Role of MEK1 and DIAPH3 expression in colorectal adenoma-carcinoma sequence
Abstract
BACKGROUND:
It is well established that most colorectal carcinomas arise from conventional adenomas through the adenoma-carcinoma sequence (ACS) model. mitogen-activated protein kinases (MAPKs) pathway has been reported as a crucial player in tumorigenesis. The MAPK signaling pathway is activated by different extracellular signals involving the “mitogen-activated/extracellular signal-regulated kinase 1 (MEK1)”, and this induces the expression of genes involved in proliferation and cellular transformation. Diaphanous-related formin-3 (DIAPH3) acts as a potential metastasis regulator through inhibiting the cellular transition to amoeboid behavior in different cancer types.
OBJECTIVE:
The aim of the study was to investigate the pattern of immunohistochemical expression of MEK1 and DIAPH3 in colorectal adenoma (CRA) and corresponding colorectal carcinoma (CRC) specimens.
METHODS:
The immunohistochemical expression of DIAPH3 and MEK1 was examined in 43 cases of CRC and their associated adenomas using tissue microarray technique.
RESULTS:
MEK1 was overexpressed in 23 CRC cases (53.5%) and in 20 CRA cases (46.5%). DIAPH3 was overexpressed in 11 CRA cases (about 29%) which were significantly lower than CRC (22 cases; 58%) (P = 0.011). Both MEK1 and DIAPH3 overexpression were significantly correlated in CRC (P = 0.009) and CRA cases (P = 0.002). Tumors with MEK1 overexpression had a significantly higher tumor grade (P = 0.050) and perineural invasion (P = 0.017).
CONCLUSIONS:
Both MEK1 and DIAPH3 are overexpressed across colorectal ACS with strong correlation between them. This co- expression suggests a possible synergistic effect of MEK1 and DIAPH-3 in colorectal ACS. Further large-scale studies are required to investigate the potential functional aspects of MEK1 and DIAPH3 in ACS and their involvement in tumor initiation and the metastatic process.
1Introduction
Conventional adenoma are well established precursor of colorectal carcinomas as detailed in the widely accepted model of the adenoma-carcinoma sequence (ACS), which represents the major pathway in colorectal carcinogenesis [1]. Adenomas exhibit a gradual accumulation of genetic and epigenetic alterations which is the leading cause of tumor development. These alterations, either single or combined, result in the development of tumors with varying clinicopathological characteristics [2].
The mitogen-activated protein kinases (MAPKs) pathway has been reported as a well-established player in tumorigenesis. MAPKs controls key signal transduction mechanisms that regulate cellular growth, differentiation and apoptosis, it also play a central role in the initiation and progression of cancers [3]. The MAPKs family’s first member, “Extracellular signal-regulated protein kinase (ERK)”, is activated by MAPK/ERK kinase (MEK). MEK1 and MEK2 possess a significant role in cancer pathogenesis as promoting mutations in the cDNAs encoding MEK1 and MEK2 can induce cell transformation and contribute to carcinogenesis [4].
Diaphanous-related formin-3 (DIAPH3) has an important function in nucleation, elongation and bundling of linear actin filaments and regulates microtubule stability through direct interaction with microtubules [5, 6]. Thus, DIAPH3 could act as regulator of metastasis through inhibiting the cells from gaining the amoeboid behavior in different types of cancers. DIAPH3 silencing resulted in destabilized microtubules and prompted endosomal accumulation of epidermal growth factor receptor (EGFR), and hyper-activation of EGFR/MEK/ERK signaling human carcinoma cells. DIAPH3 silencing promotes cell proliferation, migration, colony formation, epithelial-mesenchymal transition, and metastasis through activation of beta-catenin/TCF pathway [7]. It has also been reported that DIAPH3 deficiency enhances cellular motility, invasion which are important pillars in the metastatic cascade, and in human cancers, decreased DIAPH3 expression significantly correlates with aggressive behavior and metastatic events [8]. Aberrant activations of RAS-ERK and Wnt/β-catenin pathways are common occurrences in a variety of human malignancies including CRC [9]. Therefore, we hypothesized that MEK1 and DIAPH3 expressions may have a significant interplay at the process of colorectal carcinogenesis. A correlated overexpression of DIAPH3 and MEK1 was previously observed in colorectal carcinoma [6]. So far as we know, the involvement of MEK1 and DIAPH3 in adenoma-carcinoma sequence has not been studied yet. In the current study, we aimed to investigate the pattern of immunohistochemical expression of MEK1 and DIAPH3 in colorectal adenomas (CRAs) and corresponding CRCs arising on top of these adenomas using tissue microarray technique through correlating the expression of those proteins with the clinicopathological parameters of the studied cases.
2Materials and methods
2.1Samples
Colorectal carcinoma cases arising on a background of adenomas were searched for at the records of surgical pathology laboratory at Gastroenterology Center, Mansoura University, Egypt. Forty-three cases were found in the period from January 2007 to January 2012. The Patients included in the study had no history of neoadjuvant therapy whether radiotherapy or chemotherapy.
2.2Clinical data and histopathological evaluation
Clinicopathological data (including the H&E stained slides) were reviewed. This includes: gender, age, size, site, shape, histological type, depth of invasion (T), grade, pushing or infiltrating tumor edges, perineural and lymphovascular invasion, intra- and peri- tumoral lymphocytic infiltration, nearby and distant mucosa, neutrophilic infiltrate extent, metastases in lymph nodes (N), distant metastasis (M), TNM staging as per the latest WHO classification of CRC [10], state of surgical cut margins, related schistosomiasis or any additional abnormalities. The pathological findings of the associated adenomas were also reported especially degree of dysplasia, associated inflammatory cell infiltrate and associated familial polyposis coli.
2.3Tissue microarray construction
H&E-stained sections were reviewed to select representative areas for tissue microarray (TMA) construction. High density manual tissue microarray blocks were created using a previously described modified mechanical pencil tip technique [11]. For each case, three 0.8 mm diameter cores were cut-out from representative areas of the paraffin blocks. Routine 4 μm H&E sections were prepared from the TMA blocks. Sections for immunohistochemistry were prepared using positive charged slides.
2.4Immunohistochemistry
5 μm sections were cut and mounted on super frost plus slides. Sections were immunostained with 0.1 ml of anti- DIAPH3 Antibody (Novus Biologicals cat # NBP1-89099) and anti- MEK1 [p Ser221] Antibody (Novus Biologicals cat # NBP1-41823). The Power-Stain™ 1.0 Poly HRP DAB Kit for Mouse + Rabbit (Genemed Biotechnologies, inc, cat#52-0017) detection kit was used. Immunostaining was done according to the manufactures’ protocol. Slides were incubated at 57°C for 10 minutes in an oven followed by a dewaxing step through immersing the slides in warm xylene for 10 minutes and afterwards they were immersed for additional 10 minutes in room temperature xylene. After the last step a rehydration step was performed, slides were immersed in declining concentrations of alcohol followed by tap water. Antigen retrieval step was performed through immersion of slides in Citrate buffer pH6 in a standard microwave (Power 800 W) for 20 minutes till the point of boiling. After that, the slides were rinsed two times 10 minutes each by PBS-T (phosphate buffered saline+ 5% Triton-X100). To block endogenous peroxidase activity, a few droplets of alcohol based hydrogen peroxidase were added on the slides for 20 minutes in a humidity chamber, followed by 3 washes by PBS-T, 3 minutes each wash. MEK1 antibody concentration of 1:200 was used, and 1:100 for DIAPH3 (concertation of an antibody was chosen after optimization). The slides were incubated in a humidity chamber at room temperature with the diluted antibodies for an hour. Slides were then washed 3 times by PBS-T, 3 minutes each wash. The secondary antibody was applied and incubated with the slides for 30 minutes, then washed three times by PBS-T times, 3 minutes per wash. DAB-chromogen was added for 3-5 minutes, which was followed by counterstaining using Mayer’s hematoxylin.
2.5Immunohistochemical analysis
H-score from 0 to 300 was used to give a score for each core. H-score is achieved by multiplying the value of the intensity of cytoplasmic staining (0 = negative; 1 = weak; 2 = moderate; 3 = strong) by the proportion of positive cells (0 to 100%). The mean value was calculated for every case. An arbitrary cut-off point was chosen, that is the median value of H-scores for MEK1 and DIAPH3. Tumors were classified as either “Negative/low expression”; negative/less than the median value, and “high expression”; equal to/greater than the median value. During the procedure of slide preparation and staining, occasional cores were lost which may lead to minor variability in number of cases analyzed at each statistical test.
2.6Statistical analysis
Data analysis was done using SPSS for Windows version 20.0 (SPSS Inc, IBM, Chicago, Illinois). Chi-square test (χ2) was utilized to assess differences in the categorical variables among various groups. In any of the tests used, a 2-tailed p value of≤0.05 was considered significant.
2.7Ethical approval
The study was approved by the members of the Medical Research Ethics Committee, Institutional Review Board (IRB), Faculty of Medicine, Mansura University, Mansura, Egypt, with Code No.: R.22.12.1991.
3Results
3.1Clinicopathological features of CRC and CRA cases
43 cases of CRC and their associating adenomas were evaluated. Patients’ age range was 27 to 80 years (mean, 56.22 years). Tissues from 29 males and 14 females were included in the study. 27 cases (62.8%) were of non-mucinous adenocarcinoma (NMA) and 16 cases of mucinous adenocarcinoma type (MA). The clinicopathological characteristics the cases are listed in Table 1.
Table 1
Clinicopathological features of 43 patients with colorectal adenomas
| Variable | No. of patients (%) |
| Association with FAP* | |
| - Negative | 37 (86.0) |
| - Positive | 6 (14.0) |
| Degree of dysplasia of adenoma | |
| - Mild to moderate (low grade) | 20 (46.5) |
| - Severe (high grade) | 23 (53.5) |
| Chronic inflammatory cell infiltrate of adenoma | |
| - No/mild | 23 (53.5) |
| - moderate/marked | 20 (46.5) |
*FAP = familial adenomatous polyposis syndrome.
3.2MEK1 expression in CRC and CRA
The median value of MEK expression in CRA and CRC cases that was used to divide cases into negative/low expression and high expression groups was 188 in this study. Cytoplasmic MEK1 was overexpressed in 23 CRC cases out of the 43 analyzed cases (about 53.5% of the cases) (17 cases “63% ” of NMA and 6 cases “37.5% ” of MA), which was not statistically significant (P = 0.106). MEK1 was overexpressed in 20 CRA cases (46.5%) which was also not significantly different from CRC (P = 0.518, 0.780, 0.446 for all cases, NMA cases and MA cases respectively) (Table 2) (Fig. 1a and b).
Table 2
MEK1 expression in CRC cases and corresponding adenomas
| MEK1 expression | Chi-square | P value | ||
| Negative/Low | High | |||
| - All carcinomas (n. = 43) | 20 (46.5%) | 23 (53.5%) | 0.419 | 0.518 |
| - All adenomas | 23 (53.5%) | 20 (46.5%) | ||
| - Non-mucinous carcinomas (n. = 27) | 10 (37.0%) | 17 (63.0%) | 0.078 | 0.780 |
| - Corresponding adenomas | 11 (40.7%) | 16 (59.3%) | ||
| - Mucinous carcinomas (n. = 16) | 10 (62.5%) | 6 (37.5%) | 0.582 | 0.446 |
| - Corresponding adenomas | 12 (75.0%) | 4 (25.0%) | ||
Fig. 1
MEK1 and DIAPH3 expression in colorectal carcinoma and adenoma. (A) Marked MEK1 cytoplasmic staining in a case of grade II CRC (B) Moderate MEK1 cytoplasmic staining in moderately dysplastic epithelium of adenoma. (C) Moderate DIAPH3 cytoplasmic staining in in a case of grade II CRC. (D) Negative DIAPH3 staining in moderately dysplastic adenoma. (x 200).
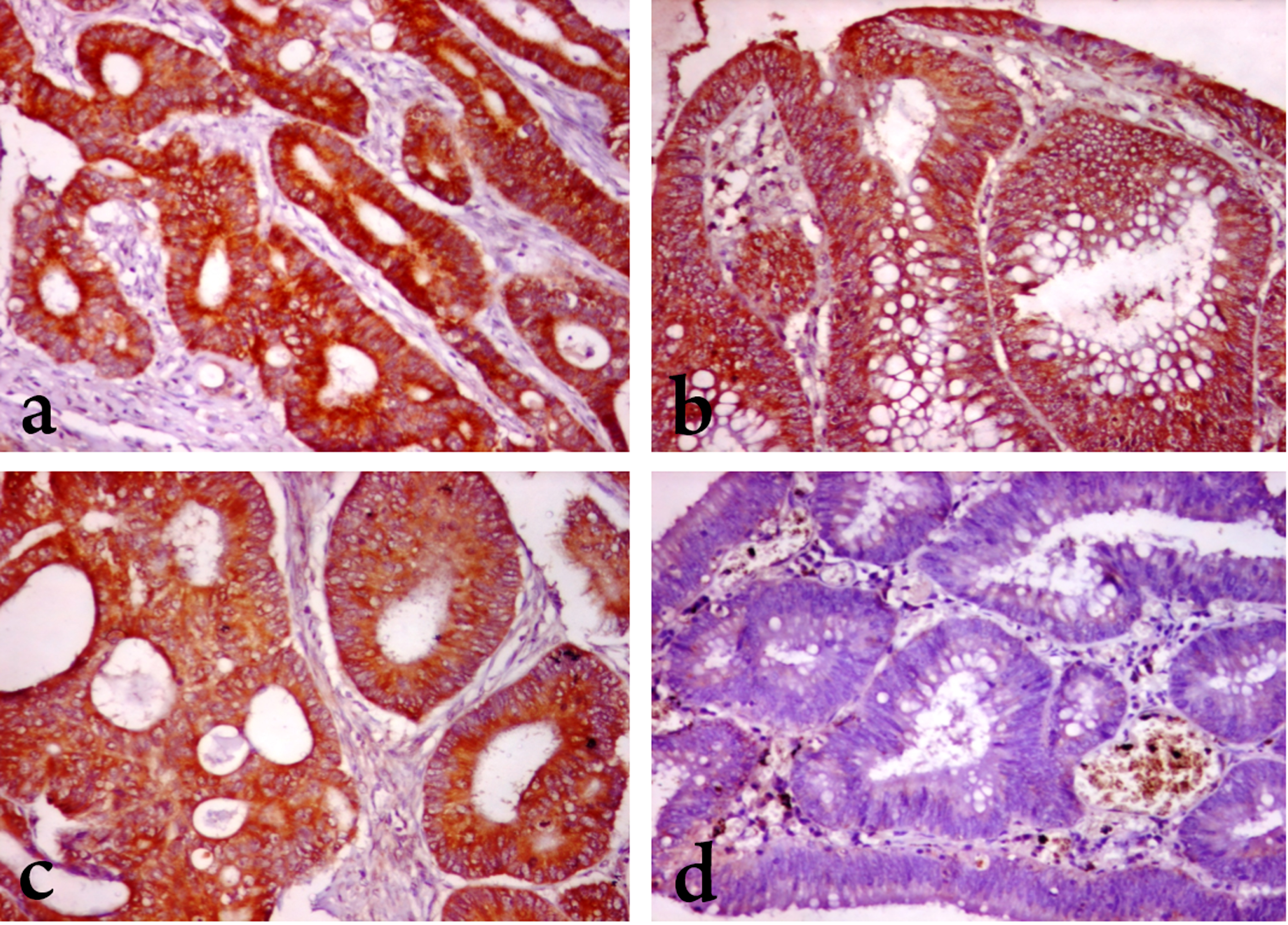
3.3DIAPH3 expression in CRC and CRA
The tissue cores of 5 cases of CRA were lost during IHC, so the corresponding CRC cases was discarded from the analyses, and so 38 cases were included in various DIAPH3 expression analyses. The median value of DIAPH3 expression in CRA and CRC cases that was used to divide cases into negative/low expression and high expression groups was 100 in this study. Cytoplasmic DIAPH3 was overexpressed in 22 CRC cases out of the 38 analyzed cases (about 58% of the cases) (16 cases “64% ” of NMA and 6 cases “46.2% ” of MA), which was not statistically significant (P = 0.280). DIAPH3 was overexpressed in 11 CRA cases (about 29%) which was significantly lower than CRC, especially NMA (P = 0.011 and 0.024 respectively). For MA cases, DIAPH expression didn’t show any statistically significant difference between CRC and CRA (P = 0.216) (Table 3) (Fig. 1 c and d).
Table 3
DIAPH3 expression in CRC cases and corresponding adenomas
| DIAPH3 expression | Chi-square | P value | ||
| Negative/Low | High | |||
| - All carcinomas (n. = 38) | 16 (42.1%) | 22 (57.9%) | 6.481 | 0.011* |
| - All adenomas | 27 (71.1%) | 11 (28.9%) | ||
| - Non-mucinous carcinomas (n. = 25) | 9 (36.0%) | 16 (64.0%) | 5.128 | 0.024* |
| - Corresponding adenomas | 17 (68.0%) | 8 (32.0%) | ||
| - Mucinous carcinomas (n. = 13) | 7 (53.8%) | 6 (46.2%) | 1.529 | 0.216 |
| - Corresponding adenomas | 10 (76.9%) | 3 (23.1%) | ||
*P≤0.05 is significant.
3.4Interrelation between MEK1 and DIAPH3 expression in CRA and CRC
In CRC, about 80% of the cases that showed MEK1 overexpression showed also concomitant DIAPH3 high expression. MEK1 and DIAPH3 over-expressions were significantly interrelated in CRC (P = 0.009). Likewise, in CRA, about 90% of cases with negative/low MEK1 expression revealed concurrent negative/low expression of DIAPH3, while about 56% of the cases showed concomitant MEK1 and DIAPH3 overexpression, this was also statistically significant (P = 0.002) (Table 4).
Table 4
Interrelation between MEK1 and DIAPH3 expressions in CRC and CRA
| MEK1 expression | Chi-square | P value | |||
| Negative/Low | High | ||||
| CRC cases (N = 38) | DIAPH3 expression | ||||
| -Negative/low | 12 (63.2%) | 4 (21.1%) | |||
| -High | 7 (36.8%) | 15 (78.9%) | 6.909 | 0.009* | |
| CRA cases (N = 38) | DIAPH3 expression | ||||
| -Negative/low | 20 (90.9%) | 7 (43.8%) | |||
| -High | 2 (9.1%) | 9 (56.2%) | 10.016 | 0.002* | |
*P≤0.05 is significant.
3.5Expression of MEK1 and DIAPH3 in relation to clinicopathological parameters in CRC cases
In CRC cases, 80% of grade I cases showed negative/low MEK1 expression, while 71% of grade II cases and 41% of grade III cases showed MEK1 overexpression. MEK1 overexpression was significantly associated with higher tumor grade (P = 0.050). About 90% of CRC cases with perineural invasion revealed overexpression of MEK1, which was statistically significant (P = 0.017). On the contrary, DIAPH3 overexpression was not significantly related to any of the tested clinicopathological and histological findings in CRC (data not shown).
3.6Expression of MEK1 and DIAPH3 in relation to clinicopathological parameters in CRA cases
In CRA cases, the only significant relation between studied clinicopathological and histological parameters and MEK1 and DIAPH3 expressions was between MEK1 overexpression and the degree of dysplasia. Seventy-five percent of adenomas with high grade dysplasia showed MEK1 overexpression (P = 0.008) (Tables 5&6).
Table 5
Characteristics of MEK1 expression in 43 cases of CRAs
| MEK1 expression | Chi-square | P value | ||
| Negative/Low | High | (X2) | ||
| Degree of dysplasia | ||||
| - Mild to moderate | 15 (65.2%) | 5 (25.0%) | ||
| - Severe | 8 (34.8%) | 15 (75.0%) | 6.955 | 0.008* |
| Chronic inflammatory cell infiltrate | ||||
| - No/mild | 11 (47.8%) | 12 (60.0%) | ||
| - moderate/marked | 12 (52.2%) | 8 (40.0%) | 0.637 | 0.425 |
| Association with FAP | ||||
| - Negative | 19 (82.6%) | 17 (89.5%) | ||
| - Positive | 4 (17.4%) | 2 (10.5%) | 0.400 | 0.527 |
*P value≤0.05 is significant; FAP = familial adenomatous polyposis syndrome.
Table 6
Characteristics of DIAPH3 expression in 38 cases of CRAs
| DIAPH3 expression | Chi-square | P value | ||
| Negative/Low | High | (X2) | ||
| Degree of dysplasia | ||||
| - Mild to moderate | 14 (51.9%) | 5 (45.5%) | ||
| - Severe | 13 (48.1%) | 6 (54.5%) | 0.128 | 0.721 |
| Chronic inflammatory cell infiltrate | ||||
| - No/mild | 14 (51.9%) | 5 (45.5%) | ||
| - moderate/marked | 13 (48.1%) | 6 (54.5%) | 0.128 | 0.721 |
| Association with FAP | ||||
| - Negative | 24 (88.9%) | 8 (72.7%) | ||
| - Positive | 3 (11.1%) | 3 (27.3%) | 1.535 | 0.215 |
FAP = familial adenomatous polypsis syndrome.
4Discussion
The intracellular protein kinase MAPK signaling pathway can be activated by extracellular signals through MEK1 which induces upregulated expression of genes controlling proliferation as well as cellular transformation [12]. Bai and colleagues [13] reported a significantly increased expression of MEK1 in primary colorectal cancer compared to normal mucosa, suggesting that the MAPK activation is potential player in colorectal carcinogenesis. Although MEK1 expression in colorectal carcinoma has been studied before, this is the first study to analyze its expression in samples that represent the adenoma-carcinoma sequence in CRC carcinogenesis. In the current study, MEK1 was found to be expressed in nearly half of the cases of CRA and CRC which supports this suggestion. This up-regulation of MEK1 in the colon adenoma-carcinoma sequence suggests that MEK1 may be associated with early disease progression in colorectal cancer.
In the present study, MEK1 overexpression showed a significant association with higher tumor grade and with perineural invasion in CRC cases. Notably, in CRA cases MEK1 overexpression was significantly associated with higher degree of dysplasia. The expression of MEK1 in dysplastic and malignant cells through the ACS indicates a possible role in early tumor progression and/or tumor cells survival. Similarly, Bai and colleagues [13] demonstrated a significantly higher positivity of MEK1 in poorly differentiated colorectal adenocarcinoma and in mucinous adenocarcinoma compared to adenocarcinoma of well or moderate differentiation, which supported that MEK1 expression was linked to the degree of differentiation of malignant cells.
Activation of the EGFR induced by the small G-protein RAS, together with the protein kinase RAF activates MAPK cascade [14]. Overexpression and activation of this receptor are commonly detected in colorectal cancer [15]. MEK1 can increase cell proliferation through EGF-EGFR-Ras-MEK-MAPK cascade [16]. In concordance with our results of MEK1 expression in ACS, Williet and colleagues [17] have also demonstrated that there is an increasing EGFR expression in the process of colorectal carcinogenesis, from adenomas showing low grade dysplasia (10% overexpression), and high grade dysplasia (77.8%), to carcinoma (100%). Similarly, another study [18] observed that EGFR copy number progressively increases, from highly dysplastic adenomas to early invasive and locally advanced adenocarcinomas, proposing that deregulation of EGFR may be implicated in tumor initiation and also correlate with tumor progression. These findings denote that EGFRs have a fundamental contribution in the transformation and progression of colorectal carcinoma and may suggest that MAPK activation through EGFR and possibly MEK-1 may be associated with disease progression in colonic cancer.
Furthermore, the activity of MEK1-ERK pathway can be influenced by some RNA-binding proteins (RBPs) [19]. From those, insulin-like growth factor 2 mRNA-binding protein 3 (IMP3) was reported amongst the most frequently dysregulated RBPs in CRC. Recently, it was reported that only IMP3 tends to increase from normal tissue to tumor tissue, and additionally increase from tumor tissue to metastatic liver tissue [20]. Thus, these observations may suggest a potential therapeutic significance for CRC treatment via inhibiting IMP3 in combination with the MEK1 inhibitors.
DIAPH3 has been found to inhibit the cellular transition to amoeboid tumor phenotypes [8]. As amoeboid behavior allows cells to move across gaps in the fibrillary matrix, it is well established that amoeboid cells have a greater tendency for dissemination and metastasis [21]. In the present study, DIAPH3 was significantly overexpressed in colorectal carcinomas especially non-mucinous adenocarcinomas compared to adenomas. A possible mechanism is that that expression of DIAPH3 occurs at a later stage potentially after MEK1 in colorectal adenoma-carcinoma sequence. In contrast, Lin and colleagues [22] reported increased expression of DIAPH1, but not DIAPH3, in colonic carcinoma cells. Recently, a study done by Huang and colleagues [23] concluded that DIAPH3 functions as a tumor suppressor in CRC as DIAPH3 depletion is associated with tumor progression and worse prognosis. Also, DIAPH3 increased expression was seen in prostate and breast cancer cells. Some previous studies reported that DIAPH3 mRNA levels were raised in hepatocellular carcinoma (HCC) compared to non-cancerous tissues [7]. On the other hand, other studies reported that DIAPH3 protein levels did not show significant change in benign tumors and organ-confined carcinoma whereas they noticed a substantial decrease in the levels of DIAPH3 protein in metastatic lesions compared to normal tissue and organ-confined tumors [8].
DIAPH3 enhances the proliferation, migration, colony formation, epithelial-mesenchymal transition, and metastatic potential of tumor cells via activation of beta-catenin/TCF signaling [7]. Many human cancers; including CRC, harbor aberrant mutations of the RAS-ERK and Wnt/β-catenin pathways. Concurrent aberrant mutations of these pathways result in colorectal tumorigenesis enhancement from the initiation till metastasis [9]. In our study, a significant interrelation between expressions of MEK-1 and DIAPH-3 in both CRA and CRC cases was observed suggestive of a potential synergistic role of MEK1 and DIAPH-3 in ACS. Our results showed that MEK1 expression is involved in early colorectal carcinogenesis, while DIAPH3 expression has a more delayed role. Such co-expression of early and late ACS transition markers may be highly predictive of the progression of adenoma-carcinoma sequence, and can be the basis for development of risk reduction or early therapeutic intervention approaches. There are currently no studies that evaluated the functional attributes of interplay of MEK-1 and DIAPH-3 expression in ACS, and so far as we know, this study is the first to investigate these correlations. Therefore, we recommend further research to elucidate the potential joint functional roles of MEK-1 and DIAPH-3 in the initiation and progression of CRC and additionally patients’ prognosis, usage of additional techniques of detection like Western Blot analysis ... etc. is highly recommended. Further studies are needed to reveal the relation of MEK-1 and DIAPH-3 expression to the consensus molecular subtypes (CMS) classificationof CRC.
5Conclusions
In conclusion, both MEK1 and DIAPH3 are overexpressed across colorectal ACS with strong correlation between them. This co-expression supports a possible synergistic function of MEK1 and DIAPH-3 in colorectal ACS. More researches are required on a larger scale to investigate the potential functional aspects of MEK1 and DIAPH3 in ACS and their involvement in tumorigenesis and the metastatic process.
Acknowledgments
The authors would like to express gratitude to all the staff that provided administrative and technical support, particularly Dr. Mohamed Abdelhamid Hasan Ahmed Pathology department, Faculty of Medicine, Suez Canal University, Egypt for his extraordinary efforts.
Author contributions
All authors read and approved the manuscript.
CONCEPTION: AAMF, KE and WME
INTERPRETATION OR ANALYSIS OF DATA: AAMF, KE and WME, AKE and ETE
PREPARATION OF THE MANUSCRIPT: AAMF, ETE, and AKE
REVISION FOR IMPORTANT INTELLECTUAL CONTENT: AAMF, AKE, KE, WME and ETE.
SUPERVISION: AAMF, and AKE
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | Parkin DM . Global cancer statistics in the year 2000. Lancet Oncol. (2001) ;2: (9):533–43. doi: 10.1016/S1470-2045(01)00486-7. Erratum in: Lancet Oncol. 2001;2(10):596. |
[2] | Rodriguez-Salas N , Dominguez G , Barderas R , Mendiola M , García-Albéniz X , Maurel J , Batlle JF . Clinical relevance of colorectal cancer molecular subtypes, Crit Rev Oncol Hematol. (2017) ;109: :9–19. doi: 10.1016/j.critrevonc.2016.11.007. |
[3] | Deng H , Ravikumar TS , Yang WL . Bone morphogenetic protein-4 inhibits heat-induced apoptosis by modulating MAPK pathways in human colon cancer HCT116 cells, Cancer Lett. (2007) ;256: (2):207–17. doi: 10.1016/j.canlet.2007.06.008. |
[4] | Caunt CJ , Sale MJ , Smith PD , Cook SJ . MEK1 and MEK2 inhibitors and cancer therapy: The long and winding road, Nat Rev Cancer. (2015) ;15: (10):577–92. doi: 10.1038/nrc4000. |
[5] | Gaillard J , Ramabhadran V , Neumanne E , Gurel P , Blanchoin L , Vantard M , Higgs HN . Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules, Mol Biol Cell. (2011) ;22: (23):4575–87. doi: 10.1091/mbc.E11-07-0616. |
[6] | Foda A , Ahmed M , Elkalla H , El-Zahaf E , Abdallah H , Wagih H , Sami M . Role of MEK1 and DIAPH3 Expression in Colorectal Carcinoma, Research in Oncology. (2018) ;14: (2):75–82. doi: 10.21608/resoncol.2018.4042.1059. |
[7] | Dong L , Li Z , Xue L , Li G , Zhang C , Cai Z , Li H , Guo R . DIAPH3 promoted the growth, migration and metastasis of hepatocellular carcinoma cells by activating beta-catenin/TCF signaling, Mol Cell Biochem. (2018) ;438: (1-2):183–90. doi: 10.1007/s11010-017-3125-7. |
[8] | Hager MH , Morley S , Bielenberg DR , Gao S , Morello M , Holcomb IN , Liu W , Mouneimne G , Demichelis F , Kim J , Solomon KR , Adam RM , Isaacs WB , Higgs HN , Vessella RL , Di Vizio D , Freeman MR . DIAPH3 governs the cellular transition to the amoeboid tumour phenotype, EMBO Mol Med. (2012) ;4: (8):743–60. doi: 10.1002/emmm.201200242. |
[9] | Jeong WJ , Ro EJ , Choi KY . Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway, NPJ Precis Oncol. (2018) ;2: (1):5. doi: 10.1038/s41698-018-0049-y. |
[10] | Hamilton SR , Bosman FT , Boffetta P , et al. Tumours of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, et al. (eds.). WHO Classification of Tumours of the Digestive System, 5th edition. IARCPress, Lyon; (2019) . pp. 132–182. |
[11] | Foda AA . No-cost manual method for preparation of tissue microarrays having high quality comparable to semiautomated methods, Appl Immunohistochem Mol Morphol. (2013) ;21: (3):271–4. doi: 10.1097/PAI.0b013e318268a93f. |
[12] | Tsuchiya T , Tsuno NH , Asakage M , Yamada J , Yoneyama S , Okaji Y , Sasaki S , Kitayama J , Osada T , Takahashi K , Nagawa H . Apoptosis induction by p38 MAPK inhibitor in human colon cancer cells, Hepatogastroenterology. (2008) ;55: (84):930–5, PMID: 18705300. |
[13] | Bai X , Yu B , Su H , et al. Expression of MEK1 in the colorectal cancer and its clinical implication, Chin. -Ger. J. Clin. Oncol. (2012) ;11: :142–145. https://doi.org/10.1007/s10330-011-0942-0. |
[14] | Benvenuti S , Sartore-Bianchi A , Di Nicolantonio F , Zanon C , Moroni M , Veronese S , Siena S , Bardelli A . Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies, Cancer Res. (2007) ;67: (6):2643–8. doi: 10.1158/0008-5472.CAN-06-4158. |
[15] | Spano JP , Lagorce C , Atlan D , Milano G , Domont J , Benamouzig R , Attar A , Benichou J , Martin A , Morere JF , Raphael M , Penault-Llorca F , Breau JL , Fagard R , Khayat D , Wind P . Impact of EGFR expression on colorectal cancer patient prognosis and survival, Ann Oncol. (2005) ;16: (1):102–8. doi: 10.1093/annonc/mdi006. |
[16] | Zhou YW , Li R , Duan CJ , Gao Y , Cheng YD , He ZW , Zeng JX , Zhang CF . Expression and clinical significance of C14orf166 in esophageal squamous cell carcinoma, Mol Med Rep. (2017) ;15: (2):605–12. doi: 10.3892/mmr.2016.6056. |
[17] | Williet N , Petcu CA , Rinaldi L , Cottier M , Del Tedesco E , Clavel L , Dumas O , Jarlot C , Bouarioua N , Roblin X , Peoc’h M , Phelip JM . The level of epidermal growth factor receptors expression is correlated with the advancement of colorectal adenoma: Validation of a surface biomarker, Oncotarget. (2017) ;8: (10):16507–17. doi: 10.18632/oncotarget.14961. |
[18] | Flora M , Piana S , Bassano C , Bisagni A , De Marco L , Ciarrocchi A , Tagliavini E , Gardini G , Tamagnini I , Banzi C , Bisagni G . Epidermal growth factor receptor (EGFR) gene copy number in colorectal adenoma-carcinoma progression, Cancer Genet. (2012) ;205: (12):630–5. doi: 10.1016/j.cancergen.2012.10.005. |
[19] | Sugiura R , Kita A , Shimizu Y , Shuntoh H , Sio SO , Kuno T . Feedback regulation of MAPK signalling by an RNA-binding protein, Nature. (2003) ;424: (6951):961–5. doi:10.1038/nature01907. |
[20] | Zhang M , Zhao S , Tan C , Gu Y , He X , Du X , Li D , Wei P . RNA-binding protein IMP3 is a novel regulator of MEK1/ERK signaling pathway in the progression of colorectal Cancer through the stabilization of MEKK1 mRNA, J Exp Clin Cancer Res. (2021) ;40: (1):200. doi: 10.1186/s13046-021-01994-8. |
[21] | Sanz-Moreno V , Marshall CJ . The plasticity of cytoskeletal dynamics underlying neoplastic cell migration, Curr Opin Cell Biol. (2010) ;22: (5):690–6. doi: 10.1016/j.ceb.2010.08.020. |
[22] | Lin YN , Windhorst S . Diaphanous-related formin 1 as a target for tumor therapy, Biochem Soc Trans. (2016) ;44: (5):1289–93. doi: 10.1042/BST20160120. |
[23] | Huang R , Wu C , Wen J , Yu J , Zhu H , Yu J , Zou Z . DIAPH3 is a prognostic biomarker and inhibit colorectal cancer progression through maintaining EGFR degradation, Cancer Med. (2022) ;11: (23):4688–702. doi: 10.1002/cam4.4793. |




