Effect of rmEGF combined with ELF-EMF on promoting wound healing in rats
Abstract
BACKGROUND:
The process of wound healing is complex, and expediting it remains a challenge. The advantages of extremely low frequency electric and magnetic fields (ELF-EMF) are its non-invasive treatment, promotes healing and promotes myogenesis of C2C12 cells. Epidermal growth factor (EGF) is known to play a vital role in promoting wound healing, so a combination of ELF-EMF and EGF can have far-reaching significance.
OBJECTIVE:
To study the effect of recombinant murine epidermal growth factor (rmEGF) combined with ELF-EMF on wound healing.
METHODS:
Thirty-six rats were randomly divided into three groups: normal control group, EGF group, and ELF-EMF
RESULTS:
The wound healing rate of EGF
CONCLUSIONS:
rmEGF combined with ELF-EMF significantly promotes wound healing in SD rats.
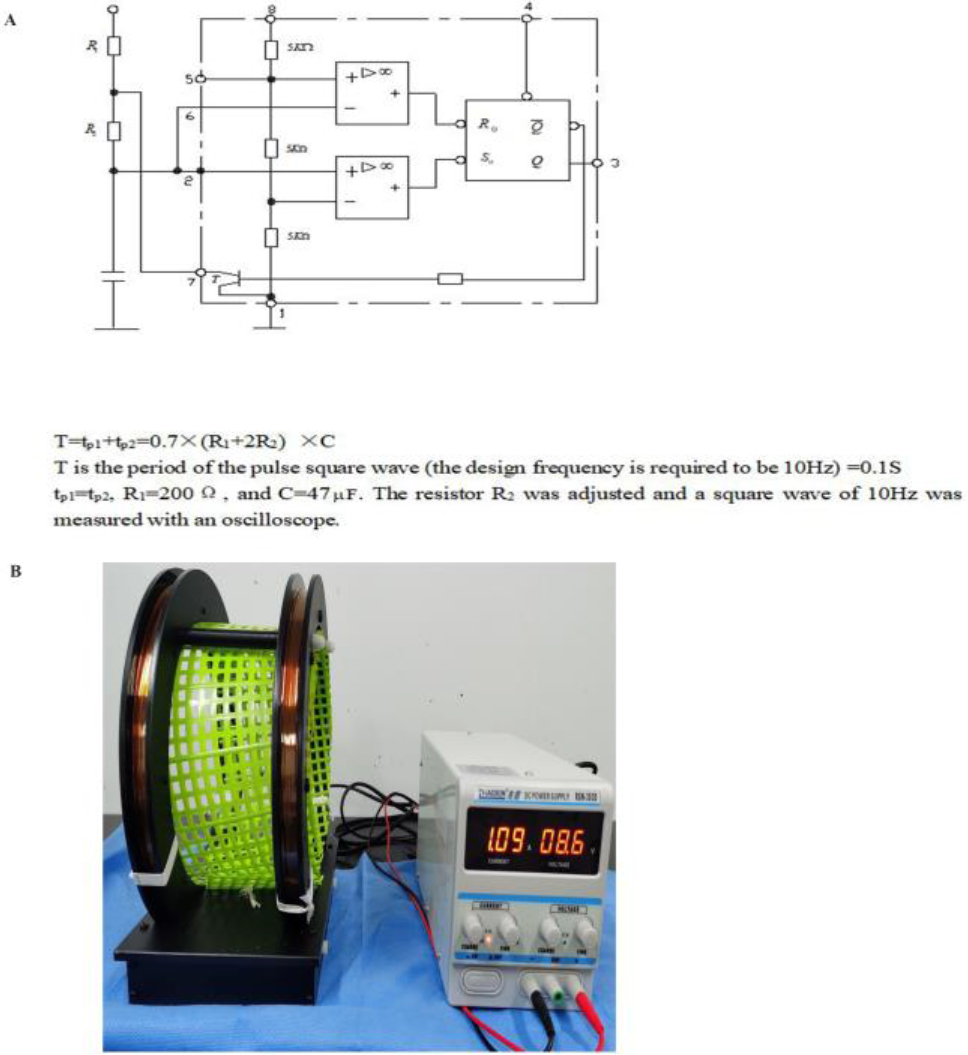
Figure 1.
Schematic representation of ELF-EMF equipment. A. Circuit diagram of ELF-EMF; B. ELF-EMF instrument with the intensity of 10 Hz/1.5 mT in operation.
1.Introduction
Due to a prolonged time in the healing of wounds, wound infection may get further aggravated, and osteomyelitis or sepsis may occur in severe cases [1, 2, 3, 4]. Therefore, the rate of wound healing remains an important concern. Currently, there are many treatment methods, but they all have their own problems and shortcomings; hence, there is an urgent need for effective adjuvant treatment methods [5]. Epidermal growth factor is known to promote wound healing but in a steady manner. Literature studies have shown that ELF-EMF can produce positive biological effects on the body and is a non-toxic and non-invasive method [6, 7, 8]. Therefore, this study envisaged the application of recombinant murine epidermal growth factor combined with ELF-EMF was used to treat the wound in SD rats. The wound healing was measured at different time points, and the morphological changes of each group were observed by HE staining, so as to study the effect of rmEGF combined with ELF-EMF on wound healing.
2.Material and methods
2.1ELF-EMF equipment
The ELF-EMF equipment used in the study is shown in Fig. 1. The frequency of the magnetic field was 10 Hz, and a Helmholtz coil was used to ensure the uniformity of the magnetic field strength in the coil.
2.2Animal model
Anesthesia was induced by injecting 10 ml/kg of avertin (1.25%) intraperitoneally. After successful anesthesia, the rats were shaved, disinfected, and placed with bedding. A dorsal wound 20 mm
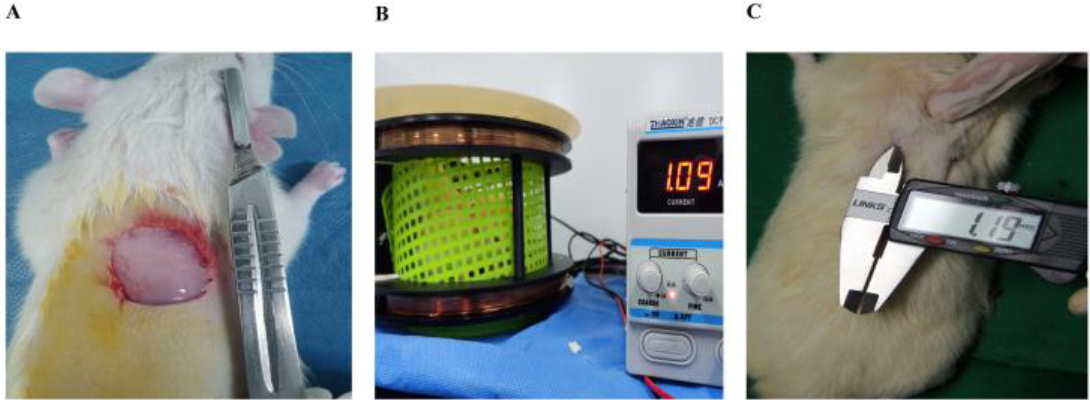
Figure 2.
Establishment of the animal model and the magnetic field intervention after grouping. A is wound establishment; B is SD rats exposed to 10 Hz/1.5 mT stimulation; C is measurement of wound healing.
2.3HE staining of wound tissue
The original wound on the back of the rats was harvested with a size of about 20 mm
2.4Statistical analysis
All experiments were repeated three or more times and analyzed by GraphPad Prism9.4.1(681) software. The data between multiple groups were tested for homogeneity of variance, and the data between groups were analyzed by one-way analysis of variance. If the homogeneity of variance test was not obtained, the rank-sum test was used to analyze the data between groups. *P< 0.05, ** P< 0.01 indicates that the difference was statistically significant.
3.Results
3.1Calculation of wound healing rate
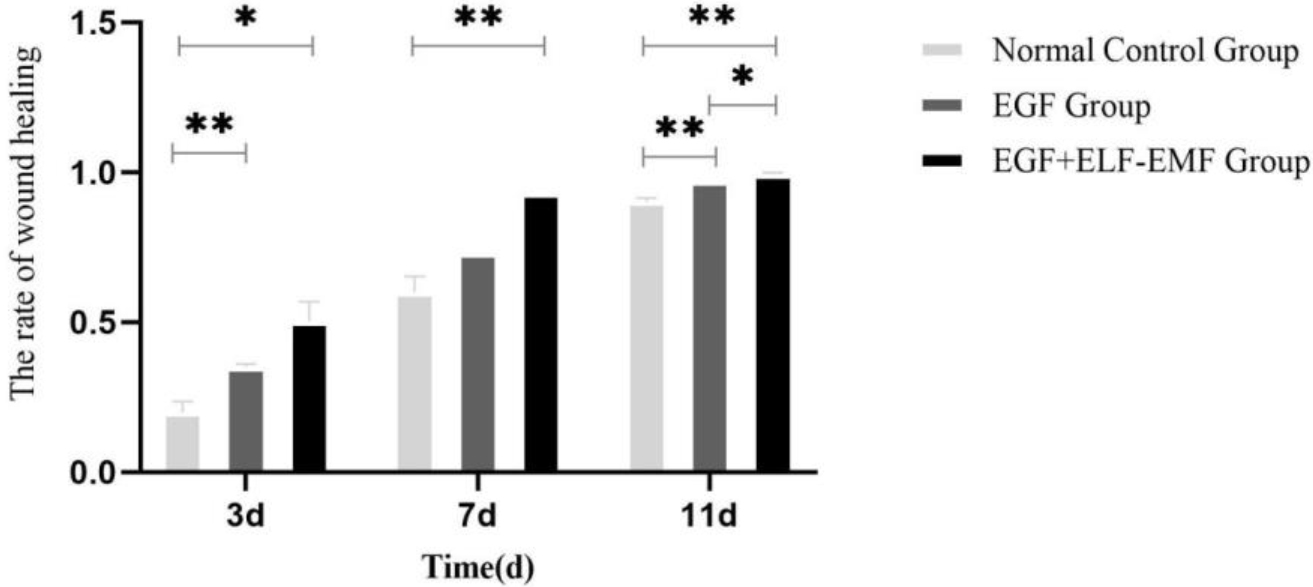
On the third day of wound healing, the average wound healing rate was 20% in normal control group, 35% in EGF alone group, and 50% in EGF combined with ELF-EMF group. On the 7th day of wound healing, the average wound healing rate of normal control group was 60%, the average wound healing rate of EGF alone group was 73%, and the average wound healing rate of EGF
Figure 3.
Wound healing rate of different experimental groups.
3.2HE staining of wound tissue
On the third day of wound healing in the normal control group, an obvious inflammatory cell infiltration was noted with no evident endothelial cell proliferation. Further, the number of fibroblasts was less than other groups, and the arrangement was disordered (Fig. 4-3d-A). On the 3rd day after injury, there were proliferation of vascular endothelial cells, less capillary formation, more inflammatory cells, larger cell bodies of fibroblasts, and round and oval nuclei in granulation tissue in the EGF alone group, which were less than those in EGF combined with ELF-EMF group (Fig. 4-3d-B). At the same time, in the EGF combined with ELF-EMF group, the new granulation tissue showed proliferation of vascular endothelial cells, neovascularization with inflammatory cell infiltration, obvious proliferation of fibroblasts, and round or oval nuclei with deep staining (Fig. 4-3d-C).
Figure 4.
Wound healing at different time intervals in different groups. Group A is the normal control group, Group B is the EGF group, and Group C is ELF-EMF
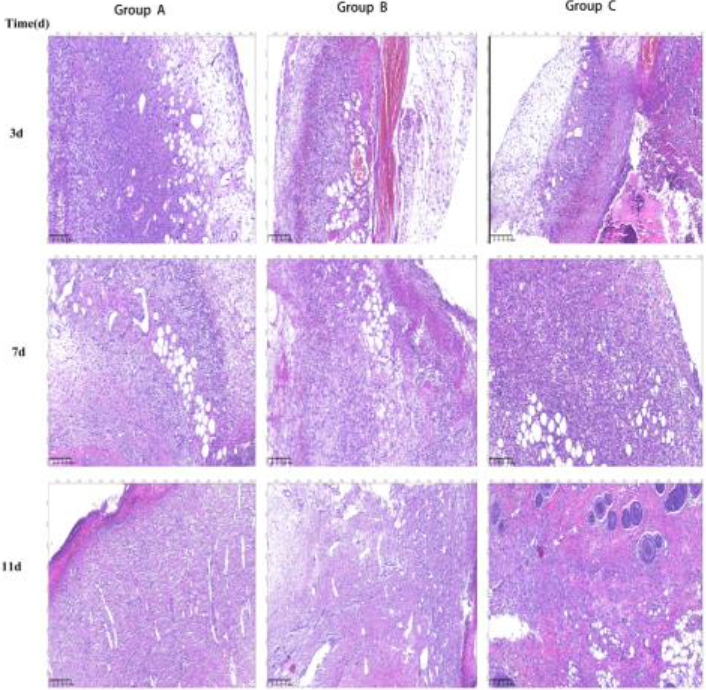
On the 7th day of wound healing, in the normal control group, there were a few capillaries formed in the granulation tissue, the cells were mainly inflammatory cells, the fibroblasts were more than before, but less than other groups, and the capillaries were rare (Fig. 4-7d-A). In EGF alone group, there were more fibroblasts with darker color, active angiogenesis, and fewer inflammatory cells on the 7th day after injury (Fig. 4-7d-B). In the EGF combined with ELF-EMF group, a reduced inflammatory cell infiltration, a large number of fibroblasts, dark staining, and more capillary components were noted (Fig. 4-7d-C).
On the 11th day of wound healing, in the normal control group, the inflammatory cells were gradually reduced, the fibroblasts were significantly increased and arranged in a vortex disorderly shape (Fig. 4-11d-A). In the EGF alone group, the number of capillaries was reduced, the number of mature fibroblasts was increased, cells were arranged in bundles, and the arrangement between bundles was disordered (Fig. 4-11d-B). In the EGF combined with ELF-EMF group, the number of new capillaries and inflammatory cells was reduced, the number of mature fibroblasts was increased, the cells were arranged in bundles, and the red matrix components were uniform (Fig. 4-11d-C).
3.3Observation of wound healing in each group
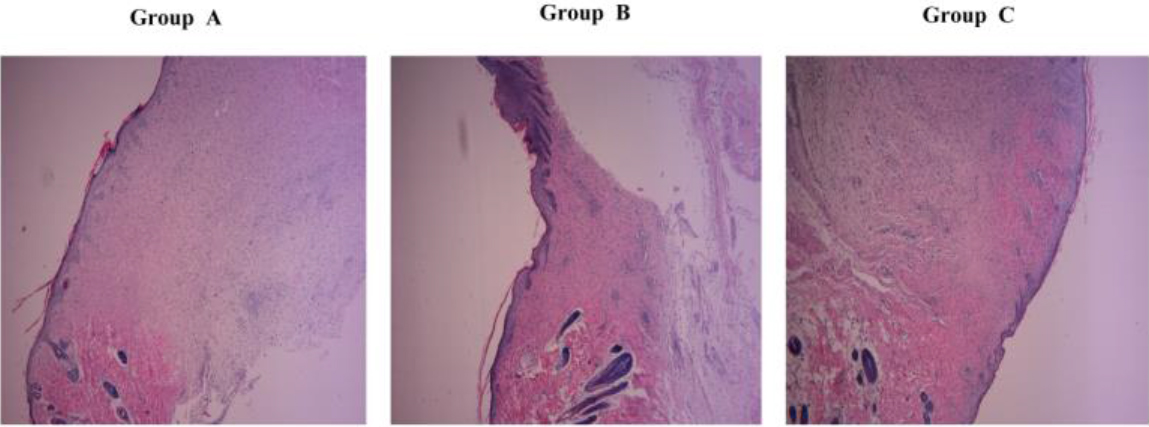
After all the wounds healed (15th day), a large number of fibroblasts were observed in the wound specimens of EGF combined with ELF-EMF group. The cell body of fibroblasts was large, the nuclei were regular, the fibers were arranged in an orderly manner, and the skin epidermis was thickened. The skin appendages were significantly better than the other groups (Fig. 5-A/B/C).
Figure 5.
Differences in HE staining of fibrous tissue within the healed wound on the 15th day.Group A is the normal control group, Group B is the EGF group, and Group C is ELF-EMF
4.Conclusion
This study was aimed to study the effect of recombinant murine epidermal growth factor (rmEGF) combined with ELF-EMF on wound healing in SD rats. The wound healing rate of EGF
5.Discussion
Wound healing is an extremely complex and orderly process [9]. EGFR is expressed in almost all kinds of cells, such as epidermal cells, fibroblasts, vascular endothelial cells, etc. [10]. EGF combines with EGFR to exert its biological activity, which can promote the formation of granulation tissue, proliferation of fibroblasts, and induce them to synthesize collagen fibers [11]. EGF acts on TGF-
There are exhaustive studies on the combination of recombinant epidermal growth factor with other drugs or treatment methods to accelerate wound healing; however, the synergistic effect was not significant. A large number of studies [18, 19, 20] have shown that extremely low frequency and low intensity electromagnetic fields can produce significant biological effects on organisms with little or no radiation, which lays the feasibility of this experimental study. Moreover, previous experiments have confirmed that 10 Hz/1.5 mT ELF-EMF has significant biological effects on proliferation and differentiation of C2C12 cells [21]. This is the reason for selecting the magnetic field parameter of 10 Hz/1.5 mT. Electromagnetic fields are known to regulate the inflammatory response of wounds and promote wound healing by regulating the activity of immune cells like neutrophils and macrophages [22]. It may also affect the wound healing process by up-regulating the expression of anti-inflammatory cytokines and down-regulating the expression of pro-inflammatory cytokines in the wound. In addition, the time of electromagnetic field exposure is also a hotly discussed issue. According to the literature [1, 2, 3], the irradiation time was different in different studies with different irradiation time, but the effect of 2-6 h irradiation was better, so 2 h was selected as the irradiation time in this experiment.
The combined application of rmEGF and ELF-EMF in wound healing has the advantages of a “non-thermal effect” of the ELF-EMF, along with fewer side effects on organisms due to the small radiation employed. At present, the mechanism of non-thermal effects is supported by the resonance theory, cyclotron resonance theory, etc. In this study, on the 7th day of wound healing, EGF combined with ELF-EMF group healed faster than other groups, and EGF combined with ELF-EMF group promoted the wound healing more rapidly. On the 11th day of wound healing, EGF combined with ELF-EMF group exhibited the best wound healing. The HE staining results also showed that the number of capillaries and fibroblasts in EGF combined with ELF-EMF group were better than those in other groups, and the cells were arranged in an orderly manner.
This study found that 10 Hz/1.5 mT combined with EGF could accelerate wound healing and exhibited a certain positive effect. However, the specific mechanism is not clear. It may possibly promote the migration and proliferation of keratinocytes and accelerate wound re-epithelialization, since electromagnetic fields are known to promote skin and soft tissue wound healing by promoting neovascularization and relaxation of local blood vessels [23, 24]. This needs to be confirmed by subsequent studies. In addition, the effects of adding a simple magnetic field group or adding different intensities of 10 Hz on wound healing need to be considered, which can make this experimental study more convincing and reflect the rationality of the experimental design.
Acknowledgments
This research was supported by the Heilongjiang Postdoctoral Fund of China (LBH-Z23256).
Conflict of interest
None of the authors have any conflicts of interest to report.
References
[1] | Fitzsimmons RJ, Strong DD, Mohan S, Baylink DJ. Low-amplitude, low-frequency electric field-stimulated bone cell proliferation may in part be mediated by increased IGF-II release. J Cell Physiol. (1992) Jan; refvol150(1): 84-9. doi: 101002/jcp.1041500112.. |
[2] | Matsumoto H, Ochi M, Abiko Y, Hirose Y, Kaku T, Sakaguchi K. Pulsed electromagnetic fields promote bone formation around dental implants inserted into the femur of rabbits. Clin Oral Implants Res. (2000) Aug; 11: (4): 354-60. doi: 10.1034/j.1600-0501.2000.011004354.x. |
[3] | Patruno A, Costantini E, Ferrone A, Pesce M, Diomede F, Trubiani O, Reale M. Short ELF-EMF Exposure Targets SIRT1/Nrf2/HO-1 Signaling in THP-1 Cells. Int J Mol Sci. (2020) Oct 2; 21: (19): 7284. doi: 10.3390/ijms21197284. |
[4] | Phillipson M, Kubes P. The Healing Power of Neutrophils. Trends Immunol. (2019) Jul; 40: (7): 635-647. doi: 10.1016/j.it.2019.05.001. |
[5] | Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. (2019) Jun; 146: : 209-239. doi: 10.1016/j. |
[6] | Henschenmacher B, Bitsch A, de Las Heras Gala T, Forman HJ, Fragoulis A, Ghezzi P, Kellner R, Koch W, Kuhne J, Sachno D, Schmid G, Tsaioun K, Verbeek J, Wright R. The effect of radiofrequency electromagnetic fields (RF-EMF) on biomarkers of oxidative stress in vivo and in vitro: A protocol for a systematic review. Environ Int. (2022) Jan; 158: : 106932. doi: 10.1016/j. |
[7] | Gholipour Hamedani B, Goliaei B, Shariatpanahi SP, Nezamtaheri M. An overview of the biological effects of extremely low frequency electromagnetic fields combined with ionizing radiation. Prog Biophys Mol Biol. (2022) Aug; 172: : 50-59. doi: 10.1016/j. |
[8] | Piacentini R, Ripoli C, Mezzogori D, Azzena GB, Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J Cell Physiol. (2008) Apr; 215: (1): 129-39. doi: 10.1002/jcp.21293. |
[9] | Freedman BR, Hwang C, Talbot S, Hibler B, Matoori S, Mooney DJ. Breakthrough treatments for accelerated wound healing. Sci Adv. (2023) May 19; 9: (20): eade7007. doi: 10.1126/sciadv.ade7007. |
[10] | Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. (2015) Aug; 173: (2): 370-8. doi: 10.1111/bjd.13954. |
[11] | Tanaka A, Nagate T, Matsuda H. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J Vet Med Sci. (2005) Sep; 67: (9): 909-13. doi: 10.1292/jvms.67.909. |
[12] | Park JS, Kim JY, Cho JY, Kang JS, Yu YH. Epidermal growth factor (EGF) antagonizes transforming growth factor (TGF)-beta1-induced collagen lattice contraction by human skin fibroblasts. Biol Pharm Bull. (2000) Dec; 23: (12): 1517-20. doi: 10.1248/bpb.23.1517. |
[13] | Matsumoto Y, Kuroyanagi Y. Development of a wound dressing composed of hyaluronic acid sponge containing arginine and epidermal growth factor. J Biomater Sci Polym Ed. (2010) ; 21: (6-7): 715-26. doi: 10.1163/156856209X435844. |
[14] | Wu W, Zeng N, Peng YY, Lu XH, Li CY, Wang ZC. The effects of recombinant human epithelialgrowth factor and protein-free calf blood extract for recovery of corneal mechanical epithelial defects healing and neovascularization. Eur Rev Med Pharmacol Sci. (2014) ; 18: (22): 3406-11. |
[15] | Khanbanha N, Atyabi F, Taheri A. Fatemeh. Hea1ing efficacy of an EGF impregnated triple gel based wound dressing: in vitro and in vivo studies. Biomed Res Int. (2014) ; 2014: : 4932-37. doi: 10.1155/2014/493732. |
[16] | Ogino S, Morimoto N, Sakamoto M, Jinno C, Taira T, Suzuki S. Efficacy of gelatin gel sheets sustaining epidermal growth factor for murine skin defects. J Surg Res. (2016) Apr; 201: (2): 446-54. doi: 10.1016/j.jss.2015.11.027. |
[17] | Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. (2014) 22: (3): 313-25. doi: 10.1111/wrr.12173. |
[18] | Baureus Koch CL, Sommarin M, Persson BR, et al. Interaction between weak low frequency magnetic fields and cell membranes. Bioelectromagnetics. (2003) ; 24: (6: 395-402. doi: 10.1002/bem.10136. |
[19] | Korpinar MA, Kalkan MT, Tuncel H. The 50 Hz (10 mT) sinusoidal magnetic field: effects on stress-related behavior of rats. Bratislava Medical Journal. (2012) ; 113: (9): 521-524. doi: 10.4149/bll_2012_117. |
[20] | Sunkari VG, Aranovitch B, Portwood N, et al. Effects of a low-intensity electromagnetic field on fibroblast migration and proliferation. Electromagn Biol. (2011) ; 30: : 80-85. doi: 10.3109/15368378.2011.566774. |
[21] | Han FJ, Yin S, Wu H, Zhou CL, Wang XT. Effect on Myoblast Differentiation by Extremely Low Frequency Pulsed Electromagnetic Fields. Journal of Mechanics in Medicine and Biology. (2022) ; 22: : 1-14. |
[22] | Pesce M, Patruno A, Speranza L, et al. Extremely low frequency electromagnetic field and wound healing: implication of cytokines as biological mediators. Eur Cytokine Netw. (2013) ; 24: (1): 1-10. doi: 10.1684/ecn.2013.0332. |
[23] | Barati M, Darvishi B, Javidi MA, Mohammadian A, Shariatpanahi SP, Eisavand MR, Madjid Ansari A. Cellular stress response to extremely low-frequency electromagnetic fields (ELF-EMF): An explanation for controversial effects of ELF-EMF on apoptosis. Cell Prolif. (2021) Dec; 54: (12): e13154. doi: 10.1111/cpr.13154. |
[24] | Patruno A, Costantini E, Ferrone A, Pesce M, Diomede F, Trubiani O, Reale M. Short ELF-EMF Exposure Targets SIRT1/Nrf2/HO-1 Signaling in THP-1 Cells. Int J Mol Sci. (2020) Oct 2; 21: (19): 7284. doi: 10.3390/ijms21197284. |




