Patterns of mutations in nine cancer-related genes and PAF development among smoking male patients diagnosed with bladder cancer
Abstract
BACKGROUND:
Smoking is one of the most popular risk factors provoking bladder cancer (BC). This research intended to estimate cigarette smoking effect involving PAF signs between smoking patients with BC and non-smoking patients with same diagnosis to define relations with pathological characteristics and their prognosis on zero-relapse and disease-associated recovery.
METHODS:
Two groups of smokers (n = 54) and non-smokers (n = 62) were selected. Both cohorts of patients had BC. They were evaluated utilizing NGS on 9 cancer-related genes and confirmed through the Sanger DNA sequencing and histopathological tests based on H&E staining. The factor of smoking and impact of PAF development by ELISA assay and PAF-R manifestation in terms of immunochemical evaluation on BC areas comparing to a control group (n = 30) was examined involving healthy contributors, including the use of well-designed statistical trials.
RESULTS:
The multivariate evaluation showed considerable rise in mutation patterns related to smoking among BC patients (group 3), increase in PAF development (***P<0.001) and vivid signs of PAF-R contrasted to non-smokers with BC (group 2) and control group (group 1). All the identified biological changes (gains/losses) were recorded at the same locations in both groups. Patients from group 3 held 3-4 various mutations, while patients from group 2 held 1-3 various mutations. Mutations were not identified in 30 respondents from control group. The most repeated mutations were identified in 3 of 9 examined genes, namely TP53, PIK3CA and PTEN, with highest rates of increase in Group 3. Moreover, histopathological tests revealed barely identifiable and abnormal traits in BC tissues, i.e. were without essential histopathological changes between groups 2 and 3.
CONCLUSION:
Smoking of cigarettes provokes PAF development due to urothelial inflammation and rise of mutations in 9 cancer-related genes. These are indicative factors of inducing BC.
Abbreviations
BC: | bladder cancer |
BSA: | bovine serum albumin |
CNAs: | copy number aberrations |
mg/ml: | milligram/ milliliter |
nm: | nanometer |
PAF: | platelet-activating factor |
PBS: | phosphate buffered saline |
SNVs: | single nucleotide variants |
TLC of lipids: | Thin-layer chromatography |
v/v: | volume per volume |
vol: | volume |
μM: | micromolar |
SSV: | sanger sequencing validation |
NGS: | next generation sequencing |
1Introduction
Bladder cancer (BC) is one of the most prevalent global health concerns. It is the sixth most widespread type of cancer, representing high mortality rates; for example, 16,870 deaths from BC have been registered just this year [1, 2]. BC is characterized by several critical symptoms, e.g., blood in the urine, regular urination triggers, and urination-related pain [2]. BC is also associated with the following risk factors: inheritance and family history, previous bladder complications, history of radiation therapy, and smoking habits [2].
In Saudi Arabia, high rates of smoking (48–70.7%) are indicative of huge health problems for the local population, which is especially relevant in young adults, particularly males [3]. Despite clear evidence that smoking can provoke various types of cancer and shorten life, a considerable number of male adults continue to smoke [4, 5]. To date, one of the leading causes of BC development is tobacco smoking [6]. Previous research revealed that smoking contributes to elevated platelet-activating factor (PAF) development and PAF receptor (PAF-R) manifestation, thus increasing chances of tumors and metastasis formation among smokers [1, 7]. PAF has been demonstrated to play a key part in the inflammatory process, thereby affecting endothelium with inflammation. Other studies have shown PAF’s principal role in provoking tumors and metastasis [8–13].
As urothelial cells are biologically vulnerable to cigarette smoke, smoking initiates the release of PAF, suppresses hydrolysis functions, and intensifies PAF-R manifestation [14]. Experiments with mice and long-term cigarette smoking showed signs of damage to urothelial function and high concentration of PAF within the bladder [1, 14].
This study focused on the impact of cigarette smoke regarding nine cancer-related genes, utilizing next-generation sequencing (NGS) technology. The data was confirmed via the Sanger sequencing method, which was applied to both smokers and non-smokers diagnosed with BC. Moreover, smoking and PAF accumulation with PAF-R manifestation in BC areas was studied with immunohistochemistry and histopathological tests, based on H&E staining.
2Materials and methods
2.1Ethics, participants and compliance
Referring to the tenets and standards of the Deanship of Scientific Research for Princess Nourah Bint Abdulrahman University, all clinical trials and studies have been performed in compliance. The local ethics board, KACST, Riyadh, KSA has approved the study under the following credentials: study number H-01-R059, IRB LOG number 20-0242. In addition, before taking any samples for analysis, the official written permissions from patients have been retrieved.
2.2Design and clinical analysis
The research has been performed by attracting 151 male participants. To get demographic data and medical records, each participant has been assessed through self-reporting technique. Participants have given their informed consent with no exceptions. Based on clinical evaluations, 121 patients aged between 57 and 72 have been finally selected for the research project. They have been further divided into two groups.
The first cohort represented the control group and involved 30 healthy male individuals with no smoking history. The second cohort included 67 male participants diagnosed with BC yet having no smoking history. Finally, the third cohort of 54 male participants diagnosed with BC with smoking history was formed. Diagnosing was made prior to chemotherapy/radiotherapy procedures (Table 1).
Table 1
Demographic data of male participants involved in the research
| Covariate | Group 1: Control patients | Group 2: Non-smokers with BC | Group 3: Smoking patients with BC |
| No. of participating patients | 30 | 62 | 54 |
| Age during diagnosis | |||
| <60 years | 19 | 22 | 13 |
| ≥60 years | 11 | 40 | 41 |
| Other related disease | |||
| Nephropathy | 0/ 30 (0%) | 29/ 62 (46.77%) | 35/54 (64.81%) |
| Urinary tract disease | 0/ 30 (0%) | 32/ 62 (51.61%) | 37/ 54 (68.51%) |
| Urinary tract infection | 2/ 30 (6.66%) | 21/ 62 (33.87%) | 24/ 54 (44.44%) |
| Renal failure | 0/ 30 (0%) | 0/ 62 (0%) | 2/ 54 (3.70%) |
| Peripheral arterial disease | 0/ 30 (0%) | 31/ 62 (50.00%) | 52/ 54 (96.29%) |
| Hypertension | 0/ 30 (0%) | 9/ 62 (14.51%) | 48/ 54 (88.88%) |
| Diabetes mellitus | 0/ 30 (0%) | 10/ 62 (16.12%) | 50/ 54 (92.59%) |
| Smoking history | |||
| Never | 0/ 30 (0%) | 0/ 62 (0%) | 0/ 54 (0%) |
| Exposed to secondhand smoke | 0/ 30 (0%) | 0/ 62 (0%) | 17/ 54 (31.48%) |
| Former (15–20 years) | 0/ 30 (0%) | 0/ 62 (0%) | 7/ 54 (12.96%) |
| Former (≥20 years) | 0/ 30 (0%) | 0/ 62 (0%) | 47/ 54 (87.03%) |
| Urinalyses (Mean±SD) | |||
| PH | 5.9±2.08 | 6.8±1.36 | 7.6±2.14 |
| Uric acid (mg/day) | 789.3±3.25 | 802.7±2.18 | 811.6±2.06 |
| Ammonia (mmol/day) | 42.2±3.61 | 54.4±4.22 | 62.1±3.43 |
| Urea (g/day) | 10.4±2.12 | 14.8±3.07 | 15.6±2.14 |
2.2.1DNA extraction
DNA extraction from all three-group male participants’ blood samples was performed with a help of Qiagen DNA isolation toolkit (Cat No. 69506, Quigen, Hilden, Germany) in compliance with the manual. Samples’ concentration and quality was evaluated utilizing the NanoDrop Spectrophotometer complex (Thermo Scientific, United States).
2.2.2Targeted next-generation sequencing
For NGS technology, a self-developed amplicon panel (known as TruSeq Custom Amplicon v1.5 developed and supplied from Illumina, San Diego) has been utilized embracing all coded exons of 9 cancer-associated genes among smoking and non-smoking male participants diagnosed with BC. The following genes were covered: ARID1A, CDKN2A, FGFR3, KRAS, NRAS, PIK3CA, PTEN, RB1, TP53. Database has been prepared in compliance with the manufacturer’s standards and requirements. MiSeq® benchtop sequencer was applied to ensure sequencing procedure. Raw inputs have been analyzed right on the MiSeq software (MiSeq Control Software, v2.6, Real-Time Analysis software, v1.18.54). For variant identification and alignment, we applied the SeqNext Module which is a part of the Sequence Pilot program (version 4.4.0, JSI medical systems GmbH, Ettenheim, Germany). To avoid possible germline options, variants having an allele frequency below 1% in open population libraries (gnomAD, [15]) have been excluded before direct evaluation of the options left.
High-quality CNAs have been defined based on amplicon data thanks to a newly designed algorithm. Identification was made based on the efficiency value of PCR exponential output of single amplicons in every sample studied [16]. The special web-platform was used to design oncoprints and visualize potential variants –http://cbioportal.org.
2.2.3Sanger sequencing method
To confirm the most prevalent mutations identified during NGS, specific exons of the studied genes (namely CDKN2A (exons 1, 2), FGFR3 (exons 10–18), KRAS (exon 2), NRAS (exons 2, 3), PIK3CA (exons 9, 20), PTEN (exon 8), TP53 (exon 9)) were initially enhanced with a help of PCR. Enhanced amplicons were further sequenced using an ABI 3730XL sequencer developed by Life Technologies (Carlsbad, CA, United States) in compliance with standards [19–21].
2.2.4PAF development
Each participant was able to provide 3 ml of blood for samples in the study. Blood samples obtained were put in a storage at room temperature for 30 minutes to clot. Further, they were centrifuged for 10 minutes at the speed of 3000 rpm. Afterwards, the serum samples have been selected and evaluated using the ELISA kit (Catalog Number E-EL-H2199, Elabscience Houston, TX, USA), exploiting the Biomek 4000 ELISA microplate liquid reagent dispensing automated instrument (Beckman Coulter, Brea, CA, USA) and applying EL405LS ELISA microplate automatic washing device (developed by BioTek Instruments, Winooski, VT, USA).
Furthermore, the absorption capacity of every well was interpreted at a wavelength of 450 nm utilizing a Multiskan FC plate-reader (developed by Thermo Scientific, Waltham, MA, USA). The average zero standard optical density has been taken away from all absorption cases. Eventually, a standard curve has been designed by applying a four-parameter logistic (4-PL) curve system. The level of concentration in the sample studied has been measured via insertion along the standard curve created by multiplying the outcomes and by the dilution variables mentioned before [22].
2.2.5Immunohistochemistry and histological tests
Paraffin blocks of biopsy samples related to cigarette smoking and BC diagnosis were taken from the male participants during surgical procedures and prior to chemotherapy/ radiotherapy practices. Approximately, 3–5μ sections were cut to pass traditional histopathology test and immunohistochemical analysis. Two slides have been provided from each block. Specifically, Eosin and Haematoxylin stains have been used on a slide to achieve the entire tissue. Moreover, the stained areas have been scanned at high magnitude (20X) to evaluate the histopathological sections. Then, these sections have been taken on photo using a photomicroscope. In addition, immunohistochemical analysis has been performed on the second slide from each block applying PAF-R antibody in compliance with a standard described in the previous studies [19].
2.2.6Statistical analysis
To ensure analysis of data, the Graph Pad Prism 9.0 (developed by Graph Pad Software Inc., San Diego, CA) was used. Afterwards, the quantitative results of analysis have been presented as standard deviations and mean values. P values < 0.05 were determined in the study as statically valid and relevant.
3Results
3.1Analysis of biological mutations
DNA of 116 male participants (54 smokers and 62 non-smokers) diagnosed with BC has been sequenced to embrace each coding exon of 9 cancer-associated genes utilizing NGS technology. They were later evaluated for biological (somatic) mutations contrasting data to the control group. NGS technology revealed that 13 biological mutations were identified in 7 of 9 cancer-associated genes of the DNA samples regarding both smoking and non-smoking groups of male participants with BC. All the identified biological mutations (both gains/losses) were placed at repeated sites with alternative patterns/frequencies of the deviations defined, comparing between Group 2 and Group 3 (Fig. 1). Group 3 of smokers with BC demonstrated a considerable rise in biological mutations among the selected genes contrasted to Group 2 of non-smokers with BC. Moreover, male participants from Group 3 held between 3-4 exclusive mutations compared to 1-3 exclusive mutations from Group 2 male participants.
Fig. 1
Identified somatic/biological mutations noticed in all coding exons of 9 cancer-associated genes through NGS technology; derived from non-smoking (green lines) and smoking (black lines) male participants diagnosed with BC.
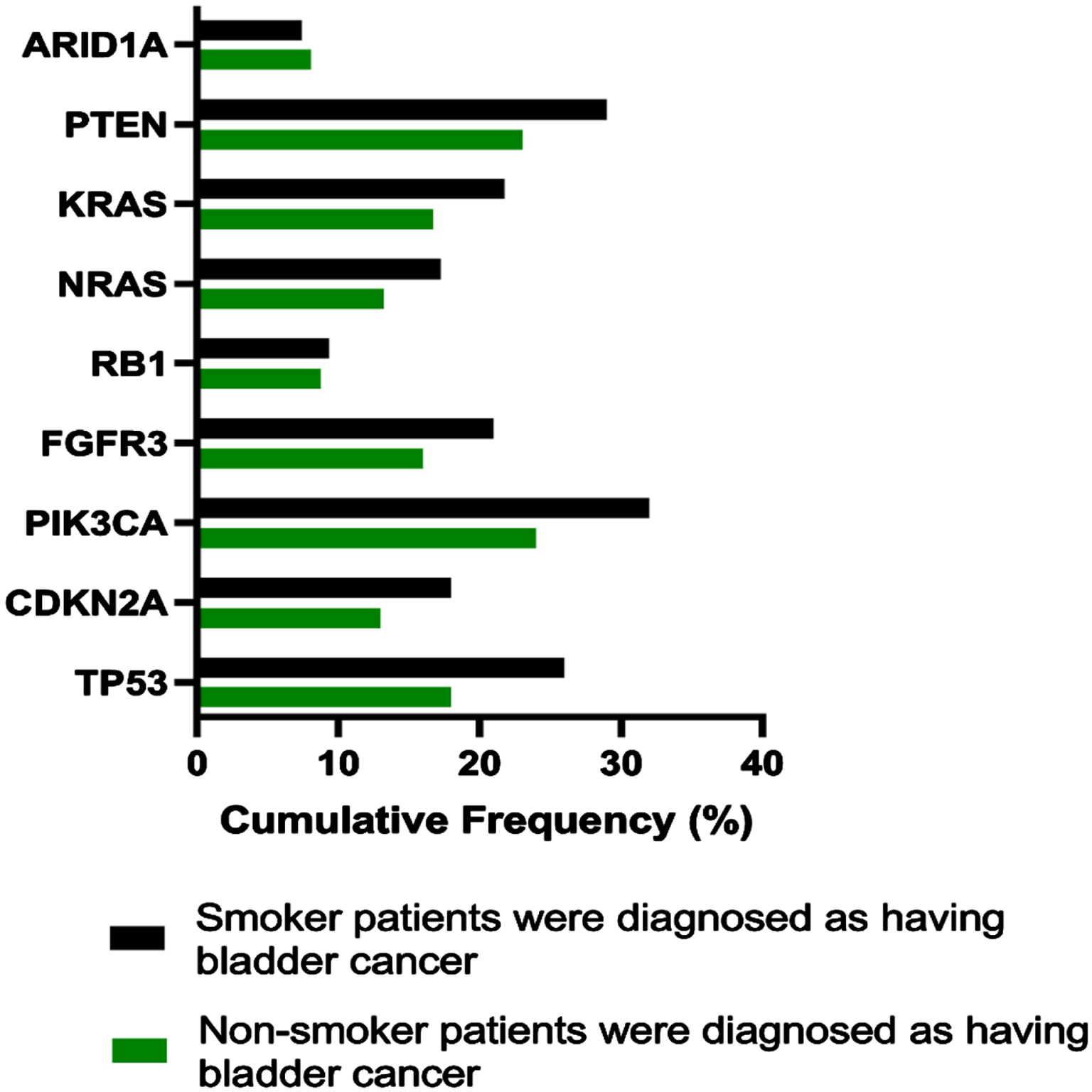
Observable mutations have been detected in ARID1A gene (9.71%), RB1 gene (10.34%), CDKN2A gene (13.01%), NRAS (13.22%), KRAS (16.73%), FGFR3 gene (16.00%), TP53 gene (18.01%), PTEN gene (23.02%) and PIK3CA gene (24.00%) in Group 2 male participants.
Moreover, the prevalent mutations have been identified among Group 3 male participants, such as ARID1A gene (12.71%), RB1 gene (14.31%), CDKN2A gene (18.11%), NRAS (17.19%), KRAS (21.62%), FGFR3 gene (21.03%), TP53 gene (26.12%), PTEN gene (29.10%) and PIK3CA gene (32.04%).
Based on findings from NGS procedure, most frequently mutated genes were 7 of 9 selected genes, specifically TP53, PIK3CA, FGFR3, CDKN2A, NRAS, KRAS and PTEN. Furthermore, genes identified have been confirmed on validity with a help of SSV (Fig. 2). Two biological mutations were identified as frameshift pathologies presented in exon 2 for genes KRAS & NRAS with a considerable amount of mutation patterns in Group 3 compared to Group 2.
Fig. 2
Patterns of various mutations have been detected in TP53, CDKN2A, PIK3CA, FGFR3, HRAS, NRAS, KRAS and PTEN genes with a help of SSV; they were observed in non-smoking participants (green lines) and smoking participants (black lines) diagnosed with BC.
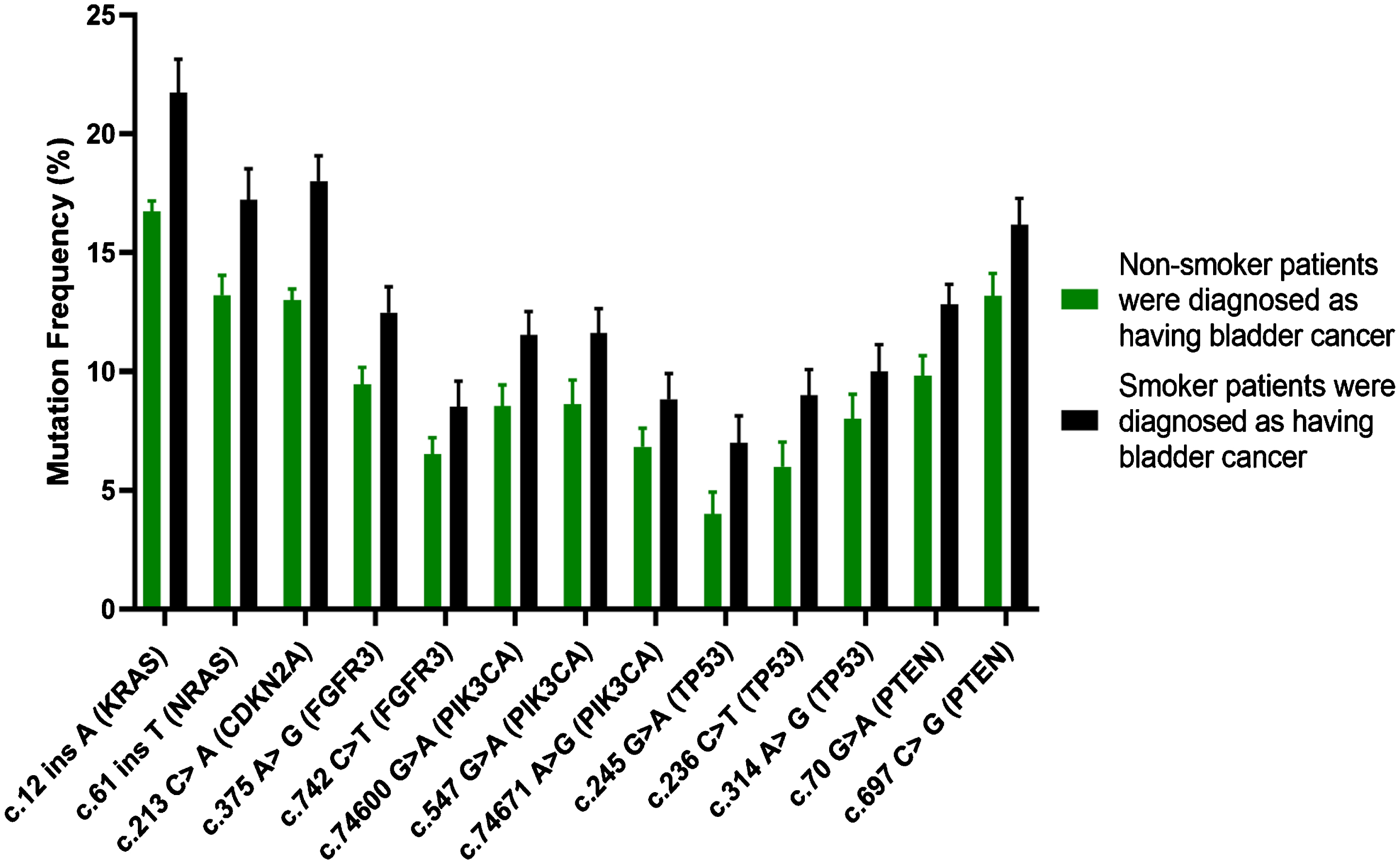
Changes of SSCA in exon 2 within KRAS gene have been recorded in 16/62 (16.12%) among non-smoking participants diagnosed with BC (Group 2); meanwhile, they have been identified in 12/54 (22.2%) among smoking participants with BC (Group 3) because of 12 ins A mutation. Moreover, small changes of SSCA in exon 2 of NRAS gene were also identified in 8/62 (12.9%) among non-smoking participants diagnosed with BC (Group 2). Respectively, they have been recorded in 9/54 (16.66%) among smoking participants with BC (Group 3) because of 61 ins T mutation.
SSV revealed that the most regularly developing mutations (>25%) were observable in 3 of 9 studied genes (namely TP53, PIK3CA and PTEN); moreover, a considerable rise in number of mutations was registered in Group 3 contrasted to Group 2 (Fig. 3). In comparison, all 3 identified genes were associated with frequently developing mutations ranged 18–24% in Group 2 (Fig. 1).
Fig. 3
Electropherograms derived through the Sanger sequencing technique indicated of biological mutations in such genes as TP53, CDKN2A, PIK3CA, FGFR3, NRAS, KRAS and PTEN genes.
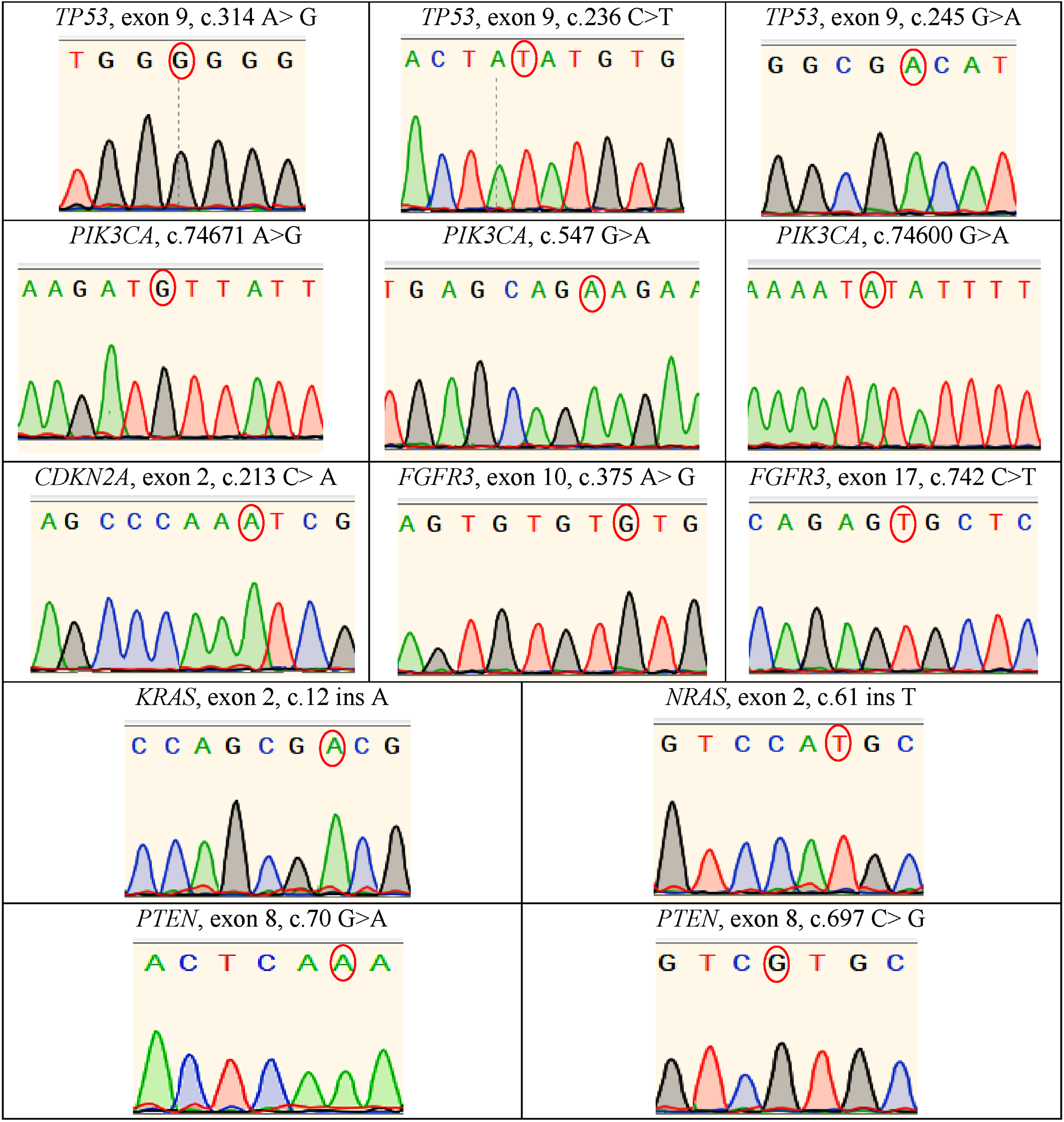
Moreover, regularly developing mutations in 2 of 9 selected genes (namely, ARID1A and RB1) were associated with the lowest values (>10%) in both testing groups which was confirmed by NGS (Fig. 1). Importantly, the nucleotide sequencing of such genes as TP53, PIK3CA, FGFR3, CDKN2A and PTEN demonstrated no presence of frameshift mutations and pathologies in above-mentioned genes. Similarly, there were no mutations identified above in the study’s control group.
3.1PAF production
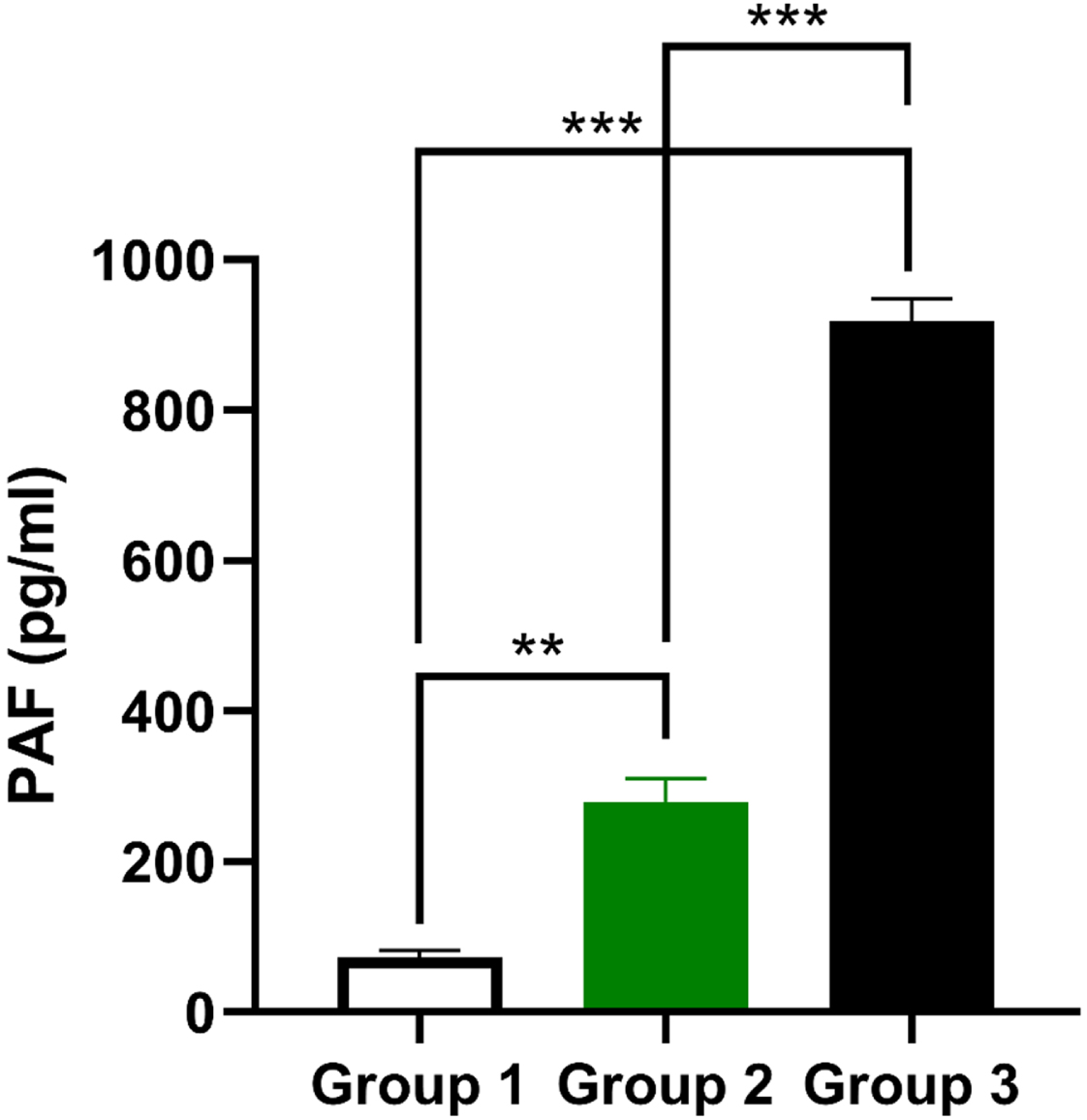
The extent of PAF is considered one of the vital signs of the inflammation. Figure 4 showed a striking increase of PAF production (**P<0.01) in Group 2 consisting of non-smokers with BC as compared to the control group. PAF production levels also elevated noticeably (***P<0.001) in Group 3 of smoking male participants with BC contrasting to Group 1 and Group 2.
Fig. 4
PAF production in three groups of male participants with normal and abnormal levels of PAF. They were calculated referring to the blood serum taken from non-smoking participants (green lines) and smoking participants (black lines) diagnosed with BC. Vlues were presented as means±SEM (n = 30) of three trials. ***P<0.001, **P<0.01, *P<0.05 comparing to the values of the control Group 1.
3.3Histopathology and immunohistochemistry
Outcomes of histopathological tests have been evaluated by a professional histopathologist. They were later graded by three separate tumor scores (Table 2). Each section of carcinoma affecting human bladder revealed barely identifiable characteristics of BC tissues from Group 2 and Group 3. In addition, a distinct rise in mitotic numbers, sizes, abnormal extent of proliferation rates in covering typical tissues with a minor number of nuclei and nuclear pleomorphism tendencies has eventually increased the composition of RBCs with making veins overfilled, causing necrosis, initiating intrusion of intracellular and extracellular elements, and leading to damage of cellular polarity, yet without considerable histopathological differences between Group 2 and Group 3 (Fig. 5, A and B).
Table 2
Categories of tumor for patients with BC in Group 2 and Group 3
| Covariate | Group 2: Non-smoker patients were diagnosed as having bladder cancer | Group 3: Smoker patients were diagnosed as having bladder cancer |
| No. pathologic grade | ||
| Low (1 + 2 grades) | 51/62 (82.25%) | 18/ 54 (33.33%) |
| High (3 grade) | 11/ 62 (17.74%) | 36/ 54 (66.66%) |
| No. lymph node status | ||
| Positive | 3/ 62 (4.47%) | 31/ 54 (57.40%) |
Fig. 5
Microscopic histopathology provides images of cross sections of BC obtained from both Group 2 and Group 3 and analyzed under light microscopy, magnitude 100μm. H&E stain was added to A and B samples demonstrating imbalance in bladder cells, showing atypical sizes and forms for normal cells. Section A was obtained from Group 2; section B was obtained from Group 3, both demonstrating occasional textures of bladder tissues showing abnormal mitotic signs, decreased nucleoli, necrosis effects, presence of intracellular/extracellular elements and elevated level of RBCs with several overfilled veins. Sections C and D demonstrated immunity to stains by releasing PAF-R antibody. Section C was obtained from Group 2 demonstrating weak yet positive staining of PAF-R protein in non-smoking male participants with BC; meanwhile, Section D was obtained from Group 3 demonstrating strong and positive staining of PAF-R protein in male smokers with BC.
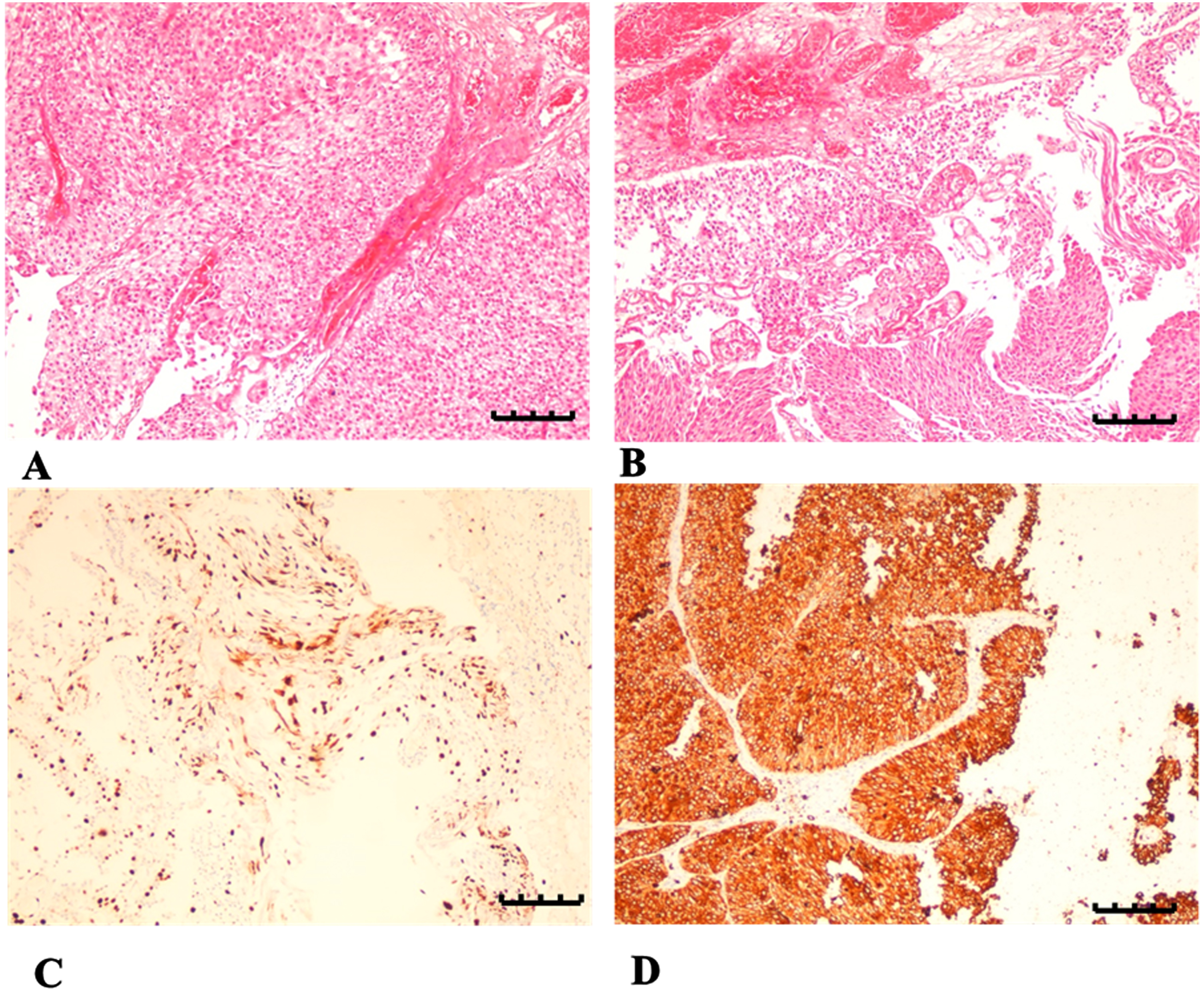
Later, immunohistochemistry evaluations indicated about nuclear staining in approximately 20% responses for weak positivity in relation to PAF-R antibody, which was observable in Group 2 (Fig. 5, C). Additionally, manifestation of PAF-R was identified as positive above 50% in all the rest bladder tissues affected by tumors (Fig. 5, D). It should also be mentioned that the practice of antigen sampling has been measured and performed in compliance with the latest registered quantitative H-scores for each case. It was completed by multiplying the values in relation to the following established categories: above 50% (robust), 20–40% (medium), 1–20% (weak), and 0% (none).
4Discussion
Current research relied on clinicopathological findings from different groups of participants (Table 1). This confirmed that smoking cigarettes raised health risks associated with BC development, which was supported by findings from another study that identified pyroptosis of urothelial cells via the ROS/NLRP3/caspase-1 signaling channel, provoked by cigarette smoke [23–25]. Moreover, the relation between nephropathy, renal failure, and BC has been confirmed. In addition, other research suggested that BC could be provoked among smokers and non-smokers depending on their medical history, e.g., hypertension, peripheral arterial disease, and diabetes [26–29]. Smoking also contributes to the progression of BC in late stages of life. As such, age remains a critical risk factor for eliciting umbilical cord blood (UCB) and accelerating the organism’s degradation with an official diagnosis [30]. Nonetheless, our data from Table 1 revealed that the diagnosis of BC is not differentiated by age among patients, although it was shown that various carcinogens (tobacco and smoking chemicals) contribute to its development, with a patient’s genetic risks and inheritance, independent of age [30]. In context of a patient’s smoking history, it was found that non-smoking patients have less active and severe forms of BC compared to their smoking counterparts, as shown in Table 2. Recent research showed that smoking may directly provoke development of invasive cancer cells with a much higher likelihood [30, 31, 34, 36]. Previous studies reported that the population-attributable risk with BC with tobacco smoking is 4 times higher in men than women, and current cigarette smoke triples BC risk compared to never smoking [30, 37].
The process of how cigarette smoke provokes metastasis, specifically in the lungs, has not yet been studied [32]. Smoking, despite being a factor of high morbidity and mortality, is still a popular habit at 22.1%, with Saudis using cigarettes each day. Statistically, 43.7% of smokers are males, with the female population of smokers representing 16.5% [33]. An average cigarette contains approximately 7,000 chemicals, and more than 70 are scientifically categorised as carcinogenic. This means that these chemicals can damage the cellular structure of DNA and initiate the process of tumorigenesis [34]. This not only damages DNA, but in mice studies it decreased recovery capacity in the lungs, heart, and bladder; in addition, similar degradation effects were noted in human studies in context of lung and bladder cells [35, 36].
Moreover, cancer-associated genes (comprising oncogenes and tumor suppressor genes) are mutated, causing cells to grow rapidly, thus leading to cancer. Among many cancer-associated genes, nine BC-associated genes were selected as the most common in this study [24, 35, 36, 38], relying on NGS technology, which identified thirteen observable mutations in 7 of 9 cancer-associated genes of DNA samples among non-smoking and smoking participants with BC. These mutations were confirmed by structural signature variation (SSV), detecting a similar number of mutation patterns in the studied genes. Other research determined growing mobility of tumor cells in the bladder, which increased urothelial cell damage with cigarette smoke’s chemicals [1, 14, 16, 24, 29, 35, 36]. These findings uncover biological mechanisms by which smoke may generally induce BC progression, although details of that process are still unclear. Nonetheless, given that metastasis (not always the tumor itself) leads to 90% of deaths in cases with a BC diagnosis, with scientific efforts directed to study mechanisms of how cigarette smoke can prompt metastasis that in turn leads to cancer complications [32].
Other research demonstrated that PAF triggers pro-inflammatory cells, including macrophages and neutrophils through selected inflammatory channels; this was claimed to contribute to diverse biological processes, such as epithelial apoptosis, growth of vascular permeability, and smooth muscle contractions [33, 36, 39, 40]. In connection with reproductive function, anti-apoptosis, and DNA repair, it was suggested as a potential cause of cancer progression [36]. The research found that PAF suppression decreased adhesion to endothelial cells, reducing metastasis in melanoma and colon cancer cells [39, 40].
The current study presented outcomes of the ELISA assay: they identified that PAF was increasingly generated in the blood serum of participants from Groups 2 and 3, unlike participants from the control group. Specifically, the highest values of PAF (***P<0.001) were found in Group 3, or smokers with BC. Moreover, immunohistochemistry tests revealed a decline of PAF-R protein manifestation in BC samples among non-smokers (Group 2) in contrast to BC areas among smokers (Group 3). As histopathological tissues were stained with H&E, it was demonstrated that there were no considerable differences between Groups 2 and 3. With a close tie to cigarette smoking, inflammatory channels of spread are essential preconditions of BC development, with elevated PAF release potentially contributing to cancer cell development, spread, and tumor tissue production, confirmed and described by several past studies [11, 12, 24, 32, 36, 38–40].
In the end, it may be argued that smoking cigarettes activates PAF development and results in the urothelial inflammation process, which is further related to serious risks of BC and chances of mutation in nine cancer-related genes (shown in the data of this study, in which PAF levels were almost more than three times that of Group 3, compared to PAF levels in Group 2). These risk factors can be initiated in various signaling channels and pathways to eventually create the invasion of cancer cells in the bladder’s environment. In fact, the evaluation of nine cancer-related genes is a good prediction tool that defines risks of BC development in smokers, with a focus on PAF development and receptor (PAF-R) manifestation due to urothelial inflammation. Future studies must focus on other cancer-related genes to discover new somatic and biological mutations in smokers with a BC diagnosis to be able to compare them with non-smoking patients who have BC.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R227), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author contributions
CONCEPTION: EA, AMA, TMA, MAA and FAS
DATA CURATION: EA, AMA, TMA, MAA, FAS, LMA, ASJ, ISA, MAA, MAA and FAA
ANALYSIS OF DATA: EA, AMA, TMA, MAA, FAS, LMA, ASJ, ISA, MAA, MAA and FAA
PREPARATION OF THE MANUSCRIPT: WSA
REVISION FOR IMPORTANT INTELLECTUAL CONTENT: EA, AMA, TMA, MAA, FAS, LMA, ASJ, ISA, MAA, MAA and FAA
Conflict of interest
The authors declare no conflict of interest.
Data availability
All relevant data are within the paper.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R227), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
References
[1] | Kispert S , Marentette J , McHowat J Cigarette smoking promotes bladder cancer via increased platelet-activating factor. Physiological Reports. (2019) ;7: (3):e13981. DOI: 10.14814/phy2.13981 |
[2] | Howlader N , Noone AM , Krapcho M , Miller D , Brest A , Yu M , Ruhl J , Tatalovich Z , Mariotto A , Lewis DR , Chen HS . SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD, USA. (2020) . |
[3] | Nasser AM , Geng Y , Al-Wesabi SA . The prevalence of smoking (cigarette and waterpipe) among university students in some Arab countries: a systematic review. Asian Pacific Journal of Cancer Prevention: APJCP. (2020) ;21: (3):583. DOI: 10.31557/APJCP.2020.21.3.583 |
[4] | Crowley DM , Hill LG , Kuklinski MR , Jones DE . Research priorities for economic analyses of prevention: Current issues and future directions. Prevention Science. (2014) ;15: (6):789–98. DOI: 10.1007/s11121-013-0429-z |
[5] | Sheffer CE , Stein JS , Petrucci C , Mahoney MC , Johnson S , Giesie P , Carl E , Krupski L , Tegge AN , Reid ME , Bickel WK . Tobacco dependence treatment in oncology: Initial patient clinical characteristics and outcomes from Roswell Park Comprehensive Cancer Center. International Journal of Environmental Research and Public Health. (2020) ;17: (11):3907. DOI: 10.3390/ijerph17113907 |
[6] | World Health Organization. International Agency for Research on Cancer. 2019. |
[7] | Lordan R , Tsoupras A , Zabetakis I . The potential role of dietary platelet-activating factor inhibitors in cancer prevention and treatment. Advances in Nutrition. (2019) ;10: (1):148–64. DOI: 10.1093/advances/nmy090 |
[8] | Montrucchio G , Alloatti G , Camussi G . Role of platelet-activating factor in cardiovascular pathophysiology. Physiological Reviews. (2000) ;80: (4):1669–99. DOI: 10.1152/physrev.2000.80.4.1669 |
[9] | Bussolati B , Biancone L , Cassoni P , Russo S , Rola-Pleszczynski M , Montrucchio G , Camussi G . PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. The American Journal of Pathology. (2000) ;157: (5):1713–25. DOI: 10.1016/S0002-9440(10)64808-0 |
[10] | Denizot Y , Desplat V , Drouet M , Bertin F , Melloni B . Is there a role of platelet-activating factor in human lung cancer? Lung Cancer. (2001) ;33: (2-3):195–202. DOI: 10.1016/S0169-5002(01)00197-0 |
[11] | Melnikova VO , Mourad-Zeidan AA , Lev DC , Bar-Eli M . Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. Journal of Biological Chemistry. (2006) ;281: (5):2911–22. DOI: 10.1074/jbc.M508683200 |
[12] | Melnikova V , Bar-Eli M . Inflammation and melanoma growth and metastasis: the role of platelet-activating factor (PAF) and its receptor. Cancer and Metastasis Reviews. (2007) ;26: (3):359–71. DOI: 10.1007/s10555-007-9092-9 |
[13] | McHowat J , Gullickson G , Hoover RG , Sharma J , Turk J , Kornbluth J . Platelet-activating factor and metastasis: calcium independent phospholipase A2β deficiency protects against breast cancer metastasis to the lung. American Journal of Physiology-Cell Physiology. (2011) ;300: (4):C825–32. DOI: 10.1152/ajpcell.00502.2010 |
[14] | Kispert SE , Marentette J , Campian EC , Isbell TS , Kuenzel H , McHowat J . Cigarette smoke-induced urothelial cell damage: potential role of platelet-activating factor. Physiological Reports. (2017) ;5: (5):e13177. DOI: 10.14814/phy2.13177 |
[15] | Karczewski KJ , Francioli LC , Tiao G , Cummings BB , Alföldi J , Wang Q , Collins RL , Laricchia KM , Ganna A , Birnbaum DP , Gauthier LD . The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. (2020) ;581: (7809):434–43. DOI: 10.1038/s41586-020-2308-7 |
[16] | Maurer A , Ortiz-Bruechle N , Guricova K , Rose M , Morsch R , Garczyk S , Stöhr R , Bertz S , Golz R , Reis H , Bremmer F . Comparative genomic profiling of glandular bladder tumours. Virchows Archiv. (2020) ;477: (3):445–54. DOI: 10.1007/s00428-020-02787-8 |
[17] | Gao J , Aksoy BA , Dogrusoz U , Dresdner G , Gross B , Sumer SO , Sun Y , Jacobsen A , Sinha R , Larsson E , Cerami E . Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science Signaling. (2013) ;6: (269):p11. DOI: 10.1126/scisignal.2004088 |
[18] | Cerami E , Gao J , Dogrusoz U , Gross BE , Sumer SO , Aksoy BA , Jacobsen A , Byrne CJ , Heuer ML , Larsson E , Antipin Y . The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discovery. (2012) ;2: (5):401–4. DOI: 10.1158/2159-8290.CD-12-0095 |
[19] | Suliman RS , Algebaly AS , Alqahtani WS . Role of Human PTEN and TP53 Sequence Mutations in the Etiology of Breast Cancer in Saudi Patients. Pakistan Journal of Biological Sciences. (2020) ;23: (3):321–30. DOI: 10.3923/pjbs.2020.321.330 |
[20] | Arsenic R , Treue D , Lehmann A , Hummel M , Dietel M , Denkert C , Budczies J . Comparison of targeted next-generation sequencing and Sanger sequencing for the detection of PIK3CA mutations in breast cancer. BMC Clinical Pathology. (2015) ;15: (1):1–9. DOI: 10.1186/s12907-015-0020-6 |
[21] | Platz A , Hansson J , Månsson-Brahme E , Lagerlöf B , Linder S , Ringborg U , Lundqvist E , Sevigny P , Inganäs M . Screening of germline mutations in the CDKN2A and CDKN2B genes in Swedish families with hereditary cutaneous melanoma. Journal of the National Cancer Institute. (1997) ;89: (10):697–702. DOI: 10.1093/jnci/89.10.697 |
[22] | Li G , Wu W , Zhang X , Huang Y , Wen Y , Li X , Gao R . Serum levels of tumor necrosis factor alpha in patients with IgA nephropathy are closely associated with disease severity. BMC Nephrology. (2018) ;19: (1):1–9. DOI: 10.1186/s12882-018-1069-0 |
[23] | Vermeulen SH , Hanum N , Grotenhuis AJ , Castano-Vinyals G , Van Der Heijden AG , Aben KK , Mysorekar IU , Kiemeney LA . Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. British Journal of Cancer. (2015) ;112: (3):594–600. DOI: 10.1038/bjc.2014.601 |
[24] | Wu Z , Liu Q , Zhu K , Liu Y , Chen L , Guo H , Zhou N , Li Y , Shi B . Cigarette smoke induces the pyroptosis of urothelial cells through ROS/NLRP3/caspase-1 signaling pathway. Neurourology and urodynamics. (2020) ;39: (2):613–24. DOI: 10.1002/nau.24271 |
[25] | Van den Brand JA , van Dijk PR , Hofstra JM , Wetzels JF . Cancer risk after cyclophosphamide treatment in idiopathic membranous nephropathy. Clinical Journal of the American Society of Nephrology. (2014) ;9: (6):1066–73. DOI: 10.2215/CJN.08880813 |
[26] | Tuccori M , Filion KB , Yin H , Oriana HY , Platt RW , Azoulay L . Pioglitazone use and risk of bladder cancer: population based cohort study. BMJ. 2016;352. DOI: 10.1136/bmj.i1541 |
[27] | Fang H , Yao B , Yan Y , Xu H , Liu Y , Tang H , Zhou J , Cao L , Wang W , Zhang J , Zhao L . Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technology & Therapeutics. (2013) ;15: (11):914–22. DOI: 10.1089/dia.2013.0131 |
[28] | Kok VC , Zhang HW , Lin CT , Huang SC , Wu MF . Positive association between hypertension and urinary bladder cancer: epidemiologic evidence involving 79,236 propensity score-matched individuals. Upsala Journal of Medical Sciences. (2018) ;123: (2):109–15. DOI: 10.1080/03009734.2018.1473534 |
[29] | Bashaweeh RK , Alfaraj GA , Alwatban JJ , Allabboudy LM , Alghabban SA , Alkhateeb S . Urothelial carcinoma of the bladder with abnormal inguinal metastasis: A case report. Urology Case Reports. (2021) ;35: :101535. DOI: 10.1016/j.eucr.2020.101535 |
[30] | Shariat SF , Sfakianos JP , Droller MJ , Karakiewicz PI , Meryn S , Bochner BH . The effect of age and gender on bladder cancer: a critical review of the literature. BJU International. (2010) ;105: (3):300–8. DOI: 10.1111/j.1464-410X.2009.09076.x |
[31] | Jiang X , Castelao JE , Yuan JM , Stern MC , Conti DV , Cortessis VK , Pike MC , Gago-Dominguez M . Cigarette smoking and subtypes of bladder cancer. International Journal of Cancer. (2012) ;130: (4):896–901. DOI: 10.1002/ijc.26068 |
[32] | Kispert SE , Marentette JO , McHowat J . Enhanced breast cancer cell adherence to the lung endothelium via PAF acetylhydrolase inhibition: a potential mechanism for enhanced metastasis in smokers. American Journal of Physiology-Cell Physiology. (2014) ;307: (10):C951–6. DOI: 10.1152/ajpcell.00218.2014 |
[33] | Alzahrani WA , Mohammed MA , Harthi AA , Alshaikh AM , Almubaddil MS , Darwish NB . The socioeconomic and cultural impact of tobacco and smoking in Saudi Arabia. International Journal of Medicine in Developing Countries. (2020) ;4: (8):1276–80. DOI: 10.24911/IJMDC.51-1575925960 |
[34] | Lushniak BD , Samet JM , Pechacek TF , Norman LA , Taylor PA . The health consequences of smoking—50 years of progress: A report of the Surgeon General. 2014. |
[35] | Lee HW , Park SH , Weng MW , Wang HT , Huang WC , Lepor H , Wu XR , Chen LC , Tang MS . E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proceedings of the National Academy of Sciences. (2018) ;115: (7):E1560–9. DOI: 10.1073/pnas.1718185115 |
[36] | Boeri L , Soligo M , Frank I , Boorjian SA , Thompson RH , Tollefson M , Quevedo FJ , Cheville JC , Karnes RJ . Cigarette smoking is associated with adverse pathological response and increased disease recurrence amongst patients with muscle-invasive bladder cancer treated with cisplatin-based neoadjuvant chemotherapy and radical cystectomy: a single centre experience. BJU International. (2019) ;123: (6):1011–9. DOI: 10.1111/bju.14612 |
[37] | Freedman ND , Silverman DT , Hollenbeck AR , Schatzkin A , Abnet CC . Association between smoking and risk of bladder cancer among men and women. JAMA. (2011) ;306: (7):737–45. DOI: 10.1001/jama.2011.1142 |
[38] | Goel A , Ward DG , Noyvert B , Yu M , Gordon NS , Abbotts B , Colbourne JK , Kissane S , James ND , Zeegers MP , Cheng KK . Combined exome and transcriptome sequencing of non-muscle-invasive bladder cancer: associations between genomic changes, expression subtypes, and clinical outcomes. Genome Medicine. (2022) ;14: (1):1–6. DOI: 10.1186/s13073-022-01056-4 |
[39] | Mannori G , Barletta E , Mugnai G , Ruggieri S . Interaction of tumor cells with vascular endothelia: role of platelet activating factor. Clinical & Experimental Metastasis. (2000) ;18: (1):89–96. DOI: 10.1023/A:1026548700247 |
[40] | Kispert SE , Marentette JO , McHowat J . Enhanced breast cancer cell adherence to the lung endothelium via PAF acetylhydrolase inhibition: a potential mechanism for enhanced metastasis in smokers. American Journal of Physiology-Cell Physiology. (2014) ;307: (10):C951–6. DOI: 10.1152/ajpcell.00218.2014 |




