Cervical cancer risk in association with TNF-alpha gene polymorphisms in Bangladeshi women
Abstract
BACKGROUND:
Tumor necrosis factor-alpha (TNF-α) is among the vital pro-inflammatory cytokines that potentially exerts a significant influence on the immune response, hence potentially regulating the advancement of cervical lesions.
OBJECTIVE:
Our study objective was to examine the relationship between two single nucleotide polymorphisms (SNPs) (rs1799724 and rs1800629) of TNF-α and the risk of cervical cancer in women from Bangladesh.
METHODS:
We recruited 133 patients with cervical cancer and 126 healthy individuals for this study. Genotyping was performed using real-time PCR SNP genotyping assay. Multivariate logistic regression analysis was used to determine the odds ratio (OR) along with 95% confidence intervals (CI) and p-values.
RESULTS:
For rs1799724 (C > T) polymorphism, TT mutant homozygous genotype carried 3.26 times increased risk of developing cervical cancer (OR = 3.26, 95% CI = 1.15–9.28, p = 0.027). Polymorphism of rs1800629 (G > A) was also related to an elevated risk of cervical cancer. Individuals with the AG heterozygous genotype (OR = 2.85, 95% CI = 1.20–6.74, p = 0.017) and AA mutant homozygous genotype (OR = 4.55, 95% CI = 1.24–16.60, p = 0.022) also had a higher likelihood of having cervical cancer. Moreover, we found that injectable contraceptives increase the risk of cervical cancer. Individuals who smoked and/or had first-degree relatives with cancer were more likely to carry the risk allele, which increases the likelihood of developing cervical cancer.
CONCLUSION:
TNF-α polymorphisms in rs1799724 and rs1800629 increase the susceptibility of developing cervical cancer in women from Bangladesh.
1Introduction
Cervical cancer is characterized by an uncontrolled growth of abnormal cells in the cervix [1]. The cervix is located at the lower part of the uterus which is a component of the female reproductive system [2]. According to Global Cancer Statistics 2020 (GLOBOCAN), cervical cancer is the seventh most frequently diagnosed cancer in humans. Among women, it ranks fourth as the most prevalent diagnosed cancers and it is also the fourth most common cause of cancer-related death [3]. In Bangladesh, it was found to be the second most frequently diagnosed cancer among females, resulting in 8,268 new cases and 4,971 deaths in 2020 [4]. Infection of the cervix by Human papillomavirus (HPV) is a necessary but not a standalone factor in cervical cancer [5]. Other factors, such as genetic and epigenetic factors contribute to disease progression [2, 6]. About 5% of cervical tumors are not found to be associated with human papillomavirus (HPV) [5].
Persistent inflammation of the cervix due to various inflammatory mediators can cause tumorigenesis [7]. Tumor necrosis factor-α (TNF-α) is a multifunctional proinflammatory cytokine [8], which is mostly produced by monocytes or macrophages and plays a very important role in the regulation of inflammation, immunity, and defense system of host cells [9]. However, increased levels of TNF-α can lead to organ dysfunctions and death [8]. Studies have demonstrated that increased levels of TNF-α in different types of cancer cells are accountable for the process of cell transformation [9]. Overall, TNF-α promotes tumor angiogenesis, tumor cell proliferation, invasion, and metastasis [9].
TNF-α is located in the short arm of chromosome 6 (region p21.3) in the major histocompatibility complex class III region [10], which is about 2.76 kb long and contains 3 introns and 4 exons (Fig. 1) [9]. TNF-α is known to have pleiotropic effects and most of the polymorphisms of this gene are located in its promoter region [11]. Polymorphisms of this gene are suspected to contribute to various disease prognoses including cervical cancer [10].
Fig. 1
Schematic representation of the location of the TNF genes within the Major Histocompatibility Complex (MHC) on chromosome 6. TNF includes lymphotoxin-α (LT-α), tumor necrosis factor (TNF-α), and lymphotoxin-β (LT-β). The positions of the two SNPs, rs1799724 (-857 C>T), and rs1800629 (-308 G>A) in the TNF-α gene are indicated.
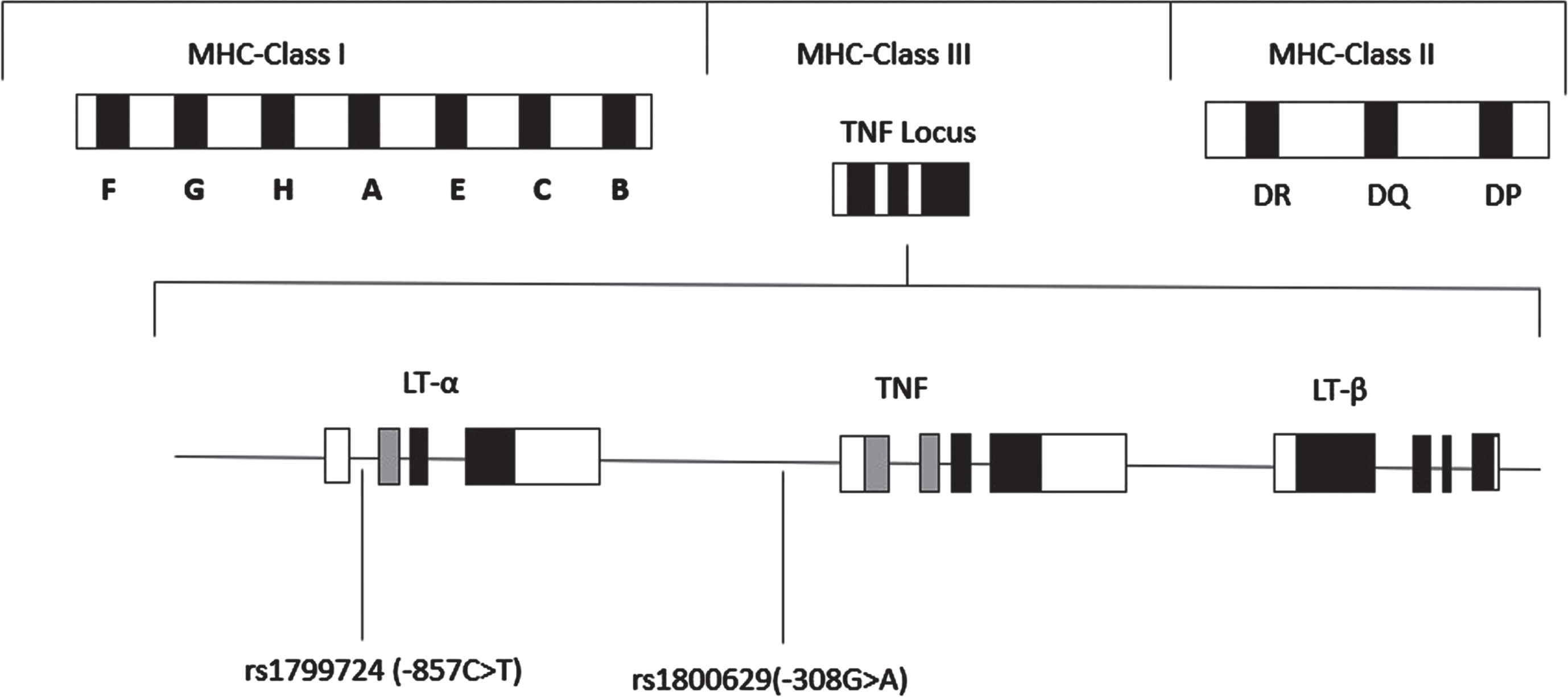
In this study, we investigated two single nucleotide polymorphisms (SNPs) of the TNF-α gene promoter, rs1800629 (–308 G>A) and rs1799724 (–857 C>T), which have been associated with several types of cancer [12–14], including cervical cancer [15–17]. Studies showed that polymorphism of rs1800629 (G > A) can result in the formation of two types of alleles named TNF1 and TNF2. The more common allele TNF1 is defined by the presence of guanine whereas the substitution of guanine with adenosine leads to the formation of TNF2 which results in the alteration of TNF-α expression [18–21]. Furthermore, the rs1799724 polymorphism (–857 C>T), positioned in the promoter region, modifies the immunological response by enhancing the transcription of the TNF-α cytokine. This alteration contributes to the persistent inflammation accompanying Human papillomavirus (HPV) 16 infection, a significant risk factor for cervical cancer [22]. Several case-control studies in the Han Chinese population and Hispanic women reported that polymorphism at this site may increase the risk of cervical cancer [16, 17]. However, a study in Mexican and Indian women revealed that the T allele provides a protective effect rather than conferring risk for cervical cancer [22, 23]. Yet, no significant association for this polymorphism with cervical cancer was reported in Mexican women [24]. Overall, the association of this SNP with cervical cancer is still not conclusive.
Polymorphism of rs1800629 (–308 G>A) and its relation with cervical cancer has been investigated in populations with different ethnic backgrounds and significant positive association was reported in Caucasian, Indian, and Han Chinese women [9, 25–27]. Another study has found that the presence of A allele increased the risk of cervical cancer in Iranian women [15]. A previous meta-analysis reported that rs1800629 polymorphism was significantly associated with an elevated risk of cervical cancer in Caucasian women but not in Asians [28]. In another meta-analysis, A allele of rs1800629 polymorphism was reported to have an association with cervical cancer in women of Chinese, Indian, and Caucasian ethnicities [29]. However, a negative association of this polymorphism (rs1800629) with cervical cancer also has been reported in Chinese and Thai women [30, 31]. A study conducted on Han Chinese women reported that both rs1800629 and rs1799724 polymorphisms were significantly associated with an increased risk of cervical cancer [16].
Overall, the association between these two TNF-α gene polymorphisms rs1800629 (–308 G>A) and rs1799724 (–857 C>T) is not consistent. Hence, our study sought to assess the association between these two SNPs of the TNF-α gene and cervical cancer in women from Bangladesh. Moreover, we investigated the association of these SNPs with the clinicopathological characteristics of the patients.
2Materials and methods
2.1Selection of study population
We included 133 cervical cancer patients and 126 age-matched healthy controls from two hospitals located in Dhaka named Dhaka Cancer and General Hospital and Dhaka Medical College and Hospital, Dhaka during the period of February 2022 to May 2023. Post hoc power calculation was performed for the sample size using G*Power software. The recruited sample achieved a power, (1-β) = 90%, to detect medium effect size, d = 0.4, with an α level of 0.05.
Following the histological diagnosis, the patients were classified based on the staging guidelines set by the International Federation of Gynecology and Obstetrics [32]. The medical records of the patients were reviewed, and it was found that none of them had been infected with HPV and all of them abstained from consuming alcohol during their whole lives. Following a comprehensive medical examination, healthy individuals were recruited as controls. However, healthy controls having a history of brain injury, mental illness, post-traumatic stress disorder, pregnancy, alcohol consumption, and substance abuse were excluded from the study. Written informed consent forms were signed by all study participants, and the study was conducted in compliance with the Declaration of Helsinki and its later revisions [33]. The study protocol (IRB/DU/2021/185-7) was approved by the institutional review board of the University of Dhaka.
2.2DNA extraction and genotyping
3 ml of blood was collected by venipuncture from each study participant in potassium EDTA tubes (BD Vacutainer® blood collection tubes, Becton and Dickinson and Company, USA). Genomic DNA was extracted from 200 μL of blood using the FavorPrep™ blood genomic DNA extraction mini kit (Favorgen Biotech Corp. Taiwan). The quality of the extracted DNA was examined by a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Genotyping of rs1799724 and rs1800629 was carried out using TaqMan SNP genotyping assay products C_11918223_10, C_7514879_10, respectively (Applied Biosystems, Foster City, California, USA) by allelic discrimination assay using fluorogenic probes. Real-time PCR was performed on ABI 7500 Fast Real-Time PCR system in a 10 μL reaction mix containing 40 ng of DNA template, 5 μL of TaqMan® Universal PCR Master Mix, 0.3 μL of 40x assay mixture. The reaction condition was initial incubation at 60°C for 1 minute and 95°C for 10 min, followed by 50 cycles of denaturation at 92°C for 30 s, and annealing/extension at 60°C for 1 min. For quality control, 5% of the samples were randomly selected and genotyped, and the results showed no discrepancy.
2.3Statistical analysis
The statistical analyses were conducted using two-tailed tests. To compare categorical variables, we used a chi-squared test while an independent samples t-test was used for the continuous variables. The Hardy-Weinberg equilibrium was used to find the deviation in the genotype frequencies between the patients’ group and healthy control group. Multivariate logistic regression was used to calculate the adjusted odds ratio (OR) with 95% confidence interval (CI), controlling for age, menstruation status, and tobacco use. A p-value less than 0.05 was considered to be statistically significant. The statistical analyses were conducted using SPSS version 23.
3Results
3.1Characteristics of the study population
Table 1 provides detailed demographical and clinicopathological characteristics of the cases and controls. Our study included a cohort of 259 women, consisting of 133 individuals diagnosed with cervical cancer and 126 healthy controls. A statistically significant difference (p = 0.025) was found between the patients and controls in terms of injectable contraceptives (IC) use, with a higher percentage (11.3%) in the case group compared to the controls (3.2%). This aligns with the findings from previous research articles where users of IC were at higher risk of having cervical cancer [34–36]. Nevertheless, there were no statistically significant differences in terms of age, menopausal state, dwelling status, parity, postmenopausal status, and family history of cancer.
Table 1
Distribution of demographic characteristics of cervical cancer patients and controls
| Characteristics | Cases | Controls | χ2 | p value | |
| (n = 133) (%) | (n = 126) (%) | ||||
| Age | >45 | 71 (53.4) | 55 (43.7) | 2.45 | 0.117 |
| ≤45 | 62 (46.6) | 71 (56.3) | |||
| Family history of cancer | Yes | 17 (12.8) | 21 (16.7) | 0.78 | 0.377 |
| No | 116 (87.2) | 105 (83.3) | |||
| Dwelling | Urban | 28 (21.1) | 21 (16.7) | 0.81 | 0.368 |
| Rural | 105 (78.9) | 105 (83.3) | |||
| Menstrual status | Post-menopause | 58 (43.6) | 64 (50.8) | 1.34 | 0.247 |
| Pre-menopause | 75 (56.4) | 62 (49.2) | |||
| Smoking | Smokers | 30 (22.6) | 18 (14.3) | 2.93 | 0.087 |
| Non-smokers | 103 (77.4) | 108 (85.7) | |||
| Parity | > 7 | 4 (3.0) | 6 (4.8) | 0.54 | 0.464 |
| 0–7 | 129 (97.0) | 120 (95.2) | |||
| Contraception | None | 49 (36.8) | 47 (37.3) | ||
| Oral pills | 57 (42.9) | 68 (53.9) | 0.64 | 0.422 | |
| Injection | 15 (11.3) | 4(3.2) | 5.00 | 0.025* | |
| Others# | 12 (9.0) | 7 (5.6) | 0.94 | 0.334 | |
| Stage of cancer | IA-IB | 27 (20.3) | |||
| IIA-IIB | 70 (52.6) | ||||
| IIIa-IIIB | 36 (27.1) | ||||
| Histopathology | Squamous cell carcinoma | 108 (81.2) | |||
| Adenocarcinoma | 19 (14.3) | ||||
| Others | 6 (4.5) | ||||
| Tumour grade | I | 15 (11.3) | |||
| II | 102 (76.7) | ||||
| III | 16 (12.0) |
#Others: Oral pills + condom(male), Oral pills + combined injectable contraceptives (CIC). *p < 0.05.
3.2Analysis of genotype frequencies of rs1799724 and rs1800629 of TNF-α gene
Table 2 provides a summary of the distribution of genotype frequencies in cases and healthy controls for both of the investigated SNPs. For rs1799724, the frequency of both CT heterozygous genotype and TT mutant homozygous genotype was upregulated in patients compared to controls. Multivariate logistic regression analysis revealed a 3.26-fold increase in the risk of having cervical cancer in individuals with the TT genotype (adjusted OR = 3.26, 95% CI = 1.15–9.28, p = 0.027). In contrast, the CT heterozygous genotype did not show any statistically significant association (p = 0.166). The dominant model (CT+TT vs CC) model also showed a statistically significant association with cervical cancer (adjusted OR = 2.42, 95% CI = 1.20–4.91, p = 0.014). In the allelic model analysis, the presence of the T allele increased the risk of cervical cancer by 2.58 times (adjusted OR = 2.58, 95% CI = 1.45–4.60, p = 0.013).
Table 2
Genotype frequencies of TNF-α gene polymorphisms in cervical cancer patients and controls
| Genotypes | Cases | Controls | Adjusted | 95% Cl | p value | |
| n = 133(%) | n = 126(%) | odds ratio | ||||
| rs1799724 | CC | 104 (78.2) | 113 (89.7) | ref | ref | ref |
| CT | 14 (10.5) | 8 (6.3) | 1.90 | 0.77–4.72 | 0.166 | |
| TT | 15 (11.3) | 5 (4.0) | 3.26 | 1.15–9.28 | 0.027* | |
| CT+TT | 29 (21.8) | 13 (10.3) | 2.42 | 1.20–4.91 | 0.014* | |
| T Allele | 44 (16.6) | 18 (7.2) | 2.58 | 1.45–4.60 | 0.013* | |
| rs1800629 | GG | 101 (76.0) | 115 (91.3) | ref | ref | ref |
| AG | 20 (15.0) | 8 (6.3) | 2.85 | 1.20–6.74 | 0.017* | |
| AA | 12 (9.0) | 3 (2.4) | 4.55 | 1.24–16.60 | 0.022* | |
| AG+AA | 32 (24.1) | 11 (8.7) | 3.31 | 1.59–6.91 | 0.001** | |
| A Allele | 44 (16.6) | 14 (5.6) | 3.37 | 1.80–6.32 | 0.0002** |
*p < 0.05. **p < 0.001.
For rs1800629, the frequencies of AG heterozygous and AA mutant homozygous were greater in patients in comparison to controls (15% vs. 6.3% and 9% vs. 2.4%, respectively). The AA mutant homozygous genotype confers 4.55 times more odds (adjusted OR = 4.55, 95% CI = 1.24–16.60, p = 0.022) of developing cervical cancer. Moreover, the AG heterozygous genotype was also associated with a significantly higher risk (2.85 times) of having cervical cancer (adjusted OR = 2.85, 95% CI=1.20–6.74, p = 0.017). The dominant model (AG+AA vs GG) also demonstrated a significant association (adjusted OR = 3.31, 95% CI = 1.59–6.91, p = 0.001). The presence of the A allele also increased the risk of cervical cancer by 3.37 times (adjusted OR = 3.37, 95% CI = 1.80–6.32, p = 0.0002).
3.3Association of TNF-α polymorphisms with clinicopathological characteristics in patients
The analysis of rs1799724 in cervical cancer patients with varied clinicopathological features revealed that patients with first-degree relatives with cancer had significantly higher odds (p < 0.0001) of carrying the risk allele T compared to those who had no family history of cancer (Table 3). This phenomenon was again found for rs1800629, where among patients with a positive family history of cancer there were more carriers of the mutant A allele (p = 0.0004) compared to those without any first-degree relative with cancer (Table 4). In addition, patients who smoke were more prone (p = 0.0001) to carry the risk A allele and thus had greater odds of developing cervical cancer.
Table 3
Correlation of rs1799724 polymorphisms with clinicopathological characteristics of the patients
| Characteristics | rs1799724 Carrier n = 29 (%) | rs1799724 Non-carrier n = 104 (%) | Odds ratio (OR) | 95% Cl | p Value | |
| Age (years) | ≤45 | 16 (55.2) | 46 (44.2) | ref | ref | – |
| > 45 | 13 (44.8) | 58 (55.8) | 0.64 | 0.28–1.47 | 0.298 | |
| Family history of cancer | No | 17 (58.6) | 99 (95.2) | ref | ref | – |
| Yes | 12 (41.4) | 5 (4.8) | 13.98 | 4.37–44.73 | <0.0001* | |
| Dwelling status | Rural | 21 (72.4) | 84 (80.8) | ref | ref | – |
| Urban | 8 (27.6) | 20 (19.2) | 1.6 | 0.62–4.13 | 0.332 | |
| Menstrual status | Pre-menopause | 16 (55.2) | 59 (56.7) | ref | ref | – |
| Post-menopause | 13 (44.8) | 45 (43.3) | 1.07 | 0.47–2.43 | 0.881 | |
| Tobacco use | Non-smokers | 20 (69.0) | 83 (79.8) | ref | ref | – |
| Smokers | 9 (31.0) | 21 (20.2) | 1.78 | 0.71–4.47 | 0.220 | |
| Parity | 0–7 | 28 (96.6) | 101 (97.1) | ref | ref | – |
| > 7 | 1 (3.4) | 3 (2.9) | 1.20 | 0.12–12.01 | 0.875 | |
| Contraception | None | 12 (41.4) | 37 (35.6) | ref | ref | – |
| Oral pills | 12 (41.4) | 45 (43.3) | 0.82 | 0.33–2.04 | 0.674 | |
| Injection | 3 (10.3) | 12 (11.5) | 0.77 | 0.19–3.20 | 0.720 | |
| Others# | 2 (6.9) | 10 (9.6) | 0.62 | 0.19–3.22 | 0.566 |
#Others: Oral pills + condom(male), Oral pills + combined injectable contraceptives (CIC). *p < 0.05.
Table 4
Correlation of rs1800629 polymorphisms with clinicopathological characteristics of the patients
| Characteristics | rs1800629 Carrier n = 32 (%) | rs1800629 Non-carrier n = 101 (%) | Odds ratio (OR) | 95% Cl | p Value | |
| Age (years) | ≤45 | 16 (50) | 46 (45.5) | Ref | Ref | – |
| >45 | 16 (50) | 55 (54.5) | 0.84 | 0.38-1.85 | 0.660 | |
| Family history | No | 21 (65.6) | 95 (94.1) | Ref | Ref | – |
| Yes | 11 (34.4) | 6 (5.9) | 7.33 | 2.43-22.11 | 0.0004* | |
| Dwelling status | Rural | 27 (84.4) | 78 (77.2) | Ref | Ref | – |
| Urban | 5 (15.6) | 23 (22.8) | 0.63 | 0.22–1.82 | 0.390 | |
| Menstrual status | Pre-menopause | 17 (53.1) | 58 (57.4) | Ref | Ref | – |
| Post-menopause | 15 (46.9) | 43(42.6) | 1.19 | 0.54–2.64 | 0.669 | |
| Tobacco use | Non-smokers | 16 (50) | 87 (86.1) | Ref | Ref | – |
| Smokers | 16 (50) | 14 (13.9) | 6.21 | 2.54–15.19 | 0.0001* | |
| Parity | 0–7 | 31 (96.9) | 98 (97.1) | Ref | Ref | – |
| >7 | 1 (3.1) | 3 (2.9) | 0.95 | 0.09–9.45 | 0.965 | |
| Contraception | None | 8 (25.0) | 41 (40.6) | Ref | Ref | – |
| Oral pills | 19 (59.4) | 38 (37.6) | 2.56 | 1.00–6.54 | 0.049 | |
| Injection | 2 (6.2) | 13 (12.9) | 0.79 | 0.15–4.19 | 0.780 | |
| Others# | 3 (9.4) | 9 (8.9) | 1.71 | 0.34–7.74 | 0.487 |
#Others: Oral pills+condom(male), Oral pills + combined injectable contraceptives (CIC). *p < 0.05.
4Discussion
We aimed to evaluate the association between two TNF-α gene polymorphisms and the susceptibility to cervical cancer in Bangladeshi women. Our investigation revealed that both rs1799724 and rs1800629 SNPs polymorphisms were significantly associated with cervical cancer. Additionally, we observed that the use of injectable contraceptives may be a significant factor in the development of cervical cancer.
In our analysis, we found that the CT genotype of the SNP rs1799724 had 1.71 times more risk to develop cervical cancer, whereas individuals with the TT genotype had a 3.26-fold increased risk. Our findings are in alignment with previous case-control studies which described that the presence of T allele increased the risk of developing cervical cancer in Han Chinese and Hispanic women [16, 17]. However, our findings contradict the findings from a case-control study with Mexican population which reported no significant association of this polymorphism with cervical cancer [24]. Interestingly a study with the Indian population revealed that T allele has a protective effect rather than conferring risk [23]. As a result, the effect of this polymorphism on cervical cancer is still inconsistent. As far as we are aware, this is the first case-control study conducted in the Bangladeshi population where we have found cervical cancer risk effect of rs1799724 in women from Bangladesh. Therefore, to corroborate our findings, larger sample sizes and a wider range of ethnicities must be studied in the future.
Regarding the SNP rs1800629, we have found a statistically significant association with cervical cancer. The AG and AA genotypes upregulated the risk of cervical cancer by 2.81 and 4.55 times, respectively. Our results are consistent with a number of earlier genetic epidemiology studies that reported polymorphism at this site imparts a significant risk of developing cervical cancer in Chinese, Portuguese, Argentine, Indian, Polish, and Iranian women [8, 9, 15, 16, 25–27, 37]. This finding was further supported by several meta-analysis results which indicated a significant association between the TNF-α gene rs1800629 polymorphism and the risk of developing cervical cancer [28, 29, 38–41]. In contrast, a lack of association was found between rs1800629 polymorphism and cervical cancer in the Thai and South African populations [30, 42]. The discrepancy may have arisen from ethnic differences which can modify the expression of the TNF-α gene in women from diverse ethnicities. Both rs1799724 and rs1800629 are situated in the promoter region of TNF- α and polymorphisms in this site increase the risk developing of cervical cancer.
In addition, cervical cancer may be influenced by various environmental factors. Our study found that women who preferred injectable contraceptives as birth control had a higher susceptibility (p = 0.025) for developing cervical cancer. Similar findings were observed in Latin American and South African women where long-term usage of injectable contraceptives imparts an increased risk of cervical cancer [34, 36]. The risk was even greater in recent users of injectable contraceptives compared to those who never used [36]. The use of oral contraceptives is considered a prime risk factor for cervical cancer [43]. In our study, self-reported data on injectable contraceptives were collected from both patients and controls and we could not get information if they were using injectable progestin contraceptives or a combination of progestin-estrogen contraceptives, thus we cannot confirm which contraceptive confers risk of cervical cancer. Further studies are required to fully understand the precise mechanism by which injectable contraceptives induce risk for cervicalcancer.
Next, our analysis of the genetic polymorphisms in the context of distinct clinicopathological features yielded intriguing findings. We found a higher prevalence of T risk allele of rs1799724 (p < 0.0001) and A risk allele of rs1800629 (p = 0.0004) in patients who had a family history (first-degree relatives) of cancer. Similarities in genetic profile and shared environmental factors may contribute to this vulnerability. Family history was found to be a contributing factor for carrying risk alleles of rs1800795 of the IL-6 gene in a separate group of Bangladeshi cervical cancer patients [44]. However, Moosazadeh et al. reported no statistically significant association between a family history of cancer with cervical cancer [45]. Further genetic linkage studies with cervical cancer patients and their families are thus warranted.
Additionally, we also discovered that the smokers were more often carriers of the risk A allele of rs1800629 (p < 0.0001). Several studies found a strong association between smoking and the risk of developing cervical cancer [46, 47]. Sugawara et al. conducted a meta-analysis among Japanese women and reported that smokers had a higher risk of developing cervical cancer than nonsmokers [48].
There were some limitations in our study. We did not include cervical cancer patients with HPV infection. Therefore, we cannot ascertain whether comparable results will be obtained in individuals who are afflicted with HPV. Furthermore, we had a relatively small sample size and thus it would only detect large effects. Moreover, the power to detect genotype–disease association may be insufficient. Due to the absence of gene or protein expression data, we were unable to ascertain whether the polymorphism in these two SNPs has any cis-regulatory influence on gene or protein expression.
5Conclusion
In conclusion, polymorphisms at rs1799724 and rs1800629 on the TNF-α gene were found to be significantly associated with cervical cancer in women from Bangladesh. To validate our findings, more research with a broader sample size and a range of ethnicities is required.
Acknowledgments
The authors would like to thank the study participants, doctors, and nurses for their contribution in the study. Moreover, the authors acknowledge the full support of the Department of Pharmaceutical Sciences, North South University during the study.
Author contributions
CONCEPTION: M.M., N.A.
DATA CURATION: Z.T., S.S., M.P., S.M., A.F., M.R., T.S., N.C., N.A.
ANALYSIS OF DATA: Z.T., S.S., M.P.
PREPARATION OF THE MANUSCRIPT: Z.T., S.S., M.P., S.M.,
REVISION FOR IMPORTANT INTELLECTUAL CONTENT: M.H., T.S., N.C., N.A.
SUPERVISION: M.M.
Conflict of interest
The authors declare no conflict of interest.
Data availability
All data related to the manuscript are provided in the manuscript main file, figure, and tables. The corresponding author will provide additional information on a valid request, if required.
Funding
The authors did not receive any specific funding for this work.
References
[1] | Wang H , Lakshmipriya T , Chen Y , Gopinath SCB . Squamous Cell Carcinoma Biomarker Sensing on a Strontium Oxide-Modified Interdigitated Electrode Surface for the Diagnosis of Cervical Cancer. Biomed Res Int. (2019) ;2019: . https://doi.org/10.1155/2019/2807123. |
[2] | Ramachandran D , Dörk T Genomic risk factors for cervicalcancer. Cancers (Basel). (2021) ;13. https://doi.org/10.3390/cancers13205137. |
[3] | Sung H , Ferlay J , Siegel RL , Laversanne M , Soerjomataram I , Jemal A , et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2021) ;71: :209–49. https://doi.org/10.3322/caac.21660. |
[4] | The Global Cancer Observatory. Cancer Fact Sheet Bangladesh. (2021) ;745: :1–2. |
[5] | Fernandes A , Viveros-Carreño D , Hoegl J , Ávila M , Pareja R Human papillomavirus-independent cervical cancer. Int J Gynecol Cancer. (2022) ;32: :1–7. https://doi.org/10.1136/ijgc-2021-003014. |
[6] | Revathidevi S , Murugan AK , Nakaoka H , Inoue I , Munirajan AK APOBEC: A molecular driver in cervical cancer pathogenesis. Cancer Lett. (2021) ;496: :104–16. https://doi.org/10.1016/j.canlet.2020.10.004. |
[7] | Mosaffa F , Kalalinia F , Lage H , Afshari JT , Behravan J Pro-inflammatory cytokines interleukin-1 beta, interleukin 6, and tumor necrosis factor-alpha alter the expression and function of ABCG2 in cervix and gastric cancer cells. Mol Cell Biochem. (2012) ;363: :385–93. https://doi.org/10.1007/s11010-011-1191-9. |
[8] | Barbisan G , Pérez LO , Contreras A , Golijow CD TNF-αand IL-10 promoter polymorphisms, HPV infection, and cervical cancerrisk. Tumor Biology. (2012) ;33: :1549–56. https://doi.org/10.1007/s13277-012-0408-1. |
[9] | Yang J , Wang Y , Zhang S , Li Y , Li C , Liu W , et al. The Associationof TNF-α Promoter Polymorphisms with Genetic Susceptibility to Cervical Cancer in a Chinese Han Population. Int J Gen Med. (2022) ;15: :417–27. https://doi.org/10.2147/IJGM.S350263. |
[10] | El-Tahan RR , Ghoneim AM , El-Mashad N TNF-α genepolymorphisms and expression. Springerplus. (2016) ;5.https://doi.org/10.1186/s40064-016-3197-y. |
[11] | Lee E , Ouzounova M , Piranlioglu R , Thu M , Mustafa M , Daniela G , et al. The pleiotropic effects of TNF α in breast cancersubtypes is regulated by TNFAIP3 / A20. Oncogene. (2018) . https://doi.org/10.1038/s41388-018-0472-0. |
[12] | Abdalhabib EK , Algarni A , Saboor M , Alanazi F , Ibrahim IK , Alfeel AH , et al. Association of TNF-α rs1800629 with Adult Acute B-Cell Lymphoblastic Leukemia. Genes (Basel). (2022) ;13: :1–8. https://doi.org/10.3390/genes13071237. |
[13] | Mao YQ , Dong SQ , Gao M Association between TNF-α rs1799724 and rs1800629 polymorphisms and the risk of Crohn’s disease. Genetics and Molecular Research. (2015) ;14: :15811–21. https://doi.org/10.4238/2015.December.1.33. |
[14] | Shi LX , Zhang L , Zhang DL , Zhou JP , Jiang XJ , Jin YL , et al. Association between TNF-α G-308A (rs1800629) polymorphism and susceptibility to chronic periodontitis and type 2 diabetes mellitus: A meta-analysis. J Periodontal Res. (2021) ;56: :226–35. https://doi.org/10.1111/jre.12820. |
[15] | Behboodi N , Farazestanian M , Rastgar-Moghadam A , Mehramiz M , Karimi E , Rajabian M , et al. Association of a variant in the tumor necrosis factor alpha gene with risk of cervical cancer. Mol Biol Re. (2021) ;48: :1433–7. https://doi.org/10.1007/s11033-021-06185-4. |
[16] | Li X , Yin G , Li J , Wu A , Yuan Z , Liang J , et al. The correlation between TNF-α promoter gene polymorphism and genetic susceptibility to cervical cancer. Technol Cancer Res Treat. (2018) ;17: :1–7. https://doi.org/10.1177/1533033818782793. |
[17] | Yin G , Zhu T , Li J , Wu A , Liang J , Zhi Y CXCL12 rs266085 and TNF-α rs1799724 polymorphisms and susceptibility to cervical cancer in a Chinese population. Int J Clin Exp Pathol. (2015) ;8: :5768–74. |
[18] | Elahi MM , Asotra K , Matata BM , Mastana SS Tumor necrosis factor alpha - 308 gene locus promoter polymorphism: An analysis of association with health and disease. Biochim Biophys Acta Mol Basis Dis. (2009) ;1792: :163–72. https://doi.org/10.1016/j.bbadis.2009.01.007. |
[19] | Ibrahim A , Rahman HA , Khorshied M , Sami R , Nasr N , Khorshid O Tumor necrosis factor alpha-308 and Lymphotoxin alpha+252 genetic polymorphisms and the susceptibility to non-Hodgkin lymphoma in Egypt. Leuk Res. (2012) ;36: :694–8. https://doi.org/10.1016/j.leukres.2011.11.016. |
[20] | Šedý J , Bekiaris V , Ware CF Tumor necrosis factor superfamily in innate immunity and inflammation. Cold Spring Harb Perspect Biol. (2015) ;7. https://doi.org/10.1101/CSHPERSPECT.A016279. |
[21] | Takeuchi S , Takeuchi N , Tsukasaki K , Bartram CR , Zimmermann M , Schrappe M , et al. Genetic polymorphisms in the tumour necrosis factor locus in childhood acute lymphoblastic leukaemia. Br J Haematol. (2002) ;119: :985–7. https://doi.org/10.1046/J.1365-2141.2002.03964.X. |
[22] | Deshpande A , Nolan JP , White PS , Valdez YE , Hunt WC , Peyton CL , et al. TNF-α promoter polymorphisms and susceptibility to human papiflomavirus 16-associated cervical cancer. Journal of Infectious Diseases. (2005) ;191: :969–76.https://doi.org/10.1086/427826. |
[23] | Kohaar I , Hussain S , Kumar A , Singhal P , Choudhury S , Das B , et al. Impact of Haplotype TNF-LTA Locus with Susceptibility to Cervical Cancer in Indian Population. Obstetrics & Gynecology: An International Journal. (2014) :1–18.https://doi.org/10.5171/2014.831817. |
[24] | Nieves-Ramirez ME , Partida-Rodriguez O , Alegre-Crespo PE , Tapia-Lugo M del C , Perez-Rodriguez ME Characterization of Single-Nucleotide Polymorphisms in the Tumor Necrosis Factor α Promoter Region and in Lymphotoxin α in Squamous Intraepithelial Lesions, Precursors of Cervical Cancer. Transl Oncol. (2011) ;4: :336–44.https://doi.org/10.1593/tlo.11226. |
[25] | Roszak A , Misztal M , Sowińska A , Jagodziński PP TNF-α -308G/A as a Risk Marker of Cervical Cancer Progression in the Polish Population. Mol Diagn Ther. (2015) ;19: :53–7.https://doi.org/10.1007/s40291-015-0130-y. |
[26] | Sousa H , Oliveira S , Santos AM , Catarino R , Moutinho J , Medeiros R Tumour necrosis factor alpha 308G/A is a risk marker for the progression from high-grade lesions to invasive cervical cancer. Tumor Biology. (2014) ;35: :2561–4. https://doi.org/10.1007/s13277-013-1337-3. |
[27] | Thakre TR , Singh A , Mitra M Association of TNF-308 gene polymorphism with Cervix Cancer susceptibility among women of Chhattisgarh. Res J Pharm Technol. (2019) ;12: :2339–42. |
[28] | Li M , Han Y , Wu TT , Feng Y , Wang HB Tumor Necrosis Factor Alpha rs1800629 Polymorphism and Risk of Cervical Lesions: A Meta-Analysis. PLoS One. (2013) ;8. https://doi.org/10.1371/journal.pone.0069201. |
[29] | Wang Y , Yang J , Huang J , Tian Z Tumor Necrosis Factor-αPolymorphisms and Cervical Cancer: Evidence from a Meta-Analysis. Gynecol Obstet Invest. (2020) ;85: :153–8.https://doi.org/10.1371/journal.pone.0069201. |
[30] | Chinchai T , Homchan K , Sopipong W , Chansaenroj J , Swangvaree S , Junyangdikul P , et al. Lack of associations between TNF-α polymorphisms and cervical cancer in Thai women. Asian Pacific Journal of Cancer Prevention. (2016) ;17: :953–6.https://doi.org/10.7314/APJCP.2016.17.3.953. |
[31] | Wang N , Yin D , Zhang S , Wei H , Wang S , Zhang Y , et al. TNF-Alpha rs1800629 Polymorphism Is Not Associated with HPV Infection or Cervical Cancer in the Chinese Population. PLoS One. (2012) ;7: :2–6. https://doi.org/10.1371/journal.pone.0045246. |
[32] | Hee SK , Yong SS International Federation of Gynecology and Obstetrics (FIGO) staging system revised: What should be considered critically for gynecologic cancer? J Gynecol Oncol. (2009) ;20: :135–6. https://doi.org/10.3802/jgo.2009.20.3.135. |
[33] | World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects JAMA. (2013) ;310: :2191–4. https://doi.org/10.1001/JAMA.2013.281053. |
[34] | Herrero R , Brinton LA , Reeves WC , Brenes MM , De Britton RC , Tenorio F , et al. Injectable contraceptives and risk of invasive cervical cancer: Evidence of an association. Int J Cancer. (1990) ;46: :5–7. https://doi.org/10.1002/IJC.2910460103. |
[35] | Iversen L , Fielding S , Lidegaard Ø , Hannaford PC Contemporary hormonal contraception and cervical cancer in women of reproductive age. Int J Cancer. (2021) ;149: :769–77. https://doi.org/10.1002/IJC.33585. |
[36] | Urban M , Banks E , Egger S , Canfell K , O’Connell D , Beral V , et al. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black south african women: Case-control study. PLoS Med. (2012) ;9: :1–11. https://doi.org/10.1371/journal.pmed.1001182. |
[37] | Du G-H , Wang J-K , Richards JR , Wang J-J Genetic polymorphisms in tumor necrosis factor alpha and interleukin-10 are associated with an increased risk of cervical cancer. Int Immunopharmacol. (2019) ;66: :154–61. https://doi.org/10.1016/j.intim2018.11.015. |
[38] | Ding B , Fu S , Wang M , Yue C , Wang W , Zhou D , et al Tumor necrosis factor α -308G>A polymorphisms and cervical cancer risk: Ameta-analysis. International Journal of Gynecological Cancer. (2012) ;22: :213–9. https://doi.org/10.1097/IGC.0b013e3182375aed. |
[39] | Farbod M , Karimi-Zarchi M , Heiranizadeh N , Seifi-Shalamzari N , Akbarian-Bafghi MJ , Jarahzadeh MH , et al. Association of TNF-α -308G>A Polymorphism with Susceptibility to Cervical Cancer and Breast Cancer - a Systematic Review and Meta-analysis. Klinicka Onkologie. (2019) ;32: :170–80.https://doi.org/10.14735/amko2019170. |
[40] | Hamadani S , Kamali M , Hantoushzadeh S , Tabatabaee RS , Neamatzadeh H , Foroughi E , et al. Association of the s1799724 and rs1800629 Polymorphisms of the TNF-α Gene with Susceptibility to Cervical Cancer: a Systematic Review and Meta-Analysis based on 24 Case-Control Studies. Asian Pacific Journal of Cancer Care. (2017) ;2: :29. https://doi.org/10.31557/apjcc.2017.2.2.29. |
[41] | Liu L , Yang X , Chen X , Kan T , Shen Y , Chen Z , et al Association between TNF-α polymorphisms and cervical cancer risk: Ameta-analysis. Mol Biol Re. (2012) ;39: :2683–8. https://doi.org/10.1007/s11033-011-1022-9. |
[42] | Govan VA , Constant D , Hoffman M , Williamson AL The allelic distribution of -308 Tumor Necrosis Factor-alpha gene polymorphism in South African women with cervical cancer and control women. BMC Cancer. (2006) ;6: :1–6. https://doi.org/10.1186/1471-2407-6-24. |
[43] | Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. (2007) ;370: :1609–21. https://doi.org/10.1016/S0140-6736(07)61684-5. |
[44] | Shaswati M , Oeishy FH , Mumu SB , Zahid MZI , Hossain M , Haque MA , et al. Polymorphisms of the interleukin-6 (IL-6) gene contribute to cervical cancer susceptibility in Bangladeshi women: A case-control study. Health Sci Re. (2023) ;6. https://doi.org/10.1002/hsr2.1238. |
[45] | Moosazadeh M , Karimi A , Zaboli E , Hedayatizadeh-Omran A , Reza A , Kheradmand M Risk of ovarian and cervical cancer in women with positive cancer family history: Results of tabari cohort study. Clin Cancer Investig J. (2021) ;10. |
[46] | Fujita M , Tase T , Kakugawa Y , Hoshi S , Nishino Y , Nagase S , et al. Smoking, earlier menarche and low parity as independent risk factors for gynecologic cancers in Japanese: A case-control study. Tohoku Journal of Experimental Medicine. (2008) ;216: :297–307. https://doi.org/10.1620/tjem.216.297. |
[47] | Hirose K , Hamajima N , Takezaki T , Kuroishi T , Kuzuya K , Sasaki S , et al. Smoking and dietary risk factors for cervical cancer at different age group in Japan. J Epidemiol. (1998) ;8: :6–14. https://doi.org/10.2188/jea.8.6. |
[48] | Sugawara Y , Tsuji I , Mizoue T , Inoue M , Sawada N , Matsuo K , et al. Cigarette smoking and cervical cancer risk: An evaluation based on a systematic review and meta-analysis among Japanese women. Jpn J Clin Oncol. (2019) ;49: :77–86. https://doi.org/10.1093/jjco/hyy158. |




