Effects of therapeutic instrumental music performance and motor imagery on chronic post-stroke cognition and affect: A randomized controlled trial
Abstract
BACKGROUND:
The burden of post-stroke cognitive impairment, as well as affective disorders, remains persistently high. With improved stroke survival rates and increasing life expectancy, there is a need for effective interventions to facilitate remediation of neurocognitive impairments and post-stroke mood disorders.
OBJECTIVE:
To investigate the effects of Therapeutic Instrumental Music Performance (TIMP) training with and without Motor Imagery on cognitive functioning and affective responding in chronic post-stroke individuals.
METHODS:
Thirty chronic post-stroke, community-dwelling participants were randomized to one of three experimental arms: (1) 45 minutes of active TIMP, (2) 30 minutes of active TIMP followed by 15 minutes of metronome-cued motor imagery (TIMP+cMI), (3) 30 minutes of active TIMP followed by 15 minutes of motor imagery without cues (TIMP+MI). Training took place three times a week for three weeks, using a selection of acoustic and electronic instruments. Assessments, administered at two baselines and post-training, included the Trail Making Test (TMT) - Part B to assess mental flexibility, the Digit Span Test (DST) to determine short-term memory capacity, the Multiple Affect Adjective Checklist - Revised (MAACL-R) to ascertain current affective state, and the General Self-Efficacy Scale (GSE) to assess perceived self-efficacy. The Self-Assessment Maniqin (SAM) was also administered prior to and following each training session.
RESULTS:
Thirty participants completed the protocol, ten per arm [14 women; mean age = 55.9; mean time post-stroke = 66.9 months]. There were no statistically significant differences between pooled group baseline measures. The TIMP+MI group showed a statistically significant decrease in time from pre-test 2 to post-test on the TMT. The TIMP group showed a significant increase on MAACL sensation seeking scores, as well as on the Valence and Dominance portions of the SAM; TIMP+cMI showed respective increases and decreases in positive and negative affect on the MAACL, and increases on the Valence, Dominance, and Arousal portions of the SAM. No statistically significant association between cognitive and affective measures was obtained.
CONCLUSIONS:
The mental flexibility aspect of executive functioning appears to be enhanced by therapeutic instrumental music training in conjunction with motor imagery, possibly due to multisensory integration and consolidation of representations through motor imagery rehearsal following active practice. Active training using musical instruments appears to have a positive impact on affective responding; however, these changes occurred independently of improvements to cognition.
1Introduction
Stroke is a global health concern and a leading cause of disability (Johnson et al., 2019). Amongst all neurological disorders, it contributes the largest proportion of disability-adjusted life-years (Feigin et al., 2019). Life expectancies are increasing in most regions (Kyu et al., 2018), and as the prevalence of neurological disorders rises with age, there will be an ongoing need for prevention and rehabilitation services to support affected individuals (Feigin et al., 2019).
Post-stroke sequelae may include cognitive and emotional, as well as motor, visual spatial perceptual and communication disorders (Teasell, Hussein, Viana, Donaldson, & Madady, 2020). Data from an inner-city multiethnic population revealed a cognitive impairment prevalence rate of 22%, which remained relatively unchanged from three months to 5 years post-stroke (Douiri, Rudd, & Wolfe, 2013). Using multiple contact strategies to reach persons lost to clinic follow-up, a five-year cumulative incidence of 29% was found for post-stroke dementia (Pendlebury et al., 2015). A systematic review and meta-analysis revealed that four in ten patients exhibited levels of impairment that did not meet dementia criteria within one year post-stroke (Sexton et al., 2019). In addition, a pooled frequency estimate indicated 31% of individuals experience depression at any time point up to five years following a stroke (Hackett & Pickles, 2014), while anxiety disorders and symptoms occur in 24% of chronic post-stroke individuals (Burton et al., 2012). A study comparing point prevalence of anxiety in persons 20-months post-stroke with control participants of similar age and sex found a higher burden of anxiety in the stroke survivors (Cumming, Blomstrand, Skoog, & Linden, 2016).
Vascular cognitive impairment, a term used to de-scribe persons with cognitive difficulties induced by vascular etiologies (Hachinski, 1994), encompasses a spectrum from normal functioning to dementia. The categories Mild Vascular Cognitive Disorder and Vascular Dementia or Major Vascular Cognitive Disor-der, with diagnostic thresholds, have been proposed (Sachdev et al., 2014). Cognitive domains assessed in vascular cognitive disorders include attention and processing speed, frontal-executive function (including mental flexibility, working memory, planning, decision making, and response to feedback), and learning and memory (Sachdev et al., 2014). Deficits may encompass any cognitive domain; however, fro-ntal executive function, attention and processing speed are most commonly impacted (Lanctôt et al., 2019). Evidence suggests mental flexibility and immediate verbal retrieval may be disproportionately affected in persons with mild vascular cognitive impairment (Garrett et al., 2004). Higher levels of frontal executive functioning impairment were found in patients with vascular dementia relative to patients with Alzheimer’s Disease matched for age, education and severity (Looi & Sachdev, 1999). Post-stroke depression may also negatively impact functional outcomes, including cognition (Salter et al., 2018), and may impede conscious attentional processing (Maier et al., 2019). While moderate levels of depression appear to decrease with time, levels of anxiety seem to be more persistent and stable (Morrison, Pollard, Johnston, & MacWalter, 2005), and may be a stronger predictor than depression of overall health related quality of life (HRQoL) (Morris, van Wijck, Joice, & Donaghy, 2013).
Cognitive impairment and affective disorders have direct effects on quality of life and independence (Cumming, Marshall, & Lazar, 2013; Raju, Sarma, & Pandian, 2010). Associations have been found between cognitive impairment, depression and anxiety, and greater dependence in activities of daily living (ADL) (Claesson, Lindén, Skoog, & Blomstrand, 2005; Sturm et al., 2004), leading to higher health care costs (Claesson et al., 2005; Husaini et al., 2013). Functional recovery may be indirectly impacted (Cumming et al., 2013). Post-stroke depression appears to increase disability (Paolucci et al., 2019), and executive dysfunction has been found to negatively impact physiotherapy rehabilitation (Hayes, Donnellan, & Stokes, 2015). However, vascular cognitive impairment manifestations that are mild or slow and progressive may not be apparent to the person affected, their healthcare provider, or to informal caregivers (Lanctôt et al., 2019). While asymptomatic individuals may not demonstrate cognitive impairment, they may be at increased risk for decline and therefore merit preventative measures (Sachdev et al., 2014).
Interventions to address post-stroke vascular cognitive impairment and affective disorders have met with modest success. A systematic review found limited evidence to support use of remedial training and compensatory interventions to reduce the effects of executive impairments post-stroke (Poulin, Korner-Bitensky, Dawson, & Bherer, 2012). A Cochrane Review (Chung et al., 2013) found insufficient high-quality evidence to support generalized conclusi-ons regarding efficacy of executive dysfunction rehabilitation following stroke. A further review of post-stroke memory rehabilitation approaches re-ported short term improvements in subjective memory functioning but no clear long-term effects (das Nair, Cogger, Worthington, & Lincoln, 2017). Furthermore, a systematic review of non-randomized controlled studies of psychological interventions to address post-stroke cognitive impairment obtained non-significant results for executive function, memory, processing speed and IQ (Merriman et al., 2019). Psychological therapy was found to have a small but significant effect in improving mood and preventing depression (Hackett, Anderson, House, & Halteh, 2008). However, results did not support its effecti-veness in treating post-stroke depression (Hackett, Anderson, House, & Xia, 2008); researchers found that the proportion of individuals experiencing dep-ression following a stroke has remained relatively stable (Hackett & Pickles, 2014). In addition, a Cochrane Review found only limited, low-quality evidence for psychological and/or pharmacological treatment of post-stroke anxiety disorders or symptoms (Knapp, 2019).
Studies using auditory stimuli targeting motor re-habilitation in post-stroke participants have had some secondary effects on cognition and affect. Significant improvements on neuropsychological evaluations of visual attention, speed of processing, and rate of lea-rning were obtained following four weeks of music-supported therapy training (Ripollés et al., 2015). Significant changes to positive affect on the Positive and Negative Affect Schedule, and to the valence and arousal portions of the Self-Assessment Maniqin (SAM) were also obtained. Using the Trail-Making Test (TMT) -Part B, researchers found significant improvements in music-group participants following five weeks of music-supported therapy interventions, as well as significant reductions on the negative affect portion of the Positive and Negative Affect Schedule following five and ten weeks of music-supported therapy training (Fujioka et al., 2018).
Therapeutic Instrumental Music Performance (TIMP) is a neurologic music therapy (NMT) technique which uses carefully selected and positioned acoustic and electronic instruments in upper extremity rehabilitation (M.H. Thaut, 2005). Previous research has demonstrated a significant effect of neurologic music therapy interventions directly targeting executive functioning in individuals with traumatic brain injury (M.H. Thaut et al., 2009). The current study investigates the hypothesis that TIMP interventions, with and without motor imagery, may improve cognitive and affective outcomes relative to baseline performances in individuals at the chronic post-stroke stage.
2Materials and methods
2.1Participants
Thirty community-dwelling volunteers (14 fem-ales, mean age 55.9) began and completed the protocol (Fig. 1). Inclusion criteria were hemiparesis following a unilateral stroke sustained > 6 months prior to enrollment, at least minimal volitional movement of the affected limb, age ranging from 30–79 years, as well as ability to understand and follow simple instructions. Participants who had a comorbid neurological disorder or who were currently participating in an upper extremity rehabilitation program were excluded. Demographic characteristics of participants are indicated in Table 1. Ethics approval was provided by the Social Sciences, Humanities and Education Research Ethics Board of the University of Toronto, and by the University Health Network Research Ethics Board. All procedures were conducted in accordance with the Declaration of Helsinki. Volunteers were recruited through posters and by word of mouth. The study was also registered at ClinicalTrials.gov (ID# NCT03246217).
Fig. 1
Consort diagram for participant screening and enrollment.
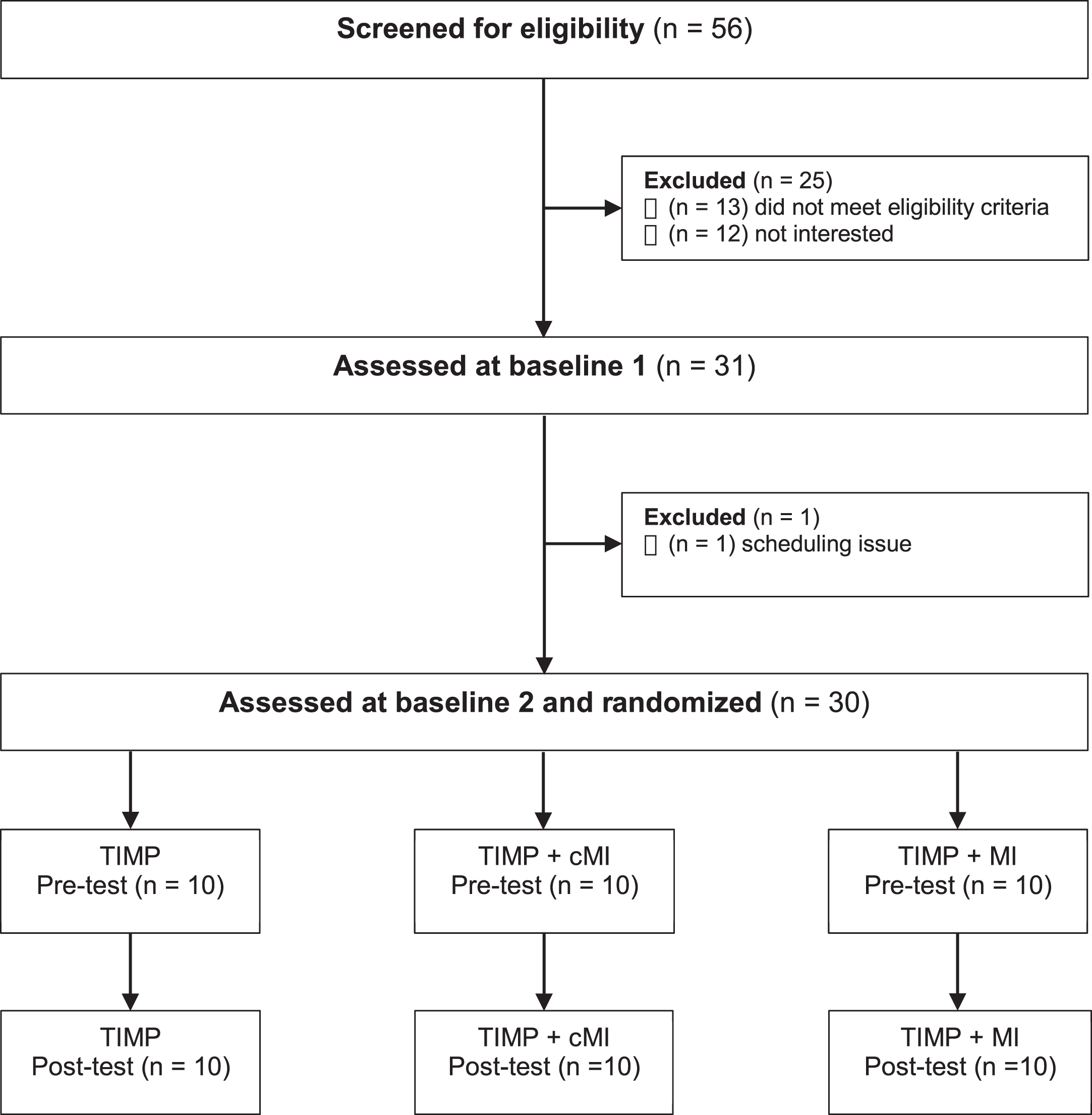
Table 1
Demographic characteristics
| TIMP (n = 10) | TIMP + cMI (n = 10) | TIMP + MI (n = 10) | |
| Age, ya | 54.7 (10.76) | 55.5 (15.01) | 57.6 (11.14) |
| Males/Females | M: 5, F: 5 | M: 5, F: 5 | M: 6, F: 4 |
| Years of educationa | 15.4 (3.24) | 16 (1.83) | 17.4 (2.68) |
| MoCA scorea | 26.8 (3.74) | 26.8 (2.90) | 26.8 (3.01) |
| Music trainingb | 1:6, 2:2, 3:2 | 1:6, 2:2, 3:2 | 1:1, 2:5, 3:4 |
| Months since stroke onseta | 71.7 (69.13) | 50.7 (45.53) | 78.3 (67.46) |
| Type of stroke, ischemic/hemorrhagic | H: 3, I: 7 | H: 3, I: 7 | H: 5, I: 5 |
| Side of lesion, right/left | R: 7, L: 3 | R: 7, L: 3 | R: 3, L: 7 |
| Location of lesion | Frontal lobe: 3 | Frontal lobe: 2 | Frontal lobe: 2 |
| Brainstem: 2 | Brainstem: 0 | Brainstem: 4 | |
| MCA: 4 | MCA: 4 | MCA: 2 | |
| Basal ganglia: 1 | Basal ganglia: 2 | Basal ganglia: 1 | |
| Left thalamus, left occipital, right cerebellum: 1 | M1, PMC, IFG: 1 | ||
| Lacunar stroke in right corona radiata: 1 |
aData represented as mean (SD). bMusical background: 1 = None; 2 = Limited, informal; 3 = Some formal training (>1 yr). MoCA (Montreal Cognitive Assessment); MCA (middle cerebral artery); M1 (primary motor cortex); PMC (premotor cortex); IFG (inferior frontal gyrus).
2.2Procedure
Participants were randomly assigned to one of three experimental arms by a blinded independent allocator, using blocked randomization to ensure groups were of equal size. The investigator who obtained informed consent and the assessors were blinded to group assignment. Two baseline assessments were administered a minimum of one week apart, and there was one final post-training assessment. Training, which took place three times a week for three weeks, was conducted by qualified Neurologic Music Therapists. Group 1 participants received 45 minutes of active TIMP training; Group 2 participants received 30 minutes of TIMP followed by 15 minutes of cued motor imagery (TIMP+cMI), which involved listening to a metronome beat set to the participant’s preferred tempo for each exercise while engaging in motor imagery; Group 3 participants received 30 minutes of TIMP followed by 15 minutes of motor imagery without external cues (TIMP+MI). Exercises were designed to train gross and fine motor control using acoustic and electronic instruments which were selected and positioned to meet individual needs. The participant’s preferred tempo for each activity was determined using a metronome with a tap feature; slow tempi were subdivided to facilitate entrainment. The metronome cue provided a stable temporal reference point throughout training in addition to the therapist’s musical support.
The mental flexibility component of executive functioning was assessed using the Trail Making Test (TMT) -Part B (Lezak, Howieson, & Loring, 2004). The TMT-Part B has been shown to have excellent test-retest reliability for persons with stroke (Goldstein & Watson, 1989). This is a timed test and the score was based on how long it took for the person to accomplish the task. Results are reported in seconds, with lower scores indicative of less impairment. Participants completed the “trail” by drawing a line alternating between letters and numbers in ascending order. Errors were pointed out and affected the score by adding to completion time. The forward Di-git Span Test (DST), a subtest from the Wechsler Adult Intelligence Scale III (Wechsler, 1997), was used to assess short-term memory capacity. A score was assigned based on the longest span of numbers the participant was able to repeat from memory in sequential order, with a maximum score of 16. In addition, perceived self-efficacy was assessed using the General Self-Efficacy Scale (GSE), which measures sense of competence in managing new and challenging situations. Internal consistency for the scale has been reported ranging from 0.75 to 0.91 (Scholz, Doña, Sud, & Schwarzer, 2002). Current affect was assessed using the state form of the Multiple Affect Adjective Check List-Revised (MAACL-R) (Zuckerman et al., 1983). A positive affect composite score is generated from combined positive affect and sensation seeking subscales, while anxiety, depression, and hostility subscales are combined into a summary score for dysphoria. Adequate internal reliability has been shown for all but the sensation seeking subscale (Lubin et al., 1986). Valence, arousal and dominance were assessed using the Self-Assessment Manikin (SAM). The instrument was designed to provide an accessible, non-verbal affective measure, and has been found to correlate highly with a verbal semantic differential scale (Bradley & Lang, 1994).
2.3Statistical analysis
The Statistical Package for Social Sciences (SPSS) software, version 26, was used for all analyses. As the data did not meet assumptions for parametric analyses, non-parametric equivalents were used instead. For the TMT, the distribution of differences was not symmetrical and the Sign Test was used. The Wilcoxon signed-rank test was used to analyze the DST, GSE scale, MAACL-R, and SAM data. The Kruskal-Wallis test was run to assess between-group differences. In addition, correlation analyses were run on pooled group data using Kendall’s tau-b to compare cognitive and affective change scores. Significance was assessed at the 0.05 level.
3Results
There were no statistically significant between-group differences on any demographic variables. Pooled baseline measures did not significantly differ (Table 2).
Table 2
Comparison of baseline measures
| Pre-test 1 | Pre-test 2 | P Value | |
| Trail Making Test - Part Ba | 109.87 (48.86) | 99.03 (48.22) | 0.066 |
| Digit Span Test | 9.9 (3.06) | 10.2 (3.43) | 0.402 |
| General Self Efficacy Scale | 32.17 (4.46) | 32.70 (4.76) | 0.193 |
| Multiple Affect Adjective | 7.60 (6.51) | 8.47 (6.47) | 0.252 |
| Checklist (MAACL) | |||
| MAACL - Positive affect | |||
| MAACL - Sensation seeking | 5.07 (1.64) | 5.20 (1.85) | 0.846 |
| MAACL - Positive | 12.67 (7.50) | 13.67 (7.78) | 0.154 |
| affect composite | |||
| MAACL - Anxiety | 1.03 (2.08) | 1.03 (1.45) | 0.888 |
| MAACL - Depression | 1.33 (2.15) | 0.90 (1.54) | 0.456 |
| MAACL - Hostility | 0.53 (1.20) | 0.59 (1.05) | 0.662 |
| MAACL - Dysphoria | 2.90 (4.71) | 2.50 (3.01) | 0.920 |
Wilcoxon signed-rank test using pooled group of 30 participants. Means (standard deviation), 2-sided asymptotic probability. aSign test.
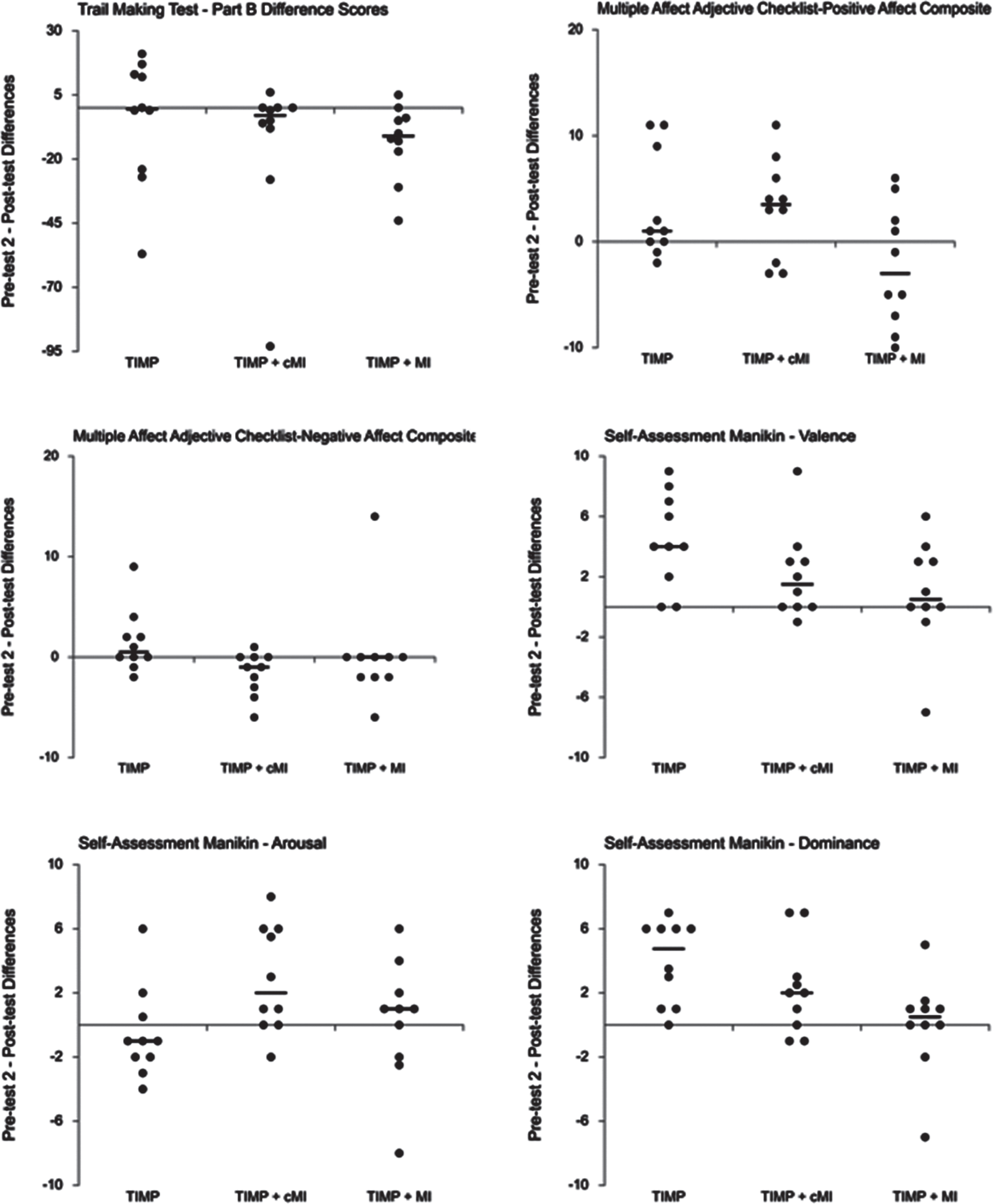
The Trail Making Test - Part B (TMT) was admi-nistered to assess effects of interventions on cognitive flexibility. TIMP participants had no statistically significant decrease in time taken to complete the TMT from pretest 2 (Mdn = 84.50) to post-test (Mdn = 77.50), median difference (0.50), z = 0.00, p = 1.00. Similarly, there was a non-significant decrease in time taken for participants in TIMP+cMI: pretest 2 (Mdn = 88.50), post-test (Mdn = 72.00), median difference (3.00), z = 1.51, p = 0.125. However, there was a statistically significant decrease in time for TIMP+MI participants from pretest 2 (Mdn = 87.50) to post-test (Mdn = 71.00), median difference (11.00) z = 2.00, p = 0.039*, r = 0.47. Eight participants in this group completed the test in less time, one took longer, and one stayed the same. Table 3 provides gr-oup means and standard deviations, while Fig. 2 shows individual change scores and group medians. There was no statistically significant difference be-tween the three groups as assessed by an independent-samples Kruskal-Wallis test of percent change between pre-test 2 and post-test, H(2) = 2.621, p = 0.270.
Table 3
Changes in cognition and state affect
| Treatment condition | Pre-test 2 | Post-test | P value | |
| Trail Making Test - Part Ba | TIMP | 90.70 (37.43) | 86.00 (37.88) | 1.000 |
| TIMP + cMI | 106.80 (62.47) | 93.30 (56.55) | 0.125 | |
| TIMP + MI | 99.60 (45.20) | 86.50 (51.22) | 0.039* | |
| Digit Span Test - Forward | TIMP | 10.30 (4.35) | 10.70 (4.37) | 0.459 |
| TIMP + cMI | 10.20 (3.01) | 10.20 (3.23) | 0.865 | |
| TIMP + MI | 10.00 (3.16) | 9.70 (2.83) | 0.669 | |
| General Self-Efficacy Scale | TIMP | 33.60 (3.31) | 34.30 (3.27) | 0.202 |
| TIMP + cMI | 32.60 (6.35) | 34.50 (4.50) | 0.098 | |
| TIMP + MI | 31.90 (4.51) | 32.00 (4.85) | 1.000 | |
| MAACL - Positive affect | TIMP | 7.00 (7.75) | 8.90 (7.78) | 0.261 |
| TIMP + cMI | 11.50 (6.11) | 14.20 (6.44) | 0.102 | |
| TIMP + MI | 6.90 (4.72) | 5.20 (3.39) | 0.235 | |
| MAACL - Sensation seeking | TIMP | 4.50 (1.90) | 5.80 (1.93) | 0.042* |
| TIMP + cMI | 5.70 (2.00) | 6.10 (2.08) | 0.364 | |
| TIMP + MI | 5.40 (1.58) | 4.80 (1.55) | 0.323 | |
| MAACL - Positive affect composite | TIMP | 11.50 (9.44) | 14.70 (9.15) | 0.105 |
| TIMP + cMI | 17.20 (7.30) | 20.30 (8.11) | 0.045* | |
| TIMP + MI | 12.30 (5.59) | 10.00 (4.16) | 0.261 | |
| MAACL - Anxietya | TIMP | 1.10 (1.85) | 1.50 (1.72) | 0.336 |
| TIMP + cMI | 1.30 (1.42) | 0.40 (0.70) | 0.041* | |
| TIMP + MI | 0.70 (1.06) | 0.80 (1.32) | 0.785 | |
| MAACL - Depressiona | TIMP | 1.20 (2.04) | 2.10 (3.21) | 0.144 |
| TIMP + cMI | 1.10 (1.60) | 0.40 (0.84) | 0.223 | |
| TIMP + MI | 0.40 (0.70) | 0.80 (1.87) | 1.000 | |
| MAACL - Hostilitya | TIMP | 0.50 (1.08) | 0.70 (1.57) | 1.000 |
| TIMP + cMI | 0.33 (0.71) | 0.30 (0.67) | 1.000 | |
| TIMP + MI | 0.90 (1.29) | 0.60 (1.58) | 0.498 | |
| MAACL - Dysphoriaa | TIMP | 2.80 (4.18) | 4.30 (6.17) | 0.147 |
| TIMP + cMI | 2.70 (2.36) | 1.10 (0.88) | 0.041* | |
| TIMP + MI | 2.00 (2.40) | 2.20 (4.57) | 0.492 |
Wilcoxon signed-rank test, means (standard deviation), 2-sided asymptotic probability. aLower scores on these measures indicate improved functioning. MAACL (Multiple Affect Adjective Checklist). *p < 05.
Fig. 2
Cognitive and affective change scores and group medians (Weissgerber, Garovic, Savic, Winham, & Milic, 2016).
The Digit Span Test (DST) was administered to understand the effects of interventions on short-term memory capacity. There was no significant dif-ference between pretest 2 and post-test for any of the three groups: TIMP pretest 2 (Mdn = 10.00), post-test (Mdn = 12.00), z = 0.74, p = 0.459; TIMP + cMI pretest 2 (Mdn = 10.00), post-test (Mdn = 10.50), z = 0.17, p = 0.865; TIMP+MI pretest 2 (Mdn = 11.50), post-test (Mdn = 10.00), z = 0.43, p = 0.669. A Kruskal-Wallis H test of median post-test scores across groups yielded a non-significant difference, H(2) = 0.83, p = 0.662.
The General Self-Efficacy scale (GSE) provided a measure of participants’ perceived sense of self-efficacy. There was a nonsignificant difference in medians from pretest 2 to post-test for each of the groups: TIMP pretest 2 (34.00), post-test (34.00), median difference (0.50), z = 1.28, p = 0.202; TIMP+ cMI pretest 2 (35.00), post-test (35.00), median difference (1.50), z = 1.65, p = 0.098; TIMP+MI pretest 2 ((30.00), post-test (30.50), median difference (0), z = 0.00, p = 1.00. An independent-samples Kruskal-Wallis test of percent change between pre-test 2 and post-test, was non-significant, H(2) = 1.244, p = 0.537.
The state form of the MAACL-R, used to assess current affect, yielded mixed results. For the positive affect composite score, TIMP + cMI participants had a statistically significant change from pretest 2 (Mdn = 16.00) to post-test (Mdn = 24.50), z = 2.00, p = 0.045*, r = 0.45, while TIMP participants had a non-significant increase from pretest 2 (Mdn = 8.50) to post-test (Mdn = 12.00), z = 1.62, p = 0.105, and TIMP+MI participants had a non-significant decrease from pretest 2 (Mdn = 13.50) to post-test (Mdn = 9.50), z = 1.13, p = 0.261. A Kruskal-Wallis test found a significant between-group difference at post-test, H(2) = 6.44, p = 0.040*. Pairwise comparisons were performed with a Bonferroni correction for multiple comparisons. Adjusted p-values are presented. Post hoc analysis revealed statistically significant differences in mean rank scores between TIMP+cMI (20.80) and TIMP+MI (10.90), p = 0.035*. There were no statistically significant differences involving TIMP (mean rank = 14.80). A Kruskal-Wallis test conducted on between-group percent change scores, however, yielded no significant differences, H(2) = 3.19, p = 0.203. On the sensation seeking subscale, the TIMP group had a statistically significant change from pretest 2 (Mdn = 4.00) to post-test (Mdn = 5.50), z = 2.03, p = 0.042*, r = 0.64, while results were non-significant for TIMP+cMI (p = 0.364) and for TIMP+MI (p = 0.323).
On the MAACL-R negative affect composite scale, there was a significant decrease in dysphoria for participants in TIMP+cMI (pretest 2 Mdn = 2.50, post-test Mdn = 1.00, z = 2.04, p = 0.041*, r = 0.55). TIMP participants reported a non-significant change from pretest 2 (Mdn = 1.00) to post-test (Mdn = 2.00), z = 1.45, p = 0.147, while TIMP+MI had a non-significant decrease from pretest 2 (Mdn = 1.50) to post-test (Mdn = 1.00), z = 0.687, p = 0.492. Median post-test scores were not statistically significantly different between groups, as assessed by a Kruskal-Wallis test, H(2) = 2.341, p = 0.310. On the anxiety subscale, there was a statistically significant decre-ase for TIMP+cMI participants from pretest 2 (Mdn = 1.00) to post-test (Mdn = 0.00), z = 2.04, p = 0.041*, r = 0.65, while changes for TIMP (p = 0.336) and TIMP+MI (p = 0.785) were non-significant. No significant changes were obtained for any of the groups on subscales for depression and hostility.
The Self-Assessment Manikin (SAM) provided a subjective measure of changes in valence, arousal, and dominance before and after each of the nine intervention sessions. There was a statistically significant improvement in valence for participants in TIMP and TIMP+cMI: TIMP pre-test (Mdn = 30.50), post-test (Mdn = 37.50), z = 2.53, p = 0.011*, r = 0.90; TIMP+cMI pre-test (Mdn = 33.50), post-test (Mdn = 35.00), z = 2.12, p = 0.034*, r = 0.80. There was a non-significant change for participants in TIMP+MI: pre-test (Mdn = 36.50), post-test (Mdn = 36.50), z = 0.93, p = 0.351, r = 0.35, and a non-statistically significant percent change between groups as determined by a Kruskal-Wallis test, H(2) = 5.481, p = 0.065. Table 4 indicates pre- and post-training group means and standard deviations. Figure 2 shows individual change scores and group medians.
Table 4
Valence, arousal, and dominance changes pre- and post-training sessions
| Treatment condition | Pre-training | Post-training | P - Value | |
| SAM - Valence | TIMP | 32.60 (8.07) | 37.00 (6.90) | 0.011* |
| TIMP + cMI | 34.85 (7.17) | 36.95 (5.98) | 0.034* | |
| TIMP + MI | 34.80 (6.30) | 35.70 (5.25) | 0.351 | |
| SAM - Arousal | TIMP | 26.90 (8.60) | 26.35 (6.92) | 0.282 |
| TIMP + cMI | 37.45 (6.76) | 40.30 (4.03) | 0.035* | |
| TIMP + MI | 31.60 (6.17) | 31.85 (6.84) | 0.721 | |
| SAM - Dominance | TIMP | 29.75 (7.55) | 33.70 (7.35) | 0.007* |
| TIMP + cMI | 33.65 (8.49) | 35.90 (8.20) | 0.028* | |
| TIMP + MI | 29.55 (6.18) | 29.60 (5.30) | 0.733 |
*p < 0.05. SAM (Self-Assessment Manikin).
There was a statistically significant change in arousal for participants in TIMP+cMI from pre-test (Mdn = 38.00) to post-test (Mdn = 41.00), z = 2.11, p = 0.035*, r = 0.74. TIMP and TIMP+MI results were non-significant: TIMP pre-test (Mdn = 26.50), post-test (Mdn = 26.00), z = -1.08, p = 0.282, r = -0.34; TIMP+MI pre-test (Mdn = 30.00), post-test (Mdn = 30.50), z = 0.357, p = 0.721, r = 0.12. There was a non-statistically significant percent change between groups, H(2) = 4.480, p = 0.106.
There was a statistically significant change in dominance for participants in TIMP and TIMP+cMI: TIMP pre-test (Mdn = 27.00), post-test (Mdn = 31.00), z = 2.70, p = 0.007*, r = 0.90; TIMP+cMI pre-test (Mdn = 32.50), post-test (Mdn = 38.50), z = 2.20, p = 0.028*, r = 0.73. There was a non-significant change for TIMP+MI: pre-test (Mdn = 28.00), post-test (Mdn = 28.50), z = 0.341, p = 0.733, r = 0.13. A Kruskal-Wallis H test was run to determine if there were differences between groups. Differences in percent change scores were statistically significant, H(2) = 7.359, p = 0.025. Pairwise comparisons were performed with a Bonferroni correction for multiple comparisons, with adjusted p-values presented. Post hoc analysis revealed statistically significant differences between the median percent change for TIMP (0.14) and TIMP+MI (0.02), p = 0.020, but there were no statistically significant differences between TIMP+cMI (Mdn = 0.06) and any other group.
Analyses using pooled group data found no correlations between cognitive and affective change scores (Table 5). However, a strong, positive correlation was found between valence and dominance change scores on the SAM.
Table 5
Correlation analyses using pooled group data
| Kendall’s tau-b | P value | |
| TMT - Part B; MAACL - Positive affect composite | τ= 0.105 | p = 0.430 |
| TMT Part B; MAACL - Dysphoria | τ= –0.023 | p = 0.868 |
| TMT Part B; SAM - Valence | τ= 0.069 | p = 0.611 |
| TMT Part B, SAM - Arousal | τ= –0.089 | p = 0.505 |
| MAACL - Positive affect composite; SAM - Valence | τ= 0.022 | p = 0.870 |
| SAM - Valence; SAM - Arousal | τ= 0.066 | p = 0.635 |
| SAM - Valence; SAM - Dominance | τ= 0.390 | p = 0.005* |
| SAM - Arousal; SAM - Dominance | τ= 0.122 | p = 0.374 |
Paired difference scores, comparing pre-test 2 –post-test change scores between cognitive and affective measures, and within affective measures. SAM (Self-Assessment Manikin); TMT (Trail Making Test); MAACL (Multiple Affect Adjective Checklist). *p < 0.05.
4Discussion
This study examined the effects of TIMP training, with and without motor imagery, on cognitive and affective outcomes in persons at the chronic post-stroke stage. Results indicated improvements on the TMT-Part B measure of mental flexibility. There was a statistically significant reduction in completion time for the TIMP+MI group. The TIMP and TIMP+cMI groups had non-significant changes. There were no statistically significant between-group differences. All results obtained on the DST, a measure of short-term memory capacity, were non-significant. Results also indicated statistically significant improvements in positive affect in the TIMP and TIMP+cMI groups, as measured by the MAACL-R and SAM. Furthermore, TIMP+cMI showed some significant decreases in negative affect on the MAACL-R. No association was found between changes in cognition and affective responding.
Participants in the TIMP+MI condition received 30 minutes of active TIMP training followed by motor imagery without any external cues. In contrast, participants in the TIMP+cMI condition listened to a metronome cue during motor imagery that was set to their preferred tempo for executing a particular activity. In the emulation theory of motor imagery (Grush, 2004), efferent motor centres of the brain drive a body emulator which maps efference copy to probable proprioceptive and kinesthetic signals to produce motor imagery. The emulator is a flexible system that evolves according to its own internal dynamic without external sensory feedback correction. The external cue provided during the TIMP+cMI condition may have reduced the flexibility and responsiveness of the emulator, possibly hindering formation of adaptive representations. In the TIMP+MI condition the emulator operated without external input, providing maximal flexibility for formation of representations. Meta-analyses of the neural correlates of motor imagery, action execution, and action observation revealed consistent recruitment of the dorsolateral prefrontal cortex only during motor imagery (Hardwick, Caspers, Eickhoff, & Swinnen, 2018). Research suggests that the dorsolateral prefrontal cortex supports cognitive flexibility by selecting relevant abstract rules in a changing environment (Mansouri, Matsumoto, & Tanaka, 2006). The TIMP+MI condition may have enabled participants to form and reform novel motor representations without input and restrictions from external stimuli. Nevertheless, the TIMP+cMI group had the highest initial mean score on the TMT, indicating greater challenges with executive functioning, and in the absence of an assessment to determine motor imagery ability, outcomes may also have been affected by group composition and heterogeneity. Active rehabilitative practice without subsequent motor imagery rehearsal to consolidate representations did not appear to enhance cognitive flexibility.
The DST measure of auditory short-term memory capacity yielded no significant between-group or timepoint changes. Researchers using the MST protocol found small, non-significant post-treatment improvements on the DST (Grau-Sánchez et al., 2018; Ripollés et al., 2015). Serrien and colleag-ues (2008), using a synchronization-continuation paradigm, found increased involvement of mesial-central regions, irrespective of task complexity, during unpaced relative to paced performance, suggestive of increased abstract processing of temporal information. Researchers found that inserting a de-lay interval between pacing and continuation tasks activated the prefrontal-parietal-temporal network, associated with working memory, during the continuation phase (Jantzen, Oullier, Marshall, Steinberg, & Kelso, 2007). This may reflect recruitment of working memory during the delay period, providing explicit mnemonic support in the absence of an external cue, which may otherwise drive implicit representations of the temporal interval. Evidence of subliminal entrainment has been found that dynamically replicates changing periodic structures during experimentally-manipulated rhythmic sensorimotor synchronization tasks, suggesting the presence of subconscious physiological auditory-motor entrainment mechanisms (M.H. Thaut, Kenyon, Schauer, & McIntosh, 1999; M.H. Thaut, Miller, & Schauer, 1998; M.H. Thaut, Tian, & Azimi-Sadjadi, 1998). TIMP study interventions involved auditory-motor coupling to an isochronous beat set to participants’ preferred tempi for each exercise, which may have contributed to subliminal entrainment. MST exerci-ses, on the other hand, are modelled for particip-ants; however, rhythmic facilitation is not provided throughout (Schneider, Schönle, Altenmüller, & Münte, 2007), potentially increasing working memory requirements. Using functional magnetic resonance imaging (fMRI), Chen and colleagues (2008) noted greater activation of working memory regions in musicians presented with progressively more complex rhythms, reflecting increased reliance on top-down processing in more challenging synchronization tasks. Use of a readily discernible, predictable beat for entrainment purposes in the TIMP study, in contrast, may have reduced the need to recruit additional neural resources subserving working memory networks.
Current measures of well-being (positive affect and sensation seeking) as well as dysphoria (anxiety, depression, and hostility) were obtained using the state form of the MAACL-R. The positive affect subscale assessed passive aspects of well-being, while the sensation seeking subscale evaluated active, energetic aspects. Participants in the TIMP group, who had 45-minute active training sessions without motor imagery, scored significant gains on the sensation seeking scale. TIMP group participants also had significant gains on the valence and dominance portions of the SAM, completed before and after each training session. Active practice using individually-tailored TIMP interventions appears to enhance positive affective responding. Ripolles and colleagues (2015) found twenty sessions of active music training led to significant improvements on the positive portion of the Positive and Negative Affect Scale as well as on the valence and arousal portions of the SAM. Self-ratings of mood using a Faces Scale showed significant improvements during the course of music-supported training (Van Vugt et al., 2016; Van Vugt, Ritter, Rollnik, & Altenmuller, 2014). An eight-week community-based rehabilitation program using rhythmic auditory stimulation and incorporating percussion instrument playing resulted in significant improvements in mood states as measured by a Korean version of the Profile of Mood States (Jeong & Kim, 2007).
Participants in the TIMP + cMI group, who had 30 minutes of active practice followed by 15 minutes of metronome-cued motor imagery, scored sign-ificant gains on the positive affect composite scale of the MAACL-R, as well as significant increases in valence, arousal, and dominance on the SAM. In addition, there were significant decreases on the MAACL-R negative affect composite scale and the anxiety subscale. Other studies have reported decreases in negative affect following music-based rehabilitative interventions. Following music-supported therapy training, significant reductions in negative affect were found on the short form of the Profile of Mood States (Van Vugt et al., 2016; Van Vugt et al., 2014), on the Beck Depression Inventory (Ripollés et al., 2015), and on the negative affect portion of the Positive and Negative Affect Schedule in the music group after five and ten weeks of training (Fujioka et al., 2018). In a systematic review of motor imagery in upper limb rehabilitation, using the PETTLEP model (Physical, Environment, Task, Timing, Learning, Emotion, and Perspective), the authors noted that the emotion category has received little attention (Harris & Hebert, 2015). Participants in TIMP+cMI condition listened to a metronome cue set to their preferred pace for executing an action while practicing motor imagery; hence, there was no external input during the cued motor imagery condition that would have enhanced affective responding. However, results suggest the participants found the active practice interventions to be emotionally meaningful. This affective engagement may have continued during the subsequent motor imagery practice.
While the TIMP+MI group scored a significant improvement on the TMT-Part B assessment of mental flexibility, there were no significant changes to affect. In addition, no significant associations were found between pooled group cognitive and affective change scores. Depression has been found to impact top-down attentional processing, acting as an additional cognitive load (Maier et al., 2019); however, all three groups scored low on the MAACL-R depression subscale, suggesting depression was not a factor in cognitive performance. The TIMP+MI group had non-significant decreases from baseline 2 to post-test in positive affective responding as measured by the MAACL-R. It is difficult in the absence of formal interviews to ascertain the reasons for this. Anecdotally, a number of participants expressed regret that the intervention sessions were ending. TIMP+MI participants scored particularly strong gains on functional assessments (data to be reported in a future publication), and this may have contributed to a sense of regret, manifested in the current state affect measures, that sessions were ending.
While pre-test 2 - post-test results on the GSE were non-significant, effect sizes for TIMP and TIMP+cMI were in the small-medium range (r = 0.37). Furthermore, a Friedman test using pooled group data indicated a significant effect of time, χ2(2) = 11.77, p = 0.003. Post hoc analysis revealed a statistically significant increase from pre-test 1 (Mdn = 32.00) to post-test (Mdn = 34.00), p = 0.007, r = 0.46, showing a positive trend in perceived self-efficacy over the duration of the study. Scores on the four-point scale tended to be confirmatory, in the “moderately true” to “exactly true” range. A sample of 367 Canadians had a mean GSE score of 3.12 (Scholz et al., 2002), whereas the means for this study sample were consistently higher (pre-test 1, M = 3.22; pre-test 2, M = 3.27; post-test, M = 3.36), indicating a generally strong sense of self-efficacy. The mean age (56 years) for participants in this study was younger than the average age identified for participants in stroke rehabilitation studies (M = 64 years) (Gaynor, Geoghegan, & O’Neill, 2014). Wulf and colleagues (2012) noted that older adults may be negatively impacted by beliefs in reduced ability due to age, limiting their performance and learning. A systematic review found a negative association for persons with stroke between self-efficacy and depression, and a positive association with activities of daily living and health-related quality of life (Korpershoek, van der Bijl, & Hafsteinsdóttir, 2011). TIMP study interventions, targeting both impairment and simulation of functional movements, were individually tailored, designed to consolidate and build on existing capacities as well as provide participants with sufficient challenge to cultivate and maintain a sense of personal accomplishment. Tasks viewed as acquirable skills have been shown to strengthen self-efficacy and a sense of personal attainment, leading to heightened performance levels (Jourden, Bandura, & Banfield, 1991).
5Conclusion
While results of the current study appear to indicate positive effects of TIMP interventions on cognition and affect, this study had a number of limitations. It was a relatively small clinical trial, with heterogeneous group compositions, and there were only nine intervention sessions over a three-week period. Originally the plan was to include 12 training sessions over four weeks; however, due to slow initial recruitment and logistical issues the time frame had to be abbreviated. In addition, a planned assessment to determine motor imagery ability had to be discontinued due to time constraints, and there was no provision for retention assessments. Nevertheless, significant cognitive and affective gains were made in a relatively short period of time. TIMP+MI appears to enhance mental flexibility in chronic post-stroke participants, possibly due to consolidation of representations through motor imagery following active practice. In addition, active training using musical instruments appears to have a positive impact on affective responding, although these changes occurred independently of changes to cognition. Further trials with larger sample sizes, more extensive neuropsychological evaluations, and retention assessments will be required to confirm these preliminary results.
Conflict of interest
None to report.
Acknowledgments
The authors would like to thank the individuals who participated in this study, as well as S. Duane and K. Kang for conducting assessments, and Neurologic Music Therapists N. Richard, L. Ho, B. Li, C. Paleshi, and S. Lavigne for providing training.
References
1 | Bradley, M. M. , & Lang, P. J. ((1994) ). Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behaviour Therapy and Experimental Psychiatry, 25: (1), 49–59. doi: https://doi.org/10.1016/0005-7916(94)90063-9 |
2 | Burton, C. A. C. , Murray, J. , Holmes, J. , Astin, F. , Greenwood, D. , & Knapp, P. ((2012) ). Frequency of anxiety after stroke: A systematic review and meta-analysis of observational studies. International Journal of Stroke, 8: (7), 545–559. doi: https://doi.org/10.1111/j.1747-4949.2012.00906.x |
3 | Chen, J. L. , Penhune, V. B. , & Zatorre, R. J. ((2008) ). Moving on time: Brain network for auditory-motor synchronization is modulated by rhythm complexity and musical training. Journal of Cognitive Neuroscience, 20: (2), 226–239. doi: https://doi.org/10.1162/jocn.2008.20018 |
4 | Chung, C. S. Y. , Pollock, A. , Campbell, T. , Durward, B. R. , Hagen, S. , & Chung, C. S. Y. ((2013) ). Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database of Systematic Reviews, 2013: (4), CD008391. doi: https://doi.org/10.1002/14651858.CD008391.pub2 |
5 | Claesson, L. , Lindén, T. , Skoog, I. , & Blomstrand, C. ((2005) ). Cognitive impairment after stroke - impact on activities of daily living and costs of care for elderly people. Cerebrovascular Diseases, 19: (2), 102–109. doi: https://doi.org/10.1159/000082787 |
6 | Cumming, T. B. , Blomstrand, C. , Skoog, I. , & Linden, T. ((2016) ). The high prevalence of anxiety disorders after stroke. American Journal of Geriatric Psychiatry, 24: (2), 154–160. doi: http://dx.doi.org/10.1016/j.jagp.2015.06.003 |
7 | Cumming, T. B. , Marshall, R. S. , & Lazar, R. M. ((2013) ). Stroke, cognitive deficits, and rehabilitation: Still an incomplete picture. International Journal of Stroke, 8: (1), 38. doi: https://doi.org/10.1111/j.1747-4949.2012.00972.x |
8 | das Nair, R. , Cogger, H. , Worthington, E. , & Lincoln, N. B. ((2017) ). Cognitive rehabilitation for memory deficits after stroke: An updated review. Stroke, 48: (2), e28–e29. doi: https://doi.org/10.1161/STROKEAHA.116.015377 |
9 | Douiri, A. , Rudd, A. G. , & Wolfe, C. D. A. ((2013) ). Prevalence of poststroke cognitive impairment: South London Stroke Register 1995-2010. Stroke, 44: , 138–145. doi: https://doi.org/10.1161/STROKEAHA.112.670844 |
10 | Feigin, V. L. , Nichols, E. , Alam, T. , Bannick, M. S. , Beghi, E. , Blake, N. ,... Car, M. ((2019) ). Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology, 18: (5), 459. doi: http://dx.doi.org/10.1016/S1474-4422(18)30499-X |
11 | Fujioka, T. , Dawson, D. R. , Wright, R. , Honjo, K. , Chen, J. L. , Chen, J. J. ,... Ross, B. ((2018) ). The effects of music-supported therapy on motor, cognitive, and psychosocial functions in chronic stroke. Annals of the New York Academy of Sciences, 1423: (1), 264–274. doi: https://doi.org/10.1111/nyas.13706 |
12 | Garrett, K. D. , Browndyke, J. N. , Whelihan, W. , Paul, R. H. , DiCarlo, M. , Moser, D. J. ,... Ott, B. R. ((2004) ). The neuropsychological profile of vascular cognitive impairment—no dementia: Comparisons to patients at risk for cerebrovascu-lar disease and vascular dementia. Archives of Clinical Neuropsychology, 19: (6), 745–757. doi: https://doi.org/10.1016/j.acn.2003.09.008 |
13 | Gaynor, E. J. , Geoghegan, S. E. , & O’Neill, D. ((2014) ). Ageism in stroke rehabilitation studies. Age and Ageing, 43: (3), 429–431. doi: https://doi.org/10.1093/ageing/afu026 |
14 | Goldstein, G. , & Watson, J. R. ((1989) ). Test-retest reliability of the halstead-reitan battery and the WAIS in a neuropsychiatric population. The Clinical Neuropsychologist, 3: (3), 265–272. doi: https://doi.org/10.1080/13854048908404088 |
15 | Grau-Sánchez, J. , Duarte, E. , Ramos-Escobar, N. , Sierpowska, J. , Rueda, N. , Redón, S. ,... Rodríguez-Fornells, A. ((2018) ). Music-supported therapy in the rehabilitation of subacute stroke patients: A randomized controlled trial. Annals of the New York Academy of Sciences, 1423: (1), 318–328. doi: https://doi.org/10.1111/nyas.13590 |
16 | Grush, R. ((2004) ). The emulation theory of representation: Motor control, imagery, and perception. Behavioural and Brain Sciences, 27: , 377–442. doi: https://doi.org/10.1017/S0140525X04000093 |
17 | Hachinski, V. ((1994) ). Vascular dementia: A radical redefinition. Dementia, 5: (3-4), 130–132. |
18 | Hackett, M. L. , Anderson, C. S. , House, A. , & Halteh, C. ((2008) ). Interventions for preventing depression after stroke. Cochrane Database of Systematic Reviews, 2008: (3), CD003689. doi: https://doi.org/10.1002/14651858.CD003689.pub3 |
19 | Hackett, M. L. , Anderson, C. S. , House, A. , & Xia, J. ((2008) ). Interventions for treating depression after stroke. Cochrane Database of Systematic Reviews (4), CD003437 - CD003437. doi: https://doi.org/10.1002/14651858.CD003437.pub3 |
20 | Hackett, M. L. , & Pickles, K. ((2014) ). Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. International Journal of Stroke, 9: (8), 1017–1025. doi: https://doi.org/10.1111/ijs.12357 |
21 | Hardwick, R. M. , Caspers, S. , Eickhoff, S. B. , & Swinnen, S. P. ((2018) ). Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neuroscience and Biobehavioral Reviews, 94: , 31–44. doi: https://doi.org/10.1016/j.neubiorev.2018.08.003 |
22 | Harris, J. E. , & Hebert, A. ((2015) ). Utilization of motor imagery in upper limb rehabilitation: A systematic scoping review. Clinical Rehabilitation, 29: (11), 1092–1107. doi: https://doi.org/10.1177/0269215514566248 |
23 | Hayes, S. , Donnellan, C. , & Stokes, E. ((2015) ). Executive dysfunction post-stroke: An insight into the perspectives of physiotherapists. Disability and Rehabilitation, 37: (20), 1817–1824. doi: https://doi.org/10.3109/09638288.2014.980915 |
24 | Husaini, B. , Levine, R. , Sharp, L. , Cain, V. , Novotny, M. , Hull, P. ,... Moonis, M. ((2013) ). Depression increases stroke hospitalization cost: An analysis of 17,010 stroke patients in 2008 by race and gender. Stroke Research and Treatment, 2013: , 1–7. doi: http://dx.doi.org/10.1155/2013/846732 |
25 | Jantzen, K. J. , Oullier, O. , Marshall, M. , Steinberg, F. L. , & Kelso, J. A. S. ((2007) ). A parametric fMRI investigation of context effects in sensorimotor timing and coordination. Neuropsychologia, 45: (4), 673–684. doi: https://doi.org/10.1016/j.neuropsychologia.2006.07.020 |
26 | Jeong, S. , & Kim, M. T. ((2007) ). Effects of a theory-driven music and movement program for stroke survivors in a community setting. Applied Nursing Research, 20: , 125–131. doi: https://doi.org/10.1016/j.apnr.2007.04.005 |
27 | Johnson, C. O. , Nguyen, M. , Roth, G. A. , Nichols, E. , Alam, T. , Abate, D. ,... Javanbakht, M. ((2019) ). Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology, 18: (5), 439–458. doi: http://dx.doi.org/10.1016/S1474-4422(19)30034-1 |
28 | Jourden, F. J. , Bandura, A. , & Banfield, J. T. ((1991) ). The impact of conceptions of ability on self-regulatory factors and motor skill acquisition. Journal of Sport and Exercise Psychology, 13: (3), 213–226. doi: https://doi.org/10.1123/jsep.13.3.213 |
29 | Knapp, P. ((2019) ). How effective are treatments for anxiety after stroke? A Cochrane Review summary with commentary. NeuroRehabilitation, 44: , 457–458. doi: https://doi.org/10.3233/NRE-189006 |
30 | Korpershoek, C. , van der Bijl, J. , & Hafsteinsdóttir, T. B. ((2011) ). Self-efficacy and its influence on recovery of patients with stroke: A systematic review. Journal of Advanced Nursing, 67: (9), 1876–1894. doi: https://doi.org/10.1111/j.1365-2648.2011.05659.x |
31 | Kyu, H. H. , Abate, D. , Abate, K. H. , Abay, S. M. , Abbafati, C. , Abbasi, N. ,... Vos, T. ((2018) ). Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for countries and territories, 1990-2017 : A systematic analysis for the Global Burden of Disease Study 2017. The Lancet (British edition), 392: (10159), 1859–1922. doi: https://doi.org/10.1016/S0140-6736(18)32335-3 |
32 | Lanctôt, K. L. , Lindsay, M. P. , Smith, E. E. , Sahlas, D. J. , Foley, N. , Gubitz, G. ,... Swartz, R. H. ((2019) ). Canadian Stroke Best Practice Recommendations:Mood, cognition and fatigue following stroke, 6th edition update 2019. International Journal of Stroke, 1-21. doi: https://doi.org/10.1177/1747493019847334 |
33 | Lezak, M. D. , Howieson, D. B. , & Loring, D. W. ((2004) ). Neuropsychological Assessment. New York, NY: Oxford University Press. |
34 | Looi, J. C. L. , & Sachdev, P. S. ((1999) ). Differentiation of vascular dementia from AD on neuropsychological tests. Neurology, 53: (4), 670–678. doi: https://doi.org/10.1212/WNL.53.4.670 |
35 | Lubin, B. , Zuckerman, M. , Hanson, P. G. , Armstrong, T. , Rinck, C. M. , & Seever, M. ((1986) ). Reliability and validity of the Multiple Affect Adjective Check List - Revised. Journal of Psychopathology and Behavioral assessment, 8: (2), 103–117. doi: https://doi.org/10.1007/BF00963575 |
36 | Maier, M. A. , Low, S. C. , Ballester, B. R. , Banuelos, N. L. , Oller, E. D. , & Verschure, P. F. M. J. ((2019) ). Depression modulates attentional processing after stroke. Biosystems and Biorobotics, 21: , 702–706. doi: https://doi.org/10.1007/978-3-030-01845-0_140 |
37 | Mansouri, F. A. , Matsumoto, K. , & Tanaka, K. ((2006) ). Prefrontal cell activities related to monkeys’ success and failure in adapting to rule changes in a Wisconsin Card Sorting Test analog. The Journal of Neuroscience, 26: (10), 2745–2756. doi: https://doi.org/10.1523/JNEUROSCI.5238-05.2006 |
38 | Merriman, N. A. , Sexton, E. , McCabe, G. , Walsh, M. E. , Rohde, D. , Gorman, A. ,... Hickey, A. ((2019) ). Addressing cognitive impairment following stroke: Systematic review and meta-analysis of non-randomised controlled studies of psychological interventions. BMJ Open, 9: (2), e024429. doi: https://doi.org/10.1136/bmjopen-2018-024429 |
39 | Morris, J. H. , van Wijck, F. , Joice, S. , & Donaghy, M. ((2013) ). Predicting health related quality of life 6 months after stroke: The role of anxiety and upper limb dysfunction. Disability and Rehabilitation, 35: (4), 291–299. doi: https://doi.org/10.3109/09638288.2012.691942 |
40 | Morrison, V. , Pollard, B. , Johnston, M. , & MacWalter, R. ((2005) ). Anxiety and depression 3 years following stroke: Demographic, clinical, and psychological predictors. Journal of Psychosomatic Research, 59: (4), 209–213. doi: https://doi.org/10.1016/j.jpsychores.2005.02.019 |
41 | Paolucci, S. , Iosa, M. , Coiro, P. , Venturiero, V. , Savo, A. , De Angelis, D. , & Morone, G. ((2019) ). Post-stroke depression increases disability more than 15% in ischemic stroke survivors: A case-control study. Frontiers in Neurology, 10: . doi: https://doi.org/10.3389/fneur.2019.00926 |
42 | Pendlebury, S. T. , Chen, P.-J. , Welch, S. J. V. , Cuthbertson, F. C. , Wharton, R. M. , Mehta, Z. , & Rothwell, P. M. ((2015) ). Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (ii) Effect of attrition on follow-up. Stroke, 46: (6), 1494–1500. doi: https://doi.org/10.1161/STROKEAHA.115.009065 |
43 | Poulin, V. , Korner-Bitensky, N. , Dawson, D. R. , & Bherer, L. ((2012) ). Efficacy of executive function interventions after stroke: A systematic review. Topics in Stroke Rehabilitation: Moving Evidence Into Practice in Stroke Rehabilitation, 19: (2), 158–171. doi: https://doi.org/10.1310/tsr1902-158 |
44 | Raju, R. S. , Sarma, P. S. , & Pandian, J. D. ((2010) ). Psychosocial problems, quality of life, and functional independence among Indian stroke survivors. Stroke (1970), 41: (12), 2932–2937. doi: https://doi.org/10.1161/STROKEAHA.110.596817 |
45 | Ripollés, P. , Rojo, N. , Grau-Sanchez, J. , Amengual, J. L. , Cámara, E. , Marco-Pallarés, J. ,... Rodriguez-Fornells, A. ((2015) ). Music supported therapy promotes motor plasticity in individuals with chronic stroke. Brain Imaging and Behavior, 10: (4), 1289–1307. doi: https://doi.org/10.1007/s11682-015-9498-x |
46 | Sachdev, P. , Kalaria, R. , O’Brien, J. , Skoog, I. , Alladi, S. , Black, S. E. ,... Institutionen för neurovetenskap och, f. ((2014) ). Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer disease and associated disorders, 28: (3), 206–218. doi: https://doi.org/10.1097/WAD.0000000000000034 |
47 | Salter, K. , Mehta, S. , Wiener, J. , Cotoi, A. , Teasell, R. , Foley, N. , & Speechley, M. ((2018) ). Post-stroke depression and mood disorders. In Evidence-based review of stroke rehabilitation (pp. 1-84). Retrieved from http://www.ebrsr.com/sites/default/files/ch18_v19_2020.pdf |
48 | Schneider, S. , Schönle, P. W. , Altenmüller, E. , & Münte, T. F. ((2007) ). Using musical instruments to improve motor skill recovery following a stroke. Journal of Neurology, 254: (10), 1339–1346. doi: https://doi.org/10.1007/s00415-006-0523-2 |
49 | Scholz, U. , Doña, B. G. , Sud, S. , & Schwarzer, R. ((2002) ). Is general self-efficacy a universal construct? Psychometric findings from 25 countries. European Journal of Psychological Assessment, 18: (3), 242–251. doi: https://doi.org/10.1027//1015-5759.18.3.242 |
50 | Serrien, D. J. ((2008) ). The neural dynamics of timed motor tasks: Evidence from a synchronization - continuation paradigm. European Journal of Neuroscience, 27: (6), 1553–1560. doi: https://doi.org/10.1111/j.1460-9568.2008.06110.x |
51 | Sexton, E. , McLoughlin, A. , Williams, D. J. , Merriman, N. A. , Donnelly, N. , Rohde, D. ,... Bennett, K. ((2019) ). Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. European Stroke Journal, 4: (2), 160–171. doi: https://doi.org/10.1177/2396987318825484 |
52 | Sturm, J. W. , Donnan, G. A. , Dewey, H. M. , Macdonell, R. A. L. , Gilligan, A. K. , & Thrift, A. G. ((2004) ). Determinants of handicap after stroke: The North East Melbourne Stroke Incidence Study (NEMESIS. Stroke, 35: (3), 715–720. doi: https://doi.org/10.1161/01.STR.0000117573.19022.66 |
53 | Teasell, R. , Hussein, N. , Viana, R. , Donaldson, S. , & Madady, M. ((2020) ). Stroke Rehabilitation Clinician Handbook. In Evidence-Based Review of Stroke Rehabilitation (pp. 1-27). Retrieved from www.ebrsr.com |
54 | Thaut, M. H. ((2005) ). Rhythm, music, and the brain: Scientific foundations and clinical applications. New York, NY: Routledge. |
55 | Thaut, M. H. , Gardiner, J. C. , Holmberg, D. , Horwitz, J. , Kent, L. , Andrews, G. ,... McIntosh, G. ((2009) ). Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Annals of the New York Academy of Sciences, 1169: , 406–416. doi: https://doi.org/10.1111/j.1749-6632.2009.04585.x |
56 | Thaut, M. H. , Kenyon, G. P. , Schauer, M. I. , & McIntosh, G. C. ((1999) ). The connection between rhythmicity and brain function: Implications for therapy of movement disorders. IEEE Engineering in Medicine and Biology Magazine, 18: , 101–108. doi: https://doi.org/10.1109/51.752991 |
57 | Thaut, M. H. , Miller, R. A. , & Schauer, L. M. ((1998) ). Multiple synchronization strategies in rhythmic sensorimotor tasks: Phase vs period correction. Biological Cybernetics, 79: (3), 241–250. doi: https://doi.org/10.1007/s004220050474 |
58 | Thaut, M. H. , Tian, B. , & Azimi-Sadjadi, M. R. ((1998) ). Rhythmic finger tapping to cosine-wave modulated metronome sequences: Evidence of subliminal entrainment. Human Movement Science, 17: (6), 839–863. doi: https://doi.org/10.1016/S0167-9457(98)00031-1 |
59 | Van Vugt, F. T. , Kafczyk, T. , Kuhn, W. , Rollnik, J. D. , Tillmann, B. , & Altenmüller, E. ((2016) ). The role of auditory feedback in music-supported stroke rehabilitation: A single-blinded randomised controlled intervention. Restorative Neurology and Neuroscience, 34: (2), 297–311. doi: https://doi.org/v10.3233/RNN-150588 |
60 | Van Vugt, F. T. , Ritter, J. , Rollnik, J. D. , & Altenmuller, E. ((2014) ). Music-supported motor training after stroke reveals no superiority of synchronization in group therapy. Frontiers in Human Neuroscience, 8: (MAY). doi: http://dx.doi.org/10.3389/fnhum.2014.00315 |
61 | Wechsler, D. ((1997) ). Wechsler Adult Intelligence Scale - III. San Antonio, TX: The Psychological Corporation. |
62 | Weissgerber, T. L. , Garovic, V. D. , Savic, M. , Winham, S. J. , & Milic, N. M. ((2016) ). From static to interactive: Transforming data visualization to improve transparency. PLoS Biology, 14: (6), e1002484. doi: https://doi.org/10.1371/journal.pbio.1002484 |
63 | Wulf, G. , Chiviacowsky, S. , & Lewthwaite, R. ((2012) ). Altering mindset can enhance motor learning in older adults. Psychology and Aging, 27: (1), 14–21. doi: https://doi.org/10.1037/a0025718 |
64 | Zuckerman, M. , Zuckerman, M. , Lubin, B. , Lubin, B. , Rinck, C. M. , & Rinck, C. M. ((1983) ). Construction of new scales for the Multiple Affect Adjective Checklist. Journal of Behavioral Assessment, 5: (2), 119–129. doi: https://doi.org/10.1007/BF01321444 |




