Effects of constraint-induced movement therapy for the lower extremity among individuals post-stroke: A randomized controlled clinical trial
Abstract
BACKGROUND:
Stroke often leads to lower extremity impairments that significantly hinders functional recovery.
OBJECTIVE:
To investigate the effectiveness of constraint-induced movement therapy for the lower extremity (CIMT-LE) for improving balance and ambulation among people post-stroke.
METHODS:
A randomized controlled, single-blinded clinical trial was conducted. Participants were recruited and randomized into one of two groups: CIMT-LE group and control. Outcome measures were the Fugl-Meyer assessment of lower extremity, Berg balance scale, ten-meter walk test and six-minute walk test. Outcome measures were collected at baseline, following the conclusion of the therapeutic programs and after three months.
RESULTS:
38 participants were enrolled in the study (19 in each group). No significant differences were found between groups at baseline. At the conclusion of therapeutic programs, both groups showed significant changes compared to baseline. However, changes seen in the CIMT-LE were clinically significant. Further, at three months following the conclusion of the program, the recorded improvements were retained by participants.
CONCLUSION:
A CIMT-LE program compared to an intensity-matched conventional program yielded significant clinical improvements among people post-stroke. These improvements were seen in lower extremity motor recovery, postural balance and gait speed. Furthermore, these improvements were retained three months following the conclusion of the therapeutic program.
1Introduction
Patients post-stroke commonly suffer from upper extremity (UE) and lower extremity (LE) dysfunction (Hendricks, van Limbeek, Geurts, & Zwarts, 2002). Four out of five stroke survivors initially present with UE and LE hemiparesis (Hendricks et al., 2002). Further, at six months post stroke over 80% of patients will demonstrate persistent functional deficits (e.g., impairment of hand manual dexterity, muscle weakness, loss of postural control and abnormal gait), especially those with significant impairment at onset (Kwakkel, Kollen, & Lindeman, 2004). Despite rehabilitation efforts, only 5% of stroke survivors regain full function of the affected UE and LE (Staines, McIlroy, & Brooks, 2009; Whitall, McCombe Waller, Silver, & Macko, 2000). These findings strongly support the need to develop more effective therapeutic interventions for the paretic UE and LE.
Constraint-induced movement therapy (CIMT) is a common intervention for the treatment of the post-stroke paretic upper extremity (UE) (Lindsay et al., 2008). The development of CIMT as a therapeutic intervention was derived from the work of Edward Taub and colleagues (Taub et al., 1994). This intervention was targeted to overcome the phenomenon known as ‘learned non-use’. CIMT is comprised of four components: (i) administering intense, daily therapeutic exercises over consecutive days; (ii) utilizing a ‘shaping’ technique for providing function-oriented, supervised exercises for the paretic limb; (iii) behavioral strategies, also called a transfer package that facilitate the transfer of the learned skills from this intervention into everyday activities; and (iv) strategies to facilitate the use of the paretic limb (e.g., restraint for the non-paretic limb in UE CIMT) (Morris, Taub, & Mark, 2006; Taub et al., 1994; Taub et al., 2006; Taub et al., 2013). Various clinical trials and systematic reviews have investigated the efficacy of CIMT for improving the function of the paretic UE post-stroke. The results of these studies indicate that CIMT yields positive effects as seen in the improved function of the paretic UE post-stroke (Chiu & Ada, 2016; Corbetta, Sirtori, Castellini, Moja, & Gatti, 2015; Etoom et al., 2016; Fleet et al., 2014; Peurala et al., 2012; Sirtori, Corbetta, Moja, & Gatti, 2009; Stevenson, Thalman, Christie, & Poluha, 2012; Wolf et al., 2010; Wolf et al., 2006; Wolf et al., 2008).
As CIMT has been shown to yield positive functional effects for the UE post-stroke, studies have attempted to assess the therapeutic benefits of a CIMT program for the LE impairments and locomotion deficits post-stroke (Abdullahi, Aliyu, et al., 2021; Aruin, Hanke, Chaudhuri, Harvey, & Rao, 2000; Bonnyaud et al., 2013; Choi, Shin, Bang, & Choi, 2017; Dos Anjos, Morris, & Taub, 2020; e Silva et al., 2017; Fritz, Pittman, Robinson, Orton, & Rivers, 2007; Hase, Suzuki, Matsumoto, Fujiwara, & Liu, 2011; Kallio, Nilsson-Wikmar, & Thorsen, 2014; Marklund & Klassbo, 2006; Numata, Murayama, Takasugi, & Oga, 2008; Regnaux et al., 2008; Rodriguez & Aruin, 2002; Vearrier, Langan, Shumway-Cook, & Woollacott, 2005; Zhu et al., 2016). These studies speculated that a CIMT program for the LE may yield positive changes in balance and ambulation for people post-stroke. However, the bulk of these studies are small scale case reports and lack robust experimental evidence to indicate if CIMT is effective for reducing LE impairments and functional deficits. Further, the majority of these studies did not include all the elements (e.g., shaping techniques and transfer package) recommended for a successful CIMT program. Moreover, a number of studies have utilized a restraint device for the LE, which maybe a safety concern and may induce further coordination abnormalities.
The positive effects of CIMT are not necessarily related to the use of a restraint. Ample evidence suggests that the restraint alone does not yield similar functional improvement in the UE post-stroke, as does a complete CIMT program (Brogardh, Vestling, & Sjolund, 2009; Corbetta et al., 2015; Kwakkel, Veerbeek, van Wegen, & Wolf, 2015; Uswatte, Taub, Morris, Barman, & Crago, 2006). Further, various studies have reported positive effects following a CIMT program that did not use a restraint device for the UE (Brogardh et al., 2009; Corbetta et al., 2015; Kwakkel et al., 2015; Uswatte et al., 2006). CIMT program for LE (CIMT-LE), shows great potential for improving balance and ambulation for people post-stroke and further studies are needed to ascertain its effectiveness for improving LE function among people post-stroke. Therefore, the aim of the current study is to investigate the effectiveness of a CIMT-LE program for improving balance and ambulation among people post-stroke.
2Methods
2.1Design
A randomized controlled, single-blinded clinical trial (RCT) was conducted. Subjects were recruited and randomized into two groups. The first group was a control group. This group received dose-matched, usual and customary care. The second group was the experimental group which received the CIMT-LE protocol. For this study, an experienced evaluator who is blinded to group assignments administered all assessments. The local Committee of Health Research Ethics at Qassim University approved all procedures (Approval#: 20-09-04). All participants provided a written informed consent. All experimental procedures were in accord with the Declaration of Helsinki of 1964 and its later amendments.
2.2Sample size
The main outcome measure used in this study was the Fugl-Meyer assessment of lower extremity (FMA-LE). Using an effect size of (0.4) reported in a previous study (Abdullahi, Aliyu, et al., 2021), and assuming a significance level of (α= 0.05) with a power of (β= 0.8), we arrive at a required sample size of 34 individuals (17 for each group). An additional 10% (total sample = 38) were added the required sample size in anticipation for any technical difficulties, consent withdrawn or not showing for follow-up.
2.3Participants
Participants were recruited from the local healthcare centers and from the community through advertisements and word of mouth. The inclusion criteria were: (i) clinical diagnosis of no more than one stroke (either ischemic or hemorrhagic) experienced more than 1 year prior to study enrollment, resulting in LE hemiparesis; (ii) age≥18 years; (iii) a score of≥24 on the Mini-Mental State Examination; (iv) ability to walk independently for 10 meters (with or without a walking aid); (v) a score of≥35 on the Berg balance scale (BBS); and (vi) ability to participate in the study and adhere to the therapeutic sessions. Exclusion criteria included: (i) excessive pain in the more-affected LE (defined as > 4/10 on the visual analog scale); (ii) increased hypertonia in the hemiparetic LE (defined as≥3 on the Modified Ashworth Scale) (Bohannon & Smith, 1987); (iii) currently enrolled in a physical rehabilitation program; and (iv) diagnosis of terminal illness, life-threatening co-morbidity or concomitant neurological or psychiatric illness.
Participants were randomized into two groups using a computerized (block) randomization scheme. Pre-stratification was done according to the participant’s pre-morbid footedness and also the participant’s score on the main outcome measure (FMA-LE), either a score of less than or equal to 20 or more than 20. This was done to ensure that both groups are comparable in LE function prior to therapy. Randomization was conducted following the consent and pre-treatment assessments. Randomization was administered by an independent researcher who was not involved in the treatment or the assessment of participants.
2.4Procedures
Following the assignment of participants’ into their respective groups, baseline assessments were collected from all participants. Regardless of group assignment, therapeutic sessions for all participants were 3.5 hours, five days per week for a duration of 2 weeks. For the CIMT-LE group, three hours of the session were allocated for physical exercises and the reminder of the session time (30 minutes) was dedicated for the transfer package (TP). The physical exercises provided to participants were functionally-oriented and supervised. These exercises were targeted towards the more effected lower extremity and administered using a shaping technique. These shaping tasks were selected by the therapist based on the targeted movements (e.g., knee extension). Subsequently, the difficulty of exercises are determined based on the participant’s level and progressed periodically. Frequent feedback was provided to participants during task practice. Each task was performed for 10 trials, with each trial lasting 30–45 seconds. For example, one of the tasks given to the CIMT-LE group was step-up exercises. In this exercise the participant is asked to step up on a stool with the more affected lower extremity and then return to the starting position with both feet on level floor. Progressing the task would include increasing the height of the stool, increasing number of repetitions and/or increasing the distance between the stool and the participant.
The TP includes a number of techniques, including a behavioural contract. This contract was signed by all participants in the CIMT-LE group on the first day of the program. It included an agreement to commit to the therapeutic program and provided a list of activities that are typically performed daily and emphasized the use of the more affected lower extremity. Further, the behavioural contract included a number of home-exercises that should be performed daily while at home (outside treatment session and during weekends) and following the conclusion of the therapeutic program.
For the control group, participants received a conventional post-stroke rehabilitation program. The program included range of motion and stretching exercises, balance, walking and endurance training. Further, participants in the control group were provided with transfer training, rehabilitation education, and encouraged to practice some of the exercises at home and following the conclusion of the program. Following the conclusion of the therapeutic program, assessments were collected from all participants. Further, after 3 months from the end of the program, participants were invited to come in again for a follow-up assessment.
2.5Outcome measures
Outcome measures were collected from participants prior to the therapeutic program, immediately after the program and at 3 months follow-up. The primary outcome measure was the FMA-LE, which has excellent validity and reliability for assessing motor recovery post-stroke (Gladstone, Danells, & Black, 2002). The FMA-LE includes 17 items divided into two subscales (lower extremity and speed/coordination). Each item on the FMA-LE is scored on a 3-level (0–2) ordinal scale, for a total possible score of 34 points.
The secondary outcome measures for this study are the BBS, the 10-meter walk test (10MWT) and the 6 minute walk test (6MWT). The BBS is a measure used to assess an individual’s skill to balance safely during a number of predetermined tasks. The BBS includes 14 items with each item graded on a five-point (0–4) ordinal scale for an overall possible score of 56. The BSS is a valid and reliable measure of balance for people post-stroke (Alghadir, Al-Eisa, Anwer, & Sarkar, 2018; Berg, Wood-Dauphinee, & Williams, 1995; Mao, Hsueh, Tang, Sheu, & Hsieh, 2002; Wang, Hsueh, Sheu, Yao, & Hsieh, 2004). The 10MWT assesses walking speed in meters per second over a short distance (10 meter). This measure gives an overall indication of an individual’s gait skill. The 10MWT has excellent validity and reliability among people post-stroke (Flansbjer, Holmback, Downham, Patten, & Lexell, 2005; Lin, Hsu, Hsu, Wu, & Hsieh, 2010; Wolf et al., 1999). The 6MWT is used to assess walking endurance by determining the distance walked (meters) over a short period (6 minutes). The 6MWT has excellent validity and reliability among individuals post-stroke (Fulk, Echternach, Nof, & O’Sullivan, 2008).
2.6Data analysis
Statistical analyses were performed using SPSS (version 23) statistical package software. The baseline measures for participants were compared between groups using independent t-tests to ensure that participants are not significantly different at baseline. Further, separate repeated measures analyses of variance (ANOVA) were conducted for all outcome measures with time of assessment as a within-subjects factor and group assignment as a between-subjects factor. Post-hoc analyses were performed using Bonferroni corrections. The significance level for all statistical analyses was set to α= 0.05.
3Results
The number of individuals assessed for eligibility were 53, of those 15 were excluded either for not meeting the inclusion criteria or did not wish to participate in the study. The number of participants who were randomized and allocated in their respective groups were 38 individuals. All the included individuals received the intervention program, attended the follow-up session and were included in the final analysis of the study (Fig. 1).
Fig. 1
Flowchart of participant recruitment.
Summary of participants’ characteristics is shown in Table 1. Results of independent t-tests for baseline measures showed that both groups were not significantly different at the outset of the study across all four outcome measures (FMA-LE), BBS, 10MWT and 6MWT. The overall adherence to the program was good for both groups, with an average of 3.3 hours/session for the CIMT group and an average of 3.2 hours/session for the control group. Adverse events were uncommon with 5 adverse event recorded for the CIMT group, and 3 for the control group. There were no missing data for all outcome measures.
Table 1
Participants’ Characteristics
| Demographics | CIMT Group | Control Group |
| N (males/females) | 19 (10/9) | 19 (9/10) |
| Age - mean (SD) | 60.1 years (10.8) | 59.3 years (11.4) |
| Stroke type (ischemic/hemorrhagic) | 16/3 | 15/4 |
| Stroke side (right/left) | 13/6 | 11/8 |
| Time since stroke in months - mean (SD) | 30.2 (13.9) | 36.8 (19.5) |
| MMSE - median (min-max) | 27 (24–30) | 28 (24–31) |
| Pre-morbid footedness (right/left) | 16/3 | 17/2 |
CIMT: Constraint-induced movement therapy, SD: Standard deviation, MMSE: Mini-mental state examination.
The results of the ANOVA analyses show that both therapeutic programs are effective as seen across all outcome measures. A summary of outcome measures results overtime according to group is presented in Table 2. As both therapeutic programs progressed, FMA-LE scores, BBS score, 10MWT and 6MWT increased. Furthermore, a significant difference was found between groups as CIMT program yielded better improvement (as seen in outcome measures post-intervention) compared to control group program. In addition, retention of therapeutic effect was maintained among groups as seen in three-months follow-up assessment results.
Table 2
Outcome Measures Over Time According to Group*
| Outcome | Baseline | Program End | 3 Months Follow-up | |||
| Measures | CIMT | Control | CIMT | Control | CIMT | Control |
| FMA-LE | 21.95 (2.6) | 21.42 (1.8) | 27.37 (1.9) | 25.68 (1.7) | 26.58 (1.7) | 25.53 (1.6) |
| BBS | 40.3 (3.3) | 40.4 (3.2) | 47.5 (1.8) | 44.7 (2.4) | 48.1 (1.7) | 44.6 (3.1) |
| 10MWT | 0.26 (0.04) | 0.26 (0.05) | 0.40 (0.04) | 0.36 (0.07) | 0.40 (0.03) | 0.37 (0.05) |
| 6MWT | 90.5 (4.4) | 90.8 (4.2) | 96.3 (4.9) | 93.4 (4.1) | 96.2 (4.8) | 93.9 (4.4) |
*Figures are means and standard deviations. FMA-LE: Fugl-Meyer assessment lower extremity, BBS: Berg balance scale, 10MWT: Ten meter walk test (m/s), 6MWT: Six minute walk test (meters), CIMT: Constraint-induced movement therapy.
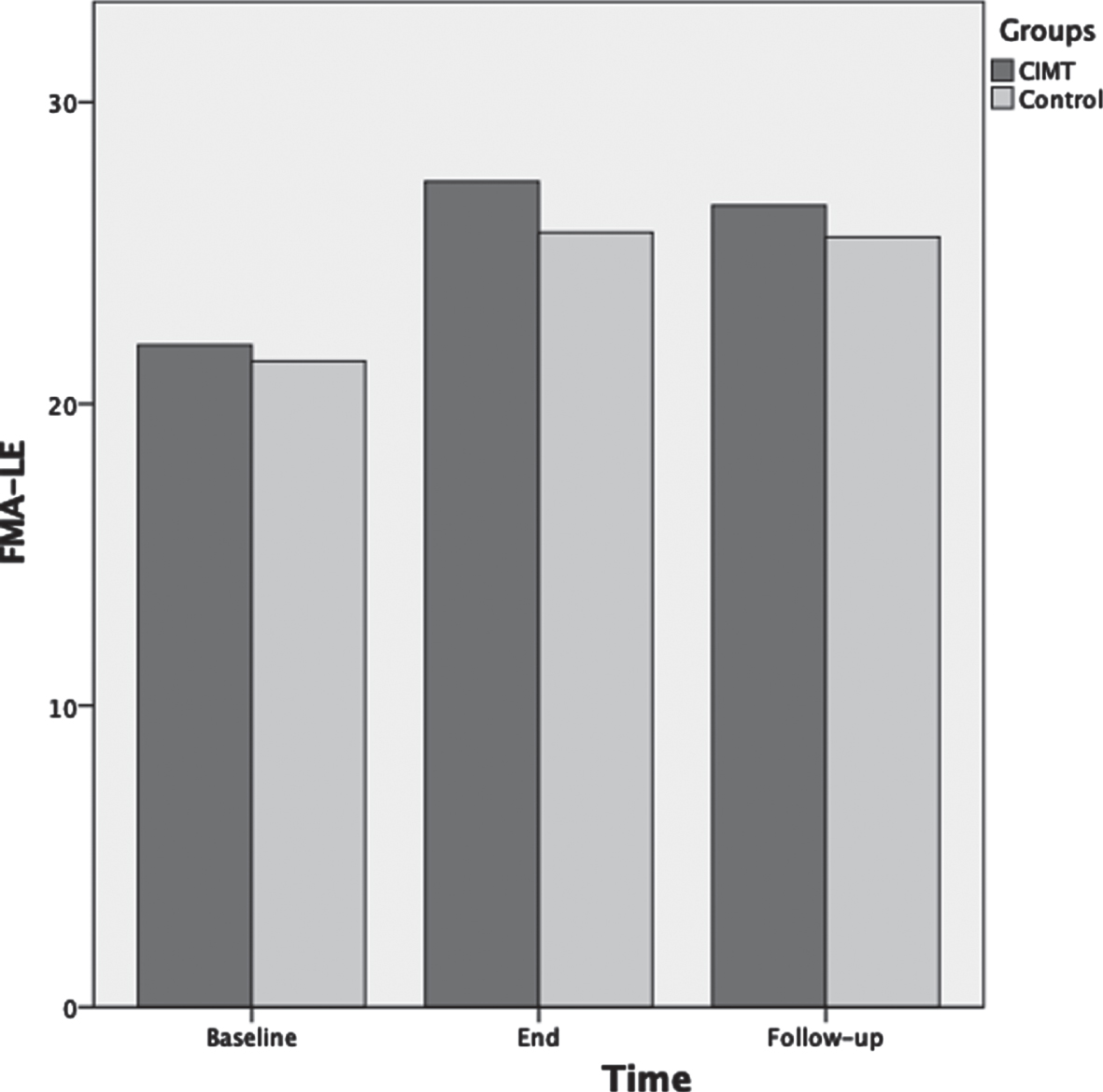
The ANOVA results for FMA-LE showed that as the therapeutic program progressed, the FMA-LE significantly increased F(2,72) = 89.9, p < 0.001, ηp2 = 0.71 (Fig. 2). Test of between-subjects effects indicated that the CIMT group had significantly higher scores compared to the control group F(1,36) = 6.6, p = 0.015, ηp2 = 0.15. Post-hoc analyses showed that FMA-LE was not significantly different between groups at baseline (p = 0.48); however, FMA-LE was significantly different between groups at the end of the therapeutic program (p = 0.007) and approached significance at three months follow-up (p = 0.056).
Fig. 2
Mean scores of the Fugl-Meyer assessment-lower extremity (FMA-LE) as a function of time for both groups: CIMT and control. The time points at which outcome measures were collected from participants were: baseline (pre-intervention), end (post-intervention) and follow-up (3 months following the program conclusion).
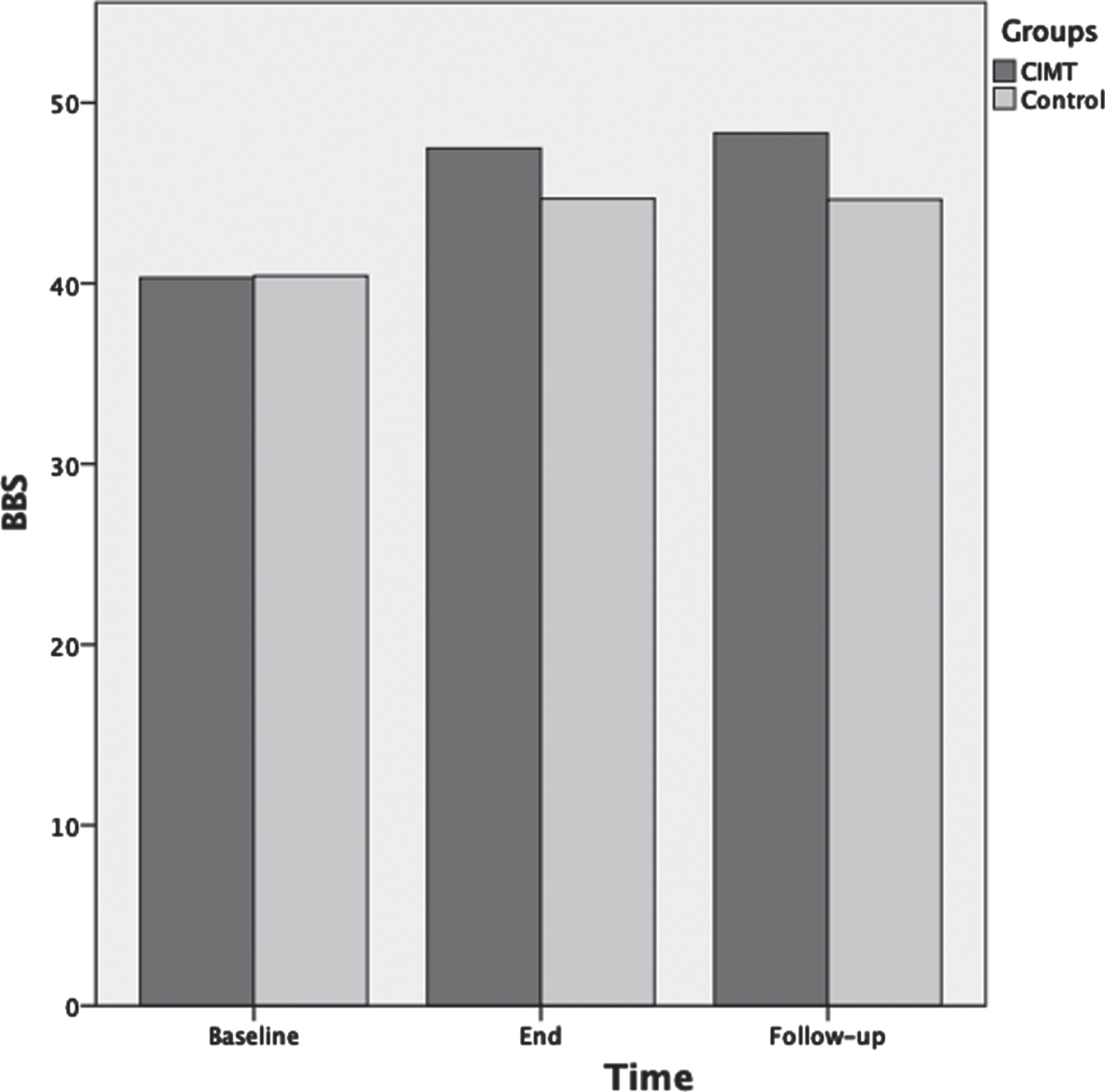
For BBS scores ANOVA results showed that as the therapeutic program progressed, the BBS scores significantly increased F(2,72) = 125.3, p < 0.001, ηp2 = 0.78 (Fig. 3). Main effects for time of assessment were superseded by time of assessment by group interaction F(2,72) = 10.5, p < 0.001, ηp2 = 0.23. Test of between-subjects effects indicated that the CIMT group had significantly higher scores compared to the control group F(1,36) = 8.5, p = 0.006, ηp2 = 0.19. Post-hoc analyses showed that BBS was not significantly different between groups at baseline (p = 0.92); however, BBS was significantly different between groups at the end of the therapeutic program (p = 0.001) and at three months follow-up (p < 0.001).
Fig. 3
Mean scores of the Berg balance scale (BBS) as a function of time for both groups: CIMT and control. The time points at which outcome measures were collected from participants were: baseline (pre-intervention), end (post-intervention) and follow-up (3 months following the program conclusion).
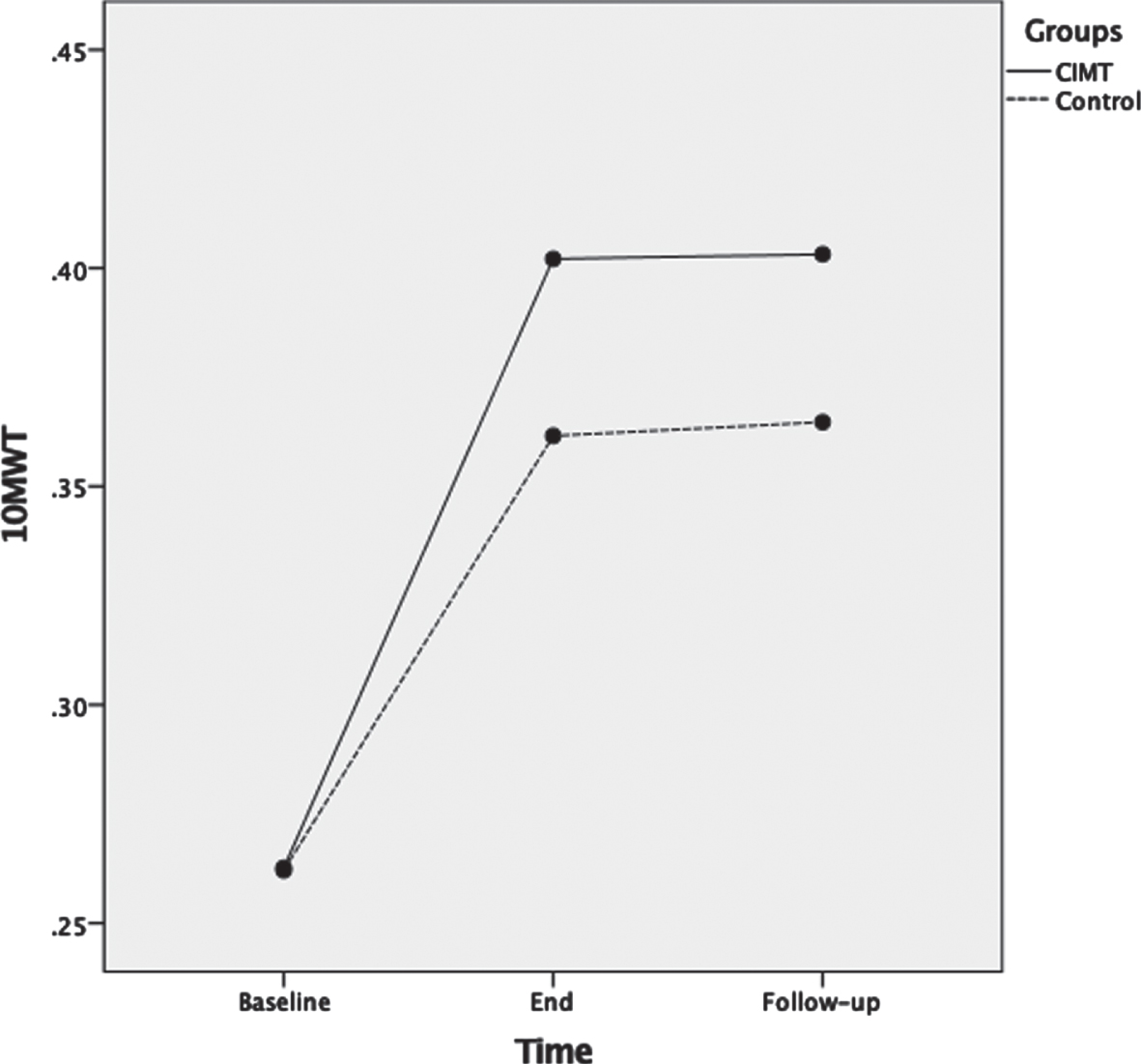
For 10MWT, ANOVA results showed that as the therapeutic program progressed, the 10MWT scores significantly increased F(2,72) = 159.1, p < 0.001, ηp2 = 0.82 (Fig. 4). Main effects for time of assessment were superseded by time of assessment by group interaction F(2,72) = 4.2, p = 0.02, ηp2 = 0.12. Test of between-subjects effects indicated that the CIMT group had significantly higher scores compared to the control group F(1,36) = 4.7, p = 0.04, ηp2 = 0.12. Post-hoc analyses showed that 10MWT was not significantly different between groups at baseline (p = 0.97); however, 10MWT was significantly different between groups at the end of the therapeutic program (p = 0.02) and at three months follow-up (p = 0.005).
Fig. 4
Mean scores of the ten meter walk test (10MWT) measured in m/s as a function of time for both groups: CIMT and control. The time points at which outcome measures were collected from participants were: baseline (pre-intervention), end (post-intervention) and follow-up (3 months following the program conclusion).
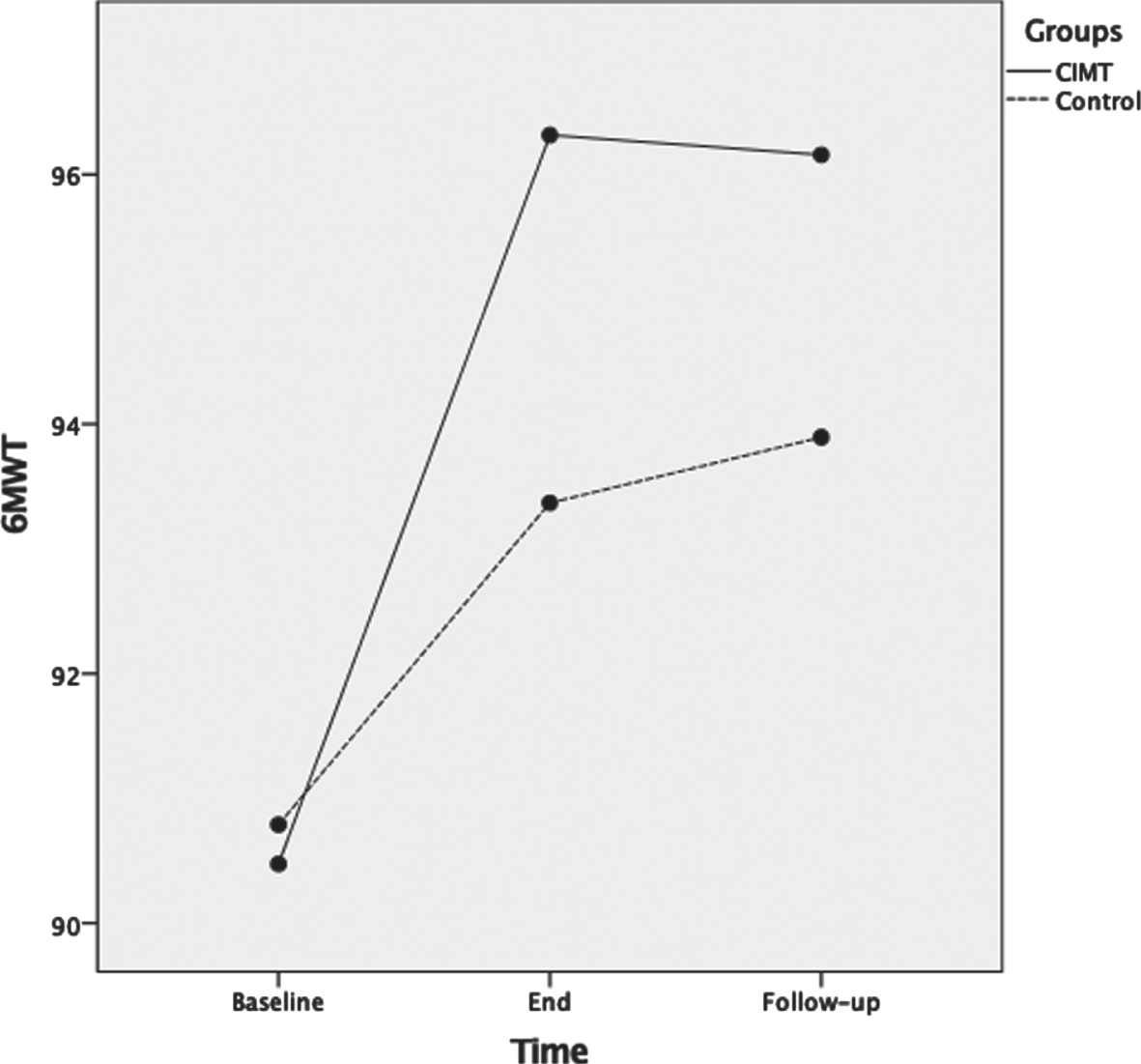
For 6MWT, ANOVA results showed that as the therapeutic program progressed, the 6MWT scores significantly increased F(2,72) = 54.4, p < 0.001, ηp2 = 0.60 (Fig. 5). Main effects for time of assessment were superseded by time of assessment by group interaction F(2,72) = 6.5, p = 0.003, ηp2 = 0.15. Test of between-subjects effects indicated no significant difference (p = 0.23) between groups. Post-hoc analyses showed that 6MWT was not significantly different between groups at baseline (p = 0.82); and at the end of the therapeutic program the p-value approached significance (p = 0.053) but was not significantly different between the two groups. Lastly, at three months follow-up there were no significant differences between groups (p = 0.14).
Fig. 5
Mean scores of the six minute walk test (6MWT) measured in meters as a function of time for both groups: CIMT and control. The time points at which outcome measures were collected from participants were: baseline (pre-intervention), end (post-intervention) and follow-up (3 months following the program conclusion).
4Discussion
The current study examined the effectiveness of a CIMT-LE program compared to conventional post-stroke rehabilitation. The results indicate that at the end of the therapeutic programs both interventions yielded beneficial functional effects as measured by the FMA-LE, BBS, 10MWT and 6MWT. However, the CIMT program was more effective than the dose-matched, conventional post-stroke rehabilitation. These findings are in congruent with previous studies that examined CIMT for the lower extremity among people post-stroke (Abdullahi, Aliyu, et al., 2021; Aruin et al., 2000; Bonnyaud et al., 2013; Choi et al., 2017; Dos Anjos et al., 2020; e Silva et al., 2017; Fritz et al., 2007; Hase et al., 2011; Kallio et al., 2014; Marklund & Klassbo, 2006; Numata et al., 2008; Regnaux et al., 2008; Rodriguez & Aruin, 2002; Vearrier et al., 2005; Zhu et al., 2016). Providing individuals post-stroke with effective rehabilitation is clinically important and warrants further research, especially with the increasing number of people who continue to suffer from post-stroke impairments.
Previous studies that have examined CIMT for the LE have primarily focused on providing intensive LE rehabilitation and did not include, or only included some of the elements of CIMT. For studies that involve the upper extremity, ample evidence suggests that the TP amplifies the beneficial effects of CIMT in comparison to programs that did not include TP (Taub et al., 2013; Uswatte & Taub, 2013). Moreover, in a recent case report, the authors reported that the use of TP for a CIMT-LE program led to added improvements in gait and mobility among people post-stroke (Dos Anjos et al., 2020). It is still unknown if the addition of TP yields superior improvements compared to a CIMT-LE program without TP. Previous studies have reported positive benefits of a CIMT-LE without the use of TP (Abdullahi, Aliyu, et al., 2021; Aruin et al., 2000; Choi et al., 2017; e Silva et al., 2017).
The intervention protocol for CIMT-LE in the current study omitted the use of a restraint for the non-hemiparetic LE. The rationale for omitting a restraint is two-fold. One, to avoid the safety concerns as a restraint may increase the risk of falling. Two, the use of a restraint may prompt an abnormal gait or postural control pattern for participants during the intervention. The results of the study indicated that even with the absence of a restraint device the CIMT-LE protocol was successful in yielding positive functional benefits. This findings corroborates previous studies that concluded there are no differences between a CIMT-LE intervention with or without a LE restraint (Dos Anjos et al., 2020; e Silva et al., 2017). Similarly, previous studies investigating CIMT for the upper extremity have reported positive effects following a CIMT program that did not use a restraint device for the upper extremity (Brogardh et al., 2009; Corbetta et al., 2015; Kwakkel et al., 2015; Uswatte et al., 2006).
In a recent systematic review and meta-analysis, the authors reviewed 16 studies with different designs that utilized CIMT-LE (Abdullahi, Truijen, et al., 2021). The authors reported that the majority (n = 10) of studies included in their review were rated as level II evidence. The authors concluded that CIMT-LE improves motor function, balance, functional mobility and gait among people post-stroke. Further, the authors indicated that CIMT-LE is superior to conventional therapy in improving quality of life for people post-stroke. In a recent RCT, the authors aimed to compare the difference between two CIMT-LE protocols, one that focused on duration of therapy time while the other focused on number of task practice repetitions. The results of the study showed that both means of intervention delivery were effective in improving motor function, balance and functional mobility (Abdullahi, Aliyu, et al., 2021). Similarly, a previous RCT examined the efficacy of a modified CIMT-LE for improving gait parameters and center of mass displacement. The results showed that a modified CIMT-LE intervention was effective in improving the center of mass displacement both in the sagittal and frontal planes and also led to gait improvements.
In the current study participants from both groups showed significant differences following their respective interventions. However, the changes seen among the CIMT-LE group were clinically significant as seen by the improvements in the most of the study’s outcome measures. For FMA-LE, participants in the CIMT-LE group showed a change of six points in their average scores following the interventions compared to four points for the control group (Table 2). The change in average scores on the FMA-LE for the CIMT group is equal to the reported minimal clinically important difference (MCID) of six points (Pandian, Arya, & Kumar, 2016). Similarly, for the BBS both groups showed a significant improvement in their scores following their respective interventions. However, the change of 7 points for the CIMT-LE group (compared to 4 points for the control group) surpasses the reported MCID for the BBS of 5 points (Tamura, Miyata, Kobayashi, Takeda, & Iwamoto, 2021). For the 10MWT, the improvement seen among the CIMT-LE group of (0.14 m/s) is a substantial meaningful change as reported in a previous study (Perera, Mody, Woodman, & Studenski, 2006). For the 6MWT, although the change following the intervention was significant, it did not reach clinical significance.
The follow-up results at three months show that both groups retained the functional improvements following the intervention. There were no statistical significant differences in all outcome measures between the end of the therapeutic program and at three months follow-up. The retention of the therapeutic effects could be explained by the fact that all participants were given home instructions and exercises to continue treatment at home. This finding is similar to previous research that have reported that the beneficial effects of CIMT-LE are retained at three and six months following the conclusion of therapeutic training (Aruin et al., 2000; Marklund & Klassbo, 2006). With the increasing number of people who suffer from post-stroke impairment, it is essential to administer therapeutic programs that are effective in improving the functional status of individuals and are retained long after the program ends. Thus, enhancing the rehabilitation services provided to people post-stroke, and help alleviate the high loads that rehabilitation specialists face (Clarke et al., 2018).
The current study is not without limitations. Blinding of the clinicians administering the interventions was not possible; however, bias was minimized by blinding participants to study hypothesis. Another limitation was that the participants included in the current study were people with chronic stroke. As such, the findings may not be generalizable to other individuals in different phases of post-stroke recovery. However, specifying individuals post-stroke in the chronic stage was intentional from the outset of the study. Any recorded improvement seen following the intervention would more likely be attributed to the therapeutic program and not due to spontaneous recovery (Cassidy & Cramer, 2017).
5Conclusion
An intensive CIMT-LE program compared to an intensity-matched conventional program yielded significant clinical improvements among people post-stroke. These improvements were seen in LE motor recovery, postural balance and gait speed. Furthermore, these improvements were retained after three months of the therapeutic program conclusion. Therefore, these findings provide further support to the application of CIMT-LE for facilitating the recovery of people post-stroke.
Conflict of interest
The author reports no conflict of interest.
Funding
The author gratefully acknowledges Qassim University, represented by the Deanship of Scientific Research, for the financial support for this research (number 20007-fcohsb-2020-1-1-W) during the academic year 1442AH/2021AD.
References
1 | Abdullahi, A. , Aliyu, N. U. , Useh, U. , Abba, M. A. , Akindele, M. O. , Truijen, S. , & Saeys, W. ((2021) ) Comparing Two Different Modes of Task Practice during Lower Limb Constraint-Induced Movement Therapy in People with Stroke: A Randomized Clinical Trial, Neural Plast 2021: , 6664058. doi: 10.1155/2021/6664058. |
2 | Abdullahi, A. , Truijen, S. , Umar, N. A. , Useh, U. , Egwuonwu, V. A. , Van Criekinge T. , & Saeys, W. ((2021) ) Effects of Lower Limb Constraint Induced Movement Therapy in People With Stroke: A Systematic Review and Meta-Analysis, Front Neurol 12: , 638904. doi: 10.3389/fneur.2021.638904. |
3 | Alghadir, A. H. , Al-Eisa, E. S. , Anwer, S. , & Sarkar, B. ((2018) ) Reliability, validity, and responsiveness of three scales for measuring balance in patients with chronic stroke, BMC Neurol 18: (1), 141. doi: 10.1186/s12883-018-1146-9. |
4 | Aruin, A. S. , Hanke, T. , Chaudhuri, G. , Harvey, R. , & Rao, N. ((2000) ) Compelled weightbearing in persons with hemiparesis following stroke: the effect of a lift insert and goal-directed balance exercise.. Retrieved from, J Rehabil Res Dev 37: (1), 65–72 https://www.ncbi.nlm.nih.gov/pubmed/10847573. |
5 | Berg, K. , Wood-Dauphinee, S. , & Williams, J. I. ((1995) ) The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke, Scand J Rehabil Med 27: (1), 7792547. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/. |
6 | Bohannon, R. W. , & Smith, M. B. ((1987) ) Interrater reliability of a modified Ashworth scale of muscle spasticity., Physical Therapy 67: (2), 3809245. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/. |
7 | Bonnyaud, C. , Pradon, D. , Zory, R. , Bussel, B. , Bensmail, D. , Vuillerme, N. , & Roche, N. ((2013) ) Effects of a gait training session combined with a mass on the non-paretic lower limb on locomotion of hemiparetic patients: a randomized controlled clinical trial, Gait Posture 37: (4), 627–630. doi: 10.1016/j.gaitpost.2012.09.010. |
8 | Brogardh, C. , Vestling, M. , & Sjolund, B. H. ((2009) ) Shortened constraint-induced movement therapy in subacute stroke - no effect of using a restraint: a randomized controlled study with independent observers, J Rehabil Med 41: (4), 231–236. doi: 10.2340/16501977-0312. |
9 | Cassidy, J. M. , & Cramer, S. C. ((2017) ) Spontaneous and Therapeutic-Induced Mechanisms of Functional Recovery After Stroke, Transl Stroke Res 8: (1), 33–46. doi: 10.1007/s12975-016-0467-5. |
10 | Chiu, H. C. , & Ada, L. ((2016) ) Constraint-induced movement therapy improves upper limb activity and participation in hemiplegic cerebral palsy: a systematic review, J Physiother 62: (3), 130–137. doi: 10.1016/j.jphys.2016.05.013. |
11 | Choi, H. S. , Shin, W. S. , Bang, D. H. , & Choi, S. J. ((2017) ) Effects of Game-Based Constraint-Induced Movement Therapy on Balance in Patients with Stroke: A Single-Blind Randomized Controlled Trial, Am J Phys Med Rehabil 96: (3), 184–190. doi: 10.1097/PHM.0000000000000567. |
12 | Clarke, D. J. , Burton, L. J. , Tyson, S. F. , Rodgers, H. , Drummond, A. , Palmer, R. , & Forster A. ((2018) ) Why do stroke survivors not receive recommended amounts of active therapy? Findings from the ReAcT study, a mixed-methods case-study evaluation in eight stroke units, Clin Rehabil 32: (8), 1119–1132. doi: 10.1177/0269215518765329. |
13 | Corbetta, D. , Sirtori, V. , Castellini, G. , Moja, L. , & Gatti, R. ((2015) ) Constraint-induced movement therapy for upper extremities in people with stroke, Cochrane Database Syst Rev (10), CD004433. doi: 10.1002/14651858.CD004433.pub3. |
14 | Dos Anjos S. , Morris, D. , & Taub, E. ((2020) ) Constraint-Induced Movement Therapy for Lower Extremity Function: Describing the LE-CIMT Protocol, Phys Ther 100: (4), 698–707. doi: 10.1093/ptj/pzz191. |
15 | Silva E. M. G. S. , Ribeiro, T. S. , da Silva T. C. C. , Costa, M. F. P. , Cavalcanti, F. , & Lindquist, A. R. R. ((2017) ) Effects of constraint-induced movement therapy for lower limbs on measurements of functional mobility and postural balance in subjects with stroke: a randomized controlled trial, Top Stroke Rehabil 24: (8), 555–561. doi: 10.1080/10749357.2017.1366011. |
16 | Etoom, M. , Hawamdeh, M. , Hawamdeh, Z. , Alwardat, M. , Giordani, L. , Bacciu, S. , & Foti, C. ((2016) ) Constraint-induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: systematic review and meta-analysis, Int J Rehabil Res 39: (3), 197–210. doi: 10.1097/MRR.0000000000000169. |
17 | Flansbjer, U. B. , Holmback, A. M. , Downham, D. , Patten, C. , & Lexell, J. ((2005) ) Reliability of gait performance tests in men and women with hemiparesis after stroke, J Rehabil Med 37: (2), 75–82. doi: 10.1080/16501970410017215. |
18 | Fleet, A. , Che, M. , Mackay-Lyons, M. , Mackenzie, D. , Page, S. , Eskes, G. , & Boe, S. ((2014) ) Examining the use of constraint-induced movement therapy in canadian neurological occupational and physical therapy, Physiother Can 66: (1), 60–71. doi: 10.3138/ptc.2012-61. |
19 | Fritz, S. L. , Pittman, A. L. , Robinson, A. C. , Orton, S. C. , & Rivers, E. D. ((2007) ) An intense intervention for improving gait, balance, and mobility for individuals with chronic stroke: a pilot study, J Neurol Phys Ther 31: (2), 71–76. doi: 10.1097/NPT.0b013e3180674a3c. |
20 | Fulk, G. D. , Echternach, J. L. , Nof, L. , & O’Sullivan, S. ((2008) ) Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke, Physiother Theory Pract 24: (3), 195–204. doi: 10.1080/09593980701588284. |
21 | Gladstone, D. J. , Danells, C. J. , & Black, S. E. ((2002) ) The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties, Neurorehabil Neural Repair 16: (3), 232–240. doi: 10.1177/154596802401105171. |
22 | Hase, K. , Suzuki, E. , Matsumoto, M. , Fujiwara, T. , & Liu, M. ((2011) ) Effects of therapeutic gait training using a prosthesis and a treadmill for ambulatory patients with hemiparesis, Arch Phys Med Rehabil 92: (12), 1961–1966. doi: 10.1016/j.apmr.2011.07.005. |
23 | Hendricks, H. T. , van Limbeek J. , Geurts, A. C. , & Zwarts, M. J. ((2002) ) Motor recovery after stroke: a systematic review of the literature, Arch Phys Med Rehabil 83: (11), 12422337.Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/. |
24 | Kallio, K. , Nilsson-Wikmar, L. , & Thorsen, A. M. ((2014) ) Modified constraint-induced therapy for the lower extremity in elderly persons with chronic stroke: single-subject experimental design study, Top Stroke Rehabil 21: (2), 111–119. doi: 10.1310/tsr2102-111. |
25 | Kwakkel, G. , Kollen, B. , & Lindeman, E. ((2004) ) Understanding the pattern of functional recovery after stroke: facts and theories., Restorative Neurology and Neuroscience 22: (3-5), 15502272. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/. |
26 | Kwakkel, G. , Veerbeek, J. M. , van Wegen, E. E. , & Wolf, S. L. ((2015) ) Constraint-induced movement therapy after stroke, Lancet Neurol 14: (2), 224–234. doi: 10.1016/S1474-4422(14)70160-7. |
27 | Lin, J. H. , Hsu, M. J. , Hsu, H. W. , Wu, H. C. , & Hsieh, C. L. ((2010) ) Psychometric comparisons of 3 functional ambulation measures for patients with stroke, Stroke 41: (9), 2021–2025. doi: 10.1161/STROKEAHA.110.589739. |
28 | Lindsay, P. , Bayley, M. , McDonald, A. , Graham, I. D. , Warner, G. , & Phillips, S. ((2008) ) Toward a more effective approach to stroke: Canadian Best Practice Recommendations for Stroke Care, CMAJ 178: (11), 1418–1425. doi: 10.1503/cmaj.071253. |
29 | Mao, H. F. , Hsueh, I. P. , Tang, P. F. , Sheu, C. F. , & Hsieh, C. L. ((2002) ) Analysis and comparison of the psychometric properties of three balance measures for stroke patients, Stroke 33: (4), 1022–1027. doi: 10.1161/01.str.0000012516.63191.c5. |
30 | Marklund, I. , & Klassbo, M. ((2006) ) Effects of lower limb intensive mass practice in poststroke patients: single-subject experimental design with long-term follow-u, Clin Rehabil 20: (7), 568–576. doi: 10.1191/0269215506cr973oa. |
31 | Morris, D. M. , Taub, E. , & Mark, V. W. ((2006) ) Constraint-induced movement therapy: characterizing the intervention protocol.. Retrieved from, Europa Medicophysica 42: (3), 257–268 http://www.ncbi.nlm.nih.gov/pubmed/17039224. |
32 | Numata, K. , Murayama, T. , Takasugi, J. , & Oga, M. ((2008) ) Effect of modified constraint-induced movement therapy on lower extremity hemiplegia due to a higher-motor area lesion, Brain Inj 22: (11), 898–904. doi: 10.1080/02699050802425436. |
33 | Pandian, S. , Arya, K. N. , & Kumar, D. ((2016) ) Minimal clinically important difference of the lower-extremity fugl-meyer assessment in chronic-stroke, Top Stroke Rehabil 23: (4), 233–239. doi: 10.1179/1945511915Y.0000000003. |
34 | Perera, S. , Mody, S. H. , Woodman, R. C. , & Studenski, S. A. ((2006) ) Meaningful change and responsiveness in common physical performance measures in older adults, J Am Geriatr Soc 54: (5), 743–749. doi: 10.1111/j.1532-5415.2006.00701.x. |
35 | Peurala, S. H. , Kantanen, M. P. , Sjogren, T. , Paltamaa, J. , Karhula, M. , & Heinonen, A. ((2012) ) Effectiveness of constraint-induced movement therapy on activity and participation after stroke: a systematic review and meta-analysis of randomized controlled trials, Clin Rehabil 26: (3), 209–223. doi: 10.1177/0269215511420306. |
36 | Regnaux, J. P. , Pradon, D. , Roche, N. , Robertson, J. , Bussel, B. , & Dobkin, B. ((2008) ) Effects of loading the unaffected limb for one session of locomotor training on laboratory measures of gait in stroke, Clin Biomech (Bristol, Avon) 23: (6), 762–768. doi: 10.1016/j.clinbiomech.2008.01.011. |
37 | Rodriguez, G. M. , & Aruin, A. S. ((2002) ) The effect of shoe wedges and lifts on symmetry of stance and weight bearing in hemiparetic individuals, Arch Phys Med Rehabil 83: (4), 478–482. doi: 10.1053/apmr.2002.31197. |
38 | Sirtori, V. , Corbetta, D. , Moja, L. , & Gatti, R. ((2009) ) Constraint-induced movement therapy for upper extremities in stroke patients, Cochrane Database Syst Rev (4), CD004433. doi: 10.1002/14651858.CD004433.pub2. |
39 | Staines, W. R. , McIlroy, W. E. , & Brooks, D. ((2009) ) Functional Impairments Following Stroke: Implications for Rehabilitation, Current Issues in Cardiac Rehabilitation and Prevention 17: (1), 5–8. |
40 | Stevenson, T. , Thalman, L. , Christie, H. , & Poluha, W. ((2012) ) Constraint-Induced Movement Therapy Compared to Dose-Matched Interventions for Upper-Limb Dysfunction in Adult Survivors of Stroke: A Systematic Review with Meta-analysis, Physiother Can 64: (4), 397–413. doi: 10.3138/ptc.2011-24. |
41 | Tamura, S. , Miyata, K. , Kobayashi, S. , Takeda, R. , & Iwamoto, H. ((2021) ) The minimal clinically important difference in Berg Balance Scale scores among patients with early subacute stroke: a multicenter, retrospective, observational study, Top Stroke Rehabil 1–7. doi: 10.1080/10749357.2021.1943800. |
42 | Taub, E. , Crago, J. E. , Burgio, L. D. , Groomes, T. E. , Cook, E. W. , 3rd DeLuca S. C. , & Miller, N. E. ((1994) ) An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping, J Exp Anal Behav 61: (2), 281–293. doi: 10.1901/jeab.1994.61-281. |
43 | Taub, E. , Uswatte, G. , King, D. K. , Morris, D. , Crago, J. E. , & Chatterjee, A. ((2006) ) A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke, Stroke 37: (4), 1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. |
44 | Taub, E. , Uswatte, G. , Mark, V. W. , Morris, D. M. , Barman, J. , Bowman, M. H. , & Bishop-McKay S. ((2013) ) Method for enhancing real-world use of a more affected arm in chronic stroke: transfer package of constraint-induced movement therapy, Stroke 44: (5), 1383–1388. doi: 10.1161/STROKEAHA.111.000559. |
45 | Uswatte, G. , & Taub, E. ((2013) ) Constraint-induced movement therapy: a method for harnessing neuroplasticity to treat motor disorders, Prog Brain Res 207: , 379–401. doi: 10.1016/B978-0-444-63327-9.00015-1. |
46 | Uswatte, G. , Taub, E. , Morris, D. , Barman, J. , & Crago, J. ((2006) ) Contribution of the shaping and restraint components of Constraint-Induced Movement therapy to treatment outcome.. Retrieved from, NeuroRehabilitation 21: (2), 147–156. https://www.ncbi.nlm.nih.gov/pubmed/16917161. |
47 | Vearrier, L. A. , Langan, J. , Shumway-Cook, A. , & Woollacott, M. ((2005) ) An intensive massed practice approach to retraining balance post-stroke, Gait Posture 22: (2), 154–163. doi: 10.1016/j.gaitpost.2004.09.001. |
48 | Wang, C. H. , Hsueh, I. P. , Sheu, C. F. , Yao, G. , & Hsieh, C. L. ((2004) ) Psychometric properties of 2 simplified 3-level balance scales used for patients with stroke, Phys Ther 84: (5), 15113276. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/. |
49 | Whitall, J. , McCombe Waller, S. , Silver, K. H. , & Macko, R. F. ((2000) ) Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke, Stroke; a Journal of Cerebral Circulation 31: (10), 11022069. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/. |
50 | Wolf, S. L. , Catlin, P. A. , Gage, K. , Gurucharri, K. , Robertson, R. , & Stephen, K. ((1999) ) Establishing the reliability and validity of measurements of walking time using the Emory Functional Ambulation Profile, Phys Ther 79: (12), 10630281. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/. |
51 | Wolf, S. L. , Thompson, P. A. , Winstein, C. J. , Miller, J. P. , Blanton, S. R. , Nichols-Larsen, D. S. , & Sawaki, L. ((2010) ) The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy, Stroke 41: (10), 2309–2315. doi: 10.1161/STROKEAHA.110.588723. |
52 | Wolf, S. L. , Winstein, C. J. , Miller, J. P. , Taub, E. , Uswatte, G. , Morris, D. , & Investigators, E. ((2006) ) Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial, JAMA 296: (17), 2095–2104. doi: 10.1001/jama.296.17.2095. |
53 | Wolf, S. L. , Winstein, C. J. , Miller, J. P. , Thompson, P. A. , Taub, E. , Uswatte, G. , & Clark, P. C. ((2008) ) Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial, Lancet Neurol 7: (1), 33–40. doi: 10.1016/S1474-4422(07)70294-6. |
54 | Zhu, Y. , Zhou, C. , Liu, Y. , Liu, J. , Jin, J. , Zhang, S. , & Wu, Y. ((2016) ) Effects of modified constraint-induced movement therapy on the lower extremities in patients with stroke: a pilot study, Disabil Rehabil 38: (19), 1893–1899. doi: 10.3109/09638288.2015.1107775. |




