Pressure ulcers acquired during inpatient rehabilitation after spinal cord injury, characterization and predictors: A 15-years’ experience
Abstract
BACKGROUND:
Most studies focus on the risk factors associated with the development of pressure ulcers (PUs) during acute phase or community care for individuals with spinal cord injury (SCI).
OBJECTIVES:
This study aimed to i) compare clinical and demographic characteristics of inpatients after SCI with PUs acquired during rehabilitation vs inpatients without PUs and ii) evaluate an existing PU risk assessment tool iii) identify first PU predictors.
METHODS:
Individuals (n = 1,135) admitted between 2008 and 2022 to a rehabilitation institution within 60 days after SCI were included. Admission Functional Independence Measure (FIM), American Spinal Injury Association Impairment Scale (AIS) and mEntal state, Mobility, Incontinence, Nutrition, Activity (EMINA) were assessed. Kaplan-Meier curves and Cox proportional hazards models were fitted.
RESULTS:
Overall incidence of PUs was 8.9%. Of these, 40.6% occurred in the first 30 days, 47.5% were sacral, 66.3% were Stage II. Patients with PUs were older, mostly with traumatic injuries (67.3%), AIS A (54.5%), lower FIM motor (mFIM) score and mechanical ventilation. We identified specific mFIM items to increase EMINA specificity. Adjusted Cox model yielded sex (male), age at injury, AIS grade, mFIM and diabetes as PUs predictors (C-Index = 0.749).
CONCLUSION:
Inpatients can benefit from combined assessments (EMINA + mFIM) and clinical features scarcely addressed in previous studies to prevent PUs.
1Introduction
Spinal cord injuries (SCIs) are a leading cause of permanent disability, affecting up to 500,000 people across the world each year (McGrath et al., 2019). Comprehensive inpatient rehabilitation from multiple disciplines (nursing, physical therapy, internal medicine, occupational therapy, psychology) is essential to support persons with SCI (Maribo et al., 2020). Such inpatients have a high risk of developing pressure ulcers (PUs) due to motor and sensory impairments (Mathew et al., 2013), immobility or changes in skin composition (Šín et al., 2022). The treatment and management of PUs is one of the most challenging clinical problems amongst inpatients with SCI, with recognized costly and lifelong implications (Vecin et al., 2022). Although its appearance is not a causal factor of mortality (González-Méndez et al., 2018), it is associated with other complications during inpatient treatment such as increased length of stay, risk of infection, workload for nursing staff and healthcare costs (Coleman et al., 2013; Jiang et al., 2014). In fact, in persons with SCI, the presence of a PU was recently reported to entail a fourfold increase in the annual cost of care over a one-year period (Gourlan et al., 2020).
Nevertheless, a recent systematic review (Shiferaw et al., 2020) revealed that about one in three patients with SCI still develop PUs. This highlights the need to develop specific preventive strategies to reduce the burden of PUs in this population.
As recently reported (Brienza et al., 2018), most studies focus on the risk factors associated with the development of PUs during community care for individuals with SCI, however less address the risk factors in this population during hospitalization. Around 30–40% of individuals with SCI develop PUs during the acute and rehabilitation phases (Vecin et al., 2022). Furthermore, PUs represent a primary cause of re-hospitalization during the first five years after SCI (diPiro et al., 2022).
Studies suggest that risk factors for PUs in persons with SCI include duration after SCI, age (older age), sex (being male), poor nutritional status, tetraplegia, comorbidity, weight (low body mass index), higher-level spinal cord injuries, lower level of education, lack of an intimate partner (Shiferaw et al., 2020) or a low score on the Functional Independence Measure (FIM) motor score (Verschueren et al., 2011). Nevertheless, most studies focus on acute care, e.g. Verschueren and colleagues analyzed 193 patients where the occurrence of PUs was 36.5% during acute care phase and 39.4% during functional rehabilitation (Verschueren et al., 2011). Having had a PU during acute rehabilitation phase was reported as the strongest risk factor. Analyses considering PUs acquired during subacute rehabilitation are therefore, scarce. The scarcity of studies analyzing PUs acquired in subacute rehabilitation settings hinders our ability to effectively prevent and manage these debilitating injuries, potentially impacting patient outcomes and increasing healthcare costs.
Another important factor yet to receive adequate study during post-acute rehabilitation after SCI are ulcer risk assessment scales. Most PUs are identified as avoidable; therefore, management primarily focuses on prevention (García-Fernández et al., 2014). Most clinical practice guidelines recommend PU risk assessment for all individuals when entering an acute or longer-term care facility; however, consensus concerning implementation of the best instrument or procedure for PU risk assessment is lacking (Heikkilä et al., 2022). In a previous systematic review, pooled analysis revealed that the Braden, EMINA (mEntal state, Mobility, Incontinence, Nutrition, Activity), Norton, Cubbin-Jackson, and Waterlow scales achieved the most robust PU predictive capacity (García-Fernández et al., 2014). The EMINA scale has been tested in various health care settings (Roca-Biosca et al., 2015). The validity indicators and predictive capacity are good, the scale is easy to use, with clearly defined factors, but scarcely analyzed when assessing inpatients after SCI.
Therefore, we hypothesized that: H1) medical factors such as presence of diabetes, dyslipidemia, hypertension or mechanical ventilation, as well as demographic factors such as gender, age and severity of injury measured by the American Spinal Injury Association Impairment Scale (AIS), would be associated with increased risk for the formation of PUs in newly injured individuals with traumatic or non-traumatic SCI during post-acute inpatient rehabilitation. H2) Empirical data will support the use of EMINA risk assessment. H3) post-acute PUs predictors will emerge using traditional survival analysis techniques. If confirmed, H1 and H2 could be used to support the decisions for specific mitigation strategies and H3 could in turn be used as a first step towards specific future model developments.
This study aimed to retrospectively analyze a cohort of inpatients with SCI in a rehabilitation setting to i) compare clinical and demographic characteristics of inpatients who experienced a PU acquired during rehabilitation vs inpatients who did not experience a PU event; ii) evaluate an extensively used PU risk assessment tool in such context; and iii) develop a model to identify first PU predictors.
2Methods
2.1Study design
A retrospective observational cohort study was conducted, analyzing inpatients admitted to the Rehabilitation Unit of Institut Guttmann Hospital, Barcelona, Spain. The period under study was December 2008 –December 2022. This study conforms to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines (STROBE, 2023; Supplemement).
2.2Participants and setting
Eligible participants were adults (≥18 years at the date of admission) with the diagnosis of SCI, receiving inpatient rehabilitation within 60 days after injury, at Institut Guttmann Hospital, between December 2008 –December 2022. Patients were excluded due to SCI in the context of severe multi-impairment. Patients who acquired PUs in acute care prior to their admission to inpatient rehabilitation were also excluded.
All persons admitted to the rehabilitation unit were referred from different acute care setting hospitals and fulfilled the hospitals criteria for admission which included SMART (Specific, Measurable, Achievable, Relevant and Time-bound) (Wade et al., 2009) objectives and support for discharge in case of severe disability. The rehabilitation program included five daily hours of intensive treatment oriented towards physical, behavioral and psychosocial problems as well as training in activities of daily life living.
2.3Standardized assessment instruments
FIM motor scores were categorized into 3 levels (Brock et al., 2007): good, fair, and poor. A “good” outcome was defined as score of 65 or above, scores under 46 indicate a large physical burden of care (poor outcome). Each FIM item is rated on a scale from 1 (complete dependence) to 7 (complete independence); higher ratings representing greater functional independence (summed scores range from 18 to 126). Item scores of 1 and 2 represent patients that are completely dependent (CD) on a helper at two levels of assistance, item scores of 3–5 represent patients that have modified dependence (MD) on a helper at three levels of assistance and item scores of 6 and 7 represent patients that do not require a helper and have either modified or complete independence (IND) (Graham et al., 2014).
Acceptable to high internal consistency, intra-rater and inter- rater reliability of the FIM items in separate groups of patients with SCI and stroke was reported (Küçükdeveci et al., 2013). The concurrent validity was satisfactory through the correlation of the FIM with ASIA and Brunnstrom motor scales (Küçükdeveci et al., 2013).
The Hospital Anxiety and Depression Scale (HADS) is considered one of the most popular clinical and research instruments used to screen for anxiety and depression, with extensive applications in SCI. It contains 14 items and consists of two subscales: anxiety and depression. Each item is rated on a four-point scale, giving maximum scores of 21 for anxiety and depression (Zigmond, 1983). The anxiety and depression subscales were both reported as reliable (r = .72,.82) in SCI (Müller et al., 2012). Concurrent validity has been evaluated mainly using the Beck Depression Inventory and the State-Trait Anxiety inventory reporting a range of correlations from.49 to.83 (Bjelland et al., 2002).
The EMINA scale was developed and validated in patients in acute-care and medium-stay hospitals in Spain, and it is currently used in several Spanish-speaking countries. The five criteria that make up the scale are: mental status, mobility, incontinence, nutrition, and activity. Each criterion is scored from 0 to 3, therefore, the total score of the scale ranges from 0 to 15 : 0 for patients at no risk and 15 for those at highest risk. EMINA score identifies three PU risk levels: low risk (0≤EMINA≤3), medium risk (4≤EMINA≤7) and high risk (EMINA≥8) (Roca-Biosca et al., 2015). EMINA interobserver reliability was evaluated by an intraclass correlation coefficient for the total score of the scale of 0.93. The kappa index for each of the five scale criteria ranged from 0.72 to 0.92. For the validity of the criteria a ROC curve was made with an area under the curve of 0.82 (0.79–0.85) (Gallego, 2001).
2.4Pressure ulcers events
A PU is defined in related literature as “localized damage to the skin and underlying soft tissue usually over a bony prominence or related to a medical or other device. It can present as intact skin or an open ulcer and occurs as a result of intense and/or prolonged pressure, or pressure in combination with shear” (Shiferaw et al., 2020). PUs most commonly occur over bony prominences but can develop on any part of the body (Vecin et al., 2022).
In 2008, the Nursing Department of Institut Guttmann Hospital implemented a PU management program that uses a digital form to systematically record specific variables of each PU, such as the severity of the event, its location, measures and other variables of interest. The PU management program includes a four-stage PU classification and staging system, as recommended by the European Pressure Ulcer Advisory Panel (EPUAP).
Through the PU management program, education and training is provided to both the inpatient and the family and is recorded in the Education folder of the inpatient’s electronical health records. The PUs management program is being assessed at the Institut Guttmann Hospital by the Joint Commission International since 2008, with the last recertification obtained in 2022.
2.5Sample size calculation
We performed an apriori statistical power analyses using G*Power version 3.1 test for multiple regression analyses (Faul et al., 2009) to compute the required sample size. We applied the following effect sizes conventions: f2 = 0.02 small effects, f2 = 0.15 medium effects, f2 = 0.35 large effects (the smallest the effect sizes, the largest the sample size). We aimed for an effect size f2 = 0.025, alfa error probability = 0.05 and statistical power = 0.95, with a number of predictors ranging from 20 to 25 and a number of tested predictors also ranging from 15 to 25. We obtained an optimal sample size of n = 1,127. The detailed G*Power calculation is presented in the Supplement.
2.6Statistical analyses
Data analysis was performed using the R environment for statistical computing and graphics (R Foundation, Vienna, Austria –Version 4.0.4).
Absolute and relative frequencies were reported for categorical variables. Non-parametric statistics with median and interquartile range (IQR), were used for those continuous variables not normally distributed. Differences between groups were analyzed with Student t-test, Mann–Whitney U-test and Chi2 test (χ2). Fisher exact test was used for variables with <5 cases. Spearman’s rank correlation coefficient was used to investigate relations between variables.
Kaplan-Meier analysis with the Log Rank test for statistical significance was used to investigate the time to first PU event. The time period from rehabilitation admission to the first occurrence of a PU event, or the end of inpatient stay, was used to measure event-free time. Patients who did not experience a PU event were censored at discharge.
Univariable and multivariable Cox proportional hazards models were used to identify independent predictors of PUs. Univariable models were used to select the predictors to be entered in multivariable models: only variables resulting as significant in univariable models at P < 0.10 were entered into the multivariable model, as in related research (Beghi et al., 2018). Risks were computed as hazards ratios with 95% confidence intervals (95% CIs).
Included candidate predictors were: age at injury, sex, time since injury to rehabilitation admission, risk factors such as diabetes, dyslipidemia, hypertension, body mass index. Clinical assessments such as AIS grade, FIM and HADS at admission, injury level (paraplegia or tetraplegia), cause of injury (traumatic or non-traumatic), the persons they were living with at admission (Alone, Intimate partner, Parents or close family, Others) and educational level (High school, College, or university).
The incidence rate or incidence density was calculated as the ratio between the number of PUs developed during the rehabilitation stay and the sum of the lengths of stay for each individual throughout the period under study for every 1,000 days of stay.
Statistical significance was set at the 5% level (P < 0.05). Missing data was handled using the listwise deletion method. In the absence of consistent findings from previous research in SCI to calculate the sample size, the decision was taken to include all available inpatients as initial source population.
2.7Ethical considerations
The study was approved by the Institut Guttmann Ethics Committee of Clinical Research. The participants were anonymized and non-identifiable. Participants provided informed written consent to be included in research studies addressed by Institut Guttmann Hospital.
3Results
The source population (n = 2,011) were all adult inpatients (≥18 at the moment of admission) with the diagnosis of SCI, receiving inpatient rehabilitation at Institut Guttmann Hospital between December 2008 –December 2022. Admitted patients with no complete FIM assessments performed within 7 days at rehabilitation admission and discharge were excluded (n = 310). Inpatients who acquired PUs during acute care (n = 133) were excluded as well as inpatients with time since injury to admission longer than 60 days (n = 394). Cases of SCI in the context of severe multi-impairment were also excluded (n = 39) leaving 1135 inpatients included in the study, with 101 of them acquiring PUs during inpatient rehabilitation, giving an incidence of 8.9%.
The incidence rate was 10.01 PUs for 1,000 days of stay, with a 95% CI: 8.16–12.28. The median time in days to PU onset was 72 days (25th percentile = 39; 75th percentile = 102), with a minimum of 1 day and a maximum of 199 days with a length of stay ranging from one week to 200 days, and a median length of stay of 79 (45–109) days. In a similar context Verschueren et al. reported a median time since injury to rehabilitation admission of 35 days (range 25–61 days), in their case the median duration of functional rehabilitation phase was 191 days (range 132–290 days) (Verschueren et al., 2011).
Supplementary Figure 1 shows the total number of inpatients included in the study by year highlighting those who acquired a PU and those who did not.
Forty point six per cent of PUs appeared in the first 30 days after admission and 61.4% appeared in the first 60 days (details are presented in Supplementary Table 3).
3.1PU events: characteristics for inpatients who developed a PU vs. inpatients who did not
Table 1 compares characteristics for inpatients who developed a PU vs. inpatients who did not. A larger proportion of inpatients who developed PUs presented with a traumatic SCI (67.3% v 50.2%), AIS A injury (54.5% v 26.5%) and had a higher average LOS (114 v 75 days) and a lower average FIM motor score (25 v 39 points).
Table 1
Clinical and demographic characteristics for inpatients with and without ulcers
| No-ulcer | Ulcer | All | p | |
| (N = 1034) | (N = 101) | (N = 1135) | ||
| Age at injury | 49 (35–62) | 52 (41–64) | 49 (35–62) | 0.041 |
| Age at injury ranges | 0.092 | |||
| 18–30 | 184 (17.8%) | 13 (12.9%) | 197 (17.4%) | |
| 31–45 | 268 (25.9%) | 17 (16.8%) | 285 (25.1%) | |
| 46–60 | 291 (28.1%) | 35 (34.7%) | 326 (28.7%) | |
| 61–75 | 256 (24.8%) | 33 (32.7%) | 289 (25.5%) | |
| 76+ | 35 (3.4%) | 3 (3.0%) | 38 (3.3%) | |
| Sex | 0.029 | |||
| Female | 368 (35.6%) | 25 (24.8%) | 393 (34.6%) | |
| Male | 666 (64.4%) | 76 (75.2%) | 742 (65.4%) | |
| AIS | <0.001 | |||
| A | 274 (26.5%) | 55 (54.5%) | 329 (29.0%) | |
| B | 111 (10.7%) | 15 (14.9%) | 126 (11.1%) | |
| C | 222 (21.5%) | 18 (17.8%) | 240 (21.1%) | |
| D | 427 (41.3%) | 13 (12.9%) | 440 (38.8%) | |
| Level | 0.096 | |||
| Paraplegia | 650 (62.9%) | 55 (54.5%) | 705 (62.1%) | |
| Tetraplegia | 384 (37.1%) | 46 (45.5%) | 430 (37.9%) | |
| Completeness of injury | <0.001 | |||
| Complete | 274 (26.5%) | 55 (54.5%) | 329 (29.0%) | |
| Incomplete | 760 (73.5%) | 46 (45.5%) | 806 (71.0%) | |
| NLI | 0.071 | |||
| C1-C4 | 227 (22.0%) | 34 (33.7%) | 261 (23.0%) | |
| C5-C8 | 157 (15.2%) | 13 (12.9%) | 170 (15.0%) | |
| L1-S5 | 169 (16.3%) | 10 (9.9%) | 179 (15.8%) | |
| T1-T6 | 200 (19.3%) | 20 (19.8%) | 220 (19.4%) | |
| T7-T12 | 281 (27.2%) | 24 (23.8%) | 305 (26.9%) | |
| Cause of injury | 0.001 | |||
| Non-traumatic | 515 (49.8%) | 33 (32.7%) | 548 (48.3%) | |
| Traumatic | 519 (50.2%) | 68 (67.3%) | 587 (51.7%) | |
| Diabetes | 93 (9%) | 14 (13.9%) | 107 (9.4%) | 0.110 |
| Dyslipimia | 146 (14.1%) | 10 (9.9%) | 156 (13.7%) | 0.240 |
| Hypertension | 239 (23.1%) | 24 (23.8%) | 263 (23.2%) | 0.883 |
| Heart failure | 53 (5.1%) | 5 (5.0%) | 58 (5.1%) | 0.939 |
| BMI | 24.3 (21.7–27.6) | 24.1 (21.1–27.0) | 24.3 (21.6–27.6) | 0.306 |
| BMI Categories | 0.533 | |||
| Underweight | 45 (4.4%) | 7 (7.1%) | 52 (4.6%) | |
| Normal | 535 (51.8%) | 52 (52.5%) | 587 (51.9%) | |
| Overweight | 302 (29.3%) | 29 (29.3%) | 331 (29.3%) | |
| Obese | 150 (14.5%) | 11 (11.1%) | 161 (14.2%) | |
| Mechanical ventilation | 37 (3.6%) | 10 (9.9%) | 47 (4.1%) | 0.002 |
| Canula | 14 (1.4%) | 8 (7.9%) | 22 (1.9%) | <0.001 |
| TSI, days | 44 (24–48) | 43 (29–49) | 45 (25–49) | 0.834 |
| LOS | 75 (43–104) | 114 (81–142) | 79 (45–109) | <0.001 |
| FIM at admission | ||||
| FIM Total | 74 (57–98) | 59 (48–72) | 72 (55–96) | <0.001 |
| FIM Cognitive | 35 (34–35) | 35 (33–35) | 35 (34–35) | 0.008 |
| FIM Motor | 39 (24–63) | 25 (14–39) | 38 (22–62) | <0.001 |
| FIM Motor stratification | <0.001 | |||
| Poor | 583 (56.4%) | 82 (81.2%) | 665 (58.6%) | |
| Fair | 205 (19.8%) | 13 (12.9%) | 218 (19.2%) | |
| Good | 246 (23.8%) | 6 (5.9%) | 252 (22.2%) | |
| FIM at discharge | ||||
| FIM Total | 108 (83–115) | 77 (55–102) | 106 (78–114) | <0.001 |
| FIM Cognitive | 35 (35–35) | 35 (34–35) | 35 (35–35) | 0.005 |
| FIM Motor | 73 (50–80) | 43 (20–67) | 72 (44–80) | <0.001 |
| HADS at admission | ||||
| Anxiety | 4 (2–7) | 4 (2–6) | 4(2–7) | 0.534 |
| Normal | 773 (74.8%) | 78 (77.2%) | 851 (75.0%) | 0.838 |
| Borderline | 118 (11.4%) | 11 (10.9%) | 129 (11.4%) | |
| Abnormal | 143 (13.8%) | 12 (11.9%) | 155 (13.7%) | |
| Depression | 3 (2–6) | 3 (2–6) | 3 (2–6) | 0.671 |
| Normal | 823 (79.6%) | 81 (80.2%) | 904 (79.6%)) | 0.939 |
| Borderline | 87 (8.4%) | 9 (8.9%) | 96 (8.5%) | |
| Abnormal | 124 (12.0%) | 11 (10.9%) | 135 (11.9%) | |
| Living with | 0.800 | |||
| Alone | 150 (14.5%) | 17 (16.8%) | 167 (14.7%) | |
| Parents or other family | 235 (22.7%) | 19 (18.8%) | 254 (22.4%) | |
| Intimate partner | 589 (57.0%) | 59 (58.4%) | 648 (57.1%) | |
| Living with others | 60 (5.8%) | 6 (5.9%) | 66 (5.8%) | |
| Years of education | 0.275 | |||
| Illiterate (<1) | 10 (1.0%) | 3 (3.0%) | 13 (1.1%) | |
| Read and write (< 2 years) | 65 (6.3%) | 3 (3.0%) | 68 (6.0%) | |
| Primary (2–5 years) | 500 (48.4%) | 49 (48.5%) | 549 (48.4%) | |
| High school (6–12 years) | 274 (26.5%) | 26 (25.7%) | 300 (26.4%) | |
| University (> 13 years) | 185 (17.9%) | 20 (19.8%) | 205 (18.1%) | |
| EMINA score(**) | 4 (3–6) | 5 (4–7) | 5 (3–6) | < 0.001 |
| EMINA categorization | < 0.001 | |||
| Low risk | 272 (27.2%) | 4 (4.0%) | 276 (25.1%) | |
| Mid risk | 672 (67.2%) | 86 (86.0%) | 758 (68.9%) | |
| High risk | 56 (5.6%) | 10 (10.0%) | 66 (6.0%) |
FIM: Functional Independence Measure; HADS: Hospital Anxiety and Depression Scale; AIS: American Spinal Injury Association Impairment Scale; LOS: Length of inpatient stay; TSI: time since injury; NLI: Neurological level of injury; BMI: body mass index; EMINA mEntal state, Mobility, Incontinence, Nutrition, Activity. (**) EMINA assessment was performed at rehabilitation admission in 1000 of the 1034 patients with no ulcer (96.7%) and in 100 of the 101 patients with ulcers (99.0%).
Figure 1 maps out the admission motor FIM with the time of the event of the first PU from admission and from discharge. As in Table 1, this figure shows that the majority of inpatients who developed a PU had a low FIM score at admission (<46).
Fig. 1
Total PUs analyzed in this study (n = 101) presented as a scatter plot, each point represents a PU with days after admission in the x axis and days before discharge in the y axis, the color of each point indicates the motor FIM level (the closer to green, the better motor FIM).
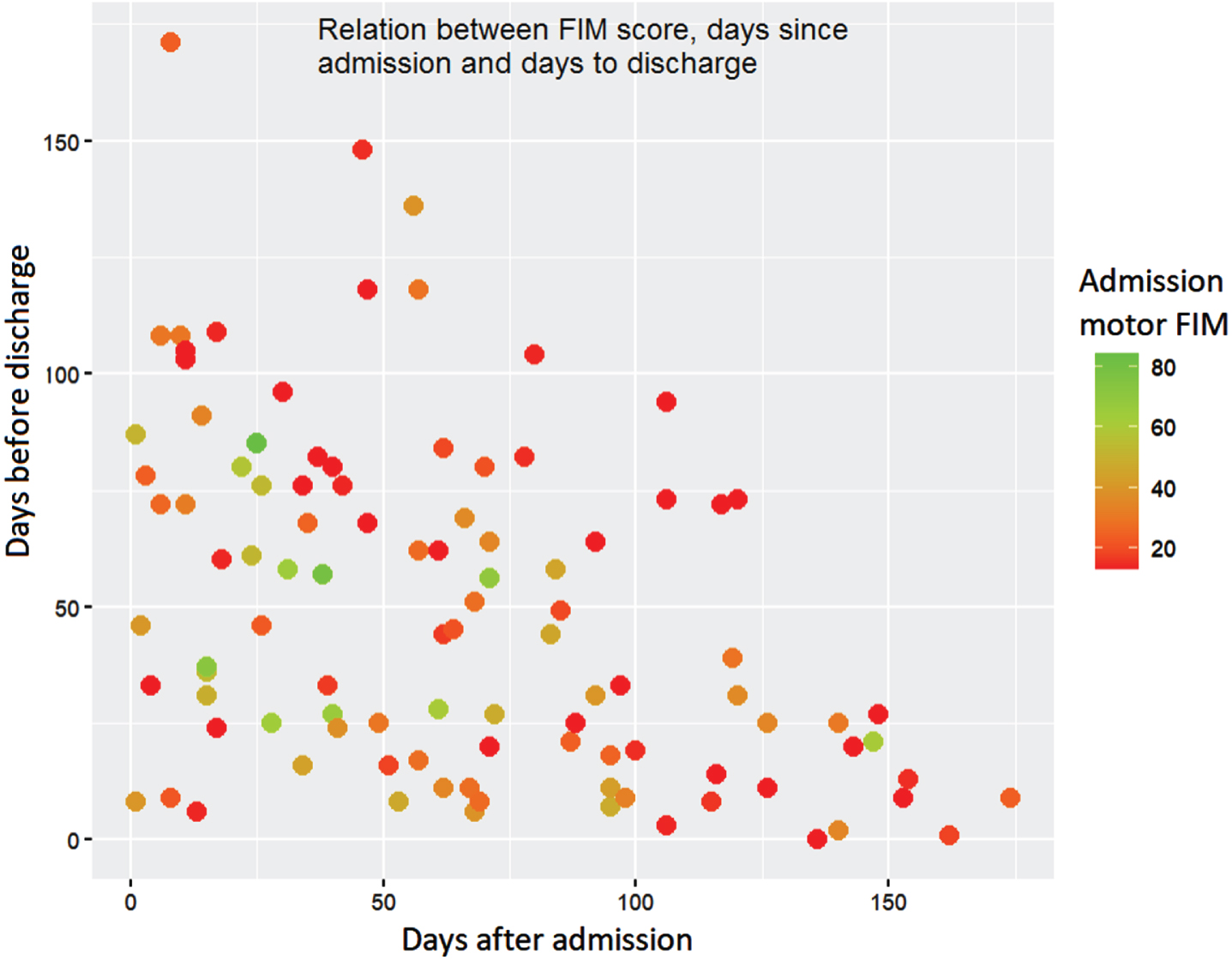
Table 2
First occurrence of pressure ulcers during inpatient rehabilitation (n = 101)
| Stage I | Stage II | Stage III | Total | p | |
| Cases | 29 (28.7%) | 67 (66.3%) | 5 (4.9%) | 101 | |
| Location | < 0.001 | ||||
| Heel | 2 (6.9%) | 7 (10.4%) | 1 (20.0%) | 10 (9.9%) | |
| Ankle | 2 (6.9%) | 3 (4.5%) | 0 (0.0%) | 5 (5.0%) | |
| Trochanter | 1 (3.4%) | 3 (4.5%) | 0 (0.0%) | 4 (4.0%) | |
| Ischium | 0 (0.0%) | 7 (10.4%) | 1 (20.0%) | 8 (7.9%) | |
| Sacrum | 6 (20.7%) | 39 (58.2%) | 3 (60.0%) | 48 (47.5%) | |
| Torso/Back | 6 (20.7%) | 0 (0.0%) | 0 (0.0%) | 6 (5.9%) | |
| Other | 12 (41.4%) | 8 (11.9%) | 0 (0.0%) | 20 (19.8%) | |
| Size in cm | |||||
| Length | 10 (5–20) | 10 (5–20) | 20 (20–60) | 10 (5–20) | 0.05 |
| Width | 15 (6–30) | 10 (8–20) | 40 (24–40) | 12 (8–24) | 0.041 |
| Time since admission | 28 (12–64) | 55 (27–79) | 62 (51–76) | 46 (21–76) | 0.045 |
| to ulcer, days |
Supplementary Table 1 describes PUs acquired in patients during acute care (n = 133). Regarding the onset of the first PU, 33 (24.8%) PUs presented in Stage I, 48 (36.1%) in Stage II and 36 (27.0%) in Stage III. In contrast to PUs acquired during inpatient rehabilitation, 16 (12.0%) Stage IV PUs were observed. PUs in the sacrum were also most prevalent, accounting for 74 (55.6%) of all PUs and notably the majority of PUs in Stage II (75.0%), Stage III (55.6%) and Stage IV (56.2%). Stage I PUs were most commonly located on the torso/back (33.3%).
Supplementary Table 2 compares clinical and demographic characteristics for patients who acquired PUs during acute treatment (n = 133) vs patients who acquired PUs during rehabilitation (n = 101).
3.2PU risk assessment tool: EMINA
Table 1 presents EMINA assessments performed at rehabilitation admission in 1000 of the 1034 patients who did not acquire a PU (96.7%) and in 100 of the 101 patients who acquired a PU (99.0%).
As detailed in Table 1 most of the patients (68.9%) are categorized by EMINA at admission as mid-risk of acquiring a PU. Nevertheless, of those classified as mid-risk only 11.4% (86/758) actually acquired a PU. The relationship between EMINA categorizations and acquired PUs is further illustrated in Fig. 2. Each point in the plot represents the EMINA score assessed at admission for each patient, with red plots showing patients who acquired a PU and blue points representing patients who did not acquire a PU. Dashed lines represent the EMINA cutoff points: low-risk of PU occurrence (0≤EMINA≤3), mid-risk (4≤EMINA≤7) and high-risk (EMINA≥8) (Roca-Biosca et al., 2015; Gallego, 2001). Thus, for patients categorized as low-risk, Fig. 2 shows four red points (two of them between 2010 and 2015, one between 2015 and 2020 and the other one after 2020).
Fig. 2
EMINA assessments at admission presented as scatter plot, each point represents an EMINA assessment with dotted lines showing risk cut-off values (0–3: low risk; 4–7: medium risk and≥8 high risk).
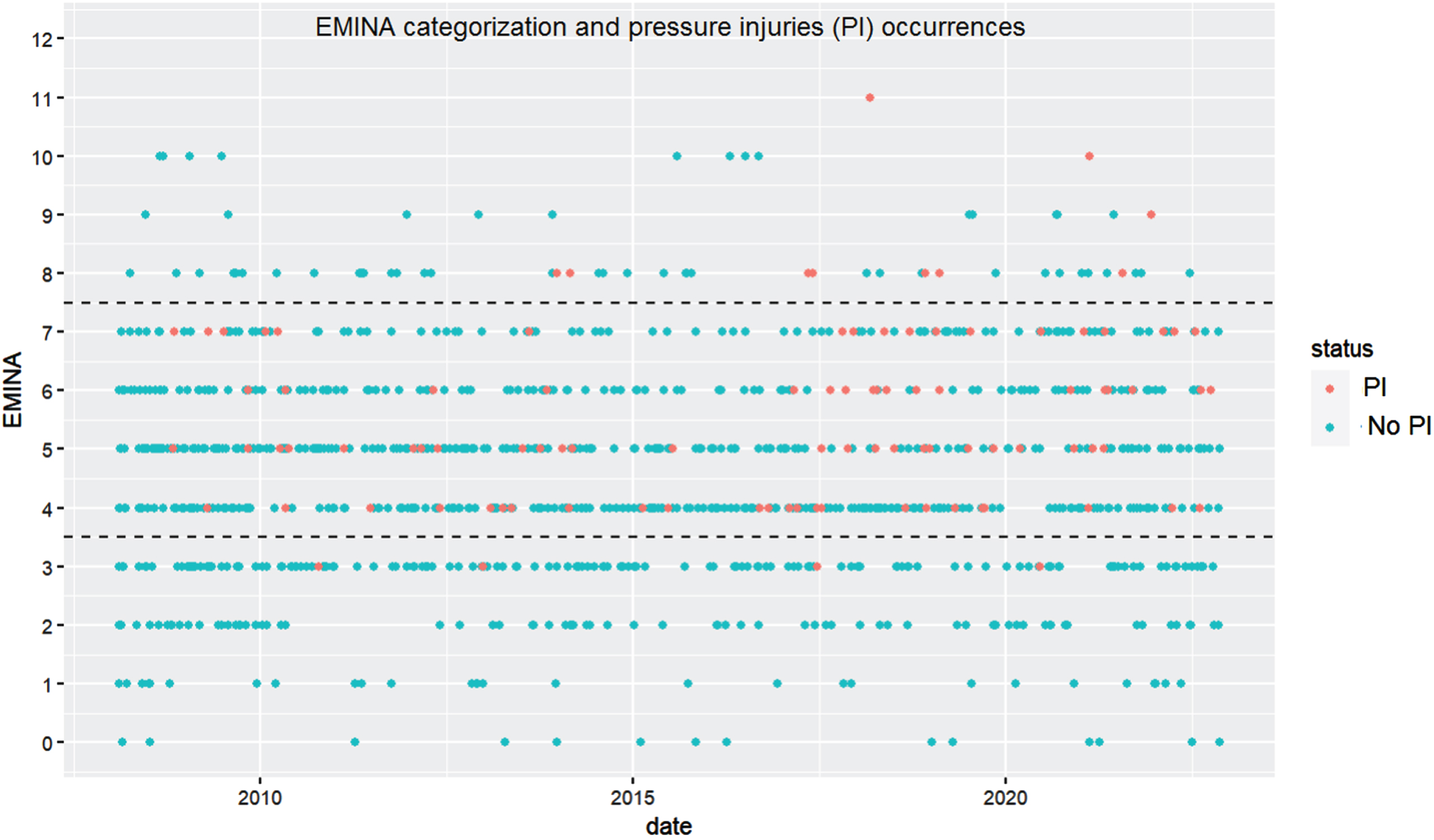
To ensure that all patients at risk of developing PUs receive preventive measures, those classified as mid-risk by the EMINA scale should be considered to be at high-risk. This will result in a high sensitivity (0.76), meaning that most patients who would go on to develop a PU are identified. However, it will also result in a very low specificity (<0.10), meaning that many patients who would not go on to develop a PU are treated as high risk, which may be a drain on resources.
Therefore, other patient characteristics should also be considered for patients classified as mid-risk in order to make more accurate risk assessments.
Table 3 describes patients’ characteristics for those classified as mid-risk on the EMINA scale who acquired a PU vs those who did not acquire a PU. A larger proportion of inpatients who developed a PU presented with a traumatic SCI (68.6% v 51.9%), AIS A injury (55.8% v 31.1%) and had a lower average FIM motor score at admission (24 v 35).
Table 3
Clinical and demographic characteristics for inpatients with and without ulcers and EMINA mid-risk
| No-ulcer | Ulcer | All | p | |
| (N = 672) | (N = 86) | (N = 758) | ||
| Age at injury | 48 (34–63) | 52 (44–65) | 49 (35–63) | 0.043 |
| Age at injury ranges | 0.048 | |||
| 18–30 | 122 (18.2%) | 11 (12.8%) | 133 (17.5%) | |
| 31–45 | 179 (26.6%) | 13 (15.1%) | 192 (25.3%) | |
| 46–60 | 172 (25.6%) | 31 (36.0%) | 203 (26.8%) | |
| 61–75 | 175 (26.0%) | 28 (32.6%) | 203 (26.8%) | |
| 76+ | 24 (3.6%) | 3 (3.5%) | 27 (3.6%) | |
| Sex | 0.035 | |||
| Female | 233 (34.7%) | 20 (23.3%) | 253 (33.4%) | |
| Male | 439 (65.3%) | 66 (76.7%) | 505 (66.6%) | |
| AIS | <0.001 | |||
| A | 209 (31.1%) | 48 (55.8%) | 257 (33.9%) | |
| B | 81 (12.1%) | 12 (14.0%) | 93 (12.3%) | |
| C | 165 (24.6%) | 17 (19.8%) | 182 (24.0%) | |
| D | 217 (32.3%) | 9 (10.5%) | 226 (29.8%) | |
| Neurological level | 0.045 | |||
| Cervical | 250 (37.2%) | 43 (50.0%) | 293 (38.7%) | |
| Thoracic | 323 (48.1%) | 36 (41.9%) | 359 (47.4%) | |
| Lumbar | 99 (14.7%) | 7 (8.1%) | 106 (14.0%) | |
| Injury | 0.039 | |||
| Paraplegia | 421 (62.6%) | 44 (51.2%) | 465 (61.3%) | |
| Tetraplegia | 251 (37.4%) | 42 (48.8%) | 293 (38.7%) | |
| Completeness of injury | <0.001 | |||
| Complete | 209 (31.1%) | 48 (55.8%) | 257 (33.9%) | |
| Incomplete | 463 (68.9%) | 38 (44.2%) | 501 (66.1%) | |
| Cause of injury | 0.004 | |||
| Non-traumatic | 323 (48.1%) | 27 (31.4%) | 350 (46.2%) | |
| Traumatic | 349 (51.9%) | 59 (68.6%) | 408 (53.8%) | |
| FIM at admission | ||||
| FIM Total | 69 (54–89) | 58 (48–70) | 67 (53–87) | < 0.001 |
| FIM Motor | 35 (21–54) | 24 (13–37) | 34 (19–53) | < 0.001 |
| FIM Motor stratification | 0.003 | |||
| Poor | 441 (65.6%) | 72 (83.7%) | 513 (67.7%) | |
| Fair | 132 (19.6%) | 9 (10.5%) | 141 (18.6%) | |
| Good | 99 (14.7%) | 5 (5.8%) | 104 (13.7%) | |
| EMINA score | 5 (4–6) | 5 (4–6) | 5 (4–6) | 0.076 |
FIM: Functional Independence Measure; AIS: American Spinal Injury Association Impairment Scale.
We can now re-calculate sensitivity and specificity for patients classified by EMINA as mid-risk by introducing cause of injury and defining traumatic cause as high risk of PU and non-traumatic cause as low risk of PU, this yields to sensitivity = 0.68 and specificity = 0.48.
If we instead consider completeness of injury and define complete injury as high risk of PU and incomplete injury as low risk of PU, this yields to sensitivity = 0.55 and specificity = 0.68.
Table 4 describes FIM items for patients classified as mid-risk on the EMINA scale who acquired a PU vs those who did not acquire a PU. A significantly larger proportion (p < 0.001) of inpatients who developed PUs were rated as CD in the FIM items of bathing (79.1% v 56.4%), toileting (87.2% v 66.2%), bed/chair transfers (75.6% v 52.8%), tub/shower transfers (84.9% v 63.1%), toilet transfers (81.4% v 60.6%) and wheelchair mobility (59.7% v 36.9%). Differences in proportions of patients rated as CD for bladder (81.4% v 67.7%) and bowel (83.7% v 67.4%) were significant (p = 0.02) and (p = 0.006) respectively.
Table 4
FIM items for inpatients with and without ulcers and EMINA mid risk
| Item | Level | No ulcer | Ulcer | Total | p |
| (N = 673) | (N = 86) | (N = 758) | |||
| Eating | CD | 139 (20.7%) | 33 (38.4%) | 172 (22.7%) | < 0.001 |
| MD | 119 (17.7%) | 15 (17.4%) | 134 (17.7%) | ||
| IND | 414 (61.6%) | 38 (44.2%) | 452 (59.6%) | ||
| Grooming | CD | 187 (27.8%) | 37 (43.0%) | 224 (29.6%) | 0.014 |
| MD | 153 (22.8%) | 15 (17.4%) | 168 (22.2%) | ||
| IND | 332 (49.4%) | 34 (39.5%) | 366 (48.3%) | ||
| Bathing | CD | 379 (56.4%) | 68 (79.1%) | 447 (59.0%) | <0.001 |
| MD | 203 (30.2%) | 14 (16.3%) | 217 (28.6%) | ||
| IND | 90 (13.4%) | 4 (4.7%) | 94 (12.4%) | ||
| Dress upper | CD | 290 (43.2%) | 50 (58.1%) | 340 (44.9%) | 0.011 |
| MD | 158 (23.5%) | 20 (23.3%) | 178 (23.5%) | ||
| IND | 224 (33.3%) | 16 (18.6%) | 240 (31.7%) | ||
| Dress lower | CD | 435 (64.7%) | 67 (77.9%) | 502 (66.2%) | 0.045 |
| MD | 145 (21.6%) | 13 (15.1%) | 158 (20.8%) | ||
| IND | 92 (13.7%) | 6 (7.0%) | 98 (12.9%) | ||
| Toileting | CD | 445 (66.2%) | 75 (87.2%) | 520 (68.6%) | < 0.001 |
| MD | 147 (21.9%) | 8 (9.3%) | 155 (20.4%) | ||
| IND | 80 (11.9%) | 3 (3.5%) | 83 (10.9%) | ||
| Bladder | CD | 455 (67.7%) | 70 (81.4%) | 525 (69.3%) | 0.029 |
| MD | 87 (12.9%) | 8 (9.3%) | 95 (12.5%) | ||
| IND | 130 (19.3%) | 8 (9.3%) | 138 (18.2%) | ||
| Bowel | CD | 453 (67.4%) | 72 (83.7%) | 525 (69.3%) | 0.006 |
| MD | 91 (13.5%) | 8 (9.3%) | 99 (13.1%) | ||
| IND | 128 (19.0%) | 6 (7.0%) | 134 (17.7%) | ||
| Bed/chair | CD | 355 (52.8%) | 65 (75.6%) | 420 (55.4%) | < 0.001 |
| MD | 224 (33.3%) | 14 (16.3%) | 238 (31.4%) | ||
| IND | 93 (13.8%) | 7 (8.1%) | 100 (13.2%) | ||
| Toilet | CD | 407 (60.6%) | 70 (81.4%) | 477 (62.9%) | < 0.001 |
| MD | 191 (28.4%) | 11 (12.8%) | 202 (26.6%) | ||
| IND | 74 (11.0%) | 5 (5.8%) | 79 (10.4%) | ||
| Tub/shower | CD | 424 (63.1%) | 73 (84.9%) | 497 (65.6%) | < 0.001 |
| MD | 182 (27.1%) | 9 (10.5%) | 191 (25.2%) | ||
| IND | 66 (9.8%) | 4 (4.7%) | 70 (9.2%) | ||
| Walk | CD | 64 (51.6%) | 5 (55.6%) | 69 (51.9%) | 0.940 |
| MD | 41 (33.1%) | 3 (33.3%) | 44 (33.1%) | ||
| IND | 19 (15.3%) | 1 (11.1%) | 20 (15.0%) | ||
| Wheelchair | CD | 202 (36.9%) | 46 (59.7%) | 248 (39.7%) | < 0.001 |
| MD | 175 (31.9%) | 21 (27.3%) | 196 (31.4%) | ||
| IND | 171 (31.2%) | 10 (13.0%) | 181 (29.0%) | ||
| Stairs | CD | 630 (93.8%) | 83 (96.5%) | 713 (94.1%) | 0.588 |
| MD | 30 (4.5%) | 2 (2.3%) | 32 (4.2%) | ||
| IND | 12 (1.8%) | 1 (1.2%) | 13 (1.7%) |
CD: completely dependent, MD: modified dependence; IND: independent.
We can now re-calculate sensitivity and specificity for patients classified by EMINA as mid-risk by introducing, for example, the FIM Eating item and defining CD as high risk of PU, MD and IND as low risk of PU, this yields to sensitivity = 0.38 and specificity = 0.79.
3.3Pressure ulcer event predictors
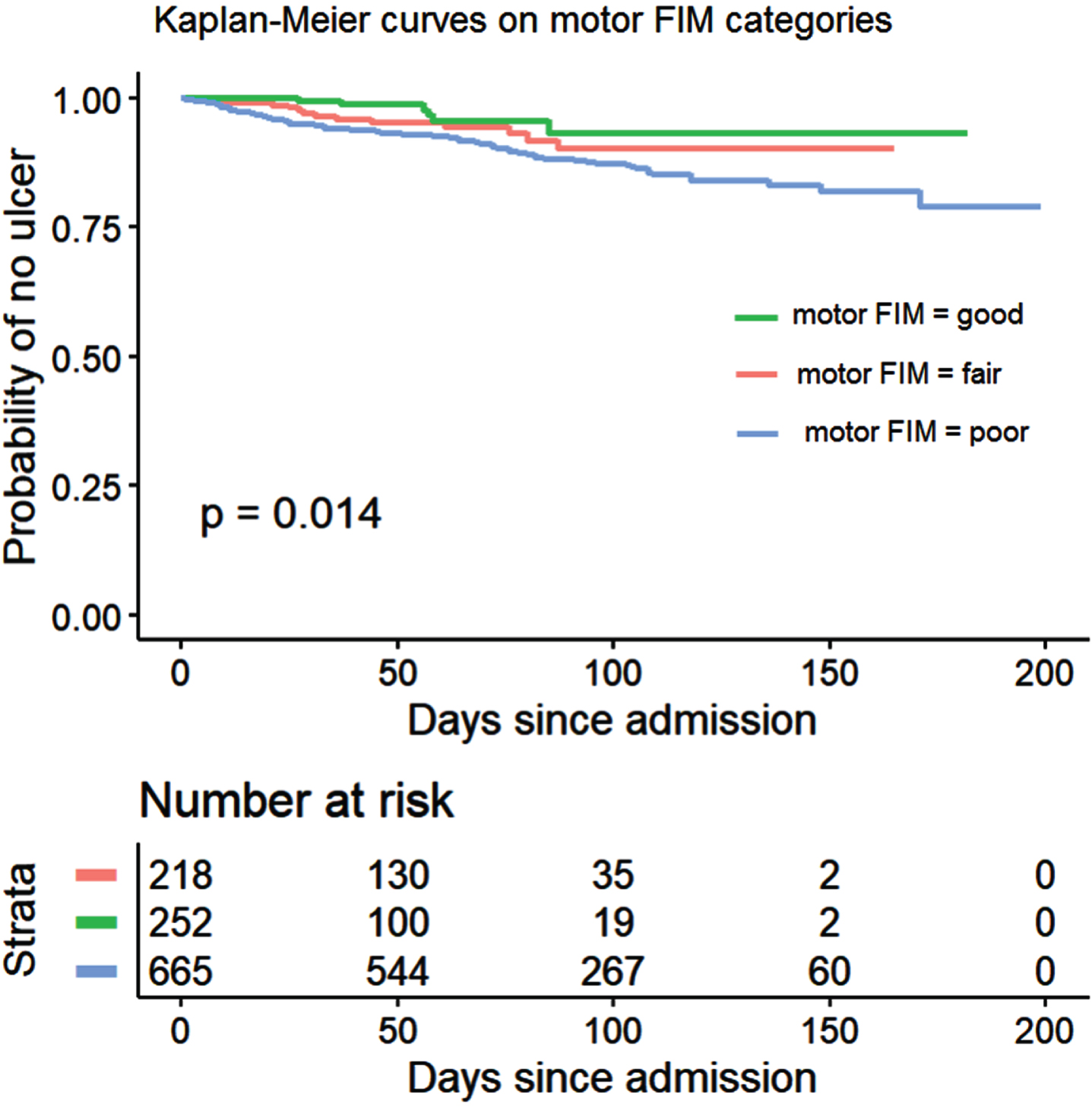
Kaplan–Meier analysis showed a probability of developing a PU during inpatient rehabilitation that follows the AIS grades, with results being lower in individuals with AIS grades B-D and highest for AIS grade A (p < 0.001), especially after the first 60 days (Fig. 3). Similarly, inpatients with “good” motor FIM at admission showed a lower probability of acquiring PUs than those with “fair” motor FIM which in turn show lower probability than those with “poor” motor FIM at admission (p = 0.014) as shown in Fig. 4.
Fig. 3
Kaplan-Meier curves on time to first PU for inpatients (n = 1135) classified according to their AIS grades.
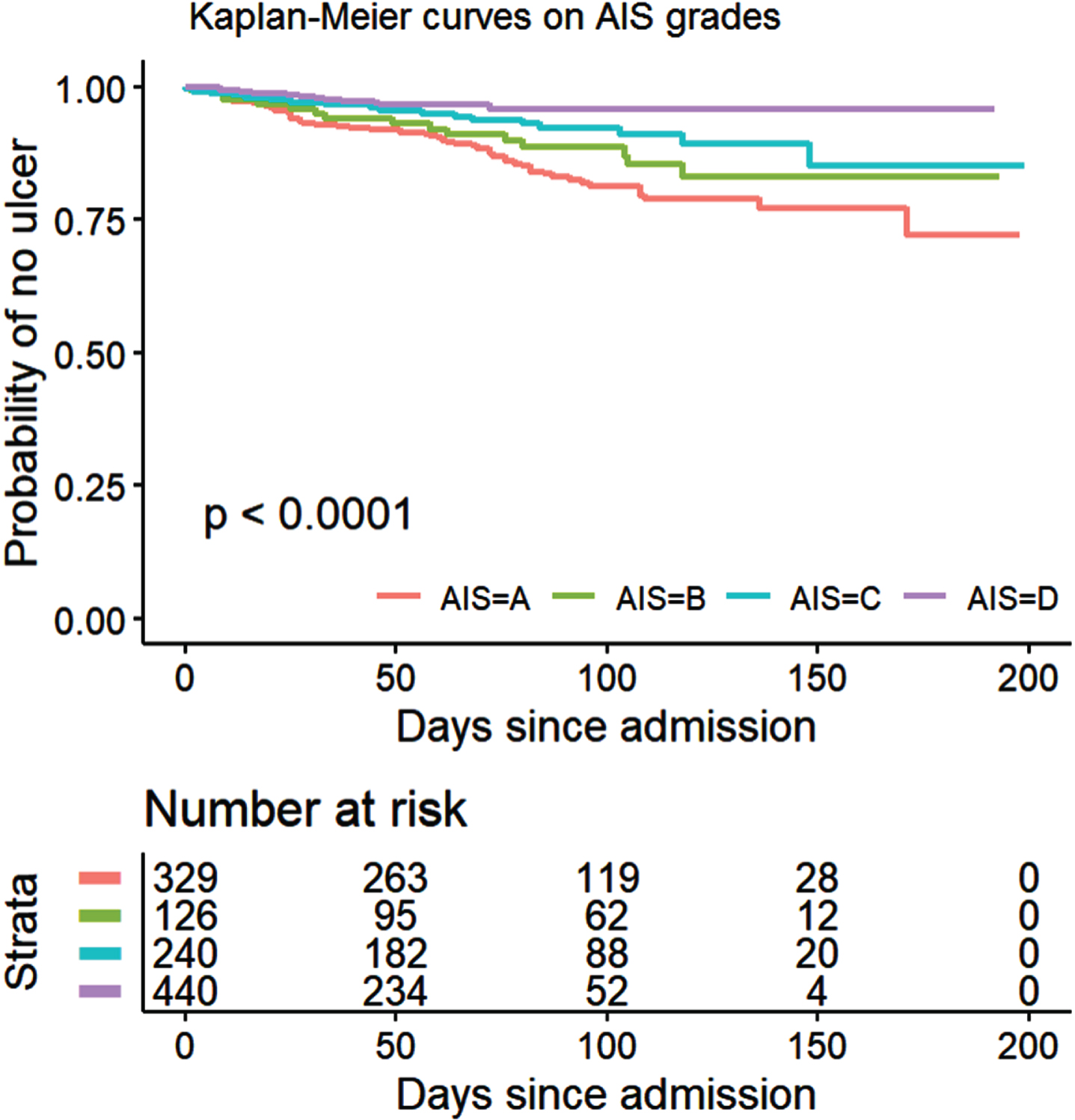
Fig. 4
Kaplan-Meier curves on time to first PU for inpatients (n = 1135) classified according to their motor FIM at admission.
In Supplementary Figure 2 we present Kaplan–Meier analysis for patients with and without diabetes mellitus at admission, highlighting that those without diabetes presented a lower probability of acquiring PUs (p = 0.026). Throughout the inpatient rehabilitation length of stay, differences were larger between 50 and 100 days after admission.
The univariable Cox model (Table 5) identified several independent factors (p < 0.1) to be included in the multivariable model: sex (male), age at injury, AIS grades, traumatic cause of injury, motor and cognitive FIM at admission, diabetes, use of mechanical ventilation and use of cannula. Those not identified by univariable analysis as significant predictors of risk of PUs were time since injury to rehabilitation admission, neurological level of injury, paraplegia or tetraplegia, HADS anxiety or depression scores, body mass index, dyslipidemia, hypertension, heart failure, social situation and level of education.
Table 5
Cox proportional hazards: Univariable and multivariable models
| Univariable | Multivariable | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | ||||
| Female (reference) | ||||
| Male | 1.52 (0.97–2.40) | 0.064 | 1.69 (1.04–2.74) | 0.031 |
| Age at injury | 1.01 (1.00–1.02) | 0.018 | 1.02 (1.01–1.04) | < 0.001 |
| TSI to admission | 0.99 (0.98–1.00) | 0.554 | ||
| AIS | ||||
| A (reference) | ||||
| B | 0.70 (0.39–1.24) | 0.228 | 0.72 (0.40–1.29) | 0.273 |
| C | 0.46 (0.27–0.79) | 0.004 | 0.38 (0.21–0.66) | < 0.001 |
| D | 0.25 (0.14–0.47) | < 0.001 | 0.21 (0.11–0.41) | < 0.001 |
| NLI | ||||
| C1-C4 (reference) | ||||
| C5-C8 | 0.60 (0.32–1.15) | 0.128 | ||
| L1-S5 | 0.59 (0.29–1.21) | 0.154 | ||
| T1-T6 | 0.76 (0.44–1.33) | 0.352 | ||
| T7-T12 | 0.71 (0.42–1.20) | 0.208 | ||
| Level | ||||
| Paraplegia (reference) | ||||
| Tetraplegia | 1.16 (0.78–1.72) | 0.452 | ||
| Cause of injury | ||||
| Non-traumatic (reference) | ||||
| Traumatic | 1.47 (0.96–2.23) | 0.069 | 1.16 (0.72–1.86) | 0.536 |
| Motor FIM admission | 0.97 (0.96–0.99) | < 0.001 | 0.98 (0.97–0.99) | 0.035 |
| Cognitive FIM admission | 0.95 (0.92–0.99) | 0.016 | ||
| HADS Depression admission | 0.98 (0.92–1.03) | 0.496 | ||
| HADS Anxiety admission | 0.96 (0.91–1.02) | 0.214 | ||
| BMI admission | 0.98 (0.94–1.02) | 0.484 | ||
| Diabetes | 1.87 (1.06–3.30) | 0.028 | 1.74 (0.95–3.18) | 0.04 |
| Dyslipidemia | 0.77 (0.40–1.49) | 0.447 | ||
| Hypertension | 1.11 (0.70–1.75) | 0.655 | ||
| Heart failure | 1.02 (0.41–2.51) | 0.957 | ||
| Mechanical ventilation | 1.91 (0.99–3.69) | 0.052 | 0.70 (0.29–1.67) | 0.433 |
| Cannula | 3.19 (1.53–6.62) | 0.002 | 2.32 (0.88–6.10) | 0.08 |
| Living with | ||||
| Alone (reference) | ||||
| Intimate partner | 1.04 (0.60–1.79) | 0.873 | ||
| Parents or other family | 0.80 (0.41–1.55) | 0.520 | ||
| Others | 0.99 (0.39–2.52) | 0.991 | ||
| Educational level | ||||
| College (reference) | ||||
| High school | 0.98 (0.61–1.57) | 0.955 | ||
| University | 1.16 (0.69–1.94) | 0.563 |
C-Index = 0.749 (se = 0.026). FIM: Functional Independence Measure; HADS: Hospital Anxiety and Depression Scale; AIS: American Spinal Injury Association Impairment Scale; NLI: Neurological level of injury; TSI: Time since injury, BMI: body mass index.
The final multiple-variable Cox model confirmed five independent predictors: sex (male), age at injury, AIS grade (C and D), motor FIM at admission and diabetes.
4Discussion
This retrospective study identified several characteristics for persons with SCI who developed a PU during inpatient rehabilitation, compared to those who did not, adding to the scarce literature. In relation to our H1) medical factors such as presence of diabetes, dyslipidemia, hypertension or mechanical ventilation and demographic factors such as gender, age and severity of injury measured by American Spinal Injury Association (AIS) were associated with the risk of the formation of PUs in newly injured individuals with traumatic or non-traumatic SCI during subacute inpatient rehabilitation. Empirical data highlighted the limitations of EMINA risk assessment (H2) and we proposed clinical and demographic variables comparing patients at mid-risk who acquired a PU vs those who did not. Furthermore, sub-acute PUs predictors emerged using traditional survival analysis techniques (H3).
Persons with SCI have multiple comorbidities, all of which increase their risk of developing a PU, such as neurogenic orthostatic hypotension, autonomic dysfunction, neurogenic restrictive and obstructive lung disease, neuropathic pain, spasticity, neurogenic bladder, neurogenic bowel, neurogenic obesity, sarcopenia, and metabolic syndrome (including diabetes mellitus, hypertension, dyslipidemia, and systemic inflammation) (Brienza et al., 2018). These factors are more prevalent in those with a complete injury and are compounded by the paralysis and sensory loss below the level of the injury that does not allow the person to detect skin changes (Fryer et al., 2022). Previous studies have also identified that those with AIS A injuries have a higher prevalence of PUs during inpatient rehabilitation, also finding the same in those with traumatic injuries (Verschueren et al., 2011; Fryer et al., 2023). Although previous work has highlighted that obesity can increase the risk of PUs due to the risk of impaired cutaneous wound healing, fascial dehiscence, surgical site infections, and vascular disease (Vecin et al., 2022), our study was unable to find any significant differences in the proportions of inpatients who developed PU vs those who did not in regard to BMI. Previous studies have highlighted that having had a pressure ulcer during the acute rehabilitation phase was the strongest risk factor for developing a PU during inpatient rehabilitation (Verschueren et al., 2011). However, our results report on those who had their first PU during inpatient rehabilitation, with the intention of understanding what factors contributed to this first PU not addressed in previous research. It is notable from our data that no stage 4 PUs occurred during inpatient rehabilitation, compared to 12.0% in the acute phase (as shown in Supplementary Table 1). This is likely due to the patients being more medically stable in the rehab stage, without the requirement to be on bed rest as is often the case in the acute stages, particularly after a traumatic SCI (Fryer et al., 2023). Previous studies however have reported that stage 4 ulcers accounted for as many as 6.3% of PU in functional rehabilitation (Verschueren et al., 2011). In relation to PUs location, in our case the majority were located at the sacrum (55.6%) in concordance with a previous study (Verschueren et al., 2011). Development of a PU in the sacrum is often associated with long periods in a lying or reclined position (Vecin et al., 2022) while those acquired in sitting usually include ischium heels and feet (Fryer et al., 2023). Therefore our high incidence of sacral PU can be explained by the higher proportion of patients with traumatic (67.3%) and AIS A (54.5%) injuries who developed a PU. Results from our study do contrast with reports for persons admitted with SCI in Barcelona, Spain, that show the highest incidence at the ischium (27%), followed by sacrum (21%) and trochanters (20%) (Vecin et al., 2022). Trochanter PU incidence was low in the acute (0.8%) and rehabilitation (4.0%) stages, as also seen in a previous study (Verschueren et al., 2011). The majority of PUs in our study were shown to develop within the first 30–60 days after admission.
Another important factor yet to receive adequate study after SCI is the use of PU assessment tools. Various risk assessment instruments have been developed, including the Braden, Cubbin and Jackson, CBO, Norton, Ramstadius, and Waterlow scales, of which the Braden Scale has been the most tested in a variety of care settings. It has been validated in several studies and has been found to be the best for identifying the patient’s PU risk as well as being found to have good sensitivity and moderate specificity and moderate predictive validity. However, the Braden Scale has not been identified to help nursing professionals in preventive work on PU as recently reported (Heikkilä et al., 2022).
In this study we analyzed the EMINA scale, which is extensively used in Spanish-speaking countries, but scarcely analyzed when assessing inpatients after SCI. Our results showed that the EMINA scale had good reliability for highlighting risk for developing a PU during their rehabilitation admission if inpatients were rated as high-risk or low-risk. Considering only patients in these two risk categories (276 + 66, as shown in Table 1), the EMINA presents with good sensitivity (0.71) and good specificity (0.82). However, increased specificity is required for those rated as mid-risk, with only 86/672 developing a PU. To implement preventive measures, if all patients classified as mid-risk are considered as high risk, the specificity is too low (<0.20). Previous studies have found significant differences in FIM items between groups of persons with SCI who develop PU, compared to those who do not, specifically from the domains of self-care, continence, transfers and locomotion (Verschueren et al., 2011). In our study, significant differences were also found between PU and no PU groups for FIM items from the domains of self-care (eating, bathing, toileting), continence (bladder, bowel), transfers (bed/chair, toilet, tub/shower) and locomotion (wheelchair). Although items from these FIM domains have shown significant differences, a previous study also highlighted that the greatest predictive power was found in total FIM motor score, rather than individual items, being shown to have an accurate percentage prediction of 76% in their multivariate logistic regression model (Verschueren et al., 2011). In our study we used specific clinical variables (such as cause of injury) and also FIM items (such as Eating), to increase specificity of EMINA for those patients assessed as mid-risk. Findings from our Cox proportional hazard models presented in Table 5 continue to show a link between AIS grade and low motor FIM at admission.
Over the 15-year study period care aspects for inpatients have developed, with one of the key changes coming in the form of greater attention to patient nutrition. Since 2018 after screening and assessment of nutritional status at admission to Institut Guttmann Hospital became standard practice, specific supplements which included energy-enriched supplements of protein alone and mixed supplements of protein, carbohydrate, lipids, vitamins, and minerals were included in patients’ diets as evaluated by the dietician. This change in intervention may have contributed to the downward trend in PU incidence between 2018 and 2022 (Supplementary Figure 1), however, future work should aim to more specifically study the effect of such nutritional interventions on PU incidence.
In relation to the implications of our results to clinical practice, for the first time in the context of rehabilitation after SCI, this study identified several characteristics for inpatients who developed a PU during inpatient rehabilitation, compared to those who did not, adding to the scarce literature. The majority of PUs in our study were shown to develop within the first 30–60 days after admission with 40.6% appearing in the first 30 days. A larger proportion of inpatients who developed PUs presented with an older age at injury (especially 46–60 years), traumatic SCI (67.3% v 50.2%), AIS A injury (54.5% v 26.5%), lower FIM motor score (25 v 39 points) and mechanical ventilation (9.9% v 3.6%). Our results suggest focusing special preventive measures on patients presenting with such characteristics at admission. In relation to PUs location, the majority were located at the sacrum, which is often associated with long periods in a lying or reclined position. In this study we analyzed the EMINA scale, which is extensively used in Spanish-speaking countries, but scarcely analyzed when assessing inpatients after SCI. Our results showed that the EMINA scale had good reliability for highlighting risk for developing a PU during rehabilitation if inpatients were rated as high-risk or low-risk. Nevertheless, EMINA is not useful in practice for those classified as mid-risk. For those cases, our results suggest that heightened staff awareness of the greater risk of PU development should be applied to persons who present with a traumatic SCI, AIS A grade and a low total FIM score (<46) at admission. An additional finding from our results to consider in nursing practice was the significance of the presence of diabetes as predictor for acquiring a PU. Diabetes is an inconsistently considered factor within PU risk scales. It is present only in the Waterlow, CBO and SCIPUS scales, but not included in the Norton, Braden, SCIPUS-A or EMINA scales (Verschueren et al., 2011; Gallego, 2001). Diabetes is associated with hypoxia that impairs wound healing and angiogenesis through decreased levels of VEG-F, as well as hyperglycemia, which adds to oxidative stress. Diabetes has previously been suggested as a factor associated with PUs, but the findings were mixed, and the evidence was insufficient (Li et al., 2016).
Limitations of this research include the potential compromised determination of the complete spectrum of the circumstances of the PUs. We have not included all factors previously identified as related to the risk of PU. Not having the nutritional parameters for all patients included impedes us from extracting definitive conclusions in this regard. No data were collected on the frequency of postural changes or other preventive strategies, either of the type of special pressure relief surface used. Standard PU prevention recommendations are provided both verbally and in writing through educational materials. In addition, in cases where it is necessary, individualized recommendations are provided to the inpatient according to identified needs (i.e. gait training and/or transfers). This information was not included in this analysis, leaving room for future work. The duration of the study along with the low incidence of patients with PUs could make identification of other possible risk factors difficult, as could have appeared with a larger sample as in the case of malnutrition risk evaluation or alcohol abuse, not included in this analysis.
We have not identified causal associations between variables, predictors of PUs are not also the cause of PUs, in order to address causation a different design needs to be considered (e.g. propensity score methods, inverse probability treatment weighting, marginal structural models) which requires a specific analysis and is left for future work.
Further, retrospective cohort studies are more prone to bias than prospective observational or experimental studies. Thus, our results may not be directly generalizable to other institutions, nevertheless we applied standardized assessment tools (AIS, FIM, HADS) and PUs were reported using a Joint-Commission International certified management system.
The Institut Guttmann Hospital protocols for PU treatment and management have been certified by the Joint Commission International during the whole period under study. In relation to the patients’ characteristics, the lack of a national SCI register prevents us from having a better knowledge of the epidemiological characteristics of these patients (Avellanet et al., 2017). After a specific report of the situation in 2005, the Ministry of Health implemented a national protocol in 2010 which likely contributed to the lower rates of complete injuries observed over time, with our Supplementary Figure 3 showing a decrease starting in 2018. Similar to other countries, there is also a progressive increase in the mean age of patients with SCI which is likely linked to the shift from traumatic to nontraumatic cases (Avellanet et al., 2017). The decrease of traumatic injuries is also observed in our study, notably between 2008–2010 and maintained during through until 2015 (Supplementary Figure 5). Regarding the independence in ADLs at admission no variations in trends are particularly observed in our patients during the whole period under study (Supplementary Figure 7).
Finally, given that this was a 15-year study, treatment and management of PUs have evolved over time, continuously changing with the demands and resources available. In relation to the hospital physical infrastructures, during the entire period under study (2008–2022) the Institut Guttmann Hospital had 148 articulating beds with electrical lifting systems by compass and double auto-regression that minimizes the pressure on pelvic area. The mattresses evolved from polyurethane foam (100% of the beds in 2008) to viscoelastic foam (100% of the beds in 2018). During the 2008–2012 period, 60% of polyurethane foams were replaced by viscoelastic, the remaining 40% were replaced during the 2013–2018 period. In relation to patients’ repositioning in bed, related research shows conflicting results and insufficient evidence for optimal bed and seated positioning for PU prevention (Goah et al., 2015). Generally, patients mostly received a repositioning every 2–4 hours in bed (on both polyurethane and viscoelastic mattresses) and more frequently when positioned in a chair, following related guidelines (Fryer et al., 2023). At admission all patients were provided with an inflatable wheelchair cushion during the whole period under study. Regarding the Nursing personnel the number of professionals and shifts remained stable during the whole period under study, organized in three shifts (07 : 00–14 : 59; 15 : 00–22 : 59 and 23 : 00–06 : 59).
5Conclusions
For the first time in the context of rehabilitation after SCI, this study identified several characteristics and predictors for inpatients who were admitted with no PUs and developed a PU during inpatient rehabilitation. According to our results collected during a 15 years period, almost 10% of inpatients will acquire a PU and preventive measures can benefit from characteristics that shown mixed results in previous research, such as the presence of diabetes mellitus as well as the use of clinical and demographic variables routinely assessed at rehabilitation admission (e.g. age, FIM or AIS grades) to complement and increase reliability of risk assessment tools such as EMINA.
Funding
None to report.
Conflict of interest
The authors report no conflict of interest.
Acknowledgments
Special thanks to Olga Araujo from Institut Guttmann –Documentation Department for her continuous support in providing access to related publications.
Data availability
The data that support the findings of this work are available from the corresponding author upon reasonable request.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/NRE-230234.
References
1 | Avellanet, M. , Gonzalez-Viejo, M. A. ((2017) ) People with Spinal Cord Injury in Spain, American Journal of Physical Medicine and Rehabilitation 96: (2 Suppl 1), S112–S115. doi: 10.1097/PHM.0000000000000636. |
2 | Beghi, E. , Gervasoni, E. , Pupillo, E. , Bianchi, E. , Montesano, A. , Aprile, I. , Agostini, M. , Rovaris, M. , Cattaneo, D. ((2018) ) Prediction of Falls in Subjects Suffering From Parkinson Disease, Multiple Sclerosis, and Stroke, Archives of Physical Medicine and Rehabilitation 99: (4), 641–651. doi: 10.1016/j.apmr.2017.10.009. |
3 | Bjelland, I. , Dahl, A. A. , Tangen Haug, T. , Neckelmann, D. ((2002) ) The validity of the Hospital Anxiety and Depression Scale: An updated literature review, Journal of Psychosomatic Research 52: , 69–77. |
4 | Brienza, D. , Krishnan, S. , Karg, P. , Sowa, G. , Allegretti, A. L. ((2018) ) Predictors of pressure ulcer incidence following traumatic spinal cord injury: A secondary analysis of a prospective longitudinal study, Spinal Cord 56: (1), 28–34. doi: 10.1038/sc.2017.96. |
5 | Brock, K. A. , Vale, S. J. , Cotton, S. M. ((2007) ) The effect of the introduction of a case-mix-based funding model of rehabilitation for severe stroke: An Australian experience, Archives of Physical Medicine and Rehabilitation, 88: , 827–832. doi: 10.1016/j.apmr.2007.04.001. |
6 | Coleman, S. , Gorecki, C. , Nelson, E. A. , Closs, S. J. , Defloor, T. , Halfens, R. , Nixon, J. ((2013) ) Patient risk factors for pressure ulcer development: Systematic review, International Journal of Nursing Studies 50: , 974–1003. https://doi.org/10.1016/j.ijnurstu.2012.11.019. |
7 | DiPiro, N. D. , Murday, D. , Corley, E. H. , Krause, J. S. ((2022) ) The primary and secondary causes of hospitalizations during the first five years after spinal cord injury. Spinal Cord, 60: (6), 574–579. doi: 10.1038/s41393-022-00750-9. |
8 | Faul, F. , Erdfelder, E. , Buchner, A. , Lang, A.-G. ((2009) ) Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses, Behavior Research Methods 41: , 1149–1160. |
9 | Fryer, S. , Caggiari, S. , Major, D. ((2023) ) Continuous pressure monitoring of inpatient spinal cord injured patients: Implications for pressure ulcer development, Spinal Cord 61: , 111–118. |
10 | Gallego, C. ((2001) ) Validación de la escala EMIN Un instrumento de valoración del riesgo de desarrollar úlceras por presión en pacientes hospitalizados, Enfermerı a Clínica 11: (3), 1130–8621. https://doi.org/10.1016/S1130-8621(01)73696-0. |
11 | García-Fernández, F. P. , Pancorbo-Hidalgo, P. L. , Agreda, J. J. ((2014) ) Predictive capacity of risk assessment scales and clinical judgment for pressure ulcers: A meta-analysis, Journal of Wound, Ostomy and Continence Nursing 41: (1), 24–34. doi: 10.1097/01.WON.0000438014.90734.a2. |
12 | González-Méndez, M. I. , Lima-Serrano, M. , Martín-Castaño, C. , Alonso-Araujo, I. , Lima-Rodríguez, J. S. ((2018) ) Incidence and risk factors associated with the development of pressure ulcers in an intensive care unit, Journal of Clinical Nursing 27: (5-6), 1028–1037. doi: 10.1111/jocn.14091. |
13 | Gourlan, M. , Pellechia, A. , Robineau, S. , Foulon, B. , Gault, D. , Lefort, M. , Goossens, D. , Mathieu, S. , Laffont, I. , Dupeyron, A. , Ninot, G. , Gelis, A. ((2020) ) What pressure ulcers mean to me?” Representations of pressure ulcer in persons with spinal cord injury: A qualitative study, J Tissue Viability 29: (4), 324–330. doi: 10.1016/j.jtv.2020.07.002. |
14 | Graham, J. E. , Granger, C. V. , Karmarkar, A. M. , Deutsch, A. , Niewczyk, P. , Divita, M. A. , Ottenbacher, K. J. ((2014) ) The Uniform Data System for Medical Rehabilitation: Report of follow-up information on patients discharged from inpatient rehabilitation programs in 2002–2010, American Journal of Physical Medicine & Rehabilitation 93: (3), 231–244. https://doi.org/10.1097/PHM.0b013e3182a92c58. |
15 | Groah, S. L. , Schladen, M. , Pineda, C. G. , Hsieh, C. H. ((2015) ) Prevention of pressure ulcers among people with spinal cord injury: A systematic review, Physical Medicine and Rehabilitation 7: (6), 613–636. doi: 10.1016/j.pmrj.2014.11.014. |
16 | Heikkilä, A. , Kotila, J. , Junttila, K. ((2022) ) Validation of the Helsinki University Hospital prevent pressure Injury Risk Assessment Tool: A prospective observational study, BMC Nursing 21: (1), 18. doi: 10.1186/s12912-021-00799-6. |
17 | Jiang, Q. , Li, X. , Qu, X. , Liu, Y. , Zhang, L. , Su, C. , Wang, J. ((2014) ) The incidence, risk factors and characteristics of pressure ulcers in hospitalized patients in China, International Journal of Clinical and Experimental Pathology 7: , 2587–2594. |
18 | Küçükdeveci, A. A. , Kutlay, Ş. , Yıldızlar, D. , Öztuna, D. , Elhan, A. H. , Tennant, A. ((2013) ) The reliability and validity of the World Health Organization Disability Assessment Schedule (WHODAS-II) in stroke, Disabilility and Rehabilitation 35: (3), 214–220. doi: 10.3109/09638288.2012.690817. |
19 | Li, C. , DiPiro, N. , Cao, Y. ((2016) ) The association between metabolic syndrome and pressure ulcers among individuals living with spinal cord injury, Spinal Cord 54: , 967–972. |
20 | Mathew, A. , Samuelkamaleshkumar, S. , Radhika, S. , Elango, A. ((2013) ) Engagement in occupational activities and pressure ulcer development in rehabilitated South Indian persons with spinal cord injury, Spinal Cord 51: (2), 150–155. doi: 10.1038/sc.2012.112. |
21 | Maribo, T. , Jensen, C. M. , Madsen, L. S. , Handberg, C. ((2020) ) Experiences with and perspectives on goal setting in spinal cord injury rehabilitation: A systematic review of qualitative studies, Spinal Cord 58: (9), 949–958. doi: 10.1038/s41393-020-0485-8. |
22 | McGrath, R. , Hall, O. , Peterson, M. , DeVivo, M. , Heinemann, A. , Kalpakjian, C. ((2019) ) The association between the etiology of a spinal cord injury and time to mortality in the United States: A 44-year investigation, Journal of Spinal Cord Medicine, 42: (4), 444–452. doi: 10.1080/10790268.2018.1505311. |
23 | Müller, R. , Cieza, A. , Geyh, S. ((2012) ) Rasch analysis of the Hospital Anxiety and Depression Scale in spinal cord injury, Rehabilitation Psychology 57: (3), 214–223. https://doi.org/10.1037/a0029287. |
24 | Roca-Biosca, A. , Garcia-Fernandez, F. P. , Chacon-Garcés, S. , Rubio-Rico, L. , Olona-Cabases, M. , Anguera-Saperas, L. , Garcia-Grau, N. , Tuset-Garijo, G. , de Molina-Fernández, I. , Velasco-Guillen, M. C. ((2015) ) Validation of EMINA and EVARUCI scales for assessing the risk of developing pressure ulcers in critical patients, Enfermerıa Intensiva 26: (1), 15–23. doi: 10.1016/j.enfi.2014.10.003. |
25 | (R Foundation, Vienna, Austria). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.URLhttps://www.R-project.org/ (last access 20/07/2023). |
26 | Shiferaw, W. S. , Akalu, T. Y. , Mulugeta, H. , Aynalem, Y. A. ((2020) ) The global burden of pressure ulcers among patients with spinal cord injury: A systematic review and meta-analysis, BMC Musculoskeletical Disorders 21: (1), 334. doi: 10.1186/s12891-020-03369-0. |
27 | Šín, P. , Hokynková A. , Marie, N. , Andrea, P. , Krč R. , Podroužek, J. ((2022) ) Machine learning-based pressure ulcer prediction in modular critical care data, Diagnostics (Basel) 12: (4), 850. doi: 10.3390/diagnostics12040850. |
28 | STROBE Strengthening the Reporting of Observational Studies in Epidemiologyhttps://www.equator-network.org/reporting-guidelines/strobe/.(last access 20/01/2023) |
29 | Vecin, N. M. , Gater, D. R. ((2022) ) Pressure Injuries and Management after Spinal Cord Injury, Journal of Personalized Medicine 12: (7), 1130. doi: 10.3390/jpm12071130. |
30 | Verschueren, J. H. , Post, M. W. , de Groot, S. , van der Woude, L. H. , van Asbeck, F. W. , Rol, M. ((2011) ) Occurrence and predictors of pressure ulcers during primary in-patient spinal cord injury rehabilitation, Spinal Cord 49: (1), 106–112. doi: 10.1038/sc.2010.66. |
31 | Wade, D. T. ((2009) ) Goal setting in rehabilitation: An overview of what, why and how, Clinical Rehabilitation 23: (4), 291–295. doi: 10.1177/0269215509103551. |
32 | Zigmond, A. S. , Snaith, R. P. ((1983) ) The hospital anxiety and depression scale, Acta Psychiatrica Scandinavica 67: (6), 361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. |




