Added-value of spasticity reduction to improve arm-hand skill performance in sub-acute stroke patients with a moderately to severely affected arm-hand
Abstract
BACKGROUND AND OBJECTIVE:
Stroke patients with a moderately to severely affected hand may be impeded in exploiting their full arm-hand training potential during rehabilitation due to spasticity. Reducing early signs of spasticity in sub-acute stroke patients may lead to improvements in arm-hand-function and arm-hand-skill-performance.
METHODS:
Single-case-experimental-design and meta-analysis. Ten sub-acute stroke patients (Modified-Ashworth-Scale:1 + to 3) participated. Training: 2x6 weeks, using a well-described arm-hand regime (therapy-as-usual). Botulinum-toxin was administered once within 5 weeks after onset of therapy-as-usual. Measures: Action-Research-Arm-Test, ABILHAND, Fugl-Meyer-Assessment, grip-strength, Motricity-Index.
RESULTS:
At group level, after baseline trend correction, adjusting for spontaneous recovery and therapy-as-usual effects, the added-value of botulinum-toxin-A on arm-hand-function and arm-hand-skill-performance was not confirmed. However, non-detrended data revealed significant improvements over time on arm-hand-function and arm-hand-skill-performance level (p≤0.037). Conversely, at individual level, after baseline trend correction, 7/10 patients improved on arm-hand-function: Fugl-Meyer-Assessment (N = 4; p≤0.019), grip-strength (N = 3; p≤0.014), Motricity-Index (N = 4; p≤0.002), whereas 6/10 patients improved on arm-hand-skill-performance: Action-Research-Arm-Test (N = 3; p≤0.042), ABILHAND (N = 5; p≤0.034).
CONCLUSION:
Application of botulinum-toxin-A may have an added-value in a substantial part of sub-acute stroke patients suffering from spasticity early post-stroke and who, at the point of therapy admission, display no dexterity. It may improve their arm-hand performance when combined with a well– defined therapy-as-usual.
1Introduction
The presence of spasticity in (sub-acute) stroke survivors is acknowledged as a hindrance in eliciting voluntary movement in the affected arm and hand, and may impede both arm-hand function and arm-hand skill performance enhancing interventions (Baker & Pereira, 2015; G. Sheean, Lannin, Turner-Stokes, Rawicki, & Snow, 2010).
In contrast to patients with a mildly impaired hand, patients with a moderately to severely affected hand show an uncertain, non-linear trend regarding arm-hand recovery (Prabhakaran et al., 2008; Winters, Kwakkel, Nijland, & van Wegen, 2016). In some of these patients improvements in arm-hand function (AHF) and arm-hand skill performance (AHSP) is observed due to e.g. spontaneous recovery and the therapy they receive, especially in the early phase post-stroke (J.A. Franck, Smeets, & Seelen, 2017; K. Hayward, Kuys, Barker, & Brauer, 2014). A substantial part of these patients experience moderate to severe grades of spasticity (Modified Ashworth Scale (MAS) scores 1 + to 3 (Ashworth, 1964; Bohannon & Smith, 1987) in the sub-acute phase post stroke (Lundstrom, Smits, Terent, & Borg, 2010; Sunnerhagen, 2016; Wissel et al., 2015). Due to a combination of muscle weakness and spasticity in the affected arm and hand, sub-acute stroke patients with a moderately to severely affected arm-hand may be unable to attend functional rehabilitation training programs, which may lead to a delay in their functional recovery, or in failure to achieve specific treatment goals in arm hand rehabilitation (Demetrios et al., 2014; Esquenazi & Mayer, 2004). Eventually, this may reduce their, already limited, possibilities to use their affected arm-hand in daily activities.
Spasticity occurring in the affected arm and hand can be reduced by using botulinum toxin (BoNT) injections (Royal College of Physicians, 2018; G. Sheean et al., 2010). In the past, BoNT injections were often applied as a single (pharmacological) intervention. However, effective management of reducing spasticity and enhancing hand function demands a holistic, interdisciplinary approach in which spasticity management interventions are integrated in an overall rehabilitation program (Baker & Pereira, 2015; Bhakta, 2000; Devier, Harnar, Lopez, Brashear, & Graham, 2017; Esquenazi, Novak, Sheean, Singer, & Ward, 2010; Prazeres et al., 2018; Royal College of Physicians, 2018; Takekawa et al., 2013; Wolf et al., 2012). Nowadays, BoNT is more frequently applied in combination with other forms of therapy, like, for instance, physical or occupational therapy (Devier et al., 2017; Kinnear, Lannin, Cusick, Harvey, & Rawicki, 2014; Royal College of Physicians, 2018). When BoNT is applied adjunct to arm-hand rehabilitation interventions, one may first observe a decrease of spasticity well before improvement of AHF. During this time frame, based on motor relearning principles, patients are trained to learn how to use their upper limb muscles with reduced muscle tone within arm-hand function and arm-hand skill performance tasks (Francis et al., 2004).
In the past decade, a substantial number of therapy approaches were developed in which botulinum toxin was provided adjunct to therapy targeting deficits in AHF and AHSP (Demetrios et al., 2014; Devier et al., 2017; Kinnear et al., 2014; Kuo & Hu, 2018; Monaghan et al., 2011; Royal College of Physicians, 2018; Ward et al., 2014). However, reports on the effects of these approaches have been ambiguous (Baker & Pereira, 2015; Dong, Wu, Xiaohua, & Wang, 2017; Foley et al., 2013; Kinnear et al., 2014; Prazeres et al., 2018; Royal College of Physicians, 2018; Turner-Stokes, Fheodoroff, Jacinto, & Maisonobe, 2013; Wolf et al., 2012). Significant though modest results regarding active AHF after arm-hand rehabilitation combined with BoNT were reported in the systematic reviews by Foley et al. (2013) and Baker et al. (2015). Also Takekawa et al. (Takekawa et al., 2013) and Devier et al. (Devier et al., 2017) demonstrated improved AHF in chronic stroke patients with a moderately to mildly impaired arm-hand who received botulinum toxin in combination with a tailored arm-hand rehabilitation program. However, Shaw et al. (Shaw et al., 2011), Prazeres et al. (Prazeres et al., 2018) and Wolf et al. (Wolf et al., 2012) found no added-value of the injection of BoNT versus placebo both immediately followed by an arm-hand rehabilitation program with respect to AHF in chronic stroke patients. Furthermore, a recently published meta-analysis of Andringa and colleagues reported lack of effects of BoNT on arm-hand capacity (Andringa et al., 2019).
Important factors that may explain the ambiguity regarding the demonstration of functional improvements in AHF and AHSP after the application of arm-hand rehabilitation combined with botulinum-toxin are:
– First, the diversity regarding (often undefined) therapy type and therapy intensity applied in conjunction with botulinum toxin across the studies (Demetrios et al., 2014; Foley et al., 2013). Studies of interventions combined with botulinum-toxin have tended to focus on single treatment modalities as for instance the application of electrical stimulation (Hesse, Reiter, Konrad, & Jahnke, 1998), constraint-induced movement therapy (CIMT) (Sun et al., 2010), and task-specific practice (Weber et al., 2010). The set-up of these studies deviates from arm-hand interventions delivered in day-to-day arm-hand rehabilitation settings which normally consist of a complex array of interventions, adjusted to the patient’s individual needs.
– Secondly, the diversity in patient characteristics like post-stroke time and stroke location (Fransisco, 2007; Picelli et al., 2014; Wissel et al., 2015): The majority of the studies published, included chronic stroke patients with a mildly to moderately affected arm-hand.
– Thirdly: the different pathophysiological mechanisms leading to spasticity and how the latter affects neuromuscular control (Lieber, Roberts, Blemker, Lee, & Herzorg, 2017). Given their biomechanical properties, muscles need time to change after having been injected with botulinum toxin, and one may assume that the course of this process may differ considerably between subjects.
The most effective combination of therapy approaches, to be applied in conjunction with the application of BoNT, has not been identified yet (Demetrios et al., 2014; Kinnear et al., 2014). This especially holds in sub-acute stroke patients with a moderately to severely affected arm-hand. Considering the severity of the disability that has to be overcome, and in order to achieve a clinically important change in AHF and ASHP, it is essential to evaluate the patient’s full potential within the limited time-window of recovery. However, the optimal type (approach, setting and modalities) and intensity of therapy to improve AHF and AHSP in this particular group of stroke patients is unclear and is often based on expert opinion only.
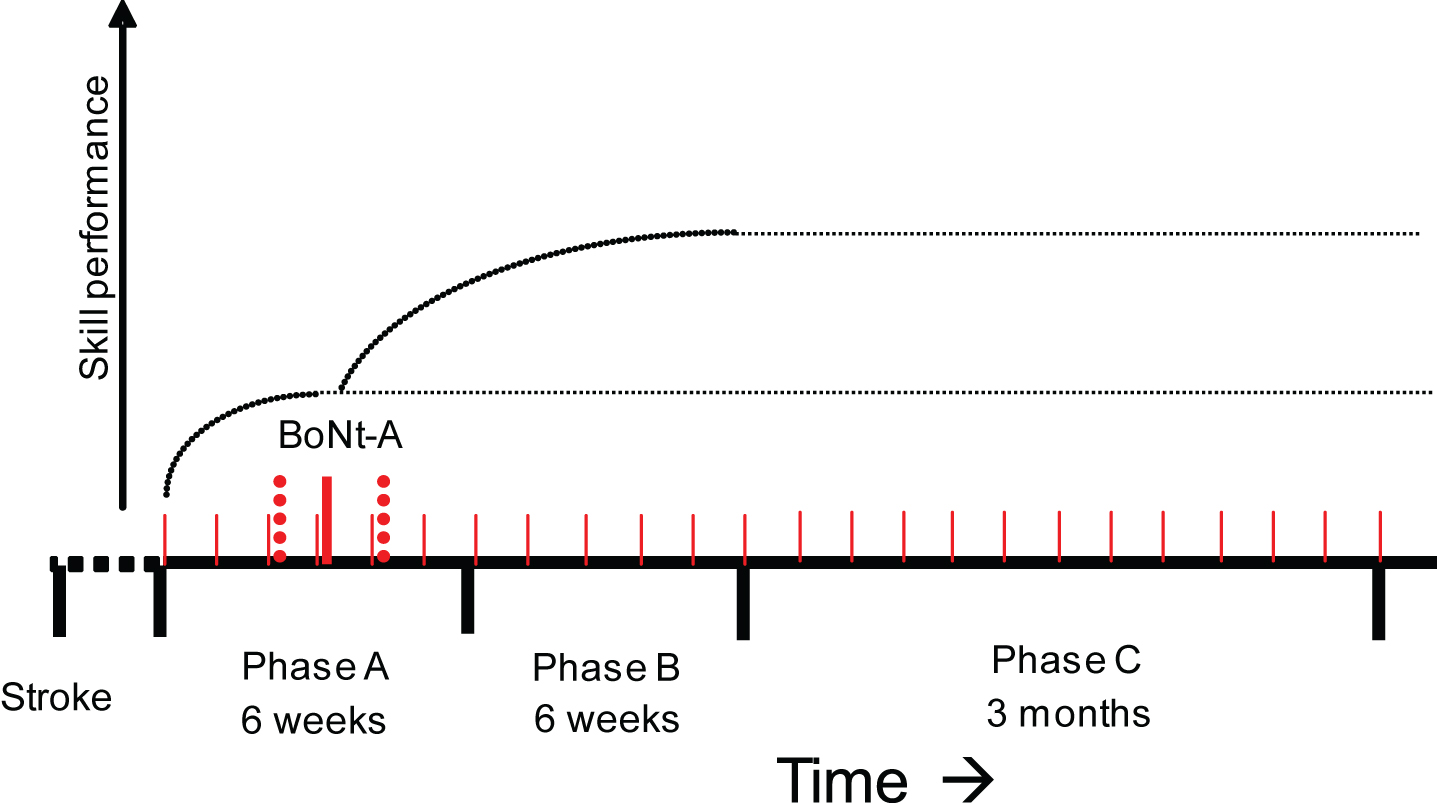
CARAS (acronym for: Concise Arm and hand Rehabilitation Approach in Stroke) (J.A. Franck, Halfens, Smeets, & Seelen, 2015) is a well-defined arm-hand rehabilitation treatment for stroke survivors with a moderately to severely affected arm-hand. The CARAS approach provides clinicians with clear rationales to assist a broad range of sub-acute stroke patients who cope with hand dexterity problems towards attaining a certain level of AHF and AHSP (J.A. Franck et al., 2015). In CARAS, patients are allocated to one of three training programs, classified according to the UAT (Utrecht Arm-hand Test) scores (Kruitwagen-van Reenen, Post, Mulder-Bouwens, & Visser-Meily, 2009). Program 1 is for persons with a severely impaired AHF (UAT 0-1), whereas Program 2 consists of a ‘gross motor grip performance training’, designed for persons with a moderately impaired AHF (UAT 2-3). Program 3 targets stroke patients with a mildly impaired AHF (UAT 4– 7). Program 1 and program 3 cover a training period of six consecutive weeks. Due to their moderate level of arm-hand impairment at the initial phase of their rehabilitation period, patients admitted to Program 2 participate in a 12-week during training period, consisting of 2×6 consecutive weeks, called ‘training episode 1’ and ‘training episode 2’, graphically presented in Fig. 1.
Fig. 1
Schematic representation of the study design. ABoNt-A = application of ABoNt-A. Phase A = 3 - 6 weeks of training in CARAS, program 2. Phase B = CARAS training in program 2 after ABoNt-A injections. Phase C = Measurement moments from 2 till 12 weeks after CARAS. Dotted line: experimental stimulus or intervention. Dark dots: measurements of outcome variable X.
The aim of the present study is to investigate the added-value of reduction of early signs of spasticity on improving arm-hand function (AHF) and functional arm-hand skill performance (AHSP) in sub-acute stroke patients with either a severely or moderately affected arm-hand (Utrechtse Arm-hand Test (UAT) (Kruitwagen-van Reenen et al., 2009) score 1– 3) and moderate to severe grades of spasticity, i.e. Modified Ashworth Scale (MAS) score 1 + to 3 (Ashworth, 1964) adjunct to therapy-as-usual.
Our research hypothesis is:
In sub-acute post-stroke patients with a moderately to severely affected arm-hand (UAT score 1– 3) and moderate to severe grades of spasticity, reduction of spasticity in the shoulder, arm and hand muscles, adjuvant to a well-defined arm-hand rehabilitation approach leads to significant and clinically relevant improvements in arm-hand function and arm-hand skill performance.
2Methods
The present study featured 1) a multiple baseline single case experimental design (Barlow, 2008) involving 10 individuals in the sub-acute phase after a stroke, and 2) a meta-analysis or group analysis of the data of all these 10 single cases. To correct for improvements caused by e.g. spontaneous recovery and/or other treatment received, all-time series per subject were linearly detrended for any baseline trends. As presented in Fig. 1, the study covered three phases (A, B and C) in which each participant underwent sequential observations and measurements, generating a time series per patient per outcome measure.
Measurements were repeatedly performed at baseline, with a time interval of one week. Baseline length randomly varied between 3– 6 weeks across subjects during the first training period, i.e. phase A, in which CARAS was applied, the rationale of which has been reported by Franck et al., (Franck et al., 2015). Consecutive to phase A, phase B started once the Abobotulinum toxin-A (ABoNt-A) was injected, which was administered adjunct to CARAS. Both phases together encompassed 12 weeks. Measurements were continued using the 1-week intervals until the end of the second 6-weeks training period (phase B). Finally, measurements performed during the ensuing 3 months follow-up (phase C) were interspaced by two weeks. A detailed description of this study protocol has been presented by Franck et al., (Franck, Smeets, Renders, & Seelen, 2018)
This study received ethical approval from the Medical Ethics Committee of Maxima Medical Centre in Veldhoven, the Netherlands (METC reference number: W16.027; CCMO code: 56494.015.16). This study was conducted according to the principles of the Declaration of Helsinki (version October 2013) and in accordance with the Dutch Medical Research Involving Human Subjects Act (Wet medisch-wetenschappelijk onderzoek met mensen (WMO) (Nederlandse Rijksoverheid [Dutch Government]).
2.1Study population
First, sub-acute stroke patients admitted to the department of Brain Injury Rehabilitation at Adelante rehabilitation centre in Hoensbroek, the Netherlands, were informed about the content and purpose of the study. Subsequently, they were asked to participate in this study. Written informed consent was obtained from all participants prior to the start of their participation in this study.
Patients with a moderately to severely affected arm-hand (UAT score; 1– 3) who developed early signs of spasticity in the arm and/or hand, i.e. within 5 weeks after start of arm-hand treatment (CARAS), remained in the study. In patients who had a severe paretic arm and hand (UAT score 1– 3) at admission to the rehabilitation centre, but who did not develop early signs of spasticity within 5 weeks after start of arm-hand treatment (thereby not being in the target group), were excluded from the study and measurements used in the study ceased. However, they continued their arm-hand rehabilitation in program 2 as ‘therapy-as-usual’ combined with their regular therapy-related measurements. Any research data of the latter patient group recorded for the sole purpose of the research was discarded.
2.2Inclusion criteria
In order to be eligible to participate in this study, a subject had to meet all of the following criteria: Age> = 18 years; stroke; sub-acute phase after stroke, i.e. between 2 weeks and 3 months post-stroke; moderate to severe paretic arm and hand (UAT score 1– 3); functional disabling spasticity in the upper extremity: Modified Ashworth Scale (MAS) score 1 + to 3 (developing within 5 weeks after the start of arm-hand treatment); Eligible to participate in the CARAS program for a period of 12 weeks; Able to understand the questionnaires and measurement instructions.
2.3Exclusion criteria
A subject who met any of the following criteria was excluded from participation in this study: severe non-stroke related co-morbidity that may interfere with arm-hand function; additional complaints that may interfere with the execution of the measurements; no informed consent.
2.4Procedures
Patients with a moderately to severely affected arm-hand were asked to participate in the study before the start of the arm-hand treatment regime, i.e. program 2 (gross motor grip performance training) of CARAS (Franck et al., 2015). Once admitted, measurements started according to the study protocol (Franck et al., 2018).
The training duration of CARAS’ program 2 contained 2×6 weeks. A single week of training consisted of 3 days of 1.5 hours training time. All training sessions contained the following structure: Patients started with training on a personal goal for 5 – 10 minutes, followed by 45 minutes of training fitted to arm-hand motor control issues, sub-goals and the patient’s current performance level, which was determined by therapists prior to admission. After these 45 minutes of training, the patient worked 5 – 10 minutes towards his or her personal goal again. CARAS is the standard therapy (therapy-as-usual) provided by physiotherapists and occupational therapists to stroke patients with arm-hand problems who are admitted to Adelante rehabilitation centre for treatment. Once enrolled in CARAS’ program 2, the patient’s level of impairment and personal needs were determined, and tailored interventions were applied (Franck et al., 2015).
Patients who showed early signs of spasticity (MAS score 1 + to 3) within the first 5 weeks of training episode 1 of CARAS were treated with ABoNt-A. The latter occurred within 1 week after the severity of spasticity was determined. Target muscles in the shoulder, arm and forearm were identified using echography. In order to avoid muscles getting excessively weakened, thereby losing their ability to facilitate movements, ABoNt-A dosages were limited to 50% of the prescribed amount related to the target muscle. (Bakheit et al., 2001; Ipsen, 2016; Suputtitada & Suwanwela, 2005).
In patients who had a moderately to severely affected arm and hand at point of admission but who did not develop spasticity (MAS score 1 + to 3) within 5 weeks after the start of the arm-hand treatment (thereby not being in the target group), measurements ceased.
2.5Outcome measures
2.5.1Primary outcome measures
Changes in patient’ arm-hand skill performance capacity was measured using the Action Research Arm test (ARAT). The ARAT is a valid and reliable instrument, sensitive to change in measuring upper limb capacity at activity level in patients with stroke (Hsieh, Hsueh, Chiang, & Lin, 1998; Van der Lee, Roorda, Beckerman, Lankhorst, & Bouter, 2002; Yozbatiran, Der-Yeghiaian, & Cramer, 2008). The 19 items are scored on a 4-point scale, with a total score ranging from 0 to 57.
2.5.2Secondary outcome measures
Perceived performance was measured by the ABILHAND, a Rasch-analyzed test, which measures the level of manual ability in terms of the difficulty perceived by patients with hand impairments in their daily life (Ashford, Slade, Malaprade, & Turner-Stokes, 2008). It focuses on 23 bimanual activities that are representative for a person’s daily activities (Penta, Tesio, Arnould, Zancan, & Thonnard, 2001; Penta, Thonnard, & Tesio, 1998), using a 3-level ordinal rating scale: impossible (0), difficult (1), and easy (2) to perform. The ABILHAND is valid, responsive and clinically useful (Ashford et al., 2008; Penta et al., 2001).
At function level, the Fugl-Meyer Motor Assessment (FMA), Motricity Index (MI) (Demeurisse, Demol, & Robaye, 1980) and JAMAR hand-held dynamometer (grip strength) were used. The FMA (part upper extremity) is a reliable and valid instrument to measure AHF in stroke patients (Gladstone, Danells, & Black, 2002; Salter, Teasell, Foley, & Jutai, 2007), with a score ranging from 0 to 66. The JAMAR hand-held dynamometer was used to measure grip strength of the hand (in kgf) (Hamilton, McDonald, & Chenier, 1994).
2.6Data processing and statistical analysis
2.6.1Handling of missing values
When 1 or 2 (temporally adjacent) value(s) in a time series of data were missing, these missing value(s) were estimated by linear interpolation using the two valid adjacent values in the time series. In case of the final time series’ observation missing, the ‘last-observation-carried-forward’ principle was used. In case of 3 or more missing values, the whole case was discarded.
2.6.2Data analysis
An in-depth overview of the different data analyses techniques used in this study as well as their rationale have been reported by Franck et al. (J. A. Franck et al., 2018).
First, all-time series per subject were linearly detrended for any baseline trends, using a least squares method, to (partially) compensate for improvements caused by e.g. spontaneous recovery and/or other treatment received. This was done for the time series of the ARAT, ABILHAND, FMA, JAMAR and MI. The residuals, i.e. the detrended (and thereby rendered mutually independent) data, were subsequently analysed for each participant. Furthermore, mean residual data per subject per measure (FMA, MI, grip strength, ARAT and ABILHAND) were calculated for the baseline phase (Phase A), for the treatment phase after application of the spasticity reducing therapy (Phase B), and for the follow-up period (Phase C). These data were analysed at group level.
2.6.3Group level data
At group level, first, mean data per subject per measure (FM, MI, grip strength, ARAT and ABILHAND) were calculated for the baseline phase (Phase A), for the treatment phase after application of the spasticity reducing therapy (Phase B), and for the follow-up period (Phase C). Statistical (within-group) analysis of these data included Friedman two-way analysis of variance by ranks, followed by multiple comparison using Wilcoxon signed ranks tests in a Bonferroni approach. The latter was done to compensate for spurious false positive findings (Siegel & Castellan, 1988).
Next, all-time series per subject were linearly detrended for any baseline trends, using a least squares method, to (partially) compensate for improvements caused by e.g. spontaneous recovery and/or other treatment received. This was done for the ARAT, ABILHAND, FM, JAMAR and MI. The residuals, i.e. the detrended (and thereby rendered mutually independent) data, were subsequently analysed at group level. Statistical (within-group) analysis of these data included Kruskal-Wallis one-way analysis of variance tests and multiple comparison involving Mann-Whitney U-tests, again in a Bonferroni approach.
2.6.4Individual level data
At individual level, mean baseline trend-corrected data, i.e. the residuals, per subject per measure (FM, MI, grip strength, ARAT and ABILHAND) for all three phases were used in the statistical analyses. The latter included Kruskal-Wallis one-way analysis of variance tests and multiple comparison involving Mann-Whitney U-tests in a Bonferroni approach.
MAS results are reported descriptively.
3Results
3.1Patient characteristics and error analysis
Thirteen patients entered the study. According to the protocol, three participants left the study within the first 5 weeks because they did not develop spasticity in the shoulder, arm or hand. No further drop-outs of study participants occurred. Ten patients (all males) completed the study. No baseline values or final follow-up values were missing. Missing values were minimal (0.37%) and these data were estimated using linear interpolation based on the two valid adjacent values in the time series.
Two serious adverse events were reported. One patient underwent a one-day admission to the hospital because of low blood sugar levels. One patient experienced a recurrent (minor) stroke during the follow-up phase, between the 3rd and the 4th measurement point during the follow-up phase. None of these events were in any way related to the study. Due to logistical reasons, one patient was treated with ABoNt-A on the Monday of week 7, instead of the originally planned Friday of week 6 of Phase A.
Demographic and clinical baseline data of all participants are presented in Table 1. Table 2 provides details of the muscles treated with ABoNt-A and the dosage of ABoNt-A per target muscle, expressed in Units. Table 2 also presents the patients’ level of spasticity as measured using the Modified Ashworth Scale in phase A, phase B and phase C.
Table 1
Demographic and baseline characteristics of the study participants
| P1 | P3 | P5 | P6 | P7 | P8 | P9 | P10 | P12 | P13 | Mean (sd) | |
| Participants | |||||||||||
| Age (year) | 50 | 62 | 70 | 51 | 66 | 77 | 49 | 65 | 31 | 42 | 56.3 (14.1) |
| Stroke Type | Isch | Isch | Isch | Isch | Isch | Isch | Isch | Isch | Isch | Hem | |
| Lesion Site | Lac | ACM | ACM | RHns | ACM | ACM | RHns | Lac | Lac | BG | |
| Impaired side | L | R | R | L | R | R | L | L | R | L | |
| Dominant side | L | R | R | R | R | L | R | R | R | R | |
| Days post-stroke | 60 | 44 | 31 | 45 | 40 | 66 | 51 | 43 | 69 | 62 | 51.1 (12.6) |
| Measurements | |||||||||||
| UAT | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| ARAT | 3 | 10 | 9 | 6 | 20 | 3 | 0 | 6 | 3 | 9 | 6.9 (5.6) |
| ABILHAND | 0.364 | – 1.797 | – 1.044 | – 0.327 | – 0.853 | 0.048 | – 1.804 | – 0.791 | 0.008 | 0.263 | – 0.5933(0.797) |
| FM | 17 | 17 | 34 | 26 | 39 | 23 | 11 | 17 | 15 | 25 | 22.4 (8.8) |
| Grip-strength(kg) | 0 | 1.7 | 4 | 0 | 5 | 0 | 0 | 1.3 | 5.7 | 0.3 | 1.8 (2.3) |
| MI | 58 | 39 | 49 | 61 | 64 | 44 | 34 | 33 | 49 | 50 | 48.1 (10.8) |
| MAS Elbow | 2 | 1+ | 1+ | 1+ | 1 | 1+ | 1+ | 1+ | 0 | 1+ | |
| Wrist | 0 | 0 | 0 | 0 | 1+ | 2 | 0 | 2 | 1+ | 0 | |
| Hand | 0 | 0 | 0 | 0 | 1+ | 2 | 1+ | 2 | 2 | 0 | |
| Baseline length (in weeks) | 4 | 4 | 4 | 5 | 5 | 7 | 6 | 4 | 3 | 5 | 4.7 (1.2) |
Isch = Ischemic. Hem = Hemorrhagic. Lac = Lacunar. ACM = Artery Cerebri Media. RHns = Right Hemisferic, not specified. BG = Basal Ganglia. UAT = Utrechtse Arm Hand Test. MAS = Modified Ashworth Scale. MI = Motricity Index. FM = Fugl Meyer. ARAT = Action Research Arm Test. SD = Standard Deviation.
Table 2
Dosage of ABoNt-A per muscle (in Units), injection sites of target muscles, and grade of spasticity, measured using the MAS
| Participants | P1 | P3 | P5 | P6 | P7 | P8 | P9 | P10 | P12 | P13 | |
| Muscles Injected: | FDP | 125 | 150 | 100 | 100 | 125 | 125 | 100 | |||
| FDS | 150 | 125 | 100 | 100 | 150 | 125 | 100 | 100 | |||
| FPL | 100 | ||||||||||
| FCR | 125 | 125 | 100 | 125 | 125 | 150 | 75 | ||||
| FCU | 125 | 100 | 125 | 150 | |||||||
| PrT | 75 | ||||||||||
| BBra | 50 | 200 | |||||||||
| Total Dose of | |||||||||||
| BoNT-A (U): | 500 | 300 | 200 | 275 | 200 | 300 | 500 | 500 | 500 | 250 | |
| MAS Score: | PA PB PC | PA PB PC | PA PB PC | PA PB PC | PA PB PC | PA PB PC | PA PB PC | PA PB PC | PA PB PC | PA PB PC | |
| 1 + 1+1+ | 2 1 1 | 1 + 1 1+ | 1 + 0 1 | 1 + 0 1 | 1 + 0 2 | 1 + 1+2 | 2 2 2 | 1 + 1+1+ | 1 + 1+1 |
3.1.1Group level data: General improvement over time
Mean group values of the ARAT, ABILHAND, FM, grip strength and MI for all three phases, i.e. phase A, phase B and phase C are presented below.
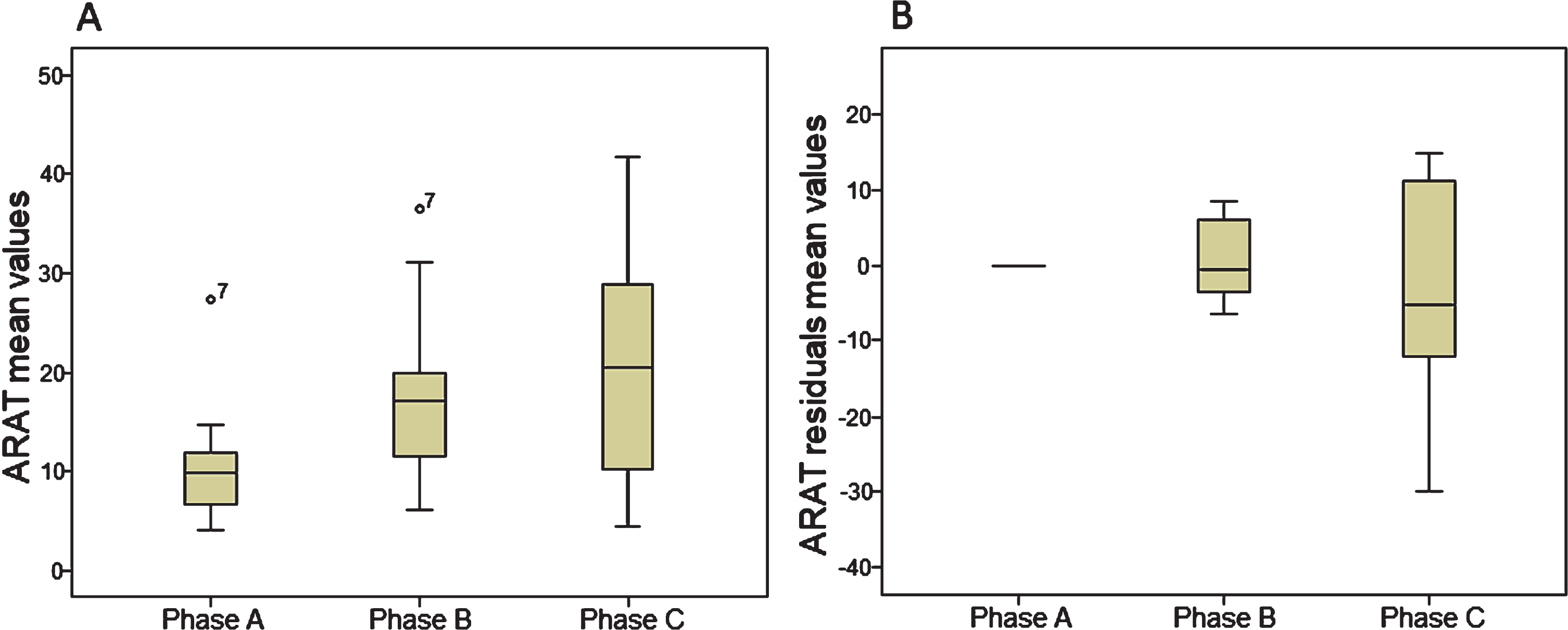
ARAT: Overall, on average, all patients improved over time on the ARAT (p = 0.002) across all three phases. Furthermore, a multiple comparison analysis revealed that mean ARAT values were significantly improved in phase B, relative to the baseline data, i.e. phase A (p = 0.005). Also, the mean ARAT values calculated in phase C were significantly higher compared to the baseline data (p = 0.013). No statistical differences were found for the mean ARAT data between phase B and C (p = 0.221). Boxplots of ARAT results are presented in Fig. 2a.
Fig. 2
Boxplots of Action Research Arm Test mean values (2a) and residuals mean values (2b). ARAT = Action Research Arm Test; Circles = outlier value.
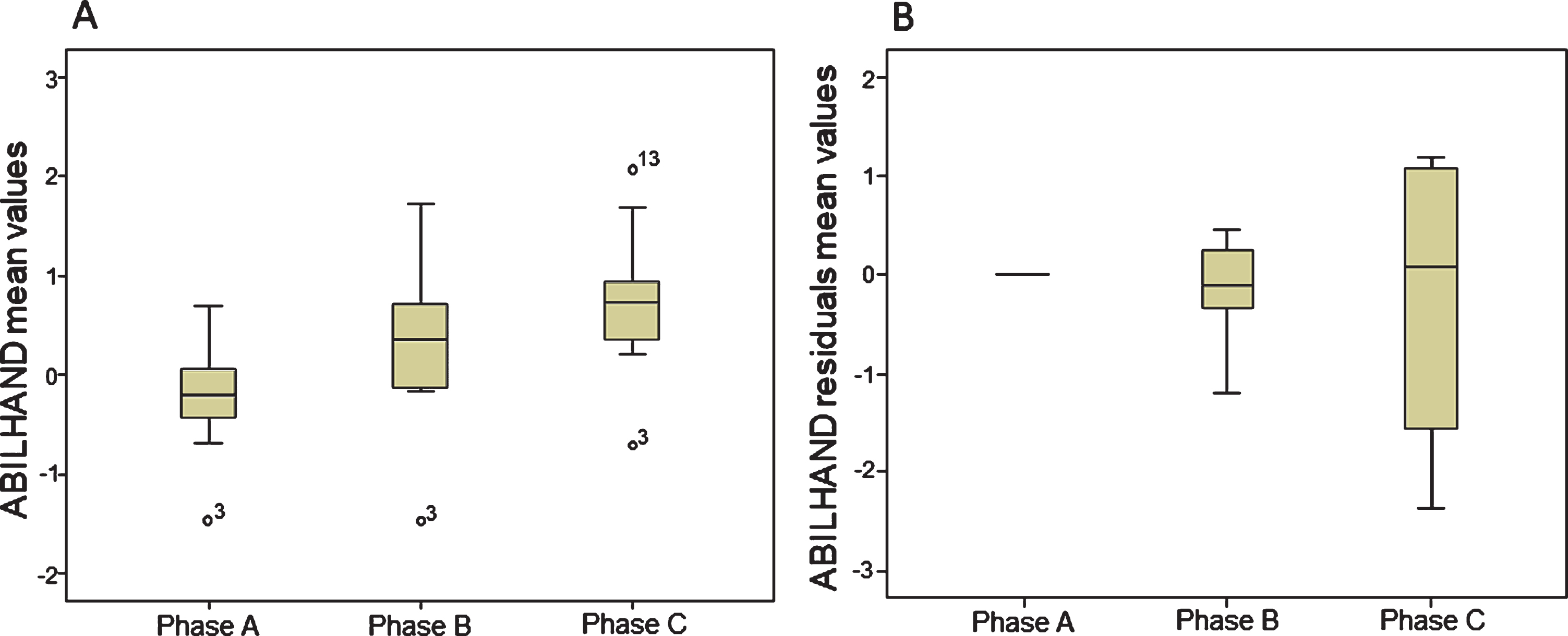
ABILHAND: Overall, on average, patients improved over time on the ABILHAND (p = 0.001). Multiple comparison revealed that ABILHAND results were higher both in phase B and phase C, relative to baseline data (p = 0.017 and p = 0.005 respectively). No significant differences were found between phase B and C (p = 0.047). Boxplots of ABILHAND results are presented in Fig. 3a.
Fig. 3
Boxplots of ABILHAND mean values (3a) and residuals mean values (3b). Circles = outlier value.
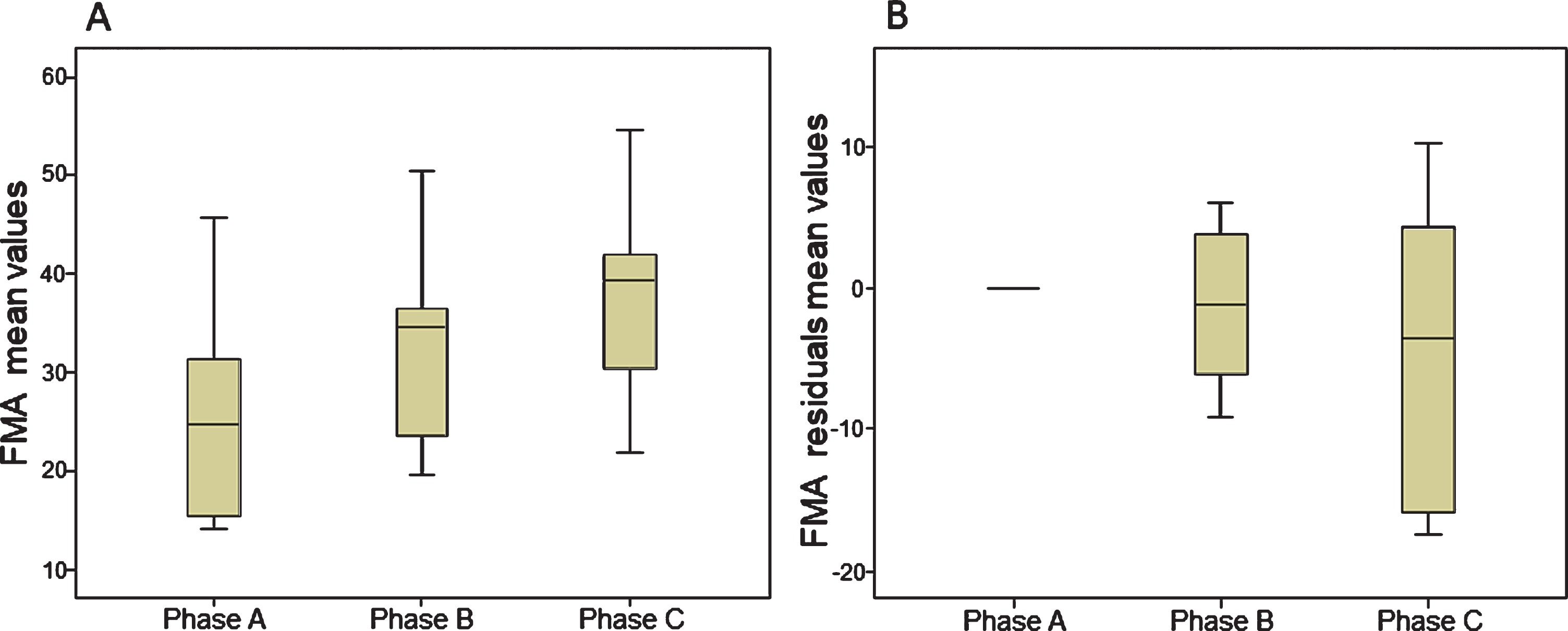
Fugl-meyer motor assessment: Overall, on average, patients improved over time on the FMA (p = 0.001). Multiple comparison showed significant changes between two of the three phases; between phase A and phase B (p = 0.008), and phase A and C (p = 0.005). No significant differences were found between phase B and C (p = 0.037). Boxplots of FMA results are presented in Fig. 4a.
Fig. 4
Boxplots of FMA mean values (4a) and residuals mean values (4b). FMA = Fugl-Meyer Motor Assessment.
Grip strength: Overall, on average, patients changed significantly over time regarding grip strength (p = 0.001). Multiple comparison showed substantial changes between phase B and phase C (p =0.007) and phase A and phase C (p = 0.037). No statistical differences were found between phase A and phase B (p = 0.093). Boxplots of grip-strength results are presented in Fig. 5a.
Fig. 5
Boxplots of Grip strength mean values (5a) and residuals mean values (5b). Circles = outlier value.
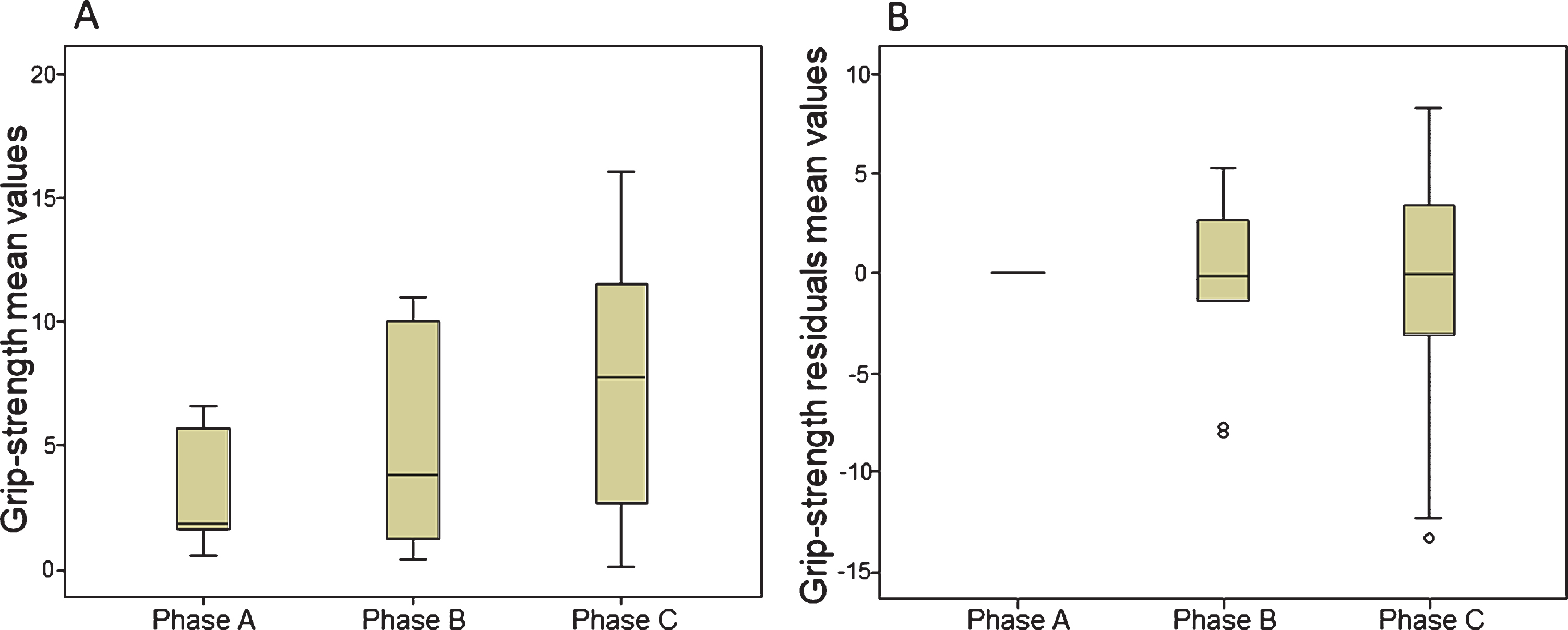
Motricity index: Overall, on average, patients improved over time on strength measured using the Motricity Index (p = 0.014). Multiple comparison showed significant improvements between all three phases; phase A and B (p = 0.013), phase B and phase C (p = 0.028) and phase A and phase C (p = 0.013). Boxplots of MI results are presented in Fig. 6a.
Fig. 6
Boxplots of MI mean values (6a) and residuals mean values (6b).
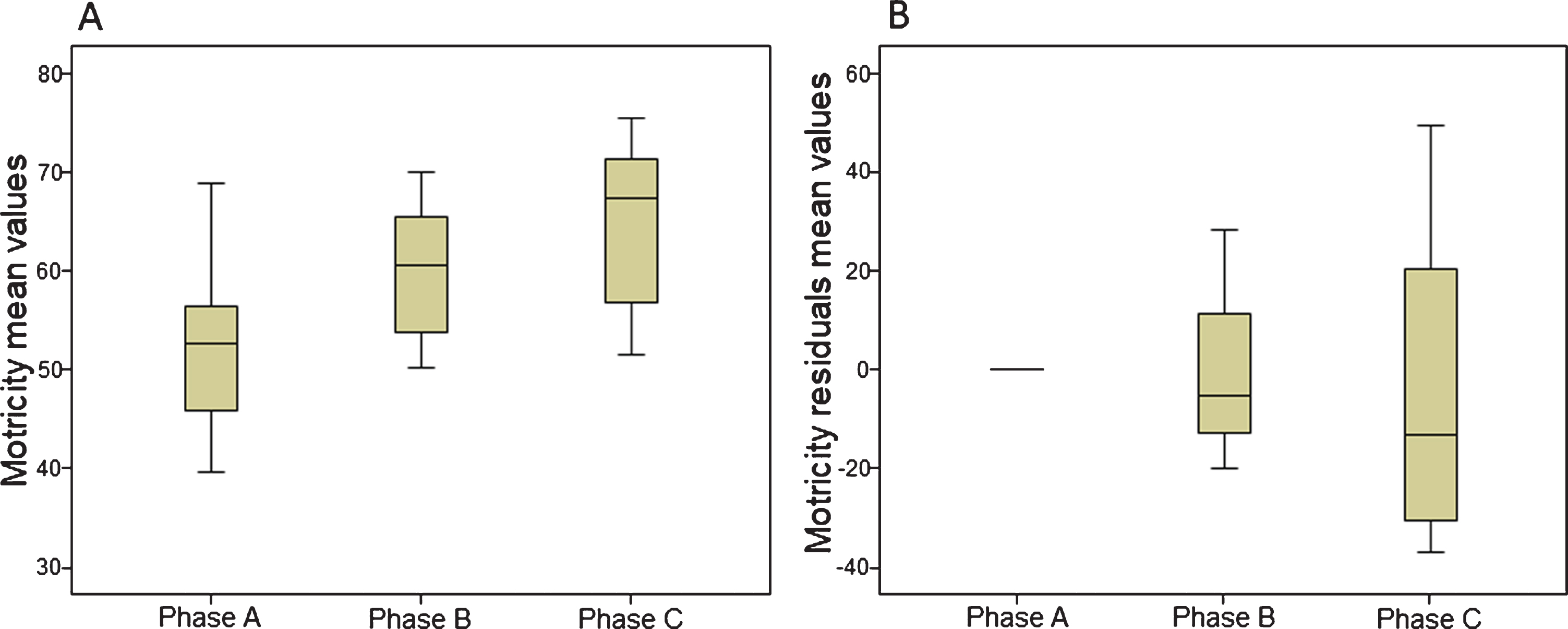
3.1.2Group level data: Changes over time, corrected for baseline trends
Mean group values of the linearly baseline detrended data of the ARAT, ABILHAND, FM, grip strength and MI, are presented above.
Boxplots of the mean linearly baseline trend-corrected ARAT, ABILHAND, FM, grip strength and MI values for all 10 participants, for all three phases, are presented in Fig. 2b, 3b, 4b, 5b and 6b respectively. No significant differences in the residuals values of the ARAT, ABILHAND, FM, grip strength and MI were found between either one of the three phases (p > = 0.419).
3.1.3Individual level data: Baseline trend corrected time series for individual participants
Single case time series: Regarding changes in arm-hand capacity, for each participant, boxplots of ARAT time series residuals for phase A, B and C are presented in Fig. 7.
Fig. 7
Boxplots of Action Research Arm Test residuals. Action Research Arm Test within-subject residuals for all subjects for Phase A, Phase B and Phase C.
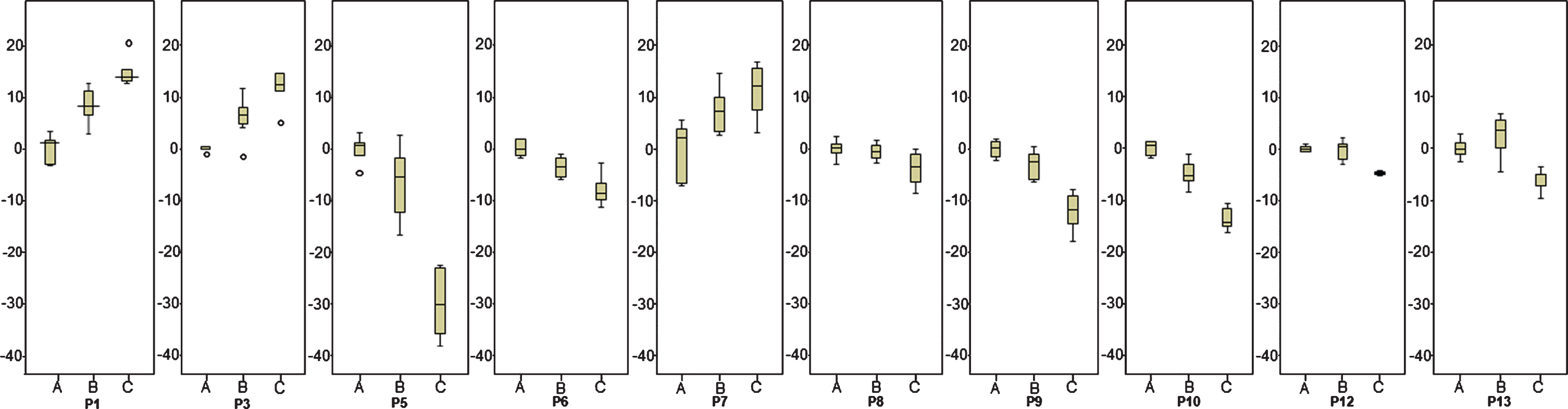
In three patients (P1, P3 and P7) overall mean ARAT residuals were higher in the follow-up phase relative to the baseline phase (p < = 0.012). In the remaining seven patients (P6, P8, P9, P10, P12 and P13) no statistically significant improvements in mean ARAT residuals across phases were observed. In one patient (P5) a statistically significant decrease in mean ARAT residuals was observed between phase A and phase C (p = 0.002).
P1 and P3 showed improvements over time across phase A, B and C: Residuals from phase B were higher compared to phase A, although this difference did not attain statistical significance. In phase C a significant improvement was observed relative to phase B (p < = 0.004). ARAT residuals calculated in phase C were significantly higher than those in phase A (p < = 0.006). In P7 results were statistically significantly different between phase A and C (p < = 0.010). No significant differences were observed between phase A and B (p < = 0.025). In phase C, in three participants, P1, P9 and P10 a return of spasticity in wrist-and hand muscles was observed.
With respect to perceived arm-hand capacity (ABILHAND) and to arm-hand function (FMA, grip strength, MI) median and interquartile range of the within-subject results of all measurements are shown in Table 3a. Both the Kruskal-Wallis p-values and the subsequent multiple comparison p-values regarding phase A, B and C are presented in Table 3b.
Table 3a
Mean residuals data per subject per phase for the ABILHAND, FM, grip strength and Motricity Index. Significant results are printed in bold
| Measures | P1 | P3 | P5 | P6 | P7 | P8 | P9 | P10 | P12 | P13 | |
| Abilhand | Overall | 0.002 | 0.034 | 0.005 | 0.012 | 0.459 | 0.002 | 0.531 | 0.002 | 0.004 | 0.011 |
| Phase A – Phase B | 0.062 | 0.291 | 0.337 | 0.200 | 0.522 | 0.062 | 0.935 | 0.012 | 0.174 | 0.873 | |
| Phase B – Phase C | 0.003 | 0.015 | 0.010 | 0.025 | 0.631 | 0.019 | 0.273 | 0.010 | 0.005 | 0.010 | |
| Phase A – Phase C | 0.006 | 0.100 | 0.004 | 0.010 | 0.200 | 0.002 | 0.391 | 0.006 | 0.011 | 0.010 | |
| FM | Overall | 0.525 | 0.003 | 0.004 | 0.001 | 0.000 | 0.019 | 0.001 | 0.014 | 0.004 | 0.002 |
| Phase A – Phase B | 0.570 | 0.006 | 0.126 | 0.004 | 0.004 | 0.042 | 0.007 | 0.028 | 0.007 | 0.150 | |
| Phase B – Phase C | 0.617 | 0.003 | 0.015 | 0.004 | 0.004 | 0.831 | 0.085 | 0.283 | 0.053 | 0.004 | |
| phase A – Phase C | 0.234 | 0.408 | 0.004 | 0.004 | 0.004 | 0.012 | 0.003 | 0.008 | 0.011 | 0.004 | |
| Grip Strength | Overall | 0.001 | 0.010 | 0.006 | 0.002 | 0.014 | 0.272 | 0.278 | 0.134 | 0.003 | 0.001 |
| Phase A – Phase B | 0.004 | 0.061 | 0.055 | 0.109 | 0.010 | 0.734 | 0.223 | 0.291 | 0.126 | 0.025 | |
| Phase B – Phase C | 0.010 | 0.046 | 0.078 | 0.006 | 0.522 | 0.201 | 0.784 | 0.063 | 0.003 | 0.004 | |
| Phase A – Phase C | 0.006 | 0.010 | 0.004 | 0.004 | 0.016 | 0.156 | 0.153 | 0.273 | 0.011 | 0.004 | |
| Motricity Index | Overall | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 |
| Phase A – Phase B | 0.012 | 0.012 | 0.025 | 0.004 | 0.010 | 0.007 | 0.007 | 0.004 | 0.006 | 0.006 | |
| Phase B – Phase C | 0.003 | 0.004 | 0.010 | 0.004 | 0.025 | 0.033 | 0.011 | 0.004 | 0.017 | 0.004 | |
| Phase A – Phase C | 0.006 | 0.006 | 0.004 | 0.004 | 0.006 | 0.002 | 0.003 | 0.006 | 0.011 | 0.004 |
Table 3b
Interquartile range (IQR) and median scores of the ABILHAND, Fugl-Meyer Motor Assessment (FMA), grip strength (Gr str) and Motricity Index (Motricity I) for all subjects of phase A, phase B and phase C
| Measures | P1 Median | P3 Median | P5 Median | P6 Median | P7 Median | P8 Median | P9 Median | P10 Median | P12 Median | P13 Median |
| [IQR] | [IQR] | [IQR] | [IQR] | [IQR] | [IQR] | [IQR] | [IQR] | [IQR] | [IQR] | |
| Abilhand | ||||||||||
| Phase A | 0.002 | 0.998 | – 0.107 | 0.087 | 0.008 | 0.092 | 0.022 | – 0.174 | – 0.006 | – 0.175 |
| [– 0.250, 0.250] | [– 0.304, 0.255] | [– 0.373, 0.475] | [– 0.389, 0.483] | [– 0.208, 0.159] | [– 0.110, 0.127] | [– 0.585, 0.539] | [– 0.459, 0.546] | [– 0.035, 0.041] | [– 0.301, 0.282] | |
| Phase B | 0.185 | – 0.235 | – 0.297 | – 0.456 | – 0.148 | 0.247 | 0.317 | – 1.308 | 0.463 | – 0.052 |
| [0.156, 0.446] | [– 0.373, 0.136] | [– 0.929, 0.136] | [– 0.594, 0.030] | [– 0.204, – 0.003] | [0.083, 0.586] | [– 0.016, 0.366] | [– 1.841, –.549] | [0.025, 0.712] | [– 0.386, 0.276] | |
| Phase C | 1.189 | 0.359 | – 0.2.621 | – 1.662 | – 0.214 | 0.992 | 0.377 | – 2.272 | 1.208 | – 1.016 |
| [0.813, 1.394] | [0.047, 0.545] | [– 3.215, 1.491] | [– 2.122, – 1.050] | [– 0.459, 0.094] | [0.796, 1.495] | [0.233, 0.506] | [– 2.570, – 1.836] | [1.105, 1.269] | [– 1.195, – 0.395] | |
| FM | ||||||||||
| Phase A | – 0.40 | – 0.20 | 0.50 | 0.34 | – 0.67 | – 0.13 | 0.43 | 0.80 | 0.15 | 1.33 |
| [– 2.40, 2.60] | [– 1.45, 1.55] | [– 2.0, 1.25] | [– 2.38, 2.30] | [– 1.92, 2.58] | [– 0.90, 1.85] | [– 0.57, 1.43] | [– 2.40, 2.00] | [– 0.98, 0.83] | [– 4.86, 3.37] | |
| Phase B | – 1.40 | 5.30 | – 6.0 | – 8.86 | – 8.16 | 3.88 | – 3.57 | 3.20 | 5.65 | – 2.04 |
| [– 2.00, 4.40] | [3.30, 7.80] | [– 11, 0.50] | [– 11.8, – 6.73] | [– 9.16, – 3.66] | [1.02, 6.73] | [– 5.57, – 2.57] | [2.0, 5.2] | [3.57, 8.90] | [– 6.68, -0.28] | |
| Phase C | 3.30 | 0.80 | – 17 | – 17.1 | – 14.1 | 4.38 | – 7.07 | 4.30 | 10.1 | – 14.4 |
| [– 1.80, 4.80] | [–.45, 1.93] | [– 20.3, – 14.8] | [– 18.8, – 15.9] | [– 19.6, – 13.7] | [1.85, 6.60] | [– 13.1, – 3.57] | [2.70, 6.05] | [9.27, 11.47] | [– 19.05, – 10.3] | |
| Grip Str | ||||||||||
| Phase A | – 0.47 | – 0.60 | – 0.20 | – 0.24 | 0.086 | – 0.62 | 1.38 | 1.07 | – 0.45 | – 0.70 |
| [– 3.30, 3.53] | [– 1.27, 1.56] | [– 1.09, 1.35] | [– 2.42, 3.15] | [– 2.12, 2.58] | [– 1.32, 1.57] | [– 3.48, 4.14] | [– 3.22, 2.68] | [– 2.14, 2.59] | [– 1.90, 1.97] | |
| Phase B | – 14.5 | 3.73 | – 2.21 | – 3.35 | 3.44 | – 1.38 | – 3.19 | – 0.47 | 1.90 | – 2.64 |
| [– 25.5, – 8.13] | [2.07, 5.73] | [– 2.95, – 0.411] | [– 7.09, – 0.61] | [2.73, 4.87] | [– 2.82, 2.65] | [– 4.0, – 0.52] | [– 4.63, 1.13] | [.27, 3.38] | [– 7.74, – 1.68] | |
| Phase C | – 49.8 | 8.48 | – 4.18 | – 11.72 | 4.30 | 1.53 | – 2.90 | 3.70 | 6.82 | – 10.33 |
| [58.9, 41.4] | [6.11, 9.61] | [– 6.17, – 3.43] | [– 17.8, – 10.45] | [2.43, 5.98] | [0.39, 2.10] | [– 6.06, – 0.20] | [– 0.53, 5.02] | [5.85, 9.03] | [– 13.9, – 9.92] | |
| Motricity I | ||||||||||
| Phase A | 1.40 | 1.40 | 4.44 | – 0.57 | – 1.30 | 0.50 | 1.04 | – 0.80 | 0.25 | – 0.32 |
| [– 5.35, 4.65] | [– 3.50, 2.80] | [– 12.4, 8.00] | [– 4.82, 5.79] | [– 2.93, 3.72] | [– 2.86, 1.33] | [– 5.61, 4.63] | [– 3.25, 3.65] | [– 1.63, 1.38] | [– 1.26, 1.90] | |
| Phase B | 9.20 | – 11.0 | – 24.3 | 27.8 | – 4.67 | 10.66 | – 10.7 | – 12.0 | 15.8 | – 5.38 |
| [7.90, 9.50] | [– 17.8, – 7.60] | [– 27.5, – 7.43] | [21.03, 37.2] | [– 7.03, – 3.58] | [8.29, 15.11] | [– 23.7, – 8.23] | [– 12.50, – 8.10] | [12.8, 26.5] | [– 6.58, – 3.40] | |
| Phase C | 20.8 | – 26.2 | – 35.10 | 48.7 | – 13.1 | 18.1 | – 30.0 | – 37,4 | 30.5 | – 12.8 |
| [14.8, 23.0] | [– 28.7, – 21.3] | [– 40.1, – 31.0] | [44.1, 55.5] | [– 19.1, – 7.64] | [14.5, 21.5] | [– 35.3, – 26.4] | [– 43.6, – 32.0] | [27.6, 33.7] | [– 16.2, – 10.34] |
4Discussion
The aim of the present study was to investigate the added-value of the reduction of early signs of spasticity on improving arm-hand function and functional arm-hand skill performance in sub-acute stroke patients with either a severely or moderately affected arm-hand (UAT score 1– 3) and moderate to severe grades of spasticity, i.e. MAS scores between 1 + to 3 adjunct to a well-defined arm-hand rehabilitation approach (Franck et al., 2015).
To discern between spontaneous recovery and therapy-as-usual effects on the one hand, and the spasticity reducing treatment on the other hand, two methodological approaches were used. Firstly, a ‘multiple baseline single experimental design’ was used. Secondly, the time series of each subject were ‘detrended’ for any baseline trends to investigate the added-value of BoNt-A on changes in arm-hand function (AHF) and arm-hand skill performance (AHSP) in sub-acute stroke patients with a moderate to severely affected arm-hand.
At group level, on average, participants improved significantly regarding both AHF and AHSP in phase B and C, relative to phase A, except for grip strength. In contrast, after baseline trend correction, data at group level did not confirm that the application of ABoNt-A resulted in an additional improvement of AHF and AHSP adjunct to therapy-as-usual, i.e. CARAS. However, the application of linear detrending using the within-subject data baseline values measured in phase A of the study may have led in some cases to a) an overestimation of spontaneous recovery and therapy-as-usual effects in phase B and C, and could therefore have led to an underestimation of any unique effect of the ABoNt-A as applied in phase B (administration of ABoNt-A).
At the individual level, as to each individual patient’s baseline detrended time series, data showed that the injection of ABoNt-A in three out of the ten participants resulted in significant additional improvements in arm-hand capacity, as measured with the ARAT. Five patients improved at the level of self-perceived performance, as measured with the ABILHAND. Seven out of ten patients demonstrated significant improvements in arm-hand function (AHF). Four patients improved on motor function measured with the FM, the Motricity Index and six patients improved on (grip)-strength. In three patients no beneficial effects from the contribution of ABoNt-A during arm-hand rehabilitation were observed. In one patient a decrease in ARAT residuals was observed between the trainings phase and follow-up phase.
To achieve the desired effect, i.e. a long lasting improvement of AHF and AHSP in stroke patients who suffer from spasticity, many authors recommend to apply a person-tailored approach using a distinct arm-hand rehabilitation intervention in conjunction with botulinum toxin, coupled to relevant AHF and AHSP outcome measures (Demetrios et al., 2014; Devier et al., 2017; Foley et al., 2013; Kinnear et al., 2014; Mills, Finlayson, Sudol, & O’Connor, 2016; Picelli et al., 2014; Royal College of Physicians, 2018; G. Sheean et al., 2010). However, only a minority of studies actually did combine rehabilitation and the application of botulinum toxin including an arm-hand rehabilitation program which is tailored to the patients’ individual characteristics and adaptable to changes in AHF and AHSP level throughout the study period. For example, Devier and colleagues combined botulinum toxin during a well described, patient-tailored arm-hand rehabilitation program. They observed AHF improvements in chronic stroke patients with a mildly affected hand (Devier et al., 2017).
Despite the fact that at group level no significant results as to the added-value of ABoNt-A adjunct to therapy-as-usual were found, the individual data show that several individuals showed significant improvements on AHSP and even more on AHF. With respect to these findings some remarks have to be made. First, the study participants who demonstrated higher baseline values on the Fugl-Meyer wrist and hand section improved significantly at arm-hand skill capacity level during phase B and/or C, in contrast to participants with lower initial FMA values. FMA outcome values are associated with cortico-spinal tract integrity and recovery of the affected arm-hand (Jang et al., 2003). Our results suggest that the former subgroup may have benefitted more from spontaneous recovery in the distal part of the arm during phase A, compared to the latter subgroup and therefore may have obtained higher levels of arm-hand capacity during phase B and C. Maybe especially in the group of patients who displayed significant improvements, the application of botulinum toxin may have facilitated the re-occurrence of voluntary movements that were hampered by spasticity before.
Secondly, the application of the linear baseline detrending of the time series may have led to an underestimation of the potential effects of the botulinum toxin in three study participants regarding both AHSP and AHF level during and after their treatment. Due to the rapidity with which spontaneous recovery combined with arm-hand training interventions occurred during the first (baseline) phase of rehabilitation, the linear detrending may have caused the aforementioned underestimation of any singular added-value of the botulinum toxin application.
Three patients did not attain a significant arm-hand capacity level because they showed MAS scores between 1 + and 2 in the wrist and hand combined with a low level of motor recovery in the distal part of the arm, as measured with the FMA. The combination of both a lack of strength in the wrist/hand and the presence of spasticity in wrist and hand muscles may have hampered progression at the level of arm-hand capacity.
In these three patients, who coped with a very low level of motor output in the distal part of their arm, the flexor muscles of the wrist and/or fingers were treated with ABoNt-A. However, patients who received ABoNt-A in the wrist muscles and/or hand muscles may experience a temporarily delay in regaining hand function and the course of re-learning how to use the affected hand. This is caused by the combination of reduced muscle tone, changes in spasticity and changes in muscle function of the already weakened wrist-and hand muscles (Francis et al., 2004; Fransisco, 2007). This phenomenon, i.e. the loss of muscle function, may explain the lack of significant grip strength improvement between the baseline phase and the intervention phase in these three patients. Ultimately, this could have led to no statistically significant differences regarding arm-hand capacity being found, as a certain level of grip strength is required to observe any progression at arm-hand capacity level as measured with the ARAT.
Progression in AHF and AHSP may be hampered by co-morbidity, especially in stroke survivors with a moderately to severely affected arm-hand. Early post-stroke spasticity is highly correlated with a low motor ability level due to severe muscle weakness (Francis et al., 2004), and a high level of ADL dependency (Leathley et al., 2004; Lundstrom et al., 2010; Opheim, Danielsson, Alt Murphy, Persson, & Sunnerhagen, 2015; Pundik, McCabe, Skelly, Tatsuika, & Daly, 2018; Wissel et al., 2015), shoulder pain (Lindgren, Jonsson, Norrving, & Lindgren, 2007; Ratnasabapathy et al., 2003) or edema (Boomkamp-Koppen, Visser-Meily, Post, & Prevo, 2005). These symptoms were also present in the majority of the patients who participated in the present study. In order to regain control over goal-directed voluntary movements of the hand as efficiently as possible, depending on underlying sensory, motor or cognitive deficits, the aforementioned symptoms were tackled using a set-up of interventions aimed to the specific needs and abilities of each participant. However, the multitude of symptoms within a single subject affecting AHF and AHSP outcome to a certain extent, may have obscured the unique contribution of ABoNt-A applied in this study.
The application of botulinum toxin is considered an adjunct intervention with temporary effects (De Paiva, Meuniere, Molgo, Aoki, & Dolly, 1999) that provides a window of opportunity by temporarily reducing spasticity (Demetrios et al., 2014). In the follow-up phase of this study, a minority of the participants experienced a return of spasticity in muscles who were previously treated with ABoNt-A, a phenomenon that negatively influenced progression regarding AHF and AHSP. Besides the temporary effect of botulinum toxin, patients with a low motor ability level experience more spasticity and associated reactions (Bhimani & Anderson, 2014). These associated reactions often appear and increase when patients become more mobile when, for example, they get out of their wheelchair and start walking longer distances. This may have led to an increase in tone and, eventually, in biomechanical and (neuro-)physiological changes of the different tissues in the affected arm-hand (G. Sheean, 2001) during the training phase, thus negatively influencing AHF and AHSP.
The overall results at group level of this study are in line with Baker et al. who found significant improvements in AHSP as measured by the ARAT in sub-acute and chronic stroke patients with an affected arm-hand who received BoNT in conjunction with arm hand training (Baker & Pereira, 2015). Furthermore, the studies of Turner Stokes et al. (2013) and Demetrios et al. (2015) reported substantial improvements in AHF in moderately to mildly impaired chronic stroke patients who participated in an unspecified (high intensity) arm-hand rehabilitation program (Demetrios et al., 2014; Turner-Stokes et al., 2013). However, at group level, the baseline trend-corrected data of our study did not confirm that the application of ABoNt-A leads to an additional improvement in moderately to severely impaired sub-acute stroke patients.
This is in contrast with Cousins et al. (2010) who showed a significant positive change in AHSP after applying botulinum toxin in stroke individuals with no arm function (Cousins, 2010).
However, in that study an undefined form of arm-hand training and botulinum toxin was provided simultaneously. This may have led to difficulties to ascertain the added-value of the botulinum toxin.
A recently published systematic review found no evidence that BoNT is effective in regaining arm-hand use (Andringa et al., 2019). The major part of this meta-analysis included studies containing chronic stroke patients. Multiple studies included in review did not offer adjunctive rehabilitation therapies after botulinum toxin has been applied in order to optimize voluntary control. Also information with respect to the content and dose of arm-hand therapy offered adjunctive to botulinum toxin was not clearly described. These aforementioned factors make it difficult to compare their results with our study, involving stroke patients in the sub-acute phase, who received ABoNt-A and participated in a defined high-intensity arm-hand therapy regime.
Research concerning sub-acute stroke patients who suffer from a non-functional hand (UAT 0– 3) is scarce in literature (Hayward, Barker, & Brauer, 2010; Oujamaa, Relave, Froger, Mottet, & Pelissier, 2009). From a clinical point of view, exploring the possibilities for training methods for this particular group, especially in early post-stroke phase, is of utmost importance because this could make the difference between either no dexterity or regaining and maintaining dexterity in patients. A further study with more focus on why some individuals respond well to the combined intervention of arm-hand therapy and ABoNt-A in terms of AHF and AHSP progressions, while others do not respond well to the combined intervention, is warranted.
5Limitations of the study
The present study is not without limitations. The application of linear detrending may probably have led to an underestimation of effects of the ABoNt-A applied within the arm-hand training provided in phase B. The latter may have been the case in at least three participants. Future research should also focus on identifying other, non-linear models that may be used to describe effects of spontaneous recovery and therapy-as-usual in stroke patients, that may then be used to gauge the added-value of adjunct interventions like the use of ABoNt-A,
Using the single case experimental design is a valid and efficient way to capture clinically relevant clinical questions rapidly and convert them into a research format. However, creating baseline stability regarding the dependent variables before the intervention to be investigated is applied, is difficult due to, among others, spontaneous recovery processes and therapy-as-usual offered during the baseline phase.
6Conclusion
Combining early post-stroke spasticity reduction with a well-defined therapy-as-usual may improve arm-hand performance in sub-acute stroke patients suffering from spasticity and who display no dexterity at the point of therapy admission.
Patients with a moderately to severely affected hand who benefitted from spontaneous recovery in the distal part of the arm may benefit from the application of botulinum toxin adjunct to arm-hand therapy
Acknowledgments
We are grateful to all patients who participated in this study and to all therapists who contributed to this study.
Conflict of interest
We have the following interests: JAF, JEL, KR and HAMS are employed by Adelante. RS is employed by Maastricht University. There are no patents, products in development or marketed products to declare.
References
1 | Andringa, A. , Port, I. , van Wegen, E. , Ket, J. , Meskers, C. , & Kwakkel, G. ((2019) ). Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity PoststrokeOver Different ICF Domains: a Systematic Review and Meta-Analysis. Arch Phys Med Rehabil. doi:doi.org/10.1016/j.apmr.2019.01.016 |
2 | Ashford, S. , Slade, M. , Malaprade, F. , & Turner-Stokes, L. ((2008) ). Evaluation of functional outcome measures for the hemiparetic upper limb: a systematic review. J Rehabil Med, 40: (10), 787–795. |
3 | Ashworth, B. ((1964) ). Preliminary trial of carisoprodol in multiple sclerosis. Partictioner, 192: , 540–542. |
4 | Baker, A. , & Pereira, G. ((2015) ). The efficacy of Botulinum Toxin A for limb spasticity on improving activity restriction and quality of life: a systematic review and meta-analysis using the GRADE approach. Clin Rehabil, 30: (6), 549–558. |
5 | Bakheit, A. M. , Pittock, S. , Moore, A. P. , Wurker, M. , Otto, S. , Erbguth, F. , & Coxon, L. ((2001) ). A randomized, double-blind, placebo-controlled study of the efficacy and safety of botulinum toxin type A in upper limb spasticity in patients with stroke. European Journal of Neurology, 8: (6), 559–565. |
6 | Barlow, D. , Nock, M. K. , & Hersen, M. ((2008) ). Single case experimental designs: strategies for studying behavior change. Cambridge: Pearson Publishing. |
7 | Bhakta, B. ((2000) ). Management of spasticity in stroke. Br Med Bull, 56: (2), 476–485. |
8 | Bhimani, R. , & Anderson, L. ((2014) ). Clinical Understanding of Spasticity: Implications for Practice. Rehabilitation Research and Practice, 2014: , 1–10. |
9 | Bohannon, R. W. , & Smith, M. B. ((1987) ). Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther, 67: , 206–207. |
10 | Boomkamp-Koppen, H. G. , Visser-Meily, J. M. , Post, M. W. , & Prevo, A. J. ((2005) ). poststroke hand swelling and oedema: prevalence and relationship with impairment and disability. Clin Rehabil, 19: (5), 552–559. |
11 | Cousins, E. W. , Roffe, A. , Rimington, C. , & L. Pandyan, A. ((2010) ). Does low-dose botulinum toxin help the recovery of arm function when given early after stroke? A phase II randomized controlled pilot study to estimate effect size. Clin Rehabil, 24: , 501–513. |
12 | De Paiva, A. , Meuniere, F. , Molgo, J. , Aoki, K. , & Dolly, J. ((1999) ). Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. J Proc Natl Acad Sci, 96: , 3200–3205. |
13 | Demetrios, M. , Gorelik, A. , Louie, J. , Brand, C. , Baquley, I. J. , & Khan, F. ((2014) ). Outcomes of ambulantory rehabilitation programmes following botulinum toxin for spasticity in adults with stroke. J Rehabil Med, 46: , 730–737. |
14 | Demeurisse, G. , Demol, O. , & Robaye, E. ((1980) ). Motor evaluation in vascular hemiplegia. European Neurology, 19: (6), 382–389. |
15 | Devier, D. , Harnar, J. , Lopez, L. , Brashear, A. , & Graham, G. ((2017) ). Rehabilitation plus OnabotulinumtoxinA Improves Motor Function over OnabotulinumtoxinA Alone in Post-Stroke Upper Limb Spasticity: A Single-Blind Randomized Trail. Toxins, 9: (216), 1–9. |
16 | Dong, Y. , Wu, T. , Xiaohua, H. , & Wang, T. ((2017) ). Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: a systematic review with meta-analysis and trial sequential analysis. Eur J Phys Rehabil Med, 53: (2), 256–267. |
17 | Esquenazi, A. , & Mayer, N. ((2004) ). Botulinum toxin for the management of muscle overactivity and spasticity after stroke. Curr Atheroscler Rep, 3: , 295–298. |
18 | Esquenazi, A. , Novak, I. , Sheean, G. , Singer, B. J. , & Ward, A. B. ((2010) ). International consensus statement for the use of botulinum toxin treatment in adults and children with neurological impairments–introduction. European Journal of Neurology, 2: , 1–8. |
19 | Foley, N. , Pereira, S. , Salter, K. , Fernandez, M. , Speechley, M. , Sequeira, K. ,... Teasell, R. ((2013) ). Treatment With Botulinum Toxin Improves Upper-Extremity Function Post Stroke: A Systematic Review and Meta-Analysis. Archives of Physical Medical Rehabilitation, 94: , 977–989. |
20 | Francis, H. P. , Wade, D. T. , Turner-Stokes, L. , Kingswell, R. S. , Dott, C. S. , & Coxon, E. A. ((2004) ). Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. Journal of Neurology Neurosurgery Psychiatry, 75: , 1547–1551. |
21 | Franck, J. A. , Halfens, J. A. M. , Smeets, R. J. E. M. , Seelen, H. A. M. ((2015) ). Concise Arm and hand Rehabilitation Approach in Stroke (CARAS): A practical and evidence-based framework for clinical rehabilitation management. The Open Journal of Occupational Therapy, 3: (4), Article 10. doi: 10.15453/2168-6408.1164 |
22 | Franck, J. A. , Smeets, R. J. E. M. , Renders, K. , & Seelen, H. A. M. ((2018) ). Added-value of Early Post-stroke Spasticity Reduction during Arm-hand Rehabilitation in Improving Functional Arm-hand Skill Performance: A Multiple Baseline Single Case Experimental Design Study. Internation Journal of Neurorehabilitation, 5: (4). |
23 | Franck, J. A. , Smeets, R. J. E. M. , & Seelen, H. ((2017) ). Changes in arm-hand function and arm-hand skill performance in patients after stroke during and after rehabilitation. PLoS One, 12: (6), 1–18. |
24 | Fransisco, G. ((2007) ). Botulinum Toxin for Post-stroke Spastic Hypertonia: A Review of its Efficacy and Application in Clinical Practice. Ann Acad Med Singapore, 36: , 22–30. |
25 | Gladstone, D. J. , Danells, C. J. , & Black, S. E. ((2002) ). The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair, 16: (3), 232–240. |
26 | Hamilton, G. F. , McDonald, C. , & Chenier, T. ((1994) ). Meting van de grijpkracht: waarde en betrouwbaarheid van de bloeddrukmeter en de ‘Jamar grijpdynamometer’. Stimulus, 13: (3), 164–165. |
27 | Hayward, K. , Barker, R. , & Brauer, S. ((2010) ). Interventions to promote upper limb recovery in stroke survivors with severe paresis: a systematic review. Disabil Rehabil, 32: (24), 1973–1986. |
28 | Hayward, K. , Kuys, S. , Barker, R. , & Brauer, S. ((2014) ). Can stroke survivors with severe upper arm disability achieve a clinically important change in arm function during inpatient rehabilitation? A multicentre, prospective, observational study. NeuroRehabilitation, 35: (1), 17–23. doi: 10.3233/nre-141096 |
29 | Hesse, S. , Reiter, F. , Konrad, M. , & Jahnke, M. ((1998) ). Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double-blind, placebocontrolled trail. Clin Rehabil (12), 381-388. |
30 | Hsieh, C. L. , Hsueh, I. P. , Chiang, F. M. , & Lin, P. H. ((1998) ). Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing, 27: (2), 107–113. |
31 | Ipsen, F. ((2016) ). summary of product characteritics. Dysport RVG 17505. |
32 | Jang, S. H. , Kim, Y. H. , Cho, S. H. , Lee, J. H. , Park, J. W. , & Kwon, Y. H. ((2003) ). Cortical reorganization induced by task-oriented training in chronic hemiplegic stroke patients. Neuroreport, 14: (1), 137–141. |
33 | Kinnear, B. , Lannin, N. , Cusick, A. , Harvey, L. , & Rawicki, B. ((2014) ). Rehabilitation Therapies After Botulinum Toxin-A Injection to Manage Limb Spasticity: a Systematic Review. Phys Ther, 94: , 1569–1581. |
34 | Kruitwagen-van Reenen, E. T. , Post, M. W. , Mulder-Bouwens, K. , & Visser-Meily, J. M. ((2009) ). A simple bedside test for upper extremity impairment after stroke: validation of the Utrecht Arm/Hand Test. Disabil Rehabil, 31: (16), 1338–1343. |
35 | Kuo, C. L. , & Hu, G. C. ((2018) ). Post-stroke spasticity: A Review of Epidemiology, Pathophysiology, and Treatments. International Journal of Gerontology, 1-5. |
36 | Leathley, M. J. , Gregson, J. M. , Moore, A. P. , Smith, T. L. , Sharma, A. K. , & Watkins, C. L. ((2004) ). Predicting spasticity after stroke in those surviving to 12 months. Clin Rehabil, 18: , 438–443. |
37 | Lieber, R. , Roberts, T. , Blemker, S. , Lee, S. , & Herzorg, W. ((2017) ). Skeletal muscle mechanics, energetics and plasticity. J Neuroeng Rehabil, 14: (108), 1–16. |
38 | Lindgren, I. , Jonsson, A. C. , Norrving, B. , & Lindgren, A. ((2007) ). Shoulder pain after stroke: a prospective population-based study. Stroke, 38: (2), 343–348. |
39 | Lundstrom, E. , Smits, A. , Terent, A. , & Borg, J. ((2010) ). Time-course and determinants of spasticity during the first six months following first-ever stroke. J Rehabil Med, 42: , 296–301. |
40 | Mills, P. B. , Finlayson, H. , Sudol, M. , & O’Connor, R. ((2016) ). Systematic review of adjunct therapies to improve outcomes following botulinum toxin injection for treatment of limb spasticity. Clin Rehabil, 30: (6). |
41 | Monaghan, K. , Horgan, F. , Blake, C. , Cornall, C. , Hickey, P. P. M. , Lyons, B. E. , & Langhorne, P. ((2011) ). Physical treatment interventions for managing spasticity after stroke. Cochrane Database of Systematic Reviews (7). |
42 | Nederlandse Rijksoverheid [Dutch Government]. http://wetten.overheid.nl/BWBR0009408/2017-03-01 |
43 | Opheim, A. , Danielsson, A. , Alt Murphy, M. , Persson, H. C. , & Sunnerhagen, K. S. ((2015) ). Early prediction of long-term upper limb spasticity after stroke: Part of the SALGOT study. Neurology, 85: , 873–880. |
44 | Oujamaa, L. , Relave, I. , Froger, J. , Mottet, D. , & Pelissier, J. Y. ((2009) ). Rehabilitation of arm function after stroke. Literature review. Ann Phys Rehabil Med, 52: (3), 269–293. |
45 | Penta, M. , Tesio, L. , Arnould, C. , Zancan, A. , & Thonnard, J. L. ((2001) ). The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke, 32: (7), 1627–1634. |
46 | Penta, M. , Thonnard, J. L. , & Tesio, L. ((1998) ). ABILHAND: a Rasch-built measure of manual ability. Arch Phys Med Rehabil, 79: (9), 1038–1042. |
47 | Picelli, A. , Tamburin, S. , Gajofatto, F. , Zanette, G. , Praitano, M. , Saltuari, L. ,... Smania, N. ((2014) ). Association between Severe Upper Limb Spasticity and Brain Lesion Location in Stroke Patients. Biomed Research International, 1-6. |
48 | Prabhakaran, S. , Zarahn, E. , Riley, C. , Speizer, A. , Chong, J. Y. , Lazar, R. M. ,... Krakauer, J. W. ((2008) ). Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair, 22: (1), 64–71. doi: 10.1177/1545968307305302 |
49 | Prazeres, A. , Lira, M. , Aquiar, P. , Monteiro, L. , Vilasboas, I. , & Melo, A. ((2018) ). Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: A randomized, double-blinded, placebo-controlled trial. Neurology International, 10: (7385). |
50 | Pundik, S. , McCabe, J. , Skelly, M. , Tatsuika, C. , & Daly, J. ((2018) ). Association of spasticity and motor dysfunction in chronic stroke. Ann Phys Rehabil Med, 1207: , 1–6. |
51 | Ratnasabapathy, Y. , Broad, J. , Baskett, J. , Pledgern, M. , Marshallm, J. , & Bonita, R. ((2003) ). Shoulder pain in people with a stroke: a population-based study. Clin Rehabil, 17: , 304–311. |
52 | Royal College of Physicians, B. S. o. R.M., The Chartered Society of Physiotherapy, Association of Chartered Physiotherapists in Neurology and the Royal College of Occupational Therapists. (2018). Spasticty in adults: management of botulinum toxin national guidelines 2018. |
53 | Salter, K. L. , Teasell, R. W. , Foley, N. C. , & Jutai, J. W. ((2007) ). Outcome assessment in randomized controlled trials of stroke rehabilitation. Am J Phys Med Rehabil, 86: (12), 1007–1012. |
54 | Shaw, L. C. , Price, C. , van Wijck, F. , Shackely, P. , Steen, N. , Barnes, M. ,... Rodgers, H. ((2011) ). Botulinum Toxin for the Upper Limb After Stroke (BoTULS) Trial Effect on Impairment, Activity Limitation, and Pain. Stroke, 42: , 1371–1379. |
55 | Sheean, G. ((2001) ). Botulinum treatment of spasticity: why is it so difficult to show a functional benefit? Curr Opin Neurol, 14: , 771–776. |
56 | Sheean, G. , Lannin, N. A. , Turner-Stokes, L. , Rawicki, B. , & Snow, B. J. ((2010) ). Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: international consensus statement. Eur J Neurol, 17: (2), 74–93. |
57 | Siegel, S. , & Castellan, N. ((1988) ). Nonparametric statistics. New York: McGraw-Hill International Editions. |
58 | Sun, S. F. , Hsu, C. W. , Sun, H. P. , Hwang, C. W. , Yang, C. L. , & Wang, J. L. ((2010) ). Combined botulinum toxin type A with modified constraint-induced movement therapy for chronic stroke patients with upper extremity spasticity: a randomized controlled study. Neurorehabil Neural Repair, 24: , 34–41. |
59 | Sunnerhagen, K. S. ((2016) ). Predictors of spasticity after stroke. Curr Phys Med Rehabil Rep, 4: , 182–185. |
60 | Suputtitada, A. , & Suwanwela, N. C. ((2005) ). The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil Rehabil, 27: , 176–184. |
61 | Takekawa, T. , Abo, M. , Ebihara, K. , Taguchi, K. , Sase, Y. , & Kakuda, W. ((2013) ). Long-term effects of injection of botulinum toxin type A combined with home-based functional training for post-stroke patients with spastic upper limb hemiparesis. Acta Neurol Belg, 113: (4), 469–475. |
62 | Turner-Stokes, L. , Fheodoroff, K. , Jacinto, J. , & Maisonobe, P. ((2013) ). Results from the Upper Limb International Spasticity Study-II (ULISII): A large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real-life clinical management. BMJ Open. |
63 | Van der Lee, J. H. , Roorda, L. D. , Beckerman, H. , Lankhorst, G. J. , & Bouter, L. M. ((2002) ). Improving the Action Research Arm test: a unidimensional hierarchical scale. Clin Rehabil, 16: (6), 646–653. |
64 | Ward, A. B. , Wissel, J. , Borg, J. , Ertzgaart, P. , Herrmann, C. , Kulkarni, J. ,... Fulford-Smith, A. ((2014) ). Functional goal achievement in post-stroke spasticity patients: the botox economic spasticity trail (BEST. Journal of Rehabilitation Medicine, 46: , 504–513. |
65 | Weber, D. J. , Skidmore, E. R. , Niyonkuru, C. , Chang, C. L. , Huber, L. M. , & Munin, M. C. ((2010) ). Cyclic functional electrical stimulation does not enhance gains in hand grasp function when used as an adjunct to onabotulinumtoxin A and task practice therapy: a single-blind, randomized controlled pilot study. Archives of Physical Medical Rehabilitation, 91: , 679–686. |
66 | Winters, C. , Kwakkel, G. , Nijland, R. , & van Wegen, E. ((2016) ). When Does Return of Voluntary Finger Extension Occur Post-Stroke? A Prospective Cohort Study. PLoS One, 11: (8), 1–12. doi: 10.1371/journal.pone.0160528 |
67 | Wissel, J. , Verrier, M. , Simpson, D. , Charles, D. , Guinto, P. , Papapetropoulos, S. , & Sunnerhagen, K. ((2015) ). Post-stroke spasticity: predictors of early development and considerations for therapeutic intervention. Physical medicine and Rehabilitation, 7: , 60–67. |
68 | Wolf, S. , Milton, B. , Reiss, A. , Easley, K. , Shenvi, N. , & Clark, P. ((2012) ). Further Assessment to Determine the Additive Effect of Botulinum Toxin Type A on an Upper Extremity Exercise Program to Enhance Function Among Individuals With Chronic Stroke but Extensor Capability. Archieves of Medical Rehabilitation, 93: , 578–587. |
69 | Yozbatiran, N. , Der-Yeghiaian, L. , & Cramer, S. C. ((2008) ). A standardized approach to performing the action research arm test. Neurorehabil Neural Repair, 22: (1), 78–90. |




