Association between vitamin D receptor (APAI rs7975232) genotypes and vitamin D serum levels in Iraqi multiple sclerosis patients
Abstract
BACKGROUND:
Multiple sclerosis (MS) is a long-term condition characterized by chronic inflammation, damage to the myelin sheath, and progressive nerve cell degeneration. It is a heterogeneous and multifactorial disease. The aim of the present investigation was to analyze the connection between variations in the vitamin D receptor gene. (APAI rs7975232) and vitamin D serum levels among MS patients.
METHODS:
Blood samples were collected from 75 Iraqi patients with MS (33 male, 42 female), and 75 control group volunteers who appeared to be in good health with an age range of 20–50 years. Vitamin D receptor (VDR) gene polymorphism was detected by HRM RT-PCR and vitamin D serum levels were assessed by ELISA.
RESULTS:
Detection of VDR gene polymorphism in MS patients discovered that the wild genotype was C/C 15 (20%), the heterozygous genotype CA was 27(36%), and the homozygous genotype AA was 33(44%), whilst allele C occurrence was 57(38%) and allele A was 93(62%), compared per control genotype C/C was 40(53.3%), CA genotype was 20(26.6%), AA genotype was 15(20%), C allele frequency was 100(66.6%) and A allele was 50(33.3%) with highly significant difference (P≤0.001). Analysis of vitamin D serum levels showed much higher levels in the control group (43.40±0.85 pg/ml) than in the MS patients group (15.46±0.93 pg/ml; P≤0.001). Result of relationship between Vitamin D serum level with genotype of VDR among individuals with MS was found to be significant decrease (5.3±0.52) at AA genotype of MS patients, followed by (11.79±0.68) in CA genotype and finally (15.52±0.93) in CC genotype, all highly significant (P≤0.01).
CONCLUSION:
There was a notable correlation observed with VDR (APAI rs7975232) genotypes and Vitamin D serum level in MS Iraqi patients.
1Introduction
Multiple sclerosis (MS) is a pathological condition that arises when the immune system attacks the myelin sheath, which encompasses and safeguards nerve cells [1]. Research has shown that the risk of MS is greatly influenced by both hereditary and environmental factors [2]. It has been postulated that the immune system may be positively influenced by vitamin D (vit D), such that MS is less likely when the body maintains sufficient amounts of this vitamin. Hence, it is vital for specialists to appreciate the possible impacts of vit D fully. Numerous studies have investigated the influence of vit D on the formation and maturation of immune cells, such as macrophages, dendritic cells (DCs), T cells, and B cells [3, 4]. Vit D binds to nuclear vit D receptors (VDRs), which belong to the steroid/thyroid hormone receptor superfamily. The VDR gene is located on chromosome 12q12–14 in humans [5]. Specific variations of the VDR gene have been shown to be associated with alterations in the uptake and functioning of vit D. The single nucleotide polymorphism (SNP) ApaI (rs7975232), located inside intron 8 of the 3’ untranslated region (UTR), has been shown to have a correlation with the stability of VDR mRNA, in conjunction with many other polymorphisms [6]. The generation of variant alleles due to ApaI polymorphism has the potential to weaken the activities of VDR, hence increasing MS vulnerability via an imbalance in vit D levels [3]. Vit D comprises two separate forms, known as D2 and D3, which function as hormones. Synthesis of D3 occurs in the skin upon exposure to sunshine. Epidemiological studies have provided evidence indicating that there is a probable link between low levels of vit D and the risk of developing MS. This relationship is believed to be primarily attributed to the anti-inflammatory properties of vit D and its possible impact on cytokine levels [3, 7]. Given that sun exposure stimulates the production of vit D3 in the skin, it is postulated that early childhood exposure to sunlight is correlated with a decrease in the likelihood of developing MS [8]. Research has shown that vit D has a vital role in enhancing regulatory function to manage autoimmune diseases. It has been noted that individuals with MS often have reduced levels of vit D in their blood [9, 10]. MS is a very consequential health condition of considerable importance. The primary objective of this research was to explore the correlation between the polymorphism of the vit D receptor gene (ApaI rs7975232) and the blood level of vit D in individuals diagnosed with MS.
2Materials and methods
The study was approved by the Ethics Committee of our institution (Reference number: IGEBGS.H.2.1303, at 15-5-2022) and informed consent obtained. Venous blood (3 mL) was drawn from each of the 75 patients diagnosed with MS (30 men and 45 women). The patients were aged between 20 years and 50 years. They were age- and gender-matched with 75 apparently healthy volunteers, who served as a control group. The provided samples were partitioned into two parts.; first 1 ml of blood was transferred into EDTA anti-coagulant tubes, mixed slightly, and stored at (–20 ° C) until the DNA purification kit (Promega/USA) was used to extract DNA from the sample according to Mohammed [11, 12] for the detection of VDR (rs975232) polymorphism by using the High-resolution melting (HRM) method by RT- PCR. The amplification of DNA fragmentation was carried out utilizing the primers (T-RW=CTAGGGGTGGTGGGATTGAGCAGTGAAGT, G-RW=TGCGGGGTGGTGGGATTGAGCAGTGAAGG and Common Forwards = GAAGGCACAGGAGCTCTCAGCTGGGC). The thermal procedure for HRM involved initiating Taq Polymerase activation at 95 °C for 3 minutes, followed by 45 cycles of denaturation at 95 °C for 25 seconds, annealing at 62 °C for 30 seconds, and a final extension at 72 °C for 20 seconds. Following PCR amplification, HRM was conducted within the temperature range of 60–95 °C.
The remaining 2 mL were transferred into a gel tube and left to rest for 10–30 min to facilitate serum clotting. Following this, the tube was centrifuged at 3000 rpm for 10 min, and the obtained serum was stored at –20 °C until it was utilized for evaluation of vit D via ELISA.
The effects of multiple factors on the study parameters were determined using the Statistical Analysis System (SAS) program [13]. The Chi-square test was used to compare the distribution of patients and control subjects according to gender and autoimmune disease as well as genotype frequencies and alleles between sick and control groups. T-test was used to compare the mean gene expression of patients and control for significant differences between percentages (at probabilities of 0.05 and 0.01). The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the risk of MS with variable genotypes.
3Results
The age distribution of MS patients revealed that the greatest prevalence occurred in the 30–39-year-old age bracket, comprising 37 cases (49.3%). The 40–49- and 20–29-year-old age groups exhibited equivalent proportions of 16 cases each (21.3%). Conversely, the 50 + age group demonstrated the lowest proportion, with six cases (8%). These variations were statistically significant (p≤0.01), as shown in (Table 1).
Table 1
Comparison between patient’s age
| Age (years) | Patients No. (%) |
| Twenty -twenty nine | 16 (21.3%) |
| Thirty -thirty nine | 37 (49.3%) |
| Forty- forty nine | 16 (21.3%) |
| Fifty ≤ | 6 (8%) |
| Chi-Square | 32.0 |
| P-value | 0.001 |
**(P≤0.01).
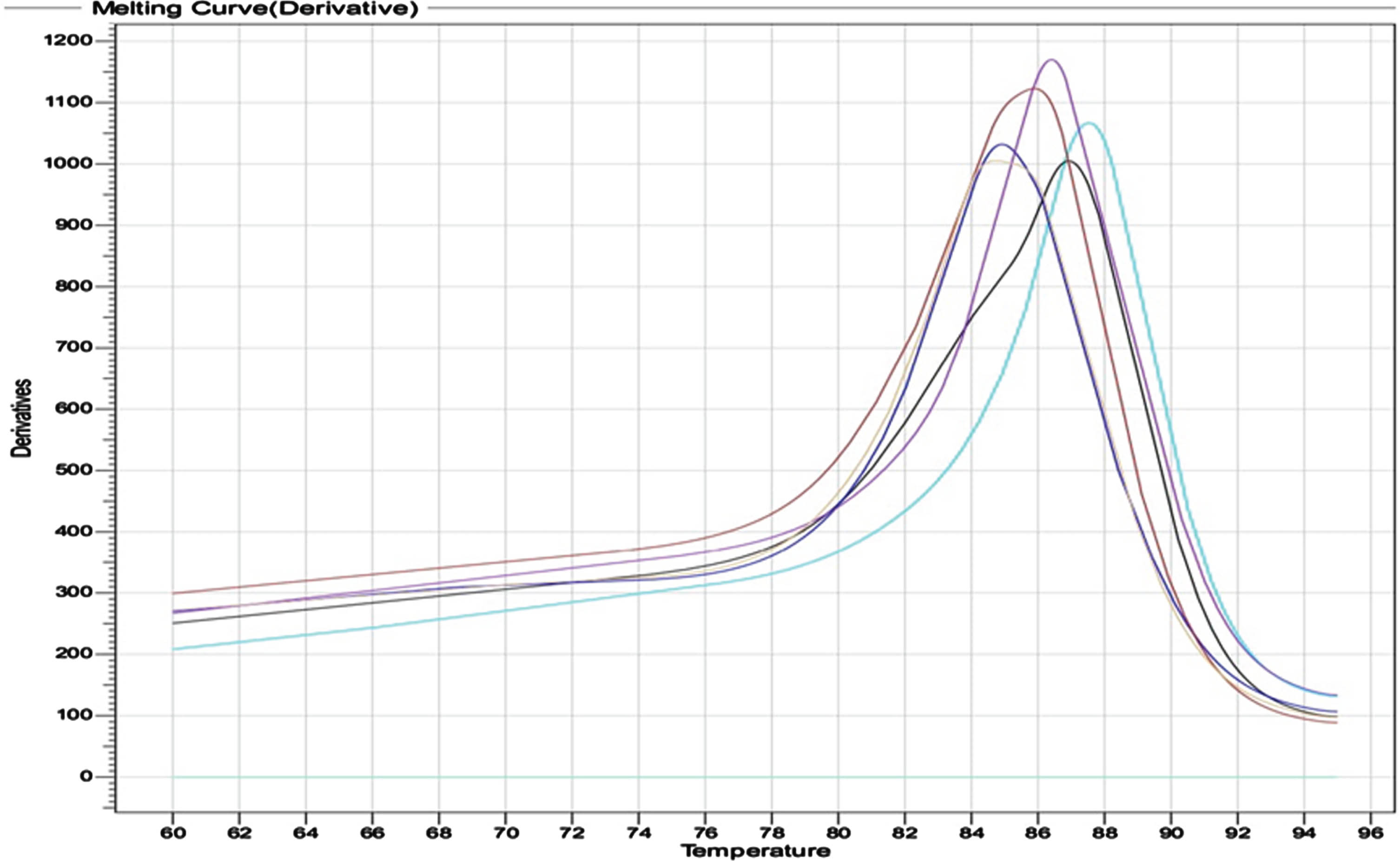
Outcomes of genotypes distribution and allele frequency VDR gene (APA rs975232) were achieved by using HRM real time PCR. The output of thermocycler for the three genotypes; wild CC, mutant AA and hetero CA are shown in (Fig. 1). The melting curve of primers specificity is illustrated in (Fig. 2).
Fig. 1
Output of HRM real time for the three genotypes: The Wild CC showing in sky color, AA Mutant in green, and CA hetero in violet.
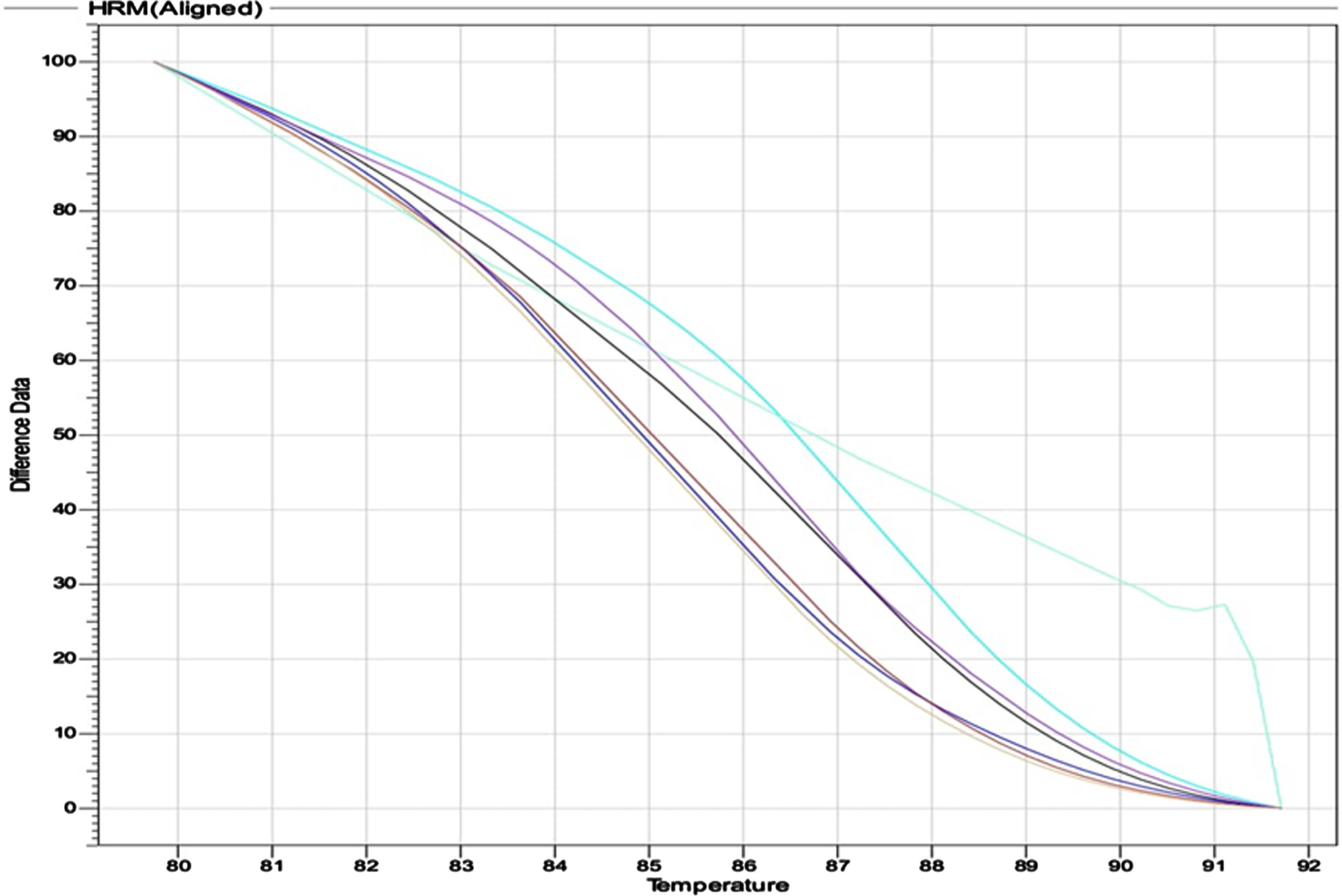
Fig. 2
The melting curve of primers which explain the high specificity for the primer.
The results of genotype distribution in the MS patients and control groups show that the wild genotype CC occurred in 15 patients (20.0%), CA the heterogeneous was 27 (36.0%), and the homogenous genotype AA, was 33 (44.0%), and the incidence of the (C) allele was 57 (38.0%) with OR (0.34). The (A) allele incidence was 93 (62.0%) with OR (2.9) in MS patient group, while in control group, wild genotype CC was 40 (53.3%), heterozygous genotype CA was 20 sample (26.6%), homozygous genotype AA was 15 (20. %). The frequency of C allele was 100 (66.6%), A allele frequency was 50 (3.33%) with highly significant difference as shown by a p-value 0.01 (Table 2).
Table 2
Genotypes spreading and allele frequency of VDR SNP (APA rs7975232 C > A) In both the patient and control groups
| Genotype C/A | Patients No. (%) | Control No. (%) | Chi-Square (χ2) | O.R. (C.I.) | P-value |
| Wild: CC | 15 (20%) | 40 (53.3%) | 6.84 | 0.27 (0.13–0.55) | 0.0004** |
| heterozygous: CA | 27 (36%) | 20 (26.6%) | 5.82 | 1.19 (0.60–2.35) | 0.016* |
| mutant homozygous: AA | 33 (44%) | 15 (20%) | 15.32 | 3.14 (1.51–6.49) | 0.0001** |
| Total | 75 (100%) | 75 (100%) | |||
| Chi-square | 3.84 | 6.24 | |||
| P-value | 0.04 | 0.04 | |||
| Allele | Frequency | Chi-square | O.R. (C.I.) | P-value | |
| C | 57(38%) | 100 (66.6%) | 22.36 | 0.34 (0.21–0.55) | 0.0001** |
| A | 93(62%) | 50 (33.3%) | 20.28 | 2.9 (1.81–4.63) | 0.0001** |
| Chi-square | 4.17 | 7.0 | |||
| P-value | 0.01* | 0.001** | |||
*(P≤0.05); **(P≤0.01); CI: Confidence Interval; O.R: Odd Ratio.
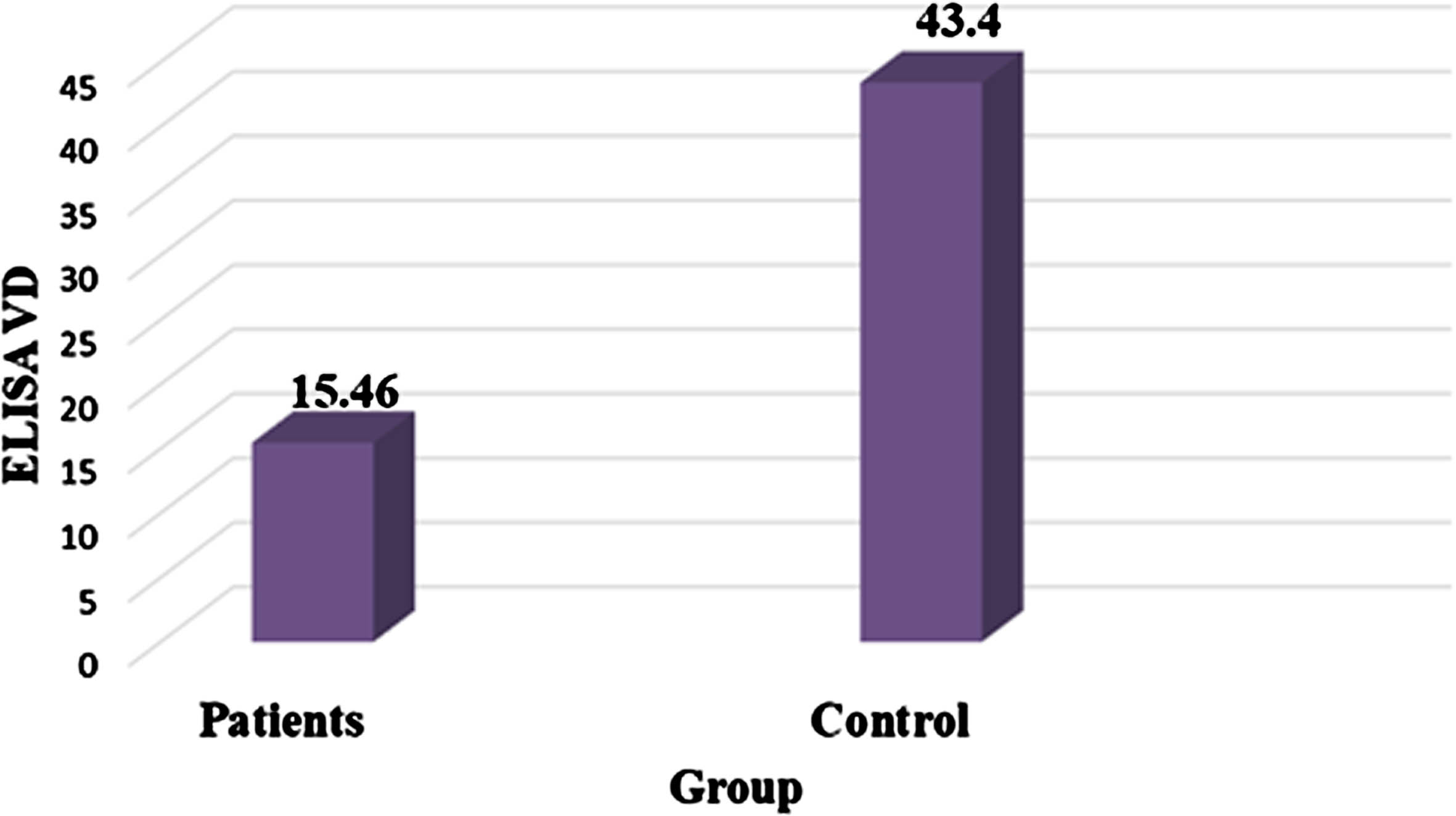
The results comparing serum vit D levels indicate significantly greater levels in the control (mean±standard deviation = 43.4±0.85 pg/mL) than in the MS group (15.5±0.93 pg/mL). The statistical analysis demonstrates high significance, with p≤0.01 (Table 3, Fig. 3).
Table 3
Different between serum VD level in MS patients and control groups
| Group | Mean±SE of serum VDR (pg/ml) |
| Patients | 15.46 + 0.93 |
| Control | 43.40 + 0.85 |
| T-test | –22.02 |
| P-value | 0.0001** |
**(P≤0.01).
Fig. 3
Comparison between serum VD level in MS patients and control group.
The relationship between serum vit D level with VDR genotypes in MS patients, revealed that a substantial decrease in serum vit D level (5.3 + 0.52 pg/ml) at AA genotype, followed by (11.8 + 0.68 pg/ml) in CA genotype, and finally (15.5 + 0.93 pg/ml) in CC genotype individuals with highly statistical significance, p-value of less≤ (Table 4).
Table 4
Relationship between VDR SNP (APA rs7975232 C > A) and VD serum level
| Geno type | Mean±SE VD serum (pg/ml) |
| CC | 15.52 + 0.93 |
| CA | 11.79 + 0.68 |
| AA | 5.3 + 0.52 |
**(P≤0.1).
4Discussion
This study found that the highest incidence of MS occurred in the 30–39-year-old age group. This phenomenon may be linked to modern lifestyle choices, including a heavy dependence on fast food that lacks essential nutrients. Moreover, prolonged sedentary habits, marked by excessive time spent using TVs, computers, and phones, might play a role, as individuals may neglect outdoor pursuits and regular exercise. Similar findings have been shown in other research, indicating that the occurrence of MS is prevalent among individuals in their thirties. These results align with the research conducted by Alroughani, who also observed a high prevalence of MS among individuals in this age group [14–17]. The findings of the current study contradict the research conducted by Khademi et al. [18], which observed a lower prevalence of MS in individuals aged 35 to 32 compared to older age groups. There are several reasons for this difference, including the severity and progression of the disease, the effectiveness of treatment at younger ages, and individual variations in pathology among patients.
Results of genotypes and allele incidence of VDR SNP (APAI rs7975232) in those with MS and control groups, indicated that individuals with at least one copy of the (A) allele had an elevated susceptibility to MS. Among the genetic variations studied for disease susceptibility, the most extensively investigated are single-nucleotide polymorphisms (SNPs). Of particular functional significance is the APA-I polymorphism, characterized by a C/A allele variation located within intron 8 of the 3’ un translated region (UTR) of the VDR gene. Furthermore, an intriguing interaction was observed concerning the impact of the VDR APA-I polymorphism on MS risk and the nutritional consumption of vit D. The C allele of the APA SNP was related with declined 25(OH) [18]. Alignment with prior research is seen in our findings, echoing earlier studies that identified a significant correlation between the APA-I polymorphism and MS in both the alleles (CA) and (AA). This correlation was particularly noteworthy when compared to other studies. Moreover, a link was revealed between the presence of the A allele in the APA-I polymorphism and reduced levels of 25(OH)D. Noteworthy associations were also established between polymorphisms in the VDR gene and the severity and progression of MS [19–21]. Vit D impacts the activity of microglia, which are known for releasing inflammatory substances that contribute to myelin damage in central nervous system autoimmunity. Treating mice with calcitriol immediately after inducing autoimmunity results in reduced activation of microglia, less oxidative stress, and decreased permeability of the blood-brain barrier [22]. Deleting a portion of the vit D receptors in young mice lessens microglia activation, reducing both the occurrence and severity of autoimmunity. In various central nervous system diseases and injuries, vit D has been observed to regulate the behavior and oxidative stress of microglia [23]. Consequently, vit D seems to effectively shift microglia from an inflammatory M1 state to a reparative M2 state, thereby decreasing inflammation and limiting demyelination [24]. The correlation between the severity of MS and vit D levels can be elucidated by the potent immune-modulating properties of vit D. The activity of proinflammatory cells is suppressed by this vitamin, which simultaneously promotes the activity of anti-inflammatory cells and cytokines [9].
The correlation between serum vit D levels and the VDR genotype in MS patients shows that those with at least one (A) allele are at an elevated risk of MS. Additionally, the presence of the mutant (A) allele in heterozygous (CA) and homozygous (CC) forms is linked to the vit D status among MS patients. Based on our result we emphasize the need for future studies with greater sample size to enhance the generalizability of the findings.
5Conclusions
The VDR (APAI rs7975232) genotype plays in important role in immune responses and, consequently, MS susceptibility. A higher risk of MS was observed in patients that had at least one copy of the (A) allele, and there was an association between vit D serum level and the presence of the mutant (A) allele in heterozygous (CA) and homozygous (CC) genotypes in Iraqi MS patients.
Acknowledgments
The authors would like to thank the staff of Institute of Genetic Engineering and Biotechnology for Post Graduate Studies for their support. We also want to thank the referees for their thoughtful understanding of the manuscript and their insightful remarks.
Authors’ contributions
Zahraa Kadhim Lafi: Conception, Interpretation or analysis of data, Preparation of the manuscript, Revision for important intellectual content.
Bushra Jasim Mohammed: Preparation of the manuscript, Supervision.
Conflict of interest
The authors declare that they have no conflict of interest
Funding
The authors have no funding to report
References
[1] | Leray E. , Moreau T. , Fromont A. , Edan G. , Epidemiology of multiple sclerosis, Revue Neurologique 172: (1) ((2016) ), 3–13. |
[2] | McKay K.A. , Tremlett H. , Epidemiology of Multiple Sclerosis and Environmental Risk Factors, Neuroimmunology: Multiple Sclerosis, Autoimmune Neurology and Related Diseases (2021), 137–153. |
[3] | Brownlee W.J. , Hardy T.A. , Fazekas F. , Miller D.H. , Diagnosis of multiple sclerosis: Progress and challenges, The Lancet 389: (10076) ((2017) ), 1336–1346. |
[4] | Gado K.H. , Gado T.H. , Samie R.M.A. , Khalil N.M. , Emam S.L. , Fouad H.H. , Clinical significance of vitamin D deficiency and receptor gene polymorphism in systemic lupus erythematosus patients, The Egyptian Rheumatologist 39: (3) ((2017) ), 159–164. |
[5] | Szodoray P. , Nakken B. , Gaal J. , Jonsson R. , Szegedi A. , Zold E. , Szegedi G. , Brun J. , Gesztelyi R. , Zeher M. , The complex role of vitamin D in autoimmune diseases, Scandinavian Journal of Immunology 68: (3) ((2008) ), 261–269. |
[6] | Thirunavukkarasu R. , Chitra A. , Asirvatham A. , Jayalakshmi M. , Association of Vitamin D Deficiency and Vitamin D Receptor Gene Polymorphisms with Type 1 Diabetes Risk: A South Indian Familial Study, J Clin Res Pediatr Endocrinol (2023). |
[7] | Mohammed B.J. , TNF-alpha gene polymorphism and its relation to vitamin D, calcium, alkaline phosphatase and ferritin status in Iraqi beta thalassemia patients, Biomedicine 42: (5) ((2022) ), 906–911. |
[8] | Hedström A.K. , Olsson T. , Kockum I. , Hillert J. , Alfredsson L. , Low sun exposure increases multiple sclerosis risk both directly and indirectly, Journal of Neurology 267: ((2020) ), 1045–1052. |
[9] | Gombash S.E. , Lee P.W. , Sawdai E. , Lovett-Racke A.E. , Vitamin D as a risk factor for multiple sclerosis: Immunoregulatory or neuroprotective? Frontiers in Neurology 13: ((2022) ), 796933. |
[10] | Lateef A.N. , Mohammed B.J. , Effect of Age on Apoptosis and Necrosis of Peripheral Blood Lymphocytes in Sample of Iraqi Type 2 Diabetes Patients, Iraqi Journal of Biotechnology 22: (1) ((2023) ) . |
[11] | Lafi Z.K. , Mohammed B.J. , Mohammed, Relationship between vitamin D receptor genotypes (FOK1rs2228570) and IL18 gene expression in sample of multiple sclerosis Iraqi patients, Human antibodies. |
[12] | Luaibi H.A. , Mohammed B.J. , Does TNF-α 308 G/A (rs1800629) gene polymorphism associate with liver and pancreas disorders in Iraqi adults with beta thalassemia major? Human Antibodies (Preprint) (2023), 1–7. |
[13] | Cary N. , Statistical analysis system, User’s guide. Statistical. Version 9, SAS. Inst. Inc. USA (2012). |
[14] | Abdulhameed S.A. , Mohammed B.J. , The Relationship of Gene Expression between TNF and TNF-Like Cytokine 1A Genes in Sample of Multiple Sclerosis Iraqi Patients, Iraqi Journal of Biotechnology 21: (2) ((2022) ), 88–95. |
[15] | Alroughani R. , Ahmed S. , Behbehani R. , Khan R. , Thussu A. , Alexander K. , Ashkanani A. , Nagarajan V. , Al-Hashel J. , Increasing prevalence and incidence rates of multiple sclerosis in Kuwait, Multiple Sclerosis Journal 20: (5) ((2014) ), 543–547. |
[16] | Hammood F.S. , Mohammed B.J. , Genetic Identifications for Sample of Multiple Sclerosis Iraqi Patients, Iraqi Journal of Biotechnology 21: (2) ((2022) ), 225–235. |
[17] | Yahya R.N. , Kasim A.A. , Al Gawwam G.A. , Comparing the quality of life among patients with relapsing remitting multiple sclerosis in Iraq using different disease modifying therapies, Iraqi Journal of Pharmaceutical Sciences (P-ISSN 1683-3597 E-ISSN 2521-3512) (2018), 102–114. |
[18] | Khademi M. , Dring A.M. , Gilthorpe J.D. , Wuolikainen A. , Al F. , Nimer, R.A. Harris, M. Andersson, L. Brundin, F. Piehl and T. Olsson, Intense inflammation and nerve damage in early multiple sclerosis subsides at older age: A reflection by cerebrospinal fluid biomarkers, PloS one 8: (5) ((2013) ), e63172. |
[19] | Abdollahzadeh R. , Fard M.S. , Rahmani F. , Moloudi K. , Azarnezhad A. , Predisposing role of vitamin D receptor (VDR) polymorphisms in the development of multiple sclerosis: A case-control study, Journal of the Neurological Sciences 367: ((2016) ), 148–151. |
[20] | Tizaoui K. , Kaabachi W. , Hamzaoui A. , Hamzaoui K. , Association between vitamin D receptor polymorphisms and multiple sclerosis: Systematic review and meta-analysis of case–control studies, Cellular & Molecular Immunology 12: (2) ((2015) ), 243–252. |
[21] | Ad’hiah A.H. , Atiyah N.S. , Fadhil H.Y. , Qualitative and Quantitative Molecular Analysis of Epstein-Barr Virus in Iraqi Patients with Relapsing-Remitting Multiple Sclerosis, Iraqi Journal of Science (2023), 127–137. |
[22] | De Oliveira L.R.C. , Mimura L.A.N. , Fraga-Silva T.F.D.C. , Ishikawa L.L.W. , Fernandes A.A.H. , Zorzella-Pezavento S.F.G. , Sartori A. , Calcitriol prevents neuroinflammation and reduces blood-brain barrier disruption and local macrophage/microglia activation, Frontiers in Pharmacology 11: ((2020) ), 161. |
[23] | Lee P.W. , Selhorst A. , Lampe S.G. , Liu Y. , Yang Y. , Lovett-Racke A.E. , Neuron-specific vitamin D signaling attenuates microglia activation and CNS autoimmunity, Frontiers in Neurology 11: ((2020) ), 19. |
[24] | Cui C. , Xu P. , Li G. , Qiao Y. , Han W. , Geng C. , Liao D. , Yang M. , Chen D. , Jiang P. , Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: Role of renin-angiotensin system, Redox Biology 26: ((2019) ), 101295. |




