Neurocognitive outcomes in adults following cerebral hypoxia: A systematic literature review
Abstract
BACKGROUND:
Hypoxic ischemic brain injury (HIBI) occurs as a result of complete or partial disruption of cerebral oxygen supply. The physical and cognitive sequelae of adults following hypoxia varies widely.
OBJECTIVE:
To systematically review studies exploring the neuropsychological outcomes following hypoxic brain insult in adults.
METHODS:
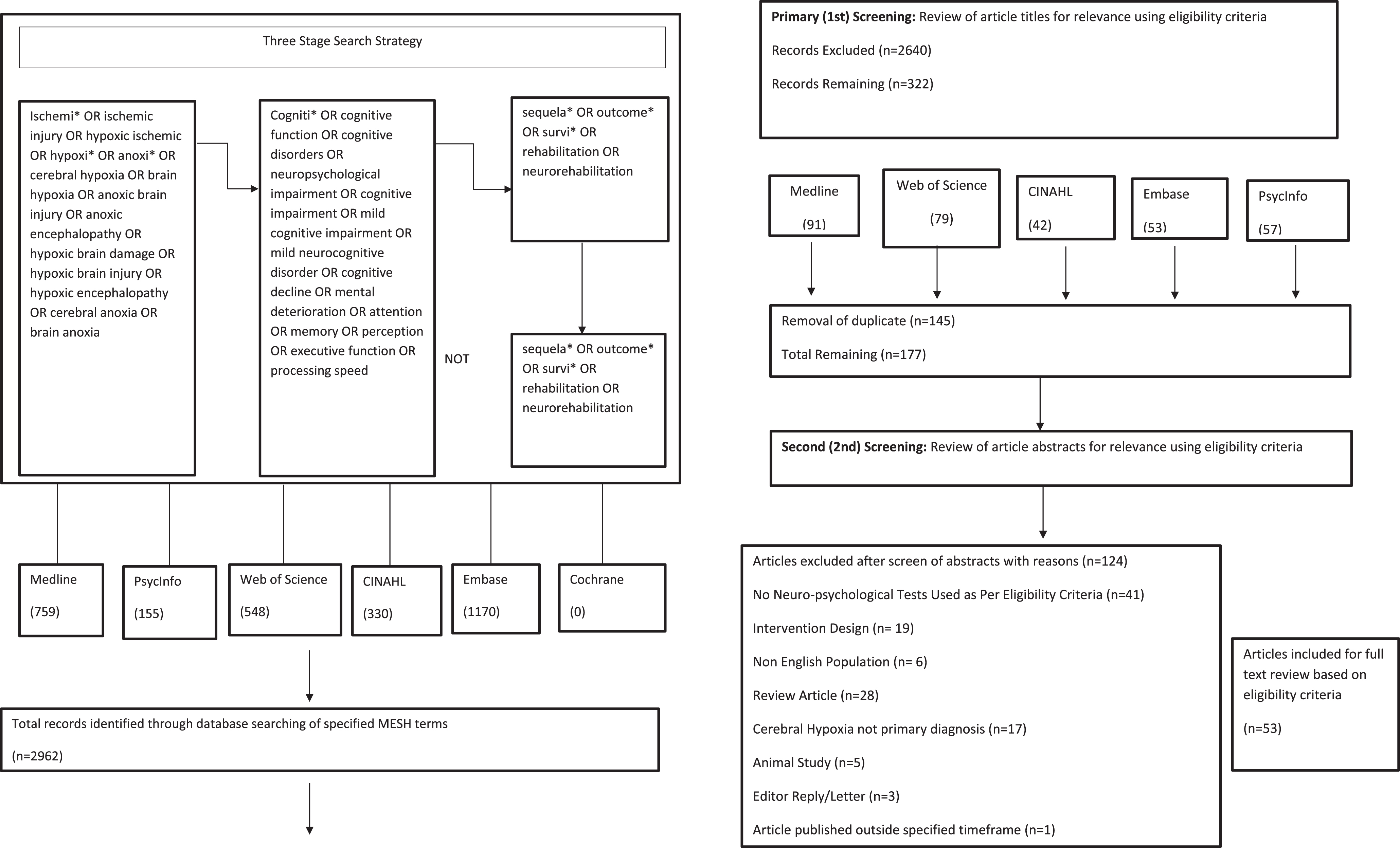
Data was sourced using six databases (CINAHL, Cochrane, Embase, Medline, PsycInfo and Web of Science). Initial MESH terms identified 2,962 articles. After a three-stage independent review process, 18 articles, 9 case studies and 9 group studies were available for data synthesis from 1990-2012. Case study data was converted to standardised scores and compared to available test norms. Cohen’s d was calculated to permit group data interpretation.
RESULTS:
Intellectual decrement was observed in some studies although difficult to delineate given the lack of use of measures of premorbid ability. Cognitive sequelae varied albeit with predominant disturbance in verbal memory, learning ability and executive function observed across studies. Wechsler Memory Scale Revised (WMS-R) visual memory was comparable to normative data. Impaired Rey Osterrieth Complex Figure (ROCFT) performance was found among group studies. Across visuo-constructional and attention domains, performance varied, although no significant difference relative to reported means was observed.
CONCLUSIONS:
Future studies should consider the use of standardised assessment protocols, which include measures of premorbid functioning and performance validity.
Abbreviations
BNT | Boston Naming Test |
RAVLT | Rey Auditory Verbal Learning Test |
RBMT | Rivermead Behavioural Memory Test |
ROCFT | Rey Osterrieth Complex Figure Test |
TMT | Trail Making Test A & B |
WAIS-R | Wechsler Adult Intelligence Scale Revised |
WMS-R | Wechsler Memory Scale Revised |
WCST | Wisconsin Card Sorting Task (WCST) |
1Introduction
The brain requires a continuous supply of oxygen to permit normal function (Caine & Watson, 2000; Ferdinand & Roffe, 2016). Cerebral hypoxia results from interruption to cerebral blood flow and blood oxygenation (Huang & Castillo, 2008). Oxygen deprivation can have a significant and often permanent impact on an individual’s brain functioning (Hopkins & Bigler, 2012) and has been considered a non-traumatic form of brain injury (Stock, Cowie, Chan et al., 2016). Parkin, Miller & Vincent (1987) described the typical pattern of effect as diffuse, resulting in global deterioration in neuropathological and cognitive processes. Others have proposed brain regions are damaged at different rates, with ‘watershed’ regions of the cortex particularly vulnerable due to their dependence on cerebral arteries for an effective and continuous vascular supply (Caine & Watson, 2000). Studies have also specified brain regions in the temporal lobes including the hippocampus that are most susceptible to the effects of a hypoxic event due to their higher metabolic activity (Raphael, Elkharrat, Jars-Guincestre et al., 1989; Hopkins, Weaver & Kesner, 1993; Hopkins, Gale, Johnston et al., 1995).
1.1Aetiology and prognosis
Hypoxia can develop as a result of anaesthetic accidents, carbon monoxide (CO) poisoning (Quinn, McGahee, Polittle et al., 2009), cardiac and respiratory arrest (Perez, Samudra & Aiyagari, 2016), pulmonary injuries and cerebral blood loss injuries (Schultz, Sepehry & Greer, 2018), following stroke (Ferdinand & Roffe, 2016), near drowning (Nucci, Lukasova, Sato & Amaro, 2016) and attempted suicide via hanging (Tazopoulou, Milijkovitche, Trucelle et al., 2016) as well as conditions such as COPD. Suffice to state, the pathophysiology and neuropathology is most clearly established for hypoxia following cardiac arrest, CO poisoning and stroke (Busl & Greer, 2010; Ferdinand & Roffe, 2016). In a recent study exploring the long-term prognosis of individuals following hypoxic injury, Heinz and Rollnik (2015) described the variability in outcome among survivors, acknowledging it can be either poor or good. Historically others have agreed, referring to the inconsistent pattern as representing a bimodal pattern, with a moderate skew in the direction of a poorer outcome (Bachman & Katz, 1997). Notwithstanding, significant periods of brain hypoperfusion are generally associated with poorer clinical outcomes (Busl & Greer, 2010).
In attempting to understand and predict the seemingly unpredictable pattern of outcome, some have proposed that structural, pathophysiology and cognitive changes following hypoxia are most dependent upon the nature and severity of the injury (Greer, 2006). In an extensive literature review published a decade ago, Anderson & Arciniegas (2010) identified the total duration of unconsciousness after hypoxic brain injury relates to long-term cognitive prognosis. Others including Caine and Watson (2000) strongly argued that impairment is related to the site and brain structures affected by the hypoxic event. Earlier studies however, reported no relationship between age, gender, cause, site of onset or severity of injury as predictive of long-term outcome (Levy, Caronna & Singer, 1985).
1.2Cognitive outcomes following hypoxic ischemic brain injury
A considerable amount of literature exists documenting the neurological, cognitive, behavioural and psychosocial outcomes of Hypoxic Ischaemic Brain Injury (HIBI). Among this patient group, parkinsonism, dystonia, seizures, decline in processing speed, memory, executive function and depression have all been reported (Allen, Tranel, Bruss & Damasio, 2006; Hopkins & Bigler, 2012; Porter, Hopkins, Weaver et al., 2002; Wilson, Staniforth, Till et al., 2014). The specific neurobehavioral and neurocognitive changes which arise following cerebral hypoxia vary from brain death to coma and minimally conscious states to varying degrees of cognitive impairment but nevertheless well described in the existing literature (Anderson & Arciniegas, 2010; Caine & Watson, 2000; Hopkins, Gale & Weaver, 2006; Lu-Emerson & Khot, 2010; Schultz, Sepehry & Greer, 2018; O’Connor, Verfaelli & Cermak, 1995; Wilson, 1996).
In an earlier seminal review (1966-1998) Caine and Watson (2000) observed memory deficits in over half (54%) of individuals. Changes in personality and behaviour were also described in 31.3% of cases. Sauve, Lewis, Blankenbiller, Rickabaugh, & Pressler (1996) employed a case-controlled design to examine cognitive impairment in chronic heart failure patients compared with matched healthy controls on a number of domains including orientation, attention, memory, executive function and speed of information and motor processing. Those with chronic heart failure scored poorer on all cognitive domains compared to age and gender matched healthy controls. Single case studies have also documented cognitive deterioration post injury. Parkin et al. (1987) observed on the effects of anoxic encephalopathy following cardiac arrest on a 43-year old female and describe memory loss, dysphasia and an overall decline in general cognitive function compared to premorbid ability. Similarly, in a case study reporting two adolescents following suicide attempts by hanging, Zabel, Slomine, Brady, & Christensen, (2005) document impairments in multiple cognitive domains including attention, expressive and receptive language, processing speed, visuospatial abilities, memory as well as executive functioning.
1.3Rationale for current review
With advances in acute medical treatment and improved resuscitation practice, survival rates are increasing among individuals who sustain even severe oxygen deprivation (Anderson & Arciniegas, 2010; Knot & Tirschwell, 2009). However, significant morbidity persists and increasingly individuals require longer-term support beyond acute care. Nevertheless, the potential physical, cognitive and psychological consequences of cerebral hypoxia remain variable. To date there has been no published systematic review documenting the cognitive outcomes of adult survivors following Hypoxic Ischaemic Brain Injury (HIBI). Four extensive albeit non-systematic reviews have been published (Anderson & Arciniegas, 2010; Caine & Watson, 2000; Ferdinand & Roffe, 2016; Schultz, Sepehry & Greer, 2018). In addition, one unpublished thesis study which although systematic had identifiable gaps in terms of search terminology and subsequent output (Crooks, 2014). Accordingly, systematic data synthesis could provide an enhanced understanding of the impairment patterns observed among individuals with varying aetiology and course as well as facilitated improved prognostics in subacute and post-acute/rehabilitation settings. This paper aims to systematically review the peer reviewed published literature on cerebral hypoxia related to observed neurocognitive outcomes in adults.
2Methodology
2.1Search strategy
This systematic review was prospectively registered with Prospero (2017: CRD42017054692) in keeping with PRISMA-P 2015 statement (Moher, Shamseer, Clarke et al., 2015). Relevant literature was sourced via a systematic search of six common databases namely Medline, PsycINFO, Web of Science, CINAHL, Embase and Cochrane. Studies were identified using MESH headings. Limits placed on the search specified time (January 1970-December 2016), language (English), population (humans) and age (>18 years). Dissertation and conference proceedings were excluded, and duplicates removed. The following search terms were employed: Ischemi* OR ischemic injury OR hypoxic ischemic OR hypoxi* OR anoxi* OR cerebral hypoxia OR brain hypoxia OR anoxic brain injury OR anoxic encephalopathy OR hypoxic brain damage OR hypoxic brain injury OR hypoxic encephalopathy OR cerebral anoxia OR brain anoxia AND Cogniti* OR cognitive function OR cognitive disorders OR neuropsychological impairment OR cognitive impairment OR mild cognitive impairment OR mild neurocognitive disorder OR cognitive decline OR mental deterioration OR attention OR memory OR perception AND sequela* OR outcome* OR survi* OR rehabilitation OR neurorehabilitation NOT sequela* OR outcome* OR survi* OR rehabilitation OR neurorehabilitation.
The search involved three sub-searches (see Fig. 1). A total of 2,962 articles were identified through database searching comprising 759 articles from Medline, 155 from PsycInfo, 548 from Web of Science, 330 from CINAHL and 1170 from Embase. A primary screen of article titles for relevance based on Eligibility Criteria 1, resulted in the exclusion of 2,640 articles due to their use of experimental design (medication or therapy intervention), focus on neurochemical status or neuroimaging. Of the remaining 322 articles, duplicate studies were removed (n = 145) leaving 177 articles reviewed by abstract. Using Eligibility Criteria 2, of the 127 abstracts reviewed, 41 were excluded on the basis that no standardised neuropsychological tests were used to measure cognitive outcomes, 19 were experimental design with very limited comparative data, 6 used a non-English speaking population, 28 were review articles, 17 did not specify hypoxia as the primary diagnosis, 5 were animal studies, 3 were editorial correspondences, 3 involved individuals under 18 years, 1 article was published outside of the specified timeframe and 1 was only available as an abstract.
Fig. 1
Systematic Search Strategy.
2.2Eligibility Criteria 1: Criteria applied at title review
Inclusion Criteria: (1) Studies involving human, adult populations; (2) Papers published in peer reviewed journals from 1st January 1970; (3) Studies which examined neurocognitive outcomes using validated, objective and performance-based measures.
Exclusion Criteria: (1) Papers which were review articles, conference proceedings and letters to the editor; (2) Studies involving non-human populations; (3) Individuals aged <18 years.
The full text of the remaining 54 articles underwent screening by two independent reviewers based on Eligibility Criteria 2 outlined below.
2.3Eligibility Criteria 2: Applied at full text and abstract review
Inclusion Criteria: (1) Studies involving human, adult populations; (2) Papers published in peer reviewed journals since 1970; (3) Studies which examined neurocognitive outcomes using validated, objective and performance based measures.
Exclusion Criteria: (1) Studies involving populations with neurodegenerative condition including sleep apnoea and hypoxic injury as a result of high altitude climbing; (2) Treatment or experimental studies; (3) Studies using self-report measures or rating scales of cognitive function; (4) Studies which used translated measures originally normed on a UK or American population; (5) Studies involving non-English speaking participants; (6) Studies which administered neuropsychological tests which cannot be compared; (7) Studies reporting data which cannot be compared with other available normative data.
Of the 54 articles, 13 were excluded as they involved a non-English speaking population, 10 did not use objective measures of cognitive function, 1 used neuropsychological tests which were not comparable to any other studies, 1 was a descriptive paper only and 1 was a comments paper from a conference. The measured weighted Cohen’s Kappa for the two independent raters was high (k = 0.93). There was a discrepancy between reviewers on the relevance of two papers (Browne, Halligan, Wade & Taggart, 2003; Fitzgerald, Aditya, Prior, McNeill & Pentland, 2010) due to availability of data in the articles however this was resolved by a third independent reviewer.
Two independent reviewers agreed that 28 articles would be included in the final synthesis based on the eligibility criteria outlined for full article review. It was not possible however, to extract the data from ten papers due to the method used to report the outcomes of cognitive testing (composite scores) (Alexander, Lafleche, Schnyer et al., 2011; Allen, Tranel, Bruss & Damasio, 2006; Grubb, Fox, Smith et al., 2000; Fitzgerald, Aditya, Prior et al., 2010; Lim, Alexander & LaFleche, 2004; Lim, Verfaellie, Schnyer et al., 2014; Myers, Hopkins, Deluca et al., 2008; O’Reilly, Grubb & O’Carroll, 2003; Porter et al., 2002; Sauve, Lewis, Blankenbiller et al., 2009; Speach, Wong, Cattarin et al., 1998). Where possible the raw data was requested from the authors, but this was either not available or the data that was provided was not amenable to comparison with the data extracted from the remaining articles. The primary data was not documented in one paper (Browne et al., 2003), however it was available from an earlier paper (Taggart, Browne, Halligan & Wade, 1999) and therefore included in data extraction. Accordingly, a total of eighteen studies (9 case studies; 9 group studies) were included in the final synthesis.
2.4Data extraction
Where studies reported standardised scores, it was possible to extract data for analysis. For case studies the scores were converted to z-scores based on the population mean and standard deviation as reported for each test. Comparability of group studies was facilitated by converting reported mean performance to Cohen’s d representing the overall effect size. Prior to analysis and in order to interpret results, scores of neuropsychological tests which fell equal to or more than 2 S.D below the mean of the normative population were defined as having significant impairment in that domain, while impaired functioning was indicated for performance equal to or greater than 1 S.D. For those case studies which employed a longitudinal design, the latest available time point was included in data analysis. Where relevant, results were considered significant at the 0.01 level.
3Results
3.1Quality appraisal
The Newcastle-Ottowa Scale adapted for Cross-Sectional Studies (Wells, Shea, O’Connell, Peterson, Welch, Losos & Tugwell, 2008) was employed as a framework to assess the overall quality of articles included in the final synthesis. A second reviewer also independently assessed the quality of articles and the measured weighted Cohen’s Kappa for the raters was high (k = 0.90). When the quality criteria were applied to each study, the most frequently fulfilled quality requirement of the Newcastle-Ottowa Scale related to the method of assessment outcome employed during the study and the statistical analysis used (see Table 1).
Table 1
Quality Appraisal of Included Studies
| Author | n | Study Design | Age at Testing (Years) | Interval from Index Event to Assessment | Cerebral Hypoxia Aetiology | Quality Assurance Rating (Months) | |
| 1 | Auerbach et al. (1990) | 1 | Case Study | 18 | 28 | Chlorine Gas Poisoning | 5* |
| 2 | Medalia et al. (1991) | 2 | Case Study | 24 | 24 | Attempted Hanging | 5* |
| 26 | 36 | Attempted Hanging | |||||
| 3 | Hopkins et al. (1995) | 3 | Case Study | 34 | 6 | CO Poisoning | 5* |
| 31 | 12 | Cardiorespiratory Arrest | |||||
| 49 | 12 | Asthmatic Attack | |||||
| 4 | Hopkins et al. (1998) | 2 | Case Study | 56 | 12 | Hantavirus Pulmonary Syndrome | 5* |
| 67 | 13 | Hantavirus Pulmonary Syndrome | |||||
| 5 | Kaplan (1999) | 1 | Case Study | 49 | <2 | Cardiac Arrest | 5* |
| 6 | Armengol (2000) | 5 | Case Study | 19 | 28 | Near Drowning | 6* |
| 45 | 13 | Cardiac Arrest | |||||
| 27 | 15 | Anaesthesia | |||||
| 20 | 31 | Anaesthesia | |||||
| 34 | 9 | Asthmatic Attack | |||||
| 7 | Wilson et al. (2003) | 6 | Case Study | 46 | <12 | Cardiac Arrest | 7* |
| 40 | <12 | CO Poisoning | |||||
| 32 | <12 | Attempted Hanging | |||||
| 56 | <12 | Respiratory Arrest | |||||
| 29 | <12 | Respiratory Arrest | |||||
| 41 | <12 | Respiratory Arrest | |||||
| 8 | Baggett et al. (2003) | 1 | Case Study | 36 | 9 | Cardiac Arrest | 5* |
| 9 | Warren et al. (2012) | 1 | Case Study | 48 | <12 | Status Epilepticus | 5* |
| 10 | Wilson (1996) | 14 | Retrospective Cohort Study | 30.22 (13.7) | Not Specified | 5 CO Poisoning | 6* |
| 4 Cardiac Arrest | |||||||
| 2 Anaesthetic Accident | |||||||
| 1 Respiratory Arrest following Pulmonary Embolus | |||||||
| 1 Hanging | |||||||
| 1 Status Epilepticus | |||||||
| 11 | Moser et al. (1999) | 93 | Retrospective Matched Cohort | 60.94 (10.74) | Not Specified | CHD | 5* |
| 12 | Gale et al. (1999) | 21 | Retrospective Cohort Study | 39.0 (7.0) | Not Specified | CO Poisoning | 7* |
| 13 | Hopkins et al. (1999) | 55 | Prospective Cohort Study | 45.4 (16.00) | >12 | Acute Respiratory Distress Syndrome | 7* |
| 14 | Taggart et al. (1999) | 50 | Prospective Cohort Study | 58.9 (10.4) | 3 | Coronary Artery Bypass Grafting with Cardiopulmonary Bypass | 5* |
| 15 | Drysdale et al. (2000) | 10 | Retrospective Cohort Study | 70.0 | 36 | Out of Hospital Cardiac Arrest | 7* |
| 16 | Porter et al. (2000) | 62 | Longitudinal Cohort | 37.0 (7.0) | 6 | CO Poisoning | 6* |
| 17 | Hopkins et al. (2005) | 10 | Retrospective Matched Cohort Study | 40.8 (9.0) | 24.6 | CO Poisoning | 8* |
| 18 | Hopkins et al. (2006) | 66 | Observational Cohort Study | 39.2 (18.2) | >12 | Acute Respiratory Distress Syndrome | 7* |
3.2Recruitment and sample information
A summary of the eighteen papers included in the final review is provided in Table 1. For case studies (n = 9), age at testing ranged from 18–67 years with a mean age of 37.66 (S.D. = 13.59). The average age range of those who participated in group studies (n = 9) was 30.22–70 years with a mean age of 48.82 (S.D. = 13.29). Time interval from the hypoxic event to cognitive assessment varied among the studies ranging from 1 month to 28 months. Aetiology differed among the studies and included heart failure, anterior communicating artery (ACoA), acute respiratory distress syndrome, respiratory arrest, carbon monoxide poisoning, in hospital cardiac arrest, attempted hanging, coronary artery bypass surgery, near downing, status epilepticus, anaesthetic accident, hantavirus pulmonary syndrome, anaphylactic reaction and chlorine gas poisoning.
All eighteen studies used standardised neuropsychological assessment to examine cognitive outcomes. Specifically, 14 used the Wechsler Adult Intelligence Scale Revised (WAIS-R) (Wechsler, 1987), 10 used the Rey Osterrieth Complex Figure Test (ROCFT) (Rey & Osterrieth, 1993), 9 used Trail Making Test (TMT) version A and B (Reitan & Wolfson, 1985; Reitan & Wolfson, 1995), 8 used the Rey Auditory Verbal Learning Test (RAVLT) (Schmidt, 1996) and 7 administered the Wechsler Memory Scale –Revised (WMS-R) (Wechsler, 1987). The remainder of the neuropsychological measures used varied and included The Boston Naming Test (BNT) (Kaplan, Goodglass & Weintrab, 1983), The Wisconsin Card Sorting Test (WCST) (Heaton, Chelune, Talley et al., 1981), The Rivermead Behavioural Memory Test (RBMT) (Wilson, Cockburn & Baddeley, 1985) and the WAIS-R Digit Span subtest (Wechsler, 1987).
3.3Overall intellectual functioning
Intellectual functioning was assessed using the WAIS-R in 19 cases and 5 group studies (n = 166). In the main, studies reported relatively preserved function with some exceptions of marked impairments. The pooled mean FSIQ for the group studies was 98.7 (S.D. = 1.15) and comparable to normative data (d = 0.12). The mean FSIQ of the case studies was 83.95 (S.D. = 15.50) indicating impairment relative to the general population with a large effect size calculated (d = 1.05). Analysis using a One Sample t Test showed a significant difference in mean FSIQ [t (18) = –4.15, p = 0.001], VIQ [t (18) = –3.89, p = 0.001] or PIQ [t (18) = –2.736, p = 0.014] of the reviewed studies. It is probable some of the reported decline in cognitive function observed in the case studies may be accounted for by lack of use of standardised measures of premorbid ability (Medalia, Merriam & Ehrenreich, 1991; Wilson, Harpur, Watson & Morrow, 2003). Notwithstanding, the observed decline in cognitive function within the remaining studies was not in keeping with premorbid estimates (Auerbach & Hodnett, 1990; Hopkins, Gale, Johnston et al., 1995; Armengol, 2000).
3.4Assessment of language
Language ability was measured using the Boston Naming Test in two case studies (n = 4) (Armengol, 2000; Baggett, Kelly & Korenman, 2003) and one group study (n = 93) (Moser, Cohen & Clark, 1999). In the case studies, all but one individual showed severely impaired ability. The overall mean for the case studies was 36.25 (S.D. = 20.0) with a large effect size (d = 1.27) well below the normative mean. For the group studies, a small effect size was observed when performance was compared to the study’s control group (matched on age and gender) (d = 0.29) relative to the normative sample (d = 0.37). Despite the variability between the case and group studies, a One Sample t Test showed no significant difference in overall ability ([t (3) = –1.60, p = 0.208]).
3.5Memory and learning
On formal neuropsychological measures of memory function, the results showed varied cognitive sequelae with predominant disturbance in verbal ability observed across all studies. Using the RAVLT, four case studies (n = 12) demonstrated rapid loss of verbal memory and overall performance below the normative population on a learning task (M = 4.75; S.D. = 1.76) and delayed recall (M = 1.44; S.D. = 2.19). Comparatively, the mean performance on the learning trial of the RAVLT among the five group studies (N = 202), was below the normative mean (M = 5.7; S.D. = 0.33) and reflected a medium-large effect size (d = 0.74). For studies which administered the recognition trial of the RAVLT all individuals performed better on recognition than delayed free recall (Hopkins, Gale, Johnston et al., 1995; Hopkins, Larson-Lohr, Weaver et al., 1998; Hopkins, Weaver, Pope et al., 1999). Four cases also assessed verbal memory (n = 8) using the WMS-R and had a mean performance of 84.5 (S.D. = 25.0) reflecting ability below the normed mean with a medium effect size (d = 0.74) observed. For three group studies (n = 141) pooled mean performance on verbal memory tasks of the WMS-R was 90.5 (S.D. = 5.84) demonstrating ability below the normative population with a large effect size calculated (d = 0.82).
Visual memory ability as assessed by the Wechsler Memory Scale Revised (WMS-R) was comparable to normative data among the case studies (d = 0.21) and group studies (d = 0.01). However, impaired performance was observed among group studies on the Rey Osterrieth Complex Figure Test (ROCFT). Two group studies administered the ROCFT immediate visual memory task (n = 121) with a mean group performance of 15.2 (S.D. = 1.15), reflecting impairment below normed data with a large effect size observed (d = 0.95). On delay, impairment persisted with a mean of 13.4 (S.D. = 3.4) below the general population and reflecting a medium effect size (d = 0.67).
Among studies which employed the Rivermead Behaviour Memory Test (RBMT), impaired performance was observed in both case and group studies. Both case studies (d = 3.02) and group studies (d = 2.23) yielded a large effect size, with performance below the test norms. Finally, as Table 3 illustrates, the One Sample t Test showed no statistically significant difference between the mean performance across case studies and the group studies on all tasks of memory, with exception of RAVLT delayed verbal recall.
Table 2
Summary of Data Synthesis
| Cognitive Domain | Number of Studies | Total Pooled Sample Size | Case Studies Pooled Mean (S.D.) | Effect Size (d) | Group Studies Pooled Mean (S.D.) | Effect Size (d) |
| FSIQ | 14 | 185 | 83.95 (15.50) | 1.05 | 98.7 (1.15) | 0.12 |
| RAVLT (Learning) | 8 | 154 | 4.75 (1.76) | 1.07 | 5.7 (0.33) | 0.74 |
| RAVLT (Delay) | 7 | 161 | 1.44 (2.19) | 3.80 | 8.50 (0.37) | 1.25 |
| WMS-R (Verbal Memory) | 7 | 139 | 84.50 (25.0) | 0.74 | 90.50 (5.84) | 0.82 |
| WMS-R (Visual Memory) | 7 | 139 | 94.63 (31.69) | 0.21 | 99.78 (0.22) | 0.01 |
| ROCFT (Immediate) | 6 | 126 | 18.80 (6.10) | 0.22 | 15.23 (1.15) | 0.95 |
| ROCFT (Delay) | 10 | 174 | 14.56 (9.28) | 0.57 | 13.41 (3.44) | 0.67 |
| RBMT | 2 | 16 | 7.83 (6.43) | 3.02 | 14.6 (4.4) | 2.23 |
| WAIS-R Digit Span | 6 | 101 | 7.50 (2.89) | 0.78 | 8.83 (0.56) | 0.45 |
| TMT A | 9 | 270 | 39.40 (9.30) | 1.12 | 34.19 (4.44) | 0.31 |
| TMT B | 9 | 270 | 96.00 (22.05) | 1.93 | 84.23 (10.72) | 1.61 |
| WCST | 3 | 18 | 32.25 (23.40) | 1.39 | 10.46 (10.29) | 0.12 |
| ROCFT (Copy) | 8 | 172 | 28.50 (9.33) | 0.62 | 30.81 (2.09) | 0.28 |
| BNT | 3 | 99 | 32.25 (20.02) | 1.27 | 52.25 (7.75) | 0.37 |
Table 3
Difference in Mean Performance among Case and Group Studies
| One Sample t Test | ||
| Cognitive Domain | t | p |
| WMS-R Verbal Memory | –0.68 | 0.529 |
| WMS-R Visual Memory | –0.46 | 0.659 |
| ROCFT Immediate Memory | 1.31 | 0.261 |
| ROCFT Delayed Recall | 0.35 | 0.736 |
| RAVLT New Learning (Trial 1) | –1.83 | 0.095 |
| RAVLT Encoding (Trail 5) | –3.77 | 0.003 |
| RAVLT Delayed Recall | –9.68 | 0.000 |
| RAVLT Recognition | –1.89 | 0.118 |
| RBMT | –2.58 | 0.050 |
3.6Attention and concentration
Formal assessment of attention was limited across studies and employed the WAIS-R digit span subtest. Three case studies (n = 4) had an overall mean performance of 7.5 (S.D. = 2.9), with a medium effect size (d = 0.78) below the normative mean. The pooled mean performance for three group studies (n = 97) using the digit span was 8.8 (S.D. = 0.56), reflecting a medium effect size (d = 0.45) below the mean performance of the normative sample. There was no significant difference in the mean performance of the case studies and group studies ([t (4) = –0.921, p = 0.425]).
3.7Visuospatial construction abilities
The copy subtest of the ROCFT was used to assess visuo-spatial and constructional abilities in three cases (n = 6) and five group studies (n = 166). The mean performance of the case studies was 28.5 (S.D. = 9.3) with a medium effect size calculated (d = 0.62) and reflected performance below normative data. The mean performance of the group studies was 30.8 (S.D. = 2.09), with a small effect size calculated compared to the normative population. In the main, overall performance reflected ability in the normal range, although there were some individual exceptions which showed impairment. Notwithstanding, One Sample t Test analysis showed no significant difference in mean performance between the case and group studies ([t (5) = –0.606, p = 0.571]).
3.8Executive function
Formal assessment of executive function using the TMT, showed ability below the normative mean on Trails A, a simple task of visual search and sequencing and impaired performance on Trails B, a task of attentional switching. Three case studies among five individuals had a mean performance on TMT A of 39.4 (S.D. = 9.3) with large effect size calculated (d = 1.12) illustrating performance below the normative sample. For six group studies (n = 265) the pooled mean performance on TMT A was (M = 34.19; S.D. = 4.44) with a medium effect size calculated (d = 0.31) again indicating performance below the normative population. On TMT B the case studies (d = 1.93) and group studies (d = 1.61) showed a large effect size, with ability below the normative mean. One Sample t Test revealed no significant difference in performance between the case studies and group studies on either the TMT A ([t (6) = –3.104, p = 0.021]) or TMT B [t (6) = –2.904, p = 0.027]).
Executive function was also assessed using the original Wisconsin Card Sorting Test (WCST) among three case studies (n = 6) and 2 group studies (n = 35). The pooled mean number of perseverative errors among the case studies was 32.25 (S.D. = 23.4) with a large effect size (d = 1.39) compared to normative data. For group studies the mean number of perseverative errors was 10.46 (S.D. = 10.29), and comparable to the normative population (d = 0.12). A One Sample t Test revealed no significant difference between the mean performance of case studies and group studies ([t (3) = 1.86, p = 1.60]).
4Discussion
This systematic review examined the published literature on the neuropsychological outcomes among adult survivors following cerebral hypoxia, with the aim of establishing the extent or otherwise of domain specific cognitive impairment relative to normative data sources. Results show a varied picture across cognitive domains examined with the greatest disturbance in delayed verbal recall and executive function. During performance on the Rey AVLT, individuals showed deficits in terms of delayed verbal recall. However, in all studies which administered the Rey AVLT recognition subtest, the sample’s performance benefitted from cueing, which is suggestive of a retrieval deficit (Anderson & Arciniegas, 2010; Khan, 2012) or alternatively maybe due to faulty encoding. On tasks of executive function, impaired performance of varying degrees was also observed across all studies. Difficulties performing tasks of executive function could denote decline in a variety of cognitive processes including visuospatial function, psychomotor processing speed, planning and organisation, problem solving, attention, switching and anticipation (Lezak, Howieson & Loring, 2004; Strauss & Sherman, 2006). Poorer performances on tasks of executive function which involve a combination of complex cognitive processes has been described in previous studies (Sauve et al., 2009; Speech et al., 1998; Wilson et al., 2003).
In the main, relatively preserved overall intellectual functioning was observed among the cohort with some exceptions of marked impairments relative to normative data. Some studies noted impairments relative to expected pre-morbid functioning (Auerbach & Hodnett, 1990; Medalia et al., 1991; Wilson, Harpur, Watson & Morrow, 2003) although some remained within normal limits (Baggett et al., 2003; Hopkins, Gale & Weaver, 2006). Some other studies offered no specific details on premorbid ability despite observable impairment (Armengol, 2000; Hopkins, Gale, Johnston et al., 1995).
Visual memory performance on the WMS-R was comparable to age matched normative data, whilst ROCFT impairment was found across all studies. For two studies (n = 16) assessing everyday memory using the RBMT severe impairment in performance was observed among all individuals. Performance on visuospatial constructional abilities and attention varied among the studies, although not significantly. Most showed intact ability on both with some exceptions of marked impairments (Auerbach & Hodnett, 1990; Gale et al., 1999; Hopkins, Gale, Johnston et al., 1995; Hopkins, Gale & Weaver, 2006; Wilson, 1996).
The findings of the present systematic review are consistent with Bachman & Katz’s (1997) view, that neuropsychological outcomes following hypoxia can present a bimodal pattern of cognitive outcome, in which individuals can experience markedly varying degrees of clinical recovery. Over time, for some there can be limited long-term cognitive disturbance, while for others the effects can result in debilitating impairment which has the potential to impact on daily function and lead to significant disability (Alexander, Lafleche, Schnyer & Verfaellie, 2011; O’Connor, Verfaelli & Cermak, 1995).
Previous studies have proposed an amnesic picture of cognitive impairment as the prototypical outcome of hypoxia (Volpe & Hirst, 1983) which results from localised, focal damage (reduced grey matter) to hippocampal regions of the brain (DiPaola, Caltagrione, Fadda et al., 2008). In this systematic review, impaired verbal memory particularly the rapid loss of verbal memory overtime was observed across all studies. Deficits in ability to acquire new information and recall after delay is also described in previous reviews, which report greater incidence of anterograde amnesia than retrograde amnesia among this population (Anderson & Arciniegas, 2010). Previous case and group studies have also reported impairments in delayed recall (O’Reilly, Grubb & O’Carroll, 2003; Sauve et al., 2009). In addition, impairment was also noted across all studies which administered the RMBT. It is probable that such impairment will impact on individual’s ability to return to activities of daily living as documented by O’Reilly et al. (2003) among IHCA and OHCA survivors.
While there is a high level of memory impairment among survivors, it is nevertheless not inevitable (Hopkins, Larson-Lohr, Weaver & Bigler, 1998). Further, the results do not conclusively agree with the amnesic syndrome theory and demonstrate that disturbances in memory typically co-occur with impairments in other cognitive domains, a finding reported in previous studies (Lim, Alexander & Lafleche, 2004). This might suggest a more diffuse pattern of damage following hypoxia (Baggett et al., 2003; Shah, Al-Adawi, Dorvlo et al., 2004). Reports of multiple cognitive domain level impairments following hypoxia are consistent with previous reviews (Anderson & Arciniegas, 2010; Caine & Watson, 2000). Indeed, Caine et al. (2000) concluded that watershed regions and the basal ganglia may be more vulnerable to the effects of oxygen deprivation during a hypoxic event than the hippocampus. Further, the finding that memory performance among the examined sample benefitted from cueing is notable in the context of the classical hippocampus functions, given that retrieval has been considered a cognitive process associated with frontal regions and largely independent of the hippocampus (Frankland & Bontempi, 2005).
Beyond memory disturbance individuals also showed impaired performance in executive function and varying ability in domains of attention, language and visuospatial abilities. Whilst an earlier review (Caine & Watson, 2000) acknowledged disturbance in executive function following hypoxia, it identified that among the studies reviewed, few formal assessment measures were administered and instead studies largely relied on clinical observation of personality and behavioural changes. It is notable that the present review observed notable executive function impairment on objective testing. Similarly, although variable, observed attentional impairments have been previously documented although assessment of attention is frequently overlooked in clinical reports (Anderson & Arciniegas, 2010; Wilson, Harpur, Watson & Morrow, 2003). The outcome on language ability is also consistent with previous studies which is reportedly is less disturbed than other cognitive domains (Caine & Watson, 2000) albeit that expressive language is more affected than receptive abilities (Corkin, Cohen, Sullivan et al., 1985; Meyer, 1956).
4.1Review limitations
While this review provides a systematic synthesis of the neurocognitive outcomes in adults following cerebral hypoxia, the reliability and generalisability of the results should be carefully considered in light of a number of notable limitations. Firstly, the quality assessment performed using the Newcastle Ottawa Quality Assessment Scale noted that included articles employed standardised age normed neuropsychological assessments. Nonetheless, review findings were derived from small sample case or group studies. Thus, it is possible that included studies may not be representative of the larger HIBI clinical population particularly those presenting following severe cerebral anoxia. In addition, few studies utilised any measure of estimated premorbid cognitive ability. Furthermore, studies often did not offer details on the method of recruitment and selection and where studies had non-respondents, no details were provided to allow for comparability.
Heterogeneity in relation to age, aetiology and severity of the hypoxia and the time interval between hypoxic incident and neuropsychological assessment, further restrict the comparisons which could be made among the studies. Of note, none of the included studies utilised measures of performance validity which would now be commonly employed in routine clinical practice (British Psychological Society practice guidelines, 2009; Bush, Ruff, Tröster et al., 2005). In addition, in terms of age, there was a significant difference between included group and case studies. Previous research has considered age a contributor to the degree of impairment following cerebral hypoxia (Hopkins, Gale, Johnston et al., 1995) and recommended younger individuals (<30 years) are most appropriate for rehabilitative interventions post hypoxia (Grosswasser, Cohen & Costeff, 1989). However, age related effects have not been reported unequivocally (Levy et al., 1985; Greer, 2006). With varied aetiology of injury, previous reviews identified that the extent of cognitive impairment following hypoxia is related to the cause of the injury (Anderson & Arciniegas, 2010; Busl & Greer, 2010). However, others have argued that severity and aetiology of hypoxia is not associated with long-term neuropsychological outcomes (Hopkins et al., 1995). Hopkins et al. (1995) noted remarkably different outcomes among the three individuals described in a case study and yet reported comparable patterns and severity of initial brain insult. Armengol (2000) reported the length of coma was not predictive of impairments in neuropsychological or psychosocial outcomes and Gale et al (1999) proposed anatomical changes post injury visualised by imaging were related to degree of impairment rather than aetiology of insult. Comparatively, by exploring the outcomes of those with a traumatic brain injury (TBI) and those with an anoxic brain injury, Hopkins, Tate & Bigler (2005) observed no significant difference in overall cognitive sequelae, which it could be argued highlights the limited influence of aetiology on neuropsychological outcomes.
Interval from injury to testing also varied considerably among the studies and it is possible those tested in an earlier, more acute phase could show greater impairment on testing, which may not be reflective of their neuropsychological function overtime. However, previous studies have estimated that greatest recovery takes place in the initial three months post hypoxic injury, which may suggest the differences in interval time among the studies in the present review had limited influence (Allen, Tranel, Bruss & Damasio, 2006; Lim, Alexander & LaFleche, 2004).
Findings are also constrained by the varied use of neuropsychological tests across the studies. While some used extensive neuropsychological batteries, others employed briefer screening assessments which may be less sensitive at detecting impairment of higher cognitive functions (Porter, Hopkins, Weaver et al., 2002). It is also possible that inaccessible data could have contributed further to clarifying the overall picture of neuropsychological outcome following hypoxia. Finally, while the method of calculating effect size offered a means of comparing to normative data, some have cautioned that the use of qualitative categories as described by Cohen (1981) can result in the underestimation of impact of what Cohen considered a small effect size (Glass, McGaw & Smith, 1981).
5Conclusions
A considerable body of literature already exists which documents the neurological, cognitive, behavioural and psychosocial consequences of cerebral hypoxia (Anderson & Arciniegas, 2010; Allen, Tranel, Bruss & Damasio, 2006; Caine & Watson, 2000; Hopkins & Bigler, 2012; Porter et al., 2002; Wilson et al., 2014). This review provides a systematic synthesis of published neuropsychological outcomes from 1990–2012 following cerebral hypoxia but not cerebral anoxia. The results demonstrate the varied cognitive sequelae, which can exist among this clinical population. While some individuals present with significant impairment which results in functional disturbance, others, after the initial acute phase of recovery, display limited residual cognitive effects and may show neurocognitive functioning largely equivalent to their premorbid ability. Results also show that neuropsychological outcomes following hypoxia are not isolated to memory impairment, characterised in the literature as an amnesic syndrome (Cummings, Tomiyasu, Read & Benson, 1984; Volpe & Hirst, 1983; Volpe, Holtzman & Hirst, 1986). Overall, combined data sources indicated that impairment following cerebral hypoxia can manifest as executive function difficulties and also in some cases, decline in attention, language as well as visuo-constructional abilities.
The results of the review have important clinical implications for specialists working within subacute medical and rehabilitation settings. The complex and highly variable outcomes of cerebral hypoxia as described in the studies reviewed demonstrates that a one size fits all model cannot be applied to this highly distinct clinical group and supports the use of tailored neuropsychological assessment to inform rehabilitative planning and optimise recovery and long-term outcomes. Finally, planned future clinical studies should consider the use of standardised assessment protocols, which include measures of premorbid functioning and performance validity. Thereafter, there may also be clinical merit in establishing an international register for this population.
Conflict of interest
The authors did not receive any third-party financial support during the completion of this systematic review and therefore declare no conflicts of interest.
Acknowledgments
The authors would like to thank Dr Donncha Hanna, Lecturer, Department of Psychology, Queen’s University Belfast for his expertise and guidance on the statistical analysis and interpretation of data.
References
1 | Alexander, M. P. , Lafleche, G. , Schnyer, D. , Lim, C. & Verfaellie, M. ((2011) ). Cognitive and functional outcome after out of hospital cardiac arrest, Journal of International Neuropsychological Society 17: , 364–368. |
2 | Allen, J. S. , Tranel, D. , Bruss, J. & Damasio, H. ((2006) ). Correlations between regional brain volumes and memory performance in anoxia, Journal of Clinical & Experimental Neuropsychology 28: , 457–476. |
3 | Anderson, C. A. & Arciniegas, D. B. ((2010) ). Cognitive sequelae of hypoxic-ischemic brain injury: A Review, Neurorehabilitation 26: , 47–63. |
4 | Armengol, C. G. ((2000) ). Acute oxygen deprivation: neuropsychological profiles and implications for rehabilitation, Brain Injury 14: , 237–250. |
5 | Auerbach, V. & Hodnett, C. ((1990) ). Neuropsychological follow-up in a case of severe chlorine gas poisoning, Neuropsychology 4: , 105–112. |
6 | Bachman, D. & Katz, D. I. ((1997) ). Anoxic-hypotensive brain injury and encephalitis. In Mills, V. M. and Katz, D. I. (Ed.) (1997). Neurologic Rehabilitation: A Guide to Diagnosis, Prognosis and Treatment Planning, pp. 145-176. Blackwell Science, Malden, M. A. |
7 | Baggett, M. R. , Kelly, M. P. & Korenman, L. M. ((2003) ). Neuropsychological deficits of a U.S army pilot following an anoxic event as a function of cardiac arrest, Military Medicine 9: , 769–771. |
8 | British Psychological Society (2009). Assessment of Effort in Clinical Testing of Cognitive Functioning for Adults. Leicester (UK): The British Psychological Society. |
9 | Browne, S. M. , Halligan, P. W. , Wade, D. T. & Taggart, D. P. ((2003) ). Postoperative hypoxia is a contributory factor to cognitive impairment after cardiac surgery, Cardiopulmonary Support and Physiology 126: , 1061–1064. |
10 | Bush, S. S. , Ruff, R. M. , Tröster, A. I. et al. ((2005) ). Symptom validity assessment: Practice issues and medical necessity, Archives of Clinical Neuropsychology 20: , 419–426. DOI: 10.1016/j.acn.2005.02.002 |
11 | Busl, K. M. & Greer, D. M. ((2010) ). Hypoxic-ischemic brain injury: Pathophysiology, Neuropathology and mechanisms, NeuroRehabilitation 26: , 5–13. |
12 | Caine, D. & Watson, J. D. G. ((2000) ). Neuropsychological and neuropathological sequelae of cerebral anoxia: A critical review, Journal of the International Neuropsychological Society 6: , 86–99. |
13 | Cohen, J. ((1992) ). A power primer, Psychological Bulletin 112: , 155–159. |
14 | Corkin, S. , Cohen, N. J. , Sullivan, E. V. , Clegg, R. A. , Rosen, T. J. & Ackerman, R. H. ((1985) ). Analyses of global memory impairments of different aetiologies, Annals of the New York Academy of Science 444: , 10–40. |
15 | Crooks, S. ((2014) ). Long-term neuropsychological and psychosocial outcomes of hypoxic-ischemic brain injury. Unpublished doctoral dissertation. Queen’s University Belfast, Northern Ireland. |
16 | Cummings, J. L. , Tomiyasu, U. , Read, S. & Benson, D. F. ((1984) ). Amnesia with hippocampal lesions after cardiopulmonary arrest, Neurology 34: , 679–681. |
17 | DiPaola, M. , Caltagrione, C. , Fadda, L. , Sabatini, U. , Serra, L. & Carlesimo, G. A. ((2008) ). Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia, Hippocampus 18: , 719–728. |
18 | Ferdinand, P. & Roffe, C. ((2016) ). Hypoxia after stroke: a review of experimental and clinical evidence, Experimental and Translational Stroke Medicine 8: , 9. DOI: 10.1186/s13231-016-0023-0 |
19 | Fitzgerald, A. , Aditya, H. , Prior, A. , & McNeill Pentland, B. ((2010) ). Anoxic brain injury: Clinical patterns and functional outcomes. A study of cases, Brain Injury 24: , 1311–1323. |
20 | Frankland, P. W. & Bontempi, B. ((2005) ). The organisation of recent and remote memories, Nature Review of Neuroscience 6: , 119–130. |
21 | Gale, S. D. , Hopkins, R. O. , Weaver, L. K. , Bigler, E. D. , Booth, E. D. & Blatter, D. D. ((1999) ). MRI, Quantitative MRI, SPECT, and neuropsychological findings following carbon monoxide poisoning, Brain Injury 13: , 229–243. |
22 | Glass, G. V. , McGaw, B. & Smith, M. L. ((1981) ). Meta-Analysis in Social Research, pp. 104. Sage, Beverly Hills. |
23 | Greer, D. M. ((2006) ). Mechanisms of injury in hypoxic-ischemic encephalopathy: implications to therapy, Seminars in Neurology 26: , 373–379. |
24 | Grosswasser, Z. , Cohen, M. & Costeff, H. ((1989) ). Rehabilitation outcomes after anoxic brain damage, Archives of Physical Medicine and Rehabilitation 70: , 186–188. |
25 | Grubb, N. R. , Fox, K. A. A. , Smith, K. , Best, J. , Blane, A. , Ebmeier, K. P. , Glabus, M. F. & O’Carroll, R. E. ((2000) ). Memory impairment in out-of-hospital cardiac arrest survivors is associated with global reduction in brain volume, Stroke 31: , 1509–1514. |
26 | Heaton, R. K. , Chelune, G. J. , Talley, J. L. , Kay, G. G. & Curtiss, G. ((1981) ). Wisconsin Card Sorting Test Manual Revised and Expanded, pp. 21-30. Psychological Assessment Resources Inc. U.S. |
27 | Heinz, U. E. & Rollnik, J. D. ((2015) ). Outcome and prognosis of hypoxic brain damage patients undergoing neurological early rehabilitation, BMC Research Notes 8: , 243. DOI: 10.1186/s13104-015-1175-z |
28 | Hopkins, R. O. & Bigler, E. D. ((2012) ). Neuroimaging of anoxic injury: Implications for neurorehabilitation, Neurorehabilitation 31: , 319–329. |
29 | Hopkins, R. O. , Gale, S. D. & Weaver, L. K. ((2006) ). Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome, Brain Injury 20: , 263–271. |
30 | Hopkins, R. O. , Gale, S. D. , Johnston, S. C. , Anderson, C. V. , Bigler, E. D. , Blatter, D. D. & Weaver, L. K. ((1995) ). Case Study: Severe anoxia with and without concomitant brain atrophy and neuropsychological impairments, Journal of International Neuropsychological Society 1: , 501–109. |
31 | Hopkins, R. O. , Larson-Lohr, V. , Weaver, L. & Bigler, E. ((1998) ). Case Study: Neuropsychological impairments following hantavirus pulmonary syndrome, Journal of the International Neuropsychological Society 4: , 190–196. |
32 | Hopkins, R. O. , Tate, D. F. & Bigler, E. D. ((2005) ). Anoxic versus traumatic brain injury: Amount of tissue loss, not aetiology, alters cognitive and emotional function, Neuropsychology 19: , 233–242. |
33 | Hopkins, R.O. , Weaver, L. K. & Kesner, R. P. ((1993) ). Long term memory impairments and hippocampal magnetic resonance imaging in carbon monoxide poisoned subjects, Abstracts, Undersea, Hyperbaric Society Annual Scientific Meeting 20: , 15. |
34 | Hopkins, R. O. , Weaver, L. K. , Pope, D. , Orme, J. F. , Bigler, E. D. & Larson-Lohr, V. ((1999) ). Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome, American Journal of Respiratory Critical Care Medicine 160: , 50–56. |
35 | Huang, B. Y. & Castillo, M. ((2008) ). Hypoxic-Ischemic Brain Injury: Imaging Findings from birth to adulthood, Radiographic 28: , 2. doi: http://dx.doi.org/10.1148/rg.282075066. |
36 | Kaplan, E. , Goodglass, H. & Weintrab, S. ((1983) ). The Boston Naming Test. Lea & Febiger, Philadelphia. |
37 | Khan, A. U. ((2012) ). Clinical Disorders of Memory, pp. 121–128. Plenum Medical Book Company, New York. |
38 | Knot, S. & Tirschwell, D. L. ((2009) ). Long-term neurological complications after hypoxic-ischemic encephalopathy, Seminars in Neurology 26: , 422–431. |
39 | Levy, D. E. , Caronna, J. J. Singer, B. H. et al. ((1985) ). Predicting outcome from hypotoxic-ischemic coma, JAMA 253: , 1420–1426. |
40 | Lezak, M. D. , Howieson, D. B. & Loring, D. W. ((2004) ). Neuropsychological Assessment Fourth Edition, pp. 611–645611-645. Oxford University Press, New York. |
41 | Lim, C. , Alexander, M. P. & LaFleche, G. ((2004) ). The neurological and cognitive sequelae of cardiac arrest, Neurology 63: , 1774. DOI: 10.1212/01.WNL.0000144189.83077.8E |
42 | Lim, C. , Verfaellie, M. , Schnyer, D. , Lafleche, G. & Alexander, M. P. ((2014) ). Recovery, long-term cognitive outcome and quality of life following out-of-hospital cardiac arrest, Journal of Rehabilitation Medicine 46: , 691–697. |
43 | Lu-Emerson, C. & Khot, S. ((2010) ). Neurological sequelae of hypoxic-ischemic brain injury, Neurorehabilitation 26: , 35–45. |
44 | Medalia, A. A. , Merriam, A. E. & Ehrenreich, J. H. ((1991) ). The neuropsychological sequelae of attempted hanging, Journal of Neurology, Neurosurgery, & Psychiatry 54: , 546–548. |
45 | Meyer, A. ((1956) ). Neuropathological aspects of anoxia, Proceedings of the Royal Society of Medicine 49: , 619–622. |
46 | Moher, D. , Shamseer, L. , Clarke, M. et al. ((2015) ). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement, Systematic Reviews 4: , 1. Doi: 10.1186/2046-4053-4-1 |
47 | Moser, D. J. , Cohen, R. A. & Clark, M. M. ((1999) ). Neuropsychological functioning among cardiac rehabilitation patients, Journal of Cardiopulmonary Rehabilitation 2: , 91–97. |
48 | Myers, C. E. , Hopkins, R. O. , Deluca, J. , Moore, N. B. , Wolansky, L. J. , Sumner, J. M. & Gluck, M. A. ((2008) ). Learning and generalization deficits in patients with memory impairments due to anterior communicating artery aneurysm rupture or hypoxic brain injury, Neuropsychology 22: , 681–686. |
49 | Nucci, M. P. , Lukasova, K. , Sato, J. R. & Amaro, E. ((2016) ). Brain injury after moderate drowning: subtle alterations detected by functional magnetic resonance imaging. Brain Imaging and Behavior, Doi: 10.1007/s11682-016-9619-1 |
50 | O’Connor, M. , Verfaelli, M. & Cermak, L. S. ((1995) ). Clinical Differentiation of Amnesic Subtypes in: Baddley, A. D., Wilson, B. A., & Watts, F. N. (Eds). Handbook of Memory Disorders, pp. 64-66. John Wiley & Sons, Chichester. |
51 | O’Reilly, S. M. , Grubb, N. R. & O’Carroll, R. E. ((2003) ). In-hospital cardiac arrest leads to chronic memory impairment, Resuscitation 58: , 73–79. |
52 | Parkin, A. J. , Miller, J. & Vincent, R. ((1987) ). Multiple neuropsychological deficits due to anoxic encephalopathy: A case study, Cortex 23: , 655–65. |
53 | Perez, C. A. , Samudra, N. & Aiyagari, V. ((2016) ). Cognitive and functional consequences of cardiac arrest, Current Neurology and Neuroscience Reports 16: , 70: Doi: 10.1007/s11910-016-0669-y |
54 | Porter, S. S. , Hopkins, R. O. , Weaver, L. K. , Bigler, E. D. & Blatter, D. D. ((2002) ). Corpus callosum atrophy and neuropsychological outcome following carbon monoxide poisoning, Archives of Clinical Neuropsychology 17: , 195–204. |
55 | Quinn, D. K. , McGahee, S. M. , Politte, L. C. , Duncan, G. N. , Cusin, C. , Hopwood, C. J. & Stern, T. A. ((2009) ). Complications of carbon monoxide poisoning: A case discussion and review of the literature, The Primary Care Companion to the Journal of Clinical Psychiatry 11: , 74–79. |
56 | Raphael, J. D. , Elkharrat, D. , Jars-Guincestre, M. C. , Chastang, C. , Chasles, V. , Vercken, J. B. & Gajdos, P. ((1989) ). Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication, Lancet 19: , 414–419. |
57 | Reitan, R. M. & Wolfson, D. ((1985) ). The Halstead-Reitan Neuropsychological Test Battery. Neuropsychological Press, Arizona. |
58 | Reitan, R. M. & Wolfson, D. ((1995) ). Category Test and Trail Making Test as measures of frontal lobe functions, Clinical Neuropsychologist 9: , 50–56. |
59 | Rey, A. & Osterrieth, P. A. ((1993) ). Translations of excerpts from Andre Rey’s “Psychological examination of traumatic encephalopathy” and P. A. Osterrieth’s “The complex figure copy test”. The Clinical Neuropsychologist, 7: , 3–21 (Originally published in 1941 and 1944). |
60 | Sauve, M. J. , Lewis, W. R. , Blankenbiller, M. , Rickabaugh, B. & Pressler, S. J. ((2009) ). Cognitive impairments in chronic heart failure: A case-controlled study, Journal of Cardiac Failure 15: , 1. |
61 | Schmidt, M. ((1996) ). Rey Auditory and Verbal Learning Test. A Handbook, pp. 12-14. Western Psychological Services, L.A. |
62 | Schultz, I. Z. , Sepehry, A. A. & Greer, S. C. ((2018) ). Anoxia-Hypoxia in Forensic Neuropsychological Assessment: Cognitive Impact of Pulmonary Injuries, Respiratory Distress, Cerebral Blood Hypoperfusion and Major Surgeries, Psychological Injury and Law 11: ((2)), 153–170. |
63 | Shah, M. K. , Al-Adawi, S. , Dorvlo, A. S. S. , et al. ((2004) ). Functional outcomes following anoxic brain injury: a comparison with traumatic brain injury, Brain Injury 14: , 237–250. |
64 | Speach, D. P. , Wong, T. M. , Cattarin, J. A. & Livecchi, M. A. ((1998) ). Case Study: Hypoxic brain injury with motor apraxia following an anaphylactic reaction to hymenoptera venom, Brain Injury 12: , 239–244. |
65 | Stock, D. , Cowie, C. , Chan, V. , Colantonio, A. , Wodchis, W. P. , Alter, D. , & Cullen, N. ((2016) ). Determinants of alternate-level-of-care delayed discharge among acute care survivors of hypoxic-ischemic brain injury: a population-based cohort study, CMAJ Open 4: , 689–697. |
66 | Strauss, E. , & Sherman, E. M. S. ((2006) ). A Compendium of Neuropsychological Tests: Administration, Norms and Commentary, Third Edition, Oxford (UK): Oxford University Press. |
67 | Taggart, D. P. , Browne, S. M. , Halligan, P. W. & Wade, D. T. ((1999) ). Is cardiopulmonary bypass still the cause of cognitive dysfunction after cardiac operations? Journal of Thoracic Cardiovascular Surgery 118: , 414–421. |
68 | Tazopoulou, E. , Milijkovitche, R. , Trucelle, J. L. , Schnitzler, A. , Onillon, M. , Zucco, T. , Hawthorne, G. & Montreuil, M. ((2016) ). Rehabilitation following cerebral anoxia. An assessment of patients, Brain Injury 30: , 95–103. |
69 | Volpe, B. T. & Hirst, W. ((1983) ). The characterisation of an amnesic syndrome following hypoxic ischemic injury, Archives of Neurology 40: , 436–440. |
70 | Volpe, B. T. , Holtzman, J. D. & Hirst, W. ((1986) ). Further characterisation of patients with amnesia after cardiac arrest, Neurology 36: , 408–411. |
71 | Wechsler, D. ((1987) ). Wechsler Adult Intelligence Scale –Revised, pp. 16-28. The Psychological Corporation, US. |
72 | Wechsler, D. ((1987) ). Wechsler Memory Scale –Revised, pp. 43-57. The Psychological Corporation, US. |
73 | Wells, G. A. , Shea, B. , O’Connell, D. , Peterson, J. , Welch, V. , Losos, M. & Tugwell, P. ((2008) ). The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp |
74 | Wilson, B. A. ((1996) ). Cognitive functioning of adult survivors of cerebral hypoxia, Brain Injury 12: , 863–874. |
75 | Wilson, B. A. , Cockburn, J. , Baddeley, A. , ((1985) , (2003) a). The Rivermead Behavioural Memory Test. Thames Valley Test Company, England. |
76 | Wilson, F. C. , Harpur, J. , Watson, T. & Morrow, J. J. ((2003) ). Adult survivors of severe cerebral hypoxia: Case series survey and comparative analysis, Neurorehabilitation 17: , 1–8. |
77 | Wilson, M. , Staniforth, A. , Till, R. , das Nair, R. & Vesey, P. ((2014) ). The psychosocial outcomes of anoxic brain injury following cardiac arrest, Resuscitation 85: , 795–800. |
78 | Zabel, A. T. , Slomine, B. , Brady, K. & Christensen, J. ((2005) ). Neuropsychological profile following suicide attempt by hanging: two adolescent case reports, Child Neuropsychology 11: , 373–388. |




