The influence of gait training combined with portable functional electrical stimulation on motor function, balance and gait ability in stroke patients
Abstract
BACKGROUND:
Problems with motor functions, balance and gait ability commonly occur in stroke patients and cause asymmetric posture imbalance and gait patterns.
OBJECTIVE:
We examined the effects of gait training (GT) combined with portable functional electrical stimulation (FES) on motor functions, balance and gait ability of stroke patients.
METHODS:
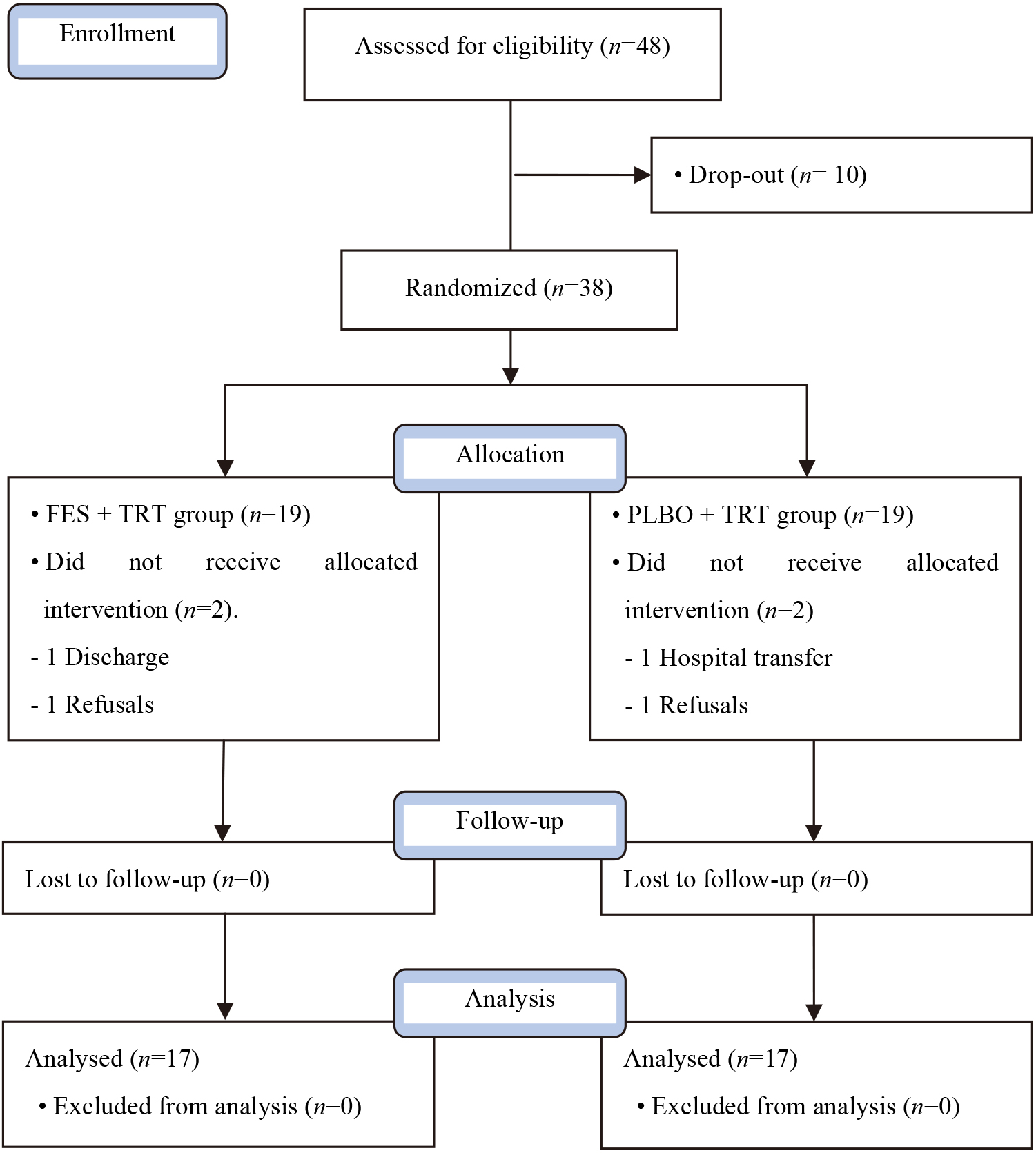
A single blind, randomized control trial was conducted with 34 post stroke patients who were randomly allocated to two groups: 1) FES
RESULTS:
Both groups showed significant improvements in motor function, balance and gait ability. The FES
CONCLUSION:
It was found that the gait training applied with FES is effective in improving the motor function, balance and gait abilities of stroke patients.
1.Introduction
Stroke patients commonly experience various complications such as hemiplegia, sensory impairment, damages to motor functions and muscle strength problems in accordance with the section and degree of the damage [1]. Muscle strength imbalance occurs when there are problems in balance ability while in the standing position to describe asymmetric gait patterns [2].
Several studies relating to gait training (GT) have demonstrated the results of improved lower extremity function in post-stroke patients [3]. Gait is considered the most important factor determining functional independence in daily life activities [4]. Treadmill training, which is widely used as part of a gait training, can help with loading by controlling the patient’s weight, and the following effects are expected: improvement of muscle strength, re-awareness of balance, and gait improvement [5].
Functional electrical stimulation (FES) is used in various rehabilitation fields and applied to GT, reduction of edema [6], improvement and maintenance of the range of motion [5], prevention of muscular atrophy [7], and improvement of ankle dorsiflexor strength [8]. However, a disadvantage of FES is that it does not involve the active will of the patient, and it is simply repetitive and considered less effective in relearning motor functions, which is a crucial factor for recovery in stroke patients. Therapists conducted GT in stroke patients by artificially inducing FES [9].
Foot drop is so frequent that it affects nearly 20% of stroke patients [10]. Foot drop makes it difficult for the heel to touch the ground when walking and causes the foot to be dragged, leading to abnormal gait patterns such as swinging pattern gait and hip flexion gait [11]. Such abnormal gait not only decreases the quality of life by reducing the gait/walking speed but also causes several other problems, such as exposure to the risk of falling [12].
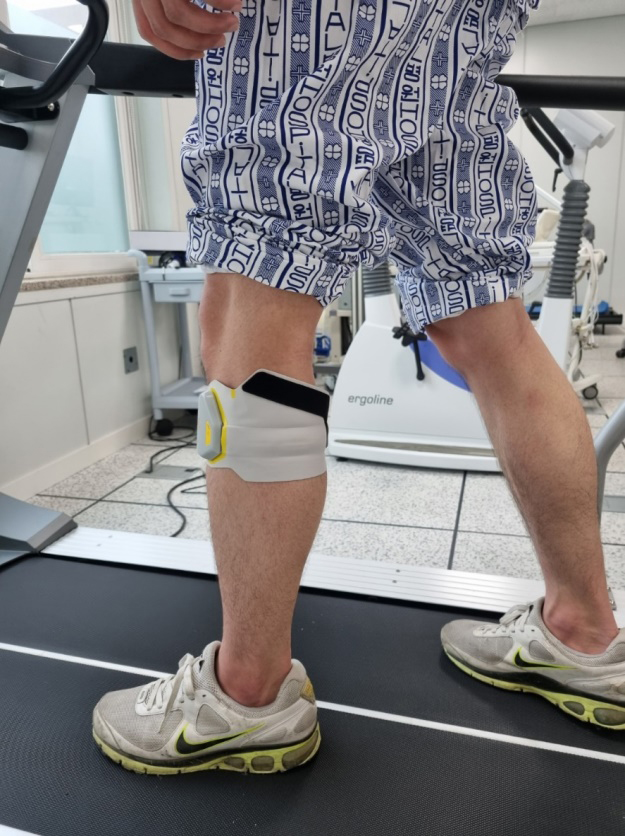
“Walkami,” a FES treatment equipment, is a gait assisting device that supports dorsal flexion while walking by inducing electrical stimulation for ankle movement. The FES generally has less contact area with the skin; it does not restrict ankle movement and hence does not hamper the gait of a person, facilitating effective contraction of the muscles [13]. According to a previous study, when FES is applied to the dorsiflexor and hamstring, it can be more effective in controlling the lower extremities than when applied to only the dorsiflexor [14].
Recent study have found the treadmill exercise as an effective intervention method to improve balance and gait in various cases. Reportedly, after conducting treadmill exercise for six weeks, significant effects could be seen on gait and balance [15, 16]. In et al. reported that treadmill GT with thera-band effectively improves on motor functions of lower limb, balance and gait in post-stoke patients [17]. Bao et al. reported that body weight supported treadmill plus FES could significantly improve lower extremity function, balance, spasticity and gait in post-stroke [18]. Hakakzadeh et al. argued that bilateral multi joint FES and treadmill exercise showed statistically significant difference in spasticity and gait in poststroke [19]. Furthermore, Ray et al. indicated that combined treadmill control with FES showed a statistically significant increase in gait speed in post-stroke patients [20]. As noted above, it is important to find a treatment method combining treadmill GT and FES to improve motor functions, balance and gait of stroke patients. Therefore, this study aimed to examine the effect of GT
2.Method
2.1Research design
The present study was conducted with the randomized clinical trial pretest-posttest design. By using the single-blind test, a therapist with over 5 years of clinical experience and no knowledge of the groups, conducted the evaluation, analysis and each training. The basis of calculation for the number of subjects was calculated by conducting the statistical evaluation with G*power Version 3.1.9.4. The effect size was calculated based on pilot study of 6 stroke patients. Effects size was 1.01,
2.2Participants
The present study conducted experiments on both female and male stroke patients hospitalized at B hospital in Gyeonggi-do and the details regarding the selection of patients are as follows. The inclusion criteria as follows: (1) first episode of unilateral stroke with hemiplegia caused by brain damage, (2) above 21 points from the Mini Mental State Korea (MMSE-K), (3) enabled to communicate, (4) had no allergic reactions to FES and had no gait problems with ankle joint contracture. The exclusion criteria as follows: (1) cerebellum-related diseases, (2) sight and hearing impairments, (3) cardiovascular system problems. This study was approved by the judging committee at Gimcheon University (GU-201805-HRa-03-02-P) and each subject signed the informed consent form.
Figure 1.
Flow diagram of this study.
2.3Experimental procedure
The motor function balance and gait abilities of patients before the experiment and four weeks afterward were evaluated. The selected individuals participated in the study after receiving detailed explanations of the study procedure. Using a lottery method, those who picked odd numbers were set as the FES
2.4Intervention
In this study, GT refers to treadmill GT. The GT
Figure 2.
FES equipment.

Figure 3.
Task-related training combined with portable functional electrical stimulation.
The PLBO
2.5Outcome measures
Fugl-Meyer assessment (FMA) was performed to evaluate the motor functions of the lower extremities. It is composed of 17 items and the score range is 0–34. The intra-rater reliability for stroke patients was
2.6Statistical analysis
The overall statistical analysis of this study was conducted by using the SPSS 21.0. The test of normality for variables was conducted through the Shapiro-Wilk test. For the comparison of general characteristics of subjects and the pre-homogeneity of two groups, independent
Table 1
General characteristics of subjects
| Variable | FES | PLBO | |
| Gender | |||
| Male | 7 | 8 | 0.730 |
| Female | 10 | 9 | |
| Height (cm) | 166.07 (4.07) | 163.18 (5.79) | 0.276 |
| Weight (kg) | 67.38 (6.51) | 64.46 (8.67) | 0.831 |
| Age (year) | 52.24 (11.39) | 53.24 (7.04) | 0.761 |
| Stroke type | |||
| Infarction | 7 | 10 | 0.303 |
| Hemorrhage | 10 | 7 | |
| Affected side | |||
| Left | 6 | 9 | 0.300 |
| Right | 11 | 8 | |
| MMSE-K (score) | 27.00 (0.87) | 27.35 (0.86) | 0.242 |
| Post-stroke duration (month) | 9.24 (2.02) | 9.00 (1.54) | 0.705 |
Table 2
Chances of the FMA
| Parameter | FES | PLBO | Between groups | ||||
|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | Change | Pre-test | Post-test | Change | ||
| FMA (score) | 21.12 (2.12) | 25.53 (1.62) | 4.41 (1.33) | 21.53 (2.15) | 22.76 (1.99) | 1.24 (0.75) | |
Table 3
Chances of the POMA
| Parameter (score) | FES | PLBO | Between groups | ||||
|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | Change | Pre-test | Post-test | Change | ||
| Balance | 14.06 (0.83) | 14.42 (0.71) | 1.35 (0.49) | 14.06 (0.90) | 14.82 (0.73) | 0.76 (0.46) | 0.003 |
| Gait | 8.06 (0.75) | 9.35 (0.61) | 1.29 (0.45) | 8.12 (0.60) | 9.00 (0.79) | 0.88 (0.60) | 0.033 |
| Total | 22.12 (1.17) | 24.76 (0.97) | 2.65 (0.61) | 22.17 (1.13) | 23.82 (0.73) | 1.65 (0.70) | |
3.Results
The general characteristics of the 34 stroke patients are show in Table 1. In the FMA score test was more significantly increased in the FES
Table 4
Chances of the gait ability
| Parameter | FES | PLBO | Between groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | Change | Pre-test | Post-test | Change | ||||||||
| SL (cm) | 64.91 | (7.35) | 68.99 | (6.61) | 4.08 | (3.17) | 65.34 | (8.23) | 67.21 | (7.64) | 1.87 | (1.72) | 0.017 |
| GC (step/sec) | 2.38 | (0.45) | 1.91 | (0.35) | (0.26) | 2.32 | (0.36) | 2.14 | (0.35) | 0.18 | (0.21) | 0.001 | |
| Cadence (step/sec) | 0.53 | (0.10) | 0.60 | (0.08) | 0.07 | (0.08) | 0.54 | (0.07) | 0.56 | (0.07) | 0.02 | (0.03) | 0.038 |
| TDS (%) | 34.55 | (3.51) | 31.86 | (3.09) | 2.69 | (1.06) | 33.44 | (2.42) | 31.75 | (2.66) | 1.68 | (1.09) | 0.011 |
| ASL (cm) | 37.38 | (6.30) | 41.84 | (5337) | 4.46 | (3.02) | 37.90 | (6.26) | 39.99 | (6.07) | 2.09 | (1.11) | 0.007 |
| ASS (%) | 33.91 | (2.76) | 35.81 | (2.81) | 1.90 | (1.64) | 35.36 | (1.71) | 36.25 | (2.14) | 0.89 | (1.14) | 0.046 |
| ASp (m/s) | 0.59 | (0.18) | 0.71 | (0.18) | 0.11 | (0.07) | 0.61 | (0.16) | 0.66 | (0.15) | 0.05 | (0.04) | 0.008 |
| AST (m/s) | 1.27 | (0.31) | 0.97 | (0.18) | (0.22) | 1.26 | (0.29) | 1.10 | (0.26) | 0.16 | (0.15) | 0.034 | |
| AS (m/s) | 0.60 | (0.23) | 0.74 | (0.21) | 0.13 | (0.10) | 0.65 | (0.22) | 0.70 | (0.23) | 0.04 | (0.06) | 0.004 |
During the OptoGait assessment, stride length (SL) and gait cycle (GC) significantly increased in the FES
4.Discussion
In generally, the ultimate rehabilitation goal for stroke patients is enable them to execute daily activities independently. This study looked at the influence of a four-week therapy session with portable FES combined with GT on motor functions, balance and gait in stroke patients. The study was conducted every day for 30 minutes, five times a week, for four weeks. The purpose was to confirm the usefulness of treadmill GT with Walkami.
The FES treatment is used as an accessory for GT and improving conditions for gait disabilities, strengthening affected muscles in patients recovering from a stroke, and treating injuries to the central nerves such as spinal cord injuries [28]. The effects of FES application to the tibialis anterior muscle show considerable improvements in Fugl-Meyer score, gait speed, stride length, security, reduction of physiological energy consumption efficiency, and stiffness in plantar flexion [8, 24]. Lindquist et al. state that FES revitalizes the tibialis anterior muscles on the affected side, leading to increased muscular contraction, accelerated dorsal flexion, and movement relearning [23].
Thus, this study helped provide GT to participants through treadmill GT for electrical stimulation of the tibialis anterior muscle that causes dorsal flexion of the ankle, which is important in improving motor functions, balance, and gait in recovering stroke patients based on prior research.
To examine the influence that FES and treadmill training have on motor functions, the FMA changes were studied. In our study, the FES
The present study examined the changes in balance and gait ability to examine the influence of FES
In this study, In this study, 4 weeks of intervention were conducted. In a previous study, mirror therapy training combined with functional electrical stimulation was performed 5 times a week for 4 weeks. This study identified an effective intervention method for motor function, balance, and gait. Combined training of gait and FES stimulation led to significant improvements in balance and gait [33]. Such improvement could be attributed to the induction of nerve and muscle reorganization by providing additional afferent stimulation and repetitive gait training [34]. Therefore, this study also found that the electrical stimulation of tibialis anterior muscle is considered to have influenced the posture control of stroke patients as the muscle recovery of tibialis anterior muscle moved the weight to the affected side to act as the stabilizer of the ankle joint and induced appropriate alignment of the affected lower extremities. As for the improvement of gait, the occurrence of toe drag was reduced due to the FES of Walkami, which shortened the offering period of the affected lower limbs [35]. The gait speed is considered to have increased as the movements of the lower extremities accelerated through the muscle recovery of the tibialis anterior muscle [29].
Furthermore, Walkami improved the weight-bearing ability by allowing users to efferently control the affected ankle during the stance phase in the affected leg by assisting dorsal flexion and reducing the time of stance phase by moving to one-foot support from two feet support [36]. Furthermore, it helped by lengthening the swing phase time to create a more relaxed forward stepping and considerably improving the gait. The FES led to the contraction of the tibialis anterior muscle from the terminal stance of the affected side, thereby improving insufficient dorsal flexion and preventing toe drag. Moreover, the application of FES during the dorsal flexion of the affected side increased the joint movement range of the knee and hip joints during the swing phase, thereby improving gait [37].
There are a few limitations to this study. The number of participants was insufficient, and a tracer study was not conducted to confirm whether the effects were lasting. Tracer studies can help identify the long-term effects of the training and biomechanical elements such as energy consumption efficiency, joint angle, muscle activity, and muscle exhaustion.
5.Conclusion
The present study exhibited that treadmill GT combined with portable FES showed significantly improved results in the motor functions, balance and gait ability of the lower extremities when compared to the treadmill gait training combined with placebo FES. Moreover, it was found that the treadmill training applied with FES on the tibialis anterior muscle and the common peroneal nerve was effective in improving the motor functions, balance and gait abilities of stroke patients. Therefore, it can be considered as an element that can increase the recovery abilities when planning programs for enhancing functions of stroke patients.
Acknowledgments
This work was supported by a Gimcheon University Research Grant.
Conflict of interest
The authors declare that there were no conflicts of interest.
References
[1] | Mercier L, Audet T, Hébert R, Rochette A, Dubois MF. Impact of motor, cognitive, and perceptual disorders on ability to perform activities of daily living after stroke. Stroke. (2001) ; 32: (11): 2602-8. |
[2] | Ikai T, Kamikubo T, Takehara I, Nishi M, Miyano S. Dynamic postural control in patients with hemiparesis. Am J Phys Med Rehabil. (2003) ; 82: (6): 463-9. |
[3] | Ng SS, Hui-Chan CW. Transcutaneous electrical nerve stimulation combined with gait training improves lower limb functions in subjects with chronic stroke. Stroke. (2007) ; 38: (11): 2953-9. |
[4] | Shankaranarayana AM, Gururaj S, Natarajan M, Balasubramanian CK, Solomon JM. Gait training interventions for patients with stroke in India: A systematic review. Gait Posture. 83: : 132-40. |
[5] | Dobkin BH. Short-distance walking speed and timed walking distance: Redundant measures for clinical trials. Neurology. (2006) ; 66: (4): 584-586. |
[6] | Burgess LC, Immins T, Swain I, Wainwright TW. Effectiveness of neuromuscular electrical stimulation for reducing oedema: A systematic review. J Rehabil Med. (2019) ; 51: (4): 237-43. |
[7] | Dirks ML, Wall BT, Snijders T, Ottenbros CL, Verdijk LB, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. (2014) ; 210: (3): 628-41. |
[8] | Sabut SK, Sikdar C, Kumar R, Mahadevappa M. Functional electrical stimulation of dorsiflexor muscle: Effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation. (2011) ; 29: (4): 393-400. |
[9] | Costantino C, Pedrini M, Petraglia F, Pedrazzi G. Effectiveness of single functional electrical stimulation in neurological patients with ankle-foot orthoses. J Nov Physiother. (2016) ; 6: (280): 2. |
[10] | Laufer Y, Hausdorff JM, Ring H. Effects of a foot drop neuroprosthesis on functional abilities, social participation, and gait velocity. Am J Phys Med Rehabil. (2009) ; 88: (1): 14-20. |
[11] | Simonsen EB, Moesby LM, Hansen LD, Comins J, Alkjaer T. Redistribution of joint moments during walking in patients with drop-foot. Clin Biomech. (2010) ; 25: (9): 949-52. |
[12] | Dunning K, O’Dell MW, Kluding P, McBride K. Peroneal stimulation for foot drop after stroke: A systematic review. Am J Phys Med Rehabil. (2015) ; 94: (8): 649-64. |
[13] | Kesar TM, Perumal R, Reisman DS, Jancosko A, Rudolph KS, Higginson JS, et al. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: Effects on poststroke gait. Stroke. (2009) ; 40: (12): 3821-7. |
[14] | Springer S, Vatine JJ, Lipson R, Wolf A, Laufer Y. Effects of dual-channel functional electrical stimulation on gait performance in patients with hemiparesis. ScientificWorldJournal. (2012) ; 2012: : 530906. |
[15] | Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: Results of the STEPS randomized clinical trial. Phys Ther. (2007) ; 87: (12): 1580-602. |
[16] | Yang S, Hwang WH, Tsai YC, Liu FK, Hsieh LF, Chern JS. Improving balance skills in patients who had stroke through virtual reality treadmill training. Am J Phys Med Rehabil. (2011) ; 90: (12): 969-78. |
[17] | In T, Jin Y, Jung K, Cho HY. Treadmill training with Thera-Band improves motor function, gait and balance in stroke patients. NeuroRehabilitation. (2017) ; 40: (1): 109-14. |
[18] | Bao X, Luo JN, Shao YC, Tang ZQ, Liu HY, Liu H, Tan JW. Effect of functional electrical stimulation plus body weight-supported treadmill training for gait rehabilitation in patients with poststroke: A retrospective case-matched study. Eur J Phys Rehabil Med. (2020) ; 56: (1): 34-40. |
[19] | Hakakzadeh A, Shariat A, Honarpishe R, Moradi V, Ghannadi S, Sangelaji B, et al. Concurrent impact of bilateral multiple joint functional electrical stimulation and treadmill walking on gait and spasticity in post-stroke survivors: A pilot study. Physiother Theory Pract. (2019) ; 30: : 1-9. |
[20] | Ray NT, Reisman DS, Higginson JS. Combined user-driven treadmill control and functional electrical stimulation increases walking speeds poststroke. J Biomech. (2021) ; 124: : 110480. |
[21] | Kim KJ, Kim KH. Progressive treadmill cognitive dual-task gait training on the gait ability in patients with chronic stroke. J Exerc Rehabil. (2018) ; 14: (5): 821-8. |
[22] | Kim KH, Lee KB, Bae Y-H, Fong SS, Lee SM. Effects of progressive backward body weight suppoted treadmill training on gait ability in chronic stroke patients: A randomized controlled trial. Technol Health Care. (2017) ; 25: (5): 867-76. |
[23] | Lindquist AR, Prado CL, Barros RM, Mattioli R, Da Costa PHL, Salvini TF. Gait training combining partial body-weight support, a treadmill, and functional electrical stimulation: Effects on poststroke gait. Phys Ther. (2007) ; 87: (9): 1144-54. |
[24] | Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, et al. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. (2011) ; 33: (2): 309-13. |
[25] | Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. (1993) ; 73: (7): 447-54. |
[26] | Canbek J, Fulk G, Nof L, Echternach J. Test-retest reliability and construct validity of the tinetti performance-oriented mobility assessment in people with stroke. J Neurol Phys Ther. (2013) ; 37: (1): 14-9. |
[27] | Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. (2005) ; 37: (2): 75-82. |
[28] | Kralj A, Bajd T, Turk R, Benko H, eds. Results of FES application to 71 SCI patients. Proceedings of the RESNA 10th Annual conference; (1987) : Resna Press Washington (DC). |
[29] | Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. (2010) ; 32: (19): 1594-603. |
[30] | Sharif F, Ghulam S, Malik AN, Saeed Q. Effectiveness of Functional Electrical Stimulation (FES) versus Conventional Electrical Stimulation in Gait Rehabilitation of Patients with Stroke. J Coll Physicians Surg Pak. (2017) ; 27: (11): 703-6. |
[31] | Tong RK, Ng MF, Li LS. Effectiveness of gait training using an electromechanical gait trainer, with and without functional electric stimulation, in subacute stroke: A randomized controlled trial. Arch Phys Med Rehabil. (2006) ; 87: (10): 1298-304. |
[32] | Sheffler LR, Taylor PN, Bailey SN, Gunzler DD, Buurke JH, IJzerman MJ, et al. Surface peroneal nerve stimulation in lower limb hemiparesis: Effect on quantitative gait parameters. Am J Phys Med Rehabil. (2015) ; 94: (5): 341-57. |
[33] | Lee DG, Lee GH. Effect of afferent electrical stimulation with mirror therapy on motor function, balance, and gait in chronic stroke survivors: A randomized controlled trial. Eur J Phys Rehbil Med. (2019) ; 55: (4): 442-9. |
[34] | Qian JG, Rong K, Qian Z, Wen C, Zhang S. Effects of a multichannel dynamic functional electrical stimulation system on hemiplegic gait and muscle forces. J Phys Ther Sci. (2015) ; 27: (11): 3541-4. |
[35] | Park J, Lee D, Oh D. Immediate effect of foot drop stimulator in outpatients with chronic stroke: A mixed method study. J Int Acad Phys Ther Res. (2020) ; 11: (1): 1992-8. |
[36] | Park JS, Lee SH, Yoo WG, Chang MY. Immediate effect of a wearable foot drop stimulator to prevent foot drop on the gait ability of patients with hemiplegia after stroke. Assist Technol. (2019) ; 16: : 1-5. |
[37] | Kim JH, Chung Y, Kim Y, Hwang S. Functional electrical stimulation applied to gluteus medius and tibialis anterior corresponding gait cycle for stroke. Gait Posture. (2012) ; 36: (1): 65-7. |




