Relationship between anxiety and concussion symptoms among adolescents enrolled in a randomized controlled trial of aerobic exercise
Abstract
BACKGROUND:
Affective symptoms, specifically, anxiety, are often overlooked after sport-related concussion (SRC), and may contribute to prolonged recovery.
OBJECTIVE:
To examine the impact of anxiety during clinical recovery among adolescents (13–18y) enrolled in a randomized trial of aerobic exercise for SRC.
METHODS:
Patients at three sites were randomized into aerobic exercise or stretching arms, and enrolled in the 4-week intervention. The relationship between PROMIS Anxiety score at initial visit and time to symptom resolution was evaluated with survival analysis. The relationship between weekly PROMIS Anxiety score and Post-concussion Symptom Inventory (PCSI) score was evaluated with Linear Mixed Models. Analyses adjusted for study arm and baseline covariates.
RESULTS:
Among 54 adolescents (median age = 15.8y, initial visit PCSI score = 32, pre-injury PROMIS Anxiety score = 2), median time to symptom resolution was 10 days (25th-75th percentiles: 6-24) in the Low-PROMIS Anxiety group and 12 days (25th-75th percentiles: 5-21) in the High-PROMIS Anxiety group (p = 0.62). Each additional unit of PROMIS Anxiety score corresponded to a 1.52-unit higher PCSI total score (p < 0.01). Neither effect varied by aerobic exercise/stretching group.
CONCLUSION:
Higher initial PROMIS Anxiety score was not significantly associated with delayed symptom resolution. However, over time, PROMIS Anxiety score was significantly associated with elevated PCSI score, regardless of exercise/stretching group.
1Introduction
1.1Background
There is growing interest among clinicians and researchers to investigate the role of mental health on recovery following concussion. Affective symptoms, anxiety in particular, represent an important and often overlooked symptom domain, and a potentially modifiable risk factor for prolonged recovery (Harmon et al., 2019). Just as providers evaluate and manage concussion-related vestibular, vision, headache, and nausea symptoms, clinical recognition and treatment of affective symptoms and anxiety may improve concussion treatment outcomes (Ellis et al., 2015; Kapadia et al., 2019).
Investigations into the role of anxiety and depression after concussion have been limited and focused predominantly on college-aged athletes, and on cognitive performance and return-to-play outcomes (Ali et al., 2021; Covassin et al., 2013; D’Alonzo et al., 2022; Thomas, Guty, et al., 2021). Findings suggest a positive correlation between anxiety and depressive symptoms, and overall concussion symptom burden (Edmed & Sullivan, 2012; Thomas, Riegler, et al., 2021). Athletes with anxiety and depressive symptoms also performed worse on attention and processing speed measures in neurocognitive outcomes compared to healthy controls (Thomas, Riegler, et al., 2021), and experienced longer timelines to symptom resolution and return-to-play (D’Alonzo et al., 2022). Regarding youth specifically, one study reported positive correlations between pre-existing anxiety disorders and symptom burden following concussion (Martin et al., 2020), and a second study reported relationships between pre-existing emotional distress and delayed recovery outcomes (Rosenbaum et al., 2020).
Research is warranted to better understand anxiety and the possible relationship with symptoms after concussion in the pediatric population. The lifetime prevalence of at least one diagnosed concussion is 20% (Veliz et al., 2017), and is increasing (Veliz et al., 2021), and by age 16, roughly 10% of children and adolescents meet criteria for an anxiety disorder (Costello et al., 2003; Kessler et al., 2012). Importantly, it is estimated that only 5-15% of children and adolescents utilize services for mental health problems, including anxiety (Eijgermans et al., 2021). This study focuses on pediatric athletes in particular, where sport-related concussions (SRC) occur relatively often (Halstead et al., 2010; Veliz et al., 2017; Yaramothu et al., 2019); and account for approximately 15% of injuries among high school athletes (Meehan et al., 2011). While recent studies have examined the influence of symptom burden and symptom type on clinical recovery (Ali et al., 2021; Putukian et al., 2021; Schilling et al., 2020; Sicard et al., 2021) and return-to-play trajectories (D’Alonzo et al., 2022), it is unclear how certain symptom domains, including physical, cognitive, emotional, and sleep factors respond to current approaches to treatment following concussion. Given the burden of SRC and increasing efforts to inform and create multidimensional clinical care teams (Polinder et al., 2018) to treat concussion symptoms in this population, adolescent athletes are well-positioned as an appropriate group in which to study these complex relationships.
1.2Objective
The purpose of this study was to determine the nature and impact of reported anxiety on clinical recovery in the context of a cohort of adolescent athletes with SRC, enrolled in a randomized trial of aerobic exercise. Our approach to evaluate these relationships was two-fold. First, we explored the effect of adolescents’ reported anxiety at the initial visit after SRC on time to symptom resolution. We hypothesized that higher initial anxiety would be associated with longer time to symptom resolution. Second, we investigated how reported anxiety over time related to symptom burden over time, hypothesizing that higher anxiety during follow-up would be associated with higher overall symptom burden. We also evaluated whether study arm assignment – randomization to aerobic exercise compared to stretching– affected either analysis.
2Methods
2.1Data source
We conducted a secondary analysis of data from a parallel-design, multi-center randomized controlled (RCT) trial of aerobic exercise, funded by the American Medical Society for Sports Medicine (AMSSM). The purpose of the RCT was to examine the effect of prescribing sub-threshold aerobic exercise early after injury as a treatment approach for adolescent athletes with SRC (Leddy et al., 2021). From July 2018 to April 2020 (Clinicaltrials.gov: NCT02959216), patients aged 13–18 years with SRC were enrolled from three sites with concussion programs in Buffalo, NY (University at Buffalo, UB), Philadelphia, PA (Children’s Hospital of Philadelphia, CHOP), and Boston, MA (Boston Children’s Hospital, BCH) (Leddy et al., 2021). The study was approved by the Institutional Review Board (IRB) of the Children’s Hospital of Philadelphia with reliance of the Boston Children’s and University at Buffalo IRBs on the CHOP IRB. All eligible patients, and the parents of minors (aged 13–17 years), provided written informed consent to participate in the study.
Across sites, male and female adolescents (aged 13–18 years) presenting within 10 days of injury and diagnosed with SRC by an experienced sports medicine physician using standardized criteria were invited to participate in the trial (Leddy et al., 2021; McCrory et al., 2017). Exclusion criteria consisted of the following: (1) 3-point or less difference between current and pre-injury symptoms as measured by the PCSI (Sady et al., 2014); (2) moderate or severe TBI as indicated by a score < 13 on GCS, lesion on CT/MRI, and/or focal neurologic signs consistent with intracerebral lesion; (3) injury involving loss of consciousness for≥30 minutes or post-traumatic amnesia for≥24 hours; (4) inability to exercise because of lower-extremity orthopedic injury, significant vestibular or visual dysfunction, or increased cardiac risk; (5) pre-existing comorbidities that prevent participation in active testing and/or rehabilitation; (6) history of more than 3 diagnosed concussions; (7) currently on medications that affect autonomic function, such as ADHD medications or mood stabilizers; (8) active substance abuse/dependence; (9) unwillingness to participate in research; and (10) limited English proficiency.
Participants were randomized into prescribed sub-threshold aerobic exercise or stretching exercise arms, and the intervention lasted up to 4 weeks after injury with weekly visits, and a 4-month post-injury follow-up visit. In the aerobic exercise arm, participants received a new target HR exercise prescription each week based on reassessment of exercise tolerance until recovered. If a participant did not recover by the conclusion of the study 4 weeks post-injury, further comprehensive clinical treatment was provided. In addition to the clinical characteristics collected at each weekly visit, concussion symptoms were collected three times per day using ecological momentary assessment (EMA) via the LifeData smartphone application platform, ReCoUPS (Recovering Concussion Update on Progression of Symptoms) (Wiebe et al., 2021), which prompted participants to complete the Post-Concussion Symptom Inventory (PCSI; scored 0–120) (Rabinowitz & Fisher, 2020; Sady et al., 2014).
The primary study outcome of the AMSSM trial was clinical recovery, defined as 1) a return to pre-injury symptoms (two or fewer new mild symptoms on the current PCSI compared with pre-injury), 2) exercise tolerance without exacerbation of concussion symptoms, and 3) a normal visio-vestibular physical examination.
2.2Study sample
The sample for this analysis consisted of a subset of the AMSSM study population: out of a total 118 adolescents recruited into the trial, 54 male and female adolescents with SRC with complete initial visit anxiety data from the PROMIS Anxiety pediatric short form. This instrument was introduced one year into the AMSSM trial. A retrospective recall of pre-injury PROMIS Anxiety pediatric short form score was also collected at initial visit and included in this analysis. Symptom data were collected via daily symptom scores via EMA using the PCSI, as described earlier. Both the PROMIS Anxiety pediatric short form and PCSI are established and validated instruments for measuring anxiety and concussion symptoms in the pediatric population (Bevans et al., 2018; Iverson, Marsh, et al., 2021; Sady et al., 2014).
2.3Variables and definitions
PROMIS Anxiety pediatric short form score. The primary exposure in the present analysis and main measure of anxiety in the study was the PROMIS Anxiety pediatric short form, which consists of 8 items that assess the domain of anxiety in children and adolescents in the past 7 days on a 5-point scale (Bevans et al., 2018). The PROMIS Anxiety pediatric short form was administered to participants at each of the study visits. Initial visit (IV), follow-up visit #1 (F1), follow-up visit #2 (F2) and 4-week post-injury follow-up (F4) only were included in the analysis since very few in the sample (<5%) attended a third follow-up visit or completed a 4-month post-injury follow-up visit. Participants were included in the current analysis if they completed the PROMIS Anxiety pediatric short form during at least 75% of the four visits.
PCSI symptoms. The primary outcomes for this analysis were based on the Post- Concussion Symptom Inventory (PCSI), where responses for each of 20 symptoms can range from 0–6. Scores are summed and reported as total PCSI, on a scale of 0–120. For this analysis, we selected the maximum total PCSI score reported through ReCOUPS on each day for each participant. From these scores, we selected the score that each participant reported one day following the day that PROMIS Anxiety pediatric short form score was collected at each weekly study visit date.
Covariates of interest for this analysis were study site (UB, CHOP, BCH), intervention group (aerobic exercise or stretching), sex, age, pre-injury PROMIS Anxiety pediatric short form score (collected retrospectively), initial PCSI score, concussion history (≥1 previous concussions), history of diagnosed anxiety, family history of diagnosed anxiety, and time from injury (in days) to initial study visit. Characteristics were identified as covariates, potential confounders, and effect modifiers prior to analysis based on earlier work and clinical expertise of the study investigators (Leddy et al., 2021; Wiebe et al., 2021) (Fig. 1).
Fig. 1
Directed Acyclic Graph (DAG) to demonstrate the relationships between PROMIS Anxiety pediatric short form score (exposure) and PCSI score (outcome), and potential covariates and confounders to be included in the model.

2.4Statistical analysis
Descriptive statistics of the study sample at initial visit were conducted with proportions for categorical variables, using chi-square and Fisher’s exact test, and median and 25th-75th percentiles for continuous variables using Wilcoxon rank-sum test. Our threshold for statistical significance was p < 0.05, and we also examined effect estimates, standard errors, and confidence intervals. We considered model assumptions and prior contextual and clinical knowledge to be instrumental throughout our analysis and interpretation. Analysis was performed using Stata17 (StataCorp, 2021).
2.4.1Survival analysis
Survival analysis was conducted to examine the relationship between initial PROMIS Anxiety pediatric short form score and time to symptom resolution. Survival time was defined as time (in days) from intervention start to symptom resolution date, with time origin as the date of the initial visit. Participants experienced a failure if they achieved symptom resolution during the study period, defined as the first date when the participant reported two or fewer new symptoms on the PCSI for two consecutive days. Participants were considered (right-censored) if they did not have symptoms resolve within the four-week intervention period, that is, before their intervention end date. The primary exposure of interest was PROMIS Anxiety pediatric short form score at the initial visit. We examined the overall distribution of the PROMIS Anxiety pediatric short form scores and split this exposure into 2-High/Low categories at the median. The purpose of this analysis was to compare time from intervention start to symptom resolution among those with High compared to Low PROMIS Anxiety pediatric short form scores at the initial visit.
Kaplan Meier curves are presented by PROMIS Anxiety pediatric short form score at initial visit and also stratified by intervention group (aerobic exercise versus stretching). The overall difference in survival between groups was tested with Log-rank tests. We then modeled the relationship with Cox proportional hazards regression to estimate the hazard ratio (i.e., rate ratio) for symptom resolution, adjusting for potential confounders and covariates of interest (Fig. 1). We conducted post-hoc tests to evaluate the assumption of proportional hazards by examining observed-predicted plots and results of the Schoenfeld test, and model fit by estimating the cumulative hazard and examining Cox-Snell residuals plot (see Appendix).
2.4.2Longitudinal analysis
Standard analyses such as logistic or linear regression models provide us with cross-sectional effects and associations, and assume the outcome measure of interest is independent. However, longitudinal data, or data collected over time, are by nature correlated and between-subject differences, within-subject biological variability, and measurement error are all sources that can lead to potential variability in data.
In our study, in order to examine the relationship between PROMIS Anxiety pediatric short form and PCSI scores, accounting for individual trajectories over time, we needed to use linear models with random effects. By doing this, we were able to interpret the models in terms of effects and associations over time (i.e., rates). We used Linear Mixed Model (LMM) with unstructured covariance structure to model the relationship between PROMIS Anxiety pediatric short form score weekly during follow-up and total PCSI score weekly during follow-up (assessed one day after each PROMIS Anxiety pediatric short form score was obtained, creating a one-day lag between exposure and outcome).
To identify the best-fitted model for our data, we modeled the random effect terms with random intercept, and also with random intercept and random slope for time. This means that in the random intercept model, we allowed for between-individual variance in total PCSI at initial visit, and in the model with random intercept and slope, we allowed for between-individual variance of the rates of change of total PCSI over visits/time. We performed an unadjusted LMM, and adjusted for covariates including group, sex, age, concussion history, initial PCSI score, and site. We also tested for effect modification by study arm (i.e., aerobic exercise versus stretching exercise). We selected our final model, the LMM with random intercept and random slope, as this model resulted in the lowest AIC (Akaike information criterion) value, representative of model fit (Liang et al., 2008). The LMM model is valid under the assumption that data are Missing at Random (MAR), meaning that the probability of missing data is independent of missing values, but could depend on observed values. We believed this to be a reasonable assumption and took necessary steps.
To help address missing data, we used Generalized Estimating Equations (GEE) with unstructured covariance structure to examine the association between the outcome, missing PCSI symptom score, and covariates of interest: group, sex, age, and initial PCSI score, accounting for correlation among repeated measures for the same patient. GEE models are valid under the assumption that all missing data are Missing Completely at Random (MCAR), meaning that the probability of missing is independent of observed and missing values. The goal of the GEE analysis was to identify covariates that may help to explain the missingness of our outcome variable, total PCSI score over time. We set statistical significance at p < 0.05, and also examined effect estimates, and confidence intervals, and considered prior contextual and clinical knowledge in deciding whether to include covariates in the LMM. We chose to model our missing data with GEE as the most parsimonious way to identify factors that would inform our main model of the outcome, PCSI, permitting us to adjust for these factors in the LMM, which allows us to reduce bias due to missingness.
3Results
3.1Description of sample
Our sample consisted of 54 adolescents with SRC. The majority of participants were recruited from the concussion clinics at the University at Buffalo (50.9%) and Children’s Hospital of Philadelphia (41.8%). 60% participated in the aerobic exercise regimen and 61.8% were male. The median age at enrollment was 15.8 years (25th-75th percentiles: 14.4-16.7). Nearly half (49.1%) had experienced at least one previous concussion. The median time between injury and enrollment in the study was 6 (25th-75th percentiles: 4-9) days. Median PROMIS Anxiety pediatric short form score at initial visit was 3 (25th-75th percentiles: 0-8). Overall, median pre-injury PROMIS Anxiety pediatric short form score differed significantly between the two PROMIS Anxiety pediatric short form scores at initial visit groups (p < 0.001), as did age (p = 0.03). No other characteristics of interest demonstrated statistically significant differences between the two groups. Complete descriptive characteristics are presented in Table 1.
Table 1
Description of sample by initial visit PROMIS Anxiety pediatric short form score
| PROMIS Anxiety pediatric short form score at initial visit | ||||
| Characteristic, n(%) | Overall | Low,≤3 | High,>3 | p-value* |
| Total | 54(100.0) | 29(53.7) | 25(46.3) | |
| Clinical characteristics | ||||
| Pre-injury PROMIS Anxiety pediatric short form score (med, 25th-75th percentiles) | 2(0-4) | 0(0-0) | 4(3-6) | p < 0.001 |
| Pre-injury PCSI score (med, 25th-75th percentiles) | 1(0-3) | 1(0-3) | 2(0-3) | 0.43 |
| Initial visit PCSI score (med, 25th-75th percentiles) | 32(18-48) | 27(16-45) | 39(22-54) | 0.16 |
| Sex, male | 34(61.8) | 20(60.6) | 13(39.4) | 0.20 |
| Age (years; med, 25th-75th percentiles) | 15.8(14.4-16.7) | 15.1(14.3-16.1) | 16.1(15.6-17.3) | 0.03 |
| Mechanism of injury | ||||
| Fall | 9(16.4) | 3(37.5) | 5(62.5) | |
| Struck by person | 22(40.0) | 12(54.6) | 10(45.5) | 0.70 |
| Struck by or against object | 24(43.6) | 14(58.3) | 10(41.7) | |
| Lost consciousness, yes | 5(9.1) | 2(40.0) | 3(60.0) | 0.65 |
| Concussion history (1+) | 27(49.1) | 15(57.7) | 11(42.3) | 0.57 |
| Med history, diagnosed anxiety | 2(3.6) | 0(0) | 2(100.0) | 0.21 |
| Family med history, diagnosed anxiety | 14(25.5) | 6(42.9) | 8(57.1) | 0.37 |
| Study characteristics | ||||
| Injury to 1st visit (days; med, 25th-75th percentiles) | 6(4-9) | 6(4-8) | 6(4-9) | 0.26 |
| Study site | 0.18 | |||
| CHOP | 23(41.8) | 3(33.3) | 6(66.7) | |
| Boston Children’s | 4(7.3) | 1(33.3) | 2(66.7) | 0.59 |
| Univ Buffalo | 28(50.9) | 15(50.0) | 19(50.0) | |
| Intervention group, aerobic exercise | 33(60.0) | 17(51.5) | 16(48.5) | 0.67 |
*Chi-square test, Fisher’s Exact test, Wilcoxon rank-sum.
3.2Survival analysis results
Overall, 28 participants achieved symptom resolution during the 4-week study period and 11 were censored, in that they did not reach symptom resolution prior to the end of the study period, either due to persisting symptoms or loss to follow-up. Median time to symptom resolution was 10 days (25th-75th percentiles: 6-24) among the Low-PROMIS Anxiety pediatric short form score group and 12 days (25th-75th percentiles: 5-21) in the High-PROMIS Anxiety pediatric short form score group (Fig. 2). The difference in overall time to symptom resolution was not statistically significant (p = 0.62). Median time to symptom resolution was longest among participants in the High-PROMIS/Stretching (median = 20; 25th-75th percentiles: 12-21 days) and Low-PROMIS/Stretching groups (median = 20; 25th-75th percentiles: 8-26 days), followed by the High-PROMIS/Aerobic group (median = 9; 25th-75th percentiles: 5-12 days). Those with initial High-PROMIS Anxiety pediatric short form scores and randomized to the Exercise group exhibited the shortest time to symptom resolution (median = 7; 25th-75th percentiles: 5-17 days; Fig. 3). These differences were not statistically significant (p = 0.57).
Fig. 2
Kaplan Meier plot displaying time from concussion injury to symptom resolution, stratified by PROMIS Anxiety level at the initial visit.
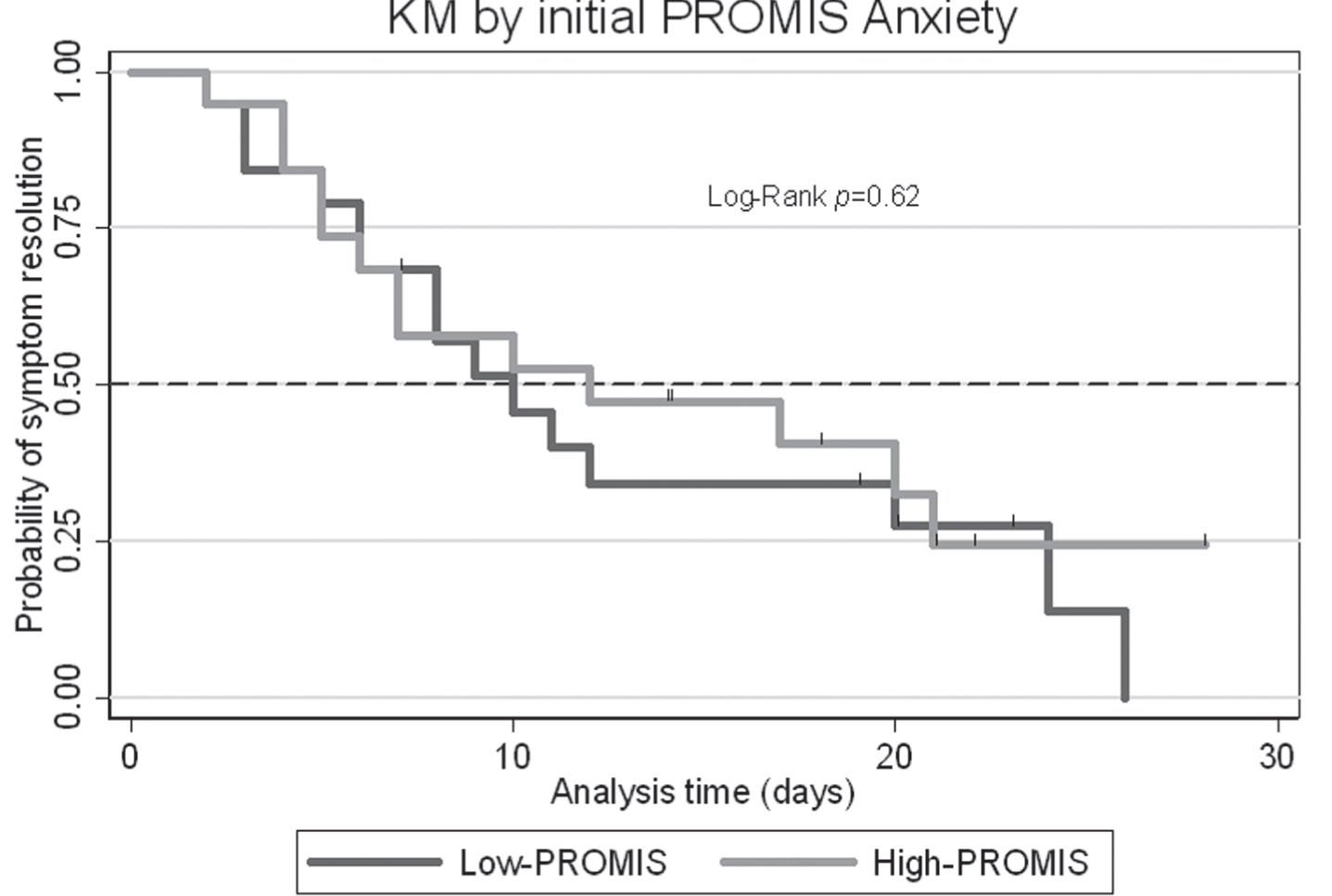
Fig. 3
Kaplan Meier plot displaying time from concussion injury to symptom resolution, stratified by PROMIS Anxiety level at the initial visit and randomization group.

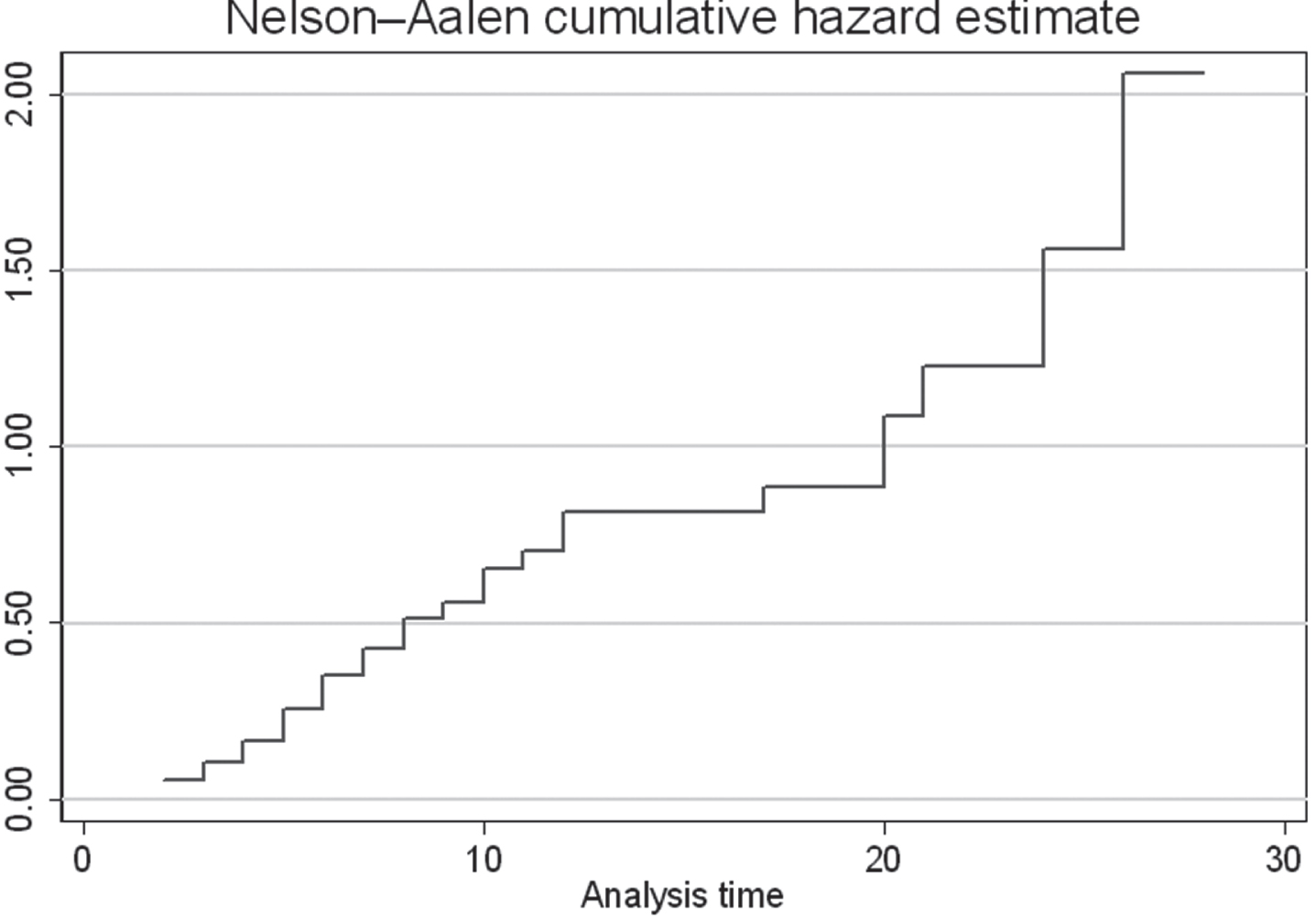
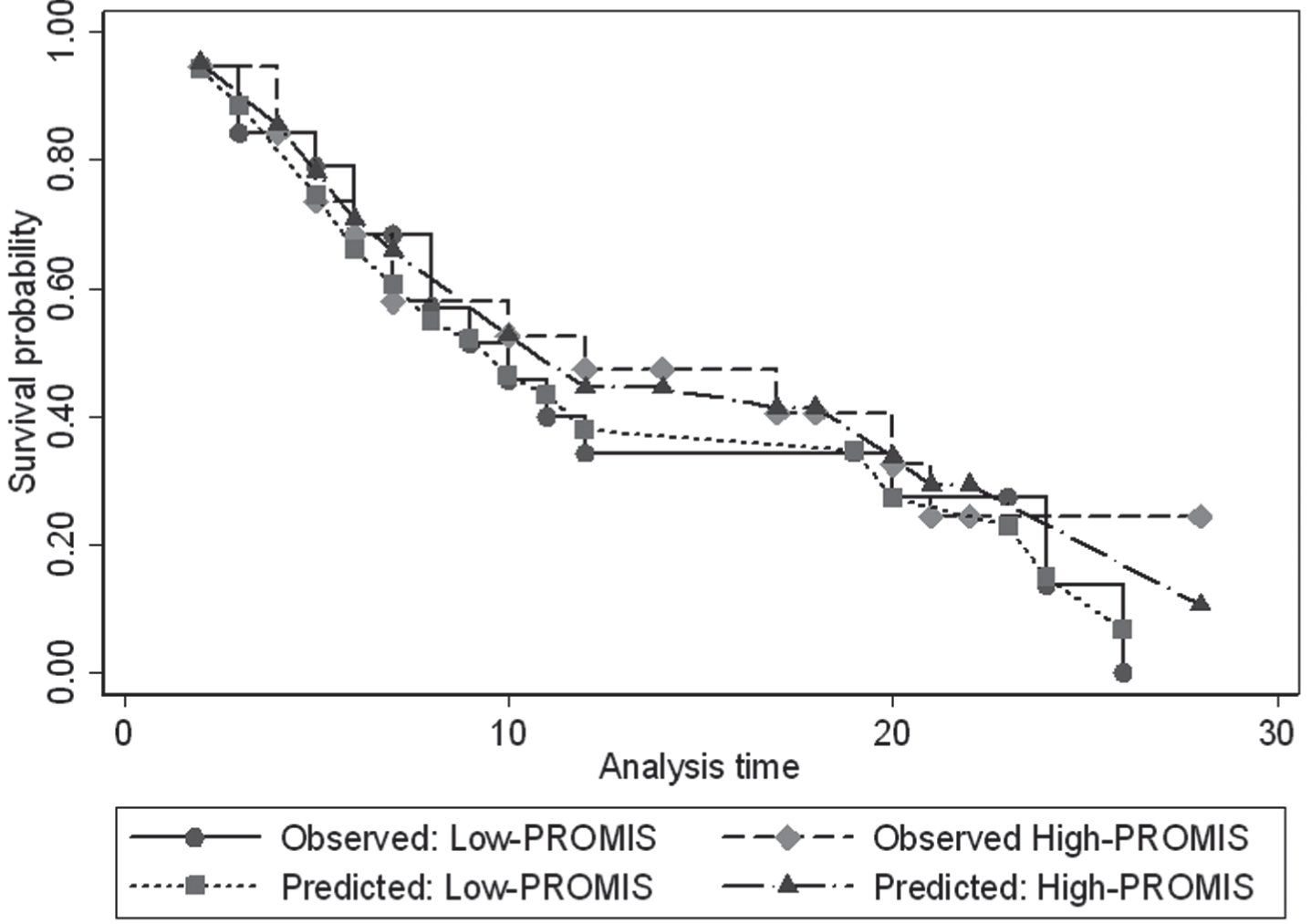
In the unadjusted Cox regression, the hazard of symptom resolution was lower for High-PROMIS Anxiety pediatric short form score at initial visit as compared to Low-PROMIS Anxiety pediatric short form score at initial visit, although not statistically significant; HR = 0.83, 95% CI 0.39-1.76, p = 0.63). The hazard of symptom resolution was higher for High-PROMIS Anxiety pediatric short form initial visit score as compared to Low-PROMIS Anxiety pediatric short form initial visit score, adjusting for initial visit PCSI score, pre-injury PROMIS Anxiety pediatric short form score, randomization group, sex, injury to time to initial visit, concussion history (1+), and study site, although not statistically significant; HR = 1.44, 95% CI 0.40-5.29. Results of the observed-predicted plot (Appendix Figure 1) and Schoenfeld test revealed no violations to the assumption of proportional hazards (p > 0.05, Global test p = 0.95). The cumulative hazard Cox-Snell residuals plot indicated reasonable model fit (Appendix Figure 2).
3.3Longitudinal analysis results
PROMIS Anxiety pediatric short form scores and PCSI scores for individuals in the study over time are depicted in Fig. 4. Higher PROMIS Anxiety pediatric short form scores corresponded to higher PSCI total scores on the subsequent day, with each additional unit of PROMIS Anxiety pediatric short form score corresponding to a 1.52- unit higher PCSI total score (95% CI 0.08-1.61), adjusting for study assignment group, sex, age, concussion history, time from concussion to initial visit, PCSI total score at IV, and study site (p = 0.03). The variance among participants at IV is 160.99, and across time is 121.06. 52.8% of the total variance at baseline is due to variability between-participants. Complete LMM results are displayed in Table 2.
Fig. 4
Individual PROMIS Anxiety and PCSI scores over time. Note: IV = initial visit, F1 = follow-up visit #1, F2 = follow-up visit #2, F4 = week-4 follow-up visit.
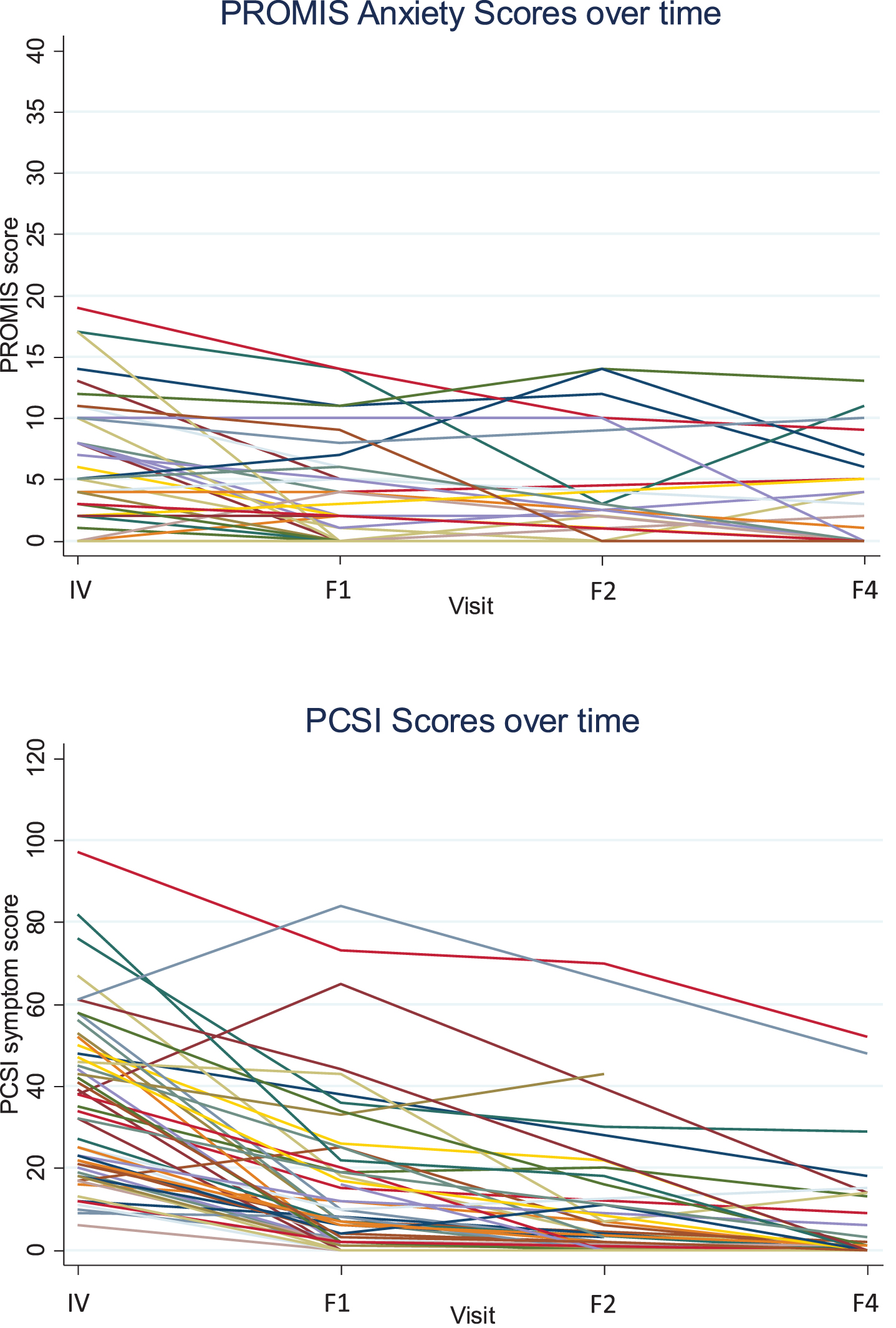
Table 2
Linear mixed effects model for total PCSI score with two random effects (random intercept and\\ random slope for time)
| Variable | β | 95% CI | p |
| PROMIS Anxiety pediatric short form score | 1.52 | 0.08-1.61 | <0.01 |
| Aerobic exercise group | 3.04 | –7.16-13.24 | 0.56 |
| Male sex | –0.73 | –11.13-9.68 | 0.89 |
| Age | –6.74 | –11.00- –2.48 | <0.01 |
| Concussion history (1+) | –8.59 | –19.77-2.59 | 0.13 |
| Time injury to initial visit (days) | –1.33 | –3.50-0.84 | 0.23 |
| Initial PCSI score | 0.74 | 0.44-1.03 | <0.001 |
| Site | |||
| Boston Children’s | 1.57 | –18.66-21.80 | 0.88 |
| University at Buffalo | 4.93 | –6.69-12.56 | 0.41 |
The proportion of missingness in the outcome, total PCSI score, increased over time from 42% at the initial visit to 94% by the week-4 follow-up visit. The odds of missing outcome PCSI score for participants in the aerobic exercise group over time was – 0.48 times that of the stretching exercise group, and was not statistically significant (p = 0.54). The odds of missing outcome PCSI score for male participants over time was 1.21 times that of females, and was not statistically significant (p = 0.11). The associations between missingness over time and higher age and baseline PCSI score were also not statistically significant.
4Discussion
By examining the presence and influence of anxiety symptoms both at initial visit and with follow-up over time, this study makes novel contributions to the literature on pediatric concussion and the role of anxiety during SRC recovery. We explored these relationships in the context of a robust, multi-site randomized trial of adolescents with SRC.
Our results suggest that anxiety symptoms reported at the initial visit (here, within 10 days of injury) may not be a clear prognostic indicator for recovery duration among adolescents following SRC, at least in the context of an overall PROMIS Anxiety pediatric short form score. This finding aligns with the work of Ali et al. (2021), who found that, among those with diagnosed depression or anxiety, the existence of pre-morbid depression or anxiety alone did not influence post-injury cognitive or symptom recovery. Current studies have focused on the acute course of concussion-related anxiety, while few have considered the role of pre-injury anxiety or the influence of anxiety on long-term symptoms (Arnett et al., 2019; Gornall et al., 2021). The nature of our study allowed us to examine these relationships longitudinally, with measurements of daily PCSI and weekly PROMIS Anxiety symptom data. We observed an association between reported anxiety and concussion symptom burden, where higher PROMIS Anxiety pediatric short form scores corresponded to higher PCSI total scores on the subsequent day, adjusting for covariates of interest. This finding builds upon recent studies, reporting greater overall concussion symptom burden among adolescents with an anxious profile (Champigny et al., 2021; Iverson, Greenberg, et al., 2021), and supports a potential relationship between anxiety and symptom burden over time. As discussed previously, literature shows that children and adolescents with higher symptom burden experience longer recovery (Houck et al., 2019; Schilling et al., 2020). This area warrants continued study to identify how anxiety over time may reflect increased symptom burden or function as an effect modifier in the relationship between symptom burden and clinical recovery.
An important finding from our longitudinal analysis is the high variance among adolescents at initial visit and over time. Even after adjustment for factors that may influence concussion symptom burden, over half of the total variance at baseline was due to variability between participants, suggesting that unmeasured confounding is likely present. Still, our results indicate anxiety, measured at initial visit and over time contributes to concussion symptom burden and therefore may be a modifiable risk factor that could be addressed in a comprehensive approach to treatment of concussion, with appropriate screening and management of anxiety conceivably improving recovery outcomes.
Our findings also have implications for identifying anxiety symptoms in adolescents after concussion. Without the use of screening and management protocols that have been shown to work well in this population, adolescents with anxiety symptoms may go undiagnosed and, therefore, untreated. Our findings demonstrate the importance of implementing anxiety screening measures within concussion treatment models. The relatively low PROMIS Anxiety pediatric short form scores reported by our participants also should inspire future work and closer examination of adolescents with lower levels of anxiety that may still be clinically meaningful.
We hope that our analysis informs and motivates future studies to, not only better estimate prevalence, but also examine the nature and source of anxiety for adolescents following concussion. Future studies should also seek to identify and test novel treatments. Observational and experimental designs utilizing mixed-methods could be leveraged to explore these complex relationships. In the present study, the potential effect of aerobic exercise on anxiety after concussion was limited by sample size. Social support has been suggested to be an effective strategy to reduce anxiety after concussion. Specifically, Covassin et al. (2014) found that concussed athletes with greater satisfaction with social support had lower state-anxiety levels, particularly during return to play. Todd et al. (2018) published additional qualitative evidence of this, showing that ice hockey players cited support from peers, family, and health care providers as mitigating factors in their experience of compromised identify, isolation, and anxiety following concussion. Integrating social support and other psychological interventions into concussion management may be beneficial and should be studied further.
4.1Limitations
There are several limitations to this analysis. The overall sample size was small for several reasons; namely, PROMIS Anxiety pediatric short form was added to the study protocol and began being collected from enrolling participants nearly one year into the RCT. In addition, recruitment and enrollment for the RCT was stopped in March 2020 due to the COVID-19 pandemic. Finally, sample size varied significantly across sites, where there were challenges to recruitment. This study also had a sizeable amount of missing data. We used a GEE model to help us identify covariates that predict missingness in our data and included these in our main LMM. Still, the threat of unmeasured confounding may exist. Date of symptom resolution during the study period was not taken into account in this analysis, and future analyses should adjust for this since participants may have chosen to stop reporting PCSI symptoms when their symptoms had improved or resolved.
Overall, study participants experienced relatively low levels of anxiety as measured via the pediatric PROMIS Anxiety pediatric short form. However, the field has yet to understand or establish which levels of anxiety are statistically significant versus clinically meaningful during concussion recovery; thus, this project contributes new knowledge. Finally, there is a potential for selection bias given that inclusion criteria for the trial required participants be enrolled within 10 days of concussion (median = 6 days); that is, this criterion may select for adolescents whose symptoms have already largely resolved in the days after their injury or adolescents who are experiencing prolonged symptoms, and are still symptomatic.
5Conclusion
Higher anxiety at initial visit, measured here with the PROMIS Anxiety pediatric short form, was not significantly associated with time to symptom resolution in this analysis. As the nature of anxiety and affective symptoms is changeable, we examined the relationship of anxiety to overall symptom burden over time. In this sample, PROMIS Anxiety pediatric short form score was significantly associated with a modest increase in PCSI symptoms over time following SRC. The difference between aerobic and stretching groups was not statistically significant. Future work should aim to better understand the relationship between concussion symptoms and the influence of the affective symptom domain upon recovery, in addition to the effect of aerobic exercise.
Conflict of interest
DJW provides consulting services on the topic of concussion/TBI epidemiology to the NCAA. JJL discloses grant support: NIH, DoD, AMSSM; Scientific Advisory Board: Neuronasal, Highmark Innovations, Noggin Health, and Quadrant Biosciences; Stock options in Highmark Innovations, Noggin Health, and 360 Concussion Care. BAD, CLM, MCC and BSW have no conflict of interest to report.
Ethics statement
A common institutional review board (IRB) was used through SmartIRB, with the University at Buffalo and Boston Children’s Hospital relying on the Children’s Hospital of Philadelphia IRB for oversight of the study (ethics approval granted via IRB number 15-012491).
Informed consent
All eligible patients, and the parents of minors (aged 13–17 years), provided written informed consent in a setting compliant with the Health Insurance Portability and Accountability Act.
Funding
This study was funded by the American Medical Society for Sports Medicine.
Author contributions
BAD and DJW conducted the analysis and drafted the original version of the manuscript. CLM, MC, BSW and JLL contributed to revised versions of the manuscript. All authors reviewed and edited the manuscript and approved the final version.
Acknowledgments
We thank Anna Brilliant and Rebecca Daniels (Boston Children’s Hospital, Boston, MA, USA), Julia Orchinik, Bernadette D’Alonzo, and Theresa Soya (Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA), Eileen Storey, Fairuz Mohammed, and Shelly Sharma (Children’s Hospital of Philadelphia, Philadelphia, PA, USA), and Emily Horn, Scott Darling, Heidi Suffoletto, and Michael Freitas (SUNY Buffalo, Buffalo, NY, USA) for their assistance with subject recruitment and data collection for this study. Finally, we thank all study participants.
References
1 | Ali, M. , Asghar, N. , Li, A. , Hannah, T. , Spiera, Z. , Marayati, N. F. , Dreher, N. , Durbin, J. , Gometz, A. , Lovell, M. , & Choudhri, T. ((2021) ) Incidence of concussion and recovery of neurocognitive dysfunction on ImPACT assessment among youth athletes with premorbid depression or anxiety taking antidepressants. J Neurosurg Pediatr, 1–7. https://doi.org/10.3171/2020.11.PEDS20821 |
2 | Arnett, P. A. , Guty, E. , & Bradson, M. ((2019) ) Assessment of depression and anxiety in sports concussion evaluations. In Neuropsychology of sports-related concussion, (pp. 71–94). American Psychological Association. https://doi.org/10.1037/0000114-004 |
3 | Bevans, K. B. , Gardner, W. , Pajer, K. A. , Becker, B. , Carle, A. , Tucker, C. A. , & Forrest, C. B. ((2018) ) Psychometric Evaluation of the PROMIS(R) Pediatric Psychological and Physical Stress Experiences Measures. J Pediatr Psychol 43: (6), 678–692. https://doi.org/10.1093/jpepsy/jsy010 |
4 | Champigny, C. , Roberts, S. D. , Terry, D. P. , Maxwell, B. , Berkner, P. D. , Iverson, G. L. , & Wojtowicz, M. ((2021) ) Acute Effects of Concussion in Adolescent Athletes With High Preseason Anxiety. Clinical Journal of Sport Medicine. https://doi.org/10.1097/jsm.0000000000000963 |
5 | Costello, E. J. , Mustillo, S. , Erkanli, A. , Keeler, G. , & Angold, A. ((2003) ) Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry 60: (8), 837–844. https://doi.org/10.1001/archpsyc.60.8.837 |
6 | Covassin, T. , Crutcher, B. , Bleecker, A. , Heiden, E. O. , Dailey, A. , & Yang, J. ((2014) ) Postinjury anxiety and social support among collegiate athletes: a comparison between orthopaedic injuries and concussions. J Athl Train 49: (4), 462–468. https://doi.org/10.4085/1062-6059-49.2.03 |
7 | Covassin, T. , Elbin, R. J. , Bleecker, A. , Lipchik, A. , & Kontos, A. P. ((2013) ) Are there differences in neurocognitive function and symptoms between male and female soccer players after concussions? Am J Sports Med 41: (12), 2890–2895. https://doi.org/10.1177/0363546513509962 |
8 | D’Alonzo, B. A. , Bretzin, A. C. , & Wiebe, D. J. ((2022) ) The Role of Reported Affective Symptoms and Anxiety in Recovery Trajectories After Sport-Related Concussion. Am J Sports Med. https://doi.org/10.1177/03635465221098112 |
9 | Edmed, S. , & Sullivan, K. ((2012) ) Depression, anxiety, and stress as predictors of postconcussion-like symptoms in a non-clinical sample. Psychiatry Res 200: (1), 41–45. https://doi.org/10.1016/j.psychres.2012.05.022 |
10 | Eijgermans, D. G. M. , Raat, H. , Jansen, P. W. , van de Looij-Jansen, P. M. , Hillegers, M. H. J. , & Jansen, W. ((2021) ) Children’s use of psychosocial care in a population-based longitudinal study: less likely for girls, children with a non-Western background and children with a high quality of life. Eur Child Adolesc Psychiatry. https://doi.org/10.1007/s00787-021-01737-2 |
11 | Ellis, M. J. , Ritchie, L. J. , Koltek, M. , Hosain, S. , Cordingley, D. , Chu, S. , Selci, E. , Leiter, J. , & Russell, K. ((2015) ) Psychiatric outcomes after pediatric sports-related concussion. J Neurosurg Pediatr 16: (6), 709–718. https://doi.org/10.3171/2015.5.PEDS15220 |
12 | Gornall, A. , Takagi, M. , Morawakage, T. , Liu, X. , & Anderson, V. ((2021) ) Mental health after paediatric concussion: a systematic review and meta-analysis. Br J Sports Med 55: (18), 1048–1058. https://doi.org/10.1136/bjsports-2020-103548 |
13 | Halstead, M. E. , Walter, K. D. , Councilon Sports, M., & Fitness. ((2010) ) American Academy of Pediatrics. Clinical report–sport-related concussion in children and adolescents. Pediatrics 126: (3), 597–615. https://doi.org/10.1542/peds.2010-2005 |
14 | Harmon, K. G. , Clugston, J. R. , Dec, K. , Hainline, B. , Herring, S. , Kane, S. F. , Kontos, A. P. , Leddy, J. J. , McCrea, M. , Poddar, S. K. , Putukian, M. , Wilson, J. C. , & Roberts, W. O. ((2019) ) American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med 53: (4), 213–225. https://doi.org/10.1136/bjsports-2018-100338 |
15 | Houck, Z. , Asken, B. , Bauer, R. , & Clugston, J. ((2019) ) Predictors of post-concussion symptom severity in a university-based concussion clinic. Brain Inj 33: (4), 480–489. https://doi.org/10.1080/02699052.2019.1565897 |
16 | Iverson, G. L. , Greenberg, J. , & Cook, N. E. ((2021) ) Anxiety Is Associated With Diverse Physical and Cognitive Symptoms in Youth Presenting to a Multidisciplinary Concussion Clinic. Front Neurol 12: , 811462. https://doi.org/10.3389/fneur.2021.811462 |
17 | Iverson, G. L. , Marsh, J. M. , Connors, E. J. , & Terry, D. P. ((2021) ) Normative Reference Values, Reliability, and Item-Level Symptom Endorsement for the PROMIS(R) v2.0 Cognitive Function-Short Forms 4a, 6a and 8a. Arch Clin Neuropsychol 36: (7), 1341–1349. https://doi.org/10.1093/arclin/acaa128 |
18 | Kapadia, M. , Scheid, A. , Fine, E. , & Zoffness, R. ((2019) ) Review of the Management of Pediatric Post-Concussion Syndrome-a Multi-Disciplinary, Individualized Approach. Curr Rev Musculoskelet Med 12: (1), 57–66. https://doi.org/10.1007/s12178-019-09533-x |
19 | Kessler, R. C. , Petukhova, M. , Sampson, N. A. , Zaslavsky, A. M. , & Wittchen, H. U. ((2012) ) Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21: (3), 169–184. https://doi.org/10.1002/mpr.1359 |
20 | Leddy, J. J. , Master, C. L. , Mannix, R. , Wiebe, D. J. , Grady, M. F. , Meehan, W. P. , Storey, E. P. , Vernau, B. T. , Brown, N. J. , Hunt, D. , Mohammed, F. , Mallon, A. , Rownd, K. , Arbogast, K. B. , Cunningham, A. , Haider, M. N. , Mayer, A. R. , & Willer, B. S. ((2021) ) Early targeted heart rate aerobic exercise versus placebo stretching for sport-related concussion in adolescents: a randomised controlled trial. Lancet Child Adolesc Health 5: (11), 792–799. https://doi.org/10.1016/S2352-4642(21)00267-4 |
21 | Liang, H. , Wu, H. , & Zou, G. ((2008) ) A Note on Conditional AIC for Linear Mixed-Effects Models. Biometrika 95: (3), 773–778. https://doi.org/10.1093/biomet/asn023 |
22 | Martin, A. K. , Petersen, A. J. , Sesma, H. W. , Koolmo, M. B. , Ingram, K. M. , Slifko, K. B. , Nguyen, V. N. , Doss, R. C. , & Linabery, A. M. ((2020) ) Concussion symptomology and recovery in children and adolescents with pre-existing anxiety. J Neurol Neurosurg Psychiatry 91: (10), 1060–1066. https://doi.org/10.1136/jnnp-2020-323137 |
23 | McCrory, P. , Meeuwisse, W. , Dvorak, J. , Aubry, M. , Bailes, J. , Broglio, S. , Cantu, R. C. , Cassidy, D. , Echemendia, R. J. , Castellani, R. J. , Davis, G. A. , Ellenbogen, R. , Emery, C. , Engebretsen, L. , Feddermann-Demont, N. , Giza, C. C. , Guskiewicz, K. M. , Herring, S. , Iverson, G. L. , &... Vos, P. E. ((2017) ) Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 51: (11), 838–847. https://doi.org/10.1136/bjsports-2017-097699 |
24 | Meehan, W. P. 3rd , d’Hemecourt, P. , Collins, C. L. , & Comstock, R. D. ((2011) ) Assessment and management of sport-related concussions in United States high schools. Am J Sports Med 39: (11), 2304–2310. https://doi.org/10.1177/0363546511423503 |
25 | Polinder, S. , Cnossen, M. C. , Real, R. G. L. , Covic, A. , Gorbunova, A. , Voormolen, D. C. , Master, C. L. , Haagsma, J. A. , Diaz-Arrastia, R. , & von Steinbuechel, N. ((2018) ) A Multidimensional Approach to Post-concussion Symptoms in Mild Traumatic Brain Injury. Front Neurol 9: , 1113. https://doi.org/10.3389/fneur.2018.01113 |
26 | Putukian, M. , Riegler, K. , Amalfe, S. , Bruce, J. , & Echemendia, R. ((2021) ) Preinjury and Postinjury Factors That Predict Sports-Related Concussion and Clinical Recovery Time. Clin J Sport Med 31: (1), 15–22. https://doi.org/10.1097/JSM.0000000000000705 |
27 | Rabinowitz, A. R. , & Fisher, A. J. ((2020) ) Person-Specific Methods for Characterizing the Course and Temporal Dynamics of Concussion Symptomatology: A Pilot Study. Sci Rep 10: (1), 1248. https://doi.org/10.1038/s41598-019-57220-1 |
28 | Rosenbaum, P. E. , Locandro, C. , Chrisman, S. P. D. , Choe, M. C. , Richards, R. , Pacchia, C. , Cook, L. J. , Rivara, F. P. , Gioia, G. A. , & Giza, C. C. ((2020) ) Characteristics of Pediatric Mild Traumatic Brain Injury and Recovery in a Concussion Clinic Population. JAMA Netw Open 3: (11), 2021463. https://doi.org/10.1001/jamanetworkopen.2020.21463 |
29 | Sady, M. D. , Vaughan, C. G. , & Gioia, G. A. ((2014) ) Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol 29: (4), 348–363. https://doi.org/10.1093/arclin/acu014 |
30 | Schilling, S. , Mansour, A. , Sullivan, L. , Ding, K. , Pommering, T. , & Yang, J. ((2020) ) Symptom Burden and Profiles in Concussed Children with and without Prolonged Recovery. Int J Environ Res Public Health 17: (1). https://doi.org/10.3390/ijerph17010351 |
31 | Sicard, V. , Harrison, A. T. , & Moore, R. D. ((2021) ) Psycho-affective health, cognition, and neurophysiological functioning following sports-related concussion in symptomatic and asymptomatic athletes, and control athletes. Sci Rep 11: (1), 13838. https://doi.org/10.1038/s41598-021-93218-4 |
32 | StataCorp. ((2021) ) Stata Statistical Software: Release 17. In StataCorp, LLC. |
33 | Thomas, G. A. , Guty, E. T. , Riegler, K. E. , & Arnett, P. A. ((2021) ) Comorbid Affective Symptomatology and Neurocognitive Performance in College Athletes. J Int Neuropsychol Soc, 1–11. https://doi.org/10.1017/S1355617721000412 |
34 | Thomas, G. A. , Riegler, K. E. , Guty, E. T. , & Arnett, P. A. ((2021) ) Relationship Between Self-Reported Concomitant Depressive and Anxiety Symptoms and the Post-Concussion Symptoms Scale (PCSS). J Int Neuropsychol Soc, 1–11. https://doi.org/10.1017/S135561772100134X |
35 | Todd, R. , Bhalerao, S. , Vu, M. T. , Soklaridis, S. , & Cusimano, M. D. ((2018) ) Understanding the psychiatric effects of concussion on constructed identity in hockey players: Implications for health professionals. PLoS ONE 13: (2), 0192125. https://doi.org/10.1371/journal.pone.0192125 |
36 | Veliz, P. , McCabe, S. E. , Eckner, J. T. , & Schulenberg, J. E. ((2017) ) Prevalence of Concussion Among US Adolescents and Correlated Factors. JAMA 318: (12), 1180–1182. https://doi.org/10.1001/jama.2017.9087 |
37 | Veliz, P. , McCabe, S. E. , Eckner, J. T. , & Schulenberg, J. E. ((2021) ) Trends in the Prevalence of Concussion Reported by US Adolescents, 2016-2020. JAMA 325: (17), 1789–1791. https://doi.org/10.1001/jama.2021.1538 |
38 | Wiebe, D. J. , Storey, E. P. , Orchinik, J. O. , Grady, M. F. , Leddy, J. J. , Willer, B. S. , Haider, M. N. , Mannix, R. , Meehan, W. P. , Vernau, B. , & Master, C. L. ((2021) ) Measuring Recovery With Ecologic Momentary Assessment in a Randomized Trial of Exercise After Sport-Related Concussion, Clin J Sport Med, 32: (4), 345–353, July 2022. | DOI: 10.1097/JSM.0000000000000946 |
39 | Yaramothu, C. , Goodman, A. M. , & Alvarez, T. L. ((2019) ) Epidemiology and Incidence of Pediatric Concussions in General Aspects of Life. Brain Sci 9: (10). https://doi.org/10.3390/brainsci9100257 |
Appendices
Appendix figures
Appendix Fig. 1
Observed-predicted plot.
Appendix Fig. 2
Cumulative Hazard plot.