The improvement of pain symptoms in patients with burning mouth syndrome through combined laser and medication therapy
Abstract
BACKGROUND:
The etiology of Burning Mouth Syndrome (BMS) remains unclear.
OBJECTIVE:
To explore the differences in the therapeutic efficacy of pain improvement between medication therapy and laser therapy in patients with BMS.
METHODS:
45 BMS patients were randomly divided into three groups: The Combination therapy group (Group A,
RESULTS:
All three groups (A, B, and C) showed a significant reduction in NRS scores after treatment, with statistically significant differences observed among the different groups. Group A exhibited the most significant improvement, with a statistically significant difference before and after treatment.
CONCLUSION:
Laser and medication therapy are effective methods for reducing oral burning pain * symptoms, and their combined use yields more significant therapeutic effects.
1.Introduction
Burning Mouth Syndrome (BMS) is a mucosal disorder characterized by a burning sensation in the oral cavity without any organic lesions [1, 2]. The prevalence of BMS ranges from 0.7% to 5%, with most cases occurring in perimenopausal women, with a male-to-female ratio of 1:7. The etiology of BMS remains unclear. Still, current theories suggest that neural and psychological factors, endocrine changes, local oral irritation, and systemic factors primarily cause it. Patients with BMS often experience increased psychological stress and decreased quality of life [3, 4, 5, 6, 7, 8, 9].
Semiconductor laser, also known as low-level laser or near-infrared laser, belongs to the category of low-energy lasers [10, 11]. The application of low-level laser not only produces “systemic effects,” meaning that effects are observed not only at the point of irradiation but also in the surrounding areas, but it is also particularly effective in local treatments, significantly improving therapeuticoutcomes [12]. Semiconductor laser therapy for oral diseases primarily focuses on conditioning effects, improving blood circulation, anti-infection, and anti-inflammatory and analgesic effects. On the one hand, laser therapy can inhibit the development of traumatic tissues, increase tissue permeability, and improve the microcirculation system of the body, which is conducive to cellular regeneration. On the other hand, when a laser beam is applied to biological tissues, it generates biological stimulation, photochemical, photothermal, and other related effects.
Consequently, the laser utilizes these effects and characteristics to achieve anaesthesia, pain relief, vaporization, cauterization, cutting, and coagulation effects [13]. Laser therapy is categorized into low-energy and high-energy lasers based on their different energy levels, with the former being primarily used in the treatment of BMS. Experimental studies have shown that low-level lasers (LLL) have biological stimulation and regulation effects and have been widely used in the treatment of organic or functional diseases of peripheral nerves. LLL is also being explored for symptom control in BMS [14].
Most treatment methods for BMS aim to alleviate clinical symptoms such as dry mouth, pain, nerve regeneration and repair, nutritional deficiencies, endocrine imbalances, and psychological disorders. Clinical treatments mainly include rinsing medications, neurotrophic drugs, antipsychotic drug combinations, and low-level laser treatment (LLLT) [15]. This study focuses on different treatments for middle-aged and elderly patients with Burning Mouth Syndrome in Kunming, Yunnan Province. It conducts a cross-sectional and longitudinal comparative analysis of the data from the three treatment methods to compare their differences in therapeutic efficacy.
2.Data and methods
2.1Case selection
A total of 45 patients diagnosed with Burning Mouth Syndrome (BMS) who sought treatment at the Comprehensive Department of Kunming Medical University Affiliated Stomatological Hospital from May 2022 to February 2023 were selected as the study subjects. All participants were aged 50 or above, including six males and 39 females, representing the age and population group most commonly affected by BMS. Each participant provided informed consent and signed an informed consent form. According to the diagnostic criteria of the International Headache Society (IHS) [16], patients with oral lesions or any other local changes, such as those accompanied by dry syndrome, trauma, allergic reactions, or clinically diagnosed etiological diseases, were excluded. The participants were randomly divided into three groups, namely, the Combined Treatment Group (Group A,
While including more control groups for detailed subgroup analyses is feasible, it may present challenges in obtaining ethical approval. In this trial, it is necessary to ensure improvement in patients’ pain outcomes, and implementing a placebo control may not be practical. Therefore, we have chosen to use clinically feasible treatment methods as controls, which can ensure patient efficacy while allowing for experimental research and analysis. The sample size calculation for this trial was based on the prevalence of burning mouth syndrome in our hospital, which was determined to be around 3–4%. Using the sample size calculation formula and considering a reasonable dropout rate, it was determined that 45 participants would be required for this study.
Prior to the commencement of the trial, we strictly adhered to the current clinical inclusion and exclusion criteria for burning mouth syndrome, ensuring the exclusion of patients with pre-existing psychiatric disorders or those who had previously taken psychotropic medications, which was done to maintain homogeneity among the enrolled patients.
2.2Symptom evaluation
The pain severity of the patients was assessed using the Numeric Rating Scale (NRS), which is a scale that measures the intensity of pain and considers its impact on psychological well-being and daily life. The pain level and its impact were rated on a scale of 1–10, with higher scores indicating greater severity. Three rounds of NRS evaluations and questionnaire completion were conducted: before treatment, one week after treatment, and one month after treatment.
2.3Medication treatment
The medication treatment included the following: Methylcobalamin dispersible tablets, 0.5 mg per dose, three times a day, orally taken for one month; Xylitol Chlorhexidine Lozenges, one tablet per dose, three times a day, orally taken for one month; 2% Sodium Bicarbonate Solution, 3–5 mL per rinse, rinsing for 3 minutes after meals, three times a day, continuously used for one month.
2.4Laser teatment
A semiconductor laser therapy device was used continuously with an output power of 0.5 W, an emission wavelength of 1064 nm, a pulse energy of 60–80 mJ, and a tip diameter of 1 mm. The patients received one laser irradiation session per week for four weeks. The laser beam was targeted at the areas where the burning sensation was most prominent, with the laser fiber tip positioned 1 mm away from the mucosa. Each point was irradiated for 10 seconds.
2.5Statistical analysis
Statistical analysis of the data was performed using SPSS 20.0 software. Non-parametric tests were conducted to compare the three groups’ NRS scores before and after treatment. A total of 45 valid cases were included in this study. The pre-treatment NRS scores for all groups ranged from 5 to 7, with no significant differences (
3.Results
3.1Basic information of patients
The pre-treatment NRS scores of the 45 participants ranged from 5 to 7, with no significant differences (
Table 1
Differences in NRS scores of each group
| Combination treatment | Medical treatment | Laser treatment | ||||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | |
| Mean | 5.6000 | 2.8000 | 5.8000 | 3.7500 | 6.0000 | 4.2000 |
| Number | 10 | 10 | 10 | |||
| 0.001 | 0.000 | 0.000 | ||||
Figure 1.
CONSORT 2010 flow diagram.
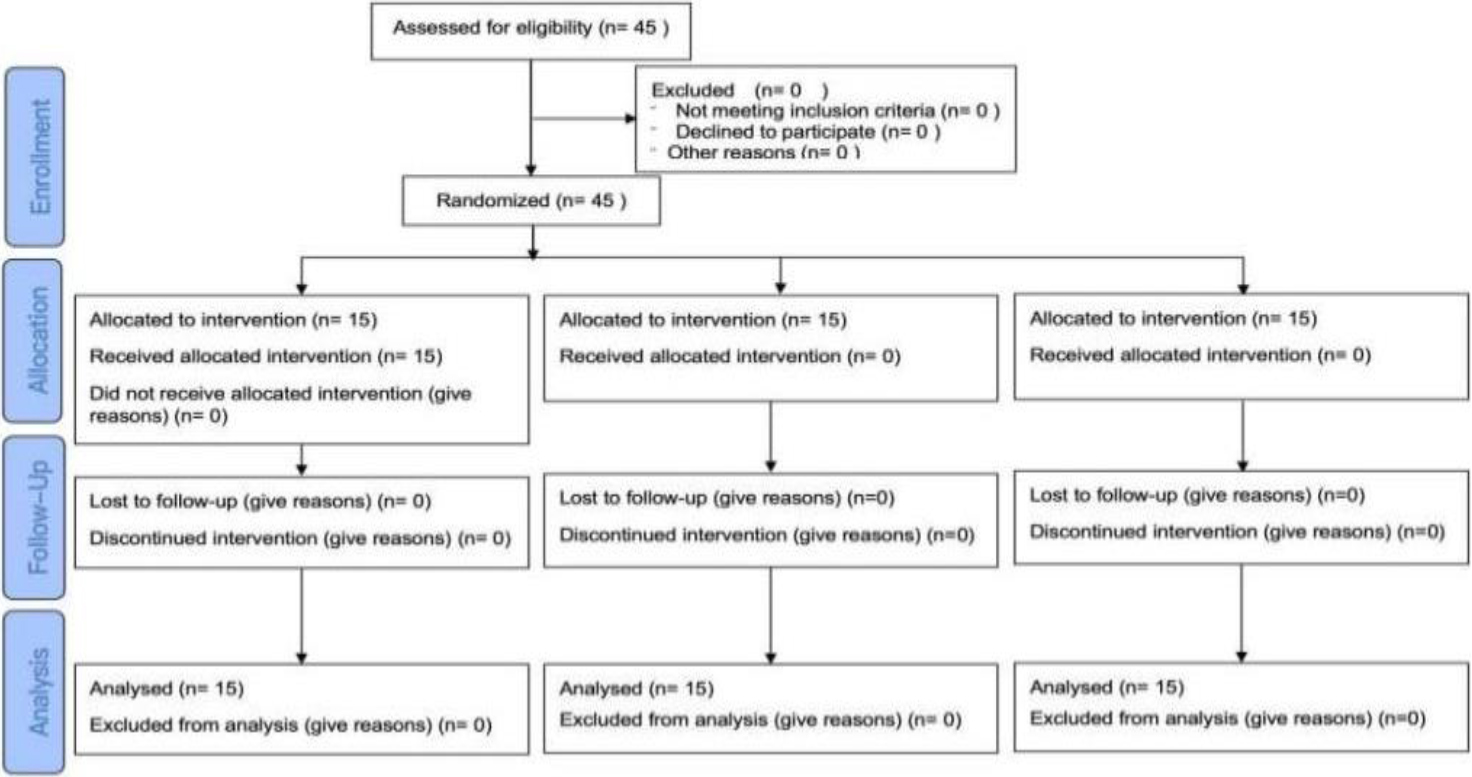
3.2Pre-experiment
Before the formal study, a pre-experiment was conducted to ensure the feasibility and authenticity of the experimental design.10 BMS patients with pre-treatment NRS scores ranging from 5 to 7 were randomly assigned to receive respective treatments and their scores were compared after seven days of treatment. A paired samples t-test was used for statistical analysis. As shown in Table 1, after seven days of treatment, the NRS scores in Groups A, B, and C all decreased, and there were statistically significant differences before and after treatment (
Table 2
Tests of normality
| Patient numbers | Standard error | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Combination treatment | Medical treatment | Laser treatment | Combination treatment | Medical treatment | Laser treatment | Combination treatment | Medical treatment | Laser treatment |
| Pre-treatment | 15 | 15 | 15 | 0.290 | 0.251 | 0.343 | 0.001 | 0.012 | 0.000 |
| Second week | 15 | 15 | 15 | 0.240 | 0.144 | 0.212 | 0.240 | 0.200* | 0.069 |
| One month | 15 | 15 | 15 | 0.208 | 0.234 | 0.164 | 0.208 | 0.026 | 0.200* |
*This is a lower bound of true significance.
Table 3
Hypothesis test summary(intra-group)
| Groups | Check day | Samples | VAS (Mean | |
|---|---|---|---|---|
| Combination treatment | 1-Pre-Treatment | 15 | 5.8000 | 3-2:0.296 |
| 2-First Check(14 day) | 15 | 3.1333 | 3-1:0.000 | |
| 3-Second Check(1 month) | 15 | 1.8667 | 2-1:0.001 | |
| Medical treatment | 1-Pre-Treatment | 15 | 5.8667 | 3-2:1.00 |
| 2-First Check(14 day) | 15 | 3.2000 | 3-1:0.00 | |
| 3-Second Check(1 month) | 15 | 2.6667 | 2-1:0.01 | |
| Laser treatment | 1-Pre-Treatment | 15 | 5.8667 | 3-2:1.000 |
| 2 First Check(14 day) | 15 | 4.4667 | 3-1:0.003 | |
| 3-Second Check(1 month) | 15 | 3.6667 | 2-1:0.042 |
3.3Efficacy evaluation of each group
Since the post-treatment NRS scores of Groups A, B, and C did not follow a normal distribution in this study (Table 2), non-parametric tests were used for data analysis. As shown in Table 3, after one treatment course, the NRS scores in Groups A, B, and C all decreased, with Group A showing the most significant improvement, and the differences were statistically significant (
Figure 2.
Differences in NRS scores of each group.
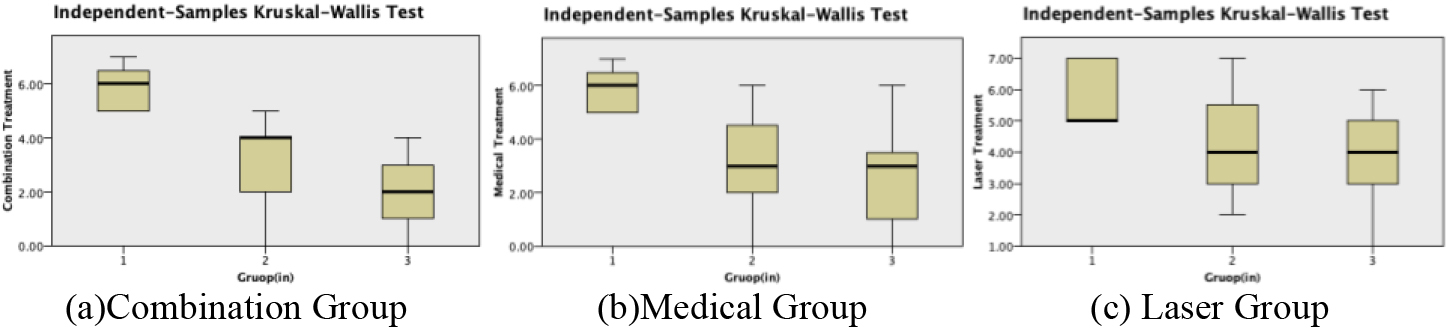
During the pilot study, we initially used the Visual Analog Scale (VAS) for pain assessment. However, considering the cultural and linguistic differences among patients and their varying levels of comprehension, we chose to use the Numeric Rating Scale (NRS) as it has equivalent testing power and is relatively simpler to administer. Additionally, we ensured that the assessment of pain relief in patients during the trial was not deliberately guided to be entirely patient self-reported, taking into account potential limitations in their ability to complete the assessment independently.
Figure 3.
Hypothesis test summary(inter-group).
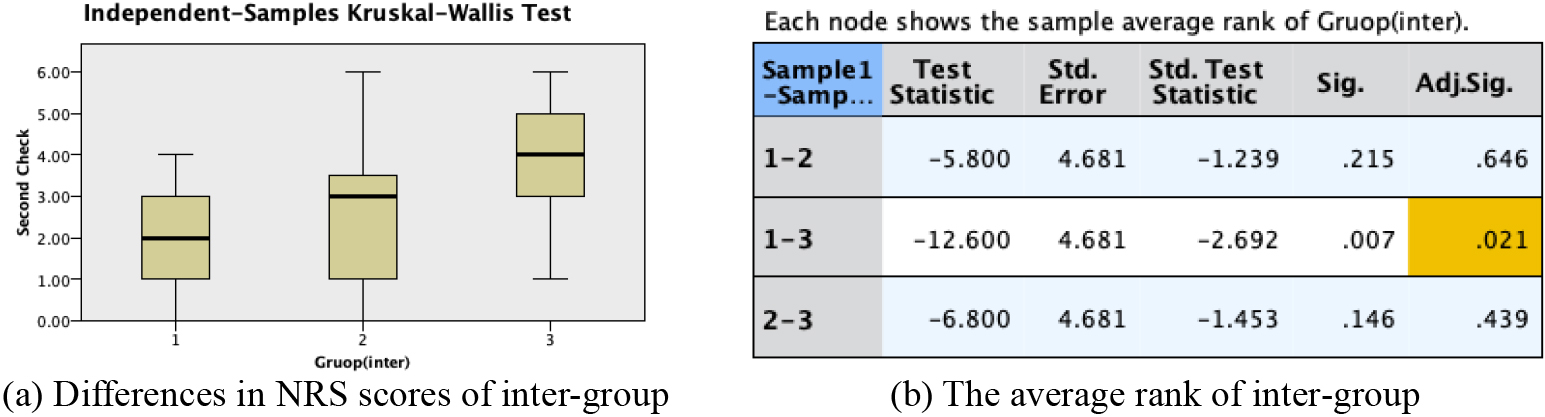
3.4Comparison of treatment efficacy among groups
The post-treatment NRS scores after one treatment course were analyzed to compare the treatment efficacy among the three groups. As shown in Fig. 3, the NRS scores in Group A were significantly lower than those in Group B and Group C (
4.Discussion
BMS is primarily typical in menopausal females, and patients often have or are accompanied by other subjective sensory abnormalities, among which the symptom of pain described as a burning sensation account for a large proportion. The patients in this study are over 50 years old, with females outnumbering males. [16, 17, 18, 19] About 80% of patients have different degrees of sensation of dry or burning pain in the mouth [2, 20] which is consistent with the results of the current study.
Low-level laser treatment (LLLT) is a widely accepted non-invasive patient therapy. LLLT is widely used in the medical field due to its safety and lack of side effects. It has been found in the experiments of Li Bohan et al. that DENLAS-10BM is effective and safe in the treatment of oral soft tissue laser diode [21]. Low-energy laser therapy has been applied to the symptom control of BMS in many countries, including ours. The main therapeutic effect of LLLT on BMS is conditioning, in which, through laser penetration, tissue plays a biological stimulation and biological regulation, that is, the induction of analgesia, anti-inflammatory and repair. Unlike high-energy lasers used for ablation and cutting, low-energy lasers do not have any significant thermal effects on tissues [22]. (The wavelength range of light used for LLLT is from 600 to 1,070 nm, and most studies have focused on the median value of this wavelength range, whereas this clinical study used a 1,064 nm semiconductor laser and achieved significant therapeutic results.) The mechanism of LLLT therapeutic effect occurs through a variety of processes; so far, LLLT has been shown to be effective in treating BMS [23, 24]. The results of this study showed that the NRS score in the laser group after treatment was significantly lower than that before treatment, and there was a statistical difference (
Mecobalamin has good transmissibility to nerve tissue, participates in the metabolism of nucleic acid, protein, and lipids through methylation function, and has the function of nourishing and maintaining nerves [25]. Cetylpyridinium chloride buccal tablets belong to quaternary ammonium surfactant, which can adsorb on the surface of oral Candida cell membrane to induce protein denaturation and finally plays a strong antibacterial and bactericidal effect [26]. Sodium bicarbonate gargle has weak alkalinity, which can neutralize acid decomposition, residual curd and sugar and inhibit the growth of fungi. In this study, the NRS scores of patients also decreased significantly after simple drug treatment, which once again proved the crucial clinical significance of the above drugs in the treatment of burning mouth syndrome.
Research has shown that, currently, low-level laser therapy (LLLT) is mainly used in conjunction with other treatment methods for treating burning mouth syndrome (BMS). Laser therapy alone is not as effective as combined therapy, and the effectiveness of laser therapy may vary depending on the wavelength and irradiation frequency [27]. Recent studies by Spanemberg et al. [28] have demonstrated satisfactory therapeutic effects of LLLT in some BMS patients and suggest that low-level laser therapy in combination with psychotropic medication is a better treatment approach. The results of this study indicate that the combined treatment group, compared to the group receiving laser therapy alone, showed a more significant reduction in Numeric Rating Scale (NRS) scores at the end of treatment. The data between the two groups showed statistical differences, suggesting that in the treatment of BMS, the combined treatment group has better efficacy than laser therapy alone, consistent with current research findings both domestically and internationally [29]. In a study conducted by Spanemberg et al. [30] in 2015, three different wavelengths of low-level laser were reported: IR1W (830 nm, 100 mW, 5 J, 176 J/cm2, 50 s); IR3W (830 nm, 100 mW, 5 J, 176 J/cm2, 50 s); red laser (685 nm, 35 mW, 2 J, 72 J/cm2, 58 s). It was demonstrated that low-level laser therapy with different wavelengths had significant therapeutic effects on BMS, with statistically significant differences in NRS scores before and after treatment (
Meanwhile, Škrinjar I [31] also pointed out that there is currently no standardized low-level laser for treating BMS, and the commonly used wavelength range for low-level lasers is from 600 to 1070 nm. Most studies focus on selecting the midpoint of this wavelength range, while this clinical study used a 1064 nm semiconductor oral laser therapy device, which is rarely studied for its efficacy. In this study, the laser therapy device had an output power of 0.5 W, an emission wavelength of 1064 nm, a pulse energy of 60–80 mJ, a laser-mucosa distance of 1 mm, a 10-second irradiation at each site, a tip diameter of 1 mm, one treatment per week, a treatment duration of 4 weeks. The short treatment duration facilitates patient acceptance and provides insights for clinical application. Both the medication treatment group and the combined treatment group significantly reduced NRS scores before and after treatment, demonstrating that both are effective methods for treating BMS symptoms. Although there was no significant statistical difference in the final efficacy comparison, the combined treatment group had a shorter duration of medication use, fewer adverse reactions from patients, better efficacy, and higher safety, which is beneficial for clinical application.
In summary, this study indicates that drug therapy combined with laser therapy is an effective treatment for Burning Mouth Syndrome. The use of a 1064 nm semiconductor oral laser therapy instrument can achieve exact efficacy according to the course of treatment and therapy in the paper, and the therapy has no apparent adverse reactions, few side effects, no trauma, high safety performance and high patient acceptance, providing a new scheme for the clinical treatment of BMS. The specific mechanism of laser therapy and drug therapy in treating BMS and the long-term adverse effects on the body are the direction of future research.
In the subsequent studies, we will conduct further research on patients with burning mouth syndrome who exhibit different clinical manifestations, types of onset, and varying degrees of pain. We will include additional control groups to objectively evaluate the treatment outcomes of combination therapies using more objective assessment scales.
Conflict of interest
None to report.
References
[1] | Dym H, Lin S, Thakkar J. Neuropathic pain and burning mouth syndrome: an overview and current update. Dent Clin North Am. (2020) ; 64: (2): 379-399. doi: 10.1016/j.cden.2019.12.009. |
[2] | Tavee J, Zhou L. Small fiber neuropathy: a burning problem. Cleve Clin J Med. (2009) ; 76: (5): 297-305. doi: 10.3949/ccjm.. |
[3] | Chen QM. Analysis of Oral Mucosal Diseases. 2nd ed. Beijing: People’s Medical Publishing House; 2019: : 269. |
[4] | Souza FT, Santos TP, Bernardes VF, et al. The impact of burning mouth syndrome on health-related quality of life. Health Qual Life Outcomes. (2011) ; 9: : 57. |
[5] | Klasser GD, Epstein JB, Villines D, et al. Burning mouth syndrome: A challenge for dental practitioners and patients. Gen Dent. (2011) ; 59: (3): 210-220. |
[6] | Honda M, Iida T, Kamiyama H, et al. Mechanical sensitivity and psychological factors in patients with burning mouth syndrome. Clin Oral Investig. (2019) ; 23: (2): 757-762. doi: 10.1007/s00784-018-2488-9. |
[7] | Lee YH, An JS, Chon S. Sex differences in the hypothalamic-pituitary-adrenal axis in patients with burning mouth syndrome. Oral Dis. (2019) ; 25: (8): 1983-1994. doi: 10.1111/odi.13195. |
[8] | Kolkka-Palomaa M, Jääskeläinen SK, Laine MA, et al. Pathophysiology of primary burning mouth syndrome with special focus on taste dysfunction: a review. Oral Dis. (2015) ; 21: (8): 937-948. doi: 10.1111/odi.12345. |
[9] | Magri LV, Carvalho VA, Rodrigues FCC, Bataglion C, Leite-Panissi CRA. Non-specific effects and clusters of women with painful TMD responders and non-responders to LLLT: a double-blind randomized clinical trial. Lasers Med Sci. (2018) ; 33: (2): 385-92. doi: 10.1007/s10103-017-2406-4. |
[10] | Braun A, Kettner M, Berthold M. Efficiency of soft tissue incision with a novel 445-nm semiconductor laser. Lasers Med Sci. (2018) ; 33: (1): 27-33. |
[11] | Sindel A, Dereci Ö, Hatipoğlu M, et al. Evaluation of temperature rise following the application of diode and ErCr: Ysgg lasers: an ex vivo study. Eur Oral Res. (2018) ; 52: (3): 131-136. |
[12] | Wei X, Li M, Li J, et al. Current status, challenges, and reflections on several new system semiconductor lasers and related industries. Chinese Journal of Engineering Science. (2020) ; 22: (3): 21-28. |
[13] | Wang DJ, Teng SS. Application and research progress of semiconductor lasers in oral clinical medicine. Continuing Medical Education. (2022) ; 36: (9): 153-156. |
[14] | Chen Q, Yan YY, Lu SL. Research progress of laser therapy in the treatment of oral mucosal diseases. Chinese Journal of Geriatric Oral Medicine. (2017) ; 15: (3): 185-188+192. |
[15] | Fang H. Observation on the effects of chlorhexidine mouthwash containing xylitol on the levels of IL-6 and SOD in serum and saliva of patients with periodontitis. World Latest Medical Information Abstracts. (2017) ; 22: (72): 50. |
[16] | Adamo D, Celentano A, Ruoppo E, Cucciniello C, Pecoraro G, Aria M et al. The relationship between sociodemographic characteristics and clinical features in burning mouth syndrome. Pain Med. (2015) ; 16: : 2171-2179. |
[17] | Danhauer SC, Miller CS, Rhodus NL, Carlson CR. Impact of criteria – based diagnosis of burning mouth syndrome on treatment outcome. Orofac Pain. (2002) ; 16: : 305-311. |
[18] | Lima ENA, Barbosa NG, Santos ACS, Lemos TMAM, Souza CM, Trevilatto PC et al. Comparative analysis of psychological, hormonal, and genetic factors between burning mouth syndrome and secondary oral burning. Pain Med. (2016) ; 1-10. |
[19] | Kohorst JJ, Bruce AJ, Torgerson RR, et al. The prevalence of burning mouth syndrome: a population-based study. Br J Dermatol. (2014) ; 172: (6): 1654-6. |
[20] | Mu DD, Su S, Jin JQ, et al. A case-control study on the association between dry mouth symptoms of burning mouth syndrome and anxiety and depression symptoms. Chinese Journal of Mental Health. (2016) ; 30: (4): 253-257. |
[21] | Li BH, Zou SQ, Wang X. Study on the effectiveness and safety of DENLAS-10BM semiconductor laser in the treatment of oral soft tissue diseases. Chinese Journal of Geriatric Oral Medicine. (2020) ; 18: (1): 21-25. doi: 10.19749/j.cn.cjgd.1672-2973.2020.01.006. |
[22] | Mussttaf RA, Jenkins DFL, Jha AN. Assessing the impact of low level laser therapy ( LLLT ) on biological systems: a review. Int J Radiat Biol. (2019) ; 95: (2): 120-143. |
[23] | Al-Maweri SA, Javed F, Kalakonda B, et al. Efficacy of low level laser therapy in treating burning mouth syndrome: A systematic review. Photodiagnosis Photodyn Ther. (2017) ; 17: : 188-193. |
[24] | Spanemberg JC, Figueiredo MA, Cherubini K, et al. Low-level Laser Therapy: A Review of Its Applications in the Management of Oral Mucosal Disorders. Altern Ther Health Med. (2016) ; 22: (6): 24-31. |
[25] | Zhou HY, Xiao JH, Hu JP, et al. Efficacy of alpha-lipoic acid combined with methylcobalamin in treating diabetic neuropathy and its influence on oxidative stress and hs-CRP. Chinese Journal of Hospital Pharmacy. (2012) ; 32: (11): 868-870. |
[26] | Meir J, Hartmann E, Eckstein MT, et al. Identification of Candida albicans regulatory genes governing mucosal infection. Cellular Microbiology. (2018) ; 20: (8): e12841. |
[27] | Dai XT, Lu SL. Research progress on treatment methods for burning mouth syndrome. Chinese Journal of Geriatric Oral Medicine. (2022) ; 20: (2): 118-123. doi: 10.19749/j.cn.cjgd.1672-2973.2022.02.012. |
[28] | Spangenberg JC, Segura-Egea JJ, Rodríguez-de Rivera-Campillo E, Jané-Salas E, Salum FG, López-López J. Low-level laser therapy in patients with Burning Mouth Syndrome: A double-blind, randomized, controlled clinical trial. J Clin Exp Dent. (2019) ; 11: (2): e162-9. doi: 10.4317/jced.55517. |
[29] | Ma L. Efficacy study of low-level laser therapy for postmenopausal women with burning mouth syndrome. [dissertation]. Xinjiang Medical University; (2023) . |
[30] | Spanemberg JC, López JL, de Figueiredo MA, Cherubini K, Salum FG. sEfficacy of low-level laser therapy for treating burning mouth syndrome: a randomized, controlled trial. J Biomed Opt. (2015) ; 20: (9): 098001. doi: 10.1117/1.JBO. 20.9.098001: . |
[31] | Škrinjar I, Lončar Brzak B, Vidranski V, Vučićević Boras V, Rogulj AA, Pavelić B. Salivary Cortisol Levels and Burning Symptoms in Patients with Burning Mouth Syndrome before and after Low-Level Laser Therapy: a Double-Blind Controlled Randomized Clinical Trial. Acta Stomatol Croat. (2020) ; 54: (1): 44-50. doi: 10.15644/asc54/1/5. PMID: 32523156; PMCID: PMC7233125. |




