Motorist disorientation syndrome; clinical features and vestibular findings
Abstract
BACKGROUND:
Motorist Disorientation Syndrome (MDS) is a term used to describe patients who primarily experience symptoms of dizziness/disorientation whilst in a motor car [21]. There is uncertainty about the relevance of vestibular dysfunction and whether this disorder could instead be a visually induced dizziness (VV) or part of a functional disorder similar to Persistent postural perceptual dizziness (PPPD).
OBJECTIVE:
We present the largest case-series to date of patients whose main complaint is of illusions of movement of self/vehicle when driving, characterising features of this group.
METHODS:
18 subjects underwent detailed clinical assessment including validated questionnaires. A subset of patients underwent vestibular function testing.
RESULTS:
Mean onset age was 42 years, with no gender preponderance. Mean symptom duration was 6.39 years and was significantly longer in women. 50% reported moderate or severe handicap. Vestibular abnormalities were found in 60% of subjects tested. There was no significant difference in VSS total score between those with MDS and vestibular migraine (p = 0.154) with both having higher scores than healthy controls (p = 0.002, 0.000 respectively).
CONCLUSIONS:
MDS represents consistent symptoms, with high symptom burden, comparable to vestibular migraine. Vestibular deficits were not a consistent feature and similarities to VV and PPPD exist.
1Introduction
Since the 1980 s there has been recognition of a group of patients who primarily experienced symptoms of dizziness/disorientation whilst in a motor car. The original description from Page and Gresty described six patients presenting with difficulty while driving a motor car, including illusions of the car turning or veering to one side [21]. Symptoms were exacerbated in certain situations such as driving on open, featureless roads, going over the brow or descending hills, or going around corners. In a later review published in a report on spatial disorientation in military vehicles, the authors grouped symptom presentation into three main categories; “it feels as if the car is i) veering on wide open roads such as motorways; ii) turning in towards overtaking vehicles; iii) about to turn over when descending a hill or rounding a bend” [10].
The term ‘Motorist’s Vestibular Disorientation Syndrome’ was coined for this situation specific dizziness. Reduced visual input, combined with abnormal vestibular findings, was initially postulated as a mechanism [21]. Subsequent work by Bronstein introduced the concept of visual vertigo (VV) [1]. VV was initially thought to be due to a mismatch between visual and vestibular inputs after peripheral vestibular dysfunction, however subsequent work showed that it may be due to increased reliance on visual clues for orientation in some patients (termed visual dependence) [8]. VV was combined with clinical characteristics of phobic postural vertigo, space-motion discomfort and chronic subjective dizziness by the Barany society to form the clinical entity of PPPD, a chronic functional neurological disorder where symptoms are exacerbated by motion and exposure to moving visual stimuli [23]. This raised the question as to whether the motion and complex visual stimuli whilst driving in patients with visual dependence could be responsible for MDS, making it a type of functional vestibular disorder.
Since the initial descriptions, little research has been done since to further characterise the clinical presentation and vestibular findings and impact on this group of patients who present with dizziness primarily when driving. We present the largest case series of patients to date with these presenting symptoms and describe the clinical features, vestibular findings and impact in this group, referencing vestibular migraine, to help clarify the aetiology of this disorder and guide clinicians onmanagement.
2Methods
Our Institutional Ethical Review Board (National Hospital for Neurology and Neurosurgery Ethics Committee) approved the study. All patients gave written informed consent to participation.
2.1Patients
Patients were identified retrospectively from case notes and prospectively from neuro-otology clinics over a period of 1 year. If not recruited from clinic they were invited to participate by post. Potential participants who did not respond to the original invitation were contacted after six weeks to check the information had been received.
Patients were included if they were aged over 18 years and complained of symptoms of disorientation or illusions of movement of self or vehicle, primarily when driving a car. They were excluded if there was a more likely diagnosis for their symptoms.
2.2Controls
Control subjects were recruited for the nestled case control study. Normal subjects were partners or friends of those attending the clinic in whom there were no symptoms or history of a vestibular disorder. Patients with vestibular migraine were identified in the neuro-otology clinic, according to standardised criteria [19].
2.3Interview
All subjects underwent a standardised detailed interview with an experienced physician (LM) either in person or by telephone. Data were collected on age, sex, duration of symptoms, provoking situations, avoidance behaviour, associated symptoms and associated diagnoses. The presence of migraine according to the International Headache Society criteria was recorded.
2.4Questionnaires
Subjects completed standardised validated questionnaires including the Dizziness Handicap Inventory (DHI) [13] and Vertigo Symptom Scale (VSS) [25]. The DHI is a validated questionnaire, completed by the patient, which assesses self-perceived handicap from dizziness. Total score of 0–30 reflects mild, 31–60 moderate, and 61–100 severe handicap. The Vertigo Symptom Scale is a validated questionnaire, completed by the patient, which assesses the frequency of autonomic and anxiety symptoms (VSS-AA) and vertigo/balance symptoms (VSS-VB) as well as giving a total score (VSS-T). Subjective response to treatment wasrecorded.
2.5Vestibular testing
A vestibular test battery was performed, as deemed appropriate by the clinician. Available tests were ENG eye movement testing, sinusoidal rotation, vestibulo-ocular reflex suppression, impulsive rotation, optokinetic stimulation and caloric testing. Due to the retrospective nature of the data VHIT records were not available or unreliable so where excluded from analysis.
For eye movement recordings, horizontal direct current electro-oculography (ENG) was carried out according to a standard protocol: gaze testing was performed in central and lateral gaze (+/–30°) to assess for nystagmus with and without fixation; for smooth pursuit testing, subjects were required to track a laser-projected target moving in a sinusoidal fashion at 0.2, 0.3 and 0.4 Hz. Horizontal saccadic testing was performed using an projected target moving randomly over a 5 to 30 degree arcSinusoidal rotation was carried out in the dark using a motorised chair driven at a frequency of 0.2 Hz, peak velocity of +/–30°/s for a duration of approximately eight cycles. Ability to suppress the vestibulo-ocular reflex was then tested by repeating the sinusoidal stimulus and asking the patient to fixate on a target that moved with them (i.e. stationary with respect to the patient) for approximately four cycles. Impulsive rotation comprised of velocity steps at +/–60°/s until the nystagmus subsided (approximately 45 seconds, maximum of 100 seconds, approximate acceleration/deceleration (–140°/s2). In full field optokinetic testing the subject was stationary whilst the surrounding striped curtain revolved around the patient’s vertical axis at a speed of 40°/s, alternating direction every 5–10 s for approximately 30 s. Optokinetic after nystagmus was recorded on cessation of the optokinetic stimulus.
For bithermal water caloric testing, a 40-second irrigation in each ear at 44°C and 30°C according to the Fitzgerald-Hallpike technique was performed. Canal paresis was calculated using nystagmus duration according to Jongkees’ formula: [(Right 30°C + Right 44°C) - (Left 44°C + left 30°C)]/(Right 30°C + Left 44°C + Right 44°C + Left 30°C). Likewise, directional preponderance was calculated as [(Right 30°C + Left 44°C) - (Right 44°C + left 30°C)]/(Right 30°C + Left 44°C + Right 44°C + Left 30°C). Due to the retrospective nature of this study, video head impulse testing was not available.
MRI imaging of the brain/internal auditory meati was performed where clinically indicated by the history, examination and investigation findings following standard clinical recommendations to exclude structural abnormalities.
Statistical analysis was carried out using SPSS for windows version 24. Descriptive statistics were used to summarize the data collected. Mann-Whitney U tests were used to compare the difference of means of data that was not normally distributed whilst independent samples t tests were used for normally distributed data. Analysis of variance testing (ANOVA) was used to analyse whether there were any statistically significant differences between the means of 3 or more groups. Fishers exact test (for smaller groups) or Chi2 tests were used to test for associations in categorical variables. All data were quoted to 3 significant figures and a p value of <0.05 was considered significant.
3Results
28 patients with a diagnosis of Motorist’s Disorientation Syndrome were identified prospectively through clinic and retrospectively by case note searching. One patient declined to participate, four were uncontactable and three were excluded as the diagnosis was thought to be wrong at interview (with symptoms primarily of visually triggered vertigo, but when outside a car rather than when driving). 2 patients were excluded on the basis of MRI imaging which indicated a structural abnormality which was primarily thought to be the cause of their symptoms: one patient had moderate small vessel disease in the brainstem that could contribute to symptoms and confound investigation interpretation, one patient was found to have an acoustic neuroma.
3.1Demographic data
i) Subjects: In total data from 18 participants was included (9 male and 9 female). The mean age of onset of symptoms was 41.7 years (SD = 10, range 20–56 years). An independent samples Mann-Whitney U test showed that the age at onset distribution was not significantly different for men and women (p = 0.258).
ii) Controls: Age and gender matched groups of healthy controls (n = 12) and patients with a diagnosis of vestibular migraine (VM) (n = 11) were compiled.
3.2Reported characteristics
All 18 patients experienced a feeling of disorientation or illusions of movement whilst driving. The onset was described as acute by 12 patients. 72.2% (n = 13) experienced a unidirectional sense of pulsion (see Table 1). 72.2% (n = 13) of patients only experienced symptoms on roads with high speed limits such as motorways and dual carriageways. 33.3% (n = 6) reported that their symptoms were worse on bumps/hills. 64.7% (n = 11) of subjects also reported symptoms whilst being a passenger. 44% (n = 8) of patients also experienced these symptoms when not in a car.
Table 1
Summary table ofthe 18 subjects detailing key symptoms and vestibular testing findings. There were deemed to be no abnormal findings in the tests performed if there was no significant directional preponderance or canal paresis on caloric testing, no spontaneous or gaze evoked nystagmus with or without fixation on ENG, no abnormalities on gain, phase or symmetry on smooth pursuit ENG, no abnormalities on latency, velocity and accuracy of horizontal involuntary saccade testing, normal values for gain, phase and symmetry for sinusoidal rotation, normal gain and time constant for impulsive rotation, normal gain and symmetry for OKN and time constant and gain for OKAN. All measurements were compared to the normative values obtained for departmental testing
| Subject | Gender | Age at | Duration of | Feeling of | Symptoms | Unidirectional | Symptoms | Symptoms | Abnormal |
| onset | symptoms | disorientation or | only on fast | pulling | outside | also when a | findings on | ||
| (years) | illusions of | moving roads | sensation | of car | passenger | vestibular | |||
| movement | testing | ||||||||
| whilst driving | |||||||||
| 1 | M | 44 | 3 | yes | yes | no | no | yes | nil |
| 2 | F | 34 | 10 | yes | yes | yes-R | no | yes | nil |
| 3 | M | 36 | 7 | yes | yes | yes-L | no | yes | Insufficient testing |
| 4 | F | 41 | 8 | yes | no | yes -other | yes | yes | nil |
| 5 | M | 51 | 3 | yes | yes | yes-L | no | no | Right DP (IR) abnormal OKAN |
| 6 | F | 47 | 3 | yes | no | yes-L | yes | no | Insufficient testing |
| 7 | M | 51 | 7 | yes | yes | yes -L | no | yes | Right DP (SR). Left CP with Right DP on caloric |
| 8 | F | 36 | 8 | yes | yes | no | no | no | Abnormal OKAN |
| 9 | F | 27 | 16 | yes | yes | yes- backwards | yes | yes | nil |
| 10 | M | 46 | 4 | yes | no | no | yes | yes | Right DP (SR) |
| 11 | F | 20 | 11 | yes | no | no | no | yes | Abnormal OKN (right DP with normal gain) |
| 12 | F | 56 | 5 | yes | yes | yes-L | no | no | Insufficient testing |
| 13 | M | 50 | 4 | yes | yes | yes | no | yes | Abnormal OKN (right) |
| 14 | F | 30 | 17 | yes | yes | yes-L | no | yes | nil |
| 15 | F | 52 | 2 | yes | no | no | yes | no | Bilateral vestibular hypofunction (SR) |
| 16 | M | 51 | 3 | yes | yes | yes -backwards | yes | yes | Right DP (SR and IR) |
| 17 | M | 34 | 3 | yes | yes | yes-L | yes | no | Bilateral hypofunction (SR) abnormal OKN |
| 18 | M | 44 | 1 | yes | yes | yes-R | yes | no | nil |
(M = male, F = female, L = left, R = right, DP = directional preponderance, CP = canal paresis, OKN = optokinetic nystagmus, OKAN = optokinetic after nystagmus, SR = sinusoidal rotation, IR = impulsive rotation.
The mean length of symptoms was 6.39 years (SD = 4.63, range 1–17 years) with a mean duration of 3.89 years in men (SD = 1.97, range 1–7 years) and 8.89 in women (SD = 5.26, range = 2–17 years). An Independent Mann-Whitney U test analysis shows that the duration of symptoms was significantly longer for the women than for the men in our sample (U = 16, N1 = 9, N2 = 9 two tailed p = 0.03). Half of the patients (n = 9) had a stable course.
38.9% (n = 7) of subjects reported feelings of acute anxiety or panic associated with their symptoms. 2 patients reported that first degree relatives had also experienced similar symptoms. 50% (n = 9) met the criteria for a diagnosis of migraine, of these 5 had self defined motion sickness.
Symptoms were severe enough to cause 17 patients (94.4%) to change their driving habits: 6 subjects (33%) stopped driving completely and 11 (61%) stopped driving on provoking roads. Only 1 patient continued to drive as usual.
3.3Questionnaires
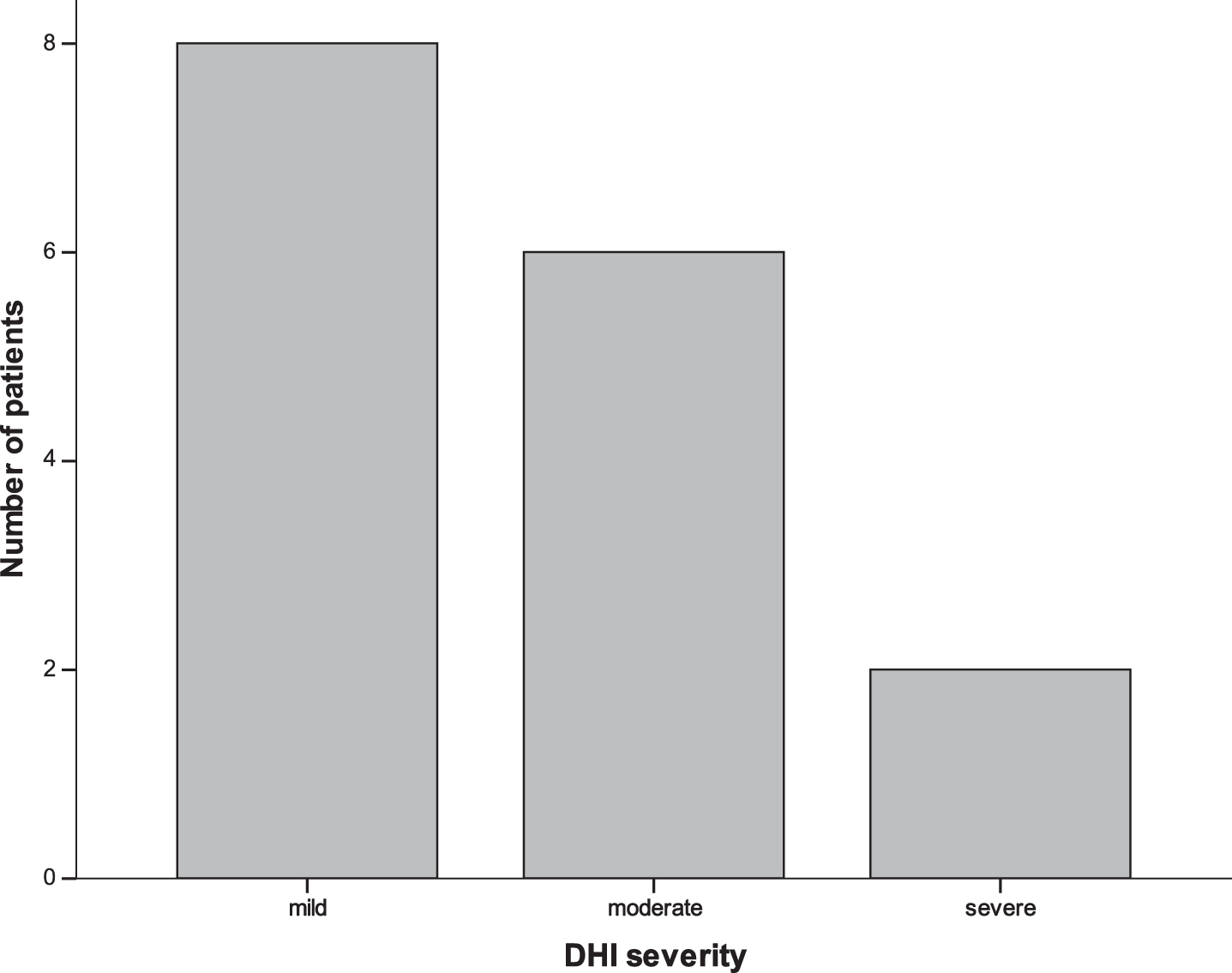
The DHI was administered to 16 patients. The total score (DHI-T) was a mean of 34.63 (SD = 21.796). From the 16 subjects 8 had a score indicative of mild handicap, 6 moderate and 2 severe (Fig. 1). The mean response to the emotional subset was 11.75 (SD = 7.60), to the functional subset was 12.88 (SD = 12.8) and to the physical subset was 10.00 (SD = 7.866).
Fig. 1
Histogram to show the total DHI scores indicating overall severity of self-perceived handicap from dizziness. A total score of 0–30 reflects mild, 31–60 moderate, and 61–100 severe handicap.
The VSS was completed by 12 patients, There was no significant difference between the VSS-AA scores for men (M = 24.80) and women (M = 25.14) (t = 0.43, df = 10, p = 0.966).
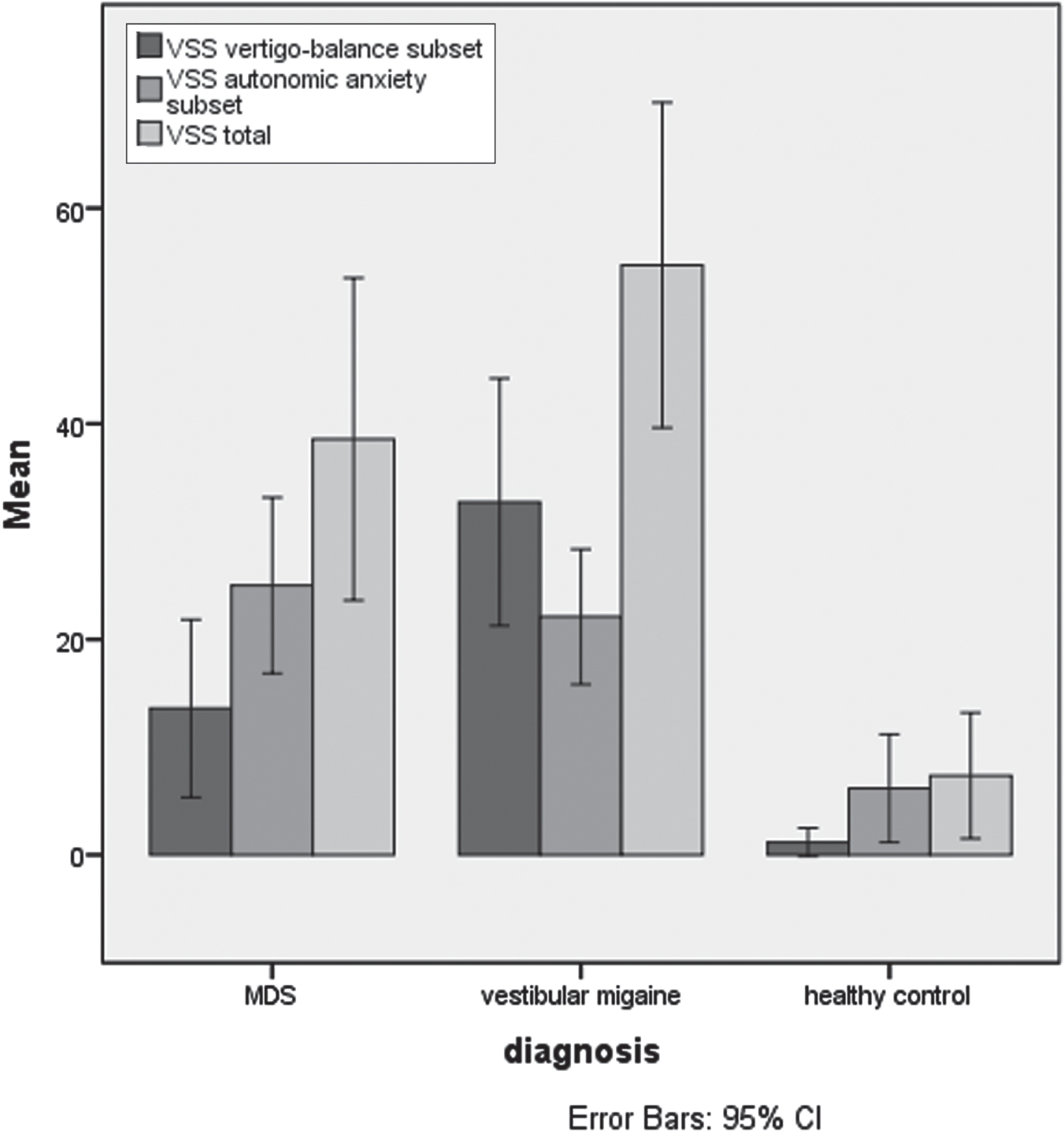
The MDS patients’ VSS scores were compared to the 2 control groups: 11 with VM and 12 normal controls (see Fig. 2). A one-way ANOVA showed an overall significant difference for mean VSS-AA scores (p = 0.000, F = 39.5), and the mean total VSS scores (p = 0.002, F = 24), with the MDS group having a higher score in both instances.
Fig. 2
Bar chart to show the VSS scores in the MDS, vestibular migraine and healthy control groups
3.4Vestibular testing
15 patients had complete vestibular testing. The remaining 3 patients were excluded from the analysis as compliance issues resulted in incomplete test results and it was deemed that they had not had sufficient testing to determine vestibular function. Due to the retrospective nature of the data video head impulse testing records were not available or unreliable.
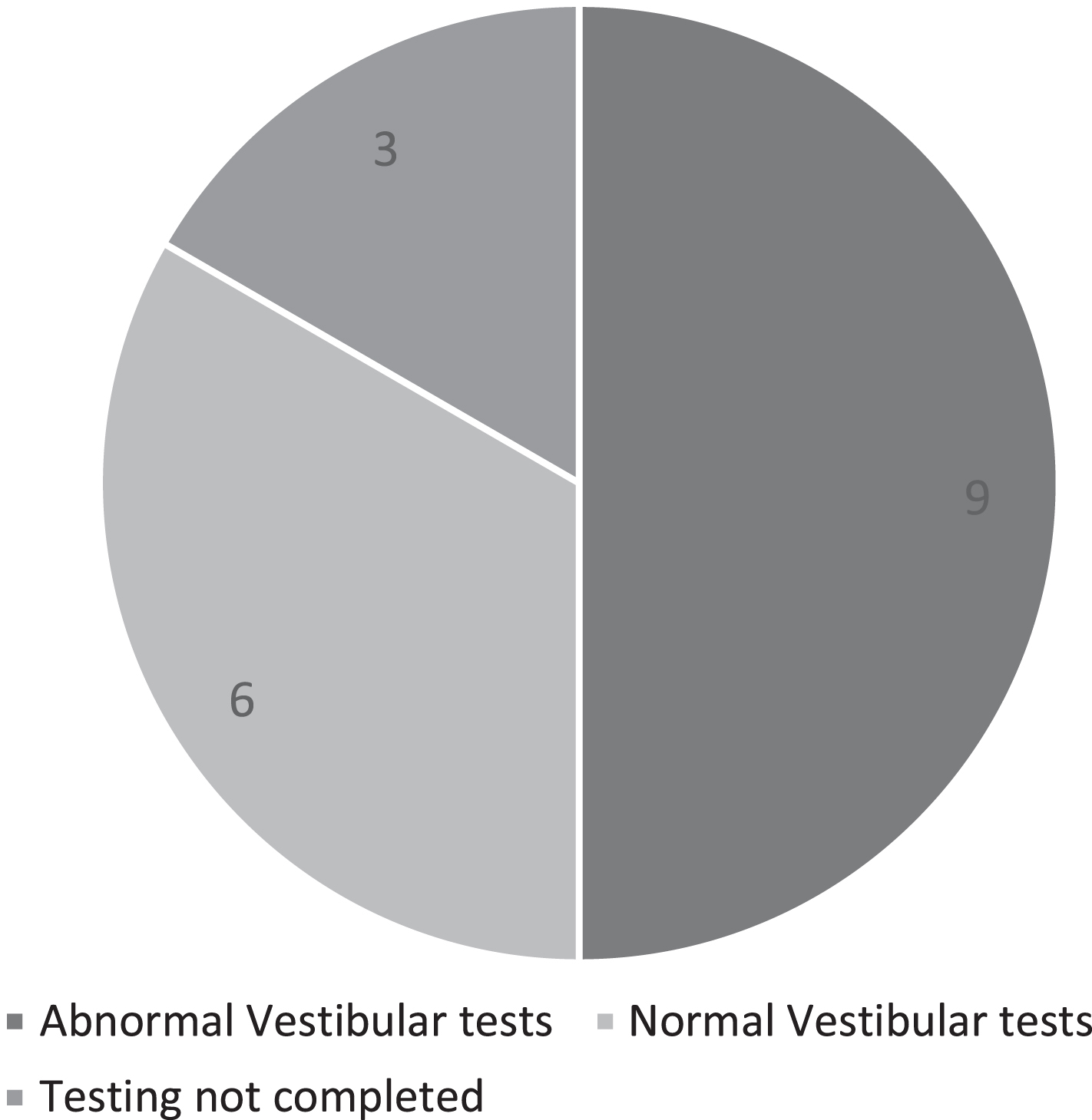
In total 9/15 (60%) patients were found to have abnormal vestibular test results with a mixture of peripheral and central vestibular findings indicating vestibular tonal asymmetry (see Fig. 3). 3 patients had evidence of peripheral vestibular dysfunction (canal paresis +/– directional preponderance on caloric/rotation testing) with a further 2 patients demonstrating bilateral vestibular dysfunction. 2 patients had a directional preponderance on OKN testing, 1 patient had a directional preponderance on impulsive rotation and abnormal OKAN and 1 patient had isolated abnormal OKAN.
Fig. 3
Pie chart showing vestibular test results.
Fisher exact test analysis revealed that there was no significant association between the presence of vestibular abnormalities and the acute/chronic onset of symptoms (two-tailed Fisher exact p = 1.000), the presence of symptoms as a passenger (two-tailed Fisher exact p = 0.580) and the presence of symptoms outside of the car (two-tailed Fisher exact p = 0.315). There was no significant association between the presence of symptoms only on motorways (two-tailed Fisher exact p = 0.136) or symptoms made worse by bumps etc. (two-tailed Fisher exact p = 1.000) and the presence of vestibular abnormalities. There was also no significant relationship between the presence of vestibular test abnormalities and the reporting of unidirectional pulling symptoms (two-tailed Fisher exact p = 0.58).
An independent t-test showed that there was a significant difference between mean total DHI scores (DHI-T) between the group of patients with normal vestibular tests (mean = 52.40) and those without (mean = 29.78) (p = 0.049). The group with normal vestibular tests gave higher mean scores.
3.5Vestibular rehabilitation
8 patients (44.4%) received vestibular rehabilitation treatment for their symptoms. Of these 5 were treated with traditional vestibular rehabilitation gaze stabilisation exercises alone (GS), 4 of whom found that these didn’t help, and 1 found them subjectively useful. A further 3 were treated with a combination of Visual Vertigo (VV) and gaze stabilisation exercises. All of these found the gaze stabilisation exercises not useful but the visual vertigo exercises useful. 2 patients had used ‘as required’ travel sickness medication. Only 1 found this useful.
An independent samples t-test on the limited treatment data obtained showed that there was a significant difference between the age of the patient and whether the treatment helped (M = 37.75, SD = 6.076) or not (M = 50.80, SD = 6.573), with treatment more likely to help those who are younger (t = 3.057, df = 7, two-tailed p = 0.018). Chi2 analysis showed that there was no significant association between whether treatment helped and the presence of vestibular abnormalities (X2 = 0.565, df = 2, p = 0.754).
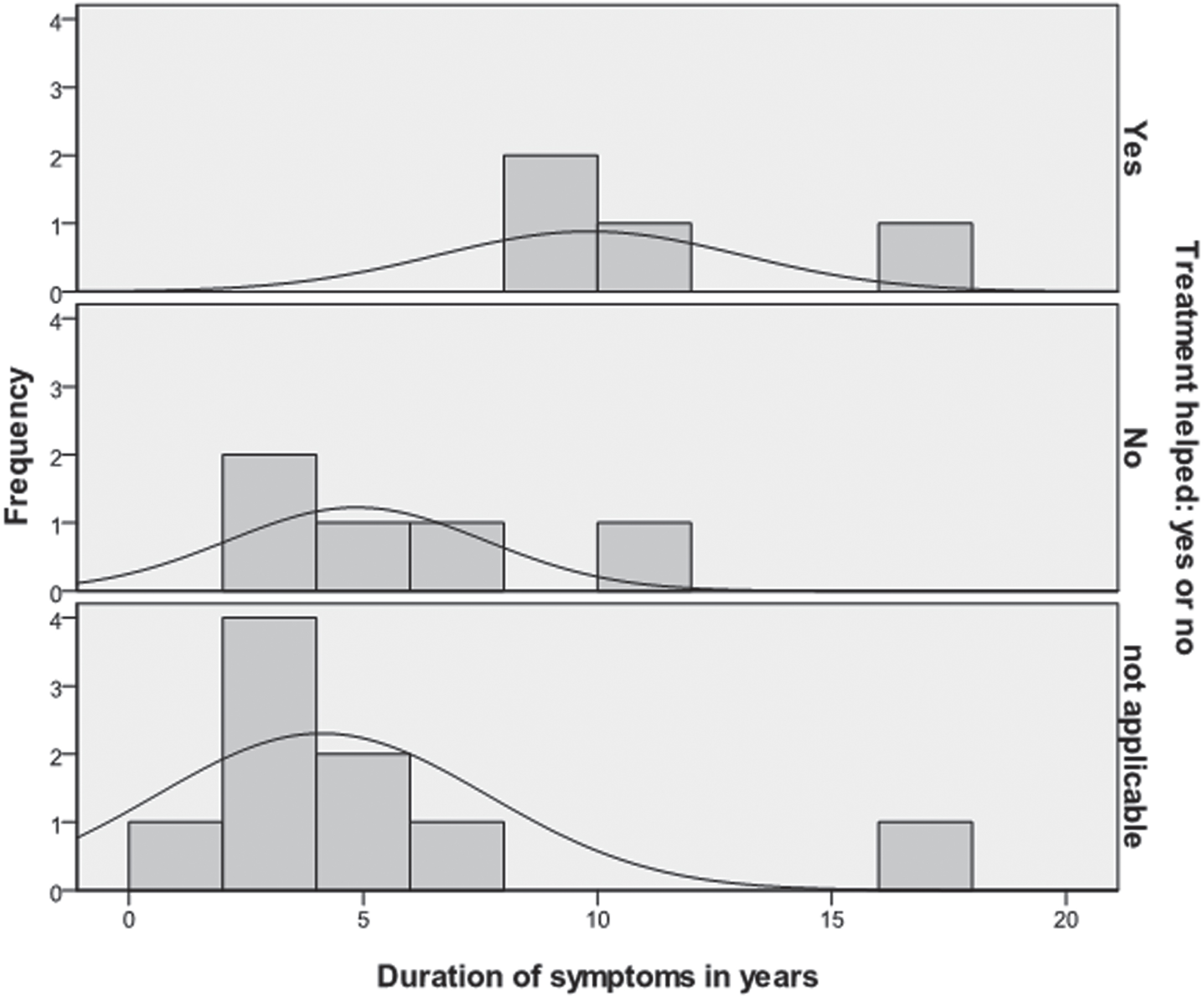
An independent samples t-test showed that there was no significant difference between the mean duration of symptoms and whether treatment helped (M = 10.8) or not (M = 5.60) (t = 2.30, df = 7, p = 0.06) (see Fig. 4).
Fig. 4
Histogram to show the relationship between the duration of symptoms and whether treatment helped.
4Discussion
Within recent years, there has been increasing interest in the complex integration of input signals that control spatial orientation, retinal stability and balance in response to a changing visual environment such as in a moving car.
Within a static environment, input signals from the visual system, proprioceptive system and vestibular system must be integrated and modulated by the higher cognitive networks, including the memory and emotional centres. When the surrounding environment is dynamic, such as in a moving car, these signals must be processed at a speed likely to be dependent on the speed of the moving car, in order to provide real time information about spatial orientation within the environment. The complexity of the signals is also expected to vary depending on the road condition e.g. proprioceptive information will be increased on bumpy or uneven roads compared to smooth roads, or the characteristics of the visual scenarios e.g. tree-lined roads or motorways compared with the featureless, open tops of hills.
Patients with vestibular hypofunction may have unreliable or inaccurate input signals from the periphery, leading to inaccurate central integration outputs, and hence experience an impaired sense of self-orientation. This may be the basis for the illusion of the car veering/turning. Within the existing literature, the significance of vestibular hypofunction as a cause of these symptoms has been debated. In the original descriptions by Page and Gresty [21], evidence of either central or peripheral vestibular findings was described in all patients. Their explanation centres around vestibular deficits, within either the otolith organs, semi-circular canals or a combination of the two, causing abnormal sensations of lateral acceleration or tilt. Interestingly, Gresty was later to observe that the vestibular abnormalities found in the original paper were slight [10]. Brookler [6] described a case study of a 24-year-old man with a 2 month history of feeling as if he were ‘on a roller coaster’ whilst driving at high speed who had a bi-thermal caloric testing showing a 33% directional preponderance to the left, suggesting a degree of vestibular tonal imbalance. In contrast to the studies above, but in line with Bronstein et al. [2] who discounted vestibular pathology as the sole mechanism for MDS, 40% of our patients did not have evidence of vestibular dysfunction and in those that did, the vestibular findings were in some cases non-specific and showed poor directional correlation to their symptoms. Interpretive caution must be applied before discounting vestibular dysfunction as a mechanism however as detection is limited by the testing modality sensitivity. Two patients had evidence of bilateral vestibular hypofunction. This relatively high number is interesting in view of the finding that those with bilateral vestibular hypofunction are less likely to get motion sickness, another condition where sensory conflict is felt to be important [16].
In some cases of vestibular hypofunction, a process occurs where there is an alteration in the relative weighting given to the input signals that provide information about balance and self-orientation. In these patients, visual input is prioritised as the most reliable input (visual dependence). In evolutionary terms, this shift in the relative weighting of the input signals makes sense as it allows for substitution of a more reliable information stream to allow for balance and orientation to be maintained. However, it also leaves these patients vulnerable in scenarios where optic information is complex and unreliable. These scenarios can trigger symptoms known clinically as ‘visually induced dizziness.’ Within a dynamic moving environment, there is increased potential for complexity of these input signals. Guerraz et al. [11] recognised that the optic flow information perceived by a driver whilst moving in a car could potentially be a trigger for visually induced dizziness and this may be the basis for the MDS presentation. There is also a variation in the degree of visual dependence in normal individuals, with those with higher visual dependence being more symptomatic after vestibular insult, [8] potentially predisposing them to MDS in this scenario.
In the scenario where a patient is reliant on visual information in order to maintain self-orientation, a sudden lack of such input information, such as when going over the brow of a hill, is likely to cause disorientation. Symptoms that were triggered by hills, bumps and dips were a key feature in Page and Gresty’s original description of MDS and have been cited by other authors as a feature of MDS. Around a third of our patients found that they were a triggering factor. Symptoms were present in a relatively high proportion of patients also as passengers. This is likely to reflect that similar visual inputs are seen as a passenger, although information about back seat passengers, who would potentially be exposed to a different /reduced visual input, was not collected and may be an interesting line of research for the future. 44% of our patients also experienced similar symptoms, albeit to a lesser extent when not in a car, suggesting either poor compensation from vestibular hypofunction or a degree of visual dependence/ VV in other similar scenarios.
Although MDS does not share all the clinical symptoms that define PPPD, VV has been incorporated as part of the core symptoms of PPPD [23] and thus MDS shares some similarities as well as some differences. The main similarity is the exacerbation by movement and exposure to visually complex or moving stimuli, including in particular full field visual stimuli, such as might be experienced in a moving car. It does appear to cause significant distress and functional impairment, and patients avoid provoking scenarios. However, the symptoms described, such as pulling feelings, are recognised as not typical for PPPD. MDS does appear to have a chronic course, however it varies from PPPD in that symptoms only appear to be provoked predominately in a driving scenario, rather than having a more persistent presence. MDS also differs to PPPD in gender differences. Our results showed a 50:50 split between males and females with the symptoms of MDS. This is consistent with the other reported case studies [3, 21] where there did not appear to be a significant gender preponderance, but not with the female preponderance seen in early reports of PPPD [23].
The fact that these symptoms are provoked purely whilst in a car raises the possibility that MDS may be a form of motion sickness. Diagnostic criteria for motion sickness have been produced by the Barany society [7]. One of the criteria is that physical or visual motion produces dizziness and/or vertigo, further delineated as including visual motion illusions, potentially correlating with the symptoms of pulsion described by our patients. Individuals with migraine are known to have greater susceptibility to motion sickness [7] 50% (n = 9) of our patients met the criteria for a migraine diagnosis. However the role of migraine may be multifactorial, as it also confers an increased susceptibility to VV [15] and is recognised as a trigger for PPPD [23].
An accurate picture of the incidence and prevalence of these reported symptoms is difficult to determine from the literature, partly due to the lack of a formal definition or diagnostic criteria. There is a paucity of reports of patients with these symptoms which may be due to an under recognition of the condition [3]. Bronstein et al. [2] described that 1% of patients referred to a tertiary centre report motorists’ disorientation, which makes approximately five individuals each year. These were adults of both sexes. Gresty and Ohlmann [10] describe seeing an average of 10 patients out of 200 in a neuro-otological clinic with MDS (5% of patients). Our patients were recruited, both retrospectively and prospectively in a tertiary neuro-otology clinic, therefore the numbers are unlikely to reflect the prevalence of patients with these symptoms presenting in non-tertiary clinics. However, as has been noted by other studies, there is likely to be under-recognition of these symptoms as a clinical entity. Patients may simply avoid driving rather than present to medical attention. Our prevalence amongst a tertiary clinic population is certainly less than the 1 in 20 patients reported by Gresty and Ohlman [10] but above Bronstein’s reported prevalence of approximately 5 patients per year presenting in his clinic [3]. It should be noted, however, that the lack of formal diagnostic criteria of what constitutes MDS might be the cause of thesedifferences.
The mean age of onset in our study, at 42 years, with a range of 20–56 years agrees with Bronstein’s statement that the patients are typically young and middle aged [3] This age presentation profile would fit with the reported average age of presentation of PPPD that is the mid-40 s, with a range from adolescence to late adulthood [23]. However it should be borne in mind that those younger than 17 years are not legally allowed to drive in the UK.. Also, the UK National Travel Survey shows that this is age bracket (40–49) who make the most trips per year which may be a confounding factor [18].
The duration of the symptoms, with a range of 1 to 17 years (mean = 6.39) would categorise MDS as a chronic condition [17]. Our results showed that the duration of symptoms was significantly longer for the women than for the men in our sample (despite the age of onset not being significantly different), by definition increasing the prevalence in women. The reason for this longer duration of symptoms is not clear. Gender differences in processing conflicting visuo-vestibular information as recognised by other authors [24], may be an explanation. Another explanation may relate to gender differences in anxiety manifestations; however, our data did not indicate this. Gender differences in trips per person per year also do not appear to explain this[18].
In Page and Gresty’s original description the impact the symptoms had on the subjects was mentioned. Two of the patients who had illusions of being pulled had their cars checked for mechanical problems. Patients were described as having their daily activities restricted whist others as having to limit their driving speed or routes. Bronstein [3] describes a case of the feeling of veering causing a taxi driver to take such inappropriate countermeasures that she ended up on other side of the road, clearly presenting a safety issue. Our results agree with other authors that patients are often significantly affected by their symptoms and that this is not a complaint to be dismissed lightly, indeed 94.4% of our subjects altered their driving habits. Driving phobia has been defined as ‘either complete elimination of all driving or severe restriction of all driving,’ driving reluctance, as ‘avoidance of all discretionary (driving for pleasure) driving or riding’, and avoidance as driving ‘limited to the accident site or certain classes of driving situations’ [12]. Based on these definitions 61% of our subjects would have driving reluctance and 33% driving phobia as a result of their symptoms.
The use of self-reported measures of the impact of dizziness is important as the results of vestibular function tests have been shown to correlate poorly with perceived impact on quality of life for the patient [14]. At least a mild handicap was reported on the DHI in all patients, 50% reporting a mild handicap, 37.5% a moderate handicap and 12.5% perceiving their handicap to be severe. It was noticeable that the VSS– total and VSS-AA scores were significantly higher in the MDS group compared to healthy controls indicating the severity of the clinical symptoms. They were not significantly different to the vestibular migraine group, which in itself is indicative of severity, as migraine has been shown to have a significant impact on quality of life [20].
A range of treatment was used, perhaps reflecting the fact that treatment for MDS is acknowledged as difficult [7] and a lack of evidence base within this area. Half felt a subjective improvement in their symptoms with either GS exercises alone or a combination of GS and VV exercises, suggesting, at present, this is a reasonable course of action. However, the numbers treated were small in this study therefore it would be unwise to conclude about treatment efficacy.
Other studies have proposed that the mainstay of treatment should be directed towards desensitisation to moving environments, based on treatment strategies used with patients with visual vertigo [11]. For example, Gresty and Ohlman [10] proposed desensitisation strategies, starting with simple environments and moving to increasingly unstable and ambiguous environments with Bronstein suggesting a similar framework of desensitization and retraining [5]. Some novel approaches could also be examined in the future such as the role of a tactile vibration system to overcome spatial disorientation, trialled in aircraft [22].
Within our small group, treatment appeared to be more effective in those who are younger, in line with other vestibular disorders [9], although these patients may have improved anyway, as they are less likely to have co-morbid factors that would affect compensation.
5Conclusion
We have presented the largest case series of patient with symptoms of disorientation and illusions of movement of self or the vehicle, primarily when driving a motorcar. Despite the limitations of this type of study, common features have been elicited and described.
Further thought is needed as to where this constellation of symptoms fits within the diagnostic criteria already produced for other conditions such as VM, PPPD and motion sickness, to help clinicians further understand and manage this condition. It may be more complex than a peripheral vestibular deficit, as was initially thought, with functional reweighting within the central vestibular pathways such as occurs in functional vestibular disorders, a possible explanation.
This study provides data which can contribute to discussion about where MDS should be placed within the current diagnostic framework for diagnosis of vestibular disorders. In turn, improved recognition and appropriate classification of this symptom complex will help clinicians to recognise the specific features of the condition, enabling further research into the role of potential factors such as visual dependency and facilitating further research into treatment.
Supplementary information
Statistical analysis for age and gender controls:
A one-way unrelated analysis of variance (ANOVA) confirmed there was no significant difference between the mean age for the MDS group (M = 46.17 SD = 8.255), the VM group (M = 42.55 SD = 10.113) and the normal controls (M = 45.67, SD = 7.808) (p = 0.570). Chi squared analysis confirmed that there was no significant difference between the observed and expected frequency of men and women in each group (X2 = 3.457. df = 1, p = 0.063).
References
[1] | Bronstein A.M. , Visual vertigo syndrome: Clinical and posturography findings, J Neurol Neurosurg Psychiatry 59: ((1995) ), 472–476. |
[2] | Bronstein A.M. , Golding J.F. and Gresty M.A. , Vertigo and dizziness from environmental motion: Visual vertigo, motion sickness, and drivers’ disorientation, PhD Semin Neurol 33: ((2013) ), 219–230. |
[3] | Bronstein A.M. , Oxford Textbook of Vertigo and Imbalance, Oxford University Press, 2013. |
[4] | Bronstein A.M. and Lempert T. , Dizziness, A Practical Approach toDiagnosis and Management Second Edition, Cambridge University Press, 2017. |
[5] | Bronstein A.M. , Golding J.F. , Gresty M.A. , Visual vertigo, motionsickness and disorientation in motor vehicles, Sem Neurol 40: (1) ((2020) ), 116–129. |
[6] | Brookler K.H. , Electronystagmography in a patient who could notdrive more than 5 minutes at highway speeds Ear, Nose & Throat Journal 81: (4) ((2002) ). |
[7] | Cha Y. , Golding J.F. , Keshavarz B. , Furman J. , Kim J. , Lopez-Escamez J.A. , Magnusson M. , Yates B. and Lawson B. , Motion sickness diagnostic criteria: Consensus Document of the Classification Committee of the Bárány Society, J Vestib Res 31: (5) ((2021) ), 327–344. |
[8] | Cousins S. , Cutfield N.J. , Kaski D. , Palla A. , Seemungal B.M. , Golding J.F. , Staab J.P. and Bronstein A. , Visual dependency and dizziness after vestibular neuritis, PLoS One 9: (9) ((2014) ), e105426. 18. |
[9] | Davies R. , Luxon L. , Bamiou D. and Shorvon S. , Neuro-Otology: Problems of Dizziness, Balance and Hearing in: C. Clarke, R. Howard and M. Rossor, et (ed). Neurology, A Queen’s Square Textbook, Second Edition, Wiley Blackwell, 2009. |
[10] | Gresty M.A. and Ohlmann T. , Motorists Vestibular Disorientation Syndrome Revisited, In: Spatial Disorientation in Military Vehicles: Causes, Consequences and Cures, held in La Coruña, Spain, Published in RTO-MP-086, 2002. |
[11] | Guerraz M. , Yardley L. , Bertholon P. , Pollak L. , Rudge P. , Gresty M.A. and Bronstein A.M. , Visual vertigo: Symptom assessment, spatial orientation and postural control, Brain 124: (Pt 8) ((2001) ), 1646–1656. |
[12] | Hickling E.J. and Blanchard E.B. , The private practice psychologist and manual-based treatments: Post-traumatic stress disorder secondary to motor vehicle accidents, Behaviour Research and Therapy 35: (3) ((1997) ), 191–203. |
[13] | Jacobson G.P. , The development of the dizziness handicap inventory, PhD Arch Otolaryngol Head Neck Surg 116: (4) ((1990) ), 424–427. |
[14] | Jacobson G.P. , Newman C.W. , Hunter L. and Balzer G.K. , Balance function test correlates of the Dizziness Handicap Inventory, J Am Acad Audiol 2: ((1991) ), 253–260. |
[15] | Lempert T. , Vestibular migraine, Semin Neurol 33: ((2013) ), 212–218. |
[16] | Murdin L. , Golding J. and Bronstein A. , Managing motion sickness, BMJ ((2011) ), 343. |
[17] | National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) https://www.cdc.gov/chronicdisease/about/index.htm. |
[18] | National Travel Survey: 2018 Statistics covering personal travel within Great Britain by English residents. https://www.gov.uk/government/statistics/national-travel-survey-2018. |
[19] | Neuhauser H. and Lempert T. , Vertigo and dizziness related to migraine: A diagnostic challenge, Cephalalgia 24: ((2004) ), 83–91. |
[20] | Neuhauser H.K. , Radtke A. , von Brevern M. , Feldmann M. , Lezius F. , Ziese T. and Lempert T. , Migrainous vertigo: Prevalence and impact on quality of life, Neurology 67: (6) ((2006) ), 1028–1033. |
[21] | Page N.G. and Gresty M.A. , Motorist’s vestibular disorientation syndrome, J Neurol Neurosurg Psychiatry 48: ((1985) ), 729–735. |
[22] | Paillard A.C. , Quarck G. and Denise P. , Sensorial countermeasures for vestibular spatial disorientation aviation, Space, and Environmental Medicine 85: ((2014) ). |
[23] | Staab J.P. , Eckhardt-Henn A. , Horii A. , Jacob R. , Strupp M. , Brandt T. and Bronstein A. , Diagnostic criteria for persistentpostural-perceptual dizziness (PPPD): Consensus document of thecommittee for the Classification of Vestibular Disorders of theBárány Society, J Vestib Res 27: (4) ((2017) ), 191–208. |
[24] | Viaud-Delmon I. , Ivanenko Y.P. , Berthoz A. , et al., Sex, lies and virtual reality, Nature Neuroscience 1: ((1998) ), 1. |
[25] | Yardley L. , Masson E. , Verschuur C. , Haacke N. and Luxon L. , Symptoms, Anxiety and handicap in dizzy patients: Development of the vertigo symptom scale, Journal of Psychosomatic Research 36: (8) ((1992) ), 731–741. |



