Does paravertebral ozone injection have efficacy as an additional treatment for acute lumbar disc herniation? A randomized, double-blind, placebo-controlled study
Abstract
OBJECTIVE:
In this study we investigate the effects of paravertebral ozone injections (POI), which have been used as a new treatment approach for lower back pain in recent years, on pain and physical activity in patients with acute lumbar disc herniation (LDH) as an additional treatment.
METHODS:
Thirty-eight patients were assigned into the ozone therapy (OT) group (
RESULTS:
A significant improvement was seen in the VAS and ODI scores in the final follow-up (V4) as compared with the baselines scores (V1) in both groups (
CONCLUSION:
As an additional treatment combined with conservative treatment, lumbar POI can lessen pain and disability in patients with acute LDH.
1.Introduction
Acute low back pain (LBP) is an important cause of disability and may frequently occur at any stage of life, disrupting activities of daily living [1]. It is reported that lumbar disc disruption is present in approximately 73% of acute LBP cases, and approximately half of these are associated with lumbar disc herniation (LDH) [2, 3, 4]. In acute LDH, conservative (medication, physical therapy [PT], and exercises) and minimally invasive (epidural steroid injection [ESI]), nonsurgical treatments yield satisfactory results [2, 5, 6]. However, certain patients may not respond to these treatments or experience relapse [6]. Sometimes, the use of these treatments may not be possible because of side effects or contraindications [2, 5]. Short-term use of non-steroidal anti-inflammatory drugs and analgesics is recommended because of the risk of side effects [7]. Although PT modalities (ultrasound, laser, traction, TENS, and thermotherapy) are commonly used in clinical practice, there is no consensus in the literature on their effectiveness [8]. Epidural steroid injections (ESI) are known to be effective in short-term pain relief in acute LDH [9]. However, the use of ESI may be limited because of potential side effects and contraindications associated with the drugs used (corticosteroid, local anesthetic, and contrast agent) or injection [10].
Although the success rate of surgical treatment is high, it is only recommended to patients who have progressive neurological deficits or are unresponsive to conservative treatments due to probability of relapse and complications. Therefore, it has been suggested that minimally invasive, well-tolerated, and low-cost ozone therapy (OT) can be used as a complementary treatment for patients with LDH who do not respond to conservative treatments before resorting to surgery or when surgery is not possible [2, 11, 12, 13, 14]. OT can be administered through intradiscal, epidural/intraforaminal, and paravertebral intramuscular injections. Intradiscal or epidural ozone injections can be performed under sedation and with the help of radiological imaging (fluoroscopy and computed tomography) [15]. On the contrary, paravertebral ozone injections (POI) are a relatively easy and less invasive technique that can be performed in outpatient clinic conditions without the need for sedation and radiological imaging and may therefore be preferred [2, 16, 17]. However, to the best of our knowledge, no study comparing these methods in terms of efficacy was found in the existing literature.
Ozone is an unstable form of oxygen, and free radicals formed upon contact with body tissues cause light and transient oxidative stress, thereby stimulating the upregulation of the antioxidant system, the modulation of the immune system and the suppression of inflammatory processes. Thus, it is used as a complementary modality in the treatment of many degenerative, inflammatory, vascular, or infectious diseases [12, 18]. By injecting ozone directly into paravertebral muscles intradiscally or indirectly in LDH, it has been reported that it can reduce the disc volume and have anti-inflammatory and analgesic effects [19, 20, 21, 22]. Although ozone injections in many European and Asian countries are used with increasing popularity in LDH, there are few studies on their efficacy, many of which are based on the results of intradiscal and intraforaminal ozone injections [13, 15, 20, 23]. There are no placebo-controlled studies; however, there are a few studies with active control groups among a small number of studies using POI [2, 5, 7, 24]. Therefore, the authors believe that this randomized controlled trial (RCT) conducted to determine the efficacy of POI as an additional treatment in decreasing the pain and disabilities in patients with acute LDH will have significant contributions to the literature.
2.Materials and methods
2.1Study design
A randomized controlled study design was employed and reported in accordance with the CONSORT principles. The study protocol was approved by the Clinical Research Ethics Committee of Istanbul Gelisim University (meeting date: November 18, 2019; Decision No. 20–22). This study was conducted in accordance with the rules of the Declaration of Helsinki, and all subjects provided written informed consent prior to participation.
2.2Participants
This study included patients who were 18 to 60 years old, had LBP (radicular or non-radicular pain) (with a VAS score of 5 and above) complaints for the first time and for 4 weeks and less, and were diagnosed with acute LDH. The diagnosis of LDH was made in the presence of clinical findings (manual muscle testing, positive supine straight leg raise test, dermatomal pain, sensory deficits, and reflex deficits) consistent with protrusion or extrusion image at the L3-L4, L4-L5 or L5-S1 levels reported by the radiologist in lumbar magnetic resonance imaging (MRI).
Figure 1.
Study flowchart.
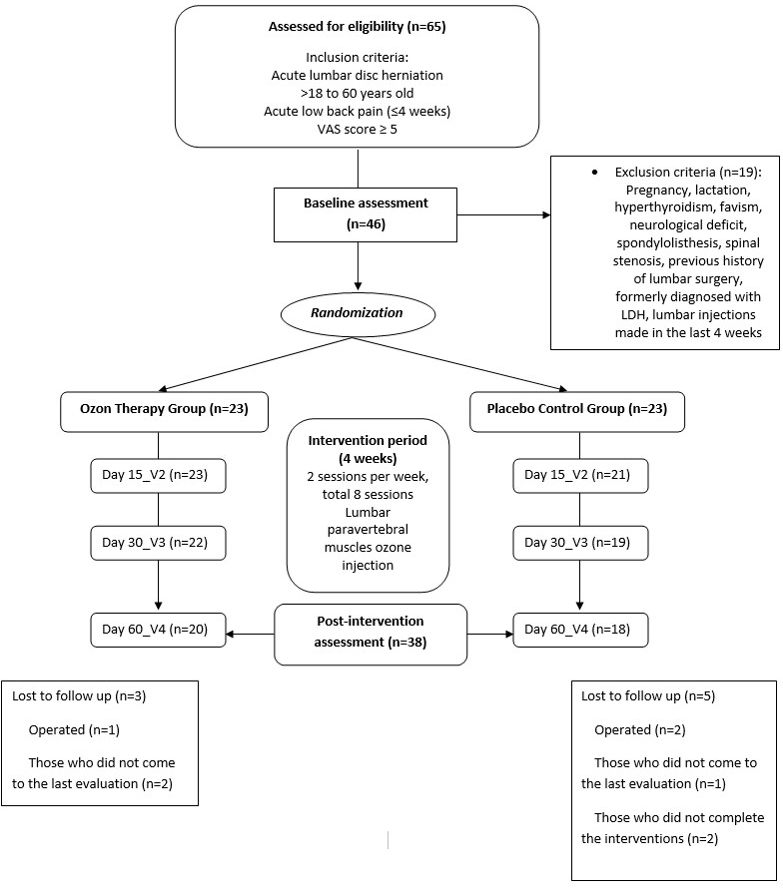
The study was conducted between December 2019 and May 2020 at the PT and rehabilitation clinic of Private Nisa hospital. The exclusion criteria in terms of OT included pregnancy, lactation, hyperthyroidism, favism (glucose-6 phosphate dehydrogenase enzyme deficiency), sickle cell anemia, hypoglycemia, hypotension, and exogenous antioxidant vitamins use (vitamins A, C, E, and B9). The exclusion criteria in terms of LDH included spondylolisthesis, spinal stenosis, muscle weakness due to radiculopathy, previous history of lumbar surgery, and lumbar paravertebral or intradiscal or epidural injections (ozone, steroid, local anesthetic, prolotherapy, acupuncture, and dry needling) in the last 4 weeks.
2.3Randomization
Randomization was done by a physician who did not participate in the recruitment and treatment of the participants. The participants were asked to choose a number between 1 and 10. Those who selected odd numbers were included in the PC group and those who selected an even number were included in the OT group (Fig. 1).
Table 1
Patients’ main clinical features
| Variable | Ozone therapy group | Placebo control group | |
|---|---|---|---|
| Total | 23 (100) | 23 (100) | |
| Sex | 0.765 | ||
| Female | 13 (56.5) | 14 (60.9) | |
| Male | 10 (43.5) | 9 (39.1) | |
| Pain | 0.546 | ||
| Lumbar | 15 (65.2) | 13 (56.5) | |
| Radicular pain | 8 (34.8) | 10 (43.5) | |
| Physical therapy program | 15 (65.2) | 14 (60.9) | 0.760 |
| Medication | |||
| Diclofenac sodium | 13 (56.5) | 12 (52.2) | 0.552 |
| Naproxen | 8 (34.8) | 9 (39.1) | 0.546 |
| Paracetamol | 10 (43.5) | 12 (52.2) | 0.555 |
| Thiocolchicoside | 14 (60.9) | 15 (65.2) | 0.760 |
| Tramadol hydrochloride | 6 (26.1) | 7 (30.4) | 0.534 |
| Mean (SD) | Mean (SD) | ||
| Age (years) | 45.5 (8.2) | 46.9 (8.6) | 0.579 |
| BMI (kg/m | 27.3 (5.2) | 27.2 (3.7) | 0.966 |
| VAS | 7.6 (1.5) | 7.5 (1.5) | 0.769 |
| ODI | 71.8 (17.4) | 72.9 (17.0) | 0.826 |
2.4Interventions
The number of sessions, doses, and concentrations in the OT were determined based on the Madrid Declaration on Ozone Therapy (MDOT) [25] and the recommendations of Società Scientifica di Ossigeno-OzonoTerapia (SIOOT) [26]. Intramuscular ozone injections to bilateral lumbar paravertebral muscles (15 mL for each side, for a total of 30 ml) were administered by the same physician, who had received formal training in OT, using 22-gauge needles through an extraspinal approach (from 2 cm lateral to the spine to a depth of 4 cm) for a total of 8 sessions (2 sessions per week for 4 weeks) under sterile conditions. On the vertebral level where LDH is, the injections were slowly administered on 3 points each on the right and left sides (5 ml for each point) and frequently to the areas corresponding to L3–L4, L4–L5, and L5–S1 without prior anesthesia in the polyclinic. The administered ozone concentrations (ozone/oxygen gas) were 20
The PT program included hot pack (20 minutes), ultrasound (10 minutes and 1.5 Watt/cm
In medical treatment, the patients used one or more of the drugs diclofenac sodium (150 mg/day), naproxen (1000 mg/day), paracetamol
2.5Evaluations
The ages, sexes, and body mass indexes of the patients were recorded. The patients were evaluated using the visual analog scale (VAS) [27] and the approved Turkish version of Oswestry Disability Index (ODI) [28]. The patients were assessed for pain (VAS) and disability related to the LBP (ODI) at the scheduled visits before the treatment (V1), during the treatment period (15 (V2) and 30 (V3) days after the treatment started), and after the treatment ended (one month (V4)). Evaluations were made by face to face interview. The physician and patient who performed the assessment, administered the POI, planned the PT program and medication, were blinded to the OT doses. A different physician adjusted the ozone concentration from the generator and drew it into the syringe. Additionally, only this physician knew the ozone dose to be applied to the patient. In addition, the adverse effects (load and fullness sensation and pain at the injection site) of the OT were recorded.
2.5.1VAS
VAS is a measurement tool used to determine the intensity or frequency of symptoms [29]. The VAS assessment, which was used in this study to measure the patients’ levels of pain, was made using figures from “0” to “10,” marked equally in a 10-cm line. The authors explained to the patients that “0” means no pain, “5” means moderate pain, and “10” means unbearable pain, and the patients were asked to mark the appropriate score on the line that described best their own pain [27].
2.5.2ODI
ODI is one of the most commonly recommended outcome measures for spinal cord disorders and is recognized as the “gold standard” of lumbar functional outcome instruments. It consists of 10 physical activity sections rated from 0 to 5. The total score is calculated by adding up all the points marked in each section. The total possible score is calculated by multiplying the number of marked episodes by 5, and the maximum possible score is 50. ODI total score (%) is calculated by dividing the total score by the total possible score and multiplying the quotient by 100. High scores indicate that the individual is more affected by the disease [30].
2.6Statistical analyses
All statistical analyses were performed using the Statistical Package for the Social Sciences version 20.0 (International Business Machines Corporation (IBM), Chicago, IL, USA). All outcome analyses were performed according to the principle of intention-to-treat. The intention-to-treat analysis was conducted according to a “worst-case-scenario” analysis: subjects who did not complete the treatment or had not undergone follow-up assessments were assigned a poor outcome, corresponding to the final average change recorded in per-protocol in the PC group [31]. The descriptive statistics were presented as the mean
The sample size was calculated under the assumption that 20% of the patients randomly assigned to the PC group and 55% to the OT group would be pain-free at the end of the treatment. On this basis, the minimum number of patients to be enrolled in each treatment arm would be 23 with at least 80% of power and 5% significance.
3.Results
Of the 65 patients with acute LDH evaluated in the study, 46 met the study criteria and were randomly divided into two groups. Twenty of the 23 patients in the OT group and 18 of the 23 patients in the PC group completed the study. 8 patients (OT: 3 (13%); PC: 5 (21.7%)) discontinued the study before completion (Fig. 1). Table 1 summarizes the clinical features of the patients. There was no significant difference in the baseline (V1) VAS and ODI scores between the two groups (
Fifteen (65.2%) patients in the OT group and fourteen (60.9%) patients in the PC group received the PT program (
The patients in the OT group had lower VAS scores in V2, V3, and V4 periods compared with those in the PC group. Starting from V3, this difference became significant and reached its peak in V4 (mean difference 2.2) (
Figure 2.
Mean VAS scores at different follow-up in OT group (black bars) and PC group (gray bars). Lower values correspond to clinical improvement.
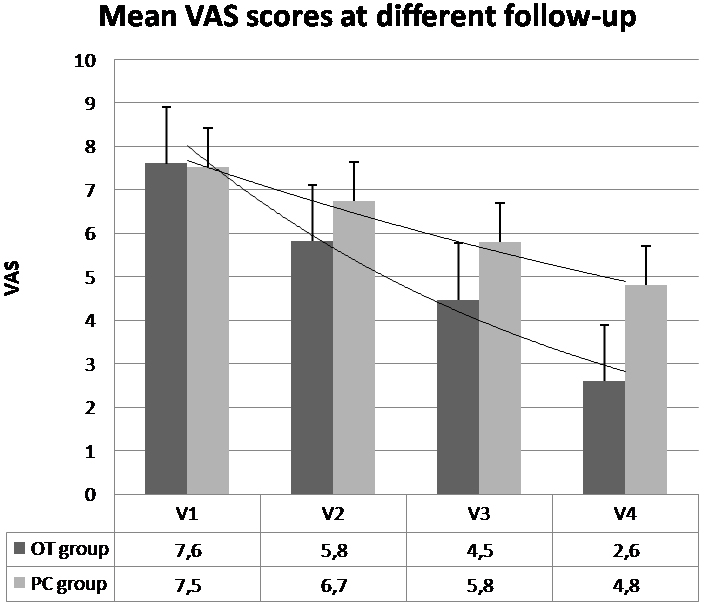
Figure 3.
Mean ODI scores at different follow-up in OT group (black bars) and PC group (gray bars). Lower values correspond to clinical improvement.
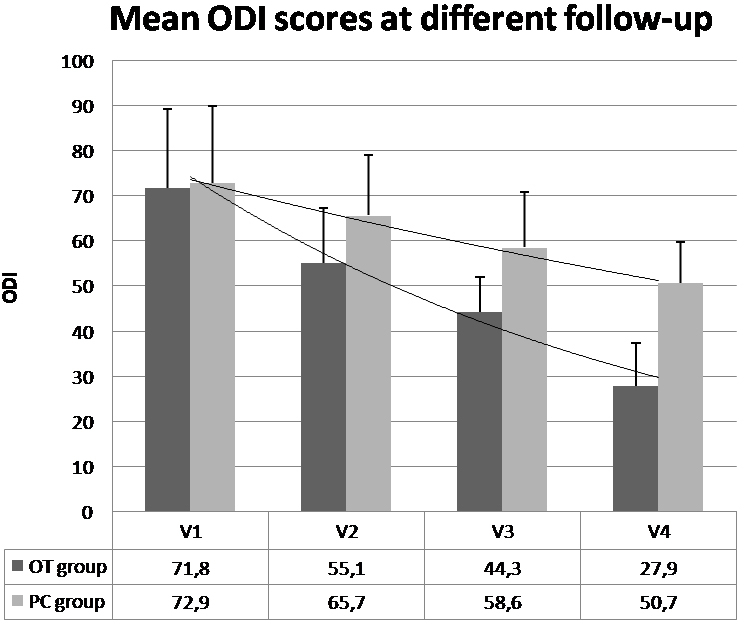
When compared to the baseline (V1) ODI scores, there was a significant improvement in the V2 (mean difference
At the end of the follow-up, the VAS scores fell below 5 in 100% of the patients in the OT group and in 38.8% of the patients in the PC group. Furthermore, 3 patients became completely pain-free in the OT group.
Load and fullness sensation and mild pain in the lumbar region lasting several hours during the ozone injections were observed in all the patients, more pronounced in the patients of the OT group. These adverse effects, which were more common during the first sessions, gradually decreased in the subsequent sessions. No other side effects were observed during the follow-up period. Since the follow-up process was short, no patient underwent a control MRI.
4.Discussion
In this study OT was used as an additional treatment in patients with acute LDH who were diagnosed with LDH for the first time and had no absolute surgical indications. There were significant improvements in the pain and disability scores of the patients in the OT group compared with those in the PC group.
Minimally invasive OT methods (intradiscal, epidural, and paravertebral), which have been widely used in treating many diseases in recent years, are used in treating LDH [2, 11, 12, 13]. In treating acute LDH, there are other effective conservative therapies (PT modalities, exercises, and ESI) [2, 5, 7]. However, in cases where these treatments cannot be performed or no response is obtained or surgery is not possible, POI, a minimally invasive OT application, may be required because it has some advantages.
PT modalities (ultrasound, laser, traction, TENS, and thermotherapy) are commonly used in clinical practice in treating acute LDH [32]. Tailored therapeutic exercises and spinal manipulation can be effective options [8, 9]. Most therapists consider these interventions to be effective [32, 33]. Although there are studies that support this [32, 34], there is insufficient evidence in the treatment guidelines to make a recommendation for or against the use of these modalities [8, 9]. Because of this contradiction between the use of PT applications in clinical practice and the recommendations in the guidelines, the effectiveness of PT is still under discussion. Despite these discussions, this study also used PT modalities and therapeutic exercises in terms of their effectiveness in treating LDH, and results that support the complementary role of the additional POI application have been reached.
ESI is recommended in LDH treatment guidelines because of its pain-relieving properties in patients who do not respond to other conservative treatments [8, 9]. Although this intervention is generally shown to be safe, it is known that it has certain risks [10]. These risks are potential side effects arising from the injection itself (headache because of dural perforation, bleeding, infection, and vasovagal reaction) or the pharmacological agents used (allergic reactions, increased blood sugar, mood changes, and insomnia) [10]. The use of ESI may be limited by such side effects and contraindications such as uncontrolled diabetes mellitus [10]. In such cases, POI, which is a more superficial percutaneous injection that does not have any corticosteroid or local anesthetic side effects and is not related to dura, may be preferred. Furthermore, POI may be preferred over ESI because it can be applied relatively easily in outpatient clinic conditions without the requirement for sedation and imaging assistance [2, 16, 17].
In LDH, intradiscal, intraforaminal, and POI exhibit some direct and indirect mechanical and anti-inflammatory effects [2, 11, 12, 13, 14]. It is reported that ozone has an effect on activating proteoglycans, on reducing ischemia and venous stasis, on reducing the release of pro-inflammatory cytokines (interleukin (IL)-1, IL-2, IL-8, IL-12, IL-15, interferon-
Intradiscal and intraforaminal ozone injections have been used in the treatment of LDH for over 20 years [23, 38]. In a few studies on these procedures in the literature, clinical success of up to 70–80% was reported [23, 38]. In their review, Magalhaes et al. [20] reported the evidence level of intradiscal OT for long-term pain relief as strong recommendation and low quality. In the same review, the evidence level of paravertebral OT was reported as strong recommendation and medium quality. However, data regarding OT are limited because of the absence of a placebo-controlled study in this meta-analysis and the fact that the two paravertebral ozone RCTs [2, 5] were performed with an active control group [20]. To the best of the authors’ knowledge, this study was the first POI study conducted with a PC group. In most studies conducted, POI were administered to patients with chronic LBP. To the best of the authors’ knowledge, this study is the third one conducted in an acute LDH setting [2, 24].
In the literature, authors found three RCTs [2, 5, 24], one prospective non-RCT [7], and three retrospective [11, 14, 17] studies on the treatment of LBP with POI. In these studies, with non-PC groups, POI were compared to analgesic and anti-inflammatory drugs [24], simulated therapy [2], global postural re-education [17] and epidural steroid [5] administration as active control groups, and they all found more significant improvements in the pain and disability scores in favor of OT. In this study, improvements in the pain and disability scores of the patients in the OT group were more significant compared with those of the patients in the PC group. Melchionda et al. [24] reported that the success rate of POI compared with analgesic and anti-inflammatory drugs was 50% vs. 16.6% in the second week and 80% vs. 50% in the 6-month follow-up. Paoloni et al. [2] found that 61% of the patients in the paravertebral ozone group and 33% in the simulated therapy group became pain-free after 6 months of follow-up. Out of 351 patients with chronic LBP, Zambello et al. [5] treated 171 patients with epidural steroid and 180 patients with POI. Total or near-total remission of pain was observed in 88.2% of patients in the OT group in the short term (three weeks) and 77.1% in the long term (six months). These rates were 59% and 47.3%, respectively, in the epidural steroid group.
There is no consensus in the literature on the standard ozone doses and concentrations in POI. Paoloni et al. [2] administered POI at a concentration of 20
Side effects reported in POI are extremely rare. Infection, a common risk for any invasive procedure, has been reported in POI. There is one case of death in the literature becaus of pyogenic muscle involvement and fulminant septicemia following POI [39], and one case of paravertebral and intraabdominal abscess that developed two months after POI and was treated with antibiotic therapy [40]. These infections may have been caused by the lack of sterility in the injection area during the procedure and that close attention should be paid to sterility. The authors performed the procedures in this study under sterile conditions and did not encounter any infection during the follow-up period. Moreover, it is known that ozone has antimicrobial and disinfectant properties [41]. Except for the possible risk of infection in POI, no significant side effects were reported except for mild pain in the injection area and fullness sensation and transient ecchymosis, which were reported in the literature and observed in our study [2, 11, 12]. This supports the idea that POI can be safely administered at the recommended dose range after the necessary sterility conditions are met.
4.1Study limitations
The low number of cases and short follow-up period (two months) are the most important limitations of this study. Although the lack of a standard treatment protocol for POI in the literature is a general limitation, the authors have considered widely accepted consensus recommendations in this study. Another limitation that made patient comparison more difficult was that some patients received various medications and PT programs other than the ozone therapy. However, there was no statistical difference between the groups of the patients who received the same PT program and similar medications. Finally, because there was no cost-effectiveness analysis in our study, no conclusion could be drawn on this issue. However, it can be concluded that this minimally invasive procedure is a relatively inexpensive form of treatment because it can be performed in an outpatient clinic without any radiological imaging aid, as in this study.
5.Conclusion
Although there are other effective conservative treatments in acute LDH, it can be said that, as an additional treatment combined with conservative treatment, lumbar POI can lessen pain and disability. It can be concluded that these minimally invasive ozone injections can be used as an effective complementary intervention to treat acute LDH. However, RCTs with a higher number of patients and longer follow-up periods are still needed.
Author contributions
HS: Conceptualization, methodology, writing – review and editing, data curation, formal analysis. NS: Methodology, data curation
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors would like to thank all patients in the study. They furthermore thank the Private Nisa Hospital Physical Medicine and Rehabilitation Clinic for their contributions, and Enago (https://www.enago.com.tr/ ceviri/) for their assistance in manuscript translation and editing.
Conflict of interest
The authors declare that they have no conflict of interest.
References
[1] | Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a US national survey. Spine. (1995) ; 20: : 11-9. |
[2] | Paoloni M, Di Sante L, Cacchio A, Apuzzo D, Marotta S, Razzano M, et al. Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: a multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine (Phila Pa 1976). (2009) ; 34: (13): 1337-44. |
[3] | Crock HV. Internal disc disruption: a challenge to disc prolapses fifty years on. Spine. (1986) ; 11: : 650-3. |
[4] | Hyodo H, Sato T, Sasaki H, Tanaka Y. Discogenic pain in acute nonspecific low-back pain. Eur Spine J. (2005) ; 14: (6): 573-7. |
[5] | Zambello A. Epidural steroid injection vs paravertebral O2O3 infiltration for symptomatic herniated disc refractory to conventional treatment: a prospective randomized study. Rivista Italiana di Ossigeno-Ozonoterapia. (2006) ; 5: : 123-7. |
[6] | Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. BMJ. (2003) ; 327: (7410): 323. |
[7] | Alyan S, Zaghlol R, Mustafa Shimaa A. Efficacy of combined paravertebral ozone (O2O3) therapy with physiotherapy in patients with chronic mechanical low back pain. Egypt Rheumatol Rehabil. (2018) ; 45: : 106-11. |
[8] | North American Spine Society. Clinical guidelines for multidisciplinary spine care diagnosis and treatment of lumbar disc herniation with radiculopathy. Burr Ridge, IL: North American Spine Society, (2012) . |
[9] | Luchtmann M, Firsching R. Lumbar disc herniation: evidence-based guidelines – a review. The Indian Practitioner. (2016) ; 69: (3): 36-41. |
[10] | Weinstein SM, Herring SA; NASS. Lumbar epidural steroid injections. Spine J. (2003) ; 3: (3 Suppl): 37S-44S. |
[11] | Özcan Ç, Polat Ö, Çelik H, Uçar BY. The effect of paravertebral ozone ınjection in the treatment of low back pain. Pain Pract. (2019) ; 19: (8): 821-5. |
[12] | Bocci V, Borrelli E, Zanardi I, Travagli V. The usefulness of ozone treatment in spinal pain. Drug Des Devel Ther. (2015) ; 9: : 2677-85. |
[13] | Costa T, Linhares D, Ribeiro da Silva M, Neves N. Ozone therapy for low back pain. A systematic review. Acta Reumatol Port. (2018) ; 43: (3): 172-81. |
[14] | Biazzo A, Corriero AS, Confalonieri N. Intramuscular oxygen-ozone therapy in the treatment of low back pain. Acta Biomed. (2018) ; 89: (1): 41-6. |
[15] | Das G, Ray S, Ishwarari S, Roy M, Ghosh P. Ozone nucleolysis for management of pain and disability in prolapsed lumber intervertebral disc. A prospective cohort study. Interv Neuroradiol. (2009) ; 15: (3): 330-4. |
[16] | Diracoğlu D. Ozone-oxygen therapies in musculoskeletal diseases. Turk J Phys Med Rehab. (2016) ; 62: (2): 183-91. |
[17] | Apuzzo D, Giotti C, Pasqualetti P, Ferrazza P, Soldati P, Zucco GM. An observational retrospective/horizontal study to compare oxygen-ozone therapy and/or global postural re-education in complicated chronic low back pain. Funct Neurol. (2014) ; 29: (1): 31-9. |
[18] | Bocci VA. Scientific and medical aspects of ozone therapy: the state of the art. Arch Med Res. (2006) ; 37: : 425-35. |
[19] | Buric J, Rigobello L, Hooper D. Five and ten year follow up on intradiscal ozone injection for disc herniation. Int J Spine Surg. (2014) ; 8: : 17. |
[20] | Magalhaes FN, Dotta L, Sasse A, Teixera MJ, Fonoff ET. Ozone therapy as a treatment for low back pain secondary to herniated disc: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. (2012) ; 15: (2): E115-29. |
[21] | Smith NL, Wilson AL, Gandhi J, Vatsia S, Khan SA. Ozone therapy: an overview of pharmacodynamics, current research, and clinical utility. Med Gas Res. (2017) ; 7: : 212-9. |
[22] | Iliakis E, Valadakis V, Vynios DH, Tisiganos CP, Agapitos E. Rationalization of the activity of medical ozone on intervertebral disc: a histological and biochemical study. Riv Neuroradiol. (2001) ; 14: (suppl 1): 23-30. |
[23] | D’Erme M, Scarchilli A, Artale AM, Pasquali Lasagni M. Ozone therapy in lumbar sciatic pain. See comment in PubMed Commons below Radiol Med. (1998) ; 95: (1-2): 21-4. |
[24] | Melchionda D, Milillo P, Manente G, Stoppino L, Macarini L. Treatment of radiculopathies: a study of efficacy and tollerability of paravertebral oxygen-ozone injections compared with pharmacological anti-inflammatory treatment. J Biol Regul Homeost Agents. (2012) ; 26: (3): 467-74. |
[25] | Madrid Declaration on Ozone Therapy [homepage on the Internet]. Madrid: 2015 [updated 2015 July 12; cited 2020 Sep 17]. Available from: https://isco3.org/madrid-declaration-2nd-edition/. |
[26] | Coclite D, Napoletano A, Barbina D, Guerrera D, Guerra R, Paoloni M, et al, (Eds). Conferenza di consenso. Ossigeno-ozono terapia nel trattamento delle lombosciatalgie da ernia discale con tecnica iniettiva intramuscolare paravertebrale. Istituto Superiore di Sanità Roma, 20 novembre 2006. Roma: Istituto Superiore di Sanità; 2008. (Rapporti ISTISAN 08/9). Available at: http://www.iss.it/binary/publ/cont/08-9%20web.1208510331.pdf. |
[27] | Dixon JS, Bird HA. Reproducibility along a 10 cm vertical visual analogue scale. Ann Rheum Dis. (1981) ; 40: (1): 87-9. |
[28] | Yakut E, Düger T, Oksüz C, Yörükan S, Ureten K, Turan D, et al. Validation of the turkish version of the oswestry disability index for patients with low back pain. Spine (Phila Pa 1976). (2004) ; 29: (5): 581-5. |
[29] | Dauphin AP, Guillemin F, Virion JM, Briançon S. Bias and precision in visual analogue scales: a randomized controlled trial. American Journal of Epidemiology. (1999) ; 150: (10): 1117-27. |
[30] | Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. (2000) ; 25: : 2940-53. |
[31] | Hollis S, Campbell F. What is meant by intention-to-treat analysis? Survey of a published randomized controlled trials. BMJ. (1999) ; 319: : 670-4. |
[32] | Unlu Z, Tasci S, Tarhan S, Pabuscu Y, Islak S. Comparison of 3 physical therapy modalities for acute pain in lumbar disc herniation measured by clinical evaluation and magnetic resonance imaging. J Manipulative Physiol Ther. (2008) ; 31: (3): 191-8. |
[33] | Li CL, Bombardier. Physical therapy management of low back pain: an exploratory survey of therapist approaches. Phys Ther. (2001) ; 81: : 1018-28. |
[34] | Karimi N, Akbarov P, Rahnama L. Effects of segmental traction therapy on lumbar disc herniation in patients with acute low back pain measured by magnetic resonance imaging: a single arm clinical trial. J Back Musculoskelet Rehabil. (2017) ; 30: (2): 247-53. |
[35] | Brown MD. The source of low back pain and sciatica. Semin Arthritis Rheum. (1989) ; 18: (suppl 2): 67-72. |
[36] | Haro H, Shinomiya K, Komori H, Okawa A, Saito I, Miyasaka N, et al. Upregulated expression of chemokines in herniated nucleus pulposus resorption. Spine. (1996) ; 21: : 1647-52. |
[37] | Bocci V. Oxygen-Ozone Therapy. A Critical Evaluation. Kluwer Academic Publishers: Dordrecht; (2002) . |
[38] | Muto M, Avella F. Percutaneous treatment of herniated lumbar disc by intradiscal oxygen-ozone injection. Interv Neuroradiol. (1998) ; 4: (4): 279-86. |
[39] | Gazzeri R, Galarza M, Neroni M, Esposito S, Alfieri A. Fulminating septicemia secondary to oxygen-ozone therapy for lumbar disc herniation: case report. Spine (Phila Pa 1976). (2007) ; 32: : E121-3. |
[40] | Menéndez P, García A, Peláez R. Absceso paravertebral e intraabdominal secundario a ozonoterapia por lumbalgia. Rev Esp Cir Ortop Traumatol. (2014) ; 58: : 125-7. |
[41] | Sharma M, Hudson JB. Ozone gas is an effective and practical antibacterial agent. Am J Infect Control. (2008) ; 36: (8): 559-63. |




