Regulatory role of BNP in the spinal center of rats with nonbacterial prostatitis
Abstract
BACKGROUND:
A growing body of evidence has shown that activating spinal cord glial cells (typically astrocytes and microglial cells) is closely related to hyperpathia and persistent pain.
OBJECTIVE:
To investigate the expression of GFAP and CR3/CD11b in cornu dorsale medullae spinalis of rats with nonbacterial prostatitis, to explore the therapeutic efficacy and action mechanism of intrathecal injection of BNP alleviating chronic neuropathic pain.
METHODS:
Eighteen male SPF SD rats were randomly divided into sham operation control group, nonbacterial prostatitis group (NBP) and intrathecal injection BNP group, the NBP model was established by intraprostatic injection of CFA, and the spinal cord of L6-S1 segment was extracted seven days after intrathecal injection of BNP; The expression of GFAP and CR3/CD11b in dorsal horn of spinal cord were detected by immunofluorescence and Western blot.
RESULTS:
The cumulative optical density values of GFAP and CR3/CD11b immunofluorescence assay in the NBP group were higher than those in the sham operation group, with statistical significance (
CONCLUSION:
Intrathecal injection of BNP can down-regulate the expressions of GFAP and CR3/CD11b in L6-S1 spinal cord of NBP rat model and to further inhibit chronic pain caused by NBP.
1.Introduction
The traditional view holds that glial cells only have neurotrophic and neuroprotective effects, but do not participate in neural intracellular signal transduction. As the research deepens, a growing body of evidence has shown that activating spinal cord glial cells (typically astrocytes and microglial cells) is closely related to hyperpathia and persistent pain [1, 2, 3]. Neuroglial cells not only exert neuroprotective and neurotrophic effects on the central nervous system, but also offer a site for metabolism of the excitatory neurotransmitters. Furthermore, the activated glial cells can release excitatory neurotransmitters (glutamic acid and aspartic acid), contributing to pathological pain [4]. Our preliminary experiments and several other studies have confirmed an enhanced activation of the glial cells in the dorsal horn of the spinal cord in rats with nonbacterial prostatitis (NBP) [5]. Once activated, glial cells release various cytokines (e.g., TNF-a) and glutamic acid to maintain the intracellular pain signaling and promote generalized pain, thereby participating in the transduction and modulation of pain [6, 7]. Thus, the expression of biomarkers for the activation of glial cells can be measured indirectly to reflect pain severity.
Prostate pain may be accompanied by abnormalities in the conductive pathway and neuro-modulatory mechanisms and are related to the secondary lesions in the L6-S1 spinal cord innervating the prostate [8, 9]. Brain natriuretic peptide (BNP), a member of the natriuretic peptide family, can activate the natriuretic peptide receptor A (NPR-A). BNP is mainly expressed in the ventricle, inhibits ventricular fibrosis, and can be used as an important biomarker for the diagnosis and prognosis of heart failure [10]. According to the latest findings, BNP and NPR-A are involved in the regulation of inflammatory pain [14]. In the present study, BNP was injected into the L6-S1 spinal cord segment of rat. The induced changes in the expressions of glial fibrillary acidic protein (GFAP) in astrocytes in the dorsal horn of the spinal cord and the expression of complement receptor 3 (CR3/CD11B) in microglial cells were measured. Our results can assist to validate the therapeutic effect of BNP in NBP and provide an experimental basis NBP treatment using BNP.
2.Materials and methods
2.1Laboratory animals
Eighteen healthy male SD rats of SPF grade of age four weeks, with an average weight
of (250
2.2Reagents and equipment
Complete Freund’s adjuvant (CFA, Sigma, USA), BNP (Sigma, USA), Mouse Monoclonal Anti-GAPDH (TA-08, Beijing Zhongshan Golden Bridge Biotechnology, 1/2000), Rabbit Polyclonal Anti- GFAP (ab33922, Abcam, 1/2000), Rabbit Polyclonal Anti- CR3 (ab32512, Abcam, 1/500), HRP-labeled goat anti- mouse IgG (H+L, ZB-2305, Beijing Zhongshan Golden Bridge Biotechnology), HRP-labeled goat anti-rabbit IgG (H+L, ZB-2301, Beijing Zhongshan Golden Bridge Biotechnology), RIPA cell lysate (C1053, Beijing Pulilai Gene Technology Co., Ltd.), PVDF membrane (IPVH00010, Millipore), Supersensitive luminescent liquid (RJ239676, Thermo), Fluorescence microscope (CKX53, OLYMPUS), Protein vertical electrophoresis apparatus (DYY-6C, Beijing Liuyi Instrument Factory), Ultra-high sensitivity chemiluminescence Imaging system (Chemi DocTM XRS+, BOLE Life Medical Products (Shanghai) Co., Ltd.), and intelligent artificial climate incubator (ingbo Jiangnan Instrument Factory) were employed for the study.
3.Experimental methods
3.1Laboratory animals
Eighteen male SD rats of SPF grade were first acclimatized for one week. Six rats were randomly selected to form sham operation group. The remaining twelve rats received an intraprostatic injection of CFA to develop the NBP model. Six rats were included in the NBP model group. The other six rats were included in the NBP model
3.2Construction of the animal model of NBP
All the rats did not eat for 8 hours and did not drink for 4 hours before surgery. The rats were anesthetized by subcutaneous injection of 10% chloral hydrate at 0.3 ml/100 g. After effective anesthesia, the rats were immobilized to the operating table in a supine position. The rats were dehaired using sodium sulfide, and the skin was prepared. The abdominal skin was disinfected with iodophor, and a sterile draping towel was placed. A longitudinal incision (about 1 cm) was made in the middle of the abdomen under sterile conditions. The bladder was located after entering the abdominal cavity and lift gently with a cotton swab. In this way, the prostate was fully exposed, and the ventral lobe of the prostate was pulled out using ophthalmic bent forceps. In the model group, 0.1 ml of CFA was injected via the ventral lobe of the prostate. In the sham operation group, 0.1 ml of normal saline was injected via the ventral lobe of the prostate. The prostate and bladder were returned to the abdominal cavity. The incision was closed layer by layer using absorbable sutures, and the skin was disinfected again. The rats were then placed in the intelligent artificial climate incubator, followed by intramuscular injection of penicillin sodium at 25 thousand units/100 g and the addition of antibiotics in water to prevent infections. The rats were allowed to drink water at eight hours post-operative and to eat at twenty four 24 hours post-operative. The breeding temperature was 20–25∘C, and the relative humidity was 50%–60%.
3.3Intrathecal injection
The procedures of intrathecal injection were modified based on those described in the literature (J Pharmacol Toxicol Methods. 1994, 32(4): 197–200). The intrathecal injection was administered in the following steps: The rats were placed in an anesthesia chamber and anesthetized by inhaling isoflurane. After effective anesthesia was established, the rats were immobilized in a prone position with an elevated lower abdomen. A blunt projection was located with the left hand in the lower spine on the left side of the rat, and its horizontal position corresponded to L6. The left thumb and the middle finger were positioned on each side of the L6-S1 disc spaces, respectively, tightening the skin outwards. The left index finger located the disc space where the puncture would be performed, while the right hand held a microsyringe that was vertically and slowly inserted through the disc space. A sensation of hollowness was felt when the needle was inserted into the subarachnoid space. When the needle was removed, a small amount of cerebrospinal fluid was visible in the syringe. The puncture was considered successful if the rat tail quivered or swung sideways. In that case, BNP was slowly injected, and the injection was completed within 10 s.
3.4Spinal cord extraction
The procedure of spinal cord extraction was modified based on that described in the literature (Brain Res Bull. 2005, 66(3): 259–267), and consisted of the following steps: Subcutaneous injection of atropine (0.04 mg/kg) was given, and 5 min later, the rats were anesthetized by subcutaneous injection of 10% chloral hydrate at 0.3 ml/100 g. After the anesthesia, the rats were immobilized on the small animal test bench in a prone position. The skin was prepared and disinfected, and a sterile surgical towel was placed. Subsequently, a blunt dissection was performed for the thoracolumbar spine with the spinous process and the transverse process. The resected spine was then placed in 100% O2-saturated DMEM. Under an operating microscope, the nerves connected to the spinal cord are carefully removed using precision corneal scissors and hairspring forceps, along with the connective tissue envelope, spinous process, and transverse process. The intervertebral spinal cord was exposed, carefully pulled up, and properly preserved. Six rats in each group were selected for immunofluorescence detection, and the remaining six rats were analyzed by Western Blot.
3.5Statistical analysis
Optical density values of the immunofluorescence images and the grayscale values of Western Blot images were measured using Image J (NIH, USA). Data was processed using SPSS 17.0 and expressed as mean standard deviation (X
4.Results
4.1HE staining of prostate tissues
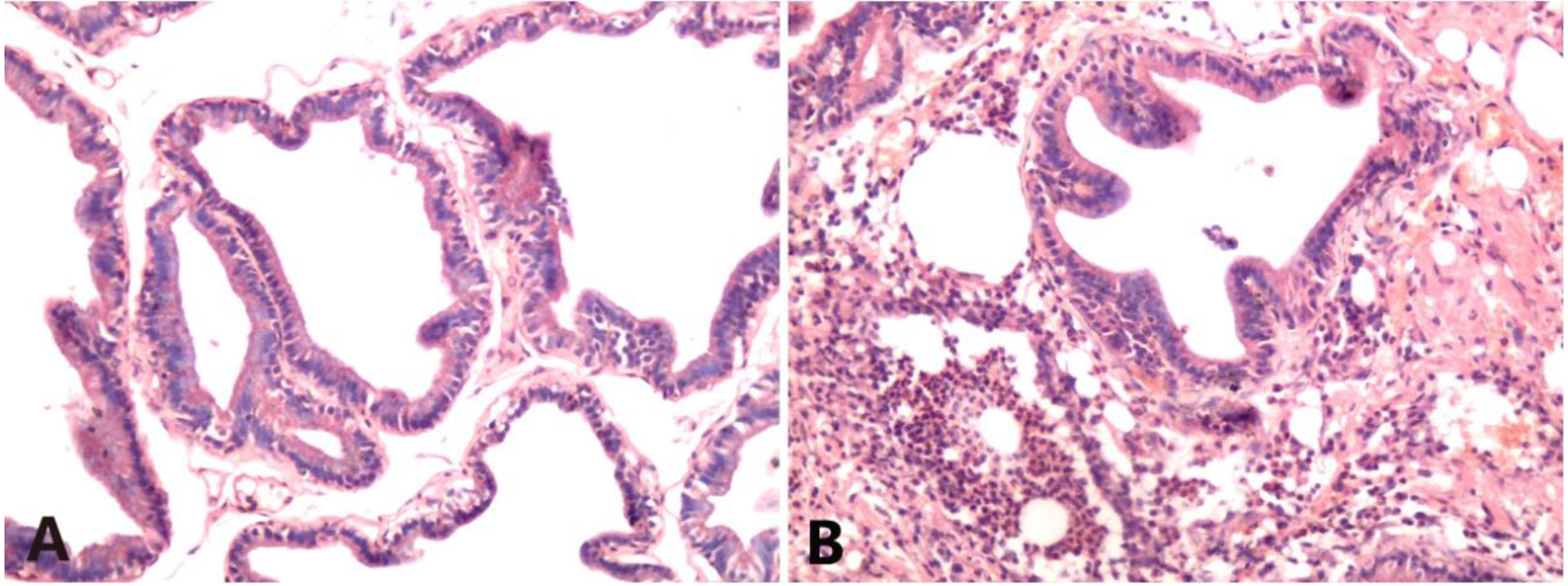
The sham operation group showed normal prostate structure, and the glandular cavity morphology was different and relatively regular. There was no interstitial dilation or edema, and no interstitial inflammatory cell infiltration was observed. In contrast, an intense inflammatory response was found in the NBP group. The glandular structure of the prostate was irregular. There was severe vasodilation and vasocongestion, and a blood vessel burst was observed. Severe perivascular neutrophilic and lymphocytic infiltration was also noted (Fig. 1).
Figure 1.
HE staining of rat prostate tissues (HE
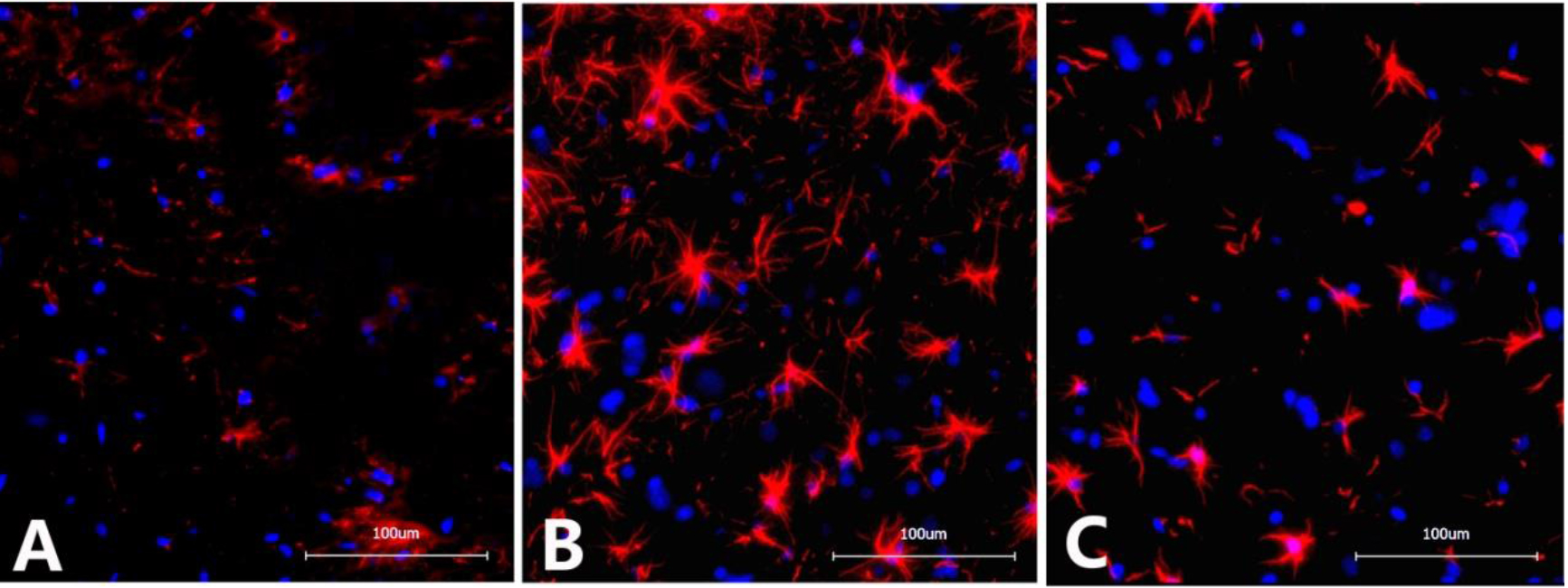
4.2Immunofluorescence detection of GFAP in the L6-S1 spinal cord
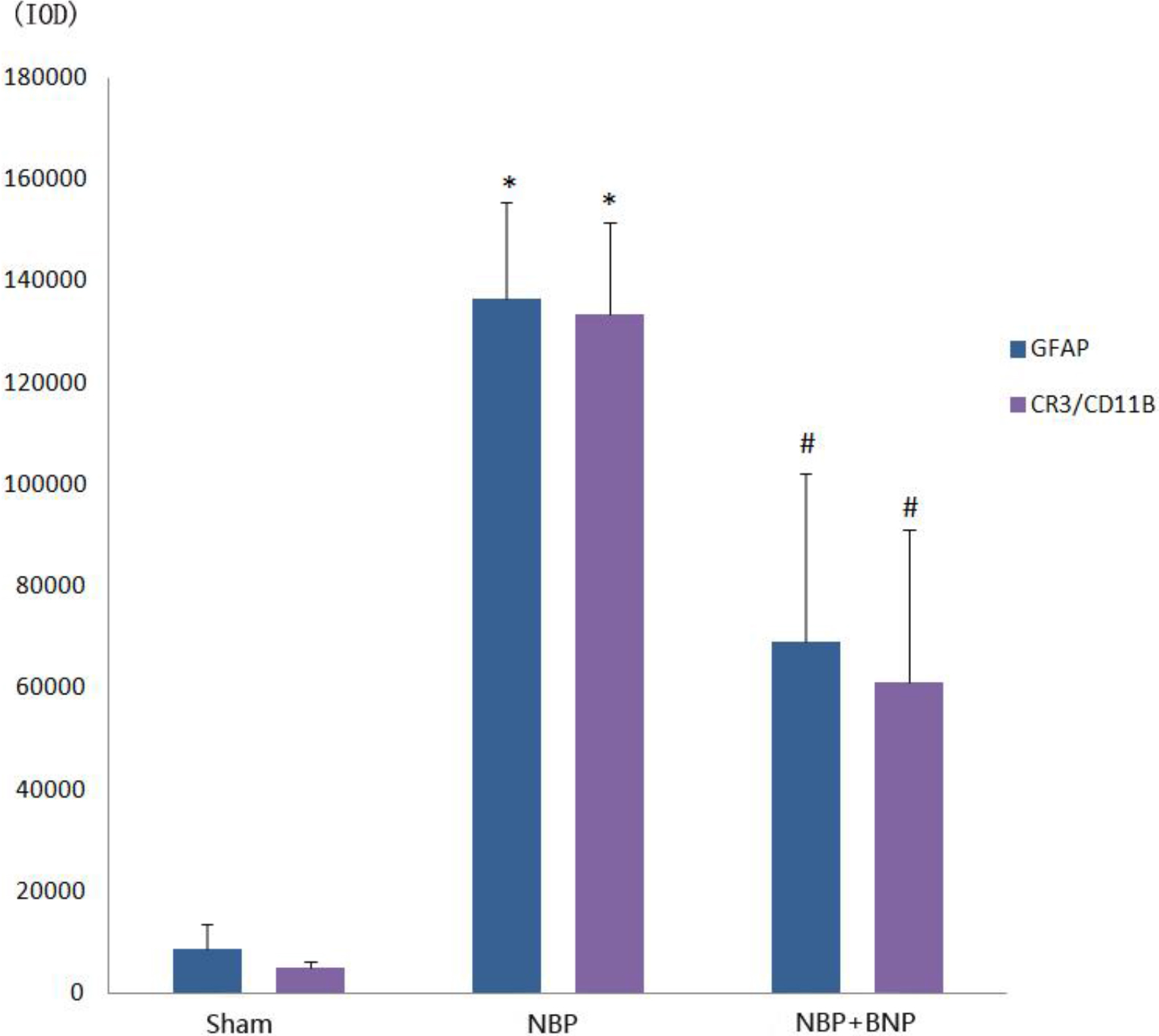
The optical density of GFAP in the spinal cord of rats from the NBP model group (136483
Figure 2.
Immunofluorescence images of GFAP in the dorsal horn of the L6-S1 spinal cord in different groups of rats. A. Sham operation group: Scattered expression of GFAP with small, inconspicuous cell bodies with slender protrusions and lightly stained chromosomes; B. NBP model group: Significantly increased GFAP expression with apparently enlarged cell bodies with thickened and elongated protrusions and darkly stained chromosomes; C. NBP
4.3Immunofluorescence detection of CR3/CD11B in the L6-S1 spinal cord
The optical density of CR3/CD11B in the spinal cord of rats from the NBP model group (133482
Figure 3.
Immunofluorescence images of CR3/CD11B in the dorsal horn of the L6-S1 spinal cord in different groups of rats. A. Sham operation group: Scattered expression of CR3/CD11B with small, inconspicuous cell bodies and lightly stained chromosomes; B. NBP model group: Significantly increased CR3/CD11B expression with apparently enlarged cell bodies and darkly stained chromosomes; C. NBP
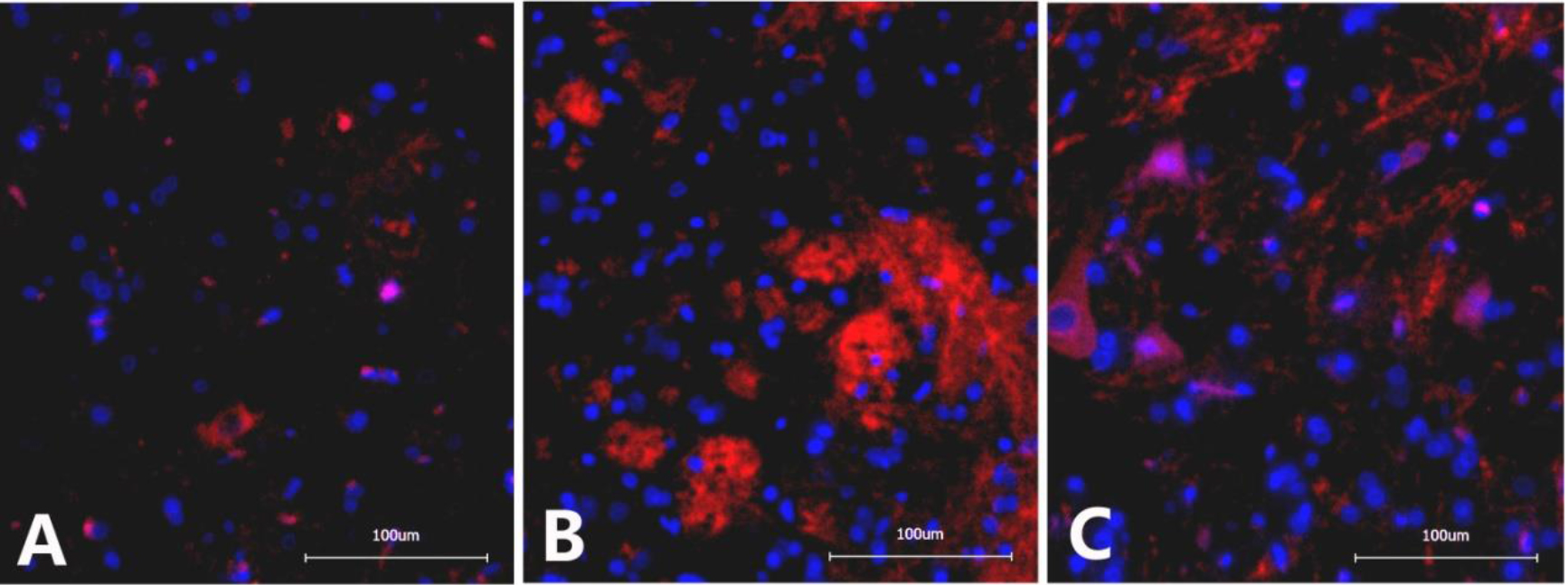
Figure 4.
Immunofluorescence IOD histogram of GFAP and CR3/CD11B in the dorsal horn of the L6-S1 spinal cord in different groups of rats.
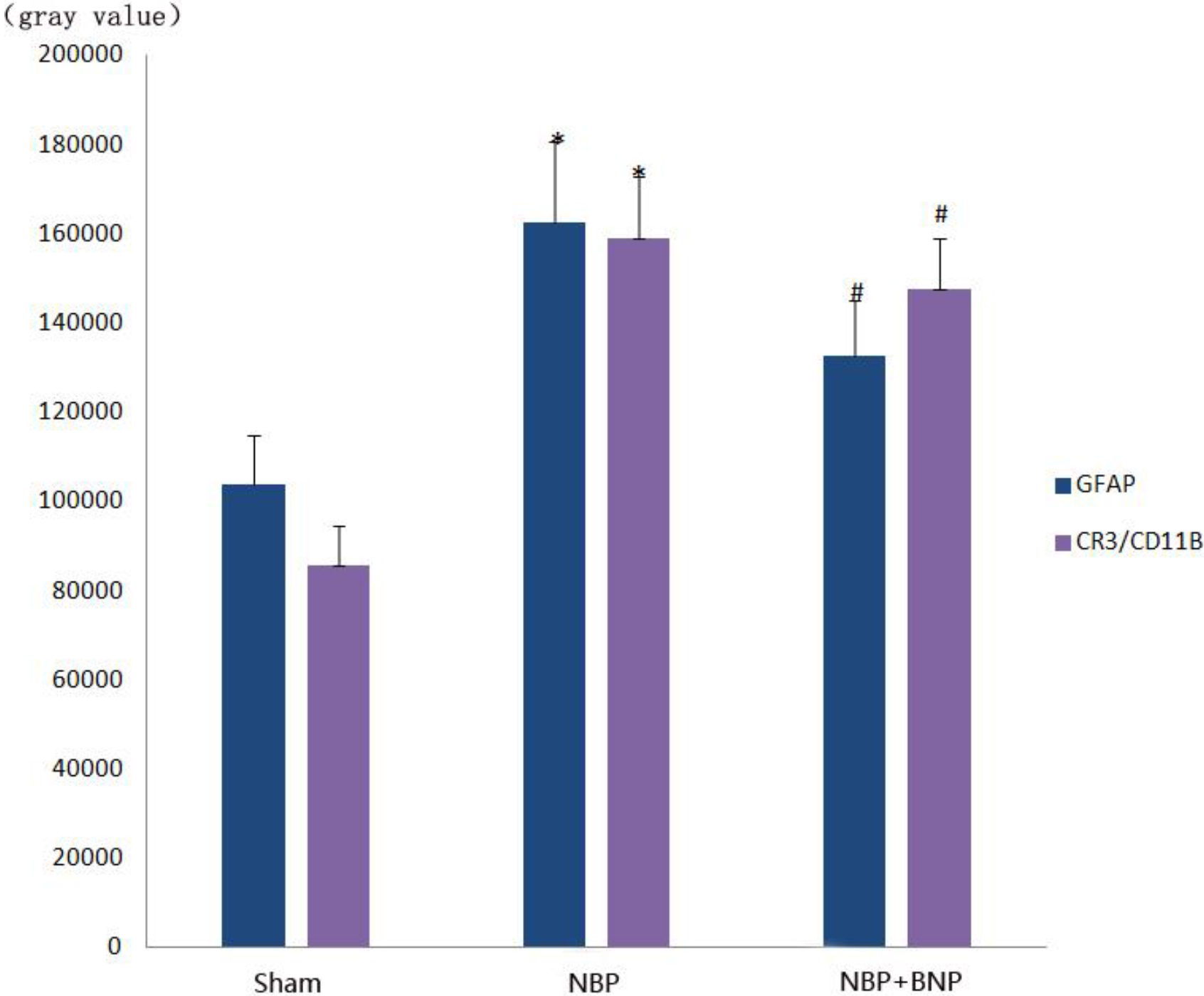
4.4Expression of GFAP and CR3/CD11B in the L6-S1 spinal cord using Western Blot analysis
The expression of CR3/CD11B, the marker of microglia and that of GFAP, and the marker of astrocytes were significantly increased in the NBP model group (
Figure 5.
Expression of GFAP and CR3/CD11B in the dorsal horn of the L6-S1 spinal cord in different groups of rats.
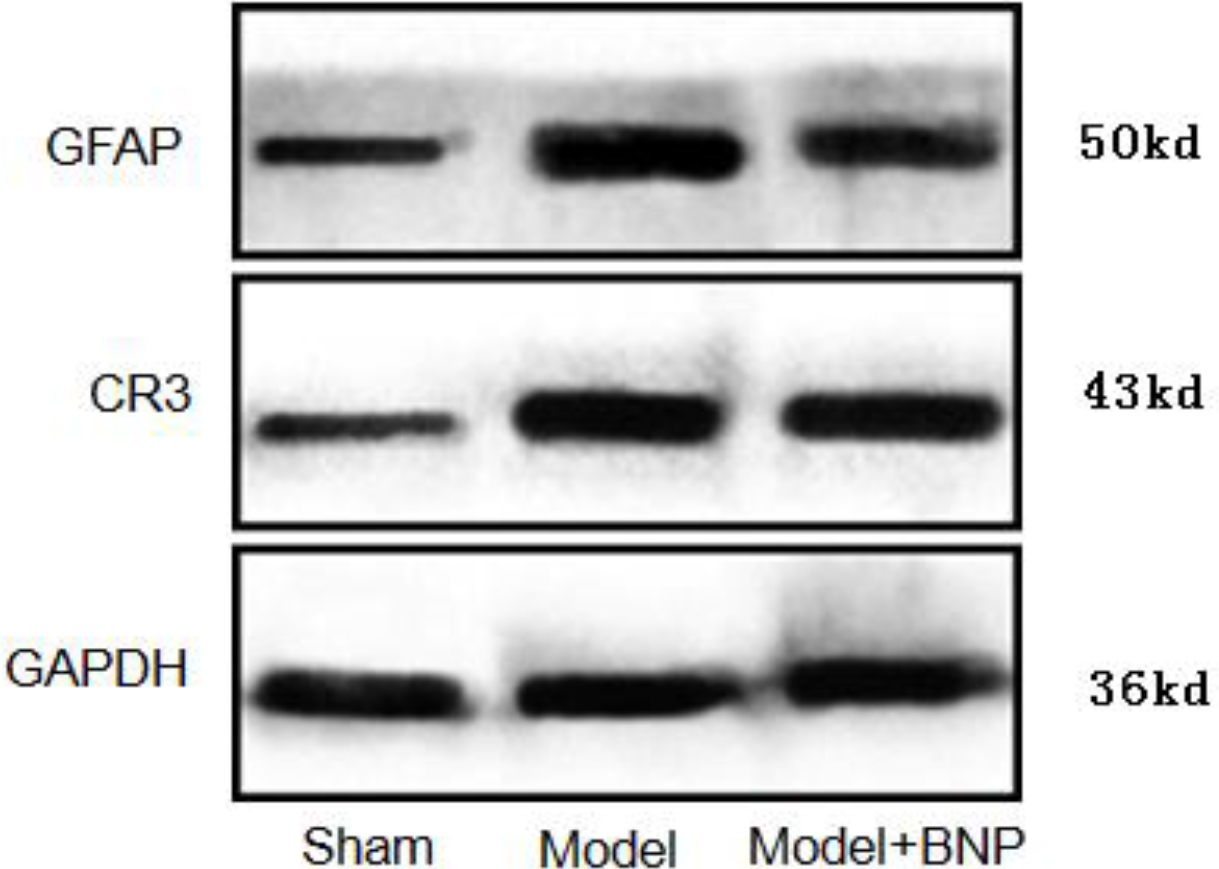
Figure 6.
Gray value histogram of GFAP and CR3/CD11B protein expression in the dorsal horn of the L6-S1 spinal cord in different groups of rats.
5.Discussion
Nonbacterial prostatitis (NBP) is a common urologic disease and the most important type of prostatitis. Pain is the main symptom of NBP and one of the important factors affecting patients’ quality of life [11]. At present, the pathogenesis of NBP remains largely unknown. It is believed that the pain associated with NBP is a neuropathic pain state resulting from the combined action of several factors [12], for example, the glial cell activation and changes in the excitability of dorsal root ganglia. Currently, the most commonly used analgesics for pain associated with NBP are non-steroidal anti-inflammatory drugs (NSAIDs) and opioids. Both have achieved good clinical efficacy, though their limitations in the mechanism of action cannot be ignored. Specifically, NSAIDs are known to cause adverse reactions to varying degrees, such as gastrointestinal reactions, gastric ulcers, gastric bleeding, and allergy [13], while opioids can be highly addictive. These defects have restricted the clinical use of either in treating NBP.
Brain natriuretic peptide (BNP) is a 32-amino acid polypeptide and an important diagnostic, therapeutic and prognostic marker for heart failure. When BNP binds to its receptor, NPR-A can elevate the cGMP level. Protein kinase G (PKG) is widely present in cells, mediating the signal transduction function of the second messenger cGMP. Recent studies have shown that the signaling pathway mediated by BNP/NPR-A plays an important role in inflammatory pain [14]. Nociceptive sensory neurons in dorsal root ganglia act as the first element in the nociceptive afferent pathway. Noxious stimulation can trigger the release of excitatory neurotransmitters, such as glutamic acid, from the afferent fibers of these neurons, which further transmit pain signals to the spinal cord and the brain. Intraspinal injection of BNP can dramatically inhibit such acute and chronic inflammatory pain. According to the above situation, BNP secreted by the nociceptive afferent fibers exerts an inhibitory effect on the excitatory synaptic transmission by activating NPR-A at the presynaptic terminals. Thus, activating the BNP/NPR-A signal transduction pathway in nociceptive sensory neurons can be a new analgesic strategy. Peripheral infections/inflammation can activate the brain-spinal cord pathway, leading to hyperpathia. The maintenance of this circulatory pathway, in turn, depends on the activation of astrocytes and microglial cells in the spinal cord. Some researchers believe that chronic pain associated with NBP may originate from a single disease, while several factors may be involved in its development into a chronic neuropathic pain state [15]. The latter is believed to implicate the action of glial cells in the spinal cord. Our study showed that CFA-induced NBP demonstrated an enhanced activation of astrocytes and microglial cells in the shallow and deep dorsal horns of the L6-S1 spinal cord. We confirmed that the activation of astrocytes and microglial cells was indeed involved in the NBP-associated pain. Intrathecal BNP injection weakened the activation of astrocytes and microglial cells in the L6-S1 spinal cord of rats with NBP, thereby inhibiting the NBP-induced pain.
6.Conclusion
The present study showed that CFA induced NBP in SD rats, which resulted in the enhanced expression of GFAP and CR3/CD11B in the L6-S1 spinal cord and caused inflammatory pain. The severity of pain caused by NBP was measured based on the expression levels of GFAP and CR3/CD11B. Intrathecal BNP injection downregulated the GFAP and CR3/CD11B expression in the L6-S1 region of the spinal cord of NBP rats. If the BNP/NPR-A mediated signaling pathway is activated, it can reduce the activation of spinal microglia in NBP rats and inhibit pain. Thus, it was inferred that BNP may act as an intrinsic pain reliever in neural inflammatory pain.
Acknowledgments
This study was supported by the Natural Science Foundation of Jiangxi (grant no. 20202BABL206023) and the Commission science and technology plan project of Jiangxi Provincial Health (grant no. 202410186).
Conflict of interest
None to report.
References
[1] | Bethea JR, Fischer R. Role of Peripheral Immune Cells for Development and Recovery of Chronic Pain. Front Immunol. (2021) ; 12: : 641588. |
[2] | Li DY, Gao SJ, Sun J, Zhang LQ, Wu JY, Song FH, Liu DQ, Zhou YQ, Mei W. Targeting the nitric oxide/CGMP signaling pathway to treat chronic pain. Neural Regen Res. (2023) ; 18: (5): 996-1003. |
[3] | Fiore NT, Debs SR, Hayes JP, Duffy SS, Taylor GM. Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol. (2023) ; 19: (4): 199-220. |
[4] | Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. (2008) ; 322: (5907): 1551-5. |
[5] | Lao YF, Li ZW, Bai YN, Li WJ, Wang J, Wang YN, Li QC, Dong ZL. Glial Cells of the Central Nervous System: A Potential Target in Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Pain Research and Management. 2023: (2061632): 7-12. |
[6] | Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. (2010) ; 9: (8): 807-19. |
[7] | Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. (2010) ; 22: (5): 499-511. |
[8] | Ishigooka M, Nakada T, Hashimoto T, Iijima Y, Yaguchi H. Spinal substance P immunoreactivity is enhanced by acute chemical stimulation of the rat prostate. Urology. (2002) ; 59: (1): 139-44. |
[9] | Chen Y, Wu X, Liu J, Tang W, Zhao T, Zhang JH. Distribution of convergent afferents innervating bladder and prostate at dorsal root Ganglia in rats. Urology. (2010) ; 76: (3): 764e1-6.. |
[10] | Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, Burnett JC. Jr. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. (2006) ; 209: (3): 943-9. |
[11] | Zhao FL, Yue M, Yang H, Wang T, Wu JH, Li SC. Health-related quality of life in Chinese patients with chronic prostatitis/chronic pelvic pain syndrome. Qual Life Res. (2010) ; 19: (9): 1273-83. |
[12] | Schaeffer AJ. Etiology and management of chronic pelvic pain syndrome in men. Urology. (2004) ; 63: (3 Suppl 1): 75-84. |
[13] | Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. (2009) ; 106: (22): 9075-80. |
[14] | Yang XR, Chen Q, Ma M, Xie WJ, Gong BB, Huang YM, Li Y, Liu SM, Hu JP, Liang SD, Chen J, Liu F, Sun T. Expression and Regulation of Brain Natriuretic Peptide and Natriuretic Peptide Receptor A (NPR-A) in L6–S1 Dorsal Root Ganglia in a Rat Model of Chronic Nonbacterial Prostatitis. Med Sci Monit. (2019) ; 25: : 90427. |
[15] | Schaeffer AJ. Etiology and management of chronic pelvic pain syndrome in men. Urology. (2004) ; 63: (3 Suppl 1): 75. |




