Does SLT combined with NIBS enhance naming recovery in post-stroke aphasia? A meta-analysis and systematic review
Abstract
BACKGROUND:
Non-invasive brain stimulation has been widely used as an adjunctive treatment for aphasia following stroke.
OBJECTIVE:
The aim of this study was to investigate the effect of non-invasive brain stimulation as an adjunctive treatment on naming function in aphasia following stroke.
METHODS:
This review included randomized controlled trials (RCTs) involving 5 databases (Web of Science, Embase, Cochrane Library, OVID and PubMed) that investigated the effects of electrical stimulation on stroke patients. The search included literature published up to November 2023.
RESULTS:
We identified 18 studies, and the standardized mean differences (SMDs) showed that the effect sizes of TMS and tDCS were small to medium. Moreover, the treatment effects persisted over time, indicating long-term efficacy.
CONCLUSION:
This study suggested that NIBS combined with speech and language therapy can effectively promote the recovery of naming function in patients with post-stroke aphasia (PSA) and that the effects are long lasting.
1Introduction
Currently, stroke remains one of the most debilitating diseases worldwide (Saini et al., 2021). In China, there are approximately 2 million new cases of stroke each year (Y.-J. Wang et al., 2022). The severe consequences of stroke are reflected not only in its high mortality rate (W. Wang et al., 2017) but also in the associated disability and sequelae. Aphasia is a common functional impairment following stroke and can occur after stroke. According to statistics, aphasia affects no less than 30% of stroke survivors (Flowers et al., 2016). Aphasia results in impairments in speech, reading, writing, and comprehension for affected individuals. Compared to stroke survivors without aphasia, aphasic patients face significant daily life challenges, including reduced social participation and lower quality of life. The damage caused by post-stroke aphasia (PSA) not only poses a threat to individuals but also imposes a significant burden on society (Jacobs & Ellis, 2023). Therefore, timely rehabilitation measures for patients are extremely necessary.
Naming disorder stands out as one of the most prevalent language impediments among individuals with aphasia, concurrently representing the most enduring symptom of this condition (Goodglass & Wingfield, 1997). The assessment of naming function also stands out as one of the most sensitive indicators for evaluating the severity of aphasia. The naming process is a complex undertaking involving numerous relatively distinct cognitive processes and psychological representations (Levelt, 2001). It serves as an application for the overall assessment of poststroke brain function. Therefore, the quality of naming function can, to a certain extent, predict the recovery of speech function in PSA patients (Meier et al., 2020).
In terms of the treatment of PSA, the most common approach to aphasia rehabilitation is behavioral speech and language therapy (SLT). However, the efficacy of SLT has been a subject of significant controversy throughout its history, and even today, there remains skepticism in the literature regarding its effectiveness in aphasia treatment (Brady et al., 2016). However, in recent years, a phase III randomized controlled trial (RCT) published in the Lancet provided explicit support for the use of impairment-based SLT in improving speech production in chronic aphasia patients (Breitenstein et al., 2017). SLT is typically given one-on-one by an SLP, but group therapy data are scarce. More sessions generally mean better outcomes, but current healthcare constraints and costs hinder increasing treatment frequency. Hence, additional therapies beyond SLT are needed.
Fortunately, in recent years, NIBS has emerged as an adjunctive intervention to SLT. Among prevalent NIBS techniques for PSA, rTMS and tDCS stand out. From a mechanistic perspective, the improvement of aphasia by NIBS is based on post-stroke neuroplastic changes, promoting language function recovery through alterations in synaptic potentials of neurons and similar mechanisms (Terao & Ugawa, 2002). rTMS induces eddy currents in neuronal membranes, thereby eliciting action or postsynaptic potentials in axons. Conversely, tDCS modulates resting membrane potentials and neuronal discharge rates by adjusting specific channels and receptors. Both methods are considered potentially effective in improving language performance when combined with SLT for PSA. Given the potential for neural reorganization post-stroke, NIBS may modulate brain plasticity by reducing maladaptive activation in the intact hemisphere or enhancing excitability near damaged language regions, thereby aiding aphasia recovery.
However, conclusive evidence regarding the superior efficacy of combined SLT and NIBS therapy over SLT alone is lacking. Yet, mainstream research underscores NIBS as a meaningful adjunctive treatment. Bucur et al.’s meta-analysis (Bucur & Papagno, 2019) indicates enhanced language functions, particularly naming, with NIBS. However, some meta-analyses suggest NIBS might lack a significant therapeutic effect on aphasia. Recent findings by You et al. (2023a) imply that tDCS has no enduring impact on noun naming or communication in PSA patients.
This study employed meta-analysis to evaluate if NIBS combined with SLT is superior to SLT alone and to assess their long-term effects. The aim was to provide robust evidence for clinical treatment, explore new therapy avenues, and facilitate the reintegration of PSA individuals into family and society.
2Methods
2.1Literature search and study selection
We systematically searched five electronic databases, namely MEDLINE (PubMed), Embase, the Cochrane Library, OVID, and Web of Science, for studies focused on the treatment of PSA. These studies utilized either rTMS or tDCS combined with aphasia SLT. The search included literature published up to November 2023.
The following keywords were used: (1) “noninvasive brain stimulation”, “NIBS”, “tDCS”, “transcranial direct current stimulation”, “transcranial magnetic stimulation”, “TMS” AND (2) “Speech Therapies” AND (3) “aphasia”, “language disorder” AND (4) “stroke”.
2.2Literature inclusion and exclusion criteria
• Inclusion criteria:
• Studies involving adult participants with PSA.
• Interventions consisted of either isolated speech therapy combined with sham stimulation or speech therapy combined with noninvasive brain stimulation.
• At least one outcome measure assessed verbal or written naming ability.
• The study design was categorized as an RCT.
• Publications available in the English language.
• Studies with a minimum of four or more participants.
• Exclusion criteria:
• Interventions targeting other types of poststroke conditions or non-stroke-induced aphasia.
• Invasive brain stimulation methods such as acupuncture, electroconvulsive therapy, or the use of NIBS alone without combined speech therapy.
• Case reports and studies involving fewer than four participants, as well as gray literature (i.e., unpublished sources such as unpublished doctoral theses or reports).
• International conference reports without specific data, opinion pieces, case reports, case series, prior reviews, or meta-analyses.
• Studies lacking sufficient information for analyzing treatment effects (i.e., when quantitative data extraction is not feasible) or when authors did not respond to requests for data.
2.3Data extraction
Two researchers (Huang and Guo) used a standardized data extraction form to assess the eligibility of studies, resolving discrepancies through consensus or referral to a third reviewer (Xiong) for resolution.
For each included study, the following information was extracted:
(1) Patient characteristics: sample size (treatment and control groups), sex, age, time since stroke, level of education, and lesion volume;
(2) rTMS stimulation protocol: target region, rTMS frequency and intensity, pulse number and duration, number of sessions, related SLT, sham stimulation, outcome measures;
(3) tDCS stimulation protocol: montage type (uni- or bipolar, anode or cathode), electrode size, stimulation area, electrode placement, hemisphere stimulation, reference electrode, current intensity, number of sessions and duration, SLT, sham stimulation, outcome measures;
(4) Study characteristics: primary objective, study design, study language, follow-up results, and authors’ conclusions.
To address the contentious issues in data extraction, we established the following criteria:
• For studies with multiple follow-up periods, we selected the longest one consistent with other studies.
• Only data on language recovery effects (e.g., aphasia battery scores, speech abilities) were extracted, excluding fMRI or blood flow data.
• One likely effective stimulation protocol per study was chosen, or the most common one in the literature if no information was available.
• Naming accuracy was prioritized as the primary outcome measure to minimize variability among studies; alternate measures included total aphasia battery scores and speech content units.
• In cases of multiple outcome measures for the same treatment, naming accuracy was prioritized as the most frequently reported measure.
We contacted the relevant principal investigators to retrieve missing data.
2.4Quality assessment
Each study underwent quality assessment by two independent reviewers (Huang and Xiong) using the risk of bias assessment method recommended by the Cochrane Collaboration, comprising seven assessment domains. Discrepancies were resolved through consensus or by consulting a third reviewer (Guo) for resolution. We contacted the trialists for clarification and to request missing information.
2.5Assessment of heterogeneity
The I2 statistic was used to assess heterogeneity. We used a random-effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity, we did not violate the preconditions of a fixed-effects model approach.
2.6Statistical analysis
The analysis was performed using RevMan 5.3 software. For all outcomes representing continuous data, we planned to enter means and standard deviations and calculate a pooled estimate of the mean difference (MD) with a 95% confidence interval (CI). To calculate the treatment effect, the standardized mean differences (SMDs) were pooled using the random-effects model regardless of the heterogeneity test results (Q or I), since there is a certain amount of variance between studies due to their particular characteristics (e.g., stimulation parameters, associated therapies, patient characteristics).
3Results
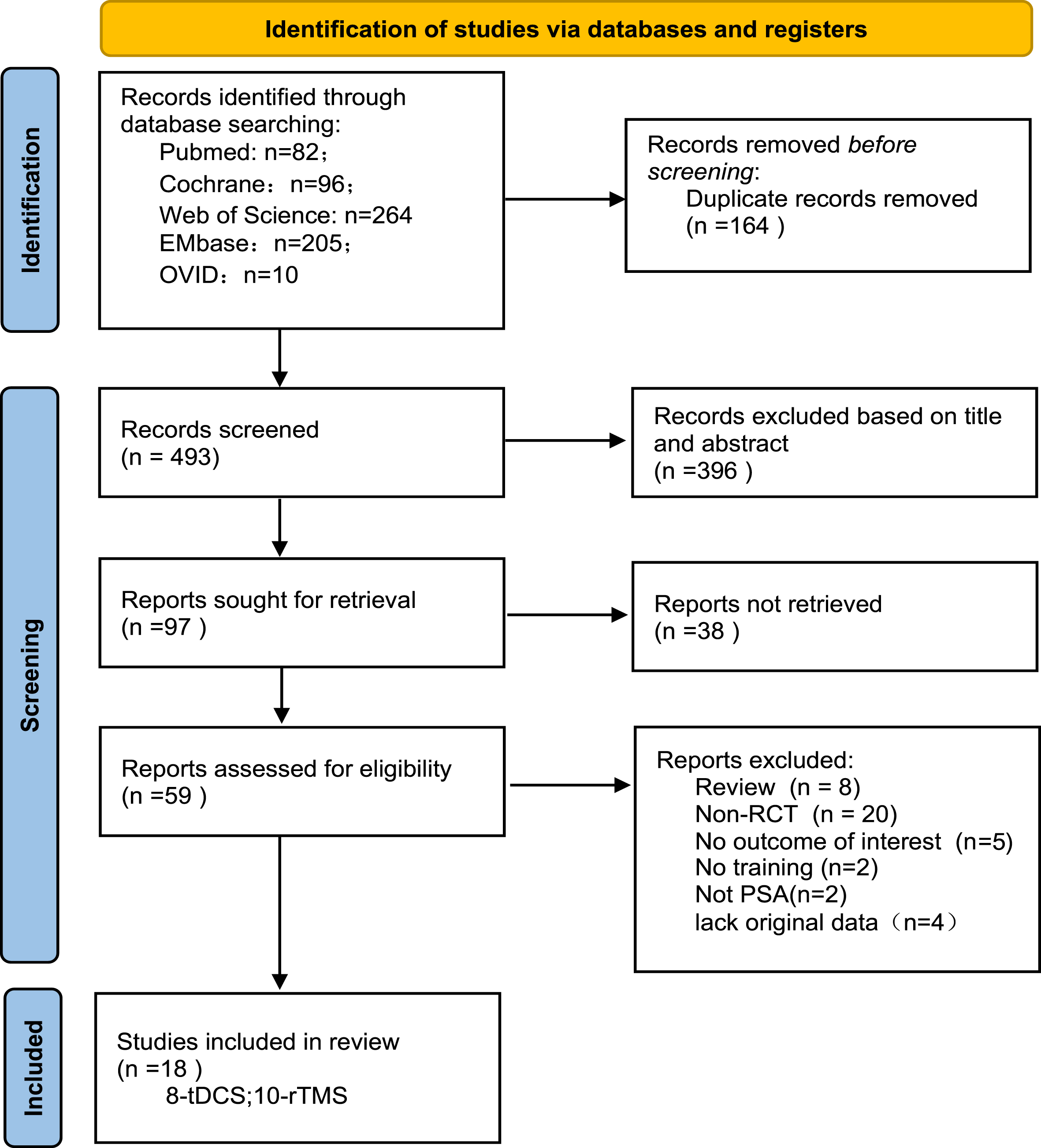
The literature search initially retrieved 657 publications, and following application of the eligibility criteria and duplicate removal, 97 studies were selected for a full-text review; ultimately, 18 studies met the inclusion criteria. Figure 1 summarizes the inclusion process.
Fig. 1
Flow diagram of the included studies in the review.
3.1Study characteristics
Eight studies used tDCS as a therapeutic treatment for aphasia (Polanowska et al., 2013; Cid-Fernandez et al., 2022; Polanowska et al., 2013; Zhao et al., 2021; Kang et al., 2011; Feil et al., 2019; Matar et al., 2022; Stockbridge et al., 2023). Eight studies investigated the effects of rTMS on aphasic patients after stroke (Rubi-Fessen et al., 2015; Waldowski et al., 2012; Hu et al., 2018; Weiduschat et al., 2011; C. P. Wang et al., 2014; Haghighi et al., 2017; Low et al., 2023; Ren et al., 2019; Yoon et al., 2015; Seniów et al., 2013). All of these studies used combined therapy, namely noninvasive brain stimulation combined with SLT.
3.2Participants included
A comprehensive review of 18 studies examined 398 stroke patients with aphasia, including 199 in the experimental group and 199 in the sham group. Of these, 246 patients underwent rTMS therapy, with 118 receiving real rTMS and 128 receiving sham stimulation. Additionally, 152 patients participated in the tDCS experiments, with 81 receiving real tDCS and 71 receiving sham tDCS.
Participant characteristics exhibited heterogeneity across the studies, particularly in aspects such as age, sex, educational level, duration poststroke, and cerebral lesion volume.
• In terms of poststroke interval, 13 studies involved patients in the subacute aphasia phase (n = 312, duration: 4-189 days poststroke). Additionally, 5 studies focused on chronic aphasia patients (n = 86, duration: 6 months to 5 years poststroke). The participants’ ages ranged between 37 and 79 years.
• Six studies provided data on the volume of stroke lesions.
The basic information of the included studies is shown in Table 1.
Table 1
Characteristics of the included participants
| ID | Study | NIBS | Total number of participants included | Intervention group | Control group | ||||||
| Sample Size | Male | Female | Age (mean±SD) | Educational level (mean±SD) | Duration of illness (mean±SD) | Lesion volume (mean±SD) | Sample size | ||||
| 1 | Susana 2022 | tDCS | 10 | 5 | 3 | 2 | 62.8±16.4 | 14.6±4.98y | >6 m | ns | 5 |
| 2 | Sarah 2019 | tDCS | 12 | 6 | 4 | 2 | 59±12 | 13±1.7y | 49±18d | 46819±61171 mm3 | 6 |
| 3 | Eun 2011 | tDCS | 10 | 5 | 4 | 1 | 62±10.07 | 14.4±2.19y | 22.88±36.02d | ns | 5 |
| 4 | Dae 2011 | tDCS | 21 | 14 | 7 | 7 | 70.43±9.24 | 10.43±3.21y | 24.71±5.22d | 67.57±26.37 cm3 | 7 |
| 5 | Katarzyna 2013 | tDCS | 24 | 14 | 7 | 7 | 56.1±10.1 | 14.6±3.4y | 59.6±48.7d | 53.4±34.6 cm3 | 10 |
| 6 | Qi Zhao 2020 | tDCS | 18 | 8 | 2 | 6 | 58.00±8.718 | 13.75±1.146y | 3.10±2.86 m | 70.14±44.02 cm3 | 10 |
| 7 | Shereen 2022 | tDCS | 6 | 3 | 1 | 2 | ns | ns | 17.67±3.83 | ns | 3 |
| 8 | Melissa 2023 | tDCS | 51 | 26 | 16 | 10 | 65.1±12.2 | 16±6.0y | 54.6±30.7d | ns | |
| 9 | Mohammad 2017 | rTMS | 12 | 6 | 3 | 3 | 61.67±7.06 | ns | subacute | ns | 6 |
| 10 | Caili 2019 | rTMS | 28 | 13 | 7 | 6 | 62.46±10.95 | ns | 50.58±23.80 | ns | 15 |
| 11 | Ilona 2015 | rTMS | 30 | 15 | 5 | 10 | 67.9±8.12 | ns | 41.47±21.51d | 23219±17395 mm3 | 15 |
| 12 | Konrad 2012 | rTMS | 26 | 13 | 6 | 7 | 62.31±11.03 | 13.3±4.06y | 26.6±12.27d | ns | 13 |
| 13 | Chih-Pin 2014 | rTMS | 30 | 15 | 13 | 2 | 62.1±12.7 | 12.2±3.9y | 15.7±8.5d | ns | 15 |
| 14 | Nora 2011 | rTMS | 10 | 6 | 1 | 5 | 66.67±9.05 | ns | 57.5±26.89 | 23012.5±25031.5 mm3 | |
| 15 | Xue-yan 2018 | rTMS | 30 | 10 | 6 | 4 | 48.5±11.2 | Primary school 1, Secondary school 7, Bachelor 2 | 7.5±3.2 m | ns | 20 |
| 16 | Trevo 2023 | rTMS | 20 | 10 | 7 | 3 | 61.5±12.2 | ns | 3.2±2.4y | ns | 10 |
| 17 | Tae 2015 | rTMS | 20 | 10 | 8 | 2 | 60.46±9.63 | ns | 6.80±2.39 m | ns | 10 |
| 18 | Joanna 2013 | rTMS | 40 | 20 | 8 | 12 | 61.8±11.8 | 13.3±3.3y | 33.5±24.1d | ns | 20 |
NIBS: noninvasive brain stimulation; tDCS: transcranial direct current stimulation; rTMS: repetitive transcranial magnetic stimulation; mean: average value; SD: standard deviation.
3.3Intervention
3.3.1Stimulation area
Based on the NIBS type, stimulation areas were categorized into rTMS and tDCS groups. All rTMS studies stimulated the contralateral (right hemisphere) anterior language areas (pars triangularis or pars opercularis), corresponding to BA 45, with one study stimulating (Waldowski et al., 2012) both the triangular and opercular parts.
In tDCS experiments, the most frequently targeted brain region was Broca’s area. In all of the included studies involving anodal tDCS (A-tDCS), the anode was placed over the left Broca’s area, while the cathode was positioned over the contralateral supraorbital margin. In one study, the cathode was placed on the deltoid muscle head.
In tDCS experiments, Broca’s area was the most frequently targeted brain region. In all studies involving A-tDCS, the anode was positioned over the left inferior frontal gyrus (LIFG) or the frontal-central area (FC5), corresponding to BA 44/45, which corresponds to Broca’s area, and the reference electrode was placed on the contralateral supraorbital margin BA 10/11. One study positioned the reference electrode at the deltoid muscle head (Zhao et al., 2021), whereas for C-tDCS (D. S. You et al., 2011), the cathode was placed on the right superior temporal gyrus BA 21/22, with the reference electrode also located at the contralateral supraorbital margin.
3.3.2Stimulation protocol
• rTMS
All studies used inhibitory stimulation protocols. For rTMS, intensities ranged from 80% to 100% of resting motor threshold (RMT), with two studies at 100% RMT, six at 90%, and two at 100%. Frequency was 1 Hz, and durations varied (10 to 30 minutes). Most studies had 10 to 15 sessions; only one had 20 sessions (Yoon et al., 2015). Magnetic pulses were delivered using figure-of-eight coils.
• tDCS
In all studies, tDCS typically used two equal-sized electrodes. One study used different dimensions: 4×5 cm for the anode and 5×7 cm for the cathode. Other studies used electrodes with areas of 25 cm2 (5 cm×5 cm) and, in some cases, 35 cm2 (5 cm×7 cm).
Stimulation intensity varied, with tDCS at either 1 mA or 2 mA. Among eight papers, three used 1 mA, and five used 2 mA, resulting in a current density of 0.028 mA/cm2. Except for one 30-minute and one 10-minute study (D. S. You et al., 2011), the remaining studies all had a duration of 20 minutes.
• SLT
In all studies, SLT was conducted concurrently or post-intervention with rTMS and tDCS. Led by speech therapists, sessions were tailored to patients’ needs. For example, sessions lasting 30 minutes, five times weekly, involved activities like picture naming, conversational therapy, word-finding exercises, and computerized aphasia treatment.
3.3.3Placebo
All RCTs were double-blind, placebo-controlled, ensuring both researchers and patients were unaware of treatment allocation. Randomization assigned patients to treatment or control groups. In rTMS, sham stimulation involved positioning the coil at a 90deg angle to the skull. In tDCS, sham stimulation was achieved by turning it off after a brief duration (15–60 seconds).
3.3.4Outcome measures
Outcome assessments, including picture naming, naming reaction time, Boston Naming Test, correct naming occurrences, fluency, grammatical accuracy, lexical selection, and general language scores from aphasia batteries (e.g., Western Aphasia Battery Aphasia Quotient), varied across studies. This review prioritized picture naming; if unavailable, naming subtest scores from the Boston Naming Test or aphasia batteries were used.
3.3.5Follow-up
Seven studies reported follow-up results, four of which were rTMS stimulation studies (Hu et al., 2018; Waldowski et al., 2012; Low et al., 2023; Seniów et al., 2013) and three of which were tDCS studies (Polanowska et al., 2013; Feil et al., 2019; Matar et al., 2022). The follow-up periods varied widely, ranging from 4 weeks to 6 months. The treatment protocols for the NIBS techniques used in each study are outlined in Tables 2 and 3.
Table 2
Characteristics of tDCS studies
| ID | Study | tDCS parameters | Follow-up | SLT | Sham stimulation | Outcome measures | |||||
| Montage Unipolar /Bipolar (A-tDCS, C-tDCS) | Stimulation area | Reference electrode | Electrode dimension | Current intensity | Course of treatment | ||||||
| 1 | Susana 2022 | A-tDCS | CP5 (BA 44) | over the RSO. (BA 22) | Anode: 20 cm2 cathode: 35 cm2 | 1mA; 20 min | Five times per week,2weeks. | ns | Behavioral Naming Training | Turned off after 30 s from the start | BNT |
| 2 | Sarah 2019 | Bipolar tDCS | F5 according to the EEG system, targeting the Broca’s area (BA 44/45) | t the F6 location of the EEG system. (BA 8) | 35 cm2 | 2mA; 20 min | Five times per week,2 weeks. | 4 weeks | Personalized Speech and Language Training | Turned off 8 s after the patient feels it | PNT |
| 3 | Eun 2011 | C-tDCS | Above F8, corresponding to the right Broca’s area (BA 44/45) | the left supraorbital area. (BA 8) | 25 cm2 | 2mA; 20 min | Once daily, for a total of five sessions. | ns | Word Retrieval Training | Current applied for 1 minute, then gradually reduced to zero | Correct Naming Count |
| 4 | Dae 2011 | A-Tdcs OR C-tDCS | Anode: n = 7, left superior temporal gyrus; Cathode: n = 7, right superior temporal gyrus (BA 44/45) | on the contralateral orbit. (BA 8) | 5 cm×7 cm | 2 mA,30 min | Five times per week,2weeks | ns | Traditional Speech and Language Therapy | Turned off after 30 s from the start | K-WAB |
| 5 | Katarzyna 2013 | A-tDCS | (intersection point between T3-Fz and F7-Cz) (BA 44) | d above the right supraorbital area. (BA 8) | 5 cm×7 cm | 1ma, 10 min ampere density:0.028 mA/cm2 | Five times per week, 3 weeks | 3months | Computerized Language Training | Turned off within a few seconds after activation | Naming Accuracy |
| 6 | Qi Zhao 2020 | A-tDCS | L-IFG, Broca’s area (BA 44/45) | on the surface of the deltoid muscle head on the right shoulder. (BA 8) | 10 cm2 | 2 mA,20 min | Five times per week, 4 weeks | ns | Individualized Speech Training | Turned off after 30 s from the start | WAB-Naming |
| 7 | Shereen 2022 | A-tDCS | L-IFG or FC5 (BA 44/45) | on the contralateral supraorbital. (BA 8) | 5×7 cm | 2mA, 20 min | Once per week,of 6 weeks | 6months | VNeST | Turned off after 30 s from the start | Picture Naming |
| 8 | Melissa 2023 | A-tDCS | L-IFG or FC5 (BA 44/45) | on the contralateral supraorbital. (BA 8) | Ns | 1mA, 20 min | Five times per week, 3 weeks | ns | Computer-Based Naming Therapy | Turned off after 30 s from the start | PNT+Naming80 |
SLT: speech and language therapy; BNT: Boston Naming Test; PNT: Philadelphia Naming Test; K-WAB: Korean version of the Western Aphasia Battery.
Table 3
Characteristics of the rTMS studies
| ID | Study | rTMS Parameters | Follow-Up | Speech Therapy | Sham Stimulation | Outcome Measures | |||||
| Stimulation area | Frequency | Intensity | Number of pulses | Duration | Session(s) | ||||||
| 1 | Mohammad 2017 | Right posterior inferior frontal gyrus (BA 44) | 1 Hz | 100% RMT | 1,200pulses | 20 min | Five times per week, 2 weeks | ns | Conventional Speech Therapy | Coil tilted at 90deg | WAB-Naming |
| 2 | Caili 2019 | Right pars triangularis of the P-IFG (BA 45) | 1 Hz | 80% RMT | 1,200pulses | 20 min | Five times per week, 3 weeks | ns | Oral Comprehension and Expression | Coil perpendicular to the scalp | WAB-Naming |
| 3 | Ilona 2015 | Right triangular part of the inferior frontal gyrus (BA 45) | 1 Hz | 90% RMT | 1,200pulses | 20 min | Five times per week, 2 weeks | ns | Behavioral Language Therapy | Coil placed at the apex of the inferior frontal gyrus | Naming Accuracy |
| 4 | Konrad 2012 | Anterior (triangular part - rptr) and posterior (opercular part - rpop); the anterior stimulation site located 2.5 cm posterior and 3 cm above the canthus-ear plane line; the posterior stimulation site located 4.5 cm behind and 6 cm above the neck-ear plane line. (BA 44/45) | 1 Hz | 90% RMT | 1,800pulses | 30 min (15 minutes each for PTr and Pop) | Five times per week,3 weeks | 15 weeks | Oral Comprehension and Expression | Sham coil | Naming Accuracy |
| 5 | Chih-Pin 2014 | Broca’s homolog (i.e., contralateral triangular part, PTr) (BA 44) | 1 Hz | 90% RMT | 1,200pulses | 20 min | Once daily, for a total of ten sessions | ns | Naming Training | Sham coil | Object Naming Accuracy |
| 6 | Nora 2011 | Right triangular region of the inferior frontal gyrus (IFG) (BA 44) | 1 Hz | 90% RMT | 1,200pulses | 20 min | Five times per week,2 weeks | ns | Individualized Speech Training | Coil placed at the apex of the inferior frontal gyrus | Picture Naming Accuracy |
| 7 | Xue-yan 2018 | F4 area on the standard EEG-10-20 system (BA 44) | 1 Hz | 80% RMT | 600 pulses | 10 min | Once daily, for a total of ten sessions | 2months | Conventional Speech Therapy | Coil tilted at 90deg | WAB |
| 8 | Trevo 2023 | Right triangular area of the inferior frontal gyrus (BA 45) | 1 Hz | 100% RMT | 1,200pulses | 20 min | Five times per week,2 weeks | 3months | Multimodal Aphasia Therapy | Sham coil | BNT |
| 9 | Tae 2015 | IFG (BA 44) | 1 Hz | 90% RMT | 1,200pulses | 20 min | Five times per week, 4 weeks | ns | Individualized Speech Training | None | K-WAB |
| 10 | Joanna 2013 | Right triangular area of the inferior frontal gyrus (BA 45) | 1 Hz | 90% RMT | 1,800pulses | 30 min | Five times per week, 3 weeks | 15 weeks | Oral Comprehension and Expression | Sham coil | BNAE |
3.4Meta-analysis results
3.4.1Postintervention efficacy of the NIBS
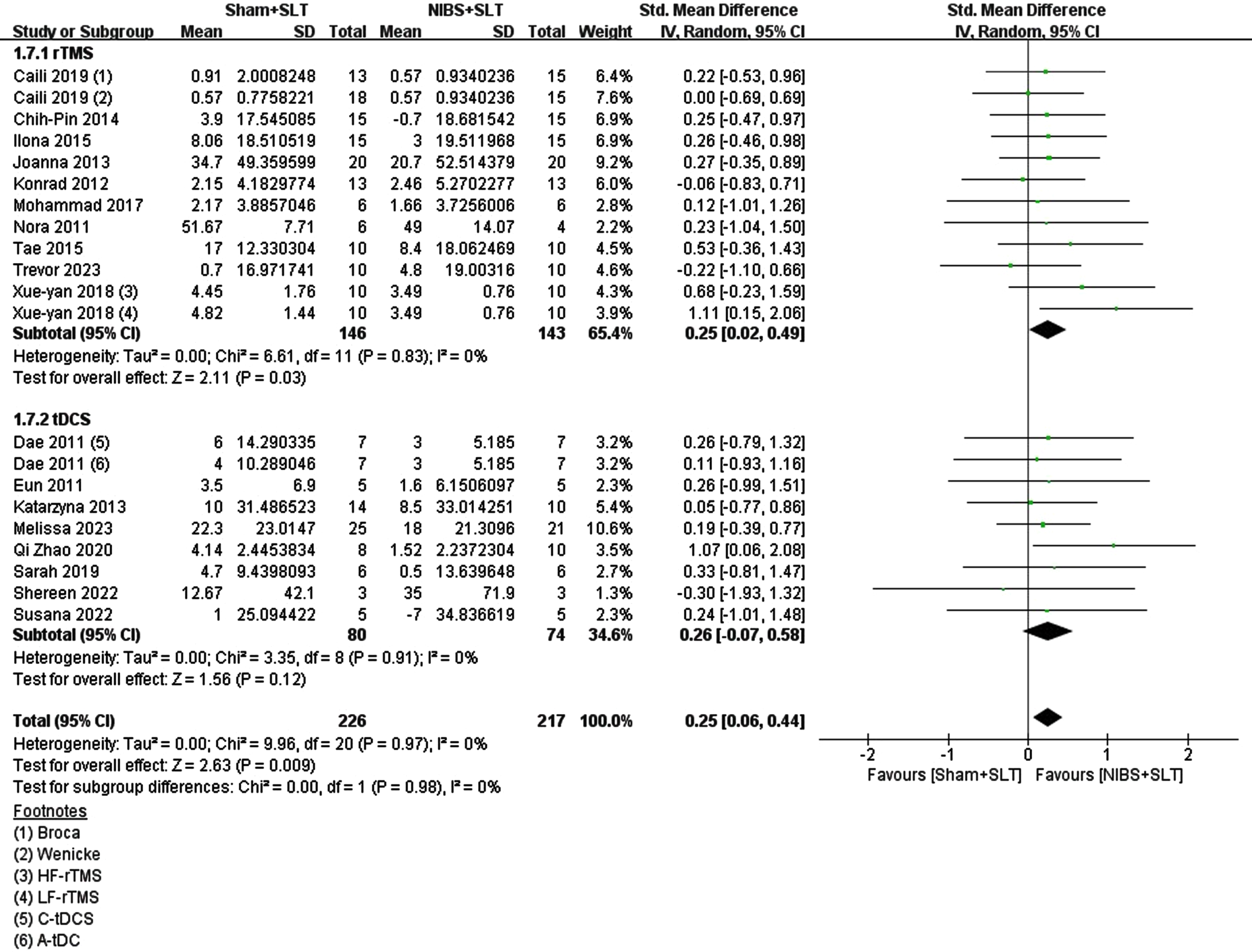
The main aim was to assess NIBS combined with SLT’s impact on naming functions. A meta-analysis of 18 RCTs found a moderate effect on post-stroke naming function recovery, statistically significant (overall SMD = 0.25; 95% CI = [0.06, 0.44], p = 0.009; I2 = 0%). However, results differed for rTMS and tDCS. rTMS showed a significant small to medium effect (SMD = 0.25; 95% CI = [0.02, 0.49], p = 0.03; I2 = 0%), while tDCS, though with a similar effect size, lacked statistical significance (SMD = 0.26; 95% CI = [–0.07, 0.58]; p = 0.12; I2 = 0%). Yet, moderating factor comparison between the two techniques showed no significant differences (Chi = 0.00, df = 1, p = 0.98, I2 = 0%). See Fig. 2.
Fig. 2
Forest plot of the effect on naming function in the included studies.
3.4.2Follow-up efficacy
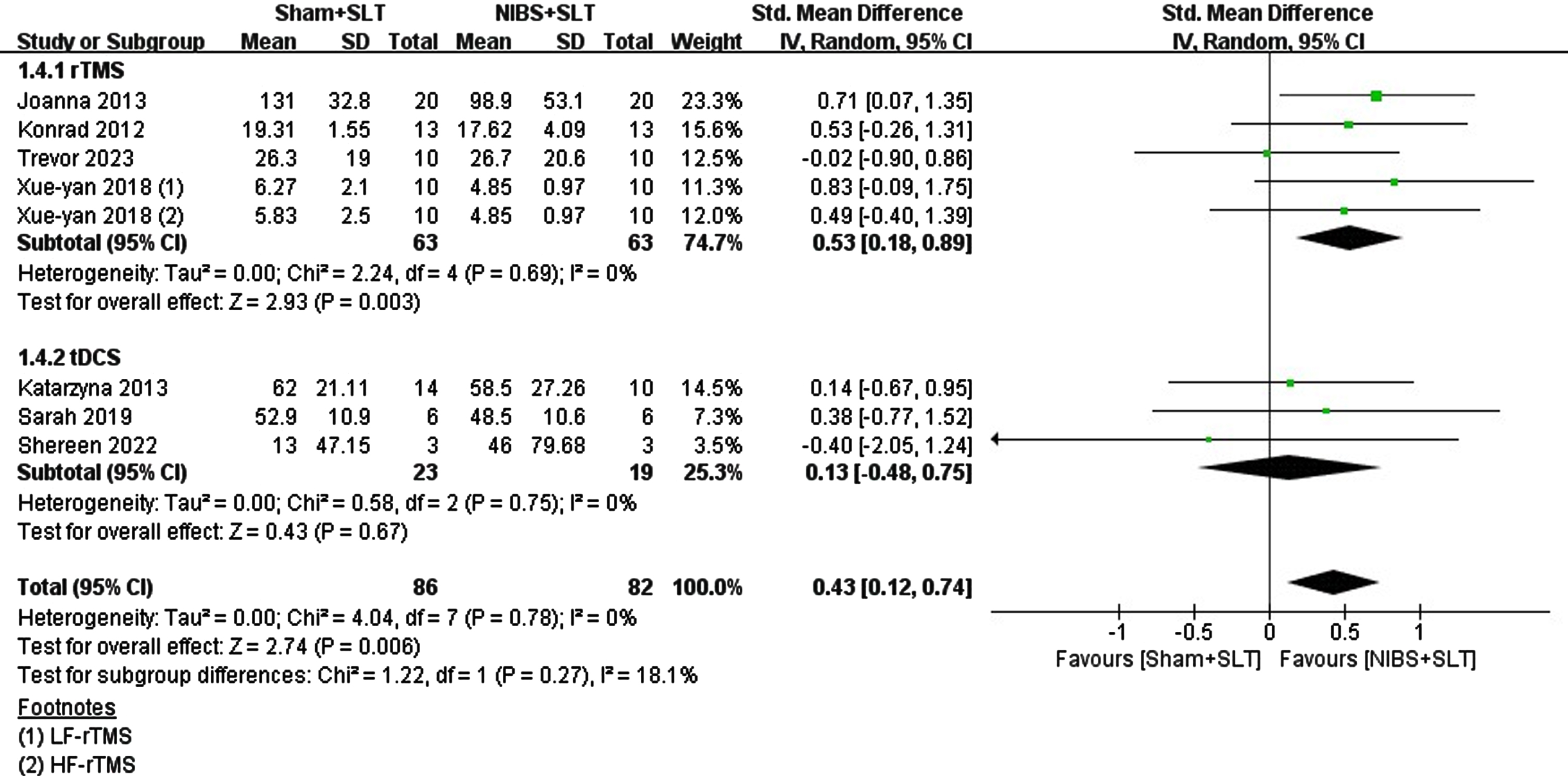
Among the 18 studies, 7 reported long-term follow-up results within 6 months. 4 combined rTMS with SLT, and 3 combined tDCS with SLT. This study aimed to explore whether NIBS stimulation exhibits long-term differences in efficacy compared to SLT alone. Meta-analysis revealed that NIBS as an adjunctive therapy had a medium effect size compared with SLT alone (SMD = 0.43; 95% CI = [0.12, 0.74], p = 0.006; I2 = 0%). Subgroup analysis of rTMS versus tDCS revealed distinct outcomes: rTMS had a significant medium effect size (SMD = 0.53; 95% CI = [0.18, 0.89], p = 0.003; I2 = 0%), while tDCS had a very small effect size (SMD = 0.13; 95% CI = [–0.48, 0.75], p = 0.67; I2 = 0%). Moderator tests comparing the two techniques showed no significant difference (Chi2 = 1.22, p = 0.27, I2 = 18.1%). See Fig. 3.
Fig. 3
Forest plot of the long-term effect of naming function in the included studies.
3.4.3Chronic vs. subacute
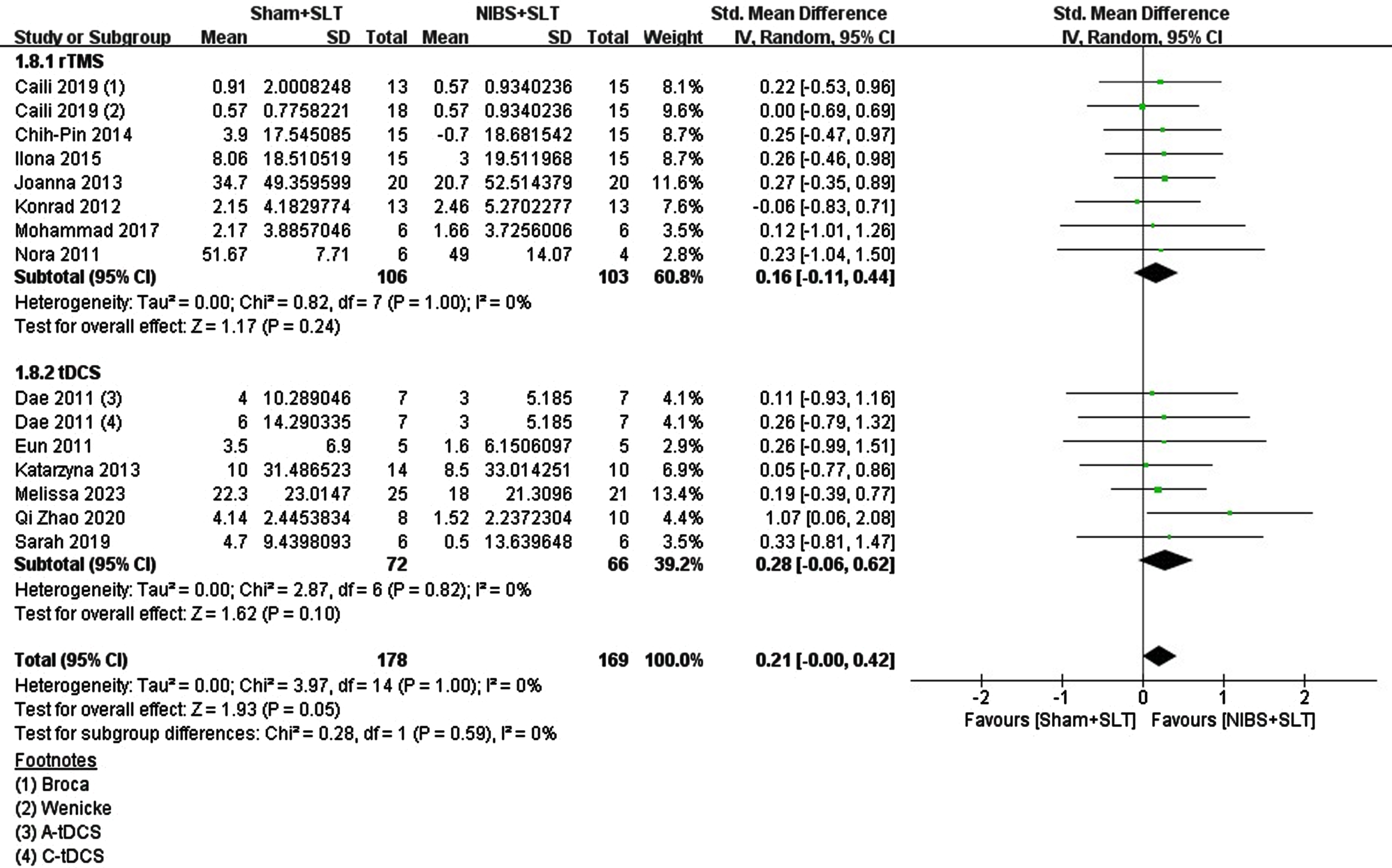
Subacute or chronic stroke phases determine brain state and plasticity, influencing treatment outcomes. The studies included chronic patients (n = 5, tDCS = 2, rTMS = 3) and subacute aphasia patients (n = 13, tDCS = 6, rTMS = 7) to compare NIBS efficacy. For subacute aphasia (<6 months poststroke), the effects of rTMS (SMD = 0.19, p = 0.20, N = 7) and tDCS (SMD = 0.28, p = 0.12, N = 6) were not significantly different. Pooled analysis revealed that NIBS combined with SLT had a small effect on naming function recovery (SMD = 0.23, p = 0.05), with no significant difference between stimulation types (Chi2 = 0.14, p = 0.70, I2 = 0%). See Fig. 4.
Fig. 4
Forest plot of the included studies on the naming recovery effect in chronic aphasia.
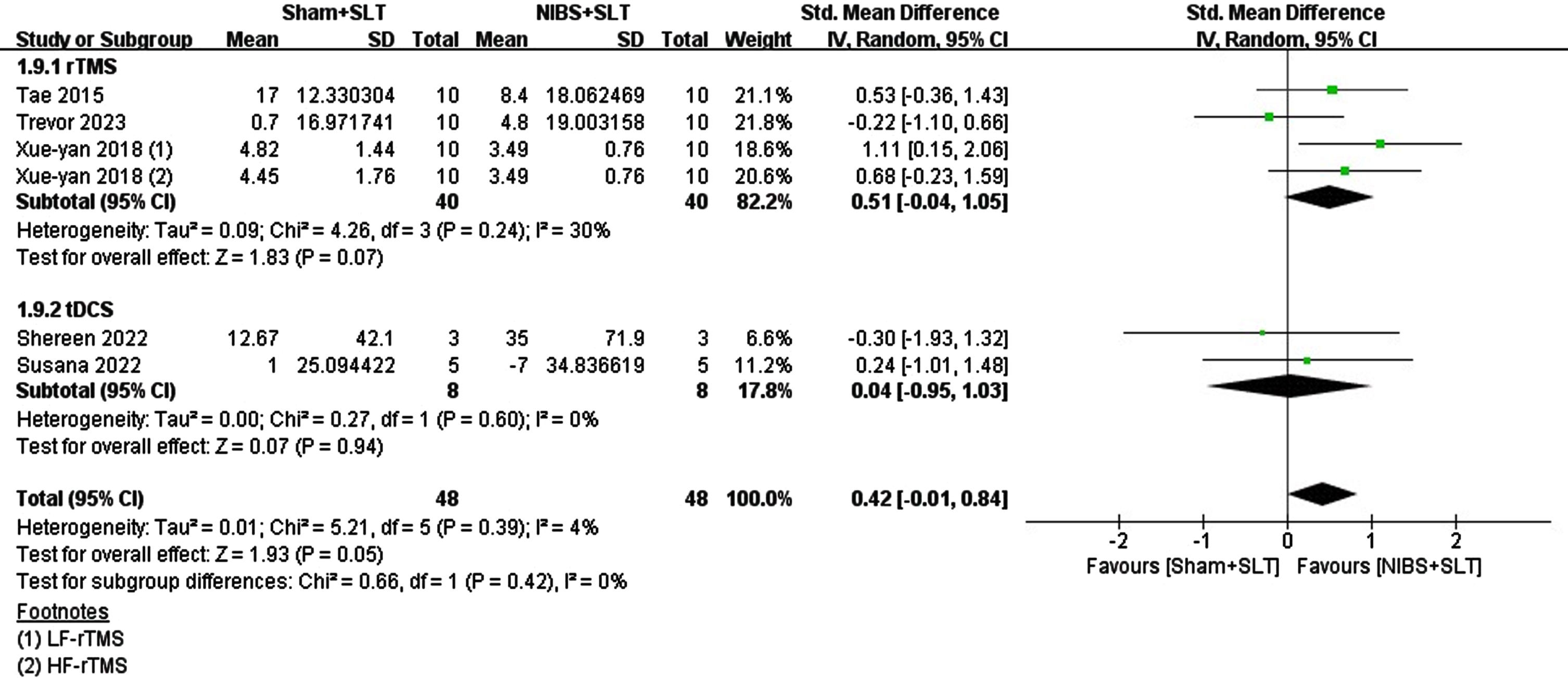
Few studies have explored the impact of NIBS on chronic aphasia (>6 months poststroke) in chronic stroke patients (rTMS = 3, tDCS = 2). rTMS had a nonsignificant effect size (SMD = 0.36, p = 0.25), while tDCS had an extremely low effect size (SMD = 0.04, p = 0.94). Pooled analysis revealed a moderate effect size for both stimulations (SMD = 0.26, p = 0.26), with no significant difference between them (Chi2 = 0.24, p = 0.62, I2 = 0%). See Fig. 5.
Fig. 5
Forest plot of the included studies on the naming recovery effect in Subacute aphasia.
3.4.4Literature quality assessment
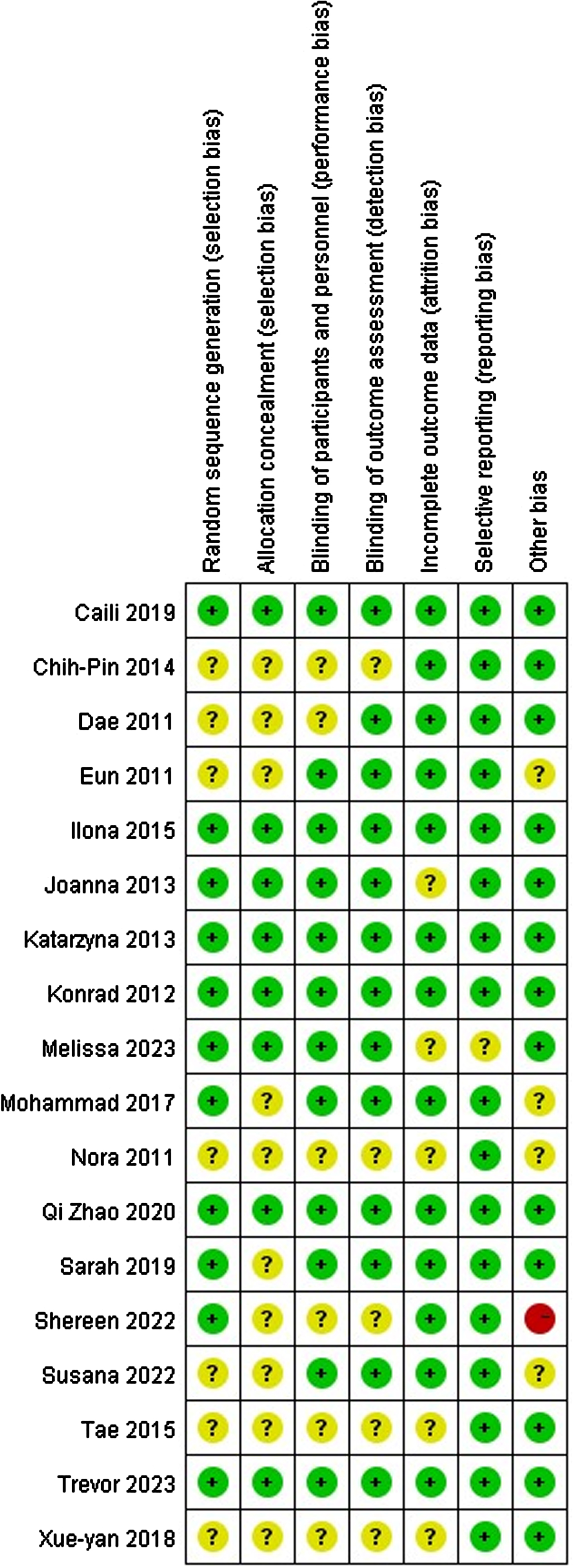
All 18 studies were RCTs, with 6 having low bias risk, 1 having high bias risk, and the remaining having moderate bias risk. One high-risk study had only 6 participants. Random number tables were used in 7 studies, opaque envelopes/mailbox methods were used in 2 studies, computer-generated sequences were used in 2 studies, and randomization was not specified in 7 studies. Allocation concealment and blinding were reported in 8 studies. All data were confirmed to be complete, with no selective reporting or bias. The risk of bias results is depicted in Fig. 6.
Fig. 6
Risk of bias legend.
4Discussion
In recent years, interest in studying tDCS or rTMS for clinical aphasia rehabilitation to improve outcomes and shorten treatment has increased. This meta-analysis aimed to assess the efficacy of NIBS (tDCS or rTMS) as an SLT adjunct for PSA naming function recovery. The goal of this study was to validate NIBS as an effective adjunctive therapy and establish the credibility of rTMS and tDCS as genuinely effective SLT add-ons. Currently, neural plasticity supports NIBS in PSA treatment. Interhemispheric inhibition and compensation models provide the primary theoretical basis (Sheng et al., 2023). The inhibition model suggests inhibiting the contralesional hemisphere enhances recovery, while the compensation model suggests the contralesional hemisphere compensates for functional loss (Mirdamadi et al., 2023). NIBS can modulate interhemispheric functional connectivity, promoting language network reconstruction post-stroke (Casula et al., 2021). Additionally, Di Pino et al. (2014) proposed a bimodal balance-recovery model linking interhemispheric balancing and functional recovery to spared structural reserves.
The meta-analysis, comprising 18 RCT studies, revealed that compared to the Sham group, NIBS had a moderate and significant impact on the recovery of naming function in PSA. This finding is consistent with current research on stroke aphasia rehabilitation, indicating the potential of rTMS and tDCS. Subgroup analyses indicated that the effects of both methods ranged from small to moderate, although the differences were not statistically significant, likely due to the small sample sizes. The PASS software was utilized to determine the sample sizes for future research, recommending a minimum of 20 individuals per group, with an estimated effect size of 0.5 for the WAB-AQ. Among the literature included in our analysis, only one study met this sample size requirement.
In the rTMS studies, two showed negative effects. One study by Trevor et al. combined rTMS with multimodal aphasia treatment (M-MAT), which involved intensive SLT. Despite the negative effects observed in the meta-analysis, individual analysis of this study revealed improvements in both the rTMS and sham treatment groups, with no statistically significant difference between them. Two possible explanations for this phenomenon are suggested: ding172 The intensive M-MAT might mask the specific improvements in naming function induced by rTMS, and ding173 rTMS might selectively impact PSA, exerting effects on specific phenotypes. We lean towards the first hypothesis, because previous meta-analyses have confirmed a correlation between the intensity of SLT and recovery outcomes in aphasia patients, indicating that greater intensity may lead to better therapeutic effects (Brady et al., 2022).
For the assessment of treatment’s long-term effects, data from seven trials with subsequent follow-up were analyzed. Overall, NIBS demonstrated a significant moderate effect compared to the Sham group. Subgroup analysis revealed a moderate effect of rTMS when combined with SLT, indicating observable long-term efficacy in naming function recovery following PSA. However, tDCS exhibited minimal and nonsignificant effects, suggesting limited long-term efficacy in naming function recovery. Our findings are consistent with recent meta-analyses (Y. You et al., 2023b), indicating a lack of distinct long-term therapeutic advantages with tDCS. Nevertheless, Bucur et al.’s meta-analysis suggested significant moderate effects of tDCS on long-term PSA recovery (Bucur & Papagno, 2019). This difference may stem from carryover effects in crossover studies, and caution should be exercised in interpreting our conclusions due to the limited number of studies with long-term tDCS follow-up.
Based on the theory of neuroplasticity, it is currently believed that the earlier the intervention, the better the prognosis (Laska et al., 2001). Therefore, we were curious whether NIBS has different effects in patients at different stages. Among the 18 studies, 13 focused on patients in the post-stroke subacute phase, which is in line with the mainstream population in current PSA rehabilitation. Post-stroke subacute aphasia has significant rehabilitation potential, especially within the first three months. However, in studies of chronic aphasia, NIBS therapy showed small to moderate effects, but not statistically significant. rTMS demonstrated a moderate effect size, while tDCS had very low effects on chronic patients. Nevertheless, due to the limited number of studies and small sample sizes, these results need to be cautiously interpreted, and further validation is warranted.
We were curious if different parameters of intervention yield distinct outcomes. In rTMS studies, all investigations utilized 1 Hz stimulation targeting the right Broca’s area. Damage to the left hemisphere disrupts inhibitory connections, increasing involvement of the contralateral right hemisphere regions and impeding neural plasticity. Low-frequency stimulation (LF-rTMS) of the right Broca’s area may reduce inhibitory input, thereby restoring post-stroke inhibitory balance. However, two studies introduced varying parameters. In the study by Hu et al., high-frequency rTMS (HF-rTMS) alongside LF-rTMS was introduced, potentially facilitating network reorganization (Jaillard et al., 2005). The results revealed that HF-rTMS also has therapeutic efficacy for PSA naming function, although not as pronounced as LF-rTMS. In the research by Ren et al., they applied LF-rTMS to different regions and found that stimulating the right pSTG contributed to improvements in auditory comprehension and repetition abilities, while stimulating the right pIFG contributed to enhancements in spontaneous speech and repetition abilities.
Although current research indicates that HF-rTMS is not as effective as LF-rTMS, it still yields significant effects. Some studies suggest applying HF-rTMS to the damaged hemisphere for optimal results. Szaflarski et al. (2011) applied HF-rTMS to the left Broca’s area and observed increased blood flow in the left Broca’s and Wernicke’s areas. Conversely, Hara et al. (2017) applied HF-rTMS to the lesioned hemisphere, resulting in increased activity in the contralateral hemisphere. Recently, Chang et al.’s (2022) study confirmed that in poststroke stable aphasia patients, language function improves and cortical-cortical interactions change when HF-rTMS is used to target the most active language-related area identified in advance by functional near-infrared spectroscopy (fNIRS). This suggests that HF-rTMS may have promising therapeutic effects on chronic aphasia patients. Further high-quality, large-sample studies are needed to determine the optimal application method and efficacy of HF-rTMS.
In tDCS research, most studies have applied anodal stimulation to the damaged side of Broca’s area. A-tDCS can enhance cortical excitability near Broca’s area, yet the exact mechanism remains unclear. However, three studies used different intervention methods: Kang et al. applied C-tDCS to reduce cortical excitability on the intact hemisphere, You et al. used two polarities of tDCS with no differences in naming function, and Feil et al. applied stimulations with two polarities, focusing on efficacy and safety without analyzing synergistic effects.
We investigated if lesion location variability affects NIBS combined with SLT outcomes. Out of 18 reviewed articles, only 7 recorded lesion locations, with one categorizing them into anterior/posterior regions. In Joanna et al.’s study, 7 patients had anterior lesions and 4 had anterior-posterior lesions. Results slightly favored anterior lesions, possibly due to targeting Broca’s homologous area. Trevo et al. suggested promoting right IFG activity over inhibiting it when left hemisphere cortex preservation is poor, aligning with current research. Unfortunately, other studies lacked relevant discussions on lesion location and prognosis correlation. We hope for more quality research on this topic.
This study is not without limitations. Firstly, studies with fewer than 4 participants were excluded because they could not produce RCTs, thus omitting such case reports and crossover studies introduces bias (Coemans et al., 2023; Fiori et al., 2011). Secondly, considering our focus on language function, excluding non-English literature that was not included may limit the comprehensiveness of the results (Abo & Kakuda, 2010). Furthermore, excluding unpublished grey literature, conference papers, yearbooks, etc., may increase the risk of publication bias. Additionally, there are differences among studies in participant characteristics, intervention measures, outcomes, and duration of follow-up. We attempted to address these differences through analysis, but may not fully eliminate confounding factors.
Our study suggests that for patients with naming function disorders post-stroke, NIBS as adjunctive intervention effectively enhances naming function recovery compared to standalone SLT, demonstrating long-term efficacy. Among adjunctive treatments, rTMS shows a moderate effect, with LF-rTMS being particularly useful and preferable as a clinical choice. Although slightly less effective than rTMS, tDCS as adjunctive therapy also demonstrates considerable efficacy. For subacute patients, both tDCS and rTMS are viable options, with a slight preference for rTMS. However, for chronic patients, we recommend LF-rTMS. In terms of long-term efficacy, rTMS shows a moderate effect, while tDCS exhibits relatively weaker effects. This suggests that the long-term efficacy of rTMS surpasses that of tDCS intervention. All studies included in our analysis were RCTs without crossover experiments, minimizing errors and confusion associated with inappropriate washout periods.
5Conclusion
The fusion of NIBS technology with speech and language training enhances naming function recovery in PSA, with long-term efficacy. In clinical aphasia treatment, LF rTMS is preferred when combined with SLT. For subacute patients, both options are viable, with a preference for rTMS. However, for chronic patients, LF-rTMS is recommended.
Acknowledgments
None to report.
Author contributions
HJ contributed to the design of the study. HJ and LBB contributed to the critical revision of the manuscript. CLS contributed to the screening of the papers, data extraction and analysis, and writing and revision of the paper. GXQ contributed to the screening of the papers, data extraction and analysis, and writing of the paper. XAL contributed to the screening of the papers and data extraction. HYS contributed to the screening of the papers and revision of the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (no. 82074512); Fujian Province Science and Technology Planning Project-social Development Guidance (Key) Project (no. 2023Y0035); and Fujian University of Traditional Chinese Medicine Collaborative Innovation Center for Rehabilitation Technology (no. X2022011).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics statement
This study, as a literature review, is exempt from Institutional Review Board approval.
Conflict of interest
The authors declare that they have no competing interests.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/NRE-240065.
References
1 | Abo, M. , Kakuda, W. ((2010) ). Neuroimaging and neurorehabilitation for aphasia]. Brain and nerve=Shinkei kenkyu no shinpo, 62: (2), 141–149. |
2 | Brady, M. C. , Ali, M. , VandenBerg, K. , Williams, L. J. , Williams, L. R. , Abo, M. , Becker, F. , Bowen, A. , Branden-burg, C. , Breitenstein, C. , Bruehl, S. , Copland, D. A. , Cranfill, T. B. , Di Pietro-Bachmann, M. , Enderby, P. , Fillingham, J. , Galli, F. L. , Gandolfi, M. , Glize, B. , ... REhabil Recovery PeopLE Aphasia St. ((2022) ). Dosage, Intensity, and Frequency of Language Therapy for Aphasia: A Systematic Review-Based, Individual Participant Data Network Meta-Analysis. STROKE, 53: (3), 956–967. https://doi.org/10.1161/STROKEAHA.121.035216 |
3 | Brady, M. C. , Kelly, H. , Godwin, J. , Enderby, P. , Campbell, P. ((2016) ). Speech and language therapy for aphasia following stroke. The Cochrane Database of Systematic Reviews, 2016: (6), CD000425. https://doi.org/10.1002/14651858.CD000425.pub4 |
4 | Breitenstein, C. , Grewe, T. , Flöel, A. , Ziegler, W. , Springer, L. , Martus, P. , Huber, W. , Willmes, K. , Ringelstein, E. B. , Haeusler, K. G. , Abel, S. , Glindemann, R. , Domahs, F. , Regenbrecht, F. , Schlenck, K.-J. , Thomas, M. , Obrig, H. , de Langen, E. , Rocker, R. , ... Baumgaertner, A. ((2017) ). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet (London, England), 389: (10078), 1528–1538. https://doi.org/10.1016/S0140-6736(17)30067-3 |
5 | Bucur, M. , Papagno, C. ((2019) ). Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? A comparative systematic review and meta-analysis on naming performance. Neuroscience and Biobehavioral Reviews, 102: , 264–289. https://doi.org/10.1016/j.neubiorev.2019.04.019 |
6 | Chang, W. K. , Park, J. , Lee, J.-Y. , Cho, S. , Lee, J. , Kim, W.-S. , Paik, N.-J. ((2022) ). Functional Network Changes After High-Frequency rTMS Over the Most Activated Speech-Related Area Combined With Speech Therapy in Chronic Stroke With Non-fluent Aphasia. Frontiers in Neurology, 13: , 690048. https://doi.org/10.3389/fneur.2022.690048 |
7 | Cid-Fernandez, S. , Angel Rivas-Fernandez, M. , Varela-Lopez, B. , Galdo-Alvarez, S. ((2022) ). Combined anodal transcranial direct current stimulation and behavioural naming treatment improves language performance in patients with post-stroke aphasia. Brain Inj, 36: (8), 1039–1045. https://doi.org/10.1080/02699052.2022.2109733 |
8 | Coemans, S. , Struys, E. , Tsapkini, K. , Paquier, P. , Vandenborre, D. , Keulen, S. ((2023) ). Case report: The effects of cerebellar tDCS in bilingual post-stroke aphasia. Frontiers in Human Neuroscience, 17: , 1173178. https://doi.org/10.3389/fnhum.2023.1173178 |
9 | Di Pino, G. , Pellegrino, G. , Assenza, G. , Capone, F. , Ferreri, F. , Formica, D. , Ranieri, F. , Tombini, M. , Ziemann, U. , Rothwell, J. C. , Di Lazzaro, V. ((2014) ). Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nature Reviews. Neurology, 10: (10), 597–608. https://doi.org/10.1038/nrneurol.2014.162 |
10 | Feil, S. , Eisenhut, P. , Strakeljahn, F. , Mueller, S. , Nauer, C. , Bansi, J. , Weber, S. , Liebs, A. , Lefaucheur, J.-P. , Kesselring, J. , Gonzenbach, R. , Mylius, V. ((2019) ). Left Shifting of Language Related Activity Induced by Bihemispheric tDCS in Postacute Aphasia Following Stroke. Frontiers in Neuroscience, 13: . https://doi.org/10.3389/fnins.2019.00295 |
11 | Fiori, V. , Coccia, M. , Marinelli, C. V. , Vecchi, V. , Bonifazi, S. , Ceravolo, M. G. , Provinciali, L. , Tomaiuolo, F. , Marangolo, P. ((2011) ). Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience, 23: (9), 2309–2323. https://doi.org/10.1162/jocn.2010.21579 |
12 | Flowers, H. L. , Skoretz, S. A. , Silver, F. L. , Rochon, E. , Fang, J. , Flamand-Roze, C. , Martino, R. ((2016) ). Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Archives of Physical Medicine and Rehabilitation, 97: (12), 2188–2201.e8. https://doi.org/10.1016/j.apmr.2016.03.006 |
13 | Goodglass, H. , Wingfield, A. ((1997) ). Anomia: Neuroanatomical and cognitive correlates. Academic Press. |
14 | Haghighi, M. , Mazdeh, M. , Ranjbar, N. , Seifrabie, M. A. ((2017) ), - Further Evidence of the Positive Influence of Repetitive Transcranial Magnetic Stimulation on Speech and Language in Patients with Aphasia after Stroke: Results from a Double-Blind Intervention with Sham Condition. 75: (4). |
15 | Hara, T. , Abo, M. , Kakita, K. , Mori, Y. , Yoshida, M. , Sasaki, N. ((2017) ). The Effect of Selective Transcranial Magnetic Stimulation with Functional Near-Infrared Spectroscopy and Intensive Speech Therapy on Individuals with Post-Stroke Aphasia. European Neurology, 77: (3-4), 186–194. https://doi.org/10.1159/000457901 |
16 | Hu, X.-Y. , Zhang, T. , Rajah, G. B. , Stone, C. , Liu, L.-X. , He, J.-J. , Shan, L. , Yang, L.-Y. , Liu, P. , Gao, F. , Yang, Y.-Q. , Wu, X.-L. , Ye, C.-Q. , Chen, Y.-D. ((2018) ). Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: A randomized, sham-controlled study. Neurological Research, 40: (6), 459–465. https://doi.org/10.1080/01616412.2018.1453980 |
17 | Jacobs, M. , Ellis, C. ((2023) ). Aphasianomics: Estimating the economic burden of poststroke aphasia in the United States. Aphasiology, 37: (1), 25–38. https://doi.org/10.1080/02687038.2021.1985426 |
18 | Jaillard, A. , Martin, C. D. , Garambois, K. , Lebas, J. F. , Hommel, M. ((2005) ). Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain: A Journal of Neurology, 128: (Pt 5), 1122–1138. https://doi.org/10.1093/brain/awh456 |
19 | Kang, E. K. , Kim, Y. K. , Sohn, H. M. , Cohen, L. G. , Paik, N.-J. ((2011) ). Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restor Neurol Neurosci, 29: (3), 141–152. https://doi.org/10.3233/RNN-2011-0587 |
20 | Laska, A. C. , Hellblom, A. , Murray, V. , Kahan, T. , Von Arbin, M. ((2001) ). Aphasia in acute stroke and relation to outcome. Journal of Internal Medicine, 249: (5), 413–422. https://doi.org/10.1046/j.1365-2796.2001.00812.x |
21 | Levelt, W. J. M. ((2001) ). Spoken word production: A theory of lexical access. Proceedings of the National Academy of Sciences, 98: (23), 13464–13471. https://doi.org/10.1073/pnas.231459498 |
22 | Low, T. A. , Lindland, K. , Kirton, A. , Carlson, H. L. , Harris, A. D. , Goodyear, B. G. , Monchi, O. , Hill, M. D. , Dukelow, S. P. ((2023) ). Repetitive transcranial magnetic stimulation (rTMS) combined with multi-modality aphasia therapy for chronic post-stroke non-fluent aphasia: A pilot randomized sham-controlled trial. Brain and Language, 236: , 105216. https://doi.org/10.1016/j.bandl.2022.105216 |
23 | Matar, S. J. , Newton, C. , Sorinola, I. O. , Pavlou, M. ((2022) ). Transcranial Direct-Current Stimulation as an Adjunct to Verb Network Strengthening Treatment in Post-stroke Chronic Aphasia: A Double-Blinded Randomized Feasibility Study. Frontiers in Neurology, 13: , 722402. https://doi.org/10.3389/fneur.2022.722402 |
24 | Meier, E. L. , Sheppard, S. M. , Goldberg, E. B. , Head, C. R. , Ubellacker, D. M. , Walker, A. , Hillis, A. E. ((2020) ). Naming errors and dysfunctional tissue metrics predict language recovery after acute left hemisphere stroke. Neuropsychologia, 148: , 107651. https://doi.org/10.1016/j.neuropsychologia.2020.107651 |
25 | Mirdamadi, J. L. , Xu, J. , Arevalo-Alas, K. M. , Kam, L. K. , Borich, M. R. ((2023) ). State-dependent interhemispheric inhibition reveals individual differences in motor behavior in chronic stroke. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 149: , 157–167. https://doi.org/10.1016/j.clinph.2023.02.177 |
26 | Polanowska, K. E. , Lesniak, M. M. , Seniow, J. B. , Czepiel, W. , Czlonkowska, A. ((2013) ). - Anodal transcranial direct current stimulation in early rehabilitation of patients with post-stroke non-fluent aphasia: A randomized, double-blind, sham-controlled pilot study, 31: (6). |
27 | Ren, C. , Zhang, G. , Xu, X. , Hao, J. , Fang, H. , Chen, P. , Li, Z. , Ji, Y. , Cai, Q. , Gao, F. ((2019) ). - The Effect of rTMS over the Different Targets on Language Recovery in Stroke Patients with Global Aphasia: A Randomized Sham-Controlled Study. 2019. |
28 | Rubi-Fessen, I. , Hartmann, A. , Huber, W. , Fimm, B. , Rommel, T. , Thiel, A. , Heiss, W.-D. ((2015) ). Add-on Effects of Repetitive Transcranial Magnetic Stimulation on Subacute Aphasia Therapy: Enhanced Improvement of Functional Communication and Basic Linguistic Skills. A Randomized Controlled Study. Arch Phys Med Rehabil, 96: (11), 1935–U375. https://doi.org/10.1016/j.apmr.2015.06.017 |
29 | Saini, V. , Guada, L. , Yavagal, D. R. ((2021) ). Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology, 97: (20 Suppl 2), S6–S16. https://doi.org/10.1212/WNL.0000000000012781 |
30 | Seniów, J. , Waldowski, K. , Leśniak, M. , Iwański, S. , Czepiel, W. , Członkowska, A. ((2013) ). Transcranial magnetic stimulation combined with speech and language training in early aphasia rehabilitation: A randomized double-blind controlled pilot study. Topics in Stroke Rehabilitation, 20: (3), 250–261. https://doi.org/10.1310/tsr2003-250 |
31 | Sheng, R. , Chen, C. , Chen, H. , Yu, P. ((2023) ). Repetitive transcranial magnetic stimulation for stroke rehabilitation: Insights into the molecular and cellular mechanisms of neuroinflammation. Frontiers in Immunology, 14: , 1197422. https://doi.org/10.3389/fimmu.2023.1197422 |
32 | Stockbridge, M. D. , Elm, J. , Breining, B. L. , Tippett, D. C. , Sebastian, R. , Cassarly, C. , Teklehaimanot, A. , Spell, L. A. , Sheppard, S. M. , Vitti, E. , Ruch, K. , Goldberg, E. B. , Kelly, C. , Keator, L. M. , Fridriksson, J. , Hillis, A. E. ((2023) ). Transcranial Direct-Current Stimulation in Subacute Aphasia: A Randomized Controlled Trial. Stroke, 54: (4), 912–920. https://doi.org/10.1161/STROKEAHA.122.041557 |
33 | Szaflarski, J. P. , Vannest, J. , Wu, S. W. , DiFrancesco, M. W. , Banks, C. , Gilbert, D. L. ((2011) ). Excitatory repetitive transcranial magnetic stimulation induces improvements in chronic post-stroke aphasia. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 17: (3), CR132–139. https://doi.org/10.12659/msm.881446 |
34 | Terao, Y. , Ugawa, Y. ((2002) ). Basic mechanisms of TMS. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 19: (4), 322–343. https://doi.org/10.1097/00004691-200208000-00006 |
35 | Waldowski, K. , Seniow, J. , Lesniak, M. , Iwanski, S. , Czlonkowska, A. ((2012) ). - Effect of low-frequency repetitive transcranial magnetic stimulation on naming abilities in early-stroke aphasic patients: A prospective, randomized, double-blind sham-controlled study. |
36 | Wang, C. P. , Hsieh, C. Y. , Tsai, P. Y. , Wang, C. T. , Lin, F. G. , Chan, R. C. ((2014) ), - Efficacy of synchronous verbal training during repetitive transcranial magnetic stimulation in patients with chronic aphasia, 45: (12). |
37 | Wang, W. , Jiang, B. , Sun, H. , Ru, X. , Sun, D. , Wang, L. , Wang, L. , Jiang, Y. , Li, Y. , Wang, Y. , Chen, Z. , Wu, S. , Zhang, Y. , Wang, D. , Wang, Y. , Feigin, V. L. ((2017) ). Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation, 135: (8), 759–771. https://doi.org/10.1161/CIRCULATIONAHA.116.025250 |
38 | Wang, Y.-J. , Li, Z.-X. , Gu, H.-Q. , Zhai, Y. , Zhou, Q. , Jiang, Y. , Zhao, X.-Q. , Wang, Y.-L. , Yang, X. , Wang, C.-J. , Meng, X. , Li, H. , Liu, L.-P. , Jing, J. , Wu, J. , Xu, A.-D. , Dong, Q. , Wang, D. , Wang, W.-Z. , ... Zhao, J.-Z. ((2022) ). China Stroke Statistics: An update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke and Vascular Neurology, 7: (5), 415–450. https://doi.org/10.1136/svn-2021-001374 |
39 | Weiduschat, N. , Thiel, A. , Rubi-Fessen, I. , Hartmann, A. , Kessler, J. , Merl, P. , Kracht, L. , Rommel, T. , Heiss, W. D. ((2011) ). Effects of Repetitive Transcranial Magnetic Stimulation in Aphasic Stroke A Randomized Controlled Pilot Study. Stroke, 42: (2), 409–415. https://doi.org/10.1161/STROKEAHA.110.597864 |
40 | Yoon, T. H. , Han, S. J. , Yoon, T. S. , Kim, J. S. , Yi, T. I. ((2015) ). Therapeutic effect of repetitive magnetic stimulation combined with speech and language therapy in post-stroke non-fluent aphasia. NeuroRehabilitation, 36: (1), 107–114. https://doi.org/10.3233/NRE-141198 |
41 | You, D. S. , Kim, D.-Y. , Chun, M. H. , Jung, S. E. , Park, S. J. ((2011) ). Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain and Language, 119: (1), 1–5. https://doi.org/10.1016/j.bandl.2011.05.002 |
42 | You, Y., Li, Y., Zhang, Y., Fan, H., Gao, Q., & Wang, L. (2023a). Long-term effects of transcranial direct current stimulation (tDCS) combined with speech language therapy (SLT) on post-stroke aphasia patients: A systematic review and network meta-analysis of randomized controlled trials. NeuroRehabilitation, 53: (3), 285–296. https://doi.org/10.3233/NRE-230099 |
43 | You, Y., Li, Y., Zhang, Y., Fan, H., Gao, Q., & Wang, L. (2023b). Long-term effects of transcranial direct current stimulation (tDCS) combined with speech language therapy (SLT) on post-stroke aphasia patients: A systematic review and network meta-analysis of randomized controlled trials. NeuroRehabilitation, 53: (3), 285–296. https://doi.org/10.3233/NRE-230099 |
44 | Zhao, Q. , Wang, J. , Li, Z. , Song, L. , Li, X. ((2021) ). Effect of Anodic Transcranial Direct Current Stimulation Combined With Speech Language Therapy on Nonfluent Poststroke Aphasia. Neuromodulation, 24: (5), 923–929. https://doi.org/10.1111/ner.13337 |




