL | Mean Change | Mean Change % | P-Value |
| ADL | 38.6 ± 3.0 | 43.5 ± 2.5 | 4.9 ± 1.0 | 14.3 ± 3.5% | 0.002 * |
| EQ-VAS | 56.1 ± 7.6 | 73.3 ± 4.3 | 17.2 ± 5.0 | 69 ± 42% | 0.027 * |
| EQ-5D | 7.2 ± 0.2 | 6.1 ± 0.2 | −1.1 ± 0.2 | −14.9 ± 3.5% | 0.015 * |
ADL difference before and after HBO2 was analyzed using two-tailed paired t-test. EQ-VAS and EQ-5D were analyzed using Wilcoxon signed ranks test. *marks statistical significance.




