Long COVID neuropsychological follow-up: Is cognitive rehabilitation relevant?
Abstract
BACKGROUND:
Duration of neuropsychological disorders caused by long COVID, and the variables that impact outcomes, are still largely unknown.
OBJECTIVE:
To describe the cognitive profile of patients with long COVID post-participation in a neuropsychological rehabilitation program and subsequent reassessment and identify the factors that influence recovery.
METHODS:
208 patients (mean age of 48.8 y.o.), mostly female, were reevaluated 25 months after their first COVID infection and 17 months after their initial evaluation. Patients underwent subjective assessment, Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS), Phonemic Verbal Fluency and Clock Drawing Tests (NEUPSILIN) for executive functions, Hospital Anxiety and Depression Scale (HADS) and WHOQol-Bref.
RESULTS:
We noted a discrete improvement of neuropsychological symptoms 25 months after the acute stage of COVID-19; nonetheless, performance was not within the normative parameters of standardized neuropsychological testing. These results negatively impact QoL and corroborate patients’ subjective assessments of cognitive issues experienced in daily life. Improvement was seen in those who participated in psychoeducational neuropsychological rehabilitation, had higher levels of education, and lower depression scores on the HADS.
CONCLUSION:
Our data reveal the persistence of long-term cognitive and neuropsychiatric disorders in patients with long COVID. Neuropsychological rehabilitation is shown to be important, whether in-person or online.
1Introduction
At the end of 2019, the SARS-CoV-2 virus, now universally known as COVID-19, took the world by surprise and caused a global pandemic. COVID-19 affected an estimated 796 million people worldwide (World Health Organization, 2023). The virus created a new population of patients who suffer many long-term consequences. These individuals are commonly called “long-haulers” (World Health Organization, 2021). Today, the cognitive and neuropsychiatric implications in the post-acute phase of the SARS-CoV-2 infection are well documented in the literature, for both hospitalized (Taquet, Geddes et al., 2021; Vanderlind, 2021) and non-hospitalized patients (Braga et al., 2022; Hellmuth et al., 2021; Johnsen et al., 2021; Woo et al., 2020).
Recent systematic reviews have confirmed COVID’s far-reaching consequences. For example, Premraj et al. (2022) analyzed the prevalence of neurological and neuropsychiatric symptoms in 11,324 adult patients 12 weeks (3 months) after the acute phase. The main cognitive complaints were brain fog, memory problems, and attention deficits, while the neuropsychiatric issues included sleep disturbances, anxiety, and depression. Badenoch et al. (2021) demonstrated that the most prevalent neuropsychiatric symptoms, in the 18,917 patients followed-up during an average period of 77 days, were sleep disturbances, objective cognitive impairment, anxiety, and Post-Traumatic Stress Disorder (PTSD). The studies cited by Schou et al. (2021) showed the presence of anxiety and/or depression, symptoms or diagnosis of PTSD, cognitive deficits, fatigue, and sleep disturbances up to seven months post-hospital discharge. A review by Crivelli et al. (2022) also noted that patients who recovered from COVID-19 had lower overall cognition compared to healthy controls, up to seven months post-infection. Pinzon et al. (2022) analyzed 9,944 participants who had contracted the virus up to six months earlier and found that the most common symptom of long COVID was fatigue, followed by cognitive impairment, among others. A meta-analysis by Houben and Bonnechère et al. (2022) explored the influence of COVID-19 on cognition levels and showed that the overall effect, expressed in standardized mean differences, was –0.41 (95% CI [–0.55;–0.27]), regardless of the severity of the infection in adulthood, stage of pathology, or patient’s age.
Similar results were reported by studies with patients at 12 or more months post-COVID, indicating that survivors still have a high rate of physical and mental sequelae. A review by Han et al. (2022) systematically synthesized evidence of post-COVID symptoms that persist for at least 12 months in 8,591 patients, among them depression, anxiety, memory loss, concentration difficulties, and insomnia, and others. The meta-analysis by Zeng et al. (2023), which involved 1,285,407 participants from 32 countries, showed that at least one symptom lingered in 50.1% of survivors for at least 12 months post-COVID infection, with the most common being memory impairment, fatigue, depression, and PTSD. Cristillo et al. (2022) evaluated 137 patients one year after they had been hospitalized during the acute stage of the infection. Those with cognitive impairment presented lower scores on the Montreal Cognitive Assessment (MoCA) and higher impact scores on the revised events scale (IES-R), Zung’s self-assessment for depression (SDS), and score for fatigue severity scale (FSS), compared to patients with no cognitive complaints.
Longitudinal studies point to the prevalence of several neuropsychological disturbances over time. For example, Ferrucci et al. (2022) investigated the cognitive function of 53 patients (22–74 years old) five to 12 months after discharge from hospital. More than half of those patients presented deficits in at least one test over five months. Compared with the five-month evaluation, verbal memory, attention, and processing speed were significantly better after one year, while visual memory did not improve. The domains most affected after one year were processing speed and long-term verbal and visuospatial memory, indicating that cognitive impairment still lingers one year after contracting the virus. In a study with three-month and 12-month follow-ups, Rass et al. (2022) showed that symptoms were still being reported one year after COVID by 59% patients, including fatigue, difficulties concentrating, memory loss, sleep disturbances, and others. Conversely, a study by Del Brutto et al. (2022) found that six months after infection, COVID-19 survivors had a significant decline in MoCA scores, which resolved at the one-year follow-up. A noteworthy study by Liu et al. (2022) followed 3,233 patients, 60 years of age and older, who had been hospitalized during the acute stage, using telephone interviews and questionnaires to chart the patients’ cognitive state over the course of one year. The incidence of cognitive deficits twelve months after discharge from hospital was 12.45%, indicating that COVID-19 was associated with an increased risk of longitudinal cognitive decline.
Some studies also showed the impact that post-COVID neuropsychological impairment has on function and daily life (Rubin, 2020; Siegelman, 2020). Miskowiak et al. (2021) demonstrated that problems with verbal learning and executive functions negatively affected work and quality of life. Similar results were seen a year later in another study by Miskowiak et al. (2022), which identified cognitive dysfunction in approximately half of the COVID-19 patients and the negative implications this had on work performance, quality of life, and mood. A review by Aiyegbusi et al. (2021) found that patients with ‘long COVID’ more commonly presented with symptoms such as cognitive impairment, memory loss, anxiety, sleep disturbances, among others, and reported a negative effect on their quality of life, overall health, and work. Poletti et al. (2022) showed that the factor most affecting cognitive performance was depression which, in turn, impacted cognitive functioning and, by extension, quality of life.
Post-COVID cognitive impairment certainly represents a great challenge for rehabilitation services, making cognitive rehabilitation in patients with COVID-19 an important aspect of recovery (Rolin et al., 2022). Long-term follow-up is necessary because the duration of cognitive, emotional, and behavioral problems is unknown (Houben & Bonnechère, 2022; Wade, 2020; Wilson, 2020). Longitudinal studies on the effects of COVID-19 more than 12 months post-infection are scarce. The factors that help or hinder improvement are not yet completely known; most studies are not totally conclusive, and some disagree entirely about the aftereffects of the disease. Few involve in-person longitudinal neuropsychological evaluations to assess outcomes one year after symptom onset. The questions persist: Are the deficits permanent, or are they transitory and resolve over time? Does cognitive rehabilitation make a difference? In a first study with 614 participants conducted an average of eight months post-COVID, we evaluated cognitive and psychiatric disorders in patients who were hospitalized and patients who were not hospitalized during the acute phase. We found persistent impairment of executive functions and a high incidence of anxiety and depression, irrespective of symptom severity (Braga et al., 2022). The aim of this present study is to describe the cognitive profile of these same patients after neuropsychological reevaluation; assess the effects of participating in cognitive rehabilitation; and identify the variables that influence neuropsychological recovery.
2Method
2.1Participants and recruitment
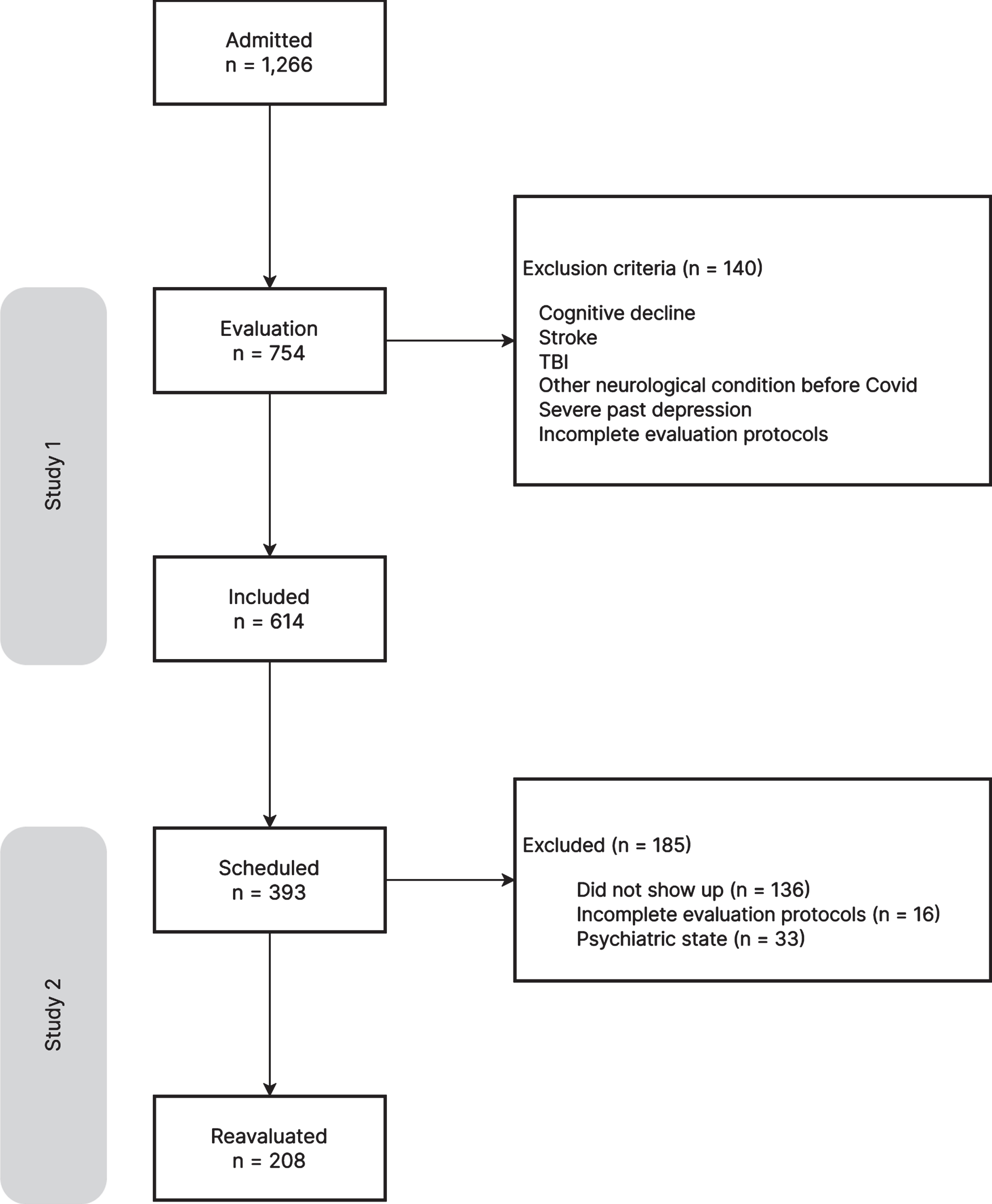
This was a longitudinal study conducted in Brazil with patients who had been referred to the long COVID program at the SARAH Network of Rehabilitation Hospitals for treatment of cognitive issues (attention, concentration, and memory difficulties, slow reasoning, blackouts, etc.) that were affecting their daily lives. Between April 2021 and January 2022, 1,266 COVID-19 survivors, who had contracted the virus between March 2020 and September 2021, underwent neuropsychological evaluation at the SARAH Network. Based on the information in patient charts (anamnesis, neurological examination, psychiatric history, and neuroimaging), the following cases were excluded: a) cognitive decline, stroke, TBI, and any other neurological conditions with compromised cognitive function existent before the COVID-19 diagnosis; b) history of severe depression; and c) incomplete evaluation protocols. Also excluded were patients who did not show up for their interviews. A total of 614 patients with a diagnosis of SARS-CoV-2 infection, confirmed by positive Polymerase Chain Reaction (PCR) test to detect viral RNA, participated in the first study. Of all the patients evaluated, 393 had scheduled in-person neuropsychological reassessments from August 2022 through June 2023, and 257 of them showed up. Patients with incomplete charts or with declining psychological states that overshadowed the cognitive issues were excluded from the study, for a final total of 208 participants (Fig. 1).
Fig. 1
Flow chart of the study.
2.2Rehabilitation program
The SARAH Network of Rehabilitation Hospitals developed a rehabilitation program for treating COVID-19 survivors who presented ongoing symptoms associated with the infection, a syndrome known as ‘long COVID’. This program comprises an interdisciplinary team that includes physicians (neurologists and geriatricians), nurses, nutritionists, speech pathologists, psychologists, and educators specializing in physical education and dance. The team conducts the intake of these patients to identify and compile their complaints and requests. All patients with subjective complaints of cognitive dysfunction post-COVID are directed to the neuropsychology team for further evaluation and instruction. Once their main cognitive complaints are fully assessed and delineated, these patients begin cognitive rehabilitation.
The neuropsychological rehabilitation program has a psychoeducational focus on cognition and emotion. Irrespective of the patient’s specific post-COVID impairments, some cognitive functions have stopped being automatic and now require conscious, intentional execution using metacognitive strategies –even if only temporarily. It is a process by which patients learn about their inner cognitive workings, identify their strengths and weaknesses, and control, monitor, organize, and adjust these processes (Flavell, 1979) so they can acquire and apply compensatory strategies and become more functional in their daily life activities. The programming includes information about cognitive functions and strategies to compensate for and manage their neuropsychological deficits. In turn, they learn to be more proactive in regaining control of their lives and solving problem. The intervention also provides emotional support by creating safe spaces for empathy and connection (Rogers, 1995; Rogers, 2021), thereby helping the patients feel seen and accepted, and their complaints understood, acknowledged, and validated.
The SARAH Network’s neuropsychological rehabilitation program lasts four consecutive weeks, with weekly two-hours group meetings that can be attended virtually or in-person, depending on the patient’s availability. A maximum of 12 participants is allowed for each group. The meetings all have a specific theme: (i) Long COVID and cognition; (ii) Executive functions; (iii) Attention and memory; and (iv) “How the mind works, let’s use it to our advantage”. At the first meeting, the patients introduce themselves and share some of their experiences with COVID and how it has affected their lives. It is a moment of listening, connecting, and empathy, during which the symptoms are “normalized”. This contributes to self-acceptance and helps reduce anxiety, an important factor for compliance with the rehabilitation program. Then the team presents the cognitive implication of long COVID, as described in current literature. The second meeting involves discussions and presentations on the executive functions, using the Socratic method for building knowledge and linking it to daily life situations, thereby promoting strategies to compensate for dysfunction or impairment. The theme of the third meeting is attention and memory, with the presentation of concepts and strategies for achieving greater functionality. During the fourth and last meeting, which is based on the precepts of the behavioral cognitive approach (Beck, 1972; Beck, 1995), the relationship between thought, emotion and behavior, cognitive distortions, mood, and anxiety are explored, within the scope of developing proactive, efficient ways to face the consequences of long COVID. Also presented are strategies for dealing with anxiety, including information about the benefits of daily relaxation, meditation, and mindfulness practices. Finally, the group is given an illustrated guide with instructions about cognitive rehabilitation for long COVID, with compensatory strategies for attaining a higher level of functioning at work, school, home, and community.
2.3Procedure
The patients were reevaluated in person by the same experienced, licensed neuropsychologists from the first study (Braga et al., 2022). Each neuropsychological evaluation took approximately one hour.
Data on sociodemographics, severity of COVID-19 symptoms, and prior comorbidities were collected from patient charts and analyzed by the SARAH multidisciplinary team. Data on hospitalization and non-hospitalization during infection, as well as inpatient treatment (intensive care unit - ICU, orotracheal intubation - OTI) were used as proxies for disease severity.
This study was approved by the SARAH Network Ethics in Research Committee (CAAE 53956921.2.0000.0022). Written informed consent was obtained from all participants.
2.4Materials
An interview protocol was created to identify the cognitive disorders reported by the patients after the first evaluation and to update changes to the patients’ socio-demographic, academic/professional, psychological, cognitive, and daily-life information. The neuropsychological assessments were performed using the same instruments from the previous evaluation (Braga et al., 2022), with the addition of the following quality-of-life measures:
(i) Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS) (Prigatano et al., 1995; Prigatano et al., 2018). The BNIS broadly and reliably assesses disorders of higher integrative mental functions. It comprises 50 items for evaluation of six functions (or subscales): speech/language, attention/concentration, orientation, visuospatial problem solving, memory, and affect. In the affect subscale, patients are asked to produce affect in their tone of voice, correctly identify affect on facial expressions, and, when shown a funny picture, react with a spontaneous smile.
(ii) NEUPSILIN subtests of Phonemic Verbal Fluency Test and Clock Drawing Test for executive functions (Fonseca et al., 2009). The Verbal Fluency Test evaluates executive functions, particularly the capacity for storing and retrieving words (Delis et al., 2001; Diamond, 2013). The Clock Drawing Test requires the integration of multiple cognitive domains, such as understanding instructions, planning, working memory, executive functions, and visuoperceptive and visuomotor processes (Hazan et al, 2018; Pinto & Peters, 2009).
(iii) Hospital Anxiety and Depression Scale (HADS) (Botega et al., 1995; Zigmond & Sanaith, 1983). HADS consists of one scale with 14 items (seven for anxiety and seven for depression). A score of 0–7 suggests that there are no signs or symptoms of anxiety. A score of 8–11 indicates possible anxiety, and scores above 11 denote probable anxiety. The same goes for scores on the depression items.
(iv) WHOQol-Bref (World Health Organization, 2004). The WHOQol-Bref contains 26 questions, of which 24 refer to the four assessment domains (physical, psychological, social relationships, and environment) and the other two address quality of life and overall health. The abbreviated questionnaire in Portuguese was standardized and validated by Fleck et al. (2020).
All of the neuropsychological tests were validated and standardized for the Brazilian population and were adjusted for subjects’ age and education. The NEUPSILIN subtests were analyzed according to the manual’s correction table for age bracket and level of education (Fonseca et al., 2009). Calculations on the BNIS overall z-score points and subscales were based on a sample of 201 healthy subjects used to validate and standardize the BNIS in Brazil (Prigatano et al., 2018).
BNIS-Gain variable was established by calculating the difference between the scores obtained at the initial assessment and again at the retest, yielding a measure of true cognitive improvement at follow-up.
2.5Statistical analysis
We conducted a descriptive and exploratory analysis of the data using statistics such as mean (M) and standard deviation (SD), range, and percentages. The correlations were evaluated with Spearman’s rank correlation coefficients.
The study considered the differences between cognitive, psychiatric, and quality of life scores across assessments using the Student Paired t-test, with Cohen’s d as the effect size measure. Additionally, these scores were dichotomized based on their normality cutoffs, and the McNemar chi-square test for paired samples was employed for further analysis.
A multiple linear regression model helped identify the factors that influenced improvement of the BNIS total z-score. The response variable was the BNIS-Gain, while the covariates included demographic characteristics (age, year of education, gender, marital status, and employment status), clinical factors (severity of COVID-19 in the acute phase, reinfection), temporal factors (time between assessments and onset), neuropsychiatric scores (anxiety and depression), and rehabilitation variables (participation in psychoeducational groups and use of compensatory strategies). The normality of the BNIS-Gain variable was assessed using the Shapiro-Wilk normality test. Forward Stepwise Regression was employed as a method for model selection.
P-values below.05 were considered statistically significant. R version 4.2.3 was used for all statistical analyses.
3Results
The sample comprised 208 participants, with mean age of 48.8years (SD = 11.9 years; range 22–77 years), most of them are females (74.0%) with 16 or more years of education (63.5%) (Table 1).
Table 1
Sociodemographic characteristics (n = 208)
| Sociodemographic characteristic | Count | Percentage | |
| Age range | 18–39 | 49 | 23.6 |
| 40–59 | 114 | 54.8 | |
| 60+ | 45 | 21.6 | |
| Gender | F | 154 | 74.0 |
| M | 54 | 26.0 | |
| Education (years) | 12–15 | 76 | 36.5 |
| 16+ | 132 | 63.5 | |
| Marital status | Married | 119 | 57.2 |
| Divorced/Separated | 33 | 15.9 | |
| Single | 46 | 22.1 | |
| Widow/er | 10 | 4.8 | |
| Employment status | Active | 154 | 74.0 |
| Retired | 33 | 15.9 | |
| Maternity license | 1 | 0.5 | |
| Unemployed | 13 | 6.2 | |
| Sick leave/Disability | 2 | 1.0 | |
Of the 208 patients, 106 (51%) had COVID for the first time in 2020 and 102 (49%) were infected in 2021. The COVID vaccine became available in Brazil in January 2021; therefore, it can be inferred that most of the sample did not yet have immunity to the virus when they contracted it.
Thirty four percent of the patients in the study had been hospitalized for severe COVID-19 symptoms, 18.8% had been in intensive care (M = 16.8 days in the ICU, SD = 11.1), and 11.1% had been intubated for oxygen support.
The patients were reevaluated, on average, 2.1 years after the first COVID-19 infection (SD = 0.38 years). The amount of time between the two evaluations ranged from eight to 25 months (M = 16.7, SD = 3.5).
3.1Neuropsychological findings
3.1.1Subjective assessment
Most of the patients (70.2%) reported that their original cognitive complaints had improved since their first neuropsychological evaluation. Specifically, the patients recounted better language (50.7%), attention (57.5%), memory (57.7%), reasoning (55.9%) and planning (54.2%) function. For neuropsychiatric issues, 43.8% of patients said their anxiety was better, 62.0% were less depressed, 54.0% saw a decrease in irritability, and 56.4% were sleeping better. Similarly, 69.9% felt that these improvements had a positive effect on their work (Table 2).
Table 2
Subjective assessment of patients’ improvement in neuropsychological and neuropsychiatric aspects, and the impact of long COVID on daily life, compared to the initial evaluation
| Subjective assessment | Patients with complaints at intake | Improved | Same | Worsened | Worsened after reinfection | Appeared after reinfection | Appeared recently |
| General evaluation | 208 | 70.2% | 14.9% | 14.9% | 0.0% | 0.0% | 0.0% |
| Cognitive aspects | |||||||
| Spatial disorientation | 44 | 45.5% | 13.6% | 29.5% | 2.3% | 0.0% | 9.1% |
| Temporal disorientation | 72 | 48.6% | 26.4% | 19.4% | 1.4% | 1.4% | 2.8% |
| Language | 142 | 50.7% | 24.6% | 19.0% | 1.4% | 0.7% | 3.5% |
| Attention | 200 | 57.5% | 24.0% | 16.0% | 2.5% | 0.0% | 0.0% |
| Memory | 208 | 57.7% | 25.0% | 13.9% | 3.4% | 0.0% | 0.0% |
| Reasoning | 170 | 55.9% | 30.0% | 11.8% | 2.4% | 0.0% | 0.0% |
| Math | 93 | 46.2% | 33.3% | 18.3% | 1.1% | 0.0% | 1.1% |
| Planning | 107 | 54.2% | 26.2% | 15.0% | 2.8% | 0.9% | 0.9% |
| Neuropsychiatric aspects | |||||||
| Anxiety | 169 | 43.8% | 29.0% | 21.9% | 2.4% | 0.0% | 3.0% |
| Depression | 137 | 62.0% | 16.1% | 15.3% | 1.5% | 1.5% | 3.6% |
| Irritability | 161 | 54.0% | 21.7% | 19.9% | 1.2% | 1.2% | 1.9% |
| Sleep | 163 | 56.4% | 20.2% | 17.2% | 2.5% | 0.0% | 3.7% |
| Appetite | 84 | 41.7% | 31.0% | 22.6% | 1.2% | 0.0% | 3.6% |
| Impact on daily life | |||||||
| Work | 156 | 69.9% | 14.7% | 12.8% | 1.9% | 0.0% | 0.6% |
| School | 101 | 57.4% | 22.8% | 17.8% | 2.0% | 0.0% | 0.0% |
| Domestic activities | 134 | 59.7% | 22.4% | 15.7% | 1.5% | 0.0% | 0.7% |
| Social/familial relationships | 102 | 55.9% | 18.6% | 20.6% | 2.9% | 0.0% | 2.0% |
Sixty-five percent of patients reported better cognitive function up to six months after the first evaluation. Improvement was mainly attributed to participation in the psychoeducational group program (55.2%), regular exercise (35.9%), and time (35.9%).
3.1.2Objective cognitive assessment
We observed several statistically significant gains on the BNIS (overall score, attention/concentration subscale, visuospatial problem-solving scale) compared to the first formal neuropsychological evaluation. The percentage of patients with results below the BNIS Total z-score cut-off (z score < –1) fell from 54% in the first evaluation to 33% at follow-up. Nevertheless, these 33% still performed below normative cutoff scores for cognitive deficit. There were also statistically significant gains (p < .05) in the phonetic verbal fluency and clock drawing (Neupsilin) subtests. However, improvement on the clock drawing test was discrete (Cohen’s d = 0.17), with 34% of the sample still making two or more mistakes (Table 3).
Table 3
Objective data: Neuropsychological evaluation and quality of life
| Evaluation | Test score (mean±SD) | Eval. 1 vs Eval. 2 | Percentage below the | McNemar’s | |||||
| normative cut-off* | Chi-squared | ||||||||
| Evaluation 1 | Evaluation 2 | t (DF) | p | Cohen’s d | Evaluation 1 | Evaluation 2 | test (p value) | ||
| BNIS (z score) | Overall score | –1.23±1.27 | –0.79±1.35 | 5.79 (207) | < 0.001 | 0.34 | 54 | 33 | < 0.001 |
| n = 208 | Speech/language | –0.58±1.54 | –0.59±1.52 | –0.05 (207) | 0.963 | 0.00 | 34 | 36 | 0.635 |
| Orientation | –0.13±1.32 | –0.31±1.75 | –1.18 (207) | 0.239 | –0.11 | 8 | 13 | 0.123 | |
| Attention/concentration | –0.45±1.09 | –0.12±1.04 | 4.31 (207) | < 0.001 | 0.31 | 38 | 25 | 0.001 | |
| Visuospatial/visual | –0.42±1.08 | –0.06±0.92 | 4.56 (207) | < 0.001 | 0.36 | 28 | 11 | < 0.001 | |
| Memory | –0.97±1.43 | –0.64±1.38 | 3.19 (207) | 0.002 | 0.23 | 38 | 32 | 0.176 | |
| Affect | –1.32±1.73 | –0.98±1.40 | 3.20 (207) | 0.002 | 0.21 | 49 | 40 | 0.051 | |
| Neupsilin | Verbal fluency (z score) | –0.55±1.06 | –0.16±1.02 | 6.20 (207) | < 0.001 | 0.38 | 35 | 25 | 0.005 |
| n = 208 | Clock test (0–5) | 3.21±1.41 | 3.44±1.23 | 2.41 (207) | 0.017 | 0.17 | 40 | 34 | 0.149 |
| HADS | Anxiety | 9.89±4.14 | 8.34±3.93 | –5.21 (207) | < 0.001 | –0.38 | 68 | 56 | < 0.001 |
| n = 208 | Depression | 8.73±3.57 | 7.25±3.88 | –5.36 (207) | < 0.001 | –0.40 | 62 | 46 | < 0.001 |
| WHOQOL-Bref | Physical | 2.98±0.67 | 3.37±0.70 | 8.49 (182) | < 0.001 | 0.62 | 76 | 55 | < 0.001 |
| n = 183 | Psychological | 3.25±0.63 | 3.51±0.63 | 5.92 (182) | < 0.001 | 0.41 | 58 | 38 | < 0.001 |
| Social relationships | 3.36±0.71 | 3.43±0.79 | 1.59 (182) | 0.113 | 0.12 | 59 | 51 | 0.051 | |
| Environmental | 3.46±0.54 | 3.53±0.60 | 2.69 (182) | 0.008 | 0.16 | 43 | 42 | 0.382 | |
| General health | 3.10±0.78 | 3.41±0.69 | 5.79 (182) | < 0.001 | 0.46 | 58 | 38 | < 0.001 | |
*Cut-off for abnormality: BNIS (z score) < –1.0; Verbal fluency (z score) < –1.0; Clock test < 4 points; HADS-Anxiety > 7 points; and HADS-Depression > 7 points; WHOQOL-Bref Domains < 3.5 points.
There were also improved scores in the anxiety and depression domains on the HADS at follow-up. Yet the percentage of patients classified as abnormal (12–21 points) was still elevated in this population, indicating levels of anxiety (56%) and depression (46%) higher than the general population (Botega, 1995) (Table 3).
There was no statistically significant correlation between acute phase COVID-19 severity and improved performance on the BNIS for neuropsychological (rs = –0.04, p = 0.524), phonetic verbal fluency (rs = –0.08, p = 0.233) and clock drawing (rs = –0.12, p = 0.087) subtests. There was also no evidence that getting COVID-19 a second time impacted performance on the BNIS (t(206) = –0.63, p = 0.528, Cohen’s d = 0.09), phonetic verbal fluency (t(206) = –0.80, p = 0.425, Cohen’s d = 0.11) and clock drawing test (t(206) = –0.83, p = 0.406, Cohen’s d = 0.12).
Despite 35.9% of the participants attributing their recovery to the passing of time, we did not find any correlation between the amount of time since the first infection and gains in the BNIS neuropsychological (rs = –0.065, p = 0.349), phonetic verbal fluency (rs = –0.05, p = 0.502) and clock drawing test (rs = –0.02, p = 0.812) scores. We saw similar results when we correlated gains with amount of time between evaluations: BNIS (rs = 0.06, p = 0.409), phonetic verbal fluency (rs = –0.02, p = 0.794) and clock drawing test (rs = –0.05, p = 0.478).
3.2Quality of life
The patients presented low scores on the WhoQOL-Bref at their first evaluation, reflecting low satisfaction across all domains. The follow-up revealed improvement in the physical and psychological domains, and general health. Despite the results indicating a statistically significant gain in the environmental domain, we noted a small effect size (Cohen’s d = 0.16) and a stable percentage of patients still below cut-off (QoL perception fair/poor) in these domains (Table 3).
The gains in the quality-of-life points were negatively correlated with anxiety and depressions scores (HADS) in all domains. Total BNIS z score correlated positively with physical, psychological, and environmental domains at follow-up: the better the BNIS score, the higher the quality-of-liferating in these domains (Table 4).
Table 4
Correlations between quality-of-life domain scores (WhoQOL-Bref), anxiety and depression scores (HADS) at follow-up, and Total BNIS z score
| WhoQOL-Bref | HADS Follow-up, rs (p-value) | Total BNIS z score, rs (p-value) | ||
| Anxiety | Depression | Baseline | Follow-up | |
| Physical | –0.57 (< 0.001) | –0.54 (< 0.001) | 0.19 (0.006) | 0.20 (0.003) |
| Psychological | –0.71 (< 0.001) | –0.71 (< 0.001) | 0.14 (0.047) | 0.14 (0.046) |
| Social relationships | –0.43 (< 0.001) | –0.52 (< 0.001) | 0.14 (0.050) | 0.04 (0.575) |
| Environmental | –0.37 (< 0.001) | –0.48 (< 0.001) | 0.14 (0.045) | 0.24 (0.001) |
| General health | –0.37 (< 0.001) | –0.46 (< 0.001) | 0.10 (0.159) | 0.13 (0.070) |
3.3BNIS and subjective assessment
The gains observed in the formal neuropsychological evaluation corroborated the findings of the subjective evaluation. In other words, patients who reported improved symptoms also presented statistically significant gains in BNIS scores, with z score averages increasing from –1.13 in the first evaluation to –0.59 in the second (t(145) = 6.27, p < 0.001, Cohen’s d = 0.43). On the other hand, those who reported no improvement of their symptoms did not present changes to their z scores between evaluations (t(61) = 1.32, p = 0.193, Cohen’s d = 0.14) (Table 5).
Table 5
Performance on Total BNIS (z score) according to confounding factors
| n | BNIS Total (z score) | Evaluation 2 vs Evaluation 1 | |||||
| Eval 1 | Eval 2 | Gain | t (DF) | p | Cohen’s d | ||
| Subjective assessment | |||||||
| Did not improve | 62 | –1.48 | –1.28 | 0.20 | 1.32 (61) | 0.193 | 0.14 |
| Improved | 146 | –1.13 | –0.59 | 0.54 | 6.27 (145) | < 0.001 | 0.43 |
| Gender | |||||||
| Male | 54 | –1.21 | –0.78 | 0.43 | 2.79 (53) | 0.007 | 0.31 |
| Female | 154 | –1.24 | –0.80 | 0.44 | 5.07 (153) | < 0.001 | 0.34 |
| Education (years) | |||||||
| 12–15 | 76 | –1.15 | –1.02 | 0.13 | 1.13 (75) | 0.262 | 0.10 |
| 16+ | 132 | –1.28 | –0.67 | 0.61 | 6.40 (131) | < 0.001 | 0.47 |
| Age range | |||||||
| 18–39 | 49 | –1.73 | –1.13 | 0.60 | 3.78 (48) | < 0.001 | 0.44 |
| 40–59 | 114 | –1.35 | –0.89 | 0.46 | 4.18 (113) | < 0.001 | 0.35 |
| 60+ | 45 | –0.41 | –0.19 | 0.22 | 1.77 (44) | 0.085 | 0.23 |
| Severity | |||||||
| Not hospitalized | 137 | –1.33 | –0.85 | 0.47 | 4.72 (136) | < 0.001 | 0.37 |
| Hospitalized | 32 | –1.11 | –0.65 | 0.45 | 2.80 (31) | 0.009 | 0.37 |
| ICU (Intensive Care Unit) | 16 | –0.92 | –0.69 | 0.23 | 1.39 (15) | 0.185 | 0.13 |
| Intubated | 23 | –1.09 | –0.72 | 0.37 | 1.59 (22) | 0.126 | 0.28 |
| Reinfection | |||||||
| No | 132 | –1.16 | –0.76 | 0.40 | 4.23 (131) | < 0.001 | 0.30 |
| Yes | 76 | –1.36 | –0.86 | 0.50 | 3.99 (75) | < 0.001 | 0.40 |
| HADS - Anxiety | |||||||
| ≤7 points | 92 | –1.04 | –0.59 | 0.44 | 3.73 (91) | < 0.001 | 0.38 |
| > 7 points | 116 | –1.39 | –0.95 | 0.44 | 4.43 (115) | < 0.001 | 0.31 |
| HADS - Depression | |||||||
| ≤7 points | 112 | –1.06 | –0.50 | 0.56 | 5.60 (111) | < 0.001 | 0.47 |
| > 7 points | 96 | –1.43 | –1.13 | 0.30 | 2.61 (95) | 0.010 | 0.22 |
| Psychoeducational groups | |||||||
| Did not participate | 47 | –1.17 | –1.00 | 0.17 | 1.03 (46) | 0.310 | 0.14 |
| 1 meeting | 28 | –1.30 | –1.18 | 0.12 | 0.57 (27) | 0.573 | 0.07 |
| 2 meetings | 21 | –1.61 | –1.09 | 0.53 | 2.95 (20) | 0.008 | 0.38 |
| 3 meetings | 36 | –1.19 | –0.67 | 0.51 | 3.10 (35) | 0.004 | 0.39 |
| 4 meetings | 76 | –1.17 | –0.50 | 0.67 | 5.14 (75) | < 0.001 | 0.56 |
| Mode of attendance | |||||||
| In-person | 83 | –1.18 | –0.76 | 0.42 | 3.30 (82) | < 0.001 | 0.28 |
| Online | 78 | –1.34 | –0.71 | 0.63 | 5.63 (77) | < 0.001 | 0.53 |
| Compensatory strategies | |||||||
| Does not use | 23 | –1.65 | –1.59 | 0.06 | 0.23 (22) | 0.821 | 0.04 |
| Uses | 185 | –1.18 | –0.70 | 0.49 | 6.15 (184) | < 0.001 | 0.38 |
| Rehabilitation strategies | |||||||
| None | 11 | –1.86 | –2.24 | –0.38 | –1.37 (10) | 0.202 | 0.27 |
| Groups and/or compensatory | 197 | –1.20 | –0.71 | 0.49 | 6.28 (196) | < 0.001 | 0.38 |
3.4Rehabilitation of long COVID
Of the 208 patients in the study, 133 (63.9%) participated in the rehabilitation program, attending at least two of the four scheduled meetings. Forty-seven (22.6%) did not attend any meeting and 28 (13.5%) were present at only one. Of the 133 participants who showed up for at least two meetings, 69 (52%) attended online.
We noted that participating in the rehabilitation program yielded a statistically significant impact on BNIS gains. Patients who attended at least two meetings changed their average z score from –1.24 at first evaluation to –0.64 at second evaluation (t(132) = 6.64, p < 0.001, Cohen’s d = 0.48). Nevertheless, those who did not attend, or attended only one meeting, did not present any gains in z scores between both evaluations (t(74) = 1.17, p = 0.246, Cohen’s d = 0.11).
BNIS scores were not affected by mode of attendance (Table 5); in other words, both in-person (M = 0.42, SD = 1.15) and online (M = 0.63, SD = 0.98) participation in the rehabilitation program yielded similar gains (t(159) = 1.25, p = 0.214, Cohen’s d = 0.20).
Patients who did not partake of the psychoeducational groups and admitted not applying any of the compensatory strategies (n = 11), had worse BNIS average total scores on the follow-up evaluation (M = –2.24, SD = 1.43) when compared to the first (M = –1.86, SD = 1.43) (Table 5).
Panel 1 details examples of patient statements before and after participating in the psychoeducational program.
Panel 1.
Perceptions before and after participating in the psychoeducational group (examples of feedback).
| Pre-group participation |
| “I’m having difficulties understanding the sequence of things, my brain can’t keep up.” |
| “I forget the time, appointments, commitments, where I put things.” |
| “I can’t focus and do two things at once, I lose steam.” |
| “I want to say the name of an object but can’t remember it.” |
| “I read a page and when I get to the end, I ask myself, ‘what was it that I just read..?”’ |
| “I’m slow to do things, I used to multitask, I want to go back to the way I was before.” |
| “I feel anxious and useless, as if I were unproductive.” |
| “I’m more tuned out.” |
| “When I’m driving, I have to be 10 times more attentive to traffic.” |
| “I go to the kitchen to get a glass of water, and halfway there I don’t know what I went there to do.” |
| “If two people are having a conversation, I can’t keep up with what they are saying.” |
| Post-group participation |
| “The group was very important for this moment of my life, and it gave me tips that I could use in my day-to-day.” |
| “I saw that I wasn’t the only one who was having problems with memory etc.” |
| “I gave me direction and knowledge to set about on the best path forward.” |
| “It’s not impossible to control my anxiety like I thought it was.” |
| “The group was very welcoming, I learned a lot, including practical strategies to help me in my daily life.” |
| “The group is very good, it helps you see your life and your problems from a different angle.” |
| “I learned how to handle my new reality.” |
| “They were very fruitful encounters with clear, important information and guidance that have helped me return to my daily routine with greater ease.” |
| “The group, the sharing, the guidance that were shared with us really help us focus on the strategies that can help us the most.” |
| “The tips we learned can really help us balance attention, cognition, anxiety.” |
| “I was made aware of easy activities that can improve my quality of life.” |
3.5Factors associated with neuropsychological outcomes
Multiple linear regression model results showed that the variables that explained improved neuropsychological performance were Baseline Total BNIS z-score, education, HADS-Depression, participation in two or more psychoeducational group meetings and use of compensatory strategies (F(5, 202) = 12.39, p < 0.001).
We noted a significant negative association between baseline Total BNIS z-score and gains in BNIS at follow-up. The worse the initial z score, the greater the BNIS gains (Table 6).
Table 6
Linear regression analysis for Total BNIS z-score improvement at follow-up
| Covariable | Model adjusted by Baseline | Final model | ||||||
| BNIS, age and education | ||||||||
| B | SE B | t | p | B | SE B | t | p | |
| Intercept | – | – | – | – | –1.92 | 0.68 | –2.84 | 0.005 |
| Baseline Total BNIS z-score | –0.29 | 0.06 | –5.07 | 0.000 | –0.34 | 0.05 | –6.19 | 0.000 |
| Age (year old) | 0.00 | 0.01 | –0.48 | 0.632 | – | – | – | – |
| Education (≥16 years of education) | 0.44 | 0.15 | 2.97 | 0.003 | 0.31 | 0.14 | 2.17 | 0.032 |
| Sex (0 = Female, 1 = Male) | 0.05 | 0.16 | 0.29 | 0.770 | – | – | – | – |
| Married (0 = No, 1 = Yes) | –0.05 | 0.14 | –0.32 | 0.749 | – | – | – | – |
| Professionally active (0 = No, 1 = Yes) | –0.16 | 0.17 | –0.94 | 0.348 | – | – | – | – |
| Hospitalization (0 = No, 1 = Yes) | 0.06 | 0.15 | 0.40 | 0.693 | – | – | – | – |
| Reinfection (0 = No, 1 = Yes) | –0.02 | 0.15 | –0.17 | 0.869 | – | – | – | – |
| Time between COVID-19 and follow-up evaluation (years) | –0.13 | 0.19 | –0.68 | 0.497 | – | – | – | – |
| Time between evaluations (months) | 0.01 | 0.02 | 0.70 | 0.484 | – | – | – | – |
| HADS-Anxiety (> 7 points) | –0.17 | 0.14 | –1.20 | 0.234 | – | – | – | – |
| HADS-Depression (> 7 points) | –0.32 | 0.14 | –2.26 | 0.025 | –0.32 | 0.14 | –2.29 | 0.023 |
| Compensatory strategies (0 = No, 1 = Yes) | 0.49 | 0.22 | 2.18 | 0.031 | 0.48 | 0.22 | 2.19 | 0.030 |
| Psychoeducational rehabilitation (2 or more meetings) | 0.41 | 0.14 | 2.81 | 0.006 | 0.35 | 0.14 | 2.46 | 0.015 |
Final model statistics: F(5,202) = 12.39, p-value < 0.001; Adjusted R-squared = 0.2157.
3.5.1Effects of education
According to the multiple linear regression model, the higher the level of education, the greater the gains in total BNIS score (Table 6). This finding corroborates the descriptive analysis, in which patients with 12 to 15 years of education did not present gains in the BNIS when the second evaluation was compared to the first.
3.5.2Effects of depression
We found a significant negative association between depression classification (HADS) and BNIS gains. Participants classified as borderline or abnormal tended towards fewer gains in BNIS (Table 6).
3.5.3Effects of rehabilitation
There was a statistically significant association between participating in the psychoeducational groups and improved neuropsychological evaluation, indicating that those who attended at least two meetings had greater BNIS gains. Better neuropsychological performance was also associated with the use of compensatory strategies, many of which were taught during these rehabilitation group meetings (Table 6).
4Discussion
This prospective longitudinal observational study comprised neuropsychological evaluations of long COVID patients post-participation in a neuropsychological rehabilitation program. Because of the relative newness of COVID-19, there is still much to learn about the long-term evolution of cognitive deficits in this population beyond 12 months. Our study showed that there were statistically significant (p < 0.01) gains in total BNIS scores in the attention/concentration and visuospatial problem-solving subscales as well as the subtests for phonetic verbal fluency and Neupsilin clock drawing test 25 months after the primary COVID-19 infection and 17 months after the initial evaluation. Nevertheless, improvement does not mean that the cognitive performance falls within the standardized neuropsychological test averages, as described by Houben and Bonnechère (2022), who showed that the standardized mean difference of long COVID patients was –0.41 (95% CI [–0.55;–0.27]), indicating significant cognitive disorders. The HADS also showed improved scores in anxiety and depression; however, patients still exhibited high levels of anxiety and depression. These findings corroborate prior publications that addressed the persistence of long-term cognitive and neuropsychiatric symptoms post-COVID; however, our results indicate that these symptoms last longer than previously reported. Ferrucci et al. (2022) explored persistent difficulties with processing speed, and verbal and visuospatial memory one year after COVID. Seeßle et al. (2022) showed that prolonged neurocognitive symptoms can linger for more than one year after the onset of COVID-19. A review by Crivelli et al. (2022) indicated that patients who recovered from COVID-19 had poorer overall cognition compared to healthy controls as much as seven months after getting infected, regardless of disease severity during the acute phase. Schou et al. (2021) found analogous results, in addition to the presence of anxiety and/or depression. Han et al. (2022) reported that symptoms such as memory loss, depression, and anxiety were still present at least 12 months after the acute phase of the disease. Similarly, a metanalysis by Zeng et al. (2023) revealed memory impairment and depression 12 months after COVID-19. Del Brutto et al. (2022) had different findings, reporting that the significant decline in MoCA scores was reversed after 18 months of follow-up.
This present study also showed that the severity of COVID-19 during the acute phase did not impact neuropsychological outcomes 25 months after infection, which is in line with our previous study (Braga et al., 2022). Consequently, neuropsychological evaluations did not indicate a difference between hospitalized and non-hospitalized patients in terms of cognitive and/or psychiatric profiles. For now, the causes are not yet entirely clear. Individuals with long COVID have elevated inflammatory markers for several months (Phetsouphanh et al., 2022). One hypothesis claims that SARS-CoV-2’s effect on the commencement and progression of neurodegenerative and neuropsychiatric diseases with neuroinflammatory etiologies should be considered a potential cause of a delayed pandemic, with great medium- and long-term repercussions on public health, and underscores the need for closely monitoring cognitive function in COVID-19 survivors (Serrano-Castro et al., 2020). It is believed that the neuroinflammatory processes and oxidative stress prevail in the propagation of long COVID neurological sequelae (Stefanou et al., 2022). The mechanisms by which they reach the brain may be associated with a rupture in the blood-brain barrier (BBB), along with the axonal transport of the virus through the trigeminal nerve, vagal nerve, or brain-gut axis (Sarubbo et al., 2022). The hippocampusmay be particularly vulnerable to infections by coronaviruses, thereby increasing the probability of compromising memory post-infection and speeding up neurodegenerative diseases (Ritchie et al, 2020). Some studies suggest that neuroinflammation and associated disorders of the central nervous system may contribute significantly to the etiopathogenesis of depressive disorders (Dabrowska et al., 2021). There is a certain degree of neuroinflammation in infected patients, even in mild cases of COVID-19, which explains some of the complaints of post-COVID syndrome (Venkataramani & Winkler, 2022). An analysis of brain imaging done before and after SARS-CoV-2 infection notes that even mild COVID-19 may be linked to cognitive impairment and changes in brain structure (Kremer & Jäger, 2022). One hypothesis is that cognitive disorders associated with mild cases are related to hypometabolic brain lesions, which are in turn likely due to neuroinflammation (Hugon, 2022). Those who experienced COVID-19 may be at greater risk of neurodegeneration and dementia. Liu et al. (2022) investigated the cognitive outcomes of long COVID patients aged 60 and older and reported an increased risk of longitudinal cognitive decline in elderly patients.
Previous studies reported the relationship between subjective assessment and objective cognitive outcomes in different pathologies, such as mild cognitive impairment, epilepsy, Parkinson’s disease, chronic fatigue syndrome, and brain tumors (Edmonds et al., 2014; Marino et al., 2009; Pranckeviciene et al., 2017; Rasouli et al., 2019). However, there is very little research exploring this relationship in the long COVID population. Our study includes subjective data, indicating that the gains observed in the formal neuropsychological evaluation corroborate the findings of the patient’s subjective assessment. Those who report improved cognitive symptoms presented statistically significant gains in BNIS scores. Similarly, patients who did not feel their symptoms were better also did not present changes in their z scores in the two evaluations. The combination of subjective and objective information provides a more complete and accurate view of the individual. Agreement between the two forms of assessment suggests that the individual has a precise understanding of their own cognitive functioning. This may indicate that the subjective reports are reliable and can be used as a guide for decision-making about the treatment and management of post-COVID cognitive disorders, concomitantly with the results of the formal neuropsychological evaluation. Together, they serve as a solid base for significant interventions, management of the patient’s cognitive challenges, and adhesion to treatment.
Most preexisting methods of investigating cognitive and neuropsychiatric dysfunction in other patient populations were adapted for patients with long COVID. There is a need for high-quality tools that can measure the patients’ self-reported progress so we can better understand the signs, symptoms, and physiopathology subjacent to the disease and in turn develop safe and effective interventions and resolutions to this large population’s day-to-day needs (De Luca et al., 2022). The BNIS proved sensitive in identifying post-COVID cognitive deficits and the subsequent improvement post-intervention. The results of the formal test corroborate the patients’ subjective perceptions about how their cognitive issues affect their daily lives. Precise, accessible evaluation of cognitive function post-COVID has relevant implications for treatment planning. A person-centered holistic approach to long COVID assessment and treatment is recommended by the National Institute for Health and Care Excellence (NICE guidelines, 2020).
An important result of our study was identifying the value of cognitive rehabilitation in long COVID outcomes. Those who improved were the ones who participated in neuropsychological rehabilitation with a psychoeducational focus on cognition and emotion, in a group setting, regardless of in-person or online attendance. Conversely, the patients who did not participate in psychoeducational groups and reported not using any compensatory strategies had worse average BNIS total scores. These results corroborate the subjective assessments, in which improved neuropsychological symptoms were attributed mainly to participation in the psychoeducational group interventions. Various rehabilitation strategies have been proposed to better the functioning and quality of life of patients with COVID-19 (Barker-Davies et al., 2020; Fugazzaro et al., 2022; Hausswirth et al., 2023; Hawke et al., 2022; Vance et al., 2021). The aim of this study was not to evaluate or defend the efficacy of one specific model of cognitive rehabilitation; nevertheless, our results show that the psychoeducational group approach is promising. The patients were responsive to this model of intervention that favors normalizing symptoms and metacognition, developing skills for dealing with emotional challenges, and proactively embracing measures to improve daily life. Most of the patients in this study used the compensatory strategies they learned in their psychoeducational groups to deal with their cognitive disorders. Using compensatory strategies minimized the impact of these impairments on the patients’ daily lives, particularly in their work environments. The patients’ verbal reports corroborate and illustrate these findings. In their pre-intervention reports, we clearly note subjective complaints about attention, memory, language, and executive functions, as well as issues dealing with the implications of these cognitive disorders. Post-intervention, however, patients spoke of learning compensatory strategies and how much these resources made a positive difference in their lives. We also noticed reduced stress and anxiety, changes in beliefs, and more proactive behaviors. The literature on neurorehabilitation specific to long COVID is incipient (De Luca et al., 2022; Mathern et al., 2022), but our results suggest that metacognitive and compensatory strategies, which are also used by people with brain injury, can be successfully applied to the population with long COVID. Moreover, the online psychoeducational group intervention model is plausible, and serves as an option for rehabilitation services.
Education also positively impacted long COVID recovery. The greater the level of education, the higher the total BNIS score. Furthermore, education is associated with cognitive reserves (Stern, 2002). Individuals with more years of formal schooling may have greater cognitive stores, which means that their brain has more resources for adapting to changes and maintaining its cognitive functioning in the face of challenges. An individual’s level of education is positively correlated with their cognitive function throughout adulthood and predicts lower risks of dementia in later life. Schooling may attenuate cognitive decline associated with aging (Lövdén et al., 2020). Education appears to influence long COVID recovery as well.
As with the first study (Braga et al., 2022), we did not find a correlation between mood and outcomes at the follow-up evaluation. However, there was a significant negative association between HADS depression classification and improvement on the BNIS. These findings lead us to believe that, despite mood was not having direct causality to cognitive disorders, neuropsychiatric issues nonetheless impact cognitive rehabilitation. It is well established in the literature that depression can affect various aspects of the patient’s cognition, social behavior, and how they perceive their quality of life (Bora & Harrison, 2012; Malhi & Mann, 2018; Rock et al., 2014).
Research has shown the implications of post-COVID cognitive impairment on quality of life (Aiyegbusi et al, 2021; Miskowiak et al, 2021; Miskowiak et al, 2022; Rubin, 2020; Seeßle et al., 2022; Siegelman, 2020), but there are few longitudinal studies that evaluate the impact of recovery from these disorders over time. Our study shows that the patients presented fair quality of life perception in all domains. On follow-up, we observed improved physical, psychological, and general health domains on the WHOQOL-Bref. We noted, also, that the worse the scores for anxiety and depression (HADS), the poorer the patient’s perception of their quality of life. Cognitive performance (BNIS) was associated with quality of life, indicating that patients with better BNIS scores also tended to have better perception of their quality of life, especially in the physical, psychological, and environmental domains. These results corroborate the study by Poletti et al. (2022) that reports depression to be the most impactful factor on cognitive performance which, in turn, influences cognitive functions that determine quality of life.
4.1Study limitations
Post-COVID syndrome involves a variety of factors, such as mechanist disorders tangential to the symptoms, including interrupted production of cellular energy due to mitochondrial dysfunction, lower oxygen supplies caused by coagulopathy, and endothelial damage and immunological dysfunction, among others (Astin et al., 2023). We exclusively addressed the neuropsychological aspects of long COVID and their outcomes over time.
Our study has some limitations, such as lack of quantitative data on cognitive function prior to COVID-19, since we evaluated adult participants who mostly did not report having cognitive issues prior to catching the virus. Nevertheless, before COVID-19, the SARAH Network was not seeing a large number of patients complaining about executive functions like attention and memory. This change occurred after the pandemic began and involves a younger population (Braga et al., 2022).
Although our study presents relevant evidence about the impact of neuropsychological rehabilitation on the cognitive recovery of patients with long COVID, it is important to underscore that this is an observational study. In our approach, possible uncontrolled confounding variables may have influenced the findings, which could limit the direct attribution of cause and effect to the rehabilitation intervention. Additionally, the neuropsychological reevaluation was conducted only once, after an average of 16.7 months, thereby hindering an analysis of potentially spontaneous improvement of symptoms in patients who were infected by COVID much earlier. Despite these limitations, the rehabilitation variables present a significant association with improved cognitive evaluations, even when assessed using a multiple linear regression model adjusted by age, education, clinical factors, and other potential confounding variables.
Another limitation is a lack of precise information about the coronavirus variants that infected these patients. We know that there was a change in symptom profiles associated with the different variants during that period (Whitaker et al., 2022). Did the different variants also determine the intensity of the post-COVID-19 sequelae? The same line of questioning can be applied to the population infected after vaccination. However, our sample comprises patients infected for the first time in 2020 and 2021. During this period, according to data by the Genomic Network Fiocruz (Fiocruz, 2023), the most prevalent strains in Brazil were B.1.1.28 and B.1.1.33, and they remained the most common until October 2020. Two main Brazil-borne variants predominantly circulated after that period, P.1 and P.2, which originated from the B.1.1.28 strain (Michelon, 2021). In 2021, the predominant variants in the country were Gama and Delta (Fiocruz, 2023). Widespread vaccination in Brazil began in January 2021. It serves to reason that by September 2021 approximately 43% of the country’s population had received two doses of the vaccine (Ministério da Saúde, 2023). Future studies are needed to better understand how the different COVID-19 strains and widespread immunization impacted the neuropsychological issues of infected individuals.
5Conclusion
Our data, collected through in-person interviews of 208 patients 25 months after the acute phase of COVID-19, add to the findings of previous studies on the persistence of long-term cognitive and neuropsychiatric post-COVID symptoms, indicating that they last for longer periods than previously reported. The results show that there was discrete neuropsychological improvement 25 months after acute COVID-19 infection; however, these results do not yet fall within the normative parameters established by neuropsychological tests. The same was seen on anxiety and depression scale scores, with a significant negative impact on how patients perceive their quality of life. The results of the formal neuropsychological evaluation corroborate the patients’ subjective assessment of how their cognitive issues affect their daily lives. To this end, the BNIS proved to be a sensitive tool. The patients who improved were the ones who participated in psychoeducational neuropsychological rehabilitation, had more years of education, and presented lower depression scores on HADS.
We recommend that these patients participate in a rehabilitation program and have their symptoms monitored over a period of time. Research on neuropsychological rehabilitation for long COVID is still nascent. However, our study showed promising results. A person-centered psychoeducational group approach that includes metacognitive and compensatory strategies to help patients with long COVID may be an effective way forward. Another important outcome of this study is mode of rehabilitation attendance. We demonstrated that virtual, online participation yielded the same positive results as in-person attendance. Both were equally effective. This finding could make access to neuropsychological rehabilitation more far-reaching and cost-effective for both providers and patients, particularly remote or underserved populations. It appears that long COVID is here to stay, so developing accessible programs founded on both objective clinical measures and reliable patient self-reports has become imperative if we are to contain the growing consequences of this recent public health emergency.
Conflict of interest
The authors declare that they have no relevant financial or personal relationships with individuals or commercial interests (entities producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients) that could inappropriately influence or bias their work.
Funding
None to report.
Acknowledgments
The authors would like to acknowledge the participation of Carolina Bahia Fonseca, Ingrid Vitória Sena de Oliveira, Isabelly Silva Santos, Sarah Ribeiro Fernandes, Olinda Paula Azevedo, and Luciana Balduino Sollaci.
References
1 | Aiyegbusi, O. L. , Hughes, S. E. , Turner, G. , Rivera, S. C. , McMullan, C. , Chandan, J. S. , Haroon, S. , Price, G. , Davies, E. H. , Nirantharakumar, K. , Sapey, E. , Calvert, M. J. TLC Study Group ((2021) ) Symptoms, complications and management of long COVID: A review, Journal of the Royal Society of Medicine 114: (9), 428–442. https://doi.org/10.1177/01410768211032850 |
2 | Astin, R. , Banerjee, A. , Baker, M. R. , Dani, M. , Ford, E. , Hull, J. H. , Lim, P. B. , McNarry, M. , Morten, K. , O’Sullivan, O. , Pretorius, E. , Raman, B. , Soteropoulos, D. S. , Taquet, M. , Hall, C. N. ((2023) ) Long COVID: Mechanisms, risk factors and recovery, Experimental Physiology 108: (1), 12–27. https://doi.org/10.1113/EP090802 |
3 | Barker-Davies, R. M. , O’Sullivan, O. , Senaratne, K. P. P. , Baker, P. , Cranley, M. , Dharm-Datta, S. , Ellis, H. , Goodall, D. , Gough, M. , Lewis, S. , Norman, J. , Papadopoulou, T. , Roscoe, D. , Sherwood, D. , Turner, P. , Walker, T. , Mistlin, A. , Phillip, R. , Nicol, A. M. , Bennett, A. N. , Bahadur, S. ((2020) ) The Stanford Hall consensus statement for post-COVID-19 rehabilitation, British Journal of Sports Medicine 54: (16), 949–959. https://doi.org/10.1136/bjsports-2020-102596 |
4 | Badenoch, J. B. , Rengasamy, E. R. , Watson, C. , Jansen, K. , Chakraborty, S. , Sundaram, R. D. , Hafeez, D. , Burchill, E. , Saini, A. , Thomas, L. , Cross, B. , Hunt, C. K. , Conti, I. , Ralovska, S. , Hussain, Z. , Butler, M. , Pollak, T. A. , Koychev, I. , Michael, B. D. , Holling, H. , Rooney, A. G. ((2021) ) Persistent neuropsychiatric symptoms after COVID-19:Asystematic review and meta-analysis, Brain Communications 4: (1), fcab297. https://doi.org/10.1093/braincomms/fcab297 |
5 | Beck, A.T. (1972). Depression: Causes and treatment. University of Pennsylvania Press. |
6 | Beck, J. S. (1995).Cognitive therapy: Basics and beyond. Guilford. |
7 | Bora, E. , Harrison, B. J. ((2012) ) Cognitive impairment in euthymic major depressive disorder: A metaanalysis, Psychological Medicine 42: (12), 2017–2026. https://doi.org/10.1017/S0033291712000179 |
8 | Botega, N. J. , Bio, M. R. , Zomignani, M. A,. , GarciaC. Jr , Pereira, W. A. B. ((1995) ) Transtornos do humor em enfermaria de clínica médica e validação de escala de medida (HAD) de ansiedade e depressão.de Pública, Revista de Saude Publica 29: (5), 355–363. |
9 | Braga, L. W. , Oliveira, S. B. , Moreira, A. S. , Pereira, M. E. , Carneiro, V. S. , Serio, A. S. , Freitas, L. F. , Isidro, H. B. L. , Souza, L. M. N. ((2022) ) Neuropsychological manifestations of long COVID in hospitalized and nonhospitalized Brazilian patients, NeuroRehabilitation 50: (4), 391–400. https://doi.org/10.3233/NRE-228020 |
10 | Cristillo, V. , Pilotto, A. , Piccinelli, S. C. , Gipponi, S. , Leonardi, M. , Bezzi, M. , Padovani, A. ((2022) ) Predictors of “brain fog” 1 year after COVID-19 disease, Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 43: (10), 5795–5797. https://doi.org/10.1007/s10072-022-06285-4 |
11 | Crivelli, L. , Palmer, K. , Calandri, I. , Guekht, A. , Beghi, E. , Carroll, W. , Frontera, J. , García-Azorín, D. , Westenberg, E. , Winkler, A. S. , Mangialasche, F. , Allegri, R. F. , Kivipelto, M. ((2022) ) Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis, Alzheimer’s & Dementia: the Journal of the Alzheimer’s Association 18: (5), 1047–1066. https://doi.org/10.1002/alz.12644 |
12 | Dabrowska, E. , Galińska-Skok, B. , Waszkiewicz, N. ((2021) ) Depressive and neurocognitive disorders in the context of the inflammatory background of COVID-19, Life (Basel) 11: (10), 1056. https://doi.org/10.3390/life11101056 |
13 | De Luca, R. , Bonanno, M. , Calabrò, R. S. ((2022) ) Psychological and cognitive effects of long COVID: A narrative review focusing on the assessment and rehabilitative approach, Journal of Clinical Medicine 11: (21), 6554. https://doi.org/10.3390/jcm11216554 |
14 | Del Brutto, O. H. , Rumbea, D. A. , Recalde, B. Y. , Mera, R. M. ((2022) ) Cognitive sequelae of long COVID may not be permanent: A prospective study, European Journal of Neurology 29: (4), 1218–1221. https://doi.org/10.1111/ene.1521 |
15 | Delis, D. C. , Kaplan, E. , Kramer, J. H. (2001). Delis-Kaplan executive function system (D-KEFS). Psychological Corporation. |
16 | Diamond, A. ((2013) ) Executive functions, Annual Review of Psychology 64: 135–168. https://doi.org/10.1146/annurevpsych-113011-143750 |
17 | Edmonds, E. , Delano-Wood, L. , Galasko, D. , Salmon, D. , Bondi, M. Alzheimer’s Disease Neuroimaging Initiative ((2014) ) Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment, Journal of the International Neuropsychological Society 20: (8), 836–847. https://doi.org/10.1017/S135561771400068X |
18 | Ferrucci, R. , Dini, M. , Rosci, C. , Capozza, A. , Groppo, E. , Reitano, M. R. , Allocco, E. , Poletti, B. , Brugnera, A. , Bai, F. , Monti, A. , Ticozzi, N. , Silani, V. , Centanni, S. , D’Arminio Monforte, A. , Tagliabue, L. , Priori, A. ((2022) ) One-year cognitive follow-up of COVID-19 hospitalized patients, European Journal of Neurology 29: (7), 2006–2014. https://doi.org/10.1111/ene.15324 |
19 | Fiocruz. (2023, August 31). Dashboard rede genômica: Vigilância genômica do SARS-Cov-2 no Brasil.https://www.genomahcov.fiocruz.br/dashboard-pt/. |
20 | Flavell, J. H. ((1979) ) Metacognition and cognitive monitoring: A new area of cognitive–developmental inquiry, American Psychologist 34: (10), 906–911. https://doi.org/10.1037/0003-066X.34.10.906 |
21 | Fleck, M. P. A. , Louzada, S. , Xavier, M. , Chamovich, E. , Vieira, G. , Santos, L. , Pinzon, V. ((2000) ) Aplicação da versão em português do instrumento abreviado de avaliação da qualidade de vida “WHOQOL-bref”, Revista de Saude Publica 34: (2), 178–183. |
22 | Fonseca, R. P. , Salles, J. F. , Parente, M. A. M. P. (2009). Neupsilin: Instrumento de avaliacao neuropsicologica breve: Manual. Vetor. |
23 | Fugazzaro, S. , Contri, A. , Esseroukh, O. , Kaleci, S. , Croci, S. , Massari, M. , Facciolongo, N.C. , Besutti, G. , Iori, M. , Salvarani, C. , Costi, S. , Emilia, R. ((2022) ) Rehabilitation interventions for post-acute COVID-19 syndrome: A systematic review, International Journal of Environmental Research and Public Health 19: (9), 5185. https://doi.org/10.3390/ijerph19095185 |
24 | Han, Q. , Zheng, B. , Daines, L. , Sheikh, A. ((2022) ) Long-term sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms, Pathogens 11: (2), 269. https://doi.org/10.3390/pathogens11020269 |
25 | Hausswirth, C. , Schmit, C. , Rougier, Y. , Coste, A. ((2023) ) Positive impacts of a four-week neuro-meditation program on cognitive function in post-acute sequelae of COVID-19 patients: A randomized controlled trial, International Journal of Environmental Research and Public Health 20: (2), 1361. https://doi.org/10.3390/ijerph20021361 |
26 | Hawke, L. D. , Nguyen, A. T. P. , Ski, C. F. , Thompson, D. R. , Ma, C. , Castle, D. ((2022) ) Interventions for mental health, cognition, and psychological wellbeing in longCOVID: a systematic review of registered trials, Psychological Medicine 52: (13), 2426–2440. https://doi.org/10.1017/S0033291722002203 |
27 | Hazan, E. , Frankenburg, F. , Brenkel, M. , Shulman, K. ((2018) ) The test of time: A history of clock drawing, International Journal of Geriatric Psychiatry 33: (1), e22–e30. https://doi.org/10.1002/gps.4731 |
28 | Hellmuth, J. , Barnett, T. A. , Asken, B. M. , Kelly, J. D. , Torres, L. , Stephens, M. L. , Greenhouse, B. , Martin, J. N. , Chow, F. C. , Deeks, S. G. , Greene, M. , Miller, B. L. , Annan, W. , Henrich, T. J. , Peluso, M. J. ((2021) ) Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients, Journal of Neurovirology 27: (1), 191–195. https://doi.org/10.1007/s13365-021-00954-4 |
29 | Houben, S. , Bonnechère, B. ((2022) ) The impact of COVID-19 infection on cognitive function and the implication for rehabilitation: A systematic review and meta-analysis, International Journal of Environmental Research and Public Health 19: (13), 7748. https://doi.org/10.3390/ijerph19137748 |
30 | Hugon, J. , Msika, E. F. , Queneau, M. , Farid, K. , Paquet, C. ((2022) ) Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex, Journal of Neurology 269: (1), 44–46. https://doi.org/10.1007/s00415-021-10655-x |
31 | Johnsen, S. , Sattler, S. M. , Miskowiak, K. W. , Kunalan, K. , Victor, A. , Pedersen, L. , Andreassen, H. F. , Jørgensen, B. J. , Heebøll, H. , Andersen, M. B. , Marner, L. , Hædersdal, C. , Hansen, H. , Ditlev, S. B. , Porsbjerg, C. , Lapperre, T. S. ((2021) ) Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients, ERJ Open Research 7: (3), 00205–2021. https://doi.org/10.1183/23120541.00205-2021 |
32 | Kremer, S. , Jäger, H. R. ((2022) ) Brain changes after COVID-19 - how concerned should we be? Nature Reviews, Neurology 18: (6), 321–322. https://doi.org/10.1038/s41582-022-00661-6 |
33 | Liu, Y. H. , Chen, Y. , Wang, Q. H. , Wang, L. R. , Jiang, L. , Yang, Y. , Chen, X. , Li, Y. , Cen, Y. , Xu, C. , Zhu, J. , Li, W. , Wang, Y. R. , Zhang, L. L. , Liu, J. , Xu, Z. Q. , Wang, Y. J. ((2022) ) One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: A longitudinal cohort study, JAMA Neurology 79: (5), 509–517. https://doi.org/10.1001/jamaneurol.2022.0461 |
34 | Lövdén, M. , Fratiglioni, L. , Glymour, M. M. , Lindenberger, U. , Tucker-Drob, E. M. ((2020) ) Education and cognitive functioning across the life span, Psychological Science in the Public Interest : A Journal of the American Psychological Society 21: (1), 6–41. https://doi.org/10.1177/1529100620920576 |
35 | Malhi, G. S. , Mann, J. J. ((2018) ) Depression, Lancet 392: (10161), 2299–2312. https://doi.org/10.1016/S0140-6736(18)31948-228 |
36 | Marino, S. E. , Meador, K. J. , Loring, D. W. , Okun, M. S. , Fernandez, H. H. , Fessler, A. J. , Kustra, R. P. , Miller, J. M. , Ray, P. G. , Roy, A. , Schoenberg, M. R. , Vahle, V. J. , Werz, M. A. ((2009) ) Subjective perception of cognition is related to mood and not performance, Epilepsy & Behavior 14: (3), 459–464. https://doi.org/10.1016/j.yebeh.2008.12.007 |
37 | Mathern, R. , Senthil, P. , Vu, N. , Thiyagarajan, T. ((2022) ) Neurocognitive rehabilitation in COVID-19 patients: A clinical review, Southern Medical Journal 115: (3), 227–231. https://doi.org/10.14423/SMJ.0000000000001371 |
38 | Michelon, C. ((2021) ) Main SARS-CoV-2 variants notified in Brazil, Brazilian Journal of Clinical Analyses 53: (2), 109–116. https://doi.org/10.21877/2448-3877.202100961 |
39 | Ministério da Saúde. (2023, August 31). Vacinometro COVID-19. https://infoms.saude.gov.br/extensions/SEIDIGI DEMAS Vacina C19/SEIDIGI DEMAS Vacina C19.html |
40 | Miskowiak, K.W., Johnsen, S., Sattler, S. M., Nielsen, S., Kunalan, K., Rungby, J., Lapperre, T., & Porsberg, C. M. (2021). Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. European Neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 46: , 39–48. https://doi.org/10.1016/j.euroneuro.2021.03.019 |
41 | Miskowiak, K. W. , Fugledalen, L. , Jespersen, A. E. , Sattler, S. M. , Podlekareva, D. , Rungby, J. , Porsberg, C. M. , Johnsen, S. ((2022) ) Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: Pattern, severity, and functional implications, European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology 59: 82–92. https://doi.org/10.1016/j.euroneuro.2022.04.004 |
42 | National Institute for Health and Care Excellence (NICE). (2020). COVID-19 rapid guideline: Managing the longterm effects of COVID-19: NICE Guideline [NG188]. https://www.nice.org.uk/guidance/ng188 |
43 | Phetsouphanh, C. , Darley, D. R. , Wilson, D. B. , Howe, A. , Munier, C. M. L. , Patel, S. K. , Juno, J. A. , Burrell, L. M. , Kent, S. J. , Dore, G. J. , Kelleher, A. D. , Matthews, G. V. ((2022) ) Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection, Nature Immunology 23: (2), 210–216. https://doi.org/10.1038/s41590-021-01113-x |
44 | Pinto, E. , Peters, R. ((2009) ) Literature review of the Clock Drawing Test as a tool for cognitive screening, Dementia and Geriatric Cognitive Disorders 27: (3), 201–213. https://doi.org/10.1159/000203344 |
45 | Pinzon, R. T. , Wijaya, V. O. , Jody, A. A. , Nunsio, P. N. , Buana, R. B. ((2022) ) Persistent neurological manifestations in long COVID-19 syndrome: A systematic review and meta-analysis, Journal of Infection and Public Health 15: (8), 856–869. https://doi.org/10.1016/j.jiph.2022.06.013 |
46 | Poletti, S. , Palladini, M. , Mazza, M. G. , De Lorenzo, R. , COVID-19 BioB Outpatient Clinic Study Group, Furlan, R, Ciceri, F. , Rovere-Querini, P. , Benedetti, F. ((2022) ) Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: Role of depression and impact on quality of life, European Archives of Psychiatry and Clinical Neuroscience 272: (5), 773–782. https://doi.org/10.1007/s00406-021-01346-9. |
47 | Pranckeviciene, A. , Deltuva, V. , Tamasauskas, A. , Bunevicius, A. ((2017) ) Association between psychological distress, subjective cognitive complaints and objective neuropsychological functioning in brain tumor patients, Clinical Neurology and Neurosurgery 163: 18–23. https://doi.org/10.1016/j.clineuro.2017.10.007 |
48 | Premraj, L. , Kannapadi, N. V. , Briggs, J. , Seal, S. M. , Battaglini, D. , Fanning, J. , Suen, J. , Robba, C. , Fraser, J. , Cho, S. M. ((2022) ) Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis, Journal of the Neurological Sciences 434: 120162. https://doi.org/10.1016/j.jns.2022.120162 |
49 | Prigatano, G. P. , Amin, K. , Rosenstein, L. (1995). Administration and scoring manual for the BNI screen for higher cerebral functions. Barrow Neurological Institute. |
50 | Prigatano, G, P. , Souza, L. M. N. , Braga, L. W. ((2018) ) Performance of a Brazilian sample on the portuguese translation of the BNI screen for higher cerebral functions, Journal of Clinical and Experimental Neuropsychology 40: (2), 173–182. https://doi.org/10.1080/13803395.2017.1325839 |
51 | Rasouli, O. , Gotaas, M. , Stensdotter, A. , Skovlund, E. , Landrø N. , Dåstøl, P. , Fors, E. ((2019) ) Neuropsychological dysfunction in chronic fatigue syndrome and the relation between objective and subjective findings, Neuropsychology 33: (5), 658–669. https://doi.org/10.1037/neu0000550 |
52 | Rass, V. , Beer, R. , Schiefecker, A. J. , Lindner, A. , Kofler, M. , Ianosi, B. A. , Mahlknecht, P. , Heim, B. , Peball, M. , Carbone, F. , Limmert, V. , Kindl, P. , Putnina, L. , Fava, E. , Sahanic, S. , Sonnweber, T. , Löscher, W. N. , Wanschitz, J. V. , Zamarian, L. , Helbok, R. ((2022) ) Neurological outcomes 1 year after COVID-19 diagnosis: A prospective longitudinal cohort study, European Journal of Neurology 29: (6), 1685–1696. https://doi.org/10.1111/ene.15307 |
53 | Ritchie, K. , Chan, D. , Watermeyer, T. ((2020) ). The cognitive consequences of the COVID-19 epidemic: Collateral damage? Brain Communications, 2: (2), fcaa069. https://doi.org/10.1093/braincomms/fcaa069 |
54 | Rock, P. L. , Roiser, J. P. , Riedel, W. J. , Blackwell, A. D. ((2014) ) Cognitive impairment in depression: A systematic review and meta-analysis, Psychological Medicine 44: (10), 2029–2040. https://doi.org/10.1017/S0033291713002535 |
55 | Rogers, C. R. (1995). On becoming a person: A therapist’s view of psychotherapy. Houghton Mifflin Harcourt. |
56 | Rogers, C. (2021). Client centered therapy (70th Anniversary Ed). Robinson. |
57 | Rolin, S. , Chakales, A. , Verduzco-Gutierrez, M. ((2022) ) Rehabilitation strategies for cognitive and neuropsychiatric manifestations of COVID-19, Current Physical Medicine and Rehabilitation Reports 10: (3), 182–187. https://doi.org/10.1007/s40141-022-00352-9 |
58 | Rubin, R. ((2020) ) As their numbers grow, COVID-19 “long haulers” stump experts, JAMA 324: (14), 1381–1383. https://doi.org/10.1001/jama.2020.17709. |
59 | Sarubbo, F. , El Haji, K. , Vidal-Balle, A. , Bargay Lleonart, J. ((2022) ) Neurological consequences of COVID-19 and brain related pathogenic mechanisms: A new challenge for neuroscience, Brain, Behavior, & Immunity-Health 19: 100399. https://doi.org/10.1016/j.bbih.2021.100399 |
60 | Serrano-Castro P.J. , Estivill-Torrús G. , Cabezudo-García P. , Reyes-Bueno J.A. , Ciano Petersen N. , Aguilar-Castillo M.J. , Suárez-Pérez J. , Jiménez-Hernández M.D. , Moya-Molina M.Á. , Oliver-Martos B. , Arrabal-Gómez C. , Rodríguez de Fonseca F. ((2020) ). Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia, 35: (4), 245–251. doi: 10.1016/j.nrl.2020.04.002 |
61 | Schou, T. M. , Joca, S. , Wegener, G. , Bay-Richter, C. ((2021) ) Psychiatric and neuropsychiatric sequelae of COVID-19 - a systematic review, Brain, Behavior, and Immunity 97: 328–348. https://doi.org/10.1016/j.bbi.2021.07.018 |
62 | Seeßle, J. , Waterboer, T. , Hippchen, T. , Simon, J. , Kirchner, M. , Lim, A. , Müller, B. , Merle, U. ((2022) ) Persistent symptoms in adult patients 1 year after Coronavirus Disease 2019 (COVID-19): A prospective cohort study, Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America 74: (7), 1191–1198. https://doi.org/10.1093/cid/ciab611 |
63 | Siegelman J. N. ((2020) ) Reflections of a COVID-19 long hauler, JAMA 324: (20), 2031–2032. https://doi.org/10.1001/jama.2020.22130 |
64 | Stefanou M.I. , Palaiodimou L. , Bakola E. , Smyrnis N. , Papadopoulou M. , Paraskevas G.P. , Rizos E. , Boutati E. , Grigoriadis N. , Krogias C. , Giannopoulos S. , Tsiodras S. , Gaga M. , Tsivgoulis, G. (2022) Neurological manifestations of long-COVID syndrome: A narrative review. Therapeutic Advances in Chronic Disease, 13, 20406223221076890. doi: 10.1177/20406223221076890 |
65 | Stern, Y. ((2002) ) What is cognitive reserve? Theory and research application of the reserve concept, Journal of the International Neuropsychological Society 8: (3), 448–460. doi:10.1017/S1355617702813248 |
66 | Taquet, M. , Geddes, J. R. , Husain, M. , Luciano, S. , Harrison, P. J. ((2021) ) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records, The Lancet. Psychiatry 8: (5), 416–427. https://doi.org/10.1016/S2215-0366(21)00084-5 |
67 | Vance, H. , Maslach, A. , Stoneman, E. , Harmes, K. , Ransom, A. , Seagly, K. , Furst, W. ((2021) ) Addressing Post-COVID symptoms: A guide for primary care physicians, Journal of the American Board Family Medicine 34: 1229–1242. https://doi.org/10.3122/jabfm.2021.06.210254 |
68 | Vanderlind, W. M. , Rabinovitz, B. B. , Miao, I. Y. , Oberlin, L. E. , Bueno-Castellano, C. , Fridman, C. , Jaywant, A. , Kanellopoulos, D. ((2021) ) A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment, Current Opinion in Psychiatry 34: (4), 420–433. https://doi.org/10.1097/YCO.0000000000000713 |
69 | Venkataramani, V. , Winkler, F. ((2022) ) Cognitive deficits in Long Covid-19, The New England Journal of Medicine 387: (19), 1813–1815. https://doi.org/10.1056/NEJMcibr2210069 |
70 | Wade, D. T. ((2020) ) Rehabilitation after COVID-19: An evidencebased approach, Clinical Medicine (London, England) 20: (4), 359–365. https://doi.org/10.7861/clinmed.2020-0353 |
71 | Whitaker, M. , Elliott, J. , Bodinier, B. , Barclay, W. , Ward, H. , Cooke, G. , Donnelly, C.A. , Chadeau-Hyam, M. , Elliott, P. ((2022) ) Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England, Nature Communications 13: (1), 6856. doi: 10.1038/s41467-022-34244-2 |
72 | Wilson, B. A. , Betteridge, S. , Fish, J. ((2020) ) Neuropsychological consequences of Covid-19, Neuropsychologycal Rehabiitation 30: (9), 1625–1628. doi: 10.1080/09602011.2020.1808483 |
73 | World Health Organization. (2004). The World Health Organization quality of life (WHOQOL) - BREF, 2012 revision. World Health Organization. https://apps.who.int/iris/handle/10665/77773 |
74 | World Health Organization. (2021). A clinical case definition of post COVID-19 condition by a Delphi consensus. https://www.who.int/publications/i/item/WHO-2019-nCoVPostCOVID-19_condition-Clinical_case_definition-2021.1 |
75 | World Health Organization. (2023). COVID-19 Dashboard. https://covid19.who.int/ |
76 | Woo, M. S. , Malsy, J. , Pöttgen, J. , Seddiq Zai, S. , Ufer, F. , Hadjilaou, A. , Schmiedel, S. , Addo, M. M. , Gerloff, C. , Heesen, C. , Wiesch, J. S. Z. , Friese, M. A. ((2020) ) Frequent neurocognitive deficits after recovery from mild COVID-19, Brain Communications 2: (2), fcaa205. https://doi.org/10.1093/braincomms/fcaa205 |
77 | Zeng, N. , Zhao, Y. M. , Yan, W. , Li, C. , Lu, Q. D. , Liu, L. , Ni, S. Y. , Mei, H. , Yuan, K. , Shi, L. , Li, P. , Fan, T. T. , Yuan, J. L. , Vitiello, M. V. , Kosten, T. , Kondratiuk, A. L. , Sun, H. Q. , Tang, X. D. , Liu, M. Y. , Lalvani, A. , Shi, J. , Bao, Y. P. , Lu, L. ((2023) ) A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: Call for research priority and action, Molecular Psychiatry 28: (1), 423–433. https://doi:10.1038/s41380-022-01614-7 |
78 | Zigmond, A. S. , Snaith, R. P. ((1983) ) The Hospital Anxiety and Depression Scale, Acta Psychiatrica Scandinavica 67: (6), 361–370. |




