The effectiveness of cognitive behaviour therapy for reducing anxiety symptoms following traumatic brain injury: A meta-analysis and systematic review
Abstract
BACKGROUND:
Anxiety is a common neuropsychological sequela following traumatic brain injury (TBI). Cognitive Behaviour Therapy (CBT) is a recommended, first-line intervention for anxiety disorders in the non-TBI clinical population, however its effectiveness after TBI remains unclear and findings are inconsistent.
OBJECTIVE:
There are no current meta-analyses exploring the efficacy of CBT as an intervention for anxiety symptoms following TBI, using controlled trials. The aim of the current study, therefore, was to systematically review and synthesize the evidence from controlled trials for the effectiveness of CBT for anxiety, specifically within the TBI population.
METHOD:
Three electronic databases (Web of Science, PubMed and PsycInfo) were searched and a systematic review of intervention studies utilising CBT and anxiety related outcome measures in a TBI population was performed through searching three electronic databases. Studies were further evaluated for quality of evidence based on Reichow’s (2011) quality appraisal tool. Baseline and outcome data were extracted from the 10 controlled trials that met the inclusion criteria, and effect sizes were calculated.
RESULTS:
A random effects meta-analysis identified a small overall effect size (Cohen’s d) of d = –0.26 (95%CI –0.41 to –0.11) of CBT interventions reducing anxiety symptoms following TBI.
CONCLUSIONS:
This meta-analysis tentatively supports the view that CBT interventions may be effective in reducing anxiety symptoms in some patients following TBI, however the effect sizes are smaller than those reported for non-TBI clinical populations. Clinical implications and limitations of the current meta-analysis are discussed.
1Introduction
Traumatic brain injury (TBI) is defined as an injury to the brain as a result of external force. There are many possible causes of TBI, but they are most commonly caused by road traffic accidents, falls and assaults (Hyder, Wunderlich, Puvanachandra, Gururaj, & Kobusingye, 2007). In the UK, reports estimate that someone is admitted to hospital every three minutes following a TBI (Headway, 2015). TBI is a significant public health concern and a leading cause of disability in the developed world (Fleminger, & Ponsford, 2005; McAllister, 2008; Roozenbeek, Mass, & Menon, 2013; Stocchetti, 2014).
TBI is associated with long-term disability, which can significantly impact daily functioning and quality of life (Hyder et al., 2007). The sequelae following TBI often includes physical and cognitive difficulties (McAllister, 2008), and an increased incidence of psychiatric illness (Deb, Lyons, Koutzoukis, Ali, & McCarthy, 1999; Koponen et al., 2002), including anxiety disorders.
1.1Anxiety disorders and TBI
Anxiety is a commonly reported psychological complaint following TBI (Coetzer, 2010) and is the most prevalent psychiatric diagnosis within the first 12 months post-injury (Gould, Ponsford, Johnston, & Schonberger, 2011). Neurobiological damage, physical and psychological adjustment, coping style, feelings of grief, loss, and uncertainty regarding the future are all considered to contribute to the aetiology of anxiety following TBI (Williams, Evans, & Fleminger, 2003). Post-injury biopsychosocial models of adjustment consider both direct and indirect influences, in addition to a variety of mediating factors (Lishman 1973; Gainotti 1993; Kendall & Terry; 1996).
Previous research examining the relationship be-tween neuroanatomical regions and specific anxiety presentations have attempted to identify brain areas of importance. Obsessive Compulsive Disorder (OCD) is frequently associated with lesions to the frontal and connected subcortical areas such as the orbito-frontal cortex, anterior cingulate gyrus and caudate nucleus (Rydon-Grange & Coetzer, 2015; Schwar-zbold et al 2008). However, as highlighted by Coetzer (2004), the difficulty of separating overlapping symptomology in this clinical population is important to consider. For example, perseverative be-haviour, which is also associated with frontal lesions, can be mistaken for repetitive behaviour in OCD. Therefore, it is important to consider cognitive factors as an alternative hypothesis for the development of such symptoms, rather than anxiety per se.
The emergence of Post-traumatic Stress Disorder (PTSD) following TBI has shown a relationship with the degree of post-traumatic amnesia (PTA). A large study (n > 1100) by Bryant et al (2009) demonstrated that individuals with a mild TBI were more likely to develop PTSD than those without a TBI. However, those with longer periods of PTA were found to have less severe intrusive thoughts, which highlighted the potentially protective nature of PTA in evolution of PTSD after TBI. Furthermore, another factor to consider is that individuals with altered levels of consciousness may have “islands of memory” whereby memories may be processed directly through the amygdala during the traumatic event. This may result in an implicit memory processes that result in an emotional or perceptual memory, without the explicit autobiographical component.
Anxiety symptomology can manifest as apprehension, worry and fear, or as a diagnosable mental health disorder (Soo & Tate, 2012). Post-TBI, individuals are considered to be at increased risk of developing anxiety disorders (Hiott & Labbate, 2002), with the prevalence estimated to range between 11%and 70%(Rao & Lykestos, 2000; Rao & Lykestos, 2002). Furthermore, those with a pre-morbid psychiatric history are likely more vulnerable to post-TBI mood disturbances, with prevalence rates of up to 75%in this sub-group (Gould, Ponsford, Johnston, & Schonberger, 2011). This wide range in prevalence is likely due to the heterogeneous nature of the population and variability in outcome measurements used across studies. In terms of specific anxiety disorders, PTSD (19%), OCD (15%), panic disorder (14%), generalised anxiety disorder (9%) and phobias (10%), are most frequently diagnosed following TBI (Hibbard, Uysal, Kepler, Bogdany, & Silber, 1998).
Post-TBI anxiety can hinder the recovery process and result in up to four times poorer functional outcomes and increased impairment (Bryant et al., 2010). Patients who experience anxiety following TBI report significantly increased disability and reduced quality of life (Fann, Katon, Uomoto, & Esselman, 1995; Whitnall, 2006). Anxiety has also been associated with the subjective over-estimation of the severity of physical and cognitive impairments (Fann et al., 1995; Byrne, Coetzer, & Addy, 2017), potentially having a further adverse effect on outcome. Effective treatment of anxiety in this population may therefore help reduce subjective reporting of physical and cognitive impairments, and as a result improve outcome and quality of life.
1.2Treatments for anxiety
In non-TBI clinical populations, additional to psychological treatments, in some patients anxiety is often managed effectively with pharmacotherapy (Murrough, Yaqubi, Sayed, & Charney, 2015; Bande-low et al., 2015). There is evidence however, that pharmacological interventions may have limited efficacy in the TBI population. Individuals with TBI may be increasingly vulnerable to negative side effects (Warden et al., 2006) and the exacerbation of cognitive difficulties (Perna, Bordini, & Newman, 2001). The development of effective alternative, non-pharmacological treatments, including psychological interventions to augment existing approaches to rehabilitation, are therefore important to consider.
1.2.1Non pharmacological interventions
Despite the high prevalence of anxiety disorders following TBI and the negative impact they have on rehabilitation outcomes, in comparison to the general clinical population, there has been relatively little research into potential treatments. Within the TBI population, the evidence-base for psychological interventions for anxiety has been steadily expanding over the last 20 years. To date, the intervention that has had the most research within this population is Cognitive Behaviour Therapy (CBT). CBT is ultimately based on the premise that cognitions influence behaviour and emotions, and a change in one of these areas will bring about reciprocal change in the others. It is beyond the scope of this meta-analysis to provide a detailed description of CBT. Beck (1995; 1998) provides a more detailed description of the development and application of CBT.
Over recent years there has been increased interest in developing and adapting alternative interventions for use within the TBI population. Such interventions that have been considered, include Acceptance and Commitment Therapy (ACT) and Mindfulness Based Cognitive Therapy (MBCT), which have shown promising results (Kangas & McDonald, 2011; Whiting, Deane, Simpson, & McLeod, 2017; Bedard et al., 2012). The role of exercise as an intervention to reduce anxiety symptoms has also been considered, and results are promising (Gordon et al., 1998; Rzezak et al., 2015; Weinstein, et al., 2017).
1.2.2CBT for anxiety in non-TBI populations
In the general population CBT is a recommended intervention for the treatment of a range of anxiety disorders (National Institute for Health and Care Excellence [NICE], 2011) There is a wealth of empirical evidence supporting the efficacy of CBT for reducing anxiety symptoms, including several reviews of high-quality meta-analyses (Deacon & Abramowitz, 2004; Norton & Price, 2007). Hoffman, Asnaani, Vonk, Sawyer and Fang (2012) conducted a large-scale review to examine CBT as a treatment for a variety of disorders, including anxiety. Large effect sizes for the treatment of OCD and medium effect sizes for social anxiety disorder, PTSD and panic disorder were reported consistently (Hoffman et al., 2012; Carpenter et al., 2018). In another meta-analysis of 108 clinical trials, Norton and Price (2007) considered the efficacy of CBT across a range of anxiety disorders. CBT resulted in significantly larger effect sizes in comparison to no treatment or control conditions across all the anxiety disorders, particularly generalised anxiety disorder and PTSD.
1.2.3CBT in TBI populations
Over recent years, CBT has been increasingly used as a treatment within TBI populations. It has been argued that its highly structured and goal-oriented approach, in addition to a focus on concrete thoughts and behaviours, means that it is an appropriate intervention for individuals with cognitive impairments (Hodgson, McDonald, Tate, & Gertler, 2005; Doering & Exner, 2011). Additional adaptations may also be beneficial to ensure that CBT is accessible to the TBI population. A recent review by Gallagher, McLeod and McMillan (2016) reported that increased socialisation to the CBT model and utilising external memory aids were the most common adaptations used.
In 2007, Soo and Tate conducted a systematic review of the available randomised control trials (RCTs) to investigate the efficacy of psychological treatment for anxiety following TBI. At the time, there were only three RCTs that met the inclusion criteria for their systematic review, examining the efficacy of CBT (Bryant, Moulds, Guthrie & Nixon, 2005;Tiersky et al., 2005) and interpersonal process recall therapy (IPRT; Helffenstein & Wechsler, 1982). They found evidence in support of the effectiveness of CBT for the treatment of acute stress disorder post-TBI and for the combination of CBT and neuro-rehabilitation as an intervention for general anxiety symptoms following mild to moderate TBI. They reported limited evidence for the efficacy of IPRT and identified significant flaws in the methodology of this study. Soo and Tate (2007) highlighted the complexity of assessing anxiety within TBI populations; specifically, due to difficulties with differential diagnoses and diagnostic overshadowing.
Much of the current evidence-base was derived from research with individuals who have experienced acquired brain injury (ABI), which includes TBI as well as cerebrovascular accidents (CVA). This is often due to difficulties with recruitment within relatively small local TBI populations approached during clinical research projects. A meta-analysis using a mixed ABI population reported effect sizes ranging from 0 to 0.42 when investigating the efficacy of CBT on reducing anxiety symptoms (Waldron, Casserly & O’Sullivan., 2013). Although often resulting in similar neuropsychiatric sequalae, the aetiology and neuropathology of TBI and CVA are very different (Tateno, Murata, & Robinson, 2002; Werner & Engelhard, 2007), therefore, the nature and cause of anxiety, as well as response to treatment may differ between these populations. For this reason the present meta-analysis will focus specifically on TBI populations only.
The current evidence-base examining the efficacy of treatments for anxiety post-TBI is conflicted and equivocal, with studies utilising a variety of sample sizes, outcome measures, severity of TBI and focus of the intervention. As a result, it is difficult to make comparisons across studies and there is a need to synthesise current research. There have been no previous meta-analyses of controlled trials investigating specifically CBT as the primary psychological intervention to treat anxiety following TBI. The current meta-analysis therefore aims to answer the following question: Is CBT an effective intervention to reduce anxiety symptoms following TBI?
2Method
2.1Identification and selection of studies
Three electronic databases (Web of Science, PubMed and PsycInfo) were searched for eligible studies up to May 2020, using the following search te-rms: (“Cognitive Behav* Therapy” OR “CBT”) AND (“anxiety” OR “stress”) AND (“traumatic brain injury” OR “TBI” OR “brain injury” OR “head tr-auma” OR “head injury” OR “brain damage”). The search was limited to English language articles, published since 1990. An ancestral search of the ide-ntified articles was also conducted. This search me-thod, using three databases and an ancestral search, was considered a comprehensive approach to gaining access to relevant articles. Articles were screened initially via examination of title and abstract, after which full text articles were assessed according to the following eligibility criteria:
I. Participants must be 18 years or over
II. The sample must contain participants who have sustained a TBI of any severity (i.e. mild, moderate or severe)
III. Studies must be controlled trials (i.e. must contain both an intervention group and a control group)
IV. Interventions must specifically have used CBT as an intervention. For the purpose of this meta-analysis, studies were included if the intervention targeted both cognitive and behavioural processes or was stated to use an intervention that was underpinned by CBT principles.
V. Studies must include an anxiety related outcome measure.
VI. Study data must be quantitative.
2.2Assessment of study quality
The quality of each study was assessed using Reichow, Volkmar and Cicchetti’s (2008) criteria, a method with strong psychometric properties. Each individual study was initially appraised for quality using Reichow’s (2011) primary and secondary indicators (e.g. participant characteristics, statistical analysis, randomised assignment, social validity) and each indicator was assigned a quality rating of high, acceptable or unacceptable. An overall strength rating of strong, adequate or weak, was then determined for each study (Reichow et al., 2008). Quality ratings were independently checked by the second author (CB). Quality ratings are listed in Table 1.
Table 1
Quality appraisal ratings using reichow’s (2011) criteria
| Ashman | Bell | Bryant | Cooper | Hsieh | Nguyen | Ponsford | Potter | Silverberg | Tiersky | |
| et al. (2014) | et al. (2016) | et al. (2003) | et al. (2017) | et al. (2012) | et al. (2017) | et al. (2015) | et al. (2016) | et al. (2013) | et al. (2005) | |
| Primary Indicators | ||||||||||
| Participant characteristics | High | High | High | High | High | High | High | High | High | High |
| Independent variable | High | High | High | High | High | High | High | High | High | High |
| Comparison condition | High | High | High | High | High | Adequate | High | High | High | High |
| Dependent variable | High | High | High | High | High | High | High | High | High | High |
| Link between research question and data analysis | High | High | High | High | High | High | High | High | High | High |
| Statistical analysis | Adequate | High | Adequate | High | Adequate | Adequate | High | High | High | Adequate |
| Secondary Indicators | ||||||||||
| Random assignment | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Interobserver agreement | No | No | No | No | No | No | No | No | No | No |
| Blind raters | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fidelity | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Attrition | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Generalisation/maintenance | No | Yes | Yes | Yes | No | Yes | Yes | No | No | No |
| Effect size | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Social validity | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall quality rating | Adequate | Strong | Adequate | Strong | Adequate | Adequate | Strong | Strong | Strong | Adequate |
2.3Data extraction and analysis
The Metafor package for the statistical software environment, R (The R Foundation, 2018; Viechtbauer, 2010) was used to analyse all data in this meta-analysis. Data from anxiety related measures were extracted from each article by the first author. Email requests and reminders were sent for unreported data if necessary. Wherever possible, data from intention to treat (ITT) analyses were used as this is considered to provide a more pragmatic and unbiased comparison between conditions (Soares & Carneiro, 2002).
The mean change in anxiety score, from pre to immediately post-CBT intervention, divided by the baseline standard deviation, was used to calculate the effect sizes for each RCT. The difference between the effect sizes for the intervention and control group of each study were then analysed (Viechtbauer, 2010). For each outcome measure, correlation coefficients (test re-test reliability) were extracted from the current evidence-base.
Due to the potential heterogeneity of CBT interventions, and variability in methodological rigour within the identified studies, a random effects meta-analysis model was used. This model is based on the assumption that the true effect size varies between studies and therefore predicts the overall standardised mean change (SMC; Borenstein, Hedges, Higgins & Rothstein, 2010). Negative effect sizes would indicate an average reduction in anxiety scores from pre to post-intervention. Each study’s effect size was then weighted by its sample size, and pooled to provide an overall effect size for the effectiveness of CBT interventions in reducing anxiety symptoms. Using Cohen’s (1988) criteria, an effect size of 0.2 is considered to be a small effect, 0.5 a medium effect, and 0.8 a large effect.
3Results
An initial screening process yielded 938 articles. Following title and abstract examination 871 were excluded as they were found not to be relevant to the research question. The remaining 67 full-text articles were assessed and 11 were found to satisfactorily meet the above inclusion criteria. Unfortunately, one author did not respond to requests for data, therefore 10 studies were included in the meta-analysis. The selection of studies followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Moher, Liberatti, Tetlzaff & Altman, 2009). See Figure 1 for the PRISMA diagram demonstrating the search process. All 10 of the included studies were RCTs.
3.1Study characteristics
3.1.1Methodological quality
The quality of the included studies was considered to be ‘Adequate’ (Ashman, Cantor, Tsaousides, Spielman, & Gordon, 2014; Bryant, Moulds, Guthrie, & Nixon, 2003; Hsieh et al., 2012; Nguyen et al., 2017; Tiersky et al., 2005) or ‘Strong’ (Bell et al., 2016; Cooper et al., 2017; Ponsford et al., 2016; Potter, Brown, & Fleminger, 2016; Silverberg et al., 2013). Out of the 10 articles included, eight stated that they utilised ITT analysis. Tiersky et al. (2005) did not appear to use ITT and Potter et al. (2016) lost one participant to follow up but did not attempt to impute missing data.
3.1.2Participants
All participants included in the current meta-analysis were over the age of 18 and gave informed consent to participate in the individual studies. All participants were recruited from community samples, and had sustained TBIs of varying severity (i.e. mild, moderate or severe). The studies by Bell et al. (2016) and Cooper et al. (2017) used military samples, including only active service members.
Eight of the studies recruited from rehabilitation services, where TBI diagnoses and severity were confirmed by clinicians (Ashman et al., 2014; Bell et al., 2016; Bryant et al., 2003; Cooper et al., 2017; Hsieh et al., 2012; Ponsford et al., 2016; Potter et al., (2016); Silverberg et al., 2013). Nguyen et al. (2017) and Tiersky et al. (2005) relied on self-reported symptoms of loss of consciousness and PTA to confirm TBI.
All the included studies recruited participants that had experienced a TBI at least six months prior to participating in the study, with the exception of the studies by Silverberg et al. (2013) who recruited at six weeks and Bryant et al. (2003) who recruited at two weeks post-injury. In total, 359 participants were randomised to a CBT based intervention and 342 were randomised to a control condition. Several of the included studies required participants to have a diagnosed psychological disorder including anxiety (Hsieh et al., 2012; Ponsford et al., 2016), depression (Ashman et al., 2014; Ponsford et al., 2016), acute stress disorder (Bryant et al., 2003) or be at risk of developing postconcussion syndrome (PCS; Potter et al., 2016).
3.1.3Trial design
All of the studies included in the current meta-analysis were RCTs, where participants were randomly allocated to either an intervention or control arm of the trial. Seven of the studies utilised a two-group parallel trial (Ashman et al., 2014; Bell et al., 2016; Bryant et al., 2003; Nguyen et al., 2017; Potter et al., 2016; Silverberg et al., 2013; Tiersky et al., 2005) where participants were randomised to a CBT condition or a control condition. Hsieh et al. (2012) and Ponsford et al. (2015) utilised a three-group parallel trial, adding motivational interviewing (MI) or non-directive counselling (NDC) prior to CBT, in comparison to a control condition. To capture the effect of the CBT, data was extracted from the NDC and CBT condition and the control condition, pre and post-CBT (in the study by Ponsford et al., (2016) data were extracted from week three and week 12). Cooper et al. (2017) utilised a four-group parallel trial, comparing psychoeducation, to computerised cognitive rehabilitation, therapist implemented cognitive rehabilitation and CBT. Pre and post-data were extracted from the psychoeducation and the CBT condition for this study.
3.1.4Control conditions
Three of the studies utilised a wait list control (WLC; Potter et al., 2016; Ponsford et al., 2015; Tiersky et al., 2005), three utilised a treatment as usual (TAU) condition (Hsieh et al., 2012, Nguyen et al., 2017; Silverberg et al., 2013), two utilised a psychoeducation condition; face-to-face (Cooper et al., 2017) or via telephone (Bell et al., 2016), and three studies used various forms of face-to-face counselling or psychotherapy (Ashman et al., 2014; Bryant et al., 2003).
3.1.5Intervention type
The studies all administered a CBT-based intervention, however, they varied in terms of session length, frequency and format of delivery. All the interventions were manualised, to ensure treatment fidelity. All interventions were conducted individually and face-to-face, except for the studies by Cooper et al. (2017) who used a combination of individual and group interventions, and Bell et al. (2016) who conducted their CBT informed intervention via telephone call. The length of the interventions varied between 5 and 33 sessions delivered over a period of between 5 weeks and 6 months.
The primary focus of the CBT interventions included depression (Ashman et al., 2014; Ponsford et al., 2015), anxiety (Hsieh et al., 2012; Ponsford et al., 2015), acute stress disorder (Bryant et al., 2003), cognitive functioning (Bell et al., 2016; Cooper et al., 2017); postconcussional complaints (Potter et al., 2016; Silverberg et al., 2013), sleep disturbance and fatigue (Nguyen et al., 2017) and psychological symptoms (Bell et al., 2016; Tiersky et al., 2005).
Despite the differing primary focus of interventions, all incorporated the basic underlying principles of CBT including; psychoeducation, cognitive restructuring, behavioural activation, problem solving and relapse prevention. All studies incorporated structure weekly homework activities, to support participants in the practice and generalisation of skills between sessions.
3.1.6Adaptations
The studies by Ashman et al. (2014), Hsieh et al. (2012), Nguyen et al. (2017), Ponsford et al. (2016) and Potter et al. (2016) clarified the adaptations made to CBT interventions, to ensure accessibility for TBI populations. Adaptations included incorporating compensatory strategies such as written handouts, external memory aids, simplifying complex concepts, providing organisational support, implementing new strategies in vivo where possible. With the exception of Bell et al. (2016) and Cooper et al. (2017), all of the studies stated that their CBT interventions were delivered by professionals who had experience in delivering CBT to TBI populations.
3.1.7Follow up
Five of the included studies included a follow up to determine maintenance effects. Follow ups took place at two months (Nguyen et al., 2017), 12 and 18 weeks (Cooper et al., 2017) and six months (Bell et al., 2016; Bryant et al., 2003). At 21 weeks, Ponsford et al. (2016) provided a top up CBT session to participant and then re-administered outcome measures at 30 weeks.
3.1.8Outcome measures
All the studies included in the current meta-an-alysis utilised anxiety related outcome measures. These included the Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983), the State Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988), the Symptom Checklist-90-R, (SCL-90-R; Derogatis, 1994) and the PTSD checklist-military version (PCL-M; Weathers, Huska, & Keane, 1991). In the event that multiple anxiety measures were administered, measures were prioritised in the following order, according to frequency of use across the studies to maximise the consistency of extracted data and improve homogeneity; HADS, BAI, STAI, SCL-90; PCL-M. The main characteristics of the 10 articles included in this meta-analysis are summarised in Table 2 and Table 3.
Table 2
Main characteristics of studies included in the meta-analysis
| Author (year) | Design | TBI severity | Anxiety measures | Other outcome measure(s) | CBT intervention (led by) | Focus of the CBT intervention | Setting (location) |
| Ashman et al. (2014) | RCT | Mild– Severe | STAI | BDI-II, Life-3, ISEL, LES | 16 weekly sessions of manualised individual CBT based on CBT techniques for treating depression (postdoctoral fellows in clinical neuropsychology) | Depression | Community (USA) |
| Bell et al.(2016) | RCT | Mild | PCL-M | BSI-18, RPQ, EuroQol, PSQI,PHQ-9, CD-RISC, B-IFE, AUDIT, SDS, SF-12, CSC | 12 bi-weekly telephone sessions of problem-solving therapy based upon CBT principles (Master’s level counsellors) | Psychological symptoms | Community, military sample (USA) |
| Bryant et al.(2003) | RCT | Mild | BAI | ASDI, IES, BDI, CAPS | 5 weekly sessions of manualised individual CBT (clinical psychologists) | Acute stress disorder | Community (Australia) |
| Cooper et al.(2017) | RCT | Mild | SCL-90 PCL-M | PASAT, KBCI | 10 weekly sessions of manualised individual and group integrated cognitive rehabilitation and CBT. Focus on cognitive restoration and anxiety/depression symptoms (doctoral level psychologists) | Cognitive difficulties. | Community, military sample (USA) |
| Hsieh et al. (2012) | RCT | Moderate–Severe | HADS-A DASS | CSA, SPRS-2, SADI, | 12 weekly sessions of individual manualised CBT (clinical neuropsychologists) | Anxiety | Community (Australia) |
| Nguyen et al. (2017) | RCT | Mild–Severe | HADS-A | PSQI, ISI, BFI, FSS, ESS | 8 weekly sessions of individual manualised CBT (clinical neuropsychologist) | Sleep Disturbance | Community (Australia) |
| Ponsford et al. (2015) | RCT | Mild–Severe | HADS-A DASS | SPRS-2 | 9 weekly sessions of manualised CBT (clinical psychologist or neuropsychologist) | Anxiety and depression | Community (Australia) |
| Potter et al.(2016) | RCT | Mild–Moderate | HADS-A STAI | RPQ, BICRO-39, QOLAS,IES-R, CIS20R, MPQ,STAXI-2, EuroQol | 12 weekly sessions of individual manualised CBT (clinical neuropsychologist) | Post-concussion complaints | Community (UK) |
| Silverberg et al.(2013) | RCT | Mild | HADS-A | RPQ, M2PI, IPQ | 6 weekly sessions of individual manualised CBT (doctoral level psychologists with neuropsychology experience) | Post-concussion complaints | Community (Canada) |
| Tiersky et al. (2005) | RCT | Mild –Moderate | SCL-90R Attention Questionnaire, CRI, SCL-90, CIQ | PASAT, RAVLT, ACFI, | Individual CBT and cognitive remediation three times a week for 11 weeks (33 sessions) (clinical psychologist with experience in brain injury). | Psychosocial symptoms | Community (USA) |
ACFI–Aged Care Funding Instrument; ASDI–Acute Stress Disorder Interview; AUDIT–Alcohol Use Disorders Identification Test; BAI–Beck Anxiety Inventory; BDI–Beck Depression Inventory; BICRO-39–Brain Injury Community Rehabilitation Outcome Scale; BDI-II–Beck Depression Inventory-II; B-IFE–Brief inventory for Functioning Evaluation; BSI-18–Brief Symptom Inventory-18; CAPS–Clinician Administered PTSD Scale; CD-RISC–Connor-Davidson Resilience Scale-10; CIQ–Community Integration Questionnaire; CIS20R–Checklist of Individual Strength; CRI–Coping Response Inventory; CSA–Coping Scale for Adults; CSC–Client Satisfaction Scale; DASS–Depression Anxiety Stress Scales; ESS–Epworth Sleepiness Scale; EuroQol–European Quality of Life; GOSE–Glasgow Outcome Scale; HADS–Hospital Anxiety and Depression Scale; HISC–Head Injury Symptom Checklist; FSS–Fatigue Severity Scale; IES-R–Impact of Event Scale-Revised; ISEL–Interpersonal Support Evaluation List; IPQ-R–Illness Perception Questionnaire- Revised; ISI–Insomnia Severity Index; KBCI–Key Behaviour Change Inventory; LES–Life Experiences Survey; M2PI–Mayo-Portland Participation Index; MPQ–McGill Pain Questionnaire; PASAT–The Paced Auditory Serial Addition Task; PHQ-9–Patient Health Questionnaire-9; PSQI–Pittsburgh Sleep Quality Index; QOLAS–Quality of Life Assessment Schedule; RAVLT–Rey Auditory Verbal Learning Test; RCT–Randomised Controlled Trial; RPQ–Rivermead Postconcussion Symptoms Questionnaire; SADI–The Self-Awareness of Deficits Interview; SPRS-2–The Sydney Psychosocial Reintegration Scale; STAI–State-Trait Anxiety Inventory; STAXI-2–State-Trait Anger Expression Inventory-2 SCL-90-R–Symptom Checklist-90-R; SDS–Sheehan Disability Scale; SF-12–Short Form Health Survey; TBI–Traumatic brain injury; UCL–Utrechtse Coping List.
Table 3
Methodological characteristics and findings of articles included in the meta-analysis
| Intervention Group | Control Group | |||||||||||
| Author | N | N | Age | Gender | Time Since | Control | N | N | Age | Gender | Time Since | Findings |
| (Year) | (pre) | (post) | (M, SD) | (%M) | Injury(M, SD) | Condition | (pre) | (post) | (M, SD) | (%M) | Injury(M, SD) | |
| Ashman et al. (2014) | 39 | 22 | 47.1(10.6) | 37.8% | 13.3(16.7) years | Supportive psychotherapy (SPT) | 38 | 21 | 48.1(10.2) | 48.6% | 11.8(16.9) years | Significant time effects for the BDI, STAI and QOL outcome measures, but no group effect. No significant difference between CBT and SPT intervention groups post-intervention. |
| Bell et al. (2016) | 178 | 132 | 29.25(7.20) | 93.26% | Not reported | Psycho- education | 178 | 160 | 29.44(7.27) | 93.36 % | Not reported | Post-intervention the PST/CBT group demonstrated greater reductions in psychological distress, and PTSD symptoms; but effects not sustained at 12m follow up. |
| Bryant et al. (2003) | 12 | 12 | 29.42(13.93) | 33.3% | <2 weeks | Supportive counselling (SC) | 12 | 12 | 33.00 (14.37) | 33.3% | <2 weeks | Significantly fewer participants in the CBT group met criteria for PTSD post-treatment than the SC group (8 %vs 58%respectively). Significant reduction on the BAI for the CBT group. |
| Cooper et al. (2017) | 32 | 25 | 32.03(8.98) | 93.8% | 306.63(193.15)days | Psycho-education | 32 | 25 | 30.09(7.61) | 91.2% | 290.71(161.08) days | Integrated CR and CBT reduced functional cognitive symptoms compared to education only. No statistical analysis for anxiety measure. |
| Hsieh et al.(2012) | 10 | 10 | 36.4 (14.1) | 70% | 50.4(89.7) months | Treatment as usual (TAU) | 8 | 8 | 35.6(9.8) | 87.5% | 23.0(18.5) months | Significant reduction in HADS and DASS scores for the CBT groups compared to TAU. |
| Nguyen et al. (2017) | 13 | 11 | 45.53 (13.87) | 69.23% | 795.15(714.23) days | Treatment as usual (TAU) | 11 | 10 | 41.90 (12.95) | 63.64% | 2093.36(2192.62) days | Significant improvement in sleep quality and reduction in fatigue for CBT group compared to TAU. Secondary improvements were significant on the HADS. |
| Ponsford et al. (2015) | 26 | 26 | 39.88 (14.24) | 76.9% | 3.58 (5.87) years | Waitlist control (WLC) | 23 | 23 | 39.87 (12.88) | 73.9% | 2.61(3.68) years | Significantly greater reduction in HADS scores for the CBT groups compared to WLC. |
| Potter et al. (2016) | 26 | 25 | 40.1 (10.3) | 58% | 23% 6–12 m 23%12–24 m 54%>24 m | Waitlist control (WLC) | 20 | 20 | 43.1 (13.1) | 50%M | 35% 6–12 m 15%12–24 m 50%>24 m | Significant increase in quality of life and reduction on anxiety for the CBT group compared to WLC. |
| Silverberg et al.(2013) | 15 | 13 | 40.4(13.5) | 40% | 23.13(7.0) days | Treatment as usual (TAU) | 13 | 11 | 37.5(10.0) | 38% | 25.4(9.1) days | Significantly fewer participants in the CBT group experienced PCS symptoms. Reduction anxiety scores on the HADS (no statistical analysis). |
| Tiersky et al. (2005) | 14 | 11 | 47.55(11.78) | 54.5% | 5.01(5.46) years | Wait list | 15 | 9 | 46.00(9,35) | 32.3% | 22.2(2) years | Significant reduction on the SCL 90-R control (WLC) anxiety subscale for the CBT group compared to WLC. |
3.2Effect of CBT at reducing anxiety symptoms
A random-effects model allowed the meta-analysis to predict the overall SMC, based upon the distribution of true effect sizes (Viechtbauer, 2010). See Figure 2 for the forest plot illustrating the meta-analysis of the included 10 studies, for the anxiety outcome measure, following the completion of a CBT informed intervention. The pooled SMC was –0.26 (95%CI –0.41 to –0.11). This represents a small overall effect size of CBT in the reduction of anxiety symptoms following TBI.
Fig. 2
Forest Plot of the Effect size (ES) and 95%Confidence Intervals (CIs) in the 10 Included Studies.
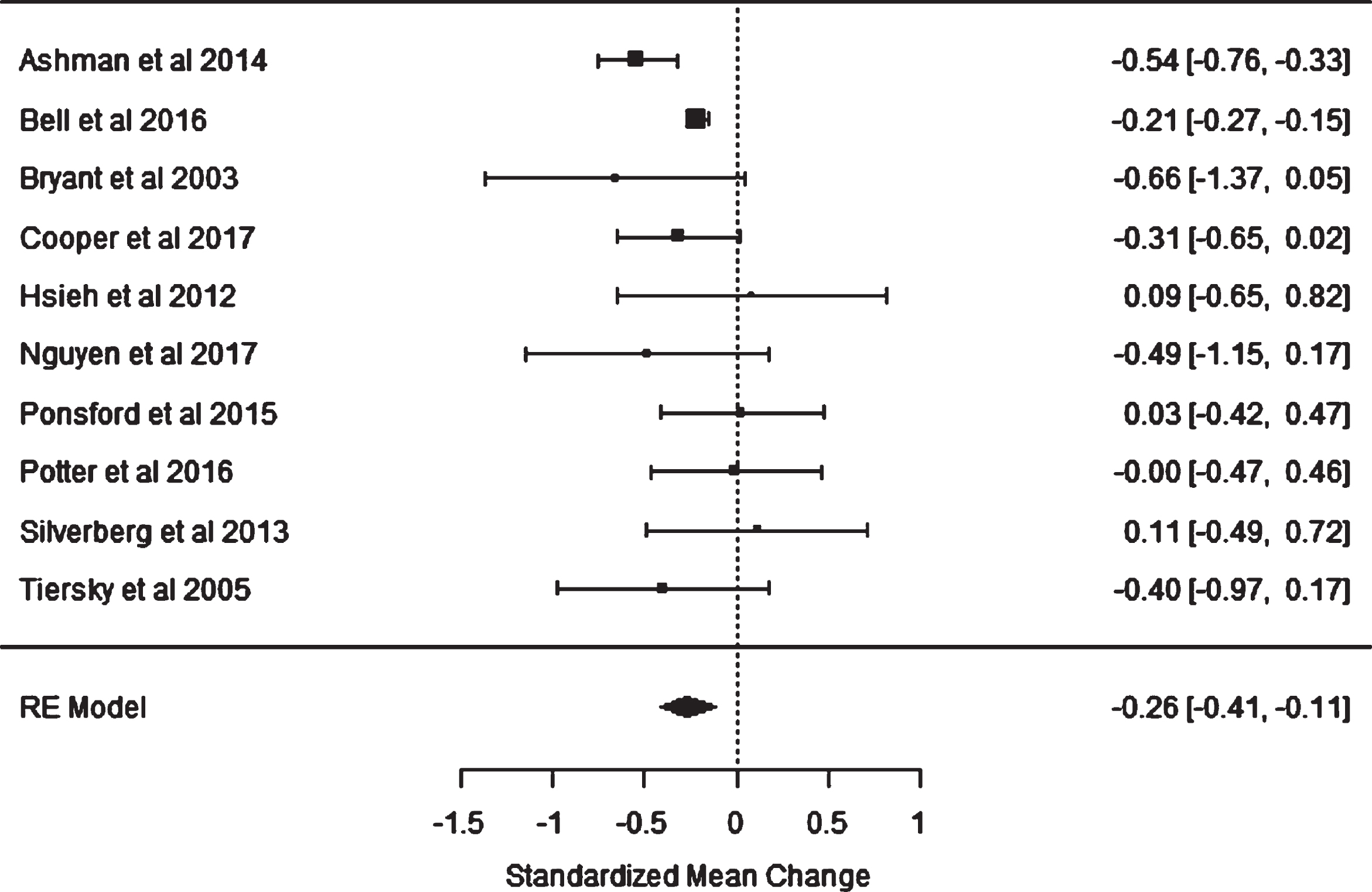
The 95%confidence intervals of the overall effect size do not cross the zero threshold, which indicates that the results are statistically significant; however, it could be argued that the margin is close. A Cochrane’s Q test of heterogeneity was completed and was found to be non-significant (p = .09), indicating that the combined estimate is a meaningful description of the included studies.
A further conservative analysis was conducted, excluding the studies which did not clearly identify using an ITT analysis (Potter et al., 2016; Tiersky et al., 2005). This resulted in a SMC of –0.27 (95%CI –0.45 to –0.10).
The forest plot demonstrated that the greatest effect size was found byBryant et al. (2003), which compared CBT to supportive counselling. This study had a very small sample size and large CIs, which cross the line of null effect, therefore indicating a lack of precision and a non-statistically significant result. Two of the studies reported statistically significant effect sizes; Ashman et al. (2014) and Bell et al. (2016). The CBT interventions utilised in these studies were delivered over the longest time periods (16 weeks and 24 weeks respectively). Bell et al. (2016) was the largest study in the meta-analysis which involved telephone interventions within a military sample. The 95%CIs of the remaining eight studies crossed the line of null effect, indicating that a null effect could have been a true effect. Many of the smaller studies had large CIs and were likely underpowered due to small samples.
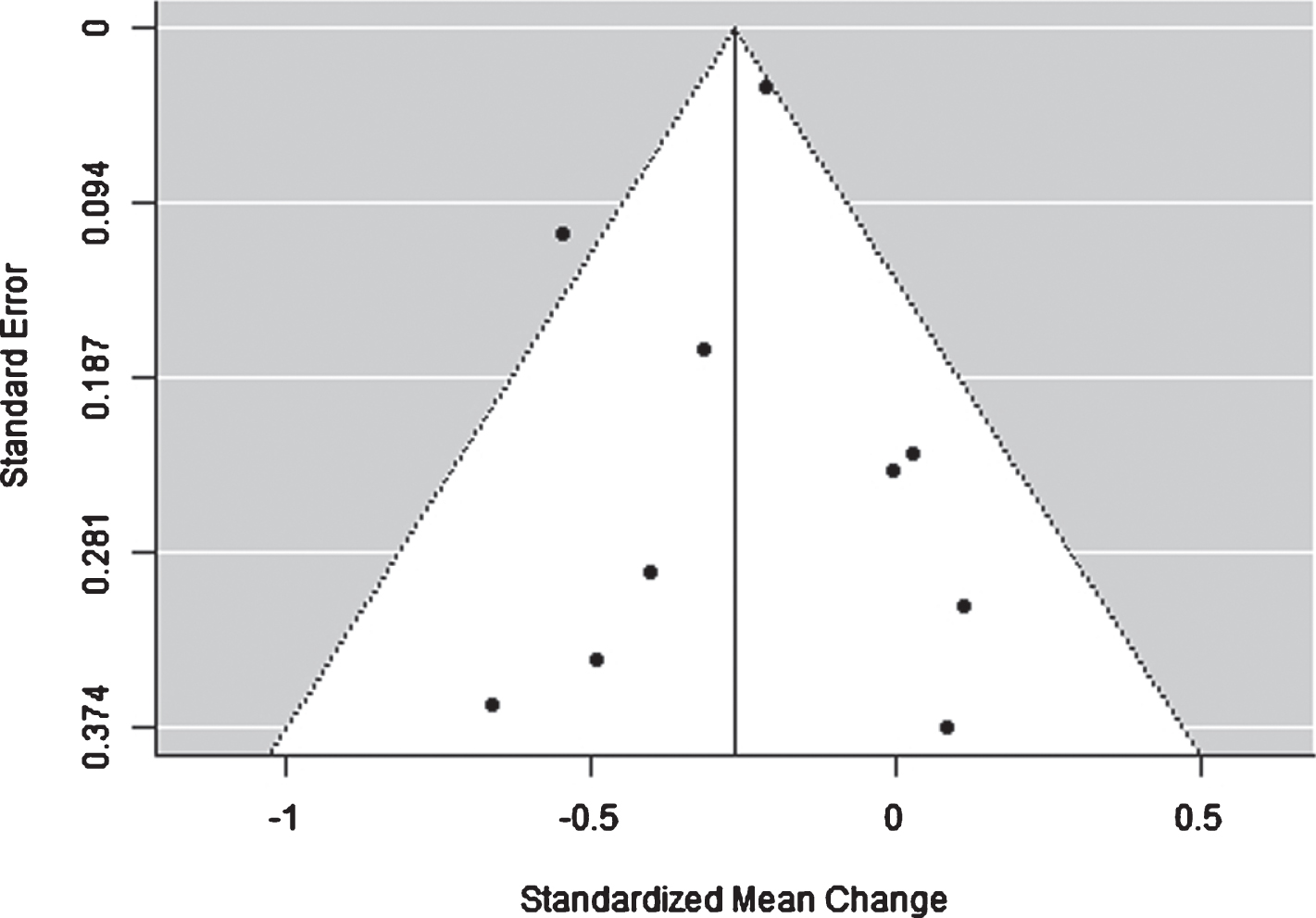
3.3Publication bias
To assess for publication bias, a funnel plot of the included studies was created (see Figure 3). An asymmetrical funnel plot would indicate the presence of publication bias. Visual inspection of the funnel plot revealed no obvious evidence of publication bias, given the relatively symmetrical pattern around the SMC. There was evidence of a wide distribution of effect sizes amongst the smaller studies, indicating that smaller studies with small or non-significant results have been published.
Fig. 3
Funnel Plot to Assess for Publication Bias.
4Discussion
The current meta-analysis synthesized the available controlled trials literature on the effectiveness of CBT for reducing anxiety symptoms following TBI, and found a small, but significant effect size (SMC = –0.26). This finding suggests that following TBI, CBT interventions result in a small reduction in anxiety symptoms in comparison to control conditions, indicating that CBT is the mechanism for change, not just contact with clinicians. The overall effect size found in this meta-analysis falls within the confidence intervals of each included study. In addition, the confidence intervals of all studies overlap, indicating homogeneity and increased reliability of the finding.
The findings from the current meta-analysis are supported by Waldron, Casserly and O’Sullivan’s (2013) meta-analysis, which was conducted within an ABI (not exclusively TBI) population. Waldron and colleagues reported effect sizes ranging from 0 to 0.42 when investigating the efficacy of CBT on reducing anxiety symptoms, with various focuses of the CBT intervention (e.g. social skills, coping, etc.). The average effect size was 0.17, which is similar to the small effect size reported in this meta-analysis. The overall effect size reported in this meta-analysis is smaller than the medium to large effect sizes that have been reported in non-TBI clinical populations. This could suggest that CBT is not as effective at reducing symptoms of anxiety within the TBI population; possibly due to the presence of cognitive impairment acting as a barrier to treatment effectiveness.
In comparison to pharmacological interventions, CBT has a negligible side effect profile (Schermuly-Haupt, Linden, & Rush, 2018), and was generally well tolerated across the studies, with 82%of participants who started CBT completing the intervention. The manualised nature of CBT meant that treatment fidelity was high, and it was feasible to administer widely across TBI populations. CBT is also considered to be a more cost-effective approach than pharmacological interventions alone, with costs of CBT offset by reduced access to healthcare (Myhr & Payne, 2006).
As with all meta-analyses, the overall effect size of the present meta-analysis appear to be driven by the larger studies. In this meta-analysis, studies by Ashman et al. (2014) and Bell et al. (2016) are the primary studies driving the effect size. Bell et al. (2016) was the largest study within this meta-analysis, with a sample of 356 military service personnel. Participants received 12 bi-weekly telephone calls, of either an education only intervention, or a CBT informed problem-solving therapy (PST). Post-treatment, the PST group significantly improved on the PCL-M compared to the control group (p = .04, treatment difference 2.89). Results however were not maintained at a 6 month follow up. The authors consider whether these effects were specific to the PST intervention, or whether improved problem solving resulted in a generalised feeling of improved wellbeing. Additionally, potential qualitative differences within military samples, and compared to civilians, need to be taken into consideration.
Similarly, Bryant et al. (2003) found that receiving five sessions of CBT within two weeks of injury, resulted in significantly fewer instances of PTSD than supportive counselling (SC; 8%vs 58%). Although this finding could be explained by rapid early spontaneous recovery, which occurs shortly after TBI (Nudo, 2013). Additionally, in comparison to the SC group, the CBT group reported a significant reduction in anxiety (p = .05); however, these effects did not persist at the six-month follow up. It would be important for future research to include robust follow up periods to determine the maintenance effect of CBT interventions.
Ponsford et al. (2016) reported a significant improvement in anxiety in their study. The current meta-analysis did not identify a significant effect. It must be noted however that for this meta-analysis, to maximise consistency, data was extracted immediately pre and post-intervention (at 3 and 12 weeks). The positive effect size reported by Ponsford et al. (2016) was found at 21 weeks, following a booster session of CBT; the effect of which was not considered within this meta-analysis.
Within the study by Ashman and colleagues (2016) a third of participants met the diagnostic criteria for an anxiety disorder at baseline, which reduced to 20%post-intervention; this difference was not found to be statistically significant. This meta-analysis only used the trait scale of the STAI and found a statistically significant difference between the CBT and SPT groups. This suggests that there was significant reduction on the trait scale of the STAI, but this did not translate into a significant reduction in diagnosable anxiety disorders.
The distinction between a statistically significant effect size and a clinically significant reduction in anxiety symptoms needs to also be considered also. It is therefore important to question what an effect size of –0.26 would look like in terms of reduction of anxiety symptoms. Four out of the five studies that administered the HADS, did not report post-intervention scores that were below the clinical threshold (Hsieh et al., 2012; Ponsford et al., 2016; Potter et al., 2016; Silverberg et al., 2013). The mean post-intervention score from Nguyen et al. (2017) was below the clinical threshold, however it was not above clinical threshold at pre intervention. This suggests that although reductions in HADS scores were identified, scores did not reduce to below clinical thresholds, and it is not known whether symptom reductions were clinically observable, or meaningful.
Whelan-Goodinson, Ponsford and Schönberger (2009) reported that within TBI populations, clinical thresholds of the HADS do not strongly correspond with clinical diagnoses of anxiety. The anxiety subscale had a sensitivity of 75%and a specificity of 69%. The authors recommend using a structured clinical interview, such as in the Diagnostic and Statistical Manual (DSM-5; American Psychiatric Association, 2013) to assess for anxiety post-TBI. Further research should therefore consider the validity of the anxiety measure utilised and use more comprehensive assessment measures.
It is worth noting that the current meta-analysis only looked at the reduction in anxiety symptoms using one anxiety outcome measure. Some of the included studies, where anxiety was a secondary outcome, did report significant changes in other areas. In the study by Silverberg et al. (2013) significantly fewer participants in the CBT group experienced symptoms of post-concussion syndrome (54%vs 91%). In the study by Nguyen at al. (2017) there was a significant improvement in sleep quality and reduction in fatigue for CBT group compared to TAU and Tiersky et al. (2005) reported reduced emotional distress for the CBT group. Hsieh et al. (2012) and Ponsford et al. (2016) both considered the effect of MI compared to NDC prior to the CBT intervention. The findings by Hsieh et al. (2012) demonstrated that MI and CBT resulted in a significantly greater reduction in anxiety than NDC and CBT, however Ponsford et al. (2016) did not find a significant difference.
4.1Limitations
There were several limitations to the current meta-analysis. Firstly, it is important to note that this review was not prospectively registered, which would have allowed for valuable peer feedback on the quality of the review protocol. It was not possible to control for the variation in the severity of TBI, the location of damage and the time since injury within the sample. There was also variation in the severity of anxiety symptoms of the sample included; with some studies only including participants with a diagnosed psychiatric disorder. However, the variation in TBI topography, and symptom profile, is reflective of the heterogeneous TBI population, and therefore difficult to control.
Additionally, due to the current lack of research into CBT interventions specifically targeting anxiety post-TBI, the current meta-analysis included a range of CBT interventions, which further increases the heterogeneity of the sample. In Waldron and colleagues’ (2013) meta-analysis, when their CBT intervention was specifically targeting anxiety, larger effect sizes were reported (average effect size of 1.04). The authors concluded that CBT is more effective when aimed at a specific difficulty, and these specific improvements do not necessarily generalise to have a significant therapeutic effect on anxiety. It could however be argued, that CBT addresses anxiety, regardless of the primary focus, for example by targeting catastrophizing cognitions, automatic negative thoughts, or acting upon safety behaviours. Despite predicted heterogeneity within the sample, tests of heterogeneity were not significant.
Due to the small number of studies within this meta-analysis that included a follow up, it was not possible to conduct further meaningful analysis to consider the maintenance effect of CBT. It is important that future research considers the long-term effect of such interventions and whether improvements are maintained.
As with all meta-analyses, the risk of publication bias needs be taken into consideration. There may be a tendency to publish statistically significant findings and not non-significant results (Zakzanis, 2001); which was coined by Rosenthal (1979) as the “file-drawer problem”. Visual inspection of the forest plot produced in this meta-analysis suggested that there were a number of small studies reporting small and non-significant effect sizes; reducing the possibility that publication bias was present. It is possible that within TBI populations there is less chance of publication bias, due to general difficulties recruiting within this population.
Additionally, the interpretation of individual effect sizes must be considered carefully, as multiple factors can influence a given effect size; particularly different types of control conditions. For example, studies that compared CBT to a wait list control condition may be more likely to report a statistically significant effect size, compared to studies that used an alternative or comparable intervention. Within the current meta-analysis however, the studies with a non-significant effect size utilised a variety of control groups, including both TAU/WLC and other forms of active intervention. Despite these limitations, the current meta-analysis has hopefully contributed to increasing our understanding of the role of CBT in the rehabilitation of patients who presents with anxiety after TBI.
5Conclusion
Anxiety is highly prevalent, debilitating and negatively impacts rehabilitation and recovery following TBI. This is the first meta-analysis to consider the specific question pertaining to the effect of using CBT informed interventions to reduce anxiety in the TBI population, by using evidence from RCTs. The results of this meta-analysis indicate that CBT results in a small, but potentially significant reduction in anxiety symptoms for individuals who have sustained a TBI.
This meta-analysis provides tentative support for the use of CBT to treat anxiety symptoms following TBI, also considering the easy to administer nature and negligible side effect profile of CBT, compared to stand-alone pharmacological interventions. It is however important that the clinical significance in addition to the statistical significance of the intervention is considered.
Future research with CBT specifically targeting anxiety in the TBI population needs to be conducted, in order to further determine its efficacy and allow increased homogeneity across studies. Additionally, in light of recent developments into other psychological interventions to treat anxiety post-TBI, including MBCT and ACT, further well-controlled research should continue investigating these alternatives to CBT, to determine the most efficacious and feasible psychological intervention in this population.
Conflict of interest
This research paper was submitted in partial fulfilment of the requirements for a Doctorate Degree in Clinical Psychology (AL). None of the authors have any personal or financial affiliations with any individuals or organisations that could influence the outcome of this meta-analysis. Accordingly, the authors report no conflict of interest.
Acknowledgments
The authors are grateful for the encouragement and support they received from colleagues at the North Wales Brain Injury Service, and Bangor University during the project
References
1 | American Psychiatric Association. ((2013) ). Diagnostic and Statistical Manual of Mental Disorders DSM-5. (5th Ed).Washington DC, American Psychiatric Association. |
2 | Ashman, T , Cantor, J. B , Tsaousides, T , Spielman, L , Gordon, W. ((2014) ). Comparison of cognitive behavioral therapy and supportive psychotherapy for the treatment of depression following traumatic brain injury: a randomised controlled trial. Journal of Head Trauma Rehabilitation, 29: (6), 467–478. |
3 | Bandelow, B , Reitt, M , Röver, C , Michaelis, S , Görlich, Y , Wedekind, D. ((2015) ). Efficacy of treatments for anxiety disorders. International Clinical Psychopharmacology, 30: (4), 183–192. |
4 | Bedard, M , Felteau, M , Marshall, S , Dubois, S , Gibbons, C , Klein, R , Weaver, B. ((2012) ). Mindfulness-based cognitive therapy: benefits in reducing depression following a traumatic brain injury. Advances in Mind-body Medicine, 26: (1), 14–20. |
5 | Beck, J.G. ((1995) ). Cognitive Behavioural Therapy: Basics and Beyond. Guildford Press, New York: USA. |
6 | Beck, A. ((1998) ). the Past and Future of Cognitive Behavioural Therapy. The Journal of Psychotherapy Practice and Research, 6: , 276–284. |
7 | Beck, A. T , Epstein, N , Brown, G , Steer, R. A. ((1988) ). An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology, 56: (6), 893–897. |
8 | Bell, K. R , Fann J. R , Brockway, J. A , Cole, W. R , Bush, N. E , Dikmen, S , . . .Temkin, N. ((2016) ). Telephone problem solving for service members with mild traumatic brain injury: a randomized, clinical trial. Journal of Neurotrauma, 34: (2), 313–321. |
9 | Borenstein, M , Hedges, L. V , Higgins, J. P , Rothstein, H. R. ((2010) ). A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods, 1: (2), 97–111. |
10 | Bryant, R. A , Moulds, M , Guthrie, R , Nixon, R. D. ((2003) ). Treating acute stress disorder following mild traumatic brain injury. American Journal of Psychiatry, 160: (3), 585–587. |
11 | Bryant, R. A , Creamer, M , O’Donnell, M , Silove, D , Clark, C. R , McFarlane, A. C. ((2009) ). Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. J Int Neuropsychol Soc, 15: (6), 862–867. |
12 | Bryant, R. A , O’Donnell, M. L , Creamer, M , McFarlane, A. C , Clark, C. R. , Silove, D. ((2010) ). The psychiatric sequelae of traumatic injury. American Journal of Psychiatry, 167: , 312–320. |
13 | Byrne, C , Coetzer, R , Addy, K. ((2017) ). Investigating the discrepancy between subjective and objective cognitive impairment following acquired brain injury: The role of psychological affect. NeuroRehabilitation, 41: (2), 501–512. |
14 | Carpenter, J. K , Andrews, L. A , Witcraft, S. M , Powers, M. B , Smits, J. A. J , Hofmann, S. G. ((2018) ). Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depression and Anxiety, 35: (6), 502–514. |
15 | Coetzer, R. ((2010) ). Anxiety and mood disorders following traumatic brain injury: clinical assessment and psychotherapy. Karnac Books, London, UK. |
16 | Coetzer, B. R. ((2004) ). Obsessive-Compulsive Disorder following Brain Injury: A Review. The International Journal of Psychiatry in Medicine, 34: (4), 363–377. |
17 | Cohen, J. ((1988) ), Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum. |
18 | Cooper, D. B , Bowles, A. O , Kennedy, J. E , Curtiss, G , French, L. M , Tate, D. F , Vanderploeg, R. D. ((2017) ). Cognitive rehabilitation for military service members with mild traumatic brain injury: A randomized clinical trial. Journal of Head Trauma Rehabilitation, 32: (3), E1–E15. |
19 | Deacon, B. J , Abramowitz, J. S. ((2004) ). Cognitive and behavioral treatments for anxiety disorders: A review of meta-analytic findings. Journal of Clinical Psychology, 60: (4), 429–441. |
20 | Deb, S , Lyons, I , Koutzoukis, C , Ali, I , McCarthy, G. ((1999) ). Rate of psychiatric illness 1 year after traumatic brain injury. American Journal of Psychiatry, 156: (3), 374–378. |
21 | Derogatis, L. ((1994) ). Administration, scoring and procedures manual for the SCL–90–R; symptom checklist-90-R. Minneapolis, Minn: National Computer Systems. |
22 | Doering, B , Exner, C. ((2011) ). Combining neuropsychological and cognitive-behavioral approaches for treating psychological sequelae of acquired brain injury. Current Opinion in Psychiatry, 24: , 156–161. |
23 | Fann, J. R , Katon, W. J , Uomoto, J. M , Esselman, P. C. ((1995) ). Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. American Journal of Psychiatry, 152: (10), 1492–1499. |
24 | Fleminger, S , Ponsford, J. ((2005) ). Long term outcome after traumatic brain injury. BMJ (Clinical Research ed.), 331: (7530), 1419–1420. |
25 | Gainotti, G. ((1993) ). Emotional and psychosocial problems after brain injury. Neuropsychological Rehabilitation, 3: , 259–277. |
26 | Gallagher, M , McLeod, H. J , McMillan, T. M. ((2016) ). A systematic review of recommended modifications of CBT for people with cognitive impairments following brain injury. Neuropsychological Rehabilitation, (1), 1–21. |
27 | Gordon, W. A , Sliwinski, M , Echo, J , McLoughlin, M , Sheerer, M. S , Meili, T. E. ((1998) ). The benefit of exercise in individuals with traumatic brain injury: a retrospective study. Journal of Head Trauma Rehabilitation, 13: (4), 58–67. |
28 | Gould, K. R , Ponsford, J. L , Johnston, L. , Schonberger, M. ((2011) ). The nature, frequency and course of psychiatric disorders in the first year after traumatic brain injury: a prospective study. Psychological Medicine, 41: , 2099–2109. |
29 | Headway. (2015). Acquired Brain Injury: The numbers behind the hidden disability. Retrieved from https://www.headway.org.uk/media//acquired-brain-injury-the-numbers-behind-the-hidden-disability.pdf. |
30 | Helffenstein, D. A , Wechsler, F. S. ((1982) ). The use of interpersonal process recall (IPR) in the remediation of interpersonal and communication skill deficits in the newly brain-injured. Clinical Neuropsychology, 4: (3), 139–142. |
31 | Hibbard, M. R , Uysal, S , Kepler, K , Bogdany, J , Silver, J. ((1998) ). Axis I Psychopathology in Individuals with Traumatic Brain Injury. Journal of Head Trauma Rehabilitation, 13: (4), 24–39. |
32 | Hiott, D. W , Labbate, L. ((2002) ). Anxiety disorders associated with traumatic brain injuries. Neurorehabilitation, 17: (4), 345–355. |
33 | Hodgson, J , McDonald, S , Tate, R , Gertler, P. ((2005) ). A randomised controlled trial of a cognitive-behavioural therapy programme for managing social anxiety after acquired brain injury. Brain Impairment, 6: (3), 169–180. |
34 | Hoffman, S. G , Asnaani, A , Vonk. I. J , Sawyer, A. T , Fang, A. ((2012) ). The efficacy of cognitive behavioural therapy: a review of meta-analyses. Cognitive Therapy and Research, 36: , 427–440. |
35 | Hsieh, M , Ponsford, J , Wong, D , Schönberger, M , Taffe, J , McKay, A. ((2012) ). Motivational interviewing and cognitive behaviour therapy for anxiety following traumatic brain injury: a pilot randomised controlled trial. Neuropsychological Rehabilitation, 22: (4), 585–608. |
36 | Hyder, A. A , Wunderlich, C. A , Puvanachandra, P , Gururaj, G , Kobusingye, O. C. ((2007) ). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation, 22: , 341–353. |
37 | Kangas, M , McDonald, S. ((2011) ). Is it time to act? The potential of acceptance and commitment therapy for psychological problems following acquired brain injury. Neuropsychological Rehabilitation, 21: (2), 250–276. |
38 | Kendall, E , Terry, D.J. ((1996) ). Psychosocial adjustment following closed head injury. Neuropsychological Rehabilitation, 6: , 101–132. |
39 | Koponen, S , Taiminen, T , Portin, R , Himanen, L , Isoniemi, H , Heinonen, H , . . . Tenovou, O. ((2002) ). Axis I and II psychiatric disorders after traumatic brain injury: A 30-year follow-up study. The American Journal of Psychiatry, 159: (8), 1315–1321. |
40 | Lishman, W. A. ((1973) ). The psychiatric sequelae of head injury: a review. Psychological Medicine, 3: , 304–318. |
41 | McAllister, T. W. ((2008) ). Neurobehavioral sequalae of traumatic brain injury: evaluation and management. World Psychiatry, 7: , 3–10. |
42 | Moher, D , Liberati, A , Tetzlaff, J , Altman, D. G. ((2009) ). Preferred reporting items for systematic reviews and metaanalyses: The PRISMA statement. PLoS Med, 6: (7): e1000097. |
43 | Murrough, J. W , Yaqubi, S , Sayed, S , Charney, D. S. ((2015) ). Emerging drugs for the treatment of anxiety. Expert Opinion on Emerging Drugs, 20: (3), 393–406. |
44 | Myhr, G , Payne, K. ((2006) ). Cost-effectiveness of cognitive-behavioural therapy for mental disorders: implications for public health care funding policy in Canada. The Canadian Journal of Psychiatry, 51: (10), 662–670. |
45 | National Institute for Health and Care Excellence (NICE) (2011). NICE Guidelines (CG123). Common mental health problems: identification and pathways to care. https://www.nice.org.uk/guidance/cg/ |
46 | Nguyen, S , McKay, A , Wong, D , Rajaratnam, S. M , Spitz, S , Williams, G , . . .Ponsford, J. L. ((2017) ). Cognitive behavior therapy to treat sleep disturbance and fatigue following traumatic brain injury: a pilot randomised controlled trial. Archives of Physical Medicine and Rehabilitation, 98: (8), 1508–1517. |
47 | Norton, P. J , Price, E. C. ((2007) ). Meta-analytic review of adult cognitive-behavioral treatment outcomes across the anxiety disorders. The Journal of Nervous and Mental Disease, 195: (6), 521–531. |
48 | Nudo, R. J. ((2013) ). Recovery after brain injury: mechanisms and principles. Frontiers in Human Neuroscience, 7: (887), 1–14. |
49 | Perna, R. B , Bordini, E. J , Newman, S. A. ((2001) ). Pharmacological treatment considerations in brain injury. Journal of Cognitive Rehabilitation, 19: (1), 4–7. |
50 | Ponsford, J , Lee, N. K , Wong, D , McKay, A , Haines, K , Always, Y , . . . O’Donnell, M. L. ((2016) ). Efficacy of motivational interviewing and cognitive behavioural therapy for anxiety and depression symptoms following traumatic brain injury. Psychological Medicine, 46: , 1079–1090. |
51 | Potter, S. D , Brown, R. G , Fleminger, S. ((2016) ). Randomised, waiting list controlled trial of cognitive-behavioural therapy for persistent postconcussional symptoms after predominantly mild-moderate traumatic brain injury. Journal of Neurology, Neurosurgery and Psychiatry, 87: , 1075–1083. |
52 | R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ |
53 | Rao, V , Lykestos, C. G. ((2000) ). Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics, 41: , 95–103. |
54 | Rao, V , Lykestos, C. G. ((2002) ). Psychiatric aspects of traumatic brain injury. Psychiatric Clinics of North America, 25: (1), 43–69. |
55 | Reichow, B. ((2011) ). Development, procedures, and application of the evaluative method for determining evidence-based practices in autism. Evidence-Based Practices and Treatments for Children with Autism (pp. 25-39) Springer. |
56 | Reichow, B , Volkmar, F. R , Cicchetti, D. V. ((2008) ). Development of the evaluative method for evaluating and determining evidence-based practices in autism. Journal of Autism and Developmental Disorders, 38: (7), 1311–1319. |
57 | Rosenthal, R. ((1979) ). The file drawer problem and tolerance for null results. Psychological Bulletin, 86: (3), 638–641. |
58 | Roozenbeek, B , Maas, A. L , Menon, D. K. ((2013) ). Changing patterns in the epidemiology of traumatic brain injury. Nature Reviews Neurology, 9: (4), 231–236. |
59 | Rzezak, P , Caxa, L , Santolia, P , Antunes, H. K , Suriano, I , Tufik, S , de Mello, M. T. ((2015) ). Affective responses after different intensities of exercise in patients with traumatic brain injury. Frontiers in Psychology, 6: , 839. |
60 | Rydon-Grange, M. R. , Coetzer, R. ((2015) ). Editorial. What do we know about Obsessive- |
61 | Compulsive Disorder following Traumatic Brain Injury? CNS Spectrums, (iFirst, 25 August, pp. 1-3). |
62 | Schermuly-Haupt, M. L , Linden, M , Rush, A. J. ((2018) ). Unwanted events and side effects in cognitive behavior therapy. Cognitive Therapy and Research, 42: (3), 219–229. |
63 | Schwarzbold, M , Diaz, A , Martins, E. T , Rufino, A , Amante, L. N , Thais, M. E , Quevedo, J , Hohl, A , Linhares, M. N , Walz, R. ((2008) ). Psychiatric disorders and traumatic brain injury. Neuropsychiatric Disease and Treatment, 4: (4), 797–816. |
64 | Silverberg, N , Hallam, B. J , Rose, A. B , Underwood, H , Whitfield, K , Thornton, A. E , Whittal, M. L. ((2013) ). Cognitive-Behavioral prevention of postconcussion syndrome in at-risk patients: a pilot randomised controlled trial. Journal of Head Trauma Rehabilitation, 28: (4), 313–322. |
65 | Soares, I , Carneiro, A. V. ((2002) ). Intention-to-treat analysis in clinical trials: principles and practical importance. Revista Portuguesa de Cardiologia, 21: (10), 1191–1198. |
66 | Soo, C , Tate R. L. ((2007) ). Psychological treatment for anxiety in people with traumatic brain injury. Cochrane Database of Systematic Reviews, 2012: (3), DOI: 10.1002/14651858.CD005239.pub2 |
67 | Spielberger, C. D , Gorsuch, R. L , Lushene, R, , Vagg, P. R , Jacobs, G. A. ((1983) ). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. |
68 | Stocchetti, N. ((2014) ). Traumatic brain injury: problems and opportunities. The Lancet Neurology, 13: (1), 14–16. |
69 | Tateno, A , Murata, Y , Robinson, R. G. ((2002) ). Comparison of cognitive impairment associated with major depression following stroke versus traumatic brain injury. Psychosomatics, 43: (4), 295–301. |
70 | Tiersky, L. A , Anselmi, V , Johnston, M. V , Kurtyka, J , Roosen, E , Schwarts, T , DeLuca, J. ((2005) ). A trial of neuropsychologic rehabilitation in mild-spectrum traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 86: , 1565–74. |
71 | Viechtbauer, W. ((2010) ). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36: (3), 1–48. |
72 | Waldron, B , Casserly, L. M , O’Sullivan, C. ((2013) ). Cognitive behavioural therapy for depression and anxiety in adults with acquired brain injury. What works for whom? Neuropsychological Rehabilitation, 23: (1), 64–101. |
73 | Warden, D. L , Gordon, B , McAllister, T. W , Silver, J. M , Barth, J. T , Bruns, J , . . . Zitnay, G. ((2006) ). Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. Journal of Neurotrauma, 23: (10), 1468–1501. |
74 | Weathers, F , Huska, J , Keane, T. ((1991) ). The PTSD checklist military version (PCL-M). National Centre for PTSD: Boston, MA. |
75 | Weinstein, A. A , Chin, L. M , Collins, J , Goel, D , Keyser, R. E , Chan, L. ((2017) ). Effect of aerobic exercise training on mood in people with traumatic brain injury. Journal of Head Trauma Rehabilitation, 32: (3), E49–E56. |
76 | Werner, C , Engelhard, K. ((2007) ). Pathophysiology of traumatic brain injury. British Journal of Anaesthesia, 99: (1), 4–9. |
77 | Whelan-Goodinson, R , Ponsford, J , Schönberger, M. ((2009) ). Validity of the Hospital Anxiety and Depression Scale to assess depression and anxiety following traumatic brain injury as compared with the Structured Clinical Interview for DSM-IV. Journal of Affective Disorders, 114: (1-3), 94–102. |
78 | Whiting, D. L , Deane, F. P , Simpson, G. K , Ciarrochi, J , McLeod, H. J. ((2017) ). Acceptance and Commitment Therapy delivered in a dyad after a severe traumatic brain injury: A feasibility study. Clinical Psychologist, 22: (2), 230–240. |
79 | Whitnall, L. ((2006) ). Disability in young people and adults after head injury: 5-7 year follow up of a prospective cohort study. Journal of Neurology, Neurosurgery & Psychiatry, 77: (5), 640–645. |
80 | Williams, W. H , Evans, J. J , Fleminger, S. ((2003) ). Neurorehabilitation and cognitive-behaviour therapy of anxiety disorders after brain injury: an overview and a case illustration of obsessive-compulsive disorder. Neuropsychological Rehabilitation, 13: (1-2), 133–148. |
81 | Zakzanis, K. K. ((2001) ). Statistics to tell the truth, the whole truth, and nothing but the truth Formulae, illustrative numerical examples, and heuristic interpretation of effect size analyses for neuropsychological researchers. Archives of Clinical Neuropsychology, 16: (7), 653–667. |
82 | Zigmond, A. S , Snaith, R. P. ((1983) ). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67: (6), 361–370. |