Technology-assisted balance assessment and rehabilitation in individuals with spinal cord injury: A systematic review
Abstract
BACKGROUND:
Balance is a crucial function of basic Activities of Daily Living (ADL) and is often considered the priority in Spinal Cord Injury (SCI) patients’ rehabilitation. Technological devices have been developed to support balance assessment and training, ensuring an earlier, intensive, and goal-oriented motor therapy.
OBJECTIVE:
The aim of this systematic review is to explore the technology-assisted strategies to assess and rehabilitate balance function in persons with SCI.
METHODS:
A systematic review was conducted in the databases PubMed, Scopus, IEEE Xplore, Cochrane Library, and Embase. Full reports on Randomized Clinical Trials (RCTs) of parallel-group or cross-over design and non-RCTs were included according to the following criteria: i) publication year from 1990 to 2021; ii) balance considered as a primary or secondary outcome; iii) population of individuals with SCI with age over 18 years old, regardless of traumatic or non-traumatic lesions, Time Since Injury, lesion level, Asia Impairment Scale score and gender. The methodological quality was determined for each included study according to the recognized Downs and Black (D&B) tool.
RESULTS:
Nineteen articles met the inclusion criteria and were included in the analysis. Four articles focused on balance assessment while 15 targeted rehabilitation interventions to improve balance by using Treadmill-Based Devices (TBD), OverGround Devices (OGD) and Tilt Table Devices (TTD). Statistically significant effects on balance can be found in TBD subcategory, in the hip-knee guidance subcategory of OGD and in the study of TTD category.
CONCLUSION:
Although different studies reported positive effects, improvements due to technology-assisted rehabilitation were not greater than those obtained by means of other rehabilitation therapies. The heterogeneity, low methodological quality, and the small number of the studies included do not allow general conclusions about the usefulness of technology-assisted balance assessment and training in individuals with SCI, even if significant improvements have been reported in some studies.
1Introduction
Even if a definition of human balance has not been universally accepted (Pollock, 2000), the term is often used, from the biomechanical perspective, to identify the preservation of the vertical projection of the body’s Centre Of Mass (COM) within the base of support (Hof, 2005; Meyer, 2006; Tamburella et al., 2014). Postural control, defined as the act of achieving, maintaining, or restoring a state of balance during any posture or activity (Pollock, 2000), is managed by the Central Nervous System by means of several afferent inputs (visual, vestibular, and somatosensory information) (Tramontano et al., 2019a; Tramontano et al., 2019b). These inputs are integrated and converted into efferent motor outputs and pass down to the spinal cord along different motor tracts (F.B. Horak, 2006; R.J. Peterka,, 2018; Tamburella et al., 2014). Individuals with Spinal Cord Injury (SCI) present motor and sensory impairments below their injury site due to a total or partial deficit of sensory and motor pathways. The impaired somatosensory afferents and the decreased voluntary motor control can consequently influence postural control in these individuals, thus resulting in balance disorders (Ilha et al., 2020), such as increased postural sway, reduced precision during bodyweight shifting, and delayed responses to external perturbations (Meyer-Heim & van Hedel, 2013).
Since balance is a crucial function of basic Activities of Daily Living (ADL), such as transfers, dressing or wheelchair handling (Chisholm et al., 2017; Harel et al., 2013; Ilha et al., 2020), it is often considered a priority to enhance the Quality of Life (QOL) in SCI population. Anderson et al. (Anderson, 2004) determined which areas of functional recovery have priority for the SCI population to achieve positive effects on their QOL. Results indicated that, from the patients’ point of view, the priorities depend on the different lesion levels. Indeed, for individuals with cervical lesions the importance of balance capability is secondary only to the upper limb and sexual functions, while for individuals with paraplegia only to sexual, bowel and bladder functions (Anderson, 2004). Moreover, from the patients’ perspective, the walking ability has less priority than balance control (Anderson, 2004).
The relationship between balance and gait has been investigated in a few studies. It has been demonstrated that balance is one of the most important factors affecting walking in individuals with SCI (Bach Baunsgaard et al., 2018; Harel et al., 2013; Scivoletto, 2008): proper balance control allows walking with fewer aids and enables patients to walk faster and for a longer distance (Scivoletto, 2008). Furthermore, balance-oriented training, enhanced with visual performance feedback, has been demonstrated to allow not only balance improvements but also a consequent gait enhancement in individuals with SCI (Tamburella et al., 2013). On the other hand, gait-oriented rehabilitation, even aided by robotic gait devices (Nam et al., 2017), leads not only to walking improvements but also to balance control enhancements.
This evidence suggests that the improvement of balance function can be achieved not only in the case of dedicated balance training (direct balance rehabilitation), but also as a secondary outcome when walking training is primarily targeted (indirect balance rehabilitation).
Technological devices have been introduced to aid the traditional balance training with the possibility to perform goal-oriented repetitive tasks, expanding the types and numbers of exercises, and quantitatively measuring motor performance and patients’ progress along the therapy.
These devices may be quite heterogeneous and can be used both for an active training of balance or for the assessment of balance recovery indicators. Some examples include (Shirota et al., 2017): i) perturbation platforms, i.e. actuated standing surfaces providing controlled destabilizing actions to challenge standing (Shirota et al., 2017; Van Asseldonk, 2007); ii) mobile robotic gait trainers, i.e. robots connected to the user at the pelvis, lower- or upper-trunk, while being mounted on a wheeled platform to provide body weight and/or posture support as well as safety during overground gait and balance training (Olensek et al., 2012); iii) mobile self-balancing platforms for balance training, i.e. devices with a standing surface mounted on two wheels with an upright handgrip, that are able to self-balance on their two wheels and to tilt forward and backward to challenge the standing balance [Toyota Motor Corporation Official Global Website]; iv) Body Weight Support (BWS) devices, i.e. generators of a constant or controlled vertical supportive force to provide safety and weight support during balance and gait training overground (Hidler et al., 2011) or on a treadmill (Frey et al., 2006); v) force platforms and stabilometric plates, i.e. flat sensorized surfaces providing continuous visual feedback on posturography-related quantities, e.g. Centre of Pressure (COP) position, foot pressure distributions, ground reaction forces amplitude and direction, etc.; vi) motion wearable sensors and virtual reality, i.e. devices measuring body kinematics information and providing a real-time feedback to users for an immersive balance training (Kalron et al., 2016); vii) wearable robotic gait trainers, i.e. systems including treadmill-based or overground exoskeletons, challenging and supporting walking and indirectly balance (Font-Llagunes et al., 2020; Shin et al., 2014).
Despite the strong diffusion of technology-assisted balance rehabilitation, to the best of our knowledge, no review has been systematically performed on its clinical usefulness in balance assessment and rehabilitation in individuals with SCI. For this reason, the aim of this review is to explore the technology-assisted strategies to assess and rehabilitate balance function in individuals with SCI. We systematically reported the studies in which balance assessment and rehabilitation were technologically-assisted.
2Methods
This systematic review was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Page et al., 2021).
2.1Search strategy and eligibility criteria
The following databases were scanned to identify studies published from inception to December 1, 2021: MED-LINE, Embase, Scopus, Cochrane Library and IEEE Xplore. The following search terms were used in each database: ("spinal cord” AND (injur* OR diseas*)) AND balance AND (robot* OR device OR technolog*) AND (rehabilit* OR assessement*). English language and studies on humans were used as restriction criteria. In addition, hand searches, starting from reference lists in the retrieved articles as well as in previous published reviews or meta-analysis, were performed. Full reports of Randomized Clinical Trials (RCTs) of parallel-group or cross-over design and non-RCTs (n-RCTs) such as cohort studies, case series, case-control, case report and pilot studies were included. Records based on individuals with SCI, regardless of type of lesion (traumatic or non-traumatic), Time Since Injury (TSI), lesion level, Asia Impairment Scale (AIS) score, age and gender were selected. Trials that recruited a mixed population suffering from SCI and other neurological conditions (e.g., stroke, multiple sclerosis) were included.
2.2Study selection and data collection process
Duplicate records were identified and removed with the support of the EndNOTE software. Two independent co-authors (G. G. and A. F.) carried out the study eligibility assessment and data extraction process. The opinion of a third author (F. T. or M. L.) was used in case of any disagreement. The first selection of studies was initially conducted on the basis of titles and abstracts and thereafter full-text articles were reviewed.
The summary of results was reported following the PRISMA statement (Page et al., 2021). A predefined data extraction form was used by two independent authors (G. G. and A. F.) to extract the following features of the included studies: authors; title; year of publication; country where the study was conducted; study design; participant features (number, gender, age, TSI, aetiology, AIS score, lesion level); device type; interventions data (total treatment duration, single session duration, frequency and total number of sessions); outcome measures (clinical scales and/or instrumental assessments); results; presence/absence of follow up assessment; number of drop-out and/or adverse events.
The methodological quality was determined for each included study according to the recognized Downs and Black (D&B) tool (Downs & Black, 1998), which is organized in different subsections: reporting, external validity, internal validity (bias) and internal validity (confounding) and power. The total score ranges from 0 to 28; a higher score indicates a higher methodological quality (Saunders et al., 2003). According to Singh et al. (2018) D&B assessment, a score below 11 points indicates “poor” quality; a score in the range 11–19 points indicates “moderate” quality while a score greater than 19 points is an indicator of “good” quality. Two independent authors (G. G. and A. F.) reviewed each article and determine the D&B score. Scoring discrepancies were resolved through a discussion with a third researcher (F. T. or M. L.).
The results reported in this review were organized following the PICO framework. Based on the purpose of this review, the devices were classified as technology-assisted strategies for balance function rehabilitation or assessment. In the former case, further subdivisions were made in order to differentiate technologies on the basis of the usage modality and the device guidance. For the balance function assessment technologies, the classification was based on the position maintained by individuals with SCI during assessment.
3Results
3.1Identification of studies
Figure 1 represents the PRISMA flow diagram of the study selection process. A total of 383 articles was identified from all the considered databases: PubMed (N = 147), Scopus (N = 180), IEEE Xplore (N = 12), Cochrane Library (N = 35), Embase (N = 9) and any article from other sources was included as additional record. Among these publications, 89 were excluded because they were duplicates. Titles and abstracts were screened for the remaining 294 articles: 260 records were excluded because they didn’t satisfy the prescribed inclusion criteria while 34 articles were retained as potentially relevant studies and a full-text review was conducted on them. Among these articles, 15 were further excluded based on criteria reported in Fig. 1. Finally, 19 articles were considered for full analysis.
Fig. 1
PRISMA flow diagram of the study selection process (Page et al., 2021).
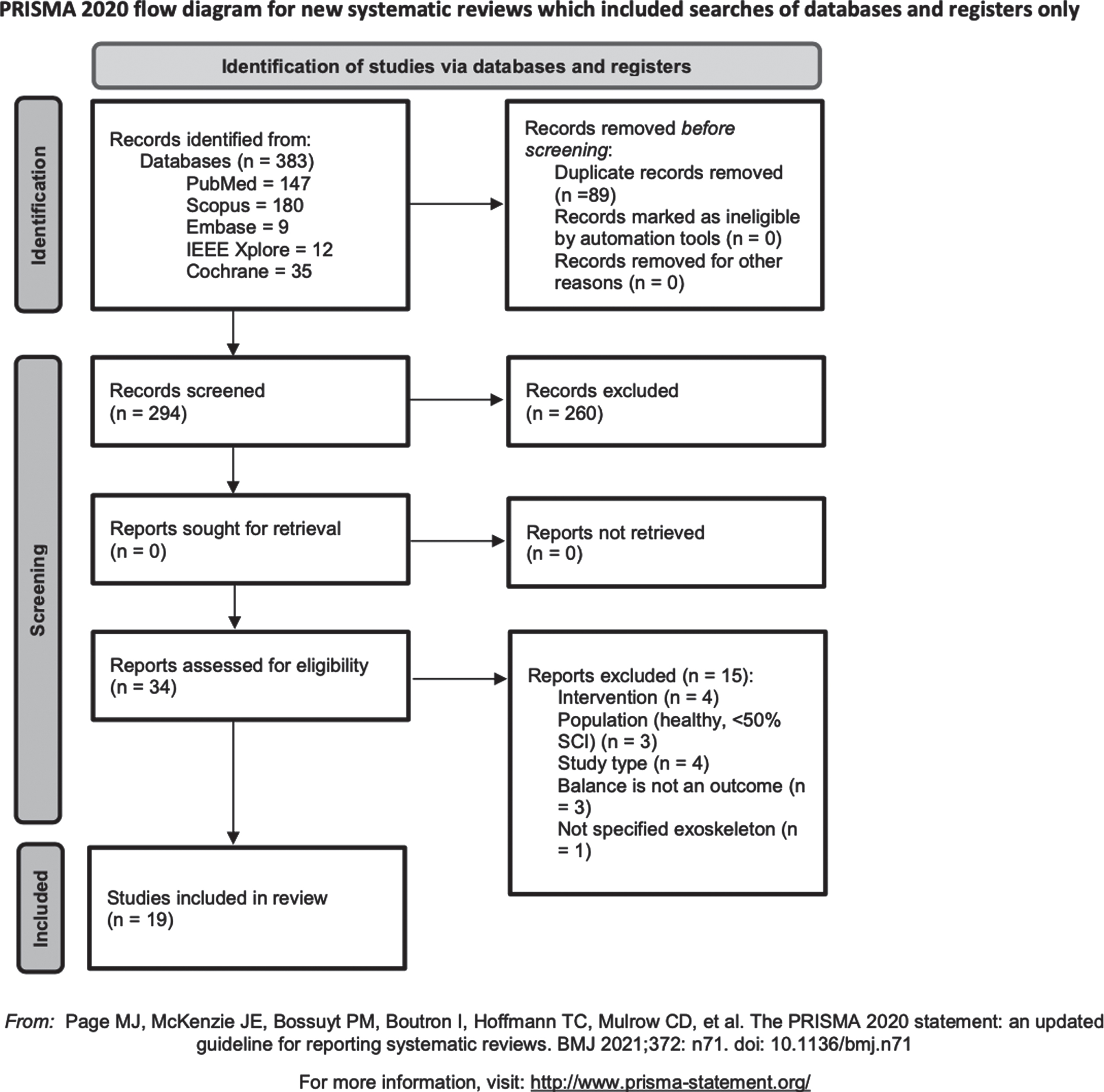
Table 1
Downs and Black tool scores reported for each rehabilitative or assessment study
| Technology-assisted rehabilitative studies | |||||||
| 1st Author, publication year | Study design | Downs and Black tool | |||||
| Reporting | External validity | Internal validity -bias | Internal validity -confounding | Power | Total score | ||
| Piira, 2019 | Randomized clinical trial | 8 | 2 | 5 | 3 | 0 | 18 |
| Labruyère, 2014 | Randomized clinical trial | 7 | 2 | 4 | 4 | 0 | 17 |
| Khan, 2019 | Cohort study | 9 | 2 | 5 | 1 | 0 | 17 |
| Martinez, 2019 | Crossover trial | 5 | 2 | 5 | 3 | 0 | 15 |
| Bach Baunsgaard, 2018 | Cohort study | 9 | 1 | 3 | 1 | 0 | 14 |
| Covarrubias-Escudero, 2019 | Descriptive study | 8 | 1 | 4 | 1 | 0 | 14 |
| Daunoraviciene, 2018 | Case series | 9 | 1 | 3 | 0 | 0 | 13 |
| Wu, 2012 | Crossover study | 7 | 0 | 3 | 3 | 0 | 13 |
| Okawara, 2020 | Non randomized clinical trial | 7 | 0 | 3 | 2 | 0 | 12 |
| Chisholm, 2017 | Case report | 7 | 0 | 3 | 2 | 0 | 12 |
| Hornby, 2005 | Case report | 7 | 1 | 2 | 1 | 0 | 11 |
| Wu, 2019 | Not reported | 7 | 0 | 3 | 1 | 0 | 11 |
| Moreh, 2009 | Case report | 6 | 1 | 2 | 1 | 0 | 10 |
| Bishop, 2012 | Case report | 6 | 0 | 2 | 0 | 0 | 8 |
| Font-Llagunes, 2020 | Case report | 5 | 0 | 2 | 0 | 0 | 7 |
| Technology-assisted assessment studies | |||||||
| 1st Author, publication year | Study design | Downs and Black tool | |||||
| Reporting | External validity | Internal validity -bias | Internal validity -confounding | Power | Total score | ||
| Lemay, 2013 | Correletional study | 9 | 2 | 2 | 1 | 0 | 14 |
| Harel, 2013 | Cross sectional study | 8 | 1 | 3 | 1 | 0 | 13 |
| Shin, 2013 | Cohort study | 9 | 1 | 3 | 0 | 0 | 13 |
| Tamburella, 2014 | Cross sectional study | 7 | 0 | 3 | 0 | 0 | 10 |
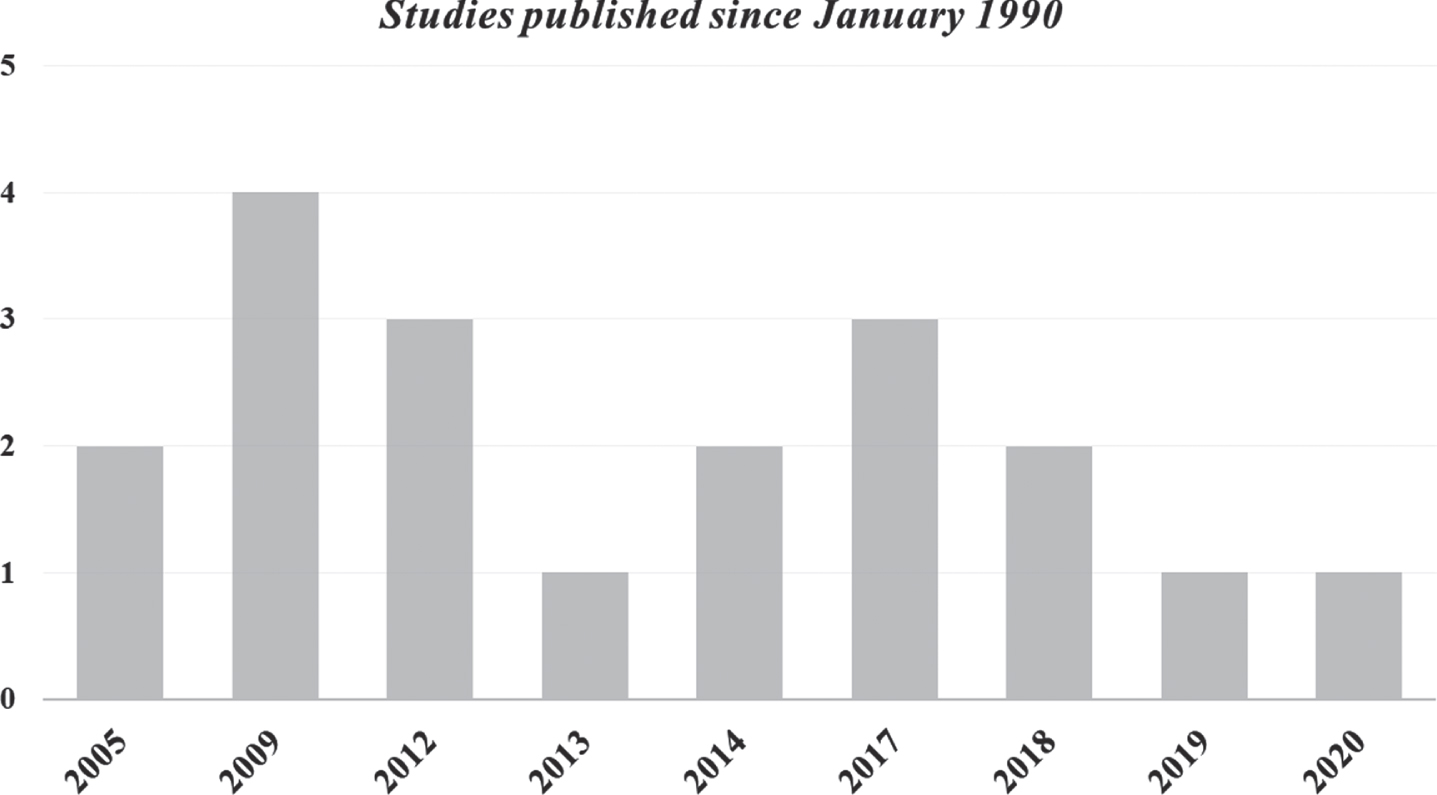
Of the 19 studies included: 15 focused on technology-assisted rehabilitation and 4 used technologies only for balance assessment. One out of 15 studies (Chisholm et al., 2017) addressed the direct balance rehabilitation (focusing on sitting balance) while all the other studies considered balance as an indirect benefit coming from a primary focus on walking rehabilitation. We categorized the studies based on the adopted technology and we analysed in depth clinical and instrumental rehabilitation outcomes. Most of the included studies were published within the last 10 years and no studies were published prior to 2005 (Fig. 2). Most of the studies were conducted in USA or Canada.
Fig. 2
Number of studies published for from January 1990 until 1 December 2021.
3.2Methodological quality
The D&B tool scores for the evaluation of methodological quality are reported in Table 1. None of the included studies was rated with “good” score. Most of the studies (Bach Baunsgaard et al., 2018; Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Daunoraviciene et al., 2018; Harel et al., 2013; Hornby et al., 2005; Khan et al., 2019; Labruyere & van Hedel, 2014; Lemay & Nadeau, 2013; Martinez et al., 2018; Okawara et al., 2020; Piira et al., 2019; Shin & Sosnoff, 2013; Wu et al., 2012; 2019) reached a “moderate” quality score (D&B score: 13.8±2.14), while the remaining 4 studies (Bishop et al., 2012; Font-Llagunes et al., 2020; Moreh et al., 2009; Tamburella et al., 2014) were classified as “poor” (D&B score: 8.75±1.5).
3.3Participants
327 participants were enrolled in the selected studies, including: 54 healthy individuals (16 females and 38 males), 270 individuals with SCI and 3 individuals who suffered a stroke. The mean age of participants for each study was classified according to different age ranges. Any study was available on individuals with age under 18 or over 65 years. The age of healthy individuals was in three studies (Covarrubias-Escudero et al., 2019; Harel et al., 2013; Wu et al., 2019) in the range 25–44 years and in one study (Shin & Sosnoff, 2013) in the range 19–24 years.
In several papers epidemiological and clinical features of individuals with SCI were reported only for those who completed the trials (N = 255); in one study (Daunoraviciene et al., 2018), in which both individuals with stroke and SCI were evaluated, epidemiological features were not reported separately. Hence, all the subsequent data will refer to a sample of 258 individuals (179 males and 79 females). Most of the studies (N = 10) (Bach Baunsgaard et al., 2018; Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Font-Llagunes et al., 2020; Harel et al., 2013; Hornby et al., 2005; Khan et al., 2019; Martinez et al., 2018; Moreh et al., 2009; Okawara et al., 2020) recruited individuals with age in the range 25–44 years. Seven studies (Daunoraviciene et al., 2018; Labruyere & van Hedel, 2014; Lemay & Nadeau, 2013; Piira et al., 2019; Tamburella et al., 2014; Wu et al., 2012; 2019) focused on individuals with age in the range 45–64 years while for two studies individuals’ age was in the range 19–24 years (Bishop et al., 2012; Shin & Sosnoff, 2013).
Information about the SCI severity and the lesion level is reported in Table 2. Three studies (Martinez et al., 2018; Piira et al., 2019; Wu et al., 2019) did not report this information for participants who dropped out (N = 15). Among the 255 individuals with SCI for which complete data were available, 132 participants sustained a traumatic SCI and 54 participants a non-traumatic one. The aetiology information was not reported for the remaining 69 individuals. The SCI severity, measured per the AIS scale, reported a total of 68 individuals with a motor complete SCI (AIS A or B) and 158 with an incomplete SCI (AIS C or D). Furthermore, one study (Font-Llagunes et al., 2020) did not report information about SCI severity of the single participant included. Considering lesion level, 78 individuals suffered a cervical lesion, 76 a thoracic lesion and 22 a lumbar lesion. Two papers (Bach Baunsgaard et al., 2018; Daunoraviciene et al., 2018) classified the SCI as thoraco-lumbar lesion for a total of 39 individuals (with not clear distinction between thoracic and lumbar lesions). No details about lesion level for 12 individuals with SCI were reported in one study (Wu et al., 2019).
Table 2
Clinical and demographic characteristics of the enrolled participants
| Technology-assisted rehabilitative studies | ||||||||||
| Study | Individuals with SCI | Control | ||||||||
| N Participants | Gender: M, F | Age: | TSI: | Aetiology: T, NT | AIS level: | LOI: | N | Gender | Age: | |
| mean±sd | w, m, y | A, B, C, D, | c, t, l, t/l | Participants | mean±sd | |||||
| A+B, C+D | ||||||||||
| Piira, 2019 | 24 (19) | M = 9, | Intervention: | >2 y | T = 12, NT = 7 | C = 6, | c = 10, t = 9 | 0 | NA | NA |
| F = 10 | 55±8; | D = 13 | ||||||||
| Control: | ||||||||||
| 46±15 | ||||||||||
| Labruyère, 2014 | 9 (9) | M = 5, | 59±11 | 50±56 m | T = 4, NT = 5 | D = 9 | c = 5, t = 4 | 0 | NA | NA |
| F = 4 | ||||||||||
| Khan, 2019 | 12 (12) | M = 8, | 37.5±13.7 | 7.6±8.1 y | T = 12 | A = 6, B = 2, | c = 3, t = 9 | 0 | NA | NA |
| F = 4 | C = 3, D = 1 | |||||||||
| Martinez, 2019 | 21 (12) | M = 9, | 40.33±8.18 | 1–23 y | T = 10, NT = 2 | A = 1, B = 1, | c = 3, t = 9, | 0 | NA | NA |
| F = 3 | C = 7, D = 3 | |||||||||
| Bach Baunsgaard, 2018 | 52 (52) | M = 36, | 35.8* | n = 25:0.3 y | T = 43, F = 9 | A+B = 25, | c = 14, | 0 | NA | NA |
| F = 16 | n = 27:5.5 y | C+D = 27 | t/l = 38 | |||||||
| Covarrubias-Escudero, 2019 | 17 (17) | M = 14, | 43.5±3.7 | 13–48 m | NR | C = 10, D = 7 | c = 11, | 17 healthy | M = 13, F = 4 | 37.5±8.9 |
| F = 3 | t = 5, | |||||||||
| l = 1 | ||||||||||
| Daunoraviciene, 2018 | 6 (3) | M = 5, | 58.83±14.19 | NR | NR | B = 2, C = 1 | t = 1, | 3 stroke | NA | NA |
| F = 1 | l = 1, | |||||||||
| t/l = 1 | ||||||||||
| Wu, 2012 | 10 (10) | M = 8, | 47±7 | 5.8±3.8 y | NR | D = 10 | c = 8, t = 2 | 0 | NA | NA |
| F = 2 | ||||||||||
| Okawara, 2020 | 20 (20) | M = 15, | 43.3±17 | 80.4±129 m | T = 15, NT = 5 | A = 2, B = 4, | c = 10, | 0 | NA | NA |
| F = 5 | C = 8, D = 6 | t = 9, | ||||||||
| l = 1 | ||||||||||
| Chisholm, 2017 | 3 (3) | M = 2, | 40.66±1.52 | 18–25 y | T = 3 | A = 2, B = 1 | c = 1, | 0 | NA | NA |
| F = 1 | t = 2 | |||||||||
| Hornby, 2005 | 3 (3) | M = 2, | 32±25.16 | 6 w; 5 w, 18 m | T = 2, NT = 1 | C = 3 | c = 2, | 0 | NA | NA |
| F = 1 | t = 1 | |||||||||
| Wu, 2019 | 13 (12) | M = 10, | 56±9 | NR | NR | D = 12 | NR | 12 | M = 9, F = 3 | 41±15 |
| F = 2 | ||||||||||
| Moreh, 2009 | 1 (1) | M = 1 | 42 | 3 w | NR | C = 1 | t = 1 | 0 | NA | NA |
| Bishop, 2012 | 1(1) | F = 1 | 22 | 4 y | T = 1 | D = 1 | c = 1 | 0 | NA | NA |
| Font-Llagunes, 2020 | 1(1) | F = 1 | 41 | NR | NR | NR | t = 1 | 0 | NA | NA |
| Technology-assisted rehabilitative studies | ||||||||||
| Study | Individuals with SCI | Control | ||||||||
| N individuals completing | Gender: | Age: | TSI: | Aetiology: | AIS level: | LOI: | N individuals | Gender | Age: | |
| M, F | mean±sd | d, w, m, y | T, NT | A, B, C, D, | c, t, l, t/l | completing | mean±sd | |||
| trial | A+B, C+D | trial | ||||||||
| Harel, 2013 | 7 (7) | M = 6, F = 1 | 41.85±13.08 | 1.5–15 y | NR | A = 6, B = 1 | t = 7 | 7 | M = 6, F = 1 | 34±13.27 |
| Shin, 2013 | 18 (18) | M = 10, F = 8 | 21.5±2.47 | NR | NR | A = 15, C = 2, D = 1 | t = 10, d = 8 | 18 | M = 10, F = 8 | 22.14±3.07 |
| Lemay, 2013 | 32 (32) | M = 25, F = 7 | 47.9±12.8 | 77.2±44.3 d | T = 21, NT = 11 | D = 32 | c = 17, t = 10. l = 5 | 0 | ||
| Tamburella, 2014 | 23 (23) | M = 14, F = 9 | 48.27±15.94 | 16.43±19.03 | T = 9, NT = 14 | D = 23 | c = 8, t = 9, l = 6 | 0 | ||
AIS: ASIA Impairment Scale; c: cervical; d: days; F: female; l: lumbar; LOI: level of injury; M: male; m: months; NR: not reported; NT: non-traumatic; SCI: Spinal Cord Injury; SD: standard deviation; T: traumatic; t: thoracic; t-l: thoraco-lumbar; TSI: Time Since Injury; w: weeks; y: years; *: SD not available; NA: not applicable.
Moreover, the studies focused on individuals for which a different TSI was elapsed. Many of the studies (N = 11) were conducted on chronic participants (Bishop et al., 2012; Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Harel et al., 2013; Khan et al., 2019; Labruyere & van Hedel, 2014; Martinez et al., 2018; Okawara et al., 2020; Piira et al., 2019; Tamburella et al., 2014; Wu et al., 2012). Three studies were conducted on a mixed population of individuals with both subacute and chronic SCI (Bach Baunsgaard et al., 2018; Hornby et al., 2005; Lemay & Nadeau, 2013), while one study involved a single individual with an acute SCI (Moreh et al., 2009). TSI information in the remaining 4 studies (Daunoraviciene et al., 2018; Font-Llagunes et al., 2020; Shin & Sosnoff, 2013; Wu et al., 2019) was not reported.
The sample size was variable among studies, ranging from 1 to 52. For details see Table 2. A total of 32 dropouts were reported by 7 studies (Bach Baunsgaard et al., 2018; Daunoraviciene et al., 2018; Khan et al., 2019; Martinez et al., 2018; Piira et al., 2019; Wu et al., 2012; 2019). Three studies reported adverse events, for a total of 81 among the participants (Bach Baunsgaard et al., 2018; Daunoraviciene et al., 2018; Khan et al., 2019) and 1 adverse event for a trainer (Khan et al., 2019). In 2 studies (Martinez et al., 2018; Piira et al., 2019) adverse events were reported without specifying the number of occurrences.
3.4Intervention
In this systematic review, 19 studies addressed the use of technological devices for balance rehabilitation (N = 15) (Bach Baunsgaard et al., 2018; Bishop et al., 2012; Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Daunoraviciene et al., 2018; Font-Llagunes et al., 2020; Hornby et al., 2005; Khan et al., 2019; Labruyere & van Hedel, 2014; Martinez et al., 2018; Moreh et al., 2009; Okawara et al., 2020; Piira et al., 2019; Wu et al., 2012; Wu et al., 2019) and assessment (N = 4) (Harel et al., 2013; Lemay & Nadeau, 2013; Shin & Sosnoff, 2013; Tamburella et al., 2014).
The technological devices used for balance rehabilitation were conveniently grouped into three main categories: Treadmill-Based Devices (TBD), OverGround Devices (OGD) and Tilt Table Devices (TTD) (see Fig. 4 and Table 3). It is worth specifying that this classification cannot be considered universally valid, but it was only introduced and adopted in the context of this work. This choice was due to the need of proposing a categorization of the specific devices presented in the papers that were retained based on our systematic search. Many other technological devices (robotics- and/or sensors-based), commercially available or present in the scientific literature, were not identified in selected studies and these systems may possibly fall outside the classification adopted here.
Table 3
Devices used addressed for each rehabilitative or assessment study
| Technology-assisted rehabilitative studies | ||
| Study | Device type | Device name |
| Chisholm, 2017 | TBD and OGD: Hip-knee guidance | Lokomat |
| Ekso | ||
| Covarrubias-Escudero, 2019 | TBD: No guidance | Cosmos treadmill+airwalk ap |
| Wu, 2019 | TBD: Pelvis guidance | Agility Trainer |
| Piira, 2019 | TBD: Hip-knee guidance | Lokomat |
| Labruyère, 2014 | TBD: Hip-knee guidance | Lokomat |
| Martinez, 2019 | TBD: Hip-knee guidance | Lokomat |
| Okawara, 2020 | TBD: Hip-knee guidance | HAL+Aeromill STM-1250+PneuWeight |
| Hornby, 2005 | TBD: Hip-knee guidance | Lokomat |
| Moreh, 2009 | TBD: Hip-knee guidance | Lokomat |
| Wu, 2012 | TBD: Lower leg guidance | CaLT: resistance modality (CaLT_R) and assistice modality (CaLT_A) |
| Khan, 2019 | OGD: Hip-knee guidance | ReWalk |
| Bach Baunsgaard, 2018 | OGD: Hip-knee guidance | Ekso |
| Bishop, 2012 | OGD: Knee guidance | Tibion Bionic Leg |
| Font-Llagunes, 2020 | OGD: Knee guidance | ABLE |
| Daunoraviciene, 2018 | TTD: Foot guidance | Erigo |
| Technology-assisted rehabilitative studies | ||
| Study | Device type | Device name |
| Harel, 2013 | Force plate | Long force plate of the Smart EquiTest CDP |
| Shin, 2013 | Force plate | AMTI |
| Lemay, 2013 | Force plate | Smart Balance Master |
| Tamburella, 2014 | Baropodometric plate | Platform BPM 120 |
CaLT: Cable-driven robotic Locomotor Trainer; HAL: Hybrid Assistive Limb; OGD: OverGround Devices; TBD: Treadmill-Based Devices; TTD: Tilt Table Devices.
In the following, additional details are provided:
• TBD: These devices are used to partially or totally guide walking over a treadmill also including a BWS system. We could identify four specific devices that we decided to categorize based on the guidance contributions, i.e. on the different body districts interested by the direct robotic intervention:
∘ No guidance: The user is totally free to walk on the treadmill with the only aid of the BWS. This is the case of the Cosmos treadmill combined with the Cosmos airwalk ap by Cosmed (Fig. 3a) (Covarrubias-Escudero et al., 2019).
∘ Pelvis guidance: The pelvis of the user is stabilized/supported by a cable-driven elastically actuated mechanism. This is reported in (Wu et al., 2019) where the Agility Trainer is presented (Fig. 3b). In (Wu et al., 2019) participants wore a trunk harness attached to a passive overhead safety support system. Lateral forces were created by using a pair of series-elastic linear motors and transmitted via cables to a pelvic harness.
∘ Hip-knee guidance: These systems include lower limb exoskeletons (also known as active robotic orthoses), i.e., wearable robots assisting directly with joint motion, typically during flexion/extension. The most widespread example of the hip-knee assistance is the Lokomat by Hocoma (Fig. 3c), which we found in (Chisholm et al., 2017; Hornby et al., 2005; Labruyere & van Hedel, 2014; Martinez et al., 2018; Moreh et al., 2009; Piira et al., 2019). The Lokomat has actuated hip and knee joints while passive foot lifters support ankle dorsiflexion during the swing phase. The trunk is connected by means of a harness to an active BWS. We could also find the case where the HAL, which was originally designed for overground walking, was used on a treadmill in combination with a BWS (Fig. 3d) (Okawara et al., 2020).
∘ Lower-leg guidance: In this case, the legs are moved by a cable-driven system thus avoiding the direct guidance of the joints and preferring an intervention on more distal limb segments. In the CaLT, Cable-driven Locomotor Trainer (Fig. 3e), used in (Wu et al., 2012), four cables, driven by motors through cable spools and pulleys, was connected to cuffs strapped to the legs around the ankles to deliver assistance/resistance forces.
• OGD: These systems include an exoskeleton assisting hip-knee motion similarly to what presented in the treadmill-based case. We found two systems suitable for hip-knee guidance, Ekso by Ekso Bionics (Fig. 3f) and ReWalk by ReWalk Robotics (Fig. 3g) (Bach Baunsgaard et al., 2018; Chisholm et al., 2017; Khan et al., 2019) also including passive springs at the ankle level, and two systems for knee guidance, ABLE (Fig. 3h) and Tibion Bionic Leg (Fig. 3i) (Bishop et al., 2012; Font-Llagunes et al., 2020). Ekso and Rewalk have a quite similar structures: they both use electrical motors to provide flexion/extension assistance at the hip and knee joints while including spring-loaded ankle joints for passive elastic support. Ekso delivers assistant as needed based on an algorithm able to estimate the physical demand of the user while ReWalk is simply position controlled. The Tibion Bionic Leg is a monolateral robotic knee orthosis providing user-initiated assistance to knee extension during weight-bearing activity as well as resistance in knee flexion.
• TTD: These systems normally are only used to re-position the body of the user at different inclinations while lying on a flat surface. In the example that we could find in (Daunoraviciene et al., 2018), the Erigo system (Fig. 3l) also includes additional functionality, i.e., a robotic stepping sub-system allowing the feet guidance on cyclic motion patterns. The Erigo can combine gradual verticalization with robotic leg movement and cyclic leg loading.
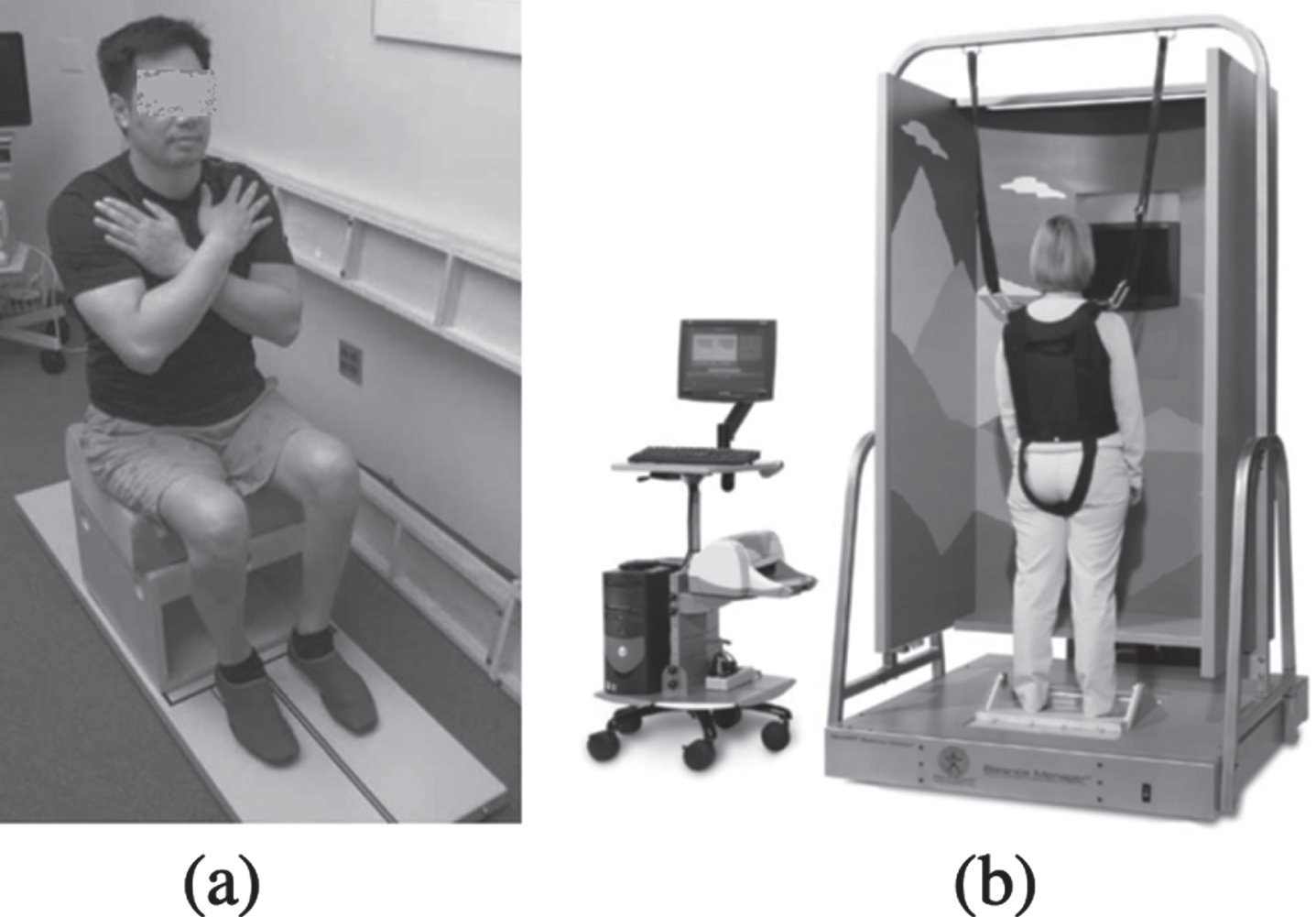
• The studies focusing on balance assessments were carried out by using different stabilometric platforms, in sitting (N = 2) (Harel et al., 2013; Shin & Sosnoff, 2013) and standing positions (N = 2) (Lemay & Nadeau, 2013; Tamburella et al., 2014). Force plates were used for the sitting balance assessment. In particular, the long force plate of the SMART EquiTest CDP by Neurocom was employed in (Harel et al., 2013) as depicted in Fig. 4a, while in (Shin & Sosnoff, 2013) participants sat with their arms by their side on a force platform by AMTI placed on a custom-built wooden box. For the standing position assessments, the Smart Balance Master by Neurocom (Fig. 4b) was used in (Lemay & Nadeau, 2013) while the BPM 120 baropodometric plate was used in (Tamburella et al., 2014).
Fig. 3
Devices used for balance rehabilitation in the studies reviewed within this paper. The devices were grouped into (a-e) TBD, (f-i) OGD and (l) TTD.
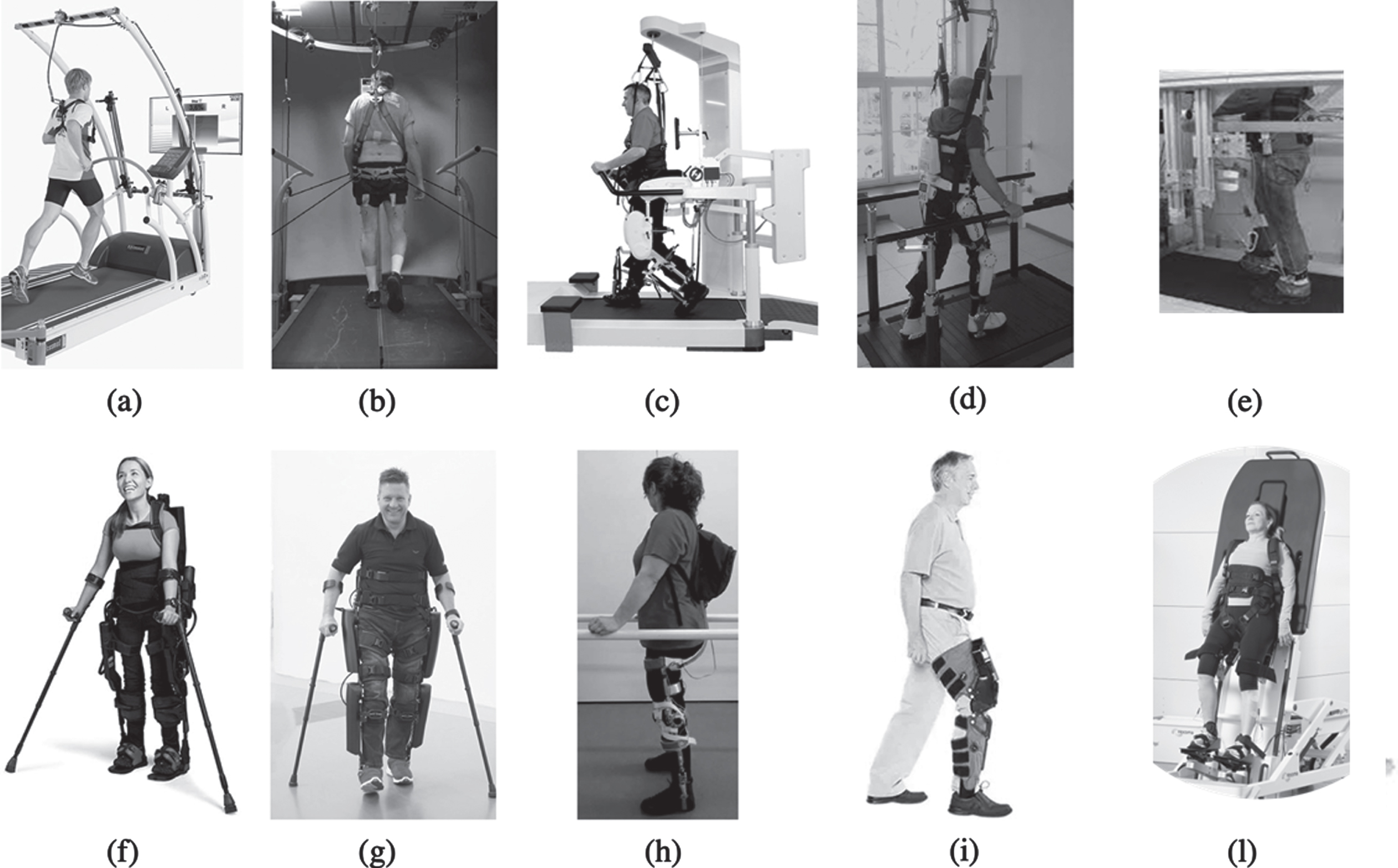
Fig. 4
(a) Force plate used in (Harel et al., 2013) (long force plate of the SMART EquiTest CDP by Neurocom); (b) Smart Balance Master by Neurocom used in (Lemay & Nadeau, 2013).
In these studies, that addressed the effects of technological devices on balance in individuals with SCI, the training protocols (number of sessions, frequency and duration) were heterogeneous and sometimes not reported (see Table 4). The total number of sessions ranged from 1 to 60 and the number of treatments per week ranged from 1 to 5. The duration of the treatment ranged from 20 to 60 minutes.
Table 4
Session therapy time frame and comparison of the included studies
| Technology-assisted rehabilitative studies | |||||||
| Device type | Session duration (minutes) | Frequency (session per week) | N of sessions | Duration (week) | Follow-up (months) | Comparison | |
| TBD and OGD | Chisholm, 2017 | 45 | 3–4 | 30 | NR | N | Ekso-Lokomat |
| Covarrubias-Escudero, 2019 | 22 | 3 | 18 | 6 w | N | N | |
| Wu, 2019 | NR | NR | 1 | NR | N | N | |
| Piira, 2019 | 48 | 3 | 24 | 8 w | N | UC | |
| Labruyère, 2014 | 45 | NR | 32 | 8 w | 6 | ST | |
| TBD | Martinez, 2019 | 30 | 3–5 | 48 | NR | 1.5 | MME |
| Okawara, 2020 | 60 | 2.6 | 20 | 8 w | N | N | |
| Hornby, 2005 | 21–30 | 1–3 | 39–60 | 16–20 w | N | N | |
| Moreh, 2009 | NR | 2 | 18 | 12 w | N | N | |
| Wu, 2012 | 45 | 3 | NR | 8 w | N | N | |
| Khan, 2019 | 60 | 3.7 | >40 | 12 w | 2–3 | N | |
| Bach Baunsgaard, 2018 | NR | 3 | 24 | 8 w | 1 | N | |
| OGD | Bishop, 2012 | 60 | NR | 7 | 2 w | N | N |
| Font-Llagunes, 2020 | 60 | NR | 7 | NR | N | Passive KAFO | |
| TTD | Daunoraviciene, 2018 | 20–40 | NR | 10 | NR | N | N |
| Technology-assisted assessment studies | |||||||
| Device type | Session duration (minutes) | Frequency (session per week) | N of sessions | Duration (week) | Follow-up (months) | Comparison | |
| Force plate | Harel, 2013 | NR | NR | NR | NR | N | NA |
| Shin, 2013 | NR | NR | NR | NR | N | NA | |
| Lemay, 2013 | 60 | NR | NR | NR | N | NA | |
| Baropodometric plate | |||||||
| Tamburella, 2014 | NR | NR | 1–12 | 1–24 w | N | NA | |
KAFO: knee ankle foot orthosis; m: months; MME: multi modal exercise; N: not in the study protocol; NA: not applicable; NR: not reported; ST: strength training; UC: usual care; w: weeks.
A total of 5 studies reported adverse events during training, even if no serious adverse event was reported. The most frequent adverse events were skin abrasions (Bach Baunsgaard et al., 2018; Khan et al., 2019; Martinez et al., 2018; Piira et al., 2019), pain (Bach Baunsgaard et al., 2018; Daunoraviciene et al., 2018) and various levels of ulceration (Bach Baunsgaard et al., 2018). Khan et al. (Khan et al., 2019) reported that two participants experienced a fall during training, but no injuries were caused to the participants thanks to the intervention of the trainer. However, the trainer reported some minor damages resulting from fall control episodes, such as minor muscle strain, sprain of the knee and bruised shin (Khan et al., 2019).
3.5Comparison
Considering the studies focused on balance rehabilitation, 5 out of 15 papers compared technological devices versus other interventions. Three studies (Labruyere & van Hedel, 2014; Martinez et al., 2018; Piira et al., 2019) compared TBD (i.e., Lokomat device) training effects versus different types of rehabilitation intervention, namely strength training (ST), multi modal exercises (MME) and usual care (UC). One out of 4 OGD studies (Font-Llagunes et al., 2020) focused on the comparison between ABLE and a passive knee ankle foot orthosis (KAFO). Lastly, a case report study combined into three different phases the use of a TBD (i.e., Lokomat device) and a OGD (i.e. Ekso device) (Chisholm et al., 2017) (see Table 4).
Follow-up assessments were performed by only 4 out of 19 studies (N = 2 for TBD; N = 2 for OGD) 1 month after the end of treatment (Bach Baunsgaard et al., 2018) or after 1.5 months (Martinez et al., 2018), 2–3 months (Khan et al., 2019) and 6 months (Labruyere & van Hedel, 2014) (see Table 4).
3.6Outcome measures
Only three studies evaluated balance as the primary outcome. Chisholm et al. (2017) focused on seated balance while Covarrubias-Escudero et al. (2019) and Wu et al. (2019) addressed standing balance with also a comparison with respect to healthy individuals. The remaining studies mainly focused on technological devices effects on gait, considering balance changes as secondary outcomes. Consequently, besides gait, other domains were addressed: balance, strength, spasticity, pain, cardiovascular parameters, QoL and ADL. Anyhow, we reported in the Appendix only data related to balance.
In the 15 studies based on technology-assisted rehabilitation, device effects on balance were analysed by means of clinical scales (N = 11) (Bach Baunsgaard et al., 2018; Bishop et al., 2012; Chisholm et al., 2017; Daunoraviciene et al., 2018; Hornby et al., 2005; Labruyere & van Hedel, 2014; Martinez et al., 2018; Moreh et al., 2009; Okawara et al., 2020; Piira et al., 2019; Wu et al., 2012) or instrumental assessment (N = 7) (Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Font-Llagunes et al., 2020; Khan et al., 2019; Labruyere & van Hedel, 2014; Martinez et al., 2018; Wu et al., 2019). Only 3 studies selected both clinical and instrumental assessments for balance analysis.
The most used clinical scale was the Berg Balance Scale (BBS; N = 9) (Bach Baunsgaard et al., 2018; Bishop et al., 2012; Daunoraviciene et al., 2018; Labruyere & van Hedel, 2014; Martinez et al., 2018; Moreh et al., 2009; Okawara et al., 2020; Piira et al., 2019; Wu et al., 2012) followed by the Timed Up and Go test (TUG; N = 4) (Bach Baunsgaard et al., 2018; Bishop et al., 2012; Hornby et al., 2005; Okawara et al., 2020) and the modified Functional Reach Test (mFRT; N = 2) (Chisholm et al., 2017; Piira et al., 2019). The Functional Reach Test (FRT) (Hornby et al., 2005), the Activities-Specific Balance Confidence scale (ABC) (Wu et al., 2019), the T-shirt test (Chisholm et al., 2017) and the Tinetti scale (Daunoraviciene et al., 2018) were used each one by single articles. For the instrumental evaluations, COP (N = 5) and COM (N = 2) data were considered. The COM data was analysed in terms of speed and lateral displacements by two studies (Font-Llagunes et al., 2020; Wu et al., 2019). Instead, the COP data was analysed in terms of Limits of Stability (LOS) (Chisholm et al., 2017; Khan et al., 2019), trajectory Root Mean Square (RMS) (Chisholm et al., 2017; Covarrubias-Escudero et al., 2019), lateral displacements (Labruyere & van Hedel, 2014), maximal excursion (Martinez et al., 2018), sway speed (Khan et al., 2019) and normalized Jerk (NJ).
For each of the 15 studies focused on technology-assisted rehabilitation we verified whether the Authors reported changes deriving from the devices usage and whether these variations were reported as statistically significant or not (see the Appendix). Therefore, we reported below the results if the Authors considered them statistically significant (p < 0.05). Six studies did not report significant changes in any balance outcome addressed (N = 1: OGD and TBD; N = 3: TBD hip-knee guidance; N = 2: OGD knee guidance).
For each one of the different TMD categories at least one study with significant changes due to training was identified. The single studies for no guidance and pelvis guidance TMD are those primarily focused on balance and the only ones with a healthy control group. For the device with no guidance (Cosmos treadmill) (Covarrubias-Escudero et al., 2019) 6 weeks of training allowed COP NJ reduction during standing balance assessment in individuals with AIS C SCI. Furthermore, results reported that COP RMS and COP NJ data were significantly different between healthy controls and individuals with SCI, particularly before the training with no guidance device. The single study focused on pelvis guidance device (Agility Trainer) (Wu et al., 2019) reported that individuals with SCI displayed a large COM speed and lateral excursion variability in response to perturbations during gait but these data reduced with practice. Contrarily, for the enrolled healthy individuals the pattern was reversed: COM excursion increased with practice. The hip-knee guidance devices (HAL and Lokomat) allowed balance changes after training. BBS score and TUG performance improved after 2 months of HAL usage. Lokomat device was tested in studies with or without comparison groups. Nevertheless, statistically significant results were reported only in the second case (N = 2). In particular, Piira et al. (2019) found a significant BBS enhancement after 2 months of Lokomat training or UC training, but the mFRT improved only after robotic training. On the contrary, Labruyere et al. (Labruyere & van Hedel, 2014) showed BBS improvements after 2 months of Lokomat training or ST training but it reached significance only for non-robotic training. The only study that included the use of a lower leg guidance device (i.e., CaLT) (Wu et al., 2012) reported a statistically significant improvement of BBS and ABC scores after 2 months of training.
The training with OGDs allowed statistically significant effects on balance only in the case of hip-knee guidance, namely in the case of Ekso and ReWalk devices usage. For the first device, individuals with SCI improved BBS score and TUG performance after two months of training, and this improvement was maintained also at the follow-up assessment (Bach Baunsgaard et al., 2018). For the latter study (Khan et al., 2019), 3 months of ReWalk device usage led to COP LOS and sway speed improvements. However, the comparison with post-training data and follow-up assessment did not show statistically significant results.
For the TTD category (Erigo device), the improvement of postural parameters in a post-acute population of both stroke and individuals with SCI was targeted. Ten sessions of training allowed statistically significant improvements in both BBS and Tinetti scale scores for stroke subjects, while only BBS enhancement was reported for individuals with SCI.
The 4 studies focusing on balance assessments addressed sitting (N = 2) (Harel et al., 2013; Shin & Sosnoff, 2013) and standing balance (N = 2) (Lemay & Nadeau, 2013; Tamburella et al., 2014). The BBS (Harel et al., 2013; Lemay & Nadeau, 2013) and the mFRT (Shin & Sosnoff, 2013) were the most used clinical outcome measures and only one study selected the Tinetti scale (Tamburella et al., 2014). For the instrumental assessments, Centre of Gravity (COG) LOS and COP data were used in two studies (Harel et al., 2013; Lemay & Nadeau, 2013). Furthermore, two studies also used the Clinical Test of Sensory Interaction on Balance (CTSIB) data or weight shifting tests.
In the two studies on seated postural control (Harel et al., 2013; Shin & Sosnoff, 2013), force plates were used and individuals with thoracic or lumbar lesion and chronic or NR TSI were enrolled. For both studies, data of impaired individuals were compared to healthy subjects’ performance with clinical and instrumental assessments. Harel et al. (2013) demonstrated that individuals with SCI presented a balance worse than healthy subjects’ one. In particular, the differences between the two groups were statistically significant for mFRT and LOS assessments but not for BBS and CTSIB. Furthermore, the Authors demonstrated that a subtest of LOS assessment (i.e., directional control) presented a statistically significant correlation with the lesion level. The second study, focusing on seated balance (Shin & Sosnoff, 2013), compared healthy subjects and individuals with SCI classified into two groups according to the lesion level: SCI at/above T10 and SCI at/below T11. Data analysis of instability index and COP Virtual Time to Contact (VTC) revealed that healthy individuals presented better balance control than SCI individuals and that the higher the lesion level, the worse is the balance control. However, functional boundary data and mFRT results indicated significant differences only in the comparison between healthy controls and individuals with high lesion levels.
Instead, the two studies focusing on standing balance assessment both included AIS D individuals with cervical, thoracic, or lumbar lesions with no comparison with healthy individuals. In Lemay et al. (Lemay & Nadeau, 2013) no statistically significant differences were pointed out between paraplegic and tetraplegic individuals considering BBS, LOS, static or weight shifting tests. Nevertheless, correlation analyses with BBS scores revealed that LOS, static or weight shifting tests were valid tools to assess balance performance in individuals with SCI. Moreover, Tamburella et al. (2014) demonstrated that, among COP parameters, velocity and trajectory length had the highest reliability, validity, and effectiveness. Furthermore, Authors found that when a stabilometric test is proposed to individuals with SCI, the testing condition with open eyes and open feet was the most valid one, whereas the testing condition with closed eyes with and open feet was the most responsive one.
4Discussion
This systematic review aimed to provide an overview of the available literature on the clinical usefulness of technological devices for balance assessment and rehabilitation in individuals with SCI. Balance is a crucial function for basic ADL and consequently for regaining the independence and improving QoL, and it is strictly interwoven with the recovery process of gait (Wirz & van Hedel, 2018). Moreover, from the patients’ viewpoint the recovery of balance is considered of primary impact on the functional capabilities after SCI (Anderson, 2004) and hence is often one of the main goals of rehabilitation programs (Caltagirone C., 2021).
Over the past two decades, technological devices have initially been developed to support traditional balance training (Nam et al., 2017) allowing individuals with SCI to improve motor functions and clinicians to monitor patients’ performance (Bishop et al., 2012; Hornby et al., 2005). Some of the available technologies allow individuals with SCI and physical therapists to benefit from different types of feedback information. In particular, the feedback for patients is extremely effective to maximize and/or compensate for remaining somatosensory information and in turn to optimize balance rehabilitation (Noamani et al., 2021).
Results of this review indicated that 15 out of 19 studies focused on technology-assisted rehabilitation, and only three of them (Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Wu et al., 2019) aimed to primarily assess the effects on balance. One study (Chisholm et al., 2017) addressed seated balance of individuals with motor complete SCI while two studies focused on standing balance of individuals with incomplete SCI. The other studies considered the balance rehabilitation as a side effect of gait training. Devices reported in the selected studies were TBDs and OGDs, with different guidance contribution, and TTDs. These systems were originally developed for walking or early verticalization and no devices specifically developed for balance rehabilitation were found based on the selected search criteria. These two observations demonstrated an apparent limited interest in the technology-assisted rehabilitation directly devoted to balance-oriented goals and seem to suggest a slight priority towards walking. Nonetheless, an interest in a direct balance assessment was found in the investigations of four studies, in which force or stabilometric plates were adopted.
Seventeen out of nineteen studies were case reports and only two were RCTs. In addition, a follow up evaluation was carried out only in four out of nineteen studies (Bach Baunsgaard et al., 2018; Khan et al., 2019; Labruyere & van Hedel, 2014; Martinez et al., 2018), with a wide range of time (from 1 to 6 months). Protocols were quite heterogeneous and each single treatment session lasted a minimum of 20 minutes up to a maximum of 60 minutes (Bishop et al., 2012; Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Daunoraviciene et al., 2018; Font-Llagunes et al., 2020; Hornby et al., 2005; Khan et al., 2019; Labruyere & van Hedel, 2014; Martinez et al., 2018; Okawara et al., 2020; Piira et al., 2019; Wu et al., 2012), with an average frequency of three times per week (Bach Baunsgaard et al., 2018; Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Hornby et al., 2005; Khan et al., 2019; Martinez et al., 2018; Moreh et al., 2009; Okawara et al., 2020; Piira et al., 2019; Wu et al., 2012). Great variability in the total number of sessions was reported, with a minimum of 5 up to 60. The total duration of the training ranged from 2 to 20 weeks, but many studies have limited the treatment duration to 8 weeks (Bach Baunsgaard et al., 2018; Labruyere & van Hedel, 2014; Okawara et al., 2020; Piira et al., 2019; Wu et al., 2012).
Furthermore, according to the D&B tool no studies were considered to have good methodological quality; most of the studies received a score of moderate (N = 15) and a few of them were scored as poor (N = 4). Limitations in the number, strength, homogeneity and quality of these studies indicate the need for further investigations and for more solid clinical protocols. The same observation can be drawn based on the reduced number of participants involved in the studies.
The age range of the involved participants was 25–65 years (Male = 80%), which is a common age range related to fall risk in patients with SCI. Since the loss of balance is considered a contributor to falls (Khan et al., 2019), the relationship between technology-assisted rehabilitation and falls after SCI is an interesting factor to be considered. However, none of the studies included in this review addressed this relationship.
Maintaining postural stability is a major challenge for individuals with incomplete SCI when they regain the ability to walk. The literature indicates that up to 75% of them experienced falls, with consequent physical injuries, decreased social involvement and developing fear of falling. These individuals considered the loss of balance as the most significant cause of their falls, implying that effective balance control is required to develop fall prevention strategies (Noamani et al., 2021). A deeper understanding of technological devices usage effects on balance rehabilitation, may clarify if direct or indirect balance training can positively affect these aspects. It could be potentially interesting to assess fall-related aspects at the follow-up examinations to understand if technological rehabilitation may reduce the number of falls even after training end.
Despite spontaneous recovery after SCI has been documented (Tamburella et al., 2019) in the subacute phase, it could be intriguing to investigate possible booster effects from the use of technological devices. However, we noticed a lack of evidence about the effects on a subacute population since the individuals analysed in the studies were mainly affected by traumatic chronic and motor incomplete lesions.
In the context of balance rehabilitation, it is interesting that only a single study, focused on ReWalk device, reported fall episodes. Nevertheless, they were controlled by the trainer and no injury occurred to participants. This suggests the crucial need for the constant presence of an expert trainer.
Despite many devices exist for balance-oriented rehabilitation, we did not find studies directly focusing on assessing their effectiveness in SCI population and in the reviewed studies different technological devices were identified and conventionally split into 3 main categories (TBD, OGD and TTD), taking into account different types of guidance.
The most used device was the Lokomat, whit all the others having a maximum of one study each. Moreover, for the few studies that included control group comparison, the types of control interventions were different. Consequently, from this set of data, it is not possible to draw general considerations and derive final conclusions about the possible effects of the use of technological devices on the recovery of balance in individuals with SCI. Only three studies targeted balance as the primary goal and two of them reported statistically significant data related to TBDs usage. It is interesting that, also, studies targeting the recovery of ambulation as a primary outcome reported statistically significant results. Different effects were noted in the three main technology categories. Among TBDs statistically, significant data are available for studies that included a population in the chronic phase with cervical or thoracic SCI classified mainly as a motor incomplete lesion. A training with variable duration (6–8 weeks) with Cosmos treadmill, Agility Trainer, HAL or CaLT allowed individuals with SCI to improve balance performance and the ability to recover from external perturbations with practice. Two studies with the Lokomat device focused on possible benefits of a 2-month training compared to ST and UC (Labruyere & van Hedel, 2014; Piira et al., 2019). In the first case (Labruyere & van Hedel, 2014) balance improved after ST training, while in the second case (Piira et al., 2019) balance enhancement (in terms of BBS score) was found after Lokomat device or UC trainings. Only for individuals who underwent Lokomat training also mFRT improved. This data indicates that technology-assisted training can indirectly improve balance after SCI, but in the case of results compared with control groups, the benefits are not greater than those from UC or muscle strength treatment. In the case of OGDs, both studies focused on hip-knee guidance devices reported balance improvements in a browed set of individuals whit different lesion severity (AIS A, B, C and D) and level (cervical, thoracic, and lumbar) after 3 months of ReWalk training or after 2 months of Ekso device training. The single study on TTD indicated that Erigo was safe and effective at improving postural control and orthostatic tolerance in individuals with thoraco-lumbar SCI (classified as AIS B or C) (Daunoraviciene et al., 2018). These results, obtained in single studies for each device, suggest the need to carry out more in-depth protocols on this topic, including both direct and indirect balance rehabilitation. Future investigations could clarify whether technology can influence balance recovery and its specific role within in the rehabilitation process for individuals with SCI.
Most of the selected studies focused on rehabilitation (N = 15) (Bach Baunsgaard et al., 2018; Bishop et al., 2012; Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Daunoraviciene et al., 2018; Font-Llagunes et al., 2020; Hornby et al., 2005; Khan et al., 2019; Labruyere & van Hedel, 2014; Martinez et al., 2018; Moreh et al., 2009; Okawara et al., 2020; Piira et al., 2019; Wu et al., 2012; 2019) rather than on the assessment (N = 4) (Harel et al., 2013; Lemay & Nadeau, 2013; Shin & Sosnoff, 2013; Tamburella et al., 2014). This observation is consistent with the clinical practice, during which rehabilitators are typically interested in technologies to restore impaired functions. Indeed, in the clinical routine the use of technological devices for the purpose of assessing patients’ performance is quite sporadic. The results of this review indicate that training efficacy was measured with both clinical scales and instrumental evaluations. The clinical scales are the most widespread because they are easy to use, even if they provide subjective assessment. In particular, the BBS is considered the gold standard for balance assessment in individuals with SCI (Lemay & Nadeau, 2010) and it is not surprising that was the most frequent clinical scale adopted in the analysed studies.
Instrumental assessment focused on seated or standing balance depending on the lesion severity and residual functional abilities of the individuals. The individuals with an incomplete SCI generally have a more stable sitting balance than those with motor complete paraplegia. Indeed, in these individuals balance becomes challenging only in more demanding postural positions, like standing. The three studies focusing on rehabilitation (Chisholm et al., 2017; Covarrubias-Escudero et al., 2019; Wu et al., 2019) aimed primarily to evaluate balance instrumental assessments based on COP or COM measurements. In the case of complete SCI, the evaluation was performed in a sitting position, while for individuals with incomplete SCI the standing position was adopted. Furthermore, for the studies based on the assessment, the two studies on seated balance (Harel et al., 2013; Shin & Sosnoff, 2013) were conducted on individuals with thoraco-lumbar lesion, and mainly AIS A SCI, while the two studies on standing balance focused on a population with AIS D SCI, including cervical, thoracic and lumbar lesions. The impact on the balance of somatosensory deficit was further addressed by testing COP stability under different sensory conditions, such as eyes open or closed. The two studies (Lemay & Nadeau, 2013; Tamburella et al., 2014) focused on standing balance reported that the balance assessment can be reliable, valid, and effective when acquiring COP velocity data, based on OF-OE and OF-CE conditions and that the LOS test should be considered as a complementary tool with respect to the BBS. In case of complete SCI, the seated posturography may represent an appealing outcome measure to evaluate the effectiveness of rehabilitation and technology usage aimed at improving seated postural control. These studies also indicate that LOS and VTC tests may represent a valid alternative to conventionally applied clinical balance tests to quantify postural instability. Despite these encouraging indications from the literature on the potentialities of instrumental balance assessments, protocols focusing on rehabilitation only rarely include quantitative measurements to objectify variations resulting from rehabilitation trainings.
4.1Limitations
The small number of studies included in this review, in combination with the small sample of individuals enrolled, the heterogeneity of technologies and protocols and the low or moderate methodological quality (only 2 RCTs) highlighted the limitations in the currently available evidence on the effectiveness of technology-assisted balance training in people with SCI suggesting the impossibility of carrying out a meta-analysis. Moreover, the reduced number of follow up evaluations does not allow to determine whether technology-assisted rehabilitation induces possible persistent changes.
5Conclusion
Although different studies reported positive effects, improvements due to technology-assisted rehabilitation were not greater than those obtained by means of other rehabilitation therapies. The low methodological quality, heterogeneity and the small number of the studies included does not allow general conclusions about the usefulness of technology-assisted balance assessment and training in individuals with SCI. Further well-designed RCTs evaluating the use of devices developed for balance assessment and rehabilitation are needed, focusing also on the missing population (e.g., sub-acute one). Finally, in the context of technology-assisted rehabilitation for the individual with SCI, a relationship between balance and gait has not been clearly explained to date.
Conflict of interest
None of the authors disclose any financial or personal relationships with other people or organizations that could inappropriately influence the work. The authors declare no conflict of interest.
Funding
Financial support was provided by the Italian Ministry of Health (Ricerca Corrente) and by GR-2019-12369207 project INTER-RO-GAIT (Patient-therapist INTERaction during RObotic GAIT rehabilitation after Spinal Cord Injury: Clinical, instrumental and hyperscanning study).
Supplementary materials
[1] The Appendix is available from https://dx.doi.org/10.3233/NRE-220060.
References
1 | Anderson, K. D. ((2004) ) Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma, 21: (10), 1371–1383. https://doi.org/10.1089/neu.2004.21.1371. |
2 | Bach Baunsgaard C. , Vig Nissen U. , Katrin Brust A. , Frotzler, A. , Ribeill, C. , Kalke, Y. B. , Leon, N. , Gomez, B. , Samuelsson, K. , Antepohl, W. , Holmstrom, U. , Marklund, N. , Glott, T. , Opheim, A. , Benito, J. , Murillo, N. , Nachtegaal, J. , Faber, W. , Biering-Sorensen, F. ((2018) ) Gait training after spinal cord injury: Safety, feasibility and gait function following 8 weeks of 1training with the exoskeletons from Ekso Bionics. Spinal Cord, 56: (2), 1371–1383. https://doi.org/10.1038/s41393-017-0013-7. |
3 | Bishop, L. , Stein, J. , Wong, C. K. ((2012) ) Robot-aided gait training in an individual with chronic spinal cord injury: A case study. J Neurol Phys Ther, 36: (3), 138–143. https://doi.org/10.1097/NPT.0b013e3182624c87. |
4 | Caltagirone C.P.F. , Imbriani P. (2021). Handbook of neurorehabilitation and principles of neurology (Vol. Therapeutic strategies and innovative rehabilitation approaches). |
5 | Chisholm, A. E. , Alamro, R. A. , Williams, A. M. , Lam, T. ((2017) ) Overground vs. treadmill-based robotic gait training to improve seated balance in people with motor-complete spinal cord injury: A case report. J Neuroeng Rehabil, 14: (1), 27. https://doi.org/10.1186/s12984-017-0236-z. |
6 | Covarrubias-Escudero, F. , Rivera-Lillo, G. , Torres-Castro, R. , Varas-Diaz, G. ((2019) ) Effects of body weight-support treadmill training on postural sway and gait independence in patients with chronic spinal cord injury. J Spinal Cord Med, 42: (1), 57–64. https://doi.org/10.1080/10790268.2017.1389676. |
7 | Daunoraviciene, K. , Adomaviciene, A. , Svirskis, D. , Griskevicius, J. , Juocevicius, A. ((2018) ) Necessity of early-stage verticalization in patients with brain and spinal cord injuries: Preliminary study, Technol Health Care, 26: (S2), 613–623https://doi.org/10.3233/THC-182508. |
8 | Downs, S. H. , Black, N. ((1998) ) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions [Article]., Journal of Epidemiology and Community 1067 Health, 52: (6), 377–384, https://doi.org/10.1136/jech.52.6.377. |
9 | Horak,F.B. ((2006) ). Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing, 35. |
10 | Font-Llagunes, J. M. , Lugris, U. , Clos, D. , Javier Alonso F. , Cuadrado, J. ((2020) ) Design, control, and pilot study of a lightweight and modular robotic exoskeleton for walking assistance after spinal cord injury [Article]. Journal of Mechanisms and Robotics, 12: (3), Article 031008. https://doi.org/10.1115/1.4045510. |
11 | Frey, M. , Colombo, G. , Vaglio, M. , Bucher, R. , Jörg, M. , Riener, R. ((2006) ) A novel mechatronic body weight support system [Article]. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 14: (3), 311–321. https://doi.org/10.1109/TNSRE.2006.881556. |
12 | Harel, N. Y. , Asselin, P. K. , Fineberg, D. B. , Pisano, T. J. , Bauman, W. A. , Spungen, A. M. ((2013) ) Adaptation of computerized posturography to assess seated balance in persons with spinal cord injury. J Spinal Cord Med, 36: (2), 127–133. https://doi.org/10.1179/2045772312Y.0000000053. |
13 | Hidler, J. , Brennan, D. , Black, I. , Nichols, D. , Brady, K. , Nef, T. ((2011) ) ZeroG: Overground gait and balance training system [Article]. Journal of Rehabilitation Research and Development, 48: (4), 287–298. https://doi.org/10.1682/JRRD.2010.05.0098. |
14 | Hof, A. L. , G M. G. J. , Sinke, W. E. (2005). The condition for dynamic stability. Journal of Biomechanics, 38, 1-8. |
15 | Hornby, T. G. , Zemon, D. H. , Campbell, D. ((2005) ) Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther, 85: (1), 52–66. https://www.ncbi.nlm.nih.gov/pubmed/15623362. |
16 | Ilha, J. , Abou, L. , Romanini, F. , Dall Pai A. C. , Mochizuki, L. ((2020) ) Postural control and the influence of the extent of thigh support on dynamic sitting balance among individuals with thoracic spinal cord injury [Article]. Clinical Biomechanics, 73: , 108–114. https://doi.org/10.1016/j.clinbiomech.2020.01.012. |
17 | Kalron, A. , Fonkatz, I. , Frid, L. , Baransi, H. , Achiron, A. ((2016) ) The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: A pilot randomized controlled trial [Article]. Journal of NeuroEngineering and Rehabilitation, 13: (1), Article 124. https://doi.org/10.1186/s12984-016-0124-y. |
18 | Khan, A. S. , Livingstone, D. C. , Hurd, C. L. , Duchcherer, J. , Misiaszek, J. E. , Gorassini, M. A. , Manns, P. J. , Yang, J. F. ((2019) ) Retrainingwalking over ground in a powered exoskeleton after spinal cord injury:Aprospective cohort study to examine functional gains and neuroplasticity. J Neuroeng Rehabil, 16: (1), 145. https://doi.org/10.1186/s12984-019-0585-x. |
19 | Labruyere, R. , van Hedel H. J. ((2014) ) Strength training versus robot-assisted gait training after incomplete spinal cord injury: A randomized pilot study in patients depending on walking assistance. J Neuroeng Rehabil, 11: , 4. https://doi.org/10.1186/1743-0003-11-4. |
20 | Lemay, J. F. , Nadeau, S. ((2010) ) Standing balance assessment in ASIA D paraplegic and tetraplegic participants: Concurrent validity of the Berg Balance Scale. Spinal Cord, 48: (3), 245–250. https://doi.org/10.1038/sc.2009.119. |
21 | Lemay, J. F. , Nadeau, S. ((2013) ) Potential of the smart balance master system to assess standing balance in people with incomplete spinal cord injury. J Rehabil Med, 45: (1), 55–60. https://doi.org/10.2340/16501977-1067. |
22 | Martinez, S. A. , Nguyen, N. D. , Bailey, E. , Doyle-Green, D. , Hauser, H. A. , Handrakis, J. P. , Knezevic, S. , Marett, C. , Weinman, J. , Romero, A. F. , Santiago, T. M. , Yang, A. H. , Yung, L. , Asselin, P. K. , Weir, J. P. , Kornfeld, S. D. , Bauman, W. A. , Spungen, A. M. , Harel, N. Y. ((2018) ) Multimodal cortical and subcortical exercise compared with treadmill training for spinal cord injury [Article]. PLoS ONE, 13: (8), Article e0202130. https://doi.org/10.1371/journal.pone.0202130. |
23 | Meyer-Heim, A. , van Hedel H. J. A. ((2013) ) Robotassisted and computer-enhanced therapies for children with cerebral palsy: Current state and clinical implementation [Article]. Seminars in Pediatric Neurology, 20: (2), 139–145. https://doi.org/10.1016/j.spen.2013.06.006. |
24 | Meyer G.A.M. , ((2006) ) Biomechanical aspects of dynamic stability. European Review of Aging and Physical Activity, 3: , 29–33. |
25 | Moreh, E. , Meiner, Z. , Neeb, M. , Hiller, N. , Schwartz, I. ((2009) ) Spinal decompression sickness presenting as partial brown-sequard syndrome and treated with robotic assisted body-weight support treadmill training [Article]. Journal of Rehabilitation Medicine, 41: (1), 88–89. https://doi.org/10.2340/16501977-0279. |
26 | Nam, K. Y. , Kim, H. J. , Kwon, B. S. , Park, J. W. , Lee, H. J. , Yoo, A. ((2017) ) Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J Neuroeng Rehabil, 14: (1), 24. https://doi.org/10.1186/s12984-017-0232-3. |
27 | Noamani, A. , Lemay, J. F. , Musselman, K. E. , Rouhani, H. ((2021) ) Characterization of standing balance after incomplete spinal cord injury: Alteration in integration of sensory information in ambulatory individuals. Gait Posture, 83: , 152–159. https://doi.org/10.1016/j.gaitpost.2020.10.027. |
28 | Okawara, H. , Sawada, T. , Matsubayashi, K. , Sugai, K. , Tsuji, O. , Nagoshi, N. , Matsumoto, M. , Nakamura, M. ((2020) ) Gait ability required to achieve therapeutic effect in gait and balance function with the voluntary driven exoskeleton in patients with chronic spinal cord injury:Aclinical study. Spinal Cord, 58: (5), 520–527. https://doi.org/10.1038/s41393-019-0403-0. |
29 | Olensek, A. , Oblak, J. , Cikajlo, I. , Novak, P. , Jere, K. , Matjacic, Z. (2012) Adaptive dynamic balance training during overground walking with assistive device. Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics. |
30 | Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hrobjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo-Wilson, E. , McDonald, S. , Moher D. ((2021) ) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372: , n71. https://doi.org/10.1136/bmj.n71. |
31 | Peterka,R.J. ((2018) ). Sensory integration for human balance control Handb Clin Neurol, 159: , 27–42. |
32 | Piira, A. , Lannem, A. M. , Sørensen M. , Glott, T. , Knutsen, R. , Jørgensen L. , Gjesdal, K. , Hjeltnes, N. , Knutsen, S. F. ((2019) ) Robot-assisted locomotor training did not improve walking function in patients with chronic incomplete spinal cord injury: A randomized clinical trial [Article]. Journal of Rehabilitation Medicine, 51: (5), 385–389. https://doi.org/10.2340/16501977-2547. |
33 | Pollock, A. S. , Durward, B. R. , Rowe, P. J. , Paul, J. P.. ((2000) ) What is balance? Clinical rehabilitation, 14: (4), 402–406. |
34 | R.J, P. ((2018) ) Sensory integration for human balance control Handb Clin Neurol, 159: , 27–42. |
35 | Saunders, L. D. , Soomro, G. M. , Buckingham, J. , Jamtvedt, G. , Raina, P. ((2003) ) Assessing the methodological quality of nonrandomized intervention studies. West J Nurs Res, 25: (2), 223–237. https://doi.org/10.1177/0193945902250039. |
36 | Scivoletto G. , R. A. , Mariotti A. , Marinucci D. , Tamburella F. , Mammone A. , Cosentino E. , Sterzi S. , Molinari M. ((2008) ) Clinical factors that affect walking level and performance in chronic spinal cord lesion patients. Spine, 33 n. 3, 259–264. |
37 | Shin, J. C. , Kim, J. Y. , Park, H. K. , Kim, N. Y. ((2014) ) Effect of robotic-assisted gait training in patients with incomplete spinal cord injury. Ann Rehabil Med, 38: (6), 719–725. https://doi.org/10.5535/arm.2014.38.6.719. |
38 | Shin, S. , Sosnoff, J. J. ((2013) ) Spinal cord injury and time to instability in seated posture. Arch Phys Med Rehabil, 94: (8), 1615–1620. https://doi.org/10.1016/j.apmr.2013.02.008. |
39 | Shirota, C. , van Asseldonk E. , Matjacic, Z. , Vallery, H. , Barralon, P. , Maggioni, S. , Buurke, J. H. , Veneman, J. F. ((2017) ) Robot supported assessment of balance in standing and walking. J Neuroeng Rehabil, 14: (1), 80. https://doi.org/10.1186/s12984-017-0273-7. |
40 | Singh, H. , Unger, J. , Zariffa, J. , Pakosh, M. , Jaglal, S. , Craven, B. C. , Musselman, K. E. ((2018) ) Robot-assisted upper extremity rehabilitation for cervical spinal cord injuries: A systematic scoping review. Disabil Rehabil Assist Technol, 13: (7), 704–715. https://doi.org/10.1080/17483107.2018.1425747. |
41 | Tamburella, F. , Moreno, J. C. , Herrera Valenzuela D. S. , Pisotta, I. , Iosa, M. , Cincotti, F. , Mattia, D. , Pons, J. L. , Molinari, M. ((2019) ) Influences of the 1219 biofeedback content on robotic post-stroke gait rehabilitation: Electromyographic vs joint torque biofeedback. J Neuroeng Rehabil, 16: (1), 95. https://doi.org/10.1186/s12984-019-0558-0. |
42 | Tamburella, F. , Scivoletto, G. , Iosa, M. , Molinari, M. ((2014) ) Reliability, validity, and effectiveness of center of pressure parameters in assessing stabilometric platform in subjects with incomplete spinal cord injury: A serial cross-sectional study. J Neuroeng Rehabil, 11: , 86. https://doi.org/10.1186/1743-0003-11-86. |
43 | Tamburella, F. , Scivoletto, G. , Molinari, M. ((2013) ) Balance training improves static stability and gait in chronic incomplete spinal cord injury subjects: A pilot study. Eur J Phys Rehabil Med, 49: (3), 353–364. https://www.ncbi.nlm.nih.gov/pubmed/23486301. |
44 | Tramontano, M. , Dell’Uomo D. , Cinnera, A. M. , Luciani, C. , Di Lorenzo C. , Marcotulli, M. , Vona, F. , Mercuro, A. , Abbruzzese, S. ((2019) a) Visual-spatial training in patients with sub-acute stroke without neglect: A randomized, single-blind controlled trial. Funct Neurol, 34: (1), 7–13. |
45 | Tramontano,M., Piermaria,J., Morone,G., Reali,A., Vergara,M., & Tamburella,F. ((2019) b). Postural Changes During Exteroceptive Thin Plantar Stimulation: The Effect of Prolonged Use and Different Plantar Localizations. Frontiers in Systems Neuroscience, 13: , 49. https://doi.org/10.3389/fnsys.2019.00049 |
46 | Van Asseldonk E. H. F. , C.M G. , Van der Helm F. C. T. , Van der Kooij H. ((2007) ) Use of induced acceleration to quantify the (De)stabilization effect of external and internal forces on postural responses. IEEE Transactions on Biomedical Engineering, 54: (12), 2284–2295. |
47 | Wirz, M. , van Hedel H. J. A. ((2018) ) Balance, gait, and falls in spinal cord injury. Handb Clin Neurol, 159: , 367–384. https://doi.org/10.1016/B978-0-444-63916-5.00024-0. |
48 | Wu, M. , Landry, J. M. , Schmit, B. D. , Hornby, T. G. , Yen, S. C. ((2012) ) Robotic resistance treadmill training improves locomotor function in human spinal cord injury: A pilot study. Arch Phys Med Rehabil, 93: (5), 782–789. https://doi.org/10.1016/j.apmr.2011.12.018. |
49 | Wu, M. M. , Brown, G. L. , Kim, K. A. , Kim, J. , Gordon, K. E. ((2019) ) Gait variability following abrupt removal of external stabilization decreases with practice in incomplete spinal cord injury but increases in non-impaired individuals. J Neuroeng Rehabil, 16: (1), 4. https://doi.org/10.1186/s12984-018-0475-7. |




