Enhancing management of agitation after traumatic brain injury: Psychiatric perspectives and quantitative assessments
Abstract
BACKGROUND:
Post-traumatic agitation is a common and problematic complication after traumatic brain injury. It may present with features consistent with psychiatric disorders, which may provide clues as to management.
OBJECTIVE:
This is a narrative review of pertinent literature and a description of a collaborative clinical approach utilizing psychiatric and brain injury rehabilitation strategies to optimize outcomes in the management of post-traumatic agitation.
METHODS:
Describe and provide evidence for a transdisciplinary clinical approach supported by existing literature and clinical experience.
RESULTS:
Given the heterogeneity of the problem and limitations in the current literature there is no standardized approach to manage post-traumatic agitation; nevertheless, a strategy is proposed that clinicians may utilize to guide treatment and assess efficacy of the chosen intervention(s).
CONCLUSION:
A clinical approach that uses quantitative assessment of targeted behavior to objectively evaluate pharmacological interventions that are generated by a collaborative approach may yield improved outcomes for managing post-traumatic agitation.
1Introduction
Post-traumatic agitation cannot be defined by one specific behavior but typically includes a spectrum of behaviors ranging from impulsivity, motor restlessness, disinhibition, irritability, emotional lability to anger, agitation, and aggression (Wiart et al., 2016). Agitation is present in 11–70% patients with traumatic brain injury (TBI), usually lasts between 1–14 days but often longer (McNett et al., 2012) and has been linked to longer acute care and rehabilitation lengths of stay, poorer recovery on functional measures and cognitive outcomes (Bogner et al., 2001). Many of the behaviors seen in post-traumatic agitation are often seen in different psychiatric disorders, and the rationale for management of post-traumatic agitation is in part based on manifestations of psychiatric conditions. There is also a bi-directional association between TBI and psychiatric disorders which may also be relevant in terms of determining clinical management. Because of this, we suggest that a collaborative approach to the management of agitation following acute brain injury that includes psychiatry as part of the treatment team may improve outcomes. Accordingly, this article is written in part to provide examples of this transdisciplinary approach that we use clinically. Similarly, the literature review was performed to identify articles that illustrate specific points, and while efforts were made to describe the quality of some of the cited studies, this is not a systematic review.
Despite the prevalence of post-traumatic agitation and the multiple clinical challenges that may result, there is no clear standard of care for addressing this problem. One reason for this is the multifactorial nature of the contributors to post-traumatic agitation. Most clinicians would agree that pre-injury characteristics, structural damage, alterations in biochemistry and concomitant neuro-medical, psychiatric and environmental conditions may all contribute to the problem.
Potentially relevant pre-injury risk factors include a history of impulsive aggression, substance use, prior arrest, premorbid affective disorders, personality disorders, substance use disorders and disorders of conduct (Kim, 2002; Vassallo et al., 2007). Risk factors associated with post-traumatic agitation include frontotemporal injury, duration of loss of consciousness, disorientation, comorbid medical conditions, and use of anticonvulsant medications (Warden et al., 2006).
Structural injury, to the frontal lobe and prefrontal cortex, particularly the ventromedial and orbitofrontal cortex, has been associated with increased hostility, impulsivity and an increase in aggressive episodes (Lauterbach et al., 2015). Furthermore, reduction in prefrontal capability to process information makes patients more prone to paranoid interpretations (Koponen et al., 2002). The neurocircuitry of aggression in TBI is crucial to understanding the mechanisms of injury and choice of pharmacological interventions to address agitation. Traumatic injury causes an abrupt and significant release of multiple neurotransmitters in the brain including serotonergic, cholinergic and catecholaminergic systems. Increased dopamine and norepinephrine may be associated with increased aggression in animal models (Eichelman, 1990) and dopamine excess is implicated in psychosis and impulse control disorders that are often associated with agitation. Impaired serotonergic functioning is a common marker for impulsive aggression and reduced serotonin levels are associated with increased levels of aggression (Brower & Price, 2001). Accordingly, serotonin specific reuptake inhibitors (SSRI’s) are often used in management of agitation following traumatic brain injury (Luauté et al., 2016).
In the early period following TBI, emotional and behavioral disturbances may be associated with post-traumatic encephalopathy, reflecting neurotrauma-induced diffuse brain dysfunction and / or manifestations of focal injuries to brain systems subserving emotional regulation and comportment. However, in some cases, emotional and behavioral dyscontrol are manifestations of other psychiatric conditions (e.g., major depressive episode, manic, hypomanic, or mixed episode, post-traumatic stress disorder, anxiety disorders, substance use disorders, psychotic disorders) (Arciniegas & Wortzel, 2014) including recurrence or exacerbation of preinjury psychiatric disorders (Silver, McAllister & Arciniegas, 2009). Thus, a greater understanding of preexisting psychiatric disorders should be considered and could alter recommendations for the initial pharmacological management of agitation.
TBI is associated with a higher risk of neuropsychiatric disorders, and psychiatric disorders are a risk factor for TBI indicating a bidirectional relationship (Rao et al., 2015). It is important to consider that psychiatric disorders may present somewhat uniquely in patients with TBI vs without TBI. For example, a person with TBI history may manifest depressive symptoms in patterns that are different from a person who did not suffer a TBI. Depressed moods are more likely to be expressed as irritability, frustration, anger, hostility, and aggression in those with TBI than as sadness (Seel, Macciocchi & Kreutzer, 2010). Further, a complex interplay of factors and underlying causes must be considered prior to treatment (see Table 1). These can be conceptualized as preinjury risk factors, those related directly related to the trauma and those indirectly related to trauma.
Table 1
Risk Factors associated with post-traumatic agitation
| Pre -injury risk factors | Factors related directly to trauma | Factors indirectly related to trauma |
| Prior psychiatric diagnosis | Aphasia, cognitive deficits | Delirium |
| Chronic substance use | Post-traumatic amnesia | Infections |
| Adult/childhood behavioral issues | Pain (uncontrolled) and sensory deficits | Metabolic disturbances |
| Poor psychosocial function | Seizures (non-convulsive status) | Sleep dysfunction, circadian rhythm changes |
| Neurotransmitter derangement | Medications (abrupt discontinuation, excessive sedation) | |
| Endocrine dysfunction |
2Treatment strategies
Guidelines for treatment include identification of target symptoms, interviewing a knowledgeable informant, along with the use of an objective scale to quantify behavior, and a differential diagnosis for the behavior other than the injury itself (review list of factors in Table 1). Non-pharmacological interventions including behavioral and environmental modifications are first line followed by pharmacological interventions. For an excellent in-depth review of non-pharmacological interventions, readers are referred to Carrier et al. (2023).
2.1Environmental modifications
Familiarizing information (family, friends), environmental reorientation (calendars, clocks), safety aides (e.g., bed rails, netted beds), surveillance systems (video monitoring, electronic bracelets).
2.2Patient program modifications
Consistent staffing, 1:1 staffing if needed, reducing care tasks, address sleep hygiene and circadian rhythm (reduced daytime naps or small scheduled naps).
2.3Behavioral modification
Preventive strategies, written behavior plans, functional assessment of behavior, physical restraints as needed (e.g., vests, mitts) (Carrier et al., 2021).
Behavioral management strategies are useful for management of aggression and include:
2.4Replacement strategies and decelerative techniques
Replacement strategies include assertiveness training (beneficial for patients who become angry when they fail to get their needs met) and differential reinforcement scheduling (to reduce rate of pre-violent behaviors). Decelerative techniques include social extinction, contingent observation, and self-controlled time outs (Arciniegas & Wortzel, 2014).
3Literature review of pharmacological management
Generally, the level of evidence to promote the use of medications for management of agitation following TBI is quite low and caution should be utilized while using medications in the management of TBI related agitation. Even though there is lack of quality evidence to support the use of antipsychotics for brain injury related agitation, it is amongst the most prevalent choice for treatment.
Typical antipsychotics and benzodiazepines are best avoided. They can delay neurobehavioral recovery, neuroplasticity, and repair post TBI (Arciniegas & Silver, 2006; Rao, Jellinek & Woolston, 1985). However, a recent study evaluating the effects of lorazepam after experimental TBI in rats showed that intermittent use of lorazepam may have an effect in improving agitation and aggression in TBI without affecting functional recovery (Cheng et al., 2018). As a general guideline, avoid medications that can worsen cognition including potent dopamine blockers (typical antipsychotics, drugs with potent D2 blocking properties like risperidone) and medications with anticholinergic side effects (paroxetine).
3.1Atypical antipsychotics
Although numerous observational studies have reported a reduction in agitation with the use of antipsychotic agents, we found no controlled studies evaluating the efficacy of antipsychotics other than olanzapine (Williamson et al., 2019). Several studies suggest that one should not use neuroleptics in the long term to treat aggressiveness after TBI except in case of prior psychiatric disease. It is essential to use certain precautions with the use of antipsychotics including epileptogenic risk, extrapyramidal side effects (lower with atypical antipsychotics) and cardiovascular risk factors (Luauté et al., 2016). There is some anecdotal evidence to support the use of quetiapine, ziprasidone and olanzapine in management of agitation following TBI (Kim & Bijlani, 2006; Nash et al., 2019). There is one case report (Kim & Bijlani, 2006) and one controlled study regarding the use of olanzapine in agitation and aggression following brain injury (Maturana Waidele & Maturana Rodillo, 2009) with one observation study showing an increase in duration of post-traumatic amnesia (Kooda et al., 2015)
3.2B blockers
B antagonists (propranolol and pindolol) have the best evidence supporting its use in the management of agitation or aggressiveness following TBI, although the number of studies and sample size of studies reviewed were relatively small (Rahmani et al., 2021). A randomized controlled study of 21 patients by Brooke et al. on 21 patients using propranolol, found it to be beneficial in reducing intensity of aggressive episodes but no change in frequency of episodes, grade B evidence (Brooke et al., 1992). Propranolol was well tolerated in doses up to 420 mg daily with reports of bradycardia and hypotension at a dose of 520 mg/day (Greendyke et al., 1986).
3.3Antiepileptic drugs
Although valproic acid is one of the most prescribed medications for management of post-traumatic agitation (Francisco et al., 2007), the evidence to support its efficacy remains limited. It appears to have a relatively benign neuropsychological profile making it cognitively safer to use in individuals with brain injury (Dikmen et al., 2000). Chatman, Showalter, and colleagues performed a retrospective chart review with 29 patients with improvement in 62% patients with a mean dose of 1257 mg/day (no objective measures were used). Uncontrolled case series have reported a reduction in agitation and aggressive behaviors with the use of valproic acid and carbamazepine (Williamson et al., 2019). There was one unpublished study of TBI patients with affective lability and alcohol dependence where valproic acid showed effectiveness in reducing weekly agitation behavioral scale (ABS) scores rated by spouse or significant other’s (Beresford et al., 2015).
A recent article by Hammond et al. did not demonstrate a difference between placebo and carbamazepine in the reduction of irritability and aggression following TBI, other than clinician ratings-which did support the use of carbamazepine. The participants (35 in each group) were all at least 6 months post-injury, with dose titrated up to 400 mg / day (Hammond et al., 2021). There is one double blind placebo-controlled trial (48 patients) supporting the use of oxcarbazepine in management of post-traumatic aggression at doses of 1200–2400/ day when compared to placebo (Mattes, 2005). There is very limited evidence to support the use of lamotrigine in post-traumatic agitation.
3.4Antidepressants
SSRI’s are used to regulate mood following TBI but there is limited evidence supporting the effectiveness of SSRI’s as a primary agent for post-traumatic agitation during acute rehabilitation. One controlled study using sertraline (100 mg/day, 11patients) vs placebo did not show significant effects on agitation (Meythaler et al., 2001) whereas an uncontrolled case series of 13 patients using sertraline 200 mg/day showed improvement in irritability and aggression after 8 weeks of treatment (Kant, Smith-Seemiller & Zeiler, 1998). A retrospective study by Mysiw et al. (1998) showed that amitriptyline may be useful as have a role in reducing the severity of directed agitation that is seen during post-traumatic amnesia (Mysiw, Jackson & Corrigan, 1988).
3.5Stimulants
There is mixed evidence related to the use of stimulants in acute post-traumatic agitation. The only two controlled studies reviewing the effects of amphetamines on agitation as a secondary outcome were nonsignificant (Francisco et al., 2007) or showed a worsening in agitation (Hart et al., 2018). A randomized single blind study using 38 patients were randomized to 30 mg of methylphenidate vs placebo showed a significant reduction in anger and aggression (Mooney & Haas, 1993).
3.6Amantadine
Three RCTs examined the impact of amantadine on irritability, aggression, and anger (Hammond et al., 2014, 2015; Neumann et al., 2017). It is postulated that amantadine may improve irritability and aggression through enhancing cognitive function and through this mechanism, may enhance cognitive appraisal and behavioral disinhibition (Hammond et al., 2014; Kraus et al., 2005). The impact of amantadine on irritability was examined in two studies (Hammond et al., 2014, 2015). Both studies used a 28-day follow-up time point, with a further 60-day follow-up also included in the 2015 study. At the 28-day follow-up, only one of the two studies found a significantly greater reduction in irritability in the treatment group (Hammond et al., 2014). However, when the results from these studies were combined in a meta-analysis, the pooled result did favor amantadine over placebo (Hammond et al., 2015).
4Assessing efficacy and tailoring the treatment plan
In terms of choice of pharmacological agents, the literature is replete with articles of all types that describe the use of medications to manage problematic behaviors after TBI, including several reviews (Caplan et al., 2021; Kalra & Watanabe, 2017; Rahmani et al., 2021), as described above. One main reason for the large number of publications is undoubtedly the fact that there is no one medication that works for all patients.
While a neuropharmacological and evidence-based approach has been described above, the clinician is still left with many pharmacological options. Given uncertain regarding efficacy, it is important to have a sound approach in determining whether a drug that has been initiated is in fact helpful. However, reliance on anecdotal or subjective reports is a common practice. Such an approach often leads to a reaction to more extreme or otherwise noteworthy observations even though such episodes may in fact be rare, potentially masking underlying improvement. The physician usually is the main clinician determining adjustments in medications, but typically spends much less time with the patient than other team members, potentially exacerbating the problem of making decisions based on limited observations. Reliance on physician assessments may also lead to an underappreciation of the previously described indirect factors that may be important elements fueling the behavioral issues.
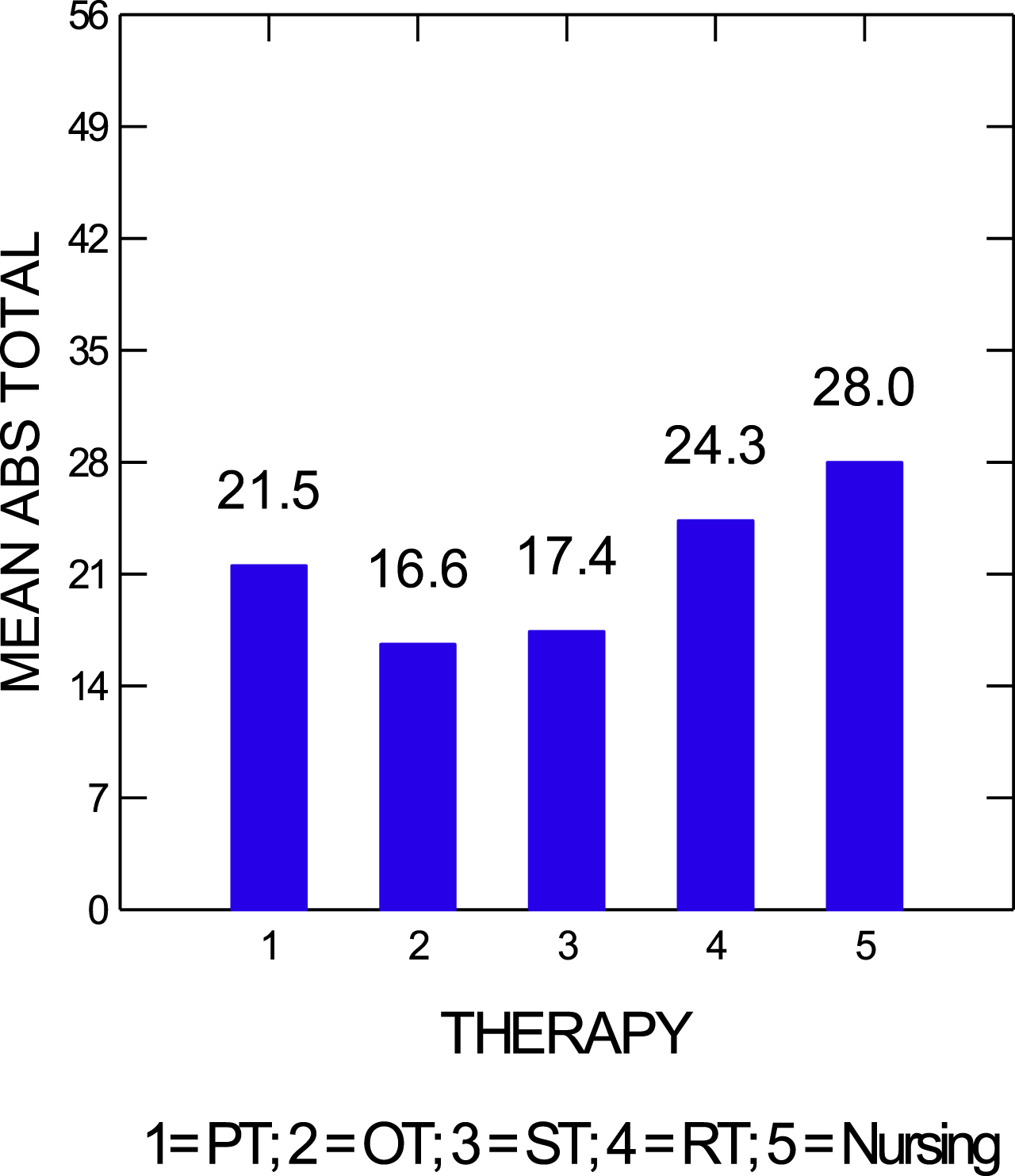
To avoid this problem, it is important to utilize objective measures when assessing problematic behaviors and determination of efficacy of interventions once they are initiated. The ABS and the Overt Aggression Scale are two tools that can be used to characterize and track such behaviors (Corrigan, 1989; Yudofsky et al., 1986). Figure 1 demonstrates improvement in mean ABS scores over time. These data can be correlated to specific interventions including, but not limited to, the initiation of a medication to address problematic behavior. When using a quantitative approach, it is strongly encouraged to obtain data at different times of the day and in different settings (e.g., in bed, during different interventions). When multiple data points are obtained, patterns may be identified that can help the team to determine whether certain changes in treatment, including medication changes, are leading to overall improvement. Trends may emerge that suggest that certain activities or interventions may be associated with worsened scores. Figure 2 supports the hypothesis that nursing interventions are associated with the elevations in ABS scores. Further assessment of more specific activities during these interventions may lead to development of strategies to lessen or modify those activities that are heightening problematic behaviors. Some of these variables may not be as easy to address than others. For example, increased ABS scores related to nursing may be due to care needs related to incontinence or medication administration. Increased ABS scores in physical therapy may be due to pain related to increased activity, or overstimulation in the therapy gym setting. Hypotheses can be generated which can be tested with further quantitative assessments, for example trialing pain medication before physical therapy.
Fig. 1
Mean agitated behavior scale (ABS) scores by day. These data can be paired with interventions to determine efficacy.
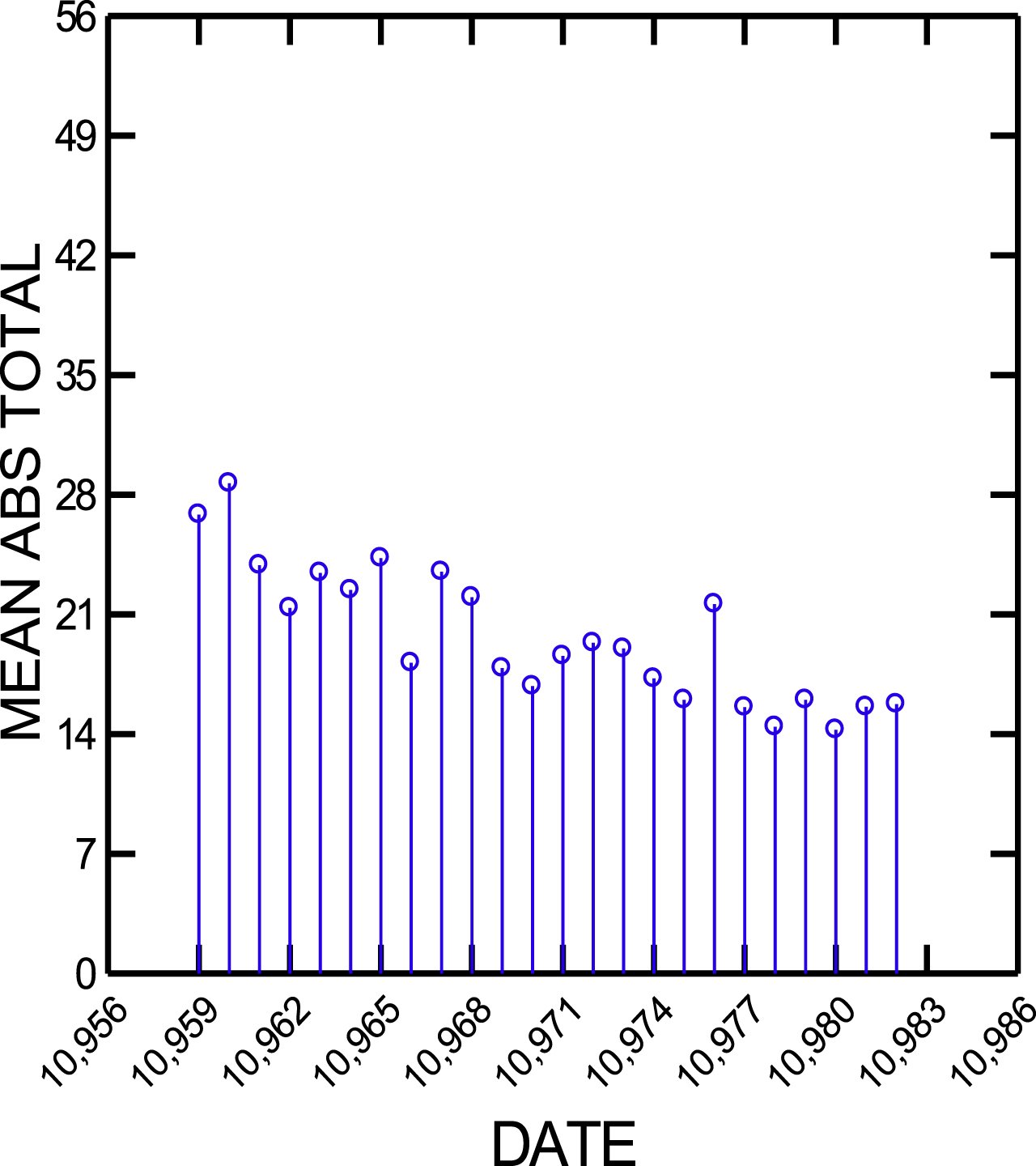
Fig. 2
Mean agitated behavior scale (ABS) scores based on type of therapy/intervention.
When focusing on problematic and challenging behaviors it may be more useful to track changes of individual items rather than total scores from scales such as the ABS. For instance, physical aggression may be the most problematic behavior, and a change in this sub-score may be diluted when using tools that summate several other behaviors that are not problematic. Using a sleep log and determining the utility of medications or other interventions such as turning off the TV or removing a smartphone at a pre-determined time to improve sleep is another, simpler example of an objective assessment. Regardless of the measures being used, this quantitative approach is based on a long history of the use of single subject designs including in TBI rehabilitation, initially developed as a research strategy, but with clear clinical applications (Whyte, DiPasquale & Vaccaro, 1999). The patients serve as their own control to assess the impact of an intervention. More data points will lead to greater clarity of the effects of time of day or interventions such as therapy, and the data can be analyzed statistically to further improve confidence in interpreting results of an intervention. However, increased data collection will increase the burden of documentation for the treating clinicians.
Tracking the use of as-needed medications might be considered a quantitative way to assess the effectiveness of scheduled medications. However, if this strategy is used, it is very important to ensure that there are well-defined parameters for administering the as-needed medications. Determining the efficacy of an as-needed medication is especially challenging not only because of the difficulty in standardizing the decisions for administration but also because it is often hard to demonstrate cause and effect. Behavioral problems associated with TBI are often relatively short-lived, and one is left determining whether clinical improvement seen after such an outburst is due to medication administration or because it has run its course, with or without the additional medicine. Incorrectly ascribing improvement to the as-needed medication may lead to inappropriate administration of a medication that may slow neurological recovery after brain injury (Roy, Vaishnavi & Rao, 2022). Because of this difficulty in demonstrating efficacy and potential risk, it is advised to employ as needed medications judiciously.
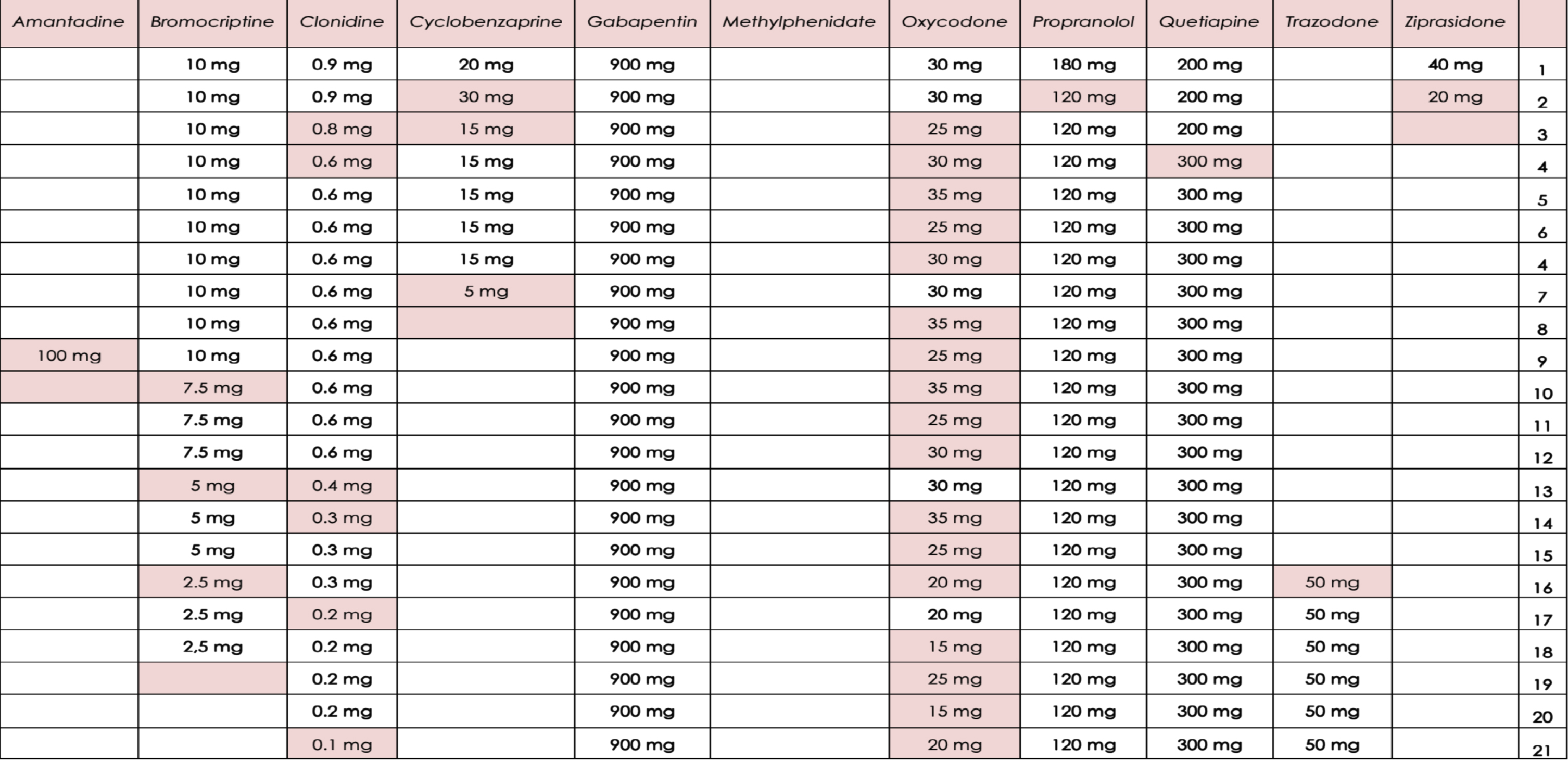
Polypharmacy is a frequent problem encountered when treating patients with significant brain injury and overall medical complexity (Cosano et al., 2016). Multiple medications that are being used to treat other medical problems may contribute to worsening of problematic behavior and make the evaluation of efficacy of a specific drug more difficult. It is often useful to systematically track these medications and periodically reconsider their necessity. They may have been started at different times in the patient’s clinical course to address a number of different conditions, and therefore the need for these medications may change over time. There may be opportunities to simplify the medication list (including times of administration) and minimize use of those medications that may be contributing to cognitive deficits. Organizing medications that are being prescribed in a table that allows the team to see when certain drugs were started and discontinued is a useful way to help interpret the efficacy of a medication to help manage problematic behavior, to consider drug interactions and to quickly review the medication profile. Figure 3 is an actual medication profile that was paired with observational data on behavior to help assess the efficacy of medication changes, in this case the reduction of bromocriptine, cyclobenzaprine and clonidine, as well as the introduction of trazodone to improve sleep. This profile is also good example of how extensive a medical profile can become, making the assessment of the clinical impact of a specific drug quite challenging.
Fig. 3
An example of part of a table used to track pharmacological management of a patient. Changes in medications are highlighted to aid the team in assessing potential medication effects on behavior.
5Conclusion
The approach to management of agitation in traumatic brain injury should include a careful assessment of preinjury psychiatric conditions. The relationship between TBI and psychiatric diagnoses and the implications for the development of behavioral problems is often under-appreciated despite the fact that this may provide a good starting point for initiation of interventions. It should be noted, however, that the evidence regarding pharmacological interventions is rather insufficient. There is some support in the literature for the use of propranolol, valproic acid and olanzapine; however, these (and other medications) need to be further studied. The use of amantadine may improve irritability whereas antipsychotics may prolong post-traumatic amnesia.
Given the fact that there are no robust guidelines to manage agitation after TBI as well as the wide variety of pathologies and clinical presentations, it is important to incorporate quantitative assessments and utilize objective measures when assessing problematic behaviors to determine efficacy of interventions. This can help govern decisions as to if and when to alter the initial treatment plan, which is necessary much more often than not. Further studies on tailored interventions throughout the acute, rehabilitation settings are needed to assess the efficacy and safety of pharmacological agents for the management of agitated behaviors.
Author contributions
Both authors made significant contributions to the conceptualization, writing and editing of the article.
Ethics statement
This study, as a literature review, is exempt from institutional review board approval and does not contain elements requiring informed consent.
Conflict of interest
The authors report no conflicts of interest.
Funding
This article did not receive any specific funding from public, commercial or non-profit funding sources.
References
1 | Arciniegas, D. B. , & Silver, J. M. ((2006) ). Pharmacotherapy of posttraumatic cognitive impairments. Behavioural Neurology, 17: (1), 25–42. doi: 10.1155/2006/460592. |
2 | Arciniegas, D. B. , & Wortzel, H. S. ((2014) ). Emotional and behavioral dyscontrol after traumatic brain injury. Psychiatric Clinics, 37: (1), 31–53. doi: 10.1016/j.psc.2013.12.001. |
3 | Beresford, T. P. , Schmidt, B. K. , Wortzel, H. , Buchanan, J. , Thumm, B. , Maravilla, F. , Temple, B. , Bartlett, S. , Kelly, J. , & Arciniegas, D. ((2015) ) A double-blind trial of divalproex sodium for affective lability and ethanol use following traumatic brain injury, [Conference abstract]. 168th Annual Meeting of the American Psychiatric Association, Toronto, Canada. |
4 | Bogner, J. A. , Corrigan, J. D. , Fugate, L. , Mysiw, W. J. , & Clinchot, D. ((2001) ). Role of agitation in prediction of outcomes after traumatic brain injury. American Journal of Physical Medicine & Rehabilitation, 80: (9), 636–644. doi: 10.1097/00002060-200109000-00002. |
5 | Brooke, M. M. , Patterson, D. R. , Questad, K. A. , Cardenas, D. , & Farrel-Roberts, L. ((1992) ). The treatment of agitation during initial hospitalization after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 73: (10), 917–921. |
6 | Brower, M. C. , & Price, B. H. ((2001) ). Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. Journal of Neurology, Neurosurgery & Psychiatry, 71: (6), 720–726. doi: 10.1136/jnn71.6.720. |
7 | Caplan, B. , Bogner, J. , Brenner, L. , Malec, J. , McKay, A. , Trevena-Peters, J. , & Ponsford, J. ((2021) ). The use of atypical antipsychotics for managing agitation after traumatic brain injury. Journal of Head Trauma Rehabilitation, 36: (3), 149–155. doi: 10.1097/HTR.0000000000000614. |
8 | Carrier, S. L. , Hicks, A. J. , Ponsford, J. , & McKay, A. ((2021) ). Managing agitation during early recovery in adults with traumatic brain injury: An international survey. Annals of Physical and Rehabilitation Medicine, 64: (5), 101532–10.1016/j.rehab.2021.101532. |
9 | Carrier, S. L. , Ponsford, J. , Phyland, R. K. , Hicks, A. J. , & McKay, A. ((2023) ). Effectiveness of non-pharmacological interventions for agitation during post-traumatic amnesia following traumatic brain injury: a systematic review. Neuropsychology Review, 33: (2), 374–392. |
10 | Cheng, J. P. , Leary, J. B. , O’Neil, D. A. , Meyer, E. A. , Free, K. E. , Bondi, C. O. , & Kline, A. E. ((2018) ). Spontaneous recovery of traumatic brain injury-induced functional deficits is not hindered by daily administration of lorazepam. Behavioural Brain Research, 339: , 215–221. doi: 10.1016/j.bbr.2017.11.039. |
11 | Corrigan, J. D. ((1989) ). Development of a scale for assessment of agitation following traumatic brain injury. Journal of Clinical and Experimental Neuropsychology, 11: (2), 261–277. doi: 10.1080/01688638908400888. |
12 | Cosano, G. , Giangreco, M. , Ussai, S. , Giorgini, T. , Biasutti, E. , Barbone, F. , & Pisa, F. E. ((2016) ). Polypharmacy and the use of medications in inpatients with acquired brain injury during post-acute rehabilitation: a cross-sectional study. Brain Injury, 30: (3), 353–362. doi: 10.3109/02699052.2015.1118767. |
13 | Dikmen, S. S. , Machamer, J. E. , Winn, H. R. , Anderson, G. D. , & Temkin, N. R. ((2000) ). Neuropsychological effects of valproate in traumatic brain injury: a randomized trial. Neurology, 54: (4), 895–902. doi: 10.1212/wnl.54.4.895. |
14 | Eichelman, B. S. ((1990) ). Neumical and psychopharmacologic aspects of aggressive behavior. Annual Review of Medicine, 41: (1), 149–158. doi: 10.1146/annurev.me.41.020190.001053 roche. |
15 | Francisco, G. E. , Walker, W. C. , Zasler, N. D. , & Bouffard, M. H. ((2007) ). Pharmacological management of neurobehavioural sequelae of traumatic brain injury: a survey of current physiatric practice. Brain Injury, 21: (10), 1007–1014. |
16 | Greendyke, R. M. , Kanter, D. R. , Schuster, D. B. , Verstreate, S. , & Wootton, J. ((1986) ). Propranolol treatment of assaultive patients with organic brain disease: a double-blind crossover, placebo-controlled study. The Journal of Nervous and Mental Disease, 174: (5), 290–294. doi: 10.1097/00005053-198605000-00005. |
17 | Hammond, F. M. , Bickett, A. K. , Norton, J. H. , & Pershad, R. ((2014) ). Effectiveness of amantadine hydrochloride in the reduction of chronic traumatic brain injury irritability and aggression. Journal of Head Trauma Rehabilitation, 29: (5), 391–399. doi: 10.1097/01.HTR.0000438116.56228.de. |
18 | Hammond, F. M. , Sherer, M. , Malec, J. F. , Zafonte, R. D. , Whitney, M. , Bell, K. , Dikmen, S. , Bogner, J. , Mysiw, J. , Pershad, R. & Amantadine Irritability Multisite Study Group, ((2015) ). Amantadine effect on perceptions of irritability after traumatic brain injury: results of the amantadine irritability multisite study. Journal of Neurotrauma, 32: (16), pp. 1230–1238. doi: 10.1089/neu.2014.3803. |
19 | Hammond, F. M. , Zafonte, R. D. , Tang, Q. , & Jang, J. H. ((2021) ). Carbamazepine for irritability and aggression after traumatic brain injury: a randomized, placebo-controlled study. Journal of Neurotrauma, 38: (16), 2238–2246. doi: 10.1089/neu.2020.7530. |
20 | Hart, T. , Whyte, J. , Watanabe, T. , & Chervoneva, I. ((2018) ). Effects of dextroamphetamine in subacute traumatic brain injury: A randomized, placebo-controlled pilot study. Journal of Neuroscience Research, 96: (4), 702–710. doi: 10.1002/jnr.2410. |
21 | Kalra, I. D. , & Watanabe, T. K. ((2017) ). Mood stabilizers for traumatic brain injury-related agitation. Journal of Head Trauma Rehabilitation, 32: (6), E61–E64. doi: 10.1097/HTR.0000000000000359. |
22 | Kant, R. , Smith-Seemiller, L. , & Zeiler, D. ((1998) ). Treatment of aggression and irritability after head injury. Brain Injury, 12: (8), 661–666. doi: 10.1080/026990598122223. |
23 | Kim, E. ((2002) ). Agitation, aggression, and disinhibition syndromes after traumatic brain injury. NeuroRehabilitation, 17: (4), 297–310. |
24 | Kim, E. , & Bijlani, M. ((2006) ). A pilot study of quetiapine treatment of aggression due to traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 18: (4), 547–549. doi: 10.1176/jn2006.18.4.547. |
25 | Kooda, K. , Aho, J. , Weber, D. , & Brown, A. ((2015) ). 1149: The effect of antipsychotic use post-traumatic brain injury on duration of post-traumatic amnesia. Critical Care Medicine, 43: (12), 289. |
26 | Koponen, S. , Taiminen, T. , Portin, R. , Himanen, L. , Isoniemi, H. , Heinonen, H. , Hinkka, S. , & Tenovuo, O. ((2002) ). Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. American Journal of Psychiatry, 159: (8), 1315–1321. doi: 10.1176/appi.aj159.8.1315. |
27 | Kraus, M. F. , Smith, G. S. , Butters, M. , Donnell, A. J. , Dixon, E. , Yilong, C. , & Marion, D. ((2005) ). Effects of the dopaminergic agent and NMDA receptor antagonist amantadine on cognitive function, cerebral glucose metabolism and D2 receptor availability in chronic traumatic brain injury: a study using positron emission tomography (PET). Brain Injury, 19: (7), 471–479. doi: 10.1080/02699050400025059. |
28 | Lauterbach, M. D. , Notarangelo, P. L. , Nichols, S. J. , Lane, K. S. , & Koliatsos, V. E. ((2015) ). Diagnostic and treatment challenges in traumatic brain injury patients with severe neuropsychiatric symptoms: insights into psychiatric practice. Neuropsychiatric Disease and Treatment, 11: , 1601–1607. doi: 10.2147/NDT.S80457. |
29 | Luauté, J. , Plantier, D. , Wiart, L. , & Tell, L. ((2016) ). Care management of the agitation or aggressiveness crisis in patients with TBI. Systematic review of the literature and practice recommendations. Annals of Physical and Rehabilitation Medicine, 59: (1), 58–67. doi: 10.1016/j.rehab.2015.11.001. |
30 | Mattes, J. A. ((2005) ). Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology, 25: (6), 575–579. doi: 10.1097/01.jc0000186739.22395.6b. |
31 | Maturana Waidele, R. , & Maturana Rodillo, R. ((2009) ). Control de la agresividad con olanzapina en pacientes post tec [aggressiveness control using olanzepine in post-TBI patients]. Ciencia & Trabajo, 31: , 22–24. |
32 | McNett, M. , Sarver, W. , & Wilczewski, P. ((2012) ). The prevalence, treatment and outcomes of agitation among patients with brain injury admitted to acute care units. Brain Injury, 26: (9), 1155–1162. doi: 10.3109/02699052.2012.667587. |
33 | Meythaler, J. M. , Depalma, L. , Devivo, M. J. , Guin-Renfroe, S. , & Novack, T. A. ((2001) ). Sertraline to improve arousal and alertness in severe traumatic brain injury secondary to motor vehicle crashes. Brain Injury, 15: (4), 321–331. doi: 10.1080/026990501750111274. |
34 | Mooney, G. F. , & Haas, L. J. ((1993) ). Effect of methylphenidate on brain injury-related anger. Archives of Physical Medicine and Rehabilitation, 74: (2), 153–160. |
35 | Mysiw, W. J. , Jackson, R. D. , & Corrigan, J. D. ((1988) ). Amitriptyline for post-traumatic agitation. American Journal of Physical Medicine & Rehabilitation, 67: (1), 29–33. doi: 10.1097/00002060-198802000-00006. |
36 | Nash, R. P. , Weinberg, M. S. , Laughon, S. L. , McCall, R. C. , Bateman, J. R. , & Rosenstein, D. L. ((2019) ). Acute pharmacological management of behavioral and emotional dysregulation following a traumatic brain injury: a systematic review of the literature. Psychosomatics, 60: (2), 139–152. doi: 10.1016/j.psym.2018.11.009. |
37 | Neumann, D. , Hammond, F. M. , Malec, J. F. , Zafonte, R. D. , Sherer, M. , Bogner, J. , Dikmen, S. , Whitney, M. P. , Bell, K. R. , Perkins, S. M. and Moser, E. A. ((2017) ). Potential impact of amantadine on aggression in chronic traumatic brain injury. Journal of Head Trauma Rehabilitation, 32: (5), 308–318. doi: 10.1097/HTR.0000000000000342. |
38 | Prais, H. A. , Nicolato, R. , & Caramelli, P. ((2009) ). Posttraumatic brain injury psychosis successfully treated with olanzapine. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 34: (1), 233–235. doi: 10.1016/j.pnpb2009.08.026. |
39 | Rahmani, E. , Lemelle, T. M. , Samarbafzadeh, E. , & Kablinger, A. S. ((2021) ). Pharmacological treatment of agitation and/or aggression in patients with traumatic brain injury: a systematic review of reviews. Journal of Head Trauma Rehabilitation, 36: (4), E262–E283. doi: 10.1097/HTR.0000000000000656. |
40 | Rao, N. , Jellinek, H. M. , & Woolston, D. C. ((1985) ). Agitation in closed head injury: haloperidol effects on rehabilitation outcome. Archives of Physical Medicine and Rehabilitation, 66: (1), 30–34. |
41 | Rao, V. , Koliatsos, V. , Ahmed, F. , Lyketsos, C. , & Kortte, K. ((2015) ). Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. In Seminars in Neurology, (Vol. 35: , No. 01, pp. 064–082). Thieme Medical Publishers. doi: 10.1055/s-0035-1544241. |
42 | Roy, D. , Vaishnavi, S. , & Rao, V. ((2022) ). Rational neuropharmacology in traumatic brain injury. In Zollman, F.S. (ed), Manual of Traumatic Brain Injury: Assessment and Management, 3rd ed., pp. 309–319. Springer Publishing Co., NY, NY. doi: 10.1891/9780826147684. |
43 | Seel, R. T. , Macciocchi, S. , & Kreutzer, J. S. ((2010) ). Clinical considerations for the diagnosis of major depression after moderate to severe TBI. The Journal of Head Trauma Rehabilitation, 25: (2), 99–112. doi: 10.1097/HTR.0b013e3181ce3966. https://doi.org/10.1007/s11065-022-09544-5. |
44 | Silver, J. M. , McAllister, T. W. , & Arciniegas, D. B. ((2009) ). Depression and cognitive complaints following mild traumatic brain injury. American Journal of Psychiatry, 166: (6), 653–661. doi: 10.1176/appi.aj2009.08111676. |
45 | Tramontana, M. G. , Cowan, R. L. , Zald, D. , Prokop, J. W. , & Guillamondegui, O. ((2014) ). Traumatic brain injury-related attention deficits: Treatment outcomes with lisdexamfetamine dimesylate (Vyvanse). Brain Injury, 28: (11), 1461–1472. doi: 10.3109/02699052.2014.930179. |
46 | Vassallo, J. L. , Proctor-Weber, Z. , Lebowitz, B. K. , Curtiss, G. , & Vanderploeg, R. D. ((2007) ). Psychiatric risk factors for traumatic brain injury. Brain injury, 21: (6), 567–573. doi: 10.1080/02699050701426832. |
47 | Warden, D. L. , Gordon, B. , McAllister, T. W. , Silver, J. M. , Barth, J. T. , Bruns, J. , Drake A. , Gentry, T. , Jagoda, A. , Katz, D. I. , Kraus, J. , Labbate, L. A. , Ryan, L. M. , Sparling, M. B. , Walters, B. , Whyte, J. , Zapaata, A. , & Zitnay G. ((2006) ). Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. Journal of Neurotrauma, 23: (10), 1468–1501. doi: 10.1089/neu.2006.23.1468. |
48 | Whyte, J. , DiPasquale, M. C. , & Vaccaro, M. ((1999) ). Assessment of command-following in minimally conscious brain injured patients. Archives of Physical Medicine and Rehabilitation, 80: (6), 653–660. doi: 10.1016/s0003-9993(99)90168-5. |
49 | Wiart, L. , Luauté, J. , Stefan, A. , Plantier, D. , & Hamonet, J. ((2016) ). Non pharmacological treatments for psychological and behavioural disorders following traumatic brain injury (TBI). A systematic literature review and expert opinion leading to recommendations. Annals of Physical and Rehabilitation Medicine, 59: (1), 31–41. doi: 10.1016/j.rehab.2015.12.001. |
50 | Williamson, D. , Frenette, A. J. , Burry, L. D. , Perreault, M. , Charbonney, E. , Lamontagne, F. , Potvin, M-J. , Giguere, J-F. , Mehta, S. , & Bernard, F. ((2019) ). Pharmacological interventions for agitated behaviours in patients with traumatic brain injury: a systematic review. BMJ Open, 9: , e029604. doi: 10.1136/bmjopen-2019-029604. |
51 | Yudofsky, S. C. , Silver, J. M. , Jackson, W. , Endicott, J. , & Williams, D. ((1986) ). The Overt Aggression Scale for the objective rating of verbal and physical aggression. The American Journal of Psychiatry, 143: , 35–39. doi: 10.1176/aj143.1.35. |




