β-catenin plus PROX1 immunostaining stratifies disease progression and patient survival in neoadjuvant-treated pancreatic cancer
Abstract
BACKGROUND:
Wnt/β-catenin signaling is a highly conserved signaling pathway that regulates the transcription factor PROX1. The role of β-catenin and PROX1 in pancreatic cancer is ambiguous, as some studies have associated their expression with tumor regression and some with tumor progression.
OBJECTIVE:
We have investigated their expression in surgically treated pancreatic cancer patients receiving neoadjuvant therapy (NAT), and patients treated upfront with surgery (US). We furthermore compared the expression of β-catenin and PROX1 between patients who had a good or poor response to NAT.
METHODS:
We evaluated β-catenin and PROX1 expression through immunohistochemistry in 88 neoadjuvant and 144 upfront surgery patients by scoring the intensity of the immunopositivity as 0–3, corresponding to negative, weak, moderate, or strong. We developed a six-tier grading scheme for the neoadjuvant responses by analyzing the remaining tumor cells in surgical specimen histological sections.
RESULTS:
Strong β-catenin immunopositivity associated with improved survival in the patients with good NAT-response (≤10% residual tumor cells) (Hazard ratio [HR] 0.26 95%, confidence interval [CI] 0.07–0.88 p = 0.030). Additionally, the combined moderate β-catenin and PROX1 expression associated with improved survival (HR 0.20 95% CI 0.05–0–76 p = 0.018) among the good responders. Among the patients with a poor NAT-response (> 10% residual tumor cells), both strong β-catenin immunopositivity and strong combined β-catenin and PROX1 associated with shorter survival (HR 2.03 95% CI 1.16–3.55 p = 0.013, and HR 3.1 95% CI 1.08–8.94 p = 0.03, respectively). PROX1 alone was not associated with survival.
CONCLUSIONS:
Strong β-catenin immunopositivity and combined strong or moderate β-catenin and PROX1 immunopositivity associated with improved survival among the good NAT-responders and worse survival among the poor NAT-responders.
1Introduction
Due to the aggressive nature of pancreatic cancer the survival rates continue to remain low, with only 6% five-year survival across all stages and 20% even in localized disease [1, 2]. Neoadjuvant therapy (NAT) offers a solution for controlling early micrometastasis, downsizing tumor, and decreasing tumor activity [3, 4]. Currently, NAT is effectively used in locally advanced and borderline resectable pancreatic cancer [5, 6]. The utilization of NAT in resectable pancreatic cancer is under debate [7].
The impacts of preoperative treatments on cell-level changes in pancreatic cancer are widely unknown. Treatment response can be evaluated histologically from postsurgical specimens by assessing the percentage of remaining viable tumor cells by objective and repeatable scale and criteria [8]. Few such scaling methods exist, but the field lacks the establishment of a single method [9–11].
β-catenin is an intracellular regulator of the Wnt signaling, [12, 13] the evolutionary conserved pathway that participates in multiple developmental events during embryonic development, including cell proliferation, stem cell renewal, and cellular differentiation [14]. During pancreas development, both inappropriate activation and inactivation lead to profound defects of the pancreas [15, 16]. In Wnt signaling, the absence of Wnt ligands leads to phosphorylation of β-catenin, which further prevents nuclear accumulation and stabilization of β-catenin and leads to repressing target genes. Wnt ligand binding results in β-catenin stabilization and accumulation in the nucleus, which further leads to transcription [17, 18].
The Wnt/β-catenin pathway has been implicated in tumorigenesis at several sites, including the colon and rectum [19], pancreas [20], and liver [21]. In normal pancreatic ductal cells, the expression of β-catenin is mainly membranous, but in pancreatic cancer β-catenin undergoes redistribution so that membranous expression decreases and aberrant cytoplasmic and nuclear expression increases [20, 22–24]. In pancreatic cancer, increased cytoplasmic, rather than membranous, expression of β-catenin is associated with metastasis [25, 26] and, in some studies, poorer survival [25, 27]. The Wnt/β-catenin plays a role in initiating the malignant process [28]. The association between the intensity of β-catenin expression and survival in pancreatic cancer is ambiguous. Saukkonen et al. showed that especially low cytoplasmic expression of β-catenin is associated with poor prognosis [24]. In another study, high β-catenin expressing cells showed more invasiveness than low expressing cells [29].
Prospero-related homeobox 1 (PROX1) is a transcriptional factor coded by the homeobox gene PROX1. In developing organs, its strongest expression has been described in lymphatic endothelial cells [30–32] but has been found in all developing germ layers in various tissues, including the central nervous system [33, 34], pancreas, and liver [34, 35]. The role of PROX1 in different malignancies is diverse, and it has been characterized as both suppressing and promoting tumor growth [23, 37–45]. In colorectal cancer (CRC), PROX1 acts as an essential downstream regulator of the Wnt/β-catenin pathway, and a high level of abnormal Wnt/β-catenin signaling increases the expression of PROX1, which is further associated with CRC tumor progression [36]. In pancreatic cancer, its role has been described mainly as tumor-suppressive [37–39]. Jin et al. found that forced PROX1 re-overexpression could reverse cell proliferation and migration of pancreatic cancer cells [39]. The site of PROX1 expression in the normal pancreatic tissue is commonly nuclear, but in pancreatic cancer, cytoplasmic staining, rather than nuclear, is frequently seen [24]. In pancreatic cancer cells, PROX1 and β-catenin expressions are generally decreased and correlate with each other [24].
The function and expression of Wnt/β-catenin signaling and PROX1 in pancreatic cancer following neoadjuvant treatment are not understood. It is not known whether the cytoplasmic expression of these markers correlates with survival in neoadjuvant-treated pancreatic cancer patients [24]. Thus, we aimed to investigate the expression of β-catenin and PROX1 and their association with survival in neoadjuvant-treated pancreatic cancer in comparison with upfront surgery (US) patients. Additionally, we aimed to analyze the differences in the expressions between the different NAT-response groups.
2Methods
We identified patients surgically treated for pancreatic ductal adenocarcinoma (PDAC) at Helsinki University Hospital between 2000 and 2016 (n = 235), finding 90 patients who received NAT. For the control group, we gathered 145 PDAC patients with upfront surgery who were propensity scored with matched age, sex, and time of surgery. The follow-up data, including time of death and local and distant disease recurrence, were collected from patient records, Statistics Finland, and the Finnish Population Registry. Patients who died of immediate (< 30 days) postoperative complications were excluded (n = 3). Due to retrospective nature of the study, the used NAT regimens vary. NATs used in the study patients are described in the Supplementary table.
2.1Tumor tissue microarray and immunohistochemistry
Formalin-fixed and paraffin-embedded post-pancreatectomy specimens were collected, and hematoxylin-eosin samples were evaluated by an experienced pathologist (JH) to confirm the diagnosis and to determine well-presented areas for the tumor tissue microarray (TMA) preparation. Six 1.0 mm cores were drilled from each tumor with an automatic tissue microarrayer (TMA Grand Master, TMA Control 3.0, 3D Histech, Hungary). TMA-blocks were cut into 4 μm sections, deparaffinized in xylene, and rehydrated through a gradually decreasing concentration of ethanol to distilled water (Tissue-Tek DRS, Sakura, Ca, USA). Slides were treated in a PreTreatment module (Agilent Dako, Ca, USA) in Tris-Hcl (pH 8.5) and Tris-EDTA (pH 9) buffer for 20 min at 98°C for antigen retrieval. Slides were immunostained in an Autostainer 480 (Lab Vision Corp., Fremont, CA, USA) by the Dako REAL EnVision Detection System, Peroxidase/DAB+, rabbit/mouse (Dako, Glostrup, Denmark). For staining, β-catenin tissues were incubated with monoclonal beta Catenin antibody (CAT-5H10 by Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA; diluted to 1:400 with Dako REAL Antibody Diluent S2022) for one hour at room temperature. PROX1 staining tissues were incubated with Human PROX1 Antibody (R&D Systems, Inc., Minneapolis, MN, USA; diluted 1:1800 with Dako REAL Antibody Diluent S2022) overnight at +4°C. A sample of colon cancer tissue served as the positive control.
All immunostained TMA samples were evaluated by two independent, blinded investigators (AE and JH). In previous studies specifically cytoplasmic expression of β-catenin and PROX1, rather than membranous or nuclear, has associated with survival [24, 40]. Hence, for evaluating the expression of β-catenin and PROX1, cytoplasmic immunostaining was used. The cytoplasmic staining intensity, in the PDAC cells, of β-catenin and PROX1 was scored from 0 to 3: 0, negative; 1, weakly positive; 2, moderately positive; and 3, strongly positive (Fig. 1). The highest score across all six TMA samples was considered representative. In case of differing scores, the consensus was reached through re-evaluation. Cytoplasmic staining of β-catenin and PROX1, as well as the mentioned antibodies, have been demonstrated before in PDAC [24]. All TMA spots of one US patient failed to include tumor; hence β-catenin could not be examined.
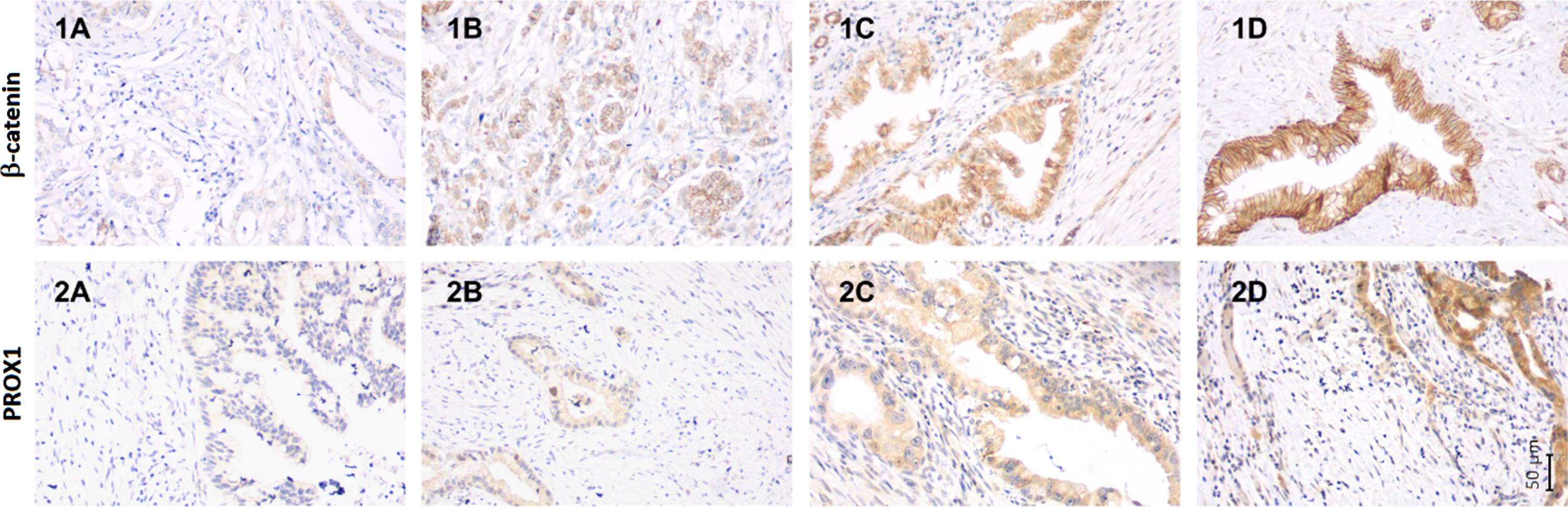
Fig. 1
The immunohistochemical staining pattern of β-catenin and PROX1 in neoadjuvant treated pancreatic ductal adenocarcinoma. The staining pattern of β-catenin using monoclonal beta Catenin antibody (CAT-5H10) (1A-1D) and PROX1 using Human PROX1 Antibody (2A-2D) in neoadjuvant treated pancreatic ductal adenocarcinoma. Representative images of β-catenin expression (1A) negative cytoplasmic expression with weak membranous positivity, (1B) weak cytoplasmic positivity, (1C) moderate cytoplasmic positivity, and (1D) strong cytoplasmic positivity. Representative images of PROX1 expression (2A) negative expression, (2B) weak cytoplasmic positivity, (2C) moderate cytoplasmic positivity, and (2D) strong cytoplasmic positivity. Magnification 200x is used in all separate images. The corresponding scale bar can be seen in lower right corner.
2.2Response to neoadjuvant therapy
Responses were determined by evaluating the percentage of remaining viable tumor cells: 0, no viable residual tumor cells (RTCs); 1, only some found with a high magnification of 200–400x (≤5% RTCs); 2, easily found with a large magnification of 200–400x (6–10% RTCs); 3, easily found with a small magnification of 20–40x (11–50% RTCs); 4, only little effect identified (51–90% RTCs); and 5, no effect identified (91–100% RTCs). Samples were evaluated by an experienced pathologist (AR). The neoadjuvant response grading has been reported before [11].
2.3Statistical analysis
For analytical purposes, in β-catenin and PROX1 analyses, we considered score 3 as a strong expression and score 0–2 as a weak expression. For analyzing the combined β-catenin and PROX1 expression, the scores were grouped as follows: strong β-catenin and PROX1 (both scored 3); either exhibiting strong staining (either scored 3); both staining weakly (both scored 0–2), corresponding to strong, moderate, and weak combined expression, respectively. In survival analyses, disease-specific survival (DSS) was applied; thus death from pancreatic cancer served as a final event, and death from other causes served as a competing event. One patient died from another cause. The follow-up time was recorded from surgery. Due to a significant statistical interaction between β-catenin and treatment response, separate survival analyses were performed for the good (≤10% residual tumor cells) and poor (> 10% residual tumor cells) NAT responders. The correlation between β-catenin and PROX1 immunostaining intensities was calculated with Spearman’s correlation using a bootstrapped confidence interval.
To compare categorical variables, we used Fisher’s exact and linear-by-linear association tests. For survival analyses, we used the Cox proportional-hazard regression and the Kaplan–Meier model with the log-rank test. In the progression-free survival (PFS) analysis, progression was defined as cancer recurrence, either local recurrence or distant metastasis, whichever occurred first. Deaths related to pancreatic cancer and other deaths were used as competing events. We considered p < 0.05 as statistically significant, and two-tailed tests were used. All statistical analyses were calculated using SPSS (version 25, IBM Corp., Armonk, TX, USA) and STATA/MP (version 16.1, StataCorp LLC, College Station, Texas, USA).
This study complied with the Declaration of Helsinki and was approved by the Surgical Ethics Committee of the Helsinki University Hospital (Dnro HUS 226/E6/06, extension TMK02 §66 17 April 2013 and 2019). The National Supervisory Authority of Health and Welfare also granted permission (Valvira Dnro 10041/06.01.03.01/2012).
3Results
NAT patients (n = 88) had significantly stronger β-catenin and PROX1 immunopositivity compared with US patients (n = 144). Overall, β-catenin immunopositivity was strong in both groups, whereas PROX1 immunopositivity was weaker (Table 1). β-catenin and PROX1 immunopositivity weakly correlated with each other in the whole study group (Spearman’s bootstrapped correlation coefficient 0.283, 95% confidence interval [CI] 0.164–0.387 p < 0.001).
Table 1
β-catenin and PROX1 immunopositivity comparison between NAT and US patients
| n (%) | NAT 88 (38) | US 144 (62) | p-value |
| β-catenin immunostaining | |||
| negative | 1 (1) | 2 (1) | |
| weak | 4 (5) | 16 (11) | |
| moderate | 36 (41) | 76 (53) | |
| strong | 47 (53) | 49 (34) | 0.004 |
| PROX1 immunostaining | |||
| negative | 4 (5) | 18 (13) | |
| weak | 26 (30) | 48 (33) | |
| moderate | 45 (51) | 70 (49) | |
| strong | 13 (15) | 8 (6) | 0.007 |
| Combined β-catenin and PROX1 | |||
| weak | 35 (40) | 92 (64) | |
| moderate | 46 (52) | 47 (33) | |
| strong | 7 (8) | 5 (4) | < 0.001 |
NAT = neoadjuvant therapy US = upfront surgery.
Among NAT patients PROX1 and β-catenin did not express correlation (Spearman’s bootstrapped correlation coefficient 0.066, 95% –0.139–0.258 p = 0.543). Patient characteristics associated with β-catenin and PROX1 were examined separately. Of the analyzed clinicopathological parameters, strong β-catenin associated with younger age (< 65 years, p = 0.005). Strong PROX1 associated with negative lymph node metastasis (p = 0.035) and vascular resection (p = 0.024) (Tables 2 and 3, respectively).
Table 2
β-catenin immunopositivity scoring and the comparison between clinicopathological parameters
| n (%) | β-catenin immunostainings | ||||
| negative | weak | moderate | strong | p-value | |
| 1 (1) | 4 (5) | 36 (41) | 47 (53) | ||
| Gender | |||||
| female | 1 (100) | 2 (50) | 20 (56) | 26 (55) | |
| male | 0 (0) | 2 (50) | 16 (44) | 21 (45) | 0.782 |
| Age | |||||
| < 65 | 0 (0) | 0 (0) | 14 (39) | 28 (60) | |
| ≥65 | 1 (100) | 4 (100) | 22 (61) | 19 (40) | 0.005 |
| Vascular resection | |||||
| Yes | 1 (100) | 2 (50) | 23 (64) | 24 (51) | |
| No | 0 (0) | 2 (50) | 13 (36) | 23 (49) | 0.270 |
| Stage | |||||
| IA | 0 (0) | 1 (25) | 3 (8) | 6 (13) | |
| IB | 1 (100) | 1 (25) | 11 (31) | 12 (26) | |
| IIA | 0 (0) | 0 (0) | 3 (8) | 4 (9) | |
| IIB | 0 (0) | 1 (25) | 11 (31) | 18 (38) | |
| III | 0 (0) | 1 (25) | 8 (22) | 6 (13) | 0.890 |
| IV* | |||||
| missing** | 1 | ||||
| T | |||||
| T1 | 0 (0) | 1 (25) | 5 (14) | 14 (30) | |
| T2 | 1 (100) | 2 (50) | 23 (64) | 21 (45) | |
| T3 | 0 (0) | 0 (0) | 7 (19) | 10 (21) | |
| T4 | 0 (0) | 1 (25) | 1 (3) | 1 (2) | 0.573 |
| missing** | 1 | ||||
| N | |||||
| N0 | 1 (100) | 3 (75) | 17 (47) | 23 (49) | |
| N1 | 0 (0) | 1 (25) | 12 (33) | 19 (40) | |
| N2 | 0 (0) | 0 (0) | 7 (19) | 5 (11) | 0.602 |
| Grade | |||||
| 1 | 1 (100) | 0 (0) | 7 (19) | 5 (11) | |
| 2 | 0 (0) | 2 (67) | 20 (56) | 33 (70) | |
| 3 | 0 (0) | 1 (33) | 8 (22) | 7 (15) | 0.377 |
| missing*** | 4 | ||||
| Neoadjuvant response | |||||
| ≤10% residual tumor cells | 0 (0) | 0 (0) | 6 (17) | 11 (23) | |
| > 10% residual tumor cells | 1 (100) | 4 (100) | 30 (83) | 36 (77) | 0.195 |
| Resection margin | |||||
| R0 | 1 (100) | 3 (75) | 27 (77) | 35 (76) | |
| R1 | 0 (0) | 1 (25) | 8 (23) | 11 (24) | 0.187 |
| missing*** | 2 | ||||
| Perineural invasion | |||||
| Yes | 1 (100) | 2 (50) | 28 (78) | 30 (64) | |
| No | 0 (0) | 2 (50) | 8 (12) | 17 (36) | 0.384 |
| Perivascular invasion | |||||
| Yes | 0 (0) | 2 (50) | 11 (31) | 12 (26) | |
| No | 1 (100) | 2 (50) | 25 (69) | 35 (74) | 0.544 |
*Stage IV patients excluded from the study. **Two patients had a complete response to NAT, one of whom had 19 mm dysplastic changes with a T1 tumor. ***Two patients had a complete response to NAT, thus the tumor grade could not be determined. Two patients had missing data. Linear-by-Linear association was applied.
Table 3
PROX1 immunopositivity scoring and the comparison between clinicopathological parameters
| n (%) | PROX1 immunostainings | ||||
| negative | weak | moderate | strong | p-value | |
| 4 (4) | 26 (30) | 45 (51) | 13 (15) | ||
| Gender | |||||
| female | 2 (50) | 14 (54) | 26 (58) | 7 (54) | |
| male | 2 (50) | 12 (46) | 19 (42) | 6 (46) | 0.844 |
| Age | |||||
| < 65 | 0 (0) | 13 (50) | 25 (56) | 4 (31) | |
| ≥65 | 4 (100) | 13 (50) | 20 (44) | 9 (69) | 0.773 |
| Vascular resection | |||||
| Yes | 2 (50) | 11 (42) | 26 (58) | 11 (85) | |
| No | 2 (50) | 15 (58) | 19 (42) | 2 (15) | 0.024 |
| Stage | |||||
| IA | 0 (0) | 3 (12) | 5 (11) | 2 (15) | |
| IB | 0 (0) | 7 (27) | 13 (30) | 5 (38) | |
| IIA | 1 (25) | 1 (4) | 4 (9) | 1 (9) | |
| IIB | 3 (75) | 13 (50) | 11 (25) | 3 (23) | |
| III | 0 (0) | 2 (8) | 11 (25) | 2 (15) | 0.373 |
| IV* | |||||
| missing** | 1 | ||||
| T | |||||
| T1 | 1 (25) | 7 (27) | 10 (22) | 2 (15) | |
| T2 | 2 (50) | 14 (54) | 22 (50) | 9 (70) | |
| T3 | 1 (25) | 5 (19) | 9 (20) | 2 (15) | |
| T4 | 0 (0) | 0 (0) | 3 (8) | 0 | 0.940 |
| missing** | 1 | ||||
| N | |||||
| N0 | 0 (0) | 11 (42) | 25 (56) | 8 (62) | |
| N1 | 4 (100) | 13 (50) | 12 (26) | 3 (23) | |
| N2 | 0 (0) | 2 (8) | 8 (18) | 2 (15) | 0.035 |
| Grade | |||||
| 1 | 0 (0) | 4 (16) | 6 (14) | 3 (25) | |
| 2 | 3 (75) | 14 (56) | 31 (72) | 7 (58) | |
| 3 | 1 (25) | 7 (28) | 6 (14) | 2 (17) | 0.820 |
| missing*** | 4 | ||||
| Neoadjuvant response | |||||
| ≤10% residual tumor cells | 1 (25) | 6 (23) | 7 (16) | 3 (23) | |
| > 10% residual tumor cells | 3 (75) | 20 (77) | 38 (84) | 10 (77) | 0.737 |
| Resection margin | |||||
| R0 | 4 (100) | 22 (88) | 30 (67) | 10 (83) | |
| R1 | 0 (0) | 3 (12) | 15 (33) | 2 (17) | 0.234 |
| missing*** | 2 | ||||
| Perineural invasion | |||||
| Yes | 3 (75) | 18 (69) | 31 (69) | 9 (69) | |
| No | 1 (25) | 8 (31) | 14 (31) | 4 (31) | 0.580 |
| Perivascular invasion | |||||
| Yes | 3 (75) | 7 (27) | 10 (22) | 5 (38) | |
| No | 1 (25) | 19 (73) | 35 (78) | 8 (62) | 0.538 |
*Stage IV patients excluded from the study. **Two patients had a complete response to NAT, one of whom had 19 mm dysplastic changes with a T1 tumor. ***Two patients had a complete response to NAT, thus the tumor grade could not be determined. Two patients had missing data. Linear-by-Linear association was applied.
A good NAT response associated with neoadjuvant radiation (p = 0.001), less advanced stage (p = 0.025), negative lymph node metastases (p = 0.029), and negative perineural invasion (p < 0.001). A strong β-catenin or PROX1 showed no association between the NAT response. Table 4 summarizes the characteristics associated with NAT-response.
Table 4
The association between different clinicopatholigcal parameters and neoadjuvant response
| n (%) | Neoadjuvant response | ||
| ≤10% residual tumor cells | > 10% residual tumor cells | p value | |
| 17 (19) | 71 (79) | ||
| Gender | |||
| female | 8 (47) | 41 (58) | |
| male | 9 (53) | 30 (42) | 0.588 |
| Age | |||
| < 65 | 7 (41) | 35 (49) | |
| ≥65 | 10 (59) | 36 (51) | 0.598 |
| Vascular resection | |||
| yes | 9 (53) | 41 (58) | |
| no | 8 (47) | 30 (42) | 0.789 |
| Radiation therapy | |||
| Yes | 11 (65) | 15 (21) | |
| no | 6 (35) | 56 (79) | 0.001 |
| Stage | |||
| IA | 3 (19) | 7 (10) | |
| IB | 7 (44) | 18 (25) | |
| IIA | 2 (13) | 5 (7) | |
| IIB | 3 (19) | 27 (38) | |
| III | 1 (6) | 14 (20) | 0.025 |
| IV* | |||
| missing** | 1 | ||
| T | |||
| T1 | 5 (29) | 15 (21) | |
| T2 | 9 (53) | 38 (54) | |
| T3 | 1 (6) | 16 (23) | |
| T4 | 1 (6) | 2 (3) | 0.139 |
| missing** | 1 | ||
| N | |||
| N0 | 12 (71) | 32 (45) | |
| N1 | 5 (29) | 27 (38) | |
| N2 | 0 (0) | 12 (17) | 0.029 |
| Grade | |||
| 1 | 2 (12) | 11 (16) | |
| 2 | 9 (53) | 46 (65) | |
| 3 | 4 (24) | 12 (17) | 0.364 |
| missing*** | 4 | ||
| Resection margin | |||
| R0 | 14 (88) | 52 (74) | |
| R1 | 2 (12.5) | 18 (26) | 0.340 |
| missing*** | |||
| Perineural invasion | |||
| yes | 12 (71) | 15 (21) | |
| no | 5 (29) | 56 (79) | < 0.001 |
| Perivascular invasion | |||
| yes | 15 (88) | 48 (68) | |
| no | 2 (12) | 23 (34) | 0.135 |
| Strong β-catenin | |||
| yes | 6 (35) | 35 (49) | |
| no | 11 (65) | 36 (51) | 0.418 |
| Strong PROX1 | |||
| yes | 14 (82) | 61 (86) | |
| no | 3 (18) | 10 (14) | 0.710 |
*Stage IV patients were excluded from the study. **Two patients experienced a complete response to NAT, one of whom had dysplastic changes and the tumor size was determined. ***Two patients had a complete response to NAT, thus the tumor grade could not be determined. Two patients had missing data. Linear-by-linear and Fisher’s exact -tests were used for this table.
Analyzing the entire NAT group, neither strong β-catenin nor strong PROX1 associated with disease-specific survival (DSS) (HR 0.03; 95% CI 0.77–1.39; p = 0.833, HR 0.89; 95% CI 0.51–1.46; p = 0.573, respectively). In the group of good responders, with≤10% of residual tumor cells, strong β-catenin associated with significantly better DSS, HR 0.26; 95% CI 0.07–0.88; p = 0.030 (Fig. 2a). Combined moderate β-catenin and PROX1 associated with better survival (HR 0.20; 95% CI 0.05–0.76; p = 0.018) (Fig. 2b) as well, but no significant association was noted with combined strong β-catenin and PROX1 (HR 0.22; 95% CI 0.03–1.64; p = 0.139) or PROX1 alone (HR 0.47; 95% CI 0.07–3.32; p = 0.446). After excluding patients with negative immunostaining in either of the markers, we found similarly that in the group of good responders a combined moderate β-catenin and PROX1 associated with better survival compared to combined weak (HR 0.21 95% CI 0.05–0.82 p = 0.025). Again, combined strong β-catenin and PROX1 did not significantly associate with survival (HR 0.24 95% CI 0.03–1.87 p = 0.170). A multivariate analysis could not be performed due to small number of patients.
Fig. 2
The disease-specific survival (DSS) analyses show the association between β-catenin, combined β-catenin and PROX1 expression and survival in the group of patients with a good neoadjuvant (NAT) response (a-b) and poor NAT response (c-d). The Kaplan-Meier method and log-rank tests were applied. a. Among patients with a good NAT response with≤10% of residual tumor cells, strong cytoplasmic β-catenin immunopositivity associated with a better DSS compared to patients with weak β-catenin. Together seven patients where censored. b. Among patients with a good NAT response, the combined moderate β-catenin and PROX1, meaning patients with either strong β-catenin or strong PROX1 immunopositivity, was associated with better survival compared to weak combined immunopositivity. Together seven patients where censored. c. Among patients with a poor NAT response with > 10% of residual tumor cells, strong β-catenin immunopositivity associated with poor survival. Together 19 patients were censored. d. Among patients with a poor NAT response, the combined strong β-catenin and PROX1, meaning both had strong immunopositivity, associated with worse survival. Furthermore, for patients with combined moderate β-catenin and PROX1, meaning either strong β-catenin or strong PROX1, the survival was better compared to patients with both markers strong, but worse compared to patients with weak combined immunopositivity, meaning neither of the markers had strong immunopositivity. Together 19 patients werecensored.
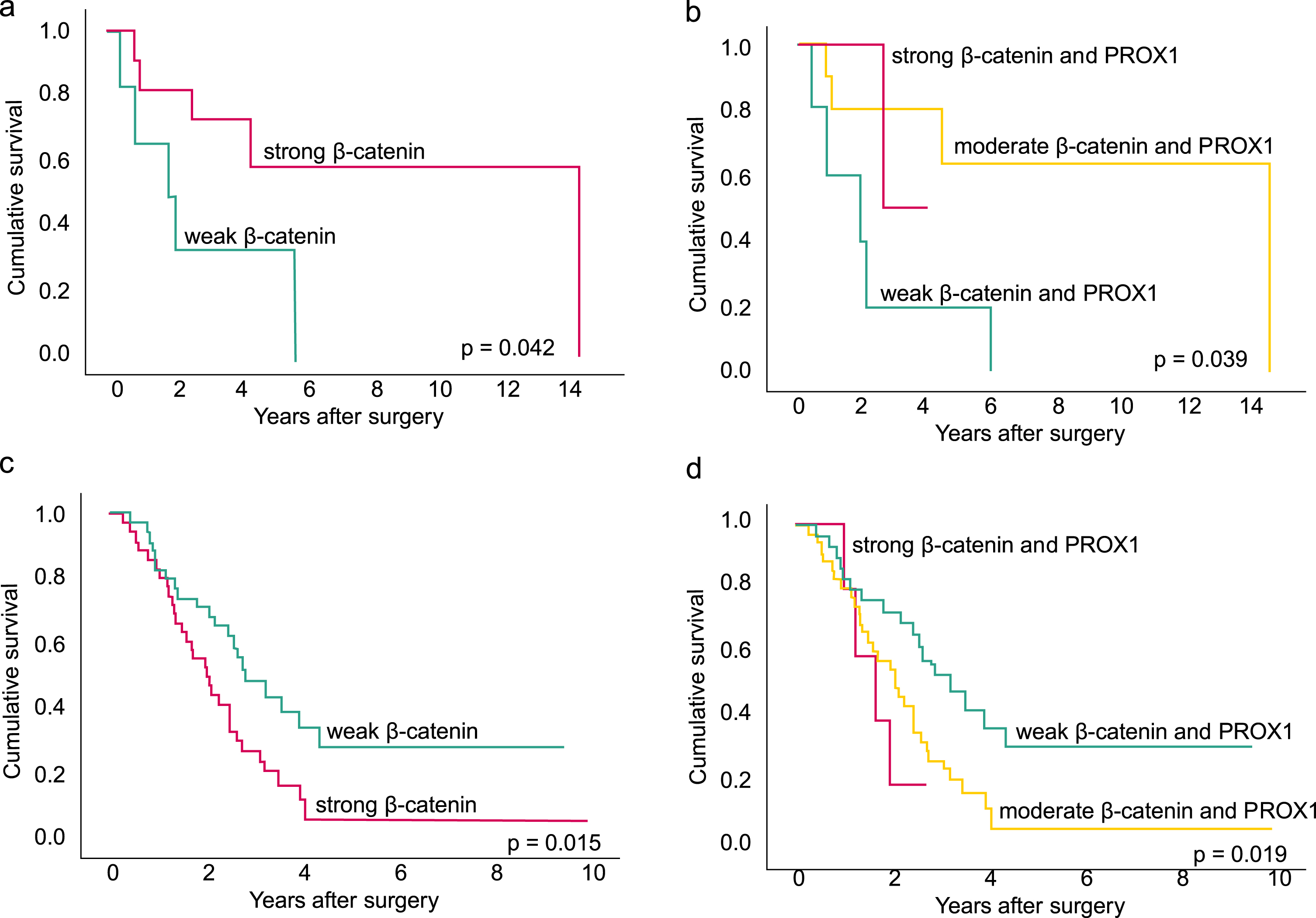
In the group of poor responders, with > 10% of residual tumor cells in contrast to the good response group, strong β-catenin associated with poor DSS: HR 2.03; 95% CI 1.16–3.55; p = 0.013 (Fig. 2c). A combined strong β-catenin and PROX1 associated with even worse survival with the following results: combined strong HR 3.1; 95% CI 1.08–8.94; p = 0.03 and combined moderate HR 2.21; 95% CI 1.21–4.05; p = 0.01 (Fig. 2d). Analysis of strong PROX1 did not yield a significant association with survival (HR 1.67; 95% CI 0.81–3.46; p = 0.168). In the multivariate DSS analysis, combined strong β-catenin and PROX1 associated with shorter survival (HR 3.73; 95% CI 1.40–9.97; p = 0.009). Table 5a describes the multivariate analysis. After excluding negative immunostainings in either of the markers results again stayed similar. Combined moderate β-catenin and PROX1 associated with worse survival with HR 2.36 95% CI 1.22–4.58 p = 0.011. Similarly, a combined strong β-catenin and PROX1 associated with even worse survival with HR 3.10 95% CI 1.04–9.18 p = 0.042. In the multivariate analysis, the results stayed the same: combined strong β-catenin and PROX1 associated with shorter DSS with HR 3.89 95% CI 1.37–11.04p = 0.011.
Table 5
The multivariate analyses describing the DSS (a) and the PFS (b) of the poor NAT response group
| a | b | |||||||
| HR | 95% CI | p –value | HR | 95% CI | p - value | |||
| Age at operation | 1.03 | 0.99 | 1.06 | 0.138 | 1.02 | 0.98 | 1.06 | 0.248 |
| Stage III | 1.97 | 0.95 | 4.11 | 0.070 | 2.26 | 44378 | 4.79 | 0.033 |
| Tumor size (mm) | 1.01 | 0.99 | 1.03 | 0.267 | 1.01 | 0.99 | 1.04 | 0.336 |
| Adjuvant therapy | 0,47 | 0.25 | 0.90 | 0.024 | 0.61 | 0.32 | 1.15 | 0.126 |
| Perivascular invasion | 0.85 | 0.41 | 1.74 | 0.654 | 0.77 | 0.41 | 1.44 | 0.412 |
| Combined β-catenin and PROX1 | ||||||||
| weak | 1 | 1 | ||||||
| moderate | 2.32 | 1.22 | 4.44 | 0.011 | 1.86 | 1.00 | 3.43 | 0.049 |
| strong | 3.73 | 1.40 | 9.97 | 0.009 | 2.38 | 1.14 | 4.97 | 0.021 |
DSS = Disease-spesific survival PFS = Progression-free survival NAT = Neoadjuvant therapy HR = Hazard ratio CI =Confidence interval.
The correlation between disease recurrence and β-catenin and PROX1 expression in the NAT group was analyzed. In the good response group, strong β-catenin and moderate combined β-catenin and PROX1 associated with improved PFS (HR 0.20; 95% CI 0.46–0.85; p = 0.029 and HR 0.07; 95% CI 0.02–2.95; p = 0.004, respectively). Strong combined β-catenin and PROX1 did not associate with PFS, nor did PROX1 analyzed alone. Again, a multivariate analysis could not be performed due to a small number of patients. In the poor response group, strong PROX1 associated with deteriorated PFS with HR 2.19 (95% CI 1.28–3.76; p = 0.004). Furthermore, combined strong β-catenin and PROX1 associated with deteriorated PFS, HR 2.11 (95% CI 1.02–4.38; p = 0.045). The multivariate analysis could be performed in the poor response group. The association of combined β-catenin and PROX1 with PFS remained: strong combined HR 2.38; 95% CI 1.14–4.97; p = 0.021 and moderate combined HR 1.86; 95% CI 1.00–3.43; p = 0.049. The factors used in the multivariate analysis are seen in Table 5b.
4Discussion
To the best of our knowledge, this is the first study to report the expression of β-catenin and PROX1 in neoadjuvant-treated pancreatic cancer. According to our results, the role of β-catenin and PROX1 in survival can be either improving or deteriorating depending on the NAT response. Among patients with good response, with less than 10% residual tumor cells, strong β-catenin and combined moderate β-catenin and PROX1 associated with improved survival. In contrast, in the poor NAT response group with more than 10% residual tumor cells, strong β-catenin and combined strong β-catenin and PROX1 associated with deteriorated survival.
According to prior studies, the association between β-catenin expression and survival in pancreatic cancer is ambiguous. Some studies have indicated that increased cytoplasmic β-catenin expression is associated with metastasis [25, 26] and poorer survival, [25, 27] whereas one study investigating pancreatic cancer patients undergoing US indicated that particularly low cytoplasmic β-catenin expression was associated with poorer survival [24]. Now, we report that the association of cytoplasmic β-catenin expression and poor or improved survival in neoadjuvant treated pancreatic cancer varies depending on the patient’s response to NAT. The underlying reasons for the variation in the survival association are unclear. In treatment of naïve tumors the Wnt/β-catenin signaling pathway function and expression may vary between the patients, which partly explains the following good or poor NAT response. Alternatively, the good treatment response may lead to changes in the tumor and the tumor microenvironment, which further cause alterations in the Wnt/β-catenin signaling. Pancreatic cancer tumors generally have strong desmoplasia and deposition of extracellular matrix where PDAC cells induce a strong response within the stroma [41]. Stromal components have been shown to affect cancer cell proliferation and survival [42, 43]. The tumor microenvironment and stromal cells have significance in whether a good treatment response is achieved [44]. The role of the microenvironment cannot be ignored when discussing the alternating role of β-catenin and PROX1 in tumor progression and survival. In our study, the strong cytoplasmic β-catenin immunopositivity had a deteriorating effect on survival in the poor response group, but overall they had stronger β-catenin expression (49% vs. 35%). The difference was not statistically significant, leaving the question if stronger expression leads to alteration in survival open.
The combined strong and moderate β-catenin and PROX1 expression similarly associated with DSS compared to strong β-catenin alone, so that in the well-responding patients, the effect seen was an improvement in survival and the poorly responding group deterioration of survival. In the NAT-patients, in either of the response groups, PROX1 alone did not statistically associate with survival. A prior study described a similar result in pancreatic cancer that β-catenin alone and combined β-catenin and PROX1 associated with survival, but no association between PROX1 and survival was seen. Only pancreatic cancer patients undergoing US were included in that study [24]. Some studies demonstrate a tumor-suppressive role of PROX1 in pancreatic cancer [37–39]. Interestingly, analyzing the whole study group, we noticed a weak correlation between β-catenin and PROX1 immunopositivity. However, while examining the NAT patients alone, the correlation could not be seen. In the NAT group, β-catenin immunopositivity was stronger compared to PROX1, whereas in the US patients, the overall staining pattern of β-catenin and PROX1 was more similar, explaining the lack of correlation in the NAT patients. Most of the strong β-catenin immunostainings among NAT patients were seen in the group of poor responders. Overall, the immunopositivity of the NAT patients was generally stronger. The difference between NAT and US patients in other tumor marker expressions by immunostaining has been reported before [10]. Especially in the poor responders’ group, the strong combined cytoplasmic β-catenin and PROX1 presented even worse survival compared with strong β-catenin alone, evoking the question of the role of PROX1 in Wnt/β-catenin signaling pathway in poorly responding NAT patients. Interestingly, moderate combined β-catenin and PROX1 associated with slightly improved survival in the good responders, compared to β-catenin alone, contradicting the role of PROX1.
When analyzing the whole NAT group, a strong β-catenin associated with younger age (< 65 years, p = 0.005) and a strong PROX1 associated with negative lymph node metastasis (p = 0.035) and vascular resection (p = 0.024). Contradictory to our results, a previous study which analyzed the association of clinicopathological parameters and β-catenin and PROX1 in US patients found a statistically significant association between low PROX1 expression and younger age and between low β-catenin expression and higher tumor grade. Analyzing the association of better NAT response and survival, the survival benefit can only be seen if the response to NAT is remarkably good, meaning≤5–10% residual tumor cells [11, 45]. The survival analysis of the NAT response groups used in this study has been reported before [11].
The findings in the PFS analysis were aligned and logically similar to DSS analysis. In the good response group, strong cytoplasmic β-catenin and moderate combined β-catenin and PROX1 associated with improved PFS, resembling the DSS analysis. In the poor response group, strong PROX1 associated with impaired PFS, and so did the strong combined β-catenin and PROX1. In the PFS analysis of poor responders β-catenin did not significantly associate with survival. Overall, these findings were aligned with DSS analysis except for the poor responders PROX1 association. The lack of PROX1 presenting statistical significance in other analyses may be due to a lack of statistical power.
To our knowledge, this is the first study to report the impact of β-catenin and PROX1 on disease progression in pancreatic cancer. The previous studies have not come to a final conclusion on whether β-catenin or PROX1 promote tumor progression or acts as a tumor suppressor, and depending on the study, results supporting both possibilities can be found. However, in pancreatic cancer most studies describe β-catenin as tumor-promoting. Similarly, the role of PROX1 in malignancy is diverse, both suppressing and promoting tumor growth [24, 37, 46–52]. In pancreatic cancer, its role has been described mainly as tumor-suppressive. Our DSS findings further support the understanding that like with β-catenin, the role of PROX1 is ambiguous and may vary depending on the patients’ good or poor treatment response.
We relied on the strengths of our study —the study cohort comprised a relatively large pool of patients with a histologically confirmed diagnosis of PDAC. The NAT and US groups were matched for age, gender, and time of surgery. Our data on survival and follow-up are reliable and precise. Multiple well-representative tumor samples were used to evaluate neoadjuvant response, and our neoadjuvant response scheme followed the recommendations of the latest tumor response consensus statements from the Amsterdam International Consensus Meeting [8]. Given these strengths, we also acknowledge limitations to our study. Only seven neoadjuvant patients presented strong combined β-catenin and PROX1 immunostaining. The TMA samples were relatively small (1 mm diameter), thus, the representativeness of cancer and immunostaining may have suffered, as the β-catenin and PROX1 expression may be unevenly distributed in the tumor. These limitations were minimized by taking several [6] TMA spots of each tumor and scoring the intensity using the strongest intensity visible in these spots. An experienced pathologist determined the location of the TMA drilling spots on the specimen. Furthermore, the staining intensity scoring was determined by two independent investigators to minimize the effect of subjectivity. Due to the retrospective nature of our study, the implementation of NAT varied between patients. This study featured a limited number of patients with a good response to NAT and, thus, we could not perform a multivariate survival analysis for those patients.
5Conclusions
In neoadjuvant-treated pancreatic cancer, the role of β-catenin and PROX1 on survival can be either improving or deteriorating depending on the treatment response, associating with significantly improved DSS and PFS in well-responding patients and with significantly impaired DSS and PFS in poorly responding patients. Overall, the expressions of β-catenin and PROX1 were stronger in the NAT patients compared to US patients. In the NAT patients, strong β-catenin associated with younger age and strong PROX1 associated with negative lymph node metastasis and vascular resection.
Acknowledgments
We thank Pia Saarinen, Jaana Koski-Alhainen, and Päivi Peltokangas for their technical assistance.
Author contributions
CONCEPTION: All authors
DATA CURATION: AE, AM, JH and AR
ANALYSIS OF DATA: HM and AE
PREPARATION OF THE MANUSCRIPT: All authors
REVISION FOR IMPORTANT INTELLECTUAL CONTENT: HS, CH, PK, KA, JH and AR
SUPERVISION: HS and CH
Conflict of interests
The authors declare no conflicts of interest.
Ethical considerations
This study complied with the Declaration of Helsinki and was approved by the Surgical Ethics Committee of the Helsinki University Hospital (Dnro HUS 226/E6/06, extension TMK02 §66 17 April 2013 and 2019). The National Supervisory Authority of Health and Welfare granted permission to use the tissue samples without requiring individual informed consent in this retrospective study (Valvira Dnro 10041/06.01.03.01/2012).
Funding
The authors gratefully acknowledge the financial support for this research from the following: the Finnish Cultural Foundation, the Sigrid Juselius Foundation, the Finnish Cancer Society, and the Helsinki University Hospital Research Fund.
Supplementary materials
[1] Data supporting the results of this study are available upon request to the corresponding author.
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200496.
References
[1] | Siegel RL , Miller KD , Fuchs HE , Jemal A . Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians. (2021) ;71: (1):7–33. doi: 10.3322/caac.21654 |
[2] | Luu AM , Braumann C , Belyaev O , Janot-Matuschek M , Rudolf H , Praktiknjo M , et al. Long-term survival after pancreaticoduodenectomy in patients with ductal adenocarcinoma of the pancreatic head. Hepatobiliary Pancreat Dis Int. (2021) ;20: (3):271–8. doi: 10.1016/j.hbpd.2020.12.006. |
[3] | Reni M . Neoadjuvant treatment for resectable pancreatic cancer: Time for phase III testing? World J Gastroenterol. (2010) ;16: (39):4883–7. doi: 10.3748/wjg.v16.i39.4883. |
[4] | Varadhachary GR , Tamm EP , Abbruzzese JL , Xiong HQ , Crane CH , Wang H , et al. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann Surg Oncol. (2006) ;13: (8):1035–46. doi: 10.1245/ASO.2006.08.011. |
[5] | Katz MHG , Shi Q , Ahmad SA , Herman JM , Marsh RDW , Collisson E , et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer alliance for clinical trials in oncology trial A021101. JAMA Surg. (2016) ;151: (8):e161137. doi: 10.1001/jamasurg.2016.1137. |
[6] | Esnaola NF , Chaudhary UB , O’Brien P , Garrett-Mayer E , Camp ER , Thomas MB , et al. Phase 2 trial of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. (2014) ;88: (4):837–44. doi: 10.1016/j.ijrob2013.12.030. |
[7] | Versteijne E , Suker M , Groothuis K , Akkermans-Vogelaar JM , Besselink MG , Bonsing BA , et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. (2020) ;38: (16):1763–73. doi: 10.1200/JCO.19.02274. |
[8] | Janssen Bv , Tutucu F , van Roessel S , Adsay V , Basturk O , Campbell F , et al. Amsterdam International Consensus Meeting: tumor response scoring in the pathology assessment of resected pancreatic cancer after neoadjuvant therapy. Mod Pathol. (2021) ;34: (1):4–12. doi: 10.1038/s41379-020-00683-9. |
[9] | Evans DB , Rich TA , Byrd DR , Cleary KR , Connelly JH , Levin B , et al. Preoperative Chemoradiation and Pancreaticoduodenectomy for Adenocarcinoma of the Pancreas. Arch Surg. (1992) ;127: (11):1335–9. doi: 10.1001/archsurg.1992.01420110083017. |
[10] | White R , Xie H , Gottfried M , Czito B , Hurwitz H , Morse M , et al. Significance of Histological Response to Preoperative Chemoradiotherapy for Pancreatic Cancer. Ann Surg Onoc. (2005) ;12: (3):214–21. doi: 10.1245/ASO.2005.03.105. |
[11] | Eurola A , Ristimäki A , Mustonen H , Nurmi AM , Hagström J , Haglund C , et al. Impact of histological response after neoadjuvant therapy on podocalyxin as a prognostic marker in pancreatic cancer. Sci Rep. (2021) ;11: (1):9896. doi: 10.1038/s41598-021-89134-2. |
[12] | Gumbiner BM , McCrea PD . Catenins as mediators of the cytoplasmic functions of cadherins. J Cell Sci Suppl. (1993) ;17: :155–8. doi: 10.1242/jcs.1993.supplement_17.22. |
[13] | Dale TC . Signal transduction by the Wnt family of ligands. Biochem J. (1998) ;329: (Pt 2):209–23. doi: 10.1042/bj3290209. |
[14] | Clevers H . Wnt/β-Catenin Signaling in Development and Disease. Cell. (2006) ;127: (3):469–80. doi: 10.1016/j.cell.2006.10.018. |
[15] | Wells JM , Esni F , Boivin GP , Aronow BJ , Stuart W , Combs C , et al. Wnt/β-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol. (2007) ;7: :4. doi: 10.1186/1471-213X-7-4. |
[16] | Heiser PW , Cano DA , Landsman L , Kim GE , Kench JG , Klimstra DS , et al. Stabilization of β-Catenin Induces Pancreas Tumor Formation. Gastroenterology. (2008) ;135: (4):1288–300. doi: 10.1053/j.gastro.2008.06.089. |
[17] | Zhan T , Rindtorff N , Boutros M . Wnt signaling in cancer. Oncogene. (2017) ;36: (11):1461–73. doi: 10.1038/onc.2016.304. |
[18] | MacDonald BT , Tamai K , He X . Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev Cell. (2009) ;17: (1):9–26. doi: 10.1016/j.devcel.2009.06.016. |
[19] | Hugh TJ , Dillon SA , O’Dowd G , Getty B , Pignatelli M , Poston GJ , et al. β-catenin expression in primary and metastatic colorectal carcinoma. Int J Cancer. (1999) ;82: (4):504–11. doi: 10.1002/(sici)1097-0215(19990812)82:4<504::aid-ijc6>3.0.co;2-6. |
[20] | Zeng G , Germinaro M , Micsenyi A , Monga NK , Bell A , Sood A , et al. Aberrant Wnt/β-catenin signaling in pancreatic adenocarcinoma. Neoplasia. (2006) ;8: (4):279–89. doi: 10.1593/neo.05607. |
[21] | de La Coste A , Romagnolo B , Billuart P , Renard CA , Buendia MA , Soubrane O , et al. Somatic mutations of the β-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. (1998) ;95: (15):8847–51. doi: 10.1073/pnas.95.15.8847. |
[22] | Pasca di Magliano M , Biankin AV , Heiser PW , Cano DA , Gutierrez PJA , Deramaudt T , et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS ONE. (2007) ;2: (11). doi: 10.1371/journal.pone.0001155. |
[23] | Cao D , Maitra A , Saavedra JA , Klimstra DS , Adsay NV , Hruban RH . Expression of novel markers of pancreatic ductal adenocarcinoma in pancreatic nonductal neoplasms: Additional evidence of different genetic pathways. Mod Pathol. (2005) ;18: (6):752–61. doi: 10.1038/modpathol.3800363. |
[24] | Saukkonen K , Hagström J , Mustonen H , Juuti A , Nordling S , Kallio P , et al. PROX1 and β-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer. (2016) ;16: :472. doi: 10.1186/s12885-016-2497-5. |
[25] | Li YJ , Wei ZM , Meng YXYX , Ji XR . β-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: Relationships with carcinogenesis and metastasis. World J Gastroenterol. (2005) ;11: (14):2117–23. doi: 10.3748/wjg.v11.i14.2117. |
[26] | Wang Z , Ma Q , Li P , Sha H , Li X , Xu J . Aberrant expression of CXCR4 and β-catenin in pancreatic cancer. Anticancer Res. (2013) ;33: (9):4103–10. |
[27] | Qiao Q , Ramadani M , Gansauge S , Gansauge F , Leder G , Beger HG . Reduced membranous and ectopic cytoplasmic expression of β -catenin correlate with cyclin D1 overexpression and poor prognosis in pancreatic cancer. Int J Cancer. (2001) ;95: (3):194–7. doi: 10.1002/1097-0215(20010520)95:3<194::aid-ijc1033>3.0.co;2-m. |
[28] | Binder ZA , SiuI-Mei IM , Eberhart CG , ap Rhys C , Bai RY , Staedtke V , et al. Podocalyxin-Like Protein Is Expressed in Glioblastoma Multiforme Stem-Like Cells and Is Associated with Poor Outcome. PLoS ONE. (2013) ;8: (10). doi: 10.1371/journal.pone.0075945. |
[29] | Nan JN , Kim OR , Lee MA . β-Catenin expression is associated with cell invasiveness in pancreatic cancer. Korean J Intern Med. (2019) ;34: (3):618–25. doi: 10.3904/kjim.2017.155. |
[30] | Wigle JT , Oliver G . Prox1 function is required for the development of the murine lymphatic system. Cell. (1999) ;98: (6):769–78. doi: 10.1016/s0092-8674(00)81511-1. |
[31] | Petrova TV , Mäkinen T , Mäkelä TP , Saarela J , Virtanen I , Ferrell RE , et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. (2002) ;21: (17):4593–9. doi: 10.1093/emboj/cdf470. |
[32] | Hong YK , Harvey N , Noh YH , Schacht V , Hirakawa S , Detmar M , et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. (2002) ;225: (3):351–7. doi: 10.1002/dvdy.10163. |
[33] | Kaltezioti V , Kouroupi G , Oikonomaki M , Mantouvalou E , Stergiopoulos A , Charonis A , et al. Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS Biol. (2010) ;8: (12):e1000565. doi: 10.1371/journal.pbio.1000565. |
[34] | Rodriguez-Niedenführ M , Papoutsi M , Christ B , Nicolaides KH , Von Kaisenberg CS , Tomarev SI , et al. Prox1 is a marker of ectodermal placodes, endodermal compartments, lymphatic endothelium and lymphangioblasts. Anat Embryol. (2001) ;204: (5):399–406. doi: 10.1007/s00429-001-0214-9. |
[35] | Oliver G , Sosa-Pineda B , Geisendorf S , Spana EP , Doe CQ , Gruss P . Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. (1993) ;44: (1):3–16. doi: 10.1016/0925-4773(93)90012-m. |
[36] | Petrova T V , Nykänen A , Norrmén C , Ivanov KI , Andersson LC , Haglund C , et al. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell. (2008) ;13: (5):407–19. doi: 10.1016/j.ccr.2008.02.020. |
[37] | Shimoda M , Takahashi M , Yoshimoto T , Kono T , Ikai I , Kubo H . A homeobox protein, Prox1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin Cancer Res. (2006) ;12: (20 Pt 1):6005–11. doi: 10.1158/1078-0432.CCR-06-0712. |
[38] | Schneider M , Büchler P , Giese N , Giese T , Wilting J , Büchler MW , et al. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int J Oncol. (2006) ;28: (4):883–90. doi: 10.3892/IJO.28.4.883. |
[39] | Jin Y , Weng Y , Wang Y , Lin J , Deng X , Shen B , et al. Mir-934 as a prognostic marker facilitates cell proliferation and migration of pancreatic tumor by targeting prox1. Onco Targets and Ther. (2020) ;13: :3389–99. doi: 10.2147/OTT.S249662. |
[40] | López-Knowles E , Zardawi SJ , McNeil CM , Millar EKA , Crea P , Musgrove EA , et al. Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol Biomarkers Prev. (2010) ;19: (1):301–9. doi: 10.1158/1055-9965.EPI-09-0741. |
[41] | Bachem MG , Schünemann M , Ramadani M , Siech M , Beger H , Buck A , et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. (2005) ;128: (4):907–21. doi: 10.1053/j.gastro.2004.12.036. |
[42] | Öhlund D , Franklin O , Lundberg E , Lundin C , Sund M . Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer. (2013) ;13: :154. doi: 10.1186/1471-2407-13-154. |
[43] | Tang D , Wang D , Yuan Z , Xue X , Zhang Y , An Y , et al. Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int J Cancer2013. (2013) ;132: (5):993–1003. doi: 10.1002/ijc.27715. |
[44] | Erkan M , Reiser-Erkan C , Michalski C , Kong B , Esposito I , Friess H , Kleeff J . The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr Mol Med. (2012) ;12: (3):288–303. doi: 10.2174/156652412799218921. |
[45] | Chatterjee D , Katz MH , Rashid A , Varadhachary GR , Wolff RA , Wang H , et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: A predictor for patient outcome. Cancer. (2012) ;118: (12):3182–90. doi: 10.1002/cncr.26651. |
[46] | Elsir T , Eriksson A , Orrego A , Lindström MS , Nistér M . Expression of PROX1 is a common feature of high-grade malignant astrocytic gliomas. J Neuropathol Exp Neurol. (2010) ;69: (2):129–38. doi: 10.1097/NEN.0b013e3181ca4767. |
[47] | Dadras SS , Skrzypek A , Nguyen L , Shin JW , Schulz MMP , Arbiser J , et al. Prox-1 promotes invasion of kaposiform hemangioendotheliomas. J Invest Dermatol. (2008) ;128: (12):2798–806. doi: 10.1038/jid.2008.176. |
[48] | Drosos Y , Neale G , Ye J , Paul L , Kuliyev E , Maitra A , et al. Prox1-Heterozygosis Sensitizes the Pancreas to Oncogenic Kras-Induced Neoplastic Transformation. Neoplasia. (2016) ;18: (3):172–84. doi: 10.1016/j.neo.2016.02.002. |
[49] | Liu Y , Ye X , Zhang JB , Ouyang H , Shen Z , Wu Y , et al. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing β-catenin expression and nuclear translocation. Oncogene. (2015) ;34: (44):5524–35. doi: 10.1038/onc.2015.7. |
[50] | Akagami M , Kawada K , Kubo H , Kawada M , Takahashi M , Kaganoi J , et al. Transcriptional factor Prox1 plays an essential role in the antiproliferative action of interferon-γ in esophageal cancer cells. Ann Surgl Oncol. (2011) ;18: (13):3868–77. doi: 10.1245/s10434-011-1683-6. |
[51] | Laitinen A , Böckelman C , Hagström J , Kokkola A , Kallio P , Haglund C . High PROX1 expression in gastric cancer predicts better survival. PLoS ONE. (2017) ;12: (8). doi: 10.1371/journal.pone.0183868. |
[52] | Park KJ , Cho SB , Park YL , Kim N , Park SY , Myung DS , et al. Prospero homeobox 1 mediates the progression of gastric cancer by inducing tumor cell proliferation and lymphangiogenesis. Gastric Cancer. (2017) ;20: (1):104–15. doi: 10.1007/s10120-015-0592-y. |




