Myofascial Trigger Points therapy decreases myotonometric tone and stiffness of trapezius muscle, benefits headaches and muscle pain in migraine
Abstract
BACKGROUND:
Migraine is a primary headache disorder. Studies have shown that 93% of people with migraine have an increased number of active Ischemic Compression Myofascial Trigger Points (IC-MTrPs) therapy.
OBJECTIVE:
To examine the effects of the IC-MTrPs therapy on: (1) mechanical properties of the upper trapezius muscle (UTM), (2) shoulder girdle and neck (SGN) muscles pain and (3) headaches characteristics in episodic migraine patients without aura.
METHODS:
Thirty-one adult, female, migraine patients without aura underwent seven IC-MTrPs therapy sessions and were tested during maximally five measurement sessions (pre- and post-1’st, post-4’th, post-7’th therapy and 1-month follow-up). Myotonometric measurements of the UTM’s tone, stiffness and elasticity, subjective SGN muscles pain, as well as headache’s level, frequency and duration were analyzed.
RESULTS:
Myotonometric tone and stiffness of the UTM significantly decreased in post-1’st, post-4’th therapy and in 1-month follow-up measurements versus pre-1’st therapy testing session. The scores for the SGN muscles’ pain significantly decreased: (i) in post-4’th and post-7’th therapy versus post-1’st therapy session, and (ii) in post-7’th versus post-4’th therapy measurements. Headache’s level, frequency and duration significantly decreased in post-7’th therapy versus pre-1’st therapy measurement session.
CONCLUSION:
IC-MTrPs therapy resulted in a decrease of upper trapezius muscle tone and stiffness, with simultaneous alleviation of shoulder girdle and neck muscle pain and the headaches characteristics in episodic migraine patients without aura.
1Introduction
Migraine is a primary headache disorder, however it is estimated that 70–76% of migraine patients report a presence of neck pain (Aquilar, Membrilla, & Diaz, 2015; Ashina et al., 2015) with muscle tenderness in this region (Mongini et al., 2004). Locations of tight muscle bands in the area of the shoulder girdle and neck (muscles: suboccipital, trapezius, sternomastoclavicular, temporal and levator scapula) (Florencio et al., 2017) contribute to chronic tension and migraine headaches (Burstein, Jakubowski, & Rauch, 2011). Especially such a large muscle as the trapezius (mainly the descending part - m. trapezius upper) takes a significant part in the function of the shoulder girdle and neck (Calandre, Hidalgo, Garcia, Leiva, & Rico-Villademoros, 2008; Travell, Simons & Simons 2018).
Studies have shown that 93% of people with migraine have an increased number of active myofascial trigger points (MTrPs) (Ferracini et al., 2017) that are in 74% in the areas of the temporal, suboccipital muscles and the shoulder girdle, what leads to a disturbance of the functional state of skeletal muscles and mobility in this region (Fernández de Las Penas et al., 2010; Tali, Menahem, Vered, & Kalichman, 2014). Travell & Simons (2018) proposed a method of ischemic compression (IC) therapy to inactivate MTrPs. Efforts have been undertaken to come up with more reliable than standard palpation (Do, Heldarskard, Kolding, Hvedstrup & Schytz, 2018) methods for identification of MTrPs, including infrared thermography, needle electromyography, elastography and also magnetic resonance imaging (Benatto et al., 2022; Luedtke, Starke, & May, 2018; Sollmann et al., 2019, 2021), that have their limitations (availability, invasive nature, complicated analysis).
Myotonometry seems to be a new promissing measurement method in this context (with usage of older Myoton-3 or newer MyotonPRO® device), which is a simple, sensitive and reliable tool of measuring the mechanical properties of human soft tissues in science, sport and medicine (Glemser et al., 2017; Marusiak et al., 2018). The myotonometric device, via its cylindrical testing end (with 3 mm diameter), generates a brief mechanical impulse to the tested muscle and consequently induces its damped oscillations in the muscle’s region in contact with the testing end. Simultaneously an accelerometer built into the divice and connected to the testing end records an acceleration of these oscillations and then this acceleration signal is used by the MyotonPRO® software to calculate the five myotonometric parameters: frequency (F, [Hz]), decrement (D, [log]), stiffness (S, [N/m]), relaxation time (R [ms]) and creep (C, [De]).
The first three parameters (i.e. myotonometric frequency, decrement and stiffness) are more often reported in the literature and their meaning for studying muscle mechanical properties is more acknowledged, therefore these parameters will be analyzed in our study. The myotonometric frequency of natural oscillations (F-MYO) characterizes non-neural muscle tone (Stefaniak, Marusiak, & Bączkowicz, 2022). The myotonometric decrement (D-MYO, [log]) reflects elasticity, understood as an ability of a muscle to restore its initial shape after deformation (Stefaniak et al., 2022). The myotonometric stiffness (S-MYO, [N/m]) indicates the ability of the muscle to resist an external force that modifies its shape (Stefaniak et al., 2022; Mroczek et al., 2018).
Previous studies presented an application of the myotonometry in an evaluation of the phenomenon of changed muscle mechanical properties in healthy asymptomatic subjects with identified MTrPs (Pérez-Bellmunt et al., 2021) and to assess in healthy individuals an effect of dry needling (Albin et al., 2020), or ischemic compression (Kisilewicz et al., 2018) on mechanical properties of MTrPs. Moreover, myotonometric measurements were conducted in patients with shoulder pain to study mechanical properties of muscles with MTrPs (Liou et al., 2018; Roch, Morin, & Gaudreault, 2020) and to assess an effect manual therapy (Gordon, Andrasik, Schleip, Birbaumer, & Rea, 2016) on mechanical characteristics of muscles with MTrPs in this group of patients.
To the best of our knowledge of relevant literature (Aoyama, 2021; De Almeida Tolentino et al., 2021; Rodríguez-Almagro et al., 2022), no study has yet presented data on the effect of ischemic compression method on myofascial trigger points therapy (IC-MTrPs) on mechanical properties of shoulder girdle and neck muscles in migraine patients without aura.
Thus, our goal was to test our hypothesis that deactivation of MTrPs with ischemic compression therapy in migraine patients without aura could result in relaxation of the upper trapezius muscle (reflected in a decreased values of myotonometric frequency, stiffness and decrement), with simultaneous alleviation of shoulder girdle and neck muscles pain as well as headaches characteristics.
2Materials and methods
2.1Subjects
Thirty-one right-handed female patients with episodic migraine without aura were recruited for the study (Table 1). The diagnosis of migraine was performed by a neurologist according to international criteria (beta ICHD-3) (Headache Classification Committee, 2018; IHS Classification ICHD - 3 Beta).
Table 1
Anthropometric and clinical data of subjects with episodic migraine without aura
| Anthropometric and clinical parameters (females, N = 31) | |
| Age [years] | 40,1 (10,5) |
| Duration of illness [years] | 17,1 (10,3) |
| Body mass [kg] | 70,0 (21,6) |
| Height [cm] | 163,0 (3,9) |
| Body Mass Index [kg/m2] | 26,4 (8,2) |
Data are expressed as mean (SD).
Inclusion criteria were: age - adults, gender - female, migraine lasting for at least 12 months diagnosed by a neurologist, no metabolic or cardiological syndromes, lack of neurological and orthopedic diseases in the area of the shoulder girdle and the cervical spine, voluntary consent to the examination in writing, and criteria according to ICHD-3 allowing to classify the symptoms as episodic migraine without aura.
Exclusion criteria were: age – under 18, gender – male, patients undergoing pharmacological treatment that cannot be discontinued, people with other headaches types, previous injuries of the musculoskeletal system in the cervical spine and cervical shoulder, skin diseases and other conditions such as deep vein thrombosis; criteria for excluding episodic migraine with aura.
The research was carried out in accordance with the Declaration of Helsinki (86/609/ECC) and the research protocol was approved by the Senate Research Ethics Committee at Jozef Pilsudski Academy of Physical Education in Warsaw (consent number: SKE 01-52 / 2017) and the Council of the Regional Center for Research and Development (RCRD) in Biala Podlaska (consent number: 14/21//10/11/2017). The study protocol has been registered as a clinical trial in the international clinical trials data base ClinicalTrials.gov (identifiter: NCT05646160).
2.2Experimental procedures
Patients suffering migraine without aura underwent a cycle of seven therapeutic sessions (encoded as Arabic numerals 1–6; as in the Fig. 1 and Table 2) of ischemic compression of myofascial trigger points (IC-MTrPs) therapy with a 3-day time intervals in between (Fig. 1). The whole therapeutic cycle lasted about 25 days, when counting from the 1’st to the 7’th IC-MTrPs therapy session (Fig. 1).
Fig. 1
Graphic time schedule of therapeutic and testing sessions with an indication of types of measurements collected during particular testing sessions. MYO –myotonometric measurent of upper trapezius muscle’s mechanical properties; PAIN –patients’ perception of shoulder girdle and neck muscles pain using the Visual Analogue Scale.
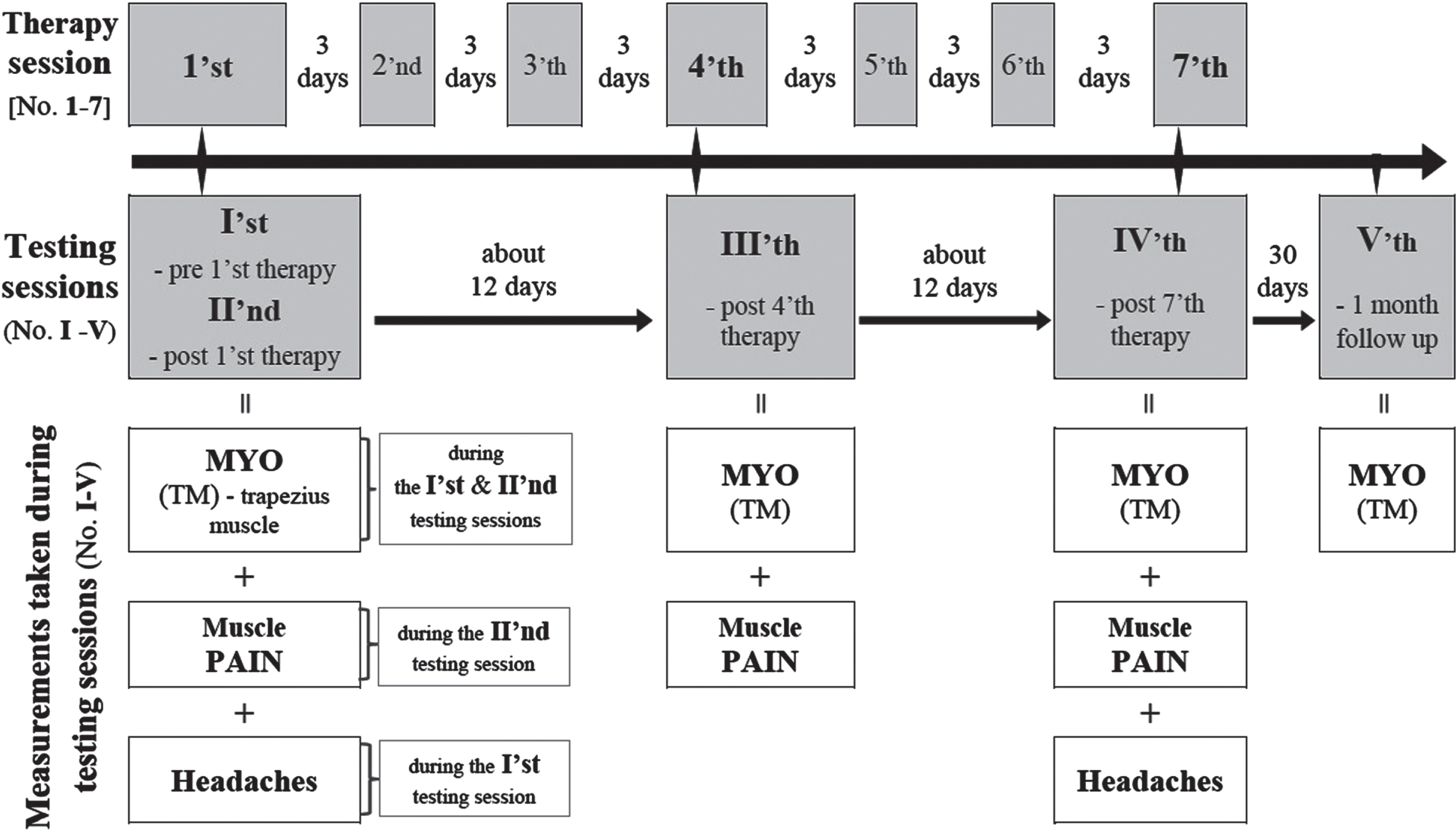
In each migraine patient the testing sessions were conducted in the following five time points (encoded as Roman numerals I–V; as in the Figs. 1, 3–6 and Table 2): I–pre-1’st therapy; II –post-1’st therapy; III –post-4’th therapy; IV –post-7’th therapy; V –one month after the 7’th therapy (1 month follow up) (Fig. 1). The myotonometric (MYO) measurements of muscle mechanical properties were performed in each of the five testing sessions: pre- and post-1’st, post-4’th, post-7’th therapy and 1 month follow up (i.e. while the testing sessions I–V; Fig. 1). Shoulder girdle and neck muscle pain was assessed during the following three testing sessions: post-1’st, post-4’th and post-7’th therapy (i.e. while the testing sessions II, III and IV; Fig. 1). Headache characteristics were evaluated twice, and namely while pre-1’st and post-7’th therapy (i.e. while the testing sessions I and IV; Fig. 1).
Fig. 3
Myotonometric frequency (F-MYO, [Hz]) values in the tested P1-P6 points at the left (A) and the right (B) side upper trapezius muscle. Data are expressed as mean (SD). * - statistically significant difference (p≤0.001) compared to the pre-1’st therapy measurement session value in the post hoc analysis.
![Myotonometric frequency (F-MYO, [Hz]) values in the tested P1-P6 points at the left (A) and the right (B) side upper trapezius muscle. Data are expressed as mean (SD). * - statistically significant difference (p≤0.001) compared to the pre-1’st therapy measurement session value in the post hoc analysis.](https://content.iospress.com:443/media/nre/2023/52-2/nre-52-2-nre220237/nre-52-nre220237-g003.jpg)
Fig. 4
Myotonometric decrement (D-MYO, [log]) values in the tested P1-P6 points at the left (A) and right (B) side upper trapezius muscle. Data are expressed as mean (SD). No significant p > 0,005.
![Myotonometric decrement (D-MYO, [log]) values in the tested P1-P6 points at the left (A) and right (B) side upper trapezius muscle. Data are expressed as mean (SD). No significant p > 0,005.](https://content.iospress.com:443/media/nre/2023/52-2/nre-52-2-nre220237/nre-52-nre220237-g004.jpg)
Fig. 5
Myotonometric stiffness (S-MYO, [N/m]) values in the tested P1-P6 points at the left (A) and right (B) side upper trapezius muscle. Data are expressed as mean (SD). * - statistically significant difference (p≤0.001) compared to the pre-1’st therapy measurement session value in the post hoc analysis.
![Myotonometric stiffness (S-MYO, [N/m]) values in the tested P1-P6 points at the left (A) and right (B) side upper trapezius muscle. Data are expressed as mean (SD). * - statistically significant difference (p≤0.001) compared to the pre-1’st therapy measurement session value in the post hoc analysis.](https://content.iospress.com:443/media/nre/2023/52-2/nre-52-2-nre220237/nre-52-nre220237-g005.jpg)
Fig. 6
Shoulder girdle and neck (SGN) muscles pain felt by subject (assessed with VAS-visual analog scale score; [cm]) during therapeutic sessions. Data are expressed as mean (SD); # - statistically significant difference (p≤0.001) compared to the post-1’st therapy measurement session value in the post hoc analysis; ** - statistically significant difference (p≤0.001) compared to the post-4’th therapy measurement session value in the post hoc analysis.
![Shoulder girdle and neck (SGN) muscles pain felt by subject (assessed with VAS-visual analog scale score; [cm]) during therapeutic sessions. Data are expressed as mean (SD); # - statistically significant difference (p≤0.001) compared to the post-1’st therapy measurement session value in the post hoc analysis; ** - statistically significant difference (p≤0.001) compared to the post-4’th therapy measurement session value in the post hoc analysis.](https://content.iospress.com:443/media/nre/2023/52-2/nre-52-2-nre220237/nre-52-nre220237-g006.jpg)
2.3Therapeutic intervention - ischemic compression of myofascial trigger points (IC-MTrPs)
Therapeutic intervention relied on deactivation of MTrPs by ischemic compression method and was carried out according to the criteria established by Travell & Simons (2018). The IC-MTrPs therapy was applied on both sides of shoulder girdle and neck region on the following muscles: upper part of trapezius muscle, sternocleidomastoid muscle, temporal muscle, levator scapulae muscle, supraspinatus muscle, suboccipital muscle (Burstein, Jakubowski, & Rauch, 2011; Fernandez de Las Penas et al., 2010; Florencio et al., 2017).
IC-MTrPs intervention was made first on the right side and then on the left side and this order was the same in each patient. This is due to the fact that the right side showed palpatively higher muscle tone. A greater number of active MTrPs was found at the right side when compared to the left side (Fernandez de Las Penas et al., 2010). In physiotherapeutic practice, the principle of developing more tense tissues is applied in order to equalize tensions between the parties (Almeida Tolentino et al., 2021; Do et al., 2018; Espi-López et al., 2018; Fernandez De Las Penas et al. 2010).
Before starting the therapeutic procedure, the MTrPs localizations were identified by palpation and pinch pressure in patients lying back on the couch. During the procedure, a qualified physiotherapist sat behind the subject’s head. The pressure was sustained for about 5 seconds with a 2-3 seconds pause. In each subject, the intervention lasted 15 minutes on the same measurement day in the morning. During such a procedure, the patient could feel pain and discomfort in the area of pressure, however the pain decreased with time of the therapy. The manual pressure intervention were performed until the treated area was noticeably relaxed (based on therapist’s palpation) and the patient’s feeling of decreased pain (Do, Heldarskard, Kolding, Hvedstrup, & Schytz, 2018; Travell, Simons, & Simons, 2018). According to Travel & Simons (2018) when no results were obtained, the procedure was stopped after 2 minutes, and in the case of exacerbation of pain symptoms, it was stopped immediately. The strong pressure on the thumb induced local ischemia, as shown by local skin paleness. The cessation of pressure causes increased blood flow and better tissue nutrition.
The subjects underwent a cycle of seven IC-MTrPs therapeutic sessions, with 3 days breaks between each session, which lasted about 3 weeks in total (25 days) (Fig. 1). We chose the 7-session therapeutic program assuming that it would be effective enough since previous studies showed that 1-session therapy as well as longer therapeutic programs (ex. 12 sessions) were effective in MTrPs deactivation and pain alleviation (Moraska et al., 2017; Fernandez de Las Penas et al., 2011; Travell, Simons & Simons, 2018).
2.4Myotonometric measurements of muscle mechanical properties
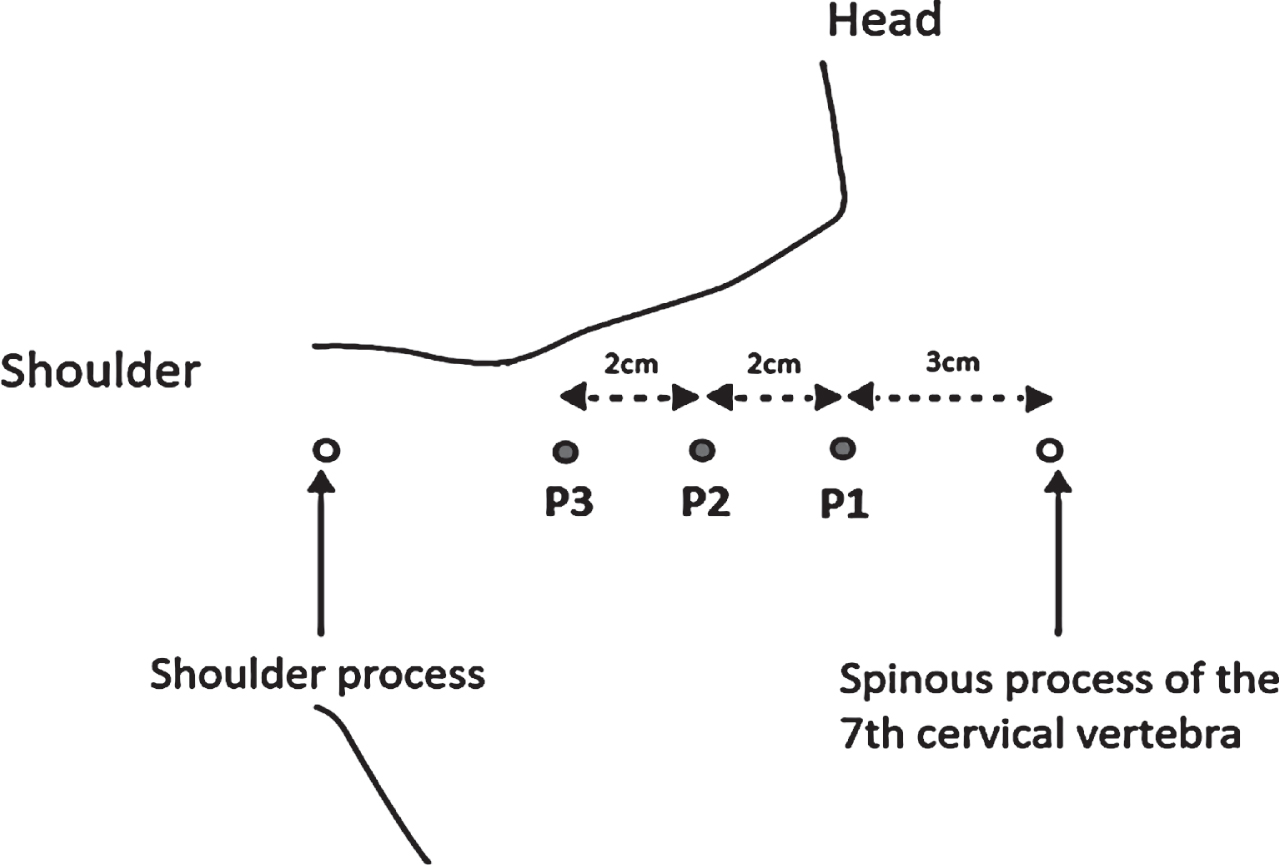
Patients were seated steadily and relaxed in a chair with full body support, hands in lap, looking straight ahead. For all patients, six measurement points were carefully indicated on both sides of upper trapezius muscle, i.e. three points on the left upper trapezius (P1, P2, P3) and three on the right upper trapezius (P4, P5, P6) (Fig. 2). The three testing points were located on a horizontal line between the cervico-thoracic junction of the spine (C7 / Th1) and the shoulder process of scapula in a distance between these points similar for each patient. Going from medial to lateral side the testing points were as follows: (i) the most medial point as P1 on the left and P4 on the right trapezius, which was distant 3 cm laterally from the cervico-thoracic junction of the spine (C7 / Th1); (ii) the next P2 or P5 point (intermediate one) was distant 2 cm laterally from the P1 or P4; (iii) and the most laterally located the P3 or P6 point was distant 2 cm from the P2 or P5 (on the left or right trapezius, respectively) (Fig. 2). The testing points were carefully marked on a skin and kept (remarked) by patients at home during the time frame of the study (about 2 months) to ensure the myotonometric measurement in the same locations for all testing sessions. In the present study, we chose only the upper trapezius muscle to be measured since in our and others opinion (Kisilewicz et al., 2018; Kisilewicz et al., 2020), when taking into account an anatomical structure and location of upper part of trapezius muscle, a selective and reliable myotonometric measurements are easier to obtain for the upper part then for the middle or lower parts.
Fig. 2
Diagram indicating locations of the three testing points (P1, P2 and P3; as the grey dots) on the left side upper trapezius muscle, where the myotonometric measurements (using MyotonPRO®) were conducted (own source). The three right side testing points (P4, P5 and P6) were located in similar manner on the right side upper trapezius muscle.
After instructions were given to the subject to relax, an investigator placed the testing end of the MyotonPRO® device on the marked testing point of the upper trapezius muscle and performed myotonometric measurements using multiscan 10 mode (10 consecutive records from which an average values for the myotonometric parameters were automatically calculated). We used the multiscan 10 mode since according to Marusiak et al., (2018) the 10 or even 5 records mode enable an excellent repeatability for the resting-condition myotonometric measurements. Agreeing with methodological guidelines (Gapeyeva, & Vain, 2008) the testing end of the MyotonPRO® device was placed in the testing point perpendicularly to the skin surface overlying the upper trapezius muscle. Myotonometric measurement’s order for the six strictly specified testing points was as follows: first P1, P2, P3 on the left side and then P4, P5, P6 on the right side. During each testing session, the whole myotonometric measurement protocol and testing conditions (temperature, light, time of the day) were identical. Myotonometric measurements were conducted by the same experienced investigator, who carried out the measurements without knowing about the measurement session number and did not participate in the analysis of the obtained data. We analyzed the following three myotonometric parameters: frequency of natural oscillations (F-MYO, [Hz]; reflecting non-neural muscle tone), decrement (D-MYO, [log]; informing about muscle elasticity) and stiffness (S-MYO, [N/m]; describing an ability of the muscle to resist an external force). The lower the F-MYO and S-MYO values, the smaller the tension and stiffness (respectively) of the examined muscle. The lower the D-MYO value the smaller the dissipation of mechanical energy during oscillation, indicating the higher the elasticity of the muscle structure (Stefaniak, Marusiak, & Bączkowicz, 2022). These parameters are more often reported in the literature and therefore their meaning for studying muscle mechanical properties is more acknowledged (Kisilewicz et al., 2018; Kisilewicz et al., 2020, Marusiak et al., 2018).
2.5Assessment of shoulder girdle and neck muscle pain using the Visual Analogue Scale (VAS)
Shoulder girdle and neck muscle pain was assessed with the graphic form of the Visual Analogue Scale (VAS), that consisted of a straight line (10 cm long) on paper with the endpoints defining extreme limits such as ‘no pain at all’ (number “0” in our study) and ‘pain as bad as it could be’ (number “10” in our study). First, the patient was asked: “How much did you feel shoulder girdle and neck muscles pain during the IC MTrPs therapy?". Then, the migraine patient was asked to mark with pen her perceived pain level on the line between the two endpoints. The distance between the two end points was measured with a ruler (Medline) to give a score of the patient’s perception of shoulder girdle and neck muscles pain, expressed in [cm]. Studies showed that an application of ruler in distance assessment increased the distance measurement reliability (Baker, Depuydt, & Thompson, 2009; Nemeth, Graham, & Harrison, 2003).
2.6Evaluation of headache characteristics
Headache characteristics were evaluated in each migraine patient before and after the 3-week IC MTrPs therapy. Average headache level while single episode was assessed using the same pain scale as it was described above in the point 2.5 (VAS score [cm]). Additionally, a frequency of headaches episodes (as number of headache episodes per month) and duration of single headache episode (as number of days) were reported by subjects in a custom-made questionnaire.
2.7Statistical analysis
The results are presented as arithmetic mean and standard deviation (mean (SD)) values for the whole tested group. Statistical differences between the testing sessions were analyzed using the repeated measures analysis of variance for Friedman ranks (due to absence of compliance with the normal distribution - Friedman ANOVA) and when a significant measurement session effect was found, post hoc for Friedman’s analysis was applied. For the inter-session comparisons of parameters measured during only two testing sessions (as in the case of headache parameters), a non-parametric test was used for two dependent samples: Wilcoxon pair wise order.
The results were considered statistically significant at the significance level of p≤0.05. We used the Statistica v. 10 software (StatSoft Inc., Krakow, Poland) for all statistical analyses.
3Results
3.1Inter-session comparisons of the myotonometric frequency (F-MYO) values in the tested points of the upper trapezius muscle
The F-MYO values, initially measured in the pre-1’st therapy session, had a clear tendency to decrease in all post therapy and 1-month follow up measurement sessions in all testing points of upper trapezius muscle (i.e. P1, P2, P3 on the left and P4, P5, P6 on the right side; Fig. 3A and 3B; respectively). However, the statistical analysis showed that compared to the pre-1’st therapy measurements the F-MYO values were significantly lower (P < 0.05) for the: (i) post-1’st therapy measurements in the left side testing point P3 (the most lateral point on the left side upper trapezius; Fig. 3A), (ii) post-4’th therapy measurements in all the three testing points on both sides of upper trapezius (i.e. P1, P2, P3 on the left and P4, P5, P6 on the right side; Fig. 3A and 3B; respectively), and (iii) 1-month follow up measurements in the left side testing point P1 (the most medial point on the left side upper trapezius; Fig. 3A) as well as in all the three right side testing points (i.e. P4, P5, P6; Fig. 3B).
3.2Inter-session comparisons of the myotonometric decrement (D-MYO) values in the tested points of the upper trapezius muscle
There was no clear tendency and any statistically significant inter-session differences (P > 0.05) in the D-MYO values in the left and right side testing points of the upper trapezius muscle (i.e. P1, P2, P3 and P4, P5, P6; Fig. 4A and 4B; respectively).
3.3Inter-session comparisons of the myotonometric stiffness (S-MYO) values in the tested points of the upper trapezius muscle
For the S-MYO measurements in the left and right side testing points of upper trapezius muscle (i.e. P1, P2, P3 and P4, P5, P6; respectively) there was a clear tendency of lower values in all post therapy and 1-month follow up measurement sessions, in reference to the pre-1’st therapy values (Fig. 5A, 5B). Nevertheless, the statistical analysis showed that compared to the pre-1’st therapy measurements the S-MYO values were significantly lower (P < 0.05) for the: (i) post-1’st therapy measurements in the left side testing point P3 (the most lateral point on the left side upper trapezius; Fig. 5A), (ii) post-4’th therapy measurements in all the three testing points on left side of upper trapezius (i.e. P1, P2, P3; Fig. 5A) and in the P4 and P6 testing points on the right side (the most medial and lateral points, respectively; Fig. 5B), and (iii) 1-month follow up measurements in the left side testing point P2 (the intermediate point on the left side upper trapezius; Fig. 5A), as well as in the P4 and P6 testing points on the right side (the most medial and lateral points, respectively; Fig. 5B).
3.4Inter-session comparisons of perceived shoulder girdle and neck muscles pain using the Visual Analogue Scale (VAS)
The initially measured VAS score for shoulder girdle and neck muscles pain in the post-1’st therapy session, significantly decreased (P < 0.05) in the post-4’th and post-7’th therapy session (Fig. 6). Also, there was a statistically significant decrease (P < 0.05) of the VAS value in the post-7’th compared to the post-4’th therapy session (Fig. 6).
3.5Inter-session comparisons of headache characteristics
All headache characteristics significantly improved (decrease of: average headache level while single episode, frequency of headache episodes and duration of single headache episode) in the post-7’th therapy session compared to the 1’st therapy session (P < 0.05; Table 2).
Table 2
Parameters assessing headache characteristics
| Measurement session name (No.) | Average headache level (VAS) while single episode [cm] | Frequency of headaches episodes [no. of days / month] | Duration of single headache episode [no. of days] |
| Pre-1’st therapy (I) | 7,55 (1,53) | 6,29 (4,95) | 2,83 (2,05) |
| Post-7’th therapy (IV) | 5,71 (2,01)* | 4,00 (3,91)* | 1,18 (1,11)* |
| Wilcoxon signed - rank test | Z = 3,78; | Z = 3,08; | Z = 3,19; |
| p = 0,0002* | p = 0,002* | p = 0,0001* |
Data are expressed as mean (SD). * - statistically significant difference (p≤0.05) compared to the pre-1’st therapy measurement session.
4Discussion
4.1Asssociation of MTrPs with migraine
Previous studies indicated that an occurrence of the MTrPs might be one of the reason that may contribute to start a migraine attack (Andreou, & Edvissone, 2019; Do, Heldarskard, Kolding, Hvedstrup, & Schytz, 2018; Sollmann et al., 2019). Additionally, several studies shown that patients with migraine have a greater number of MTrPs compared to healthy subjects and that higher number of MTrPs in neck and shoulder area correlates with muscular pain severity and duration of migraine attacks (Rezaeian, Mosallanezhad, Nourbakhsh, Ahmadi, & Nourozi, 2019; Travell, Simons, & Simons, 2018).
In contrary, studies by (Luedtke, Starke, & May, 2018; Rezaeian, Mosallanezhad, Nourbakhsh, Ahmadi, & Nourozi, 2019) reported that an inhibition of MTrPs in neck muscles (with usage of relaxation techniques including EMG biofeedback and dry needling technique) led to a decrease in frequency and severity of migraine attacks. The above mentioned study results are in agreement with our findings showing that ischemic compression therapy inactivate the MTrPs and consequently decreases headaches’ level, frequency and duration.
4.2IC-MTrPs therapy effects on the muscle hypertonia and pain
4.2.1IC-MTrPs therapy effects on the upper trapezius hypertonia as revealed by the myotonometry
We observed a clear tendency to decreased values of F-MYO and S-MYO in the trapezius muscle in all post IC-MTrPS therapy and in 1-month follow up measurement sessions, compared to initial measurements taken before starting the first IC-MTrPs therapeutic session. However, a significant decrease from the initial values of F-MYO and S-MYO were found: (i) immediately after the first IC-MTrPs therapeutic session in the most lateral point of the left side upper trapezius muscle; (ii) after the fourth IC-MTrPs therapeutic session in almost all testing points on both sides of upper trapezius muscle and (iii) one month after the cessation of the last IC-MTrPs therapy session in almost all testing points on the right side and in the most medial point (only for the F-MYO) or in the intermediate point (only for the S-MYO) on the left side upper trapezius muscle. These findings shows that the IC-MTrPs therapy caused an immediate and prolonged improvement of trapezius muscle functional state, i.e. the reduction of trapezius muscle hypertonia. There is no study so far to compare our results to other’ findings, in the context of myotonometry application to evaluate the IC-MTrPs therapy effects in migraine patients.
We suppose that this positive effect of MTrPs therapy might be related to energetic consequences of better nutrient in muscle fibers with greater blood flow in the region of the IC-MTrPs intervention, what may affect the local change of muscle fibers activity. Moraska et al. (2013), with an application microdialysis technique in the treated upper trapezius muscle, gave an experimental preliminary evidence, that IC-MTrPs regain skeletal muscle homeostasis via relaxation of the trigger point nodules. Moraska et al. (2013) showed that this effect was achieved since nutritive blood flow to the tissue is enabled, what consequently allows for increased substrate perfusion and oxygen delivery. Alternatively the above suggested mechanism might accompany the normalization of neural drive to treated muscle via inhibition of motoneurons’ activity of treated muscles. The inhibitions of treated muscle motoneurons might be caused by projections from inhibitory interneurons that receive inputs from: (i) type Ib afferents from Golgi tendon organs that might be more sensitive (and therefore send more impulses to these interneurons in spinal cord) in the state of hypertonic muscle, being mechanically deformed while the IC-MTrPs intervention; (ii) type III or IV nociceptive afferents (Lundberg, & Malmgren, 1988) that might be more active during ischemic compression intervention, which induces in treated muscle transient painful stimuli, and (iii) neocortical sensorimotor projections that send impulses to spinal cord, as an effect of repeated IC-MTrPs-induced somatosensory painful stimuli on cortical sensorimotor interaction mechanisms (Babiloni et al., 2010) responsible for activation of muscle during pain conditions.
Immediate IC-MTrPs therapy effect was limited to one testing point on the left side of the upper trapezius muscle. Probably the fact that a decrease in tone and stiffness was found only on the left side immediately after IC-MTrPs intervention might be related to the fact that the tested subjects were right-handed and engage in their life the right side more than the left one, what consequently might lead to greater hypertonia (and consequently lower immediate IC-MTrPs effect) in the right side and greater IC-MTrPs effect on the left side. It is also important to take into account a possible influence of the time course of the blood flow after IC-MTrPs. Moraska et al. (2013) showed that the IC-MTrPs induces greater blood flow in the region of the IC-MTrPs intervention and consequent better nutrient of treated muscle fibers, what leads to energetic consequences resulting in change of muscle fibers activity and consequent change of muscle tension. It is important to note in this point that the IC-MTrPs therapy always began in all subjects on the right side and then in turn the IC-MTrPs therapy was performed on the left side, but after the therapy the myotonometric measurements were always performed first on the left side and then on the right side. Probably, this blood flow course-dependent immediate IC-MTrPs effect on myotonometric tone and stiffness was easier to be captured (i.e. in shorter time) on the left side then on the right side, since there was shorter time interval between the IC-MTrPs therapy performed on the left side and consecutive myotonometric measurement conducted on this side.
Prolonged IC-MTrPs therapy effect is more spacious, what might be related with the above considered by us the repeated IC-MTrPs therapy effect on cortical mechanisms of sensory-motor interaction (Babiloni et al., 2010) and consequent neural control of muscle activity (Madeleine, & Arendt-Nielsen, 2005).
We did not find any clear tendency and significant IC-MTrPs therapy related change in D-MYO parameter of trapezius muscle in treated migraine patients. Probably, it is related to the fact that myotonometric decrement is a parameter that mostly reflects a change in viscoelastic properties of skeletal muscle that are associated mainly with structural changes, but IC-MTrPs therapy apparently leads to the functional neural activity related changes of skeletal muscle.
4.2.2IC-MTrPs therapy effects on the shoulder girdle and neck muscle pain
We found that the initially assessed level of shoulder girdle and neck muscles pain (after the first IC-MTrPs therapy session) significantly decreased while evaluation performed after the fourth and seventh therapeutic session. It is important to emphasize that the pain was assessed by patient as a response to the feeling of pain experienced by treated patient during IC-MTrPs therapy. It means that the decreased level of reported shoulder girdle and neck muscle pain was associated with lower pain-generating effect of mechanical compression during MTrPs therapy. Probably, it might be related to the fact that the treated shoulder girdle and neck muscles were less tensed (what is shown in our myotonometric results) as an effect of deactivation of MTrPs in shoulder girdle and neck muscle region. This assumption finds its confirmation in the results by Do et al. (2018), who found positive correlation between a level of muscle pain (objectively measured using pressure pain threshold - PPT) and active MTrPs in headache patients.
When taking together our myotonometric and muscle pain results we can draw a logic cause-and-effect association between the mechanical state and pain of skeletal muscle in relation to the applied IC-MTrPs therapy. In the cervical region, it has been suggested that factors such as pain and disability could be responsible for an increase in muscle tone and stiffness in spinal pain populations (Kocur, Wilski, Goliwas, Lewandowski, & Lochynski, 2019).
4.3IC-MTrPs therapy effects on the headaches’ characteristics
We assessed headache characteristics before and after 3-week period of the IC-MTrPs therapy and found a significant decrease of: (i) average headache level felt while single episode, (ii) frequency of headache episodes and (iii) duration of single headache episode. Our findings of positive effect of IC-MTrPs therapy on headaches characteristics might be caused by changed cervical spine biomechanics (associated with change in shoulder girdle and neck muscle tone) that might contribute to reduction of adverse tension in the spinal dura, that is assumed as one of the neurophysiological mechanism of headache (Alix, & Bates, 1999). This assumption is in accordance with studies by Crooks et al. (2018) and Espi-López et al. (2018), who found that soft tissue techniques and mobilization techniques may reduce headache in migraine patients.
4.4Implications and limitation of our findings, and perspectives for future research
Our findings confirm the assumption of beneficial cause-effect association between deactivation of trigger points via the IC-MTrPs therapy and an improvement of upper trapezius muscle functional state, and perceived shoulder girdle and neck muscles’ pain, with simultaneous reduction of headaches level, frequency and duration in migraine patients without aura. Findings of our uncontrolled study (case series repeated design) provide a preliminary evidence of the IC-MTrPs method’s efficacy in migraine pain therapeutic approach. This information is new and important not only from practical but also from scientific point of view. Based on our findings we can speculate about the mechanisms linking the IC-induced deactivation of MTrPs with positive changes of tonic muscle activity and consequent alleviation of muscle pain and headaches.
Unfortunately, the methodological limitations of our present study do not allow us to address some of scientific questions raised in our discussion, since we measured only the mechanical and behavioral counterparts of biochemical and neural processes that could stand behind the muscle state improvement and pain reduction in response to the IC-MTrPs therapy in migraine without aura. However, in spite of the above mentioned limitations, our current findings and considerations might be a good starting point for future controlled studies with more advanced methodology that will engage comprehensive multimodal measurements approach with using biochemical, neurophysiological and biomechanical methods.
5Conclusions
The results of our study showed that in patients suffering episodic migraine without aura the ischemic compression myofascial trigger points therapy, by deactivating MTrPs, resulted in (i) decrease of upper trapezius muscle tone and stiffness with accompanied (ii) alleviation of pain in shoulder girdle and neck muscles, and (iii) reduction of headache level as well as number and duration of headache episodes. Additionally, our study evidences that the myotonometry has the potential to be a good objective method for standard usage in physical examination of MTrPs in migraine patients.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The authors report no funding.
References
1 | Aguilar-Shea, A. L. , Membrilla, J. A. , & Diaz-de-Teran, J. ((2022) ) Migraine review for general practice. Atencion Primaria, 54: (2), 102208. DOI: 10.1016/j.aprim.2021.102208 |
2 | Albin, S. R. , Koppenhaver, S. L. , Macdonald, C. W. , Capoccia, S. , Ngo, D. , & Phippen, S. ((2020) ) The effect of dry needling on gastrocnemius muscle stiffness and strength in participants with latent trigger points. Journal of Electromyography and Kinesiology, 5: , 102479. DOI: 10.1016/j.jelekin.2020.102479 |
3 | Alix, M. E. , & Bates, D. K. ((1999) ) A proposed etiology of cervicogenic headache: The neurophysiologic basis and anatomic relationship between the dura mater and the rectus posterior capitis minor muscle. Journal of Manipulative & Physiological Therapeutics, 22: (8), 534–539. DOI: 10.1016/S0161-4754(99)70006-0 |
4 | Andreou, A. P. , & Edvissone, L. ((2019) ) Mechanism of migraine as a chronic evolutive condition. Journal of Headache and Pain, 20: (1), 117–134. DOI: 10.1186/s10194-019-1066-0 |
5 | Aoyama, N. ((2021) ) Involvement of cervical disability in migraine: A literature review. British Journal of Pain, 15: (2), 199–212. DOI: 10.1177/2049463720924704 |
6 | Ashina S. , Bendtsen L. , Lyngberg A. C. , Lipton R. , Hajiveva N. , & Jensen R. ((2015) ) Prevalence of neck pain in migraine and tension-type headache: Apopulation study. Cephalalgia, 35: , 211–2119. DOI: 10.1177/03331024145351103 |
7 | Babiloni, C. , Capotosto, P. , Del Percio, C. , Babiloni, F. , Petrini, L. , Buttiglione, M. , & Cibelli, G. ((2010) ) Sensorimotor interaction between somatosensory painful stimuli and motor sequences affects both anticipatory alpha rhythms and behavior as a function of the event side. Brain Research Bulletin, 81: (4-5), 398–405. DOI: 10.1016/j.brainresbull.2009.11.009 |
8 | Baker, P. A. , Depuydt, A. , & Thompson, J. M. ((2009) ) Thyromental distance measurement–fingers don’t rule. Anaesthesia, 64: (8), 878–882. DOI: 10.1111/j.1365-2044.2009.05985.x |
9 | Benatto, M. T. , Florencio, L. L. , Bragatto, M. M. , Dach, F. , Fernández-De-Las-Peñas, C. , & Bevilaqua-Grossi, D. ((2022) ) Neck-specific strengthening exercise compared with placebo shamultrasound in patients with migraine: A randomized controlled trial. BMC Neurology, 22: (1), 126. DOI: 10.1186/s12883-022-02650-0 |
10 | Burstein, R. , Jakubowski, M. , & Rauch, S. D. ((2011) ) The science of migraine. Journal of Vestibular Research, 21: (6), 305–314. DOI: 10.3233/VES-2012-0433 |
11 | Calandre, E. P. , Hidalgo, J. , Garcia –Leiva, J. M. , & Rico-Villademoros, F. ((2008) ) Myofascial trigger points in cluster headache patients: A case serie. Head & Face Medicine, 4: , 32. DOI: 10.1186/1746-160X-4-32 |
12 | Crooks, D. I. , Newington, K. , Pilling, L. , & Todd, M. ((2018) ) Assessing the feasibility of mobilisation of C0–C3 cervical segments to reduce headache in migraineurs. International Journal of Rehabilitation Research, 25: (8), 382–394. https://doi.org/10.12968/ijtr.2018.25.8.382 |
13 | De Almeida Tolentino, G. , Lima Florencio, L. , Ferreira Pinheiro, C. , Dach, F. , Fernández-De-Las-Peñas, C. , & Bevilaqua-Grossi, D. ((2021) ) Effects of combining manual therapy, neck muscleexercises, and therapeutic pain neuroscience education in patientswith migraine: A study protocol for a randomized clinical trial. BMC Neurology, 21: (1), 249. DOI: 10.1186/s12883-021-02290-w |
14 | Do, T. P. , Heldarskard, G. F. , Kolding, L. T. , Hvedstrup, J. , & Schytz, H. W. ((2018) ) Myofascial trigger points in migraine and tension-type headache, Journal of Headache and Pain, 10-19: (1) 84. DOI: 10.1186/s10194-018-0913-8 |
15 | Espi-López, G. V. , Ruescas-Nicolau, M. A. , Redondo, C. N. , Benítez-Martínez, J. C. , Dugailly, P. M. , & Falla, D. ((2018) ) Effect of soft tissue techniques on headache impact, disability, and quality of life in migraine sufferers: A pilot study. International of Complementary & Alternative Medicine, 24: (11), 1099–1107. DOI: 10.1089/acm.2018.0048 |
16 | Fernande de las Penas, C. , Cleland, J. A. , Palomeque-del-Cerro, L. , Caminero, A. B. , Guillem-Mesado, A. , & Jiménez-García, R. ((2011) ) Development of a clinical prediction rule for identifyingwomen with tension-type headache who are likely to achieveshort-term success with joint mobilization and muscle trigger pointtherapy. Headache, 51: (2), 246–261. DOI: 10.1111/j.1526-4610.2010.01789.x |
17 | Fernandez de Las Penas, C. , Ge, H. Y. , Alonso-Blanco, C. , González-Iglesias, J. , & Arendt-Nielsen, L. ((2010) ) Referred pain areas of active myofascial trigger points in head, neck, and shoulder muscles, in chronic tension type headache. Journal of Bodywork and Movement Therapies, 14: (4), 391–396. DOI: 10.1016/j.jbmt.2009.06.008 |
18 | Ferracini, G. N. , Florencio, L. L. , Dach, F. , Bevilaqua Grossi, D. , Palacios-Ceña, M. , et al. ((2017) ) Musculoskeletal disorders of the upper cervical spine in women with episodic or chronic migraine. European Journal of Physical and Rehabilitation Medicine, 53: (3), 342–350. DOI: 10.23736/S1973-9087.17.04393-3 |
19 | Florencio, L. L. , Ferracini, G. N. , Chaves, T. C. , Palacios-Cena, M. , & Ordas-Bandera, C. ((2017) ) Active trigger points in the cervical musculature determine the altered activation of superficial neck and extensor muscles in women with migraine. The Clinical Journal of Pain, 33: (3), 238–245. DOI: 10.1097/AJP.0000000000000390 |
20 | Gapeyeva, H. , & Vain, A. (2008). Methodical guide: Principles of applying myoton in physical medicine and rehabilitation, Muomeetria. University of Tartu Press, Tartu, Estonia. |
21 | Glemser, P. A. , Jaeger, H. , Nagel, A. M. , Ziegler, A. E. , Simons, D. , & Schlemmer, H. P. ((2017) ) 23Na MRI and myometry to compare eplerenone vs. glucocorticoid treatment in Duchenne dystrophy. Acta Myologica, 36: , 2–13. PMC5479105. |
22 | Gordon, C. M. , Andrasik, F. , Schleip, R. , Birbaumer, N. , & Rea, M. ((2016) ) Myofascial trigger point release (MTR) for treating chronic shoulder pain: A novel approach. Journal of Bodywork and Movement Therapies, 20: (3), 614–622. DOI: 10.1016/j.jbmt.2016.01.009 |
23 | Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. (2018). Cephalalgia, 38: (1), 1–211. DOI: 10.1177/0333102417738202 |
24 | IHS Classification ICHD –3 Beta. http://beta.ichd-3.org/1-migraine/1-1-migraine-without-aura/ |
25 | Kisilewicz, A. , Janusiak, M. , Szafraniec, R. , Smoter, M. , Ciszek, B , Madeleine, P. , Fernández-De-Las-Peñas, C. , & Kawczynski, A. ((2018) ) Changes in muscle stiffness of the trapezius muscle after application of ischemic compression into myofascial trigger points in professional basketball players. Journal of Human Kinetics, 15: (64), 35–45. DOI: 10.2478/hukin-2018-0043 |
26 | Kisilewicz, A. , Madeleine, P. , Ignasiak, Z. , Ciszek, B. , Kawczynski, A. , & Larsen, R.G. ((2020) ) Eccentric exercise reduces upper trapezius muscfle stiffness assessed by shear wave elastography and myotonometry. Frontiers in Bioengineering and Biotechnology, 8: , 928. DOI: 10.3389/fbioe.2020.00928 |
27 | Kocur, P. , Wilski, M. , Goliwas, M. , Lewandowski, , & Lochynski, D. ((2019) ) Influence of forward head posture on myotonometric measurements of superficial neck muscle tone, elasticity, and stiffness in asymptomatic individuals with sedentary jobs. Journal of Manipulative & Physiological Therapeutics, 42: , 195–202. DOI: 10.1016/j.jmpt.2019.02.005 |
28 | Liou, C. L. , Feng, Y. N. , Zhang, H. Q. , Li, Y. P. , Zhu, Y. , & Zhang, Z. J. ((2018) ) Assessing the viscoelastic properties of upper trapezius muscle: Intra- and inter-tester reliability and the effect of shoulder elevation. Journal of Electromyography & Kinesiolology, 43: , 226–229. DOI: 10.1016/j.jelekin.2017.09.007 |
29 | Luedtke, K. , Starke, W. , & May, A. ((2018) ) Musculoskeletal dysfunction in migraine patients. Cephalalgia, 38: (5), 865–875. DOI: 10.1177/0333102417716934 |
30 | Lundberg, A. , & Malmgren, K. ((1988) ) The dynamic sensitivity of Ib inhibition. Acta Physiologica Scandinavica, 133: (1), 123–124. DOI: 10.1111/j.1748-1716.1988.tb08389.x |
31 | Madeleine, P. , & Arendt-Nielsen, L. ((2005) ) Experimental musclepain increases mechanomyographic signal activity during sub-maximalisometric contractions. Journal of Electromyography & Kinesiology, 15: , 27–36. DOI: 10.1016/j.jelekin.2004.06.006 |
32 | Marusiak, J. , Jarocka, E. , Jaskolska, A. , & Jaskolski, A. ((2018) ) Influence of number of records on reliability of myotonometric measurements of muscle stiffness at rest and contraction. Acta of Bioengineering and Biomechanics, 20: (4), PMID: 30520445. |
33 | Mongini, F. , Ciccone, G. , Deregibus, A. , Ferrero, L. , & Mongini, T. ((2004) ) Muscle tenderness in different headache types and its relation to anxiety and depression. Pain, 112: , 58–63. DOI: 10.1016/j.pain.2004.07.025 |
34 | Moraska, A. F. , Hickner, R. C. , Kohrt, W. M. , & Brewer, A. ((2013) ) Changes in blood flow and cellular metabolism at a myofascial trigger point with trigger point release (ischemic compression): A proof-of-principle pilot study. Archives of Physical Medicine and Rehabilitation, 94: (1), 196–200. DOI: 10.1016/j.apmr.2012.08.216 |
35 | Moraska, A. F. , Schmiege, S. J. , Mann, J. D. , Butryn, N. , & Krutsch, J. P. ((2017) ) Responsiveness of myofascial trigger points to single and multiple trigger point release massages: A randomized, placebo controlled trial. American Journal of Physical Medicine & Rehabilitation, 96: (9), 639–645. DOI: 10.1097/PHM.0000000000000728 |
36 | Mroczek, D. , Superlak, E. , Konefał, M. , Maćkała, K , Chmura, P. , Seweryniak, T. , & Chmura, J. ((2018) ) Changes in thestiffness of thigh muscles in the left and right limbs during sixweeks of plyometric training in volleyball players. PolishJournal of Sport and Tourism, 25: (2), 20–24. https://doi.org/10.2478/pjst-2018-0010 |
37 | Nemeth, K. A. , Graham, I. D. , & Harrison, M. B. ((2003) ) The measurement of leg ulcer pain: Identification and appraisal of pain assessment tools. Advances in Skin & Wound Care, 16: (5), 260–267. DOI: 10.1097/00129334-200309000-00017 |
38 | Pérez-Bellmunt, A. , Simon, M. , López-De-Celis, C. , Ortiz-Miguel, S. , González-Rueda, V. , & Fernandez-De-Las-Peñas, C. ((2021) ) Effects on neuromuscularfunction after ischemic compression in latent trigger points in thegastrocnemius muscles: A randomized within-participant clinicaltrial. Journal of Manipulative & Physiological Therapeutics, S0161-4754: (20), 30156–1. DOI: 10.1016/j.jmpt.2020.07.015 |
39 | Rezaeian, T. , Mosallanezhad, Z. , Nourbakhsh, M. R. , Ahmadi, M. , & Nourozi, M. ((2019) ) The impact of soft tissue techniques in the management of migraine headache: A randomized controlled trial. Journal of Chiropractic Medicine, 18: (4), 243–252. DOI: 10.1016/j.jcm.2019.12.001 |
40 | Roch, M. , Morin, M. , & Gaudreault, N. ((2020) ) The MyotonPRO: A reliable tool for quantifying the viscoelastic properties of a trigger point on the infraspinatus in non-traumatic chronic shoulder pain. Journal of Bodywork and Movement Therapies, 24: (4), 379–385. DOI: 10.1016/j.jbmt.2020.05.002 |
41 | Rodríguez-Almagro, D. , Barassi, G. , Bertollo, M. , Obrero-Gaitán, E. , Di Iorio, A. , Prosperi, L. , Achalandabaso-Ochoa, A. , Lomas-Vega, R. , & Ibáñez-Vera, A.J. ((2022) ) Manual therapy approach to the extraocular muscles inmigraine treatment: A preliminary study. Advances in Experimental Medicine and Biology, 1375: , 29–37. DOI: 10.1007/5584_2021_704 |
42 | Sollmann, N. , Mathonia, N. , Weidlich, D. , Bonfert, M. , Schroeder, S. A. , & Badura, K. A. ((2019) ) Quantitative magnetic resonance imaging of the upper trapezius muscles - assessment of myofascial trigger points in patients with migraine, Journal of 905 Headache and Pain, 18-20: (1). DOI: 10.1186/s10194-019-0960-9 |
43 | Sollmann, N. , Schandelmaier, P. , Weidlich, D. , Börner, C. , Urban, G. , & Lang, M. ((2021) ) Patients with episodic migraine show increased T2 values of the trapezius muscles - an investigation by quantitative high-resolution magnetic resonance imaging. Cephalalgia, 41: (8), 934–942. DOI: 10.1177/0333102421996374 |
44 | Stefaniak, W. , Marusiak, J. , & Baczkowicz, D. ((2022) ) Heightened tone and stiffness with concurrent lowered elasticity of peroneus longus and tibialis anterior muscles in athletes with chronic ankle instability as measured by myotonometry. Journal of Biomechanics, 144: , 111339. DOI: 10.1016/j.jbiomech.2022.111339 |
45 | Tali, D. , Menahem, I. , Vered, E. , & Kalichman, L. ((2014) ) Upper cervical mobility, posture and myofascial trigger points in subjects with episodic migraine: Case-control study. Journal of Bodywork and Movement Therapies, 18: (4), 569–75. DOI: 10.1016/j.jbmt.2014.01.006 |
46 | Travell, J. G. , Simons, D. G. , & Simons, L. S. (2018). Travell& Simons’ myofascial pain and dysfunction: The trigger point manual. USA, Wolters&Kluwer Health, 20-75. |




