Risk identification related to exposure time of medical device derived irritant chemicals on reconstructed human epidermis
Abstract
According to the DIN EN ISO 10993-1, assessing the potential of a substance to cause skin irritation represents an essential part of the biocompatibility test portfolio for all medical devices. Traditionally, the in vivo Draize skin test is used for skin irritation hazard assessment. However, in order to improve animal welfare, considerable efforts have been undertaken to develop and validate alternative in vitro test methods for the replacement of in vivo testing. In these assays, fully differentiated three-dimensional reconstructed epidermal tissue (RhE) grown from physiological human keratinocytes in a chemically defined medium at the air liquid interface is exposed to potential irritants for defined periods of time to assess certain changes caused by inflammatory processes (e.g., viability or interleukin 1α release). Unfortunately, time periods of exposure of RhE to irritants are not defined clearly. Thus, objective of the presented study was to examine RhE viability and IL-1α release after 15 min, 24 hrs, and 48 hrs of exposure to a certain irritant. After RhE exposure to the negative control and an extract of an exemplary biomaterial (polypropylene) for 15 min and 24 hrs, Il-1α release did not exceed the threshold value and RhE viability remained unchanged. However, after 48 hrs RhE viability was reduced by 54.2% and IL-1α concentration in the cell culture medium reached 10.1 ± 1.9 pg/ml. In contrast, growth of RhE exposed to the positive control medium for 15 min caused a reduction in RhE viability of about 27.1% and a high IL-1α release (23.8 ± 13.0 pg/ml). During the following 24 hrs of exposure to the positive control medium, RhE viability was reduced by 99%. IL-1α concentration in the cell culture medium remained unchanged for the whole testing interval (48 hrs), which was probably due to the significantly lowered cellular capacity for IL-1α internalization and metabolization of RhE cells. Based on these results we suggest that a time period of 24 hrs for irritation testing of extracts of medical devices using RhE might be advantageous.
1Introduction
A mandatory requirement in the assessment of biological safety of medical devices and their constituent materials is the analysis of their potential to produce dermal irritation. This is usually defined as the production of “reversible damage to the skin following the application of a test substance for up to 4 hours” as defined by the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) [1]. This reversible local inflammatory reaction is caused by the innate (non-specific) immune system of the skin. Irritant chemicals penetrate the stratum corneum and cause impairment and cell loss of the underlying cell layers. Currently, internationally accepted test methods for skin irritation testing include the traditional in vivo animal test (Draize rabbit test) as well as in vitro test methods. All accepted in vitro test methods are based on RhE technology (reconstructed human epidermis) validated by the European Union Reference Laboratory for alternatives to animal testing (ECVAM). RhE models use physiological human keratinocytes which form a multi-layered epidermis during culturing including a stratum corneum at the top, which functions as a barrier. In contrast to using laboratory animals or excised human skin, this method has a high reproducibility due to standardized materials and processes during manufacturing.
At present it is accepted that no single in vitro test method can fully replace the in vivo test for skin irritation. However, in vitro test methods can be used as partial replacement studies for a stepwise testing strategy or as a stand-alone replacement test depending on the outcome of the study.
According to the DIN EN ISO 10993-10 the chemicals are applied to the top surface of the skin for 15 ± 5 minutes, followed by a post-treatment incubation period of 42 ± 2 hrs and subsequent assessment of their effects on cell viability and cellular IL-1α release. A test substrate is considered as skin irritant if RhE viability is less than 50% and the amount of IL-1α release is higher than 9 IU/ml. According to the norm DIN EN ISO 10993-10, 15 min are usually used as the initial exposure time of a substrate to RhE. However, for medical products tested not directly in contact with RhE but employing sample extracts, the norm allows to use a different exposure time, since concentrations of irritants in the extracts are probably lower than in the products themselves. To corroborate an adaption of exposure time, objective of this study was to compare test results obtained from a standard cell culture medium (negative control), a cell culture medium supplemented with 1 vol% of a (nonionic) surfactant (Triton X-100, positive control), and an extract of an exemplary test material (polypropylene) after 15 minutes, 24 hours, and 48 hours of exposure to RhE.
2Materials and methods
2.1Test sample
A commercially available polypropylene foil (sokufol, Germany) served as exemplary test material. The foil was sterilized by autoclaving (20 min, 121°C) prior to testing.
2.2Extraction
The sample material was extracted with cell culture medium (DMEM supplemented with 10 vol% FBS and 1 vol% of a penicillin-streptomycin mixture [penicillin 10.000 U/ml, streptomycin 10.000 μg/ml], Biozyme Scientific GmbH, Germany) for 24 ± 2 hrs at 37 ± 1°C under permanent stirring. A ratio of 6 cm2/1 ml of material to extraction medium was used.
2.3Reconstructed human epidermis
Reconstructed human epidermis (RhE) was obtained commercially (Cellsystems, Germany). RhE maintenance and growth were performed according to the instructions provided by the manufacturer.
2.4Skin irritation testing
The test was conducted according to the Directives 93/42/EEC, 90/385/EEC and the international standard DIN EN ISO/IEC 17025 : 2005. On three different RhE replicates 0.1 ml of the sample extract or of the controls (positive control: cell culture medium supplemented with 1 vol% Triton X-100 [Carl Roth, Germany]; negative control: cell culture medium) were applied topically. Then, RhE was incubated for 15 min, 24 ± 2 hrs or 48 ± 2 hrs at 37 ± 1°C in a humidified atmosphere containing 5 vol% CO2. Subsequently, RhE was rinsed with 1×PBS three times and incubated in cell culture medium for another 24 ± 2 hrs to measure the release of the inflammation marker interleukine-1α (IL-1α). IL-1α concentrations were established using an ELISA based on photometric measurement (Thermo Scientific, Germany). After another 18 hrs of incubation, activity of the intracellular dehydrogenases was measured as a means to examine RhE viability. The dehydrogenase conversion of the vital dye MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Carl Roth, Germany) into a blue formazan salt can be measured quantitatively after extraction from tissues [2]. To establish viability, RhE was incubated for 3 hrs in MTT-supplemented RhE culture medium (1 mg/ml). Subsequently, formazan was extracted by incubation with 2 ml isopropanol for 2 hrs at 37 ± 1°C and quantified spectrophotometrically at 550 nm.
2.5Statistics
Data were reported as mean value±standard deviation for continuous variables and were analyzed by Student’s t-test (paired). A p value of less than 0.05 was considered significant.
3Results
3.1Cell viability (MTT reduction)
RhE exposed to the positive control for 15 min resulted in a reduction of RhE viability of about 27.1%. After 24 hrs, RhE viability was significantly lower (–99%, p < 0,001), but not significantly different to the viability level after 48 hrs (Fig. 1).
Fig.1
Cell viability (MTT reduction) after exposure of reconstructed human epidermis to cell culture medium (DMEM supplemented with 10 vol% FBS and 1 vol% of a penicillin-streptomycin mixture; negative control, NC), cell culture medium supplemented with 1 vol% Triton X-100 (positive control, PC), or an extract of an exemplary biomaterial (TE) for 15 min, 24 ± 2 hrs or 48 ± 2 hrs at 37 ± 1°C in a humidified atmosphere containing 5 vol% CO2; means and standard abbreviation, n = 3.
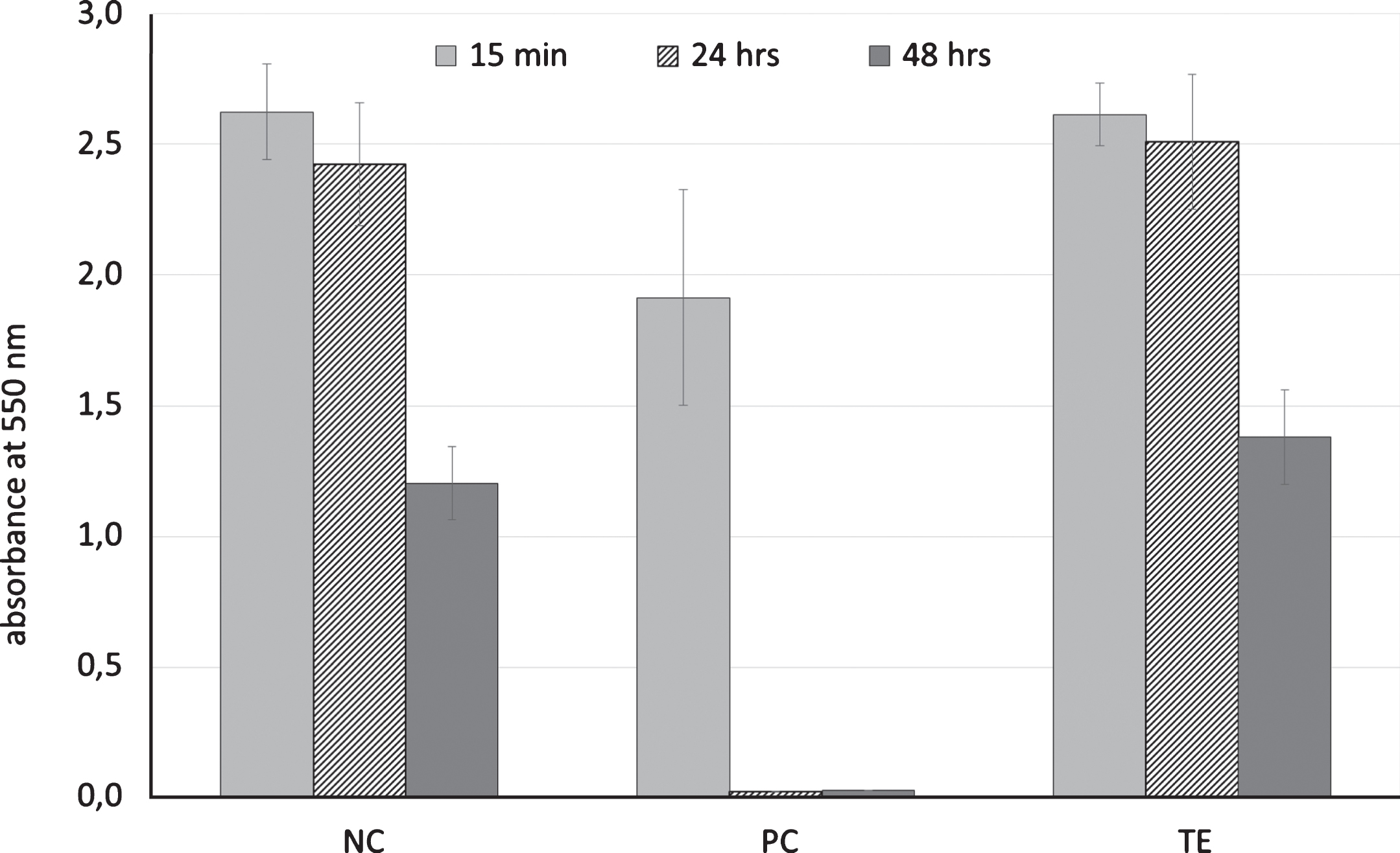
The negative control and the extract of the exemplary biomaterial showed similar results. After 15 min and 24 hrs of exposure to the RhE, viability remained unchanged After 48 hrs RhE viability was reduced by 54.2%.
3.2IL-1α-ELISA
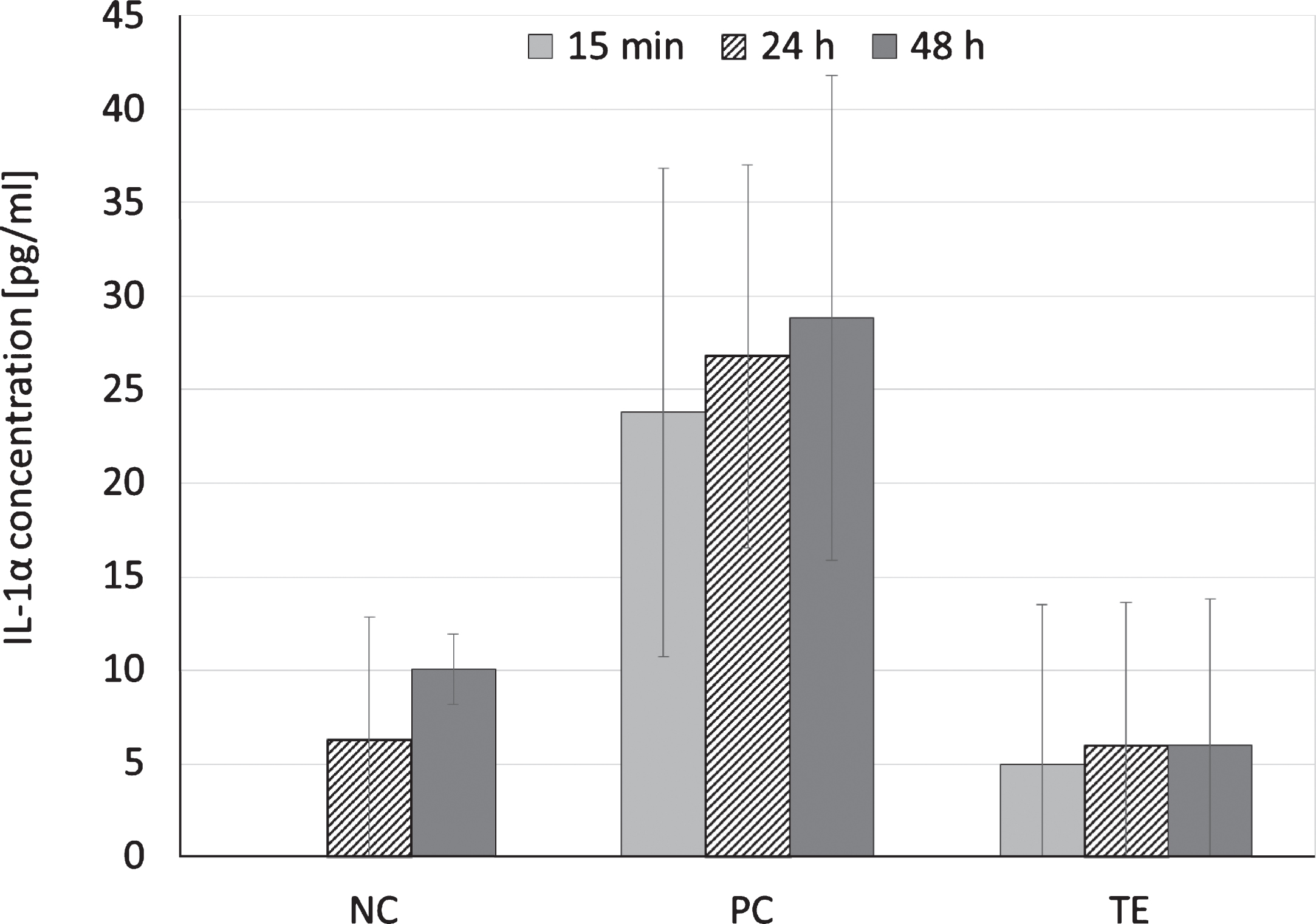
Positive control exposure to RhE resulted in an IL-1α release of 23.8 ± 13.0 pg/ml within the first 15 min and did not change for the following 48 hrs. When exposing RhE to the negative control, IL-1α release was below the analytical detection limit for the first 24 hrs. However, after 48 hrs IL-1α concentration in the cell culture medium reached 10.1 ± 1.9 pg/ml. Incubation of RhE with the extract of the test sample or the negative control resulted in similar results for 24 and 48 hrs of exposure (p > 0.05, Fig. 2).
Fig.2
IL-1α release after exposure of reconstructed human epidermis to cell culture medium (DMEM supplemented with 10 vol% FBS and 1 vol% of a penicillin-streptomycin mixture; negative control, NC), cell culture medium supplemented with 1 vol% Triton X-100 (positive control, PC), or an extract of an exemplary biomaterial (TE) for 15 min, 24 ± 2 hrs or 48 ± 2 hrs at 37 ± 1°C in a humidified atmosphere containing 5 vol% CO2; means and standard abbreviation, n = 3.
4Discussion
The availability and reproducibility of cultured skin models to establish validated biological assays has started to replace tests previously performed on living animals [3]. A rapidly increasing number of these assays have been established in order to adhere to the concept of the 3 “R”s and its realization in developed countries. RhE displays very typical patterns in terms of viability and release of interleukins 1α and 8, which can be used to differentiate a surfactant with sensitizing potential from other detergents with weak, mild, or strong irritation potential [4, 5].
According to EU classification R38, skin irritants are defined as substances and formulations causing significant inflammation of the skin. This persists for at least 24 hours after an exposure period of up to four hours established on rabbits according to the cutaneous irritation test method cited in Annex V of the Directive 67/548/EEC. An R38 class is predicted if the mean relative tissue viability is lower than 50% of the negative control.
In contrast to the 24 hours exposure time of the rabbit skin to the potential irritants in vivo, the exposure time of RhE in vitro is recommended to last at least 15 min. The time period of irritant exposure to RhE can be extended if extracts of medical devices are tested, since the concentration of irritants in extracts might be low (DIN EN ISO 10993-10).
This study showed a distinct effect of exposure time for risk identification of irritant chemicals on RhE. Both positive and negative controls confirmed the accuracy of the RhE viability test after 24 hrs and 48 hrs of RhE exposure to the substrates. However, growth of RhE with Triton X-100 supplemented cell culture medium (positive control) for 15 min resulted in a reduction of RhE cell viability by 27.1%. According to the DIN EN ISO 10993-10 and the Directive 67/548/EEC a substrate can be regarded as skin irritant (R38 class) if it causes a reduction of a mean relative tissue viability below 50% of the negative control, which applied only to the 24 and 48 hour-measurement.
RhE growth with the irritant free cell culture medium (negative control) and the extract of the exemplary biomaterial showed similar results. Both substrates had no toxic/irritant effect to RhE within the first 24 hrs, but caused a reduction of RhE viability by 50.4% after 48 hrs. This decrease in viability after two days is probably caused mainly by depletion of nutrients and an increase in metabolic products within the cell culture medium after 48 hrs. An increase in metabolic products can be toxic to the cells [6].
Measuring IL-1α release after 15 min and 24 hrs growth of RhE with the irritant free cell culture medium showed a maximum release of 6.3 pg/ml. As expected, RhE growth with the positive control caused a higher IL-1α release within 15 min, whereas IL-1α concentrations in the cell culture medium did not significantly change during the entire testing period of 48 hrs. The half life time of interleukins in vivo is very short, i.e. a few seconds, but in vitro it appeared to be prolonged. This is probably due to a low level RhE viability after 24 hrs of positive control exposure. Interleukins are polypeptid cytokines, which are synthesized as large 31-kDa precursor molecules and subsequently processed to their 17-kDa mature structure [7, 8]. Following binding to the IL-1 cell surface receptor, interleukins are internalized and accumulate within the cell [9–18]. In our study, RhE growth with the positive control resulted in a very low RhE viability (<99%) with only a few cells being able to internalize the bonded IL-1α and thus to reduce the extracellular IL-1α concentration.
5Conclusion
The study showed that RhE could be maintained up to 24 hrs with high viability. Surfactant (positive control) exposure resulted in a significant reduction of RhE viability only after 24 hrs. In addition, concentrations of the released IL-1α, which was increased after 15 min of RhE exposure to the positive control, remained unchanged during the following 48 hrs. This is probably caused by the limited capacity of RhE cells to internalize the receptor bonded Il-1α. Based on these results, an exposure time of 24 hrs may be suited best for in vitro testing of skin irritants.
References
[1] | Globally Harmonized System of Classification and Labelling of Chemicals (GHS), sixth revised edition, United Nations, (2015) . |
[2] | Mosman T . Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods (1983) ;65: :55–63. |
[3] | Poumay Y , Coquette A . Modelling the human epidermis in vitro: Tools for basic and applied research. Arch Dermatol Res (2007) ;298: (8):361–9. |
[4] | Coquette A , Berna N , Rosdy M , Vandenbosch A , Poumay Y . Differential expression and release of cytokines by an in vitro reconstructed human epidermis following exposure to skin irritant and sensitising chemicals. Toxicol In Vitro (1999) ;13: :867–77. |
[5] | Poumay Y , Dupont F , Marcoux S , Leclercq-Smekens M , Hérin M , Coquette A . A simple reconstructed human epidermis: Preparation of the culture model and utilization in in vitro studies. Arch Dermatol Res (2004) ;296: :203–11. |
[6] | John R , Masters J , Stacey GN . Changing medium and passaging cell lines. Nature Protocols (2007) ;2: :2276–84. |
[7] | Giri JG , Lomedico PT , Mizel SB . Studies on the synthesis and secretion of interleukin 1.I. A 33,000 molecular weight precursor for interleukin 1. J Immunol (1985) ;134: :343–9. |
[8] | Limjuco G , Galuska S , Chin J , Cameron P , Boger J , Schmidt JA . Antibodies of predetermined specificity to the major charged species of human interleukin 1. PNAS (1986) ;83: :3972–6. |
[9] | Grenfell S , Smithers N , Miller K , Solari R . Receptor-mediated endocytosis and nuclear transport of human interleukin 1 α. Biochem J (1989) ;264: :813–22. |
[10] | Grenfell S , Smithers N , Witham S , Shaw A , Graber P , Solari R . Analysis of mutations in the putative nuclear localization sequence of interleukin-1 beta. Biochem J (1991) ;20: :111–6. |
[11] | Grenfell S , Smithers N , Solari R . Acute upregulation of interleukin-1 receptor by ligand. Cytokine (1991) ;4: :114–24. |
[12] | Lowenthal JW , MacDonald HR . Binding and internalisation of interleukin-1 by T cells. J Exp Med (1986) ;164: :1060–74. |
[13] | Mizel SB , Kilian PL , Lewis JC , Paganelli KA , Chizzonite RA . The interleukin 1 receptor. Dynamics of interleukin 1 binding and internalization in T cells and fibroblasts. J Immunol (1987) ;138: :2906–12. |
[14] | Bird TA , Saklatvala J . Studies on the fate of receptor-bound 125I-interleukin 1 beta in porcine synovial fibroblasts. J lmmunol (1987) ;139: :92–7. |
[15] | Quarnstrom EE , Page RC , Gillis S , Dower SK . Binding, internalisation and intracellular localization of interleukin-lfl in human diploid fibroblasts. J Biol Chem (1988) ;263: :8261–69. |
[16] | Von Hoegen I , Falk W , Kojouharoff G , Krammer PH . Internalisation of interleukin 1 (ILl) correlates with ILl induced IL2 receptor expression and IL2 secretion of EL4 thymoma cells. Eur J Immunol (1989) ;19: :329–34. |
[17] | Curtis BM , Widmer MB , DeRoos P , Qwarnstrom EE . IL-1 and its receptor are translocated to the nucleus. J lmmunol (1990) ;144: :1295–1303. |
[18] | Weitzmann MN , Savage N . Nuclear internalization and DNA binding activities of interleukin-1, interleukin-1 receptor and interleukin-1/receptor complexes. Biochem Biphys Res Commun (1992) ;187: :1166–71. |




