Effects of long-term vestibular rehabilitation therapy with vibrotactile sensory augmentation for people with unilateral vestibular disorders – A randomized preliminary study
Abstract
BACKGROUND AND OBJECTIVE:
This pilot study aimed to investigate the effects of incorporating vibrotactile sensory augmentation (SA) on balance performance among people with unilateral vestibular disorders (UVD).
METHODS:
Eight participants with UVD were recruited. Participants completed 18 balance training sessions across six weeks in a clinical setting. Four participants (68.1±7.5 yrs) were randomized to the experimental group (EG) and received trunk-based vibrotactile SA while performing the balance exercises, and four participants (63.1±11.3 yrs) were assigned to the control group (CG); CG participants completed the balance training without SA. Clinical and kinematic balance performance measures were collected before training; midway through training; and one week, one month, and six months after training.
RESULTS:
All participants, regardless of group, demonstrated improvements in a subset of the clinical or balance metrics immediately following completion of the balance training protocol. The EG showed significantly greater improvements than the CG for the Activities-specific Balance Confidence Scale and postural stability during the two standing balance exercises with head movements. The EG also had larger improvements than the CG for the Sensory Organization Test (SOT), Mini Balance Evaluations Systems Test, Gait Speed Test, Dynamic Gait Index, Functional Gait Assessment, and vestibular reliance metric calculated based on the SOT.
CONCLUSIONS:
Incorporating vibrotactile SA into vestibular rehabilitation programs may lead to additional benefits that may be retained up to six months after training compared to training without vibrotactile SA. A larger study is warranted to demonstrate statistical significance between the groups.
1Introduction
From 2001 to 2004, approximately 35% of Americans aged 40 years and older experienced some form of vestibular dysfunction, which was equivalent to approximately 16 million new vestibular patients per year [1]. People with vestibular disorders have increased dizziness, reduced postural control, increased fall risk and fear of falling, increased interruptions of daily activities, and the need for additional sick leave or medical consultation [2, 28, 46]. Vestibular rehabilitation therapy (VRT) is widely prescribed to reduce dizziness and improve balance control [6, 16, 25, 26, 30, 38, 43, 54, 65, 66]. VRT is an exercise-based program that includes four major types of exercises to facilitate central compensation: gaze stability exercises, balance exercises, habituation exercises, and walking for endurance [25]. Physical therapists tailor the exercises to the individual to optimize the effectiveness of VRT [40]. However, during VRT, a subset of individuals plateau in progress and others fail to achieve complete compensation resulting in partial recovery of function and/or incomplete resolution of symptoms [9, 10, 13, 29, 33, 51].
Sensory augmentation (SA), which has been widely explored in the last few decades, provides additional cues to augment/substitute intact sensory inputs from the somatosensory, visual and vestibular systems [5]. Typical SA devices have one or multiple sensors that measure body motion and a wearable display that provides instructional cues (e.g., vibrotactile [3, 12, 36, 56, 58, 63, 64], visual [24], auditory [20, 27], electrotactile [5, 17], and multi-modal [18, 32, 35]). Numerous studies have shown that body sway during balance tasks can be reduced in a real-time manner when SA is provided [3, 5, 12, 17, 18, 20, 24, 27, 32, 35, 36, 56, 58, 63, 64].
Several studies have demonstrated that body sway reductions can be retained for hours to days following short-term (i.e., less than one week) balance training with SA [17, 18, 56]. Other studies have investigated changes in body sway or clinical outcomes following multi-session (i.e., more than 1 week) training with SA [9, 10, 14, 51]. For example, Basta et al. reported reduced body sway and improved clinical outcome measures (e.g., Sensory Organization Test (SOT) and Dizziness Handicap Inventory (DHI)) in people with vestibular disorders who trained with vibrotactile SA over a two-week (i.e., ten-session) training program, but observed no such effect in their placebo group training with a sham device which generated random SA cues [10]. Further, the balance improvements were retained at the three-month follow-up assessment [10]. Similarly, Barros et al. showed improved SOT scores among people with bilateral vestibular loss following a two-week (i.e., six-session) balance training program with electrotactile SA [9]. Brugnera et al. found that after a two-week VRT program people with vestibular disorders who trained with vibrotactile SA had improved clinical measures (e.g., SOT and Activities-specific Balance Confidence (ABC) scale), but participants who trained without vibrotactile SA showed no significant improvements [14]. In summary, previous studies have shown that balance improves when balance training is supplemented with SA, but the studies lacked either a control group that trained without SA or long-term follow-up assessments.
The aims of this study were to investigate whether 1) a six-week VRT training program (including standing, gait, and Vestibular Ocular Reflex (VOR) gaze stabilization exercises) with vibrotactile SA leads to larger reductions in body sway and clinical outcome improvements compared with VRT alone among participants with UVD who had previously completed VRT, and 2) balance improvements are retained for up to six months after the VRT program.
2Methods
2.1Participants
Sixteen people with UVD were recruited for study eligibility assessment through referrals by physical therapists and flyers at the University of Pittsburgh Medical Center. A neurologist diagnosed the participants with UVD based on the presence of a reduced vestibular response greater than or equal to 24% during caloric testing. Reduced vestibular response was determined using Jongkees’ formula; the peak slow component velocity was used to calculate side differences [37]. The recruiting period started in 2014 and ended in 2016. Participants were excluded if they had confounding neurologic or neuromuscular disorders; known pregnancy; recent lower extremity fractures/severe sprains (within the last six months); previous lower extremity joint replacement; incapacitating back or lower extremity pain; were unable to stand for three minutes without rest; a body habitus that exceeded the dimensions of the NeuroCom® EquiTest® Computerized Dynamic Posturography (waist circumference >50 inches;weight < 290 pounds); or a Montreal Cognitive Assessment score of less than 26 points (a score greater than or equal to 26 out of 30 points is considered normal cognition on the MoCA, and less than 26 points is indicative of impaired cognition [45]). People with cognitive impairments were excluded because the effects of deficits in domains such as attention on a person’s ability to attend and respond to the vibrotactile cues are unknown. Seven referrals were excluded because of low Montreal Cognitive Assessment scores. All participants had completed a standard VRT program prior to enrollment. After enrollment, participants were randomly assigned to an experimental group (EG) or a control group (CG) using a computerized randomization calculator. Participants in the EG received supervised VRT combined with vibrotactile SA (except during gait exercises), and participants in the CG received supervised VRT without vibrotactile SA. One participant in the CG dropped out of the study because of an orthopedic injury unrelated to the study. As shown in Table 1, four participants in the EG (68.1±7.5 yrs, one male) and four participants in the CG (63.1±11.3 yrs, one male) completed the study.
Table 1
Demographic information for the participants with UVD who completed the study
| ID | Age | Gender | Time from initial symptoms to start of study | Group Designation |
| 1 | 64 | M | 50 months | Control |
| 2 | 47 | F | 10.5 months | Control |
| 3 | 67 | F | 324 months | Control |
| 4 | 68 | F | 31 months | Experimental |
| 5 | 63 | F | 26.5 months | Experimental |
| 6 | 79 | M | 16 months | Experimental |
| 7 | 74 | F | 79.5 months | Control |
| 8 | 63 | F | 46 months | Experimental |
All participants provided written informed consent and the study was conducted at the university medical center in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the local institutional review board.
2.2Protocol
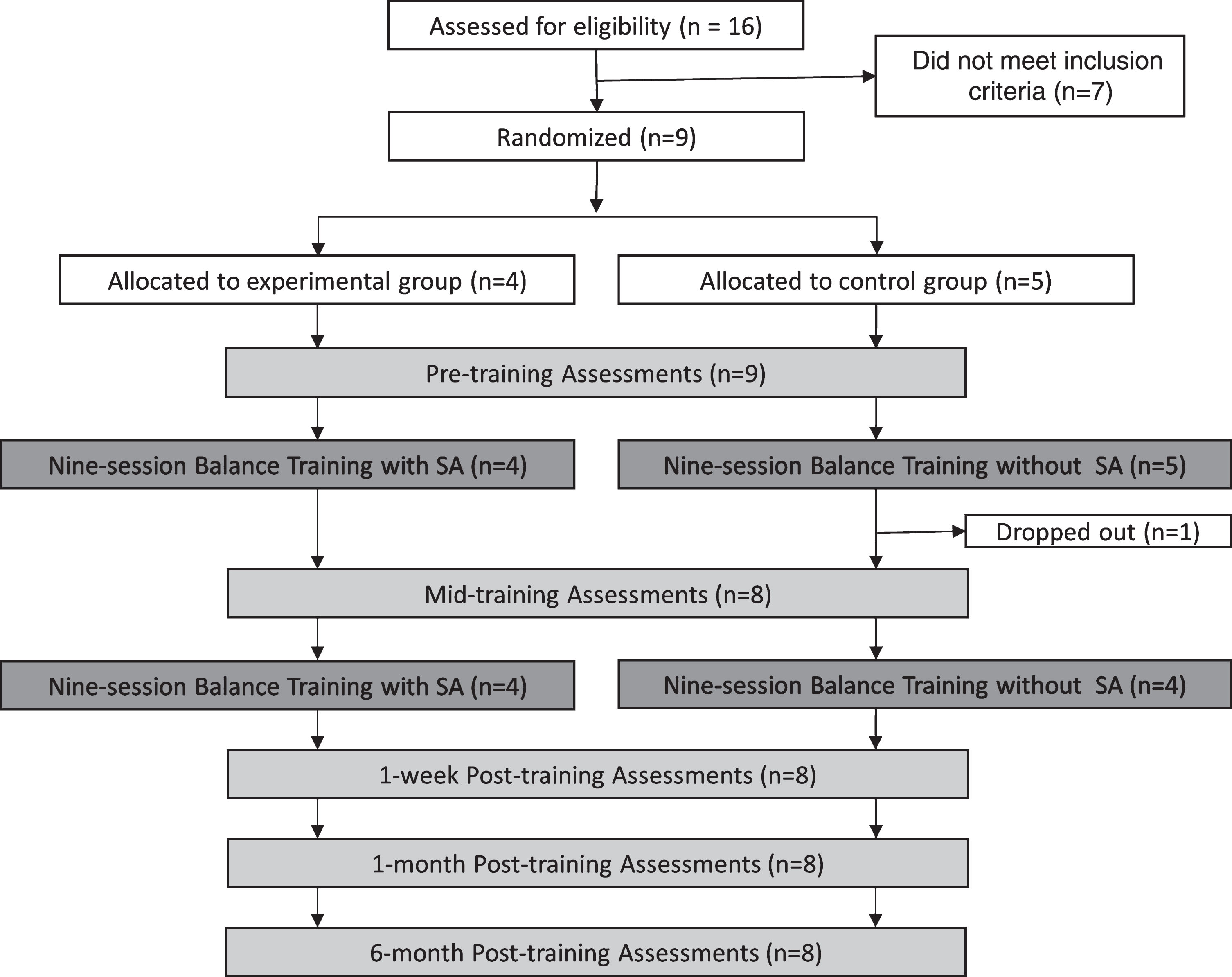
Participants completed a 6-week, 18-session VRT training program with five assessment sessions as shown in Fig. 1. The assessments were performed before training, midway through training, one week after the training, one month after the training, and six months after the training.
Fig. 1
The study protocol consisted of five assessment sessions (shown in light gray) and two nine-session rehabilitation balance training blocks (shown in dark gray).
A physical therapist, who was blinded to the participants’ group assignments, assessed each participant’s balance; the treating physical therapist was not blinded to the participants’ group assignments. The assessment included a battery of clinical tests and five standing balance exercises. Clinical tests were performed in a randomized order and included the 1) ABC Scale [41, 50], 2) DHI [34], 3) Computerized Dynamic Posturography: SOT [15], 4) Mini Balance Evaluations Systems Test (Mini-BESTest) [22], 5) Functional Reach Test (FRT) [21], 6) Gait Speed Test [47], 7) Timed Up and Go (TUG) [49], 8) Dynamic Gait Index (DGI) [55], and 9) Functional Gait Assessment (FGA) [67]. Using the results from the six SOT conditions, we calculated the participants’ reliance on the somatosensory input (ratio of SOT condition 2 score to the SOT condition 1 score), visual input (ratio of SOT condition 4 score to the SOT condition 1 score), and vestibular input (ratio of SOT condition 5 score to the SOT condition 1 score) to maintain postural stability [6, 44].
The five standing balance exercises were selected from a recently published conceptual progression framework [40]. Exercises 1–4 disturbed or removed one or more sensory inputs by varying visual, stance, head movement or standing surface conditions. Exercise 5 was a VOR gaze stabilization exercise.
Exercise 1: Feet apart stance on firm surface with eyes closed
Exercise 2: Romberg stance on firm surface with eyes closed and pitch head movements
Exercise 3: Romberg stance on foam surface with eyes open
Exercise 4: Semi-tandem Romberg stance on foam surface with eyes open
Exercise 5: Feet apart stance on firm surface with VOR gaze stabilization (maintenance of gaze on a fixed target with simultaneous horizontal head movements)
Each participant performed three trials of the five exercises, and each trial lasted for 30 seconds or to the point of loss of balance. Trunk sway in the anterior/posterior and medial/lateral directions was recorded using an inertial measurement unit. Balance performance was evaluated by three kinematic metrics including the root-mean-square (RMS) of trunk position (sway), percentage time within a one-degree zone (PZ), and the area of an elliptical fit to trunk sway (EA). Lower RMS, lower EA, and higher PZ indicate better balance performance [11, 42, 56].
During the 18 balance training sessions with the treating physical therapist, participants performed exercises from the six categories as shown in Table 2. For the weight shifting category, allparticipants were asked to shift their weight to the target tilt value in either the forward or right direction and hold their position for five seconds. Then, they were asked to shift their weight to the target tilt value in either the backward or left direction and hold their position for five seconds. For the VOR exercise category, participants completed either VOR x1 or VOR x2 gaze stabilization [25, 40]. For VOR x1, participants were asked to maintain a clear and steady gaze on a target while moving their head 30 degrees horizontally or vertically at the fastest speed possible. For VOR x2, they were asked to maintain a clear and steady gaze on a target while moving their head and the target in opposite directions (30 degrees vertically or horizontally). An Airex® Balance Pad (50 cm x 41 cm x 6 cm) was used to alter the standing surface condition.
Table 2
Exercise pool adapted from a recently published conceptual progression framework [40]. (* exercises where EG received vibrotactile SA; †weight shifting limits were pre-determined, the maximum limits were 6 deg., 3 deg., 3.5 deg. and 3.5 deg. in the forward, backward, rightward, and leftward directions, respectively; the medium limit was half of maximum limit)
| Category | Variables | |
| 1 | Standing on firm surface* | Eyes (open/closed), stance (feet apart/Romberg/semi-tandem-Romberg/tandem/single leg), head movement (none/yaw/pitch) |
| 2 | Standing on foam surface* | Eyes(open/closed), stance (feet apart/Romberg/semi-tandem-Romberg/tandem), head movement (none/yaw/pitch) |
| 3 | Weight shifting* | Eyes (open/closed), standing surface (firm/foam), shifting limit (medium/max)†, shifting speed (fast/slow), shifting direction (forward⟶backward/right⟶left) |
| 4 | Modified center of gravity* (arm raises to 90°) | Eyes(open/closed), stance (feet apart/Romberg/semi-tandem Romberg), weight in hand (none/light/heavy), arm raising speed (fast/slow), surface (firm/foam/ramp inclined 10 degrees/ramp declined 10 degrees |
| 5 | Gait | Eyes (open/closed), type of walking (normal, tandem, backward), head movement (none/yaw/pitch), walking speed (self-selected, fast slow) |
| 6 | Gaze Stabilization VOR* | Type of VOR (x1#/x2#), stance (feet apart/Romberg/semi-tandem Romberg/tandem), standing surface (firm/foam), distance to target (1 m/3 m), background of target (white/complex) |
During each balance training session, participants performed one exercise from each of the six categories. Each exercise was performed six times. Participants performed each exercise trial for 30 seconds, except for those in the weight shifting category. The physical therapist manually stopped the exercise if a participant needed to step out of position to maintain balance. For all exercise categories except for the gait category, vibrotactile SA was provided to the EG. Vibrotactile SA was provided in four randomly selected trials out of the six trials per exercise to enhance motor learning [61]. Throughout the study, both the EG and CG participants wore the vibrotactile SA device, as detailed in the next section. However, vibrotactile SA was not provided to the CG during any of the exercises.
After each exercise, the physical therapist rated the participants’ balance performance on a scale of one to five adapted from the Functional Independence Measure [39]. A rating of 1 was assigned if the participant performed the exercise independently with no body sway; a rating of 2 was assigned if supervision was needed and the participant demonstrated minimal body sway; a rating of 3 was assigned if close supervision was needed and the participant demonstrated moderate body sway; a rating of 4 was assigned if the physical therapist’s assistance was required or if the participant stepped out to maintain balance at or beyond 15 seconds within the 30 second trials; a rating of 5 was assigned if the participant fell, needed immediate assistance, or stepped out within 15 seconds. The physical therapist determined the set of exercises in the next training session using a recently published conceptual progression framework [40], ratings of the performed exercises, and clinical judgment. The treating physical therapist was blinded to participants’ pre-training and mid-training balance assessments.
2.3Instrumentation
The customized SA device shown in Fig. 2 included an inertial measurement unit (IMU, MTx-28A53G25 by Xsens Technologies B.V., Enschede, The Netherlands), four C-2 tactors (EAI Inc), a tactor controller unit (EAI Inc), a belt, a laptop (Dell Inc), and customized software written in C++. The IMU was placed on the back to record the trunk sway, and the four tactors were placed on the front, back, left, and right sides of the trunk at the L4/L5 level. The tactors operated at a frequency of 250 Hz and were driven by a sinusoidal signal generated with an RMS current of 0.225 A (peak-to-peak voltage of 4.47 V).
Fig. 2
The customized sensory augmentation device included an IMU, four C-2 tactors, a tactor controller unit, a belt, a laptop, and customized software.
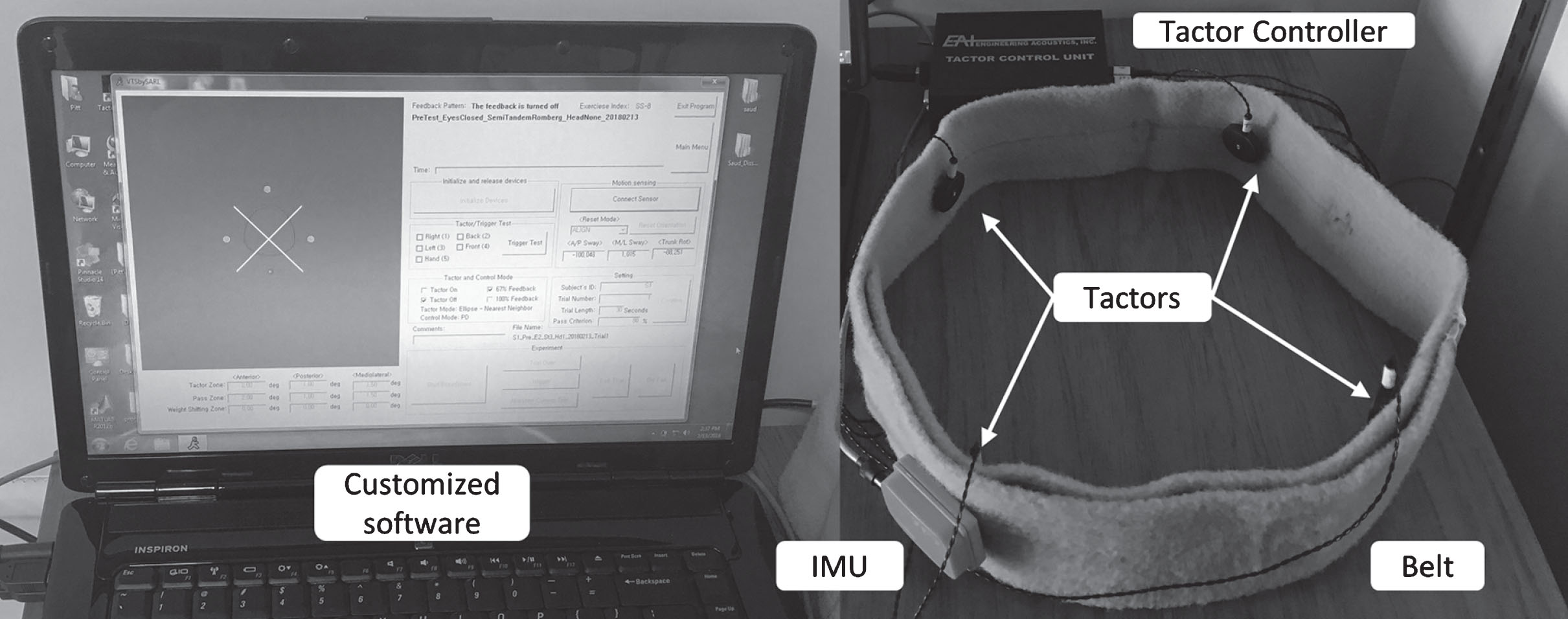
Trunk tilt plus half of the tilt rate was compared with a pre-set threshold (Table 3) to determine when to provide vibrotactile SA for firm surface standing, compliant surface standing, modified center of gravity (center of gravity was modified by means of arm raises), and VOR exercises [56, 57, 64]. For the weight shifting exercises, only trunk tilt was used to determine when to provide vibrotactile SA. The predefined tactor activation thresholds used in this study were informed by thresholds used in previously published studies [4, 52, 56] and study team expertise. Although the effects of training with narrow versus wide tactor activation thresholds on performance are unknown [8], narrow tactor activation thresholds were intentionally selected for use in this study because they provide more frequent cues. Informal usability testing of the thresholds was performed by three vestibular rehabilitation therapy experts on the study team to qualitatively confirm that the tactors were activated at appropriate postural deviations from the desired body positions, thereby providing adequate time for the participants to initiate corrective responses.
Table 3
Tactor activation thresholds
| Exercise Category | Variables | Tactor Activation Thresholds | ||
| Anterior | Posterior | Right/Left | ||
| 1 Standing on firm surface | Feet apart; Romberg; Semi-tandem-Romberg | 2.00° | 1.00° | 1.50° |
| Tandem; Single leg stance | 2.00° | 2.00° | 2.00° | |
| 2 Standing on foam surface | Feet apart; Romberg | 2.00° | 1.00° | 1.50° |
| Semi-tandem Romberg | 2.50° | 1.50° | 2.00° | |
| Tandem | 2.50° | 2.00° | 2.00° | |
| 3 Weight shifting | All conditions | 1.00° | 1.00° | 1.00° |
| 4 Modified center of gravity | All conditions | 2.00° | 4.00° | 1.50° |
| 6 Gaze Stabilization VOR | All conditions | 3.00° | 1.00° | 2.00° |
Customized software displayed the trunk motion and tactor activation information on the laptop screen, and enabled the treating physical therapist to select individual exercises for each participant. The participants did not see the screen. The SA device used in this study was considered an investigational device.
2.4Statistical analysis
The pre-training assessment differences between the EG and CG were tested using an independent samples two-tailed student’s t-test. Linear mixed effect models were used to analyze balance improvements and their persistence after the VRT training. The fixed factors were: group (EG, CG), assessment session (pre-, mid-, one week post-, one month post-, and six months post-training), and their interactions. The random effect was the difference among individual participants. For the kinematic metrics (RMS, PZ, and EA), three repeated exercise trials were averaged since there were no significant differences among the trials. The significance level was set at 0.05. All statistical analyses were performed in R (https://www.r-project.org). Due to the relatively small sample size, the minimal detectable change (MDC) for some clinical outcome measures was used to determine whether the observed improvements achieved clinical significance [69].
3Results
There were no statistical differences between the EG (n = 4) and CG (n = 4) in gender, age, clinical outcome measures, or standing balance exercise performance during pre-assessment.
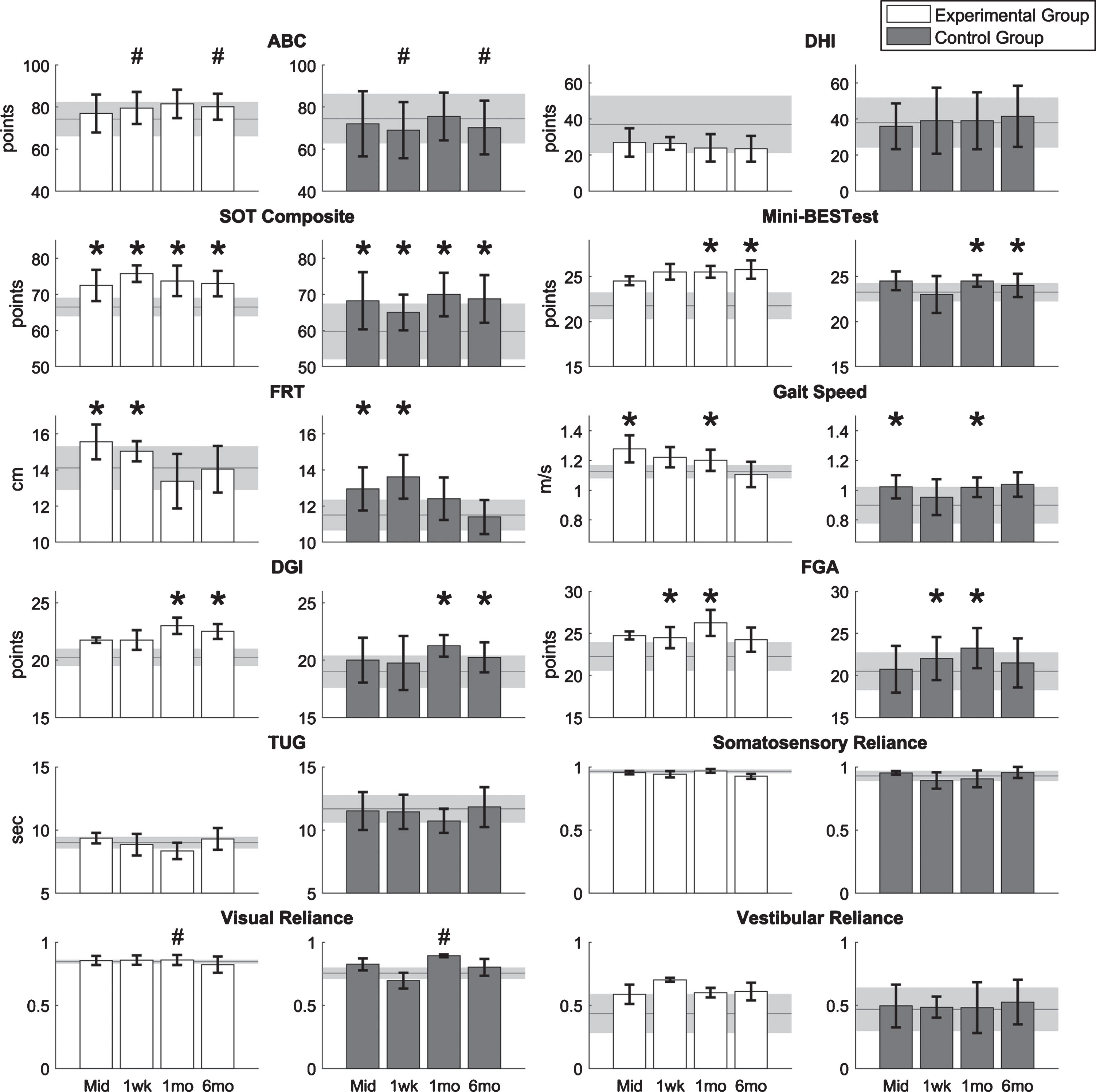
The results of the clinical outcome measures are shown in Fig. 3. There were significant main session effects (regardless of group) for SOT, FRT, and gait speed at the mid-training assessment (p < 0.05, p < 0.05, and p < 0.01, respectively); for SOT, FRT, and FGA at the 1-week post-training assessment (p < 0.05); for SOT, gait speed, DGI, FGA and Mini-BESTest at the 1-month post-training assessment (p < 0.01, p < 0.05, p < 0.001, p < 0.001, and p < 0.05, respectively); and for SOT, DGI, Mini-BESTest at the 6-month post-training assessment (p < 0.01, p < 0.05, and p < 0.05, respectively). For the ABC scale, there was a significant interaction effect during the 1-week and 6-month post-training assessments (p < 0.05); specifically, the ABC scores for the EG increased and the ABC scores for the CG decreased. For the SOT, one participant in both the EG and CG achieved an MDC of eight points [68] in the follow-up assessments. For the Mini-BESTest, one participant in the EG achieved an MDC of 3.5 points [23], but no participants in the CG achieved an MDC in the follow-up assessments.
Fig.3
Results of clinical outcome measures for eight participants with UVD. Horizontal lines and shaded areas denote the mean pre-training assessment values and standard errors of the means, respectively. Columns and error bars denote the mean mid-training (Mid), 1-week (1wk), 1-month (1mo), and 6-month (6mo) post-training assessment values and standard error of mean. An asterisk denotes the significant main session effects, and a hash mark (#) denotes the significant interaction effects between groups and sessions.
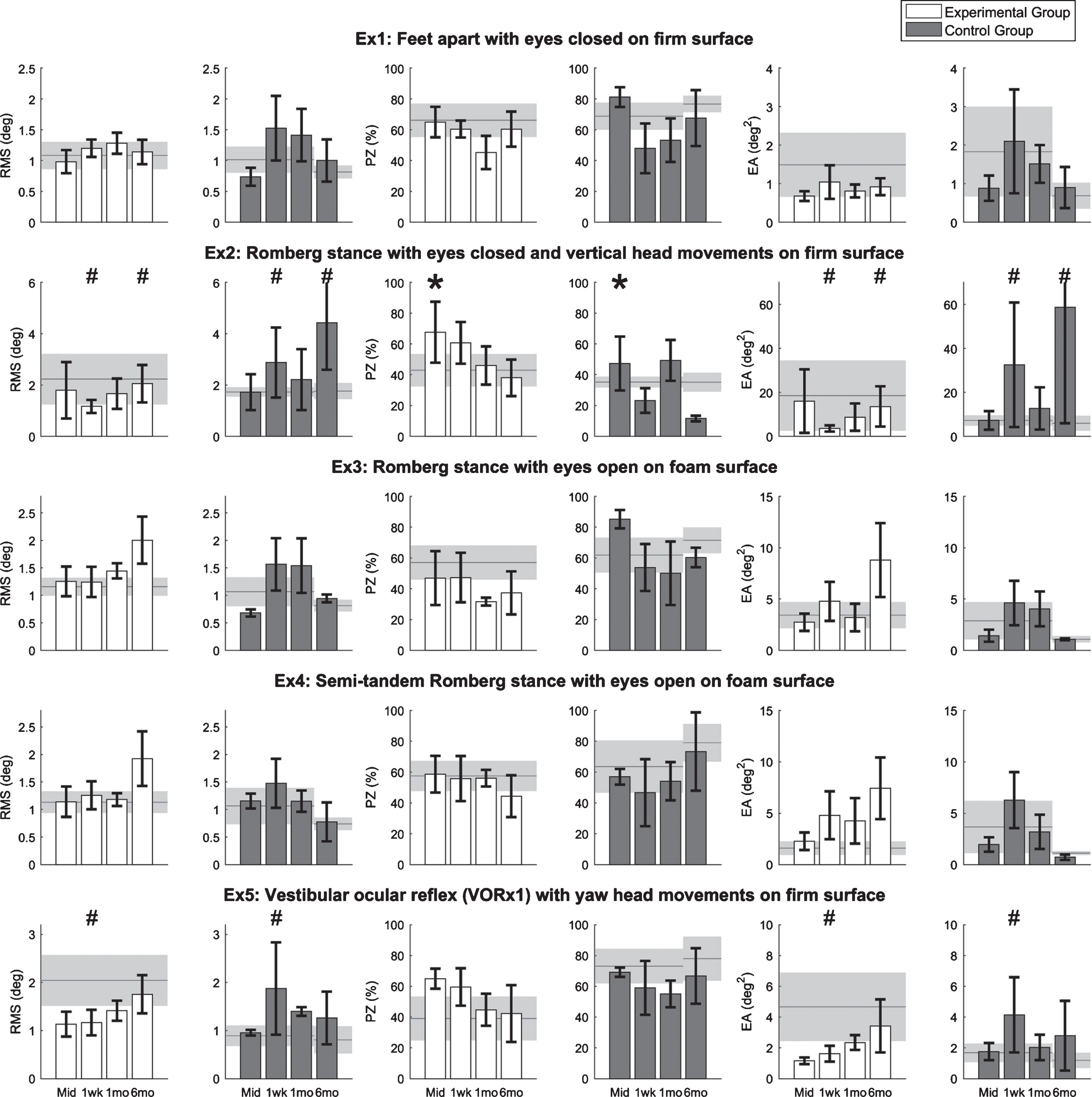
Balance performance outcomes for the five standing balance exercises are shown in Fig. 4. Due to time constraints, Participant 8 in the CG did not perform the balance exercises at the 6-month post-training assessment. For Exercises 1, 3, and 4, there were no significant main session effects or interaction effects. For Exercise 2, PZ significantly increased at the mid-training assessment regardless of group (p < 0.05). At the 1-week and 6-month post-assessments, there were significant interaction effects for the RMS and EA metrics (p < 0.05); the EG showed improvements compared to the pre-training assessment while the CG performed worse compared to the pre-training assessment. For Exercise 5, there were significant interaction effects for the RMS and EA metrics at the 1-week post-training assessments (p < 0.05); the EG showed improvements while the CG performed worse compared to the pre-training assessment.
Fig.4
Results for five standing balance exercises performed by eight participants with UVD. Horizontal lines and shaded areas denote the mean pre-training assessment values and standard errors of the means, respectively. Columns and error bars denote the mean mid-training (Mid), 1-week (1wk), 1-month (1mo), and 6-month (6mo) post-training assessment values and standard error of mean, respectively. An asterisk denotes the significant main session effects, and a hash mark (#) denotes the significant interaction effects between groups and sessions. Lower RMS, lower EA, and higher PZ indicate better balance performance.
4Discussion
All participants, regardless of group, demonstrated improvements in a subset of the clinical or balance metrics immediately following completion of the balance training protocol. Improvements in the CG reinforce the importance of continued balance training following the completion of standard VRT, and suggest that adherence to personalized balance training regimes may further improve outcomes beyond those achieved through standard VRT. Training with SA led to significantly greater improvements than training alone for the ABC scale and two standing balance exercises (i.e., Exercises 2 and 5) at the one-week post-training assessment. Furthermore, pre-/post-training differences for the ABC scale and Exercise 2 were significantly larger for the EG compared to the CG at the one-month post-training assessment.
At the one-week post-training assessment, the EG’s ABC score improved by 5.3 points and the CG’s ABC score worsened by 5.5 points. Our findings were similar to a study by Brugnera et al., which reported that the EG and CG gained balance confidence by approximately 20 points and 11 points, respectively [14]. However, the extent of the improvements and changes in balance confidence were not equivalent, which could be due to the diagnoses of participants and their pre-training performance. In the study by Brugnera et al., most of the participants were diagnosed with bilateral vestibular disorders, and the pre-training ABC scores were lower than the scores in our study (69 points vs. 74 points for the EG and 59 points vs. 74 points for the CG).
The primary differentiator between the standing balance exercises where there was a significant group difference (i.e., Exercises 2 and 5) and the standing balance exercises where there was no significant group difference (i.e., Exercises 1, 3 and 4) was the presence of head movements. This finding suggests that training with SA may be more effective at improving postural stability during activities that incorporate dynamic head movements. Given that the EG exhibited larger increases in vestibular reliance values, its participants may have been able to maintain better balance during the balance exercises that challenged the vestibular system. In addition, Exercise 5 was a VOR-based exercise; training with SA may have helped the participants to better leverage vestibular inputs to control balance during the dynamic tasks [53].
Although larger increases were observed for the SOT, Mini-BESTest, gait speed, DGI, and FGA at the one-week post-training assessment for the group that trained with SA compared with the group that trained without SA, none of the increases were statistically significant compared to the pre-training assessment values. These larger non-significant pre-/post-training differences were also observed for the Mini-BESTest, DGI and FGA at one-month and six-month post-training assessments for the EG compared with the CG. Basta et al. found that the average change in the SOT score after training with vibrotactile SA was approximately 7 points for participants with UVD [10], which is slightly smaller than the improvement in our study (9.3±3.3 points); training period duration (10 vs. 18 training sessions) and training exercises performed (six exercises vs. customized exercise progression protocol) may have contributed to the differences between Basta’s results and the results of our study. SOT and Mini-BESTest were also shown to improve following training with SA in a study involving community-dwelling healthy older adults [7].
For the gait-related measures (i.e., gait speed, DGI, FGA, and TUG), the lack of significant differences between the CG and EG may be attributed to the lack of vibrotactile SA provided during gait exercises for the EG, i.e., neither group received SA during gait exercises. The slight difference between the two groups may have resulted from carry-over effects of the standing balance training, since standing stability and locomotor performance can be highly related [19].
Inputs from the visual, vestibular, and somatosensory systems are used by the central nervous system to generate corrective torques to maintain balance [48]. During VRT, the gaze stability and balance exercises are specifically designed to disturb or remove one or more of these sensory inputs and promote reweighting of the sensory inputs [6, 25, 40]. One hypothesis for the observed improvements in balance performance following training with SA is that the use of the additional of channel of information provided by the SA device may facilitate sensory reweighting [31, 59, 60]. We observed an increase in vestibular reliance values, as calculated from the SOT protocol, for the group that trained with versus without SA. Badke et al. demonstrated a significant improvement ( 0.19 on average) in vestibular reliance among people with peripheral vestibular dysfunction after the completion of VRT [6], but in our study, the CG showed no such improvements. The CG participants in our study had previously completed standard VRT, and thus may have reached a functional plateau. On the other hand, the EG participants in our study increased their vestibular reliance with the use of SA despite also having completed standard VRT prior to participation in the study, suggesting that training with SA may facilitate sensory reweighting.
The primary limitation of this study was its small sample size. Based on the data collected in this preliminary study, approximately 20 participants or more, depending on the outcome measure of interest, would be needed to observe effect sizes (power 0.8) at the one-week post-training assessment. Second, vibrotactile SA was not provided to the EG during gait exercises. To date, of the limited studies that have investigated the use of vibrotactile SA during gait exercises [57, 59, 60, 62], most have reported reductions in trunk tilt/sway [59, 60], with minimal corrective benefits to key gait parameters such as gait velocity, step length, and step width. Furthermore, validated approaches do not exist for the full range of rehabilitative gait exercises. Third, spontaneous compensation was not controlled in this study. However, given the chronicity of the participants, the effects of spontaneous compensation may be limited. Lastly, we did not collect the information about the timing, type, number, or outcome of VRT programs completed by participants prior to the participation in this study.
5Conclusions
Both the EG and CG demonstrated improvements for a subset of the clinical or balance metrics midway through and immediately following completion of the balance training protocol. Participants that performed VRT with vibrotactile SA had larger improvements in their ABC scale scores and balance metrics for exercises involving head movements compared to participants that performed VRT without SA; these improvements were maintained up to six months following completion of the training. Vibrotactile SA may further improve rehabilitation outcomes when combined with standard VRT, however, a larger study is warranted.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
We thank Mohammed Kabeto for his assistance with statistical analysis, and Kathy Brown for her assistance with assessment tests. This work was supported by NIH (1R21DC012410-01A1).
References
[1] | Agrawal Y. , Carey J.P. , Della Santina C.C. , Schubert M.C. , Minor L.B. , Della Santina C.C. , Schubert M.C. and Minor L.B. , Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004, Arch Intern Med 169: ((2009) ) 938–944. |
[2] | Allum J.H.J. and Adkin A.L. , Improvements in Trunk Sway Observed for Stance and Gait Tasks during Recovery from an Acute Unilateral Peripheral Vestibular Deficit, Audiol Neuro-Otology ((2003) ) 8: , 286–302. |
[3] | Allum J.H.J. , Carpenter M.G. , Horslen B.C. , Davis J.R. , Honegger F. , Tang K.-S. and Kessler P. , Improving impaired balance function: real-time versus carry-over effects of prosthetic feedback, Conf Proc IEEE Eng Med Biol Soc 2011: ((2011) ), 1314–8. |
[4] | Allum J.H.J. , Carpenter M.G. , Horslen B.C. , Davis J.R. , Honegger F. , Tang K.-S. and Kessler P. , Improving impaired balance function: real-time versus carry-over effects of prosthetic feedback, Conf Proc IEEE Eng Med Biol Soc 2011: ((2011) ), 1314–8. |
[5] | Bach-y-Rita P. , Tactile sensory substitution studies, Ann N Y Acad Sci 1013: ((2004) ), 83–91. |
[6] | Badke M.B. , Miedaner J.A. , Grove C.R. , Shea T.A. and Pyle G.M. , Effects of vestibular and balance rehabilitation on sensory organization and dizziness handicap, Ann Otol Rhinol Laryngol 114: ((2005) ), 48–54. |
[7] | Bao T. , Carender W.J. , Kinnaird C. , Barone V.J. , Peethambaran G. , Whitney S.L. , Kabeto M. , Seidler R.D. and Sienko K.H. , Effects of long-term balance training with vibrotactile sensory augmentation among community-dwelling healthy older adults, J Neuroeng Rehabil 15: ((2018) ), 1–13. |
[8] | Bao T. , Kinnaird C. , Carender W.J. and Sienko K.H. , Effects of sensory augmentation activation thresholds on balance performance in people with vestibular disorders, in: Int Soc Posture Gait Res World Congr, 2019. |
[9] | Barros C.G.C. , Bittar R.S.M. and Danilov Y. , Effects of electrotactile vestibular substitution on rehabilitation of patients with bilateral vestibular loss, Neurosci Lett 476: ((2010) ), 123–6. |
[10] | Basta D. , Rossi-Izquierdo M. , Soto-Varela A. , Greters M.E. , Bittar R.S. , Steinhagen-Thiessen E. , Eckardt R. , Harada T. , Goto F. , Ogawa K. and Ernst A. , Efficacy of a vibrotactile neurofeedback training in stance and gait conditions for the treatment of balance deficits: a double-blind, placebo-controlled multicenter study, Otol Neurotol 32: ((2011) ), 1492–9. |
[11] | Basta D. , Singbartl F. , Todt I. , Clarke A. and Ernst A. , Vestibular rehabilitation by auditory feedback in otolith disorders, Gait Posture 28: ((2008) ), 397–404. |
[12] | Bechly K.E. , Carender W.J. , Myles J.D. and Sienko K.H. , Determining the preferred modality for real-time biofeedback during balance training, Gait Posture 37: ((2013) ), 391–6. |
[13] | Brown K.E. , Whitney S.L. , Wrisley D.M. and Furman J.M. , Physical therapy outcomes for persons with bilateral vestibular loss, Laryngoscope 111: ((2001) ), 1812–1817. |
[14] | Brugnera C. , Bittar R.S.M. , Greters M.E. and Basta D. , Effects of vibrotactile vestibular substitution on vestibular rehabilitation – preliminary study, Braz J Otorhinolaryngol 81: ((2015) ), 616–621. |
[15] | Clendaniel R.A. , Outcome measures for assessment of treatment of the dizzy and balance disorder patient, Otolaryngol Clin North Am 33: ((2000) ), 519–533. |
[16] | Cowand J.L. , Wrisley D.M. , Walker M. , Strasnick B. and Jacobson J.T. , Efficacy of Vestibular Rehabilitation, Otolaryngol Clin North Am 118: ((1998) ), 49–54. |
[17] | Danilov Y.P. , Tyler M.E. , Skinner K.L. and Bach-y-Rita P. , Efficacy of electrotactile vestibular substitution in patients with bilateral vestibular and central balance loss, in: IEEE EMBS Annu Int Conf, (2006) : pp. 6605–9. |
[18] | Davis J.R. , Carpenter M.G. , Tschanz R. , Meyes S. , Debrunner D. , Burger J. and Allum J.H.J. , Trunk sway reductions in young and older adults using multi-modal biofeedback, Gait Posture 31: ((2010) ), 465–72. |
[19] | Dettmann M.A. , Linder M.T. and Sepic S.B. , Relationships among walking performance, postural stability, and functional assessments of the hemiplegic patient, Am J Phys Med Rehabil 66: ((1987) ), 77–90. |
[20] | Dozza M. , Horak F.B. and Chiari L. , Auditory biofeedback substitutes for loss of sensory information in maintaining stance, Exp Brain Res 178: ((2007) ), 37–48. |
[21] | Duncan P.W. , Weiner D.K. , Chandler J. and Studenski S. , Functional reach: a new clinical measure of balance, J Gerontol 45: ((1990) ), M192–M197. |
[22] | Franchignoni F. , Horak F. , Godi M. , Nardone A. and Giordano A. , Using psychometric techniques to improve the balance evaluation systems test: The mini-bestest, J Rehabil Med 42: ((2010) ), 323–331. |
[23] | Godi M. , Franchignoni F. , Caligari M. , Giordano A. , Turcato A.M. and Nardone A. , Comparison of Reliability, Validity, and Responsiveness of the Mini- BESTest and Berg Balance Scale in Patients With Balance Disorders, Phys Ther 93: ((2013) ), 158–167. |
[24] | Halická Z. , Lobotková J. , Bučková K. and Hlavačka F. , Effectiveness of different visual biofeedback signals for human balance improvement, Gait Posture 39: ((2014) ), 410–414. |
[25] | Hall C.D. , Herdman S.J. , Whitney S.L. , Cass S.P. , Clendaniel R.A. , Fife T.D. , Furman J.M. , Getchius T.S.D. , Goebel J.A. , Shepard N.T. and Woodhouse S.N. , Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline, 2016. |
[26] | Hansson E.E. , Månsson N.-O. , Ringsberg K.A. and Håkansson A. , Falls among dizzy patients in primary healthcare: an intervention study with control group, Int J Rehabil Res 31: ((2008) ), 51–7. |
[27] | Hegeman J. , Honegger F. , Kupper M. and Allum J.H.J. , The balance control of bilateral peripheral vestibular loss subjects and its improvement with auditory prosthetic feedback, J Vestib Res 15: ((2005) ), 109–17. |
[28] | Herdman S.J. , Blatt P. , Schubert M.C. and Tusa R.J. , Falls in patients with vestibular deficits, Am J Otol 21: ((2000) ), 847–851. |
[29] | Herdman S.J. and Clendaniel R.A. , Vestibular Rehabilitation, Fourth, 2014. |
[30] | Hillier S.L. and McDonnell M. , Vestibular rehabilitation for unilateral peripheral vestibular dysfunction, Clin Otolaryngol 36: ((2011) ), 248–249. |
[31] | Horak F.B. , Postural compensation for vestibular loss and implications for rehabilitation, Restor Neurol Neurosci 28: ((2010) ), 57–68. |
[32] | Huffman J.L. , Norton L.E. , Adkin A.L. and Allum J.H.J. , Directional effects of biofeedback on trunk sway during stance tasks in healthy young adults, Gait Posture 32: ((2010) ), 62–66. |
[33] | Ites K.I. , Anderson E.J. , Cahill M.L. , Kearney J.A. , Post E.C. and Gilchrist L.S. , Balance Interventions for Diabetic Peripheral Neuropathy: A Systematic Review, J Geriatr Phys Ther 34: ((2011) ), 109–116. |
[34] | Jacobson G.P. and Newman C.W. , The development of the Dizziness Handicap Inventory, Arch Otolaryngol – Head Neck Surg 116: ((1990) ), 424–427. |
[35] | Janssen L.J.F. , Verhoeff L.L. , Horlings C.G.C. and Allum J.H.J. , Directional effects of biofeedback on trunk sway during gait tasks in healthy young subjects, Gait Posture 29: ((2009) ), 575–81. |
[36] | Janssen M. , Stokroos R. , Aarts J. , van Lummel R. and Kingma H. , Salient and placebo vibrotactile feedback are equally effective in reducing sway in bilateral vestibular loss patients, Gait Posture 31: ((2010) ), 213–7. |
[37] | Jongkees L.B.W. , Maas J.P.M. and Philipszoon A.J. , Clinical nystagmography, ORL 24: ((1962) ), 65–93. |
[38] | Jung J.Y. , Kim J.-S. , Chung P.S. , Woo S.H. and Rhee C.K. , Effect of vestibular rehabilitation on dizziness in the elderly, Am J Otolaryngol 30: ((2009) ), 295–9. |
[39] | Keith R.A. , V. C. , Granger, B.B. Hamilton and F.S. Sherwin, The functional independence measure: a new tool for rehabilitation, Adv Clin Rehabil 1: ((1987) ), 6–18. |
[40] | Klatt B.N. , Carender W.J. , Lin C. , Alsubaie S. , Kinnaird C.R. , Sienko K.H. and Whitney S.L. , A Conceptual Framework for the Progression of Balance Exercises in Persons with Balance and Vestibular Disorders, Phys Med Rehabil – Int 2: ((2015) ), 1044. |
[41] | Lajoie Y. and Gallagher S.P. , Predicting falls within the elderly community: Comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers, Arch Gerontol Geriatr 38: ((2004) ), 11–26. |
[42] | Lee B.-C. , Kim J. , Chen S. and Sienko K.H. , Cell phone based balance trainer, J Neuroeng Rehabil 9: ((2012) ), 10. |
[43] | Meli A. , Zimatore G. , Badaracco C. , De Angelis E. and Tufarelli D. , Vestibular rehabilitation and 6-month follow-up using objective and subjective measures, Acta Otolaryngol 126: ((2006) ), 259–66. |
[44] | Nashner L.M. , Black F.O. , Wall C. , Adaptation to altered support and visual conditions during stance: patients with vestibular deficits, J Neurosci 2: ((1982) ), 536–44. |
[45] | Nasreddine Z. , Charbonneau S. and Cummings J.L. , The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment, J Am Geriatr Soc 53: ((2005) ), 695–699. |
[46] | Neuhauser H.K. , Epidemiology of vertigo, Curr Opin Neurol 20: ((2007) ), 40–46. |
[47] | Perera S. , Mody S.H. , Woodman R.C. and Studenski S.A. , Meaningful change and responsiveness in common physical performance measures in older adults, J Am Geriatr Soc 54: ((2006) ), 743–749. |
[48] | Peterka R.J. , Sensorimotor Integration in Human Postural Control, J Neurophysiol 88: ((2002) ), 1097–1118. |
[49] | Podsiadlo D. and Richardson S. , The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons, J Am Geriatr Soc 39: ((1991) ), 142–148. |
[50] | Powell L.E. and Myers A.M. , The activities-specific balance confidence (ABC) scale,M28–M, Journals Gerontol Ser A Biol Sci Med Sci 50: ((1995) ), 34. |
[51] | Robinson B.S. , Cook J.L. , Richburg C.M. and Price S.E. , Use of an electrotactile vestibular substitution system to facilitate balance and gait of an individual with gentamicin-induced bilateral vestibular hypofunction and bilateral transtibial amputation, J Neurol Phys Ther 33: ((2009) ), 150–159. |
[52] | Rossi-Izquierdo M. , Ernst A. , Soto-Varela A. , Santos-Pérez S. , Faraldo-García A. , Sesar-Ignacio A. and Basta D. , Vibrotactile neurofeedback balance training in patients with Parkinson’s disease: reducing the number of falls, Gait Posture 37: ((2013) ), 195–200. |
[53] | Schubert M.C. and Minor L.B. , Vestibulo-ocular Physiology Underlying Vestibular Hypofunction, Phys Ther 84: ((2004) ), 373–385. |
[54] | Shumway-Cook A. , Horak F.B. , Yardley L. and Bronstein A.M. , Rehabilitation of balance disorders in the patient with vestibular pathology, Clin. Disord. Balanc. Posture, Gait. London, United Kingdom Arnold, Div Hodder Headl. PLC. (1996), 213–220. |
[55] | Shumway-Cook A. and Woollacott M.H. , Motor control: theory and practical applications, Lippincott Williams & Wilkins, (1995) . |
[56] | Sienko K.H. , Balkwill M.D. , Oddsson L.I.E. and Wall C. , Effects of multi-directional vibrotactile feedback on vestibular-deficient postural performance during continuous multi-directional support surface perturbations, J Vestib Res 18: ((2008) ), 273–285. |
[57] | Sienko K.H. , Balkwill M.D. , Oddsson L.I.E. and Wall C. , The effect of vibrotactile feedback on postural sway during locomotor activities, J Neuroeng Rehabil 10: ((2013) ), 93. |
[58] | Sienko K.H. , Balkwill M.D. and Wall C. , Biofeedback improves postural control recovery from multi-axis discrete perturbations, J Neuroeng Rehabil 9: ((2012) ), 53. |
[59] | Sienko K.H. , Seidler R.D. , Carender W.J. , Goodworth A.D. , Whitney S.L. and Peterka R.J. , Potential Mechanisms of Sensory Augmentation Systems on Human Balance Control, Front Neurol 9: ((2018) ). |
[60] | Sienko K.H. , Whitney S.L. , Carender W.J. and Wall III C. , The role of sensory augmentation for people with vestibular deficits: real-time balance aid and/or rehabilitation device? J Vestib Res 27: ((2017) ), 63–76. |
[61] | Subramanian S.K. , Massie C.L. , Malcolm M.P. and Levin M.F. , Does provision of extrinsic feedback result in improved motor learning in the upper limb poststroke? A systematic review of the evidence, Neurorehabil Neural Repair 24: ((2010) ), 113–124. |
[62] | Verhoeff L.L. , Horlings C.G.C. , Janssen L.J.F. , a Bridenbaugh S. and Allum J.H.J. , Effects of biofeedback on trunk sway during dual tasking in the healthy young and elderly, Gait Posture 30: ((2009) ), 76–81. |
[63] | Wall C. , Wrisley D.M. and Statler K.D. , Vibrotactile tilt feedback improves dynamic gait index: a fall risk indicator in older adults, Gait Posture 30: ((2009) ), 16–21. |
[64] | Wall III C. and Kentala E. , Effect of displacement, velocity, and combined vibrotactile tilt feedback on postural control of vestibulopathic subjects, J Vestib Res 20: ((2010) ), 61–9. |
[65] | Whitney S.L. , Alghadir A.H. and Anwer S. , Recent Evidence About the Effectiveness of Vestibular Rehabilitation, Curr. Treat. Options Neurol. 18 ((2016) ), 1–15. |
[66] | Whitney S.L. and Rossi M.M. , Efficacy of vestibular rehabilitation, Otolaryngol Clin North Am 33: ((2000) ), 659–672. |
[67] | Wrisley D.M. , Marchetti G.F. , Kuharsky D.K. and Whitney S.L. , Reliability, internal consistency, and validity of data obtained with the functional gait assessment, Phys Ther 84: ((2004) ), 906–918. |
[68] | Wrisley D.M. , Stephens M.J. , Mosley S. , Wojnowski A. , Duffy J. and Burkard R. , Learning Effects of Repetitive Administrations of the Sensory Organization Test in Healthy Young Adults, Arch Phys Med Rehabil 88: ((2007) ), 1049–1054. |
[69] | Rehabilitation Measures Database, (n.d.). sralab.org |




