A pocket companion to cell-free DNA (cfDNA) preanalytics
Abstract
The cumulative pool of cell-free DNA (cfDNA) molecules within bodily fluids represents a highly dense and multidimensional information repository. This “biological mirror” provides real-time insights into the composition, function, and dynamics of the diverse genomes within the body, enabling significant advancements in personalized molecular medicine. However, effective use of this information necessitates meticulous classification of distinct cfDNA subtypes with exceptional precision. While cfDNA molecules originating from different sources exhibit numerous genetic, epigenetic, and physico-chemical variations, they also share common features that complicate analyses. Considerable progress has been achieved in mapping the landscape of cfDNA features, their clinical correlations, and optimizing extraction procedures, analytical approaches, bioinformatics pipelines, and machine learning algorithms. Nevertheless, preanalytical workflows, despite their profound impact on cfDNA measurements, have not progressed at a corresponding pace. In this perspective article, we emphasize the pivotal role of robust preanalytical procedures in the development and clinical integration of cfDNA assays, highlighting persistent obstacles and emerging challenges.
1Introduction
The cancer genome exhibits diverse DNA mutations, epigenetic changes, and gene expressions evolving with the disease and treatment [1–4]. These molecular changes are largely conserved in cell-free DNA (cfDNA) [5], an umbrella term which refers to the total population of both mutated and wild-type DNA fragments released from diverse sources into various bodily fluids, such as blood, urine, and cerebrospinal fluid [6–8]. Among the diverse origins of cfDNA, circulating tumor DNA (ctDNA) originating from tumors and coursing through the circulatory system represents the most well-characterized biospecimen type. Molecular profiling of ctDNA as an auxiliary or substitute test for tissue biopsies (TB) is becoming an increasingly popular and versatile tool in precision oncology [9]. While TB remains the benchmark technique for various indications of lung cancer, ctDNA assays offer many unique advantages, e.g., minimally-invasive sample collection, longitudinal sampling and temporal characterization of mutations, as well as higher resolution mapping of intra- and inter-tumor genetic heterogeneity [6–9]. Numerous studies have highlighted these benefits [10–13], underscoring the potential of ctDNA profiling not only as a valuable complementary approach to TB but also a key step in rapidly transforming personalized care of lung cancer patients.
However, despite the great leaps made in ctDNA research, clinical implementation remains limited to a fraction of cases and only a small number of tests have received regulatory approval [9, 14]. Numerous obstacles impede the advancement of ctDNA assays suitable for clinical implementation. Methodological variables, technical evaluation and validation, somatic mosaicism in plasma (e.g., clonal hematopoiesis-derived cancer-associated mutations), and factors that affect variant calling and data interpretation (e.g., low ctDNA molecule counts and germline variants) pose significant challenges [9]. Another major barrier, addressed in this paper, is the diverse branching possibilities within each step of the typical preanalytical workflow, impacting both the quantitative and qualitative aspects of ctDNA measurements [8, 15–25]. The ctDNA research community has made substantial and continuous efforts to systematically identify optimal preanalytical conditions, methodologies, and products, while also attempting to establish global harmonization of standard operating procedures [8, 24, 26]. Despite some progress, ctDNA preanalytics remains an intricate and evolving matter, with persistent obstacles, gaps, and emerging challenges yet to be overcome. In this perspective paper, we aim to specifically illuminate this immense range of preanalytical factors that may affect ctDNA profiling, and in turn affect the development or implementation of clinical tests.
It is important to note that preanalytical considerations and workflows can vary substantially based on the specific clinical inquiry and application, such as (i) the type of biospecimen utilized (e.g., blood, urine, saliva), (ii) the subtype of cfDNA examined (e.g., short vs long fragments, and nuclear DNA vs mitochondrial DNA), and (iii) the analysis modalities employed (e.g., targeted vs comprehensive mutational profiling, epigenetic profiling, or extracellular vesicle characterization). However, due to the limited scope of this article, we do not aim to present a universally applicable solution for ctDNA preanalytics. Instead, we briefly address some of the most relevant factors influencing ctDNA preanalytics, which often overlap, including (1) evolving ctDNA analysis modalities and incongruous workflows; (2) an excess of commercial products; (3) uncontrolled procedural variables and divergent protocols; (4) inadequate reference materials; and (5) an incomplete understanding of biological factors that modulate the properties of ctDNA. Alongside various other unexplored variables, we present a generalized outline or “map” of the preanalytical factors to be considered (Fig. 1). While existing informative reviews have offered valuable summaries of the current literature [16–19, 21, 24, 25, 27, 28], our aim is to provide accessible reference points, which are non-exhaustive, facilitating navigation through the vast landscape of ctDNA preanalytics.
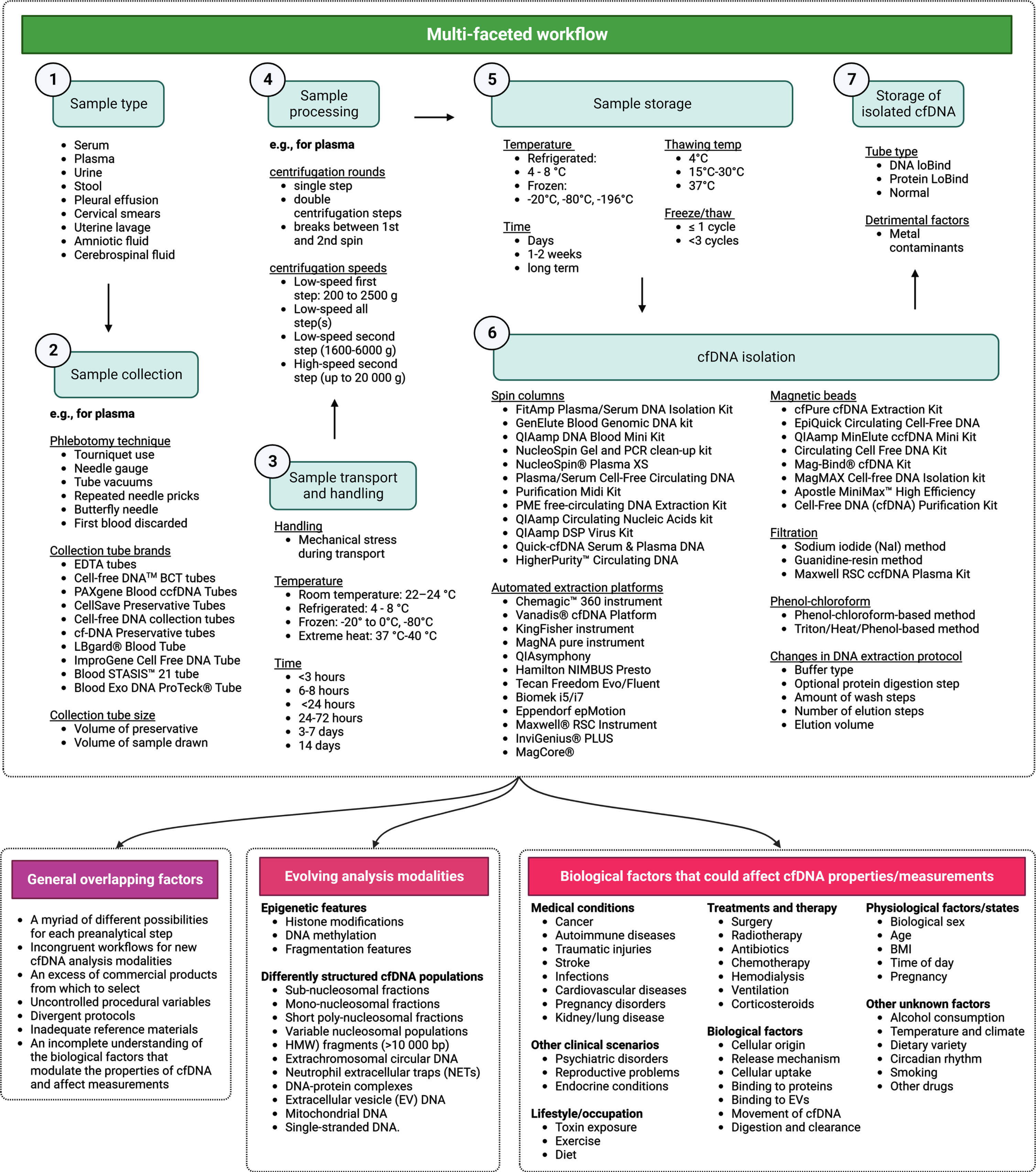
Fig. 1
Comprehensive map of cell-free DNA (cfDNA) preanalytics. This figure presents a condensed overview of essential preanalytical factors influencing the quantitative and qualitative aspects of cfDNA measurements. It emphasizes critical elements to be considered during the preanalytical phase for precise and reliable cfDNA evaluation. While a detailed investigation of each factor exceeds the paper’s scope, the text discusses pertinent factors influencing cfDNA preanalytics.
2Evolving cfDNA analysis modalities vs incongruous workflows
For several decades, the rationale and experimental methodology underlying cfDNA research have remained relatively straightforward. In clinical studies, the research framework typically consisted of the following: (a) the predominant “target” cfDNA molecules comprised mono-nucleosomes, approximately 166 bp in length, released through apoptosis, (b) isolation of cfDNA from human blood plasma or serum, and (c) profiling total cfDNA levels, point mutations, DNA sequence origins, and correlating them with specific disease indications. However, remarkable advancements in our understanding of the biological properties of cfDNA, analytical methods and technologies have recently led to a transformative shift in the field, resulting in the emergence of novel ctDNA analysis modalities and more comprehensive approaches that consider the multifaceted nature of cfDNA. This broader perspective incorporates factors such as epigenetic modifications, structural variations, diverse cellular origins, and alternative biospecimen types, recognizing that cfDNA analysis encompasses more than just plasma-derived apoptotic cfDNA fragments. Despite these notable advancements, however, certain preanalytical aspects, including technical developments, biology-informed design, and logistical solutions have lagged behind the unique requirements of next-generation ctDNA profiling. Below we present specific instances of outdated preanalytical procedures utilized in ctDNA profiling, emphasizing the necessity to reassess and modernize these protocols to ensure optimal analysis outcomes.
The notion that mono-nucleosomal cfDNA is the sole or most relevant form of cfDNA, while considering other cfDNA populations as biological noise or contamination, is challenged by a growing body of evidence [29, 30]. This evidence highlights that a wide variety of cfDNA structures hold important biological and pathological information (reviewed in [30]). These structures include (i) sub-nucleosomal (<166 bp) fractions; (ii) mono-nucleosomal and short poly-nucleosomal fractions (e.g., up to 3000 bp). The properties of these fractions are influenced by factors such as DNA sequence features, epigenetic modifications affecting DNA conformation, stochastic degradation, and regulated activity of diverse nucleases [30–33]. Interestingly, the cfDNA populations representing nucleosomal integers, e.g., mono-nucleosomes, di-nucleosomes, tri-nucleosomes, often exhibit a slightly overrepresented size-population and several differentially-sized subpopulations [34]; (iii) high molecular weight (HMW) fragments (>10 000 bp); (iv) extrachromosomal circular DNA; (v) neutrophil extracellular traps (NETs); (vi) DNA-protein complexes; (vii) extracellular vesicle (EV)-associated DNA; (viii) various forms of mitochondrial DNA, as well as (ix) various species of single-stranded DNA. In addition to the characterization of sequence-features of differently structured cfDNA fragments, a variety of epigenetic features of cfDNA are being characterized for their clinical utility as biomarkers. While some landmark studies have demonstrated higher densities of disease-defining genetic and epigenetic features in specific cfDNA subpopulations (reviewed in [29, 30, 35, 36]), large-scale systematic mapping of this phenomenon remains to be undertaken. Furthermore, while the majority of research has focused on the profiling of cfDNA in the circulatory system, cfDNA is increasingly characterized in non-blood bodily fluids. This is because a growing body of evidence indicates that bodily fluids in closer proximity to the diseased tissue or organ of interest naturally contain a higher number of absolute target molecules compared to blood [37, 38]. Evidence of enhanced representation of disease status achieved by cfDNA profiling in non-blood bodily fluids include saliva for oral cancers [39], urine for bladder cancers [40, 41], stool for colorectal cancers [42, 43], cerebrospinal fluid for brain cancer [44–46], as well as cervical smears [47], or uterine lavage [48] for gynecological cancers [49].
These new insights present both opportunities and challenges in cfDNA preanalytical procedures. Existing studies have primarily focused on optimal preanalytical procedures for the characterization of DNA mutations in mono-nucleosomal cfDNA fragments. However, it is evident that different preanalytical procedures and products (e.g., isolation of cfDNA using automated extraction using magnetic beads) may introduce bias and show varying efficiency towards differently structured cfDNA populations (reviewed in [24]). Further research is required to establish suitable preanalytical workflows for these diverse cfDNA subpopulations. Additionally, while preanalytical standardization for cfDNA profiling has primarily focused on blood specimens, it is crucial to optimize and establish preanalytical procedures for each respective bodily fluid due to the unique nature and composition of cfDNA molecules and the inherent differences among bodily fluids in terms of stability and composition.
3Excessive commercial products
The development of commercial products for ctDNA collection, purification, and profiling is driven by several factors, such as (i) the genetic and epigenetic heterogeneity of ctDNA, which necessitates the development of various methods for optimal capture and analysis of the diverse range of ctDNA variants, (ii) diverse methodological preferences of laboratories, wherein various considerations (such as ease of use, speed, robustness, automatability, scaling and cost) opens up the space for alternative products, (iii) technical advancements and rapidly evolving analysis modalities, which motivates commercial vendors to continuously innovate and deliver cutting-edge solutions and technologies, (iv) the need for customization and flexibility, which would enhance the ability of researchers and institutions to tailor preanalytical steps to their specific requirements, motivates the development of modular systems and customizable workflows, and (v) the versatility of basic scientific principles, which allows vendors to exploit different chemistries, reagents, isolation and enrichment strategies, etc., to develop unique intellectual property rights and proprietary technologies. For these reasons, there is an immensely wide range of commercial products from which to choose for various preanalytical steps. For example, there are more than 10 different blood collection tubes, more than 50 different cfDNA extraction kits (many of which are based on different purification principles), and more than 10 different automated cfDNA extraction platforms currently on the market. Individual research studies have demonstrated highly variable degrees of efficiency and bias among many of these products. However, rigorous evaluation of product performance concerning different cfDNA subtypes, different clinical settings and other confounding variables is virtually impracticable. This complicates both the selection and standardization of preanalytical workflows.
4Uncontrolled procedural variables and divergent protocols
Comparing studies that evaluate different conditions or products for a specific preanalytical step often faces challenges arising from an underappreciated mismatch in other experimental parameters. A meta-analysis of 20 publications examining various cfDNA extraction methods revealed several issues. Firstly, different methods for blood collection and processing were employed across studies. Secondly, some studies omitted important procedural information, such as sample storage temperature. Lastly, all studies employed different DNA quantification methods with varying sensitivities and biases in quantification and size estimation [24]. Consequently, differences or correlations observed between studies may not necessarily reflect the qualities of the investigated preanalytical step, method, or product, but rather may be influenced by unconsidered methodological variables. In addition to these variables, in both inter-individual and inter-laboratory contexts, subtle variations in the utilization of the same product or execution of identical methods, which may appear insignificant, can indeed have a substantial impact on measurements. For example, multiple modifiable steps have the potential to influence the perceived efficacy of a specific DNA extraction method. These include factors such as sample-thawing temperature, input volume for extraction, presence or absence of carrier RNA, utilization of vacuum pump-setups with spin-column kits, modifications in buffers, inclusion or exclusion of denaturing agents, types of storage tubes, volume of elution buffer, and the number of elution steps employed [16]. This effect is underscored by poor inter-lab concordance observed in multi-centre studies [17, 22, 50, 51], as well as several independent external quality assessment schemes (EQAs) that have not only highlighted significant variability in standard operating procedures (SOPs) but have also revealed high error rates in the analysis of identical samples across multiple laboratories [52–58].
5Inadequate reference materials
Standard reference materials (RMs) have various important roles in ctDNA preanalytics: (i) RMs can serve as benchmark materials for assessing the performance, accuracy, and reliability of assays, (ii) comparison of expected values to results obtained from RM samples with known ctDNA variants and concentration enables evaluation of accuracy, precision, sensitivity and specificity, which facilitates method validation, (iii) RM samples analyzed in parallel with patient samples in routine clinical testing enables the monitoring of important parameters, such as assay performance, accuracy of results, and potential technical errors or other issues, (iv) RMs can be used in EQA and proficiency programs to evaluate inter-laboratory consistency, determine proficiency against established benchmark methods, as well as facilitate harmonization and standardization of preanalytical workflows across different laboratories.
Despite the availability of diverse commercial vendors offering various types of RMs for specific applications of ctDNA profiling (Fig. 2), the development and selection of RMs face numerous challenges. Moreover, a consensus regarding universally agreed upon RMs for ctDNA analysis is currently lacking. These challenges arise mainly from two interconnected reasons, which contribute to the absence of widely accepted RMs in the field: First, cfDNA biospecimens often represent multi-analyte samples that are genetically, epigenetically, and structurally highly diverse and poorly characterized, which makes it very difficult to create a set of RMs with well-matched qualities. Second, even in the case of well-characterized cfDNA analytes (e.g., a 167 bp tumor-derived cfDNA molecule with a specific DNA mutation), most methods that are currently used to produce cfDNA analogues are non-commutable for certain assays. For example, the primary focus of cfDNA research to-date has been on the analysis of mono-nucleosomal cfDNA fragments (i.e., 167 bp), which can be incorporated into NGS libraries with relatively high efficiency. Many RMs that mimic these cfDNA fragments are produced by sonication of longer fragments (e.g., sonication of synthetic DNA or gDNA derived from cultured cells). However, sonication produces cfDNA fragments with a broader size distribution which incorporate into sequencing libraries with much lower efficiency [59, 60]. Moreover, sonication has been shown to damage the ends of DNA molecules, which significantly lowers the rate of adapter ligation.
Fig. 2
Overview of circulating tumor DNA (ctDNA) reference materials (RMs) provided by commercial vendors, highlighting production methods, sources, and current applications.
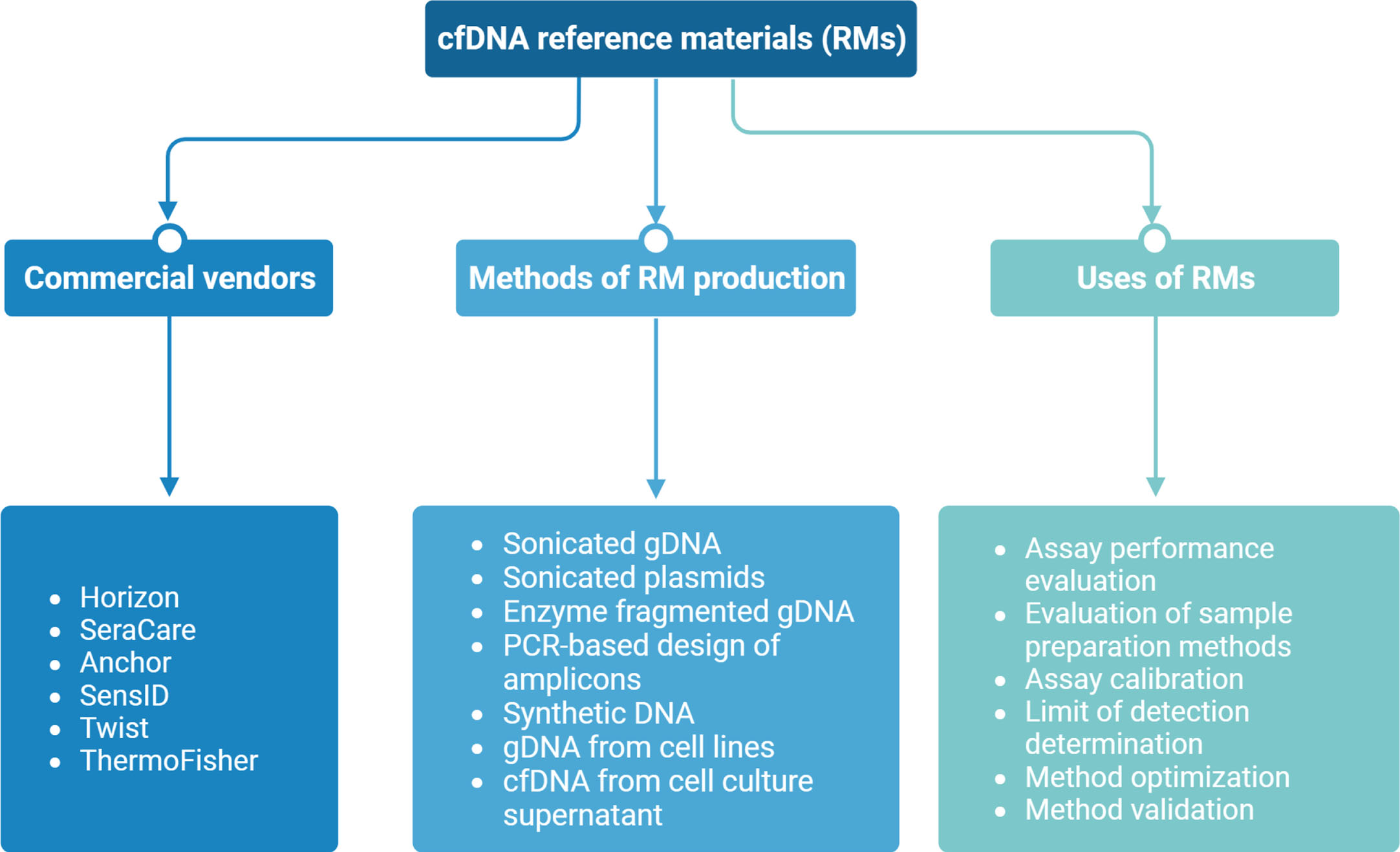
The development and selection of commutable RMs face several other challenges due to various factors related to the biological properties of cfDNA. These factors include: (1) the heterogeneity of cancer (e.g., development of RMs for pan-cancer screening vs specific cancer); (2) various marker interrogation approaches (e.g., hotspot mutations vs copy number variations); (3) distinct cfDNA types available for characterization (e.g., nuclear DNA vs mitochondrial DNA); (4) a wide range of assay methods available for cfDNA analysis, including fragment sizing (e.g., Bioanalyzer, Tapestation, Femto Pulse, Fragment Analyzer, Nanopore sequencing,), DNA quantification (e.g., qPCR, Qubit Fluorometer, Bioanalyzer), mutation analysis (e.g., ddPCR, shallow WGS, NGS panels), and epigenetic analysis (e.g., ChIP-seq, cfMeDIP-seq), which demand diverse RMs compatible with each method to ensure accurate measurements; (5) various assay platforms with different workflows and assay chemistries (e.g., ThermoFisher Oncomine, Illumina TruSight Oncology 500 ctDNA, Sysmex Inostics Plasma-SeqSensei, Roche Avenio), which may require the selection of RMs suitable for each platform to ensure compatibility and reliable results; (6) variability in preanalytical workflows employed by different laboratories, leading to variations in the behaviour of RMs under different conditions, which need to be considered when selecting RMs; (7) considerations related to matrix effects, which include differences between the RM matrix and patient samples, potential interference from DNA-binding proteins and cell-binding/uptake, pH variations, and the impact of preanalytical factors on the matrix: it is crucial to differentiate between matrix effects and lack of assay/measurement specificity; (8) diversity of RMs from different vendors, which often represent different characteristics (see Fig. 2); the selection of appropriate RMs depends on the specific requirements of the analysis; (9) various methods for RM production, each one with its own advantages and disadvantages (see Fig. 2). Considering these factors, it is evident that the biological properties of cfDNA pose significant challenges for the development and selection of suitable RMs, requiring careful consideration of the specific context and requirements of each analysis.
6Biological factors that modulate cfDNA properties
The quantitative and physico-chemical characteristics of the cfDNA population in a specific body fluid prior to sampling are influenced by a complex network of factors, which are currently poorly understood. As previously reviewed [30, 61], systematic mapping of the correlation between cfDNA characteristics and various biological, physiological, pathological and environmental factors can expand the repertoire of highly specific biomarkers and reveal strategies to enhance the detection of target cfDNA molecules by either suppressing or leveraging these correlations. Notable examples demonstrate the potential of these approaches: (i) administration of an intravenous priming agent before blood collection has been shown to increase the abundance of cfDNA in biospecimens by counteracting cellular clearance and cfDNA uptake [62]; (ii) the relative contribution of cfDNA-modulating factors, such as food intake, time-of-day, or physical activity, to the pool of target molecules versus background cfDNA remains unknown. Establishing robust correlations would enable control of patient conditions before biospecimen collection to maximize the extracellular presence of target molecules [24]; (iii) understanding the physico-chemical features of different cfDNA populations can facilitate the adaptation or development of purification methods with enhanced capacity to capture target molecules or exclude background cfDNA. Tailoring extraction and purification procedures to specific cfDNA size subpopulations or exploiting unique epigenetic properties, such as cancer-associated methylation patterns (e.g., with increased affinity for gold-nano particles [63]), can improve selectivity [64]. These advancements hold promise for optimizing cfDNA preanalytics and maximizing the detection of clinically relevant cfDNA biomarkers.
7CfDNA preanalytics in practice
Despite biological and preanalytical limitations, certain applications of cfDNA, such as qualitative or absolute detection of single or a small number of mutations in advanced stage cancers, can be sufficiently robust and reliable using standard preanalytical workflows. This depends on the sensitivity of the method employed, such as digital droplet PCR (ddPCR) or next-generation sequencing (NGS) assays with error correction. On the other hand, qualitative whole exome sequencing or whole genome sequencing of cfDNA presents challenges, but may be feasible under specific conditions, including high input cfDNA, advanced stage cancer, high method sensitivity, and parallel measurement of leukocyte DNA to mitigate misdiagnoses resulting from clonal hematopoiesis of indeterminate potential (CHIP). In the future, serial monitoring of mutation load and ctDNA will be crucial in cancer management. This includes both qualitative and quantitative assessment of ctDNA levels in the blood. However, successful implementation requires control of various variables, such as analytical, preanalytical, biological, physiological, and concomitant disease-related factors that can influence results. Moreover, the choice of quantification method, whether relative variant allele frequency (VAF) or absolute concentrations (ng/ml) of ctDNA, needs to be defined and standardized. Methodological consistency is particularly critical for protein biomarkers and even more complex for ctDNA, necessitating strict adherence to the same extraction and quantification methods to ensure accurate interpretation of serially collected data during therapy and disease monitoring. These considerations highlight the need for careful standardization and optimization of preanalytical workflows to maximize the reliability and utility of ctDNA-based diagnostics and monitoring strategies in clinical practice.
8Concluding remarks
Standardization and wide-spread harmonization of preanalytical workflows are crucial for optimizing the sensitivity, specificity, and robustness of cfDNA assays, facilitating their clinical integration. However, this is a complex task which requires careful consideration and consolidation of numerous overlapping factors and recommendations. An important step in the development of standardized protocols is the establishment of reference ranges for performance and bias for each step in a preanalytical workflow, and this process should involve thorough mapping and consideration of confounding procedural variables and divergent protocols that impact measurements. Standardized workflows will not only enable consolidation of commercial products for cfDNA analysis but also allow the integration and control of factors such as equipment availability (e.g., use of automated vs manual methods or high-speed vs low-speed centrifuges, which may depend on institutional funding), proximity to testing laboratories, as well as differences in sample handling by technicians with varying skills and training. The development and continual revision of evidence-based best-practice guidelines, along with rigorous external quality assessment schemes (EQAs) guided by clear rules for quality control measures set by regulatory authorities, such as the Guidelines of the German Federal Association (Rili-BäK), can further aid in establishing standardized workflows [26]. In line with this, other regulatory committees or consortia, such as the European Committee for Standardization (CEN), the European Organisation for Research and Treatment of Cancer (EORTC), the European consortium CANCER-ID, and Bloodpac, have proposed preanalytical guidelines for practical use, and this will have a crucial role in coordinating and guiding standardization efforts. However, it should be noted that these guidelines currently exhibit variability, limited availability, and face various other challenges including: (i) implementation variability across laboratories and institutions due to differences in equipment, sample handling, and technician expertise, leading to inconsistent practices; (ii) difficulties in widespread adoption and compliance due to existing protocols, limited resources, and resistance to change; (iii) challenges in keeping the guidelines up to date with evolving cfDNA analysis modalities, technologies and incorporating new findings; (iv) complex task of standardizing preanalytical practices across diverse platforms and technologies used in cfDNA analysis; (v) need for robust scientific evidence and validation studies to support the guidelines, considering the heterogeneity of cfDNA and complex preanalytical factors; and (vi) requirement for international consensus to achieve global harmonization and comparability. Addressing these challenges will enhance the effectiveness and practicality of preanalytical guidelines, providing clearer steps and criteria for future studies. This approach will guide researchers towards increasingly comprehensive documentation and description in studies, thereby improving the comparability of studies, which in turn will generate higher quality data for evidence-based guidelines and protocols.
Lastly, it is important to recognize the impracticability of a universally applicable preanalytical workflow. Instead, preanalytical approaches should be tailored to specific contexts rather than treated as a one-size-fits-all solution. Distinct workflows and reference materials should be developed for different biospecimens (i.e., various bodily fluids), cfDNA subtypes (e.g., short vs long fragments), and cfDNA analysis modalities (e.g., mutational vs epigenetic profiling]. Tailoring workflows will be facilitated by evidence-based approaches that stem from an improved understanding of the biological, physiological, and environmental factors influencing cfDNA properties. In this context, it is essential to acknowledge the rapid evolution of cfDNA analysis strategies and the evolving definition of cfDNA and ctDNA, necessitating ongoing adaptation and refinement of preanalytical approaches.
Author contributions
CONCEPTION: AJB and SH.
PREPARATION OF THE MANUSCRIPT: AJB.
REVISION FOR IMPORTANT INTELLECTUAL CONTENT: SH.
SUPERVISION: SH.
Conflict of interest
Stefan Holdenrieder is an Editorial Board member of Tumor Biology but had no participation in the peer review process of this paper. Abel Bronkhorst has no conflict of interest to report.
References
[1] | Stratton MR , Campbell PJ and Futreal PA . The cancer genome. Nature. (2009) ;458: (7239):719–724. doi:10.1038/nature07943 |
[2] | Vogelstein B , Papadopoulos N , Velculescu VE , Zhou S , Diaz LA Jr and Kinzler KW . Cancer genome landscapes. Science. (2013) ;339: (6127):1546–1558. doi:10.1126/science.1235122 |
[3] | Dawson MA and Kouzarides T . Cancer epigenetics: from mechanism to therapy. Cell. (2012) ;150: (1):12–27. doi:10.1016/j.cell.2012.06.013 |
[4] | Darwiche N . Epigenetic mechanisms and the hallmarks of cancer: An intimate affair. Am J Cancer Res. (2020) ;10: (7):1954–78. PMC7407342. |
[5] | Bronkhorst AJ , Ungerer V , Diehl F , Anker P , Dor Y , Fleischhacker M , et al. Towards systematic nomenclature for cell-free DNA. Hum Genet. (2021) ;140: (4):565–78. doi:10.1007/s00439-020-02227-2 |
[6] | Wan JCM , Massie C , Garcia-Corbacho J , Mouliere F , Brenton JD , Caldas C , et al. Liquid biopsies come of age: clinical applications of circulating tumour DNA. Nat Rev Cancer. (2017) ;17: (4):223–38. doi:10.1038/nrc.2017.7 |
[7] | Heitzer E , Haque IS , Roberts CES and Speicher MR . Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. (2018) :1. doi:10.1038/s41576-018-0071-5 |
[8] | Bronkhorst AJ , Ungerer V and Holdenrieder S . The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif. (2019) ;17: (March):100087. doi:10.1016/j.bdq.2019.100087 |
[9] | Heitzer E , van den Broek D , Denis MG , Hofman P , Hubank M , Mouliere F , et al. Recommendations for a practical implementation of circulating tumor DNA mutation testing in metastatic non-small-cell lung cancer. ESMO Open. (2022) ;7: (2):100399. doi:10.1016/j.esmoo2022.100399 |
[10] | Punnoose EA , Atwal S , Liu W , Raja R , Fine BM , Hughes BG , et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: Association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res. (2012) ;18: (8):2391–401. doi:10.1158/1078-0432.CCR-11-3148 |
[11] | Chabon JJ , Hamilton EG , Kurtz DM , Esfahani MS , Moding EJ , Stehr H , et al. Integrating genomic features for non-invasive early lung cancer detection. Nature. (2020) ;580: (7802):245–251. doi:10.1038/s41586-020-2140-0 |
[12] | Moding EJ , Liu Y , Nabet BY , Chabon JJ , Chaudhuri AA , Hui AB , et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer. (2020) ;1: (2):176–83. doi:10.1038/s43018-019-0011-0 |
[13] | Rolfo C , Mack PC , Scagliotti GV , Baas P , Barlesi F , Bivona TG , et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol. (2018) ;13: (9):1248–68. doi:10.1016/j.jtho.2018.05.030 |
[14] | Cisneros-Villanueva M , Hidalgo-Perez L , Rios-Romero M , Cedro-Tanda A , Ruiz-Villavicencio CA , Page K , et al. Cell-free DNA analysis in current cancer clinical trials: a review. Br J Cancer. (2022) ;126: (3):391–400. doi:10.1038/s41416-021-01696-0 |
[15] | van Dessel LF , Beije N , Helmijr JC , Vitale SR , Kraan J , Look MP , et al. Application of circulating tumor DNA in prospective clinical oncology trials - standardization of preanalytical conditions. Mol Oncol. (2017) ;11: (3):295–304. doi:10.1002/1878-0261.12037 |
[16] | Bronkhorst AJ , Aucamp J and Pretorius PJ . Cell-free DNA: Preanalytical variables.Clin Chim Acta. (2015) ;450: :243–53. doi:10.1016/j.cca.2015.08.028 |
[17] | El Messaoudi S , Rolet F , Mouliere F and Thierry AR . Circulating cell free DNA: Preanalytical considerations. Clin Chim Acta. (2013) ;424: :222–30. doi:10.1016/j.cca.2013.05.022 |
[18] | Nikolaev S , Lemmens L , Koessler T , Blouin JL and Nouspikel T . Circulating tumoral DNA: Preanalytical validation and quality control in a diagnostic laboratory. Anal Biochem. (2018) ;542: (November 2017):34–9. doi:10.1016/j.ab.2017.11.004 |
[19] | van der Pol Y , Moldovan N , Verkuijlen S , Ramaker J , Boers D , Onstenk W , et al. The Effect of Preanalytical and Physiological Variables on Cell-Free DNA Fragmentation. Clin Chem. (2022) ;68: (6):803–13. doi:10.1093/clinchem/hvac029 |
[20] | Zavridou M , Mastoraki S , Strati A , Tzanikou E , Chimonidou M and Lianidou E . Evaluation of preanalytical conditions and implementation of quality control steps for reliable gene expression and DNA methylation analyses in liquid biopsies. Clin Chem. (2018) ;64: (10):1522–33. doi:10.1373/clinchem.2018.292318 |
[21] | Meddeb R , Pisareva E and Thierry AR . Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin Chem. (2019) ;65: (5):623–33. doi:10.1373/clinchem.2018.298323 |
[22] | Lampignano R , Neumann MHD , Weber S , Kloten V , Herdean A , Voss T , et al. Multicenter Evaluation of Circulating Cell-Free DNA Extraction and Downstream Analyses for the Development of Standardized (Pre)analytical Work Flows. Clin Chem. (2020) ;66: (1):149–60. doi:10.1373/clinchem.2019.306837 |
[23] | Randeu H , Bronkhorst AJ , Mayer Z , Oberhofer A , Polatoglou E , Heinemann V , et al. Preanalytical Variables in the Analysis of Mitochondrial DNA in Whole Blood and Plasma from Pancreatic Cancer Patients. Diagnostics. (2022) ;12: (8):1905. doi:10.3390/diagnostics12081905 |
[24] | Ungerer V , Bronkhorst AJ and Holdenrieder S . Preanalytical variables that affect the outcome of cell-free DNA measurements. Crit Rev Clin Lab Sci. (2020) :1–24. doi:10.1080/10408363.2020.1750558 |
[25] | Tsui DW , Barnett E and Scher HI . Toward Standardization of Preanalytical Procedures for Cell-Free DNA Profiling. Clin Chem. (2020) ;66: (1):3–5. doi:10.1373/clinchem.2019.310854 |
[26] | Greytak SR , Engel KB , Parpart-Li S , Murtaza M , Bronkhorst AJ , Pertile MD , et al. Harmonizing cell-free DNA collection and processing practices through evidence-based guidance. Clin Cancer Res. (2020) ;26: (13):3104–9. doi:10.1158/1078-0432.CCR-19-3015 |
[27] | Fleischhacker M and Schmidt B . Pre-analytical issues in liquid biopsy–where do we stand? JLM. (2020) ;44: (3):117–42. doi:10.1515/labmed-2019-0167 |
[28] | Schmidt B and Fleischhacker M . Is liquid biopsy ready for the litmus test and what has been achieved so far to deal with pre-analytical issues? Transl Cancer Res. (2018) ;7: (S2):S130–S9. doi:10.21037/tcr.2017.12.04 |
[29] | van der Pol Y and Mouliere F . Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA. Cancer Cell. (2019) ;36: (4):350–68. doi:10.1016/j.ccell.2019.09.003 |
[30] | Bronkhorst AJ , Ungerer V , Oberhofer A , Gabriel S , Polatoglou E , Randeu H , et al. New Perspectives on the Importance of Cell-Free DNA Biology. Diagnostics. (2022) ;12: (9):2147. doi:10.3390/diagnostics12092147 |
[31] | Mouliere F . A hitchhiker’s guide to cell-free DNA biology. Neurooncol Adv. (2022) ;4: (Suppl 2):ii6–ii14. doi:10.1093/noajnl/vdac066 |
[32] | Odenheimer-Bergman A , Markus H and Murtaza M . Biology of Circulating DNA in Health and Disease. Cell-Free Circulating DNA: Purification and Analysis Techniques. World Scientific. 2022, pp.1-20. |
[33] | Thierry AR , El Messaoudi S , Gahan PB , Anker P and Stroun M . Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. (2016) ;35: (3):347–76. doi:10.1007/s10555-016-9629-x |
[34] | Ungerer V , Bronkhorst AJ , Uhlig C and Holdenrieder S . Cell-Free DNA Fragmentation Patterns in a Cancer Cell Line. Diagnostics. (2022) ;12: (8):1896. doi:10.3390/diagnostics12081896 |
[35] | Oberhofer A , Bronkhorst AJ , Uhlig C , Ungerer V and Holdenrieder S . Tracing the Origin of Cell-Free DNA Molecules through Tissue-Specific Epigenetic Signatures. Diagnostics. (2022) ;12: (8):1834. doi:10.3390/diagnostics12081834 |
[36] | Ding SC and Lo YD . Cell-Free DNA Fragmentomics in Liquid Biopsy. Diagnostics. (2022) ;12: (4):978. doi:10.3390/diagnostics12040978 |
[37] | Peng M , Chen C , Hulbert A , Brock MV and Yu F . Non-blood circulating tumor DNA detection in cancer. Oncotarget. (2017) ;8: (40):69162–73. doi:10.18632/oncotarget.19942 |
[38] | Patel KM and Tsui DWY . The translational potential of circulating tumour DNA in oncology. Clin Biochem. (2015) ;48: (15):957–61. doi:10.1016/j.clinbiochem.2015.04.005 |
[39] | Birkenkamp-Demtroder K , Christensen E , Nordentoft I , Knudsen M , Taber A , Hoyer S , et al. Monitoring Treatment Response and Metastatic Relapse in Advanced Bladder Cancer by Liquid Biopsy Analysis. Eur Urol. (2018) ;73: (4):535–40. doi:10.1016/j.eururo.2017.09.011 |
[40] | Rentsch CA , Muller DC , Ruiz C and Bubendorf L . Moving Towards Minimally Invasive Genomically Based Diagnosis and Monitoring of Bladder Cancer. Eur Urol. (2016) ;70: (1):83–4. doi:10.1016/j.eururo.2016.02.050 |
[41] | Sidransky D , Tokino T , Hamilton SR , Kinzler KW , Levin B , Frost P , et al. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. (1992) ;256: (5053):102–5. doi:10.1126/science.1566048 |
[42] | Diehl F , Schmidt K , Durkee KH , Moore KJ , Goodman SN , Shuber AP , et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. (2008) ;135: (2):489–98. doi:10.1053/j.gastro.2008.05.039 |
[43] | Bettegowda C , Sausen M , Leary RJ , Kinde I , Wang Y , Agrawal N , et al. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci Transl Med. (2014) ;6: (224):224ra24. doi:10.1126/scitranslmed.3007094 |
[44] | Jain A , Krishnamurthy PK , Landais P and Anandarajah PM . EKF for Joint Mitigation of Phase Noise, Frequency Offset and Nonlinearity in 400 Gb/s PM-16-QAM and 200 Gb/s PM-QPSK Systems, IEEE Photonics Journal. (2017) ;9: (1):1–10. doi:10.1109/jphot.2017.2649223 |
[45] | Angert RM , Leshane ES , Yarnell RW , Johnson KL and Bianchi DW . Cell-free fetal DNA in the cerebrospinal fluid of women during the peripartum period. Am J Obstet Gynecol. (2004) ;190: (4):1087–90. doi:10.1016/j.ajog.2003.10.562 |
[46] | Kinde I , Bettegowda C , Wang Y , Wu J , Agrawal N , Shih Ie M , et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. (2013) ;5: (167):167ra4. doi:10.1126/scitranslmed.3004952 |
[47] | Nair N , Camacho-Vanegas O , Rykunov D , Dashkoff M , Camacho SC , Schumacher CA , et al. Genomic Analysis of Uterine Lavage Fluid Detects Early Endometrial Cancers and Reveals a Prevalent Landscape of Driver Mutations in Women without Histopathologic Evidence of Cancer: A Prospective Cross-Sectional Study. PLoS Med. (2016) ;13: (12):e1002206. doi:10.1371/journal.pmed.1002206 |
[48] | Ross-Innes CS , Chettouh H , Achilleos A , Galeano-Dalmau N , Debiram-Beecham I , MacRae S , et al. Risk stratification of Barrett’s oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. Lancet Gastroenterol Hepatol. (2017) ;2: (1):23–31. doi:10.1016/S2468-1253(16)30118-2 |
[49] | Neumann MH , Bender S , Krahn T and Schlange T . ctDNA and CTCs in liquid biopsy–current status and where we need to progress. Comput Struct Biotechnol J. (2018) ;16: :190–5. doi:10.1016/j.csbj.2018.05.002 |
[50] | Grolz D , Hauch S , Schlumpberger M , Guenther K , Voss T , Sprenger-Haussels M , et al. Liquid Biopsy Preservation Solutions for Standardized Pre-Analytical Workflows—Venous Whole Blood and Plasma. Curr Pathobiol Rep. (2018) ;6: (4):275–86. doi:10.1007/s40139-018-0180-z |
[51] | Malentacchi F , Pazzagli M , Simi L , Orlando C , Wyrich R , Hartmann CC , et al. SPIDIA-DNA: an External Quality Assessment for the pre-analytical phase of blood samples used for DNA-based analyses. Clin Chim Acta. (2013) ;424: :274–86. doi:10.1016/j.cca.2013.05.012 |
[52] | Malentacchi F , Pizzamiglio S , Ibrahim-Gawel H , Pazzagli M , Verderio P , Ciniselli CM , et al. Second SPIDIA-DNA External Quality Assessment (EQA): Influence of pre-analytical phase of blood samples on genomic DNA quality. Clin Chim Acta. (2016) ;454: :10–4. doi:10.1016/j.cca.2015.12.032 |
[53] | Haselmann V , Ahmad-Nejad P , Geilenkeuser WJ , Duda A , Gabor M , Eichner R , et al. Results of the first external quality assessment scheme (EQA) for isolation and analysis of circulating tumour DNA (ctDNA). Clin Chem Lab Med. (2018) ;56: (2):220–8. doi:10.1515/cclm-2017-0283 |
[54] | Keppens C , Dequeker EMC , Patton SJ , Normanno N , Fenizia F , Butler R , et al. International pilot external quality assessment scheme for analysis and reporting of circulating tumour DNA. BMC Cancer. (2018) ;18: (1):804. doi:10.1186/s12885-018-4694-x |
[55] | Malentacchi F , Pizzamiglio S , Verderio P , Pazzagli M , Orlando C , Ciniselli CM , et al. Influence of storage conditions and extraction methods on the quantity and quality of circulating cell-free DNA (ccfDNA): the SPIDIA-DNAplas External Quality Assessment experience. Clin Chem Lab Med. (2015) ;53: (12):1935–42. doi:10.1515/cclm-2014-1161 |
[56] | Fairley JA , Badrick T , Denis MG , Dimitrova L , Goodall R , Maas J , et al. Implementation of circulating tumour DNA multi-target mutation testing in plasma: a perspective from an external quality assessment providers’ survey. Virchows Archiv. 2023: :1–6. doi:10.1007/s00428-023-03558-x |
[57] | Deans ZC , Williams H , Dequeker EMC , Keppens C , Normanno N , Schuuring E , et al. Review of the implementation of plasma ctDNA testing on behalf of IQN Path ASBL: a perspective from an EQA providers’ survey. Virchows Arch. (2017) ;471: (6):809–13. doi:10.1007/s00428-017-2222-z |
[58] | He HJ , Stein EV , Konigshofer Y , Forbes T , Tomson FL , Garlick R , et al. Multilaboratory assessment of a new reference material for quality assurance of cell-free tumor DNA measurements. J Mol Diagn. (2019) ;21: (4):658–76. doi:10.1016/j.jmoldx.2019.03.006 |
[59] | Vesper HW , Miller WG and Myers GL . Reference materials and commutability. Clin Biochem Rev. (2007) ;28: (4):139-47. PMC2282402 |
[60] | Bronkhorst AJ , Ungerer V , Oberhofer A , Holdenrieder S . The rising tide of cell-free DNA profiling: from snapshot to temporal genome analysis. JLM. 2022. doi:10.1515/labmed-2022-0030 |
[61] | Tabrizi S , Martin-Alonso C , Xiong K , Blewett T , Sridhar S , An Z , et al. An intravenous DNA-binding priming agent protects cell-free DNA and improves the sensitivity of liquid biopsies. BioRxiv. 2023:2023.01. 13.523947. doi:10.1101/2023.01.13.523947 |
[62] | Sina AA , Carrascosa LG , Liang Z , Grewal YS , Wardiana A , Shiddiky MJA , et al. Epigenetically reprogrammed methylation landscape drives the DNA self-assembly and serves as a universal cancer biomarker. Nat Commun. (2018) ;9: (1):4915. doi:10.1038/s41467-018-07214-w |
[63] | Mouliere F , Piskorz AM , Chandrananda D , Moore E , Morris J , Smith CG , et al. Selecting short DNA fragments in plasma improves detection of circulating tumour DNA. BioRxiv. (2017) . doi:10.1101/134437 |
[64] | Mouliere F , Chandrananda D , Piskorz AM , Moore EK , Morris J , Ahlborn LB , et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. (2018) ;10: (466):eaat4921-eaat. doi:10.1126/scitranslmed.aat4921 |




