Immediate effects of multimodal cognitive therapy in mild cognitive impairment
Abstract
BACKGROUND:
Current therapeutic evidence suggests limited efficacy of the cognitive and exercise training in mild cognitive impairment (MCI) on depression, anxiety, memory retention, comprehension, calculation, concentration, orientation, dual-task performance, and sleep disorders. Nevertheless, the immediate effects of multimodal cognitive therapy (MCT) have recently developed and its individual effects remains unknown in MCI.
OBJECTIVE:
This study aimed to compare the immediate effects of MCT on cognitive and psychological measures between young healthy and older adults with MCI.
METHODS:
Forty young healthy and older adults with MCI underwent immediate MCT (5 minutes each), including transcranial direct current stimulation (tDCS), light therapy, computerized cognitive therapy (CCT), robotic-assisted gait training (RAGT), core breathing exercises (CBE), and music therapy. Outcome measures included memory retention, comprehension, calculation, attention, orientation, dual-task performance, awareness, depression, anxiety, and sleep disorders. The Mann-Whitney U test and Friedman’s test were used at P < 0.05.
RESULTS:
Significant differences in depression, anxiety, memory retention, comprehension, calculation, attention, orientation, dual-task performance, and awareness were observed between the tDCS, CCT, and music therapy groups (P < 0.05).
CONCLUSION:
MCT was beneficial for mitigating depression, anxiety, memory retention, comprehension, calculation, attention, orientation, dual-task performance, and awareness.
1Introduction
Mild cognitive impairment (MCI) is a degenerative neurocognitive disorder characterized by difficulties in memory retention, comprehension, calculation, attention, orientation, dual-task performance, and awareness relative to peers of the same age and has been found to be associated with aging, depression, anxiety, and sleep disorders (Liew, 2019; Randhi et al., 2023). MCI is a progressive deterioration in cognitive function, including memory retention, comprehension, calculation, attention, and orientation, eventually leading to dementia due to brain atrophy (gray matter), abnormalities (white matter), and hypoactivation in the brain (frontal, parietal, and temporal lobes) (Anderson, 2019; Gu & Zhang, 2019). Previous studies have reported that dual-task performance is associated with the coordination function of episodic memory, and older adults with MCI exhibit significant impairments in dual-task performance compared with healthy age-matched individuals (Doi et al., 2017; Foley et al., 2011). Depression and anxiety are common neuropsychiatric symptoms in older adults with MCI, possibly due to the neurotoxic substances (Aβ burden of the brain) (Ma, 2020). Sleep plays a crucial role in consolidating memories by facilitating the transfer of memory traces from the hippocampus to the neocortex and may also aid in clearing metabolites from synapses (Randhi et al., 2023). Impaired self-awareness is a common symptom observed in older adults with MCI and is associated with MCI care, including treatment compliance, caregiver burden, and personal safety (Bastin et al., 2021).
To improve cognitive impairment in people with MCI, multimodal cognitive therapy (MCT), including transcranial direct current stimulation (tDCS), light therapy, computerized cognitive therapy (CCT), robot-assisted gait training (RAGT), core breathing exercises (CBE) and, music therapy have been utilized in patients with MCI, but outcome studies have produced variable results in depression, anxiety, memory retention, comprehension, calculation, concentration, orientation, dual-task performance, awareness, and sleep disorders (Calabrò et al., 2015; Han et al., 2020; Zhang et al., 2019). Calabrò et al. (2015) reported that RAGT, in addition to cognitive training, had positive effects on cognitive function with an improvement of 29% in the Mini-Mental State Examination (MMSE) (Calabrò et al., 2015). A previous meta-analysis reported that CCT improved(Khanthong et al., 2021; Riemersma-Van Der Lek et al., 2008) cognitive functions related to memory and executive function in older adults with MCI (Zhang et al., 2019). Han et al. (2020) demonstrated that cognitive intervention with musical stimuli improved memory (37%), attention (9%), and orientation (8%), and depression (8%) in 24 patients with MCI compared to the controls who received no intervention (Han et al., 2020). A previous study showed that light therapy (1000 or 300 lx) improved depression (22%), sleep efficiency (4%), and cognitive function (12%) in older adults with dementia (Riemersma-Van Der Lek et al., 2008). Khanthong et al. (2021) reported that Thai exercises, including deep breathing and holding, improved cognitive function (9%) in 71 older adults with MCI (Khanthong et al., 2021).
There is a clear need to effectively and sustainably address progressive cognitive impairments in older adults to improve depression, anxiety, sleep disorders, memory, retention, comprehension, calculation, concentration, orientation, dual-task performance, awareness, and sleep disorders of MCI. However, the perceived effects of MCT have not been explored in older adults with MCI. This clinical study aimed to investigate the effects of MCT. We hypothesized that there would be significant differences in the perceived effects on depression, anxiety, memory retention, comprehension, calculation, attention, orientation, dual-task performance, awareness, and sleep disorders between and within groups.
2Methods
2.1Participants
All sex-matched participants were recruited from community centers and provided informed consent before participating in the study. The participants completed the Korean version of the MMSE prior to interventions by psychiatrists and medical professionals. A total of forty participants were recruited for the study, consisting of twenty young healthy adults (age:25.20±3.19; 10 women) and twenty older adults diagnosed with MCI (age:79.00±8.25; 10 women). The onsite survey was performed using an online survey questionnaire with Google Forms, which was administered between August 5, 2022, and December 27, 2022. The experimental protocol was approved by the Yonsei Institutional Review Board (IRB) and Ethics Committee (approval no. 1041849-202202-BM-033-02).
Fig. 1
Flowchart of the study.
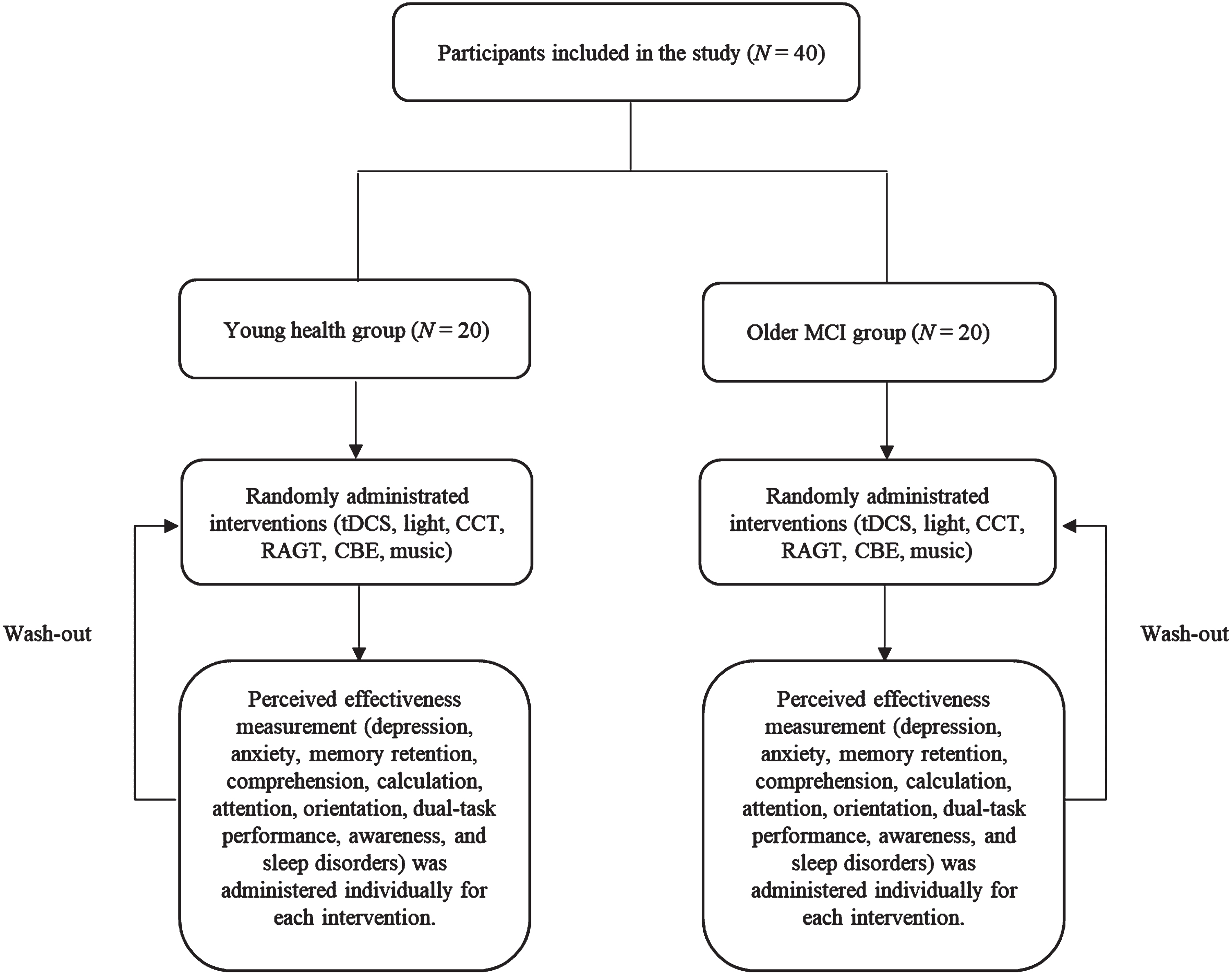
The inclusion criteria for young healthy adults were [1] age between 21 and 35 years (Nilsen & Donelson, 2001) and [2] the ability to follow verbal instructions. The exclusion criteria were [1] history of brain trauma or surgery, [2] psychiatric disorders, [3] neurological system dysfunction, and [4] neurocognitive impairments (Nilsen & Donelson, 2001). The inclusion criteria for older adults with MCI entailed [1] MMSE (between 20 to 23 points) (Gauthier et al., 2006); [2] can follow verbal instruction; [3] no significant impairment in social or occupational activities confirming the absence of dementia; [4] self-reported subjective decline in cognitive function, which was also confirmed by a family member; [5] objective evidence of cognitive deficits compared to individuals of the same age and educational level in one or more formal cognitive tests (Petersen, 2016); and [6] between 65 and 100 years old. The exclusion criteria for older adults with MCI included [1] evidence of specific abnormalities in the brain, such as focal brain lesions detected by head magnetic resonance imaging (MRI) or head computed tomography; [2] a history of mental illness or substance abuse before the onset of dementia; [3] complications of other neurological diseases or illnesses; and [4] use of antipsychotics, antidepressants, or anxiolytic drugs (Sanford, 2017).
2.2Study design
Table 1
The protocol of the multimodal cognitive therapy
| Multimodal cognitive therapy | Duration (min.) | Intensity |
| Transcranial direct current stimulation (tDCS) | 5 | 1 to 2 mA |
| Light therapy | 5 | 10000 lux |
| Computerized cognitive training (CCT) | 5 | Medium difficulty game focused on memory and attention |
| Robotic assisted gait training (RAGT) | 5 | 20% body weight support at comfortable speed |
| Core breathing exercise (CBE) | 5 | The number of breath cycles per minute was fixed at 8–10 cycles |
| Music therapy | 5 | 40 to 80 Hz |
This study deign involves patients blinded (single-blinded), quasi-randomized experimental research aimed to compare between and within group on depression, anxiety, memory retention, comprehension, calculation, concentration, orientation, dual-task performance, awareness, and sleep disorders domain in young healthy adults and older adults with MCI. Two certified physical therapists consistently performed all survey and interventions.
2.3Experimental procedure
The MCT comprises six individual interventions that are administered randomly. Following each session, participants underwent each session for a duration of five minutes, the MCT effectiveness survey was administered to assess the level of satisfaction with five-minutes washout period. Each intervention modality was implemented for 5 minutes and cumulated to 60 minutes (5 min. tDCS+5 min. light+5 min. CCT, 5 min. RAGT, 5 min. CBE, 5 min. music with 5 min. resting intervals). The flowchart of the study is shown in Fig. 1. Individual interventions included [1] the application of tDCS (Brain driver v2; The Brain Drive, Chicago, IL, US) at 1-2 mA over the cerebral cortex. [2] Participants were exposed to short-wavelength light (10000 lux over their head. [3] The CCT (CoTras-Pro; CoTras, Daegu, Republic of Korea) was conducted with participants randomly selected to play one of four games: a history game, memory game, visual perception game, or focus game. [4] The RAGT session using the Walkbot G model (P&S Mechanics, Seoul, Republic of Korea) was conducted with 20% body weight support at a comfortable speed and a stride tailored to the participant’s joint length. [5] Participants also performed core breathing exercises. [6] The participants listened to relaxing music in the frequency range of 40–80 Hz. The study protocols are presented in Table 1.
Fig. 2
Survey questionnaire for demographic characteristics and satisfaction levels.

Table 2
Demographic characteristics of participating patients (n = 40)
| Characteristics | Healthy (n = 20) | MCI (n = 20) | P-value |
| Age (years) | 25.20±3.19 | 79.00±8.25 | 0.006** |
| Sex (male/female) | 10/10 | 10/10 | 1.000 |
| Height (cm) | 169.65±9.37 | 157.25±8.45 | 0.717 |
| Weight (kg) | 67.70±13.83 | 59.40±7.30 | 0.002** |
| BMI (cm/kg2) | 23.20±3.17 | 24.01±2.26 | 0.090 |
| MMSE | 29.55±0.83 | 21.35±1.27 | 0.007** |
BMI, Body mass index; MMSE, Mini-Mental State Examination; independent t-test, *P < 0.05, **P < 0.01.
2.4Survey
A survey questionnaire was designed to examine the perceived effects of MCT among two distinct groups: young healthy adults and older adults with MCI. The questionnaire consisted of two domains comprising 84 questions, four of which were related to general demographic characteristics and ten for each of the MCT components, including tDCS, light therapy, CCT, RAGT, CBE, and music therapy. The survey utilized a Likert scale to assess the perceived (immediate) effects levels of the program, with five rating options (‘1’ for strongly disagree; ‘2’ for disagree; ‘3’ for neutral; ‘4’ for agree; ‘5’ for strongly agree. The survey questionnaire is presented in Fig. 2.
2.5Statistical analysis
Statistical analyses are presented as the mean rank. The independent t-test or Mann-Whitney U test was used to compare baseline demographic data between the healthy and MCI groups. A power analysis using G-Power software (version 3.1.9.4; Franz Faul, University of Kiel, Germany) was conducted to assess the minimum sample size requirement based on a prior pilot study. The sample size was determined to be 40 and the power (1 - β= 0.8) was based on the effect size (eta squared, η2 = 0.6). For the analysis of the Likert-scale data, the Mann-Whitney U test was used to compare the significant differences between the healthy and MCI groups. Friedman’s test was used to compare the perceived effects of MCT. If a significant difference was observed, the Wilcoxon signed-rank post hoc test was used. SPSS for Windows version 26.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. The P-value was set at 0.05.
3Results
3.1General demographic characteristics
Table 1 summarizes the demographic characteristics of the participants. Age, weight and MMSE scores were significantly different between young healthy and older adults with MCI, violating the group homogeneity at baseline. No safety issues were reported and none of the participants experienced any side effects associated with the MCT.
3.2tDCS effect between healthy and MCI groups
The effects of tDCS on memory retention (P = 0.003), comprehension (P = 0.016), calculation skills (P = 0.007), orientation (P = 0.007), dual-task performance (P = 0.000) and awareness (P = 0.011) differed significantly between the healthy and MCI groups indicating better memory retention, comprehension, calculation skills, orientation, dual-task performance, and awareness domains in MCI group than young group. Mann-Whitney U test showed no significant differences on depression, anxiety, attention, and sleep quality domains (P > 0.05) in effects between the healthy and MCI groups (Table 3).
Table 3
tDCS effect between healthy and MCI groups
| tDCS | Mean rank | U statistic | P-value | |
| Healthy (n = 20) | MCI (n = 20) | |||
| Depression | 17.02 | 23.98 | 130.50 | 0.044 |
| Anxiety | 18.23 | 22.78 | 154.50 | 0.199 |
| Memory retention | 15.18 | 25.83 | 93.50 | 0.003** |
| Comprehension | 16.20 | 24.80 | 114.00 | 0.016* |
| Calculation skills | 15.73 | 25.28 | 104.50 | 0.007** |
| Attention | 17.23 | 23.78 | 134.50 | 0.056 |
| Orientation | 15.75 | 25.25 | 105.00 | 0.007** |
| Dual-task performance | 14.35 | 26.65 | 77.00 | 0.000** |
| Awareness | 16.00 | 25.00 | 110.00 | 0.011* |
| Sleep quality | 19.05 | 21.95 | 171.00 | 0.392 |
MCI, mild cognitive impairment; tDCS, transcranial direct current stimulation; Mann-Whitney U test, *P < 0.05, **P < 0.01.
3.3Light therapy effects between healthy and MCI groups
No significant difference on depression, anxiety, memory retention, comprehension, calculation skills, attention, orientation, dual-task performance, awareness, and sleep quality domains (P > 0.05) in the effects of light therapy was observed between the healthy and MCI groups (Table 4).
Table 4
Light therapy effects between healthy and MCI groups
| Light | Mean rank | U statistic | P-value | |
| Healthy (n = 20) | MCI (n = 20) | |||
| Depression | 20.00 | 21.00 | 190.00 | 0.780 |
| Anxiety | 19.63 | 21.38 | 182.50 | 0.612 |
| Memory retention | 19.33 | 21.68 | 176.50 | 0.502 |
| Comprehension | 20.30 | 20.70 | 196.00 | 0.909 |
| Calculation skills | 19.18 | 21.83 | 173.50 | 0.459 |
| Attention | 17.70 | 23.30 | 144.00 | 0.105 |
| Orientation | 17.60 | 23.40 | 142.00 | 0.097 |
| Dual-task performance | 18.38 | 22.63 | 157.50 | 0.223 |
| Awareness | 19.90 | 21.10 | 188.00 | 0.733 |
| Sleep quality | 17.55 | 23.45 | 141.00 | 0.089 |
MCI, mild cognitive impairment; Mann-Whitney U test, *P < 0.05, **P < 0.01.
Table 5
Computerized cognitive training effects between healthy and MCI groups
| CCT | Mean rank | U statistic | P-value | |
| Healthy (n = 20) | MCI (n = 20) | |||
| Depression | 17.85 | 23.15 | 147.00 | 0.121 |
| Anxiety | 20.25 | 20.75 | 195.00 | 0.886 |
| Memory retention | 15.90 | 25.10 | 108.00 | 0.009** |
| Comprehension | 16.60 | 24.40 | 122.00 | 0.027* |
| Calculation skills | 14.60 | 26.40 | 82.00 | 0.001** |
| Attention | 14.25 | 26.75 | 75.00 | 0.000** |
| Orientation | 15.80 | 31.60 | 106.00 | 0.008** |
| Dual-task performance | 14.75 | 26.25 | 85.00 | 0.001** |
| Awareness | 16.50 | 24.50 | 120.00 | 0.022* |
| Sleep quality | 17.58 | 23.43 | 141.50 | 0.098 |
MCI, mild cognitive impairment; CCT, computerized cognitive training; Mann-Whitney U test, *P < 0.05, **P < 0.01.
Table 6
Robotic-assisted gait training effects between healthy and MCI groups
| RAGT | Mean rank | U statistic | P-value | |
| Healthy (n = 20) | MCI (n = 20) | |||
| Depression | 20.65 | 20.35 | 197.00 | 0.932 |
| Anxiety | 20.20 | 20.80 | 194.00 | 0.866 |
| Memory retention | 21.30 | 19.70 | 184.00 | 0.653 |
| Comprehension | 21.70 | 19.30 | 176.00 | 0.503 |
| Calculation skills | 21.68 | 19.33 | 176.50 | 0.510 |
| Attention | 22.20 | 18.80 | 166.00 | 0.335 |
| Orientation | 21.03 | 19.98 | 189.50 | 0.769 |
| Dual-task performance | 20.95 | 20.05 | 191.00 | 0.795 |
| Awareness | 21.58 | 19.43 | 178.50 | 0.527 |
| Sleep quality | 23.70 | 17.30 | 136.00 | 0.063 |
MCI, mild cognitive impairment; RAGT, robot-assisted gait training; Mann-Whitney U test, *P < 0.05, **P < 0.01.
3.4Computerized cognitive training effects between healthy and MCI groups
The effects of CCT on memory retention (P = 0.009), comprehension (P = 0.027), calculation skills (P = 0.001), attention (P = 0.000), orientation (P = 0.008), dual-task performance (P = 0.001), and awareness (P = 0.022) differed significantly between the healthy and MCI groups supporting that improved memory retention, comprehension, calculation skills, orientation, dual-task performance, and awareness domains. Mann-Whitney U test showed no significant differences on depression, anxiety, and sleep quality domains (P > 0.05) in effects between the healthy and MCI groups (Table 5).
3.5Robotic-assisted gait training effects between healthy and MCI groups
There were no significant differences on depression, anxiety, memory retention, comprehension, calculation skills, attention, orientation, dual-task performance, awareness, and sleep quality domains (P > 0.05) in the effects of RAGT between the healthy and MCI groups (Table 6).
Table 7
Core breathing exercise effects between healthy and MCI groups
| CBE | Mean rank | U statistic | P-value | |
| Healthy (n = 20) | MCI (n = 20) | |||
| Depression | 22.45 | 18.55 | 161.00 | 0.272 |
| Anxiety | 23.88 | 17.13 | 132.50 | 0.058 |
| Memory retention | 22.25 | 18.75 | 165.00 | 0.324 |
| Comprehension | 19.75 | 21.25 | 185.00 | 0.670 |
| Calculation skills | 21.53 | 19.48 | 179.50 | 0.565 |
| Attention | 21.90 | 19.10 | 172.00 | 0.422 |
| Orientation | 23.00 | 18.00 | 150.00 | 0.153 |
| Dual-task performance | 22.88 | 18.13 | 152.50 | 0.170 |
| Awareness | 19.15 | 21.85 | 173.00 | 0.443 |
| Sleep quality | 21.50 | 19.50 | 180.00 | 0.562 |
MCI, mild cognitive impairment; CBE, core breathing exercise; Mann-Whitney U test, *P < 0.05, **P < 0.01.
Table 8
Music therapy effects between the healthy and MCI
| Music | Mean rank | U statistic | P-value | |
| Healthy (n = 20) | MCI (n = 20) | |||
| Depression | 15.75 | 25.25 | 105.00 | 0.006** |
| Anxiety | 16.20 | 24.80 | 114.00 | 0.012* |
| Memory retention | 17.35 | 23.65 | 137.00 | 0.075 |
| Comprehension | 15.98 | 25.03 | 109.50 | 0.011* |
| Calculation skills | 16.75 | 24.25 | 125.00 | 0.037* |
| Attention | 17.50 | 23.50 | 140.00 | 0.086 |
| Orientation | 17.65 | 23.35 | 143.00 | 0.110 |
| Dual-task performance | 17.05 | 23.95 | 131.00 | 0.049* |
| Awareness | 19.13 | 21.88 | 172.50 | 0.432 |
| Sleep quality | 19.05 | 21.95 | 171.00 | 0.412 |
MCI, mild cognitive impairment; Mann-Whitney U test, *P < 0.05, **P < 0.01.
Table 9
Effects of the intervention variables in healthy group
| tDCS | Light | CCT | RAGT | CBE | Music | χ2 | P-value | |
| Depression | 3.33 | 3.00 | 3.25 | 4.13 | 3.70 | 3.60 | 5.598 | 0.347 |
| Anxiety | 3.45 | 3.08 | 3.48 | 3.70 | 3.78 | 3.53 | 2.186 | 0.823 |
| Memory retention | 3.50 | 2.83 | 3.35 | 4.13 | 3.68 | 3.53 | 6.186 | 0.289 |
| Comprehension | 3.53 | 2.93 | 3.60 | 4.13 | 3.55 | 3.28 | 5.322 | 0.378 |
| Calculation skills | 3.45 | 3.23 | 3.38 | 4.28 | 3.75 | 2.93 | 7.238 | 0.204 |
| Attention | 3.50 | 3.08 | 2.70 | 4.18 | 3.88 | 3.68 | 10.122 | 0.072 |
| Orientation | 3.55 | 2.98 | 3.40 | 3.90 | 3.80 | 3.38 | 3.998 | 0.550 |
| Dual-task performance | 3.38 | 3.08 | 2.98 | 4.33 | 3.90 | 3.35 | 9.639 | 0.086 |
| Awareness | 3.30 | 3.10 | 3.23 | 4.20 | 3.48 | 3.70 | 5.728 | 0.334 |
| Sleep quality | 3.18 | 2.98 | 3.18 | 4.28 | 4.10 | 3.30 | 10.759 | 0.056 |
tDCS, transcranial direct current stimulation; CCT, computerized cognitive training; RAGT, robot-assisted gait training; CBE, core breathing exercise; Friedman test; *P < 0.05, **P < 0.01.
3.6Core breathing exercise effects between healthy and MCI groups
No significant differences on depression, anxiety, memory retention, comprehension, calculation skills, attention, orientation, dual-task performance, awareness, and sleep quality domains (P > 0.05) were observed in the effects of CBE between the healthy and MCI groups (Table 7).
3.7Music therapy effects between healthy and MCI groups
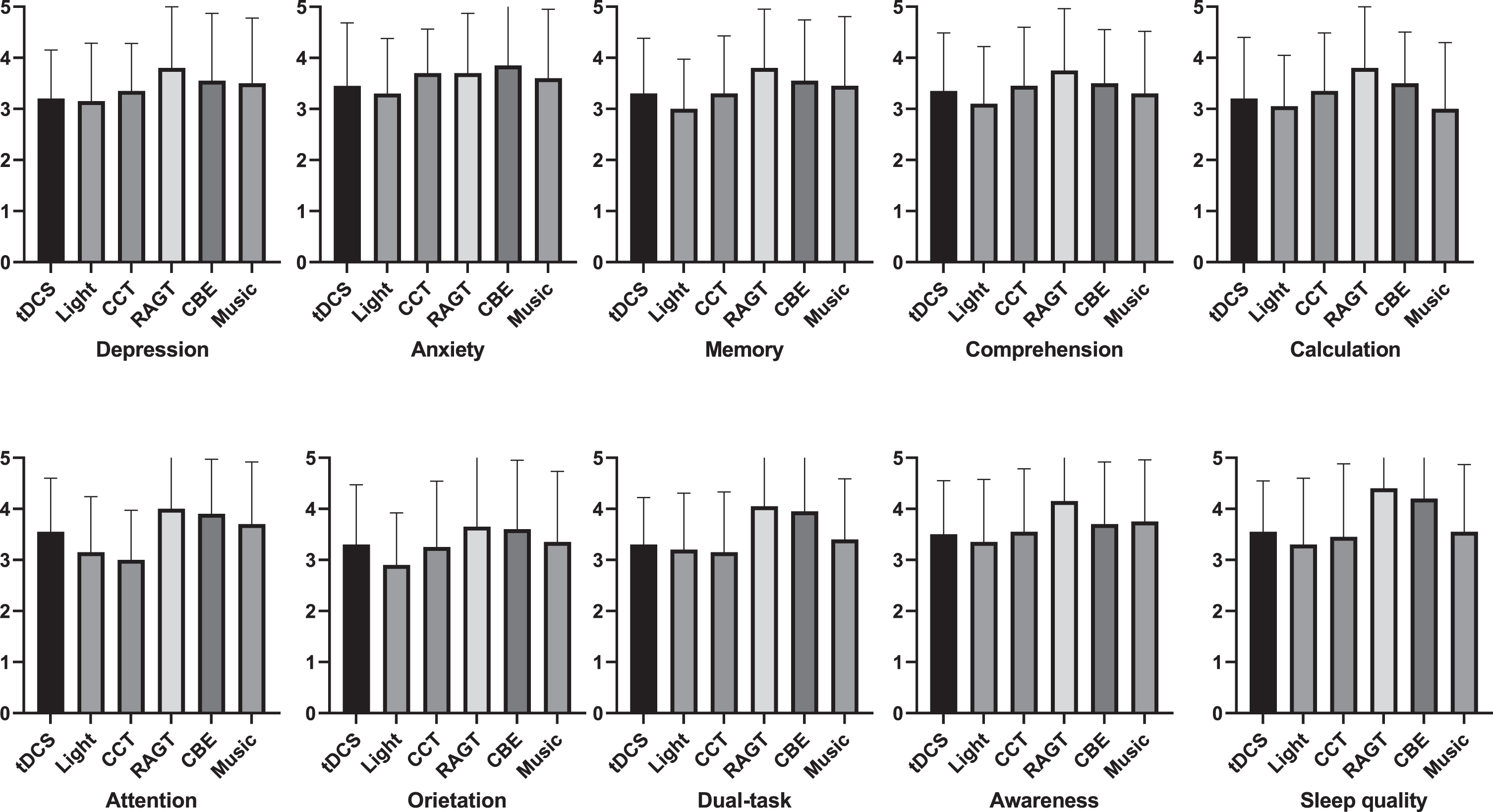
Fig. 3
Effects of the intervention variables in healthy group.
The effects of music on depression (P = 0.006), anxiety (P = 0.012), comprehension (P = 0.011), calculation skills (P = 0.037), and dual-task performance (P = 0.049) domains differed significantly between the healthy and MCI groups suggesting that depression, anxiety, comprehension, calculation skills, and dual-task performance domains. Mann-Whitney U test showed no significant differences on memory retention, attention, orientation, awareness, and sleep quality domains (P > 0.05) in effects between the healthy and MCI groups (Table 8).
3.8Effects of the intervention variables in healthy group
Friedman’s test showed no significant differences on depression, anxiety, memory retention, comprehension, calculation skills, attention, orientation, dual-task performance, awareness, and sleep quality domains (P > 0.05) in effects within groups (Table 9 and Fig. 3).
Table 10
Effects of the intervention variable in MCI group
| tDCS | Light | CCT | RAGT | CBE | Music | χ2 | P-value | |
| Depression | 3.58 | 2.93 | 3.35 | 3.53 | 2.85 | 4.78 | 18.034 | 0.003** |
| Anxiety | 3.83 | 2.98 | 3.35 | 3.40 | 2.65 | 4.80 | 20.406 | 0.001** |
| Memory retention | 4.35 | 2.70 | 4.00 | 3.25 | 2.65 | 4.05 | 19.048 | 0.002** |
| Comprehension | 3.90 | 2.58 | 4.10 | 3.18 | 2.98 | 4.28 | 16.065 | 0.007** |
| Calculation skills | 4.13 | 2.90 | 4.47 | 3.13 | 2.80 | 3.58 | 16.549 | 0.005** |
| Attention | 3.83 | 2.85 | 3.90 | 3.20 | 2.88 | 4.35 | 14.274 | 0.014* |
| Orientation | 4.43 | 2.63 | 4.33 | 3.38 | 2.53 | 3.73 | 23.748 | 0.000** |
| Dual-task performance | 4.18 | 2.75 | 4.15 | 3.58 | 2.78 | 3.58 | 15.270 | 0.009** |
| Awareness | 3.75 | 2.70 | 4.13 | 3.55 | 3.40 | 3.48 | 8.832 | 0.116 |
| Sleep quality | 3.10 | 2.90 | 4.30 | 3.53 | 3.78 | 3.40 | 9.661 | 0.085 |
tDCS, transcranial direct current stimulation; CCT, computerized cognitive training; RAGT, robot-assisted gait training; CBE, core breathing exercise; Friedman test; *P < 0.05, **P < 0.01.
3.9Effects of the intervention variables in MCI group
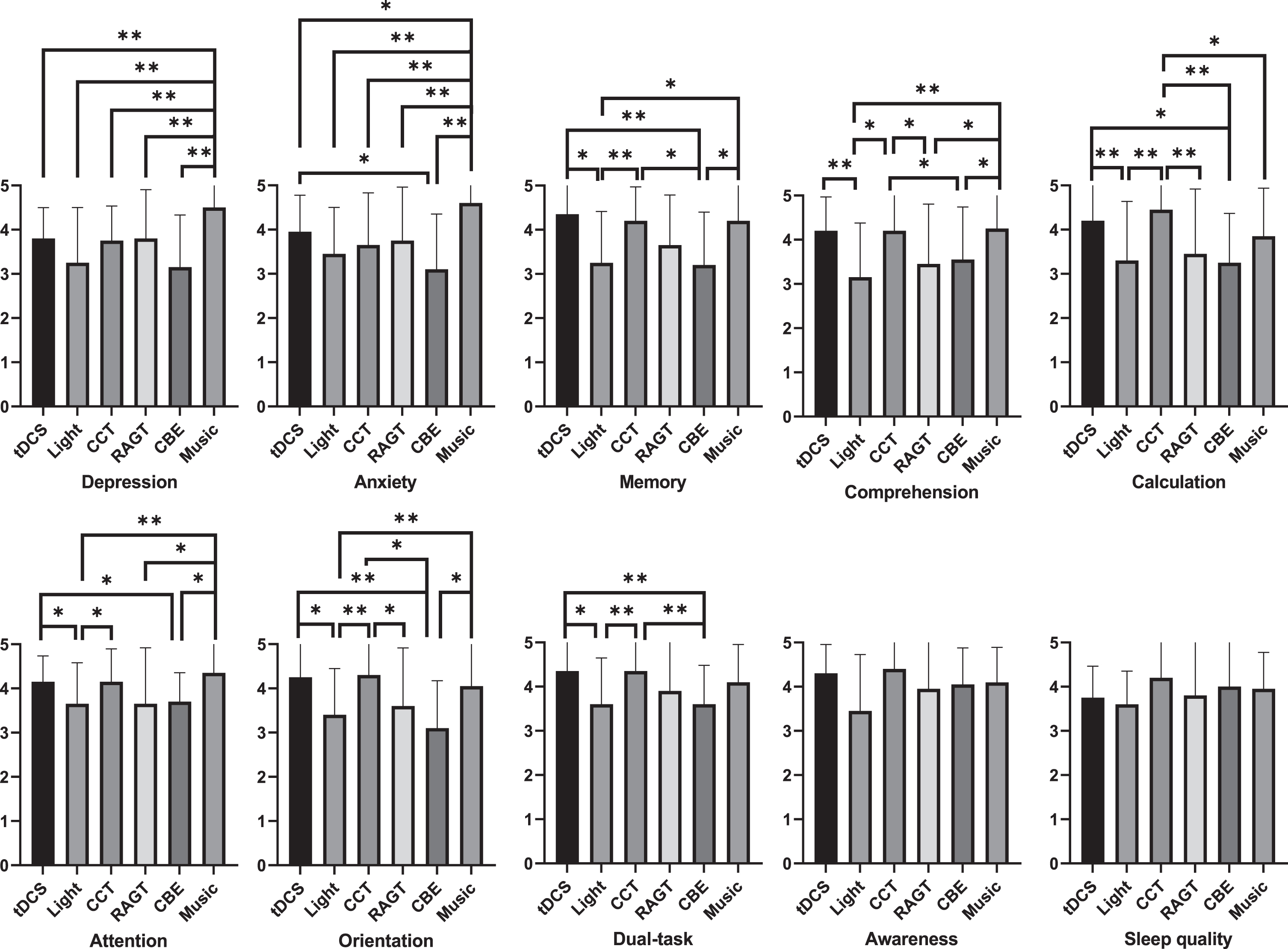
Friedman’s test showed significant differences in the effects within the group (P < 0.05) (Table 10). Post-hoc Wilcoxon signed-rank test showed that tDCS was superior to other therapeutic modalities (light or/and CBE), in improving the anxiety, memory, comprehension, calculation, attention, orientation, and dual-task domains. The CCT showed greater effectiveness than other therapeutic modalities (light, RAGT or/and CBE) in improving memory, comprehension, calculation, attention, orientation, dual-task domains. The music therapy was better than other therapeutic modalities (tDCS, light, CCT, RAGT, or/and CBE) in improving the depression, anxiety, memory, comprehension, attention, and orientation domains (Fig. 4).
Fig. 4
Effects of each therapeutic modality on specific outcome domains in the MCI group.
4Discussion
The present single-blinded quasi-randomized experimental study demonstrated the effects of MCT on cognitive (memory retention, comprehension, calculation, concentration, orientation, and awareness) and psychological function (depression, and anxiety), dual-task performance, and sleep disorders in young healthy and older adults with MCI. As anticipated, there were significant differences in the perceived effects on depression, anxiety, memory retention, comprehension, calculation, attention, orientation, dual-task performance, awareness, and sleep disorders between and within groups. In particular, MCT substantially improved cognitive function, including memory retention, comprehension, calculation, attention, and orientation, contributing to clinically meaningful outcomes in MCI rehabilitation.
The tDCS therapy-related effectiveness data revealed greater enhancements in memory retention, calculation skills, orientation, dual-task performance, and awareness domains in the MCI group than in the healthy group. In addition, tDCS therapy-related effectiveness data demonstrated that tDCS significantly increased anxiety, memory, comprehension, calculation, attention, orientation, and dual-task performance domains compared to other interventions (light and/or CBE) in the MCI group. This finding is consistent with previous results (Cruz Gonzalez et al., 2018; Gu & Zhang, 2019). Gonzalez et al. (2018) reported that tDCS resulted in an 8.13% increase in cognitive function, including executive function, memory, attention, and orientation, as measured by MoCA in older adults with MCI (Cruz Gonzalez et al., 2018). Gu et al. (2022) reported that tDCS after 20 min improved cognitive function (3.49%), memory (9.72%), and mental control (1.08%), as measured using the MoCA and Wechsler memory scales, respectively, compared with sham tDCS in 40 patients with MCI (Gu & Zhang, 2019). A possible underlying neural mechanism for such effects is that anodic tDCS results in depolarization of the resting membrane potential in neurons and enhances cortical activation through an increase in the frequency of spontaneous neuronal firing, while cathodic tDCS induces hyperpolarization of the resting membrane potential in neurons and suppresses cortical activation by reducing the firing frequency of neurons (Park et al., 2019). tDCS may increase neural activation between the amygdala (emotion and memory) and the lateral prefrontal cortex (cognition, memory, and executive functions) and, more extensively, indirect transmission via other related cortical areas, such as the anterior cingulate (motivation, decision making, and calculation) (Chen et al., 2022; da Silva et al., 2022).
The CCT therapy-related effectiveness data demonstrated greater improvements in memory retention, comprehension, calculation skills, attention, orientation, dual-task performance, and awareness domains in the MCI group than in the healthy group. Moreover, the effectiveness data pertaining to CCT indicated a notable enhancement in cognitive domains such as memory, comprehension, calculation, attention, orientation, and dual-task performance domains compared with other interventions (light therapy, CBE, and/or RAGT) in the MCI group. This finding is consistent with previous CCT findings in older adults with MCI (Herrera et al., 2012; Lee et al., 2018; Tang et al., 2019). Lee et al. (2018) demonstrated that COMCOG, a type of CCT, improved attention and memory by 64.46% and 150.98%, respectively, in a Seoul neuropsychological screening battery of 10 elderly individuals with MCI (Lee et al., 2018). Herrera et al. (2012) showed that computer-based cognitive training increased working memory, which involves remembering, understanding, and manipulating information, by 10.34% in a group of 11 elderly individuals with MCI (Herrera et al., 2012). Tang and colleagues (2019) reported that a computerized, multidomain, adaptive training program increased MoCA scores by 15.34% in a group of 30 elderly individuals with MCI (Tang et al., 2019). A possible explanation for these findings is that CCT may have an impact on neural circuits. By engaging individuals in the repetitive execution of targeted cognitive tasks, CCT enables the modification and fortification of neural circuits within the brain (Keshavan et al., 2014). This form of training has the potential to augment cognitive capabilities by promoting synaptic strengthening and establishing novel neural connections (Keshavan et al., 2014).
Music therapy-related effectiveness data showed greater positive changes in depression, anxiety, comprehension, calculation skills, and dual tasks domains in individuals with MCI than in controls. Furthermore, music therapy-related effectiveness data revealed more significant improvements in music therapy than in other interventions (tDCS, light, CCT, RAGT, and/or CBE) in the MCI group in the following domains: depression, anxiety, memory, comprehension, attention, and orientation. This result was consistent with previous data (Chu et al., 2014; Satoh et al., 2014). Previous music therapy evidence demonstrated that exposure to 40hz music resulted in an 11.2% improvement in the MMSE and a 13.4% improvement in attention among 104 older individuals with dementia (Chu et al., 2014). Satoh et al. (2014) reported that a physical exercise program combined with music had a positive effect on brain function, showing a 13.1% increase in voxel-based specific regional analysis systems for Alzheimer’s disease in the MRI scans of 80 older individuals (Satoh et al., 2014). A possible neurophysiological reason for these results is that the cognitive and emotional dimensions of auditory items are reflected in the sensory processing stages from early sensory regions to high-order prefrontal regions (Thielscher & Pessoa, 2007). Blanke (2012) insisted that stimulating the processing of sensory modalities such as auditory information can facilitate orbitofrontal (sensory integration) and insular cortex (sensory processing) activation, as well as an individual’s reactivity and adaptability to their surroundings (Blanke, 2012). Music therapy is strongly associated with cognitive and psychological function by mitigating physiological arousal, as indicated by decreased cortisol levels, release of neurotransmitters (oxytocin and endorphin), reduction in mean arterial pressure, and lowered heart rate (Salafas et al., 2020; Wakim et al., 2010).
In contrast to the light therapy-, RAGT-, and CBE-related effectiveness data, we did not find group differences in depression, anxiety, memory retention, comprehension, calculation, concentration, orientation, dual-task performance, awareness, or sleep disorders. Our findings deviate from those of previous studies, indicating a disparity in the results. Andre and colleagues (2001) reported that bright light therapy (3000 lux) improved cognitive function (18.1%), measured by MMSE, in 23 patients with Alzheimer’s disease or vascular dementia (Graf et al., 2001). Erickson et al. (2011) observed that elderly individuals with higher levels of aerobic walking fitness exhibited a larger volume of the anterior hippocampus (measured using fMRI) and displayed superior performance in spatial memory tasks (Erickson et al., 2011). Similarly, Brinke et al. (2015) demonstrated that aerobic walking exercises led to a significant increase (5.60%) in left hippocampal volume in patients diagnosed with MCI, which correlated with improved verbal memory abilities (Ten Brinke et al., 2015). The results in previous study, showed that mindfulness exercises, which focus on the breath, had increased functional connectivity (13.3%) by the fMRI, and emotional distress (44.8%) by the questionnaires, respectively, in 18 patients with cognitive impairment compared to the controls who received no intervention (Van der Gucht et al., 2020). Wong et al. (2017) reported that participation in mindfulness meditation (body scan and breathing) resulted in improved cognitive function (7.6%), depression (26.5%), and anxiety (17.1%), as measured by the MoCA and depression anxiety stress scales, respectively, among 13 older adults with MCI (Wong et al., 2017). A possible reason for this difference may be related with immediate versus long-term treatment duration.
Several research limitations should be considered in the future. One limitation of this study is that we could not determine the long-term effects of MCT. Therefore, it is necessary to examine the long-term effects of these interventions in future studies. Another limitation was the lack of homogeneity (age, weight, and MMSE scores) between the groups. Please note that we compared normal controls because no previously established normative data is available to compare. Five minutes washout periods may not be sufficient to eliminate the potential summation effect. The longer wash-out period is warranted for the future study.
5Conclusions
Our study demonstrated that tDCS, CCT, and music therapy were immediately more effective than light therapy, RAGT, and CBE in improving depression, anxiety, memory retention, comprehension, calculation, attention, orientation, dual-task performance, awareness, and sleep disorders in older adults with MCI. These results provide clinical insights when designing effective interventions to maximize the potential recovery or prevention of progressive declines in cognitive, psychological, dual-task performance, and sleep functions in MCI.
Acknowledgments
This study was supported by the Brain Korea 21 PLUS Project (Grant no. 2021-51-0151); National Research Foundation of Korea (NRF) grants (Grant nos. RS-2023-00221762 and 2021R1A2C101342313); and the Korea Institute for Robot Industry Advancement (KIRIA) (Grant no. 2023-05008).
Conflict of interest
There are no conflicts of interest to report.
References
1 | Anderson, N. D. ((2019) ). State of the science on mild cognitive impairment (MCI). CNS spectrums, 24: (1), 78–87. |
2 | Bastin, C. , Giacomelli, F. , Miévis, F. , Lemaire, C. , Guillaume, B. , Salmon, E. ((2021) ). Anosognosia in mildcognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Frontiers in Psychiatry, 12: , 631518. |
3 | Blanke, O. ((2012) ). Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews Neuroscience, 13: (8), 556–571. |
4 | Calabrò, R. S. , De Luca, R. , Leo, A. , Balletta, T. , Marra, A. , Bramanti, P. ((2015) ). Lokomat training in vascular dementia: motor improvement and beyond!. Aging clinical and experimental research, 27: , 935–937. |
5 | Chen, J. , Wang, Z. , Chen, Q. , Fu, Y. , Zheng, K. ((2022) ). Transcranial Direct Current Stimulation Enhances Cognitive Function in Patients with Mild Cognitive Impairment and Early/Mid Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Sciences, 12: (5), 562. |
6 | Chu, H. , Yang, C.-Y. , Lin, Y. , Ou, K.-L. , Lee, T.-Y. , O’Brien, A. P. , Chou, K.-R. ((2014) ). The impact of group music therapy on depression and cognition in elderly persons with dementia: a randomized controlled study. Biological research for Nursing, 16: (2), 209–217. |
7 | Cruz Gonzalez, P. , Fong, K. N. , Chung, R. C. , Ting, K.-H. , Law, L. L. , Brown, T. ((2018) ). Can transcranial direct-current stimulation alone or combined with cognitive training be used as a clinical intervention to improve cognitive functioning in persons with mild cognitive impairment and dementia? A systematic review and meta-analysis. Frontiers in Human Neuroscience, 12: , 416. |
8 | da Silva, E. R. , Rodrigues Menezes, I. R. , Brys, I. ((2022) ). Effects of Transcranial Direct Current Stimulation on Memory of Elderly People with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review. Journal of Central Nervous System Disease, 14: , 11795735221106887. |
9 | Doi, T. , Blumen, H. M. , Verghese, J. , Shimada, H. , Makizako, H. , Tsutsumimoto, K. , Hotta, R. , Nakakubo, S. , Suzuki, T. ((2017) ). Gray matter volume and dual-task gait performance in mild cognitive impairment. Brain Imaging and Behavior, 11: (3), 887–898. |
10 | Erickson, K. I. , Voss, M. W. , Prakash, R. S. , Basak, C. , Szabo, A. , Chaddock, L. , Kim, J. S. , Heo, S. , Alves, H. , White, S. M. ((2011) ). Exercise training increases size of hippocampus and improves memory. Proceedings of the national academy of sciences, 108: (7), 3017–3022. |
11 | Foley, J. A. , Kaschel, R. , Logie, R. H. , Della Sala, S. ((2011) ). Dual-task performance in Alzheimer’s disease, mild cognitive impairment, and normal ageing. Archives of Clinical Neuropsychology, 26: (4), 340–348. |
12 | Gauthier, S. , Reisberg, B. , Zaudig, M. , Petersen, R. C. , Ritchie, K. , Broich, K. , Belleville, S. , Brodaty, H. , Bennett, D. , Chertkow, H. ((2006) ). Mild cognitive impairment. The lancet, 367: (9518), 1262–1270. |
13 | Graf, A. , Wallner, C. , Schubert, V. , Willeit, M. , Wlk, W. , Fischer, P. , Kasper, S. , Neumeister, A. ((2001) ). The effects of light therapy on mini-mental state examination scores in demented patients. Biological Psychiatry, 50: (9), 725–727. |
14 | Gu, L. , Zhang, Z. ((2019) ). Exploring structural and functional brain changes in mild cognitive impairment: a whole brain ALE meta-analysis for multimodal MRI. ACS chemical neuroscience, 10: (6), 2823–2829. |
15 | Han, E. , Park, J. , Kim, H. , Jo, G. , Do, H.-K. , Lee, B. I. ((2020) ). Cognitive intervention with musical stimuli using digital devices on mild cognitive impairment: A pilot study. Healthcare, 8: (1), 45. |
16 | Herrera, C. , Chambon, C. , Michel, B.-F. , Paban, V. , Alescio-Lautier, B. ((2012) ). Positive effects of computer-based cognitive training in adults with mild cognitive impairment. Neuropsychologia, 50: (8), 1871–1881. |
17 | Keshavan, M. S. , Vinogradov, S. , Rumsey, J. , Sherrill, J. , Wagner, A. ((2014) ). Cognitive training in mental disorders: update and future directions. American Journal of Psychiatry, 171: (5), 510–522. |
18 | Khanthong, P. , Sriyakul, K. , Dechakhamphu, A. , Krajarng, A. , Kamalashiran, C. , Tungsukruthai, P. ((2021) ). Traditional Thai exercise (Ruesi Dadton) for improving motor and cognitive functions in mild cognitive impairment: a randomized controlled trial. Journal of Exercise Rehabilitation, 17: (5), 331. |
19 | Lee, G. J. , Bang, H. J. , Lee, K. M. , Kong, H. H. , Seo, H. S. , Oh, M. , Bang, M. ((2018) ). A comparison of the effects between 2 computerized cognitive training programs, Bettercog and COMCOG, on elderly patients with MCI and mild dementia: A single-blind randomized controlled study. Medicine, 97: (45). |
20 | Liew, T. M. ((2019) ). Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimer’s Research & Therapy, 11: (1), 1–8. |
21 | Ma, L. ((2020) ). Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Frontiers in aging neuroscience, 12: (9). |
22 | Nilsen, A. P. , Donelson, K. L. (2001), Literature for today’s young adults. New York: Longman. |
23 | Park, J. , Oh, Y. , Chung, K. , Kim, K. J. , Kim, C. O. , Park, J. Y. ((2019) ). Effect of home-based transcranial direct current stimulation (tDCS) on cognitive function in patients with mild cognitive impairment: a study protocol for a randomized, double-blind, cross-over study. Trials, 20: (1), 1–9. |
24 | Petersen, R. C. ((2016) ). Mild cognitive impairment. CONTINUUM: Lifelong Learning in Neurology, 22: (2 Dementia), 404. |
25 | Randhi, B. , Gutlapalli, S. D. , Pu, J. , Zaidi, M. F. , Patel, M. , Atluri, L. M. , Gonzalez, N. A. , Sakhamuri, N. , Athiyaman, S. , Hamid, P. ((2023) ). Sleep Disorders in Mild Cognitive Impairment. Cureus, 15: (3). |
26 | Riemersma-Van Der Lek, R. F. , Swaab, D. F. , Twisk, J. , Hol, E. M. , Hoogendijk, W. J. , Van Someren, E. J. ((2008) ). Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. Jama, 299: (22), 2642–2655. |
27 | Salafas, E. , Lestari, P. , Listiyaningsih, M. D. ((2020) ). The effectiveness of music therapy in reducing anxiety in third trimester of pregnancy. Anxiety, 2: (6.66), 9.53. |
28 | Sanford, A. M. ((2017) ). Mild cognitive impairment. Clinics in geriatric medicine, 33: (3), 325–337. |
29 | Satoh, M. , Ogawa, J.-i. , Tokita, T. , Nakaguchi, N. , Nakao, K. , Kida, H. , Tomimoto, H. ((2014) ). The effects of physical exercise with music on cognitive function of elderly people: Mihama-Kiho project. PloS one, 9: (4), e95230. |
30 | Tang, Y. , Xing, Y. , Zhu, Z. , He, Y. , Li, F. , Yang, J. , Liu, Q. , Li, F. , Teipel, S. J. , Zhao, G. ((2019) ). The effects of 7-week cognitive training in patients with vascular cognitive impairment, no dementia (the Cog-VACCINE study): A randomized controlled trial. Alzheimer’s & Dementia, 15: (5), 605–614. |
31 | Ten Brinke, L. F. , Bolandzadeh, N. , Nagamatsu, L. S. , Hsu, C. L. , Davis, J. C. , Miran-Khan, K. , Liu-Ambrose, T. ((2015) ). Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. British journal of sports medicine, 49: (4), 248–254. |
32 | Thielscher, A. , Pessoa, L. ((2007) ). Neural correlates of perceptual choice and decision making during fear–disgust discrimination. Journal of Neuroscience, 27: (11), 2908–2917. |
33 | Van der Gucht, K. , Ahmadoun, S. , Melis, M. , de Cloe, E. , Sleurs, C. , Radwan, A. , Blommaert, J. , Takano, K. , Vandenbulcke, M. , Wildiers, H. ((2020) ). Effects of a mindfulness-based intervention on cancer-related cognitive impairment: results of a randomized controlled functional magnetic resonance imaging pilot study. Cancer, 126: (18), 4246–4255. |
34 | Wakim, J. H. , Smith, S. , Guinn, C. ((2010) ). The efficacy of music therapy. Journal of perianesthesia nursing, 25: (4), 226–232. |
35 | Wong, W. P. , Coles, J. , Chambers, R. , Wu, D. B.-C. , Hassed, C. ((2017) ). The effects of mindfulness on older adults with mild cognitive impairment. Journal of Alzheimer’s disease reports, 1: (1), 181–193. |
36 | Zhang, H. , Huntley, J. , Bhome, R. , Holmes, B. , Cahill, J. , Gould, R. L. , Wang, H. , Yu, X. , Howard, R. ((2019) ). Effect of computerised cognitive training on cognitive outcomes in mild cognitive impairment: a systematic review and meta-analysis. BMJ open, 9: (8), e027062. |




