Effect of dancing on freezing of gait in patients with Parkinson’s disease: A systematic review and meta-analysis
Abstract
BACKGROUND:
Freezing of gait (FOG) is one of the major debilitating motor symptoms that affect Parkinson’s disease (PD) patients’ gait,
OBJECTIVE:
To investigate the effect of dancing on FOG, motor symptoms, and balance in patients with Parkinsonism.
METHODS:
Eight databases were searched for full-text English randomized control trials (RCTs). The freezing of gait (FOG) was the primary outcome while the balance and Unified Parkinson Disease Rating Scale (UPDRS-3) were the secondary outcomes. Methodological quality was evaluated by the Physiotherapy Evidence Database (PEDro) scale. Level of evidence was assessed by Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. A random-effect model of meta-analysis was used to calculate the standardized mean difference (SMD) at a 95% confidence interval (CI), and the effect size.
RESULTS:
A total of nine studies (263 patients) were included. Qualitative data related to participants, dancing type, measured outcomes, and follow-up were extracted. PEDro scale showed one fair-quality and eight high-quality studies. GRADE showed a low to very low level of evidence with moderate effect size on both UPDRS (SMD –70 [–1.04, –0.36]) and Balance (SMD 0.35 [0.08, 0.63]).
CONCLUSION:
Dance is an effective modality on improving UPDRS and balance with small effect on FOG. Further high-quality studies with high-quality of evidence are recommended to increase the confidence to the effect estimate and support the finding results.
1Introduction
Parkinson’s disease (PD) is anticipated to become a common neurological disorder by 2030. In 2020, an estimated global PD population was about 8.7 to 9.3 million (Krishnamurthi et al., 2020). The symptoms of PD are caused by the loss of dopamine-producing cells in the substantia nigra, and the etiology is unknown (Opara et al., 2017). PD is characterized by motor and non-motor symptoms (Hayes, 2019), sleep disturbance, and cognitive and neuropsychiatric problems, which are examples of non-motor symptoms (Dos Santos Delabary et al., 2018). The motor symptoms include resting tremors, bradykinesia, postural instability, and rigidity (Hayes, 2019). Freezing of gait (FOG) is one of the major debilitating motor symptoms that affect PD patients’ gait, and it leads to falls and injuries from falls, as well as disability, depression, low quality of life, and a loss of functional independence (Sawada et al., 2019). FOG episodes affect about one out of every four PD patients in the early stages of the disease, and their frequency rises to 90% in the late stages (Cosentino et al., 2020).
FOG is defined as a brief, episodic absence or marked reduction of forwarding progression of the feet despite the intention to walk (Gao et al., 2020). When patients try to go forward, they feel as if their feet were stuck to the ground. FOG typically lasts a few seconds, although it can last up to 30 s on rare occasions. The patient may be unable to create effective steps for many minutes or even longer in exceptional cases (Lord et al., 2020). Human movement relies heavily on walking; PD patients frequently freeze when they begin walking, turning, dual tasking, and in changing environments and circumstances, such as passing a doorway of a small area, changing weather, or in unexpected settings. FOG can be triggered by stress or anxiety, especially when one needs to move quickly (Son et al., 2018). Males are more likely to experience FOG than females (Macht et al., 2007).
Moreover, it is widely acknowledged that the duration length of PD is a significant risk factor for FOG, as FOG is more common in the latter stages of PD. Postural control and balance were considerably reduced in PD with FOG compared to the non-FOG PD group. The non-motor symptoms-risk factors include anxiety, depression, cognitive impairments, and sleep disturbance (Gao et al., 2020; Lord et al., 2020).
Knowledge about FOG mechanisms are inadequate, and the consideration of treating FOG is a difficult task. Several hypotheses have been developed to explain the freezing phenomena (Gao et al., 2020). The pathogenic processes are summarized in the threshold model (Plotnik et al., 2012), interference model (Lewis & Barker, 2009), and cognitive model (Vandenbossche et al., 2012).
FOG is hard to assess since the symptoms vary from patient to patient. FOG is impacted by a variety of variables, including disease severity and motor state (“On”/ “Off”), visual input, narrow alleyways, sensitivity to varied tricks, and relationship to gait patterns such as gait commencement or turns, as well as cognitive aspects such as attention, anxiety, and stress (Giladi et al., 2000). The freezing of gait questionnaire (FOG-Q) and the new Freezing of gait questionnaire (nFOGQ) are now the only recognized and reliable clinical assessments; both give a subjectively general assessment of FOG severity and its impact on patient’s quality of life in individuals with PD (Cosentino et al., 2020).
For treating FOG, there are many effective strategies like visual and auditory cueing, treadmill training, and aquatic therapy (Rutz & Benninger, 2020). In the last decade, dance has emerged as an alternative therapy for improving gait, balance, and mobility, lowering illness severity, and enhancing the overall quality of life. Dancing therapy includes different movements of the extremities, and movement of the entire body is required in most dance patterns. Stages of dance include the initiation, coordination, and cessation of movement, and the movement direction, rate, and sequencing vary. Unlike standard PD rehabilitation programs, dancing therapies can include music and a dance partner, which might contribute to additional patient advantages, such as multisensory movement signals (Kalyani et al., 2020).
A previous review reported that regular aerobic exercise like dancing has a neuroprotective impact in postponing the negative symptoms of PD, particularly in terms of balance, gait, FOG, functional mobility, and patient quality of life (Dos Santos Delabary et al., 2018). Many previous studies investigated the effect of dancing therapy on the cerebral cortex and concluded that dance might engage brain regions that are generally underactive in people with PD. When the dancing steps are done, blood flows to the cerebellum rises. While participants learned complex dance sequences, primary motor regions and motor-planning regions, including pre-motor and supplementary motor areas, were activated, suggesting that dance affects motor and pre-motor networks in the brain. It has been hypothesized that dance may change the underlying neural mechanisms in PD by improving functional connectivity in motor networks, resulting in improved motor performance, including gait parameters and balance (Brown et al., 2006; Calvo-Merino et al., 2005).
Dancing practice in conjunction appears to boost the reward system after dopamine is released via the ventral tegmental region; this means that PD patients’ anxiety, depression, mood, and cognition, in general, will be improved(Dos Santos Delabary et al., 2018); which are considered non-motor risk factors (Gao et al., 2020). Consequently, FOG will be improved as a result. Dancing has also been shown to enhance postural balance during motor gait adjustments, dynamic balance, walking speed, and pitch length in people with PD, the effects of dancing therapy lasting up to a month following the intervention. As a result, it will be utilized to enhance FOG (Pereira et al., 2019). Most dance forms include step patterns comparable to those employed in recognized PD rehabilitation programs to reduce gait freezing. A dancing partner’s shifting weight, hand positioning, or bodily contact with a person with PD, for example, might give a visual and tactile cue for the next step or signify the need for a change of balance. Dance intervention with music provides an auditory cue for movement 17. So, the purpose of the current study was to investigate the effect of dancing therapy modality on alleviating freezing of gait in Parkinsonian patients.
2Methodology
This systematic review was written according to the guidelines of preferred reporting items for systematic reviews and meta-analysis (PRISMA). The study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42022315302.
2.1Eligibility criteria
Only randomized control trials (RCTs) and pilot RCTs were included, as well as original English studies with FOG in PD as the primary or secondary outcome measured by the FOGQ or nFOGQ and in which patients were exposed to any type of dance therapy. The participants were adults with PD. The exclusion criteria were no peer review, conference abstracts, literature review with or without meta-analysis, FOG assessed only at the baseline, freezing during movements other than gait (for upper limb or foot-tapping), any exposure to any surgical, trans magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) interventions, and those in which the participants had Parkinson’s plus another syndrome.
2.2Search strategy and study selection
The search was conducted using the following databases: PubMed, MEDLINE, Cochrane Library, Web of Science, Wiley online library, and EBSCO. The search was conducted from January to April 2022, with the following keywords: [(Parkinson’s or Parkinson’s Disease or Parkinsonism) AND (freezing of gait or freezing or freeze) AND (gait or walking or balance) AND (dancing therapy or dance or dancing or ballet or tango or waltz or ballroom or Irish or Zumba)]. Only the English language filter was added, after which the previous systematic review reference was scanned for articles that may meet the eligibility criteria.
The first reviewer began the research selection process by compiling a list of article titles and abstracts that potentially meet the inclusion criteria. The EndNote tool then removed the duplicates, followed by the irrelevant studies. The second and third reviewers scanned the full paper for eligibility and collaborated to resolve disagreements.
2.3Data extraction
The following information was extracted by the reviewer: trial author, year, and place; the studies and type of dancing; the method (number of participants in each group and gender, dose, and duration) and outcome measures, with the primary outcome being gait freezing (FOGQ, nFOGQ) and secondary outcomes including balance [tested by Time up and go (TUG), Burg balance scale (BBS), and Mini Balance Evaluation Systems Test (Mini-BESTes)].
2.4Data analysis
The descriptive synthesis was provided in tables, and the quantitative analysis was done using REVMAN 5.4.1 software to do a meta-analysis, which was then shown in the forest plot. Sub-group analysis was performed to examine the different types of dance effectiveness and compared to the other intervention or no intervention of the control group for the main motor symptoms Unified Parkinson Disease Rating Scale 3 (UPDRS-3), dual-task (TUG- dual task, dual walking). The study outcome and conclusions were gathered. All gathered data were organized in a table with the following columns: author, year, place, participants, dance type, outcome measures, results, and conclusion.
2.5Evaluation of methodological quality
The PEDro scale for clinical trials was used to assess the methodological quality; it contained 11 Yes-No questions (1, Eligibility criteria; 2, Random allocation; 3, Concealed allocation; 4, Baseline comparability; 5, Blind subjects; 6, Blind therapists; 7, Blind assessors; 8, Adequate follow-up; 9, Intention-to-treat analysis; 10, Between-group comparisons; 11, Point estimates and variability). The questions were answered with Yes and No responses. The included studies were graded as being of poor quality (grade 3), good quality (4-5), or high quality (grade > 6). Two independent reviewers assessed the methodological quality for any discrepancy between the reviewers. Any discrepancy was resolved through discussion third reviewer.
2.6Quality of evidence
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method was used to assess the evidence quality. The quality of evidence was evaluated by five main categories: study limitation (risk of bias), inconsistency, indirectness, imprecision, and reporting or publishing bias. The GRADE quality of evidence was graded using the GRADE profiler 3.6 software. The evidence quality was categorized into four categories: very low, low, moderate, or high.
2.7Data analysis
The random-effects model of the meta-analysis was performed using Review Manager version 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The mean and the standard deviation (SD) were used in the meta-analysis to calculate the estimated effect size. Standardized mean difference (SMD) is presented as the effect size (ES), and the corresponding 95% CI was calculated. The estimated effect size was considered small (≤ 0.2), medium (≤ 0.5), or large (≥0.8). The I2 statistics were used to assess and quantify the potential for heterogeneity among the researchers. The heterogeneity was classified as low heterogeneity (I2 > 25%), medium heterogeneity (I2 = 26–60%), and high heterogeneity (I2 > 75%).
3Results
3.1Study selection
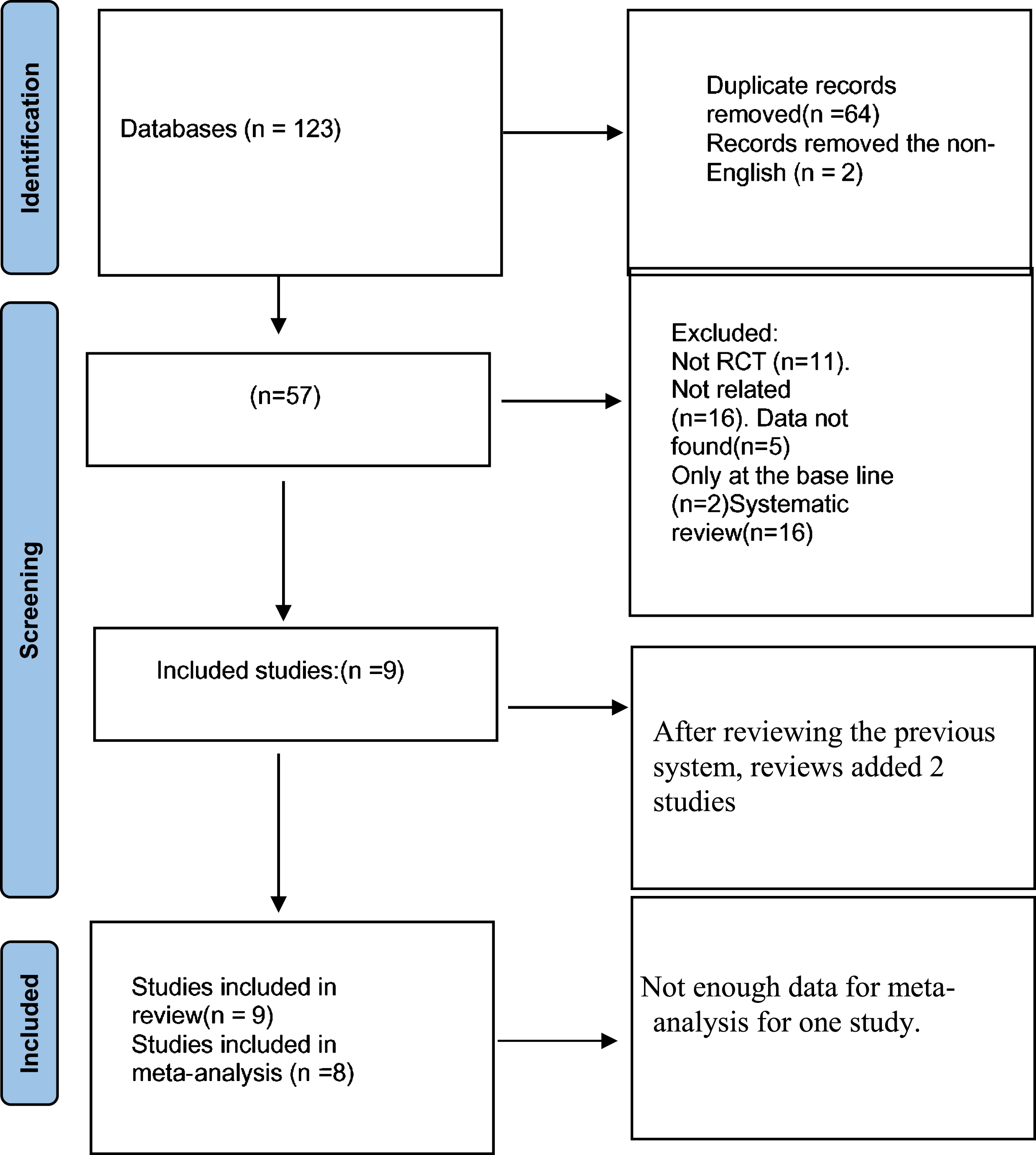
From a total of 123 studies, after removing the non-English and the duplicated articles, 57 articles remained. Then, further articles were excluded (the non-RCT, the not related articles, the article with missing data or the entire article, only assessing FOG at the start and systematic review), and the references from a previous systematic review (Alatawi, 2021; Blandy et al., 2015; Carapellotti et al., 2020; Dos Santos Delabary et al., 2018; Hasan et al., 2022; Kalyani et al., 2019; Lötzke et al., 2015; Mandelbaum & Lo, 2014; Mazzarin et al., 2017; Pereira et al., 2019; Stożek et al., 2016) were reviewed for articles that matched the eligibility criterion. Ultimately, nine articles were included in this study: seven RCTs and two pilot RCTs (Fig. 1).
Fig. 1
PRISMA flowchart of the study selection process.
3.2Subjects
A total of nine studies of dancing therapy with a total of 263 participants diagnosed with PD were included, each of the nine included a total number of patients ranging from 10 to 52, and all studies contained both: female and male participants. The frequency of dance therapy was one to two sessions per week, with the duration ranging from 3 weeks to 2 years. In addition, the follow-up time lasted up to 2 years.
3.3Intervention
The included studies used different types of dancing, most used tango dancing. Only one study used the dancing physiotherapy combined intervention method vs. regular physiotherapy exercise (Frisaldi et al., 2021). Another study used Irish dance vs. regular physiotherapy exercise (Volpe et al., 2013). Rocha et al. (Rocha et al., 2018) compared the two types of dancing (tango vs. mixed type) (Rocha et al., 2018), and Hackney et al. (Hackney & Earhart, 2009) compared the tango with Walt/foxtrot and with no intervention (Hackney & Earhart, 2009).
Two studies compared the tango with physiotherapy exercises (Hackney et al., 2007; Rios Romenets et al., 2015), and another two compared the tango with no intervention(Duncan & Earhart, 2012, 2014). One study (McKee et al. (McKee & Hackney, 2013)) compared the tango dance with an educational lecture on dancing (McKee & Hackney, 2013).
3.4Freezing of Gait
Two studies used the nFOGQ (Frisaldi et al., 2021; Volpe et al., 2013) as a measured outcome. Both studies showed a significant reduction of FOG in the dancing group compared to the exercise group. Seven studies used FOGQ for FOG assessment, while two studies (Duncan & Earhart, 2014; Rios Romenets et al., 2015) showed no significant effect of dancing compared to exercise. Duncan & Earhart (2012) reported a significant long-term effect of dance therapy after 1 year, and another study by the same group (Duncan & Earhart, 2014) showed a non-significant long-term effect of dancing therapy after 2 years on FOG. However, groups that received dance educational lectures without practicing showed non-significant results regarding FOG (McKee & Hackney, 2013).
Two studies compared the effect of two types of dance therapy (tango dance with mixed dance) (Rocha et al., 2018); the tango group showed a non-significant effect, while the mixed dance group showed a significant effect. Another study compared the tango with the waltz/foxtrot; the tango dance group showed a significant change in FOG and the waltz/foxtrot dance group showed non-significant changes (Hackney & Earhart, 2009). Other studies showed improvement with non-significant values (Duncan & Earhart, 2014; Hackney et al., 2007; McKee & Hackney, 2013; Rios Romenets et al., 2015; Rocha et al., 2018).
3.5Motor symptoms
All nine studies measured the motor symptoms in Parkinson’s through motor symptoms Unified Parkinson Disease Rating Scale 3 (UPDRS-3). Three studies compared dancing therapy to exercise and showed significant improvement in motor symptoms (Frisaldi et al., 2021; Hackney et al., 2007; Volpe et al., 2013), and two studies measured the long-term effect of dancing therapy. Duncan et al. (Duncan & Earhart, 2012) stated significant improvement in motor symptoms persisted after 1 year of tango dance. While another study by the same group confirmed significant improvement in motor symptoms after 2 years of another type of dance therapy (Duncan & Earhart, 2014).
McKee & Hackney (2013) found a significant difference in motor symptoms between the dancing therapy group and the group that received instructional lectures without practicing. One study found a non-significant improvement in motor symptoms in the dancing group compared to the exercise group (Rios Romenets et al., 2015). Motor symptoms improved significantly in the tango dance group compared to the mixed dance group (Rocha et al., 2018). Hackney & Earhart (2009) showed significant improvement in the motor symptoms in the waltz/foxtrot dancing group compared to the tango dancing therapy group. Most of the included studies indicated a significant change in favor of tango dance (Table 1).
Table 1
Characteristics of the studies
| N | Author, Year and location | Sample size and gender | Methodology | Dance type | Outcome measures | Follow-up duration | Results | Conclusion |
| 1. | Frisaldi et al., 2021 Italy | Sample size: 38 Males: | DG (19): 1 h CT and 1 h of dance. | Dance physiotherapy combined intervention called DArT method | Primary outcome: | 5 weeks | -(+SUPDRS-3) | DArT is safe, well accepted, and more effective than CT in improving motor impairment in mild PD |
| e -DG:10 | EG (19): 2 h of CT. | -UPDRS-III. | Secondary Results: | |||||
| -EG:13 | Frequency: 3/w for 5ws | -Secondary outcomes: | -(+TUG). | |||||
| Females: | -TUG. | -(+TUG-DT). | ||||||
| -DG:9 | -Mini-BESTes. | -(+NFOG-Q) | ||||||
| -EG:6 | -NFOG-Q. | -(-Mini-BESTest) | ||||||
| -TUG-DT. | -(-FES-I) | |||||||
| - (FES-I). | ||||||||
| 2. | Rocha et al., 2018 Australia | Sample size:21 | Argentine tango group (TG)(n = 10) | Argentine tango | Secondary outcome: | 8 weeks | Secondary results: | The dance programs were feasible and got important positive effect on motor outcome |
| Males: | Mixed dance group (MG)(n = 11) Frequency: for all groups 1/H, 1/w,8Ws. | Mixed dance | -TUG modified | -(-TUG), (+TUG). | ||||
| TG:4 MG:4 | –40/min home program. | - BBS | -(+BBS), (-BBS). | |||||
| Females: | -FOGQ | -(-FOG) (+FOG). | ||||||
| TG:6 MG:7 | -UPDRS-3 | -(-UPDRS-3), (+UPDRS-3). | ||||||
| 3. | Romenets et al., 2015 Canada | Sample size: 33 | - DG (n = 18): | Tango | - Primary outcome: | 12 weeks | Primary results: | Argentine tango showed an improvement of parkinsonian symptoms |
| Males: | - 1h. | UPRDS-3 | (-UPRDS-3) but got better | |||||
| -DG:12 | - EG(n = 15): | -Secondary outcome: | Secondary results: (+Mini-BESTest) | |||||
| -EG:7 | -Exercises daily Frequency: duration of treatment for 12Ws | - Mini-BESTest | (-FOGQ) | |||||
| Females: | -TUG | (-falling frequency) (-TUG, TUG -DT) | ||||||
| -DG:6 | -TUG-DT | |||||||
| -EG:7 | -Fall questioner FOGQ | |||||||
| 4. | Ryan et al., 2014 USA | Sample size: 10 | twice-weekly, 1- hour CG (n = 5): no exercise. | Argentine tango | Outcome measures: | -After 12 months | Results: | After 2 year of dancing therapy there is an improvement of motor and non- motor symptoms in Parkinson’s patients |
| Males: | -UPDRS-3. | -After 24 months | -(+UPDRS-3) | |||||
| -DG:4 | -Mini-BESTest. | - (+Mini-BESTest) | ||||||
| -CG:4 | - TUG | -(+TUG-DT) | ||||||
| Females: | TUG-DT | - (-FOGQ) and (-TUG) | ||||||
| -DG:1 | FOGQ. | |||||||
| -CG:1 | ||||||||
| 5. | Kathleen et al., 2013 Georgia | Sample size: 33 | DG (n = 24): 20 session 90-min for 10-12 Ws. | Tango | The outcome: | -After one week | Results: | Modified tango may delay the symptoms of Parkinson’s disease |
| Males: | EDG (n = 9): 1h for patients, 1/2 h for caregivers | -UPDRS-III. | -After 10-12 weeks | -(+UPDRS-3) - (+FAB) | ||||
| -DG:12 | -FAB Scale. | -(-TUG and the TUG-DT) | ||||||
| -EDG:8 | -TUG. | -(-FOG) | ||||||
| Females: | TUG-DT. | |||||||
| -DG:12 | -FOGQ. | |||||||
| -EDG:1 | ||||||||
| 6. | Volpe et al., 2013 Italy | Sample size: 24 | DG(n = 12): | Irish | Outcome: | - After 6 months | Results: | Irish dances were safe and practicable, with high adherence over a long period |
| Males: | –90 min 1/w for 6/M.EG(n = 12): 1/ w + home program for 6/M, 1.5 h. | -UPDRS-3 | - After 3 weeks | (+UPDRS-3) | ||||
| -DG:7 | -BBS | - After 6 months | (+BBS) but not significantly different from control group (+TUG) | |||||
| -EG:6 | -TUG | (+nFOGQ) | ||||||
| Females: | -nFOGQ | |||||||
| -DG:5 | ||||||||
| -EG:6 | ||||||||
| 7. | Ryan et al., 2012 USA | Sample size: 52 | -DG(n = 26): 2/W, 1 h for 12/M | Tango | Primary outcome: | - After 3 months | Results: (+UPDRS-3) | Long-term tango dance practice may slow the course of PD |
| Males: | -CG(n = 26): nothing | - UPDRS-3 | - After 6 months | - (+MiniBESTest) (+FOG) | ||||
| -DG:15 | Secondary outcome: | - After 12 months | ||||||
| -CG:15 | - MiniBESTest FOGQ. | |||||||
| Females: | ||||||||
| -DG:11 | ||||||||
| -CG:11 | ||||||||
| 8. | Madeleine et al., 2009 USA | Sample size: 31 | Tango (TG) (n = 14) | Tango, waltz/foxtrot | Outcome measures: | - After 13 weeks | Results: | Dance therapy improved the motor symptoms and motor control |
| Males: | Waltz/foxtrot (WG) (n = 17) | - UPDRS-3 | -(+UPDRS-3) | |||||
| -TG:11 | CG (n = 17): no Intervention | - BBS. | -(+BBS) | |||||
| -WG:11 | Frequency: 1 h, 2/w, 20 sessions for 13/ws | -TUG. FOGQ | -(-TUG) | |||||
| -CG:13 | -(+FOGQ) Tango | |||||||
| Females: | -(-FOGQ) Waltz/foxtrot | |||||||
| -TG:3 | ||||||||
| -WG:6 | ||||||||
| -CG:5 | ||||||||
| 9. | Madeleineet al., 2007 USA | Sample size: 19 | - DG(n = 9). | Tango | Outcome: | After 13 weeks | Results: | Tango therapy increases functional mobility in people with Parkinson’s disease |
| Males: | - EG(n = 10). | UPDRS-3 | -(+UPDRS-3) | |||||
| -DG:6 | Frequency: 21 h for 13 /Ws. | BBS | -(+BBS). | |||||
| -EG:6 | FOGQ | - (-FOG) | ||||||
| Females: | TUG | -(-TUG) | ||||||
| -DG:3 | Dual-task Walking | (- dual-walking) |
DG: dancing group, EG: exercise group, CG: control group, DG: education group, UPDRS-3: Unified Parkinson Disease Rating Scale 3, TUG: Timed Up and Go, Mini- BESTest: Mini-Balance Evaluation Systems Test, NFOG-Q: new Freezing of Gait Questionnaire, FOGQ: Freezing of Gait Questionnaire, TUG-DT: dual-task Timed Up and Go, FES-I: Falls Efficacy Scale International, BBS: Berg Balance Scale, FAB: Fullerton advanced balance scale, (+): significant change,(-):non-significant change.
3.6Balance
Eight studies applied TUG to measure balance during gait; two showed significant improvement in balance in the dancing group compared to exercise groups (Frisaldi et al., 2021; Volpe et al., 2013). However, three studies (Hackney et al., 2007; McKee & Hackney, 2013; Rios Romenets et al., 2015) report a non-significant improvement in balance in dancing groups. One study examined the long-term effect of tango dancing on balance and confirmed significant non-effect after 2 years (Duncan & Earhart, 2014). Tango dance also showed a significant effect on balance than the mixed type of dancing regarding TUG test (Rocha et al., 2018). Another study compared tango dancing with waltz/foxtrot, in which both showed a non-significant effect on balance (Hackney & Earhart, 2009). Four studies applied the mini-BES test to assess balance (Duncan & Earhart, 2012, 2014; Frisaldi et al., 2021; Rios Romenets et al., 2015); three studies examined the effect of tango dance on balance and showed significant results (Duncan & Earhart, 2012, 2014; Rios Romenets et al., 2015), while one found non-significant results (Frisaldi et al., 2021).
Four studies measured balance through BBS (Hackney & Earhart, 2009; Hackney et al., 2007; Rocha et al., 2018; Volpe et al., 2013), two studies found significant results(Hackney & Earhart, 2009; Volpe et al., 2013) while another study compared tango dance with mixed type of dance and showed an improvement of balance at the tango group with non-significant value(Rocha et al., 2018). One study recorded a significant improvement of balance through BBS score when compared to tango and waltz groups (Hackney & Earhart, 2009), and the other study applied Fullerton Advanced Balance Scale (FAB) and found a significant result (McKee & Hackney, 2013) (Table 1).
3.7Dual task
Four studies measured the dual-task ability through dual-task time up and go (TUG-DT) (Duncan & Earhart, 2012; Frisaldi et al., 2021; McKee & Hackney, 2013; Rios Romenets et al., 2015). Two studies showed significant results of dual-task ability (Duncan & Earhart, 2012; Frisaldi et al., 2021) while the other two showed non-significant results (McKee & Hackney, 2013; Rios Romenets et al., 2015). One study measured the dual-task by walking dual-task and found no significant changes (Hackney et al., 2007).
3.8Evaluation of methodological quality
Table 2
PEDro scale for assessing the methodological quality
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Overall score |
| Frisaldi et al., 2021 | √ | √ | √ | √ | × | × | √ | √ | √ | √ | √ | 8 |
| Rocha et al., 2018 | √ | √ | √ | √ | × | × | × | × | √ | √ | √ | 6 |
| Romenets et al., 2015 | √ | √ | √ | √ | × | × | × | × | √ | √ | √ | 6 |
| Kathleen, et al., 2013 | √ | × | √ | √ | × | × | √ | × | √ | √ | √ | 6 |
| Volpe et al., 2013 | √ | √ | × | √ | × | × | √ | × | × | √ | √ | 5 |
| Ryan et al., 2012 | √ | √ | √ | √ | × | × | × | × | √ | √ | √ | 6 |
| Ryan et al., 2014 | √ | √ | × | √ | × | × | √ | √ | √ | √ | √ | 7 |
| Madeleine et al., 2009 | √ | √ | × | √ | × | × | √ | √ | √ | √ | √ | 7 |
| Madeleine et al., 2007 | √ | √ | × | √ | × | × | √ | √ | √ | √ | √ | 7 |
1: Eligibility criteria,2: Random allocation,3: Concealed allocation,4: Baseline comparability,5: Blind subjects, 6: Blind therapists, 7: Blind assessors,8: Adequate follow-up,9: Intention-to-treat analysis,10: Between-group comparisons,11: Point estimates and variability.
The level of methodological quality was assessed using the PEDro scale ranges from five to eight. The included studies in the meta-analysis ranged from fair to high methodological quality, and the PEDro quality reported in Table 2. The GRADE quality of evidence scores rating from “low” to “very low”, as shown in Table 3. The downgraded score in the GRADE evaluation was due to; [1] Studies having a high risk of bias, [2] severe inconsistency with variability in findings across studies, [3] the total population size being less than 400, [4] the 95% confidence interval contains no effect and total population size is less than 400, [5] the imprecision was downgraded two levels; and [6] asymmetry in the funnel plot (diagnosed by visual inspection).
Table 3
summary of the quality of evidence for the outcome
| Quality assessment | No. of patients | Effect of estimate (SMD) | Quality | |||||||
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Dance | Control | ||
| FOG (Better indicated by lower values) | ||||||||||
| 8 | Randomized trials | Serious | No serious inconsistency | No serious indirectness | Very serious | None | 144 | 130 | –0.12 [0.44, 0.19] | ⊕○○○ VERY LOW |
| UPDRS-3 (Better indicated by lower values) | ||||||||||
| 8 | Randomized trials | Serious | Serious | No serious indirectness | Serious | None | 144 | 130 | –70 [–1.04, –0.36] | ⊕○○○ VERY LOW |
| mini-BESTest (Better indicated by higher values) | ||||||||||
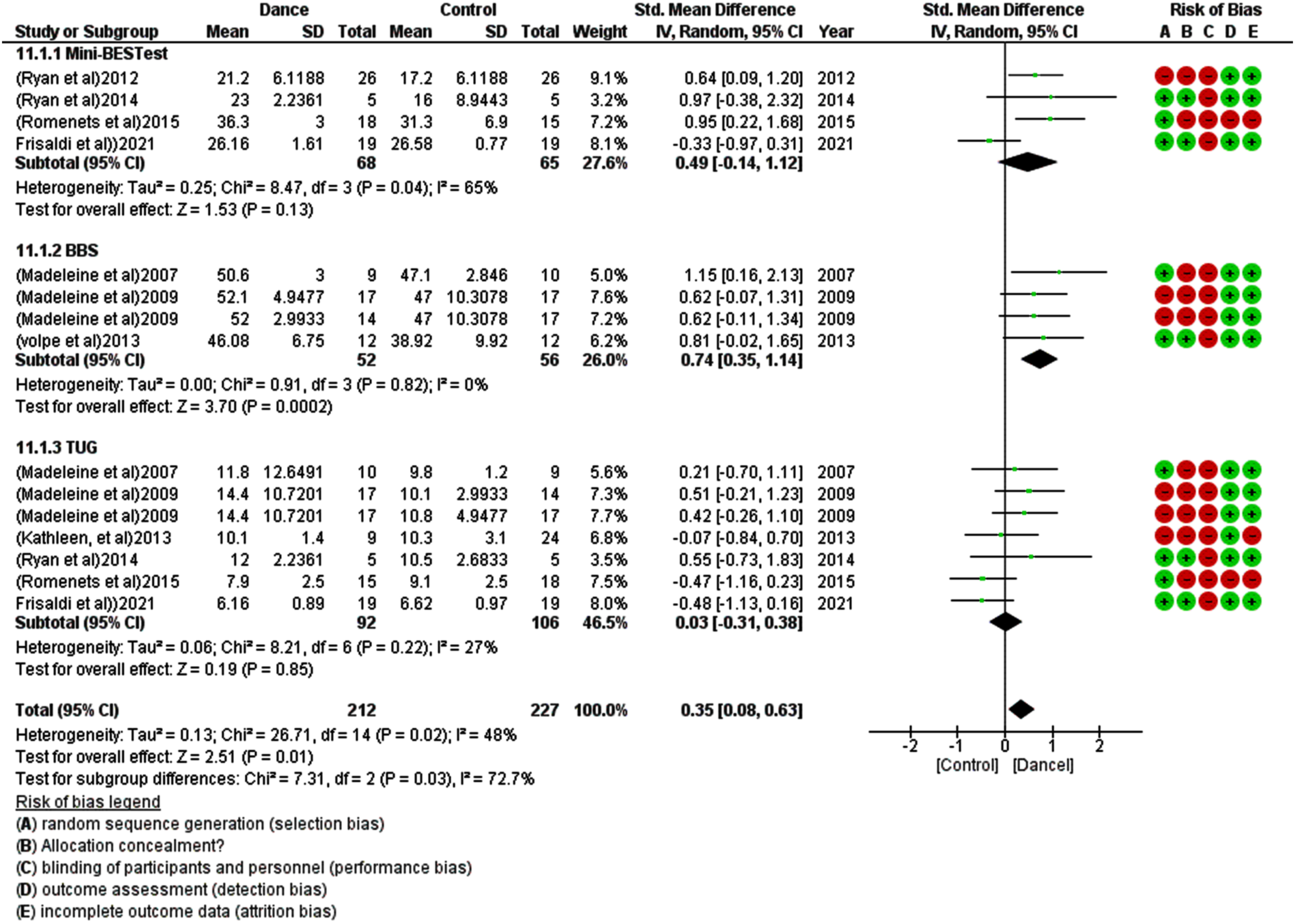
| 4 | Randomized trials | Serious | Serious | No serious indirectness | Serious | None | 68 | 65 | 0.49 [–0.14,1.12] | ⊕○○○ VERY LOW |
| BBS (Better indicated by higher values) | ||||||||||
| 3 | Randomized trials | Serious | No serious inconsistency | No serious indirectness | Serious | None | 52 | 56 | 0.74 [0.35,1.14] | ⊕⊕○○ LOW |
| TUG (Better indicated by lower values) | ||||||||||
| 6 | Randomized trials | Serious | No serious inconsistency | No serious indirectness | Very serious | Reporting bias | 106 | 92 | 0.03 [–0.31,0.38] | ⊕○○○ VERY LOW |
3.9Meta-analysis
For the meta-analysis, eight studies were included, and one was excluded due to insufficient data (i.e., mean and standard deviation for both control and treatment groups). Dancing therapy showed a non-significant effect in FOG with an overall small effect size in favor of the dance groups (SMD –0.12 [–0.44, 0.19]) and moderate heterogeneity I2 = 38% (Fig. 2). Eight studies show a significant effect of dance therapy on UPDRS-3 in patients with Parkinsonism with an overall moderate effect size compared to the other group (SMD –0.70 [–1.04, –0.36]) and Moderate heterogeneity I2 = 42% (Fig. 3). Dancing therapy showed a significant effect on balance with an overall moderate effect size in favor of the dance groups (SMD 0.35 [0.08, 0.63]) and moderate heterogeneity I2 = 48% (Fig. 4).
Fig. 2
Forest plot for the effect of dance therapy on FOG.
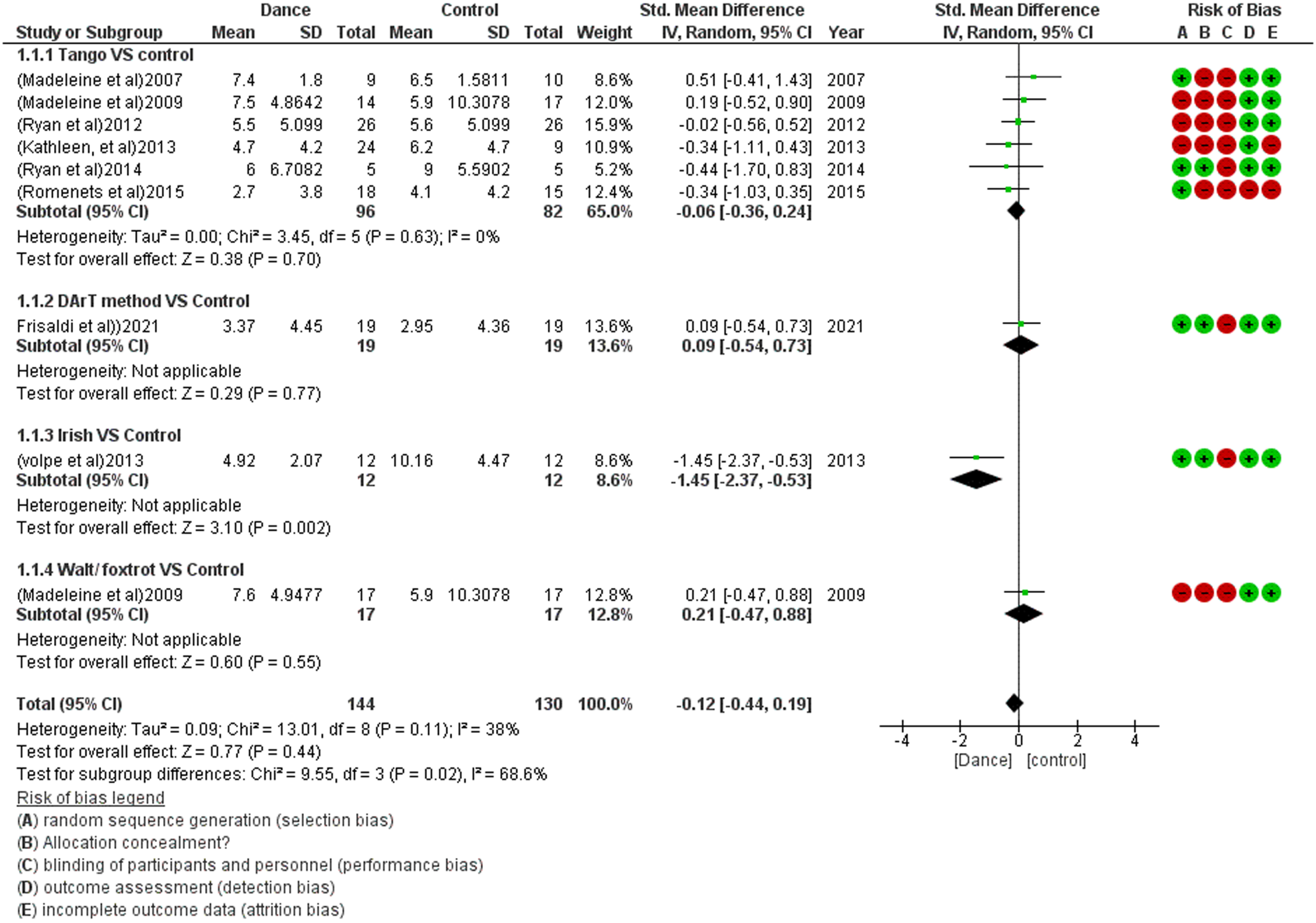
Fig. 3
Forest plot for the effect of dance therapy on UPDRS-3.
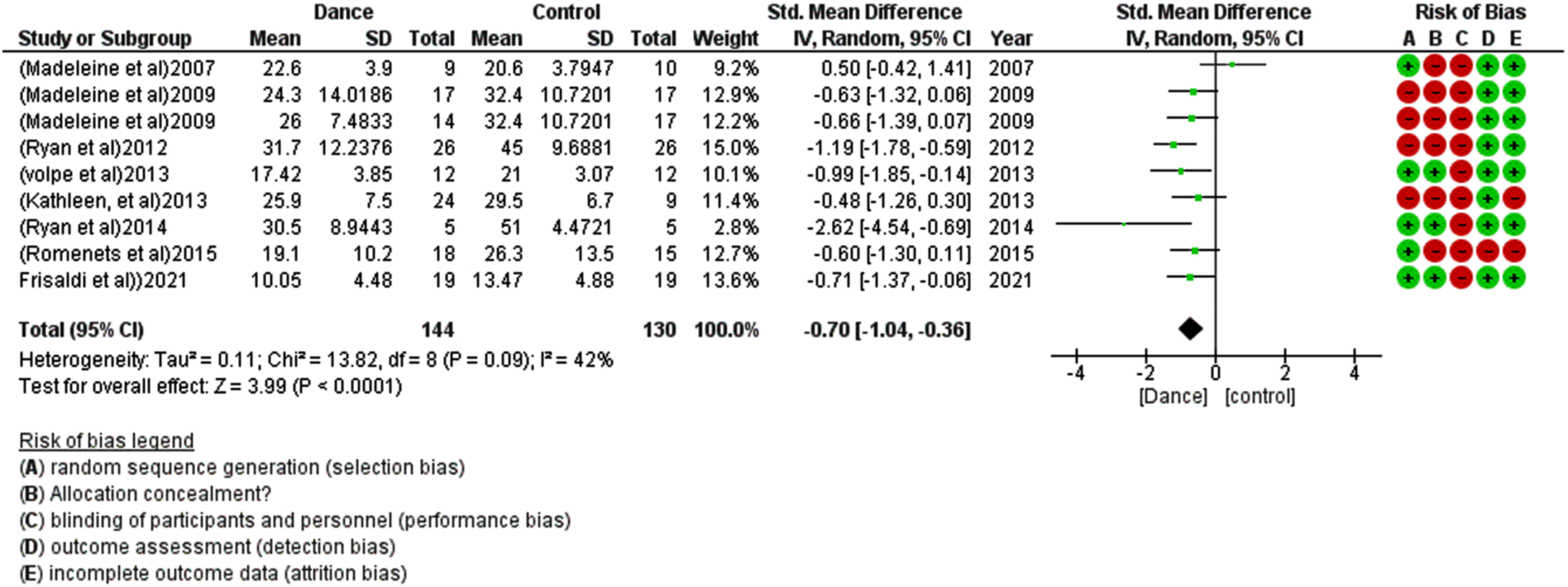
Fig. 4
Forest plot for the effect of dance therapy on balance.
4Discussion
This study aimed to investigate the effect of dancing as a therapeutic modality on alleviating the freezing of gait in Parkinsonian patients. A systematic review and meta-analysis were performed, and the current systematic review provides low to very low GRADE-based evidence and from high to fair methodological quality studies for the efficacy of dancing therapy to reduce the freezing of gait in PD patients. The included dancing types were Tango, Irish, Mixed type dance, dance physiotherapy combined intervention, and Walt /foxtrot.
The results of this study revealed no significant effect of dance therapy on FOG in PD patients. The contradictory results may be due to a different duration of dance therapy with less duration time of the dancing group than the control group, resulting in greater improvement in the control group (Frisaldi et al., 2021).
The subgroup analysis revealed a significant difference between the dancing types. This may not be the true result due to the large difference in trial numbers. Four out of five studies favored the dance group over the control group in tango dance studies. This could be due to the tactile, visual, and auditory cues that Parkinson’s patients receive from their partners and the music, as well as Parkinson’s ability to lead the dance, which provides patients with passion and excitement, increasing the sense of accomplishment and wellness and, as a result, a decrease in FOG (Lötzke et al., 2015).
Irish dance therapy had a substantial effect on FOG reduction (Rocha et al., 2018). This could be explained by the fact that dance therapy has significant improvements on mobility with music beats, balance, and gait training (Rocha et al., 2018). When the walt/foxtrot dances were compared to a control group, even though the control group received no therapy, the results favored the control group(Hackney & Earhart, 2009). This may be because the finding obtained by comparing the study’s post-treatment mean and standard deviation revealed that the control group had a lower mean and standard deviation than the dancing group at baseline. The control group, according to the study, deteriorated.
Another finding of this review was the overall significant results of UPDRS-3 for dancing therapy as an example of motor symptom improvement. Researchers found a substantial effect of tango dancing on FOG. This may be attributed to the fact that the patients during tango practice do not have to learn a complicated dance that is difficult to remember or follow physically but instead must learn to improvise spontaneous reactions, steps, and gestures in response to the music. In addition, unlike other types of dances, tango has a wide range of rhythmic diversity (Lötzke et al., 2015).
Several factors produce FOG; according to the model of the threshold theory, patients with FOG showed significant gait issues, such as reduced stride amplitude, worse gait coordination, and more step timing variability. This concept states that FOG occurs when these motor deficits accumulate to the point of motor breakdown (Plotnik et al., 2012). FOG is a severe symptom associated with greater disability, and patients with advanced PD are more likely to experience FOG, like those with postural instability and gait issues. So, FOG levels may be decreased as motor symptoms improve (Hasan et al., 2022).
Four models were explored the pathological mechanisms of FOG: a) The threshold model: This model assumes that; accumulation of gait disorders such as reduced stride amplitude, impaired gait coordination and increased variability of step timing to a point of motor breakdown, then FOG occurs (Plotnik et al., 2012). b) As dopamine neurons are mostly depleted in PD patients, concurrent processing of cognitive and/or limbic information during motor task will overload the basal ganglia capacity to the information processing, The interference between neural circuits would explain the phenomena that increasing cognitive load while performing a dual task will break down the locomotion (Plotnik et al., 2012). c) The cognitive model: patients with FOG fail to process response conflict, impose faster response decision but with greater incongruence, with triggering of FOG (Vandenbossche et al., 2012). d) The decoupling model: This model considered FOG as a disconnection between pre-planned motor program and motor response (Jacobs et al., 2009).
Despite some pharmacological treatments revealed promising results like Levo-dopa, Dopamine agonists, Monoamine oxidase B inhibitors; it is still difficult to treat FOG in PD, especially that levodopa resistant FOG. It is concluded that one drug was effective based on limited clinical trials. However, physiotherapy as Transcranial magnetic stimulation, visual, auditory, somatosensory cue, laser-shoe, showed immediate positive results. Moreover, action observation, treadmill combined with cueing, and prolonged homebased exercise trainings could be impact on FOG more than other approaches (Gao et al., 2020).
Regarding balance assessment measured in four studies (Duncan & Earhart, 2012, 2014; Frisaldi et al., 2021; Rios Romenets et al., 2015) using the Mini-Balance Evaluation Systems Test (Mini-BESTest), a meta-analysis showed a non-significant effect in favor of the dance group. Another three studies (Hackney & Earhart, 2009; Hackney et al., 2007; Rocha et al., 2018) applied the BBS and showed a significant result in favor of the dance therapy.
Six studies applied the TUG and showed non-significant results, but the overall results were in favor of the dance therapy, which could be attributed to the extra effect of visual stimulation of dancing on balance which agreed with another result of applying visual cues during the sit-to-walk action which was more successful than un-cued gait training in parkinsonian gait (Mahmoud et al., 2013), which might also justify the improvement in the TUG scale. Also, dance therapy is a physically and mentally demanding activity that involves multi-directional movement with varying rhythm and pace, as well as increasing body flexibility, muscular strength, and stretch. All these factors contributed to improved balance, gait, and functional mobility (Santos-García et al., 2020).
In the meta-analyses, the moderate heterogeneity in UPDRS-3 and balance might be due to confounding factors (the huge variation of treatment duration, different dance types, or the small sample size in the included studies). Although the GRADE system showed that the research was of low to very poor quality, dance therapy may reduce FOG in Parkinson’s patients and significantly impact UPDRS-3 and balance. As a result, further high-quality studies and research should be conducted, with a larger sample size, to distinguish between the various types of dance therapy. Furthermore, in the new trials, using defined methods will improve scientific accuracy.
4.1Limitations of the study
The use of only English-language RCTs was one of this systematic review’s limitations; there may be additional high-quality studies not published in English languages that were not included in the current study. The secondary measured variables were the motor Symptoms measured by and Balance which were measured by UPDRS-3, TUG-DT, walking dual-task, TUG, mini-BES test, BBS and FAB. Analysis of the changes among all the secondary measured variable may lead to some difficulties for the reader to monitor which symptom improved. Hence, it was considered a point of weakness.
5Conclusion
Dance therapy might effectively reduce FOG, but the included studies showed low to very low-quality evidence with little confidence in the effect estimate. Based on these findings, we recommend further high-quality trials to standardize treatment protocols and applied parameters.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work (Grant code: 22UQU4280478DSR03).
Funding
None to report.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Authorship contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by HM and ZAT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
1 | Alatawi, S. F. ((2021) ). A scoping review of the nature of physiotherapists’ role to avoid fall in people with Parkinsonism. Neurol Sci, 42: (9), 3733–3748. https://doi.org/10.1007/s10072-020-05015-y |
2 | Blandy, L. M. , Beevers, W. A. , Fitzmaurice, K. , Morris, M. E. ((2015) ). Therapeutic Argentine Tango Dancing for People with Mild Parkinson’s Disease: A Feasibility Study. Front Neurol, 6: , 122. https://doi.org/10.3389/fneur.2015.00122 |
3 | Brown, S. , Martinez, M. J. , Parsons, L. M. ((2006) ). The neural basis of human dance. Cereb Cortex, 16: (8), 1157–1167. https://doi.org/10.1093/cercor/bhj057 |
4 | Calvo-Merino, B. , Glaser, D. E. , Grèzes, J. , Passingham, R. E. , Haggard, P. ((2005) ). Action observation and acquired motor skills: an FMRI study with expert dancers. Cereb Cortex, 15: (8), 1243–1249. https://doi.org/10.1093/cercor/bhi007 |
5 | Carapellotti, A. M. , Stevenson, R. , Doumas, M. ((2020) ). The efficacy of dance for improving motor impairments, non-motor symptoms, and quality of life in Parkinson’s disease: A systematic review and meta-analysis. PLoS One, 15: (8), e0236820. https://doi.org/10.1371/journal.pone.0236820 |
6 | Cosentino, C. , Baccini, M. , Putzolu, M. , Ristori, D. , Avanzino, L. , Pelosin, E. ((2020) ). Effectiveness of Physiotherapy on Freezing of Gait in Parkinson’s Disease: A Systematic Review and Meta-Analyses. Mov Disord, 35: (4), 523–536. https://doi.org/10.1002/mds.27936 |
7 | Dos Santos Delabary M. , Komeroski, I. G. , Monteiro, E. P. , Costa, R. R. , Haas, A. N. ((2018) ). Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: a systematic review with meta-analysis. Aging Clin Exp Res, 30: (7), 727–735. https://doi.org/10.1007/s40520-017-0836-2 |
8 | Duncan, R. P. , Earhart, G. M. ((2012) ). Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair, 26: (2), 132–143. https://doi.org/10.1177/1545968311421614 |
9 | Duncan, R. P. , Earhart, G. M. ((2014) ). Are the effects of community-based dance on Parkinson disease severity, balance, and functional mobility reduced with time? A 2-year prospective pilot study. J Altern Complement Med, 20: (10), 757–763. https://doi.org/10.1089/acm.2012.0774 |
10 | Frisaldi, E. , Bottino, P. , Fabbri, M. , Trucco, M. , De Ceglia A. , Esposito, N. , Barbiani, D. , Camerone, E. M. , Costa, F. , Destefanis, C. , Milano, E. , Massazza, G. , Zibetti, M. , Lopiano, L. , Benedetti, F. ((2021) ). Effectiveness of a dance-physiotherapy combined intervention in Parkinson’s disease: a randomized controlled pilot trial. Neurol Sci, 42: (12), 5045–5053. https://doi.org/10.1007/s10072-021-05171-9 |
11 | Gao, C. , Liu, J. , Tan, Y. , Chen, S. ((2020) ). Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Transl Neurodegener, 9: , 12. https://doi.org/10.1186/s40035-020-00191-5 |
12 | Giladi, N. , Shabtai, H. , Simon, E. S. , Biran, S. , Tal, J. , Korczyn, A. D. ((2000) ). Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism & Related Disorders, 6: (3), 165–170. https://doi.org/10.1016/S1353-8020(99)00062-0 |
13 | Hackney, M. E. , Earhart, G. M. ((2009) ). Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med, 41: (6), 475–481. https://doi.org/10.2340/16501977-0362 |
14 | Hackney, M. E. , Kantorovich, S. , Levin, R. , Earhart, G. M. ((2007) ). Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther, 31: (4), 173–179. https://doi.org/10.1097/NPT.0b013e31815ce78b |
15 | Hasan, S. M. , Alshafie, S. , Hasabo, E. A. , Saleh, M. , Elnaiem, W. , Qasem, A. , Alzu’bi, Y. O. , Khaled, A. , Zaazouee, M. S. , Ragab, K. M. , Nourelden, A. Z. , Doheim, M. F. ((2022) ). Efficacy of dance for Parkinson’s disease: a pooled analysis of 372 patients. J Neurol, 269: (3), 1195–1208. https://doi.org/10.1007/s00415-021-10589-4 |
16 | Hayes, M. T. ((2019) ). Parkinson’s Disease and Parkinsonism. Am J Med, 132: (7), 802–807. https://doi.org/10.1016/j.amjmed.2019.03.001 |
17 | Jacobs, J. V. , Nutt, J. G. , Carlson-Kuhta, P. , Stephens, M. , Horak, F. B. ((2009) ). Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol, 215: (2), 334–341. https://doi.org/10.1016/j.expneurol.2008.10.019 |
18 | Kalyani, H. H. , Sullivan, K. A. , Moyle, G. M. , Brauer, S. G. , Jeffrey, E. R. , Kerr, G. K. ((2020) ). Dance improves symptoms, functional mobility and fine manual dexterity in people with Parkinson disease: a quasi-experimental controlled efficacy study. Eur J Phys Rehabil Med, 56: (5), 563–574. https://doi.org/10.23736/s1973-9087.20.06069-4 |
19 | Kalyani, H. H. N. , Sullivan, K. , Moyle, G. , Brauer, S. , Jeffrey, E. R. , Roeder, L. , Berndt, S. , Kerr, G. ((2019) ). Effects of Dance on Gait, Cognition, and Dual-Tasking in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J Parkinsons Dis, 9: (2), 335–349. https://doi.org/10.3233/jpd-181516 |
20 | Krishnamurthi, N. , Murphey, C. , Driver-Dunckley, E. ((2020) ). A comprehensive Movement and Motion training program improves mobility in Parkinson’s disease. Aging Clin Exp Res, 32: (4), 633–643. https://doi.org/10.1007/s40520-019-01236-0 |
21 | Lewis, S. J. , Barker, R. A. ((2009) ). A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord, 15: (5), 333–338. https://doi.org/10.1016/j.parkreldis.2008.08.006 |
22 | Lord, S. R. , Bindels, H. , Ketheeswaran, M. , Brodie, M. A. , Lawrence, A. D. , Close, J. C. T. , Whone, A. L. , Ben-Shlomo, Y. , Henderson, E. J. ((2020) ). Freezing of Gait in People with Parkinson’s Disease: Nature, Occurrence, and Risk Factors. J Parkinsons Dis, 10: (2), 631–640. https://doi.org/10.3233/jpd-191813 |
23 | Lötzke, D. , Ostermann, T. , Büssing, A. ((2015) ). Argentine tango in Parkinson disease–a systematic review and meta-analysis. BMC Neurol, 15: , 226. https://doi.org/10.1186/s12883-015-0484-0 |
24 | Macht, M. , Kaussner, Y. , Möller, J. C. , Stiasny-Kolster, K. , Eggert, K. M. , Krüger, H. P. , Ellgring, H. ((2007) ). Predictors of freezing in Parkinson’s disease: a survey of 6,620 patients. Mov Disord, 22: (7), 953–956. https://doi.org/10.1002/mds.21458 |
25 | Mahmoud, H. , Fayez, E. , Rahman, S. M. , Yamany, A. A. ((2013) ). Visual cues training on parkinsonian gait: A randomized controlled study. Egyptian Journal of Neurology, Psychiatry and Neurosurgery, 50: , 331–337. |
26 | Mandelbaum, R. , Lo, A. ((2014) ). Examining Dance as an Intervention in Parkinson’s Disease: A Systematic Review. American Journal of Dance Therapy, 36: , 160–175. https://doi.org/10.1007/s10465-014-9181-6 |
27 | Mazzarin, C. M. , Valderramas, S. R. , de Paula Ferreira, M. , Tiepolo, E. , Guérios, L. , Parisotto, D. , Israel, V. L. ((2017) ). Effects of Dance and of Tai Chi on Functional Mobility, Balance, and Agility in ParkinsonDisease. Topics in Geriatric Rehabilitation, 33: (4), 262–272. https://doi.org/10.1097/TGR.0000000000000163 |
28 | McKee, K. E. , Hackney, M. E. ((2013) ). The effects of adapted tango on spatial cognition and disease severity in Parkinson’s disease. J Mot Behav, 45: (6), 519–529. https://doi.org/10.1080/00222895.2013.834288 |
29 | Opara, J. , Małecki, A. , Małecka, E. , Socha, T. ((2017) ). Motor assessment in Parkinson’s disease. AnnAgric Environ Med, 24: (3), 411–415. https://doi.org/10.5604/12321966.1232774 |
30 | Pereira, A. P. S. , Marinho, V. , Gupta, D. , Magalhães, F. , Ayres, C. , Teixeira, S. ((2019) ). Music Therapy and Dance as Gait Rehabilitation in Patients With Parkinson Disease: A Review of Evidence. J Geriatr Psychiatry Neurol, 32: (1), 49–56. https://doi.org/10.1177/0891988718819858 |
31 | Plotnik, M. , Giladi, N. , Hausdorff, J. M. ((2012) ). Is freezing of gait in Parkinson’s disease a result of multiple gait impairments? Implications for treatment. Parkinsons Dis, 2012: , 459321. https://doi.org/10.1155/2012/459321 |
32 | Rios Romenets, S. , Anang, J. , Fereshtehnejad, S. M. , Pelletier, A. , Postuma, R. ((2015) ). Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med, 23: (2), 175–184. https://doi.org/10.1016/j.ctim.2015.01.015 |
33 | Rocha, P. , Aguiar, L. , McClelland, J. A. , Morris, M. E. ((2018) ). Dance therapy for Parkinson’s disease: A randomised feasibility trial. International Journal of Therapy and Rehabilitation, 25: (2), 64–72. https://doi.org/10.12968/ijtr.2018.25.2.64 |
34 | Rutz, D. G. , Benninger, D. H. ((2020) ). Physical Therapy for Freezing of Gait and Gait Impairments in Parkinson Disease: A Systematic Review. Pm r, 12: (11), 1140–1156. https://doi.org/10.1002/pmrj.12337 |
35 | Santos-García, D. , de Deus-Fonticoba, T. , Suárez Castro, E. , Á, M. A. D. , Feal-Painceiras, M. J. , Paz-González, J. M. , García-Sancho, C. , Jesús, S. , Mir, P. , Planellas, L. , García-Caldentey, J. , Caballol, N. , Legarda, I. , Hernández-Vara, J. , González-Aramburu, I. , Ávila-Rivera, M. A. , Catalán, M. J. , Nogueira, V. , Álvarez-Sauco, M. , Martinez-Martin, P. ((2020) ). The impact of freezing ofgait on functional dependency in Parkinson’s disease with regard to motor phenotype. Neurol Sci, 41: (10), 2883–2892. https://doi.org/10.1007/s10072-020-04404-7 |
36 | Sawada, M. , Wada-Isoe, K. , Hanajima, R. , Nakashima, K. ((2019) ). Clinical features of freezing of gait in Parkinson’s disease patients. Brain Behav, 9: (4), e01244. https://doi.org/10.1002/brb3.1244 |
37 | Son, M. , Cheon, S. M. , Youm, C. , Kim, Y. , Kim, J. W. ((2018) ). Impacts of freezing of gait on forward and backward gait in Parkinson’s disease. Gait Posture, 61: , 320–324. https://doi.org/10.1016/j.gaitpost.2018.01.034 |
38 | Stożek, J. , Pustułka-Piwnik, U. , Curyło, M. ((2016) ). Argentine tango in the rehabilitation ofpatients with Parkinson’s disease. Medical Rehabilitation, 20: , 33–38. https://doi.org/10.5604/01.3001.0009.2794 |
39 | Vandenbossche, J. , Deroost, N. , Soetens, E. , Coomans, D. , Spildooren, J. , Vercruysse, S. , Nieuwboer, A. , Kerckhofs, E. ((2012) ). Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Front Hum Neurosci, 6: , 356. https://doi.org/10.3389/fnhum.2012.00356 |
40 | Volpe, D. , Signorini, M. , Marchetto, A. , Lynch, T. , Morris, M. E. ((2013) ). A comparison of Irish set dancing and exercises for people with Parkinson’s disease: a phase II feasibility study. BMC Geriatr, 13: , 54. https://doi.org/10.1186/1471-2318-13-54 |




