Characteristics of persistent postural perceptual dizziness patients in a multidisciplinary dizziness clinic
Abstract
BACKGROUND:
Persistent Postural Perceptual Dizziness (PPPD) is a newly defined condition which was added to the International Classification of Vestibular Disorders in 2017. Little is known about its impact on patients.
OBJECTIVE:
The goal of this study was to analyze the symptomology, epidemiology and impact of PPPD on patients.
METHODS:
A retrospective chart review was done to identify patients who attended the Multidisciplinary Dizziness Clinic (MDC) and were diagnosed with PPPD. Responses to demographic questions, health-related quality of life surveys and several well-validated questionnaires commonly used to assess dizziness severity were analyzed.
RESULTS:
One hundred patients were diagnosed with PPPD between March 2017 and January 2019, of which 80%(80/100) were females. The average Dizziness Handicap Index score was 60.3±19.0. Responses to the Patient Health Questionnaire classified 53 patients (53/99;53.5%) as moderately to severely depressed. Sixty-four patients (64/100;64.0%) were minimally or mildly anxious according to the Generalized Anxiety Disorder scale. The average Vertigo Symptom Scale score was 24.1/60. The average Situational Vertigo Questionnaire score was 2.00. Forty-nine (49/100;49.0%) patients had migraine symptoms according to the Migraine Screen Questionnaire.
CONCLUSIONS:
In conclusion, patients with PPPD display important handicap and an elevated risk of depression, anxiety and migraines.
1Introduction
In 1986, Brandt and Dieterich described Phobic Postural Vertigo (PPV), a condition characterized by fluctuating unsteadiness and postural dizziness which did not otherwise fit the criteria of any defined neurological disorder [3]. Staab and Ruckenstein expanded on this concept by describing Chronic Subjective Dizziness (CSD), a condition characterized by persistent dizziness accompanied by subjective imbalance and worsened by moving or complex stimuli [32]. It was not until 2014 that a consensus for a defining criterion was reached in order to establish the diagnosis of Persistent Postural-Perceptual Dizziness (PPPD) and not until 2017 that this condition was added in draft form to the forthcoming 11th edition of the International Classification of Diseases by the World Health Organization (WHO) [13].
PPPD is a functional disorder characterized by dizziness, unsteadiness or non-spinning vertigo exacerbated upright posture, active or passive motion, and exposure to moving visual stimuli or complex visual patterns persisting for three months or more [33]. Although not essential for diagnosis, psychiatric comorbidities frequently accompany this diagnosis [30, 33]. This condition is often precipitated by a vestibular disorder, though any disorder that causes a bout of dizziness can be a precipitant. Typically, following the onset of such a disorder, remission of the precipitating diagnosis and compensation of the deficit occurs. However, in the case of PPPD, readaptation is inadequate and an inappropriately heightened reaction to certain postures, motions or visual stimuli ensues [32]. However, due to the novelty of PPPD and the wide array of healthcare professionals who see this disorder, challenges still remain when describing its incidence and prevalence. Still, before PPPD was defined, CSD was shown to represent 10.6%of all causes of dizziness in vertiginous patients presenting to a neuro-otology centre [26] while PPV was reported as being the most frequent form of dizziness, ranging from 22%to 26%in patients presenting to an outpatient dizziness clinic [39]. Furthermore, prospective studies have shown that 25%of individuals having suffered from an acute or episodic vestibular condition such benign paroxysmal positional vertigo will develop persistent dizziness similar to that in PPPD [33] and that most cases of functional dizziness with no identifiable vestibular or central origin are due to PPPD [35].
As PPPD is a newly defined condition with a scarcity in the literature, the goal of this study is to analyze the symptomology and epidemiology of patients affected by PPPD as well as its impacts on function, emotion and quality of life.
2Method
Written and informed consent was obtained from all participants prior to completion of the questionnaire and data collection approval was granted from our institutional Research Ethics Board (REB #20160831-01H).
2.1Multidisciplinary dizziness clinic (MDC)
In 2015, a Multidisciplinary Dizziness Clinic was formed at The Ottawa Hospital consisting of a neuro-ophthalmologist, an otolaryngologist, and other allied health professionals. This clinic accepts referrals for patients with undiagnosed dizziness from other specialists only, typically neurologists or otolaryngologists. Referrals for unspecified dizziness clearly temporally related to a head injury were in general not seen, as our centre has a separate clinic for traumatic brain injury. This resulted in a very focused, specific and homogeneous patient population of the most severe chronically dizzy patients. Patients undergo a full assessment by the team and diagnoses are then established using the patient’s symptoms, clinical examination and investigations completed prior to clinic. Full details about this clinic can be found in our previously published work [35].
Before attending the clinic, patients were invited by mail to complete a paper-based or online form consisting of demographic information, health-related quality of life surveys and several well-validated questionnaires commonly used to assess symptom severity and outcomes [8, 22]. Non-questionnaire information included gender, date of birth, symptom duration and description, duration of time off work, number of specialists consulted, investigations completed, and treatments attempted prior to this clinic. The rest of the form uses well-validated questionnaires: the Dizziness Handicap Inventory (DHI) [15, 23], the Patient Health Questionnaire (PHQ-9) [9, 18], the Generalized Anxiety Disorder scale (GAD-7) [21, 31], Vertigo Symptom Scale (VSS) [44, 46], the Situational Vertigo Questionnaire (SVQ) [10], the Migraine Screen Questionnaire (MS-Q) [19] and the Migraine Disability Assessment (MIDAS) [36, 37]. The full survey can be found in Appendix A.
2.2Questionnaire details
The DHI is a 25-item questionnaire used to assess the patient’s self-perceived physical, emotional and functional impact of dizziness. Patients may answer “No”, “Sometimes” or “Always” to every question which are weighted as 0, 2 or 4 points, respectively. The impact score is stratified into mild (≤30 points), moderate (≥31 but ≤60) and severe (≥61 points). [43]
The PHQ-9 is a 9-item questionnaire based on the symptoms and diagnostic criteria of depression as per the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). A score from 0 to 3 is assigned depending on how often the patient experiences each symptom. The total score is then collated and depression severity is classified as minimal (0–4 points), mild (5–9 points), moderate (10–14 points), moderately severe (15–19 points) and severe (20–27 points) [18].
The GAD-7 is a 7-item screening tool for diagnosis and severity of generalized anxiety disorder based on symptom frequency which patients can respond to each question ranging from “not at all” (0 points) to “nearly every day” (3 points). The total amount of points is then used to assign a score of minimal (0–5 points), mild (6–10 points), moderate (11–15 points) or severe (>15 points) anxiety [21].
The VSS-sf is a 15-item questionnaire which also utilizes Likert scale questions to evaluate the frequency of vestibular (VSS-sf-V) and anxiety (VSS-sf-A) symptoms over the last month over 8 and 7 questions, respectively. Scores are given for each answer ranging from 0 (never) to 4 (very often) and a total score ranging from 0–60 is calculated with a higher score indicating more frequent symptoms [46].
The SVQ is a 19-item questionnaire which assess features of space and motion discomfort (visual vertigo) in patients with dizziness with possible answers ranging from “not at all” (0 points) to “very much” (4 points). Final scores are tallied and then the total is divided by 19 minus the number of situations never attempted. The total score ranges from 0 to 4 with a higher score indicating a higher likelihood of visual vertigo [7].
The MS-Q is a migraine screening questionnaire which consists of 5 binary questions. When tabulating scores, a single point is given for every positive answer (yes) and none for every negative answer (no). Patients are classified into either having a score suspicious for migraine (≥4 points) or not (< 4 points) [19].
The MIDAS questionnaire consists of 7 questions to evaluate the impact of migraines by assessing the frequency of symptoms over the last 3 months and the severity of headache from 0–10. The score is calculated by adding up the total number of days which patients experience symptoms due to their headaches and patients are classified as having little (≤5 points), mild (> 5 and ≤10 points), moderate (> 10 and ≤20 points) or severe (≥21 points) disability due to migraines [36].
2.3Diagnosis of PPPD
Patients were diagnosed with PPPD if they satisfied the following diagnostic criteria set forth by the committee for the Classification of Vestibular Disorders of the Bárány Society:
A. One or more symptoms of dizziness, unsteadiness, or non-spinning vertigo are present on most days for 3 months or more.
B. Persistent symptoms occur without specific provocation, but are exacerbated by upright posture, active or passive motion without regard to direction or position, and exposure to moving visual stimuli or complex visual patterns.
C. The disorder is precipitated by conditions that cause vertigo, unsteadiness, dizziness, or problems with balance including acute, episodic, or chronic vestibular syndromes, other neurologic or medical illnesses, or psychological distress.
D. Symptoms cause significant distress or functional impairment.
E. Symptoms are not better accounted for by another disease or disorder [33]. Clinical judgement in addition to audiological and vestibular testing (including videonystagmography, video head impulse testing, vestibular evoked myogenic potentials) and imaging, were used as appropriate to satisfy this criterion.
2.4Data collection and statistical analysis
A retrospective chart review was done to identify all the patients who attended the MDC and were diagnosed with PPPD between March 2017 and January 2019. Once these patients were identified, their surveys were collected for analysis and their charts were reviewed to identify the precipitating condition which triggered the onset of PPPD as well as comorbid diagnoses that may contribute to dizziness at the time of diagnosis. For the purpose of this study, unilateral vestibulopathy as a comorbid condition was defined as anyone with demonstrable unilateral vestibular loss on bedside or vestibular testing at the time of PPPD diagnosis, independent of any clinical or laboratory measure of compensation. BPPV was considered comorbid if, at the time of PPPD diagnosis, there were ongoing clinical symptoms compatible with BPPV and typical positional nystagmus was seen on positional testing, in accordance with established diagnostic criteria [41].
Collected nominal variables were compared using Fisher’s exact test while numerical variables were compared using two-sided Student’s t-test. A p value < 0.05 was considered statistically significant. Calculations were carried out using SPSS 24 (IBM, Armonk, NY) and Excel 2010 (Microsoft, Redmond, WA).
3Results
PPPD was diagnosed in 32.9%(100/304) of patients seen at the MDC between March 2017 and June 2019. As seen in Table 1, patients were mostly female (80/100; 80.0%) and the mean age was 50.4±12.1. On average, patients had symptoms for 35.1±49.6 months, had to take 9.1±26.3 months off work, had already consulted 5.6±5.1 physicians or specialists, had undergone 3.7±1.6 diagnostic tests (Table 2) and had attempted 3.4±2.3 treatments (Table 3). There was no statistically significant difference in baseline characteristics between patients with PPPD alone and those with multiple diagnoses contributing to dizziness alongside PPPD.
Table 1
Baseline Characteristics of patients with PPPD
| Characteristic | n = 100 |
| Average age, years±SD* | 50.4±12.1 |
| Female Gender, n (%) | 80 (80.0) |
| Average duration of symptoms, months±SD | 35.1±49.6 |
| Average duration off work, months±SD | 9.1±26.3 |
| Average number of physicians or specialists seen±SD | 5.6±5.1 |
| Average number of tests done±SD | 3.7±1.6 |
| Average number of treatments done±SD | 3.4±2.3 |
*Standard deviation.
Table 2
Diagnostic evaluations most often completed prior to attending the MDC (n = 100)
| Description of symptom | Patients with symptoms n (%) |
| Brain MRI | 75 (75.0) |
| Hearing test | 74 (74.0) |
| Blood tests | 56 (56.0) |
| Brain CT | 54 (54.0) |
| Balance testing | 53 (53.0) |
| Heart testing | 37 (37.0) |
Table 3
Treatments most often attempted prior to attending the MDC (n = 100)
| Description of symptom | Patients with symptoms n (%) |
| Dimenhydrinate | 51 (51.0) |
| Betahistine | 47 (47.0) |
| Epley Maneuvers | 43 (43.0) |
| Balance physiotherapy | 43 (43.0) |
| Massage | 32 (32.0) |
| Migraine treatment | 29 (29.0) |
| Acupuncture | 29 (29.0) |
| Chiropractor | 26 (26.0) |
| Counselling | 20 (20.0) |
| Visual therapy | 18 (18.0) |
Of the 100 patients with PPPD, 55 (55/100; 55%) had an identifiable precipitating condition. Vestibular neuritis (17/55; 30.9%), vestibular migraines (11/55; 20.0%) and benign paroxysmal positional vertigo (8/55; 14.5%) were the most common precipitating conditions. (Fig. 1) Of the patients with PPPD, twenty-nine (29/100; 29.0%) had no other current disease causing dizziness while 71 (71/100; 71.0%) had other accompanying diagnoses contributing to dizziness at the time of diagnosis. Fifty-nine patients (59/100; 59.0%) had one additional diagnosis along with PPPD contributing to their symptoms while 12 patients (12/100; 12.0%) had 2 or more accompanying diagnoses with vestibular migraine (28/100; 28.0%) being the most common comorbidity (Fig. 2). Non-specific dizziness (80/100; 80.0%) was the most commonly reported symptom while feeling pushed or pulled (22/100; 22.0%) was the least common (Table 4).
Fig. 1
Precipitating conditions of PPPD (n = 55).
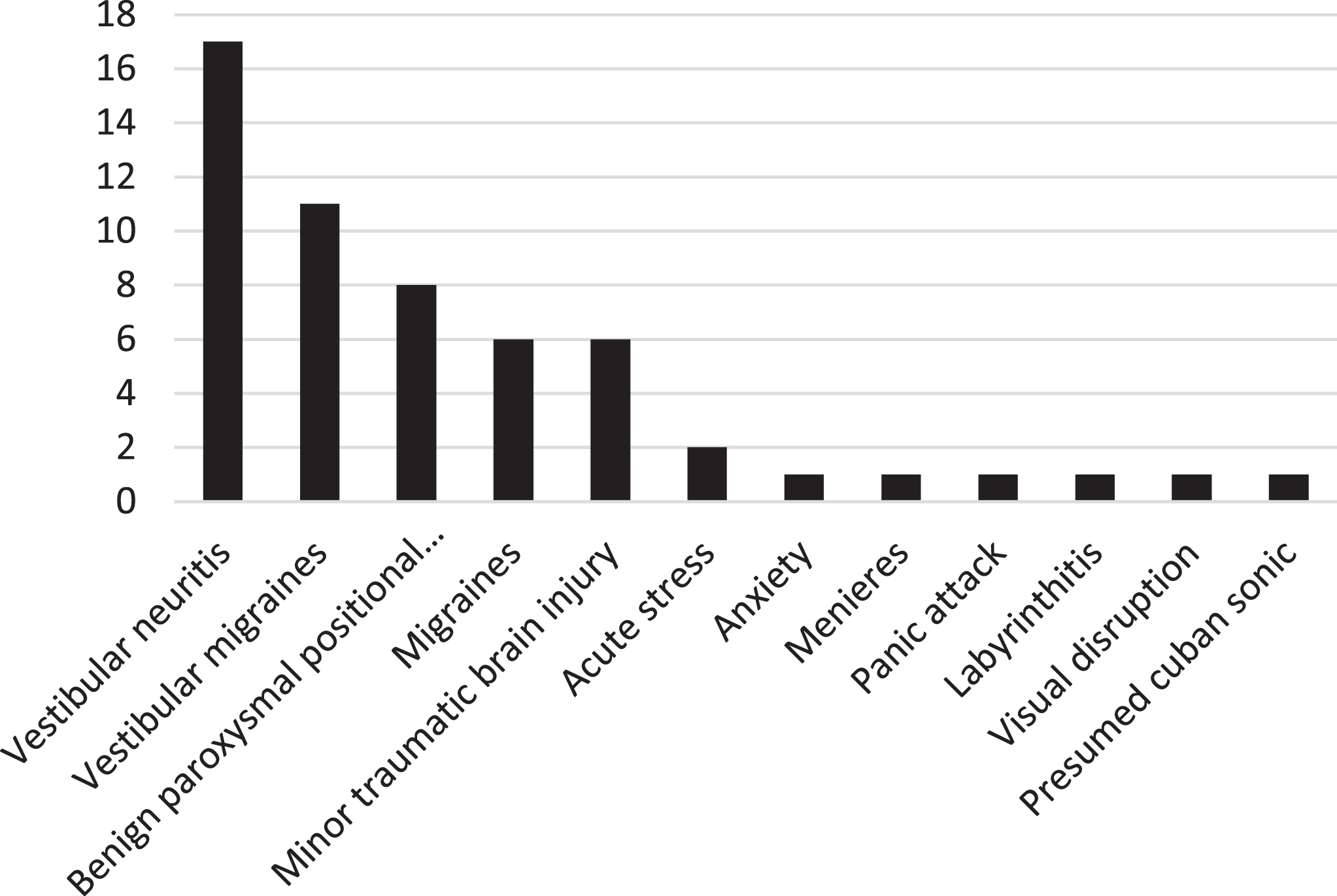
Fig. 2
Secondary diagnoses in patients who have additional diagnoses alongside PPPD contributing to their dizziness (n = 71).
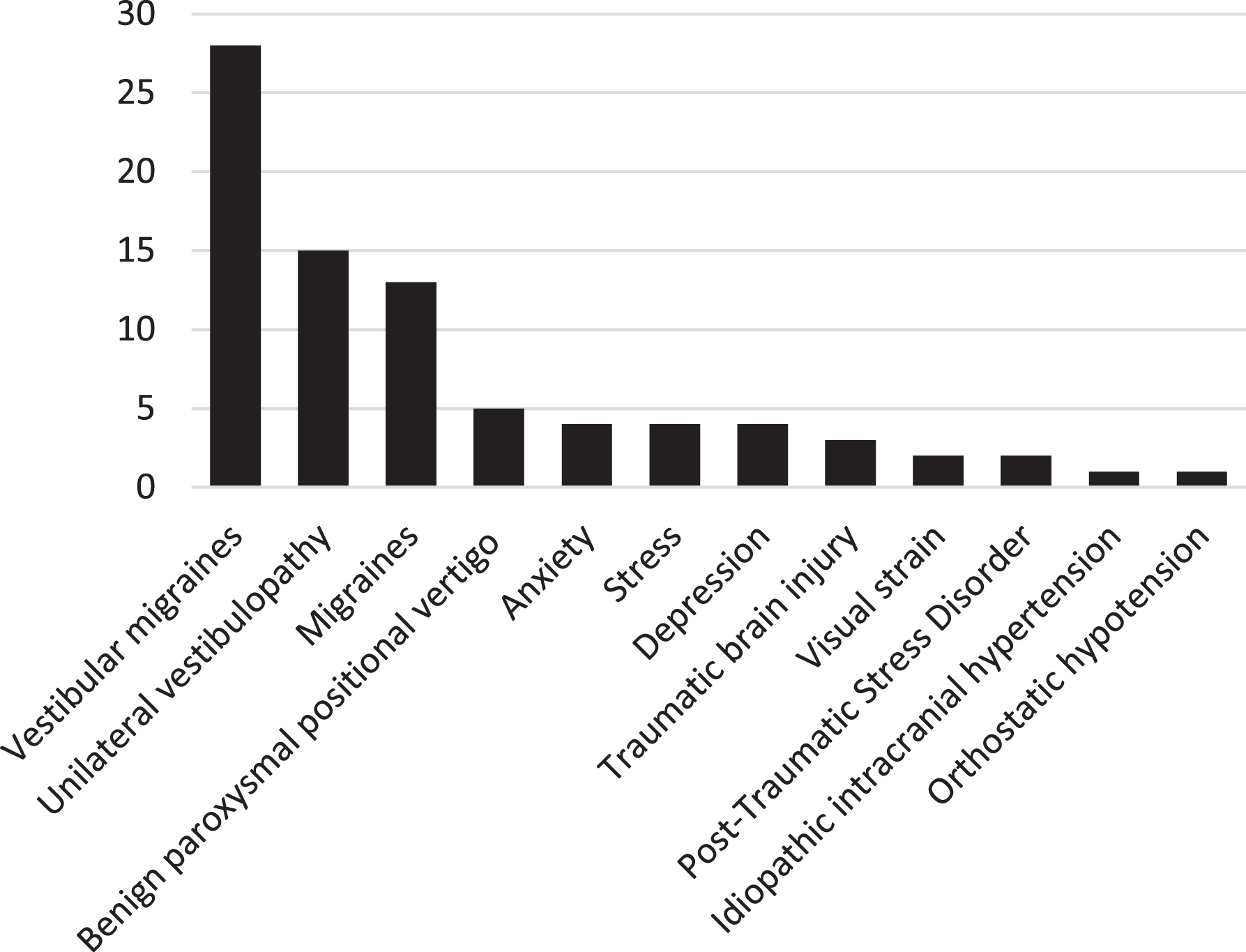
Table 4
Symptoms most often used to describe dizziness in all patients (n = 100)
| Description of symptom | Patients with symptoms n (%) |
| Dizziness | 80 (80.0) |
| Imbalance | 78 (78.0) |
| Lightheadedness | 66 (66.0) |
| Vertigo/spinning | 57 (57.0) |
| Like being drunk | 50 (50.0) |
| Fainting/Wanting to pass out | 26 (26.0) |
| Being pushed or pulled | 22 (22.0) |
Based on the DHI, patients with PPPD were most often classified as severely impacted (58/100; 58.0%) by their dizziness and had an average score of 60.3±19.0. Responses to PHQ-9 were evenly spread out across all categories with 46 patients being classified as minimally or mildly depressed (46/99; 46.5%) and 53 patients being classified as moderately to severely depressed (53/99; 53.5%). The majority of patients were minimally or mildly anxious (64/100; 64.0%) while the rest (36/100; 36.0%) were moderately or severely anxious according to the GAD-7. The average VSS-sf score was 24.1/60 and the average SVQ score was 2.00. Just under half of participants (49/100; 49.0%) had symptoms suggestive of migraine according to the MS-Q and responses to the MIDAS were bimodal with the majority of patients having either minimal or severe disability (87/100; 87.0%) due to migraines (Table 5).
Table 5
Questionnaire scores among patients diagnosed with PPPD (n = 100)
| Questionnaire | PPPD |
| DHI1 [mean (95%CI*)] | 60.3 (56.5 –64.2) |
| Mild [n (%)] | 10 (10.0) |
| Moderate [n (%)] | 32 (32.0) |
| Severe [n (%)] | 58 (58.0) |
| PHQ-92 [mean (95%CI*)] | 10.8 (9.4 –12.2) |
| Minimal [n (%)] | 21 (21.2) |
| Mild [n (%)] | 25 (25.2) |
| Moderate [n (%)] | 21 (21.2) |
| Moderately severe [n (%)] | 20 (20.2) |
| Severe [n (%)] | 12 (12.1) |
| GAD-7 score3 [mean (95%CI*)] | 7.7 (6.5 –8.9) |
| Minimal [n (%)] | 30 (30.0) |
| Mild [n (%)] | 32 (32.0) |
| Moderate [n (%)] | 17 (17.0) |
| Severe [n (%)] | 15 (15.0) |
| VSS-sf4 [mean (95%CI*)] | 24.1 (21.7 –26.5) |
| VSS-sf-V5 [mean (95%CI*)] | 15.3 (13.7 –16.9) |
| VSS-sf-A6 [mean (95%CI*)] | 8.8 (7.6 –10.0) |
| SVQ7 [mean (95%CI*)] | 2.0 (1.83 –2.19) |
| MS-Q8 [mean (95%CI*)] | 3.1 (2.7 –3.4) |
| No suspicion of migraine [n (%)] | 51 (51.0) |
| Suspicion of migraine [n (%)] | 49 (49.0) |
| MIDAS9 [mean (95%CI*)] | 49.6 (30.7 –68.4) |
| Minimal [n (%)] | 49 (49.0) |
| Mild [n (%)] | 7 (7.0) |
| Moderate [n (%)] | 8 (8.0) |
| Severe [n (%)] | 36 (36.0) |
*Data calculated with a 95%confidence interval shown as upper and lower value. 1Dizziness Handicap Inventory. 2Patient Health Questionnaire-9. 3Generalized Anxiety Disorder 7-item. 4Vertigo Symptom Scale. 5Vertigo components of the Vertigo Symptom Scale. 6Anxiety components of the Vertigo Symptom Scale. 7Situational Vertigo Questionnaire. 8Migraine Screen Questionnaire. 9Migraine Disability Assessment.
4Discussion
4.1Demographics
PPPD patients (100/304; 32.9%) comprised nearly a third of all individuals attending the MDC clinic. This is likely in part due to the fact that the MDC clinic deals exclusively with a homogenous subset of patients presenting with longstanding and undiagnosed dizziness [35]. As such, our findings may not be generalizable to all patients with PPPD presenting to a non-specialist clinic. However, this provides a distinct opportunity to collect data and study the characteristics of this unique population. PPPD was more prevalent amongst females (80/100; 80.0%) which is concordant with other studies [2, 26, 45]. The mean age of participants diagnosed with PPPD (50.4±12.1) was also consistent with what was found by other authors [2]. Furthermore, patients in this study needed to take 9.1±26.3 months off work, on average. Similarly, a study seeking to quantify the impact of chronic dizziness on productivity found that 48%of patients changed or quit their jobs as a result of their dizziness [4]. There is a study planned by the investigators in the future to attempt to quantify this lost productivity in terms of financial cost.
Of the patients who presented to this clinic, 55 patients (55/100; 55.0%) had an identifiable triggering event while the rest (45/100; 45.0%) did not have a clearly identifiable precipitating condition on chart review. This is likely due to the retrospective nature of this study. In our cohort, central and peripheral vestibular disorders were more often a triggering event (27/55; 49.1%) than the 25%recently cited by Staab et al. While panic attacks and anxiety were underrepresented with only a single case of each (1.7%) being identified as opposed to the 15–20%identified by Staab et al., vestibular migraines (11/55; 20.0%) and whiplash (6/55; 10.9%) were identified as the precipitating condition in similar frequencies [34]. This discrepancy may be due to the underrepresentation of psychiatric conditions which could be overlooked due to the lack of a psychiatrist or psychologist in our clinic and the reliance on patient-reported anxiety and depression questionnaires to identify such disorders. Furthermore, there is significant variability in the literature and more data is required to accurately determine the prevalence of these precipitating conditions [2, 45].
Most patients (71/100; 71.0%) presenting with PPPD to the MDC had a secondary diagnosis contributing to their dizziness at the time of diagnosis. The most common comorbidity was vestibular migraine (28/100; 28.0%). Bittar and Lins (2015) found hypercholesterolemia to be the most important secondary diagnosis, while Yan et al (2017) found it to be BPPV. In this study, hypercholesterolemia was not tested and only 5 patients (5/93; 5.4%) presented with BPPV as an active comorbid diagnosis. This discrepancy is potentially because patients had undergone many treatments and were seen by specialists before being referred to the MDC clinic. As a consequence, unless intractable, BPPV was already treated prior to attending this clinic and thus did not represent active disease contributing to dizziness at the time of presentation. However, both of these studies found migraines or vestibular migraines to be the second most common comorbidity.
4.2Impact of dizziness
The mean DHI scores for all patients was 60.3, which is slighty higher than what has been previously detailed in the literature regarding PPPD patients, where DHI scores ranged from 52.66–58.7 [2, 24, 47]. Most patients (90.0%) reported moderate or severe symptoms. The slightly higher DHI tendency may be explained by the stringent referral criteria to the MDC since specialist assessment and significant impact are required prior to referral. Furthermore, patients with longstanding symptoms of dizziness tend to report greater disability [40]. A preponderance in the moderate to severe DHI category was also described in a study reporting stratified DHI scores for patients with a variety of vestibular diagnoses (73%) (Whitney et al., 2004) and in patients with balance problems (61%) (Vanspauwen et al., 2016). However, the patients included in this study have a higher proportion of moderate to severe DHI scores (90%) than both studies (Vanspauwen et al., 2016; Whitney et al., 2004). As such, PPPD seems to have a more important impact on the physical, emotional and functional facets of patient lives when compard to more established causes of dizziness.
4.3Motion discomfort
While the SVQ does not have an established classification system used to estimate the severity of visual vertigo, the average score of 2.0/4 is much higher than the scores reported in patients with vertigo provoked by visual stimuli (1.59), patients with a defective labyrynth (1.14), and a control group (0.19) [10]. These findings suggest that symptoms of patients with PPPD are significantly impacted by specific disorienting environments or complex visual stimuli.
4.4Depression and PPPD
Psychiatric disorders can be precipitants of PPPD, however they do not seem to predict the onset of PPPD. In fact, it is important to recognize that PPPD is a functional disorder and not a psychiatric one [33]. While depression and anxiety were initially incorporated in the definition of PPV, they are not required for the diagnosis of PPPD. Nonetheless, studies have demonstrated depression to be prevalent among 20%of vertiginous patients with 11%suffering from severe depression [16, 17]. The PHQ-9 results in this study showed that the majority of patients were evenly distributed across minimally, mildly, moderately and moderately severe depressed classifications. Patients with CSD in particular have also been previously shown to be associated with an elevated PHQ-9 scores ranging from 7.8–8.5 while patients with PPPD had a score of 8.67 [6, 14, 29]. Both these values are only slightly lower than the average score of 10.8 reported in this study. In fact, CSD is associated with higher levels or neuroticism and greater psychiatric comorbidity when compared to healthy individuals, which may explain the predisposition of patients to higher rates of depression [14]. This may be in part due to maladaptive cognitive-behavioral responses [28].
4.5Anxiety and PPPD
Studies have shown that up to 60%of patients presenting with chronic dizziness have comorbid anxiety [32]. In fact, while, as stated earlier, PPPD is a functional disorder secondary to a vestibular precipitant, patients with pre-existing anxiety or those who respond to the event with a high degree of anxiety and psychological distress appear more likely to develop PPPD [33]. Furthermore, anxiety and related personality traits have been described as possible risk factors for the development of vestibular ailments [1, 11, 12, 27, 42, 47, 48]. In this study, patients with PPPD demonstrated symptoms of mild anxiety as evidenced by the mean VSS-sf-A score of 8.8 and the GAD-7 score of 7.7. This was consistent with other studies which identified a GAD-7 score of 8.87 in the setting of PPPD and 8–9.3 in the setting of CSD [14, 29]. These scores were also much higher than the overall population mean (2.95) [21].
4.6Migraines and PPPD
The link between migraines and vertigo is well established [20, 25]. The MIDAS questionnaire, which evaluates the impact of migraines on patients, revealed a bimodal distribution with 44.0%of patients having either moderate or severe symptoms. Appropriately, the MSQ identified 49.0%of patients with symptoms suspicious for migraine. Nonetheless, only 41 patients (41/100; 41.0%) were diagnosed with migraines. Of those patients, 13 (13/100; 13%) were diagnosed with non-vertiginous migraines and 28 (28/100; 28%) were diagnosed with vestibular migraines. Similarly, studies have shown non-vertiginous migraines to be a triggering event in 15–20%of CSD patients and all migraines to be present in up to 26%of patients with PPPD [2, 32]. In contrast, the global prevalence of migraines in the general population is 14%, reinforcing the link between PPPD and migraine [5, 38].
4.7Limitations
This study has several limitations. Due to the nature of the specialist-only referral process to the MDC, the patients represent a more homogenous set of chronically dizzy individuals without a clear diagnosis, presenting a population bias. Statistical analysis was conducted on grouped data combining patients with PPPD alone and PPPD with a secondary diagnosis. As such, certain secondary diagnoses may have acted as confounding factors. Furthermore, the majority of diagnoses were established with some subjectivity since they heavily rely on patient report of symptoms, which may also present a potential bias. Finally, since the surveys consisted mostly of self-reported questionnaires which rely on patient recall, there is potential for under or overestimation of symptoms.
5Conclusion
In summary, nearly a third of patients presenting to the MDC were diagnosed with PPPD. The majority of patients with PPPD were middle-aged females and had multiple comorbidities contributing to their symptoms, most commonly migraines. Most patients displayed significant handicap, motion discomfort, elevated depression and mild anxiety tendencies due to their dizziness. As PPPD is an emerging clinical diagnosis, it is paramount to rigorously define PPPD patient characteristics in order to inform future studies of this population and to help identify those at risk of developing PPPD. While the findings from this specialized clinic may not be generalizable to all patients with PPPD presenting to a non-specialist clinic, we hope to leverage this homogenous patient population and expansive database of symptom information to build models that both predict the development of, and help to diagnose, this common and disabling condition.
Source of funding
This project did not receive funding.
Financial Disclosure statement
None of the authors has a financial or personal interest in any of the products, devices, or drugs mentioned in this manuscript.
Ethical approval
Institutional Review Board approval was obtained for the following study.
References
[1] | Balaban C.D. and Jacob R.G. , Background and history of the interface between anxiety and vertigo, J Anxiety Disord 15: ((2001) ), 27–51. |
[2] | Bittar R.S.M. , Lins E.M.D. and Von S. , Clinical characteristics of patients with persistent postural-perceptual dizziness, Braz J Otorhinolaryngol 81: ((2015) ), 276–282. |
[3] | Brandt T.H. and Dieterich M. , Phobischer attacken-schwankschwindel, ein neues syndrome, , Med Münch Wochenschr 128: ((1986) ), 247–250. |
[4] | Bronstein A.M. , Golding J.F. , Gresty M.A. , Mandalà M. , Nuti D. and Shetye A. , The social impact of dizziness in London and Siena, J Neurol 257: ((2010) ), 183–190. |
[5] | Burch R.C. , Loder S. , Loder E. and Smitherman T.A. , The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies, Headache 55: ((2015) ), 21–34. |
[6] | Chiarella G. , Petrolo C. , Riccelli R. , Giofrè L. , Olivadese G. and Gioacchini F.M. , Chronic subjective dizziness: Analysis of underlying personality factors, J Vestib Res 26: ((2016) ), 403–408. |
[7] | Colnaghi S. , Rezzani C. , Gnesi M. , Manfrin M. , Quaglieri S. and Nuti D. , Validation of the Italian Version of the Dizziness Handicap Inventory, the Situational Vertigo Questionnaire, and the Activity-Specific Balance Confidence Scale for Peripheral and Central Vestibular Symptoms, Front Neurol 8: ((2017) ), 528. |
[8] | Enloe L.J. and Shield R.K. , Evaluation of health-related quality of life in individuals with vestibular disease using disease-specific and general outcome measures, Phys Ther 77: ((1997) ), 890–903. |
[9] | Gilbody S. , Richards S. , Brealey S. and Hewitt C. , Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis, J Gen Intern Med 22: ((2007) ), 1596–1602. |
[10] | Guerraz M. , Yardley L. , Bertholon P. , Pollak L. , Rudge P. and Gresty M.A. , Visual vertigo: symptom assessment, spatial orientation and postural control, Brain 124: ((2001) ), 1646–1656. |
[11] | Holmberg J. , Karlberg M. , Harlacher U. and Magnusson M. , Experience of handicap and anxiety in phobic postural vertigo, Acta Otolaryngol 125: ((2005) ), 270–275. |
[12] | Horii A. , Anxiety, depression, and persistent postural perceptual dizziness: International classification of vestibular disorders by Bárány Society, Equilibrium Research 76: ((2017) ), 316–322. |
[13] | ICD-11, Mortality and Morbidity Statistics n.d. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/2005792829 (accessed April 11, 2019). |
[14] | Indovina I. , Riccelli R. , Chiarella G. , Petrolo C. , Augimeri A. and Giofrè L. , Role of the Insula and Vestibular System in Patients with Chronic Subjective Dizziness: An fMRI Study Using Sound-Evoked Vestibular Stimulation, Front Behav Neurosci 9: ((2015) ). |
[15] | Jacobson G.P. and Newman C.W. , The development of the Dizziness Handicap Inventory, Arch Otolaryngol Head Neck Surg 116: ((1990) ), 424–427. |
[16] | Ketola S. , Havia M. , Appelberg B. and Kentala E. , Depressive Symptoms Underestimated in Vertiginous Patients, Otolaryngology–Head and Neck Surgery 137: ((2007) ), 312–315. |
[17] | Kim S.K. , Kim Y.B. , Park I-S. , Hong S.J. , Kim H. and Hong S.M. , Clinical Analysis of Dizzy Patients with High Levels of Depression and Anxiety, J Audiol Otol 20: ((2016) ), 174–178. |
[18] | Kroenke K. , Spitzer R.L. and Williams J.B.W. , The PHQ-9, J Gen Intern Med 16: ((2001) ), 606–613. |
[19] | Láinez M.J.A. , Domínguez M. , Rejas J. , Palacios G. , Arriaza E. and Garcia-Garcia M. , Development and validation of the Migraine Screen Questionnaire (MS-Q), Headache 45: ((2005) ), 1328–1338. |
[20] | Lempert T. and Neuhauser H. , Epidemiology of vertigo, migraine and vestibular migraine, J Neurol 256: ((2009) ), 333–338. |
[21] | Löwe B. , Decker O. , Müller S. , Brähler E. , Schellberg D. and Herzog W. , Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population, Med Care 46: ((2008) ), 266–274. |
[22] | Lynn S.G. , Driscoll C.L. , Harner S.G. , Beatty C.W. and Atkinson E.J. , Assessment of dysequilibrium after acoustic neuroma removal, Am J Otol 20: ((1999) ), 484–494. |
[23] | Mutlu B. and Serbetcioglu B. , Discussion of the dizziness handicap inventory, J Vestib Res 23: ((2013) ), 271–277. |
[24] | Nada E.H. , Ibraheem O.A. and Hassaan M.R. , Vestibular Rehabilitation Therapy Outcomes in Patients With Persistent Postural-Perceptual Dizziness, Ann Otol Rhinol Laryngol 128: ((2019) ), 323–329. |
[25] | Neuhauser H. , Leopold M. , von Brevern M. , Arnold G. and Lempert T. , Brevern, G. and T. Lempert, The interrelations of migraine, vertigo, and migrainous vertigo, Neurology 56: ((2001) ), , 436–441. |
[26] | Odman M. and Maire R. , Chronic subjective dizziness, Acta Otolaryngol 128: ((2008) ), 1085–1088. |
[27] | Oxford Textbook of Vertigo and Imbalance, Oxford, New York: Oxford University Press, ((2013) ). |
[28] | Popkirov S. , Staab J.P. and Stone J. , Persistent postural-perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness, Pract Neurol 18: ((2018) ), 5–13. |
[29] | Riccelli R. , Passamonti L. , Toschi N. , Nigro S. , Chiarella G. and Petrolo C. , Altered Insular and Occipital Responses to Simulated Vertical Self-Motion in Patients with Persistent Postural-Perceptual Dizziness, Front Neurol 8: ((2017) ), 529. |
[30] | Seemungal B.M. and Passamonti L. , Persistent postural-perceptual dizziness: a useful new syndrome, Pract Neurol 18: ((2018) ), 3–4. |
[31] | Spitzer R.L. , Kroenke K. and Williams J.B.W. , Löwe B, A brief measure for assessing generalized anxiety disorder: the GAD-7, Arch Intern Med 166: ((2006) ), 1092–1097. |
[32] | Staab J.P. , Chronic subjective dizziness,ndash, Continuum (Minneap Minn) 18: ((2012) ), 1118ndash1141. |
[33] | Staab J.P. , Eckhardt-Henn A. , Horii A. , Jacob R. , Strupp M. and Brandt T. , Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society, J Vestib Res 27: ((2017) ), 191–208. |
[34] | Staab J.P. , Persistent Postural-Perceptual Dizziness, Semin Neurol 40: ((2020) ), 130–137. |
[35] | Staibano P. , Lelli D. and Tse D. , A retrospective analysis of two tertiary care dizziness clinics: a multidisciplinary chronic dizziness clinic and an acute dizziness clinic, J Otolaryngol Head Neck Surg 48: ((2019) ), 11. |
[36] | Stewart W.F. , Lipton R.B. , Dowson A.J. and Sawyer J. , Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability, Neurology 56: ((2001) ), 20–28. |
[37] | Stewart W.F. , Lipton R.B. , Kolodner K.B. , Sawyer J. , Lee C. and Liberman J.N. , Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers, Pain 88: ((2000) ), 41–52. |
[38] | Stovner L. , Hagen K. , Jensen R. , Katsarava A. , Lipton R. and Scher A. , The Global Burden of Headache: A Documentation of Headache Prevalence and Disability Worldwide, Cephalalgia 27: ((2007) ), 193–210. |
[39] | Strupp M. , Glaser M. , Karch C. , Rettinger N. , Dieterich M. and Brandt T. , Häufigste Schwindelform im mittleren Alter: phobischer Schwankschwindel, Nervenarzt 74: ((2003) ), 911–914. |
[40] | Vanspauwen R. , Knoop A. , Camp S. , Van Dinther J. , Erwin Offeciers F. and Somers T. , Outcome evaluation of the dizziness handicap inventory in an outpatient vestibular clinic, Journal of Vestibular Research 26: ((2016) ), 479–486. |
[41] | Von Brevern M , Bertholon P. , Brandt T. , Fife T. , Imai T. , Nuti D. and Newman-Toker D. , Benign paroxysmal positional vertigo: Diagnostic criteria, Journal of Vestibular Research, J Vestib Res 25: ((2015) ), 105–117. |
[42] | Whalley M.G. and Cane D.A. , A Cognitive-Behavioral Model of Persistent Postural-Perceptual Dizziness, Cognitive and Behavioral Practice 24: ((2017) ), 72–89. |
[43] | Whitney S.L. , Wrisley D.M. , Brown K.E. and Furman J.M. , Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otol Neurotol 25: ((2004) ), 139–143. |
[44] | Wilhelmsen K. , Strand L.I. , Nordahl S.H.G. , Eide G.E. and Ljunggren A.E. , Psychometric properties of the Vertigo symptom scale - Short form, BMC Ear Nose Throat Disord 8: ((2008) ), 2. |
[45] | Yan Z. , Cui L. , Yu T. , Liang H. , Wang Y. and Chen C. , Analysis of the characteristics of persistent postural-perceptual dizziness: A clinical-based study in China, International Journal of Audiology 56: ((2017) ), 33–37. |
[46] | Yardley L. , Masson E. , Verschuur C. , Haacke N. and Luxon L. , Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale, J Psychosom Res 36: ((1992) ), 731–741. |
[47] | Yu Y-C. , Xue H. , Zhang Y-X. and Zhou J. , Cognitive Behavior Therapy as Augmentation for Sertraline in Treating Patients with Persistent Postural-Perceptual Dizziness, Biomed Res Int 2018: ((2018) ), 8518631. |
[48] | Zur O. , Schoen G. , Dickstein R. , Feldman J. , Berner Y. and Dannenbaum E. , Anxiety among individuals with visual vertigo and vestibulopathy, Disabil Rehabil 37: ((2015) ), 2197–2202. |




