Recent trends in telerehabilitation of stroke patients: A narrative review
Abstract
BACKGROUND:
Stroke is the main reason for disabilities worldwide leading to motor dysfunction, spatial neglect and cognitive problems, aphasia, and other speech-language pathologies, reducing the life quality. To overcome disabilities, telerehabilitation (TR) has been recently introduced.
OBJECTIVE:
The aim of this review was to analyze current TR approaches for stroke patients’ recovery.
METHODS:
We searched 6 online databases from January 2018 to October 2021, and included 70 research and review papers in the review. We analyzed TR of 995 individuals, which was delivered synchronously and asynchronously.
RESULTS:
Findings show TR is feasible improving motor function, cognition, speech, and language communication among stroke patients. However, the dose of TR sessions varied significantly. We identified the following limitations: lack of equipment, software, and space for home-based exercises, insufficient internet capacity and speed, unavailability to provide hands on guidance, low digital proficiency and education, high cognitive demand, small samples, data heterogeneity, and no economic evaluation.
CONCLUSIONS:
The review shows TR is superior or similar to conventional rehabilitation in clinical outcomes and is used as complementary therapy or as alternative treatments. More importantly, TR provides access to rehabilitation services of a large number of patients with immobility, living in remote areas, and during COVID-19 pandemic or similar events.
1Introduction
Stoke is the main reason for disabilities worldwide leading to prolonged functional deficits of upper and low extremities, spatial neglect, cognitive problems, aphasia and other speech pathologies, significantly reducing the quality of life despite the age of stroke survivors (Donkor, 2018; Joosup Kim et al., 2020; World Health Organization, 2011). To overcome disabilities they undergo routine conventional rehabilitation (CR), although, the results are often insufficient and unmeet the expectations of patients, their relatives, and clinicians (Johnson, Onuma, Owolabi, & Sachdev, 2016; Stinear, Lang, Zeiler, & Byblow, 2020). Moreover, CR is typically carried out for a long time at rehabilitation facilities, increasing the workload of therapists and associate healthcare costs (Bayley et al., 2012; Magwood et al., 2019). Furthermore, not all post-stroke patients access CR services due to immobility, remote living, lack of in-clinic bed space, clinicians shortage or unaffordability (Enderby et al., 2017; Kalavina, Chisati, Mlenzana, & Wazakili, 2019; Stinear et al., 2020). Apparently, the number of people with disabilities due to stroke will increase significantly over future decades because of demographic growth in developing countries (Donkor, 2018). Therefore, recovering from stroke remains a significant challenge.
Recently, telerehabilitation (TR) as a part of the telemedicine approach was introduced to overcome barriers and inequality to healthcare services in a variety of medical directions (Adams, Myers, Waddell, Spear, & Schneider, 2020; Gruska et al., 2020; Hung KN & Fong, 2019; Schröder et al., 2019; Waller & Stotler, 2018). TR means providing rehabilitation services via telemedicine (Brennan, Mawson, & Brownsell, 2009), i.e. using information and communication technologies, including video/teleconferencing, remote data-collection equipment, telemonitoring, computers, mobile phones, robotics devices, exergames, virtual reality (VR) tailored at individuals with disabilities, their families, clinicians, supervisors, and the community (Gruska et al., 2020; World Health Organization, 2011). Moreover, TR can be delivered synchronously or asynchronously depending on medical conditions, treatment plans, and patients’ needs (Morse, Biggart, Pomeroy, & Rossit, 2020).
In this research, we reviewed papers on TR of post-stroke patients and summarized most recent findings of using TR for improvements of upper and lower limbs disabilities, balance problems, spatial neglect and cognitive impairment, aphasia, and speech-language pathologies.
The novelty of the review as follows. Global challenges in epidemiological situation because of COVID-19 pandemic resulted in changing approaches to rehabilitation of stroke patients shifting to telerehabilitation. Current priorities for healthcare systems including virus diseases prevention and granting access for post-stroke patients to rehabilitation services during pandemic or related events can be achieved using telerehabilitation. Previous systematic reviews on telerehabilitation of post-stroke patients were mainly done before pandemic (Aminov et al., 2018; Mura et al. 2018; Sarfo et al., 2018; Tchero et al., 2018; Chen et al., 2019; Hung KN & Fong, 2019; Perrochon et al., 2019; Schröder et al., 2019). Therefore we reviewed most recent research on telerehabilitation of post-stroke patients.
Moreover, recent systematic reviews were aimed at reviewing on telerehabilitation services for stroke using randomized clinical trials (Laver et al., 2020) or on separate areas including aphasia (Luisa et al., 2021) and cognitive rehabilitation (Nie et al., 2021, since we summarized randomized (RCT) and non-randomized clinical trials (non-RCT) on a wider range of telerehabilitation of post-stroke patients including three main directions: motor function, cognitive, and language and speech disorders.
2Materials and methods
2.1Eligibility criteria
We used the following eligibility criteria: (1) peer-reviewed original and review English-written papers in medical journals; (2) papers investigating stroke telerehabilitation with respect to motor function disorders, spatial neglect, cognitive, memory, and speech/language rehabilitation; (3) study protocols; (4) participants (adults) diagnosed with stroke.
We excluded studies if they involved: (1) animal research; (2) conference papers, editorials, book chapters, case reports, papers with incomplete information, articles from nonmedical journals.
2.2Sources and search
We used PubMed, Cochrane Library, Wiley Online Library, Scopus, Sciencedirect, and Springerlink databases to find both research and review papers on stroke TR. The latest studies, published from January 2018 to October 2021, were included in the search query. The following keywords related to stroke TR were used with different combinations: stroke, post-stroke recovery, TR, telerehab, telemedicine, telehealth, technologies, home-based, upper limb, balance problems, spatial neglect, cognitive, aphasia, e-rehabilitation, e-rehab, exergames, VR, robotics devices.
2.3Study selection and data extraction
The authors independently screened search results and sequentially evaluated the titles and abstracts. The full papers of potentially eligible references were retrieved by one author, and then the articles eligibility was assessed by two researchers.
Figure 1 shows the PRISMA flow diagram of the results from the database searches and screening. In total, 4958 articles were identified from databases. We excluded duplicate records and remained only English-language papers. The inclusion criteria were: (a) publications in peer-reviewed journals; (b) research focusing on telerehabilitation of stroke patients with motor function disorders, spatial neglect and cognitive problems, speech and language disorders; (c) access to abstracts and full papers. The other studies were rejected after an analysis of their titles and abstracts. Remained 70 papers fulfilled all the selection criteria.
Fig. 1
PRISMA flow diagram of the results from the database searches.
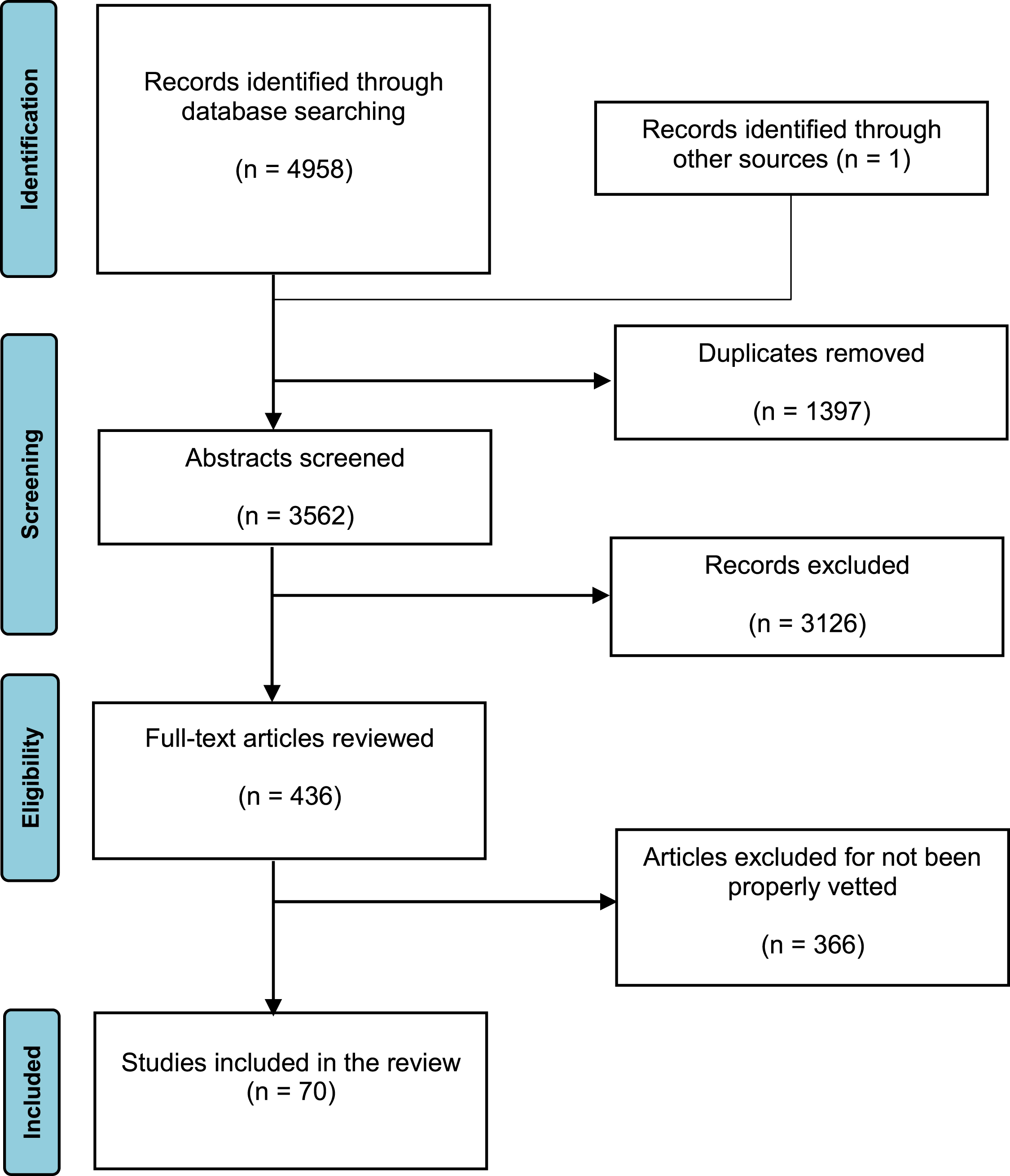
We performed data extraction and risk of biases (RoB) for all studies meeting the inclusion criteria. The information was extracted from each included trial on characteristics of participants and their number, time since stroke (TSS), type of intervention and its duration, test conditions, physiologic measures, and results. Two independent reviewers assessed the studies for RoB. We used modified checklists to meet Scottish Intercollegiate Guidelines Network criteria for RCT analysis and modified Downs and Black checklists for non-RCT. The general study characteristics and risk of bias of RCT/non-RCT are summarized in the Table 1.
Table 1
Study characteristics and risk of bias
| First author, year of publication | Number of participants | Age | Time since stroke | Duration | Intensity | RoB |
| Randomized clinical trials/research | ||||||
| Cramer, 2019 | 124 | 61(14) | 4 –36 (mean 18.7) weeks | 6–8 weeks | 36 sessions of 70 min each | low |
| Hung, 2019 | 33 | TG 56.58[45.38–64.29] | TG 29[14.5–40] months; | 12 weeks | 30 min sessions 2–3 times/week | low |
| CG 61.38[48.62–66.29] | CG 37.5[23–42.5] months | |||||
| Park, 2019 | 26 | TG 53.5; CG 51.5 | > 3 months | 4 weeks | 30 min sessions 5 days a week | low |
| Rogers, 2019 | 21 | 42–94 | 8–62 months TG 22.8(14.8); CG 30.0 (15.9) | 4 weeks | 12 sessions of 30–40 min each | medium |
| Laffont, 2020 | 51 | 55.8(14.0); 60.8(14.1) | < 3 months TG 27.5(19.4) days; CG 27.0(19.9) days | 6 weeks | 45–min session 5 days/week | low |
| Thielbar, 2020 | 20 | 21–80 59.7(10.5); 59.8(4.8) | 6.4(4.1); 6.7(5.3) | 1 month | 4 sessions of 1 h per week | medium |
| Mekbib, 2021 | 23 | TG 52.17(13.26); CG 61.00(7.69) | TG 36.9 days; CG 39.36 days | 2 weeks | 1 h/day 4 days a week | low |
| Cikajlo, 2020 | 20 | 33–65, TR 50.3(7.9); CG 51.8(15.5) | TG 4 months; CG 7.4 months | 1 week | 15 min/day during 5 days | high |
| Kannan, 2019 | 24 | TG 57.5 CG 61.0 | TR 8.9 years; CG 9.09 years | 6 weeks | 90 min 10 sessions | medium |
| Chen, 2021 | 30 | TG 61[53–68]; CG 60[52–68] | TG 2.5 (1.08–5.17) years CG 1.5 (1.08–2.33) years | 4 weeks | 12 sessions 40 min 3 times a week | high |
| Wu, 2020 | 61 | TG 56.73(11.85); CG 59.10(8.60) | Acute stroke | collaborative care model performed 2 times/week | low | |
| Withiel, 2019 | 65 | 60.9 | 41.7 months | 6 weeks | 30 min/day 5 times a week | high |
| Gil-Pagés, 2018 | 40 | > 18 | Chronic | 6 weeks | 1 h/day 5 times a week | n/a |
| Faria, 2020 | 32 | TG 59.14(11.81); CG 65.00(6.20) | > 6 months | 1 month | 12 supervised sessions | low |
| Uslu, 2020 | 100 | Adults | Chronic | 4 weeks | 2 h/day 7 days a week | n/a |
| Kim, 2021 | 80 | – | > 6 months | 5 weeks | 1 h/day during weekdays | n/a |
| Cherney, 2021 | 32 | TG 58.27; CG 55.19 | Chronic | 6 weeks | 90 min/day 6 days a week | medium |
| Øra, 2020 | 30 | 64.4 | No limits to TSS | 4 weeks | 1 h/day 5 times a week | high |
| Braley, 2021 | 32 | TG 58.9(10); CG 64.2(9.9) | TG 53 months; CG 36.1 months | 10 weeks | 30 min/day 5 days a week | high |
| Peñaloza, 2021 | 16 | TG 59.23(18.71); CG 54.63(16.73) | Chronic | 10 weeks | 2 h sessions 2 times a week | low |
| Meltzer, 2018 | 44 | TG 66.8(11.2); CG 62.9(11.6) | Chronic | 10 weeks | 1 h sessions during | low |
| Non-randomized clinical trials/research | ||||||
| Szturm, 2021 | 10 | 58(12) | 4 months –2 years | 4 months | 20–30 min 4 times a week | medium |
| Qiu, 2020 | 15 | 35–82 | Chronic | 3 months | Every weekday for at least 15 min | high |
| Smith, 2020 | 28 | 18–75 | Chronic | 6 weeks | 1 h session twice a week | high |
| Chen, 2020 | 13 | 53–86 | 4–36 weeks | 6 weeks | 36 sessions of 70 min | high |
| Kim, 2020 | 12 | 28–74 | 1–92 months | – | – | medium |
| Triandafilou, 2018 | 15 | 33–81 | > 2 years | 3 weeks | 1 h sessions 2–3 times a week | medium |
| Guillén-Climent, 2021 | 27 | 41–89 mean 63.9 | Chronic stroke | 3 weeks | 1 h sessions every day | medium |
| Sarfo, 2018 | 20 | 54.6(10.2) | Avarage TSS was 6 months | 3 months | 30–60 min 5 days a week | high |
| Escalante-Gonzalbo, 2021 | 9 | 52.67(14.76) | 6 months – 20 years | 20 weeks | 40 sessions of 45 min each, twice a week | high |
| Burgos, 2020 | 10 | 54–79 | 6–8 weeks | 4 weeks | 30 min sessions 9 times/week | medium |
| Morse, 2020 | 7 | 67.1(6.8) | 3–12 years | – | Intensity varied from rarely to daily use | high |
| Torrisi, 2019 | 40 | 55.17 | Chronic | 6 months | 50 min sessions 3 times a week | medium |
| Lawson, 2020 | 46 | > 18 | ≥ 3 months | 1.5 months | One 2 h session a week | high |
| Isernia, 2019 | 45 | 61.04(13.25); 57.77(17.17) | > 6 months | 3 months | 30–45 min/day 5 times a week | high |
| Gerber, 2019 | 15 | 53(10) | Average TSS was 444 days | – | – | high |
| Maresca, 2019 | 30 | 51.2(11.3) | Chronic | 6 months | 50 min/day 5 days a week | medium |
| Kurland, 2018 | 21 | 66.4 | Chronic | 6 months | 1 h/week | high |
| Dial, 2019 | 31 | 68.9; 61.0; 67.6; 67.8 | Chronic | – | 1–2 sessions per week | high |
| Jacobs, 2021 | 18 | 58.78(13.33) 33–96 | 1–288 months | 6 weeks | 12 sessions of 45–60 min | high |
Notes: TG –telerehabilitation group; CG –control group; Age and TSS are shown as mean(SD), median[IQR].
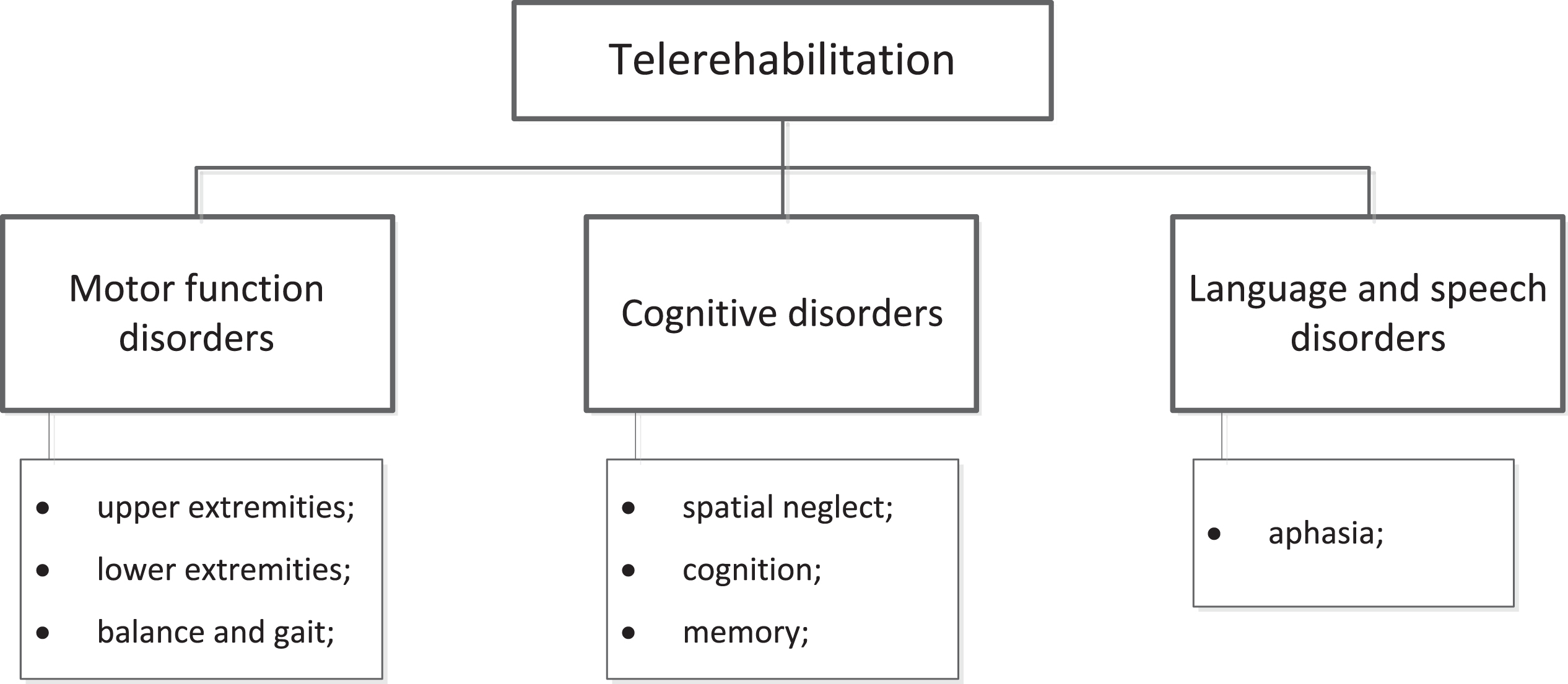
The papers were divided into three main directions depending on their topic (Fig. 2): motor function disorders, cognitive disorders, language and speech disorders.
Fig. 2
Major directions of stroke patients’ telerehabilitation.
3Results and discussion
3.1Upper and lower limbs TR, balance training
Cramer et al. (2019) explored rehabilitation of 124 stroke patients with motor impairments with a mean age (MA) of 61±14 years, experienced stroke 4–36 (mean 18.7) weeks before enrollment with Fugl-Meyer Arm Motor Scale (FM) 22–56 (mean 43). They were randomized to in-clinic (IC) or TR groups, trained during 6–8 weeks, including 36 treatment sessions (18 supervised and 18 unsupervised ones). Each session consisted of 70 min supervised activities and a 10-min break. All groups received the same therapy, exercises, and stroke education. The TR toolkit included a computer without keyboard, with gaming input devices and the internet-access, a table, and a chair. Moreover, 12 gaming devices (trackpad or PlayStation Move Motion controller (Sony)), providing functional tasks along with augmented reality, were used. During all sessions the exercises were demonstrated on the computer screen. Each supervised session began from a 30 min patient-therapist videoconferencing, containing exercises, question-answer and treatment plans reviewing activities, and study assessments.
Both groups accomplished similar gains in stroke knowledge, patients’ compliance management, and FM, exceeding minimal clinically important difference, regardless of stroke duration. Specifically, FM score change of 7.86 and 8.36 points (p < 0.001) was observed for TR and IC group from baseline to 4 weeks the research was completed. Apparently, the effect of home-based TR is attributed to a significant number of arm movement repetitions which was 1031 per day. However, the satisfaction and motivation were higher for IC group. TR is beneficial during periods of limited access to healthcare services. Moreover, it provides a basis for other musculoskeletal and neurological rehabilitation conditions, although the internet connection and digital equipment issues are still addressed to manage.
A smaller study by Szturm et al. (2021) examined a home-based TR of 10 post-stroke individuals (MA 58) with upper extremities (UE) disabilities. Initially, the participants with TSS between 4 months and 2 years attended 3–4 treatment sessions (45–60 min) at a clinical facility. The hand-arm exercises were presented to individuals to teach them doing game-assisted exercises independently at home. Then a TR consisted of the game-based exercises performed 4 times/week (20–30 min/day) for 4 months was implemented. The TR system included a laptop with a wireless inertial-based mouse which linked physical movements with interactive computer games. The mouse-device was installed in physical exercise objects (plastic ball, soccer ball, coffee mug, etc.) given to each patient by therapists to train motor function through the participant’s engagement with related computer games. All games were run on Windows operating systems. Generally, 6–8 object manipulation tasks were chosen for each patient. The games selection was based on the patient’s functional impairments and TR goals, including movement amplitude, speed, and precision adjustments. When playing computer games the patients underwent a task-specific practice of object handling and manipulation to overcome difficulties in gross and fine motor skills.
The patients and therapist communicated via email, telephone on a weekly basis. Some participants sent videos to research staff to monitor their progress and make changes in TR plans. Thus, TR was feasible improving UE function of patients. Specifically, the median time to complete the Wolf Motor Function Test (WMFT) reduced significantly from 173 s at baseline to 126 s postintervention, functional ability median WMFT score increased from 44 to 55.5, while the average change in grip strength was 2.9 kg.
Qiu et al. (2020) presented the results of UE TR using a home-based virtual reality (VR) system among 15 chronic stroke patients (aged 35–82 years). They tested the VR system every weekday (≥15 min) for 3 months. On average, the time spent with VR TR was 13.5 h. Remote clinical monitoring and limited technical support were provided via secured communication channels. The system included a patient-based platform and a cloud-based online data logging and reporting subsystem. A cross-platform of VR training application ran video games on patients’ computers. The leap motion controller consisted of two cameras and three infrared LEDs was used to capture motions of patients’ UE, which allowed them interacting and controlling the games with their hands and arms without wearable sensors. For the patients with proximal arm impairments or with significant difficulties of moving UE, an anti-gravity arm-device was introduced positioning and transporting their hands. VR platform processed kinematic data with 22 degrees of freedom for the wrist and hand, controlling the game progression using online algorithms. To train a movement pattern 12 games were used. Each game was attributed to the following categories: whole arm, hand, elbow-shoulder, and wrist. The participants played at least 3 games every weekday.
The TR of patients in a safe environment with minimal supervision showed improvements in UE functions. There was a mean increase of 5.2 (p < 0.001) on the UE FM score (UEFMA) from baseline (15–59) to post intervention (21–63), and improvements in hand kinematics. However, the severely impaired patients (UEFMA < 15) who were unable to move any of their UE joints remained out of scope.
A combination of modified Constrained Induced Movement Therapy (mCIMT) using telehealth and in-person sessions were investigated by Smith & Tomita (2020). Specifically, 28 patients aged 18–75 were divided into two groups depending on their performance on the timed portion of WMFT, including 15 patients of higher functioning and 13 lower functioning individual. Only patients who lived in their own homes, had videoconferencing devices and internet access were allowed to participate in TR. Google Hangout or Adobe Connect web-based software was installed onto their computers. Also participants were equipped with typical physical objects for fine and gross motor movements which they used during online sessions with a therapist. They wore the mitt on the unaffected UE for about 4 h/day. The internet-based sessions lasted for 1 h with the intensity of 2 times/week during 6 weeks. Additionally, in-person sessions of 1–1.5 hours occurred once a week.
Findings showed improvements in functional and quality of use in the hemiparetic UE for all participants, underlining a positive effect of a combination of mCIMT with TR and CR. WMFT functioning pre to post intervention difference was 5.481 (p = 0.00004) and 2.217 (p = 0.02335) for high and low influencing groups, while UEFMA change was 2.859 (p = 0.00638) and 2.217 (p = 0.02335). Moreover, higher attendance rate of TR (84.5%) against CR (75.3%) proves significant engagement of patients in TR. However, there were problems with internet access and low digital proficiency of individuals, which can be solved with support of technicians and family members.
Chen et al.(2020) explored a user acceptance of a home-based TR among 13 stroke patients (MA 70.52, TSS 4–36 weeks), participating in a 6-week rehabilitation. It involved therapy games, exercises, education and videoconferencing with therapists. The TR system included a computer, a monitor, a microphone, speakers, a wireless modem, the Myo Band, the Wiimote in a pistol-shaped holder, the PowerMate, PlayStation 3 Eye Move Controller, a joystick, the Logitech Trackpad, standard devices for UE therapy, a table and a folding chair. They underwent 36 sessions, including 50% of therapist-supervised through videoconferencing and the rest ones without supervision. The supervised sessions began with a 30-min work with the therapist and then the patient performed a 40-min self-administered therapy using the TR system. All unsupervised sessions started with a 5-min education consisting of prevention, recognition, response, and stroke management issues, then, stroke-tailored games and exercises were performed as pre-assigned features. The participants trained limb functions, cognitive abilities, and improved emotional well-being. Semi-structured interviews were used to analyze effectiveness of TR.
In general, videoconferencing motivated patients to stay on track during TR as they felt obliged to complete assignments. The CR group occasionally missed offline sessions. Moreover, the TR was convenient (location, time, travel issues), enhancing the dose and training intensity. Also, family members positively affected clinical outcomes. However, some patients required a progress-dependent difficulty of exercises and process visibility to keep them engaged during long-term TR. Next, they underlined the importance of technical support and the need of more space for training.
Another study by Perrochon et al. (2019) reviewed the data on using exergames by individuals with neurological diseases for upper- or lower-limb rehabilitation at home. According to their analysis, exergames showed a feasible alternative to CR. Although they highlighted benefits of TR, more research on optimal dose training is needed. Apparently, duration of TR was that one of a minimally required dose that was suggested to be of 15–16 hours. Another recommendation was using custom-designed exergames to achieve higher effectiveness of TR through targeted clinical features of neurological diseases. The task-specific approach increasing diversity of exercises and daily living activities seems to be effective.
A promising study by Kim et al. (2020) examined the feasibility of teleassessment for motor impairments of the spastic elbow for 12 post-stroke patients (aged 28–74) using a telerobotic system. It included an arm-like haptic device (master robot) that interacts with the doctor and a slave robot; the latter interacted with the patient. A doctor operated a master robot, which speed and torque were conveyed through the internet to the slave robot. A torque sensor was used to measure an elbow reaction of the patient to the movement of the slave robot, then, the signal was transferred back to the master robot. Passive range of motion, muscle strength, and spasticity tests were used in the study. To assess an elbow function remotely the master robot recreated the muscle tone of the patient’s elbow to make the doctor perceiving it via an in-person assessment. During clinical tests the doctor, medical staff and stroke patients were in different countries. They used videoconferencing to communicate with each other. The study showed the feasibility of TR. It is beneficial for patients living far from rehabilitation facilities, clinicians working remotely, on the occasions of a medical staff shortage or due to the lack of infrastructure. Importantly, the system can maintain the rehabilitation of patients during COVID-19 pandemic or similar events preventing them and medical staff from infection.
Hughes et al. (2019) evaluated a novel low-cost wearable sensor for UE kinematics measurements among 31 healthy adults (MA 24.5). A custom-built wearable sensor (outREACH sensor) was placed on the wrist of each participant and then they performed specified movements and actions. Kinematic analysis was assessed through tasks from Action Research Arm Test and Frenchay Arm Test. The results show the outREACH sensor allows to measure personalized UE movement kinematics and obtain quantitative information to enhance stroke evaluation and rehabilitation in resource constrained sub-Saharan Africa.
Another study involved 33 patients with chronic hemiplegic stroke (Hung et al., 2019). They were subdivided into an experimental group (17 individuals, MA 56.6) utilizing TR via exergames and a control group (16 patients, MA 61.4) using CR. The research lasted 3 months and consisted of 30-min sessions 2–3 times/week. The TR system included the Kinect2Scratch to track movements of the body using the Kinect sensor and transfer them to Scratch. The patients used the Kinect2Scratch to train their extremities via playing Scratch games. An occupational therapist supervised all sessions, chose the games, adjusted their difficulty according to abilities and needs of the participants, monitoring safety. The experimental group underwent 3–4 games per session, in total 8 games were tested including 2 bimanual and 8 unimanual. The control group was trained by the therapist using a variety of unilateral and bilateral UE exercises.
Findings indicate the feasibility of exergames and similar outcomes for both groups at three months follow-up. Specifically, post interventions the TR group demonstrated significant improvements in UEFMA (p = 0.001), Fugl-Meyer Assessment proximal (FM-PROX, p = 0.001) and distal (p = 0.017) UE. Moreover, there was a considerable change in WMFT-TIME (p = 0.004). The control group showed improvements in UEFMA (p = 0.014), FMA-PROX (p = 0.005), and WMFT-TIME (p = 0.006). The total activity of the affected UE and the participation level were higher in the TR group. Although there were no significant side effects observed during the interventions, most participants in TR group reported the UE soreness after training, which subsided spontaneously without additional treatment.
Triandafilou et al. (2018) introduced a promising 3D multiuser VR therapy for arm motion improvements of 15 chronic stroke individuals aged 33–81 (TSS≥2 years, mean 17.4 years). They performed one-hour training sessions for at least 2–3 days/week during 3 weeks. To simulate a home-based TR they were located in different rooms of the laboratory. The VR system allowed patients to interact within a virtual environment (VE) using digital avatars in real time. The avatar was controlled by participant and was able to manipulate virtual objects located within VE. A number of games were available to patients enhancing repetitive arm movements during collaborative and individual exercises in VE (e.g., ball bump, tea party, etc.). Technical requirements included a computer, a wireless mouse, a Kinect™ device, and an executable version of author’s code. On average, the TR allowed to increase an arm displacement in each session to 350 m. Although VR therapy showed feasibility, the involvement of relatives, friends, and clinicians facilitates health improvements. Most participants showed satisfaction, enthusiasm, and willingness to use VR, they recommended simplifying the technology and making it flexible to meet personal needs of the patients.
Another approach to VR rehabilitation presented by Park et al. (2019) in which 26 hemispheric chronic stroke patients (TSS≥2 months) participated. They tested a VR-based device, consisted of a smart board (SB) which was 104.3×63.0 cm in size, a forearm-supported controller, a display, and an android personal computer (PC). It incorporates two-dimensional planar exercises with gravitational support, preventing antigravity muscle facilitation. This feature enables involvement in TR of the patients who cannot perform hard three-dimensional exercises under gravity. The patients were divided into TR (MA 53.5) and CR (MA 51.5) group, and underwent 20 sets of rehabilitation sessions 5 days/week during 4 weeks. For TR group each session consisted of 30 min of interventions using SB and 30 min of a regular occupational therapy. The control group was treated with 1 h of CR. The patients were directed by software, which included gamified interventions among 17 training programs with respect to difficulty level, holding and moving their arms.
Findings demonstrated improvements in FMA and WMFT (p < 0.05), showed effectiveness of VR-based treatment in addition to CR, and a more pronounced effect regarding active range of motion of the proximal UE. Similar solution was obtained in a smaller study (Guillén-Climent et al., 2021), in which a single group of 9 patients (aged 41–89, MA 63.9) underwent 3-week training using TR system based on serious games, including a week of supervised training at the research institute, one more week of a homed-based supervised rehabilitation, and one week at home with remote supervision and support. The system contained a portable cost-effective robotic system ArmAssist (AA) based on exergames and the Antari Home Care platform for supervising, customizing, and managing TR remotely. It required a table space (110×68 cm), Wi-Fi access at patients’ homes. AA system included sensors measuring self-directed active movements during the games since the rehabilitation device was fastened on the forearm. The home-based TR showed usability, safety, moderate improvements in clinical outcomes (p = 0.002), and motivation among patients. Although positive changes in FMA motor function were observed, interventions did not affect the spasticity of UE.
Another study (Rogers et al., 2019) involved a rehabilitation of 21 patients aged 42–94 years with subacute unilateral stroke (TSS 8–62 days) and UE dysfunctions. The TR group (10 participants) performed VR in addition to CR, while the control group (11 patients) underwent CR. VR training included 12 individual sessions (30–40 min) during 4 weeks and was organized in a private room of the hospital. The VR system was consisted of an interactive tabletop surface display with embedded central processing unit, tangible user interfaces, software for presenting goal-directed and exploratory VE. The patients manipulated by four hand-held objects in accordance to VE presented on the touchscreen display. All groups received 3 h/day of conventional occupational and physiotherapy treatment. The findings observed a larger magnitude and rate of improvements in motor hand (p = 0.008) and cognitive (p≤0.001) function using TR than CR.
Sarfo et al. (2018) explored TR of 20 post-stroke patients (MA 54.6, TSS 6 months) using smartphones. They participated in a personalized goal-tailored TR during 5 days/week for 3 months, each session lasted 30–60 min. The TR system consisted of smartphones with preinstalled 9zest Stroke Rehab therapeutic application (The 9zest Stroke Rehab, n.d.) and internet access. To analyze ongoing rehabilitation the therapeutic exercises were administrated by the therapist via a weekly telephone conferencing. Additionally, the performance of exercises was video-recorded by participants using their smartphones and sent to the therapist. They underwent a standardized rehabilitation aimed at improving mobility through limbs strengthening exercises, dexterity enhancing fine motor movements, walking endurance, balance training via standing and seating activities. Findings demonstrated patients satisfaction and improvements in stroke levity scale scores (increased from 7.5±3.1 at enrollment to 11.8±2.2 and 12.2±2.4 at month 1 and 3, p < 0.0001). As compared to a baseline at month 3 the modified Rankin score slightly decreased from 2.2±0.6 to 1.8±0.7, while the mean baseline Barthel’s index and Montreal cognitive assessment (MoCA) score increased from 94.4% ±6.4 to 96.1% ±6.4 and from 18.2±4.3 to 22.2±7.6. The study proves the feasibility and cost-effectiveness of m-health TR. However, it is hard to estimate a natural recovery without a control group.
Laffont et al. (2020) investigated UE rehabilitation using video games (VG) against CR in patients with subacute stroke. 51 individuals (MA 58) underwent sessions of conventional occupational therapy or VG-based TR as a complementary therapy to usual rehabilitation. It included 45-min sessions 5 days/week for 6 weeks. When applied within the first month after stroke, VG TR was more efficient than CR on both sensorimotor recovery and gross grasping function.
A smaller study (20 participants) (Thielbar et al., 2020) explored both home-based multiuser (MU) and single-user (SU) VR therapy. Patients in MU (MA 59.8) and SU (MA 59.7) groups performed 4 sessions (1 hour each) treatment during 1 month. MU group involved multiple users playing VR games, since SU included a patient performing exercises via game playing. Improvements in FM UE were observed for both groups (p = 0.001). However for MU, the total arm displacement and the amount of time spent performing exercises were greater than for SU, meaning MU can extend clinical therapy to home environments.
More recently Escalante-Gonzalbo et al. (2021) explored 20 weeks TR using task-oriented video games (40 sessions of 45 min each, 2 times/week) of 9 chronic stroke patients (MA 52.67). The TR system consisted of a virtual rehabilitation platform with video games, movement sensors to perform training, and a central server. The games were personalized depending on patients’ abilities and limitations. Moreover, the therapists monitored their progress and assigned routines via the system. Findings showed significant gains in WMFT (p = 0.0039) indicating motor function recovery in a safe and enjoyable way.
Mekbib et al. (2021) explored an immersive VR TR of 12 patients (MA 52.17, TSS 36.9 days) against CR of 11 individuals (MA 61.0, TSS 39.36 days). The TR system consisted of a head-mounted display, two HTC Vive tracking stations used to track the user’s location, the leap motion tracking technology to detect and track the patient’s UE movements and transfer them onto a virtual limb in the VE, and a high graphics laptop running the software to generate the VE, store the data, etc. The TR group received 1 h/day of VR training, in addition to 1 h/day of occupational therapy 4 days/week for 2 weeks, while the control group received 2 h/day of occupational therapy. VR treatment provides the patient with tasks to perform a tabletop ball grasping, moving, and releasing using the virtual limb(s), by detecting the patient’s gestures, movements, and then graphically mimicking and displaying them in VE. Although both groups showed improvements in motor recovery and cortical reorganization, the TR group demonstrated greater improvements on UEFMA scores (p < 0.05) than the control group. It means TR can improve functional capabilities of subacute stroke patients with moderate-to-severe UE impairments.
Balance problems are other stroke consequences. Cikajlo et al. (2020) examined balance training of 20 patients, including TR (10 participants, MA 50.3, TSS 4 months) and CR (10 individuals, MA 51.8, TSS 7.4 months). The TR group underwent 3 different exergames with overall intensity of 15 min/day during 5 days in addition to CR. The TR system required 6 m2 of space to perform exercises and consisted of an LCD screen, PC, a Microsoft Kinect device, and Wii Balance Board. The Kinect was used as a hands-free user interface to interact with PC during exergames. When training, each participant stood on the board in front of the LCD screen performing exercises and motions depending on the game plan. The training included multiple exergames (balancing/standing up, single-leg exercises, and weight shifting) with a varied level of difficulty to customize rehabilitation. Although the patients perform their training on their own a physiotherapist also supervised them. Both groups showed improvements of functional balance. The TR group improved significantly in motor and balance tests, specifically the 10 m walk test (MWT) to assess gait performance (p = 0.008) and Four Step Square Test (FSST) to test dynamic balance while stepping over objects sideways, forward and backward (p = 0.009). TR demonstrated similar improvements in clinical outcomes to CR, having the advantage of the accessibility of the objective and measurable information relating to the center of the press.
Kannan et al. (2019) tested similar approach on 24 chronic stroke patients undergoing highly intensive cognitive-motor 6-week rehabilitation. Specifically, 12 individuals (MA 57.5, TSS 8.9 years) performed cognitive-motor exergames (20 sessions) since others (MA 61, TSS 9.09 years) underwent CR. Each TR session (90 min) consisted of 3 sub-sessions with a 20-min duration, containing 4 Wii-fit games and 3 cognitive tasks. During the training each person stood on a balance board and performed exercises to sense the symmetry of the body weight distribution. An assistant motivated the participants to perform exergames and cognitive tasks. The scores appeared at the end of the game provided instant feedback and then a more difficult level of the exergames and cognitive tasks was adjusted to sustain progress. Findings revealed improvements in motor function and cognition in TR group, since the CR group improved the motor function only. The authors recommend clinical implementation of cognitive-motor exergames.
More recently Chen et al. (2021) observed improvements in balance training of 30 chronic stroke patients who were equally assigned to video games TR and CR groups. They underwent 4 weeks treatment, including 12 sessions of 40 min 3 times/week. The TR consisted of game exercises performed via Kinect-based system. Video games were aimed at balance, weight bearing, strength, weight shifting, and walking tasks training. Although both groups demonstrated similar improvements in the Berg Balance Scale (BBS), the completion times of the Timed Up and Go Test significantly improved in the TR group (p = 0.005).
Another study by Burgos et al. (2020) explored TR of stroke patients (aged 54–79) with balance problems using smartphomes. They enrolled 6 patients with early subacute stroke (TSS 6–8 weeks) who underwent TR in addition to CR. The control group (4 patients) underwent CR. Their home-based TR lasted for a month and included nine 30-min sessions per week. The system consisted of 2 wireless inertial movement sensors positioned at the lumbar level and the anterior thigh of the paretic side of each patient and was connected to an Android-based smartphone, a cloud database, and exergames. To train balance the participants interacted with a custom-developed application performing task-specific exergames controlled by body motions. Moreover, they adjusted the difficulty level of exergames depending on their progress. A physical therapist daily contacted each participant using WhatsApp to keep standard interaction, increasing protocol adherence. To monitor the rehabilitation the therapist connected to the web-platform, analyzed daily games scores according to a timetable or at any convenient time. The findings revealed improvements in BBS (11.3±3.5 points), Mini-BESTest (8.3±3.01 points), and in the Barthel scale (17.5±9.87 points). The TR group showed statistically higher improvements in Barthel and Berg scales. Positive effects of TR are explained by early rehabilitation at subacute stage and high training dosage. Specifically, the TR group received 4 h 30 min/week more treatment than the control group (2 h/week). Moreover, the study demonstrates feasibility of TR system as a complementary therapy with low costs and high usability (87.5±11.61). Despite the system showed a solid performance on small samples, further research on large groups is needed.
Wu et al. (2020) explored an early rehabilitation of 61 acute stroke patients. TR group (30 individuals, MA 56.73) was treated using exercises via a home-based videoconferencing, while the control group (30 patients, MA 59.10) underwent CR. The system consisted of a computer with a preinstalled software to run the internet-based TR, a projector, a camera, and a data storage device. TR was organized on a collaborative care model and performed 2 times/week. Although both groups showed statistically significant recovery 12 weeks after intervention (p < 0.001), the TR group demonstrated greater improvements in FM (83.70±4.44), BBS (43.13±2.32) and Stroke-Specific Quality of Life Scale (SSQLS 190.57±5.09) against the control group (FM 75.29±2.89, BBS 38.29±2.70, SSQLS 175.90±5.78).
Multiple TR studies revealed improvements in FM and other tests related to motor recovery (Cramer et al., 2019; Hughes et al., 2019; Hung et al., 2019; Park et al., 2019; Perrochon et al., 2019; Rogers et al., 2019; Chen et al., 2020; Kim et al., 2020; Smith & Tomita, 2020; Qiu et al., 2020; Guillén-Climent et al., 2021; Szturm et al., 2021). Reviewed papers indicate TR is effective and comparable in clinical outcomes to CR for patients with motor function disorders. It collocates to an earlier review by Tchero et al. (2018), who found insignificant differences between TR and CR. Specifically, both groups demonstrated similarities in the Barthel Index, BBS, UEFMA, Stroke Impact Scale, Caregiver Strain Index, quality of life, and patients’ satisfaction. However, Laver et al. (2020) in their review underlined although there were similar outcomes between groups in UE functioning, balance, daily living activities, and depressive symptoms, the quality of studies varied from low to moderate evidence.
Furthermore, whereas a home-based TR offers improvements in motor skills of post-stroke patients, enhances their daily activities and typically as effective as CR, the main challenges include engagement providing external/internal motivation, the social context, technical barriers and practical issues (Chen et al., 2019; O’Neil et al., 2018).
Recent studies also show modern equipment and devices are required to design and implement TR (Bayoumy et al., 2021; Buonocunto et al., 2018; Chae et al., 2020; MacEira-Elvira et al., 2019). Apparently, the positive effect of using wearable movement sensors during TR relates to their noninvasiveness, precision, easy deployment, providing treatment therapy adjustment and targeted approach (Porciuncula et al., 2018). Morone et al. (2019) reviewed on rehabilitative devices with exergames and VR showing their positive effect to enhance motor learning via involvement of higher cognitive functions. Thus, before the development of effective e-health activities for post-stroke rehabilitation, the requirements for accessibility, usability, and content of TR with respect to the needs of patients, caregivers, and health professionals should be considered (Denham et al., 2020; Wentink et al., 2018).
3.2Spatial neglect, cognitive, and memory rehabilitation
Another stroke consequence is spatial neglect, causing significant disabilities of stroke survivors (Gammeri et al., 2020). Morse et al. (2020) explored a self-administered VR rehabilitation of 7 patients with spatial neglect (MA 67.1, TSS 3–12 years), their 3 carers or partners (MA 51), and 6 stroke clinicians (MA 44.7). A non-immersive VR system included a 40-inch monitor, a laptop, a motion-tracking sensor (Kinect), exergames, and a computerized neuropsychological rehabilitation therapy (c-SIGHT). The c-SIGHT allows patients to perform exercises on the computerized board (grasping, lifting/balancing rods) using their unaffected hands. One focus group underwent the c-SIGHT therapy since another one performed 3 exergames. A therapist monitored both activities remotely. The findings identify the self-administered VR might increase mobility and psychological wellbeing of individuals, enhancing engagement, motivation of patients, carers, and therapists. However, the study identified the following limitations: cost and availability of equipment, lack of instructions clarity, and technological awareness. Moreover, larger samples of stroke patients at different stages of their recovery and clinicians with different backgrounds are required to analyze the efficiency of VR TR in the future.
Torrisi et al. (2019) evaluated the efficacy of VR rehabilitation to improve cognitive function of 40 stroke individuals during 6 months. At the beginning of the study, the participants from the experimental group trained via VR system-Evo (3D scenarios) and the control group underwent a standard cognitive training using paper-and-pencil approach at the rehabilitation center. After discharge (in the 2nd phase) the experimental group underwent another VR session (2D scenarios) using the home tablet. The sessions lasted 50 min 3 times/week. A clinician monitored the progress of home-based TR via videoconferencing 2 times/week. Thus, TR showed more significant improvements in MOCA (p < 0.001), semantic and phonetic fluency (p < 0.001) than CR, underlining the effectiveness and importance of TR.
Lawson et al. (2020) investigated memory rehabilitation using internet videoconferencing against in-person methods. Specifically, 28 stroke patients (MA 53.36) underwent TR, whereas 18 individuals (MA 62.0) trained face-to-face during 1.5 months. TR sessions were performed 2 h/week via Zoom videoconferencing app. They included interactive exercises, memory functioning and healthy lifestyle education, internal/external compensatory memory strategies training. The cognitive training was similar to Withiel et al., who used computerized online cognitive games (Withiel et al., 2019). Additionally, homework tasks were included encouraging daily practice. Findings revealed the feasibility and effectiveness of TR providing remote training of compensatory memory skills. There was a significant increase in goal attainment scaling scores for both groups at post-intervention than at baseline (p < 0.001). Although both groups exhibited similarities in subjective measures improvements, reducing in lapses of prospective memory, the effect of TR on reducing everyday memory lapses was greater than that one of face-to-face rehabilitation. Moreover, a positive effect of booster sessions in memory functioning was observed.
Gil-Pagés et al. (2018) proposed a study protocol for a home-based computerized cognitive rehabilitation of 40 chronic stroke patients using web-platform. The TR will consist of five 1-hour sessions a week for 6 weeks and include exercises of memory, attention, and executive functions. Thereafter, the individuals will participate in sham interventions, lasting 1 hour each, including four 10 min videos and content-related quizzes appearing after them. A control group will undergo sham interventions first and then the TR. Once participants pass the initial neuropsychological assessment the results are stored in the platform. Then patients cognitive profiles (age, level of education, etc.) are processed and computerized tasks are generated by the system on a daily basis. The participants using their home-based PC perform the assigned tasks sending the results back to the server. The therapists asynchronously monitor the performance of the patients providing suitable means. A proposed approach will allow the patients to achieve improvements in cognition.
Isernia et al. (2019) investigated the TR of 45 chronic stroke patients (MA 61.04, TSS≥6 months), including 1-month training in clinic (45 min sessions 3 times/week), and then 3-months home-based TR (30–45 min sessions 5 consecutive days/week). The system incorporated PC, internet connection, motor capture devices, leap motion, the VR platform to train goal-directed movements via gaming in VE. TR was adjusted and monitored by clinicians. It included short video clips with duration of 2–9 min each. The participants repeated exercises from the film clips or performed tasks (answered content-related questions, ordered the clip sequences, etc.). They demonstrated adherence more than 80% from the 1st to 8th week of training. Most individuals indicated significant improvements on daily life functioning after TR (p < 0.001).
Another study by Faria et al. (2020) explored cognitive rehabilitation of 18 chronic stroke patients (MA 65) using web-based paper-and-pencil PDF tasks generator (TG) against VR-based intervention (14 patients, MA 59.14) consisted of paper-and-pencil tasks contextualized in different locations of a virtual city. Patients underwent 12 supervised sessions over 1 month. VR system comprised a PC with preinstalled rehabilitation software (Reh@City v2.0), a monitor, and a camera with augmented reality pattern tracking software (ARPTS). The patient worked on a tabletop, moving a customized handle with a tracking pattern on its surface interacting with VE. Movements were captured using ARPTS. VR-based training included a variety of cognitive tasks similar to daily living activities (supermarket, post office, outdoor games, reading, etc.). VR training showed improvements in general cognitive functioning, the Mann-Whitney test as assessed by the MoCA improved from baseline to post-intervention (TR: Mdn = 2, IQR = 0–3; TG: Mdn=–1.5, IQR=–3.25–2 (U = 65.00, Z = – 2.334, p = 0.020, r = 0.41)).
Bernini et al. (2021) presented a perspective study of using the HomeCoRe computer-based cognitive rehabilitation of elders with neurodegenerative diseases at home. It provides remote TR of recently discharged patients, supporting continuous recovery and overcoming a lack of healthcare professionals. Therapists set a rehabilitation plan and monitor the TR remotely. The simplicity of patient-therapist interactions via TR services result in the goal fulfillment using a non-pharmacological therapy. Remote technologies are effective means supporting the cognitive rehabilitation of stroke patients reducing their infection due to COVID-19.
Furthermore, Mantovani et al. (2020) underlined the obstacles of CR during restrictive measures (COVID-19, etc.) and other social events reducing the accessibility of healthcare, hampering prolonged cognitive rehabilitation. Since the healthcare system faces new challenges, TR can mitigate the harmful effects of quarantine and restrictions on delivering cognitive rehabilitation to patients in the future.
Cogollor et al. (2018) overviewed recent practices for cognitive rehabilitation and assessment of stroke patients. Although limitations of smart technologies for daily living were also addressed, the authors emphasized the necessity and importance of the technologies for cognitive rehabilitation. The first reason is a large number of stroke patients requiring long-term rehabilitation, the second one relates to reducing the independence of individuals due to consequences of apraxia and action disorganization syndrome, and that CR result in a slow rate of improvements. Finally, patients suffer from social exclusion. Recent developments for cognitive TR show they incorporate task-performance, monitoring, feedback features, creating the smart interactive environment to recover from stroke. Moreover, achieving a successful execution of rehabilitation tasks are in line with improvements in daily live independence, empowering rehabilitation, reducing the therapists’ workload, and providing active aging. Thus, the support in the execution of complex daily tasks, automatic error detection, home-based performance, and accessibility are among essential limitations that digital technologies should overcome to achieve TR efficiency. However, only 33% of all considered in the study (Cogollor et al., 2018) European projects meet those characteristics, hence, future e-health extensive research is required.
More recently Nie et al. (2021) reviewed on computer-assisted cognitive rehabilitation of stroke patients. They found significant improvements in global cognition of patients (p < 0.01) and their daily living activities (p < 0.05).
Our review collocates to the study (Aminov et al., 2018), summarizing findings on virtual rehabilitation of cognitive and UE stroke individuals, underlining advantages of TR over CR. The exergames are effective means for stroke recovery, improving cognitive-motor functions. This is in line with Mura et al. (2018) assuming exergames among adaptable instruments for cognitive and motor rehabilitation. They hypothesise modulation of brain regions activity is developed via active playing of video games. The clinical outcomes of exergames are estimated as complementary therapy for in-clinic and home-based environment. Although TR of neurological patients seems to be flexible and safe with a high degree of adherence, further research on large samples is required to define proper intensity, frequency, and type of therapy to achieve sustainable recovery.
3.3Speech and language rehabilitation
Another consequence of stroke is aphasia occurring in 30% of cases (Doogan et al., 2018). Meltzer et al. (2018) investigated rehabilitation of 44 chronic patients with aphasia or cognitive-linguistic communication disorders (CLCD) using TR (17 aphasia patients, MA 66.8; 5 participants with CLCD, MA 60.8) and in-person treatment (16 aphasia patients, MA 62.9; 6 CLCD individuals, MA 63.2). All patients received treatment once a week during 10 weeks. The conversational exercises (TR) were delivered via videoconferencing (WebEx) with a therapist. Most patients underwent a home-based TR, others performed from separated rooms in the hospital. Additionally, patients did self-administered homework exercises using software (TalkPath) including graded assignments in speaking, listening, reading, writing, and paralinguistic cognitive skills. The system consisted of a PC/tablet (iPad) with preinstalled software. Findings showed similar gains in language and communication skills for both groups.
Another study by Gerber et al. (2019) investigated a home-based TR of 15 aphasia patients (MA 53, TSS 444 days), in which 11 speech/language therapists (MA 28) participated. It included a digital application for tablet computers of patients and therapists, a webpage to create exercises, a database to store data, and the open source server as backend service. The language exercises (word-picture matching, word/sentence completion/repetition, anagrams, auditory and audio-visual exercises, etc.) were divided into training units of 25 tasks. The therapist assigned/adjusted units and tasks to patients, which were downloaded automatically onto their computers. Then they trained using the tablets. Also, the therapist monitored the patient’s progress. Feedback was provided automatically. The TR demonstrated simplicity, adaptability, acceptability among aphasia patients and their clinicians. They rated the TR as excellent (z = –1.90, p = 0.03) and good (z = –0.75, p = 0.04).
Maresca et al. (2019) examined a two-phase 6-month rehabilitation of 30 patients (MA 51.2±11.3 years) with aphasia acquired from hemorrhagic/ischemic stroke. They performed 50 min training 5 days/week. Half of them underwent CR since others participated in VR rehabilitation. In the first phase, the TR group was treated with experimental linguistic training using a VR system. It consisted of a touch-screen tablet to perform a variety of exercises at home, a remote workstation to control/adjust TR. The exercises were personalized according to the needs and abilities of the patients (reaction time, number of variable stimuli) and were similar to those reported by Gerber et al. (2019). A therapist organized a videoconference 2 times/week discussing outcomes, monitoring TR. The CR included the same exercises via conventional linguistic training using paper-pencil tools.
The second phase included different trajectories for experimental and control groups. The experimental group continued a home-based training with touch-screen tablets with the inbuilt protocol of linguistic exercises (Maresca et al., 2019). The offline VR training was recorded and then the data were transmitted to the control panel just after the tablet was connected to the internet. The control group underwent CR at territorial services. Thus, the CR group improved only in comprehension, depression, and quality of life whereas the VR-group showed improvements in all areas except writing. Specifically, TR influenced comprehension (p < 0.001), repetition (p < 0.001), reading (p < 0.001), naming (p < 0.01), calculation (p < 0.001), although writing effect was insignificant (p = 0.18).
Effects of tablet-based TR on aphasia patients are in line with Kurland, Liu, & Stokes (2018) who observed improvements in the naming of objects and actions among 21 chronic patients (MA 66.4). They underwent home-based training using iBooks during 6 months (1 h/week) and found applicability of the system, including patients without digital competence. It means patients can keep practicing to improve their language skills beyond a therapy discharge using a low cost TR system.
Additionally, Uslu et al. (2020) introduced a protocol for home TR using tablet-based app with combination of speech/language therapy and cognitive training. Specifically, 100 aphasia outpatients will be assigned to parallel groups. The first group will spend 80% of their time training a high-frequency TR speech/language therapy, the remaining 20% devote to cognitive TR. The control group will participate in the training in a vice versa order. Both groups will train every weekday (2 h/day) during a month.
More recently, Kim et al. (2021) proposed a protocol to support speech/language therapy of stroke patients. The TR group will utilize a tablet-based app for aphasia patients to practice exercises using evidence-based anomia treatments, phonological components, and semantic feature analysis during weekdays (1 h/day) over 5 weeks.
Another study explored a web-based oral reading for chronic aphasia patients TR (Cherney, Lee, Kim, & van Vuuren, 2021). In the beginning, 19 patients (MA 58.27) and 13 individuals (MA 55.19) were assigned to TR and control groups. All patients underwent 90 min/day unsupervised training over 6 days/week for six weeks. The TR group used a laptop, a web-camera, a headset, a digital therapist technology, providing individuals reading aloud sentences/paragraphs. First, the patients spoke simultaneously with the digital therapist, and then they performed tasks independently. The control group trained using a placebo computer game. Thus, TR group improved Western Aphasia Battery-Revised Language Quotient test (WABQT), 4.53, p < 0.001, from the baseline to 6 weeks follow-up. A mean difference between TR and control groups for pre to six-week follow-up gains was 2.7 (p < 0.05).
Another study (Øra et al., 2020) investigated whether augmented TR for 30 aphasia stroke patients (MA 64.4) was effective, feasible, and acceptable. The TR consisted of 1 h sessions 5 times/week during 1 month. The TR system included a laptop with pre-installed software and treatment materials, a speakerphone, a webcam, a wireless computer mouse facilitated participants’ control of the pointer. In the beginning, a speech-language pathologist connected to the patient’s computer using videoconferencing and remote-control software to share presentations and choose the materials for each session. Then the speech/language therapy was provided via videoconferencing. The study proves synchronous TR for aphasia patients are feasible and acceptable in addition to usual care. It showed a high satisfaction score among patients and pathologists with tolerable technical fault rates (≤15.9%). TR provides a viable service delivery model for aphasia rehabilitation. However, when developing TR services the access to clinical and technical expertise is needed.
Braley et al. (2021) explored the feasibility and clinical efficacy of a virtual speech, language, and cognitive TR for 32 individuals with aphasia against CR. Specifically, 17 patients (MA 59.8, TSS 53 months) and 15 individuals (MA 64.2, TSS 36.1 months) underwent 10 weeks treatment with intensity of 30 min/day during 5 days/week. The TR system consisted of a tablet with in-built speech, language, and cognitive therapy applications. The TR group received treatment using digital therapeutic exercises, while the control group performed home-based activities using paper workbooks. The study demonstrated the feasibility and safety of a virtual trial for patients, especially during COVID19 pandemic. The TR group showed 6.36 points higher of WABQT than the control group at follow-up to baseline (p < 0.01).
Dial et al. (2019) examined the rehabilitation of 31 patients with primary progressive aphasia. TR group (14 participants) trained via videoconferencing using a PC/tablet, a web-camera, and preinstalled software. Depending on the aphasia phenotype, three treatment protocols were utilized (2–naming impairment, 1–speech production and fluency). The control group (17 patients) underwent CR in-person using paper-pencil tests or via PC. Participants received 1–2 sessions/week. Both groups demonstrated similar outcomes.
Peñaloza et al. (2021) found insignificant differences between in-person treatment and TR of 16 chronic stroke patients with aphasia. Half of the patients (MA 59.23) underwent TR, while the rest (MA 54.63) performed in-person training. The TR system consisted of a PC, a microphone, speakers, a webcam, a mouse, the Zoom software to perform and record online sessions via videoconferencing, and internet-based Qualtrics survey software. Both groups used TR system and underwent 20 supervised sessions of 2 h each performed 2 times/week with similar treatment protocols. However, for in-person session the patient and the therapist sat together in a room of institution, since for TR group patients performed training at home.
Jacobs et al. (2021) evaluated the cost and benefits of 18 aphasia stroke patients TR using language-oriented treatment via videoconferencing. Although each one-point of improvement cost depended on the aphasia type ranged US$89–US$864, the largest outcomes were for patients with global aphasia.
Studies show TR is feasible and effective for patients with aphasia and cognitive-linguistic communication disorders, improving clinical outcomes. It implements suitable treatment plans via synchronous, asynchronous or mixed approaches gaining similar or greater improvements than CR. These effects can be due to higher intensity and duration of TR because patients train online with therapists, practice offline using applications and therapeutic VE. Those increase patients’ motivation, engagement and treatment adherence. Our observations are in line with Luisa et al. (2021) indicating similar outcomes of TR and CR for aphasia patients. Their review concluded TR facilitates access of patients to healthcare services, keeps treatment intensity at a desired level after patients’ discharge from the hospital. However, they underline low quality of the evidence among reviewed papers and recommend investigating TR on larger samples in the future.
3.4Summarizing results
To sum up, most studies prove feasibility of TR for stroke survivors and at least similar or more pronounced effects than CR, enhancing motivation and engagement, leading to sustainable recovery. TR is used for stroke patients with extremity disorders, balance problems, spatial neglect, speech, language, and memory impairments. Physical and cognitive activity of patients via systematic TR training and repetition leads to the neuroplasticity of the brain improving patients’ health and quality of life. TR can be used as complementary therapy or as an alternative to CR for patients with subacute and chronic stroke. Moreover, TR is organized synchronously or asynchronously, providing different opportunities for patients and healthcare professionals.
Another important finding is that TR can be used during pandemic or relating events. Recent COVID-19 pandemic shows the CR is hard to implement under lockdown, therefore, TR can be used instead granting access to health services of a large number of patients. Our review is in line with Iodice et al. (2021) underlining the global emergency due to COVID-19 pandemic empowered the development of digital technologies and diffusing TR for stroke patients. Moreover, TR is becoming an essential part of healthcare system reducing risks of cardiovascular diseases among patients, including those living in remote areas (Vilme et al., 2019), around the globe, and in regions with the lack of socioeconomic resources (Sarfo et al., 2018).
In our opinion, these findings would be valuable for the multidisciplinary professionals who are responsible for the management and implementation of post-stroke individuals’ rehabilitation (e.g., therapists, neuropsychologists, public health stakeholders and policymakers).
Additionally, the review works for technical and IT professionals demonstrating most currently available systems (or their parts) for TR of stroke patients. Those include a computer (PC, laptop, tablet, interactive touchscreen, etc.), a smartphone, a monitor, a web-camera, a motion-tracking sensor, a data storage system, a digital application or a web-based platform for training via videoconferencing or using exercises/exergames with non-immersive VR. Depending on the aim and tasks, a mouse, a joystick, physical exercise objects with inbuilt mouse-devices, a smart board, a computerized board, or Wii balance board are used. Fewer TR systems contain immersive VR head-mounted displays. Moreover, they are equipped with a microphone, speakers, and headsets. Also, different software and means for TR are used; general examples of them are summarized in Table 2.
Table 2
Software and means for TR of stroke patients
| Type | Name |
| Videoconferencing | Zoom, WebEx, VSee, Google Hangouts, Adobe Connect, Skype, TCMeeting v6.0, Cisco Jabber/Acano |
| Apps | 9zest Stroke Rehab, Bern Aphasia, iAphasia, iBooks HP, teleSLT, teleCT, VoiceAdapt, Constant Therapy |
| Games | Kinect, Kinect SDK, Wii, Scratch 2.0, Rapael Clinic, Reh@City v2.0, customized games |
| Systems/platforms | MERLIN system, MNVR-Rehab software system, Rehabilitation Wayout in Responsive Home Environments - REWIRE, HomeCoRe, Web-based ORLA, Antari Home Care, LANR, HEAD virtual platform |
| Support | TeamViewer, LogMeIn, WhatsApp, email, phone calls |
Recent developments in software result in diffusion of web-based platforms, applications, single/multiuser games with simple interactions or using digital avatars and VE, task-specific exercises, linguistic exercises, virtual speech-language pathologist and other self-paced training apps. This software is commercial or custom-based. Also, commercial apps for videoconferencing are widely used.
On another note, because of this review more clinicians, therapists, healthcare professionals, and patients will know possible approaches to rehabilitation via TR and ways of its organization. Moreover, they can estimate perspectives of similar rehabilitation interventions to prospective groups of patients. Before TR patients are typically provided with face-to-face guidelines, instructions and education. During TR a remote support for patients and therapists is provided via internet-based software (e.g., TeamViewer, LogMeIn), messaging apps (e.g., WhatsApp), email or phone calls.
However, for practical use of TR by therapists substantial work remains to establish the optimal dose and intensity of TR. Sheehy et al. (2019) advocated home-based TR as best practice for patients and their families to achieve healthy functioning. Their study implies a sufficient increase of patients’ functions is observed after 15 h or more of daily home-based training according to their own pace and regimen. From our review, overall dose and timing of stroke patients TR are summarized in Table 3.
Table 3
Dose and timing of stroke patients TR
| Disabilities | Clinical staging of stroke | Number of patients | Session, min | Frequency, times/week | Overal duration | References | |
| weeks | hours | ||||||
| Extremities dysfunction | Chronic | 373 | 15–70 | 1–7 | 3–20 | 4–60 | (Y. Chen et al., 2020; Cramer et al., 2019; Escalante-Gonzalbo et al., 2021; Guillén-Climent et al., 2021; Hung et al., 2019; Jonghyun Kim et al., 2020; Park et al., 2019; Qiu et al., 2020; Rogers et al., 2019; Sarfo, Adusei, et al., 2018; Smith &Tomita, 2020; Szturm et al., 2021; Thielbar et al., 2020; Triandafilou et al., 2018) |
| Subacute | 74 | 45–60 | 4–5 | 2–6 | 8–22.5 | (Mekbib et al., 2021)(Laffont et al., 2020) | |
| Balance problems | Chronic | 54 | 40–90 | 4–6 | 2–3 | 8–15 | (S. C. Chen et al., 2021; Kannan et al., 2019) |
| Subacute | 30 | 15–30 | 2–5 | 1–4 | 1.25–4.5 | (Burgos et al., 2020; Cikajlo et al., 2020) | |
| Cognitive/memory | Chronic | 228 | 30–120 | 1–5 | 6–24 | 12–60 | (Faria et al., 2020; Gil-Pagés et al., 2018; Isernia et al., 2019; Lawson et al., 2020; Torrisi et al., 2019; Withiel et al., 2019) |
| Aphasia/linguistic communication disorders | Chronic | 236 | 30–120 | 1–6 | 4–24 | 20–100 | (Braley et al., 2021; Cherney et al., 2021; Dial et al., 2019; E. S. Kim et al., 2021; Kurland et al., 2018; Maresca et al., 2019; Meltzer et al., 2018; Øra et al., 2020; Peñaloza et al., 2021; Uslu et al., 2020) |
| Total: | 995 | 15–120 | 1–7 | 1–24 | 1.25–100 | – | |
In general, the duration of treatment sessions varied significantly, 15–120 min with frequency of 1–7 times/week during 1–24 weeks. However, the duration of TR for patients with motor function disorders was less than that one for cognitive and linguistic disorders. Among individuals with motor function disorders the subacute stroke patients underwent less intensive training (8–22.5 h for extremities, 1.25–4.5 h for balance rehabilitation) than chronic patients (4–60 h for extremities, 8–15 h for balance rehabilitation). Meanwhile, observed differences might affect the results reliability, and hence it is hard to choose the best treatment approach. Moreover, some individuals with cognitive disorders claimed TR training requires a significant cognitive demand and it must be reduced to 2–3 times/week. It is also unclear whether differences in clinical outcomes varied depending on stroke sides.
Furthermore, TR showed limitations. First, the lack or high cost of specialized equipment and software, which partially can be overcome by using conventional equipment, freeware software or with the support of healthcare organizations. Then, limiting availability of devices and technologies for patients and therapists appear. Moreover, not all general commercial games are tailored for TR needs meaning special software should be developed. Also, two-dimensional VR TR limits the design of UE training. On the contrary, although the immersive VR TR is beneficial its applicability and costs need to be further explored for UE and balance TR. Some studies outline sensors connection and calibration stability need further improvement. These challenges can facilitate technological development and become opportunities for equipment and software manufacturers.
Other challenges mean insufficient internet capacity and speed requiring for TR, especially among patients living in homes with different infrastructure. Moreover, synchronous TR might face low quality connection during peak hours. PC/tablets and apps do not always allow automatically track participants usage, remote monitoring and update the tasks difficulty. That is why some studies suggest using offline applications, since the internet access is required for loading exercises, sending reports and providing feedback. Others claim it is difficult to control the environmental conditions in which participants perform the tasks and ensure weather the training was accomplished during the unsupervised sessions. If organized inappropriately asynchronous TR can bring to the lack of compliance to therapeutic dose resulting in bias.
Currently, therapists are unable to provide hands on guidance, and thus, patients must rely on verbal instructions and visual demonstrations to understand the TR exercises. However, using remote haptic therapist-patient interactions with exoskeletons and robots might help to achieve promising outcomes in the future (Baur et al., 2019; Jonghyun Kim et al., 2020).
Stroke patients are typically older people who lack of technical proficiency in using TR services. Moreover, a deficit of proficiency in digital technologies and education arises for both stroke patients and healthcare professionals, which can be managed by utilizing easy-to-learn equipment, friendly interface, clear instructions or short courses, and trials before and at the beginning of TR programs. Since most studies underline the convenience of home-based exercises, some observations report the lack of space for exercises at patients’ homes, especially for patients with motor function disorders, although, they can be performed outdoors or with the support of relatives and carers. TR might reduce the workload of therapists, although most studies indicate technical issues require ongoing attention from the support team and development of troubleshooting technologies for both patients and therapists to sustain TR 24/7.
Also, there was no systematic research on the effect of patients’ health status and clinical outcomes. Main constrains of TR it is unsuitable for severely impaired patients, although the population in the studies varied depending on health status. Some studies show participants who undergo TR are aged 50–70s, since the mean age of stroke patients is 10–20 years older. The study (Jørgensen et al., 2021) recommends not starting TR for elders just after their hospital discharge, underlining the importance of supervised home-based TR for recovering after the acute phase of stroke.
The review revealed the need for additional research on safety issues associated with unsupervised home-based TR. Interestingly, one research indicated the Kinect sensor can be used only for individuals of minimum 1.2 m height to enable tracking, reducing potential number of participants. Although TR seems to be quite flexible, studies on cognitive TR report on its limited customizing. By contrast, others evidence TR is too specific and needs generalization.
Furthermore, most studies, except Jacobs et al. (2021), did not perform any economic analysis, evaluation of cost barriers, effectiveness ratio, reimbursement, and affordability, including off-the-shelf technologies. A cost reduction of TR can be partially achieved using open software and wider diffusion of hardware. Other barriers to wide spreading of TR are privacy concerns, system security, liability, daily use suitability, and fear.
Finally, content, usability and accessibility requirements of TR for stroke patients, informal caregivers, and healthcare professionals should be included in prospective programs of TR (Wentink et al., 2019). In addition to general principles of the ideal TR described by Nuara et al. (2021), including sufficient intensity, proper repetition, motivation sustainability, and engagement, a predictive, preventive, and personalized medicine approach should be implemented to achieve improvements in TR of stroke patients.
3.5Limitations
The review has several limitations. First, the publication and language-of-publication biases are aroused because the review contains only English-published studies. Next, most reviewed studies had a small sample size, some of them lack of randomization or a control group, having high data heterogeneity restricting generalization, direct comparison, and preventing quantitative evaluation of reviewed studies.
Furthermore, the findings should be interpreted with caution because the reviewed studies involved groups of patients with different disease characteristics, used different criteria, design interventions, and approaches to TR.
3.6Implications for future research
Future studies should concentrate on systematic research on large samples, analyzing effects of stroke type, disease severity, and cost-effectiveness assessment of TR. Also the evaluation of factors affecting home-based TR of stroke patients is required.
4Conclusions
TR is feasible and used as an alternative to conventional treatment or as complementary therapy significantly improving treatment outcomes. A variety of devices and software is utilized in stroke TR. TR is delivered synchronously, asynchronously or using hybrid approach. Studies reviewed prove similar or more pronounced effect of TR on motor, cognitive functions, aphasia, and speech-linguistic patients, their engagement and motivation, granting access to rehabilitation services of a large number of patients with immobility or living in remote areas. More importantly, TR provides remote treatment of stroke patients during a pandemic, reducing social isolation, psychological problems, leading to a sustainable recovery of stroke patients.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
1 | Adams, J. L. , Myers, T. L. , Waddell, E. M. , Spear, K. L. , Schneider, R. B. ((2020) ). Telemedicine: a Valuable Tool in Neurodegenerative Diseases. Current Geriatrics Reports, 9: (2), 72–81. https://doi.org/10.1007/s13670-020-00311-z |
2 | Aminov, A. , Rogers, J. M. , Middleton, S. , Caeyenberghs, K. , Wilson, P. H. ((2018) ). What do randomized controlled trials say about virtual rehabilitation in stroke? A systematic literature review and meta-analysis of upper-limb and cognitive outcomes. Journal of NeuroEngineering and Rehabilitation, 15: (1), 29. https://doi.org/10.1186/s12984-018-0370-2 |
3 | Baur, K. , Rohrbach, N. , Hermsdörfer, J. , Riener, R. , Klamroth-Marganska, V. ((2019) ). The “beam-Me-In Strategy” - Remote haptic therapist-patient interaction with two exoskeletons for stroke therapy. Journal of NeuroEngineering and Rehabilitation, 16: (1), 85. https://doi.org/10.1186/s12984-019-0547-3 |
4 | Bayley, M. T. , Hurdowar, A. , Richards, C. L. , Korner-Bitensky, N. , Wood-Dauphinee, S. , Eng, J. J. , . . . Graham I. D. ((2012) ). Barriers to implementation of stroke rehabilitation evidence: Findings from a multi-site pilot project. Disability and Rehabilitation, 34: (19), 1633–1638. https://doi.org/10.3109/09638288.2012.656790 |
5 | Bayoumy, K. , Gaber, M. , Elshafeey, A. , Mhaimeed, O. , Dineen, E. H. , Marvel, F. A. , . . . Elshazly M. B. ((2021) ). Smart wearable devices in cardiovascular care: where we are and how to move forward. Nature Reviews Cardiology, 18: (8), 581–599. https://doi.org/10.1038/s41569-021-00522-7 |
6 | Bernini, S. , Stasolla, F. , Panzarasa, S. , Quaglini, S. , Sinforiani, E. , Sandrini, G. , . . . Bottiroli S. ((2021) ). Cognitive Telerehabilitation for Older Adults With Neurodegenerative Diseases in the COVID-19 Era: A Perspective Study. Frontiers in Neurology, 11: , 623933. https://doi.org/10.3389/fneur.2020.623933 |
7 | Braley, M. , Pierce, J. S. , Saxena, S. , De Oliveira E. , Taraboanta, L. , Anantha, V. , . . . Kiran S. ((2021) ). A Virtual, Randomized, Control Trial of a Digital Therapeutic for Speech, Language, and Cognitive Intervention in Post-stroke Persons With Aphasia. Frontiers in Neurology, 12: , 626780. https://doi.org/10.3389/fneur.2021.626780 |
8 | Brennan, D. M. , Mawson, S. , Brownsell, S. ((2009) ). Telerehabilitation: Enabling the remote delivery of healthcare, rehabilitation, and self management. Studies in Health Technology and Informatics, 145: , 231–248. https://doi.org/10.3233/978-1-60750-018-6-231 |
9 | Buonocunto, P. , Giantomassi, A. , Marinoni, M. , Calvaresi, D. , Buttazzo, G. ((2018) ). A limb tracking platform for tele-rehabilitation. ACM Transactions on Cyber-Physical Systems, 2: , 1–23. https://doi.org/10.1145/3148225 |
10 | Burgos, P. I. , Lara, O. , Lavado, A. , Rojas-Sepúlveda, I. , Delgado, C. , Bravo, E. , . . . Cerda M. ((2020) ). Exergames and telerehabilitation on smartphones to improve balance in stroke patients. Brain Sciences, 10: (11), 773. https://doi.org/10.3390/brainsci10110773 |
11 | Chae, S. H. , Kim, Y. , Lee, K. S. , Park, H. S. ((2020) ). Development and clinical evaluation of a web-based upper limb home rehabilitation system using a smartwatch and machine learning model for chronic stroke survivors: Prospective comparative study. JMIR MHealth and UHealth, 8: (7), e17216. https://doi.org/10.2196/17216 |
12 | Chen, S. C. , Lin, C. H. , Su, S. W. , Chang, Y. T. , Lai, C. H. ((2021) ). Feasibility and effect of interactive telerehabilitation on balance in individuals with chronic stroke: a pilot study. Journal of NeuroEngineering and Rehabilitation, 18: (1), 71. https://doi.org/10.1186/s12984-021-00866-8 |
13 | Chen, Y. , Abel, K. T. , Janecek, J. T. , Chen, Y. , Zheng, K. , Cramer, S. C. ((2019) ). Home-based technologies for stroke rehabilitation: A systematic review. International Journal of Medical Informatics, 123: , 11–22. https://doi.org/10.1016/j.ijmedinf.2018.12.001 |
14 | Chen, Y. , Chen, Y. , Zheng, K. , Dodakian, L. , See, J. , Zhou, R. , . . . Cramer S. C. ((2020) ). A qualitative study on user acceptance of a home-based stroke telerehabilitation system. Topics in Stroke Rehabilitation, 27: (2), 81–92. https://doi.org/10.1080/10749357.2019.1683792 |
15 | Cherney, L. R. , Lee, J. B. , Kim, K. Y. A. , van Vuuren S. ((2021) ). Web-based Oral Reading for Language in Aphasia (Web ORLA®): A pilot randomized control trial. Clinical Rehabilitation, 35: (7), 976–987. https://doi.org/10.1177/0269215520988475 |
16 | Cikajlo, I. , Rudolf, M. , Mainetti, R. , Borghese, N. A. ((2020) ). Multi-Exergames to Set Targets and Supplement the Intensified Conventional Balance Training in Patients With Stroke: A Randomized Pilot Trial. Frontiers in Psychology, 11: , 572. https://doi.org/10.3389/fpsyg.2020.00572 |
17 | Cogollor, J. M. , Rojo-Lacal, J. , Hermsdörfer, J. , Ferre, M. , Teresa Arredondo Waldmeyer M. , Giachritsis, C. , . . . María Sebastián J. ((2018) ). Evolution of cognitive rehabilitation after stroke from traditional techniques to smart and personalized home-based information and communication technology systems: Literature review. Journal of Medical Internet Research, 5: (1), e4. https://doi.org/10.2196/rehab.8548 |
18 | Cramer, S. C. , Dodakian, L. , Le, V. , See, J. , Augsburger, R. , McKenzie, A. , . . . Janis S. ((2019) ). Efficacy of Home-Based Telerehabilitation vs In-Clinic Therapy for Adults after Stroke: A Randomized Clinical Trial. JAMA Neurology, 76: (9), 1079–1087. https://doi.org/10.1001/jamaneurol.2019.1604 |
19 | Denham, A. M. J. , Wynne, O. , Baker, A. L. , Spratt, N. J. , Bonevski, B. ((2020) ). The unmet needs of carers of stroke survivors: An evaluation of Google search results. Health Informatics Journal, 26: (2), 934–944. https://doi.org/10.1177/1460458219852530 |
20 | Dial, H. R. , Hinshelwood, H. A. , Grasso, S. M. , Hubbard, H. I. , Gorno-Tempini, M. L. , Henry, M. L. ((2019) ). Investigating the utility of teletherapy in individuals with primary progressive aphasia. Clinical Interventions in Aging, 14: , 453–471. https://doi.org/10.2147/CIA.S178878 |
21 | Donkor, E. S. ((2018) ). Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Research and Treatment, 2018: , 3238165. https://doi.org/10.1155/2018/3238165 |
22 | Doogan, C. , Dignam, J. , Copland, D. , Leff, A. ((2018) ). Aphasia Recovery: When, How and Who to Treat? Current Neurology and Neuroscience Reports, 18: (12), 90. https://doi.org/10.1007/s11910-018-0891-x |
23 | Enderby, P. , Pandyan, A. , Bowen, A. , Hearnden, D. , Ashburn, A. , Conroy, P. , . . . Winter, J. ((2017) ). Accessing rehabilitation after stroke–a guessing game? Disability and Rehabilitation, 39: (7), 709–713. https://doi.org/10.3109/09638288.2016.1160448 |
24 | Escalante-Gonzalbo, A. M. , Ramírez-Graullera, Y. S. , Pasantes, H. , Aguilar-Chalé, J. J. , Sánchez-Castillo, G. I. , Escutia-Macedo, X. A. , . . . Palafox, L. ((2021) ). Safety, Feasibility, and Acceptability of a New Virtual Rehabilitation Platform: A Supervised Pilot Study. Rehabilitation Process and Outcome, 10: , 1–13. https://doi.org/10.1177/11795727211033279 |
25 | Faria, A. L. , Faria, A. L. , Faria, A. L. , Pinho, M. S. , Pinho, M. S. , Bermúdez I Badia, S. , . . . Bermúdez I Badia, S. ((2020) ). A comon of two personalization and adaptive cognitive rehabilitation approaches: A randomized controlled trial with chronic stroke patients. Journal of NeuroEngineering and Rehabilitation, 17: (1), 78. https://doi.org/10.1186/s12984-020-00691-5 |
26 | Gammeri, R. , Iacono, C. , Ricci, R. , Salatino, A. ((2020) ). Unilateral spatial neglect after stroke: Current insights. Neuropsychiatric Disease and Treatment, 16: , 131–152. https://doi.org/10.2147/NDT.S171461 |
27 | Gerber, S. M. , Schütz, N. , Uslu, A. S. , Schmidt, N. , Röthlisberger, C. , Wyss, P. , . . . Nef, T. ((2019) ). Therapist-Guided Tablet-Based Telerehabilitation for Patients With Aphasia: Proof-of-Concept and Usability Study. JMIR Rehabilitation and Assistive Technologies, 6: (1), e13163. https://doi.org/10.2196/13163 |
28 | Gil-Pagés, M. , Solana, J. , Sánchez-Carrión, R. , Tormos, J. M. , Enseñat-Cantallops, A. , García-Molina, A. ((2018) ). A customized home-based computerized cognitive rehabilitation platform for patients with chronic-stage stroke: Study protocol for a randomized controlled trial. Trials, 19: (1), 191. https://doi.org/10.1186/s13063-018-2577-8 |
29 | Gruska, M. , Aigner, G. , Altenberger, J. , Burkart-Küttner, D. , Fiedler, L. , Gwechenberger, M. , . . . Teubl, A. ((2020) ). Recommendations on the utilization of telemedicine in cardiology. Wiener Klinische Wochenschrift, 132: (23-24), 782–800. https://doi.org/10.1007/s00508-020-01762-2 |
30 | Guillén-Climent, S. , Garzo, A. , Muñoz-Alcaraz, M. N. , Casado-Adam, P. , Arcas-Ruiz-Ruano, J. , Mejías-Ruiz, M. , Mayordomo-Riera, F. J. ((2021) ). A usability study in patients with stroke using MERLIN, a robotic system based on serious games for upper limb rehabilitation in the home setting. Journal of NeuroEngineering and Rehabilitation, 18: (1), 41. https://doi.org/10.1186/s12984-021-00837-z |
31 | Hughes, C. M. L. , Louie, A. , Sun, S. , Gordon-Murer, C. , Belay, G. J. , Baye, M. , Zhang, X. ((2019) ). Development of a Post-stroke Upper Limb Rehabilitation Wearable Sensor for Use in Sub-Saharan Africa: A Pilot Validation Study. Frontiers in Bioengineering and Biotechnology, 7: , 322. https://doi.org/10.3389/fbioe.2019.00322 |
32 | Hung, J. W. , Chou, C. X. , Chang, Y. J. , Wu, C. Y. , Chang, K. C. , Wu, W. C. , Howell, S. ((2019) ). Comparison of Kinect2Scratch game-based training and therapist-based training for the improvement of upper extremity functions of patients with chronic stroke: A randomized controlled single-blinded trial. European Journal of Physical and Rehabilitation Medicine, 55: (5), 542–550. https://doi.org/10.23736/S1973-9087.19.05598-9 |
33 | Hung KN, G. , Fong, K. N. K. ((2019) ). Effects of telerehabilitation in occupational therapy practice: A systematic review. Hong Kong Journal of Occupational Therapy, 32: (1), 3–21. https://doi.org/10.1177/1569186119849119 |
34 | Iodice, F. , Romoli, M. , Giometto, B. , Clerico, M. , Tedeschi, G. , Bonavita, S. , . . . Lavorgna, L. ((2021) ). Stroke and digital technology: a wake-up call from COVID-19 pandemic. Neurological Sciences, 42: (3), 805–809. https://doi.org/10.1007/s10072-020-04993-3 |
35 | Isernia, S. , Pagliari, C. , Jonsdottir, J. , Castiglioni, C. , Gindri, P. , Gramigna, C. , . . . Baglio, F. ((2019) ). Efficiency and Patient-Reported Outcome Measures From Clinic to Home: The Human Empowerment Aging and Disability Program for Digital-Health Rehabilitation. Frontiers in Neurology, 10: , 1206. https://doi.org/10.3389/fneur.2019.01206 |
36 | Jacobs, M. , Briley, P. M. , Wright, H. H. , Ellis, C. ((2021) ). Marginal assessment of the cost and benefits of aphasia treatment: Evidence from community-based telerehabilitation treatment for aphasia. Journal of Telemedicine and Telecare, 1357633X20982773: . https://doi.org/10.1177/1357633X20982773 |
37 | Johnson, W. , Onuma, O. , Owolabi, M. , Sachdev, S. ((2016) ). Stroke: A global response is needed. Bulletin of the World Health Organization, 94: (9), 634–634A. https://doi.org/10.2471/BLT.16.181636 |
38 | Jørgensen, B. B. , Gregersen, M. , Pallesen, S. H. , Damsgaard, E. M. ((2021) ). A group-based real-time videoconferencing telerehabilitation programme in recently discharged geriatric patients: a feasibility study. European Geriatric Medicine, 12: (4), 801–808. https://doi.org/10.1007/s41999-020-00444-6 |
39 | Kalavina, R. , Chisati, E. , Mlenzana, N. , Wazakili, M. ((2019) ). The challenges and experiences of stroke patients and their spouses in Blantyre, Malawi. Malawi Medical Journal, 31: (2), 112–117. https://doi.org/10.4314/mmj.v31i2.2 |
40 | Kannan, L. , Vora, J. , Bhatt, T. , Hughes, S. L. ((2019) ). Cognitive-motor exergaming for reducing fall risk in people with chronic stroke: A randomized controlled trial. NeuroRehabilitation, 44: (4), 493–510. https://doi.org/10.3233/NRE-182683 |
41 | Kim, E. S. , Laird, L. , Wilson, C. , Bieg, T. , Mildner, P. , Möller, S. , . . . Rochon, E. ((2021) ). Implementation and effects of an information technology-based intervention to support speech and language therapy among stroke patients with aphasia: Protocol for a virtual randomized controlled trial. JMIR Research Protocols, 10: (7), e30621. https://doi.org/10.2196/30621 |
42 | Kim, Jonghyun, Sin, M. , Kim, W. S. , Min, Y. S. , Kim, W. , Paik, N. J. , . . . Park, H. S. ((2020) ). Remote Assessment of Post-Stroke Elbow Function Using Internet-Based Telerobotics: A Proof-of-Concept Study. Frontiers in Neurology, 11: , 640537. https://doi.org/10.3389/fneur.2020.583101 |
43 | Kim, Joosup, Thayabaranathan, T. , Donnan, G. A. , Howard, G. , Howard, V. J. , Rothwell, P. M. , . . . Thrift, A. G. ((2020) ). Global Stroke Statistics 2019. International Journal of Stroke, 15: (8), 819–838. https://doi.org/10.1177/1747493020909545 |
44 | Kurland, J. , Liu, A. , Stokes, P. ((2018) ). Effects of a tablet-based home practice program with telepractice on treatment outcomes in chronic aphasia. Journal of Speech, Language, and Hearing Research, 61: (5), 1140–1156. https://doi.org/10.1044/2018_JSLHR-L-17-0277 |
45 | Laffont, I. , Froger, J. , Jourdan, C. , Bakhti, K. , van Dokkum, L. E. H. , Gouaich, A. , . . . Mottet, D. ((2020) ). Rehabilitation of the upper arm early after stroke: Video games versus conventional rehabilitation. A randomized controlled trial. Annals of Physical and Rehabilitation Medicine, 63: (3), 173–180. https://doi.org/10.1016/j.rehab.2019.10.009 |
46 | Laver, K. E. , Adey-Wakeling, Z. , Crotty, M. , Lannin, N. A. , George, S. , Sherrington, C. ((2020) ). Telerehabilitation services for stroke. Cochrane Database of Systematic Reviews, 1: (1), CD010255. https://doi.org/10.1002/14651858.CD010255.pub3 |
47 | Lawson, D. W. , Stolwyk, R. J. , Ponsford, J. L. , McKenzie, D. P. , Downing, M. G. , Wong, D. ((2020) ). Telehealth Delivery of Memory Rehabilitation Following Stroke. Journal of the International Neuropsychological Society, 26: (1), 58–71. https://doi.org/10.1017/S1355617719000651 |
48 | Luisa, C. , Pawel, K. , Martina, G. , Francesca, B. , Sara, F. , Andrea, T. , Michela, A. ((2021) ). Telerehabilitation for people with aphasia: A systematic review and meta-analysis. Journal of Communication Disorders, 92: , 106111. https://doi.org/10.1016/j.jcomdis.2021.106111 |
49 | MacEira-Elvira, P. , Popa, T. , Schmid, A. C. , Hummel, F. C. ((2019) ). Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. Journal of NeuroEngineering and Rehabilitation, 16: (1), 142. https://doi.org/10.1186/s12984-019-0612-y |
50 | Magwood, G. S. , Ellis, C. , Nichols, M. , Burns, S. P. , Jenkins, C. , Woodbury, M. , Adams, R. ((2019) ). Barriers and Facilitators of Stroke Recovery: Perspectives From African Americans With Stroke, Caregivers and Healthcare Professionals. Journal of Stroke and Cerebrovascular Diseases, 28: (9), 2506–2516. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.06.012 |
51 | Mantovani, E. , Zucchella, C. , Bottiroli, S. , Federico, A. , Giugno, R. , Sandrini, G. , . . . Tamburin, S. ((2020) ). Telemedicine and Virtual Reality for Cognitive Rehabilitation: A Roadmap for the COVID- Pandemic. Frontiers in Neurology, 11: , 926. https://doi.org/10.3389/fneur.2020.00926 |
52 | Maresca, G. , Maggio, M. G. , Latella, D. , Cannavò, A. , De Cola, M. C. , Portaro, S. , . . . Calabrò, R. S. ((2019) ). Toward Improving Poststroke Aphasia: A Pilot Study on the Growing Use of Telerehabilitation for the Continuity of Care. Journal of Stroke and Cerebrovascular Diseases, 28: (10), 104303. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104303 |
53 | Mekbib, D. B. , Debeli, D. K. , Zhang, L. , Fang, S. , Shao, Y. , Yang, W. , . . . Xu, D. ((2021) ). A novel fully immersive virtual reality environment for upper extremity rehabilitation in patients with stroke. Annals of the New York Academy of Sciences, 1493: (1), 75–89. https://doi.org/10.1111/nyas.14554 |
54 | Meltzer, J. A. , Baird, A. J. , Steele, R. D. , Harvey, S. J. ((2018) ). Computer-based treatment of poststroke language disorders: a non-inferiority study of telerehabilitation compared to in-person service delivery. Aphasiology, 32: (3), 290–311. https://doi.org/10.1080/02687038.2017.1355440 |
55 | Morone, G. , Spitoni, G. F. , De Bartolo, D. , Ghanbari Ghooshchy, S. , Di Iulio, F. , Paolucci, S. , . . . Iosa, M. ((2019) ). Rehabilitative devices for a top-down approach. Expert Review of Medical Devices, 16: (3), 187–195. https://doi.org/10.1080/17434440.2019.1574567 |
56 | Morse, H. , Biggart, L. , Pomeroy, V. , Rossit, S. (2020). Exploring perspectives from stroke survivors, carers and clinicians on virtual reality as a precursor to using telerehabilitation for spatial neglect post-stroke. Neuropsychological Rehabilitation. https://doi.org/10.1080/09602011.2020.1819827 |
57 | Mura, G. , Carta, M. G. , Sancassiani, F. , Machado, S. , Prosperini, L. ((2018) ). Active exergames to improve cognitive functioning in neurological disabilities: A systematic review and meta-analysis. European Journal of Physical and Rehabilitation Medicine, 54: (3), 450–462. https://doi.org/10.23736/S1973-9087.17.04680-9 |
58 | Nie, P. , Liu, F. , Lin, S. , Guo, J. , Chen, X. , Chen, S. , . . . Lin, R. (2021). The effects of computer-assisted cognitive rehabilitation on cognitive impairment after stroke: A systematic review and meta-analysis. Journal of Clinical Nursing. https://doi.org/10.1111/jocn.16030 |
59 | Nuara, A. , Fabbri-Destro, M. , Scalona, E. , Lenzi, S. E. , Rizzolatti, G. , Avanzini, P. ((2021) ). Telerehabilitation in response to constrained physical distance: an opportunity to rethink neurorehabilitative routines. Journal of Neurology, 1–12. https://doi.org/10.1007/s00415-021-10397-w |
60 | O’Neil, O. , Fernandez, M. M. , Herzog, J. , Beorchia, M. , Gower, V. , Gramatica, F. , . . . Kiwull, L. ((2018) ). Virtual Reality for Neurorehabilitation: Insights From 3 European Clinics. S-S.. PM and R, 10: (9S2), S198–S206. https://doi.org/10.1016/j.pmrj.2018.08.375 |
61 | Øra, H. P. , Kirmess, M. , Brady, M. C. , Sørli, H. , Becker, F. ((2020) ). Technical Features, Feasibility, and Acceptability of Augmented Telerehabilitation in Post-stroke Aphasia— Experiences From a Randomized Controlled Trial. Frontiers in Neurology, 11: , 671. https://doi.org/10.3389/fneur.2020.00671 |
62 | Park, M. , Ko, M. H. , Oh, S. W. , Lee, J. Y. , Ham, Y. , Yi, H. , . . . Shin, J. H. ((2019) ). Effects of virtual reality-based planar motion exercises on upper extremity function, range of motion, and health-related quality of life: A multicenter, single-blinded, randomized, controlled pilot study. Journal of NeuroEngineering and Rehabilitation, 16: (1), 122. https://doi.org/10.1186/s12984-019-0595-8 |
63 | Peñaloza, C. , Scimeca, M. , Gaona, A. , Carpenter, E. , Mukadam, N. , Gray, T. , . . . Kiran, S. ((2021) ). Telerehabilitation for Word Retrieval Deficits in Bilinguals With Aphasia: Effectiveness and Reliability as Compared to In-person Language Therapy. Frontiers in Neurology, 12: , 589330. https://doi.org/10.3389/fneur.2021.589330 |
64 | Perrochon, A. , Borel, B. , Istrate, D. , Compagnat, M. , Daviet, J. C. ((2019) ). Exercise-based games interventions at home in individuals with a neurological disease: A systematic review and meta-analysis. Annals of Physical and Rehabilitation Medicine, 62: (5), 366–378. https://doi.org/10.1016/j.rehab.2019.04.004 |
65 | Porciuncula, F. , Roto, A. V. , Kumar, D. , Davis, I. , Roy, S. , Walsh, C. J. , Awad, L. N. ((2018) ). Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PM and R, 10: (9S2), S220–S232. https://doi.org/10.1016/j.pmrj.2018.06.013 |
66 | Qiu, Q. , Cronce, A. , Patel, J. , Fluet, G. G. , Mont, A. J. , Merians, A. S. , Adamovich, S. V. ((2020) ). Development of the Home based Virtual Rehabilitation System (HoVRS) to remotely deliver an intense and customized upper extremity training. Journal of NeuroEngineering and Rehabilitation, 17: (1), 155. https://doi.org/10.1186/s12984-020-00789-w |
67 | Rogers, J. M. , Duckworth, J. , Middleton, S. , Steenbergen, B. , Wilson, P. H. ((2019) ). Elements virtual rehabilitation improves motor, cognitive, and functional outcomes in adult stroke: Evidence from a randomized controlled pilot study. Journal of NeuroEngineering and Rehabilitation, 16: (1), 56. https://doi.org/10.1186/s12984-019-0531-y |
68 | Sarfo, F. S. , Adusei, N. , Ampofo, M. , Kpeme, F. K. , Ovbiagele, B. ((2018) ). Pilot trial of a tele-rehab intervention to improve outcomes after stroke in Ghana: A feasibility and user satisfaction study. Journal of the Neurological Sciences, 387: , 94–97. https://doi.org/10.1016/j.jns.2018.01.039 |
69 | Sarfo, F. S. , Ulasavets, U. , Opare-Sem, O. K. , Ovbiagele, B. ((2018) ). Tele-Rehabilitation after Stroke: An Updated Systematic Review of the Literature. Journal of Stroke and Cerebrovascular Diseases, 27: (9), 2306–2318. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.05.013 |
70 | Schröder, J. , van Criekinge, T. , Embrechts, E. , Celis, X. , Van Schuppen, J. , Truijen, S. , Saeys, W. ((2019) ). Combining the benefits of tele-rehabilitation and virtual reality-based balance training: a systematic review on feasibility and effectiveness. Disability and Rehabilitation: Assistive Technology, 14: (1), 2–11. https://doi.org/10.1080/17483107.2018.1503738 |
71 | Sheehy, L. , Taillon-Hobson, A. , Sveistrup, H. , Bilodeau, M. , Yang, C. , Welch, V. , . . . Finestone, H. ((2019) ). Home-based virtual reality training after discharge from hospital-based stroke rehabilitation: A parallel randomized feasibility trial. Trials, 20: (1), 333. https://doi.org/10.1186/s13063-019-3438-9 |
72 | Smith, M. A. , Tomita, M. R. ((2020) ). Combined effects of telehealth and modified constraint-induced movement therapy for individuals with chronic hemiparesis. International Journal of Telerehabilitation, 12: (1), 51–62. https://doi.org/10.5195/ijt.2020.6300 |
73 | Stinear, C. M. , Lang, C. E. , Zeiler, S. , Byblow, W. D. ((2020) ). Advances and challenges in stroke rehabilitation. The Lancet Neurology, 19: (4), 348–360. https://doi.org/10.1016/S1474-4422(19)30415-6 |
74 | Szturm, T. , Imran, Z. , Pooyania, S. , Kanitkar, A. , Mahana, B. ((2021) ). Evaluation of a Game Based Tele Rehabilitation Platform for In-Home Therapy of Hand-Arm Function Post Stroke: Feasibility Study. PM and R, 13: (1), 45–54. https://doi.org/10.1002/pmrj.12354 |
75 | The 9zest Stroke Rehab. (n.d.). Retrieved February 27, 2021, from 9zest website: https://9zest.com/stroke |
76 | Tchero, H. , Teguo, M. T. , Lannuzel, A. , Rusch, E. ((2018) ). Telerehabilitation for stroke survivors: Systematic review and meta-analysis. Journal of Medical Internet Research, 20: (10), e10867. https://doi.org/10.2196/10867 |
77 | Thielbar, K. O. , Triandafilou, K. M. , Barry, A. J. , Yuan, N. , Nishimoto, A. , Johnson, J. , . . . Kamper, D. G. ((2020) ). Home-based Upper Extremity Stroke Therapy Using a Multiuser Virtual Reality Environment: A Randomized Trial. Archives of Physical Medicine and Rehabilitation, 101: (2), 196–203. https://doi.org/10.1016/j.apmr.2019.10.182 |
78 | Torrisi, M. , Maresca, G. , De Cola, M. C. , Cannavò, A. , Sciarrone, F. , Silvestri, G. , . . . Calabrò, R. S. ((2019) ). Using telerehabilitation to improve cognitive function in post-stroke survivors: is this the time for the continuity of care? International Journal of Rehabilitation Research, 42: (4), 344–351. https://doi.org/10.1097/MRR.0000000000000369 |
79 | Triandafilou, K. M. , Tsoupikova, D. , Barry, A. J. , Thielbar, K. N. , Stoykov, N. , Kamper, D. G. ((2018) ). Development of a 3D, networked multi-user virtual reality environment for home therapy after stroke. Journal of NeuroEngineering and Rehabilitation, 15: (1), 88. https://doi.org/10.1186/s12984-018-0429-0 |
80 | Uslu, A. S. , Gerber, S. M. , Schmidt, N. , Röthlisberger, C. , Wyss, P. , Vanbellingen, T. , . . . Urwyler, P. ((2020) ). Investigating a new tablet-based telerehabilitation app in patients with aphasia: A randomised, controlled, evaluator-blinded, multicentre trial protocol. BMJ Open, 10: , e037702. https://doi.org/10.1136/bmjopen-2020-037702 |
81 | Vilme, H. , Duke, N. N. , Muiruri, C. , Wordlaw, L. S. , Skinner, A. C. ((2019) ). Using Telehealth to Disseminate Primary, Secondary, and Tertiary CVD Interventions to Rural Populations. Current Hypertension Reports, 21: (12), 92. https://doi.org/10.1007/s11906-019-0998-8 |
82 | Waller, M. , Stotler, C. ((2018) ). Telemedicine: a Primer. Current Allergy and Asthma Reports, 18: (10), 54. https://doi.org/10.1007/s11882-018-0808-4 |
83 | Wentink, M. M. , Bodegom-Vos, Van L. , Brouns, B. , Arwert, H. J. , Vliet Vlieland, T. P. M. , De Kloet, A. J. , Meesters, J. J. L. ((2018) ). What is Important in E-health Interventions for Stroke Rehabilitation? A Survey Study among Patients, Informal Caregivers, and Health Professionals. International Journal of Telerehabilitation, 10: (1), 15–28. https://doi.org/10.5195/ijt.2018.6247 |
84 | Wentink, M. , Van Bodegom-Vos, L. , Brouns, B. , Arwert, H. , Houdijk, S. , Kewalbansing, P. , . . . Meesters, J. ((2019) ). How to improve eRehabilitation programs in stroke care? A focus group study to identify requirements of end-users. BMC Medical Informatics and Decision Making, 19: (1), 145. https://doi.org/10.1186/s12911-019-0871-3 |
85 | World Health Organization. (2011), World Report on Disability 2011. https://www.who.int/disabilities/world_report/2011/report.pdf |
86 | Withiel, T. D. , Wong, D. , Ponsford, J. L. , Cadilhac, D. A. , New, P. , Mihaljcic, T. , Stolwyk, R. J. ((2019) ). Comparing memory group training and computerized cognitive training for improving memory function following stroke: A phase II randomized controlled trial. Journal of Rehabilitation Medicine, 51: (5), 343–351. https://doi.org/10.2340/16501977-2540 |
87 | Wu, Z. , Xu, J. , Yue, C. , Li, Y. , Liang, Y. ((2020) ). Collaborative Care Model Based Telerehabilitation Exercise Training Program for Acute Stroke Patients in China: A Randomized Controlled Trial. Journal of Stroke and Cerebrovascular Diseases, 29: (12), 105328. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105328 |




