Neuropsychological manifestations of long COVID in hospitalized and non-hospitalized Brazilian Patients
Abstract
BACKGROUND:
There has been a significant increase in number of patients seeking neuropsychological rehabilitation months after the acute phase of COVID-19 infection.
OBJECTIVE:
Identify the cognitive and psychiatric disorders in patients with long COVID or Post-Acute Sequelae of COVID (PASC) and explore the association between disease severity during the acute phase and persistent neuropsychological manifestations.
METHODS:
614 adults were assessed an average of eight months post-infection. Participants were, on average, 47.6 y.o., who sought rehabilitation for neuropsychological problems. Patients were evaluated using the Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS), Phonemic Verbal Fluency and Clock Drawing tests (NEUPSILIN) for executive functions, and the Hospital Anxiety and Depression Scale (HADS).
RESULTS:
The BNIS score was significantly below reference values in all subscales, especially affect and memory. Verbal Fluency and Clock Drawing subtest results were also lower. Patients with PASC tested high for anxiety/depression, but there was no statistically significant relationship between HADS and BNIS scores. Neuropsychological evaluations showed no differences in cognitive or psychiatric profiles between hospitalized and non-hospitalized patients.
CONCLUSIONS:
Neuropsychological results suggest executive function problems and high incidence of anxiety/depression, irrespective of acute-phase severity, underscoring a need for neurorehabilitation programs while providing data for public policy initiatives.
1Introduction
To date, the COVID-19 virus has infected over 456 million people worldwide. The pandemic has resulted in a global need for neurorehabilitation programs. Healthcare systems must adjust to this new reality and help COVID-19 survivors recover productive, functional lives in the workplace and in society (De Biase et al., 2020; Wade, 2020). Achieving this requires reliable data to determine the main, long-term symptoms in order to subsequently implement new public policies that support this growing population (Wilson et al., 2020).
Early studies on SARS CoV2 described psychiatric and neuropsychological symptoms in patients hospitalized during the acute phase, suggesting that COVID-19 was a systemic disease with central nervous system involvement (Almqvist et al., 2020; Mao et al., 2020; Roy et al., 2021; Vitalakumar et al., 2021). Diffuse neurological symptoms such as headaches, encephalopathies, encephalitis, confusion, dizziness and convulsions, acute cerebrovascular disease, and impaired consciousness were initially identified in COVID-19 inpatients (Mao et al., 2020). A systematic review study reported neurological complications and neuroradiological findings associated with coronavirus during the initial phase (Almqvist et al., 2020).
Over time, as our understanding of the disease grew, we began to see troubling, persistent effects: some survivors continued to present symptoms after the acute phase ended, a condition that came to be known as “long-COVID” (Halpin et al., 2020; Elkan et al., 2021; Kamal et al., 2021; Mendelson et al., 2020). Case series (Negrini et al., 2021) and quantitative studies with samples of up to 100 COVID-19 survivors (Halpin et al., 2020; Elkan et al., 2021) showed persistent neuropsychological issues. At the same time, these findings were being identified by qualitative studies using focus groups and interviews (Ladds et al., 2020).
Cognitive and psychiatric disorders were among the various symptoms of Post-Acute Sequelae of COVID (PASC) (Huang et al., 2021; Kamal et al., 2021; Miskowiak et al., 2021; Taquet, Geddes et al., 2021; Vanderlind et al., 2021). A later review also described neurological symptoms and cerebral pathogenic mechanisms (Sarubbo et al., 2022). Pathophysiological mechanisms disrupted brain homeostasis and the virus’s possible direct invasion of the brain resulted in persistent mental and cognitive symptoms (“neurocovid”) (Boldrini et al., 2021; Crunfli et al., 2020; Lee et al., 2021) associated with the inflammatory process (Zhou et al., 2020).
Since this is a new issue, there is no consolidated body of literature that addresses persistent neurocognitive deficits in non-hospitalized patients, especially those who had milder manifestations of COVID-19. A small number of studies began reporting that survivors of mild COVID-19 were experiencing lingering effects, including motor difficulties, and sensorial, neurological, cognitive, and mental health issues, in addition to persistent fatigue (Bliddal et al., 2021; Graham et al., 2021; Hampshire et al., 2021; Hellmuth et al., 2021; Johnsen et al., 2021; Woo et al., 2020). Graham et al. (2021) published a study with 50 COVID-19 survivors who had not been hospitalized yet developed neurological issues that lasted more than six weeks, the so-called “long haulers”. The most common neuropsychological problems they reported were brain fog, depression/anxiety, and reduced cognitive functioning that affected their quality of life. Mohamed-Hussein et al. (2021) concluded that non-hospitalized patients had more cognitive issues than those who had been hospitalized. In contrast, Johnsen et al. (2021) reported that three months after infection, previously hospitalized patients had more cognitive and clinical problems than non-hospitalized patients. To date, the vast majority of studies on mild COVID-19 are based on small samples, case studies, telephone interviews, and patient chart reviews. Thus, there is an urgent need for in-person neuropsychological and neuropsychiatric assessments on larger samples that can generate information to help guide programs and public policies for neurorehabilitation.
This study aims to identify cognitive dysfunction in COVID-19 survivors using a robust sample of in-person neuropsychological evaluations; explore the relationship between those disorders and anxiety and depression; and evaluate the association between persistent cognitive disorders and hospitalization or non-hospitalization during the acute phase of infection.
2Method
2.1Participants
Between April 2021 and January 2022, the Brasilia unit of the SARAH Network of Rehabilitation Hospitals saw 1,266 COVID-19 survivors who sought treatment for cognitive issues (attention, concentration, and memory difficulties, slow reasoning, black-outs, etc.) that were impacting their daily lives. All underwent in-person neuropsychological evaluations. Based on the information in patient charts (anamnesis, neurological examination, psychiatric history, and neuroimaging), the following cases were excluded from this study: a) cognitive decline, stroke, TBI, and any other neurological condition with compromised cognitive function existent before the COVID-19 diagnosis, b) severe past depression, and c) incomplete evaluation protocols. Also excluded were patients who did not show up for their interviews. The final number of study participants was 614 patients with a diagnosis of SARS-Cov-2 infection, confirmed by positive Polymerase Chain Reaction (PCR) test to detect viral RNA. This is a cross-sectional study and was approved by the SARAH Network Ethics in Research Committee (CAAE 53956921.2.0000.0022).
2.2Procedure
The neuropsychological assessments were performed using the following instruments: the Barrow Neurological Institute Screen for Higher Cerebral Functions, BNIS (Prigatano et al., 1995; Prigatano et al., 2018), the NEUPSILIN subtests of Phonemic Verbal Fluency Test and Clock Drawing Test for executive functions (Fonseca et al., 2009), and the Hospital Anxiety and Depression Scale (HADS) (Botega et al., 1995; Zigmond & Sanaith, 1983). The BNIS broadly and reliably assesses disorders of higher integrative mental functions. It comprises 50 items for evaluation of six functions (or subscales): speech/language, attention/concentration, orientation, visuospatial problem solving, memory, and affect. In the affect subscale, patients are asked to produce affect in their tone of voice, correctly identify affect on facial expressions, and, when shown a funny picture, react with a spontaneous smile.
The Verbal Fluency Test evaluates executive functions, particularly the capacity for storing and retrieving words (Delis et al., 2001; Diamond, 2013). The Clock Drawing Test requires the integration of multiple cognitive domains, such as understanding instructions, planning, working memory, executive functions, and visuoperceptive and visuomotor processes (Hazan et al., 2018; Pinto & Peters, 2009).
HADS consists of one scale with 14 items (seven for anxiety and seven for depression). A score of 0–7 suggests that there are no signs or symptoms of anxiety. A score of 8–11 indicates possible anxiety, and scores above 11 denote probable anxiety. The same goes for scores on the depression items. Eight experienced, licensed neuropsychologists administered the neuropsychological tests. All examiners attended training sessions on using the assessment protocol to ensure standardized procedures and maximize the reliability of psychometric measures. Each neuropsychological evaluation took approximately one hour. The average time between COVID-19 diagnosis and neuropsychological evaluation was eight months (SD = 4.3 months).
All of the neuropsychological tests were validated and standardized for the Brazilian population and were adjusted for subjects’ age and education. The NEUPSILIN subtests were analyzed according to the manual’s correction table for age bracket and level of education (Fonseca et al., 2009). Calculations on the BNIS overall z-score points and the subscales were based on a sample of 201 healthy subjects used to validate and standardize the BNIS in Brazil (Prigatano et al., 2018).
Data on sociodemographics, severity of COVID-19 symptoms, and prior comorbidities were collected from patient charts and analyzed by the SARAH multidisciplinary team. Data on hospitalization and non-hospitalization during infection, as well as inpatient treatment (intensive care unit - ICU, orotracheal intubation - OTI) were used as proxies for disease severity.
2.2Statistical analysis
A descriptive and exploratory analysis of the data was conducted using statistics such as mean and standard deviation (M/SD), confidence intervals, and percentages. The correlations were evaluated with Spearman’s rank correlation coefficients. The Kruskal-Wallis test quantified the association between the neuropsychological results and both the severity of COVID-19 and the HADS scores. Epsilon-squared measured the effect size and interpreted it as small (<0.08), medium (0.08 to 0.26) or large (≥0.26). Whenever statistically significant differences on the Kruskal-Wallis test were detected, a post hoc analysis using the Dunn test was utilized with p-values adjusted by the Benjamini-Hochberg method. Pearson’s Chi-squared was used to evaluate the association between HADS scores and hospitalization during the acute phase. Effect size was determined by Cramer’s V and interpreted as small, medium or large when V was 0.07, 0.21 and 0.35, respectively. P-values below 0.05 were considered statistically significant. R version 4.1.2 was used for all statistical analyses.
3Results
The sample comprised 614 participants, with mean age of 47.6 years old. (SD = 11.2). Most were female (73%). The majority (54%) had a college degree (>16 years of education), were married (55%), and were professionally active (74%) (Table 1).
Table 1
Sociodemographic characteristics (N = 614)
| Count | Percentage | ||
| Age Range | 18–39 | 141 | 23 |
| 40–59 | 383 | 62 | |
| 60+ | 90 | 15 | |
| Gender | F | 451 | 73 |
| M | 163 | 27 | |
| Education (years) | 5–8 | 31 | 5 |
| 9–11 | 34 | 6 | |
| 12–15 | 216 | 35 | |
| 16+ | 333 | 54 | |
| Marital Status | Married | 338 | 55 |
| Divorced/Separated | 77 | 13 | |
| Single | 181 | 29 | |
| Widow/er | 18 | 3 | |
| Employment Status | Active | 457 | 74 |
| Retired | 72 | 12 | |
| Unemployed | 59 | 10 | |
| Sick Leave/Disability | 26 | 4 |
The study participants had a low prevalence of comorbidities prior to contracting COVID-19; the most common were diabetes mellitus (11.1%) and cardiovascular diseases (11.2%), such as hypertension and arrhythmia. One-third of the study patients had been hospitalized for severe COVID-19symptoms, 17.3% had been in intensive care (M = 18.8 days in the ICU, SD = 13.9), and 10.6% had been intubated for oxygen support.
3.1Neuropsychological findings
All of the patients in this study complained of memory problems after COVID-19. The results of the neuropsychological evaluation indicated that patients’ performance was below reference values in all the BNIS subscales and overall scores (Fig. 1). In the BNIS, performance of –1.0 standard deviation below the mean indicates a cognitive deficit (Schoenberg et al., 2006). The average overall score on the BNIS (z score) in our sample was –1.25 (SD = 1.40), which demonstrates that these patients’ performance was, on average, lower than cutoff values for cognitive deficit. The worse scores were on the subtests for affect (M = –1.26, SD = 1.66) and memory (M = –1.00, SD = 1.44) (Table 2).
Fig. 1
Neuropsychological test results (z score) – mean and 95% confidence intervals.
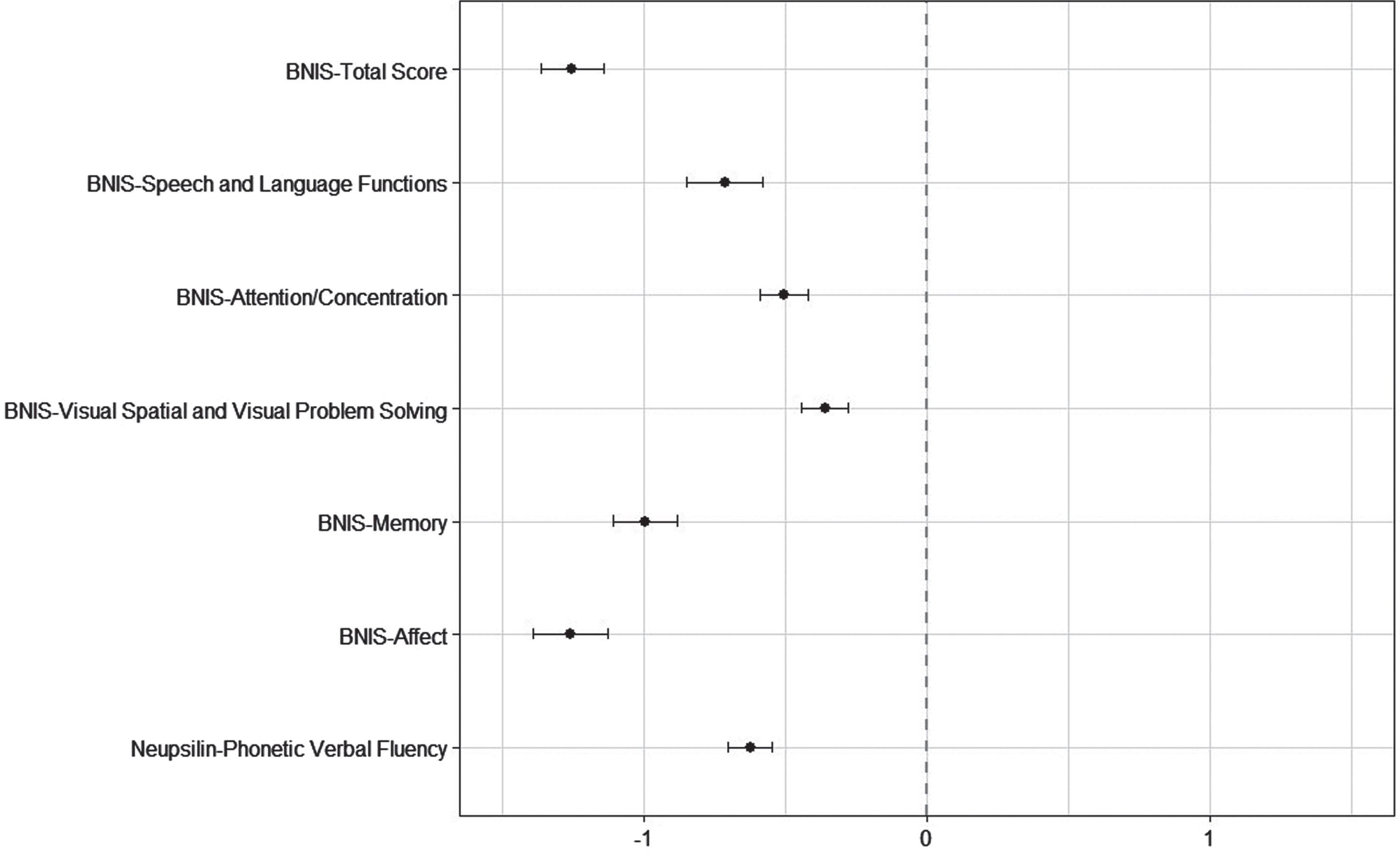
Table 2
Neuropsychological characteristics of patients with COVID-19 infection (N = 614)
| Evaluations | Raw score | z score* | ||||
| Min | Max | Mean | SD | |||
| BNIS | ||||||
| Overall score | 25 | 50 | 40.5 | 4.4 | –1.25±1.40 | (–1.37; –1.14) |
| Speech/language | 6 | 15 | 13.9 | 1.3 | –0.71±1.71 | (–0.85; –0.58) |
| Attention/concentration | 0 | 3 | 1.4 | 1.0 | –0.51±1.07 | (–0.59; –0.42) |
| Visuospatial/visual | 1 | 8 | 5.9 | 1.2 | –0.36±1.05 | (–0.44; –0.28) |
| Memory | 0 | 7 | 4.4 | 1.9 | –1.00±1.44 | (–1.11; –0.88) |
| Affect | 0 | 4 | 2.6 | 0.9 | –1.26±1.66 | (–1.39; –1.13) |
| Neupsilin | ||||||
| Phonetic Verbal Fluency | 2 | 26 | 12.6 | 4.5 | –0.63±0.99 | (–0.70;–0.55) |
| Clock Drawing Test | 0 | 5 | 3.0 | 1.5 | ||
*Mean±SD, and values in parentheses are 95% confidence interval.
The results of the Phonemic Verbal Fluency test show that post-COVID-19 patients performed below reference values (Fig. 1). Patients’ scores were also lower on the Clock Drawing Test for executive functions (M = 3.0, SD = 1.5). Almost half of the sample (45%) had difficulties with this task, committing at least two mistakes. A patient’s age did not interfere with the test’s sensitivity (rs = –0.05, p = 0.222), but we did observe that the higher the level of education, the better the performance on the test (rs = 0.24, p < 0.001).
We noted a correlation between the BNIS, Phonetic Verbal Fluency, and the Clock Drawing tests: the lower the score on the BNIS, the worse the performance on the Verbal Fluency (rs = 0.28, p < 0.001) and Clock Drawing (rs = 0.27, p < 0.001)tests.
3.2HADS findings
The results of the HAD scale showed that patients had higher scores for anxiety (abnormal = 46%) and depression (abnormal = 29%). Conversely, there was no evident association between signs of anxiety/depression and performance on neuropsychological tests, even on the BNIS affect subscale. We noted differences in Phonetic Verbal Fluency results among depression group scores, in which the Dunn multiple comparison test showed differences between the normal (0–7) and abnormal (11–21) scores. However, the effect size was very small (epsilon squared = 0.01); that is, despite the lower depression score correlating with better performance, this difference was not meaningful (Table 3).
Table 3
Means and 95% confidence intervals of neuropsychological results for HADS group scores (N = 614)
| Evaluations | Anxiety | P | Epsilon | Depression | P | Epsilon | ||||
| Normal | Borderline | Abnormal | squared | Normal | Borderline | Abnormal | squared | |||
| N = 165 | N = 186 | N = 263 | N = 213 | N = 224 | N = 177 | |||||
| BNIS (z score) | ||||||||||
| Overall score | –1.2 (–1.5; –1.0) | –1.2 (–1.4; –1.0) | –1.3 (–1.5; –1.1) | 0.739 | <0.01 | –1.2 (–1.4; –1.0) | –1.3 (–1.5; –1.1) | –1.3 (–1.5; –1.1) | 0.275 | <0.01 |
| Speech/language | –0.8 (–1.1; –0.6) | –0.6 (–0.8; –0.3) | –0.8 (–1.0; –0.5) | 0.349 | <0.01 | –0.7 (–1.0; –0.5) | –0.8 (–1.0; –0.5) | –0.6 (–0.9; –0.4) | 0.595 | <0.01 |
| Attention/concentration | –0.5 (–0.6; –0.3) | –0.4 (–0.6; –0.2) | –0.6 (–0.7; –0.5) | 0.153 | 0.01 | –0.4 (–0.5; –0.2) | –0.6 (–0.7; –0.4) | –0.6 (–0.8; –0.4) | 0.063 | 0.01 |
| Visuospatial/visual | –0.4 (–0.5; –0.2) | –0.3 (–0.5; –0.2) | –0.4 (–0.5; –0.2) | 0.976 | <0.01 | –0.4 (–0.5; –0.3) | –0.4 (–0.5; –0.2) | –0.3 (–0.5; –0.2) | 0.610 | <0.01 |
| Memory | –0.9 (–1.2; –0.7) | –1.0 (–1.2; –0.8) | –1.0 (–1.2; –0.9) | 0.925 | <0.01 | –0.9 (–1.1; –0.7) | –1.1 (–1.3; –0.9) | –1.0 (–1.2; –0.8) | 0.228 | <0.01 |
| Affect | –1.1 (–1.4; –0.9) | –1.2 (–1.5; –1.0) | –1.4 (–1.6; –1.2) | 0.355 | <0.01 | –1.2 (–1.4; –0.9) | –1.3 (–1.5; –1.0) | –1.4 (–1.6; –1.2) | 0.190 | 0.01 |
| Neupsilin | ||||||||||
| Phonetic Verbal Fluency (z score) | –0.5 (–0.7; –0.3) | –0.6 (–0.8; –0.5) | –0.7 (–0.8; –0.6) | 0.208 | 0.01 | –0.5 (–0.6; –0.3) | –0.6 (–0.8; –0.5) | –0.8 (–0.9; –0.6) | 0.015* | 0.01 |
| Clock Drawing Test | 3.0 (2.8; 3.2) | 3.1 (2.9; 3.3) | 3.1 (2.9; 3.2) | 0.843 | <0.01 | 3.1 (2.9; 3.3) | 3.0 (2.8; 3.2) | 3.1 (2.9; 3.3) | 0.959 | <0.01 |
Normal: 0–7 points; Borderline: 8–11 points; Abnormal: 12–21 points; P: P-value from Kruskal-Wallis test; Epsilon squared: effect size index. *Statistically significant pairwise comparison on normal versus abnormal group (Dunn test, P-adjusted = 0.011).
3.3Severity of COVID-19 symptoms and neuropsychological performance
We did not observe any relation between the patients’ performance on neuropsychological tests and severity of COVID-19 symptoms. Our sample’s overall score on the BNIS was lower than the reference values in more than one standard deviation and did not indicate a statistically significant difference between the hospitalized and non-hospitalized patients. The same was true for the Verbal Fluency and Clock Drawing tests (Table 4).
Table 4
Means and 95% confidence intervals of neuropsychological results for hospitalized and non-hospitalized patients (N = 614)
| Evaluations | Non-hospitalized | Hospitalized | P | Epsilon- | ||
| N = 408 | Ward N = 100 | ICU N = 41 | OTI N = 65 | squared | ||
| BNIS (z score) | ||||||
| Overall score | –1.3 (–1.4; –1.2) | –1.3 (–1.6; –1.0) | –1.2 (–1.7; –0.6) | –1.0 (–1.4; –0.6) | 0.478 | <0.01 |
| Speech/language | –0.8 (–0.9; –0.6) | –0.7 (–1.1; –0.4) | –0.6 (–1.1; –0.1) | –0.5 (–0.9; –0.1) | 0.564 | <0.01 |
| Attention/concentration | –0.5 (–0.6; –0.4) | –0.5 (–0.7; –0.3) | –0.5 (–0.9; –0.2) | –0.4 (–0.7; –0.1) | 0.865 | <0.01 |
| Visuospatial/visual | –0.4 (–0.5; –0.3) | –0.3 (–0.6; –0.1) | –0.3 (–0.7; 0.0) | –0.4 (–0.7; –0.1) | 0.879 | <0.01 |
| Memory | –1.0 (–1.1; –0.8) | –1.2 (–1.4; –0.9) | –1.0 (–1.5; –0.5) | –0.9 (–1.2; –0.5) | 0.497 | <0.01 |
| Affect | –1.4 (–1.5; –1.2) | –1.1 (–1.3; –0.8) | –1.0 (–1.4; –0.6) | –1.1 (–1.5; –0.7) | 0.216 | 0.01 |
| Neupsilin | ||||||
| Phonetic Verbal Fluency (z score) | –0.6 (–0.7; –0.5) | –0.6 (–0.8; –0.4) | –0.6 (–0.9; –0.3) | –0.7 (–1.0; –0.5) | 0.725 | <0.01 |
| Clock Drawing Test | 3.0 (2.9; 3.2) | 3.1 (2.8; 3.4) | 2.9 (2.4; 3.4) | 3.1 (2.7; 3.4) | 0.892 | <0.01 |
ICU: Intensive Care Unit; OTI: Orotracheal Intubation; P: P-value from Kruskal-Wallis test; Epsilon squared: effect size index.
According to HADS, there was no association between hospitalization during COVID-19 and scores for anxiety (X2(2, N = 614) = 1.82, p > 0.40, Cramer’s V = 0.05) and depression (X2(2, N = 614) = 3.78, p > 0.15, Cramer’s V = 0.08).
4Discussion
Our study showed that both previously hospitalized and non-hospitalized COVID-19 survivors had cognitive deficits and emotional issues that persisted an average of eight months after disease onset. It also generates original data using broader, sensitive, in-person neuropsychological testing tools that detail problems in various higher mental function domains. In addition, it is the first study to identify difficulties with affect expression and perception, regardless of the presence of anxiety/depression.
Our findings consolidate and add to existent literature on cognitive disorders in long COVID. Sample participants had low scores on the BNIS, Phonemic Verbal Fluency and Clock Drawing tests, suggesting persistent problems with executive functions. Almeria et al. (2020) reported lower performance in the attention, memory, verbal phonemic fluency and mental flexibility domains. A systematic review showed that patients with SARS-CoV-2 infection presented with low global cognitive outcomes and difficulties with memory, attention, verbal fluency, and executive functions (Daroische et al., 2021).
Our study sample exhibited significant signs of anxiety and depression, a finding that is in line with current data: neuropsychiatric issues are persistent common symptoms of neurocovid (Nakamura et al., 2021; Rogers et al., 2020; Taquet & Luciano; 2021). The study by Pistarini et al. (2021) showed that most post-COVID patients had mild or moderate depression. The same had previously been found in a systematic review by Vindegaard and Benros (2020), which noted a significantly higher level of anxiety and depression symptoms in post-COVID patients. Similarly, de Sousa Moreira et al. (2021) also ran a systematic review and found a high level of psychiatric and neuropsychiatric symptoms in COVID survivors. Higher scores in anxiety and depression were also seen in patients with cognitive complaints (Almeria et al., 2020). Contrary to expectations, our findings show that performance on neuropsychological tests was not directly impacted by high scores on anxiety and depression scales. In other words, some participants with mild cases of depression or anxiety scored low on the BNIS, while others, with severe depression or anxiety, scored high on cognitive tests. A recent study asserted that the coronavirus is systemic, capable of causing damage throughout the body, including mental health issues and neurocognitive decline (Xie et al., 2022). Research shows that COVID-19 disrupts brain homeostasis, and can directly penetrate the brain (Boldrini et al., 2021; Crunfli et al., 2020; Lee et al., 2021). We can hypothesize that, much the same way that SARS CoV2 invades brain areas associated with executive functions, it can also, independently, impact brain areas linked to mood regulation and affect.
A noteworthy finding was the fact that non-hospitalized patients exhibited neuropsychological problems similar to those who had been hospitalized, particularly regarding problems with attention, memory, and executive functions. A study by Hampshire et al. (2021) used an online self-assessment test for COVID survivors and found that cognitive disorders were present in both previously hospitalized and non-hospitalized patients. Bliddal et al. (2021) used online questionnaires and reported similar issues with memory and concentration among non-hospitalized patients with mild COVID. Woo et al. (2020) conducted telephone interviews with 18 young patients who previously had mild COVID and reported lingering difficulties with memory, attention, and concentration not linked to hospitalization. Our study is an important addition to the existent literature on long COVID, primarily concerning cognitive outcomes in mild cases. Furthermore, because of the study sample’s size and the reliability of the neuropsychological data, it contributes to current knowledge about non-hospitalizedcases.
Our findings did not show any significant differences in anxiety and depression scores among hospitalized and non-hospitalized patients. A systematic review by Vanderlind et al. (2021) reported a high incidence of anxiety and depression in hospitalized patients but did not investigate the non-hospitalized population. The literature on this topic is still emerging and these results should be explored in future research, but our results show that survivors of mild COVID also have persistent neuropsychiatricproblems.
Historically, the ratio of male to female patients has been balanced at the SARAH Network of Rehabilitation Hospitals, which treats over 1.6 million people annually. Surprisingly, when COVID-19 survivors began seeking treatment for lingering neurocognitive and neuropsychiatric problems that were affecting their daily lives, especially their return to work, the ratio shifted. In other words, a larger number of females (73%) sought assistance for COVID-related cognitive issues. Despite the literature indicating a higher rate of COVID-19 infection in males, (Aksoyalp et al., 2021; Qian et al., 2020; Toh-Manikowski et al., 2021), recent studies have begun noting a greater number of females with persistent post-COVID problems (Bucciarelli et al., 2022; Kashif et al., 2021). Our study adds to emergent literature on another aspect of long COVID; that is, its predeliction for greater neuropsychological impact on females compared with males.
4.1Study limitations
This study has limitations: One is the study design, which hinders the establishment of causality. Nevertheless, despite this being a cross-sectional study, the patients’ cognitive problems emerged only after COVID-19. Furthermore, before COVID-19, the SARAH Network was not seeing a large number of patients complaining of executive function problems, such as attention and memory. This change occurred after the pandemic began and entails a younger population (47.6 years old). Secondly, the clinical data on the patients’ acute COVID-19 phase were directly collected through in-person interviews since the SARAH Network is primarily a rehabilitation center. Another limitation is the use of information about hospitalization as a proxy for severity of COVID-19 during the acute phase, despite these criteria being compatible with those set forth by the World Health Organization (WHO, 2021). Finally, we used the HAD Scale, a self-assessing tool without psychiatric consult that measures anxiety and depression. However, HADS is sensitive to an individual’s emotional state (Brennan et al., 2010) and was able to generate important insights into the patient’s post-COVID condition. Our study found that patients with the lingering effects of long COVID may experience difficulties expressing/perceiving affect. We should be cautious in our analysis of this new finding, as there are yet no supporting studies in the literature, underscoring the need for future investigation.
5Conclusions
The strength of our study is that it presents original data and uses a robust sample of in-person neuropsychological evaluations to demonstrate that cognitive and psychiatric disorders persisted after the acute phase of COVID-19 in previously hospitalized and non-hospitalized patients. It also elucidates the independence between cognitive disorders and psychiatric problems in this population. In addition, it bears out the predominance of neuropsychological issues among the female relative to male population in long COVID.
Studies have highlighted the need for rehabilitation programs prepared to support individuals with long COVID and treat their cognitive and emotional needs, and associated disorders (Wade, 2020; Wilson et al., 2020). The results of our investigation, along with the existent literature on the subject, should assist in guiding the establishment of public policies at a time of growing demands for neurorehabilitation programs for post-COVID impairments. Ultimately, restoring function and increasing quality of life following COVID-19 infection should be a global effort.
Conflict of interest
The authors of this manuscript declare that they have no relevant financial or personal relationships with individuals or commercial interests (entities producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients) that could inappropriately influence or bias their work.
Acknowledgments
The authors would like to give a special thanks to George Prigatano and acknowledge the participation of Davi Guerra Alves, Erika Carvalho Pires Arci, Paulo Sergio Siebra Beraldo, Fabio Alexandre Costa, Ana Luisa Lourenco Moretto, Lucas de Moraes Pinto Pereira, Olinda Paula Azevedo Perez, and Luciana Balduino Sollaci.
References
1 | Aksoyalp, Z. Ş. , & Nemutlu-Samur, D. ((2021) ). Sex-related susceptibility in coronavirus disease 2019 (COVID-19): Proposed mechanisms. European Journal of Pharmacology, 912: , 174548. https://doi.org/10.1016/j.ejphar.2021.174548 |
2 | Almeria, M. , Cejudo, J. C. , Sotoca, J. , Deus, J. , & Krupinski, J. ((2020) ). Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behavior, & Immunity - Health, 9: , 100163. https://doi.org/10.1016/j.bbih.2020.100163 |
3 | Almqvist, J. , Granberg, T. , Tzortzakakis, A. , Klironomos, S. , Kollia, E. , Öhberg, C. , Martin, R. , Piehl, F. , Ouellette, R. , & Ineichen, B. V. ((2020) ). Neurological manifestations of coronavirus infections - a systematic review. Annals of Clinical and Translational Neurology, 7: (10), 2057–2071. https://doi.org/10.1002/acn3.511662 |
4 | Bliddal, S. , Banasik, K. , Pedersen, O. B. , Nissen, J. , Cantwell, L. , Schwinn, M. , Tulstrup, M. , Westergaard, D. , Ullum, H. , Brunak, S. , Tommerup, N. , Feenstra, B. , Geller, F. , Ostrowski, S. R. , Grønbæk, K. , Nielsen, C. H. , Nielsen, S. D. , & Feldt-Rasmussen, U. ((2021) ). Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Scientific Reports, 11: (1), 13153. https://doi.org/10.1038/s41598-021-92045-x |
5 | Boldrini, M. , Canoll, P. D. , & Klein, R. S. ((2021) ). How COVID- affects the brain. JAMA Psychiatry, 78: (6), 682–683. |
6 | Botega, N. J. , Bio, M. R. , Zomignani, M. A. , Garcia, C. Jr. , & Pereira, W. A. B. ((1995) ). Transtornos do humor em enfermaria de clínica médica e validação de escala de medida (HAD) de ansiedade e depressão. Revista de Sa&de Pública, 29: (5), 355–363. |
7 | Brennan, C. , Worrall-Davies, A. , McMillan, D. , Gilbody, S. , & House, A. ((2010) ). The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. Journal of Psychosomatic Research, 69: (4), 371–378. https://doi.org/10.1016/j.jpsychores.2010.04.006 |
8 | Bucciarelli, V. , Nasi, M. , Bianco, F. , Seferovic, J. , Ivkovic, V. , Gallina, S. , & Mattioli, A. V. ((2022) ). Depression pandemic and cardiovascular risk in the COVID-19 era and long COVID syndrome: Gender makes a difference. Trends in Cardiovascular Medicine, 32: (1), 12–17. https://doi.org/10.1016/j.tcm.2021.09.009 |
9 | Crunfli, F. , Carregari, V. C. , Veras, F. P. , Vendramini, P. H. , Valenca, A. G. F. , Antunes, A. S. L. M. , Brandão-Teles, C. , Zuccoli, Z. S. , Reis-de-Oliveira, G. , Silva-Costa, L. C. , Saia-Cerada, V. M. , Codo, A. C. , Parise, P. L. , Teixeira, D. A. T. , Souza, G. F. , Muraro, S. P. , Melo, B. M. S. , Almeida, D. M. , Firmino, E. M. S. ..., & Martins-de-Souza, D. ((2020) ). SARS-CoV-2 infects brain astrocytes of COVID-19 patients and impairs neuronal viability. MedRxiv, (2020.10.09.20207464). https://www.medrxiv.org/content/10.1101/2020.10.09.20207464v1 |
10 | Daroische, R. , Hemminghyth, M. S. , Eilertsen, T. H. , Breitve, M. H. , & Chwiszczuk, L. J. ((2021) ). Cognitive impairment after COVID-19-a review on objective test data. Frontiers in Neurology, 12: , 699582. https://doi.org/10.3389/fneur.2021.699582 |
11 | De Biase, S. , Cook, L. , Skelton, D. A. , Witham, M. , & Ten Hove, R. ((2020) ). The COVID-19 rehabilitation pandemic. Age and Ageing, 49: (5), 696–700. https://doi.org/10.1093/ageing/afaa118 |
12 | de Sousa Moreira, J. L. , Barbosa, S. , Vieira, J. G. , Chaves, N. , Felix, E. , Feitosa, P. , da Cruz, I. S. , da Silva, C. , & Neto, M. ((2021) ). The psychiatric and neuropsychiatric repercussions associated with severe infections of COVID-19 and other coronaviruses. Progress in Neuro-psychopharmacology & Biological Psychiatry, 106: , 110159. https://doi.org/10.1016/j.pnpbp.2020.110159 |
13 | Delis, D. C. , Kaplan, E. , & Kramer, J. H. ((2001) ). Delis-Kaplan executive function system (D-KEFS). Psychological Corporation. |
14 | Diamond, A. ((2013) ). Executive functions. Annual Review of Psychology, 64: , 135–168. doi:10.1146/annurev-psych-113011-143750. |
15 | Elkan, M. , Dvir, A. , Zaidenstein, R. , Keller, M. , Kagansky, D. , Hochman, C. , & Koren, R. ((2021) ). Patient-reported outcome measures after hospitalization during the COVID-19 pandemic: A survey among COVID-19 and non-COVID-19 patients. International Journal of General Medicine, 14: , 4829–4836. https://doi.org/10.2147/IJGM.S323316 |
16 | Fonseca, R. P. , Salles, J. F. , & Parente, M. A. M. P. ((2009) ). Neupsilin: Instrumento de Avaliação Neuropsicológica Breve: Manual. Vetor. |
17 | Graham, E. L. , Clark, J. R. , Orban, Z. S. , Lim, P. H. , Szymanski, A. L. , Taylor, C. , DiBiase, R. M. , Jia, D. T. , Balabanov, R. , Ho, S. U. , Batra, A. , Liotta, E. M. , & Koralnik, I. J. ((2021) ). Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Annals of Clinical and Translational Neurology, 8: (5), 1073–1085. https://doi.org/10.1002/acn3.51350 |
18 | Halpin, S. J. , McIvor, C. , Whyatt, G. , Adams, A. , Harvey, O. , McLean, L. , Walshaw, C. , Kemp, S. , Corrado, J. , Singh, R. , Collins, T. , O’Connor, R. J. , & Sivan, M. ((2020) ). Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. Journal of Medical Virology, 93: (2), 1013–1022. https://doi.org/10.1002/jmv.26368.12 |
19 | Hampshire, A. , Trender, W. , Chamberlain, S. R. , Jolly, A. E. , Grant, J. E. , Patrick, F. , Mazibuko, N. , Williams, S. C. , Barnby, J. M. , Hellyer, P. , & Mehta, M. A. ((2021) ). Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine, 39: , 101044. https://doi.org/10.1016/j.eclinm.2021.101044 |
20 | Hazan, E. , Frankenburg, F. , Brenkel, M. , & Shulman, K. ((2018) ). The test of time: a history of clock drawing. International Journal of Geriatric Psychiatry, 33: (1), e22–e30. https://doi.org/10.1002/gps.4731 |
21 | Hellmuth, J. , Barnett, T. A. , Asken, B. M. , Kelly, J. D. , Torres, L. , Stephens, M. L. , Greenhouse, B. , Martin, J. N. , Chow, F. C. , Deeks, S. G. , Greene, M. , Miller, B. L. , Annan, W. , Henrich, T. J. , & Peluso, M. J. ((2021) ). Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. Journal of Neurovirology, 27: (1), 191–195. https://doi.org/10.1007/s13365-021-00954-4 |
22 | Huang, C. , Huang, L. , Wang, Y. , Li, X. , Ren, L. , Gu, X. , Kang, L. , Guo, L. , Liu, M. , Zhou, X. , Luo, J. , Huang, Z. , Tu, S. , Zhao, Y. , Chen, L. , Xu, D. , Li, Y. , Li, C. , Peng, L. , Li, Y. ..., & Cao, B. ((2021) ). 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet (London, England), 397: (10270), 220–232. https://doi.org/10.1016/S0140-6736(20)32656-8 |
23 | Johnsen, S. , Sattler, S. M. , Miskowiak, K. W. , Kunalan, K. , Victor, A. , Pedersen, L. , Andreassen, H. F. , Jørgensen, B. J. , Heebøll, H. , Andersen, M. B. , Marner, L. , Hædersdal, C. , Hansen, H. , Ditlev, S. B. , Porsbjerg, C. , & Lapperre, T. S. ((2021) ). Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Research, 7: (3), 00205–2021. https://doi.org/10.1183/23120541.00205-2021 |
24 | Kamal, M. , Omirah, M. A. , M , Hussein, A. , & Saeed, H. ((2021) ). Assessment and characterisation of post-COVID-19 manifestations. International Journal of Clinical Practice, 75: (3), e13746. https://doi.org/10.1111/ijcp.13746 |
25 | Kashif, A. , Chaudhry, M. , Fayyaz, T. , Abdullah, M. , Malik, A. , Anwer, J. , Inam, S. , Fatima, T. , Iqbal, N. , & Shoaib, K. ((2021) ). Follow-up of COVID-19 recovered patients with mild disease. Scientific Reports, 11: (1), 13414. https://doi.org/10.1038/s41598-021-92717-8 |
26 | Ladds, E. , Rushforth, A. , Wieringa, S. , Taylor, S. , Rayner, C. , Husain, L. , & Greenhalgh, T. ((2020) ). Persistent symptoms after Covid-19: Qualitative study of 114 ``long Covid'' patients and draft quality principles for services. BMC Health Services Research, 20: (1), 1144. https://doi.org/10.1186/s12913-020-06001-y |
27 | Lee, M-H. , Perl, D. P. , Nair, G. , Li, W. , Maric, D. , Murray, H. , Dodd, S. J. , Koretvsky, A. P. , Watts, A. , Cheung, V. , Masliah, E. , Horkayne-Szakaly, I. , Jones, R. , Stram, M. N. , Moncur, J. , Hefti, M. , Folkerth, R. D. , & Nath, A. ((2021) ). Microvascular injury in the brains of patients with COVID-19. The New England Journal of Medicine, 384: (5), 481–483. https://doi.org/10.1056/NEJMc2033369 |
28 | Mao, L. , Jin, H. , Wang, M. , Hu, Y. , Chen, S. , He, Q. , Chang, J. , Hong, C. , Zhou, Y. , Wang, D. , Miao, X. , Li, Y. , & Hu, B. ((2020) ). Neurologic manifestations of hospitalized patients with Coronavirus disease in Wuhan, China. JAMA Neurology, 77: (6), 683–690. https://doi.org/10.1001/jamaneurol.2020.1127. |
29 | Mendelson, M. , Nel, J. , Blumberg, L. , Madhi, S. A. , Dryden, M. , Stevens, W. , & Venter, F. ((2020) ). Long-COVID: An evolving problem with an extensive impact. South African Medical Journal=Suid-Afrikaanse Tydskrif Vir Geneeskunde, 111: (1), 10–12. https://doi.org/10.7196/SAMJ.2020.v111i11.15433 |
30 | Miskowiak, K. W. , Johnsen, S. , Sattler, S. M. , Nielsen, S. , Kunalan, K. , Rungby, J. , Lapperre, T. , & Porsberg, C. M. ((2021) ). Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. European Neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology, 46: , 39–48. https://doi.org/10.1016/j.euroneuro.2021.03.019 |
31 | Mohamed-Hussein, A. , Amin, M. T. , Makhlouf, H. A. , Makhlouf, N. A. , Galal, I. , Abd-Elaal, H. K. , Abdeltawab, D. , Kholief, K. , & Hashem, M. K. ((2021) ). Non-hospitalised COVID-19 patients have more frequent long COVID-19 symptoms. The International Journal of Tuberculosis and Lung Disease: the official journal of the International Union against Tuberculosis and Lung Disease, 25: (9), 732–737. https://doi.org/10.5588/ijtld.21.0135 |
32 | Nakamura, Z. M. , Nash, R. P. , Laughon, S. L. , & Rosenstein, D. L. ((2021) ). Neuropsychiatric complications of COVID-19. Current Psychiatry Reports, 23: (5), 25. https://doi.org/10.1007/s11920-021-01237-9 |
33 | Negrini, F. , Ferrario, I. , Mazziotti, D. , Berchicci, M. , Bonazzi, M. , de Sire, A. , Negrini, S. , & Zapparoli, L. ((2021) ). Neuropsychological features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for postacute rehabilitation. Archives of Physical Medicine and Rehabilitation, 102: (1), 155–158. https://doi.org/10.1016/j.apmr.2020.09.376 |
34 | Pinto, E. , & Peters, R. ((2009) ). Literature review of the Clock Drawing Test as a tool for cognitive screening. Dementia and Geriatric Cognitive Disorders, 27: (3), 201–213. https://doi.org/10.1159/000203344 |
35 | Pistarini, C. , Fiabane, E. , Houdayer, E. , Vassallo, C. , Manera, M. R. , & Alemanno, F. ((2021) ). Cognitive and emotional disturbances due to COVID-19: An exploratory study in the rehabilitation setting. Frontiers in Neurology, 12: , 643646. https://doi.org/10.3389/fneur.2021.643646 |
36 | Prigatano, G. P. , Amin, K. , & Rosenstein, L. ((1995) ). Administration and scoring manual for the BNI screen for higher cerebral functions. Barrow Neurological Institute. |
37 | Prigatano, G. P. , Souza, L. M. N. , & Braga, L. W. ((2018) ). Performance of a Brazilian sample on the portuguese translation of the BNI screen for higher cerebral functions. Journal of Clinical and Experimental Neuropsychology, 40: (2), 173–182. https://doi:10.1080/13803395.2017.1325839 |
38 | Qian, J. , Zhao, L. , Ye, R. Z. , Li, X. J. , & Liu, Y. L. ((2020) ). Age-dependent gender differences in COVID-19 in Mainland China: Comparative study. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America, 71: (9), 2488–2494. https://doi.org/10.1093/cid/ciaa683 |
39 | Rogers, J. P. , Chesney, E. , Oliver, D. , Pollak, T. A. , McGuire, P. , Fusar-Poli, P. , Zandi, M. S. , Lewis, G. , & David, A. S. ((2020) ). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry, 7: (7), 611–627. https://doi.org/10.1016/S2215-0366(20)30203-0 |
40 | Roy, D. , Ghosh, R. , Dubey, S. , Dubey, M. J. , Benito-León, J. , & Kanti Ray, B. ((2021) ). Neurological and Neuropsychiatric Impacts of COVID-19 Pandemic. The Canadian journal of neurological sciences. The Canadian Journal of Neurological Sciences. Le Journal Canadien des Sciences Neurologiques, 48: (1), 9–24. https://doi.org/10.1017/cjn.2020.173 |
41 | Sarubbo, F. , El Haji, K. , Vidal-Balle, A. , & Bargay Lleonart, J. ((2022) ). Neurological consequences of COVID-19 and brain related pathogenic mechanisms: A new challenge for neuroscience. Brain, Behavior, & Immunity-Health, 19: , 100399. https://doi.org/10.1016/j.bbih.2021.100399 |
42 | Schoenberg, M. R. , Dawson, K. A. , Duff, K. , Patton, D. , Scott, J. G. , & Adams, R. L. ((2006) ). Test performance and classification statistics for the Rey Auditory Verbal Learning Test in selected clinical samples. Archives of Clinical Neuropsychology, 21: (7), 693–703. |
43 | Taquet, M. , Geddes, J. R. , Husain, M. , Luciano, S. , & Harrison, P. J. ((2021) ). 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet. Psychiatry, 8: (5), 416–427. https://doi.org/10.1016/S2215-0366(21)00084-5 |
44 | Taquet, M. , Luciano, S. , Geddes, J. R. , & Harrison, P. J. ((2021) ). Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet. Psychiatry, 8: (2), 130–140. https://doi.org/10.1016/S2215-0366(20)30462-4 |
45 | Toth-Manikowski, S. M. , Caldwell, J. , Joo, M. , Chen, J. , Meza, N. , Bruinius, J. , Gupta, S. , Hannan, M. , Kagalwalla, M. , Madrid, S. , Melamed, M. L. , Pacheco, E. , Srivastava, A. , Viamontes, C. , Lash, J. P. , Leaf, D. E. , Ricardo, A. C. , & STOP-COVID Investigators ((2021) ). Sex-related differences in mortality, acute kidney injury, and respiratory failure among critically ill patients with COVID-19. Medicine (Baltimore), 100: (50), e28302. https://doi.org/10.1097/MD.0000000000028302 |
46 | Vanderlind, W. M. , Rabinovitz, B. B. , Miao, I. Y. , Oberlin, L. E. , Bueno-Castellano, C. , Fridman, C. , Jaywant, A. , & Kanellopoulos, D. ((2021) ). A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Current Opinion in Psychiatry, 34: (4), 420–433. https://doi.org/10.1097/YCO.0000000000000713 |
47 | Vindegaard, N. , & Benros, M. E. ((2020) ). COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain, Behavior, and Immunity, 89: , 531–542. https://doi.org/10.1016/j.bbi.2020.05.048 |
48 | Vitalakumar, D. , Sharma, A. , Kumar, A. , & Flora, S. J. S. ((2021) ). Neurological manifestations in COVID-19 patients: A meta-analysis. ACS Chemical Neuroscience, 12: (15), 2776–2797. https://doi.org/10.1021/acschemneuro.1c00353 |
49 | Wade D. T. ((2020) ). Rehabilitation after COVID-19: an evidence-based approach. Clinical Medicine (London, England), 20: (4), 359–365. https://doi.org/10.7861/clinmed.2020-0353 |
50 | Wilson, B. A. , Betteridge, S. , & Fish, J. ((2020) ). Neuropsychological consequences of Covid-19. Neuropsychological Rehabilitation, 30: (9), 1625–1628. https://doi.org/10.1080/09602011.2020.1808483 |
51 | Woo, M. S. , Malsy, J. , Pöttgen, J. , Seddiq Zai, S. , Ufer, F. , Hadjilaou, A. , Schmiedel, S. , Addo, M. M. , Gerloff, C. , Heesen, C. , Wiesch, J. S. Z. , & Friese, M. A. ((2020) ). Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Communications, 2: (2), fcaa205. https://doi.org/10.1093/braincomms/fcaa205 |
52 | World Health Organization ((2021) ). WHO Global Clinical Platform for COVID-19: Case Report Form (CRF) for COVID-19 sequelae (“Post COVID-19 CRF”). https://apps.who.int/iris/bitstream/handle/10665/345299/WHO-2019-nCoV-Post_COVID-19_CRF-2021.2-eng.pdf |
53 | Xie, Y. , Xu, E. , & Al-Aly, Z. ((2022) ). Risks of mental health outcomes in people with covid-19: cohort study. BMJ, 376: , e068993. https://doi.org/10.1136/bmj-2021-068993 |
54 | Zhou, H. , Lu, S. , Chen, J. , Wei, N. , Wang, D. , Lyu, H. , Shi, C. , & Hu, S. ((2020) ). The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research, 129: , 98–102. https://doi.org/10.1016/j.jpsychires.2020.06.022 |
55 | Zigmond, A. S. , & Snaith, R. P. ((1983) ). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67: (6), 361–370. |




