Motor performance, motor impairments, and quality of life after eccentric resistance training in neurological populations: A systematic review and meta-analyses
Abstract
BACKGROUND:
Many overlapping factors impair motor performance and quality of life in neurological patients. Eccentric resistance training (ET) has potential benefits for improving motor performance and treating motor impairments better than some traditional rehabilitation approaches.
OBJECTIVE:
To estimate the effect of ET in neurological settings.
METHODS:
Seven databases were reviewed up to May 2022 according to PRSIMA guidelines to find randomized clinical trials involving adults with a neurological condition, who underwent ET as set by the American College of Sports Medicine. Motor performance (main outcome) was assessed as strength, power and capacities during activity. Secondary outcomes (impairments) were muscle structure, flexibility, muscle activity, tone, tremor, balance and fatigue. Tertiary outcomes were risk of fall, and self-reports of quality of life.
RESULTS:
Ten trials were included, assessed using Risk of Bias 2.0 tool, and used to compute meta-analyses. Effective effects in favour of ET were found for strength and power, but not for capacities during activity. Mixed results were found for secondary and tertiary outcomes.
CONCLUSION:
ET may be a promising intervention to better improve strength/power in neurological patients. More studies are needed to improve the quality of evidence underlying changes responsible for these results.
1Introduction
Degradation of motor performance is a major contributor to disability (WHO, 2011) and reduced quality of life in individuals with neurological conditions (Eng et al., 2009; Ramari et al., 2020; Skinner et al., 2015). Naturally, recovering motor performance is rated as a top aspiration by people after a stroke (Stroke Association, 2021), Spinal Cord Injury (SCI) (van Middendorp et al., 2016), Cerebral Palsy (CP) (Cook et al., 2022), Parkinson Disease (PD) (Bowring et al., 2022), or Multiple Sclerosis (MS) (Chiu et al., 2019).
Many overlapping factors impair motor performance in individuals with neurological conditions (Gracies, 2005a, 2005b; Graham et al., 2016; McLoughlin, 2018). Deficits in neural function and changes in motor unit properties (Klein et al., 2010; Thomas et al., 2014), involuntary muscle activity/spasms/tremor (McLoughlin, 2018), tissue remodelling and atrophy (Gorgey & Dudley, 2007; Handsfield et al., 2022; Hunnicutt & Gregory, 2017), decreases in joint mobility and muscle fascicle length due to contractures (Nuckolls et al., 2020) or rigidity (Wijemanne & Jankovic, 2019), and increased levels of fatigue (Kluger et al., 2013) worsen motor capacities over time.
Resistance training has turn become a key intervention of neuro-rehabilitation for a wide variety of conditions over time (Aravind et al., 2019; Hornby et al., 2020; National Insitute for Health and Care Excellence, 2022; Osborne et al., 2022). Despite improving strength, ‘conventional’ programs using ‘concentric’ muscle contractions might have limited effects on aforementioned motor impairments. Recently, Davis et al. (2020) highlighted the potential relevance to use ‘eccentric’ muscle contractions in neurological settings. Eccentric resistance training (ET) has a greater positive effect on neuromuscular function (e.g. muscle activation), muscle size, fascicle length and joint flexibility compared to other types of exercises in healthy individuals (Blazevich, 2019; Diong et al., 2022; Douglas et al., 2017a, 2017b). Therefore, ET has a very large spectrum of plausible benefits that could be relevant to many individuals living with consequences of a neurological condition. The practice of ET provides a unique opportunity to improve motor performance while addressing major motor impairments simultaneously. For instance, ET is assumed to produce greater gains in muscle strength and volume (hypertrophy) while it may also regulate the balance between agonist/antagonist muscle neuromechanical output (i.e. paresis and hyperactivities). Moreover, because of the greater mechanical loading involved in active lengthening, ET exercises could be relevant in treating the relative shortness in muscle fascicle length and joint contractures. ET requires less metabolic demands for the same amount of work than concentric training, making it of particular interest for patients with neurological disorders experiencing high levels of fatigue and/or cardiorespiratory issues. Evidence suggests that ET can be used safely in neurological settings (Folkerts et al., 2017; LaStayo et al., 2014). Therefore, ET holds promise for improving motor performance and simultaneously addressing major motor impairments, during neuro-rehabilitation settings. However, the scientific evidence regarding its benefits for patients is not clear, yet.
This review primary aimed to evaluate the effectiveness of ET on motor performance in neurological populations. This review also focused on the effectiveness of ET on motor impairments (secondary objective), and on patients’ health-related quality of life (tertiary objective). We also aimed to evaluate effect modifiers such as the type of disease (neurological injury, neuro-degenerative disease), the initial motor capacities, and intervention delivery (e.g. standalone/included/added to rehabilitation, compliance with the American College of Sports Medicine (ACSM) position stand for resistance training interventions (American College of Sports Medicine, 2009).
2Methodology
This systematic review was carried out according to the Cochrane Handbook (Higgins, 2022), and the Preferred Reporting Items for Systematic Reviews and Meta-analysis (Moher et al., 2009). This study, as a literature review, is exempt from Institutional Review Board approval. The protocol was pre-registered on PROSPERO database (n° CRD42021283908) for transparency.
2.1Literature search
A PICOS (Patient, Intervention, Comparison, Outcomes, Study type) framework was used to develop literature-searching strategies and keywords. Seven databases were searched to find published, ongoing or unpublished trials (grey literature) from their inception with no time restriction, initially started by PubMed, and adapted for use in the other databases (PEDro, Embase, Cochrane Library, ClinicalTrials.gov, Scopus, Web of Science). Reference lists of published systematic reviews and authors’ personal libraries were searched to confirm the retrieval of appropriate studies. An iterative process which involved team meetings was employed to define relevant search keywords that would capture relevant records. For that purpose, keywords, search strategies, and reference lists of included studies from relevant reviews from the field (resistance training in neurological populations, eccentric resistance training) were explored, so as authors’ libraries to ensure an optimal search strategy for each database. The keywords used for each criterion are listed in Table 1, and their association for each database (search strategy) is detailed in Supplementary File 1. Because all the objectives of this review might be answered by results from randomized clinical trials (RCT), we followed the recommendations of the Cochrane Handbook (Higgins, 2022) to restrict our research to RCT only. Trials were excluded if training corresponded to ‘functional or task oriented’ exercises (e.g. downhill walking), because these interventions tend to influence multiple parameters like motor control, skill, speed and endurance, which are not primary targeted by resistance training interventions.
Table 1
Criteria used for inclusion of the studies
| Category | Eligibility criteria |
| Population | Adults>18 years oldDiagnosis of a neurological injury or neuro-degenerative diseaseCorresponding keywords: “Demyelinating Autoimmune Diseases, CNS”[Mesh], “Central Nervous System Diseases”[Mesh], “Motor Neuron Disease”[Mesh], “Trauma, Nervous System”[Mesh] |
| Intervention | Resistance training including eccentric contractions, defined as a progressive exercising program by the American College of Sports Medicine (≥4 weeks, twice a week, against an external load)Corresponding keywords: Eccentric, “lengthening contraction”, “lengthening exercise” |
| Comparator | other forms of resistance programs (e.g. concentric), another form of rehabilitation (no progressive resistance program), or control (no rehabilitation)Corresponding keywords: rehab*, train*, exercise, physiother* |
| Outcomes | Motor performance (primary objective): maximal strength, maximal power, clinical exercises/tests (e.g. gait)Motor impairment (secondary objective): muscle structure, muscle activity, muscle tone, joint flexibility, fatigueHealth-related quality of life (tertiary objective): risk of fall, and self-reports of health condition/behaviourCorresponding keywords: (none, left open to ensure the greatest possibility of finding results on outcomes) |
| Design/study type | Randomized clinical trials |
| Other | Language: publications in English, French or Spanish Full/Published reports accessible for data extraction |
2.2Study selection
The selection was conducted on the open access online tool CADIMA (Kohl et al., 2018) in May 2022. Before initiating the selection, a random sample of 10 studies based on retrieved list of studies was screened by two reviewers (GLS and TL) to assess the consistency of criteria assessment, followed by a discussion between reviewers on the reasons for inclusion/exclusion, using the eligibility criteria list (Table 1). Then, the same reviewers independently removed duplicate, screened the titles and abstracts, and read the full-text of articles to determine their eligibility. At each stage of the process (titles/abstracts, full texts), every document was thoroughly examined to identify relevant information for inclusion/exclusion. In most cases, the eligibility criteria could be found in the methods section of the abstract or full-text, which provided details on the population, interventions/comparators, outcomes, and study methodology (RCT or not). At every stage of the process (duplicates, titles and abstract, full-text), a consistency check was done between both reviewers to reach a consensus. Discrepancies were resolved if required during a meeting with a third reviewer (TC) before proceeding to the next step.
2.3Data extraction and synthesis
A data collection form adapted from Cochrane Collaboration (2022) was used for collecting data from retrieved studies. Information about eligibility, method (i.e., participants, intervention/comparator, outcomes) and results were extracted by two reviewers independently (GLS and TL) and cross-checked to produce a merged collection form. If information was not available in the published papers, further precisions were requested from the corresponding author. Data extracted characterized the population, study protocol and outcomes for experimental and comparator groups. For a complete listing of collected parameters, please refer to Table 2. Because the effects of eccentric resistance training are of particular interest for a large variety of neurological conditions, and not specific to a particular situation, different populations were included and categorized as ‘neuro-injured’ (e.g. stroke) or ‘neuro-degenerative’ (e.g. multiple sclerosis). We used a customized form adapted from Hendrey et al. (2018) study to assess adherence to ACSM position stand for resistance training: frequency, intensity, type, repetitions, sets, progression, specificity and pattern, of the exercises used in the ET program (American College of Sports Medicine, 2009) (Supplementary File 5).
Table 2
Main characteristics of the included studies
| Study | Condition | Population (n of participants) | Intervention: Eccentric Training (ET) | Comparator (COMP) | Relevant outcome measures |
| Lattouf (2021) | Stroke | Setting: patients in an outpatient service (country: np)ET (19)Delay from injury: 11.6±4.1 monthsAge: 65.1±11.2 yInitial characteristics: 0.84±0.76 (MAS)Initial motor capacities: 0.46±0.69a (max-selected walking speed, 10mWT), coded ‘moderate’bCOMP (18)Delay from injury: 12.3±5.4 monthsAge: 68.7±12.4 yInitial characteristics: 0.72±0.83 (MAS)Initial motor capacities: 0.51±0.68a (max-selected walking speed, 10mWT), coded ‘moderate’b | Muscles: KE, KFFormat: added to usual rehabilitationContent: horizontal leg pressconcentric phase (both legs)+eccentric phase (paretic leg only); velocity: np.Dose:session: 2 sets x [5 reps x 40% 1RM]+1 set x [5 reps x 60% 1RM]frequency: 3/wkduration: 4 wksRange of movement for training: npVelocity of movement: npProgressivity: np | usual rehabilitation– Prevention of complications– Stimulation of motor skills– Management of spasticity– Transfers, balance displacement and functional acquisition– Therapeutic education | Participation: -Activity: gaitFunction: strength, spasticityStructure: - |
| Kadkhodaie (2020) | Parkinson’s Disease | Setting: patients in an outpatient service (Iran)ET (11)Delay from diagnostic: 61.2±38.4 monthsAge: 67.8±9.6 yInitial characteristics: 2.3±1.0 (H&Y)Initial motor capacities: -COMP (10)Delay from diagnostic: 67.2±70.8 monthsAge: 67.4±6.8 yInitial characteristics: 2.3±0.8 (H&Y)Initial motor capacities: - | Muscles: EF, WF, WEFormat: standaloneContent: weighted training balls fixed to participants hand or forearmEccentric only (concentric performed by therapist)Dose:session: 3 sets x 10 reps1 session (RPE = 10– 11)2 sessions (RPE = 12– 14)frequency: 3/wkduration: 6 wksRange of movement for training: npVelocity of movement: npProgressivity: heavier weights to keep a constant intensity level | no intervention (control) | Participation: -Activity: -Function: amplitude of hand tremorStructure: - |
| Dibble (2015) | Parkinson’s Disease | Setting: patients in an outpatient service (USA)ET (20)Delay from diagnostic: 96.0±53.8 monthsAge: 66.0±14.8 yInitial characteristics: 2.0 (2 to 4) (H&Y On)Initial motor capacities: 558.07±182.49 m (6MWT), coded ‘good’bCOMP (21)Delay from diagnostic: 68.4±50.8 monthsAge: 70.7±9.2 yInitial characteristics: 2.0 (1 to 4) (H&Y On)Initial motor capacities: 485.99±158.95 m (6MWT), coded ‘good’b | Muscles: HE, KE, AEFormat: included in a multi-component rehabilitation programContent: COMP, with eccentric training on ergometer targeting lower extremity extensorsDose:session: 15 minutesfrequency: 2/wkduration: 12 wksRange of movement for training: npProgressivity: variation in workload to maintain a RPE at 13 | multi-component rehabilitation program– warm-up exercises (bicycle or treadmill) – flexibility training (mobility exercises) – balance training (static/dynamic stability training) – upper extremity concentric resistance training (exercise machine) – concentric ergometer training (NuStep) | Participation: health statusActivity: gaitFunction: strengthStructure: quadriceps volume |
| Clark (2013) | Stroke | Setting: np (USA)ET (18)Delay from injury: 13.3±4.9 monthsAge: 63.2±10.6 yInitial characteristics: 77.3±9.4 (F-M)Initial motor capacities: 0.37±0.18 m/s (self-selected walking speed), coded ‘moderate’ bCOMP (17)Delay from injury: 12.8±4.7 monthsAge: 59.7±10.9 yInitial characteristics: 80.9±7.1 (F-M)Initial motor capacities: 0.39±0.25 m/s (self-selected walking speed), coded ‘moderate’b | Muscles: HE, HF, KE, KF, AE, AFFormat: standaloneContent: isokinetic trainingcDose:session: sets of 10 repetitionswk 1 : 9 sets (3x30°/s, 3x90°/s, 3x150°/s)wk 2 : 9 sets (3x 60°/s, 3x 120°/s, 3x180°/s)wk 3 : 12 sets (4x90°/s, 4x150°/s, 4x210°/s)wk 4 : 9 sets (3x60°/s, 3x150°/s, 3x210°/s)wk 5 : 9 sets (3x30°/s, 3x90°/s, 3x150°/s)frequency: 3/wkduration: 5 wksRange of movement for training: npVelocity of movement: see doseProgressivity: np | concentric resistance training (using the same dose as ET) | Participation: -Activity: gaitFunction: strength, power, muscle activityStructure: - |
| Hayes (2011) | Multiple Sclerosis | Setting: patients in an outpatient service (USA)ET (10)Delay from diagnostic: 149.9±113.8 monthsAge: 48.0±11.9 yInitial characteristics: 5.2±1.0 (EDSS)Initial motor capacities: 248±169 m (6MWT), coded ‘good’bCOMP (9)Delay from diagnostic: 142.2±87.6 monthsAge: 49.7±11.0 yInitial characteristics: 5.3±1.0 (EDSS)Initial motor capacities: 372±266 m (6MWT), coded ‘good’b | Muscles: HE, KE, AEFormat: added to usual rehabilitationContent: eccentric training on ergometer targeting lower extremity extensors (eccentron)Dose:session: 45 to 60 minuteswk 1 : 1– 5 min (RPE = 7)wk 2 : 1– 5 min (RPE = 9)wk 3 : 11– 14 min (RPE = 11)last 8 wks: 11– 14 min (RPE = 13)frequency: 3/wkduration: 12 wksRange of movement for training: from 10° to 90° of knee flexionVelocity of movement: pedal cadence: 15 to 20 rotations per minuteProgressivity: variation in workload to maintain a RPE from 11 to 13 | usual rehabilitation– aerobic training (NuStep, horizontal stepper)– lower extremity stretching (hamstring, quads, triceps surae)– upper extremity strength training (machines and free weights)– balance exercises (standing on a wobble board while maintaining balance for both a side-to-side and front-to-back perturbation) | Participation: -Activity: gait, risk of fallFunction: strength, balance, fatigueStructure: - |
| Dibble (2009) | Parkinson’s Disease | Setting: patients in an outpatient service (USA)ET (10)Delay from diagnostic: 73.2±46.8 monthsAge: 64.3±9.6 yInitial characteristics: 2.5±0.5 (H&Y)Initial motor capacities: 1.74±0.33 m/s (max-selected walking speed, 10mWT), coded ‘good’bCOMP (9)Delay from diagnostic: 78.0±51.6 monthsAge: 67.0±10.2 yInitial characteristics: 2.5±0.7 (H&Y)Initial motor capacities: 1.71±0.34 m/s (max-selected walking speed, 10mWT), coded ‘good’b | Muscles: HE, KEFormat: included in a multi-component rehabilitation programContent: eccentric training on ergometer targeting lower extremity extensors (eccentron)Dose:session: 5 to 30 minuteswk1: 2/wk, 3– 5 min (RPE = 7)wk2 : 3/wk, 5 min (RPE = 9)wk3 : 3/wk, 5– 10 min (RPE = 11)wk4 : 3/wk, 10– 15 min (RPE = 11– 13)wk 5– 12 : 3/wk, 15– 30 min (RPE = 13)frequency: 2– 3/wkduration: 12 wks Range of movement for training: npVelocity of movement: npProgressivity: variation in workload to obtain a RPE = 13 (wk 5 to 12) | multi-component rehabilitation program– warm-up exercises (bicycle or treadmill) – flexibility training (mobility exercises) – balance training (static/dynamic stability training) – upper extremity concentric resistance training (exercise machine) – concentric ergometer training (NuStep) | Participation: quality of lifeActivity: gait, risk of fallFunction: strengthStructure: -Dibble (2006) |
| Dibble (2006) | Parkinson’s Disease | Setting: patients in an outpatient service (USA)ET (10)Delay from diagnostic: 73.2±46.8 monthsAge: 64.3±9.6 yInitial characteristics: 2.5±0.5 (H&Y)Initial motor capacities: 575.12±142.37 m (6MWT), coded ‘good’bCOMP (9)Delay from diagnostic: 78.0±51.6 monthsAge: 67.0±10.2 yInitial characteristics: 2.5±0.7 (H&Y)Initial motor capacities: 544.72±133.13 m (6WMT), coded ‘good’b | Muscles: HE, KEFormat: included in a multi-component rehabilitation programContent: eccentric training on ergometer targeting lower extremity extensors (eccentron)Dose:session: 5 to 30 minuteswk1: 2/wk, 3– 5 min (RPE = 7)wk2 : 3/wk, 5 min (RPE = 9)wk3 : 3/wk, 5– 10 min (RPE = 11)wk4 : 3/wk, 10– 15 min (RPE = 11– 13)wk 5– 12 : 3/wk, 15– 30 min (RPE = 13)frequency: 2– 3/wkduration: 12 wksRange of movement for training: npVelocity of movement: npProgressivity: variation in workload to obtain a RPE = 13 (wk 5 to 12) | multi-component rehabilitation program– warm-up exercises (bicycle or treadmill) – flexibility training (mobility exercises) – balance training (static/dynamic stability training) – upper extremity concentric resistance training (exercise machine) – concentric ergometer training (NuStep) | Participation: -Activity: gaitFunction: strengthStructure: quadriceps volume |
| Engardt (1995) | Stroke | Setting: patients in an outpatient service (Sweden)ET (10)Delay from injury: 26.5±10.3 monthsAge: 62.2±7.6 yInitial characteristics: 81 (68 to 97) (F-M)Initial motor capacities: 0.81±0.18 m/s (self-selected walking speed, 30mWT), coded ‘good’bCOMP (10)Delay from injury: 27.8±12.0 monthsAge: 64.6±6.2 yInitial characteristics: 78 (70 to 86) (F-M)Initial motor capacities: 0.65±0.20 m/s (self-selected walking speed, 30mWT), coded ‘moderate’b | Muscles: KEFormat: standaloneContent: eccentric training on isokinetic dynamometer (exclusively on knee extensors)Dose:session: up to 15 sets of 10 repsangular velocities: pyramidal (60, 120, 180, 120, 60)frequency: 2/wkduration: 6 wksRange of movement for training: from 10° to 100° of knee flexionVelocity of movement: see doseProgressivity: variation in workload to obtain a RPE = 13 (wk 5 to 12) | concentric resistance training (using the same dose as ET) | Participation: -Activity: gaitFunction: strength, balance/posture, muscle activityStructure: - |
| Fernandez-Gonzalo (2016) | Stroke | Setting: np (Spain)ET (16)Delay from injury: 42±43.2 monthsAge: 61.2±9.8 yInitial characteristics: 28.1±1.8 (MMSE)Initial motor capacities: 20.3±14.5 s (TUG), coded ‘severe’COMP (16)Delay from injury: 51.6±58.8 monthsAge: 65.7±12.7 yInitial characteristics: 27.7±2.3 (MMSE)Initial motor capacities: 17.6±14.8 s (TUG), coded ‘severe’b | Muscles: KEFormat: standaloneContent: eccentric training on eccentric ergometer (YoYo)Dose:session: 4 sets x 7 repsAngular velocities: pyramidal (60, 120, 180, 120, 60)frequency: 2/wkduration: 12 wksRange of movement for training: from 0° to 70° of knee flexionVelocity of movement: see doseProgressivity: variation of the workload to maintain maximal intensity | no intervention (control) | Participation: quality of lifeActivity: balance, risk of fallFunction: strength, spasticityStructure: quadriceps volume |
| Lee (2013) | Stroke | Setting: patients in an inpatient service (Korea)ET (10)Delay from injury: npAge: 53.40±9.71 yInitial characteristics: npInitial motor capacities: 0.37±0.33 m/s (self-selected walking speed), coded ‘moderate’bCOMP (10)Delay from injury: npAge: 53.86±10.56 yInitial characteristics: npInitial motor capacities: 0.41±0.19 m/s (self-selected walking speed), coded ‘moderate’b | Muscles: HF, HEFormat: added to usual rehabilitationContent: Eccentric training on isokinetic dynamometerDose:session: 60 minutes (5 minutes warm up)4 sets x 8 repsfrequency: 3/wkduration: 6 wksRange of movement for training: npVelocity of movement: see doseProgressivity: np | usual rehabilitation (not described) | Participation: -Activity: risk of fall, gaitFunction: strengthStructure: - |
Abbreviations: Muscle groups: AE/AF ankle extensors/flexors, EE/EF elbow flexors/extensors, KE/KF knee extensors/flexors, HE/HF hip extensors/flexors, WE/WF wrist extensors/flexors; BBS: Berg Balance Scale; EDSS: Expanded Disability Status Scale; FAC: Functional Ambulation Category; F-M: Fugl-Meyer assessment of sensorimotor function; FSS: Fatigue Severity Scale: H&Y: Hoehn & Yahr Scale; MAS: Modified Ashworth; MDS-UPDRS III: Movement Disorders Society Unified Parkinson’s Disease Rating Scale; MMSE: mini mental state examination; MRI: Magnetic Resonance Imaging; MVC: maximum voluntary contraction; np: non precised; rep: repetitions; RM: repetition maximum; RPE: rating of perceived exertion; SF-36: Short-Form 36; TUG: timed-up and go test; VAS: visual analogue scale; wk: week; y: years; 10mWT: 10 Metres Walk Test; 30mWT: 30 Metres Walk Test; 6MWT: 6 Minutes Walk Test. a: Calculated from time values provided in the original paper (speed = 10/time). b: Previous research in neurological populations (Kubo et al., 2020; Moore et al., 2018; Quinn et al., 2013) had proposed cuts-offs to reflect ‘good’, ‘moderate’, and ‘severe’ motor performance capacities for 10mWT (‘good’>0.8 m/s, ‘moderate’ between 0.4 and 0.8 m/s, ‘severe’<0.4 m/s), 6MWT (‘good’>448 m, ‘moderate’ between 225 m and 448 m, ‘severe’<225 m), TUG (‘good’<12 sec, ‘moderate’ between 12 s and 14 s, ‘severe’<14 sec), respectively. c: The study of Clark (2013) was a staged intervention: part 1 (resistance training, 5 weeks) followed by part 2 (gait training, 3 weeks). Only data from part 1 were considered. Kubo, H., et al. (2020). Reference value of 6-minute walk distance in patients with sub-acute stroke. Top Stroke Rehabil, 27(5), 337-343. doi: 10.1080/10749357.2019.1704372. Moore, J. L., et al. (2018). A Core Set of Outcome Measures for Adults With Neurologic Conditions Undergoing Rehabilitation: A CLINICAL PRACTICE GUIDELINE. J Neurol Phys Ther, 42(3), 174-220. doi: 10.1097/NPT.0000000000000229. Quinn, L.,et al. Outcome Measures Subgroup of the European Huntington’s Disease, N. (2013). Reliability and minimal detectable change of physical performance measures in individuals with pre-manifest and manifest Huntington disease. Phys Ther, 93(7), 942-956. doi: 10.2522/ptj.20130032.
2.4Assessment of characteristics of trials
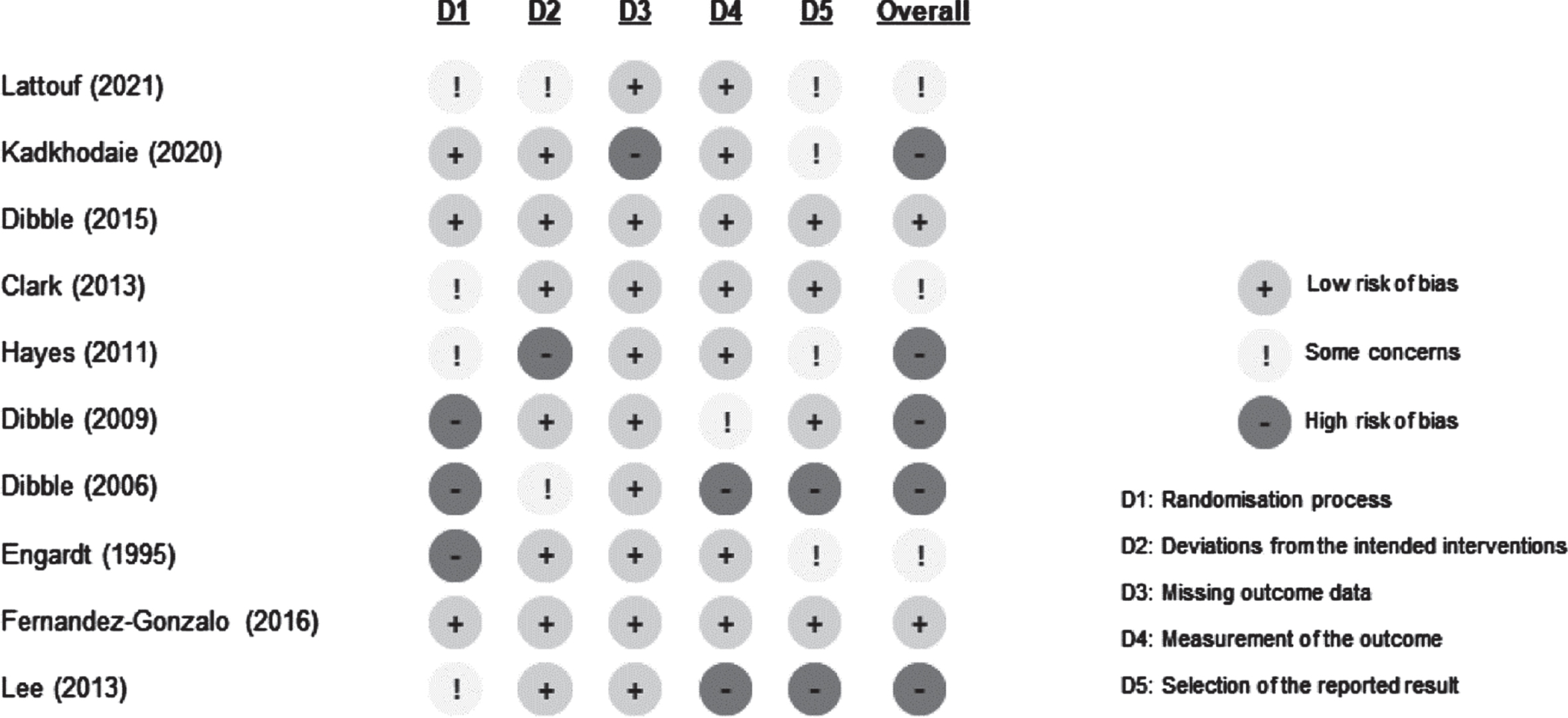
The risk of bias was assessed using The Cochrane risk of bias (RoB) 2.0 tool (Sterne et al., 2019) independently by two reviewers (GLS and TL). A third reviewer was involved (TC) if a consensus could not be reached. RoB 2.0 is structured into signalling questions evaluating five bias domains in RCTs arising from: the randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The overall RoB for the study synthetizes the bias from each domain to an overall judgment qualified as ‘some concerns’, ‘low’ or ‘high’.
2.5Data analysis
The replicability of the search strategy was assessed by the kappa statistic (κ), with levels of agreements defined as: no agreement (0.00< κ), slight (0.00≤κ≤0.19), fair (0.20≤κ≤0.39), moderate (0.40≤κ≤0.59), substantial (0.60≤κ≤0.79), almost perfect (0.80≤κ≤1) (Sim & Wright, 2005). For each outcome of each RCT, pre- and post-intervention scores were used. When multiple measures were reported for a same construct in a RCT, the one best reflecting the outcome was used (Higgins, 2022) (see Supplementary File 2). Data were converted when necessary (e.g. confidence intervals to standard deviation) using Cochrane RevMan software (The Cochrane Collaboration. Review Manager, RevMan, Version 5.4, 2020).
Data were pooled to generate random-effects meta-analyses when at least two studies could be retrieved for an outcome. The critical value for statistical significance was set at 0.05. Analyses were reported as standardised mean differences (SMD) and 95% confidence interval (95% CI). The thresholds of the effect size of generated SMD were: 0.2 (small), 0.5 (medium), 0.8 (large). Subgroup analyses were performed based on aforementioned categorization (neuro-injured, neuro-degenerative) to explore the effect of the origin of central nervous system damage on the results. Heterogeneity between trials was examined using the I2 test with values of > 25% and 50% indicative of moderate and high heterogeneity, respectively (Higgins, 2022). When SMD for a trial was large, we explored the possible causes of heterogeneity by omitting one at a time individual studies, and stratifying by participants initial motor capacities (Higgins, 2022). Results were summarised descriptively when data could not be pooled.
The quality of evidence (QoE) in relation to each outcome was rated using the approach developed by the GRADE collaboration (GRADE, 2013). Six domains are evaluated through GRADE approach: study design, RoB, indirectness, inconsistency, imprecision and publication bias. Four levels of evidence can be attributed: ‘high’, ‘moderate’, ‘low’, and ‘very low’. For each outcome, a sequential analysis system was used (Balshem et al., 2011) (described in Supplementary File 3).
3Results
The review followed the registered protocol except that effect modifiers that were examined as categorical factors in sensitivity analyses.
3.1Flow of trials through the review
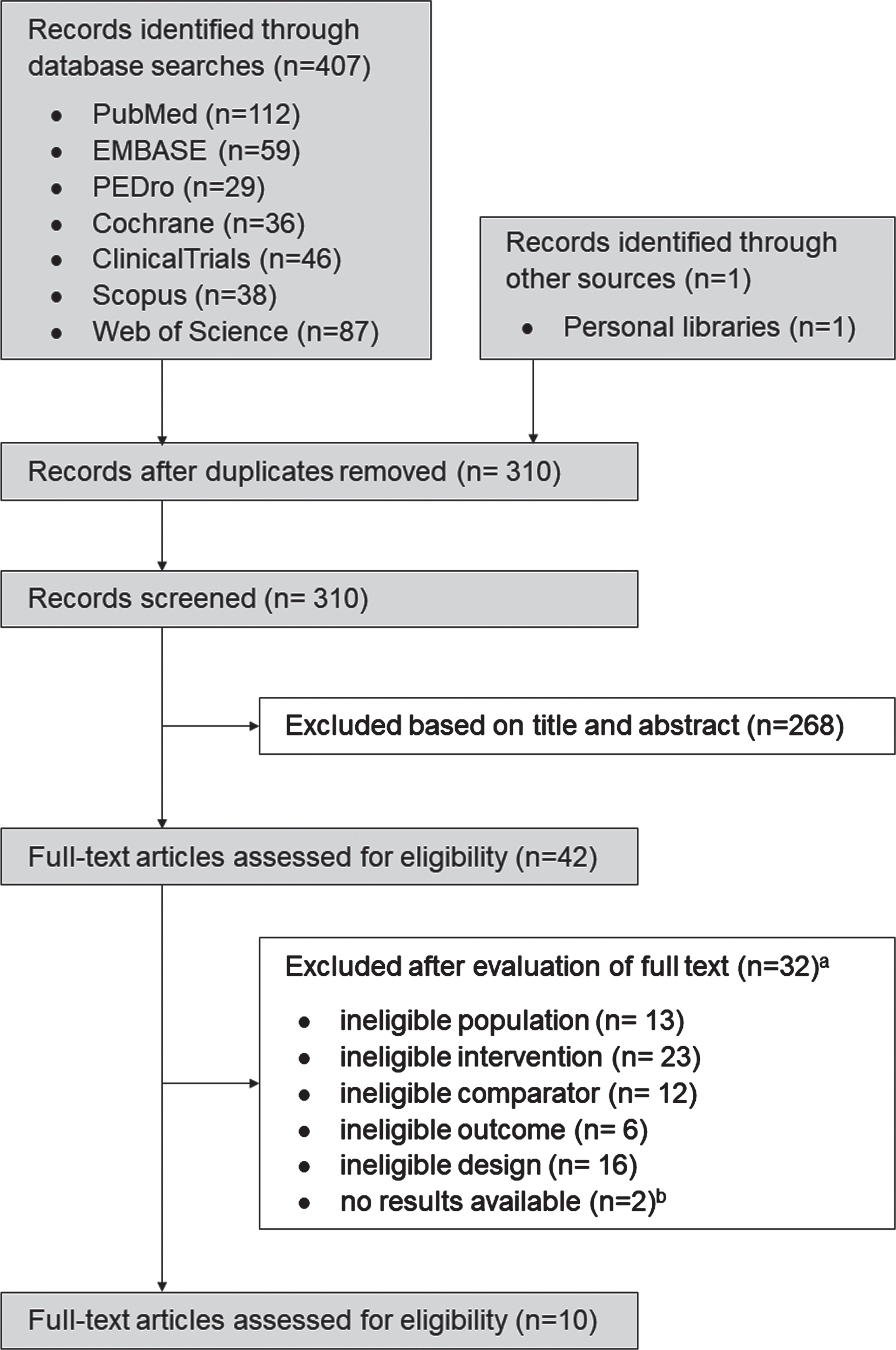
The search identified a total of n = 407 papers (310 excluding duplicates). After screening titles and abstracts, we identified 42 studies as potentially eligible (n = 3 awaiting classification) (κ=0.94). After inspecting the full reports, 10 RCTs were found eligible for inclusion (κ=0.94) (Fig. 1). The reasons for exclusion have been summarised in Supplementary File 4.
Fig. 1
Flow of trials through the review. a Some trials displayed multiple reasons for exclusion. b Authors contacted with no responses.
3.2Characteristics of the included trials
Detailed characteristics of the studies are depicted in Table 2.
3.2.1Participants
The 10 RCTs involved 260 participants, mean age ranged from 48.0 to 70.7 years, at chronic stages of their respective condition (11.6 to 149.9 months): a neurological injury (stroke, n = 141) (Clark & Patten, 2013; Engardt et al., 1995; Fernandez-Gonzalo et al., 2016; Lattouf et al., 2021; Lee & Kang, 2013); a degenerative condition: PD (n = 100) (Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009; Kadkhodaie et al., 2020), and MS (n = 19) (Hayes et al., 2011). All participants were followed in a trial during which they could be assigned prospectively to a group allocating to a single intervention (ET or comparative intervention).
3.2.2Intervention
ET targeted the lower limb in 9 studies (Clark & Patten, 2013; Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009; Engardt et al., 1995; Fernandez-Gonzalo et al., 2016; Hayes et al., 2011; Lattouf et al., 2021; Lee & Kang, 2013), and the upper limb in 1 study (Kadkhodaie et al., 2020). ET was multi-segmental in 7 studies (Clark & Patten, 2013; Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009; Hayes et al., 2011; Kadkhodaie et al., 2020; Lattouf et al., 2021), and targeted one joint in 3 studies (Engardt et al., 1995; Fernandez-Gonzalo et al., 2016; Lee & Kang, 2013). Exercises were performed on an isokinetic dynamometer in 3 studies (Clark & Patten, 2013; Engardt et al., 1995; Lee & Kang, 2013), a fitness/exercise machine in 6 studies (Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009; Fernandez-Gonzalo et al., 2016; Hayes et al., 2011; Lattouf et al., 2021), and free-weights in 1 study (Kadkhodaie et al., 2020). ET was added (Hayes et al., 2011; Lattouf et al., 2021; Lee & Kang, 2013) or included (Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009) to rehabilitation care in 6 studies, or proposed as a standalone intervention in 4 studies: compared to concentric training (Clark & Patten, 2013; Engardt et al., 1995) or to control (no intervention) (Fernandez-Gonzalo et al., 2016; Kadkhodaie et al., 2020). The ET program lasted from 4 to 12 weeks, with a frequency of 2.5 sessions/week. However, the trials remained unclear in their description and manipulation of exercise variables: repetitions of movement to failure or not, velocity and range of movement at which exercise were performed (Table 2 and Supplementary File 5).
3.2.3Outcome measures
Motor performance was assessed as maximal strength by measuring force or torque in 8 studies [isometrically (Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009; Fernandez-Gonzalo et al., 2016; Hayes et al., 2011); or dynamically (Engardt et al., 1995; Fernandez-Gonzalo et al., 2016; Lattouf et al., 2021; Lee & Kang, 2013)], power in 2 studies (Clark & Patten, 2013; Fernandez-Gonzalo et al., 2016), and gait capacities in 8 studies [distance (Dibble et al., 2015; Dibble et al., 2006; Hayes et al., 2011; Lattouf et al., 2021); velocity (Clark & Patten, 2013; Dibble et al., 2009; Engardt et al., 1995; Hayes et al., 2011; Lattouf et al., 2021; Lee & Kang, 2013)]. Regarding motor impairments, muscle structure was quantified using MRI in 3 studies as muscle size (Dibble et al., 2015) or volume (Dibble et al., 2006; Fernandez-Gonzalo et al., 2016); muscle activity using surface electromyography (sEMG) in 2 studies (Clark & Patten, 2013; Engardt et al., 1995), muscle tone in 2 studies via modified Ashworth Scale (Fernandez-Gonzalo et al., 2016; Lattouf et al., 2021), frequency of hand tremor in one study (Kadkhodaie et al., 2020), balance in 3 studies [bodyweight distribution (Hayes et al., 2011) or Berg Balance Test (Engardt et al., 1995; Fernandez-Gonzalo et al., 2016)] and fatigue in one study [Fatigue Severity Scale, Hayes et al. (2011)]. Finally, the risk of fall was measured in 4 studies using Time Up and Go test (Dibble et al., 2009; Fernandez-Gonzalo et al., 2016; Hayes et al., 2011; Lee & Kang, 2013), and 3 trials reported a self-measurement of global health status [using the generic Short-Form 36 scale Fernandez-Gonzalo et al. (2016); or disease-specific Parkinson’s Disease Questionnaire-39 (Dibble et al., 2015; Dibble et al., 2009)] (Supplementary File 2).
3.3Effect of ET on motor performance
The effect of ET on strength was examined by pooling data from 8 studies: 4 studies in persons with stroke (Engardt et al., 1995; Fernandez-Gonzalo et al., 2016; Lattouf et al., 2021; Lee & Kang, 2013), and 4 studies in neuro-degenerative conditions: 3 in PD (Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009), and 1 in MS (Hayes et al., 2011). The effect size was moderate (SMD 0.58, CI95% 0.30 to 0.87, p < 0.0001; I2=0%) in favour of ET, with no effect of the subgroup (Fig. 3A). When stratifying by the type of ET delivery, the effect of ET on strength was still significant: ET as a standalone intervention (SMD 0.79, CI95% –0.13 to 1.08, p = 0.009; I2=0%) or ET included/added into a rehabilitation program (SMD 0.52, CI95% 0.20 to 0.84, p = 0.002; I2=0%). Without the trial reporting a large effect size (Fernandez-Gonzalo et al., 2016), ET still improved strength (SMD 0.53, CI95% 0.23 to 0.84, p = 0.0005; I2 = 0%). The QoE was very low for the effect of ET on strength (Supplementary File 2).
Fig. 3
Standardised mean difference (95% CI) of effect of eccentric resistance training versus comparator (usual rehabilitation, or control) on strength (n = 194) (A) or power (n = 63) (B) immediately after the intervention (n = 194).
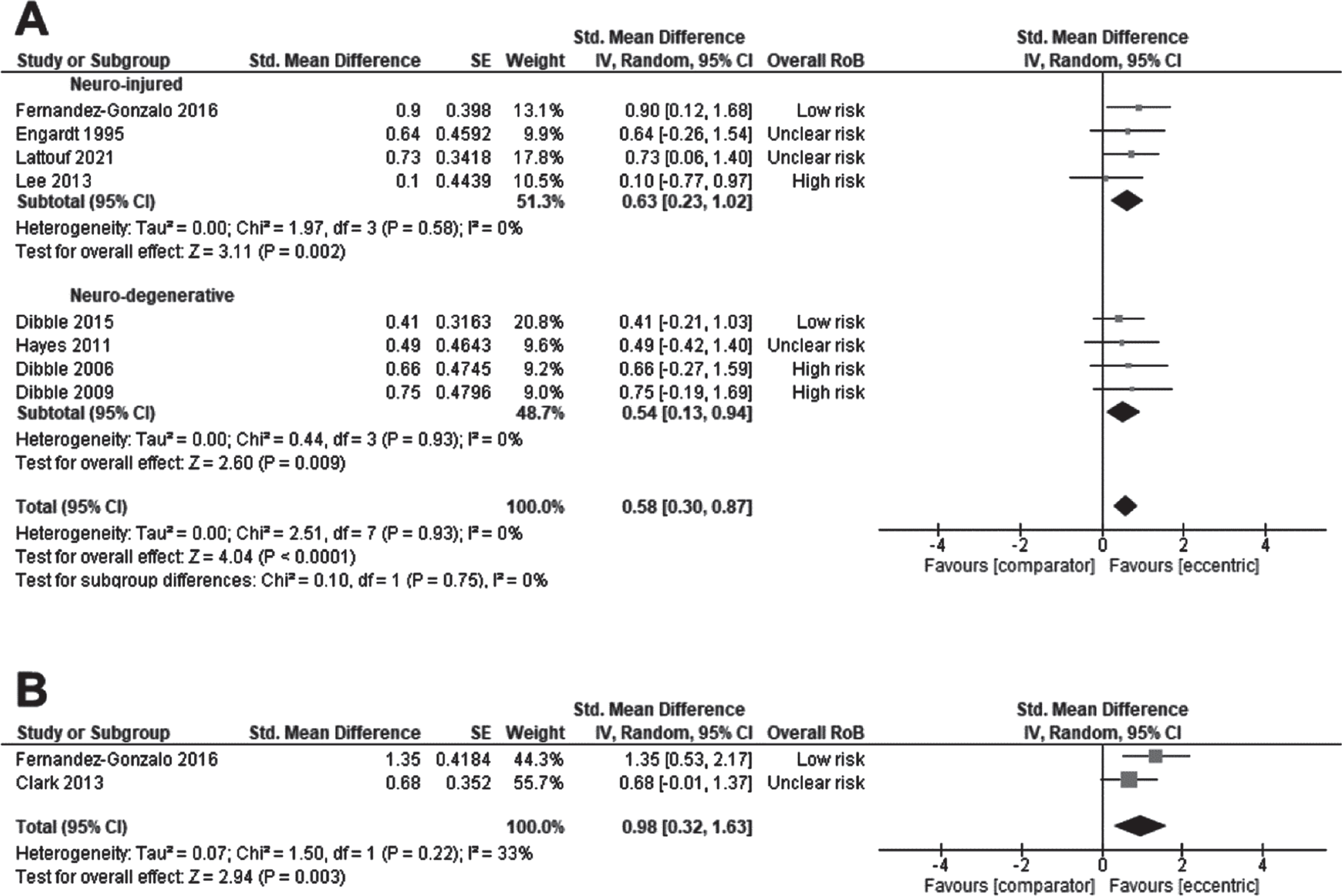
The effect of ET on power was examined by pooling outcomes from 2 studies in persons with stroke delivering ET as a standalone intervention (Clark & Patten, 2013; Fernandez-Gonzalo et al., 2016). The effect size was large (SMD 0.98, CI95% 0.32 to 1.63, p = 0.003; I2 = 0%) in favour of ET (Fig. 3B). It was not possible to test the effect of subgroup, the type of ET delivery, or initial motor capacities due to the number of studies. The QoE was rated as very low for the effect of ET on power (Supplementary File 3).
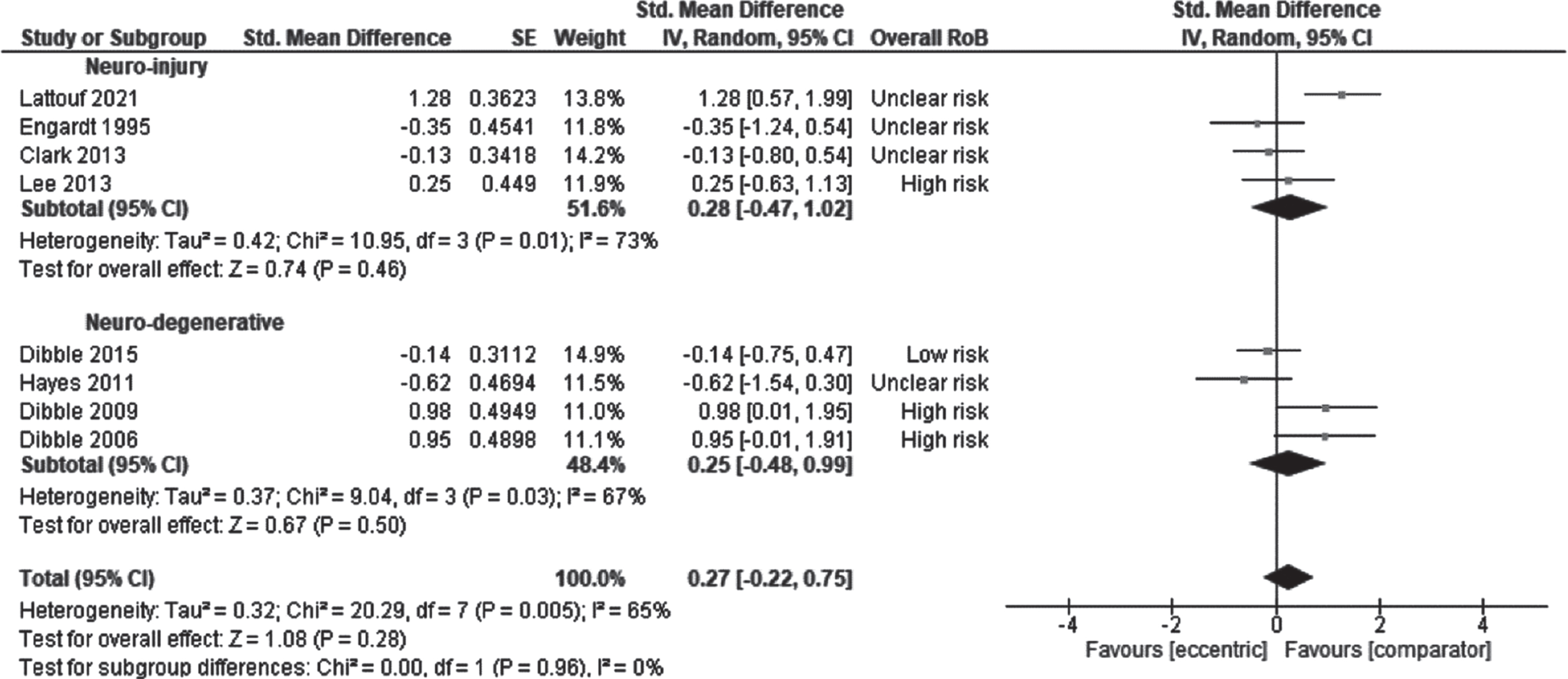
The effect of ET on gait was examined by pooling outcomes from 8 studies: 4 studies in persons with stroke (Clark & Patten, 2013; Engardt et al., 1995; Lattouf et al., 2021; Lee & Kang, 2013) and 4 studies in patients with MS (Dibble et al., 2015; Dibble et al., 2006; Dibble et al., 2009; Hayes et al., 2011). The effect size was 0.27 (CI95% –0.22 to 0.75, p = 0.28; I2 = 65%) which was not significant (Fig. 4). There was no effect of the subgroup, the initial motor capacities on the result: participants with ‘moderate’ capacities (SMD 0.28, CI95% –0.47 to 1.02, p = 0.46; I2 = 73%) or ‘good’ capacities (SMD 0.25, CI95% –0.48 to 0.99, p = 0.67; I2 = 67%). There was no effect of the type of ET delivery on the result: ET as standalone intervention (SMD –0.21, CI95% –0.74 to 0.33, p = 0.44; I2 = 70%) or ET included/added into a rehabilitation program (SMD 0.44, CI95% –0.17 to 1.05, p = 0.70; I2 = 69%). The QoE was rated as very low for the effect of ET on gait (Supplementary File 3).
Fig. 4
Standardised mean difference (95% CI) of effect of eccentric resistance training versus comparator (usual rehabilitation, or control) on gait immediately after the intervention (n = 209).
3.4Effect of ET on secondary outcomes
The effect of ET on muscle structure was examined by pooling outcomes from 3 studies in persons with stroke (Fernandez-Gonzalo et al., 2016) and with MS (Dibble et al., 2015; Dibble et al., 2006). The effect size was 0.79 (CI95% –0.02 to 1.60, p = 0.06; I2 = 66%) which was not significant. There was no effect of the subgroup on the result (Supplementary File 6A). It was not possible to test the effect of the type of ET delivery, or initial motor capacities due to the number of studies. The QoE was rated as very low for the effect of ET on muscle structure (Supplementary File 3).
The effect of ET on knee extensors sEMG activity during maximal knee extensions was examined by pooling outcomes from 2 studies in persons with stroke (Clark & Patten, 2013; Engardt et al., 1995). The effect size was 0.67 (CI95% –0.26 to 1.59, p = 0.16; I2 = 63%) which was not significant (Supplementary File 6B). It was not possible to test the effect modifiers due to the number of studies. The QoE was rated as very low for the effect of ET on muscle activity (Supplementary File 3). Additionally, the study of Engardt et al. (1995) did not find differences in knee flexors’ muscle activity during maximal knee extensions (i.e., acting as antagonists).
The effect of ET on spasticity was examined by pooling outcomes from 2 studies in persons with stroke (Fernandez-Gonzalo et al., 2016; Lattouf et al., 2021). The effect size was –0.15 (CI95% –0.63 to 0.33, p = 0.55; I2 = 0%) which was not significant (Supplementary File 6 C). It was not possible to test the effect modifiers due to the number of studies. The QoE was rated as very low for the effect of ET on muscle spasticity (Supplementary File 3). Kadkhodaie et al. (2020) did not report between-group differences on hand tremor in PD (SMD 0.83, CI95% –0.07 to 1.73).
The effect of ET on balance was examined by pooling outcomes from 3 studies in persons with stroke (Engardt et al., 1995; Fernandez-Gonzalo et al., 2016) and persons with MS(Hayes et al., 2011). The effect size was 0.66 (CI95% –1.33 to 2.66, p = 0.51; I2 = 93%) which was not significant. There was no ‘subgroup’ effect on the result (Supplementary File 6D). The QoE was rated as very low for the effect of ET on balance (Supplementary File 3).
Finally, Hayes et al. (2011) did not report between-group differences in self-perceived fatigue on daily function in people with MS (Fatigue Severity Scale, MD = 0.44, CI95% –0.36 to 1.24).
3.5Effect of ET on tertiary outcomes
The effect of ET on risk of fall was examined by pooling outcomes from 2 studies in persons with stroke (Fernandez-Gonzalo et al., 2016; Lee & Kang, 2013), 1 study in patients with PD (Dibble et al., 2009), and 1 study in patients with MS (Hayes et al., 2011). The effect size was medium (SMD –0.67, CI95% –1.11 to –0.23, p = 0.003; I2 = 0%) in favour of ET (Supplementary File 6E). There was no ‘subgroup’ effect of the on the result. The effect size was improved when participants had a ‘severe’ or ‘moderate’ motor impairment (SMD –0.82, CI95% –1.41 to –0.23, p = 0.007; I2 = 0%) and not significant when participants had ‘good’ initial motor capacities (SMD –0.49, CI95% –1.30 to 0.31, p = 0.23; I2 = 33%). When trials with a large effect size were omitted one-by-one (Dibble et al., 2009; Fernandez-Gonzalo et al., 2016; Lee & Kang, 2013), the overall result was reduced but still in favour of ET (all SMD and CI95% < –0.60, –1.13 to –0.07, all p-values<0.01). However, the result was not significant when at least two of these trials were omitted (see Supplementary File 6E for further details). The QoE was rated as very low for the effect of ET on risk of fall (Supplementary File 3).
Three trials (Dibble et al., 2015; Dibble et al., 2009; Fernandez-Gonzalo et al., 2016) assessed participants’ perceptions of their quality of life in relation to their health status. It was not possible to pool their data. Qualitatively, the ‘perception of pain’ on SF-36 scale was better improved after ET in persons with stroke (SMD 0.96, CI95% 0.16 to 1.74) (Fernandez-Gonzalo et al., 2016). In people with PD, Dibble et al. (2009) reported that ET improved the ‘global index’ subsection of Parkinson Disease Questionnaire (PDQ-39) (SMD –1.26, CI95% –2.26 to –0.25), but this result was not confirmed in a later study (no data reported) (Dibble et al., 2015).
4Discussion
This systematic review provided evidence that ET improves motor performance, especially maximal strength and power, in neurological populations. The effect of ET on secondary outcomes (motor impairments) and tertiary outcomes (health-related quality of life) were not consistent. However, the overall QoE was very low due to a high RoB, small sample sizes generating imprecisions across the studies. The moderate to large effect size for strength is in accordance with conclusions of previous reviews applying ‘conventional’ resistance training programs in neurological populations: stroke injury [SMD = 0.99, CI95% 0.28 to 1.70 Veldema and Jansen (2020); 0.98, 95% CI 0.67 to 1.29, Dorsch et al. (2018)]; CP [0.26, CI95% 0.04 to 0.48, Merino-Andres et al. (2022)]; MS [0.36, 0.18 to 0.55, Andreu-Caravaca et al. (2022); 0.45, CI95% 0.18 to 0.72, Jorgensen et al. (2017)]; PD [1.9, CI95% 0.55 to 3.24, Yang and Wang (2023)].
Also, gait was the only activity assessed in the retrieved studies of this review (n = 8/10). We did not find evidence of superiority of ET over the comparator in improving gait, which is consistent with the findings from previous reviews in neurological populations where either distance or velocity were not better improved after a ‘conventional’ resistance training program (Braz de Oliveira et al., 2021; Dorsch et al., 2018; Merino-Andres et al., 2022; Veldema & Jansen, 2020). However, a recent review in PD populations reported a better positive effect on a disease-specific impairment: freezing during gait [SMD = 0.55, CI95% 0.16 to 0.95, Yang and Wang (2023)]. We did not retrieve trials assessing changes in freezing in PD, after ET. However, when considering their initial gait capacities (distance or velocity), the participants of the trials of the present review were probably at a too ‘moderate-to-low’ degree of walking disability (Table 2) to expect a carry-over of strength on activity (Dorsch et al., 2021). Another reason might be related to the specificity of the intervention to target the most important muscle groups for walking and/or not using fast-enough muscle contractions to match the physiological needs for gait (Williams et al., 2019).
Resistance training is a complex intervention with many variables that can affect its benefits through different neural and structural adaptations. For instance, performing rapid muscle contractions might be more beneficial for optimizing neural plasticity and muscle activation while slow muscle contractions would better promote hypertrophy (Coratella, 2022; Scott et al., 2016). An optimal program would ideally fit individual needs and impairments. It is difficult to appreciate to in the present review in which extent the intervention optimized neural or structural adaptations for improving strength was related to the initial impairments of the participants. For instance, different movement velocities could be used among the retrieved studies’ protocols (Table 2). Also, motor impairments were not consistently followed in the retrieved studies (30% only). The trial of Fernandez-Gonzalo et al. (2016) in persons with stroke was the only trial assessing neural and structural outcomes in addition to strength. This study reported increases of strength and muscle volume in favor of the ET group, while muscle tone remained unchanged for both groups. These findings would mean that ET would be of particular interest to promote hypertrophy for improving strength. Dibble et al. (2006) also reported a better increase after ET in quadriceps muscle size of patients with PD in addition to strength gain. However, this result was not confirmed later (Dibble et al., 2015) and muscle activity was not measured in those trials. A trial conducted in children with CP (not included in this review) (Reid et al., 2010) reported strength gains after ET associated with a decrease in antagonist muscle co-activation. Taken together, different mechanisms (neural, structural) could be involved to improve strength after ET.
Muscle tone and balance were not be better improved after ET (Supplementary File 5), like found in ‘conventional’ resistance training programs (Merino-Andres et al., 2022; Veldema & Jansen, 2020). Surprisingly, no trials investigated joint flexibility, muscle length or stiffness despite the popularity of contractures and rigidity in individuals with neurological conditions, that are associated with changes in musculo-tendinous structure and/or mechanical properties (intrinsic level of stiffness) (Diong et al., 2012; Hoang et al., 2021; Kwah et al., 2012; Le Sant et al., 2019; Lieber & Friden, 2019). Once developed, contractures enhance the overall degradation of motor performance and disability (Ada et al., 2000; Barber et al., 2017). These features are difficult to address in neuro-rehabilitation where classical approaches using stretching exercises fail to produce clinical worthwhile results (Harvey et al., 2017; Lecharte et al., 2020; Svane et al., 2021). A recent review reported that ET offer an opportunity to improve joint range of motion and muscle fascicle length in healthy populations (Diong et al., 2022). A feasibility study in a small sample of MS patients reported clinical gains in joint flexibility after an ET (Manca et al., 2020). These preliminary results need to be confirmed in future studies, in order to identify the morphological and biomechanical adaptations underpinning the clinical outcomes.
In order to provide evidence-based strategies in clinical contexts future studies should include neurophysiological and structural outcomes in their assessments to understand how adaptations following ET: i) differ/conform to ‘conventional’ resistance training programs; ii) influence the severity of motor impairments and disability. A greater understanding of the distinct neurological and/or architectural adaptations following strength training would lead to more personalized exercise prescription for this population.
The main strength of this review was to comprehensively examine motor performance, motor impairments, and disability across different neurological conditions, by considering a person’s functioning in terms of body functions and structures, activities and participations (health-related quality of life), rather than just strength. We also considered ACSM guideline for resistance training to determine an ‘optimal’ program that would increase strength (Supplementary File 5). However, the studies focused on the lower limb (90% of the trials) and for individuals at chronic phases of their condition (Table 2). Based on that, it is not possible to conclude on the effects of ET on upper limb motor performance, for acute/subacute populations, or in patients with different conditions (e.g. CP, SCI). Moreover, despite positive results for strength after ET, the precise estimate of the mode of delivery (and comparator) is difficult to appreciate, because of the number of studies retrieved. Future trials are needed to estimate how much more (or less) effective eccentric training is than usual rehabilitation, and to determine its optimal dose-effect. Finally, we excluded trials using task-oriented exercises such as backward gait-training (Hosl et al., 2018), whose tend to influence motor control, skill, speed, endurance, and not only strength. Ballistic training offers opportunities (Williams et al., 2022) but might be more suitable when the goal of training is to improve the rate of force production (Williams et al., 2019). Also, ballistic training might not isolate the specific effect of ET which was beyond the scope of this review. Future trials should compare different types of resistance training regarding the specific needs of the activity, in order to determine to most effective approach of resistance training.
5Conclusion
This systematic review shows that ET is likely to improve motor performance in neurological conditions. Because this result is based on a very-low quality of evidence, future studies are needed to provide robust evidence and to precise the magnitude of this finding. These studies would use larger samples of participants, and test ET in acute populations and/or with more severe capacities. The effect of ET on clinical parameters that have useful implications in neurologically-impaired populations, such as joint flexibility also needs further investigation.
Conflict of interest
GLS is supported by a grant from the French Ministry of Health (PHRIP, n° PHRIP-21-0031). Funders had no role in design and conduct of the research; collection, management, data analysis and interpretation; and preparation; review, or approval of the manuscript or the decision to submit for publication. None of the other authors have any interests.
Acknowledgments
The authors have no acknowledgments.
Supplementary materials
[1] The supplementary files are available from https://dx.doi.org/10.3233/NRE-230035.
References
1 | Ada, L. , Canning, C. , & Dwyer, T. ((2000) ). Effect of muscle length on strength and dexterity after stroke. Clin Rehabil, 14: (1), 55–61. doi: 10.1191/026921500671430626 |
2 | American College of Sports Medicine. ((2009) ). American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc, 41: (3), 687–708. doi: 10.1249/MSS.0b013e3181915670 |
3 | Andreu-Caravaca, L. , Ramos-Campo, D. J. , Chung, L. H. , Martinez-Rodriguez, A. , & Rubio-Arias, J. A. (2022). Effects and optimal dosage of resistance training on strength, functional capacity, balance, general health perception, and fatigue in people with multiple sclerosis: a systematic review and meta-analysis. Disabil Rehabil, 1-13. doi: 10.1080/09638288.2022.2069295 |
4 | Aravind, N. , Harvey, L. A. , & Glinsky, J. V. ((2019) ). Physiotherapy interventions for increasing muscle strength in people with spinal cord injuries: a systematic review. Spinal Cord, 57: (6), 449–460. doi: 10.1038/s41393-019-0242-z |
5 | Balshem, H. , Helfand, M. , Schunemann, H. J. , Oxman, A. D. , Kunz, R. , Brozek, J. ,... & Guyatt, G. H. ((2011) ). GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol, 64: (4), 401–406. doi: 10.1016/j.jclinepi.2010.07.015 |
6 | Barber, L. , Carty, C. , Modenese, L. , Walsh, J. , Boyd, R. , & Lichtwark, G. ((2017) ). Medial gastrocnemius and soleus muscle-tendon unit, fascicle, and tendon interaction during walking in children with cerebral palsy. Dev Med Child Neurol, 59: (8), 843–851. doi: 10.1111/dmcn.13427 |
7 | Blazevich, A. J. ((2019) ). Adaptations in the passive mechanical properties of skeletal muscle to altered patterns of use. J Appl Physiol (1985), 126: (5), 1483–1491. doi: 10.1152/japplphysiol.00700.2018 |
8 | Bowring, F. , Welch, J. , Woodward, C. , Lo, C. , Lawton, M. , Sulzer, P. ,... & Ncer, P. D. ((2022) ). Exploration ofwhether socioeconomic factors affect the results of priority setting partnerships: updating the top 10 research priorities for the management of Parkinson’s in an international setting. BMJ Open, 12: (6), e049530. doi: 10.1136/bmjopen-2021-049530 |
9 | Braz de Oliveira, M. P. , Maria Dos Reis, L. , & Pereira, N. D. ((2021) ). Effect of Resistance Exercise on Body Structure and Function, Activity, and Participation in Individuals With Parkinson Disease: A Systematic Review. Arch Phys Med Rehabil, 102: (10), 1998–2011. doi: 10.1016/j.apmr.2021.01.081 |
10 | Chiu, C. , Park, M. , Hoffman, T. , Campbell, M. , & Bishop, M. ((2019) ). Descriptive analysis of free-text comments on healthcare priorities and experiences in a national sample of people with multiple sclerosis. Mult Scler Relat Disord, 34: , 141–149. doi: 10.1016/j.msard.2019.06.023 |
11 | Clark, D. J. , & Patten, C. ((2013) ). Eccentric versus concentric resistance training to enhance neuromuscular activation and walking speed following stroke. Neurorehabil Neural Repair, 27: (4), 335–344. doi: 10.1177/1545968312469833 |
12 | Cochrane Collaboration. (2022). Data collection form (for RCTs). In D. c. f. f. R. CIL (Ed.). |
13 | Cook, G. , Cassidy, E. , & Kilbride, C. (2022). Understanding physiotherapy and physiotherapy services: exploring the perspectives of adults living with cerebral palsy. Disabil Rehabil, 1-9. doi: 10.1080/09638288.2022.2062060 |
14 | Coratella, G. ((2022) ). Appropriate Reporting of Exercise Variables in Resistance Training Protocols: Much more than Load and Number of Repetitions. Sports Med Open, 8: (1), 99. doi: 10.1186/s40798-022-00492-1 |
15 | Davis, J. F. , Khir, A. W. , Barber, L. , Reeves, N. D. , Khan, T. , DeLuca, M. , & Mohagheghi, A. A. ((2020) ). The mechanisms of adaptation for muscle fascicle length changes with exercise: Implications for spastic muscle. Med Hypotheses, 144: , 110199. doi: 10.1016/j.mehy.2020.110199 |
16 | Dibble, L. E. , Foreman, K. B. , Addison, O. , Marcus, R. L. , & LaStayo, P. C. ((2015) ). Exercise and medication effects on persons with Parkinson disease across the domains of disability: a randomized clinical trial. J Neurol Phys Ther, 39: (2), 85–92. doi: 10.1097/NPT.0000000000000086 |
17 | Dibble, L. E. , Hale, T. F. , Marcus, R. L. , Droge, J. , Gerber, J. P. , & LaStayo, P. C. ((2006) ). High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord, 21: (9), 1444–1452. doi: 10.1002/mds.20997 |
18 | Dibble, L. E. , Hale, T. F. , Marcus, R. L. , Gerber, J. P. , & LaStayo, P. C. ((2009) ). High intensity eccentric resistance training decreases bradykinesia and improves Quality Of Life in persons with Parkinson’s disease: a preliminary study. Parkinsonism Relat Disord, 15: (10), 752–757. doi: 10.1016/j.parkreldis.2009.04.009 |
19 | Diong, J. , Carden, P. C. , O’Sullivan, K. , Sherrington, C. , & Reed, D. S. ((2022) ). Eccentric exercise improves joint flexibility in adults: A systematic review update and meta-analysis. Musculoskelet Sci Pract, 60: , 102556. doi: 10.1016/j.msks2022.102556 |
20 | Diong, J. , Herbert, R. D. , Kwah, L. K. , Clarke, J. L. , & Harvey, L. A. ((2012) ). Mechanisms of increased passive compliance of hamstring muscle-tendon units after spinal cord injury. Clin Biomech (Bristol, Avon), 27: (9), 893–898. doi: 10.1016/j.clinbiomech.2012.07.003 |
21 | Dorsch, S. , Ada, L. , & Alloggia, D. ((2018) ). Progressive resistance training increases strength after stroke but this may not carry over to activity: a systematic review. J Physiother, 64: (2), 84–90. doi: 10.1016/j.jphys.2018.02.012 |
22 | Dorsch, S. , Ada, L. , Sorial, T. , & Fanayan, E. ((2021) ). The Relationship Between Strength of the Affected Leg and Walking Speed After Stroke Varies According to the Level of Walking Disability: A Systematic Review. Phys Ther, 101: (12). doi: 10.1093/ptj/pzab233 |
23 | Douglas, J. , Pearson, S. , Ross, A. , & McGuigan, M. ((2017) a). Chronic Adaptations to Eccentric Training: A Systematic Review. Sports Med, 47: (5), 917–941. doi: 10.1007/s40279-016-0628-4 |
24 | Douglas, J. , Pearson, S. , Ross, A. , & McGuigan, M. ((2017) b). Eccentric Exercise: Physiological Characteristics and Acute Responses. Sports Med, 47: (4), 663–675. doi: 10.1007/s40279-016-0624-8 |
25 | Eng, J. J. , Lomaglio, M. J. , & Macintyre, D. L. ((2009) ). Muscle torque preservation and physical activity in individuals with stroke. Med Sci Sports Exerc, 41: (7), 1353–1360. doi: 10.1249/MSS.0b013e31819aaad1 |
26 | Engardt, M. , Knutsson, E. , Jonsson, M. , & Sternhag, M. ((1995) ). Dynamic muscle strength training in stroke patients: effects on knee extension torque, electromyographic activity, and motor function. Arch Phys Med Rehabil, 76: (5), 419–425. doi: 10.1016/s0003-9993(95)80570-2 |
27 | Fernandez-Gonzalo, R. , Fernandez-Gonzalo, S. , Turon, M. , Prieto, C. , Tesch, P. A. , & Garcia-Carreira Mdel, C. ((2016) ). Muscle, functional and cognitive adaptations after flywheel resistance training in stroke patients: apilot randomized controlled trial. J Neuroeng Rehabil, 13: , 37. doi: 10.1186/s12984-016-0144-7 |
28 | Folkerts, M. A. , Hijmans, J. M. , Elsinghorst, A. L. , Mulderij, Y. , Murgia, A. , & Dekker, R. ((2017) ). Effectiveness and feasibility of eccentric and task-oriented strength training in individuals with stroke. NeuroRehabilitation, 40: (4), 459–471. doi: 10.3233/NRE-171433 |
29 | Gorgey, A. S. , Dudley, G. A. ((2007) ). Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord, 45: (4), 304–309. doi: 10.1038/sj.sc.3101968 |
30 | Gracies, J. M. ((2005) a). Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve, 31: (5), 535–551. doi: 10.1002/mus.20284 |
31 | Gracies, J. M. ((2005) b). Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve, 31: (5), 552–571. doi: 10.1002/mus.20285 |
32 | GRADE.(2013). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. |
33 | Graham, H. K. , Rosenbaum, P. , Paneth, N. , Dan, B. , Lin, J. P. , Damiano, D. L. ,... & Lieber, R. L. ((2016) ). Cerebral palsy. Nat Rev Dis Primers, 2: , 15082. doi: 10.1038/nrd2015.82 |
34 | Handsfield, G. G. , Williams, S. , Khuu, S. , Lichtwark, G. , & Stott, N. S. ((2022) ). Muscle architecture, growth, and biological Remodelling in cerebral palsy: a narrative review. BMC Musculoskelet Disord, 23: (1), 233. doi: 10.1186/s12891-022-05110-5 |
35 | Harvey, L. A. , Katalinic, O. M. , Herbert, R. D. , Moseley, A. M. , Lannin, N. A. , & Schurr, K. ((2017) ). Stretch for the treatment and prevention of contractures. Cochrane Database Syst Rev, 1: , CD007455. doi: 10.1002/14651858.CD007455.pub3 |
36 | Hayes, H. A. , Gappmaier, E. , & LaStayo, P. C. ((2011) ). Effects of high-intensity resistance training on strength, mobility, balance, and fatigue in individuals with multiple sclerosis: a randomized controlled trial. J Neurol Phys Ther, 35: (1), 2–10. doi: 10.1097/NPT.0b013e31820b5a9d |
37 | Hendrey, G. , Holland, A. E. , Mentiplay, B. F. , Clark, R. A. , & Williams, G. ((2018) ). Do Trials of Resistance Training to Improve Mobility After Stroke Adhere to the American College of Sports Medicine Guidelines? A Systematic Review. Arch Phys Med Rehabil, 99: (3), 584–597 e513. doi: 10.1016/j.apmr.2017.06.021 |
38 | Higgins, J. P. T. (2022). Cochrane Handbook for Systematic Reviews of Interventions (6.3 (online version) ed.): Cochrane. |
39 | Hoang, P. D. , Psarakis, M. , Kwah, L. K. , Clarke, J. L. , Gandevia, S. C. , & Diong, J. ((2021) ). Brief report: Passive mechanical properties of gastrocnemius in multiple sclerosis and ankle contracture. Clin Biomech (Bristol, Avon), 84: , 105338. doi: 10.1016/j.clinbiomech.2021.105338 |
40 | Hornby, T. G. , Reisman, D. S. , Ward, I. G. , Scheets, P. L. , Miller, A. , Haddad, D. , ... & the Locomotor, C. P. G. A. T. ((2020) ). Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J Neurol Phys Ther, 44: (1), 49–100. doi: 10.1097/NPT.0000000000000303 |
41 | Hosl, M. , Bohm, H. , Eck, J. , Doderlein, L. , & Arampatzis, A. ((2018) ). Effects of backward-downhill treadmilltraining versus manual static plantarflexor stretching on muscle-joint pathology and function in children withspastic Cerebral Palsy. Gait Posture, 65: , 121–128. doi: 10.1016/j.gaitpost.2018.07.171 |
42 | Hunnicutt, J. L. , & Gregory, C. M. ((2017) ). Skeletal muscle changes following stroke: a systematic review and comparison to healthy individuals. Top Stroke Rehabil, 24: (6), 463–471. doi: 10.1080/10749357.2017.1292720 |
43 | Jorgensen, M. , Dalgas, U. , Wens, I. , & Hvid, L. G. ((2017) ). Muscle strength and power in persons with multiple sclerosis –A systematic review and meta-analysis. J Neurol Sci, 376: , 225–241. doi: 10.1016/j.jns.2017.03.022 |
44 | Kadkhodaie, M. , Sharifnezhad, A. , Ebadi, S. , Marzban, S. , Habibi, S. A. , Ghaffari, A. , & Forogh, B. ((2020) ). Effect of eccentric-based rehabilitation on hand tremor intensity in Parkinson disease. Neurol Sci, 41: (3), 637–643. doi: 10.1007/s10072-019-04106-9 |
45 | Klein, C. S. , Brooks, D. , Richardson, D. , McIlroy, W. E. , & Bayley, M. T. ((2010) ). Voluntary activation failure contributes more to plantar flexor weakness than antagonist coactivation and muscle atrophy in chronic stroke survivors. J Appl Physiol (1985), 109: (5), 1337–1346. doi: 10.1152/japplphysiol.00804.2009 |
46 | Kluger, B. M. , Krupp, L. B. , & Enoka, R. M. ((2013) ). Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology, 409–416. doi: 10.1212/WNL.0b013e31827f07be |
47 | Kohl, C. , McIntosh, E. J. , Unger, S. , Haddaway, N. R. , Kecke, S. , Schiemann, J. , & Wilhelm, R. ((2018) ). Online tools supporting the conduct and reporting of systematic reviews and systematic maps: a case study on CADIMA and review of existing tools. Environmental Evidence, 7: (1). doi: 10.1186/s13750-018-0115-5 |
48 | Kwah, L. K. , Herbert, R. D. , Harvey, L. A. , Diong, J. , Clarke, J. L. , Martin, J. H. ,... & Gandevia, S. C. ((2012) ). Passive mechanical properties of gastrocnemius muscles of people with ankle contracture after stroke. Arch Phys Med Rehabil, 93: (7), 1185–1190. doi: 10.1016/j.apmr.2012.02.009 |
49 | LaStayo, P. , Marcus, R. , Dibble, L. , Frajacomo, F. , & Lindstedt, S. ((2014) ). Eccentric exercise in rehabilitation: safety, feasibility, and application. J Appl Physiol (1985), 116: (11), 1426–1434. doi: 10.1152/japplphysiol.00008.2013 |
50 | Lattouf, N. A. , Tomb, R. , Assi, A. , Maynard, L. , & Mesure, S. ((2021) ). Eccentric training effects for patients with post-stroke hemiparesis on strength and speed gait: A randomized controlled trial. NeuroRehabilitation, 48: (4), 513–522. doi: 10.3233/nre-201601 |
51 | Le Sant, G. , Nordez, A. , Hug, F. , Andrade, R. , Lecharte, T. , McNair, P. J. , & Gross, R. ((2019) ). Effects of stroke injury on the shear modulus of the lower leg muscle during passive dorsiflexion. J Appl Physiol (1985), 126: (1), 11–22. doi: 10.1152/japplphysiol.00968.2017 |
52 | Lecharte, T. , Gross, R. , Nordez, A. , & Le Sant, G. ((2020) ). Effect of chronic stretching interventions on the mechanical properties of muscles in patients with stroke: A systematic review. Ann Phys Rehabil Med, 63: (3), 222–229. doi: 10.1016/j.rehab.2019.12.003 |
53 | Lee, S. B. , & Kang, K. Y. ((2013) ). The effects of isokinetic eccentric resistance exercise for the hip joint on functional gait of stroke patients. J Phys Ther Sci, 25: (9), 1177–1179. doi: 10.1589/jpts.25.1177 |
54 | Lieber, R. L. , & Friden, J. ((2019) ). Muscle contracture and passive mechanics in cerebral palsy. J Appl Physiol (1985), 126: (5), 1492–1501. doi: 10.1152/japplphysiol.00278.2018 |
55 | Manca, A. , Martinez, G. , Aiello, E. , Ventura, L. , & Deriu, F. ((2020) ). Effect of Eccentric Strength Training on Elbow Flexor Spasticity and Muscle Weakness in People With Multiple Sclerosis: Proof-of-Concept Single-System Case Series. Phys Ther, 100: (7), 1142–1152. doi: 10.1093/ptj/pzaa055 |
56 | McLoughlin, J. (2018). Common Impairments and the Impact on Activity. In Physical Management for Neurological Conditions (Fourth edition ed.): Elsevier Limited. |
57 | Merino-Andres, J. , Garcia de Mateos-Lopez, A. , Damiano, D. L. , & Sanchez-Sierra, A. ((2022) ). Effect of muscle strength training in children and adolescents with spastic cerebral palsy: A systematic review and meta-analysis. Clin Rehabil, 36: (1), 4–14. doi: 10.1177/02692155211040199 |
58 | Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & Group, P. ((2009) ). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ, 339: , b2535. doi: 10.1136/bmj.b2535 |
59 | National Insitute for Health and Care Excellence. (2022). Multiple sclerosis in adults: management. NICE guideline. Retrieved from https://www.nice.org.uk/guidance/ng220 |
60 | Nuckolls, G. H. , Kinnett, K. , Dayanidhi, S. , Domenighetti, A. A. , Duong, T. , Hathout, Y. ,... & Lieber, R. L. ((2020) ). Conference report on contractures in musculoskeletal and neurological conditions. Muscle Nerve, 61: (6), 740–744. doi: 10.1002/mus.26845 |
61 | Osborne, J. A. , Botkin, R. , Colon-Semenza, C. , DeAngelis, T. R. , Gallardo, O. G. , Kosakowski, H. ,... & Ellis, T. D. ((2022) ). Physical Therapist Management of Parkinson Disease: A Clinical Practice Guideline From the American Physical Therapy Association. Phys Ther, 102: (4). doi: 10.1093/ptj/pzab302 |
62 | Ramari, C. , Hvid, L. G. , David, A. C. , & Dalgas, U. ((2020) ). The importance of lower-extremity muscle strength for lower-limb functional capacity in multiple sclerosis: Systematic review. Ann Phys Rehabil Med, 63: (2), 123–137. doi: 10.1016/j.rehab.2019.11.005 |
63 | Reid, S. , Hamer, P. , Alderson, J. , & Lloyd, D. ((2010) ). Neuromuscular adaptations to eccentric strength training in children and adolescents with cerebral palsy. Dev Med Child Neurol, 52: (4), 358–363. doi: 10.1111/j.1469-8749.2009.03409.x |
64 | Scott, B. R. , Duthie, G. M. , Thornton, H. R. , & Dascombe, B. J. ((2016) ). Training Monitoring for Resistance Exercise: Theory and Applications. Sports Med, 46: (5), 687–698. doi: 10.1007/s40279-015-0454-0 |
65 | Sim, J. , & Wright, C. C. ((2005) ). The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys Ther, 85: (3), 257–268. |
66 | Skinner, J. W. , Lee, H. K. , Roemmich, R. T. , Amano, S. , & Hass, C. J. ((2015) ). Execution of Activities of Daily Living in Persons with Parkinson Disease. Med Sci Sports Exerc, 47: (9), 1906–1912. doi: 10.1249/MSS.0000000000000598 |
67 | Sterne, J. A. C. , Savovic, J. , Page, M. J. , Elbers, R. G. , Blencowe, N. S. , Boutron, I. ,... & Higgins, J. P. T. ((2019) ). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ, 366: , l4898. doi: 10.1136/bmj.l4898 |
68 | Stroke Association. (2021). The Stroke Priority Setting Partnership results for investment.. Retrieved from. |
69 | Svane, C. , Nielsen, J. B. , & Lorentzen, J. ((2021) ). Nonsurgical Treatment Options for Muscle Contractures in Individuals With Neurologic Disorders: A Systematic Review With Meta-Analysis. Arch Rehabil Res Clin Transl, 3: (1), 100104. doi: 10.1016/j.arrct.2021.100104 |
70 | Thomas, C. K. , Bakels, R. , Klein, C. S. , & Zijdewind, I. ((2014) ). Human spinal cord injury: motor unit properties and behaviour. Acta Physiol (Oxf), 210: (1), 5–19. doi: 10.1111/apha.12153 |
71 | van Middendorp, J. J. , Allison, H. C. , Ahuja, S. , Bracher, D. , Dyson, C. , Fairbank, J. ,... & Cowan, K. ((2016) ). Top ten research priorities for spinal cord injury: the methodology and results of a British priority setting partnershi. Spinal Cord, 54: (5), 341–346. doi: 10.1038/sc.2015.199 |
72 | Veldema, J. , & Jansen, P. ((2020) ). Resistance training in stroke rehabilitation: systematic review and meta-analysis. Clin Rehabil, 34: (9), 1173–1197. doi: 10.1177/0269215520932964 |
73 | WHO. (2011). World report on disability 2011. Retrieved from: https://www.who.int/publications/i/item/9789241564182 |
74 | Wijemanne, S. , & Jankovic, J. ((2019) ). Hand, foot, and spine deformities in parkinsonian disorders. J Neural Transm (Vienna), 126: (3), 253–264. doi: 10.1007/s00702-019-01986-1 |
75 | Williams, G. , Hassett, L. , Clark, R. , Bryant, A. , Olver, J. , Morris, M. E. , & Ada, L. ((2019) ). Improving Walking Ability in People With Neurologic Conditions: A Theoretical Framework for Biomechanics-Driven Exercise Prescription. Arch Phys Med Rehabil, 100: (6), 1184–1190. doi: 10.1016/j.apmr.2019.01.003 |
76 | Williams, G. , Hassett, L. , Clark, R. , Bryant, A. L. , Morris, M. E. , Olver, J. , & Ada, L. (2022). Ballistic resistance training has a similar or better effect on mobility than non-ballistic exercise rehabilitation in people with a traumatic brain injury: a randomised trial. J Physiother. doi: 10.1016/j.jphys.2022.09.004 |
77 | Yang, X. , & Wang, Z. ((2023) ). Effectiveness of Progressive Resistance Training in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Eur Neurol, 86: (1), 25–33. doi: 10.1159/000527029 |