Effects of a physical self-care support program for patients with fibromyalgia: A randomized controlled trial
Abstract
BACKGROUND:
The effects of stretching exercises in fibromyalgia (FM) deserves further study.
OBJECTIVE:
To evaluate the effectiveness of a Physical Self-Care Support Program (PSCSP), with emphasis on stretching exercises, in the treatment of FM.
METHODS:
Forty-five women with FM were randomized to the PSCSP (
RESULTS:
Nineteen and 21 patients completed the trial in PSCSP and control groups, respectively. After 10 weeks, the PSCSP group showed significantly better FIQ (difference between adjusted means,
CONCLUSIONS:
A PSCSP emphasizing stretching exercises significantly improved FIQ and SRT scores, and may be a helpful therapy for FM.
1.Introduction
Fibromyalgia (FM) is a musculoskeletal condition of unknown etiology, characterized by chronic and widespread pain lasting longer than 3 months [1, 2]. It is frequently associated with depression and anxiety [1, 2, 3]. Neurobiological mechanisms involved in the pathogenesis include greater activation of painful afferent pathways, decreased serotonin levels, and consequent reduction in the activity of the Pain Inhibitory System [1]. FM treatment aims to control pain, reduce functional limitations, and improve quality of life through comprehensive strategies involving a multidisciplinary approach [4, 5, 6, 7].
Among the non-pharmacological therapeutic approaches to FM, physical exercise is an important step to improve the disease symptoms [8, 9]. In general, evidence has favored aerobic and, to a lesser degree, strengthening exercises [8, 9]. However, stretching, a kinesiotherapeutic exercise, enables the recovery of functional muscle length, range of motion, and flexibility, relieving stress and realigning posture [10, 11]. Given their easy execution and tolerability, muscle stretching exercises can be easily included as part of a self-care strategy to obtain and maintain improvements in FM symptoms [4].
There is evidence that supported self-care, an intervention where the individual plays a central role in determining his own health care, may effectively improve symptoms of chronic diseases. This approach implies close collaboration between the health care team and the users to define the problem jointly, set the goals, institute care plans, and solve problems that arise during chronic condition management. Supported self-care utilizes group dynamics to educate through didactic actions, information, and physical means. The goal is to inform the patient about his disease, making him capable of managing his symptoms autonomously, without depending on the permanent assistance of the health team. It involves, to some extent, principles of behavioral therapies because it requires the implementation of new lifestyle habits in the daily routine to promote health [12, 13, 14].
Considering the potential benefits of supported self-care for chronic diseases and the effects of stretching exercises on musculoskeletal pain syndromes, we developed a Physical Self-Care Support Program (PSCSP) emphasizing stretching exercises, relaxing, wellness, and posture techniques for the treatment of FM. Hence, we designed a randomized controlled trial to test the effect of the PSCSP on the impact caused by the disease (measured with the Fibromyalgia Impact Questionnaire) and on measures of pain and flexibility in FM patients.
2.Methods
2.1Study design and ethical approval
This is a single-blind, parallel-group, superiority randomized controlled trial conducted from September to December 2012. The study was done in accordance with the principles of the Declaration of Helsinki, and its protocol was approved by the Research Ethics Committee of the Health Science Center at Universidade Federal de Pernambuco (UFPE) and registered under the CAAE (‘Certificado de Apresentação de Apreciação Ética’, Certificate of Presentation of Ethic Appreciation) number 00701512.7.0000.5208. This study did not receive external financial support, and the institution where the study took place (Hospital das Clínicas da Universidade Federal de Pernambuco) did not have any influence on the analysis, reporting, or interpretation of results. The study was retrospectively registered in ReBec (‘Registro Brasileiro de Ensaios Clínicos’, Brazilian Registry of Clinical Trials), identification code: RBR-6c7tns.
2.2Subjects
Potential eligible candidates for participation in the study were patients attending the Outpatient Fibromyalgia Clinic at Hospital das Clínicas da Universidade Federal de Pernambuco (HC/UFPE). They were identified by one of the principal investigators (SL) through a review of medical charts. SL enrolled the patients into the study, inviting them to participate during medical consultations or by phone contact. To be included, the patients had to meet all the following criteria: women aged 30–55 years; fulfillment of the American College of Rheumatology 1990 and 2010 criteria for diagnosis of FM [3]; residence in the metropolitan area of Recife, Pernambuco; stable psychological, physical, and drug therapy in the preceding month. Exclusion criteria were patients attending physiotherapy sessions, using gait assistance devices, suffering from associated autoimmune rheumatic diseases, subjects with physical and/or cognitive deficits, or presenting uncontrolled comorbidities.
2.3Randomization
Patients who fulfilled the eligibility criteria and agreed to participate and sign a written informed consent form were included in the study. A closed list of these patients was organized sequentially and numbered following the alphabetic order to serve as base for the random sequence application. A professional not involved with patent care and unaware of patients’ names generated a computerized random sequence (with fixed block size equal to 2) with the aid of the R software (R Foundation for Statistical Computing, Vienna, Austria), allocating patients to PSCSP or control groups, with an allocation ratio of 1:1. SL was responsible for assigning, at once, all participants to one of the groups by using the alphabetically ordered list of complete patient names and the computer generated random sequence. A post-hoc verification made by the researcher responsible for the statistical analysis (MB), checking patient names and the random sequence, confirmed that all patients were allocated correctly to the group they should have been.
2.4Interventions
All participants entered the study simultaneously and were followed during the same time frame. Over 10 weeks, patients allocated to active treatment underwent the PSCSP. The main objectives of the PSCSP were to promote knowledge of the syndrome, provide instruction on stretching techniques, and promote postural changes in the daily activities of volunteers. It consisted of 10 weekly 90-minute sessions in fixed groups of approximately 10 people and was based on active learning methodologies. Supervised and guided by the research physiotherapist (SL), the sessions usually began with chat circles where experiences were shared and questions regarding the previous session were clarified. There were moments when the floor was used as part of the practice field with mattresses made available to provide comfort and aid. At the sessions, participants were given leaflets with exercises they had learned (to keep on with the treatment at their homes), relaxing and wellness techniques, and postural guidelines for conducting everyday activities and preventing intensification of symptoms. The material was self-explanatory, containing a wealth of illustrations and written in plain language. Teaching resources and strategies included slideshows, whiteboards, demonstrations, games, and group dynamics. The PSCSP included 36 posture tips and 46 self-stretching exercises, of which 11 were for the spine, 16 for the lower limbs, 10 for the upper limbs, and 9 for body mobility and flexibility. At first, the stretching was done in 3 sets of 30 seconds holding the position. Later, this was extended to 45 seconds and afterward to 1 minute, which is the ideal duration for maximizing the technique’s benefits. Awareness-raising and/or myofascial tissue releasing practices were also presented, extending the soothing effects of stretching. Preparatory techniques for the stretching included diaphragmatic breathing, active muscle relaxation, self-massage, and warm compress. The contents of the program were progressively distributed over 10 weeks to ensure that participants learn them and become physically prepared. The PSCSP was developed in three stages, as follows: I – Information and physical preparation (sessions 1–3); II – flexibility gains and treatment of specific body areas (sessions 4–7); III – promoting independence in managing symptoms through knowledge integration (sessions 8–10). Participants received supporting material at the end of the PSCSP (self-care kits, folders, music CDs, spiky balls, tennis balls, pool noodles, bandage tapes, guides with posture tips, etc.) to maintain the benefits of the PSCSP. For further details, see Appendix Text and Appendix Table 1; the original, more detailed and illustrated description of the procedures (in Portuguese) taken in each session is available online at https://repositorio.ufpe.br/handle/123456789/11751. The minimum attendance required for the patients in the PSCSP group was 80%. Otherwise, the patient would be considered a non-completer. We offered the possibility of making up for missed sessions on subsequent days in individual meetings.
Patients allocated to the (inactive) control group were monitored at three clinical outpatient appointments with a rheumatologist (AR) to supervise the use of medications and fulfillment of the pain medication diary, but no change in treatment was made. They were told to keep their usual routine but avoid starting a new physical exercise practice of any kind. All patients in the control group were included in a waiting list and informed that, if the intervention proves effective, they would be allowed to receive the same training given to the PSCSP group. These activities took place in the following 4 months after the study ended.
2.5Outcomes
At the baseline visit, patients were evaluated using a standard interview form collecting sociodemographic and clinical data by trained members of the research team (VF or EP). On the same occasion, the Fibromyalgia Impact Questionnaire (FIQ) [15], the Visual Analogue Scale (VAS) for pain [16], and the Sit and Reach Test (SRT) [17] were also applied. The FIQ is a targeted instrument for assessing FM patients’ current health status, encompassing functional capacity, employment status, psychological distress, and physical symptoms. It consists of 19 questions organized into 10 items; higher scores (the maximum possible score is 100) indicate a worse condition [15]. The VAS for pain is a tool that evaluates the level of self-perceived pain, and scores range from 0 to 10, where zero reflects the complete absence of pain and 10 means maximum pain [16]. The Sit and Reach Test is used to assess muscular flexibility and is performed using a Wells bench. Subjects are asked to remain seated on the floor with their legs stretched straight ahead and the soles of their feet flat against the box. They are then directed to reach forward as far as possible with their hands on top of each other while breathing out, moving the bench’s scalimeter as far as they can. The maximum value obtained after three repetitions is used [17]. Patients were also asked about pain in specified body areas (left and right mandibles, shoulders, arms, forearms, hips, thighs, and legs, and cervical, dorsal, lumbar, thoracic, and abdominal regions) marking yes or no in a questionnaire. After an initial evaluation, patients received a pain medication diary, an instrument developed for this study to monitor the use of medication, where participants recorded the number of analgesic tablets taken each day.
At the end of 10 weeks, all patients were reassessed over two weeks (weeks 11 and 12) using the same instruments applied in their first evaluation. The study’s primary outcomes were the FIQ, the VAS of pain, and the SRT scores after 10 weeks of training. The FIQ sub-items measured semi-quantitatively or on VAS, the frequency of falls, the intake of analgesics, and the number of painful body areas served as secondary outcomes. The same researcher (VF or EP) who evaluated each patient at baseline applied the tests at the end of the study; these researchers were blinded to the treatment the patient was allocated to. Patients were explicitly instructed not to give any information that could permit the identification of the group they belong. All patients, independently of completing or not the training program, should return for the final evaluation. The number of analgesic tablets taken during the study period was calculated using the pain medication diaries.
2.6Statistical analysis
The sample size was calculated considering a mean FIQ score of 70 in the control group and 55 in the PSCSP group at the end of the study, with a pooled standard deviation of 15 [18]; statistical power was set at 80% for detecting a significant difference with a P value less than or equal to 0.05. Considering the possibility of a 30% loss to follow-up, the estimated sample size was 21 individuals in each group.
The data were analyzed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA), jamovi v.1.8 (The jamovi project, Sidney, Australia), and the ’rcompanion’ package of R v.4.0.1 (R foundation for statistical computing, Vienna, Austria). Quantitative variables were graphically and statistically tested (with the Kolmogorov-Smirnov goodness-of-fit test) for normality of distribution. Variables with normal distribution were presented as mean
Table 1
Baseline sociodemographic and clinical features of the participants who completed the study (numbers represent number and percentage, except where indicated otherwise)
| Variables | Group | |
|---|---|---|
| PSCSP ( | Control ( | |
| Age (years) – mean | 46.47 | 46.38 |
| Disease duration (years) – median (percentiles 25 | 5 (4, 13) | 6 (4, 10) |
| Completed basic school | 11 (57.9) | 9 (42.9) |
| Occupational status | ||
| Employed | 9 (47.4) | 10 (47.6) |
| Unemployed | 9 (47.4) | 10 (47.6) |
| On social security | 1 (5.3) | 1 (4.8) |
| Regular physical activity | 4 (21.1) | 4 (19) |
| Previously received physiotherapy | 11 (57.9) | 6 (28.6) |
| Active medications | ||
| Tricyclic antidepressants | 17 (89.5) | 14 (66.7) |
| Selective serotonin uptake inhibitors | 9 (47.4) | 11 (52.4) |
| Dual inhibitors of serotonin and norepinephrine reuptake | 3 (15.8) | 2 (9.5) |
| Anticonvulsants | 1 (5.3) | 0 (0.0) |
| Non-steroidal anti-inflammatory drugs | 6 (31.6) | 6 (28.6) |
| Total FIQ score – mean | 79.17 | 73.07 |
| VAS of pain – mean | 8.46 | 7.70 |
| Flexibility on SRT (cm) – mean | 18.25 | 23.23 |
PSCSP: Physical Self-Care Support Program; SD: standard deviation; FIQ: Fibromyalgia Impact Questionnaire; VAS: Visual Analogue Scale; SRT: Sit and Reach Test.
Missing data of continuous outcome variables were handled using complete case analysis (CCA, assuming that missing data occur completely at random [MCAR]) and multiple imputation (under the assumption that data were missing at random [MAR]). For multiple imputation, baseline variables (those reported in Table 1, except for active medications) and (non-missing) values of FIQ, VAS of pain, and Sit and Reach Test after 10 weeks were used to predict the missing values of the primary outcomes. Multiple imputation was performed separately for the PSCSP and the control groups; 20 imputed databases were generated using the Markov Chain Monte-Carlo (MCMC) method with linear regression [20], allowing a maximum of 100 iterations. In sensitivity analysis, considering that data may be missing not at random (MNAR), we performed delta-adjusted multiple imputation adding 15 to the imputed final FIQ score, assuming that non-completers would have a significant clinical worsening. In a post-hoc analysis, we categorized patients according to response (change in FIQ
3.Results
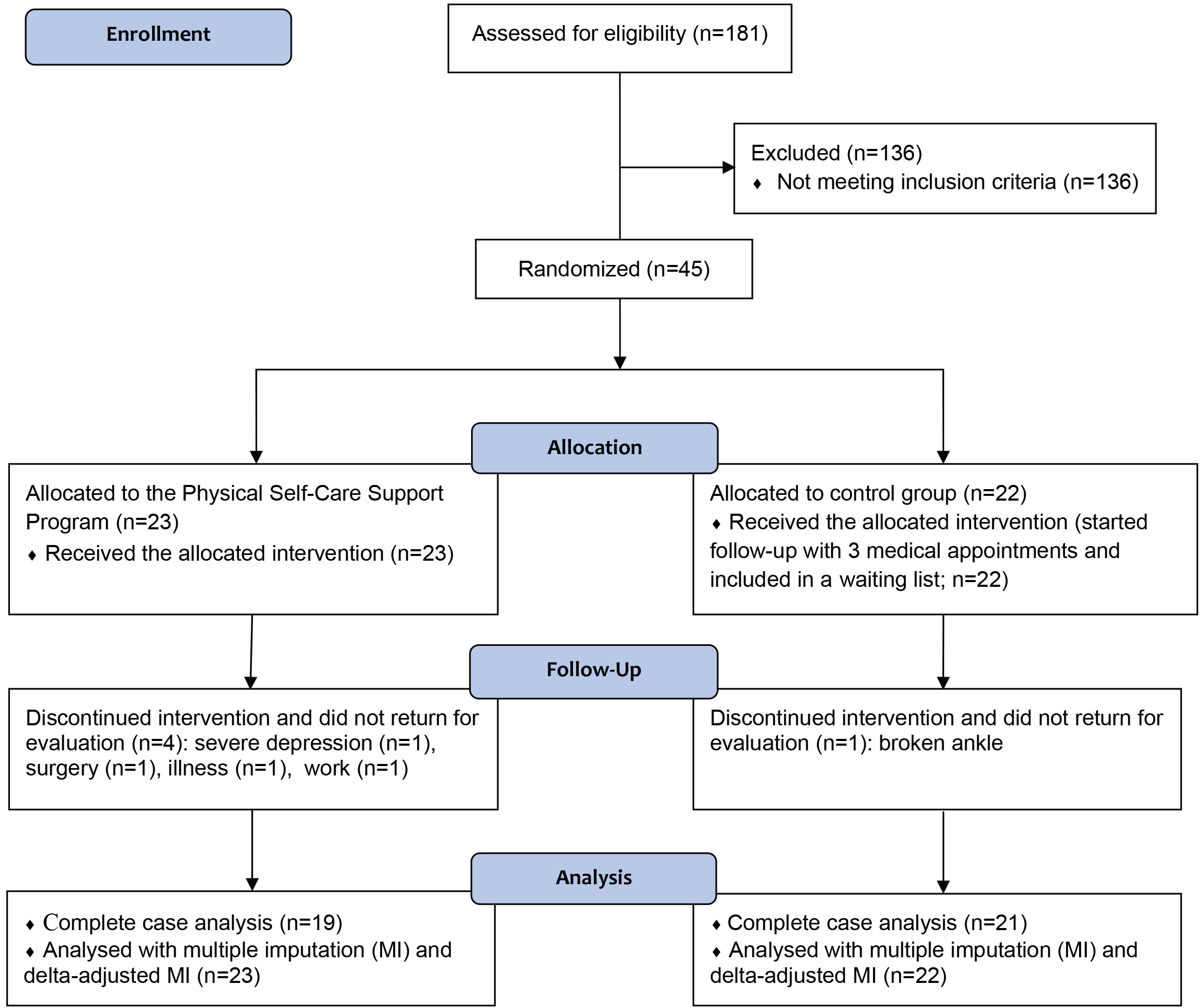
Out of 181 patients attending the Outpatient Fibromyalgia Clinic at HC/UFPE in August 2012, 45 individuals fulfilled the criteria and agreed to participate in the study; 23 were allocated to the PSCSP group and 22 to the control group. Four patients in the PSCSP group and 1 in the control group dropped out during follow-up due to reasons unrelated to the study (see Fig. 1); these individuals could not return for the evaluation of outcomes in due time, despite the efforts of the research team. The clinical and demographic features of individuals who completed the study and were included in the final analysis are depicted in Table 1. Most of these patients were middle-aged low schooling women; almost all patients (except for one individual) were on active medication treatment for FM. Half were professionally employed, and few were engaged in regular physical activity. Appendix Table 2 describes the characteristics of completers and non-completers of this study.
Table 2
Results for the primary outcomes (FIQ, VAS of pain, and flexibility at weeks 11–12) using analysis of covariance (complete case analysis)*
| Variables | Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| Between group difference (95% CI) | Effect size (95% CI)** | ||||||||
| Total FIQ score | 63.97 | 77.61 | 1.17 (0.42 to 19.3) | 0.002 | |||||||
| VAS of pain | 5.82 | 7.23 | 0.60 ( | 0.088 | |||||||
| Flexibility on SRT (cm) | 27.17 | 19.93 | 7.24 (3.12 to 11.37) | 1.23 (0.47 to 1.99) | 0.001 | ||||||
* Results were adjusted for baseline values of total FIQ score, VAS of pain, and flexibility on SRT included simultaneously in all covariance analysis models. **Measured by Cohen’s
Figure 1.
CONSORT flow diagram describing the study procedures and follow-up of patients.
The results for the primary outcomes at the end of the study (weeks 11–12) are demonstrated in Table 2. There were significantly lower FIQ and SRT scores in the PSCSP group than in the control group, but no significant difference in pain VAS was observed. The results were similar performing complete case analysis (CCA; Table 2) or using multiple imputation (see Appendix Table 3). Including the variable ‘previous experience with physiotherapy’, which presented some numerical imbalance between the study groups, in the ANCOVA models produced no significant change in the results for the FIQ and SRT scores (
In the post-hoc analysis of complete cases, a clinical response (reduction in FIQ
Table 3
Between group comparisons of the sub-items of the FIQ (higher values indicate worse outcome) at weeks 11–12 (complete case analysis)*
| Variables | Group | ||||
|---|---|---|---|---|---|
| PSCSP Estimated marginal mean | Control Estimated marginal mean | Between group difference (95% CI) | Effect size (95% CI)** | ||
| Functional capacity | 4.16 | 4.34 | 0.13 ( | 0.696 | |
| Feel good*** | 4.66 | 8.18 | 1.36 (0.64 to 2.08) | ||
| Miss work | 2.87 | 4.01 | 0.40 ( | 0.210 | |
| Difficulty with work | 7.14 | 8.68 | 0.77 (0.10 to 1.44) | 0.021 | |
| Pain | 7.25 | 8.82 | 0.78 (0.10 to 1.45) | 0.021 | |
| Fatigue | 7.51 | 9.16 | 0.93 (0.24 to 1.61) | 0.007 | |
| Unrefreshing sleep | 7.44 | 8.74 | 0.64 ( | 0.049 | |
| Stiffness | 6.84 | 8.10 | 0.64 ( | 0.050 | |
| Anxiety | 8.00 | 9.10 | 0.72 (0.05 to 1.39) | 0.031 | |
| Depression | 7.18 | 9.03 | 0.83 (0.14 to 1.53) | 0.016 | |
*Each outcome variable was adjusted only for its own baseline value using ANCOVA. **Measured by Cohen’s
The analyses comparing the scores of FIQ sub-items between the groups are presented in Table 3. Most scores were significantly lower, except for functional capacity and work absenteeism, in the PSCSP group as compared to the control group. As measured by Cohen’s
Table 4
Between group comparisons of consumption of analgesic tablets, number of falls during the study period, and number of painful body areas at weeks 11–12
| Variables | Group | ||||
|---|---|---|---|---|---|
| PSCSP | Control | Effect size (95% CI)* | |||
| Number of analgesic tablets | Weeks 1 to 5 | 11.0 (7.0, 26.0) | 25.0 (7.5, 47.5) | 0.20 ( | 0.184 |
| consumed – median (25 | Weeks 6 to 10 | 11.0 (3.0, 19.0) | 25.0 (10.5, 50.0) | 0.36 (0.07 to 0.58) | 0.016 |
| percentiles) | Entire study period | 25.0 (9.0, 37.0) | 54.0 (20.0, 87.0) | 0.30 (0.00 to 0.55) | 0.046 |
| Number of falls – median | Weeks 1 to 5 | 3.0 (1.0, 10.0) | 4.0 (1.0, 11.5) | 0.07 ( | 0.624 |
| (25 | Weeks 6 to 10 | 1.0 (0.0, 7.0) | 2.0 (0.0, 6.0) | 0.04 ( | 0.783 |
| Entire study period | 4.0 (1.0, 15.0) | 5.0 (1.5, 17.5) | 0.06 ( | 0.683 | |
| Number of painful body | Week 10 | 16 (8, 19) | 14 (12, 16) | 0.09 ( | 0.693 |
| areas – median | |||||
| (25 | |||||
*Measured by Pearson’s r coefficient, where 0.1, 0.3, and 0.5 represent the minimum values for small, medium and large effect sizes, respectively. 95% CIs were obtained with bootstrapping. **Mann-Whitney U test. PSCSP: Physical Self-Care Support Program; CI: confidence interval.
The results for other secondary outcomes are presented in Table 4. There was a significant reduction in the consumption of analgesics, especially between weeks 6 and 10, but no significant change in the number of falls or count of painful body areas. No treatment-emergent adverse effects were observed during the realization of this study.
4.Discussion
In this randomized controlled trial, the results indicated that a PSCSP with stretching exercises may effectively reduce the negative impact of FM on patients’ lives, as measured by the FIQ, in comparison to an inactive control group. Patients’ flexibility improved, but there was no significant reduction in pain. The PSCSP positively impacted well-being, reduced depression, anxiety, and the consumption of analgesics. In general, our results reinforce the importance of physical therapies in the management of FM.
The positive effects of physical exercise in treating patients with FM are widely reported in the literature [9, 21, 22], and a role for complementary and alternative therapies also has been considered for maintaining and improving the quality of life in these individuals [5, 23, 24, 25]. Substantial gaps in knowledge remain, however, particularly concerning muscle stretching exercises. Several randomized controlled trials have included some form of stretching as part of the interventions in all or most study arms [26, 27, 28, 29], and others have compared stretching with aerobic exercises [30, 31, 32, 33, 34], strengthening exercises [11, 35], Tai-Chi [36] and Ai-Chi [37]. In general, the results favor aerobic and strengthening exercises, as confirmed by two recent systematic reviews [38, 39], but there was significant heterogeneity between the included trials [39]. However, we found only one previous study comparing stretching training versus an inactive control group. Assumpção et al. [35] offered training in twice-weekly 40 minute sessions over 12 weeks. At the same time, controls continued their usual medical treatment and were included in a waiting list. The comparison of the stretching (
The variability of results in the literature on the effectiveness of stretching can also be attributed to heterogeneity in the duration of the intervention, session frequency, and exercise intensity. There is still a lack of consensus and scarce publications on therapeutic stretching in the treatment of patients with FM. However, despite the relatively poor scientific support up to now, stretching is widely used in the routine clinical treatment of patients with FM, primarily due to the belief that it acts directly reducing areas of chronic excessive fascial tension, which may lead to inflammation and pain [41].
Perhaps the effectiveness of our training program relies, at least in part, on the teaching method that was employed and on trust and motivation generated among participants in the PSCSP group. In the present study, patients underwent a comprehensive program involving not only stretching. Still, they received extensive training on the importance of self-care to manage FM symptoms, and participants played an active role in the study. The participatory methodology valued prior knowledge and physical limits of the participants, who were protagonists of the learning process. Although heterogeneous, the educational contributions from previous studies [10, 11, 27, 39, 42] demonstrated the need for awareness among subjects to facilitate treatment adherence and guarantee continuity of care as much as possible [14]. Given that active techniques to manage pain seem to be effective in FM [43] and that group environment fosters motivation for self-care [44], the PSCSP can be useful to patients coping with FM-related problems.
This study has several strengths. It is the second and largest randomized controlled to compare a program emphasizing stretching exercises with an inactive control group. Patients allocated to the control group were included in a waiting list and underwent three clinical appointments over 10 weeks. Our sample was constituted of women with, in general, a relatively low educational status, a profile similar to the average FM patient in a developing country [10, 45, 46]. We had relatively few dropouts during the study, and appropriate methods for dealing with missing data were used. The evaluators of outcomes were blinded to patients’ allocation, reducing the risk of measurement bias. Baseline features were fairly distributed between groups; nonetheless, the analyses adjusted for baseline values (including a variable distributed unevenly between groups) did not change the results significantly.
The present study also has limitations. Despite being made by a professional unaware of patients’ names, the computerized randomization sequence was not concealed in this trial. However, considering that the list of patients (where the random sequence was applied) was organized following the alphabetical order, we were able to verify that there was no intentional or unintentional manipulation of treatment assignment in this trial. Even though the number of patients in each group was stipulated by calculating the required sample size, this is a small study. So, we observed a relatively large clinical effect, but the confidence intervals are wide, and we can not exclude that a less relevant clinical effect occurs in reality. There were 4 dropouts in the PSCSP group, comparing with 1 in the control group; these patients did not return for a final evaluation despite our efforts. However, given that these patients presented worse (lower) flexibility scores, the dropouts reduced the baseline imbalance in this variable. The training program was extensive and comprehensive, and we cannot know precisely to which extent each of the interventions contained in it was responsible for the improvement of the patients. Individuals’ commitment, dedication, and understanding are an essential part of the training program, and so it may not be adequate for all FM patients. The patients could not be blinded to the treatment they were allocated to, which may favor a positive evaluation of outcomes. Our study was not planned to evaluate the long-term effects of the training strategy, and so we can not know if the observed benefits will or not be maintained over time. The results also do not allow assumptions about the efficacy of stretching exercises in comparison to aerobic or strengthening exercises.
5.Conclusions
This study evaluated the efficacy of a comprehensive PSCSP including wellness, relaxing, postural techniques, and stretching exercises, and showed that this intervention may be a valuable tool in the treatment of FM. There were significant improvements in FIQ and flexibility scores and a reduction in analgesics consumption compared with an inactive control group. Sub-analysis of items of the FIQ suggested improvements in well-being and reduction in depressive and anxiety symptoms in the PSCSP group. However, further studies in this field are still necessary to define the best strategy of exercise prescription in FM and determine which patients would get significant benefits from the PSCSP.
Author contributions
Made a substantial contribution to the concept or design of the work: SBL, ALBPD, CDLM and AR. Made a substantial contribution to the acquisition of data: SBL, VMF, EASP and AR. Made a substantial contribution to the analysis and interpretation of data and drafted the article: SBL, MB and AR. Revised it critically for important intellectual content: all authors. Approved the version to be published and take public responsibility for appropriate portions of the content: all authors.
Availability of data and materials
The datasets used for statistical analysis are available from the corresponding author on reasonable request.
Funding
This study did not receive any kind of external financial support and all expenses were provided by the investigators.
Supplementary data
The supplementary files are available from https://dx. doi.org /10.3233/BMR-191820.
Conflict of interest
The authors declare that they have no competing interests.
References
[1] | Smith HS, Harris R, Clauw D. Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician. (2011) ; 14: (2): E217-45. |
[2] | Cardoso FS, Curtolo M, Natour J, Lombardi Júnior I. Avaliação da qualidade de vida, força muscular e capacidade funcional em mulheres com fibromialgia. Rev Bras Reumatol. (2011) ; 51: (4): 338-50. |
[3] | Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg D, Katz RS, Mease P, et al. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care & Research. (2010) ; 62: (5): 600-10. |
[4] | Gür A. Physical therapy modalities in management of fibromyalgia. Curr Pharm Des. (2006) ; 12: (1): 29-35. |
[5] | Ablin J, Fitzcharles M, Buskila D, Shir Y, Sommer C, Häuser W. Treatment of Fibromyalgia Syndrome: Recommendations of Recent Evidence-Based Interdisciplinary Guidelines with Special Emphasis on Complementary and Alternative Therapies. Evid Based Complement Alternat Med. (2013) ; 2013: : 485272. |
[6] | Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. (2001) ; 4: (6): 256-62. |
[7] | World Health Organization: 2008–2013 Action Plan for the Global Strategy for the Prevention and Control of Noncommunicable Diseases. Geneva: World Health Organization; (2008) . |
[8] | Hagen KB, Dagfinrud H, Moe RH, Osteras N, Kjeken I, Grotle M, et al. Exercise therapy for bone and muscle health: an overview of systematic reviews. BMC Medicine. (2012) ; 10: : 167. |
[9] | Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. (2007) ; 17: (4): CD003786. |
[10] | Berssaneti AA. Exercícios de alongamento e fortalecimento muscular no tratamento de pacientes com fibromialgia: um ensaio clínico randomizado [tese de doutorado]. São Paulo: Faculdade de Medicina da Universidade de São Paulo. (2010) . |
[11] | Jones KD, Burckhardt CS, Clark SR, Bennett RM, Potempa KM. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. The Journal of Rheumatology. (2002) ; 29: (5): 10418: . |
[12] | Thieme K, Turk DC. Cognitive-behavioral and operant-behavioral therapy for people with fibromyalgia. Reumatism. (2012) ; 64: (4): 275-85. |
[13] | Bastos I. A técnica de grupos-operativos à luz de Pichon-Rivière e Henri Wallon. Psicólogo Informação. (2010) ; 14: (14): 160-9. |
[14] | Mendes EV. O cuidado das condições crônicas na atenção primária à saúde: O imperativo da consolidação da estratégia da saúde da família. Brasília: Organização Pan-Americana da Saúde; (2012) . |
[15] | Marques AP, Santos AMB, Assumpção A, Matsutani LA, Lage LV, Pereira CAB. Validação da versão brasileira do Fibromyalgia Impact Questionnaire (FIBROMYALGIA IMPACT QUESTIONNAIRE). Rev Bras Reumatol (2006) ; 46: (1): 24-31. |
[16] | Campbell WI, Lewis S. Visual Analogue Measurement of Pain. Ulster Med J. (1990) ; 59: (2): 149-54. |
[17] | Wells KF, Dillon EK. The sit and reach – a test of back and leg flexibility. Res Quart. (1952) ; 23: (1): 115-8. |
[18] | Carbonell-Baeza A, Ruiz JR, Aparicio VA, Ortega FB, Munguía-Izquierdo D, Alvarez-Gallardo IC, et al. Land- and water-based exercise intervention in women with fibromyalgia: the al-andalus physical activity randomised controlled trial. BMC Musculoskelet Disord. (2012) ; 13: (18): 1-11. |
[19] | Vickers AJ. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Medical Research Methodology. (2005) ; 5: : 35. |
[20] | Puma MJ, Olsen RB. Bell SH, Cristofer P. What to Do When Data Are Missing in Group Randomized Controlled Trials (NCEE 2009-0049). Washington, DC: National Center for Education Evaluation and Regional Assistance, Institute of Education Sciences, U.S. Department of Education; (2009) . |
[21] | Jones KD, Adams D, Winters-Stone K, Burckhardt CS. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988–2005). Health and Quality of Life Outcomes. (2006) ; 4: : 67. |
[22] | Busch AJ, Schachter CL, Overend TJ, Peloso PM. Exercise for Fibromyalgia: A Systematic Review. J Rheumatol. (2008) ; 35: (6): 1130-44. |
[23] | Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, et al. A Randomized Trial of Tai Chi for Fibromyalgia. N Engl J Med. (2010) ; 363: (8): 743-54. |
[24] | López-Rodríguez MM, Castro-Sánchez AM, Fernández-Martínez M, Matarán-Peñarrocha GA, Rodríguez-Ferrer ME. Comparación entre biodanza en medio acuático y stretchingen la mejora de la calidad de vida y dolor en los pacientes con fibromialgia. Aten Primaria. (2012) ; 44: (11): 641-50. |
[25] | Langhorst J, Häuser W, Bernardy K, Lucius H, Settan M, Winkelmann A, et al. Complementary and alternative therapies for fibromyalgia syndrome. Systematic review, meta-analysis and guideline. Schmerz. (2012) ; 26: (3): 311-17. |
[26] | Matsutani LA, Marques AP, Ferreira EAG, Assumpção A, Lage LV, Casarroto RA, et al. Effectiveness of muscle stretching exercises with and without laser therapy at tender points for patients with fibromyalgia. Clinical and Experimental Rheumatology. (2007) ; 25: (3): 410-15. |
[27] | Rooks DS, Gautam S, Romeling M, Cross ML, Stratigakis D, Evans B, et al. Group exercise, education, and combination self-management in women with fibromyalgia: a randomized trial. Arch Intern Med. (2007) ; 167: (20): 2192-200. |
[28] | Valencia M, Alonso B, Alvarez MJ, Barrientos MJ, Ayán C, Martín Sanchéz V. Effects of 2 physiotherapy programs on pain perception, muscular flexibility and illness impact in women with fibromyalgia: a pilot study. J Manipulative Physiol Ther. (2009) ; 32: (1): 84-92. |
[29] | Altan L, Korkmaz N, Bingol U, Gunay B. Effect of pilates training on people with fibromyalgia syndrome: a pilot study. Arch Phys Med Rehabil. (2009) ; 90: (12): 1983-8. |
[30] | McCain GA. Role of Physical Fitness Training in the Fibrositis/Fibromyalgia Syndrome. The American Journal of Medicine. (1986) ; 81: (suppl3A): 73-7. |
[31] | Valim V, Oliveira L, Suda A, Silva L, de Assis M, Barros Neto T, et al. Aerobic fitness effects in fibromyalgia. J Rheumatol. (2003) ; 30: (5): 1060-9. |
[32] | López-Rodríguez MM, Castro-Sánchez AM, Fernández-Martínez M, Matarán-Peñarrocha GA, Rodríguez-Ferrer ME. [Comparison between aquatic-biodanza and stretching for improving quality of life and pain in patients with fibromyalgia]. Aten Primaria. (2012) ; 44: (11): 641-9. |
[33] | Valim V, Natour J, Xiao Y, Pereira AF, Lopes BB, Pollak DF, et al. Effects of physical exercise on serum levels of serotonin and its metabolite in fibromyalgia: a randomized pilot study. Rev Bras Reumatol. (2013) ; 53: (6): 538-41. |
[34] | Genc A, Tur BS, Aytur YK, Oztuna D, Erdogan MF. Does aerobic exercise affect the hypothalamic-pituitary-adrenal hormonal response in patients with fibromyalgia syndrome? J Phys Ther Sci. (2015) ; 27: (7): 2225-31. |
[35] | Assumpção A, Matsutani LA, Yuan SL, Santo AS, Sauer J, Mango P, Marques AP. Muscle stretching exercises and resistance training in fibromyalgia: which is better? A three-arm randomized controlled trial. Eur J Phys Rehabil Med. (2018) ; 54: (5): 663-670. |
[36] | Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, et al. A randomized trial of tai chi for fibromyalgia. N Engl J Med. (2010) ; 363: (8): 743-54. |
[37] | Calandre EP, Rodriguez-Claro ML, Rico-Villademoros F, Vilchez JS, Hidalgo J, Delgado-Rodriguez A. Effects of pool-based exercise in fibromyalgia symptomatology and sleep quality: a prospective randomised comparison between stretching and Ai Chi. Clin Exp Rheumatol. (2009) ; 27: (5 Suppl 56): S21-8. |
[38] | Sosa-Reina MD, Nunez-Nagy S, Gallego-Izquierdo T, Pecos-Martín D, Monserrat J, Álvarez-Mon M. Effectiveness of Therapeutic Exercise in Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Biomed Res Int. (2017) ; 2017: : 2356346. |
[39] | Bravo C, Skjaerven LH, Guitard Sein-Echaluce L, Catalan-Matamoros D. Effectiveness of movement and body awareness therapies in patients with fibromyalgia: a systematic review and meta-analysis. Eur J Phys Rehabil Med. (2019) ; 55: (5): 646-657. |
[40] | Kim SY, Busch AJ, Overend TJ, Schachter CL, van der Spuy I, Boden C, et al. Flexibility exercise training for adults with fibromyalgia. Cochrane Database Syst Rev (2019) ; 9: (9): CD013419. |
[41] | Liptan GL. Fascia: A missing link in our understanding of the pathology of fibromyalgia. Journal of Bodywork & Movement Therapies. (2010) ; 14: (1): 3-12. |
[42] | Carson JW, Carson KM, Jones KD, Bennett RM, Wright CL, Mist SD. A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain. (2010) ; 151: (2): 530-9. |
[43] | Gauffin J, Hankama T, Hannonen P, Kautiainen H, Pohjolainen T, Haanpää M. Do fibromyalgia patients use active pain management strategies? A cohort study. J Rehabil Med. (2013) ; 45: (5): 477-80. |
[44] | Jung E, Erbslöh-Möller B, Gesmann M, Kühn-Becker H, Petermann F, Langhorst J. Are members of fibromyalgia syndrome self-help groups “different”? Demographic and clinical characteristics of members and non-members of fibromyalgia syndrome self-help groups. Z Rheumatol. (2013) ; 72: (5): 474-81. |
[45] | Assumpção A, Cavalcante AB, Capela CE, Sauer JF, Chalot SD, Pereira CA, et al. Prevalence of fibromyalgia in a low socioeconomic status population. BMC Musculoskelet Disord. (2009) ; 10: : 64. |
[46] | Bergman S, Herrstrom P, Hogstrom K, Petersson IF, Svensson B, Jacobsson LT. Chronic musculoskeletal pain, prevalence rates and sociodemographic associations in Swedish population study. J Rheumatol. (2001) ; 28: (6): 1369-77. |




