Efficacy and safety of onabotulinumtoxinA with standardized physiotherapy for the treatment of pediatric lower limb spasticity: A randomized, placebo-controlled, phase III clinical trial
Abstract
BACKGROUND:
Spasticity is common in cerebral palsy and can result in pain and diminished health-related quality of life.
OBJECTIVE:
To evaluate the safety and efficacy of onabotulinumtoxinA for lower limb spasticity treatment in children with cerebral palsy.
METHODS:
In this registrational phase 3, multinational, randomized, double-blind, placebo-controlled trial (NCT01603628), children (2–< 17 years) with cerebral palsy and ankle spasticity (Modified Ashworth Scale-Bohannon [MAS] score≥2) were randomized 1 : 1 : 1 to standardized physical therapy and onabotulinumtoxinA (4 or 8 U/kg), or placebo. Primary endpoint was average change from baseline at weeks 4 and 6 in MAS ankle score. Secondary endpoints included the Modified Tardieu Scale (MTS) and Global Attainment Scale (GAS).
RESULTS:
381 participants were randomized. MAS scores averaged at weeks 4 and 6 were significantly reduced with both onabotulinumtoxinA doses (8 U/kg: –1.06, p = 0.010; 4 U/kg: –1.01, p = 0.033) versus placebo (–0.8). Significant improvements in average dynamic component of spasticity, measured by MTS, and in function, measured by GAS, were observed at several time points with both onabotulinumtoxinA doses versus placebo. Most adverse events were mild or moderate.
CONCLUSIONS:
OnabotulinumtoxinA was well tolerated and effective in reducing lower limb spasticity and improving functional outcomes versus placebo in children.
1Background
Cerebral palsy (CP) is a chronic disabling condition in children that primarily affects mobility, cognitive function, and dexterity (Bax et al., 2005; Golubovic & Slavković, 2014). In the United States the prevalence of CP is estimated to range from 3.1 to 3.6 per 1000 among 8-year-old children (Christensen et al., 2014; Yeargin-Allsopp et al., 2008). Approximately 90% of all cases of CP involve some form of spasticity (Beckung et al., 2007; Reid et al., 2011). Spasticity is defined as a motor disorder characterized by a velocity-dependent increase in the tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflexes as one component of upper motor neuron syndrome (Lance, 1980). Spasticity causes muscle shortening (Lieber et al., 2004) and prevents physiologic derotation of bone because spasticity alters the normal forces affecting bony remodeling with typical ambulation resulting in poor bone development. Therefore, chronic spasticity can result in bony deformity such as coxa valga (Cho et al., 2018), and persistent femoral anteversion (Karabicak et al., 2016), requiring multiple orthopedic surgeries for joint contractures and can worsen quality of life in children with CP (Arnaud et al., 2008; Ostensjø et al., 2004; Parkinson et al., 2010).
Non-pharmacological treatments for spasticity in children with CP include physical and occupational therapy (which may include application of cold or heat as well as stretching exercises and positioning), orthotics, casting, splinting, and electrical stimulation (Awaad & Rizk, 2012; Shamsoddini et al., 2014; Strobl et al., 2015). Pharmacological treatment options include oral (baclofen, diazepam, clonazepam, dantrolene sodium, clonidine, and tizanidine), intramuscular (botulinum toxin), or intrathecal (baclofen) medications, and phenol or alcohol as nerve blocking agents (Awaad & Rizk, 2012; Delgado et al., 2010; Shamsoddini et al., 2014; Strobl et al., 2015). Surgical procedures can include selective dorsal rhizotomy and orthopedic surgery (Awaad & Rizk, 2012; Shamsoddini et al., 2014).
Botulinum toxin A has been utilized for decades as a well-tolerated and effective management tool for adults and children with spasticity. In 1994, Koman and colleagues reported that botulinum toxin A was safe and effective in treating dynamic equinus deformity in a randomized double-blind study in children with CP (Koman et al., 1994). Multiple studies have also demonstrated that botulinum toxin A can improve gait parameters, spasticity, and function (Blumetti et al., 2019; Camargo et al., 2009; Coutinho dos Santos et al., 2011; Delgado et al., 2016; Sutherland et al., 1999), and orthopedic surgeries to manage spasticity in children with CP have decreased since the introduction of botulinum toxin (Molenaers et al., 2010).
OnabotulinumtoxinA (BOTOX®; Allergan, an AbbVie company, Irvine, CA, USA) is approved for the treatment of upper and/or lower limb spasticity in adults in most countries, including the United States (Allergan plc, 2019). Recently, onabotulinumtoxinA was approved in the United States for the treatment of upper and lower limb spasticity in children (Allergan, 2020).
Here we report the results of the registrational study that led to the approval of onabotulinumtoxinA for the treatment of lower limb spasticity in children. This multicenter, randomized, double-blind, placebo-controlled study evaluated the safety, efficacy, and dose-related responses of a single treatment of onabotulinumtoxinA for lower limb spasticity in children aged 2 to < 17 years with CP also receiving standardized physical therapy (PT). This study was specifically designed to fulfill the rigors required for registration purposes with the Food and Drug Administration. It was hypothesized that the reduction in spasticity and improvement in functional measures would be significantly better in children who were treated with onabotulinumtoxinA and PT than those treated with placebo and PT. A comprehensive assessment of spasticity was provided using the Modified Ashworth Scale-Bohannon (MAS), which measures the resistance encountered to passive stretch to assess changes in muscle tone, and the Modified Tardieu Scale (MTS), which measures the difference between slow and fast range of motion.
2Methods
This international, multicenter, randomized, double-blind, placebo-controlled, 12-week, phase 3 trial (ClinicalTrials.gov identifier, NCT01603628) was conducted at 49 sites in Hungary, Italy, Philippines, Poland, Russia, South Korea, Thailand, Turkey, and the United States between 11 September 2012 and 28 June 2017. The distribution of patients across the study centers is summarized in Supplementary Table 1. The study was approved by Institutional Review Boards or Independent Ethics Committees and was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from parents/guardians along with written minor assent.
2.1Participants
Inclusion criteria included medically stable monoplegic or hemiplegic children (aged 2 to < 17 years) with a diagnosis of CP with ankle spasticity (MAS (Bohannon & Smith, 1987) score≥2 [unadjusted value]); a minimum weight of 10 kg/22 lb; Gross Motor Function Classification System Expanded and Revised (GMFCS-E&R) level I to IV; and a stable dosage regimen for any anti-spasticity/anti-epileptic medications (for≥30 days before day 1 visit).
Key exclusion criteria included patients with any medical condition that might have put the participant at increased risk with exposure to botulinum toxin type A, such as myasthenia gravis or any other significant disease that might interfere with neuromuscular function, such as muscular dystrophy; uncontrolled epilepsy; fixed contracture of the ankle joint for the study limb; botulinum toxin therapy or phenol injections for any condition within 6 months of the day 1 visit (randomization and treatment start date); and previous serial casting within 6 months of the day 1 visit, orthopedic surgeries distal to the knee, use of dynamic splint within 3 months of the day 1 visit, or planned use of these or other spasticity-reducing or orthopedic treatments during the study for the study limb.
2.2Study treatment dose and injection sites
Patients were stratified by age (≤6 and > 6 years) and baseline MAS (Bohannon & Smith, 1987) ankle score with knee extended (MAS = 2 and MAS > 2) and were centrally randomized (1 : 1 : 1) through an interactive voice or web response system to 4 U/kg (total dose for the study limb not to exceed 150 U) onabotulinumtoxinA + PT, 8 U/kg (total dose for the study limb not to exceed 300 U) onabotulinumtoxinA + PT, or placebo (0.9% sterile sodium chloride) + PT. PT was standardized for all groups. OnabotulinumtoxinA doses and injection sites were selected based on clinical trial experience in pediatric lower limb spasticity, clinical expert advice, published literature including consensus guidelines for botulinum toxin type A treatment of pediatric spasticity, and nonclinical toxicology data.
Injections were administered to four sites in the gastrocnemius muscle (both medial and lateral heads), and to two sites for both the soleus and tibialis posterior muscles. In the gastrocnemius muscle, the 4 U/kg onabotulinumtoxinA group was injected with a total of 2 U/kg per muscle (not to exceed 75 U) and the 8 U/kg group was injected with 4 U/kg per muscle (not to exceed 150 U). In both the soleus and tibialis posterior muscles, the 4 U/kg group was injected with a total of 1 U/kg per muscle (not to exceed 37.5 U) and the 8 U/kg group was injected with 2 U/kg per muscle (not to exceed 75 U).
Muscles were localized via e-stimulation, sonography, and/or electromyography. Minimal or moderate sedation in addition to local anesthesia was allowed during the procedure. Throughout the study, all patients remained on standardized PT consisting of approximately 1-hour weekly sessions from 2 weeks prior to randomization through 11 weeks post-treatment.
Participants and all site personnel, except for the Independent Drug Reconstitutor (IDR), were blinded to study drug and dose administered. To ensure that the injector remained blinded, the IDR was responsible for preparing the study medication according to specific dilution requirements depending on randomization assignment. Investigational treatments were packaged and labeled in vials that looked identical. Reconstituted study drug was delivered to the investigator in syringes containing identical volumes of study drug regardless of treatment assignment. The IDR was not involved in any study procedures other than study drug preparation and accountability (e.g., proper drug storage).
2.3Assessments and outcomes
2.3.1Primary endpoint
Change from baseline in MAS ankle score with the knee extended was assessed at weeks 2, 4, 6, 8, and 12; the primary outcome time point was the average at weeks 4 and 6. MAS scores range from 0 (no increase in muscle tone) to 4 (rigid in flexion or extension); scores reported as 0, 1, 1+, 2, 3, or 4 (Bohannon & Smith, 1987) were coded as 0, 1, 2, 3, 4, or 5 respectively for the purposes of analysis. The study did not employ a second or independent rater, but those performing the assessment were given extensive training on how to conduct the measure.
2.3.2Secondary and other endpoints
The MTS of the ankle (Boyd & Graham, 1999) with knee extended and flexed was used to measure the change from baseline in fast motion angle (R1) and slow motion angle (R2) at weeks 2, 4, 6, 8, and 12. Change from baseline in the dynamic component of spasticity of the ankle was calculated by subtracting MTS R1 values from MTS R2 values at weeks 2, 4, 6, 8, and 12.
The Clinical Global Impression (CGI) by Physician score (Guy, 1976), a 9-point scale ranging from –4 (very marked worsening) to +4 (very marked improvement), was assessed at weeks 2, 4, 6, 8, and 12; the secondary outcome time point was the average at weeks 4 and 6.
Achievement on the Goal Attainment Scale (GAS) (Clark & Caudrey, 1983) was assessed by the physician at weeks 8 and 12. This consisted of personalized active goals (e.g., walking speed, endurance, balance, and improved gait attributes) and passive goals (e.g., symptom relief such as pain, tolerance of orthotic devices, and a reduction in care needs) that were set by each participant and caregiver in collaboration with a physical therapist 2 weeks prior to treatment. Goals had to be related to a valid and reliable outcome measure and be sensitive to change. Detection of change was assessed at an individual level. After goals were set, they remained the same throughout the study. Goal achievement was assessed by the physician at weeks 8 and 12 taking into consideration input from the treating therapist, caregiver, and/or participant. GAS was scored on a 6-point scale ranging from –3 (worse than start) to +2 (improvements clearly exceeded defined therapeutic goal).
Gait analyses were performed in a subset of patients with GMFCS-E&R level I to III in selected study sites using the Edinburgh Visual Gait (EVG) total score (Read et al., 2003). Patients were videotaped while walking along a 10-meter pathway and scored on a 3-point scale (total of 11 items) ranging from 0 to 2; 0 = normal, 1 = moderate deviation, and 2 = marked deviation (Read et al., 2003). Changes from baseline in total gait scores were calculated at weeks 8 and 12.
Safety measurements included adverse events (AEs), physical examination, urine pregnancy tests (for females of childbearing potential), hematology and serum chemistry, vital signs (blood pressure, pulse rate, respiratory rate, and body temperature), body weight, immunogenicity testing (for participants weighing≥15 kg at screening), and suicide-related events (for participants aged≥6 years). AEs were defined as any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of investigational product. Treatment-related AEs were determined by the investigator and the severity of AEs was also reported.
2.3.3Statistical analysis
All efficacy, baseline, and demographic analyses were performed on the modified intent-to-treat (mITT) population according to randomization assignment. Safety analyses were performed on the safety population, which included all treated participants.
Approximately 412 patients were to be enrolled to ensure that≥351 (117 per treatment group) would complete the study. Using a two-sample t-test and a significance level of 0.05, the power for MAS change from baseline was 94%. The calculation was based on assumptions for the average of week 4 and 6 change from baseline in 8 U/kg group (–0.80) and the placebo group (–0.45) and standard deviation of 0.75. The assumptions used for the power analysis calculation for MAS were based on results from two double-blind, placebo-controlled, randomized studies in Japanese patients with post-stroke lower limb (Kaji et al., 2010a) and upper limb spasticity (Kaji et al., 2010b) who received a single treatment of onabotulinumtoxinA (300U [lower limb] or 120-240U [upper limb]).
The change from baseline in MAS, the primary endpoint, was analyzed using mixed-effect model repeated measures (MMRM) with baseline MAS ankle score as a covariate and factors of age group, treatment group, visit, treatment-by-visit interaction, pooled study center, and previous botulinum toxin exposure. The average of weeks 4 and 6 in each treatment group and difference versus placebo was estimated from the MMRM model. CGI by Physician was analyzed similarly.
GAS was analyzed using analysis of covariance and observed data with baseline ankle MAS as covariate and factors of age group, treatment group, pooled study center, and previous botulinum toxin exposure. For MTS, the analysis of covariance model was used including age group, treatment group, pooled study center, and previous botulinum toxin exposure as factors and baseline MTS of ankle with knee extended as a covariate. For EVG, the analysis of covariance model was used including baseline EVG score as a covariate and factors of age group, treatment group, pooled study center, and previous botulinum toxin exposure.
A gate-keeping approach was used to control the type I error rate for the primary endpoint (change from baseline in MAS). Specifically, 8 U/kg versus placebo was tested first, followed by the 4 U/kg versus placebo comparison, each at two-sided 0.05 level. The 4 U/kg versus placebo comparison was performed only if the test for 8 U/kg versus placebo was statistically significant. No control for multiplicity was used for the secondary endpoints.
2.3.4Ethics approval and consent to participate
Study investigators obtained approval of the study protocol from a properly constituted Institutional Review Board (IRB) or Independent Ethics Committee (IEC) prior to study initiation. The study was conducted in conformance with the International Council for Harmonisation E6 guideline for Good Clinical Practices and the principles of the Declaration of Helsinki, or the laws and regulations of the country in which the research was conducted, whichever afforded the greater protection to the individual.
Written informed consent was obtained at the first study visit. Written minor assent was obtained in accordance with local laws and IRB/IEC requirements. Written documentation was obtained in accordance with the relevant country and local privacy requirements, where applicable.
3Results
3.1Participants
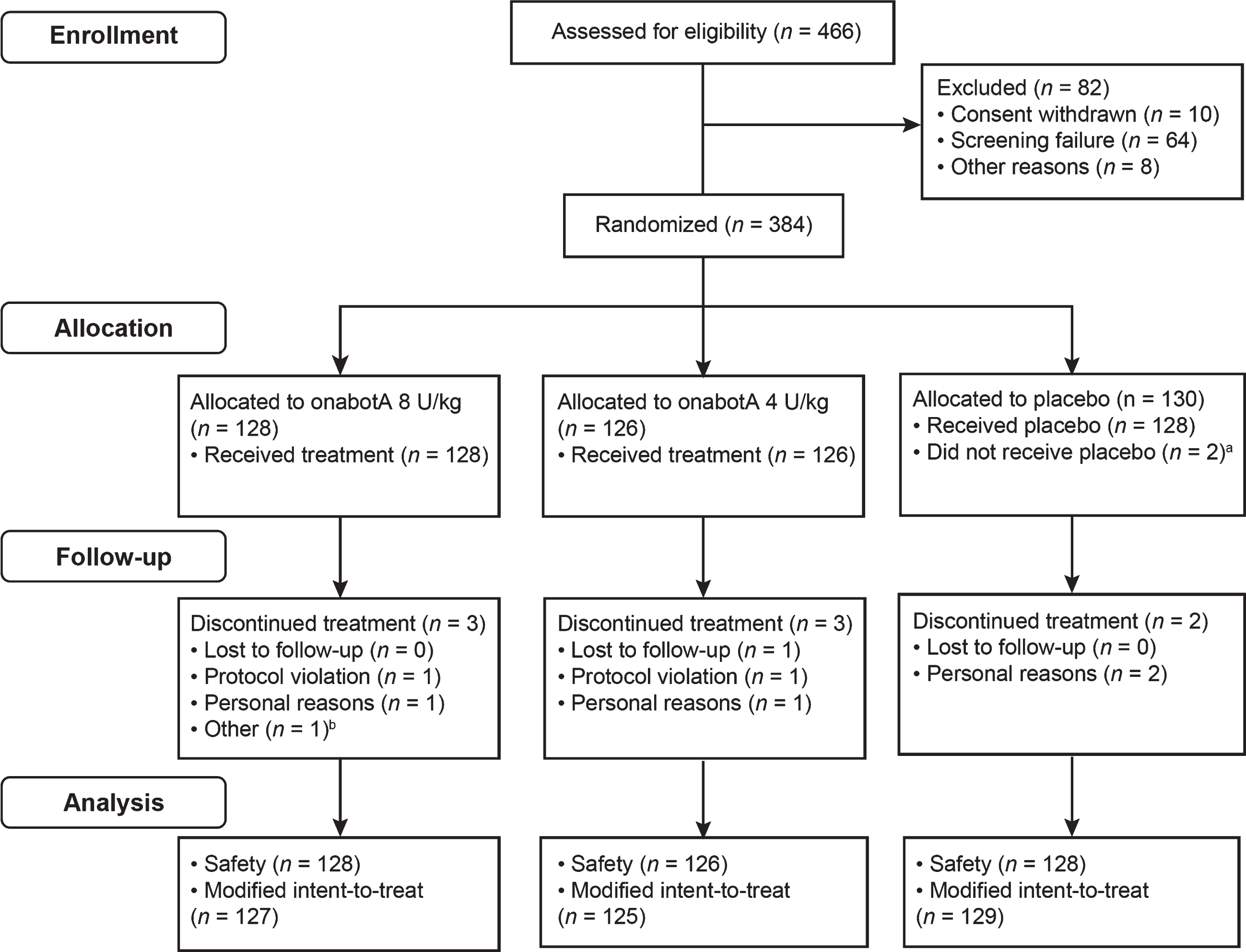
Of 466 patients screened, 384 were randomized to either onabotulinumtoxinA 8 U/kg (n = 128), onabotulinumtoxinA 4 U/kg (n = 126), or placebo (n = 130; Fig. 1). The safety population comprised 382 patients who received treatment, and the mITT population encompassed 381 patients who had a MAS ankle score at pretreatment baseline and at least one posttreatment measurement. Overall, eight patients discontinued the study; “personal reasons” was the most common cited reason, and none was related to an AE.
Fig. 1
CONSORT diagram. aOne patient withdrew consent and one was randomized to placebo but treated with 8 U/kg onabotulinumtoxinA. This participant was included in the 8 U/kg group in the safety population but in the placebo group in the modified intent-to-treat population. bFamily unable to comply with visit schedule. OnabotA: onabotulinumtoxinA.
3.2Baseline demographics and disease characteristics
Baseline demographics and disease characteristics were similar across treatment groups (Table 1). Most patients had a GMFCS-E&R of I or II; five patients had a GMFCS-E&R of IV. Over half of the patients were≤6 years of age (58.0%) and more than half were male (54.1%).
Table 1
Patient demographics
| OnabotulinumtoxinA | ||||
| 8 U/kg n = 127 | 4 U/kg n = 125 | Placebo n = 129 | Total n = 381 | |
| Age, y:mo | ||||
| Mean (SD) | 6 : 8 (3 : 11) | 6 : 5 (3 : 7) | 6 : 8 (3 : 11) | 6 : 7 (3 : 9) |
| Median (min, max) | 6.0 (2, 16) | 6.0 (2, 16) | 5.0 (2, 15) | 6.0 (2, 16) |
| ≤6, n (%) | 74 (58.3) | 73 (58.4) | 74 (57.4) | 221 (58.0) |
| Sex, n (%) | ||||
| Male | 70 (55.1) | 67 (53.6) | 69 (53.5) | 206 (54.1) |
| Race, n (%) | ||||
| White | 76 (59.8) | 76 (60.8) | 79 (61.2) | 231 (60.6) |
| Black | 2 (1.6) | 3 (2.4) | 4 (3.1) | 9 (2.4) |
| Asian | 42 (33.1) | 35 (28.0) | 37 (28.7) | 114 (29.9) |
| Hispanic | 7 (5.5) | 10 (8.0) | 6 (4.7) | 23 (6.0) |
| Other | 0 | 1 (0.8) | 3 (2.3) | 4 (1.0) |
| Topographical type, n (%) | ||||
| Hemiplegia | 110 (86.6) | 109 (87.2) | 110 (85.3) | 329 (86.4) |
| Monoplegia | 17 (13.4) | 16 (12.8) | 19 (14.7) | 52 (13.6) |
| MAS Ankle (knee extended), mean (SD)a | 3.5 (0.52) | 3.5 (0.53) | 3.5 (0.50) | ––– |
| GMFCS-E&R, n (%)b | ||||
| Level I | 69 (53.9) | 65 (51.6) | 65 (50.0) | 199 (51.8) |
| Level II | 54 (42.2) | 51 (40.5) | 58 (44.6) | 163 (42.4) |
| Level III | 5 (3.9) | 6 (4.8) | 6 (4.6) | 17 (4.4) |
| Level IV | 0 | 4 (3.2) | 1 (0.8) | 5 (1.3) |
| Medical history (≥5% total incidence), n (%) | ||||
| Seizure | 27 (21.3) | 22 (17.6) | 23 (17.8) | 72 (18.9) |
| Strabismus | 16 (12.6) | 17 (13.6) | 15 (11.6) | 48 (12.6) |
| Premature baby | 7 (5.5) | 11 (8.8) | 8 (6.2) | 26 (6.8) |
| Hydrocephalus | 13 (10.2) | 8 (6.4) | 3 (2.3) | 24 (6.3) |
| Developmental delay | 8 (6.3) | 5 (4.0) | 8 (6.2) | 21 (5.5) |
| Pneumonia | 4 (3.1) | 10 (8.0) | 7 (5.4) | 21 (5.5) |
| Upper respiratory tract infection | 8 (6.3) | 6 (4.8) | 5 (3.9) | 19 (5.0) |
aDerived MAS ankle score of 3.5 corresponds to a MAS ankle score of 2.5 since MAS scale was converted from a 4-point scale to a 5-point scale. bRandomized population. GMFCS-E&R: Gross Motor Function Classification System –Expanded and Revised; MAS: Modified Ashworth Scale-Bohannon; SD: standard deviation.
3.3Efficacy (mITT population)
3.3.1Primary endpoint
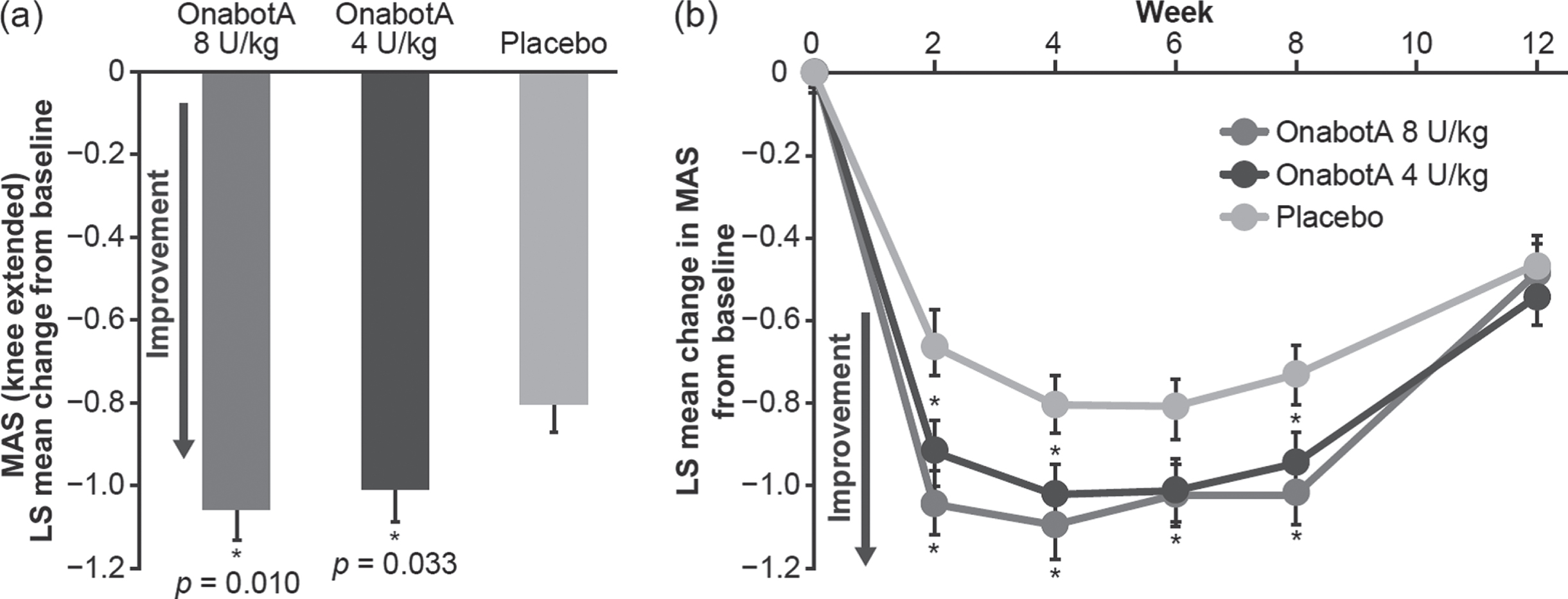
Dose-related improvements were observed in lower limb spasticity with a reduction of MAS ankle scores averaged at weeks 4 and 6 (least squares [LS] mean [standard error, SE]: 8 U/kg, –1.06 [0.07], p = 0.010; 4 U/kg, –1.01 [0.07], p = 0.033; placebo, –0.8 [0.07]; Fig. 2a). In addition, both 8 U/kg and 4 U/kg onabotulinumtoxinA doses improved the MAS scores from baseline throughout the course of the 12-week study when compared with placebo. Improvements in MAS scores were significant at weeks 2, 4, 6, and 8 for onabotulinumtoxinA 8 U/kg and at weeks 2, 4, and 8 for onabotulinumtoxinA 4 U/kg versus placebo (Fig. 2b).
Fig. 2
a Least squares (LS) mean change from baseline in ankle Modified Ashworth Scale (MAS) average of weeks 4 and 6. b LS mean change from baseline in MAS over 12 weeks (modified intent-to-treat population). *Statistically significant (p < 0.05) versus placebo. Error bars represent standard error of the mean. OnabotA: onabotulinumtoxinA.
3.3.2Secondary and other endpoints
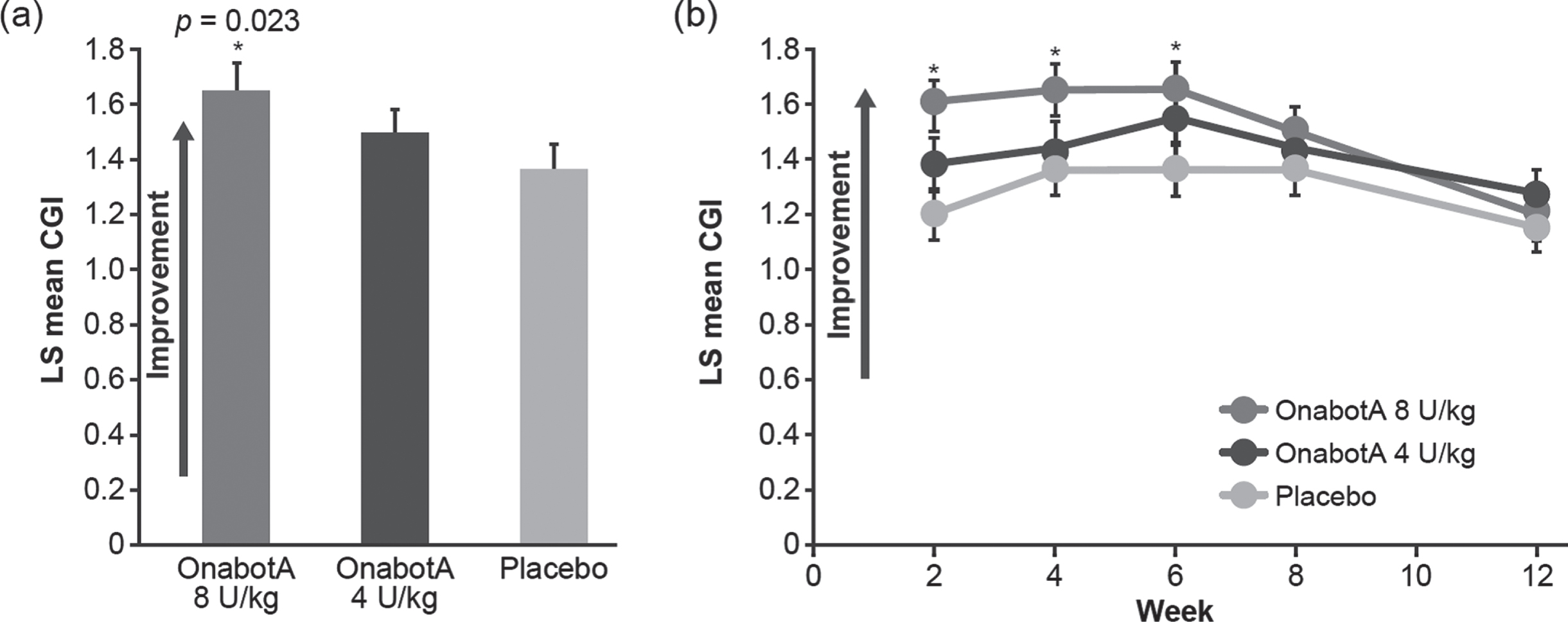
Treatment with onabotulinumtoxinA 8 U/kg significantly improved (increased) CGI scores averaged at weeks 4 and 6 versus placebo (LS mean [SE]: 1.65 [0.09] versus 1.36 [0.09]; p = 0.023, Fig. 3a). Improvements (increases) in the CGI scores were statistically significant at weeks 2, 4, and 6 with onabotulinumtoxinA 8 U/kg when compared with placebo (Fig. 3b). Treatment with onabotulinumtoxinA 4 U/kg numerically increased the CGI scores averaged at weeks 4 and 6 but did not reach significance versus placebo (LS mean [SE]: 1.49 [0.09], p = 0.299). There was a strong correlation between the MAS ankle score with knee extended and the global improvements assessed by the CGI at week 4 (p < 0.001, Spearman’s coefficient –0.552).
Fig. 3
a Least squares (LS) mean Clinical Global Impression (CGI) average of weeks 4 and 6. b LS mean CGI over 12 weeks (modified intent-to-treat population). *Statistically significant (p < 0.05) versus placebo. Error bars represent standard error of the mean. OnabotA: onabotulinumtoxinA.
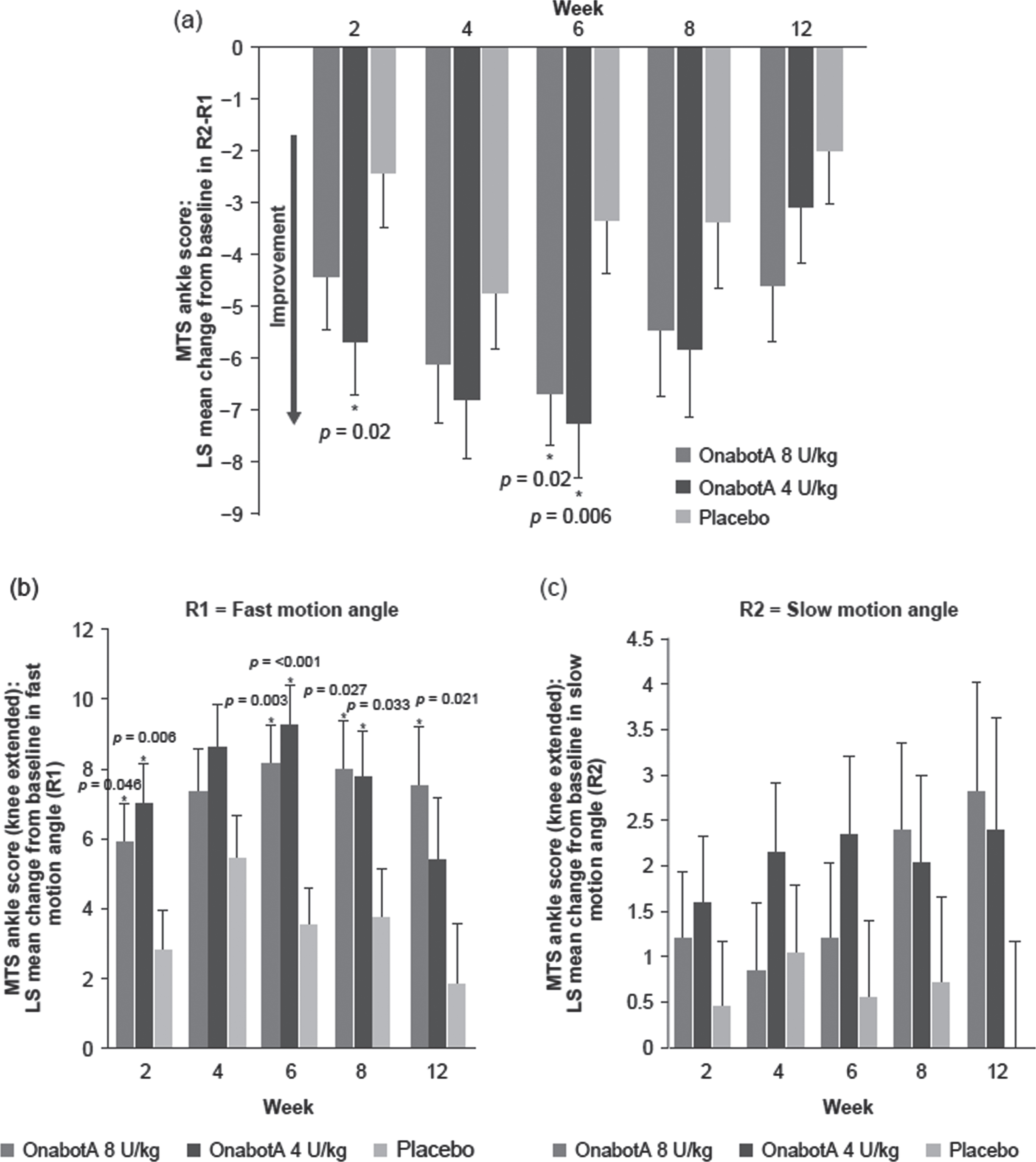
Treatment with both doses of onabotulinumtoxinA improved the average dynamic component of spasticity (R2–R1) as measured by the MTS of the ankle of the study leg with knee extended (Fig. 4a). Patients treated with onabotulinumtoxinA 8 U/kg demonstrated statistically significant improvement in MTS dynamic component (R2–R1) at week 6 (LS mean [SE], –6.65 [1.03]; p = 0.020) compared with placebo (LS mean [SE], –3.32 [1.03]). In patients treated with onabotulinumtoxinA 4 U/kg, improvements in MTS dynamic component (R2–R1) were statistically significant at weeks 2 (LS mean [SE], –5.69 [1.02]; p = 0.020) and 6 (–7.23 [1.05]; p = 0.006). Treatment with both doses of onabotulinumtoxinA improved R1 of the ankle with knee extended compared with placebo and changes were significant at most time points (Fig. 4b). Treatment with both doses of onabotulinumtoxinA numerically improved the average angle of passive range of motion (R2) when compared with placebo at most time points throughout the study, although none reached statistical significance (Fig. 4c). Similar results were seen in MTS with knee flexed (data not shown).
Fig. 4
Ankle MTS change from baseline (mITT population). a LS mean difference between knee extended MTS slow motion angle and fast motion angle (R2–R1). b LS mean change in knee extended MTS fast motion angle (R1). c knee extended MTS slow motion angle (R2). *Statistically significant (p < 0.05) versus placebo. Error bars represent standard error of the mean. LS, least squares; mITT, modified intent-to-treat; onabotA, onabotulinumtoxinA; MTS, Modified Tardieu Scale.
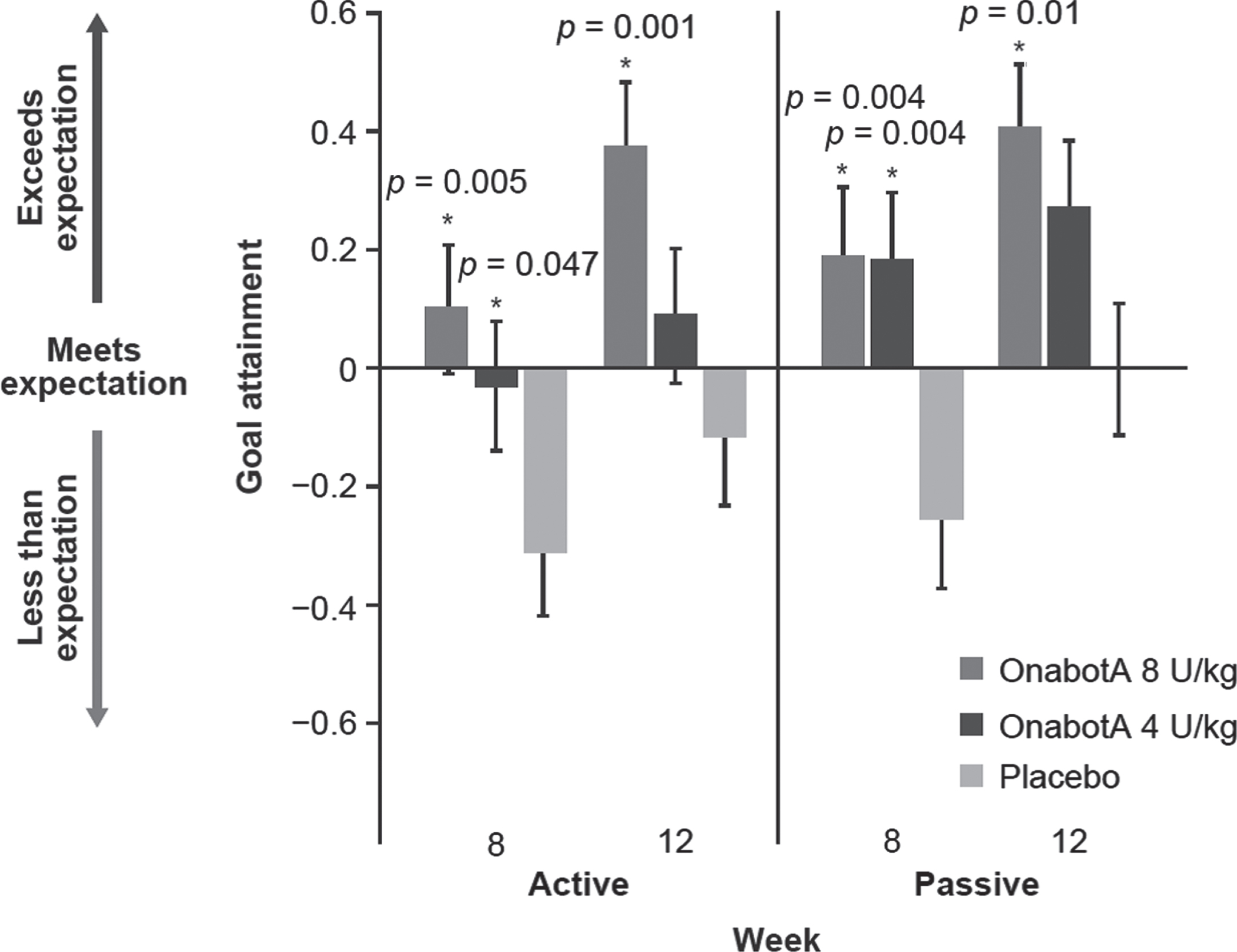
Both doses of onabotulinumtoxinA demonstrated significantly greater goal achievement scores on the physician-assessed GAS at week 8 for both active (LS mean [SE]: 8 U/kg, 0.10 [0.11], p = 0.005; 4 U/kg, –0.03 [0.11], p = 0.047) and passive (LS mean [SE]: 8 U/kg, 0.19 [0.12], p = 0.004 [0.11]; 4 U/kg, 0.18, p = 0.004) goals when compared with placebo (–0.31 [0.11] and –0.26 [0.11], respectively) (Fig. 5). At week 12, the 8 U/kg dose of onabotulinumtoxinA demonstrated significantly greater active (LS mean [SE]: 0.37 [0.11]; p = 0.001) and passive (LS mean [SE]: 0.40 [0.11]; p = 0.010) goal achievement when compared with placebo (LS mean [SE]: 0.00 [0.11]). Changes from baseline in MAS ankle score with knee extended and GAS were correlated (p < 0.001; Spearman’s coefficient –0.202 and –0.287 at week 8 for active and passive goals, respectively).
Fig. 5
Physician-assessed GAS active and passive goals (mITT population). *Statistically significant (p < 0.05) versus placebo. Error bars represent standard error of the LS mean. GAS, Goal Achievement Scale; LS, least squares; mITT, modified intent-to-treat; OnabotA, onabotulinumtoxinA.
In a subset of patients who completed the EVG assessment, dose-related numerical improvements were seen for onabotulinumtoxinA at weeks 8 and 12. The 8 U/kg dose demonstrated a statistically significant improvement from baseline to week 8 in total score (LS mean [SE]: –3.12 [0.61]; p = 0.018) when compared with placebo (LS mean [SE]: –0.86 [0.69], Fig. 6a). Improvements observed with onabotulinumtoxinA 4 U/kg did not reach statistical significance versus placebo.
Fig. 6
Edinburgh Visual Gait (EVG) total score. a LS mean change from baseline in EVG total score at weeks 8 and 12. b Still images from digital film taken during walking/running at baseline and week 8 following treatment with onabotulinumtoxinA. The left panel shows a 2-year-old male with a baseline Modified Ashworth Scale (MAS; knee extended) score of 4 who was randomized to receive 8 U/kg onabotulinumtoxinA and physical therapy (PT) and experienced a 2-point improvement. The improvement is observed in the increased elongation mobility of the ankle flexors during the EVG assessment at week 8 compared with baseline (circles). Similarly, the right panel depicts a 2-year-old female with a baseline MAS (knee extended) score of 4 who was randomized to receive 4 U/kg onabotulinumtoxinA and PT and experienced a 1-point improvement in MAS at week 8, which translated into the observed increase in mobility of the ankle flexors during the gait assessment (circles). *Statistically significant (p < 0.05) versus placebo. Error bars represent standard error of the mean. LS: least squares; MAS: Modified Ashworth Scale-Bohannon; OnabotA: onabotulinumtoxinA.
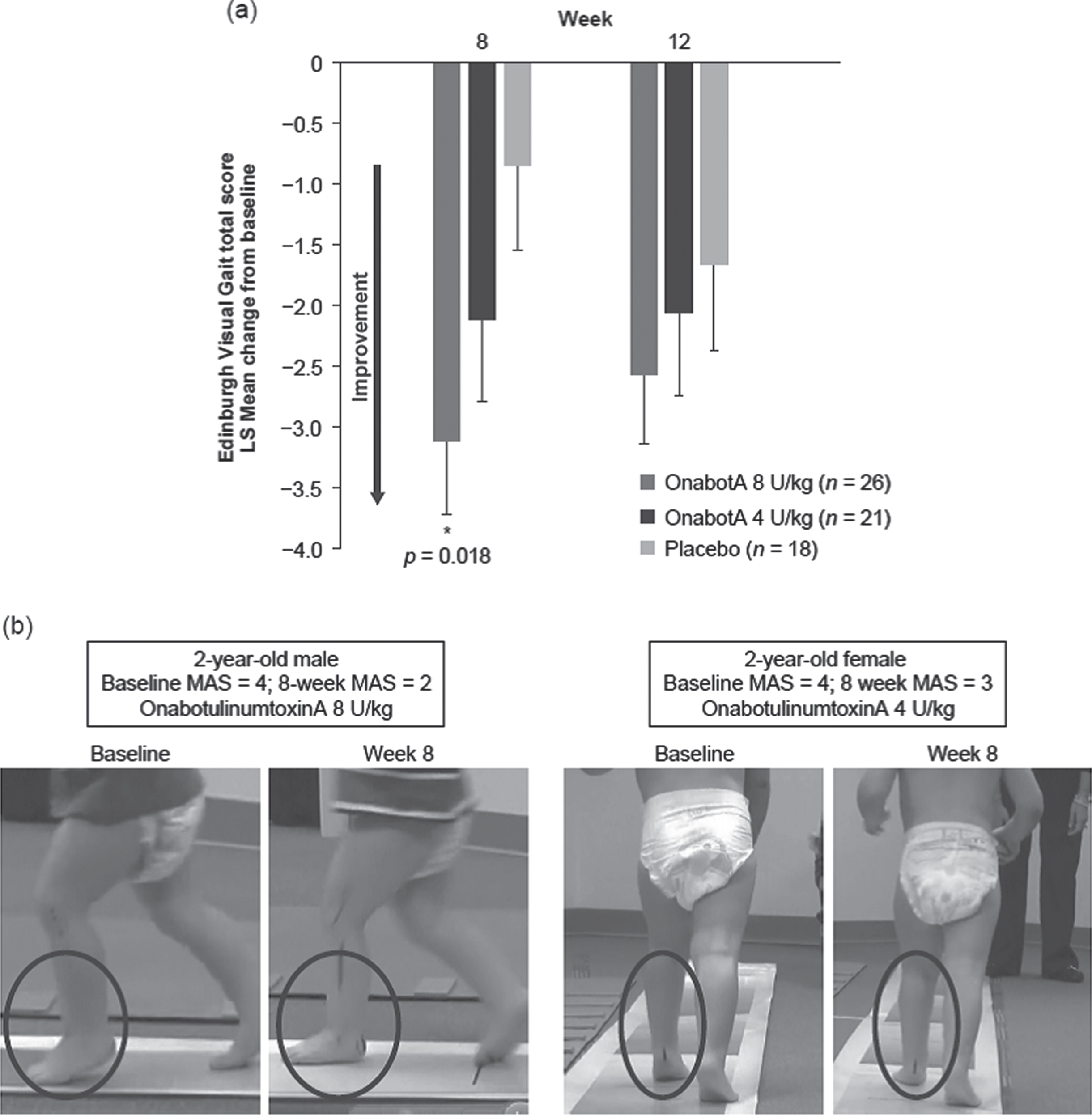
A visual representation of the EVG score improvements is shown in Fig. 6b. Still images of videos obtained at baseline and week 8 were captured during initial contact, when the heel should strike the ground to facilitate an appropriate gait pattern.
The videos from two patients (Videos 1a and 1b) clearly show an improvement in gait between baseline and week 8. It should be noted that results did vary, and these photos and videos were selected as representative based on the improvements observed in this specific measure.
3.4Safety measures
Overall, 43.8% and 42.9% of patients experienced one or more AEs following treatment with 8 U/kg and 4 U/kg onabotulinumtoxinA, respectively, and 49.2% with placebo. Most AEs seen with onabotulinumtoxinA were mild or moderate. The most frequent AEs were upper respiratory tract infections, followed by pyrexia and cough (Table 2). Although 72 patients had a history of seizure, only 6/382 patients (onabotulinumtoxinA n = 4; placebo n = 2) had a seizure during the study; of these six patients, all had a prior documented history of seizure except one patient in the placebo group. AEs classified as severe were reported for one patient (0.8%) in the 8 U/kg group (dental caries), two patients (1.6%) in the 4 U/kg group (pharyngitis and injection site pain), and three patients (2.3%) in the placebo group (pyrexia, seizure, and oropharyngeal pain/ear infection). Serious AEs occurred in no patients treated with onabotulinumtoxinA 8 U/kg, 2.4% (3/126) of patients treated with onabotulinumtoxinA 4 U/kg, and 3.1% (4/128) of patients treated with placebo; none were deemed related to study treatment. AEs assessed as treatment-related by the physician were reported in 3.1% and 2.4% of patients treated with 8 U/kg and 4 U/kg onabotulinumtoxinA, respectively, and 1.6% of patients treated with placebo. No AEs were indicative of a distant spread of toxin. No patients discontinued the study owing to AEs, and there were no deaths.
Table 2
Adverse event profile
| OnabotulinumtoxinA | ||||
| N (%) | 8 U/kg n = 128 | 4 U/kg n = 126 | All n = 254 | Placebo n = 128 |
| AEs | 56 (43.8) | 54 (42.9) | 110 (43.3) | 63 (49.2) |
| Treatment-related | 4 (3.1) | 3 (2.4) | 7 (2.8) | 2 (1.6) |
| Serious AEs | 0 | 3 (2.4) | 3 (1.2) | 4 (3.1) |
| Treatment-related | 0 | 0 | 0 | 0 |
| Discontinuations due to AE | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 |
| AEs occurring in≥2% of patients in any group | ||||
| Infection and infestations | ||||
| Viral upper respiratory tract infection | 12 (9.4) | 14 (11.1) | 26 (10.2) | 22 (17.2) |
| Upper respiratory tract infection | 8 (6.3) | 10 (7.9) | 18 (7.1) | 9 (7.0) |
| Bronchitis | 3 (2.3) | 3 (2.4) | 6 (2.4) | 4 (3.1) |
| Tonsillitis | 3 (2.3) | 2 (1.6) | 5 (2.0) | 1 (0.8) |
| Rhinitis | 3 (2.3) | 1 (0.8) | 4 (1.6) | 3 (2.3) |
| Sinusitis | 3 (2.3) | 0 | 3 (1.2) | 2 (1.6) |
| Pharyngitis | 1 (0.8) | 5 (4.0) | 6 (2.4) | 0 |
| General disorders &administration site | ||||
| Pyrexia | 5 (3.9) | 8 (6.3) | 13 (5.1) | 7 (5.5) |
| Injection site pain | 3 (2.3) | 2 (1.6) | 5 (2.0) | 0 |
| Respiratory, thoracic and | ||||
| mediastinal disorders | ||||
| Cough | 4 (3.1) | 6 (4.8) | 10 (3.9) | 3 (2.3) |
| Oropharyngeal pain | 3 (2.3) | 0 | 3 (1.2) | 1 (0.8) |
| Rhinorrhea | 1 (0.8) | 2 (1.6) | 3 (1.2) | 5 (3.9) |
| Musculoskeletal and connective tissue disorders | ||||
| Pain in extremity | 3 (2.3) | 3 (2.4) | 6 (2.4) | 3 (2.3) |
| Gastrointestinal disorders | ||||
| Diarrhea | 1 (0.8) | 4 (3.2) | 5 (2.0) | 2 (1.6) |
| Vomiting | 1 (0.8) | 2 (1.6) | 3 (1.2) | 4 (3.1) |
| Abdominal pain | 0 | 1 (0.8) | 1 (0.4) | 4 (3.1) |
| Nervous system disorders | ||||
| Seizure | 3 (2.3) | 1 (0.8) | 4 (1.6) | 2 (1.6) |
| Headache | 2 (1.6) | 1 (0.8) | 3 (1.2) | 3 (2.3) |
AE: adverse event.
4Discussion
Cerebral palsy is the most common disabling motor disorder of childhood and is a significant challenge both for the patient and caregiver involved in their rehabilitation. As survival rates continue to improve, it is expected that the number of adults with CP will continue to rise. Decline in motor function begins in childhood and deteriorates with age thus early interventions and multidisciplinary approaches are recommended to manage the consequences of CP and prevent further decline in function into adulthood (Haak et al., 2009).
In this phase 3, multicenter, randomized, double-blind, placebo-controlled clinical trial in pediatric patients with CP and lower limb spasticity, onabotulinumtoxinA treatment resulted in robust and statistically significant dose-related improvements in muscle tone (MAS scores) compared with placebo; these improvements were sustained throughout the study and were significant at the majority of timepoints assessed. The MTS, another measure of spasticity, was also significantly improved with both onabotulinumtoxinA doses in terms of the dynamic component of spasticity measure (R2–R1) at week 6 (8 U/kg) and weeks 2 and 6 (4 U/kg), and at most time points for the fast range of motion measure (R1), but not in the slow range of motion (R2). It is noteworthy that R1 is a direct measure of spasticity while R2 reflects the full range of passive motion and is influenced by a combination of muscle stiffness and underlying secondary soft tissue structural changes; therefore, the more dramatic change in R1 driving the significant changes observed in the dynamic component indicates that onabotulinumtoxinA effectively reduces muscle tone but does not appear to alter underlying structural changes after a single treatment.
Reduction in spasticity as measured by MAS and MTS was accompanied by dose-related improvements in CGI as assessed by the treating physician with onabotulinumtoxinA 8 U/kg showing significant benefit compared with placebo at the primary time point.
These results demonstrate that the 8 U/kg dose is sufficient to provide significant improvement in pediatric lower limb spasticity. Significant functional improvements were also observed in active and passive goal achievement on the GAS for both onabotulinumtoxinA groups, and in gait for the 8 U/kg group as measured by EVG.
Both doses of onabotulinumtoxinA demonstrated a safety profile similar to that of placebo. Notably, no events were reported that were indicative of a distant spread of toxin.
The reduction in spasticity and functional improvements observed in this study align with findings from other studies of botulinum toxin treatment of pediatric patients with CP (Camargo et al., 2009; Coutinho dos Santos et al., 2011) and also support the accepted clinical consensus (Delgado et al., 2010; Strobl et al., 2015).
There are a few limitations to this study. The MAS has been criticized as a primary outcome measure of spasticity because it does not take into account the velocity dependence. However, in this study the significant improvements in the dynamic component of spasticity as measured by MTS confirm the benefit of onabotulinumtoxinA on spasticity. This randomized, controlled study utilized a fixed dose and a fixed muscle injection paradigm that may not necessarily reflect the full spectrum of clinical practice. For example, injection of the soleus muscle is not always performed in clinical practice in older or ambulatory children with spasticity, and the tibialis posterior is typically treated only in patients with equinovarus (Esquenazi et al., 2017) and not pure equinus deformity. Indeed, when patients from this study were transitioned into a long-term extension study, dosing and muscle treatment paradigms were adjusted to optimize for the individual patient needs (mean dose, 6.12 U/kg), leading to further improvements with repeat treatments (Meilahn et al., 2019). Only children with a GMFCS-E&R level of I to IV were included in this study, and very few had a GMFCS level IV, so it is unknown how these results extrapolate to the more severely impacted population.
5Conclusions
In this registrational phase 3 study, significant and clinically meaningful reductions in lower limb spasticity and improvement in function were observed with onabotulinumtoxinA treatment in children with CP as reflected by improvements in MAS and MTS, CGI, GAS, and EVG. Both doses of onabotulinumtoxinA (8 U/kg and 4 U/kg) showed an acceptable safety profile in this pediatric CP population, with AEs similar to placebo.
Acknowledgments
This study and its analysis were sponsored by Allergan plc, Dublin, Ireland. We would like to thank all the participants and their families for their cooperation in this study. Editorial support for development of this manuscript was provided by Karen Pemberton, PhD, CMPP on behalf of Evidence Scientific Solutions, Inc., Philadelphia, PA, and was funded by AbbVie.
Conflict of interest
HK’s institution has received grant support from Allergan, an AbbVie company, and Ipsen, and she has served as a consultant/advisor for Allergan, an AbbVie company. JM has served as a consultant for Allergan, an AbbVie company. HGC has no disclosures to report. BAR has received honoraria from Harvard University and the American Academy of Neurology for lectures, payment for peer review from the Parkinson Study Group, and payment for serving as an advisor to the National Institute of Environmental Health Sciences. MB in the past 12 months has served as a consultant/advisor/teacher for Allergan, an AbbVie company, and Ipsen, and as an investigator for Allergan, an AbbVie company, Ipsen, and Merz. ESP has no disclosures to report. RD, EM, CL, and MFB are employees of AbbVie, and may hold AbbVie stock.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/NRE-210070.
Video 1. Videos representative of improvements seen in gait in two-year-old boy following treatment with onabotulinumtoxinA; 1a baseline and 1b onabotulinumtoxinA 8 U/kg week 8.
Video 1a. Baseline LL Spasticity: https://vimeo.com/517091220/adc24a5f5e
Video 1b. Post Treatment Visit 8: https://vimeo.com/517090331/5538ec26f8
References
1 | Allergan. (2020). Highlights of Prescribing Information. BOTOX (onabotulinumtoxinA) for injection, for intramuscular, intrade trusor, or intradermal use. Revised Sept 2020. https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190620-BOTOX-100-and-200-Units-v3-0USPI1145-v2-0MG1145.pdf |
2 | Allergan plc. (2019). Product Monograph. BOTOX® onabo-tulinumtoxinA for injection, for intramuscular, intradetrusor, or intradermal use. |
3 | Arnaud, C. , White-Koning, M. , Michelsen, S. I. , Parkes, J. , Parkinson, K. , Thyen, U. , Beckung, E. , Dickinson, H. O. , Fauconnier, J. , Marcelli, M. , McManus, V. , & Colver, A. ((2008) ) Parent-reported quality of life of children with cerebral palsy in Europe, Pediatrics, 121: (1), 54–64. |
4 | Awaad, Y. , & Rizk, T. ((2012) ) Spasticity in children, Taibah University Medical Sciences, 7: (2), 53–60. |
5 | Bax, M. , Goldstein, M. , Rosenbaum, P. , Leviton, A. , Paneth, N. , Dan, B. , Jacobsson, B. , & Damiano, D. ((2005) ) Proposed definition and classification of cerebral palsy, April, Developmental Medicine and Child Neurology, 47: (8), 571–576. |
6 | Beckung, E. , Carlsson, G. , Carlsdotter, S. , & Uvebrant, P. ((2007) ) The natural history of gross motor development in children with cerebral palsy aged to years, Developmental Medicine and Child Neurology, 49: (10), 751–756. |
7 | Blumetti, F. C. , Belloti, J. C. , Tamaoki, M. J. , & Pinto, J. A. (2019). Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy. Cochrane Database of Systematic Reviews, 10, Article CD001408. |
8 | Bohannon, R. W. , & Smith, M. B. ((1987) ) Interrater reliability of a modified Ashworth scale of muscle spasticity, Physical Therapy, 67: (2), 206–207. |
9 | Boyd, R. N. , & Graham, H. K. ((1999) ) Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy, European Journal of Neurology, 6: (S4), S23–S35. |
10 | Camargo, C. H. , Teive, H. A. , Zonta, M. , Silva, G. C. , Oliveira, M. R. , Roriz, M. M. , Brandi, I. V. , Becker, N. , Scola, R. H. , & Werneck, L. C. ((2009) ) Botulinum toxin type A in the treatment of lower-limb spasticity in children with cerebral palsy, Arquivos de Neuro-psiquiatria, 67: (1), 62–68. |
11 | Cho, Y. , Park, E. S. , Park, H. K. , Park, J. E. , & Rha, D. W. ((2018) ) Determinants of hip and femoral deformities in children with spastic cerebral palsy, Annals of Rehabilitation Medicine, 42: (2), 277–285. |
12 | Christensen, D. , Van Naarden Braun, K. , Doernberg, N. S. , Maenner, M. J. , Arneson, C. L. , Durkin, M. S. , Benedict, R. E. , Kirby, R. S. , Wingate, M. S. , Fitzgerald, R. , & Yeargin-Allsopp, M. ((2014) ) Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning –Autism and Developmental Disabilities Monitoring Network, USA, Developmental Medicine and Child Neurology, 56: (1), 59–65. |
13 | Clark, M. S. , & Caudrey, D. J. ((1983) ) Evaluation of rehabilitation services: the use of goal attainment scaling, International Rehabilitation Medicine, 5: (1), 417–415. |
14 | Coutinho dos Santos, L. H. , Bufara Rodrigues, D. C. , Simões deAssis, T. R. , & Bruck, I. ((2011) ) Effective results with botulinumtoxin in cerebral palsy, Pediatric Neurolology, 44: (5), 357–363. |
15 | Delgado, M. R. , Hirtz, D. , Aisen, M. , Ashwal, S. , Fehlings, D. L. , McLaughlin, J , Morrison, L. A , Shrader, M. W. , Tilton, A. , & Vargus-Adams, J. ((2010) ) Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society, Neurology, 74: (4), 336–343. |
16 | Delgado, M. R. , Tilton, A. , Russman, B. , Benavides, O. , Bonikowski, M. , Carranza, J. , Dabrowski, E. , Dursun, N. , Gormley, M. , Jozwiak, M. , Matthews, D. , Maciag-Tymecka, I. , Unlu, E. , Pham, E. , Tse, A. , & Picaut, P. ((2016) ) AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: a randomized controlled trial, Pediatrics, 137: (2), Article 20152830. |
17 | Esquenazi, A. , Alfaro, A. , Ayyoub, Z. , Charles, D. , Dashtipour, K. , Graham, G. D. , McGuire, J. R. , Odderson, I. R. , Patel, A. T. , & Simpson, D. M. ((2017) ) OnabotulinumtoxinA for lower limb spasticity: guidance from a Delphi Panel approach, PM& R: The Journal of Injury, Function, and Rehabilitation, 9: (10), 960–968. |
18 | Golubovic, Š. , & Slavković, S. ((2014) ) Manual ability andmanual dexterity in children with cerebral palsy, Hippokratia, 18: (4), 310–314. |
19 | Guy, W. (1976). ECDEU assessment manual for psychopharmacology. U.S. Department of Health, Education, and Welfare. |
20 | Haak, P. , Lenski, M. , Hidecker, M. J. , Li, M. , & Paneth, N. ((2009) ) Cerebral palsy and aging, Dev Med Child Neurol, 51: (Suppl 4), 16–23. |
21 | Kaji, R. , Osako, Y. , Suyama, K. , Maeda, T. , Uechi, Y. Iwasaki, M. , & Group, G. S. K. S. S. ((2010) a) Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial, J Neurol, 257: (8), 1330–1337. |
22 | Kaji, R. , Osako, Y. , Suyama, K. , Maeda, T. , Uechi, Y. , Iwasaki, M. , & Group, G. S. K. S. S. ((2010) b) Botulinum toxin type A in post-stroke upper limb spasticity, Curr Med Res Opin, 26: (8), 1983–1992. |
23 | Karabicak, G. O. , Balci, N. C. , Gulsen, M. , Ozturk, B. , & Cetin, N. ((2016) ) The effect of postural control and balance on femoral anteversion in children with spastic cerebral palsy, Journal of Physical Therapy Science, 28: (6), 1696–1700. |
24 | Koman, L. A. , Mooney, J. F. , 3rd, Smith, B. P. , Goodman, A. , & Mulvaney, T. ((1994) ) Management of spasticity in cerebral palsy with botulinum-A toxin: report of a preliminary, randomized, double-blind trial, Journal of Pediatric Orthopedics, 14: (3), 299–303. |
25 | Lance, J. W. ((1980) ) Symposium synopsis. In R. G. Feldman, R. R. Young, &W. P. Koella (Eds.), Spasticity: disordered motor control (pp. 485–495). Year Book Medical Publishers. |
26 | Lieber, R. L. , Steinman, S. , Barash, I. A. , & Chambers, H. ((2004) ) Structural and functional changes in spastic skeletal muscle, Muscle & Nerve, 29: (5), 615–627. |
27 | Meilahn, J. , Kim, H. , Racette, B. , Gul, F. , Chambers, H. G. , McCusker, E. , Chen, K. , & Dimitrova, R. (2019). Long-term safety and efficacy of open-label onabotulinumtoxinA for the treatment of lower limb spasticity in children with cerebral palsy. Presented at the Combined 73rd Academy for Cerebral Palsy and Developmental Medicine (AACPDM) and 2nd International Alliance of Academies of Childhood Disability (IAACD) triannual meeting, Anaheim, CA, USA, September 18-21, 2019. |
28 | Molenaers, G. , Van Campenhout, A. , Fagard, K. , De Cat, J. , & Desloovere, K. ((2010) ) The use of botulinum toxin A in children with cerebral palsy, with a focus on the lower limb, Journal of Children’s Orthopaedics, 4: (3), 183–195. |
29 | Ostensjø, S. , Carlberg, E. B. , & Vøllestad, N. K. ((2004) ) Motor impairments in young children with cerebral palsy: relationship to gross motor function and everyday activities, Developmental Medicine and Child Neurology, 46: (9), 580–589. |
30 | Parkinson, K. N. , Gibson, L. , Dickinson, H. O. , & Colver, A. F. ((2010) ) Pain in children with cerebral palsy: a cross-sectional multicentre European study, Acta Paediatrica, 99: (3), 446–451. |
31 | Read, H. S. , Hazlewood, M. E. , Hillman, S. J. , Prescott, R. J. , & Robb, J. E. ((2003) ) Edinburgh visual gait score for use in cerebral palsy, Journal of Pediatric Orthopedics, 23: (3), 296–301. |
32 | Reid, S. M. , Carlin, J. B. , & Reddihough, D. S. ((2011) ) Distribution of motor types in cerebral palsy: how do registry data compare? Developmental Medicine and Child Neurology, 53: (3), 233–238. |
33 | Shamsoddini, A. , Amirsalari, S. , Hollisaz, M. T. , Rahimnia, A. , & Khatibi-Aghda, A. ((2014) ) Management of spasticity in children with cerebral palsy, Iranian Journal of Pediatrics, 24: (4), 345–351. |
34 | Strobl, W. , Theologis, T. , Brunner, R. , Kocer, S. , Viehweger, E. , Pascual-Pascual, I. , & Placzek, R. ((2015) ) Best clinical practice in botulinum toxin treatment for children with cerebral palsy, Toxins (Basel), 7: (5), 1629–1648. |
35 | Sutherland, D. H. , Kaufman, K. R. , Wyatt, M. P. , Chambers, H. G. , & Mubarak, S. J. ((1999) ) Double-blind study of botulinum A toxin injections into the gastrocnemius muscle in patients with cerebral palsy, Gait &Posture, 10: (1), 1–9. |
36 | Yeargin-Allsopp, M. , Van Naarden Braun, K. , Doernberg, N. S. , Benedict, R. E. , Kirby, R. S. , & Durkin, M. S. ((2008) ) Prevalence of cerebral palsy in -year-old children in three areas of the United States in: a multisite collaboration, Pediatrics, 121: (3), 547–554. |




