Proceedings of the 14th International Newborn Brain Conference: Neonatal Neurocritical Care, seizures, and continuous aEEG and /or EEG monitoring
A comparison of treatment pathways for seizures in newborns across rural and non-rural level II and III neonatal intensive care units
Dickman J1, Carrasco McCaul M1
1University Of Wisconsin School Of Medicine And Public Health, Madison, United States
BACKGROUND: Seizures are more common during the neonatal period than at any other age, occurring at a rate of 1.10–5.5 per 1,000 live births. Neonatal seizures occur during the first 30 days of postnatal life and constitute a neurological emergency requiring prompt recognition and treatment1. Seizures in newborns differ in clinical appearance, electrographic characteristics, etiology, and management compared to seizures that occur at later stages of development2. Thus, early recognition and treatment is essential in maintaining healthy outcomes for neonates3. However, there is widespread variability on how these are initially identified, evaluated, and treated across neonatal intensive care units (NICUs)4. This study aimed to investigate the neonatal seizure treatment pathways employed by rural and non-rural Level II and III NICUs across the Midwest and Pacific Northwest to identify areas of consensus and variability.
METHODS: In total, we contacted 301 Level II and III NICUs via an electronic survey. The survey collected information regarding the evaluation of neonatal seizures, anti-seizure medication availability, electroencephalogram (EEG) monitoring methods, and if and how an official treatment pathway for neonatal seizure patients was established at their institution. Survey results will be examined using descriptive analysis.
RESULTS: With limited responses, preliminary results show that only 19% of surveyed NICUs have an established pathway for treating neonatal seizures, and only 10% have a formal definition for neonatal seizures. 43% of responding NICUs have no EEG monitoring available to them (rural NICUs make up 22% of those without monitoring). 14% of reviewed NICUs don’t have anti-seizure medications available in their NICU and instead rely on pharmacy to send them when needed.
CONCLUSIONS: Our research determined there is widespread variability in neonatal seizure detection and response from rural and non-rural Level II and III NICUs. These results highlight the lack of equitable access to neonatal seizure treatment. Specifically, our data suggests that areas of potential improvement include the implementation of a universal protocol, access to medications, and the need for education surrounding treatment pathway establishment. These developments may eventually provide earlier detection, evaluation, and treatment of seizures in newborns.
REFERENCES
[1] Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: A population-based study, California 1998-2002. J Pediatr 2009;154:24-28.e1. https://doi.org/10.1016/j.jpeds.2008.07.008.
[2] Abend NS, Jensen FE, Inder TE, Volpe JJ. 2018. Volpe‘s Neurology of the Newborn. 14: 275-321.
[3] Pisani F, Spagnoi C, Falsaperla R, Nagarajan L, Ramantani G. 2021. Seizures in the neonate: A review of etiologies and outcomes. Seizure: European Journal of Epilepsy. 85: 48-56.
[4] Keene JC, Morgan LA, Abend NS, et al. Treatment of Neonatal Seizures: Comparison of ref Pathways From 11 Neonatal Intensive Care Units. Pediatr Neurol. Published online October 11, 2021:S0887-8994(21)00222-8. doi:10.1016/j.pediatrneurol.2021.10.004.
Risk factors for later epilepsy in infants with perinatal arterial ischemic stroke (PAIS)
Gasperoni E1, Wagenaar N1, Baak L1, van der Aa N1, Harteman J1, de Vries L1, Benders M1, Tataranno M1
1Department of Neonatology, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
BACKGROUND AND PURPOSE: Infants with perinatal arterial ischemic stroke (PAIS) are at high risk of neurodevelopmental disabilities later in life, and 9-16% will develop epilepsy at pre-school age. However, risk factors associated with the development of epilepsy have not been identified yet. Early identification of infants at risk for epilepsy can lead to early intervention strategies in order to protect the child from additional developmental delay. This study aims to identify clinical, MRI, and aEEG-related risk factors in infants at risk for later epilepsy after suffering from PAIS.
MATERIALS AND METHODS: This retrospective study involves 52 term neonates, admitted to the neonatal intensive care unit (NICU) of the Wilhelmina Children’s Hospital (Utrecht, Netherlands) between February 2009 and March 2018 with seizures and MRI-confirmed PAIS.
Infants who underwent MRI with diffusion-weighted images (DWI) within 7 days after birth, had an aEEG recording up to 5 days, and at least 2 years of follow-up data were included in the study. Clinical and perinatal data were retrospectively collected. A qualitative analysis on DWI and T2-weighted images was performed to assess stroke location, size, and branch, and we quantitatively determined the stroke volume.
Moreover, aEEG traces were divided in periods of 6 hours each, out of which one hour was randomly chosen and analysed for symmetry, background pattern, and sleep-wake cycling. Clinical and electrical presence of seizures was analysed on the full length of the aEEG traces. We collected follow-up data regarding the development of epilepsy, dosage and length of anti-seizure medication (ASMs) continued after NICU discharge up to 5 years age.
RESULTS: Of 52 subjects, one infant was excluded because of a metabolic disease and two because of missing follow-up data. 49 subjects were included in the study and 4 developed epilepsy (8,2%).
The presence of hypoglycemia was significantly more common in the epilepsy vs no-epilepsy group (p=0.014). The involvement of the region of the posterior cerebral artery (PCA) (p=0.015) and a larger stroke volume were significantly more present in the epilepsy group (p=0.001).
Although not statistically significant, time to normal background pattern and to symmetry on the aEEG differed among the two groups, with a longer time observed in infants who developed epilepsy.
CONCLUSION: With this study, we made a first attempt to identify clinical, imaging, and aEEG risk factors for the development of epilepsy after PAIS. We found that infants that developed epilepsy more often had hypoglycemia, a larger stroke volume or a PCA stroke. No association was found between aEEG parameters and the development of epilepsy, however a trend was observed with time to normal background pattern and symmetry. Prospective larger cohort studies are needed to clarify the role of other risk factors on the later development of epilepsy after PAIS.
Is electrical brain activity affected by longstanding hemodynamic significant Ductus Arteriosus in Preterm infants?
Trabatti C1, Lemmers P2,3, Benders PMA2,3, van Bel F2,3, Tataranno ML2,3
1Pediatric Department, Maggiore Hospital, ASST Crema, Crema, Italy, 2Department of Neonatology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, The Netherlands, 3Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, The Netherlands
BACKGROUND AND PURPOSE: Recent studies have shown that a longstanding hemodynamically significant patent Ductus arteriosus (hsPDA) is associated with low cerebral oxygen saturations in preterm infants (rScO2) [1]. This can negatively affect brain growth [2] and the subsequent neurodevelopmental outcome. Earlier studies showed an association between electrical brain activity and rScO2 and a case report suggested less brain activity during hsPDA [3], although data are still limited. The aim of this study is to investigate changes in brain activity and its maturation in a cohort of extremely preterm infants (<28 weeks of gestation) with a prolonged hsPDA, before and after (surgical) closing.
MATERIALS AND METHODOLOGY: In 23 extremely preterm infants, born and admitted at the NICU of the Wilhelmina Children Hospital (Utrecht, The Netherlands) between 2009 and 2017, whose hsPDA failed to close noninvasively and needed surgical ligation, the pre-closure double-channel aEEG/EEG (before start of anesthesia) was visually compared with post-closure aEEG/EEG. aEEG background pattern (BGP) and sleep-wake cycling (SWC) were determined according to Hellström-Westas et al. modified criteria. Furthermore, average voltage (μV) was calculated and rScO2 was simultaneously determined using Near InfraRed Spectroscopy (NIRS).
RESULTS: Mean GA and birthweight in our cohort were 25.7 (range 24.0-27.6) weeks and 859 (range 580-1350) grams respectively. Duration of hsPDA ranged from 4 to 24 (median 13) days. Mean ±1SD pre-closure and post-closure rScO2 were 51 ±9% and 59 ±10% respectively (p<0.05). Average voltage (μV) was lower before ductal closure, with a more immature background pattern and mostly absence of SWC. Post-closure electrical activity was increased (p<0.05) and background pattern more mature. SWC were also increasingly present (see table 1).
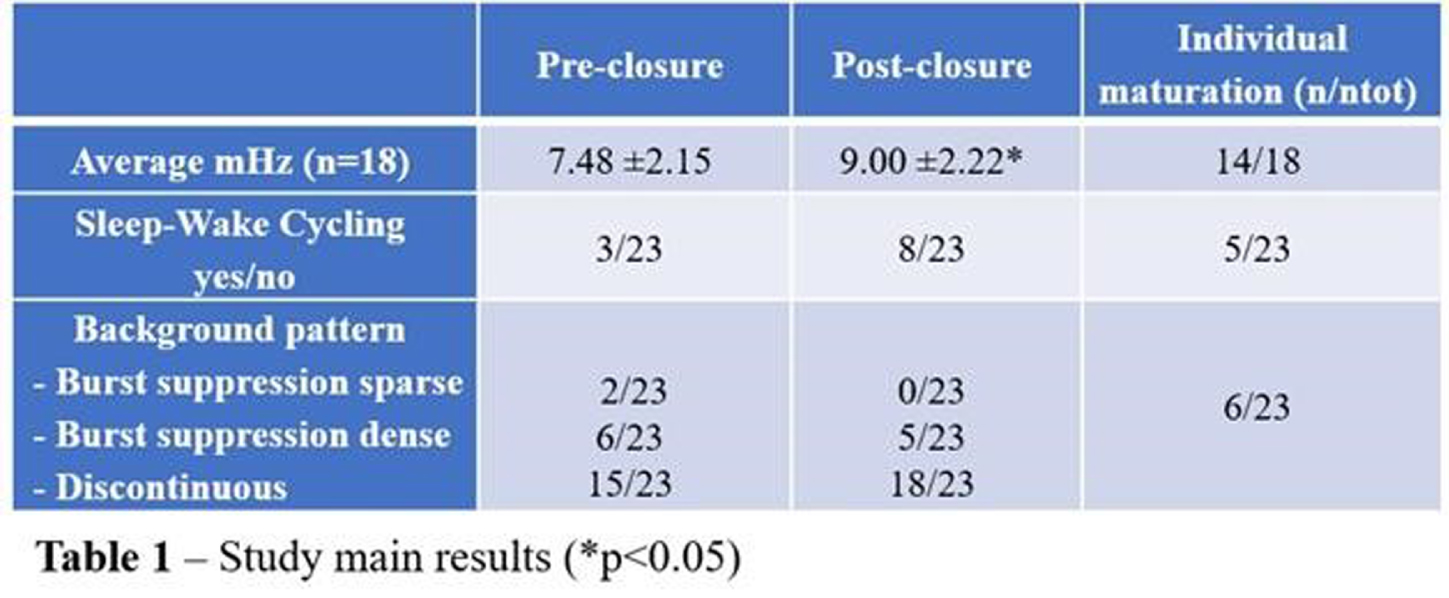
CONCLUSION: Our preliminary results indeed suggest that hsPDA negatively impacts electrical brain activity and maturation. Less optimal oxygenation of the immature brain may play a mechanistic role here. aEEG/EEG can be a useful bedside tool to monitor brain function before and after surgery in preterm infants, providing important prognostic information.
REFERENCES
[1] Lemmers PM, Molenschot MC, Evens J, Toet MC, van Bel F. Is cerebral oxygen supply compromised in preterm infants undergoing surgical closure for patent ductus arteriosus? Arch Dis Child Fetal Neonatal Ed. 2010 Nov;95(6):F429-34. doi: 10.1136/adc.2009.180117.
[2] Lemmers PM, Benders MJ, D’Ascenzo R, Zethof J, Alderliesten T, Kersbergen KJ, Isgum I, de Vries LS, Groenendaal F, van Bel F. Patent Ductus Arteriosus and Brain Volume. Pediatrics. 2016 Apr;137(4):e20153090. doi: 10.1542/peds.2015-3090.
[3] Variane GFT, Chock VY, Netto A, Pietrobom RFR, Van Meurs KP. Simultaneous Near-Infrared Spectroscopy (NIRS) and Amplitude-Integrated Electroencephalography (aEEG): Dual Use of Brain Monitoring Techniques Improves Our Understanding of Physiology. Front Pediatr. 2020 Jan 21;7:560. doi: 10.3389/fped.2019.00560.
Brain monitoring findings using a telehealth strategy in a large cohort of neonates with hypoxic-ischemic encephalopathy
Variane G1,2,3, Rodrigues D1,4, Llaguno N1,4, Magalhães M1,2, Van Meurs K5,6
1Protecting Brains & Saving Futures, São Paulo, Brazil, 2Division of Neonatology, Department of Pediatrics, Irmandade da Santa Casa de Misericórdia de São Paulo, São Paulo, Brazil, 3Networking and Communication Committee at Newborn Brain Society, Roxbury Crossing, United States of America, 4Pediatric Nursing Department, Escola Paulista de Enfermagem - Universidade Federal de São Paulo, São Paulo, Brazil, 5Stanford University School of Medicine, Stanford, United States of America, 6Lucile Packard Children’s Hospital, Palo Alto, United States of America
BACKGROUND: Hypoxic-ischemic encephalopathy (HIE) is estimated to occur in 5 to 26 newborns per 1000 live births in low and middle-income countries and is responsible for a high risk of death or disability. Neonatal seizures are often subclinical and have been associated with worse neurologic outcomes. Amplitude integrated electroencephalography combined with raw electroencephalography and video imaging (video aEEG/EEG) provides real-time screening for seizure detection. Accuracy of seizure detection using aEEG/EEG is highly dependent on the user’s experience. Telehealth may play a role in providing the necessary expertise when neurology and neurophysiology support is not available.
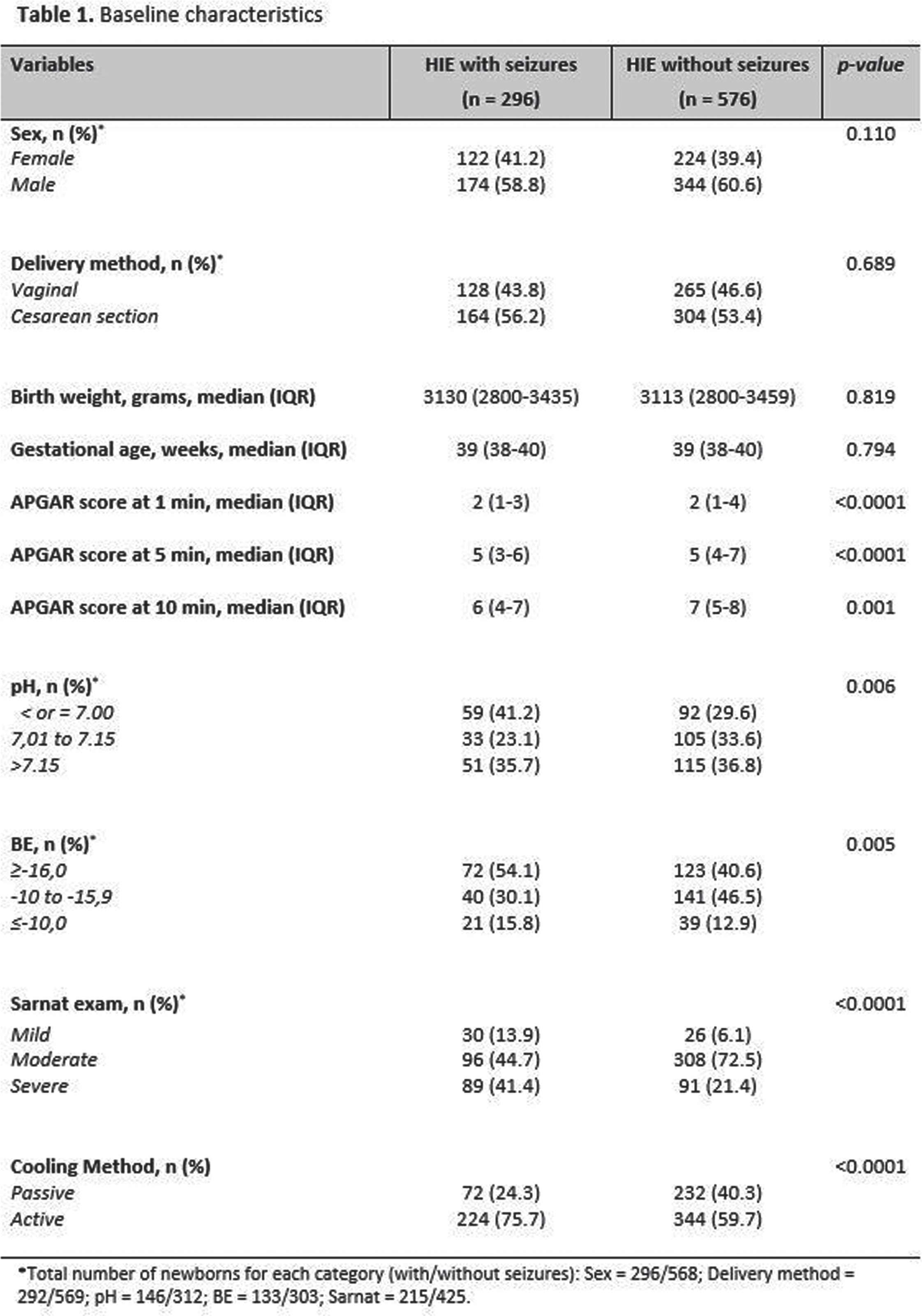
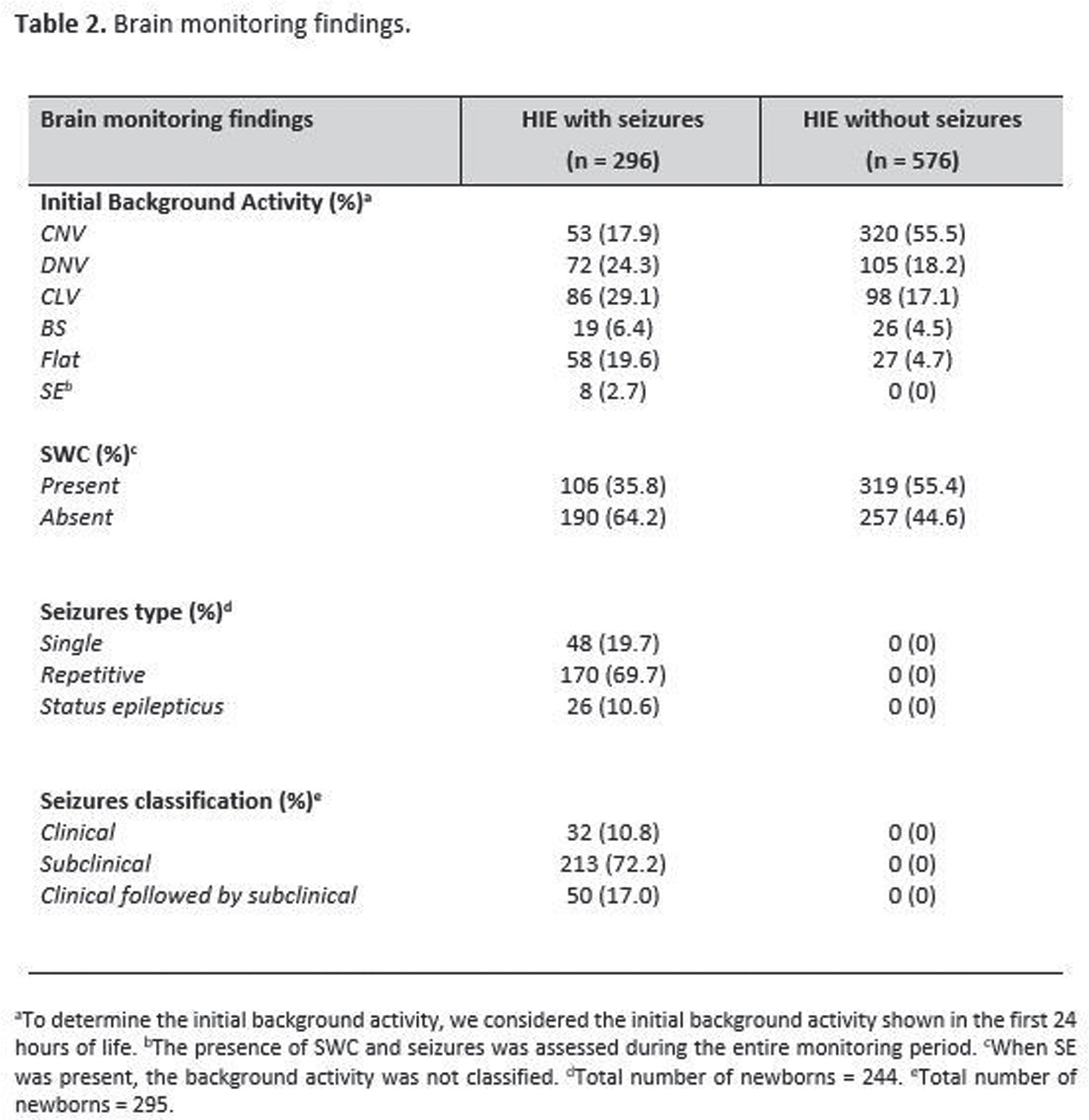
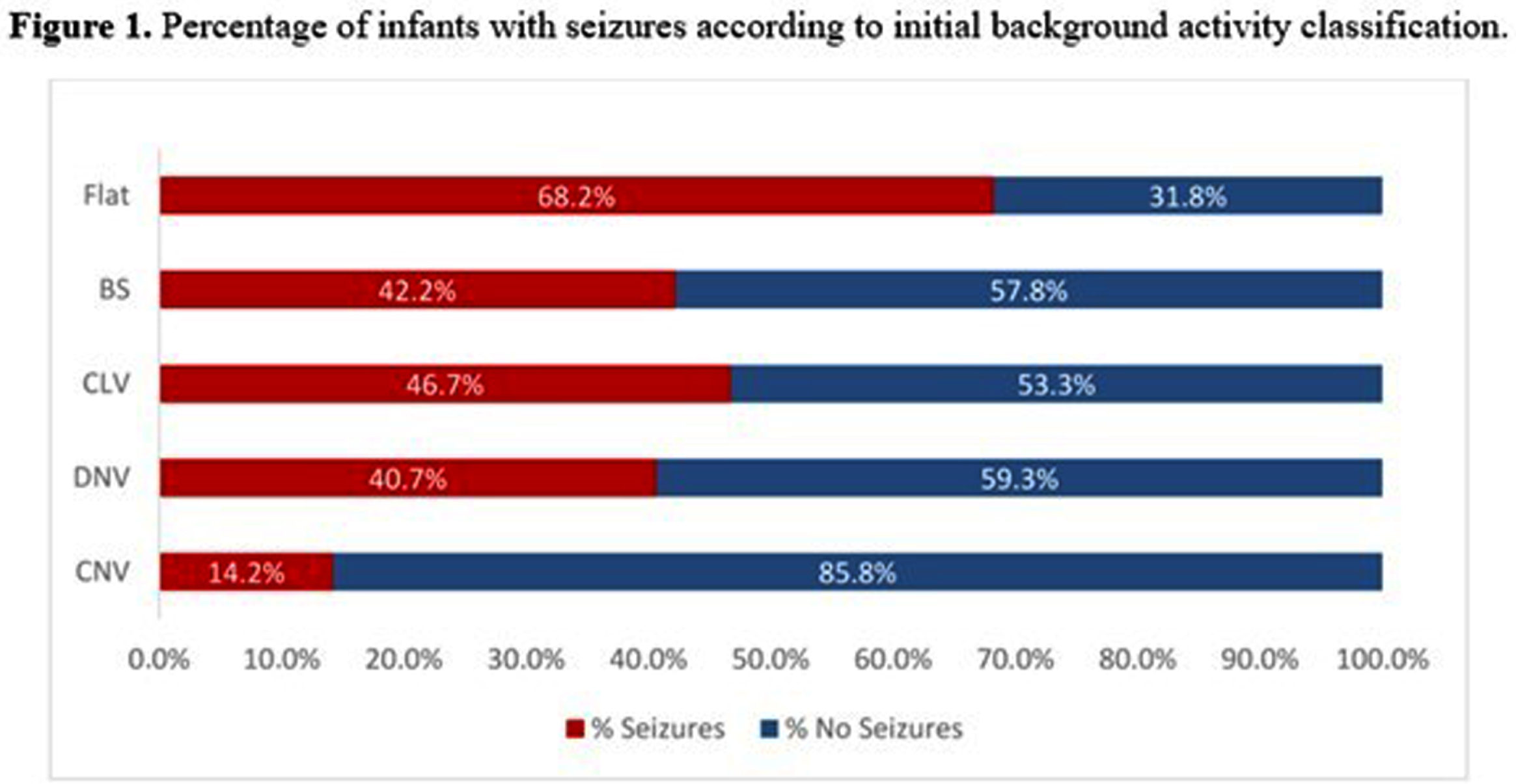
OBJECTIVES: To describe brain monitoring findings, including the onset, treatment, and evolution of seizures diagnosed with aEEG/EEG in a large cohort of newborns with HIE receiving therapeutic hypothermia (TH), assisted by a remote telemonitoring approach. Methods: Multicenter, prospective, observational study conducted from July 2017 to December 2021 at 32 hospitals in Brazil. This study included infants who met the eligibility criteria for TH and received neuromonitoring with video aEEG/EEG during cooling and rewarming. Trained neonatologists and neurologists provided aEEG/EEG interpretation in a remote monitoring center. Descriptive statistical analysis was used. Non-parametric variables were presented as median and interquartile ranges (IQR). The independent t-test, chi-square, Mann-Whitney test, and post hoc analyses were used for the associations. Results: 872 newborns from 32 hospitals were included. According to the modified Sarnat exam, 56 (6.4%) infants were classified with mild HIE, 404 (46.3%) moderate, and 180 (20.6%) severe. Infants initially classified with mild HIE received TH due to diagnosis of seizures and/or abnormal background activity on aEEG (continuous low voltage, burst suppression and/or flat trace). Baseline characteristics are shown in Table 1. Electrographic seizures were identified in 296 (33.9%) newborns, in which 26 (10.6%) had status epilepticus, 170 (69.7%) repetitive seizures and 48 (19.7%) single seizure. Sleep-wake cycling (SWC) was absent in 447 (51.3%) of newborns. A summary of the brain monitoring findings is shown in Table 2. Seizures were more common in infants with absent SWC (p<0.0001) and abnormal early background activity (p<0.0001) (Figure 1). Seizure onset was most frequent between 6 to 12 hours of life (39.7%), and 75% occurred in the first 24 hours of life (Figure 2A). The first-line antiepileptic drug (AED) used for seizure treatment was phenobarbital (99.3%) and a single AED achieved seizure control in 192 (70.6%) infants (Figure 2B).
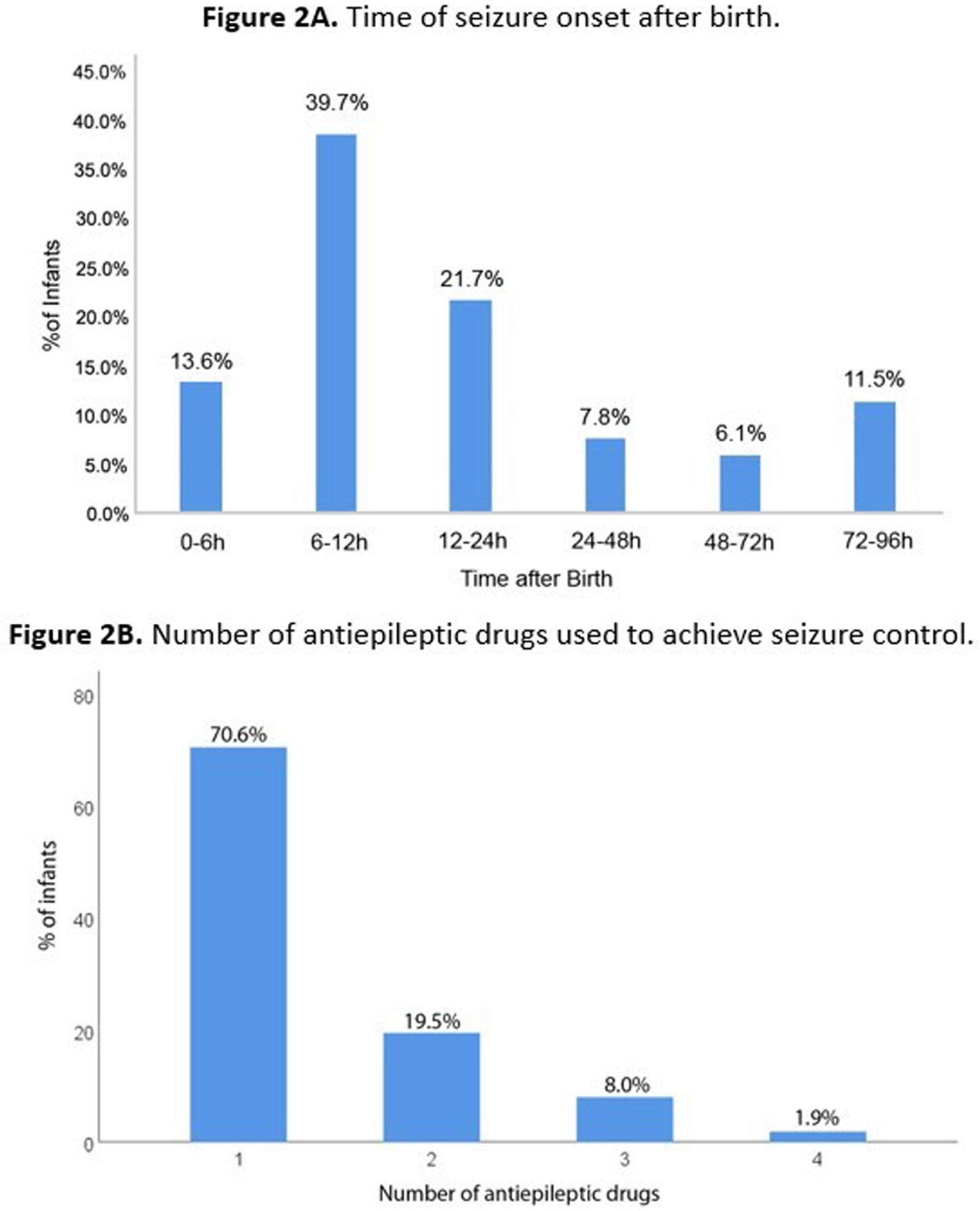
CONCLUSIONS: Seizures frequently occurred in this large cohort of newborns with HIE receiving TH and were commonly associated with early abnormal background activity and absence of SWC. Due to the high prevalence of HIE and seizures in low and middle-income countries, brain monitoring is particularly necessary. Telehealth systems may be a useful solution to improve neonatal neurocritical care in a large number of centers.
REFERENCES
[1] Abend NS, et al. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013;12(12):1170-9.
[2] Wusthoff CJ, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26(6):724-8.
Improving the usability of EEG for neonatal clinical trials: An automated artefact detection algorithm
Marchant S1, Pillay K1, van der Vaart M1, Fitzgibbon S1, Hartley C1, Slater R1
1University Of Oxford, Oxford, United Kingdom
BACKGROUND: Hospitalised infants can receive many painful procedures a day, but pharmacological analgesics are frequently not provided in part due to lack of testing in infants. Measuring noxious-evoked brain activity provides an important objective surrogate measure to assess analgesic efficacy in pre-verbal infants1. However, adopting EEG measures is technically challenging and the development of easy-to-use acquisition and analysis methods would facilitate the use of EEG in randomised clinical trials2. Contamination of EEG signal by artefact – for example by movement or muscle artefact – is common, especially when recording from infants in response to stimuli3. Usually, epochs are manually reviewed to identify epochs contaminated by artefact, but this introduces an additional source of variability, reduces the reproducibility of results, and requires expertise and time. We aimed to develop a software-based, automated method for detecting artefact in epochs of EEG signal.
METHODOLOGY: EEG data was selected from a database of previously recorded data collected at the John Radcliffe Hospital, Oxford, UK. 410 epochs (duration 1.5 seconds) were taken from EEG recordings of 160 newborn infants (postmenstrual age 28 to 43 weeks) in response to noxious, visual, auditory, and tactile stimuli and during background periods. A group of seven experienced raters independently assessed each epoch as containing artefact or not.
Features of the EEG signals, identified from existing adult literature as predictive of artefact, were calculated. A machine learning classifier was trained to predict whether any given epoch of EEG data contained artefact, with balanced accuracy assessed using leave-one-subject-out cross-validation. The ability of this automated method to identify artefact was tested on a separate, independent set of data.
RESULTS: There was good agreement between experienced raters in identifying epochs containing artefact: 24% of epochs contained artefact, mean Cohen’s Kappa: 0.51, indicating ‘fair to good’ agreement. The automated method performed well at identifying artefact with a balanced accuracy of 0.81 in the independent test set (compared to 0.84 for raters). The method performed similarly across different infant age groups and type of stimulus.
CONCLUSION: The automated artefact detector developed here performs well, with an accuracy as good as experienced EEG reviewers. Compared with manual review of EEG data, using this model enhances reproducibility, could reduce analysis time, and facilitates the application of EEG as a clinically useable tool without the need for expert EEG assessors.
REFERENCES
[1] Gursul, Deniz, et al. Nociception and the neonatal brain. Seminars in Fetal and Neonatal Medicine 24(2019):4.
[2] Baxter, Luke, et al. Using changes in brain activity to assess pain-relief in infants: Methodological considerations with Benoit et al.(2021). Early human development 157(2021):105361.
[3] Georgieva, Stanimira, et al. Toward the understanding of topographical and spectral signatures of infant movement artifacts in naturalistic EEG. Frontiers in neuroscience 14(2020):352.
Glycemic instability correlates with greater ratios of low voltage on EEG tracings in neonates with hypoxic ischemic encephalopathy
Petitpas L1, Vannasing P1, Tremblay J1, Damien J1, Balasingam T2, Marandyuk B2, Paquette N1, El Jalbout R2, Gallagher A1, Pinchefsky E2
1Neurodevelopment Optical Imaging Laboratory (LIONlab), Centre Hospitalier Universitaire Sainte-Justine, Department of Psychology, Université de Montréal, Montreal, Canada, 2Service de neurologie, Département de pédiatrie, CHU Sainte-Justine, Université de Montréal, Montreal, Canada
BACKGROUND: Hypoxic ischemic encephalopathy (HIE) is a severe newborn condition in which the underlying mechanisms still require further understanding. This clinical population is at increased risk for neonatal hypo- and hyperglycemia. Given the need to improve our understanding of metabolic functioning in HIE, this study aims to determine the association of neonatal glycemic disturbances on the brain’s background electrophysiological activity measured by electroencephalography (EEG). It was hypothesized that abnormal glucose in the first 48h of life would correlate with greater discontinuity on EEG tracings.
METHODOLOGY: Forty-nine newborns with HIE and undergoing therapeutic hypothermia were recruited at Sainte-Justine University Hospital Center. Continuous EEG monitoring using a modified neonatal 10-20 montage using 11 electrodes was started as soon as possible after admission and segments of interest containing glucose measurements in the first 48h of life were analyzed (average of 9.8h of EEG analyzed per baby). Brain activity was quantitatively assessed according to an index of discontinuity characterized by the proportion of low EEG amplitudes per segment (< 15, 12.5 and 10μV cutoffs). Glucose measurements were intermittently collected using blood samples and bedside glucometers and were retrospectively retrieved from medical charts. Participants were separated in 4 groups according to the glycemic state presented in the first 48h of life: normoglycemia (n = 14), hyperglycemia (> 8.3 mmol/L; n = 21), hypoglycemia (< 2.6 mmol/L; n = 4) and both (hyper- and hypoglycemia; n = 10).
RESULTS: The non-parametric covariance analyses revealed a significant difference between the discontinuity index for the 15μV threshold (F = 3.070, p = 0.037) when controlling for gestational age and 5 min apgar score. The pairwise comparisons showed a positive difference between the group BOTH and the NORMOGLYCEMIA group for every thresholds (15μV: t = 2.892, p = 0.006, 12.5μV: t = 2.758, p = 0.008, 10μV : t = 2.679, p = 0.010), the labile glucose group having a greater discontinuity index. A similar difference was found between the HYPERGLYCEMIA group and the NORMOGLYCEMIAl group for the 15μV (t = 2.206, p = 0.033) and 12.5μV (t = 2.100, p = 0.041) thresholds. No difference was found between the HYPOGLYCEMIA group and the NORMOGLYCEMIA group.
CONCLUSION: An abnormal glycemic profile, particularly glucose lability and hyperglycemia alone, were shown to be associated with abnormal brain activity characterized by a greater EEG discontinuity index. These results highlight the importance of monitoring and maintaining normal glucose levels in at-risk neonates with hypoxic ischemic encephalopathy.
Role of neonatal hypoglycemia and hyperglycemia in EEG spectral power and functional connectivity abnormalities in neonates with hypoxic-ischemic encephalopathy
Damien J1,2, Vannasing P1,2, Tremblay J1,2, Petitpas L1,2, Marandyuk B2, Balasingham T2, Paquette N1,2, El Jalbout R3, Gallagher A1,2, F Pinchefsky E2,4
1Neurodevelopment Optical Imaging Laboratory (LIONlab), Sainte-Justine University Hospital Center, Department of Psychology, Université de Montréal, Montreal, QC, Canada, 2Research Center, Sainte-Justine University Hospital Center, Montreal, QC, Canada,, Division of Neurology, Department of Paediatrics, Sainte-Justine University Hospital Center, University of Montreal, Montreal, Canada, 3Department of radiology, Sainte-Justine University Hospital Center, Université de Montréal, QC, Montreal, Canada, 4Division of Neurology, Department of Paedistrics, Sainte-Justine University Hospital Center, University of Montreal, Montreal, QC, Canada
BACKGROUND AND PURPOSE: Neonatal hypoglycemia and hyperglycemia have been associated with an increased risk of short and long-term sequelae on brain and visual function. Yet, there is limited evidence of this relationship in neonates with hypoxic-ischemic encephalopathy (HIE) who are at greater risk of glycemic abnormalities after birth. Therefore, we investigated how neonatal hypo- and hyperglycemia affect brain function in neonates with HIE, using continuous electroencephalography (cEEG). We hypothesized that hypo- or hyperglycemia in newborns with HIE would be associated with changes in EEG quantitative power and functional connectivity, particularly in posterior brain regions.
METHODOLOGY: In this retrospective study, 86 neonates diagnosed with HIE within the first six hours of life underwent therapeutic hypothermia and cEEG monitoring (up to 6h post-rewarming) with a modified neonatal montage including frontal (Fp1, Fp2), central (C3, C4), temporal (T3, T4) and occipital (O1, O2) electrodes. Episodes of hypoglycemia (<2.6 mmol/L) and hyperglycemia (> 8.3 mmol/L) were identified from birth until the end of the cEEG. Quantitative EEG measures of subjects with normal glycemia (n=19) were compared with neonates who had hypoglycemia only (n=7), hyperglycemia only (n=35) and episodes of both hypoglycemia and hyperglycemia (n=25). EEG spectral power measured by the area under the curve and functional connectivity using the weighted phase lag index (wPLI) were calculated for delta (1-4 Hz), theta (4-8Hz), alpha (8-12Hz), beta (12-20Hz) and total (1-20 Hz) bands. Power and functional connectivity measured during the 6h post-rewarming period were compared between groups using a one-way ANCOVA, adjusting for gestational age and clinical markers of hypoxia-ischemia severity (Apgar score at 5 minutes and umbilical artery pH), with Bonferroni-adjusted post-hoc tests.
RESULTS: Compared with normoglycemia, only neonates with both hypo- and hyperglycemia had significantly decreased total band power in central (p=.004), temporal (p<.001) and occipital (p=.020) regions (Fig.1). Delta band power was significantly lower in these neonates with both hypo- and hyperglycemia in the central (p=.002), temporal (p<.001) and occipital (p=.014) regions (Fig.2). Moreover, neonates with both hypo- and hyperglycemia showed significant wPLI increases in O1-C3 and O2-C4 connections in the delta band (p=.002 and p=.040, respectively), when compared with neonates with normal glycemia (Fig.3).
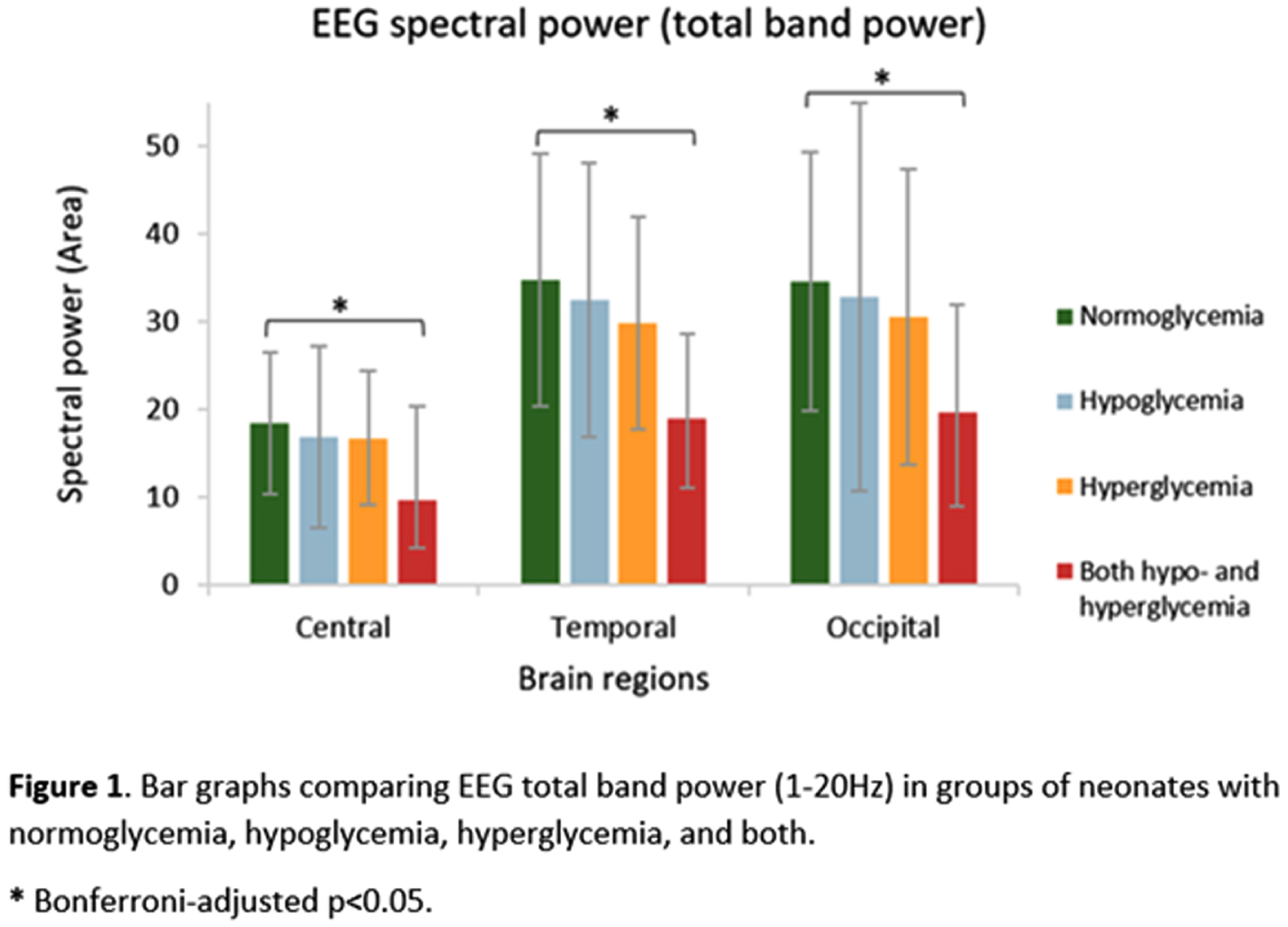
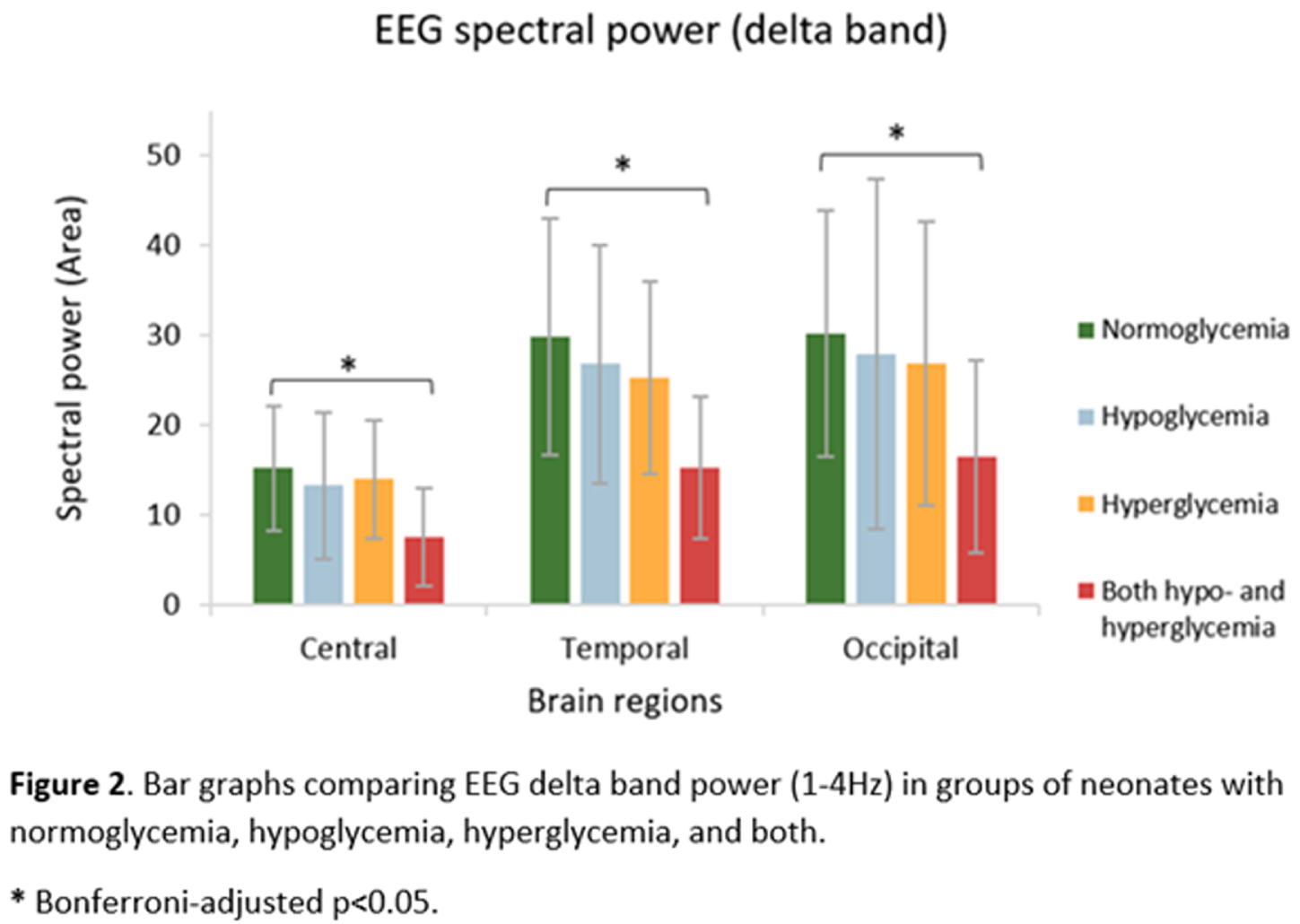
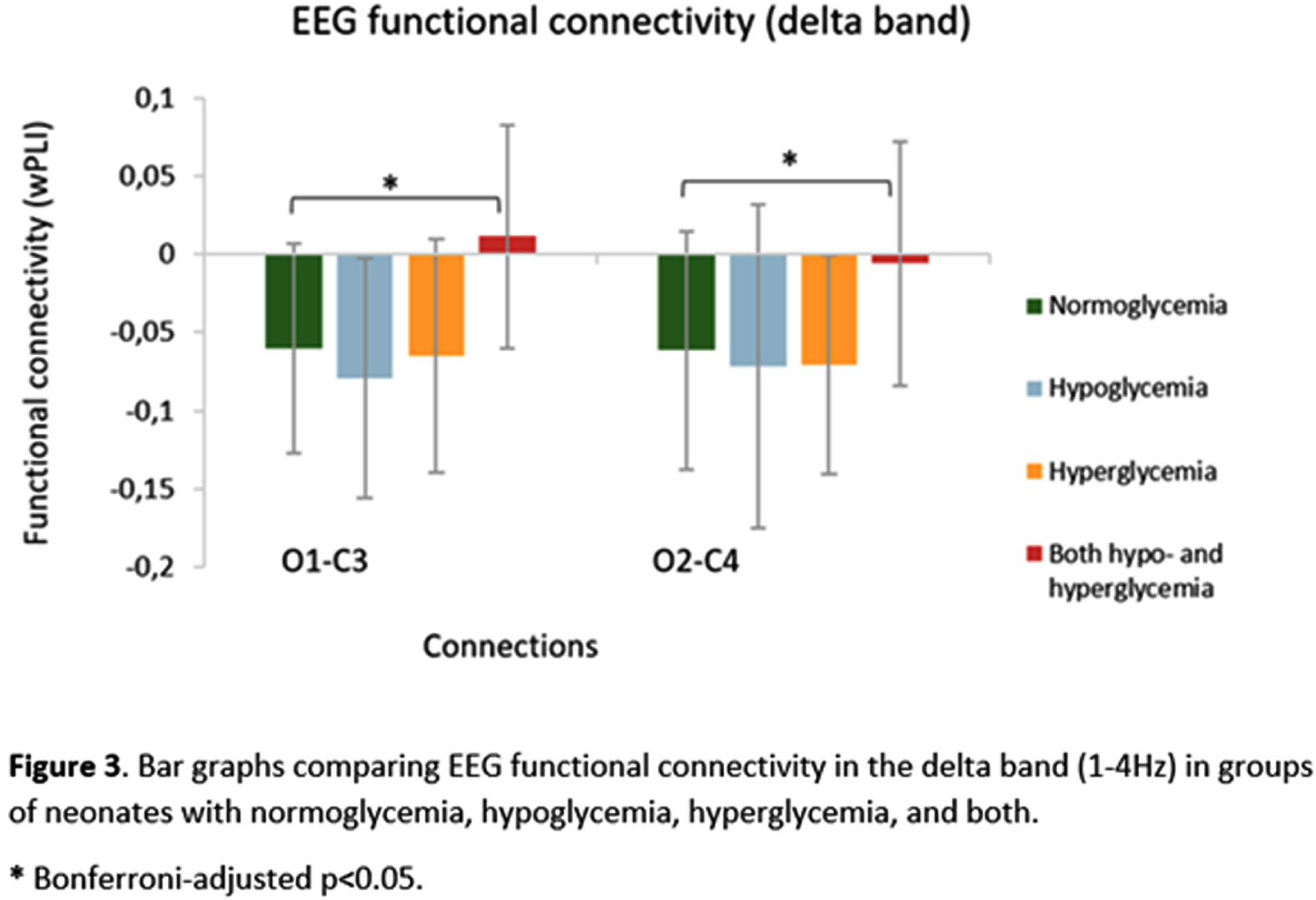
CONCLUSION/IMPACT: The occurrence of episodes of both hypo- and hyperglycemia in neonates with HIE is associated with changes in brain function after completing therapeutic hypothermia, as demonstrated by decreased EEG delta power and functional connections changes within posterior brain regions, even after adjusting for gestational age and markers of hypoxia–ischemia severity. This early detection of abnormalities in brain function highlights the importance of monitoring and maintaining normoglycemia in at-risk infants and may help guide further research on intervention protocols to better manage neonates with HIE and prevent short- and long-term neurodevelopmental consequences.
Acute provoked seizures in neonates undergoing therapeutic hypothermia are associated with alterations in pathway-specific circulating inflammatory cytokines
Numis A1,2, Juul S3, Voldal E4, Comstock B4, Wusthoff C5, Massaro A6, Bammler T7, Heagerty P3, Wu Y1,2, Glass H1,2,8
1Department of Neurology and Weill Institute for Neuroscience, University of California San Francisco, San Francisco, United States, 2Department of Pediatrics, UCSF Benioff Children’s Hospital, University of California San Francisco, San Francisco, USA, 3Department Pediatrics, University of Washington, Seattle, USA, 4Department of Biostatistics, University of Washington, Seattle, USA, 5Division of Child Neurology, Stanford University, Palo Alto, USA, 6Division of Neonatology, Children’s National Hospital, Washington D.C., USA, 7Department of Environmental & Occupational Health Sciences, Washington University, Seattle, USA, 8Department of Epidemiology & Biostatistics, University of California San Francisco, San Francisco, USA
BACKGROUND AND PURPOSE: Increasing evidence suggests neuro-inflammation is instrumental in the development and propagation of seizures. Pro-inflammatory cytokine concentrations may serve as markers of seizure severity. We aimed to measure circulating biomarker concentrations and to evaluate their association with acute provoked seizures among neonates undergoing therapeutic hypothermia (TH) for hypoxic-ischemic encephalopathy (HIE). We hypothesize that increased inflammatory cytokine and brain injury marker concentrations are associated with the presence and severity of seizures.
MATERIALS AND METHODOLOGY: This was an ancillary to the High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial of erythtropoetin (Epo) vs placebo for neonates treated with TH for HIE, and its neurophysiology sub-study (HEAL-EEG). In the HEAL trial, plasma was collected at three time points: 12-24 hours; 36-48 hours; and 74-86 hours after birth. Plasma concentrations of 28 biomarkers (including anti-inflammatory cytokines, brain-specific proteins, other growth factors, and pro-inflammatory cytokines/chemokines/peptides) were evaluated using Luminex, Meso Scale Discovery, and ELISA based platforms. Kruskal-Wallis Rank Sum Tests were used to evaluate differences in biomarker concentrations between neonates with and without seizures. Spearman’s rank correlation coefficient was used to evaluate the association between biomarker concentrations and maximum hourly seizure burden or total seizure duration. False-discovery rate was used to adjust for multiple comparisons.
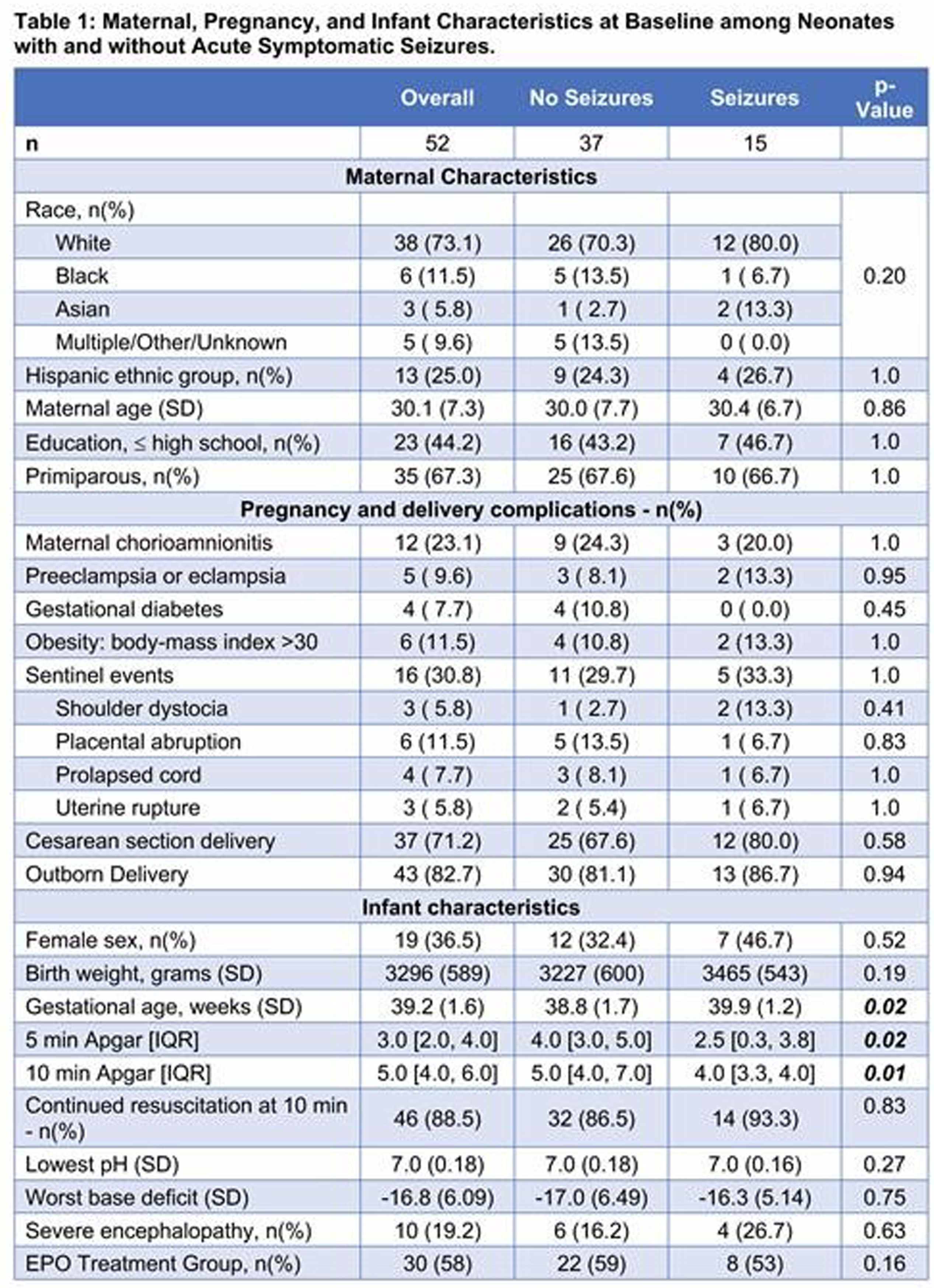
RESULTS: Among 150 participants in the HEAL-EEG cohort, 52 (35%) had biomarker concentrations measured. There was no difference in maternal and infant characteristics between neonates with and without biomarker measurements. Fifteen of 52 (29%) participants had seizures. Gestational age, 5-, and 10-minute Apgar scores differed between neonates with and without seizures (Table 1). Circulating biomarker concentrations differed between neonates with and without seizures in all categories (Figure 1). After adjustment for multiple comparisons, brain-specific proteins GFAP, s100β, and Tau concentrations were elevated at multiple time-points in neonates with seizures compared to those without seizures. Pro-inflammatory cytokines IL-12p70 and interferon-γ (IFN-γ) were elevated at 74-86 hours only. Only cytokines within the Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway (IL-12p70 and IFN-γ) were differentially expressed between groups by more than 75% (Figure 2). Significant correlations were found in biomarkers within and between the IL-1 pathway (IL-1β, IL-6, IL-17A) and the JAK/STAT pathways at all timepoints. Among neonates with seizures, biomarker concentrations did not correlate with seizure burden or total seizure duration.
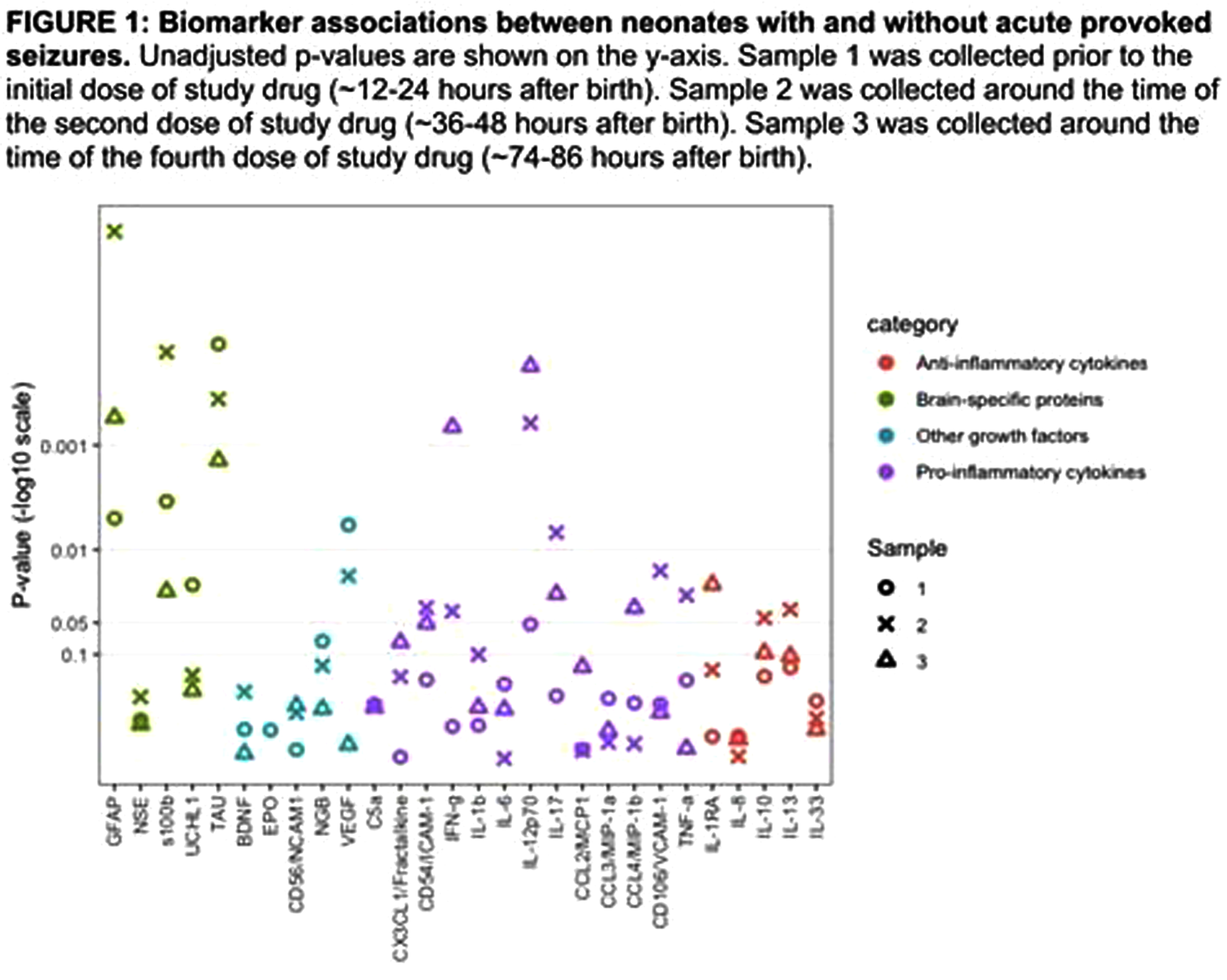
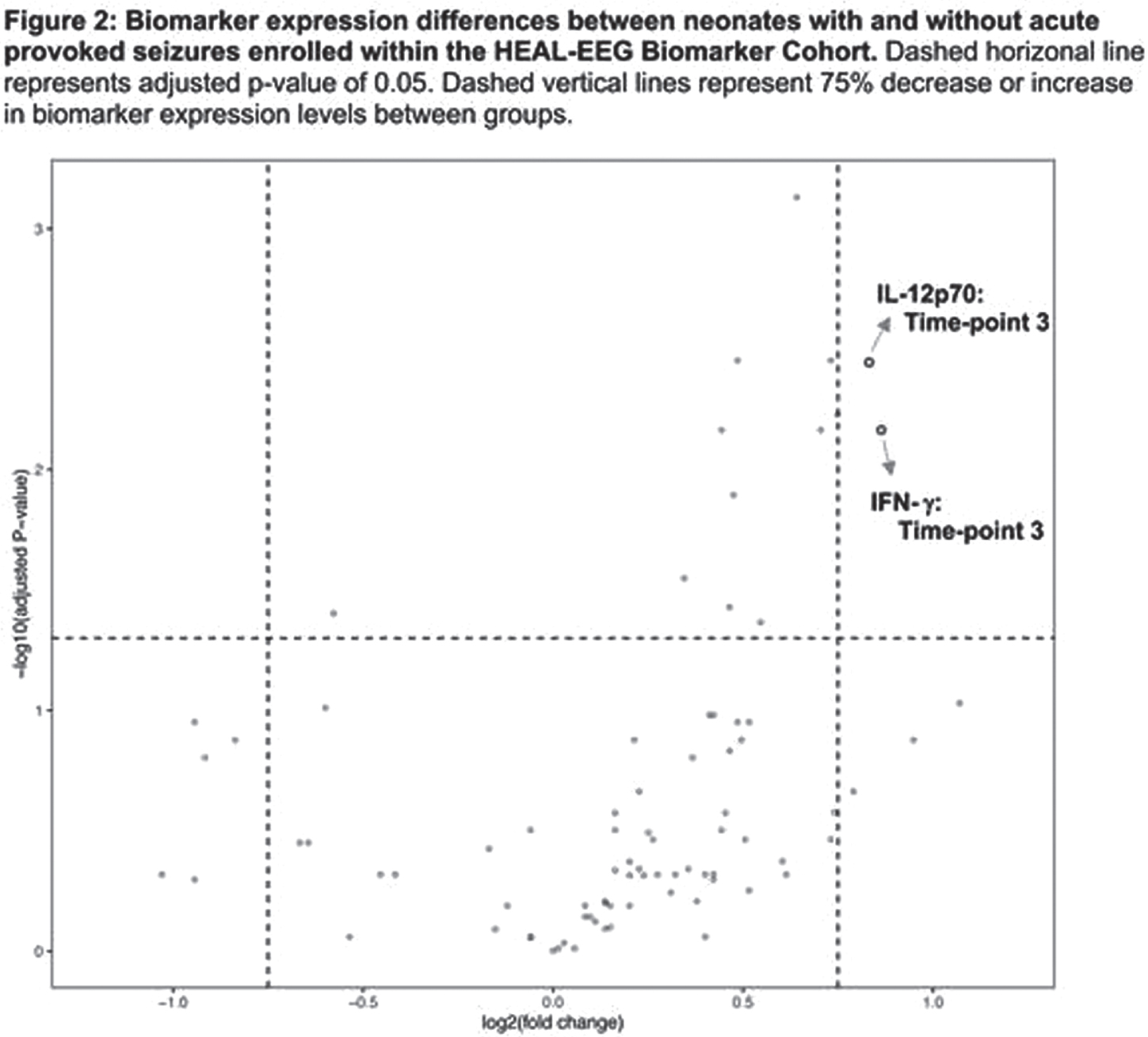
CONCLUSION/IMPACT: In neonates with HIE undergoing TH, the presence of acute provoked seizures but not seizure burden was associated with differences in inflammatory cytokine and brain injury biomarker concentrations. Cytokines within the JAK/STAT pathway had strong evidence of higher expression among neonates with seizures and correlated with changes in the IL-1β pathway. Future investigations may investigate whether therapeutics targeting the JAK/STAT pathway modify development of seizures in neonates with HIE undergoing TH.
REFERENCE
Wu Y.W., et al. Trial of Erythropoietin for Hypoxic–Ischemic Encephalopathy in Newborns. N Engl J Med 2022; 387:148-159. doi: 10.1056/NEJMoa2119660
Dexmedetomidine for Sedation of Neonates with Hypoxic Ischemic Encephalopathy Undergoing Therapeutic Hypothermia: A Single Center, Descriptive Study
Acun C, Padiyar S, Puthuraya S , Karnati S, Liu W, Aly H
1Cleveland Clinic Children’s Hospital, Cleveland, United States
BACKGROUND AND PURPOSE: Therapeutic hypothermia produces significant physiologic stress. Use of opioids for sedation in neonates with hypoxic ischemic encephalopathy (HIE) during hypothermia is a common practice, but these drugs have been associated with significant side effects. Dexmedetomidine provides sedation, prevents shivering, but does not suppress ventilation and does not cause gastric dysmotility, also neuroprotective effects reported in animal models. The objective of this study is to evaluate the safety, effectiveness, and short and long-term effects of dexmedetomidine in infants with HIE undergoing therapeutic hypothermia.
MATERIALS: This is a retrospective study included neonates ≥35 weeks of gestational age with a diagnosis of HIE undergoing therapeutic hypothermia between Jan 2014 and Dec 2021. Patients were included if they received at least 6 hours of continuous sedation only with dexmedetomidine. Neonates with lethal congenital malformations, and chromosomal anomalies were excluded. Continuous variables were described using medians and interquartile ranges, categorical variables were described using counts and percentages. Demographic and clinical characteristics were compared between patients with mild and moderate/severe HIE by using the Wilcoxon rank sum test for continuous/ordinal characteristics and the Pearson’s chi-square test or Fisher’s exact test for categorical characteristics, as appropriate.
RESULTS: Of the 97 neonates included, 46 had mild and 51 infants had moderate to severe HIE. Initial dose of dexmedetomidine was 0.2 and maximum dose was 0.4 mcg/kg/hour. Dexmedetomidine was initiated average at 5 hours of life. Average infusion duration was 77 hours and cumulative dose was 16.6 mcg/kg. Overall 40 patients (41.2%) had at least one bradycardia episode with heart rate<80/min and only 14 of them (14.4% of all patients) had heart rate <70 /min. Dexmedetomidine was decreased or discontinued in 26 (65%) of neonates with bradycardia. Only 7 patients (7.2%) had hypotension, and none of the patients had hypertension. Total fifty-five patients (56.7%) required at least one bolus of opioids during therapeutic hypothermia, 47 of them required bolus opioids in the first 24 hours of initiation of dexmedetomidine. Only 7 patients (7.2%) required at least one bolus of sedatives during therapeutic hypothermia and 5 of them required bolus sedatives in the first 24 hours of initiation of dexmedetomidine. Fifty-two of patients (53.6%) were intubated in delivery room and most of them extubated on day of life 1. Ninety-one (93.8%) patients reached full oral feeds average 6 days. Average NICU stay was 10 days. Forty-four (46.3%) patients had abnormal MRI findings. Only 3 patients had Bayley scores <70 at 8-17 months and ≥18 months age.
CONCLUSION: Most common side effect of dexmedetomidine was bradycardia. Dexmedetomidine might be used first and single agent for neonates with all degrees of HIE undergoing hypothermia.
REFERENCES
[1] Elliott M, Burnsed J, Heinan K, et al. Effect of dexmedetomidine on heart rate in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. J Neonatal Perinatal Med 2022;15(1):47-54.
[2] Cosnahan AS, Angert RM, Jano E, Wachtel EV. Dexmedetomidine versus intermittent morphine for sedation of neonates with encephalopathy undergoing therapeutic hypothermia. J Perinatol 2021;41(9):2284-2291.
[3] O’Mara K, Weiss DW. Dexmedetomidine for Sedation of Neonates with HIE Undergoing Therapeutic Hypothermia: A Single-Center Experience. Am J Perinatol Rep 2018;8:e168–e173
Table 1
Baseline characteristics
| Total HIE patients (N=97) | Mild HIE (N=46) | Moderate and severe HIE (N=51) | p-value | |
| Gender | 97 | 46 | 51 | 0.74a |
| Female | 46 (47.4) | 21 (45.7) | 25 (49.0) | |
| Male | 51 (52.6) | 25 (54.3) | 26 (51.0) | |
| Gestational Age (weeks) | 39.5 [38.3, 40.4] | 39.6 [38.3, 40.5] | 39.5 [38.1, 40.4] | 0.84b |
| Birthweight (kg) | 3.3 [3.1, 3.7] | 3.4 [3.2, 3.6] | 3.2 [2.9, 3.8] | 0.40b |
| Delivery type | 0.12a | |||
| SVD | 29 (29.9) | 11 (23.9) | 18 (35.3) | |
| Vaginal assisted | 13 (13.4) | 4 (8.7) | 9 (17.6) | |
| Cesarean section | 55 (56.7) | 31 (67.4) | 24 (47.1) | |
| Apgar scores, 1 min | 2.0 [1.0, 2.0] | 2.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 0.13b |
| 5 min | 4.0 [3.0, 5.0] | 4.0 [3.0, 5.0] | 4.0 [2.0, 5.0] | 0.31b |
| 10 min | 5.0 [4.0, 7.0] | 6.0 [5.0, 7.0] | 5.0 [4.0, 6.0] | 0.003b |
| Cord Arterial pH | 7.0 [6.8, 7.1] | 7.0 [6.9, 7.1] | 6.9 [6.8, 7.1] | 0.41b |
| bicarbonate | 18.0 [14.0, 20.0] | 17.0 [14.0, 20.0] | 18.0 [15.0, 20.0] | 0.77b |
| base deficit | 14.0 [10.0, 19.6] | 12.0 [9.0, 16.1] | 17.0 [12.8, 22.0] | 0.005b |
| < 1 hour of life blood gas | ||||
| pH | 7.2 [7.1, 7.2] | 7.2 [7.2, 7.3] | 7.1 [7.0, 7.2] | <0.001b |
| bicarbonate | 13.0 [10.0, 16.0] | 14.0 [13.0, 18.0] | 13.0 [10.0, 16.0] | 0.020b |
| base deficit | 15.0 [12.0, 18.0] | 13.0 [9.0, 15.0] | 18.0 [14.5, 21.0] | 0.002b |
| lactate | 12.6 [8.2, 15.0] | 10.2 [6.9, 14.0] | 13.2 [11.1, 17.9] | 0.014b |
| Seizures (N, %) | 21 (21.6) | 2 (4.3) | 19 (37.3) | <0.001b |
| Timing of first seizure (hours) | 14.0 [11.0, 20.0] | 4.5 [9.0, 20.0] | 14.0 [11.0, 24.0] | 0.95b |
Abbreviations: N, number of patients; HIE, Hypoxic ischemic encephalopathy; SVD, spontaneous vaginal delivery.
Statistics presented as Median [P25, P75], N (column %)
p-values: a=Pearson’s chi-square test, b=Wilcoxon Rank Sum test. Bold p-values are statistically significant.
Table 2
Medication and adverse events
| Total HIE patients (N=97) | Mild HIE (N=46) | Moderate and severe HIE (N=51) | p-value | |
| Dexmedetomidine | ||||
| Initiation, hours of life | 5.0 [4.5, 8.0] | 5.5 [5.0, 9.0] | 5.0 [4.0, 6.0] | 0.054b |
| Duration, hours | 77.0 [46.0, 87.0] | 77.0 [49.0, 86.0] | 75.0 [30.0, 90.0] | 0.77b |
| Cumulative dose - mcg/kg | 16.6 [9.9, 22.7] | 16.3 [10.2, 21.0] | 17.0 [9.2, 23.8] | 0.56b |
| Breakthrough opioid use (N, %) | 55 (56.7) | 29 (63.0) | 26 (51.0) | 0.23a |
| Doses, first 24 hours | 2.0 [1.0, 2.0] | 2.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 0.18b |
| Doses, first 80 hours | 4.0 [2.0, 5.0] | 4.0 [2.0, 6.0] | 3.0 [2.0, 4.0] | 0.15b |
| Breakthrough sedative use (N, %) | 7 (7.2) | 3 (6.5) | 4 (7.8) | >.99c |
| Doses, first 24 hours | 1.0 [0, 2.0] | 1.0 [0, 1.0] | 1.5 [0.50, 2.0] | 0.48b |
| Doses, first 80 hours | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 1.5 [1.0, 4.0] | 0.71b |
| Bradycardia < 80 bpm (N, %) | 40 (41.2) | 21 (45.7) | 19 (37.3) | 0.40a |
| 1 to 2 episodes | 29 (29.8) | 15 (32.6) | 14 (27.5) | |
| 3-4 episodes | 8 (8.3) | 4 (8.7) | 4 (7.8) | |
| >4 episodes | 3 (3.1) | 2 (4.3) | 1 (2) | |
| Intervention for bradycardia (N, %) | ||||
| No changes | 14 (35.0) | 5 (23.8) | 9 (47.4) | 0.12a |
| Dose decreased | 21 (52.5) | 10 (47.6) | 11 (57.9) | 0.52a |
| Discontinued | 23 (57.5) | 11 (52.4) | 12 (63.2) | 0.49a |
| Bradycardia < 70 bpm (N, %) | 14 (14.4) | 5 (10.9) | 9 (17.6) | 0.34a |
| Intervention for bradycardia (N, %) | ||||
| No changes | 3 (21.4) | 0 (0) | 3 (33.3) | 0.26c |
| Dose decreased | 10 (71.4) | 3 (60.0) | 7 (77.8) | 0.58c |
| Discontinued | 11 (78.6) | 3 (60.0) | 8 (88.9) | 0.51c |
| Hypotension (N, %) | 7 (7.2) | 2 (4.3) | 5 (9.8) | 0.44b |
Abbreviations: N, number of patients; HIE, Hypoxic ischemic encephalopathy; bpm, beat per minute.
Statistics presented as Median [P25, P75], N (column%)
p-values: a=Pearson’s chi-square test, b=Wilcoxon Rank Sum test, c=Fisher’s Exact test.
Table 3
Hospital Course
| Overall (N=97) | Mild (N=46) | Moderate and Severe (N=51) | p-value | |
| NICU LOS (days) | 10.0 [8.0, 14.0] | 9.0 [7.0, 12.0] | 11.0 [8.0, 18.0] | 0.010b |
| Reaching full enteral feeds (NG/OG tube), day of life | 1.0 [5.0, 7.0] | 6.0 [5.0, 7.0] | 6.0 [5.0, 8.0] | 0.29b |
| Reaching full oral feeds, day of life | 6.0 [5.0, 10.0] | 6.0 [5.0, 8.0] | 7.0 [6.0, 12.0] | 0.12b |
| Home with tube feeding (N, %) | 0.029c | |||
| No | 90 (92.8) | 46 (100.0) | 44 (86.3) | |
| NG | 4 (4.1) | 0 (0) | 4 (7.8) | |
| G-tube | 3 (3.1) | 0 (0) | 3 (5.9) | |
| Mechanical ventilation | ||||
| Number of patients (N, %) | 52 (53.6) | 19 (41.3) | 33 (64.7) | 0.021a |
| Duration, days | 1.0 [1.0, 2.0] | 1.0 [1.0, 1.0] | 1.0 [1.0, 3.0] | 0.023b |
| Non-invasive ventilation | ||||
| Number of patients (N, %) | 21 (21.6) | 6 (13.0) | 15 (29.4) | 0.051a |
| Duration, days | 4.0 [2.0, 7.0] | 1.5 [1.0, 3.0] | 4.0 [3.0, 11.0] | 0.020b |
Abbreviations: LOS, length of stay; NG, nasogastric; OG, orogastic; G-tube, gastric tube.
Statistics presented as Median [P25,P75], N (column%).
P-values: a=Pearson’s chi-square test, b=Wilcoxon Rank Sum test, c=Fisher’s Exact test.
Bold p-values are statistically significant.
Table 4
Outcomes
| Overall (N=97) | Mild (N=46) | Moderate and Severe (N=51) | p-value | |
| Died in NICU (N, %) | 1 (1.0) | 0 (0) | 1 (2.0) | >.99c |
| MRI results (N, %) | 95 (97.9) | 45 (97.8) | 50 (98.0) | |
| Normal | 51 (53.7) | 31 (68.9) | 20 (40.0) | 0.005a |
| Basal ganglia/thalamic injury | 6 (6.3) | 0 (0) | 6 (12.0) | 0.028c |
| Cortical injury | 9 (9.5) | 2 (4.4) | 7 (14.0) | 0.16c |
| White matter injury | 8 (8.4) | 2 (4.4) | 6 (12) | 0.27c |
| PLIC | 2 (2.1) | 0 (0) | 2 (4.0) | 0.50c |
| IVH | 6 (6.3) | 2 (4.4) | 4 (8.0) | 0.68c |
| Parenchymal hemorrhage | 7 (7.4) | 4 (8.9) | 3 (6.0) | 0.70c |
| Restricted diffusion/stroke | 21 (22.1) | 9 (20.0) | 12 (24.0) | 0.64a |
| Other | 6 (6.3) | 2 (4.4) | 4 (8.0) | 0.68c |
| Bayley results, | ||||
| 8-17 months (N, %) | 61 (62.9) | 27 (58.7) | 34 (66.7) | |
| Cognitive scores <85 | 4 (6.6) | 1 (3.7) | 3 (8.8) | 0.62c |
| Language scores <85 | 7 (11.5) | 3 (11.1) | 4 (11.8) | >.99c |
| Motor scores <85 | 7 (11.5) | 2 (7.4) | 5 (14.7) | 0.45c |
| Bayley results, | ||||
| 18-24 months (N, %) | 45 (46.4) | 19 (41.3) | 26 (51.0) | |
| Cognitive scores <85 | 5 (11.1) | 3 (15.8) | 2 (7.7) | 0.64c |
| Language scores <85 | 9 (20.0) | 3 (15.8) | 6 (23.1) | 0.71c |
| Motor scores <85 | 4 (8.9) | 1 (5.3) | 3 (11.5) | 0.63c |
Abbreviations: PLIC, posterior limb of internal capsule; IVH, intraventricular hemorrhage; other, non-specific MRI findings.
Statistics presented as Median [P25, P75], N (column %).
P-values: a=Pearson’s chi-square test, c=Fisher’s Exact test.
Bold p-values are statistically significant.
Monitoring EEG background state and seizure burden in neonates with neonatal encephalopathy in Uganda: A feasibility study
Mathieson S1, Nanyunya C2, Proietti J1, Mambule I2, Duckworth E4, Nakimuli A5,6, Boylan G1, Tann C2,3,4
1Infant Research Centre, University College Cork, Cork, Ireland, 2MRC/UVRI & LSHTM Research Unit, , Entebbe, Uganda, 3London School of Hygiene & Tropical Medicine, London, UK, 4University College London Hospitals NHS Trust, London, UK, 5Kawempe National Referral Hospital, Kampala, Uganda, 6Makerere University, Kampala, Uganda
BACKGROUND AND PURPOSE: Neonatal encephalopathy (NE) has the highest burden of mortality/morbidity in low-income countries (LICs),(1) however, data from these settings is sparse. Neonatal seizures are a risk factor for adverse outcomes(2), but frequently go undetected without monitoring. Continuous EEG (cEEG) is the gold standard for monitoring background activity and seizure detection, and may provide additional prognostic value(3), however is technically challenging. We report cEEG findings from a Ugandan NE cohort, to investigate background activity, neonatal seizure burden and relationships with clinical assessment and early case fatality.
MATERIALS AND METHODOLOGY: Neonates with NE recruited at Kawempe National Referral Hospital, Uganda, had cEEG and video monitoring (day 1-4). The EEG screen was masked and clinicians treated based on clinical presentation. Post-acquisition cEEG analysis included seizure annotation and grading(3) of 1hr EEG background epochs at 12, 24, 48 and 72 hours of age and at time of Thompson score. Survival to 28 days of age was recorded.
Spearman’s correlation compared EEG grade and Thompson score (day 2-5). Fisher’s exact test compared survival with status epilepticus and background score, Mann Whitney U test compared survival and seizure burden, and the Kruskal Wallis test compared seizure burden for background severity groups.
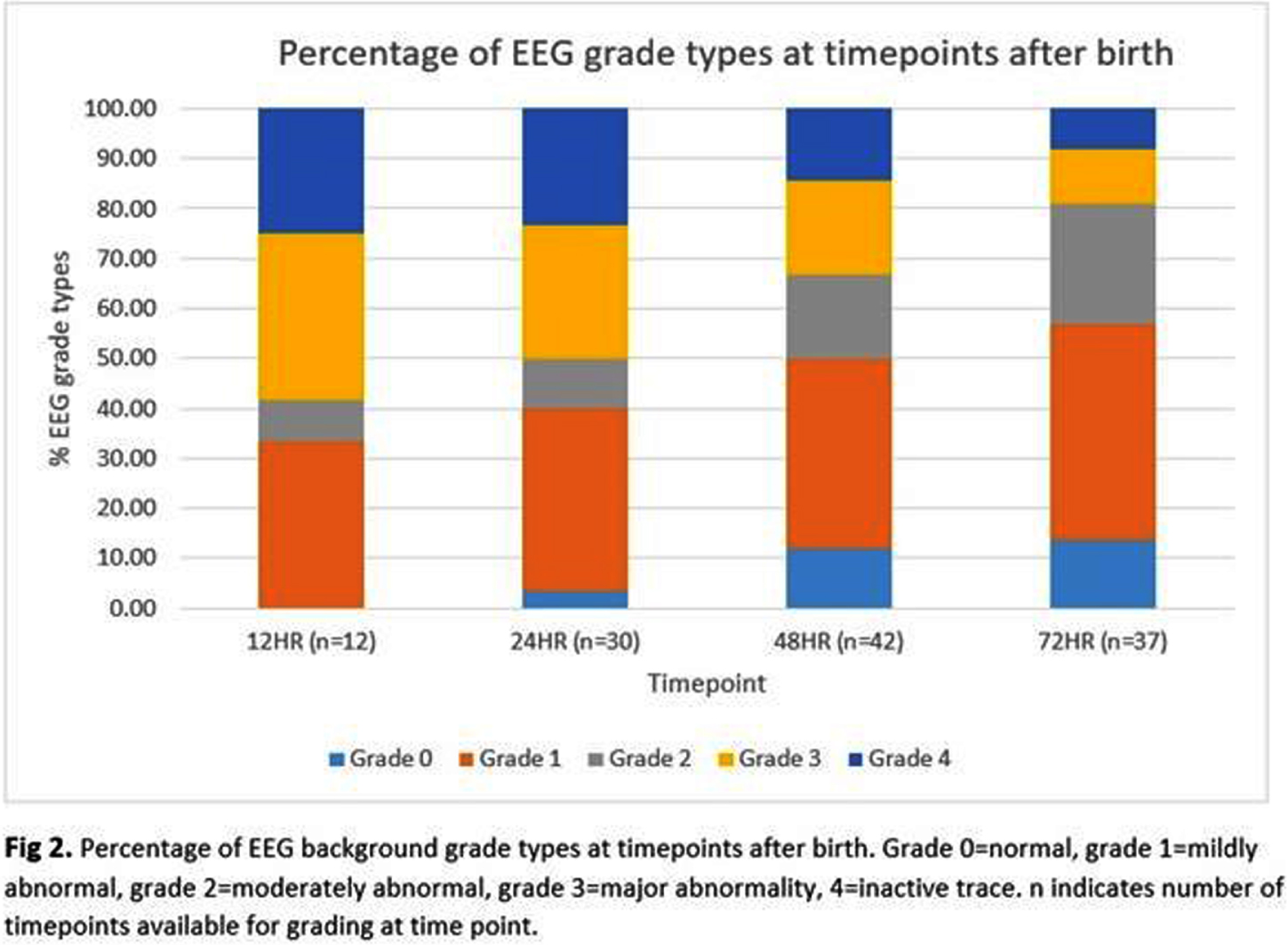
RESULTS: Of 51 recruits, 50 received monitoring of diagnostic quality. Median (IQR) duration of recording was 71.4 (52.4-72.2) hours. Electrographic seizures were recorded in 26 (52%) and, of these, 13 (50%) had status epilepticus. Neonates with seizures had a high median seizure burden of 254.8 (IQR 27.8-433.2) mins. When grouped by EEG background severity (worst grade) groups were not significantly different (Figure 1, p=0.163).
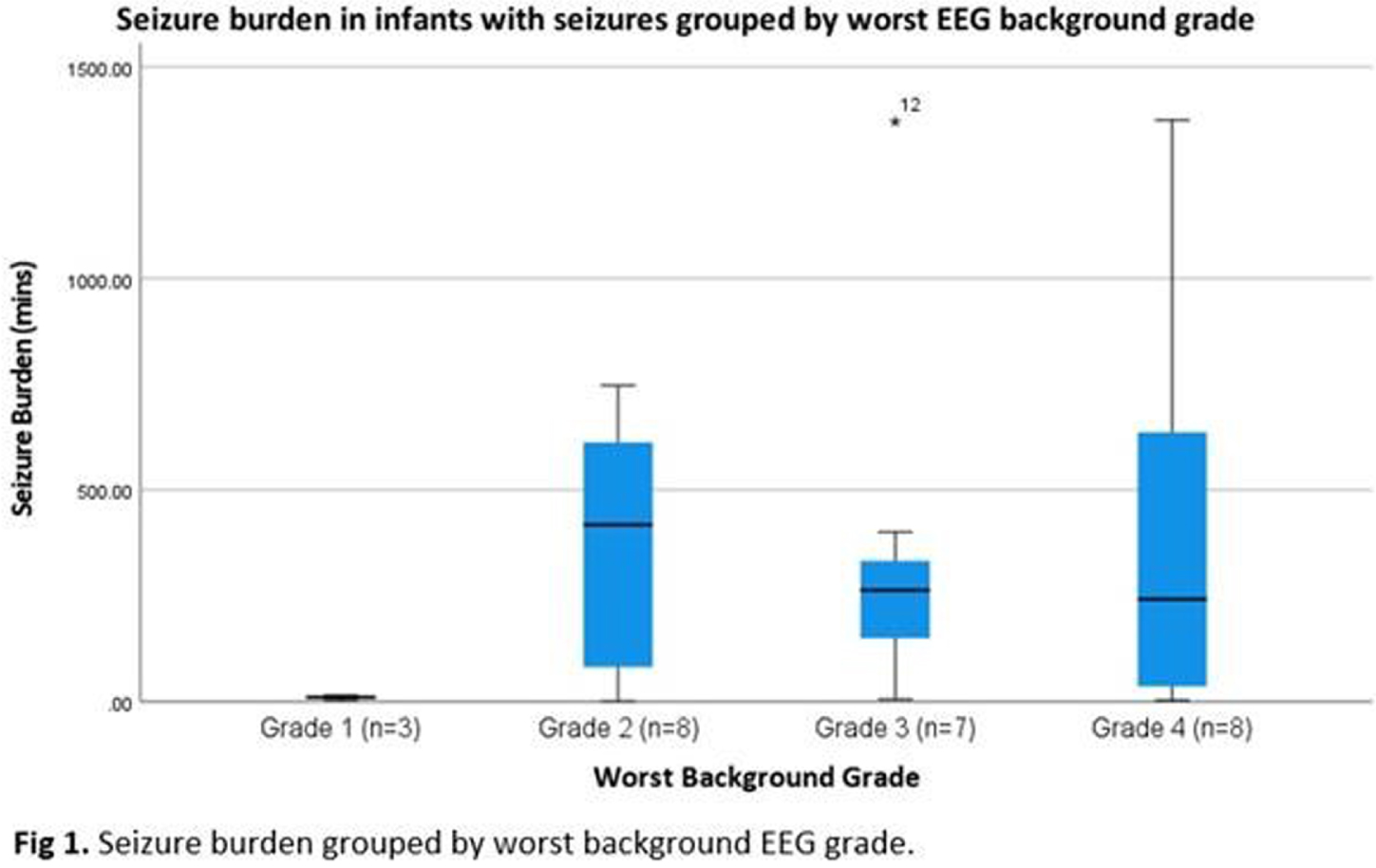
In terms of grade evolution, 14/50 infants improved over time, 28 showed no change, none showed a worsening grade and 8 had only 1 timepoint recorded (6/8 died). Figure 2 shows the proportion of different grades over time.
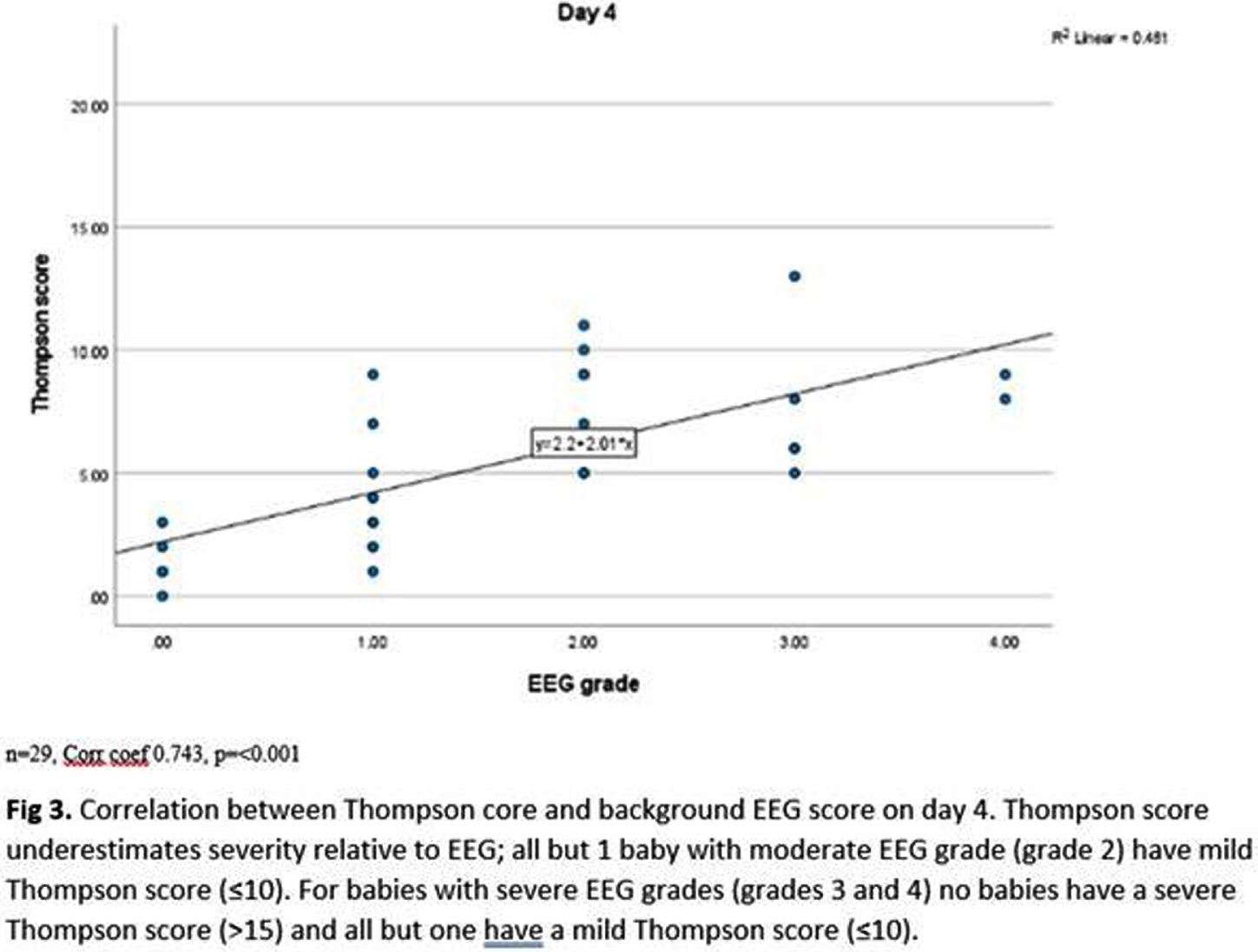
Thompson scores and EEG background scores showed strong positive correlation (coefficients day 2-5 0.620-0.743, p<0.001–0.002), however, Thompson score underestimated EEG score severity (Fig 3). Background EEG score predicted survival (p<0.01), 11/13 babies with an inactive trace died in the neonatal period, but status epilepticus (p=0.703) and seizure burden (p=0.668) did not.
CONCLUSION/IMPACT: In this Ugandan NE cohort, cEEG was a feasible and acceptable research tool. In this population, where treatment was administered by healthcare workers according to clinical status alone, electrographic seizure burden was high and may therefore contribute to adverse childhood outcomes. Whilst Thompson Score did correlate with EEG background activity, scores generally underestimated severity. In this LIC setting, without access to neonatal intensive care, an inactive EEG predicted death in the neonatal period, however status epilepticus and seizure burden did not; possibly as the most severely affected neonates had electrical brain activity that remained profoundly supressed.
1. Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74 Suppl 1:50-72.
2. Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, Ahearne CE, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2016;58(12):1242-8.
3. Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124(3):e459-67.
Neonatal encephalopathy caused by lidocaine intoxication
Van Steenis A1, Schalij N1, van Wyk L1, Tromp S1, Steggerda S1, de Vries L1
1Leiden university Medical Hospital, Albinusdreef 2, 2333 ZA Leiden, Netherlands
BACKGROUND: Encephalopathy and seizures occurring in the first hours after birth are most often caused by perinatal asphyxia. However, other causes must be considered when the clinical course is not typical for perinatal asphyxia.
MATERIALS: We describe the case of a full term, male neonate who was born after an uneventful pregnancy and presented with apnea, cyanosis, encephalopathy and seizures within an hour after birth. There were no signs of fetal distress during delivery, Apar scores and umbilical cord pH were normal. Amplitude integrated EEG (aEEG) on admission showed epileptic activity alternated by burst-suppression background pattern, see figures 1 and 2. From onset there was a broad differential diagnosis including metabolic and genetic disorders. However, as both the encephalopathy and the aEEG pattern recovered spontaneously within 24 hours these disorders became less likely. This is shown in figures 3 and 4. Additional magnetic resonance imaging revealed no abnormalities. As the mother received local anesthetics before episiotomy a toxicology screening was performed. This revealed a high plasma level of lidocaine in the newborn. It is assumed that the neonate’s scalp was accidently injected with lidocaine whilst giving mother local anesthetics.
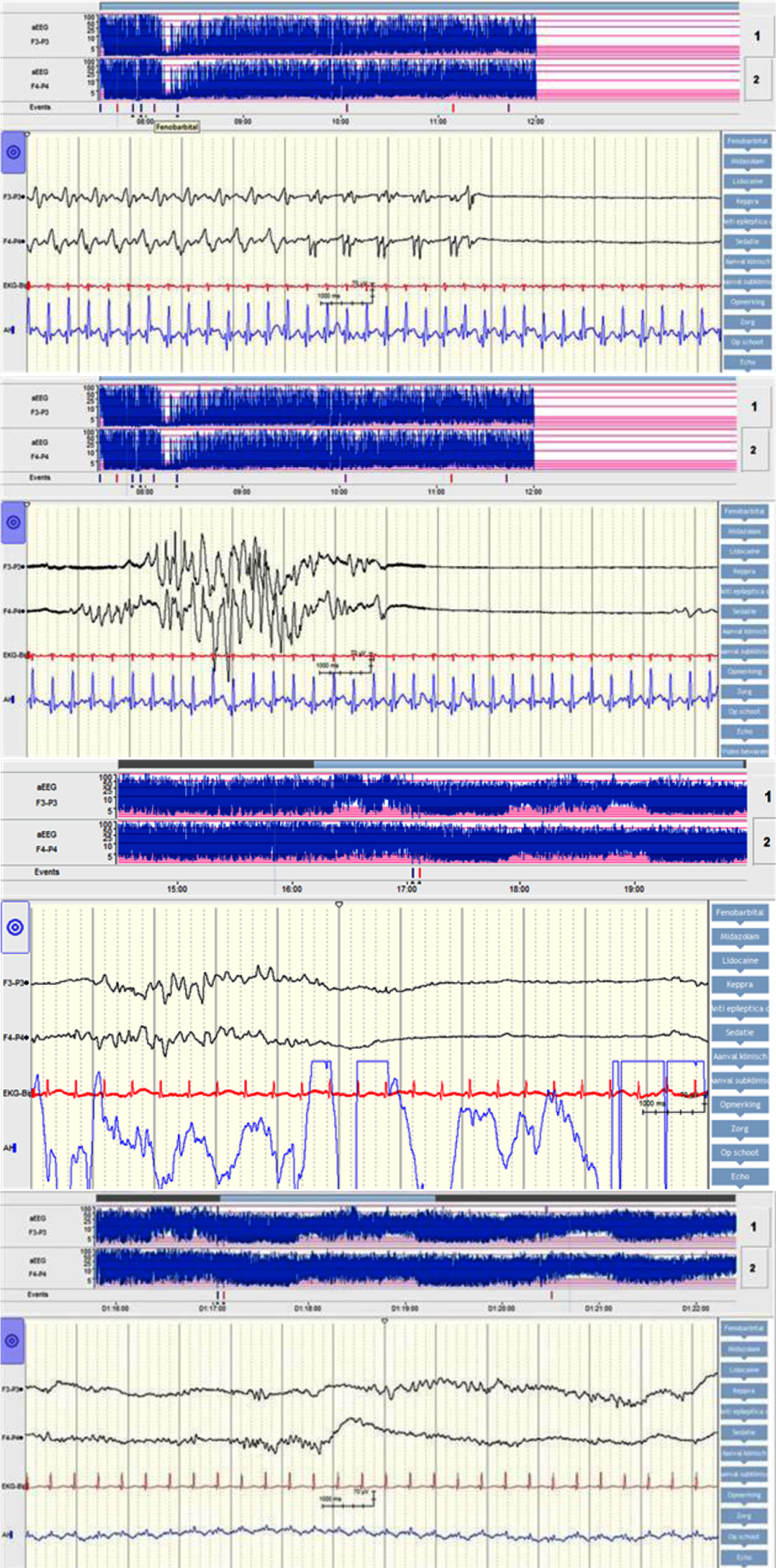
This case report describes the course of the seizures and (a)EEG background pattern. We will review the literature and emphasize the importance of toxicology screening in the work-up of unexpected early postnatal collapse, encephalopathy and seizures.
Impact of seizures on neurovascular and neurometabolic coupling in the developing brain
Harvey-jones K1, Lange F2, Verma V1, Meehan C1, Mintoft A1, Norris G1, Campbell E1, Tucker K1, Boylan G3, Robertson N1, Tachtsidis I2, Mitra S1
1University College London, London, United Kingdom, 2Department of Medical Physics and Biomedical Engineering, University College London, United Kingdom , London, United Kingdom, 3INFANT Centre, University College Cork, Cork, Ireland
BACKGROUND: Seizures are the most common neonatal neurological emergencies however limited progress has been made towards an optimal management strategy. Better understanding of seizure-induced changes is critical and previous optical neuromonitoring studies have demonstrated significant seizure effects on cerebral haemodynamics and metabolism during the peri-ictal period. Wavelet-based analysis is a promising tool to develop optical biomarkers of autoregulatory disturbance and neurovascular coupling and has been used effectively in neonatal encephalopathy to assess injury severity and predict outcomes.
Using a novel dual-optical platform combining broadband NIRS (BNIRS) and diffuse correlation spectroscopy (DCS) and wavelet-based tools for the first time in a pre-clinical seizure model, we aimed to investigate the real-time impact of seizures on neurovascular and neurometabolic coupling and the effect of phenobarbitone treatment on these parameters.
MATERIALS AND METHODOLOGY: 21 healthy newborn piglets were divided into 3 cohorts to either receive normal saline vehicles (control group A, n=6), bicuculine 4mg/kg IV (seizure group B, n= 7) to induce seizures or bicuculine 4mg/kg IV followed by phenobarbitone loading dose 20mg/kg after 10 min of continuous electrical seizures (treated seizure group C, n=8) over two time points 3h apart. All piglets underwent continuous dual-optical monitoring with BNIRS (measured changes in regional cerebral tissue saturation (StO2) and cytochrome-c-oxidase (oxCCO), and DCS (measured cerebral blood flow (BFI). Cerebral metabolic rate of oxygen consumption (CMRO2) was further calculated. Continuous EEG/aEEG and systemic monitoring occurred throughout intensive care support.
Neurovascular (aEEG-BFI, aEEG-StO2) and neurometabolic (aEEG-oxCCO, aEEG-CMRO2) coupling were measured using wavelet analysis to derive mean coherence (measure of spectral similarity) between dynamic parameter micro-oscillations (figure 1). This was performed over 1hr at four time-points (baseline, 1 hr and 2 hr after 1st seizure induction and 1 hr after 2nd seizure induction).
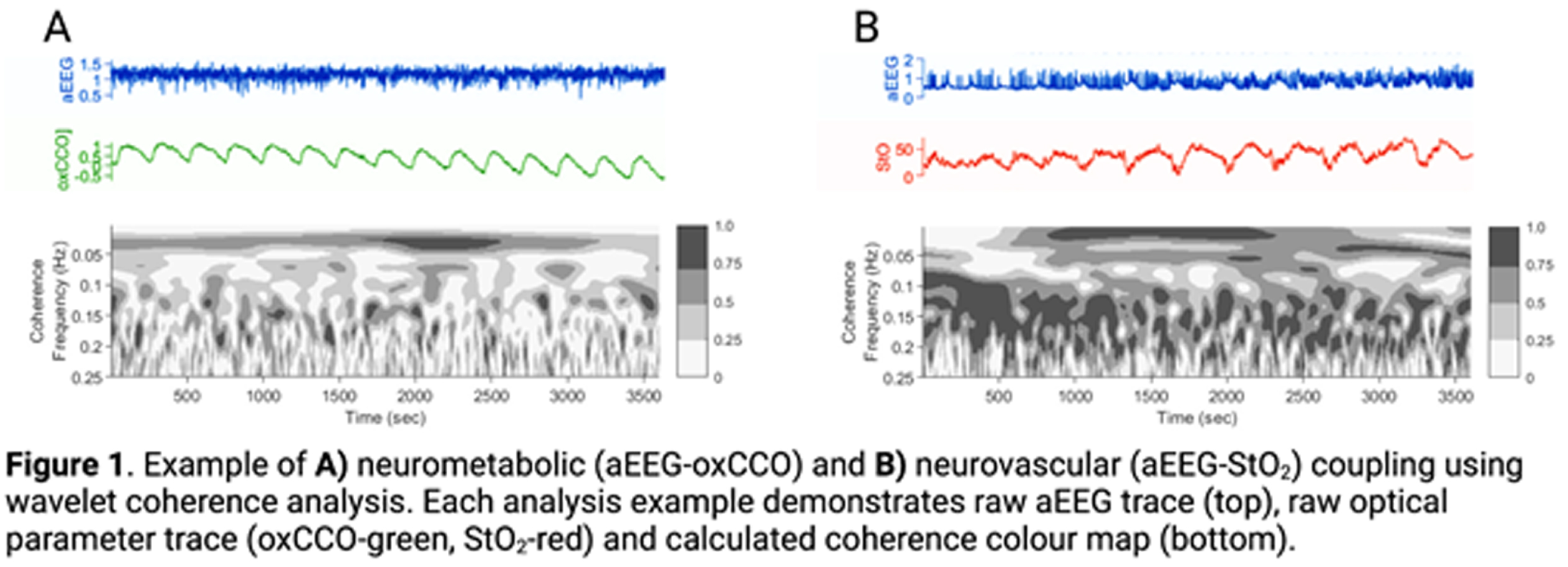
RESULTS: Mean coherence for all biomarkers (aEEG-BFI, aEEG-StO2, aEEG-oxCCO and aEEG-CMRO2) showed progressive increase in the untreated seizure group (B) during the post-ictal period when compared with the control (A) and treated seizure (C) groups (figures 2,3). aEEG-oxCCO, aEEG-BFI and aEEG-StO2 coherence significantly differentiated between groups A and B at all time-points following first seizure induction (p=0.005, 0.005, 0.007, p=0.03, 0.03, 0.01 and p=0.005, 0.02, 0.007 respectively). aEEG-CMRO2 coherence differentiated between groups A and B following 2nd seizure induction (p=0.05). In the phenobarbitone treated seizure group (C), all biomarkers showed consistent upward trends post-ictally with overall lower coherence compared to the untreated group (B), however differences between these groups did not reach significance.
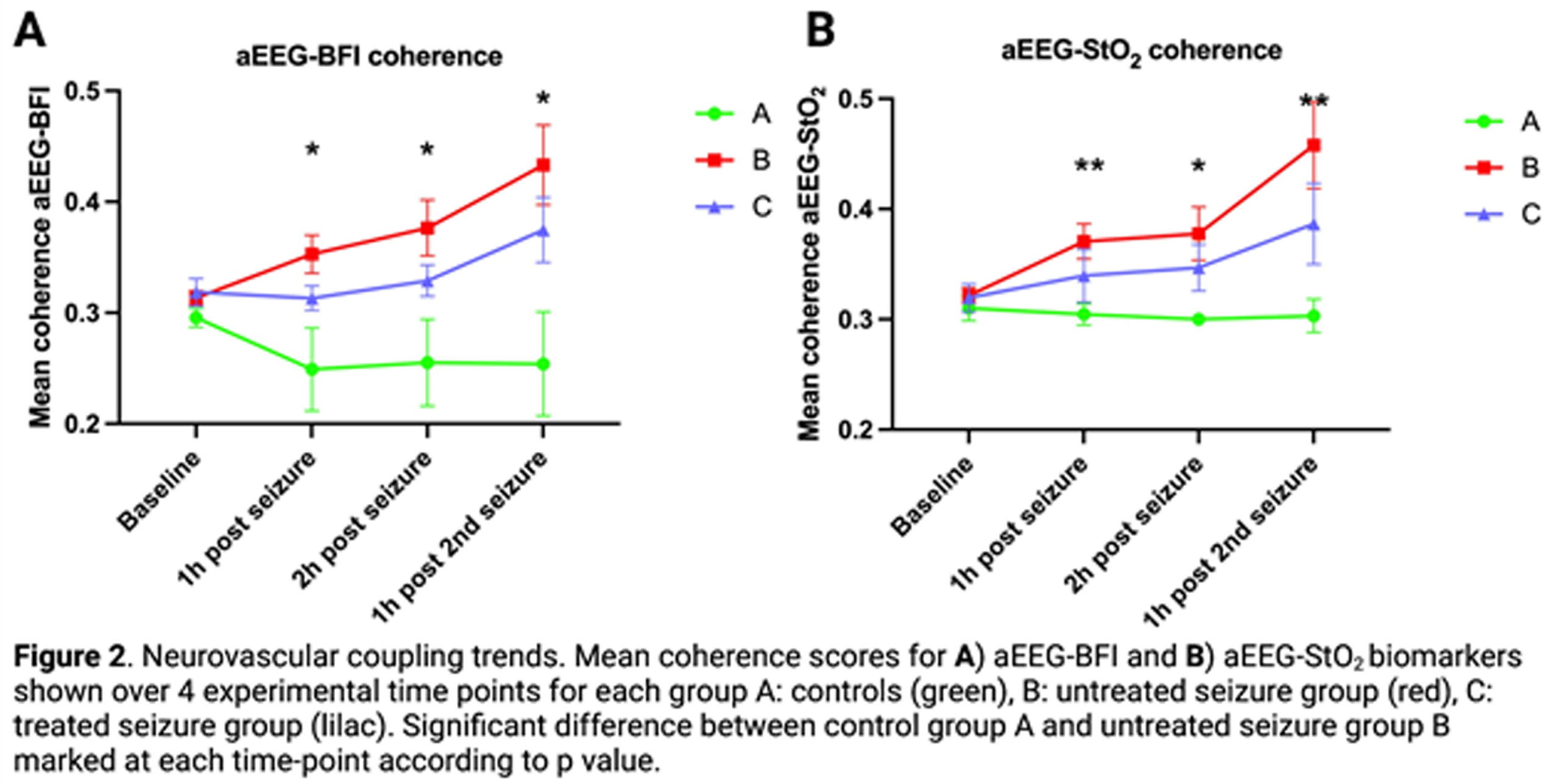
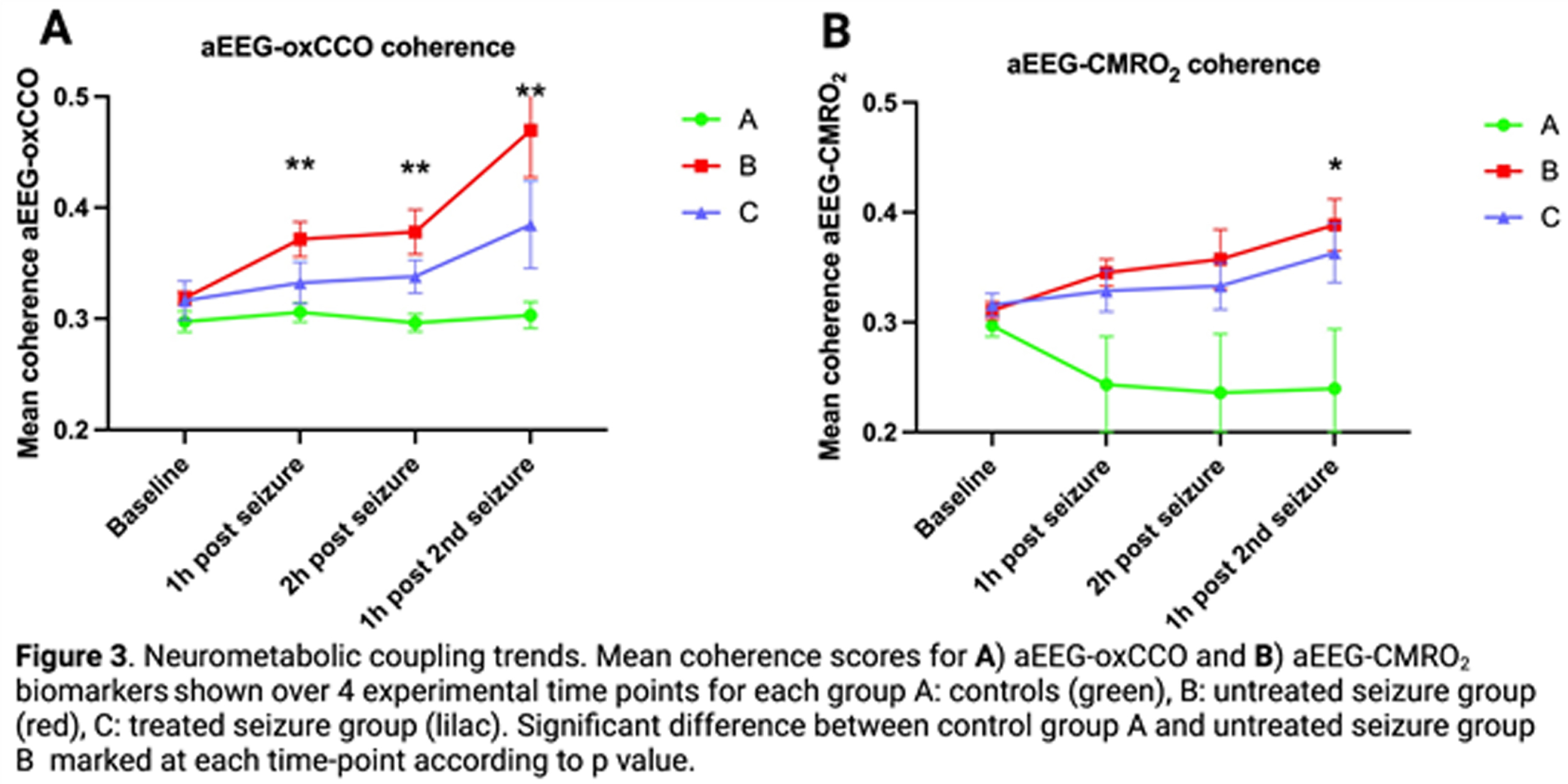
CONCLUSIONS: Induced seizures in the healthy newborn brain showed significant effects on both neurovascular and neurometabolic coupling in the post-ictal period. Effects appeared to be dampened by immediate phenobarbitone treatment. This study offers novel biomarkers that provide valuable insights into the impacts of seizures and their treatment on the neonatal brain.
The Influence of therapeutic hypothermia on seizure burden in neonates with hypoxic ischemic encephalopathy
Arad N1, Meledin I1,2, Hazan I3, Marks K1,2, Abramsky R1,2, Shany E1,2
1Ben Gurion University of the Negev, Beer Sheva, Israel, 2Cheryl And Haim Saban Children Hospital, Soroka Medical Center, Beer Sheva, Israel, 3Clinical Research Center, Soroka Medical Center, Beer Sheva, Israel
BACKGROUND AND PURPOSE: Neonatal seizures are the most frequent manifestation of neurological disorders in the newborn period, with hypoxic ischemic encephalopathy (HIE) being the main diagnosis associated with it. Based on previous publications we hypothesized that hypothermia has an antiepileptic effect.
The primary objective of this study was to compare seizure burden between newborn infants who suffered from moderate and severe HIE that were treated with therapeutic hypothermia (TH) and those that did not. Secondary objective was to compare the need for antiseizure medications (ASM) between these two groups.
MATERIALS OR METHODOLOGY: This was a retrospective study on infants born after 35 weeks gestation between 2004 and 2019, diagnosed with moderate to severe HIE, monitored with amplitude integrated EEG (aEEG) and eligible for TH according to the department protocol. Controls consisted of infants born before the implementation of TH in 2008 (non-cooled group) and cases were infants born thereafter who received TH (cooled group). Seizure burden was assessed from aEEG as total time in minutes of seizures activity per hour of recording. Other clinical and demographic data were retrieved from a prospective local database on infants with HIE. Univariable and multivariable analyses were performed to assess the association of TH with seizure burden and use of ASM.
RESULTS: Overall, 149 of 207 infants were included in the study: 112 cases and 37 controls. The two groups were comparable but for higher 5 minutes Apgar score in the cooled group (68% vs 38% with Apgar ≤5, p=0.011) (table 1). Cooled infants had a lower seizure burden overall (0.4 vs 2.3 min/h, p<0.001) (also in the moderate and severe HIE subgroups) and were also less likely to be treated with ASM (74% vs 100%, p<0.001) overall (table 2). In multivariable regression models, not undergoing TH, having a depressed aEEG background and higher Apgar scores were associated with higher seizure burden (IRR: 5.03 for non-cooled infants, p<0.001) (table 3), also, not undergoing TH was associated with a higher likelihood of multidrug ASM (OR: 4.83, p<0.001) (table 4).
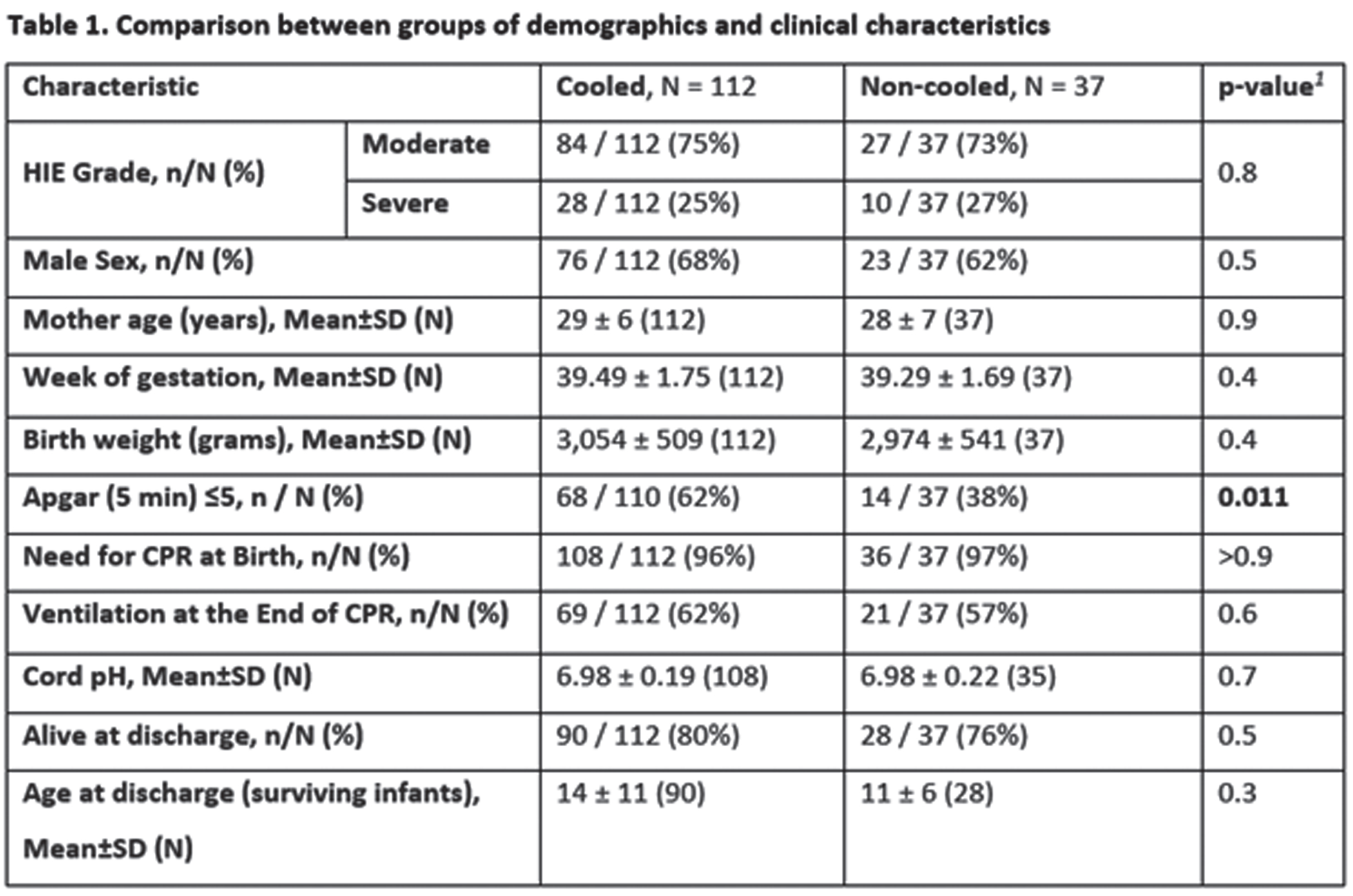
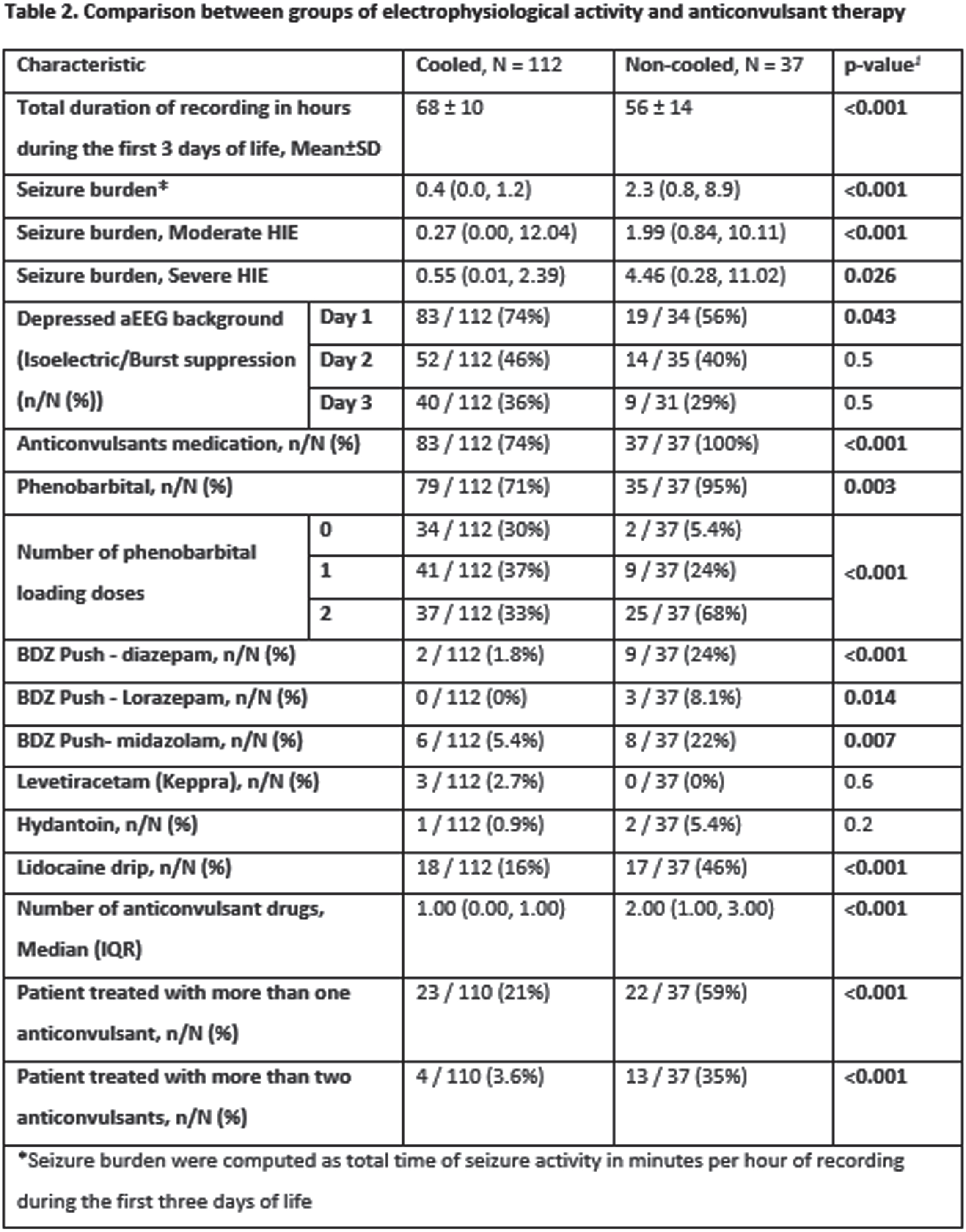
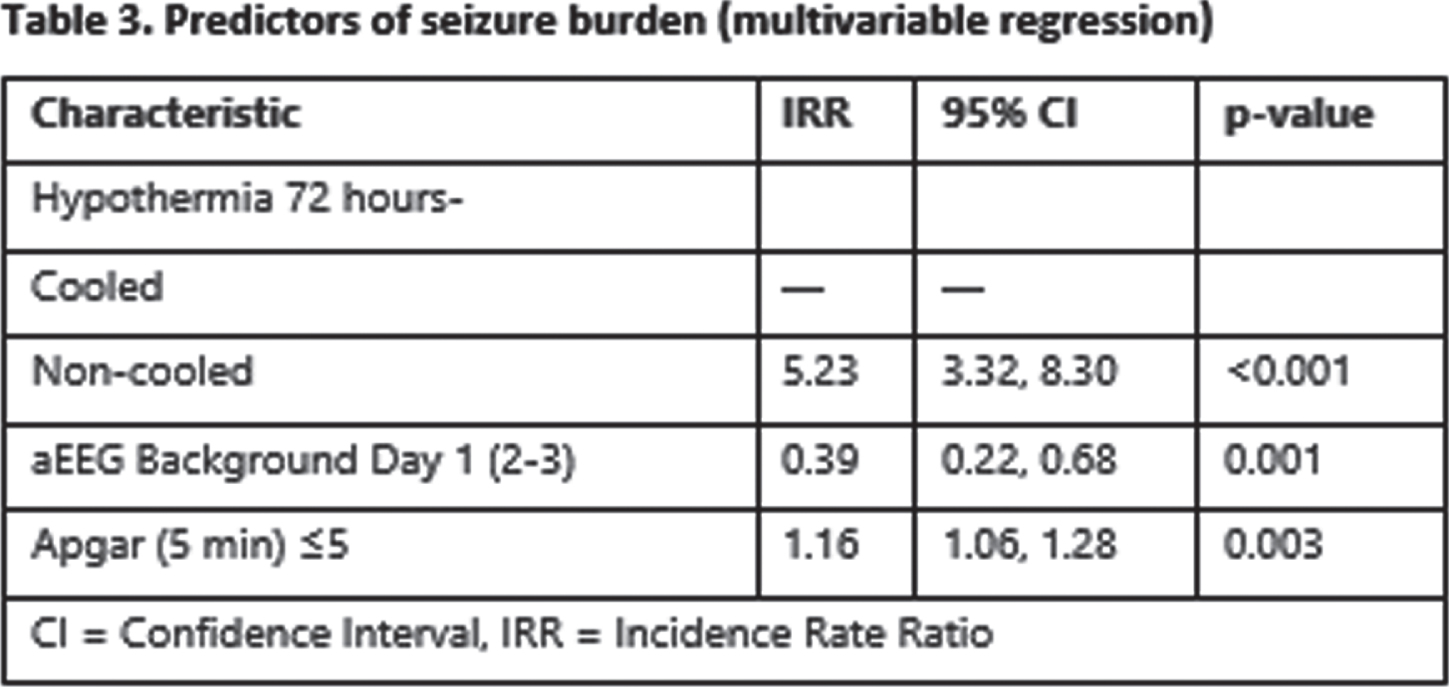
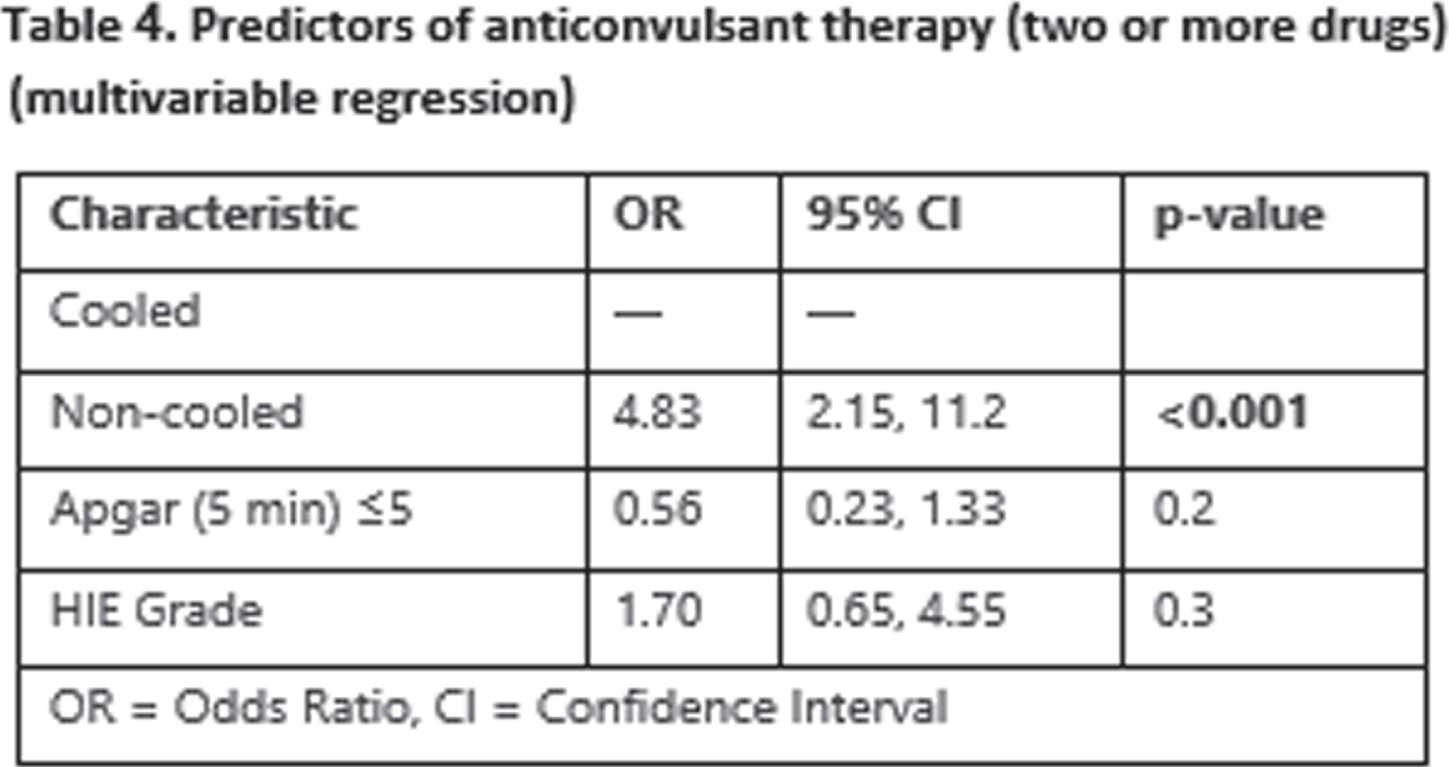
CONCLUSION/IMPACT: Therapeutic hypothermia in infants with moderate and severe HIE is associated with significant reduction of seizure burden and a significant reduction of ASM. Reduction in seizure burden as well as reduction in ASM may have a role in the beneficial influence of TH in HIE. Also, we believe the hypothermia may have a role as an add on therapy in cases of refractory seizures.
REFERENCES
[1] Glass HC et al. J Pediatr. 2009 Sep;155(3):318–23.
[2] Jacobs SE et al. Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd; 2013.
[3] Low E, et al. Arch Dis Child Fetal Neonatal Ed. 2012;97(4).
[4] Kim D-H, et al. J Epilepsy Res. 2017 Dec 31;7(2):109-114
Neonates with HIE requiring therapeutic hypothermia: Presence of seizures negatively impact developmental outcomes
Chandrashekar P1, Castri P
1Wake Forest School Of Medicine, Winston-Salem, United States
BACKGROUND AND PURPOSE: Hypoxic ischemic encephalopathy (HIE) is a serious birth complication associated with a 40–60% mortality rate and long term disabilities. Numerous randomized controlled trials indicated that therapeutic hypothermia (TH) reduces the incidence of death and severe disability following neonatal encephalopathy. However, despite TH, some babies develop seizures early in life and there is still little knowledge in the literature regarding the effect of seizures on developmental outcome.
The hypothesis for this study, suggested by a few emerging studies, was that neonates who developed seizures during therapeutic hypothermia or rewarming had worse clinical outcomes compared to the ones who were seizure free. We wanted to observe in our institution at Atrium Health Wake Forest Baptist, the developmental outcome within the first 2 years of life for babies who developed seizures and compare them to the outcomes of the ones who did not.
METHODOLOGY: Our sample group included NICU babies referred to the Neuro NICU clinic January 2017 and January 2020. Babies who were diagnosed with moderate-to-severe HIE and received therapeutic hypothermia were identified and divided into two groups: presence of seizures during hypothermia/rewarming versus no seizures. Neurological outcomes were analyzed. Demographics data was also collected including: maternal age, pregnancy complications (HTN, DM), intrapartum complications (fetal decelerations, uterine rupture, cord issues, fever in mom, shoulder dystocia, maternal hemorrhage, emergency c-section), neonatal age, sex, APGAR scores, cord pH, presence of intubation, and time of resuscitation >10 min.
RESULTS: Among the total of 22 subjects, 10 developed seizures during hypothermia or rewarming. We did not identify significant differences across demographic data in the two groups.
Subjects who did not develop seizures were more likely to have a better developmental outcome than the ones who developed seizures and overall their ASQ scores suggested a close to normal development. MRI results were normal in most of the subjects who did not develop seizures as compared to MRI showing HIE findings in most of the subjects with seizures.
CONCLUSIONS: Our data confirmed the notion that presence of seizure during hypothermia or rewarming negatively impacts developmental progress of a child in all cognitive areas. Seizures can arise at any time during hypothermia or rewarming, underlying the importance of EEG monitoring throughout the process. This gives these babies the best chances for early recognition and treatment.
Neurologic care at Colombian’s neonatal units: A national survey
Echeverria-Palacio C1, Benitez D1, Ortiz S1, Sánchez L1, Serrano-Tabares C1
1Hospital Universitario Fundacion Santa Fe de Bogota, Carrera 54C #143a-90 Apto 508, Bogota., Colombia
BACKGROUND AND PURPOSE: Neonatal neurological care field has developed considerably in the last two decades. For this reason, there are currently more tools for neuromonitoring, neuroimaging and neuroprotection, that enhance neurological assessment and treatment at neonatal units. Nevertheless, current trends in neonatal neurocritical care suppose a challenge for developing countries, in which the access to such technologies is limited. We explored the access to neurological specialized assessment, neuromonitoring, neuroimaging and neuroprotection strategies at Colombian’s neonatal units; and then we found related variables for them.
METHODOLOGY: This is a cross-sectional study from a national survey made in Colombia, South America, between May to June 2021. The survey was answered by the medical leadership for each neonatal unit through Google Forms ® (see final version in Spanish here: https://docs.google.com/forms/d/1hm4v5BVbmRacpAzXWRXi6W0kCEvGAUNycybVYp9mYPg/viewform?edit_requested=true). All data were confidential.
RESULTS: Responses from 73 neonatal units were collected, 51% of them were from university hospitals; 67% of these responses were concentrated in major cities (Bogota, Medellin, Cali, Barranquilla, and Bucaramanga). Table 1 presents characterization data, as well as the access to neurological specialized assessment, neuromonitoring, neuroimaging and neuroprotection strategies, while clinical scenarios for using these tools are shown at Table 2. Some of the highlights: the university hospitals had better access to neonatal neurological care, mainly those had neonatal fellowships. vEEG was the most frequent tool for neuromonitoring at neonatal units, but only 7% of them had real-time feedback, while 55,8% must wait for a retrospective inform (43% with therapeutic hypothermia). Additionally, in 21% of these center vEEG is interpreted by adults neurologist. In this way, only 16,3% of neonatal units had a neuromonitoring training structured strategy for neonatologists and nurses. 11% of neonatal units had trained personal for performing and interpreting cranial ultrasound, and 16% had exclusive ultrasound for NICU. Although 30,6% of neonatal units had servocontrol hypothermia, there was a heterogenous monthly patients’ volume between them: 22,7%: ≤1per month; 54,4%: 1-5; 18,2%: 6-10; and only one had more than 10 patients per month.
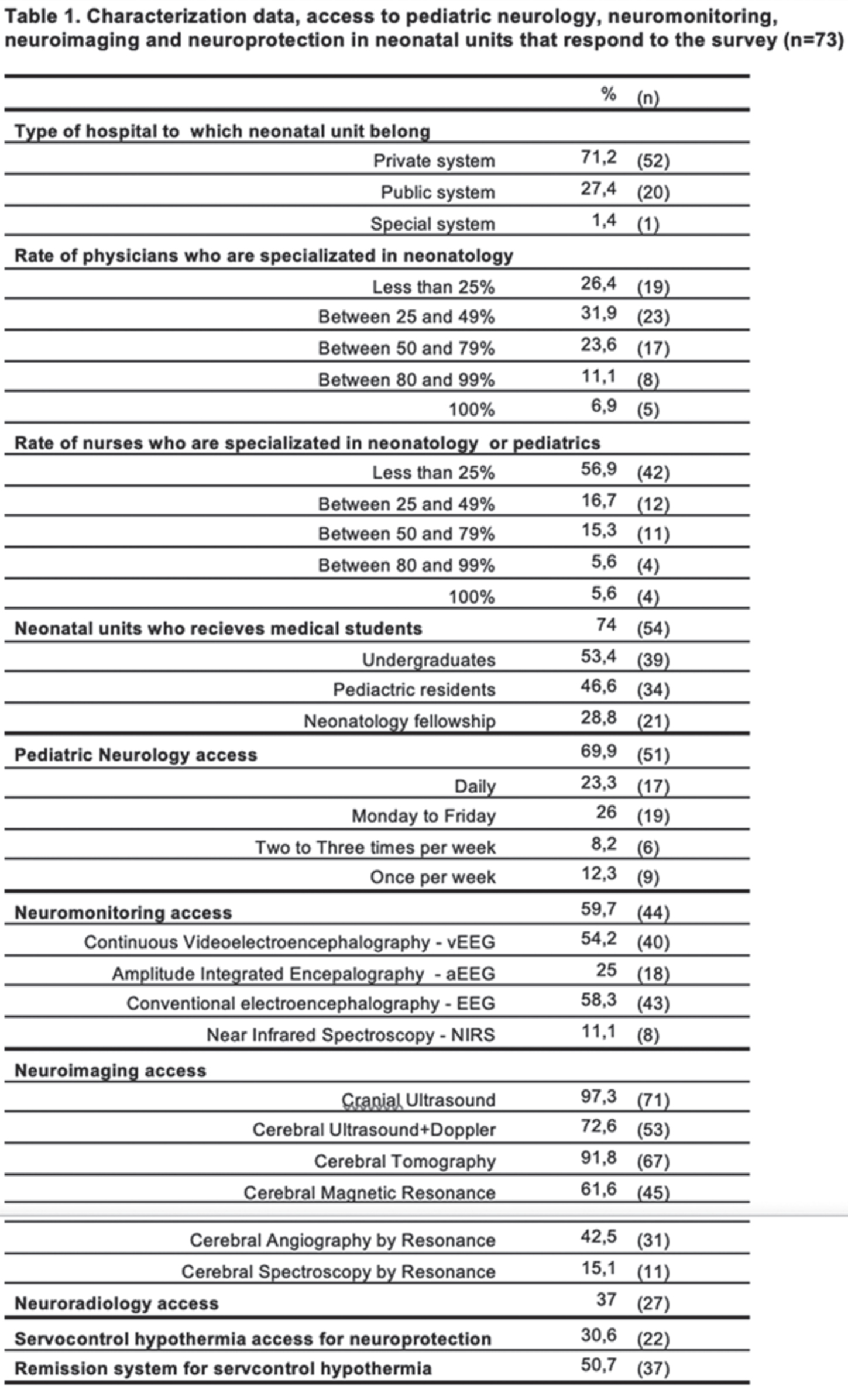
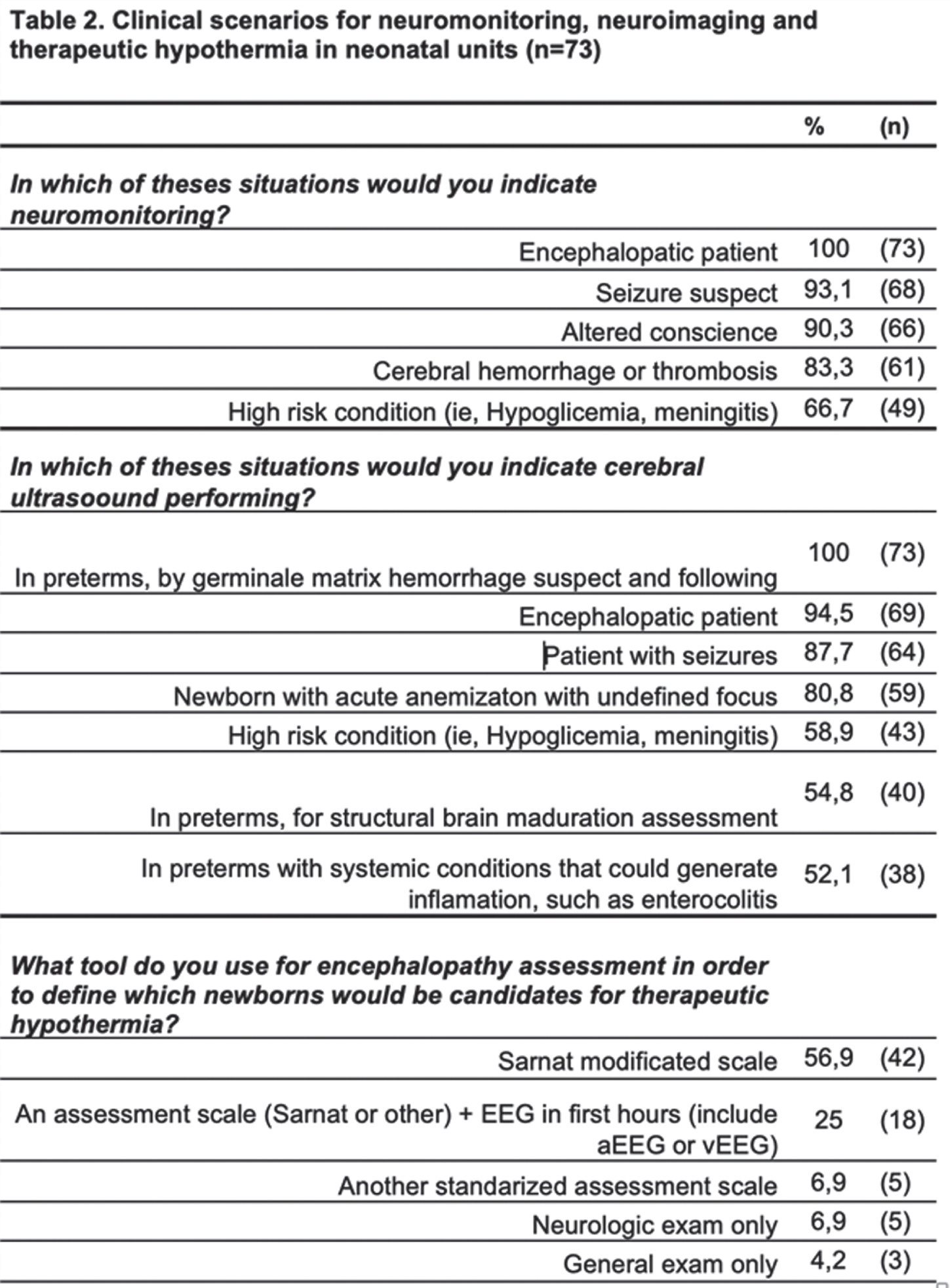
Limitations for this survey are related to self-report questionary without verification possibility, and sub-report from minor cities.
CONCLUSION/IMPACT: This is the first study that aims explore neurological care at Colombian’s neonatal units. It offers essential inputs for characterizing the access to neurological specialized assessment, neuromonitoring, neuroimaging and neuroprotection strategies. These findings can be used to achieve comprehensive strategies and integrated networks, to enhance neurocritical care at NICU, as a new frontier in developing countries.
REFERENCES
[1] Bonifacio SL, Glass HC, Peloquin S, Ferriero DM. A new neurological focus in neonatal intensive care. Nat Rev Neurol. 2011;7(9):485-94.
[2] Glass HC, Bonifacio SL, Shimotake T, Ferriero DM. Neurocritical care for neonates. Curr Treat Options Neurol. 2011;13(6):574-89.
[3] Lemus-Varela MdL, Sola A, Golombek SG, Baquero H, Dávila-Aliaga CR, Fariña D, et al. Recomendaciones terapéuticas del VII Consenso Clínico de SIBEN para la encefalopatía hipóxico-isquémica neonatal. NeoReviews. 2016;17(9):e554.
[4] Variane GF, Cunha LM, Pinto P, Brandao P, Mascaretti RS, Magalhães M, et al. Therapeutic Hypothermia in Brazil: A MultiProfessional National Survey. Am J Perinatol. 2019;36(11):1150-6.
[5] Buttle SG, Sell E, Webster R, Varin M, Lemyre B, Hahn C, et al. Continuous Electroencephalography Monitoring for Critically Ill Neonates: A Canadian Perspective. Can J Neurol Sci. 2019;46(4):394-402.
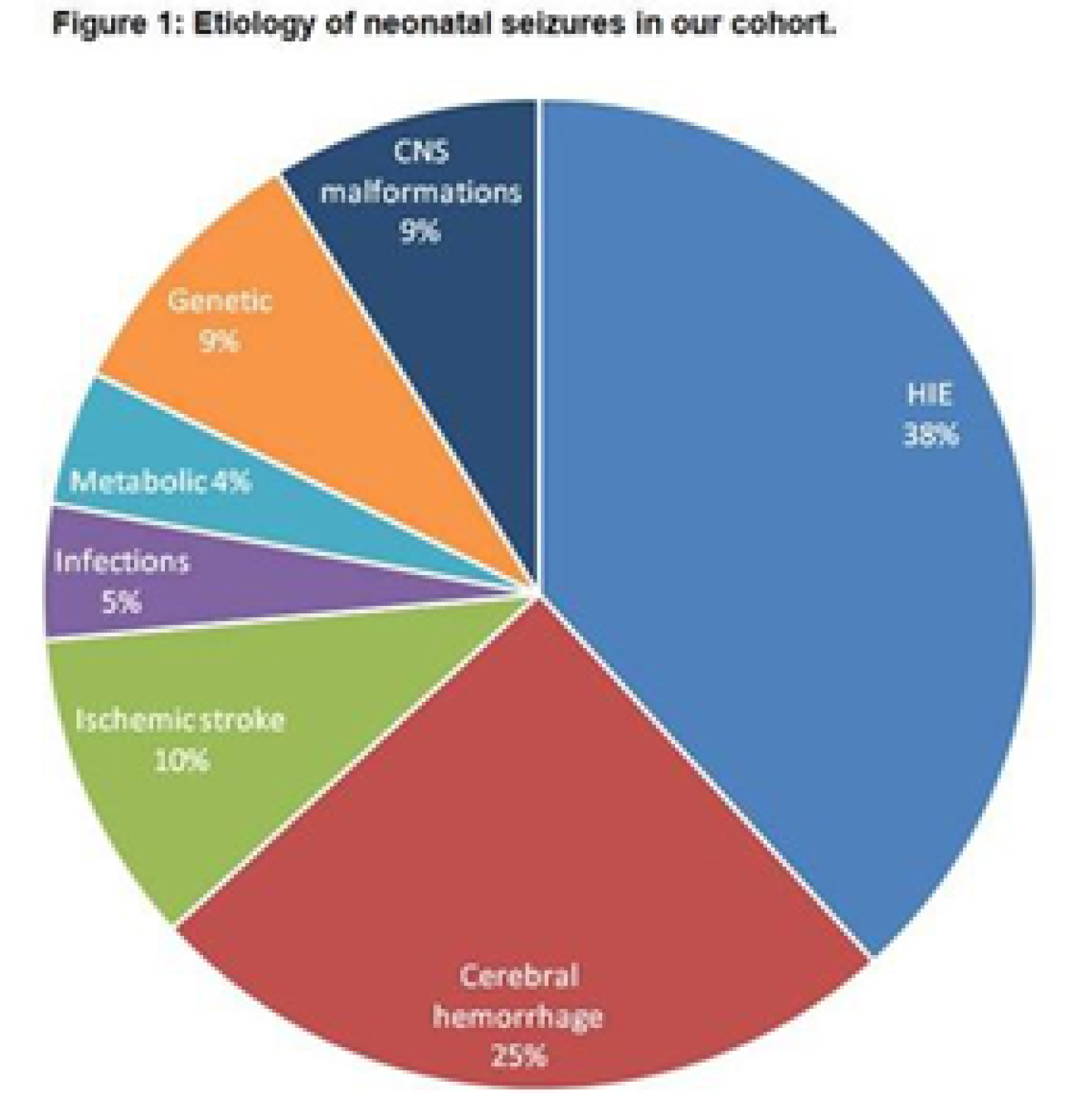
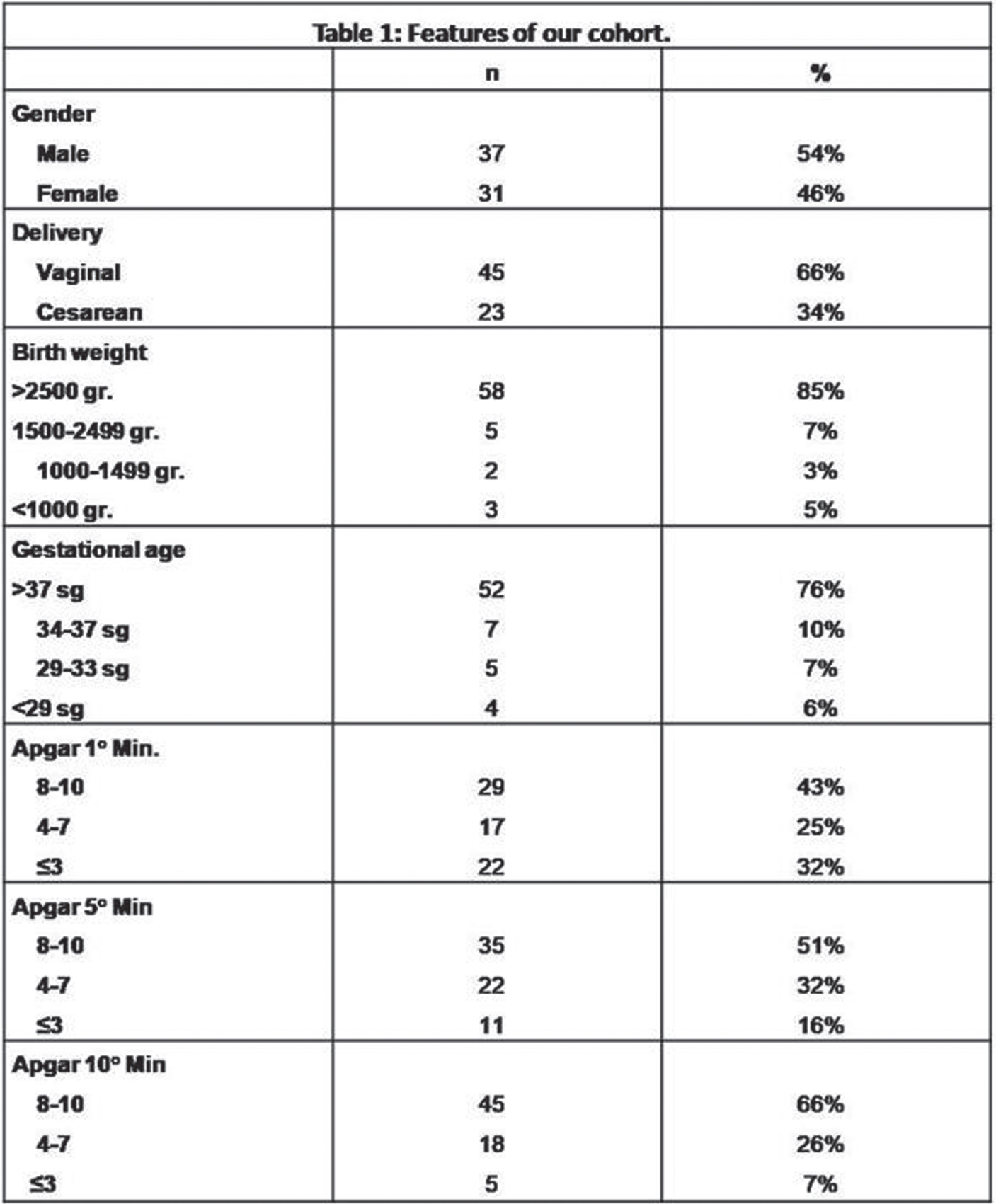
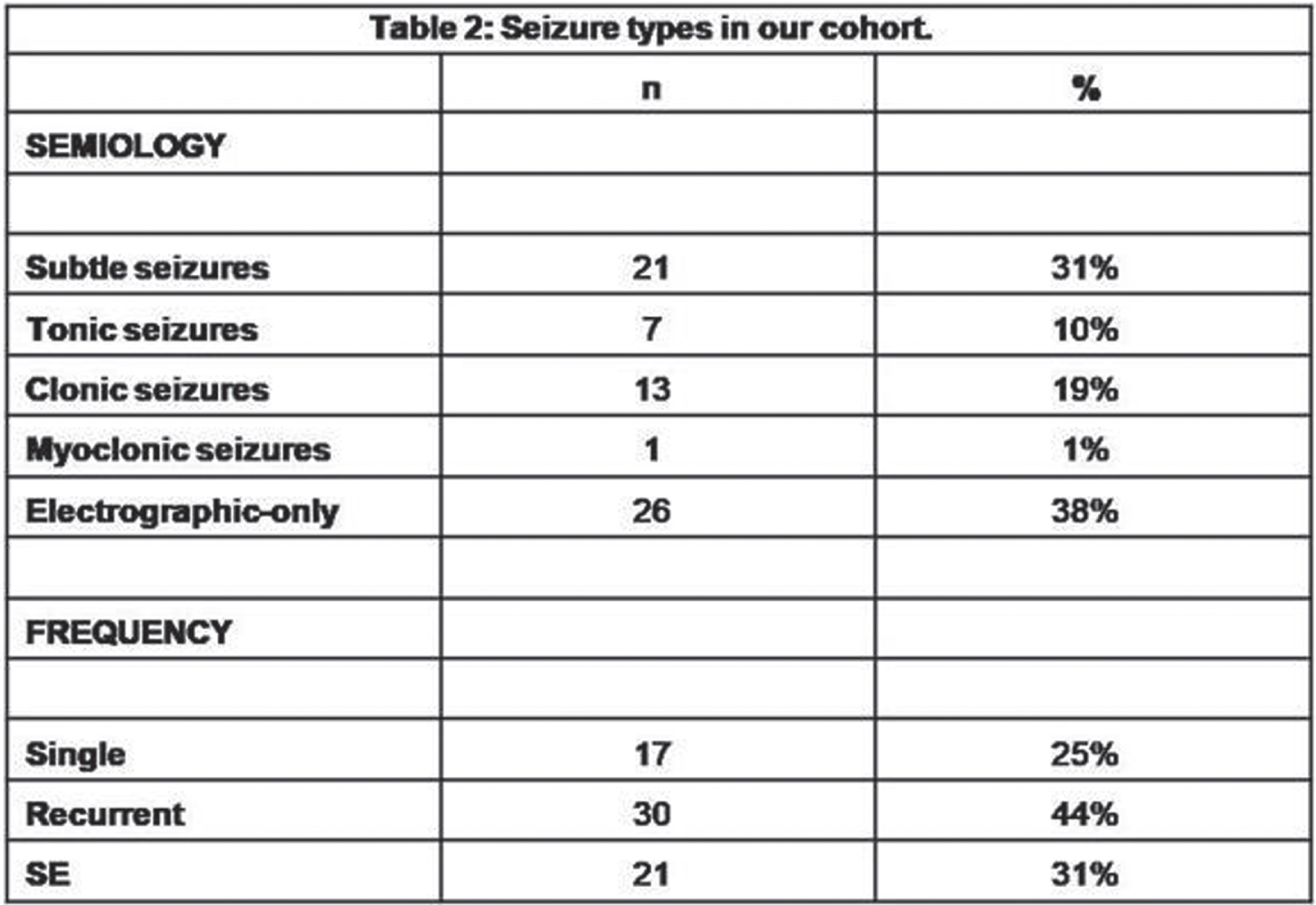
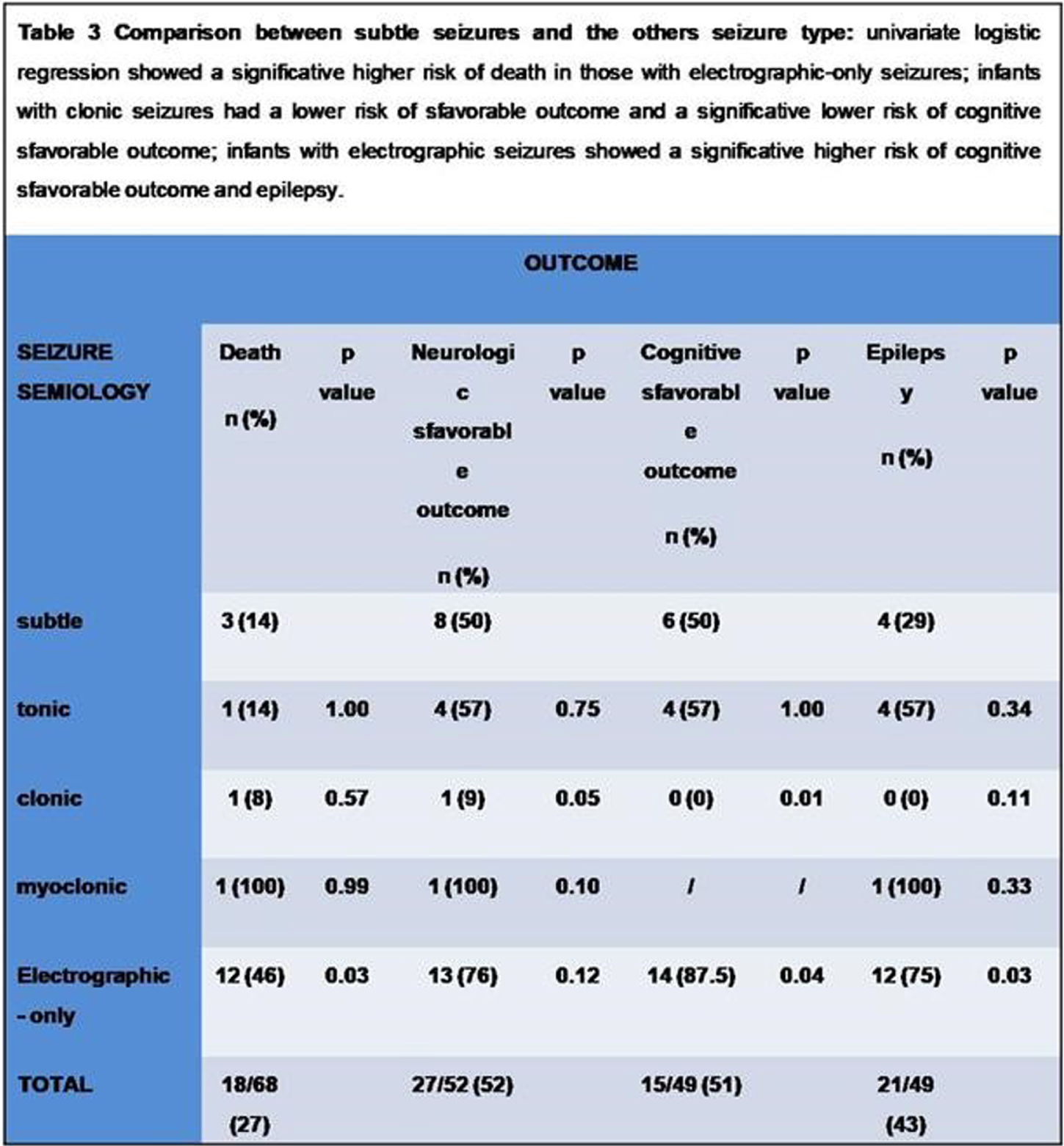
Neonatal seizures: Does semiology matter?
Boni E1, Pellegrin S1, Menzato F2, Salandin M1, Parmeggiani L1, Menna E1, Chiodin E2, Staffler A2, Ponta A1, Pavlidis E1
1Child Neurology And Neurorehabilitation Unit, Department Of Pediatrics, Regional Hospital Of Bolzano, Bolzano, Italy, 2Neonatal Intensive Care Unit - Division of Neonatology, Regional Hospital of Bolzano, Bolzano, Italy
BACKGROUND AND PURPOSE: several classifications of neonatal seizures do exist (1,2,3), nevertheless the association between semiology and outcome is still matter of debate (4,5).
MATERIALS AND METHODOLOGY: among all infants that had vEEG in the NICU of the Hospital of Bolzano between 2008 and 2020, we included those with vEEG-confirmed neonatal seizures. All infants with seizures underwent serial vEEG recordings with a complete sleep-wake cycle and/or a 1-hour duration. Semiology was classified according to Volpe’s classification (1), considering the main semiology of the seizure and including also electrographic-only seizures. Outcome was defined considering four main categories: death, neurologic outcome (sfavorable in case of cerebral palsy, deafness/blindness), cognitive outcome (clinical evaluation of the milestones and/or by means of the Griffiths Scales of Mental Development at 24 months-of-age), epilepsy. We analysed the relation between semiology and outcome, using MS Excel, Jamovi and SPSS.
RESULTS: out of the 751 infants that underwent an vEEG, 68 were included. 12/26(46%) with electrographic-only seizures and the only one with myoclonic seizures died. 20/26(76%) out of those with electrographic-only seizures and 1/13(9%) with clonic seizures showed a sfavorable neurologic outcome. 87,5% of infants with electrographic-only seizures showed a psychomotor delay at 24 months-of-age, whereas all infants with clonic seizures showed normal milestones. Infants with clonic seizures showed a lower risk of developing a sfavorable cognitive outcomey (p value=0,0137) on respect of those with subtle seizures, whereas those with electrographic-only seizures showed a higher risk (p value=0,0441). 75% of infants with electrographic-only seizures developed epilepsy, whereas none of the infants with clonic seizures showed post-neonatal epilepsy in our cohort. A proportion-Z test showed higher epilepsy rates in those infants that experienced tonic, myoclonic and electrographic-only seizures in comparison to those with clonic seizures.
CONCLUSIONS: As previously reported, electrographic-only seizures showed a relation with sfavorable outcome (4,6), likely related to the underlying severity of the brain damage. Clonic seizures, conversely, are related to more favorable outcomes (7). A proper brain monitoring during the neonatal age is recommendable, especially in those infants that show subclinical or electrographic-only seizures. Thus, newborns at risk for seizures need to be monitored in order to detect those seizures that are not visually evident. Future directions: we would like to include more infants in our cohort and classify the seizures according to the new classification for neonatal seizure (3), in order to verify the replicability of the present results.
REFERENCES
[1] Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987.
[2] Volpe JJ. Neonatal seizures: current concepts and revised classification. Pediatrics. 1989.
[3] Pressler, R.M., Cilio, M.R., Mizrahi et al. The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia. 2021.
[4] Pisani F., Piccolo B., Cantalupo G., et al. Neonatal seizures and postnatal epilepsy: a 7-y follow-up study. Pediatr Res. 2012.
[5] Boylan GB, Pressler RM, Rennie JM et al. Outcome of electroclinical, electrographic, and clinical seizures in the newborn infant. Dev Med Child Neurol. 1999.
[6] McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2020.
[7] Garfinkle J, Shevell MI. Prognostic factors and development of a scoring system for outcome of neonatal seizures in term infants. Eur J Paediatr Neurol. 2011.
Characterization of seizure semiology in neonates with encephalopathy in Uganda using continuous video-EEG monitoring
Proietti J1, Nanyunja C2, Mathieson S1, Duckworth E3, Mambule I2, Nakimuli A4,5, Tann C2,3,6, Boylan G1
1Infant Research Centre, University College Cork, Cork, Ireland, 2MRC/UVRI & LSHTM Uganda Research Unit, Entebbe, Uganda, 3University College London Hospitals NHS Trust, London, United Kingdom, 4Kawempe National Referral Hospital, Kampala, Uganda , Kampala, Uganda, 5Makerere University, Kampala, Uganda, 6London School of Hygiene & Tropical Medicine, London, United Kingdom
BACKGROUND AND PURPOSE: Neonatal encephalopathy (NE) is a leading cause of under-5 mortality and morbidity globally[1], with the majority of infants born in low-income countries where neonatal intensive care, and interventions such as therapeutic hypothermia, are often lacking. Prompt detection and treatment of NE-related seizures can be challenging, but may confer neuroprotection. We aimed to explore the electroclinical semiology and medical management of neonatal seizures amongst infants with NE in Uganda.
MATERIALS AND METHODOLOGY: Neonates with NE recruited as part of the ‘Baby BRAiN’ study[2] had continuous multichannel video-EEG-polygraphy monitoring (cEEG) over days 1-4. Local clinicians treated seizures based on clinical presentation alone. Seizure annotation in each recording was performed retrospectively[3]. Seizure semiology in cEEG segments annotated as seizures were reviewed according to the updated ILAE classification[4]. Timing of administration of an antiseizure medication loading dose (ASM) within 60 minutes of commencement of a cEEG-confirmed seizure was examined. Paroxysmal nonepileptic events occurring in the 60 minutes preceding ASM or concomitant to ECG/respiration alterations were also examined.
RESULTS: Of 51 recruits, 50 received cEEG of diagnostic quality and, of these, 26 (52%) had cEEG-confirmed seizures (Figure 1). On video review, 18/26 (70%) had a combination of electroclinical and electrographic seizures, 1 (4%) exclusively electroclinical seizures and 6 (22%) electrographic seizures only. Of those with electroclinical seizures (19), 11 (58%) displayed one semiology, and 8 (42%) more than one. The distribution of all seizure semiology types was: clonic 34%, autonomic 24%, automatisms 18%, behavioural arrest 12%, and sequential 12% (Table 1). Of those with autonomic seizures, 6 had prolonged ictal apnea (Figure 2). 64% (32/50) neonates received ASM, 7 exclusively before and 25 during monitoring (Figure 1). Of those with cEEG-confirmed seizures, only 62% (16) received ASM, and in the non-seizure group, 38% (9/24) received ASM (Figure 3). In total, ASM was administered 42 times during cEEG, of which 19 (45%) were appropriate. In the 60 minutes prior ASM administration not preceded by cEEG-confirmed seizures, generalized tonic posturing was encountered in 6 cases, jerky non-rhythmic movements during crying in 4, non-epileptic oral automatisms in 3, and tremor in 1. In addition, 13 patients also presented with abnormal non-epileptic repetitive abdominal movements, unrelated to ASM (Figure 2).
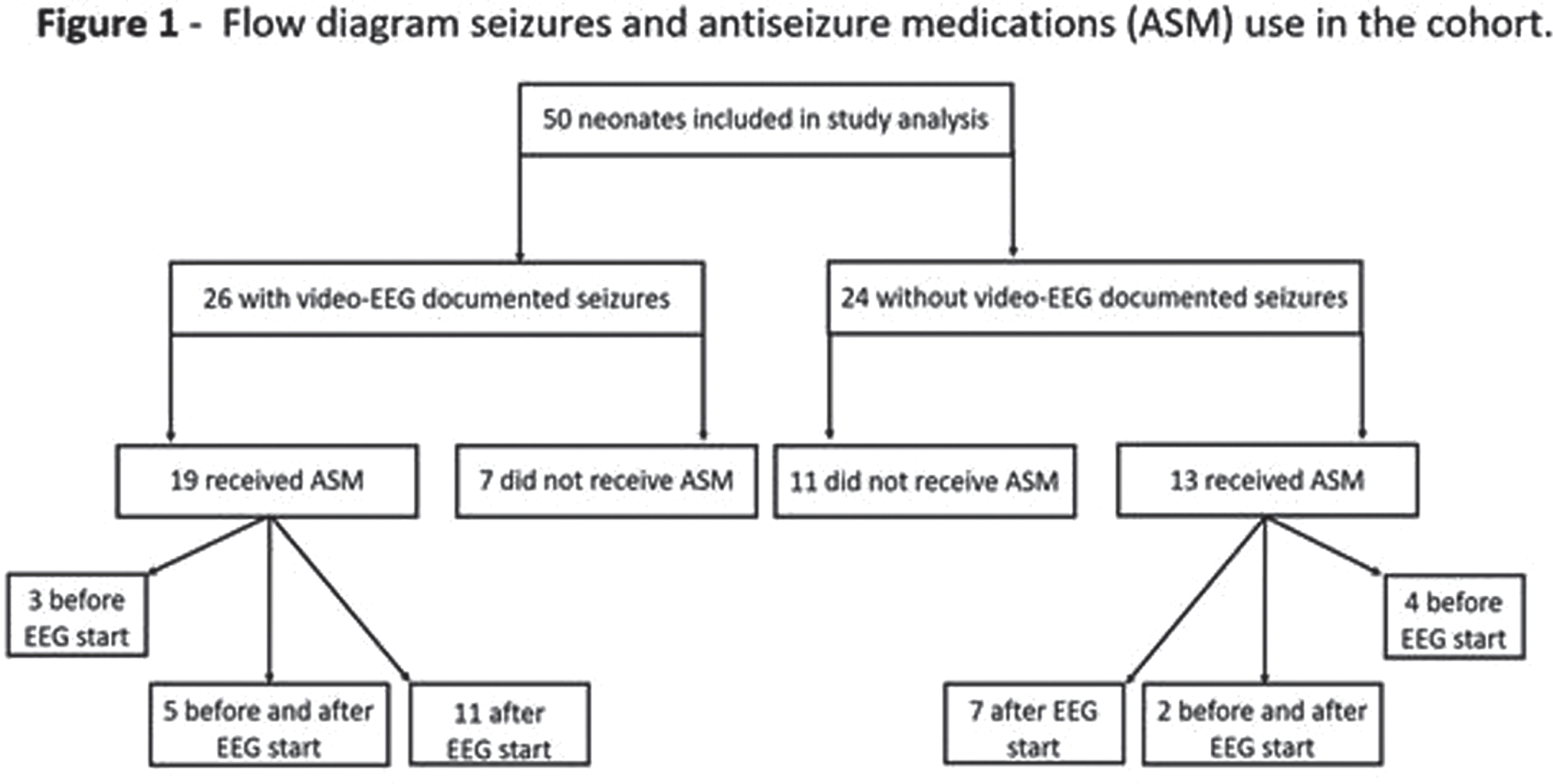
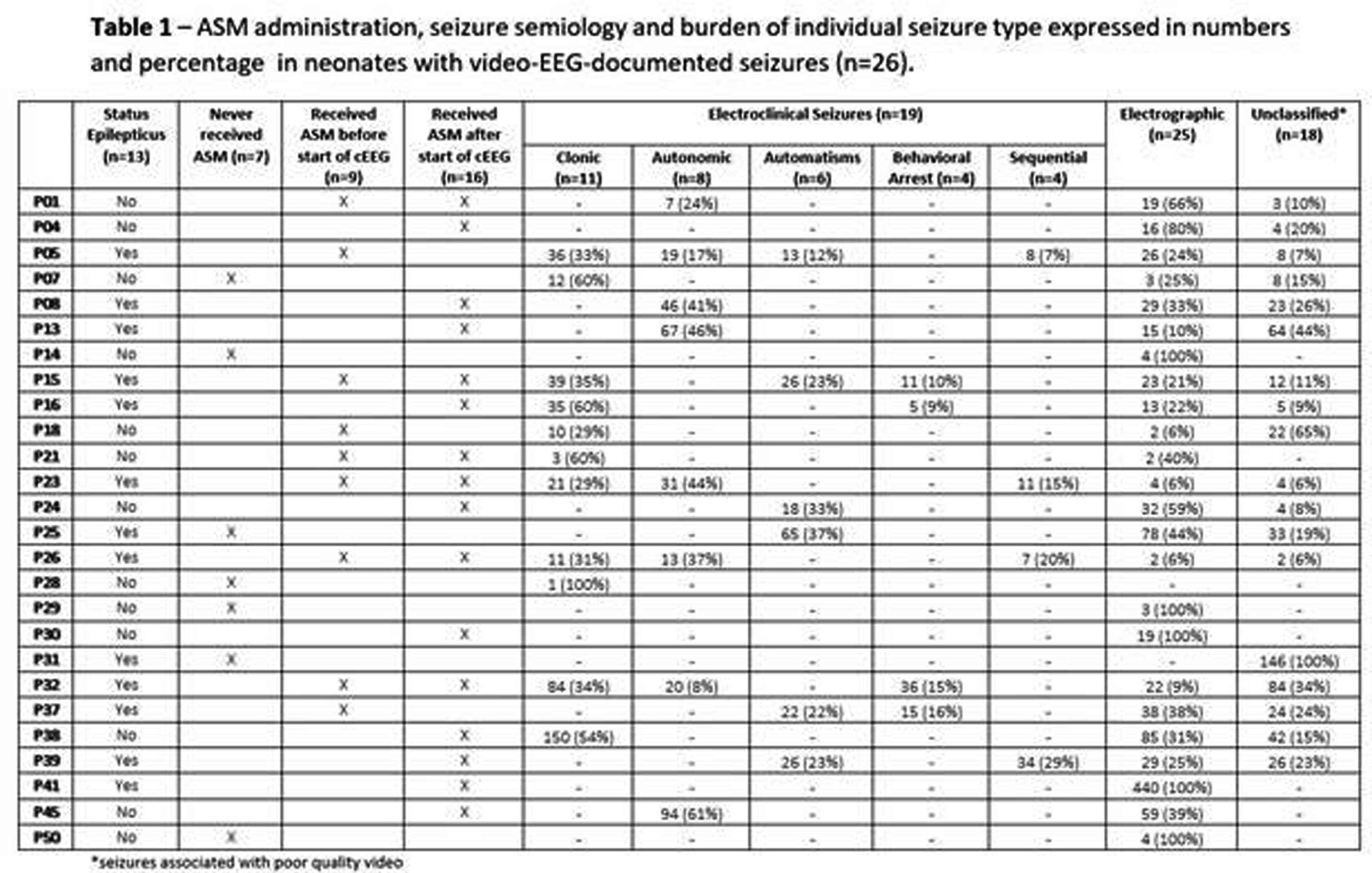
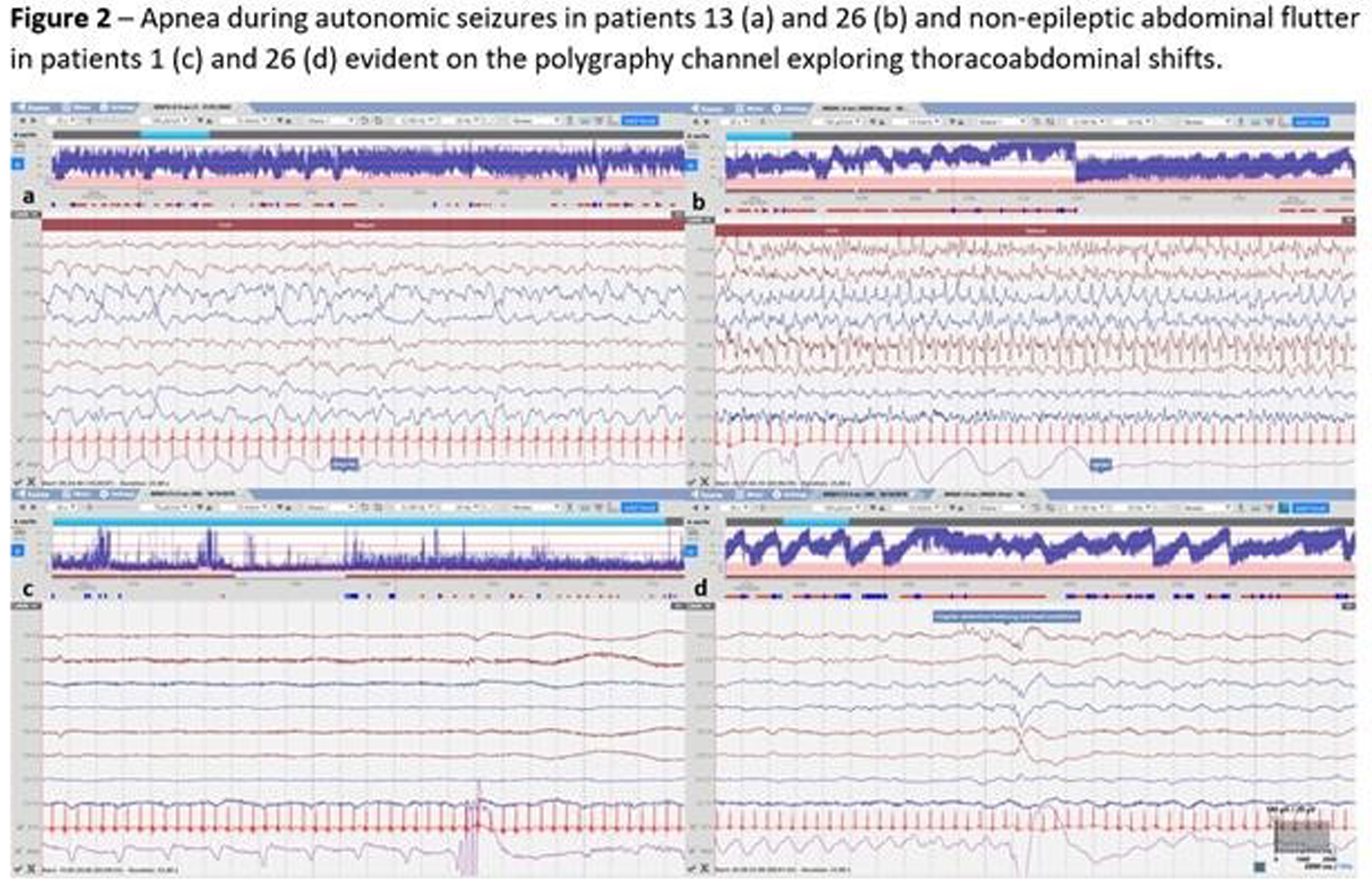
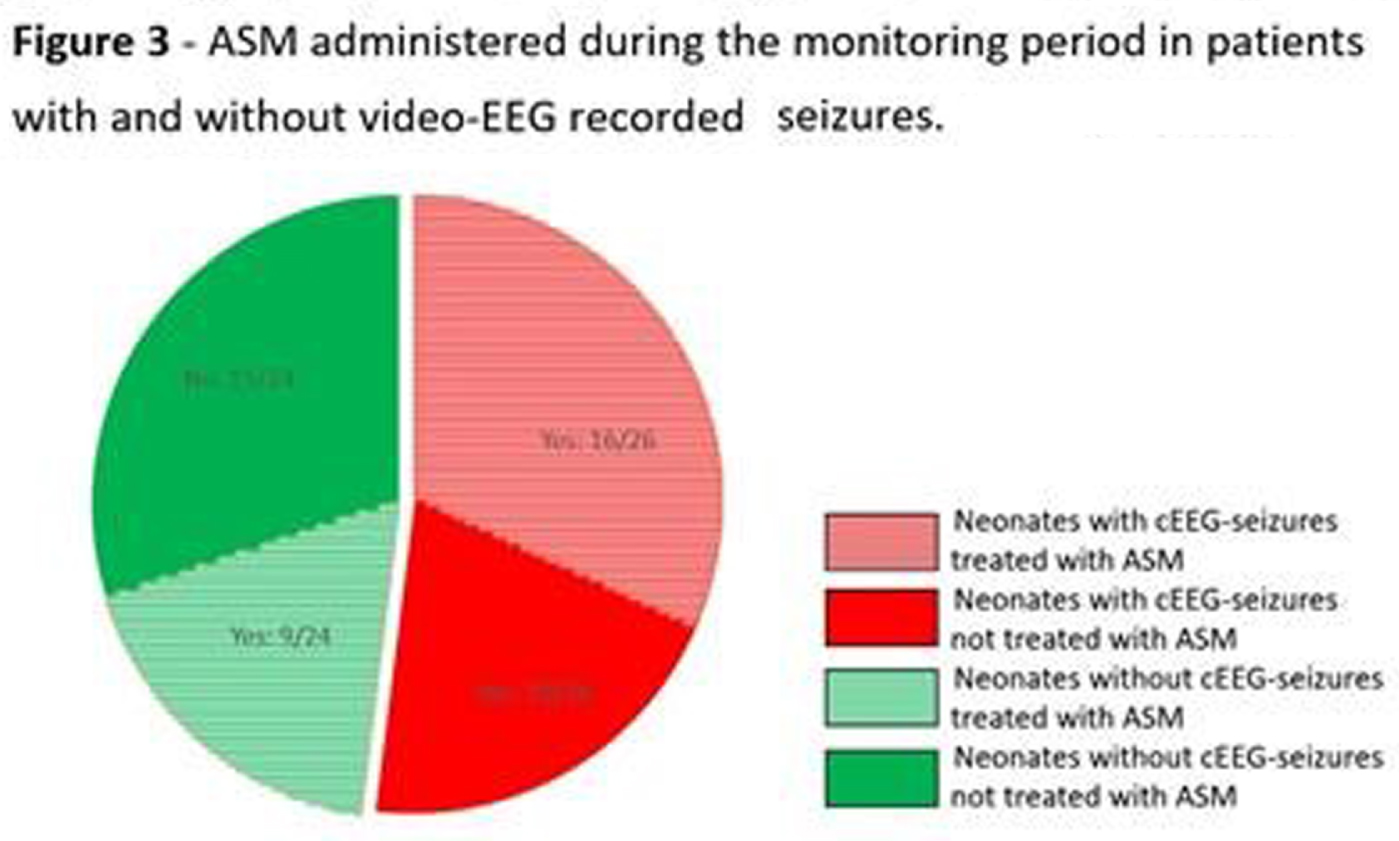
CONCLUSION: This study showed a high incidence of neonatal seizures in this NE cohort in Uganda. Clinical diagnosis proved difficult and both under and over treatment was evident. Of those with seizures, three quarters had clinical manifestation, with clonic, autonomic and automatisms representing the most commonly seen semiologies. Respiratory impairment emerged as a prominent concern, through both prolonged ictal apnea during the seizures and non-ictal abdominal flutter or gasping.
REFERENCES
[1] Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, Niermeyer S, Ellis M, Robertson NJ, Cousens S, Lawn JE. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013 Dec;74 Suppl 1(Suppl 1):50-72. doi: 10.1038/pr.2013.206. PMID: 24366463; PMCID: PMC3873711.
[2] Nanyunja C, Sadoo S, Mambule I, Mathieson SR, Nyirenda M, Webb EL, Mugalu J, et al. Protocol for the Birth Asphyxia in African Newborns (Baby BRAiN) Study: a Neonatal Encephalopathy Feasibility Cohort Study. Gates Open Res. 2022 Mar 3;6:10.
[3] Clancy RR, Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia 1987;28:537–41.
[4] Pressler RM, Cilio MR, Mizrahi EM, Moshé SL, Nunes ML, Plouin P, Vanhatalo S et al. The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia. 2021 Mar;62(3):615-628.
Enhanced neonatal encephalopathy evaluation including aEEG in the special care nursery
Patrizi S1, Elshibiny H2, El-Dib M2, Inder T3
1Newton-Wellesley Hospital, Newton, United States, 2Brigham and Women’s Hospital, Boston, United States, 3Children’s Hospital of Orange County UC, Irvine, United States
BACKGROUND: Therapeutic hypothermia (TH) improves survival and neurodevelopmental outcome in infants with neonatal encephalopathy (NE). Rigorous evaluation and timely screening are needed for accurate selection of patients eligible for TH. Several studies have reported worse outcome for outborn versus inborn infants. This difference in outcome could be attributed to differences in clinical care practices, difficulties in monitoring markers of encephalopathy, and challenges in transferring eligible newborns within the first six hours of life. Although amplitude integrated EEG (aEEG) is a bedside neuromonitoring tool that improves early detection of NE as early as three hours of life, it is not routinely used in community hospitals due to lack of resources and expertise. The aim of this study was to assess the application of standardized guidelines and the use of aEEG to optimize the evaluation of neonates at risk of NE in a community hospital special care nursery.
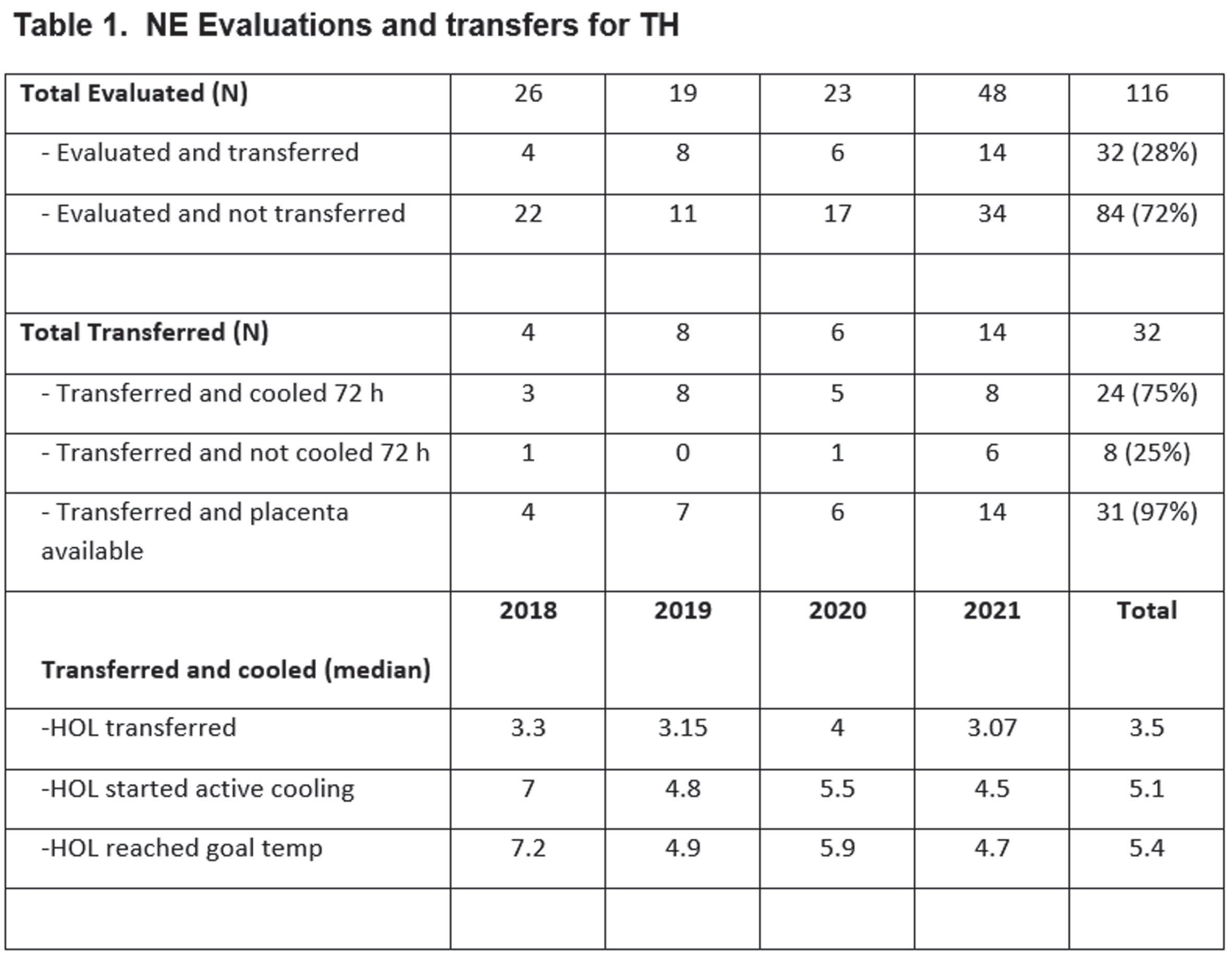
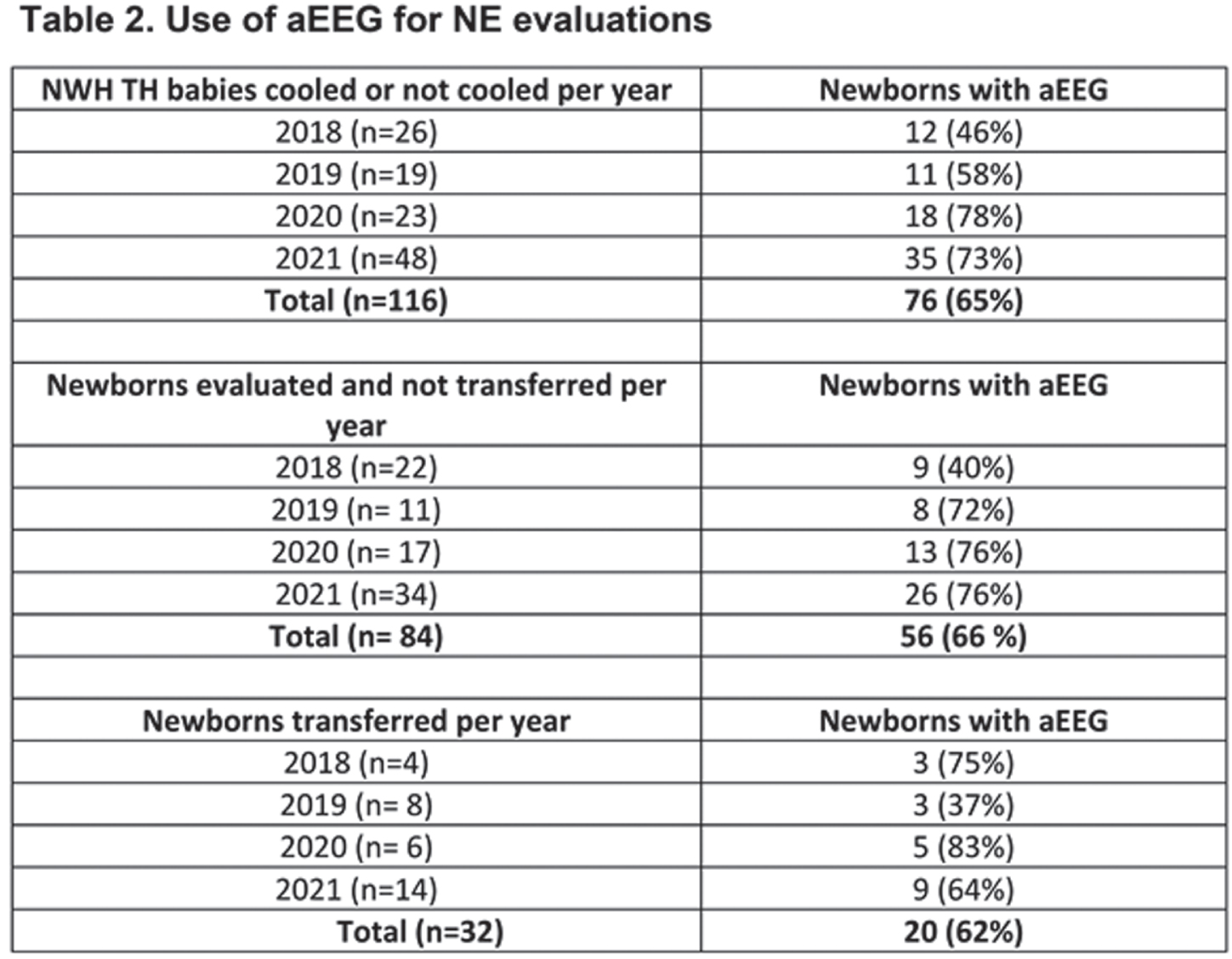
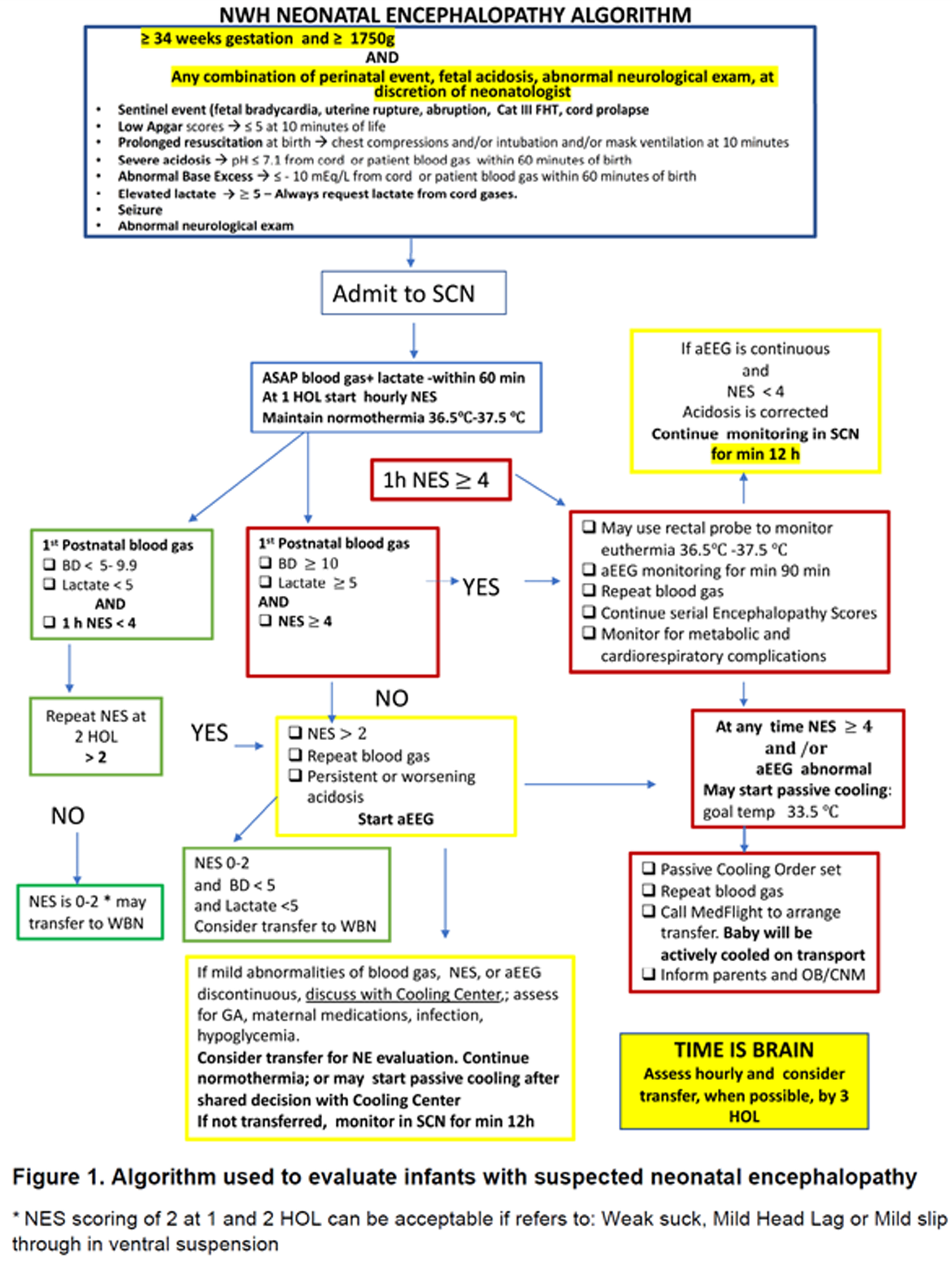
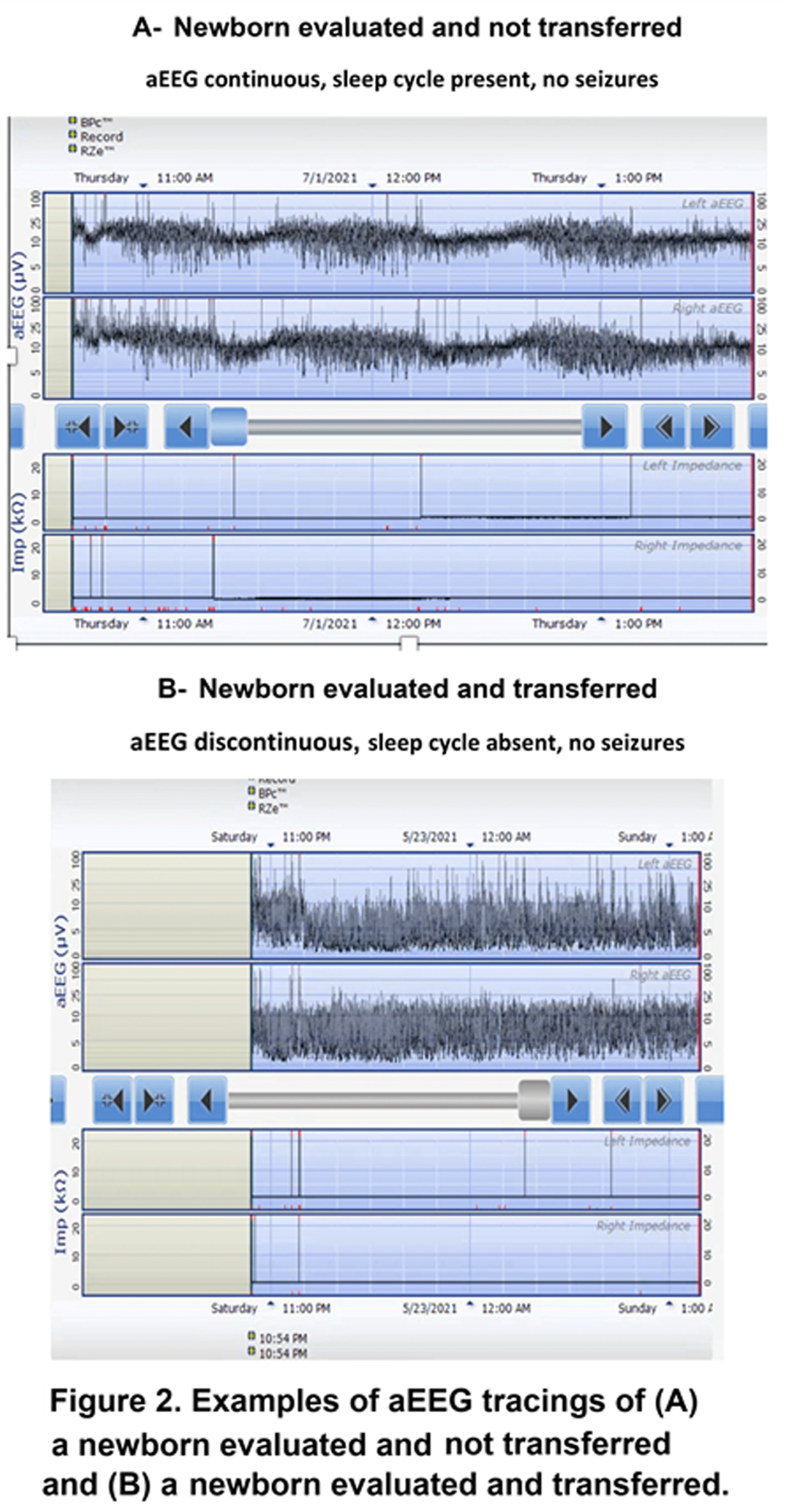
METHODS: At Newton-Wellesley Hospital (NWH), approximately 4000 deliveries occur every year, which result in 600 neonatal admissions to the level IIb Special Care Nursery. Prior to the development of NE Evaluation Guideline, most infants at risk for NE were transferred to our tertiary care center, and for those evaluated and not transferred, there was large variation in care practices. A comprehensive evaluation process which includes aEEG has been implemented since 2018. Our evaluation includes: 1. Standardized communication with obstetrical team.2 Cord gas and postnatal gas analysis guideline. 3. Standardized neurological exams 4. Two Channel aEEG (Olympic Brainz Monitor). 5 Passive cooling guidelines. 6. Rectal probe. 7. Placenta pathology. During the implementation period, we revised our guidelines, converted from using hydrogel electrodes to subdermal needles and conducted quarterly case quality reviews of all patients.
RESULTS: We evaluated a total of 116 newborns at risk for NE from April 2018 to December 2021. Of the 116 newborns, 84 (73.2) % were evaluated at NWH and not transferred, and 32 (25.5%) were transferred to the cooling center. Standardized examination was used in 100% of evaluated infants. A total of 76 (65%) newborns had aEEG recording (66% of those not transferred and 62% of those transferred). Placenta pathology was available in 97% of patients transferred for TH. Time of transfer was 3.5 hours (median). Active cooling started at 5.1 hours with target temperature achieved at 5.4 hours of life. Twenty-four (75%) of transferred newborns were eligible for TH and received 72 h cooling.
CONCLUSIONS: The use of clinical guidelines and aEEG can support clinicians in SCN to conduct accurate evaluation and selection of patients at risk of NE without delaying the start of TH. The potential benefits are early identification of patients eligible for TH and expediting transfer to cooling centers within the therapeutic window; minimize mother-newborn separation and unnecessary procedures. The use of aEEG in the community setting is feasible and offers important information with the clinical decision process in a community hospital.
REFERENCES
[1] Toet MC. Amplitude integrated aEEG 3 and 6 hours after birth in full term neonates with hypoxic ischemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999; 81; F19-F23
[2] Shalak L. Amplitude integrated Electroencephalography Coupled with an Early Neurologic Examination Enhances Prediction of Term Infants at Risk for Persistent Encephalopathy. Pediatrics. 2003;111:351-357.
[3] Merchant N. Early predictors of outcome in infants treated with hypothermia for hypoxic ischemic encephalopathy. Developmental Medicine & Child Neurology. 2015; 57; 8-16
[4] Azzopardi D. Predictive value of the amplitude integrated EEG in infants with hypoxic ischemic encephalopathy: data from a randomized trial of therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014; 99:F80-F82
[5] Al Naqeeb. Assessment of Neonatal Encephalopathy by Amplitude-integrated EEG. Pediatrics, 1999;103:1263-1271.
[6] Spitzmiller R. Amplitude-Integrated EEG is useful in Predicting Neurodevelopmental Outcome in Full-Term Infants with Hypoxic Ischemic Encephalopathy. Journal Child Neurology, 2007; 22:1069-1078.
[7] Thorensen M. Effects of Hypothermia on Amplitude-Integrated EEG in Infants with Asphyxia. Pediatrics, 2010; 126:131-139.
[8] Del Rio R. Amplitude-Integrated Electroencephalogram as a Prognostic tool in Neonates with Hypoxic Ischemic Encephalopathy. PLOS ONE; 2016:11/doi:10.1371
Outcome of therapeutic hypothermia for small-for-gestational age infants with hypoxic-ischemic encephalopathy
Kim H1, Yum S1, Kim S1, Seo Y1
1Department of Pediatrics, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea, Seoul, South Korea
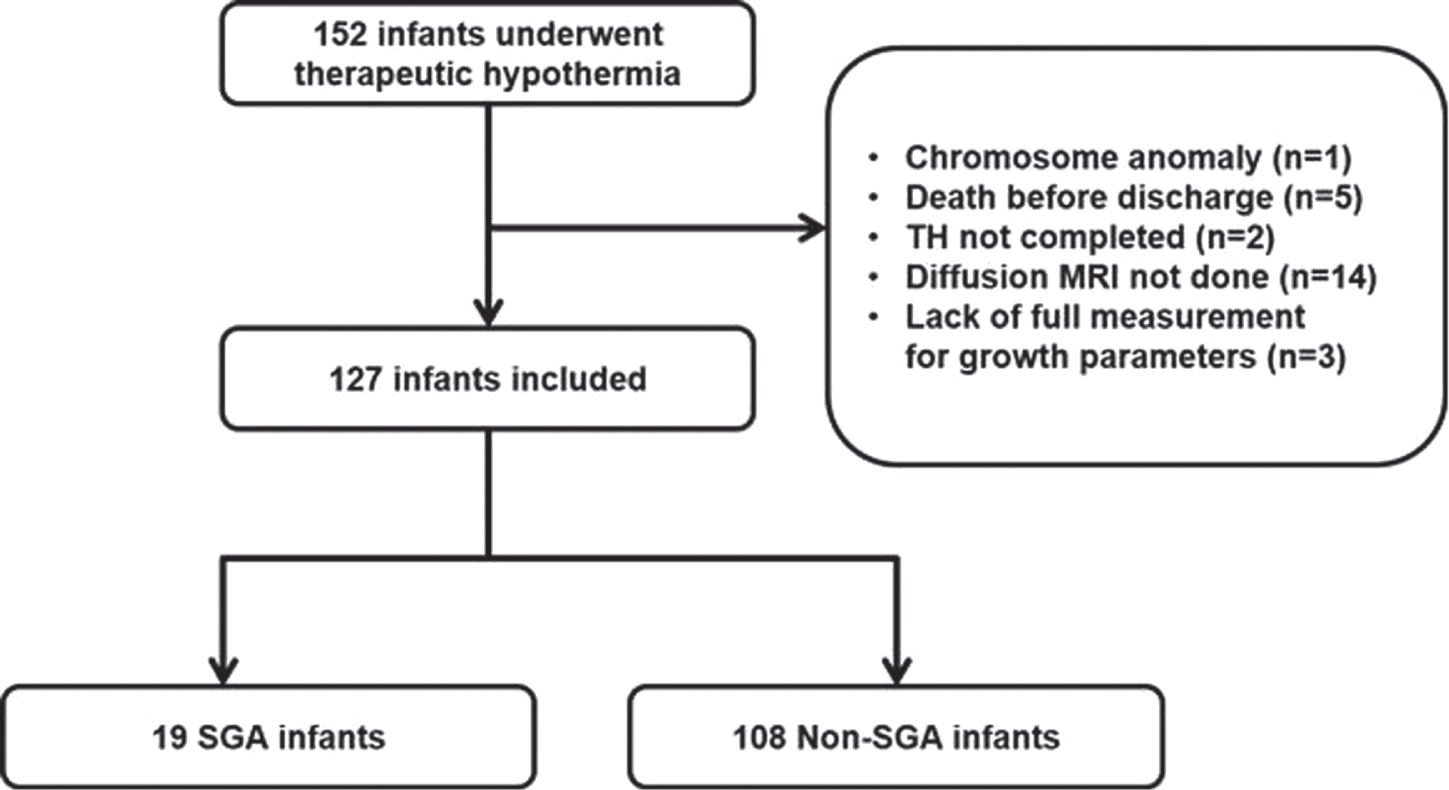
BACKGROUND AND AIM: Therapeutic hypothermia (TH) is reserved for acute hypoxic ischemic encephalopathy (HIE) infants, limiting the golden hour of treatment to 6 hours after birth. One of the contributive factors for small-for-gestational-age (SGA) is chronic intrauterine hypoxia. We aimed to evaluate whether the infants who qualified for TH had different perinatal characteristics and in-hospital outcomes depending on whether the infant was SGA or not.
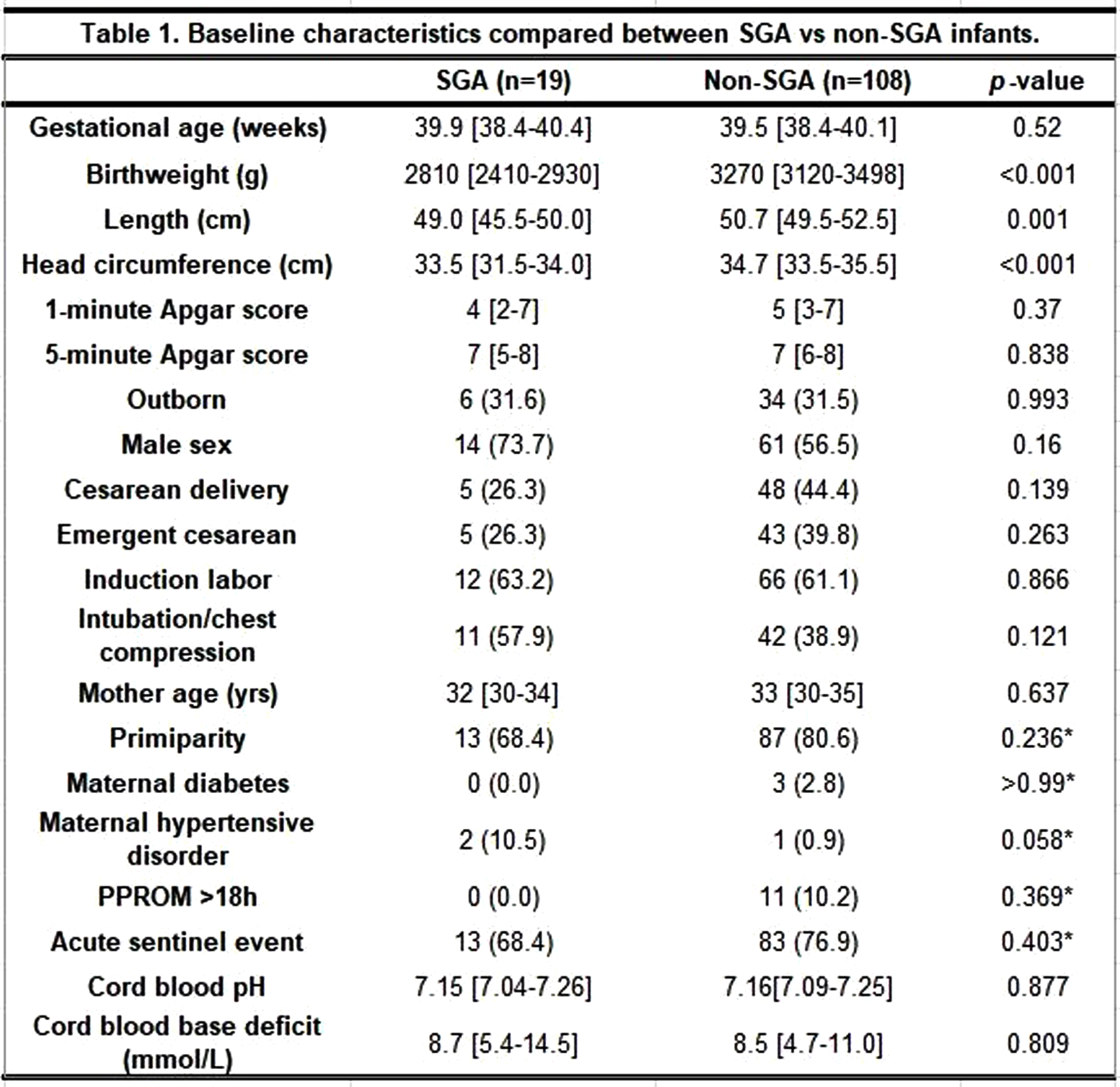
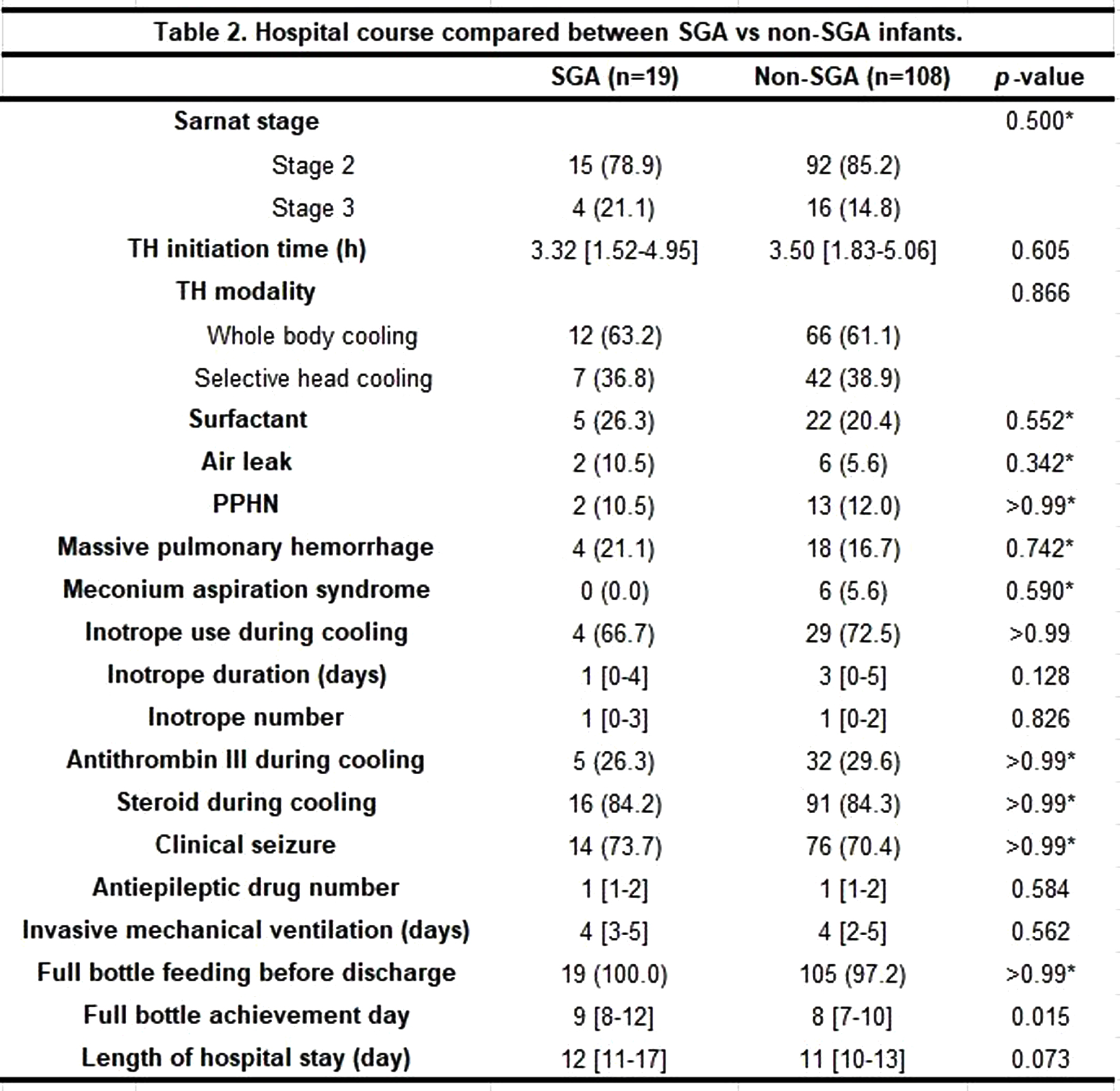
METHODS: Data from electronic medical chart was retrieved from 152 neonates who had been treated with TH. The infants with chromosomal anomaly, who died before discharge, did not complete 72 hours of TH, did not include diffusion-weighted magnetic resonance imaging (MRI), or did not have a complete measurement of growth parameters were excluded. The enrolled infants were divided into SGA vs non-SGA group, and basic characteristics and in-hospital outcome were compared.
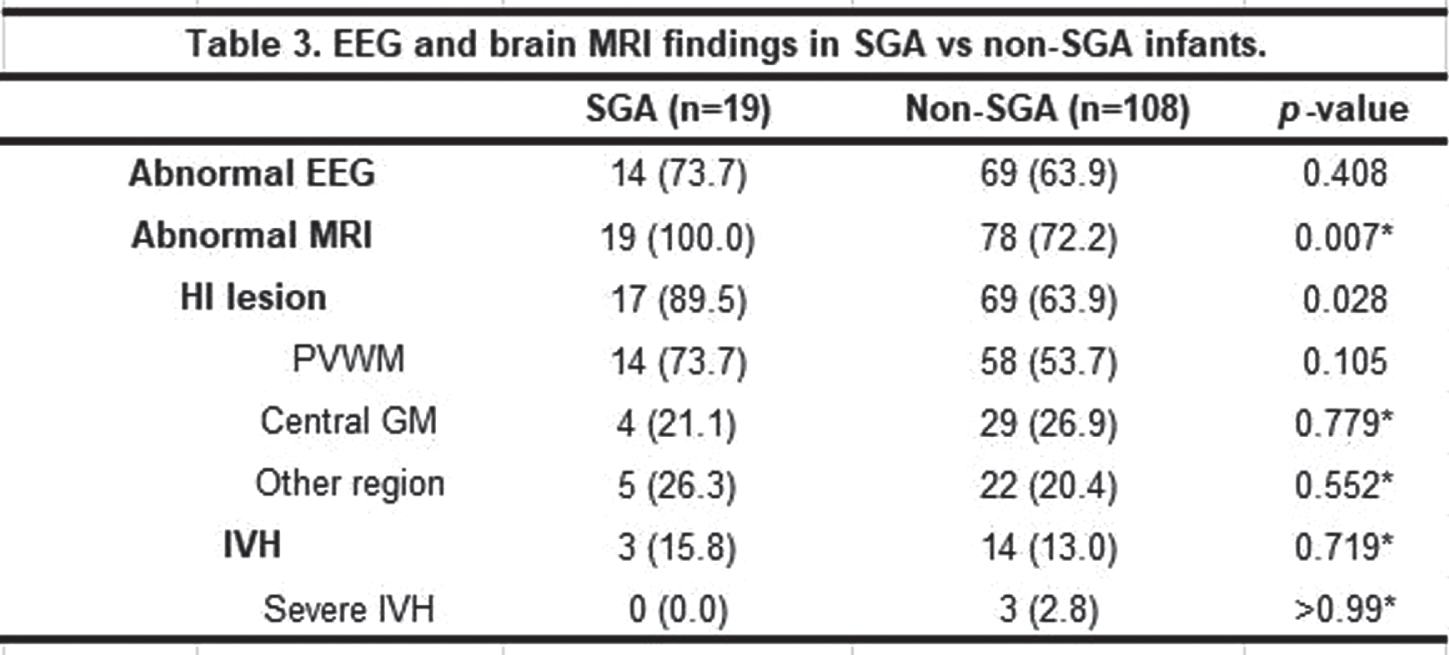
RESULTS: A total of 127 infants (19 SGA and 108 non-SGA) were included. The two groups were not significantly different concerning baseline characteristics, except for birthweight, length, and head circumference at birth. The proportion of moderate and severe HIE, TH initiation time, TH modality and other findings during TH were not different between the two groups. The SGA group achieved full bottle feeding at median 9 days compared to 8 days in the non-SGA group (p=0.015). Concerning EEG and brain MRI results, there was no significant difference in the percentage of abnormal EEG between the groups, while all SGA infants revealed abnormal MRI results compared to the non-SGA infants of whom 78 (72.2%) infants did (p=0.007). Specifically, hypoxic-ischemic lesions were found significantly more frequently in the SGA group [17 (89.5%) in the SGA group vs 69 (63.9%) in the non-SGA group (p=0.028)].
CONCLUSION: The SGA vs non-SGA infants who underwent TH did not differ in overall characteristics and clinical hospital course, except for growth parameters at birth and day achieving full bottle feeding. Among the brain evaluation results, the portion of abnormal MRI results, in particular hypoxic-ischemic lesions, was significantly greater in the SGA infants. How this result can be interpreted into future neurodevelopmental outcomes is yet to be studied.