The effect of intensified nonverbal facilitation of swallowing on dysphagia after severe acquired brain injury: A randomised controlled pilot study
Abstract
BACKGROUND:
There is little high-level evidence for the effect of the nonverbal facilitation of swallowing on swallowing ability in the subacute stage of rehabilitation following severe acquired brain injury (ABI).
OBJECTIVE:
To pilot test a randomised controlled trial to determine the effect of an intensification of the nonverbal facilitation of swallowing on dysphagia.
METHODS:
Ten patients with severe ABI and dysphagia were randomised into two groups at a highly specialised neurorehabilitation clinic.
The intervention group received an intensification of the nonverbal facilitation of swallowing and the control group received basic care of the face and mouth in addition to treatment as usual for two sessions of 20 minutes per day for three weeks.
Outcomes were Functional Oral Intake Scale (FOIS), Penetration Aspiration Scale (PAS), and electrophysiological swallowing specific parameters (EMBI).
RESULTS:
The intensified intervention was feasible. PAS and FOIS scores improved in both groups, with no differences between groups. The swallowing specific parameters reflected clinically observed changes in swallowing.
CONCLUSIONS:
PAS and FOIS are feasible instruments to measure dysphagia. It is possible and valid to measure swallowing frequency and kinematics using electromyography and bioimpedance. The definitive study should have widened inclusion criteria and optimise intervention timing to maintain patient arousal.
1Introduction
Sixty-nine million (95% CI 64-74 million) individuals worldwide are estimated to sustain a traumatic brain injury (TBI) each year (Dewan et al., 2018).
The incidence of oropharyngeal dysphagia is reported to be as high as 93% in patients with severe TBI (Hansen, Larsen, & Engberg, 2008) at admission to sub-acute rehabilitation and 37–78% in patients after stroke (Martino et al., 2005). Dysphagia is a threat to eating and drinking safely in several populations putting patients at high risk of pneumonia (Martino et al., 2005; Morgan & Mackay, 1999; Woratyla, Morgan, Mackay, Bernstein, & Barba, 1995) and malnutrition (Foley, Martin, Salter, & Teasell, 2009; Mackay, Morgan, & Bernstein, 1999).
The rehabilitation of dysphagia frequently incorporates rehabilitative exercises, aiming to improve swallowing physiology in the long term (Langmore & Pisegna, 2015). There is limited evidence that interventions such as expiratory muscle strength training, the Mendelsohn Manoeuvre or Shaker exercise improve the symptoms of dysphagia (McCullough & Kim, 2013; Shaker et al., 2002; Troche et al., 2010). Furthermore, there is a paucity of evidence relating to the dosage and intensity of therapy required (Cabib et al., 2016; Langmore & Pisegna, 2015; Robbins et al., 2008). Since patients with severe acquired brain injury (ABI) suffer from serious perceptive, sensomotory and cognitive disturbances, they are challenged to follow the therapist’s verbal guidance in executing these exercises (Cherney & Halper, 1996; Morgan & Mackay, 1999; Neumann, 1993). For patients with severe brain injury and other patients with neurological swallowing disorders, Coombes developed an interprofessional approach, the Facial Oral Tract Therapy® (F.O.T.T.), for assessing and treating problems with safe swallowing, eating, oral hygiene, breathing, voice and articulation. This concept is a complex rehabilitation intervention and uses structured tactile input and nonverbal facilitation techniques to allow for effective function in meaningful daily life activities (Hansen & Jakobsen, 2010). F.O.T.T. aims to improve function that is as normal as possible with the goal of maximum participation. For description of the methods and techniques in F.O.T.T., see Appendix.
There is, however, a lack of evidence about the active ingredients in F.O.T.T. (Hansen & Jakobsen, 2010). A pilot study investigated the effect of several interventions included in the F.O.T.T. concept on swallowing rate, alertness and swallowing ability in 10 patients with tracheostomy (Seidl, Nusser-Müller-Busch, Hollweg, Westhofen, & Ernst, 2007). Several interventions were used including positioning, oral stimulation, facilitation of swallowing, deflating and capping the tracheostomy tube. There was a statistically significant improvement in the outcome measures after an intervention period of three weeks. However, the sample was small, and the lack of a control group makes the results inconclusive.
From clinical experience, patients with severe ABI in the sub-acute stage, often show a pattern of “pumping-like” jaw movements before swallowing. The mandible moves rhythmically up and down in biting-like movements, the tongue moves anterio-posteriorly and sometimes the lips are open. It is described from patients with other neurological diagnoses, that when swallowing is preceded by “pumping-like” jaw movements, that laryngeal elevation is delayed, incomplete, or missing (Argolo, Sampaio, Pinho, Melo, & Nobrega, 2015; Daniels, 2000; Robbins, Levine, Maser, Rosenbek, & Kempster, 1993; Saito, Hayashi, Nakazawa, & Ota, 2016; Sonies, Parent, Morrish, & Baum, 1988; Veis & Logemann, 1985). This is contrary to healthy individuals, where the mandible remains stable during the pharyngeal stage of swallowing, allowing transport movements of the tongue and elevation and anterior movement of the hyoid bone and the larynx (Dodds, 1989; Palmer, Rudin, Lara, & Crompton, 1992; Shaw & Martino, 2013). Furthermore, a well-coordinated oral and pharyngeal stage contributes to a functional coordination between breathing and swallowing, which is important for protection of the airway (Bautista, Sun & Pilowsky, 2014).
To address the lack of evidence for the effect of F.O.T.T. on swallowing ability and pumping like jaw movements, we decided to perform a definitive trial. In order to design this trial, we needed to investigate the feasibility of the intervention, recruitment rate, outcome measures and trial size. The primary objectives of this pilot study were therefore: 1) to estimate the recruitment rate based on the inclusion criteria and recruitment method, 2) to determine the feasibility of intensifying existing levels of F.O.T.T. in a clinical setting, 3) to determine the feasibility of administering the outcome measures, 4) to obtain preliminary data of effect size to inform estimates of sample size for a definitive trial.
2Materials and methods
2.1Trial design
The study design is a pilot randomised controlled study.
2.2Participants
We included patients between 18 and 65 years old admitted to our clinic with severe ABI (non-sedated Glasgow Coma Scale (GCS) <9 (Teasdale & Jennett, 1974) within the first 24 hours of injury), with pumping jaw movements before swallowing saliva and clinical signs of dysphagia comprising wet voice, coughing after swallowing, low swallowing frequency, salivation and gurgling breath sound. Patients had to score ≥3 on the Penetration-Aspiration Scale (PAS) (Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996) assessed with Fiberoptic Endoscopic Evaluation of Swallowing (FEES) Langmore, 2017; Langmore, Schatz, & Olson, 1991) with at least one consistency or saliva. Exclusion criteria were formerly acquired or congenital brain damage, psychiatric diagnosis, a history of treatment for head- and neck cancer, the need for a tracheostomy tube or agitated behaviour. Since the patients were unable to give informed written consent due to their injury, informed written consent was obtained by proxy from close relatives and their GeneralPractitioner.
The study was conducted in one of two highly-specialised departments for subacute neurorehabilitation for patients with severe ABI in Denmark. The department admits patients from the Eastern part of Denmark directly from the Intensive Care Unit as soon as they ventilate spontaneously. Patients with clinical signs of dysphagia were identified by the main investigator (DJ). The patients underwent an initial FEES examination. The main investigator performed the endoscopy whilst an occupational therapist or physician gave blue-coloured food consistencies to the patient. Swallowing of all the given consistencies was video recorded: saliva, six drops of fridge-cold water, apple sauce, moderately thickened and thin liquid; bread (pieces measuring 2×2 cm) with butter. Each consistency was given three times in the order above. No facilitation of swallowing was used during the FEES procedure. Patients who scored 3 or worse on the PAS with at least one consistency were included. If patients scored 7 or 8 on the PAS, the examination was terminated to prevent further aspiration.
2.3Interventions
All participants were scheduled to receive 30 treatments over a period of three weeks (two treatments daily) in addition to their daily rehabilitation program, which included F.O.T.T. Each treatment consisted of a 10 minute rest period followed by a 20 minute intervention and a further 10 minute rest. During rests, patients were positioned in a neutral side lying position (Pickenbrock, Ludwig, Zapf, & Dressler, 2015).
The experimental treatment in the intervention group used the F.O.T.T. concept (Hansen & Jakobsen, 2010) (Appendix) with patients positioned in side lying, half sitting or sitting, depending on which position optimised the patient’s ability toswallow.
In the control group, the rest and treatment periods were the same, but the treatment comprised stimulating activities in the facial oral tract similar to those of the intervention group but without facilitation of swallowing or verbal request to swallow.
2.4Outcomes
We recorded demographics for the patients comprising age, gender, diagnosis, days from injury to inclusion, GCS and Early Functional Abilities Score (EFA) (Hankemeier & Rollnik, 2015; Heck G Schmidt T. Neurol Rehabil. 2000;6 : 125–33., 2000; Stubbs, Pallesen, Pedersen, & Nielsen, 2014). The EFA Scale represents abilities within vegetative, oro-facial, sensorimotor and cognitive functions (Stubbs et al., 2014).
Functional Oral Intake Scale (FOIS) (Crary, Mann, & Groher, 2005) scores were collected at baseline and the end of the intervention by the relevant staff who treated the patient daily. The FOIS is an ordinal scale that reflects the functional oral intake of patients with dysphagia. FOIS has been validated in 302 patients after stroke and found to have an adequate interrater reliability, validity and sensitivity to change over time (Crary et al., 2005).
Severity of dysphagia was evaluated by FEES-examination using the PAS since this is a standard tool in the field of dysphagia rehabilitation (Rosenbek et al., 1996) FEES is considered to be suitable for patients with severe neurological diseases (Langmore, 2017) The PAS has an acceptable to excellent intra- and interrater reliability (Butler, Markley, Sanders, & Stuart, 2015; Colodny, 2002). The same examination protocol used at inclusion was repeated at the end of the intervention.
Swallowing frequency and swallowing specific parameters comprising maximum laryngeal elevation, speed of laryngeal elevation and the frequency of pumping jaw movements were measured using the Electromyography-Bioimpedance Measurement System (EMBI) (Schultheiss, Schauer, Nahrstaedt, & Seidl, 2013, 2014). Bioimpedance is reduced with increase in larynx height and can therefore be used as an expression of the relative speed of laryngeal elevation (in ohm/s) and the height of laryngeal elevation (in ohms). Measurements were made using AMBU Blue Sensor ECK surface electrodes, Ref. N-00-S/25 positioned bilaterally on the processus mastoideus and the submental/suprahyoidal muscles. A reference electrode was positioned on the cheekbone (Schultheiss et al., 2014). Data was collected during both the rest and intervention periods of the treatment session. The occupational therapist used a hand switch device to record all observed spontaneous or facilitated swallows. This was necessary to validate that the measured changes in EMG and bioimpedance related to swallowing and not to other movements such as head-turning.
A record was kept of the number of interventions administered over the three-week intervention period to each patient and barriers to intensification of the intervention where the intervention plan could not be achieved. The inclusion date for the patients was also recorded to give an indication of the potential patient flow in a definitive trial.
2.5Sample size
The sample size for the study was determined by the rate of patient inclusion. The study had resources to include patients in an 18-month period.
2.6Randomisation
The participants were randomised to an intervention or a control group. Twenty sealed and numbered envelopes were prepared from a randomisation performed using MATLAB 2012b (Mathworks, Natick, USA) code by a third party. Upon patient inclusion, the principal investigator responsible for including the patients opened the envelope with the randomisation code and was subsequently no longer blinded to intervention groups. The randomisation was generated to produce a distribution of 1 : 1 between groups.
It was agreed that the intervention should be stopped if patients were unable to receive treatment for a period greater than 72 hours, for example due to infection, or if they could not comply with the protocol, for example due to restlessness or agitated behaviour.
2.7Blinding
The researchers responsible for analysis of the swallowing specific parameters and the specialist in otorhinolaryngology (ROS) scoring the PAS were blinded to group allocation together with the patient’s primary therapists, who scored EFA and FOIS. The patients and their relatives, the main investigator and the occupational therapists conducting the intervention were not blinded, due to the nature of the interventions.
2.8Statistical methods
Data concerning recruitment rate are presented as simple summary statistics. Feasibility of the intervention is presented as number of dropouts and number of treatments given.
For analysis of the scores from the PAS, we used the lowest scores from each consistency. Since most of the patients were not able to eat bread or drink thin liquid safely, we had missing data for these consistencies. Missing data for thin liquid was replaced by the scores from six drops of blue coloured water as the consistency was the same and only the volume differed.
Data was exported from the EMBI system for the analysis of the swallowing specific parameters. Number of swallows in the pre-treatment, treatment and post-treatment phases were normalised to time by calculating the number per minute for each patient.
Non-parametric statistical analysis was used throughout due to the low number of patients included and data type. We performed exploratory statistics on our primary outcomes. Wilcoxon signed rank tests were used to determine intra-group differences in FOIS and PAS from baseline to end of intervention, Mann Whitney U-tests were used to test for significant inter-group differences in change scores in PAS and FOIS.
Exploratory data analysis was performed on swallowing frequency and swallowing specific parameters in pre-intervention, intervention and post-intervention and the data presented in box plots.
Data was analysed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY). Values are presented as median and interquartile range. A p-value of less than 0.05 was considered statistically significant.
The study was approved by the Danish Capital Region Ethical Committee, registration number: H-2–2013–162 and by the Danish Data Protection Agency registration number: GLO-2014–20, I-Suite no: 02741. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
3Results
We included a total of 10 patients during the project period. There were no significant differences between groups in age, days to inclusion, EFA or GCS at baseline. There were more males in the control group than the intervention group. GCS and EFA scores show that the included patients were severely brain damaged (Table 1).
Table 1
Patient characteristics
| Control group (n = 5) | Intervention group (n = 5) | |
| Age in years | 45.6 (37.5–57.8) | 53.8 (41.8–61.4) |
| Male gender | 4 | 2 |
| Days from injury to inclusion | 70.4 (43.0) | 76.4 (21.8) |
| GCS at injury (3–15 points) | 6.8 (4.4) | 6.0 (5.2) |
| GCS at start intervention (3–15 points) | 10.6 (1.5) | 9.8 (1.8) |
| GCS at end of intervention (3–15 points) | 12.0 (2.3) | 9.8 (1.1) |
| EFA at start of intervention (20–100 points) | 47.6 (7.2) | 45.2 (9.5) |
| EFA at end of intervention (20–100 points) | 52.6 (2.9) | 50.0 (10.3) |
| Diagnosis | ||
| Aneurism | 1 | 1 |
| Hypophysis Adenoma | 1 | |
| Infarction | 1 | |
| Intracerebral haemorrhage | 2 | |
| Meningioma | 1 | |
| Subarachnoid haemorrhage | 2 | |
| Severe traumatic brain injury | 1 |
Abbreviations: EFA = Early Functional Abilities; GCS = Glasgow Coma Scale. Values for days from injury to inclusion, EFA and GCS are presented as mean (SD). Age is presented as mean (range).
Participants were enrolled over a period of 32 months. There were periods of several months where we were unable to include patients. Patients, that otherwise would have met the inclusion criteria for pumping jaw movements and PAS-score worse than 3, could not be included due to restlessness and/ or agitated behaviour, prevalent in patients in the sub-acute stage following severe ABI. These patients would not have tolerated the positioning required for measuring and intervention and they would not have tolerated the electrodes for the EMBI measurement.
Written consent was obtained for all patients fulfilling the inclusion- and exclusion criteria, no patients withdrew from the study and there were no dropouts.
The intensified intervention was feasible, one patient received the target of 30 additional treatments, one received 29, two received 27 and one received 25. The reasons for missing treatment were increased agitation or motor restlessness, deterioration of medical conditions and the transfer to another department for a single patient due to hydrocephalus.
The intervention with two extra treatments per day was very intensive for the patients, who often seemed tired. It was a challenge to incorporate the intensified treatment in an interprofessional setting whilst ensuring that the patient had adequate rest.
Table 2 shows the changes in the Penetration Aspiration Scale and Functional Oral Intake Scale for both groups. There were no significant differences in score changes between groups in these outcomes.
Table 2
Evaluation of swallowing by Penetration Aspiration Scale and Functional Oral Intake Scale at baseline and end of intervention
| Control group (n = 5) | Intervention group (n = 5) | |||||||||
| Baseline | End of intervention | Change | P-value within group | Baseline | End of intervention | Change | P-value within group | P-value between groups | ||
| PAS | Saliva | 3(3;3) | 2(1;3) | 2 (0.5;2) | 0.0833 | 3(3;4) | 1(1;1) | 2(2;2) | 0.1025 | 0.1797 |
| Apple sauce | 1(1;1) | 1(1;1) | 0 (–0.5;0.5) | 1.000 | 2.5(1.5;5.5) | 1(1;1.5) | 1(0;5.75) | 0.1615 | 0.8658 | |
| Thickened liquid | 1(1;1) | 1(1;1) | 0 (–3;1.5) | 0.8759 | 1.5(1;5) | 1(1;1) | 0(0;7) | 0.3173 | 0.3711 | |
| Thin liquid | 6(3;6) | 3(1;3) | 3 (0;4) | 0.1025 | 3(1;4) | 1(1;1) | 1(0;2.75) | 0.1615 | 0.0790 | |
| Total | 10(8;13) | 6(4;8) | 4 (–2;7.5) | 0.2185 | 8(8;9) | 4(3;4) | 5(4.5;12.5) | 0.0422* | 0.1049 | |
| FOIS | 2(2;2) | 2(2;2) | 0 (–0.5;0) | 0.3173 | 2(1;2) | 2(2;2) | 0(–1;0) | 0.1573 | 1.0000 | |
All data are presented as median and IQR; PAS: Penetration Aspiration Scale; FOIS: Functional Oral Intake Scale; *: p < 0.05.
There was a reduction in the mean value for maximum laryngeal elevation in the control group throughout the treatment (negative outcome) whereas it increased in the intervention group during facilitation when compared to the pre- and post-treatment periods (positive outcome) (Fig. 1a).
Fig.1
(a) Maximum laryngeal elevation during swallows; (b) Speed of laryngeal elevation; (c) Swallowing frequency; (d) Pumping frequency.
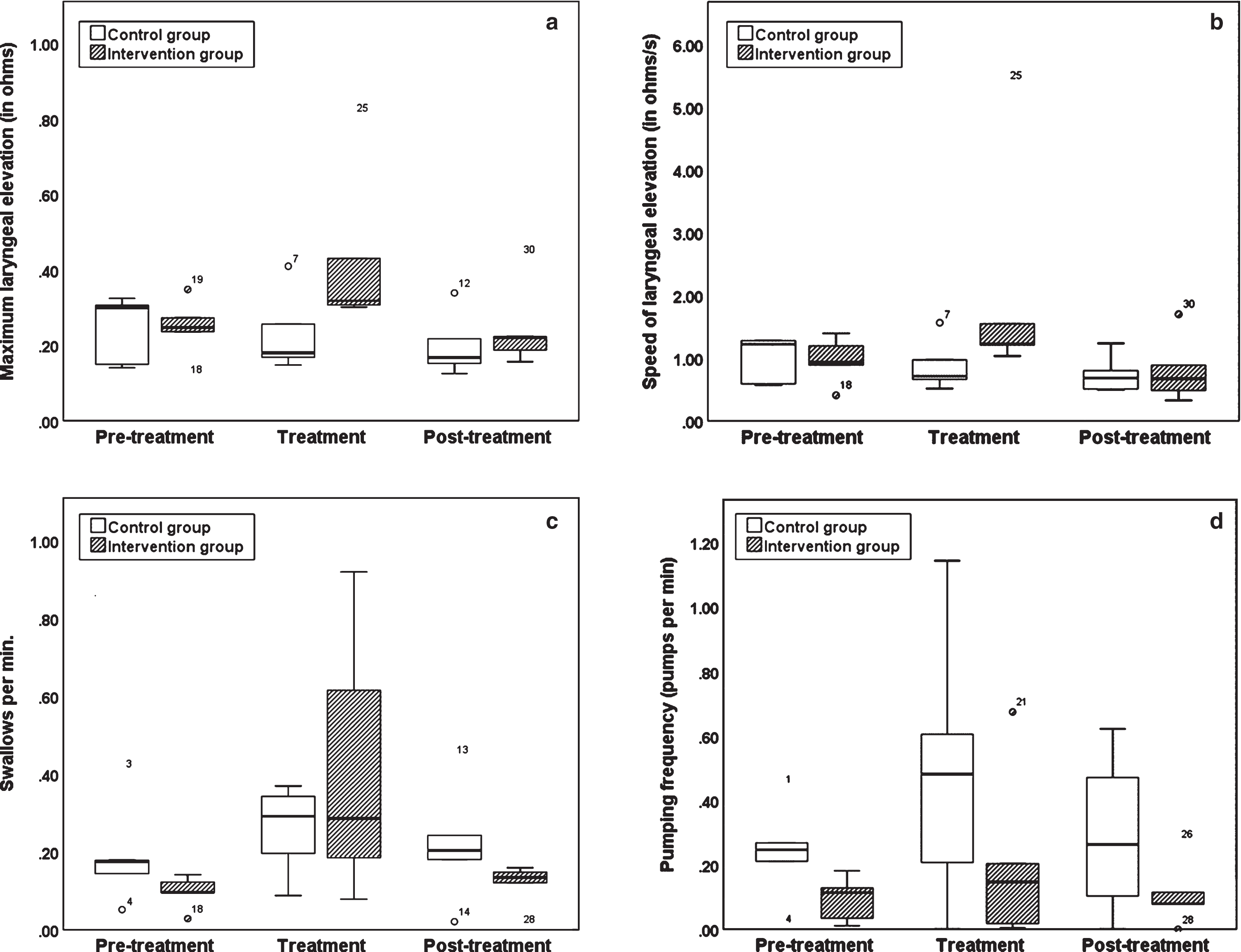
The median speed of laryngeal elevation during swallows fell during treatment and post-treatment in the control group (negative outcome), whereas it increased in the intervention group during treatment (positive outcome) compared with the pre- and post-treatment periods. (Fig. 1b).
Median swallowing frequency increased in both groups during treatment (positive outcome) compared with the pre- and post-treatment phases (Fig. 1c).
Median pumping frequency increased in both groups during treatment (negative outcome) but more greatly in the control group (Fig. 1d).
There were no observed adverse events as a result of the intervention but there was an adverse reaction in the form of increased patient fatigue during some of the interventions. There were no important changes to methods following trial commencement.
4Discussion
This study has provided evidence that PAS and FOIS are both feasible outcome measures to measure dysphagia in a definitive study. The study also demonstrated that it is possible to measure swallowing frequency and swallowing specific parameters using the EMBI system in patients with severe acquired brain damage, that are not restless or agitated. The inclusion criteria and organisational restraints limited the inclusion rate to three patients a year, which was considerably lower than anticipated. The study has additionally shown that intensifying therapy with two treatments a day is challenging both in terms of organization, but also in terms of patient fatigue and changing arousal.
One of the principal reasons explaining the slow inclusion rate was a lack of patients that met the inclusion criteria. It would improve patient inclusion if the inclusion criteria were widened by removing the age limit and patients were included at multiplecentres.
The intensification of treatment proved to be challenging both from an organizational perspective, but also from a patient perspective. The organizational challenge could be met by finding financial and human resources to provide the intensified therapy, for example during weekends and evenings. The greatest challenge is however to ensure that the patient has a high enough arousal level to benefit from the intensified therapy. Patients with severe ABI suffer from low and fluctuating arousal due to disorders of consciousness (Giacino, Fins, Laureys, & Schiff, 2014) that may inhibit or prevent motor learning (Goldfine & Schiff, 2011). Level of consciousness may confound the relationship between the amount of exercise and functional swallowing recovery.
We were able to administer the outcome measures on all patients that were included in the project. The only missing data were due to a faulty cable in the EMBI system for a single patient. FOIS and EFA require knowledge of the patient to make valid scores. This was not always the case, due to sick leave or vacation of the primary therapist and may have affected the data quality. The PAS assessment using FEES is acknowledged as gold standard for the evaluation of oropharyngeal dysphagia. Due to the invasive nature of the endoscopic examination, this outcome was only used at baseline and at the end of the intervention. A single measure, however, can vary according to the patient’s daily context and arousal and a definitive study would need to control for the patient’s level of arousal. There is some evidence, that the body position and the swallowing of different consistencies do influence swallowing specific parameters (Schultheiss, Wolter, Schauer, Nährstedt, & Seidl, 2015). Even though, the EMBI was feasible and robust in terms of patient positioning, two of the patients had difficulties staying in the neutral side lying resting position, due to muscle tone and respiratory issues. Measures made using the EMBI system in the two rest periods with different positioning may not be comparable.
The FOIS scores showed little change in few participants during the study. A scale of this type reflects relevant changes in abilities in daily life activities but would require a substantial increase in the sample size to achieve statistical significance. An adequately powered definitive randomised trial could implement this score as it more broadly reflects eating function (Crary et al., 2005). Participants in this study had more severe perceptive, cognitive and sensorimotor problems than patients with stroke in the FOIS sensitivity study (Crary et al., 2005) which may explain the relatively smaller changes in the FOIS score in this study.
The total PAS scores improved in both groups during the study period and was significantly different from baseline in the intervention group. Despite no published studies of sensitivity for this measure, it seemed to be sensitive to changes in this study. Whether the PAS is helpful in describing and measuring the severity of airway invasion when swallowing saliva or consistencies has been questioned for several reasons (Steele & Grace-Martin, 2017). The PAS produces a score for each consistency. Using only the worst scores increases the risk of skewing the score, whereas the mean value does not reflect the distribution of the scores. Furthermore, the question has been raised, whether it is more dangerous for the patient to have a PAS score of 3, where penetrated material remains above the vocal folds without being expelled (and thereby the risk for subsequent aspiration), or a score of 6, where an aspiration is followed by a subsequent effective cough. Despite these challenges in interpreting the PAS scores, this measure together with FEES remains the best available clinical tool to measure aspiration and penetration and, in the light of this study, will be a prioritised choice of secondary outcome in a future definitivestudy.
The swallowing specific parameters show a tendency for the swallowing frequency, the maximum elevation of larynx and the speed of laryngeal elevation to improve within the intervention group during the intervention phase. Furthermore, the frequency of pumping jaw movements during intervention decreased. These results appear to reflect the clinical experience of the facilitation of swallowing and these measures therefore seem to be valid in quantifying swallowing kinematics non-invasively. Future studies should investigate the relationship of these parameters to aspiration and penetration risk in patients with brain injury and whether there is a solid learning effect of the intervention reflected in an increase in eating ability measured using FOIS at a 3-month follow-up.
A controlled study in a population of patients with severe ABI in an intensive rehabilitation clinic is very challenging. The patients in our study reflected the heterogeneous nature of this patient population with varying diagnoses, symptoms and severities. All patients were medicated with pharmaceutics that can potentially influence arousal, tone and production of saliva, which might have influenced the swallowing frequency. Their ability to swallow may also vary due to other factors such as infections and level of consciousness. Some of the challenges of creating matched groups in a randomised design could be met using stratification techniques, but the greatest challenge in working with this patient group is the extent to which the intervention can be controlled in a sub-acute clinical setting.
Funding
The study was supported by the Danish Occupational Therapist Association (FF 2–13–3), the Glostrup Hospitals Research grant and Department of Neurorehabilitation, TBI Unit, Rigshospitalet.
Authors’ contributions
DJ, THP, ROS and CS modified the study design from Müller. DJ and THP initiated the study and were responsible for collection of data and monitoring the process. CS, CR and DJC analysed the data. DJ and DJC drafted the manuscript. All authors critically reviewed and contributed to the draft, revised and approved the manuscript.
Conflict of interest
Daniela Jakobsen is a certified F.O.T.T.® senior instructor and teaches F.O.T.T.® courses. The other authors declare no potential conflicts of interest with respect to the research, authorship or publication of this article.
Appendices
Appendix: The F.O.T.T.® concept
The F.O.T.T. approach was developed by Kay Coombes, a speech and language therapist in the UK, see: http://www.arcos.org.uk/services/professional-training/what-is-f-o-t-t/. It is a structured way to assess and treat problems in patients with neurogenic problems concerning four areas: Swallowing of saliva/eating and drinking, oral hygiene, breathing-voice – speech movements and facial expression. F.O.T.T. is an interprofessional concept and involves the patient’s relatives and helpers. The aim of F.O.T.T. is to integrate function and movement into daily life situations, which are relevant for the individual.
The treatment aims to provide patients with experiences of movement and meaningful tactile information in a stimulating daily life context. The goal is to reintegrate lost or impaired functions on the basis of motor learning. The concept is described in more detail by Nusser Müller Busch [1] and Hansen and Jakobsen [2].
List of therapeutic interventions in F.O.T.T.
- Creating an environment that enables the patient to respond as optimally to the treatment as possible (e.g. surroundings for the treatment, furniture, assistive devices, objects used in treatment)
- Moving and positioning the patient, facilitation of optimal alignment for breathing, swallowing and (non)-verbal communication and protection of the airway
- Integration of the hands (hand-hand, hand-eye and hand-mouth coordination in daily life context to provide helpful sensory input to the patient
- Specific tactile oral stimulation
- Facilitation of dynamic stability in the body, and for the lower jaw for functional oral movements
- Use of small amounts of food/liquid for therapeutic eating, to facilitate oral and pharyngeal transport movements
- Support breathing, phonation and protective reactions for the lower airway
- Specific facilitation of facial movements, swallowing, oral transport movements and movements relevant for oral hygiene and speech
Description of the interventions used for the treatment group
The treatment in the intervention group was conducted by means of the F.O.T.T. concept. Patients were facilitated as often as possible to swallow saliva or small amounts of cold, pureed food or thickened drink, over a period of maximum 20 minutes. Facilitation of swallowing aimed to achieve swallowing with a stable mandible instead of pumping jaw movements and a helpful position of the tongue for oropharyngeal transport. Patients were never asked to swallow. Food or drink was used when the occupational therapist considered it to be safe following the criteria: sufficient postural control for sitting or half sitting position in neutral position (using LIN®), swallowing saliva and sufficient protection of the airway in case of aspiration.
In patients who showed pumping jaw movements without a subsequent swallow, the goal was to facilitate a swallow after the pumping.
Positioning
The patient was positioned in an optimal position with respect to arousal and postural control. To standardize positioning, the LiN®-method was used, where the patient’s body sections are aligned in a neutral position. Neutral position (“0” degrees) is defined as the position of the joints in an upright standing person [3]. Patients can be positioned with that method in supine, side lying, sitting and prone. To keep the alignment of the body in the chosen position, the patient is supported with pillows, towels, bedsheets and bedcovers. The goal is to improve the patient’s mobility and facilitate active movements, by holding muscles at resting length. The assumption in the LiN®-method is that the chance to create muscle activity is greatest, when the muscles are at their optimal length. LiN® aims to avoid complications such as decubitus and contracture. Stabilizing the patient with soft material, adapted to the shape of his body, makes him feel comfortable and safe (https://lin-arge.de) [3]. The risk of aspiration was also considered when positioning. Side lying position was preferred, when the patient was at a high risk of aspirating saliva. Here, saliva could be removed from the cheek by the therapist, before it ran towards the pharynx. Half sitting in bed or sitting on the edge of the bed was used when the patient had enough postural control and vegetative stability to cope with a less supported position, and when small amounts of apple sauce or thickened liquid was used for the treatment (therapeutic eating).
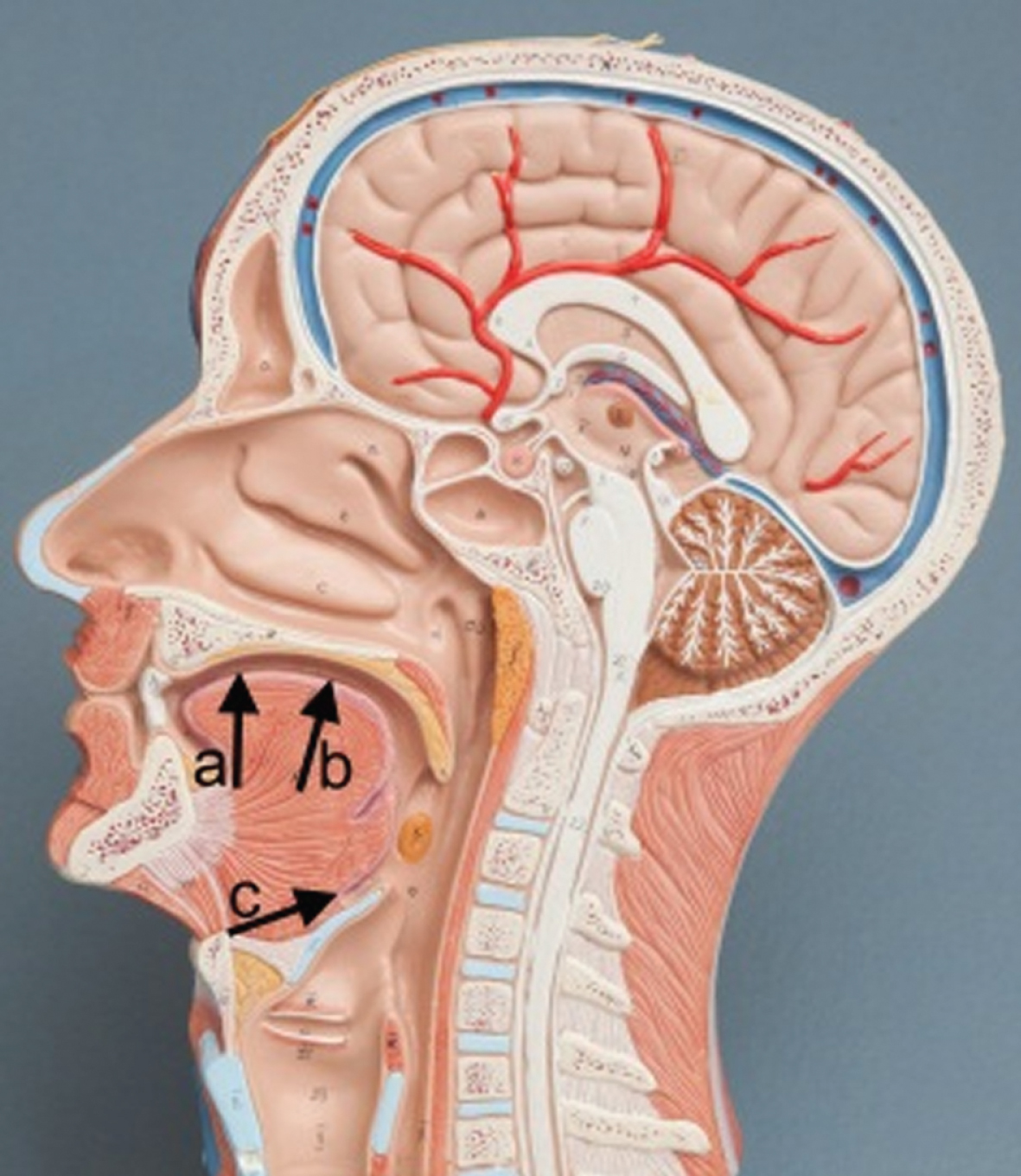
Fig.1
Direction of the therapist’s fingers to influence tongue position from the outside of the floor of the mouth.
Facilitation of swallowing
Facilitation of swallowing is a method in F.O.T.T. that aims to modulate the swallowing reaction and encourage motor learning of functional movement sequences. Facilitation in this context means to give the patient helpful, nonverbal input by specific handling, to make a movement necessary or possible, or let it happen. The facilitation aims to stabilize the head and the jaw and help the tongue to come in a functional position for oral and pharyngeal transport movements. There are several ways of facilitating swallowing, depending on the underlying causes for missing initiation of the swallow or the abnormal pattern of movement. Typically, the therapist would support the patients head and the mandible and try from the outside of the floor of the mouth to move the front of the tongue upwards towards the hard palate, or the back of the tongue towards the soft palate to initiate swallowing. Another variation is when the therapist gives sensory input by moving the tongue carefully dorso-cranially, with the fingers placed on the floor of the mouth. In this way, the posterior part of the tongue in the pharynx and the vallecular space is moved. Here, there are often remains of saliva or food/drink in patients that do not have clearing swallows due to hyposensibility in the pharynx. This intervention might help the patient to detect the remains and respond with a swallow. The facilitation of swallowing was embedded as much as possible in a meaningful everyday life context for the patient, such as oral hygiene or eating/drinking. The context was created and matched to the patient’s needs and abilities.
Fig.2
Facilitation to swallow on the floor of the mouth, combined with a grip that provides jaw-support (healthy model).

Other possibilities to facilitate swallowing could be to change the patient’s position, aiming to move remains of saliva in the pharynx, that not have been detected by the patient (fading out).
Furthermore, the therapist uses the patient’s expiration or phonation to disturb remains of saliva in the pharynx with the possibility of stimulating a swallowing response.
Context for the facilitation of swallowing
For the treatment in the intervention group, the therapist could choose to facilitate swallowing within/during the following F.O.T.T. interventions:
- Oral stimulation, involving the patient’s hands and face
- Mobilisation/activation of the tongue
- Therapeutic eating, defined as: An offer of small amounts apple sauce or liquid, with modified consistency, if necessary, in a situation that is controlled by the therapist, to work on the patient’s problems in the swallowing sequence (e.g. lack of oral/pharyngeal sensibility, discoordinated tongue movements). Therapeutic eating could be chewing food as e.g. a piece of apple, wrapped in wet gauze, licking a small amount of apple sauce from the lips, or eating a small amount from a teaspoon or drinking small amounts of liquid from a glass or cup. To use therapeutic eating, the therapist had to confirm that the patient could protect the airway sufficiently in case of aspiration.
• Support breathing, phonation and protective reactions such as coughing or clearing the throat
• Structured and therapeutic oral hygiene with focus on the swallowing reactions.
References
References
[1] | Nusser-Müller-BuschR. Die Therapie des Facio-Oralen Trakts. 4th Ed: Springer Verlag Berlin -Heidelberg (2004) (2015) . |
[2] | HansenTS, JakobsenD. A decision-algorithm defining the rehabilitation approach: ‘Facial oral tract therapy’. Disability and rehabilitation. (2010) ;32: (17):1447–60. |
[3] | PickenbrockH, LudwigVU, ZapfA, DresslerD. Conventional versus neutral positioning in central neurological disease: A multicenter randomized controlled trial. Deutsches Arzteblatt international. (2015) ;112: (3):35–42. |
Acknowledgments
We express our gratitude to all the patients and their relatives for giving their consent to participate in the study at a hard point in their lives.
We acknowledge Doris Müller, occupational therapist, F.O.T.T.® senior instructor and MSc Neurorehabilitation, Bavaria Klinik Kreischa, Germany for sharing information and experience from her pilot study and helpful discussions before and during the study period. We thank Holger Nährstedt from Technische Universität Potsdam, for his great contribution to the data analysis.
We thank the occupational therapists Jeanette Vos, Kajsa L Strøbech, Michelle Dam-Ellehammer and Maja C Nielsen who performed the interventions, and Dr. Tove Grønbæk Jensen, neurosurgeon and occupational therapist Stine Jochumsen who performed the FEES assessments. Furthermore, we thank the management of the department and the interdisciplinary staff for their practical support.
References
{ label needed for ref[@id='ref001'] } | Argolo, N. , Sampaio, M. , Pinho, P. , Melo, A. , & Nobrega, A. C. ((2015) ). Swallowing disorders in Parkinson’s disease: impact of lingual pumping. Int J Lang Commun Disord, 50: (5), 659–664. |
{ label needed for ref[@id='ref002'] } | Bautista, T. G. , Sun, Q. J. , & Pilowsky, P. M. ((2014) ). The generation of pharyngeal phase of swallow and its coordination with breathing: interaction between the swallow and respiratory central pattern generators. Prog Brain Res, 212: , 253–275. |
{ label needed for ref[@id='ref003'] } | Butler, S. G. , Markley, L. , Sanders, B. , & Stuart, A. ((2015) ). Reliability of the penetration aspiration scale with flexible endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol, 124: (6), 480–483. |
{ label needed for ref[@id='ref004'] } | Cabib, C. , Ortega, O. , Kumru, H. , Palomeras, E. , Vilardell, N. , Alvarez-Berdugo, D. , & Clave, P. ((2016) ). Neurorehabilitation strategies for poststroke oropharyngeal dysphagia: from compensation to the recovery of swallowing function. Ann N Y Acad Sci, 1380: (1), 121–138. |
{ label needed for ref[@id='ref005'] } | Cherney, L. R. , & Halper, A. S. ((1996) ). Swallowing problems in adults with traumatic brain injury. Semin Neurol, 16: (4), 349–353. |
{ label needed for ref[@id='ref006'] } | Colodny, N. ((2002) ). Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (fees) using the penetration-aspiration scale: A replication study. Dysphagia, 17: (4), 308–315. |
{ label needed for ref[@id='ref007'] } | Crary, M. A. , Mann, G. D. , & Groher, M. E. ((2005) ). Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil, 86: (8), 1516–1520. |
{ label needed for ref[@id='ref008'] } | Daniels, S. K. ((2000) ). Swallowing apraxia: A disorder of the Praxis system? Dysphagia, 15: (3), 159–166. |
{ label needed for ref[@id='ref009'] } | Dewan, M. C. , Rattani, A. , Gupta, S. , Baticulon, R. E. , Hung, Y.-C. , Punchak, M. , & Park, K. B. ((2018) ). Estimating the global incidence of traumatic brain injury. Journal of Neurosurgery, 130: (4), 1–18. |
{ label needed for ref[@id='ref010'] } | Dodds, W. J. ((1989) ). Physiology of swallowing. Dysphagia, 3: (4), 171–178. |
{ label needed for ref[@id='ref011'] } | Foley, N. C. , Martin, R. E. , Salter, K. L. , & Teasell, R. W. ((2009) ). A review of the relationship between dysphagia and malnutrition following stroke. J Rehabil Med, 41: (9), 707–713. |
{ label needed for ref[@id='ref012'] } | Giacino, J. T. , Fins, J. J. , Laureys, S. , & Schiff, N. D. ((2014) ). Disorders of consciousness after acquired brain injury: the state of the science. Nature Reviews. Neurology, 10: (2), 99–114. |
{ label needed for ref[@id='ref013'] } | Goldfine, A. M. , & Schiff, N. D. ((2011) ). What is the role of brain mechanisms underlying arousal in recovery of motor function after structural brain injuries? |
{ label needed for ref[@id='ref014'] } | Hankemeier, A. , & Rollnik, J. D. ((2015) ). The Early Functional Abilities (EFA) scale to assess neurological and neurosurgical early rehabilitation patients. BMC Neurol, 15: , 207. |
{ label needed for ref[@id='ref015'] } | Hansen, T. S. , & Jakobsen, D. ((2010) ). A decision-algorithm defining the rehabilitation approach: ’Facial oral tract therapy’®. Disability and Rehabilitation, 32: (17). |
{ label needed for ref[@id='ref016'] } | Hansen, T. S. , Larsen, K. , & Engberg, A. W. ((2008) ). The association of functional oral intake and pneumonia in patients with severe traumatic brain injury. Arch Phys Med Rehabil, 89: (11), 2114–2120. |
{ label needed for ref[@id='ref017'] } | Heck G Schmidt T. Neurol Rehabil. (2000) ;6: :125–33., S.-B. G. ((2000) ). Early Functional Abilities (EFA) – eine Skala zur Evaluation von Behandlungsverläufen in der neurologischen Frührehabilitation. 6: , 125–133. |
{ label needed for ref[@id='ref018'] } | Langmore, S. E. ((2017) ). History of Fiberoptic Endoscopic Evaluation of Swallowing for Evaluation and Management of Pharyngeal Dysphagia: Changes over the Years. Dysphagia, 32: (1), 27–38. |
{ label needed for ref[@id='ref019'] } | Langmore, S. E. , & Pisegna, J. M. ((2015) ). Efficacy of exercises to rehabilitate dysphagia: A critique of the literature. Int J Speech Lang Pathol, 17: (3), 222–229. |
{ label needed for ref[@id='ref020'] } | Langmore, S. E. , Schatz, K. , & Olson, N. ((1991) ). Endoscopic and Videofluoroscopic Evaluations of Swallowing and Aspiration. Annals of Otology, Rhinology & Laryngology, 100: (8), 678–681. |
{ label needed for ref[@id='ref021'] } | Mackay, L. E. , Morgan, A. S. , & Bernstein, B. A. ((1999) ). Swallowing disorders in severe brain injury: risk factors affecting return to oral intake. Arch Phys Med Rehabil, 80: (4), 365–371. |
{ label needed for ref[@id='ref022'] } | Martino, R. , Foley, N. , Bhogal, S. , Diamant, N. , Speechley, M. , & Teasell, R. ((2005) ). Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke, 36: (12), 2756–2763. |
{ label needed for ref[@id='ref023'] } | McCullough, G. H. , & Kim, Y. ((2013) ). Effects of the Mendelsohn maneuver on extent of hyoid movement and UES opening post-stroke. Dysphagia, 28: (4), 511–519. |
{ label needed for ref[@id='ref024'] } | Morgan, A. S. , & Mackay, L. E. ((1999) ). Causes and complications associated with swallowing disorders in traumatic brain injury. J Head Trauma Rehabil, 14: (5), 454–461. |
{ label needed for ref[@id='ref025'] } | Neumann, S. ((1993) ). Swallowing therapy with neurologic patients: results of direct and indirect therapy methods in 66 patients suffering from neurological disorders. Dysphagia, 8: (2), 150–153. |
{ label needed for ref[@id='ref026'] } | Palmer, J. B. , Rudin, N. J. , Lara, G. , & Crompton, A. W. ((1992) ). Coordination of mastication and swallowing. Dysphagia, 7: (4), 187–200. |
{ label needed for ref[@id='ref027'] } | Pickenbrock, H. , Ludwig, V. U. , Zapf, A. , & Dressler, D. ((2015) ). Conventional versus neutral positioning in central neurological disease: A multicenter randomized controlled trial. Dtsch Arztebl Int, 112: (3), 35–42. |
{ label needed for ref[@id='ref028'] } | Robbins, J. , Butler, S. G. , Daniels, S. K. , Diez Gross, R. , Langmore, S. , Lazarus, C. L. , & Rosenbek, J. ((2008) ). Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res, 51: (1), S276-300. |
{ label needed for ref[@id='ref029'] } | Robbins, J. , Levine, R. L. , Maser, A. , Rosenbek, J. C. , & Kempster, G. B. ((1993) ). Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil, 74: (12), 1295–1300. |
{ label needed for ref[@id='ref030'] } | Rosenbek, J. C. , Robbins, J. A. , Roecker, E. B. , Coyle, J. L. , & Wood, J. L. ((1996) ). A penetration-aspiration scale. Dysphagia, 11: (2), 93–98. |
{ label needed for ref[@id='ref031'] } | Saito, T. , Hayashi, K. , Nakazawa, H. , & Ota, T. ((2016) ). Clinical Characteristics and Lesions Responsible for Swallowing Hesitation After Acute Cerebral Infarction. Dysphagia, 31: (4), 567–573. |
{ label needed for ref[@id='ref032'] } | Schultheiss, C. , Schauer, T. , Nahrstaedt, H. , & Seidl, R. O. ((2013) ). Evaluation of an EMG bioimpedance measurement system for recording and analysing the pharyngeal phase of swallowing. Eur Arch Otorhinolaryngol, 270: (7), 2149–2156. |
{ label needed for ref[@id='ref033'] } | Schultheiss, C. , Schauer, T. , Nahrstaedt, H. , & Seidl, R. O. ((2014) ). Automated detection and evaluation of swallowing using a combined EMG/bioimpedance measurement system. Scientific World Journal, 2014: , 405471. |
{ label needed for ref[@id='ref034'] } | Schultheiss, C. , Wolter, S. , Schauer, T. , Nahrstaedt, H. , & Seidl, R. O. ((2015) ). [Effect of body position on coordination of breathing and swallowing]. Hno, 63: (6), 439–446. |
{ label needed for ref[@id='ref035'] } | Seidl, R. O. , Nusser-Muller-Busch, R. , Hollweg, W. , Westhofen, M. , & Ernst, A. ((2007) ). Pilot study of a neurophysiological dysphagia therapy for neurological patients. Clin Rehabil, 21: (8), 686–697. |
{ label needed for ref[@id='ref036'] } | Shaker, R. , Easterling, C. , Kern, M. , Nitschke, T. , Massey, B. , Daniels, S. , & Dikeman, K. ((2002) ). Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology, 122: (5), 1314–1321. |
{ label needed for ref[@id='ref037'] } | Shaw, S. M. , & Martino, R. ((2013) ). The normal swallow: muscular and neurophysiological control. Otolaryngol Clin North Am, 46: (6), 937–956. |
{ label needed for ref[@id='ref038'] } | Sonies, B. C. , Parent, L. J. , Morrish, K. , & Baum, B. J. ((1988) ). Durational aspects of the oral-pharyngeal phase of swallow in normal adults. Dysphagia, 3: (1), 1–10. |
{ label needed for ref[@id='ref039'] } | Steele, C. M. , & Grace-Martin, K. ((2017) ). Reflections on Clinical and Statistical Use of the Penetration-Aspiration Scale. Dysphagia, 32: (5), 601–616. |
{ label needed for ref[@id='ref040'] } | Stubbs, P. W. , Pallesen, H. , Pedersen, A. R. , & Nielsen, J. F. ((2014) ). Using EFA and FIM rating scales could provide a more complete assessment of patients with acquired brain injury. Disabil Rehabil, 36: (26), 2278–2281. |
{ label needed for ref[@id='ref041'] } | Teasdale, G. , & Jennett, B. ((1974) ). Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England), 2: (7872), 81–84. |
{ label needed for ref[@id='ref042'] } | Troche, M. S. , Okun, M. S. , Rosenbek, J. C. , Musson, N. , Fernandez, H. H. , Rodriguez, R. , & Sapienza, C. M. ((2010) ). Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurology, 75: (21), 1912–1919. |
{ label needed for ref[@id='ref043'] } | Veis, S. L. , & Logemann, J. A. ((1985) ). Swallowing disorders in persons with cerebrovascular accident. Arch Phys Med Rehabil, 66: (6), 372–375. |
{ label needed for ref[@id='ref044'] } | Woratyla, S. P. , Morgan, A. S. , Mackay, L. , Bernstein, B. , & Barba, C. ((1995) ). Factors associated with early onset pneumonia in the severely brain-injured patient. Conn Med, 59: (11), 643–647. |




