Comparison of resting tremor at the upper limb joints between patients with Parkinson’s disease and scans without evidence of dopaminergic deficit
Abstract
BACKGROUND:
A representative symptom of Parkinson’s disease (PD) is resting tremor. The clinical manifestation of scans without evidence of dopaminergic deficit (SWEDD) is similar to it of PD, though the phenomenology of SWEDD is not well known.
OBJECTIVE:
In the present study, the resting tremor of 9 SWEDD patients was quantitatively compared with that of 11 PD patients.
METHODS:
Four 3-axis gyro sensors were attached on the index finger, thumb, dorsum of the hand, and arm of the more tremulous side. Root mean square (RMS) angular speed and angular displacement as well as irregularity of angular speed and displacement were derived from the sensor data.
RESULTS:
Although disease duration and Hoehn and Yahr stages were comparable, SWEDD patients exhibited different tremor features from PD patients. Significantly faster RMS angular speed and greater RMS angular displacement (
CONCLUSION:
These results indicate that quantitative indices obtained from resting tremor task could be important biomarkers for identifying potential patients with SWEDD among patients diagnosed with PD.
1.Introduction
Resting tremor is one of the most representative manifestation of Parkinson’s disease (PD) [1] and is observed in approximately 75% of the PD population [2]. Resting tumor is an involuntary and oscillatory movement most noticeable in fingers, hands, and arms, occurring mostly at rest [3]. The Unified Parkinson Disease Rating Scale (UPDRS) rates severity from 0 (normal) to 4 (severe) and is a popular assessment tool [4]. However, UPDRS is based on subjective rating and has poor intra-rater reliability due to diversity in clinical experience [5]. Thus, more objective and reliable systems for assessment of resting tremor at upper limb would be useful for accurate evaluation of response to clinical interventions.
Many related studies have proposed quantitative measurable technologies of tremor using IMU sensors such as gyro sensors [6, 7, 8, 9] and accelerometers [8, 9]. They have developed wearable system based on IMU sensors that can be attached on fingers, hands and forearms. Quantitative variables, such as averaged angular velocity, averaged angular displacement, mean or peak frequency for quantification of tremor in PD or essential tremor (ET) patients, were analyzed. In addition, the IMU sensor-based outcome measures showed good correlation with various tremor symptoms (e.g., resting tremor, postural tremor, kinetic tremor).
Recently, some of patients (4%–14.7%) diagnosed with PD exhibited normal dopamine transporter imaging and have been categorized as scans without evidence of dopaminergic deficit (SWEDD) [10]. The typical manifestation of patients with SWEDD is similar to that of PD patients [11]; however, the phenomenology of SWEDD is challenging to describe [12]. Furthermore, patients with did not respond to L-dopa [13] and showed minimal clinical progression [14] compared with PD patients. Although dopaminergic imaging can be used for diagnosis of SWEDDs, the method remains unavailable due to its economic inefficiency [15]. Therefore, understanding clinical characterizations of SWEDD and distinguishing SWEDD from PD patients is important to avoid inappropriate interventions.
In several studies, SWEDD patients showed differences in gait patterns, handwriting, and upper-limb bradykinesia compared with PD patients [11, 16, 17]. Specifically, PD patients had a slower gait speed, smaller stride length, reduced arm swing, greater impairment in handwriting, and greater impairment in forearm movement patterns (greater and more regular average speed and higher and more regular average amplitude) than SWEDD patients. However, few studies have tried to quantitatively and kinematically measures resting tremor at upper limb in SWEDD patients. A quantitative kinematic measurement of resting tremor may provide additional information that may help differentiate SWEDD from PD. Although a triaxial accelerometer was used to compare between PD and SWEDD patients [23], previous studies did not measure resting tremor considering various sensor locations as well as directions.
In the present study, resting tremor in SWEDD patients was compared with that in PD patients; the resting tremor of the fingers, dorsum of hands, and arms in PD and SWEDD patients were quantitatively measured using 3-axis gyro sensors.
2.Methods
2.1Subjects
This study included 9 patients with SWEDD and 11 patients with PD. Patients with normal brain MRI and normal dopamine transporter imaging among patients diagnosed with PD were defined as SWEDD [18]. An experienced nuclear medicine physician visually diagnosed SWEDD. Two neurologists recruited the 9 PD patients according to the United Kingdom Parkinson’s Disease Society Brain Bank (UKBBC) [19]. Patients with stroke or any neurological disease, possible sign of atypical parkinsonism, psychiatric disorders, or using medications affecting central dopaminergic pathways were excluded from this study. SWEDD patients usually have been shown to no substantial changes in disease progression despite no dopaminergic therapy [14]. Therefore, SWEDD patients were not treated with levodopa in present study.
Table 1
Clinical profiles of the patients
| Variables | PD ( | SWEDD ( | Statistical significance |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ( | |
| Age (years) | 60.1 (13.1) | 72.0 (7.8) | 0.03 |
| Height (cm) | 159.8 (10.3) | 154.8 (9.4) | 0.27 |
| Weight (kg) | 65.6 (11.0) | 59.4 (9.7) | 0.21 |
| Disease duration (years) | 3.4 (2.5) | 3.0 (3.7) | 0.77 |
| Hoehn and Yahr stage | 1.9 (0.6) | 2.1 (0.4) | 0.39 |
| UPDRS total score | 30.7 (10.4) | 26.9 (12.7) | 0.48 |
PD, Parkinson’s disease; SWEDD, scans without evidence of dopaminergic deficit; SD, standard deviation; UPDRS, unified Parkinson’s disease rating score.
Table 1 shows subject information on age, height, weight, disease duration, Hoehn and Yahr (HY) stage, and UPDRS total score. All clinical profiles except age were not significantly different between two patient groups. Patients with early disease including HY stages 1, 2, and 2.5 (SWEDD: 2.1
2.2Experiments and analysis
A wearable measurement system based on 3-axis gyro sensors (L3G4200D, STMicroelectronics, Aschheim city, Germany) was used in this study. The reliability of this system has been proven for postural tremor [7] and action tremor [6] in patients with ET. Specifically, angular velocity was quantitatively measured postural tremor in patients with ET and averaged speed at the dorsum of hand showed strong correlation with the clinical evaluation (
Figure 1.
Quantitative measurement of resting tremor using 3-axis gyro sensors.
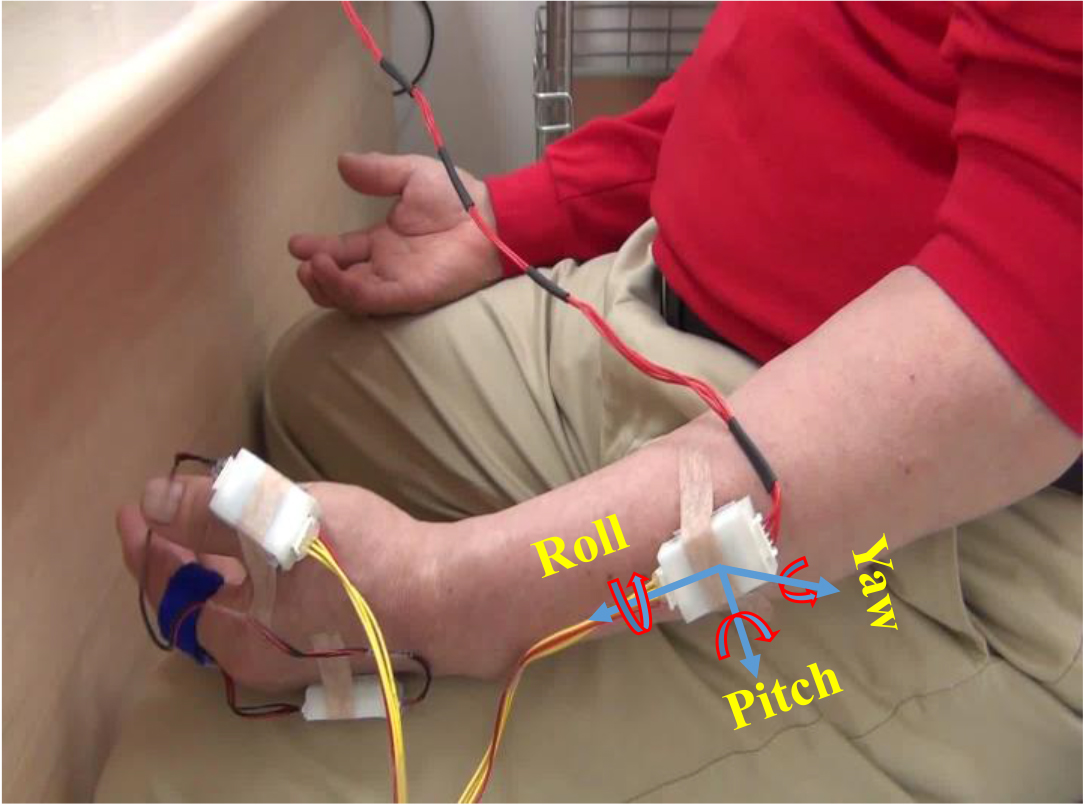
As shown in Fig. 1, the sensors were attached on the index finger, thumb, dorsum of the hand, and arm of the more tremulous side. All patients were instructed to maintain a resting position while seated in a chair, with hands relaxed and forearms placed on thighs. The sensor data were recorded for 15 s with a 100 Hz sampling frequency using a microprocessor (MSP430F5522). Measurement software was developed using LabVIEW for wireless communication with the microprocessor. After sufficient explanation to ensure that each participant was familiar with the procedure, the resting tremor task was performed 3 times. Reportedly, the frequency of resting tremor in PD mainly ranges between 4 and 7 Hz [20]. Therefore, a digital filter with a passband of 0.65–12.5 Hz was used to remove low-frequency drift and high-frequency noise [26].
RMS angular speed and angular displacement were used to represent the mean speed and mean amplitude of the involuntary and oscillatory movement caused by resting tremor, respectively [6, 7]. Coefficient of variation (CV) was used to represent the irregularity of the repetitive oscillating tremor patterns [17, 21]. In addition, peak frequency and peak power were calculated from the power spectrum of angular velocity signals.
Independent
Table 2
Comparison of time domain analysis indices between PD and SWEDD patients
| Analysis indices | Sensor position | Direction | PD Mean (SD) | SWEDD Mean (SD) | Significance | ||
|---|---|---|---|---|---|---|---|
| RMS angular speed (deg/sec) | Index finger | Roll | 14.1 | (18.2) | 2.6 | (4.1) | 0.067 |
| Pitch | 17.1 | (18.8) | 1.7 | (1.2) | 0.021 | ||
| Yaw | 13.3 | (19.5) | 2.5 | (3.8) | 0.102 | ||
| Dorsum of hand | Roll | 13.4 | (19.0) | 2.8 | (4.4) | 0.098 | |
| Pitch | 6.7 | (8.1) | 0.7 | (0.5) | 0.033 | ||
| Yaw | 6.7 | (10.5) | 1.0 | (1.3) | 0.102 | ||
| Thumb | Roll | 18.9 | (27.7) | 2.5 | (4.1) | 0.080 | |
| Pitch | 12.7 | (15.6) | 1.4 | (1.4) | 0.038 | ||
| Yaw | 12.4 | (17.9) | 1.8 | (3.0) | 0.082 | ||
| Arm | Roll | 1.9 | (2.9) | 0.3 | (0.6) | 0.072 | |
| Pitch | 1.4 | (1.7) | 0.2 | (0.3) | 0.029 | ||
| Yaw | 1.4 | (1.9) | 0.2 | (0.3) | 0.052 | ||
| RMS angular displacement (deg) | Index finger | Roll | 1.67 | (2.60) | 0.40 | (0.72) | 0.174 |
| Pitch | 2.27 | (2.83) | 0.21 | (0.16) | 0.037 | ||
| Yaw | 1.20 | (1.65) | 0.32 | (0.57) | 0.146 | ||
| Dorsum of hand | Roll | 1.35 | (2.22) | 0.40 | (0.75) | 0.237 | |
| Pitch | 1.13 | (1.48) | 0.10 | (0.07) | 0.044 | ||
| Yaw | 0.86 | (1.47) | 0.14 | (0.17) | 0.133 | ||
| Thumb | Roll | 1.89 | (2.86) | 0.31 | (0.56) | 0.102 | |
| Pitch | 1.41 | (1.68) | 0.24 | (0.31) | 0.046 | ||
| Yaw | 1.35 | (1.95) | 0.17 | (0.24) | 0.075 | ||
| Arm | Roll | 1.89 | (2.86) | 0.32 | (0.58) | 0.103 | |
| Pitch | 1.40 | (1.66) | 0.24 | (0.31) | 0.045 | ||
| Yaw | 1.35 | (1.94) | 0.17 | (0.25) | 0.074 | ||
| CV of angular speed | Index finger | Roll | 0.37 | (0.16) | 0.48 | (0.29) | 0.307 |
| Pitch | 0.38 | (0.18) | 0.59 | (0.39) | 0.120 | ||
| Yaw | 0.35 | (0.15) | 0.49 | (0.39) | 0.279 | ||
| Dorsum of hand | Roll | 0.39 | (0.17) | 0.43 | (0.17) | 0.599 | |
| Pitch | 0.36 | (0.17) | 0.38 | (0.14) | 0.855 | ||
| Yaw | 0.36 | (0.15) | 0.38 | (0.16) | 0.724 | ||
| Thumb | Roll | 0.37 | (0.17) | 0.43 | (0.13) | 0.378 | |
| Pitch | 0.41 | (0.14) | 0.48 | (0.20) | 0.346 | ||
| Yaw | 0.34 | (0.17) | 0.36 | (0.18) | 0.817 | ||
| Arm | Roll | 0.38 | (0.15) | 0.34 | (0.11) | 0.542 | |
| Pitch | 0.35 | (0.14) | 0.35 | (0.12) | 0.989 | ||
| Yaw | 0.35 | (0.10) | 0.33 | (0.14) | 0.721 | ||
| CV of angular displacement | Index finger | Roll | 0.18 | (0.18) | 0.31 | (0.29) | 0.233 |
| Pitch | 0.17 | (0.18) | 0.39 | (0.42) | 0.134 | ||
| Yaw | 0.17 | (0.12) | 0.33 | (0.42) | 0.245 | ||
| Dorsum of hand | Roll | 0.17 | (0.14) | 0.24 | (0.15) | 0.331 | |
| Pitch | 0.10 | (0.06) | 0.26 | (0.16) | 0.018 | ||
| Yaw | 0.15 | (0.10) | 0.20 | (0.15) | 0.388 | ||
| Thumb | Roll | 0.19 | (0.15) | 0.19 | (0.12) | 0.965 | |
| Pitch | 0.18 | (0.10) | 0.13 | (0.07) | 0.285 | ||
| Yaw | 0.14 | (0.10) | 0.20 | (0.09) | 0.195 | ||
| Arm | Roll | 0.19 | (0.15) | 0.19 | (0.12) | 0.960 | |
| Pitch | 0.18 | (0.11) | 0.14 | (0.07) | 0.357 | ||
| Yaw | 0.14 | (0.10) | 0.20 | (0.09) | 0.189 | ||
PD, Parkinson’s disease; SWEDD, scans without evidence of dopaminergic deficit; SD, standard deviation; RMS, root mean square, CV, coefficient of variation.
Figure 2.
Representative resting tremor signals of finger in the pitch direction in SWEDD (left) and PD (right) patients.
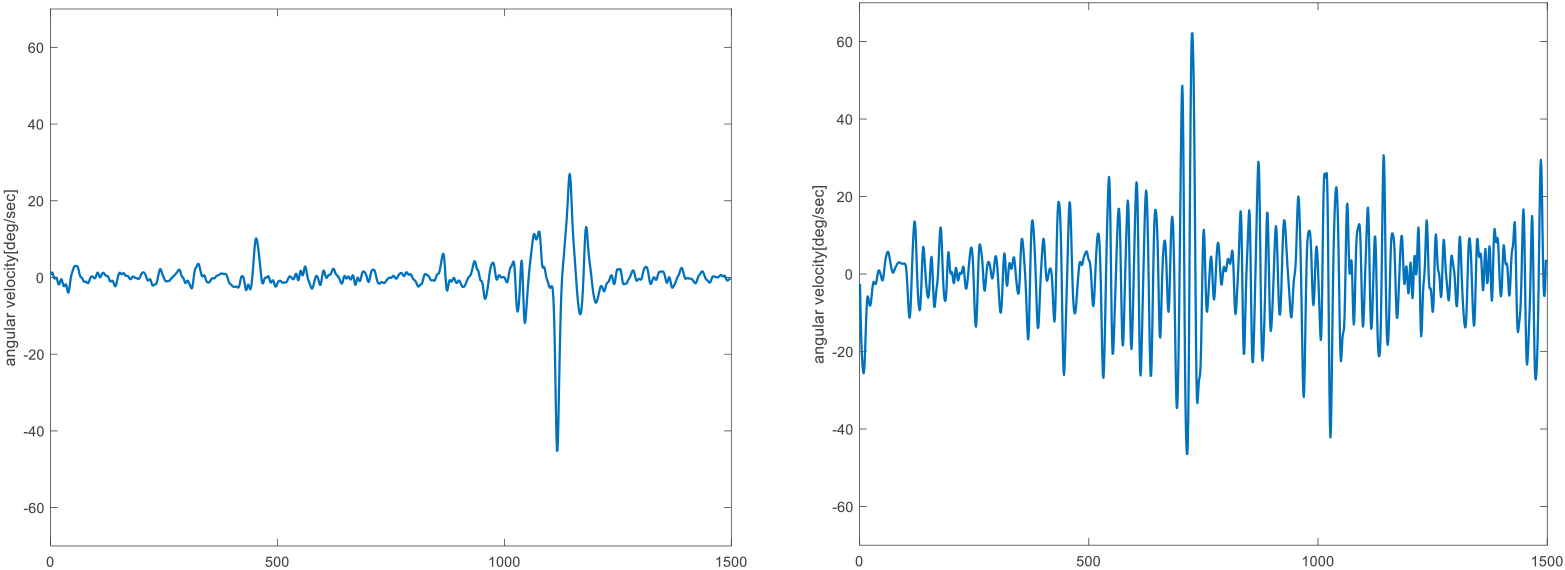
3.Results
Figure 2 shows representative resting tremor signals of index finger joint in the pitch direction for comparison between SWEDD and PD patients. PD patient showed greater angular velocity signals than SWEDD patient. Table 2 shows the differences of time domain variables between PD and SWEDD patients. The RMS angular speed of pitch direction was significantly different in all sensor attachment positions in the PD and SWEDD patients (
Table 3
Comparison of frequency domain analysis indices between PD and SWEDD patients
| Analysis indices | Sensor position | Direction | PD Mean (SD) | SWEDD Mean (SD) | Significance | ||
|---|---|---|---|---|---|---|---|
| Peak frequency (Hz) | Index finger | Roll | 5.0 | (0.9) | 5.1 | (1.5) | 0.881 |
| Pitch | 5.1 | (0.6) | 4.5 | (1.8) | 0.389 | ||
| Yaw | 5.4 | (0.8) | 4.4 | (1.4) | 0.060 | ||
| Dorsum of hand | Roll | 5.1 | (1.0) | 5.2 | (1.5) | 0.834 | |
| Pitch | 5.3 | (0.8) | 4.0 | (2.2) | 0.124 | ||
| Yaw | 5.3 | (0.7) | 4.1 | (1.8) | 0.087 | ||
| Thumb | Roll | 5.2 | (0.9) | 5.5 | (1.4) | 0.633 | |
| Pitch | 5.1 | (0.7) | 4.9 | (1.3) | 0.578 | ||
| Yaw | 5.5 | (1.0) | 4.6 | (1.3) | 0.082 | ||
| Arm | Roll | 4.8 | (0.9) | 4.8 | (1.4) | 0.864 | |
| Pitch | 5.1 | (0.9) | 4.5 | (1.6) | 0.354 | ||
| Yaw | 4.5 | (1.0) | 4.0 | (1.3) | 0.312 | ||
| Peak power (deg | Index finger | Roll | 182510 | (463889) | 2125 | (5662) | 0.226 |
| Pitch | 169253 | (310474) | 559 | (860) | 0.102 | ||
| Yaw | 127750 | (293700) | 2224 | (5193) | 0.187 | ||
| Dorsum of hand | Roll | 179541 | (459194) | 2378 | (6448) | 0.230 | |
| Pitch | 29444 | (49163) | 49 | (63) | 0.075 | ||
| Yaw | 36981 | (84945) | 205 | (486) | 0.182 | ||
| Thumb | Roll | 285482 | (568437) | 2056 | (5546) | 0.129 | |
| Pitch | 119160 | (200875) | 319 | (669) | 0.078 | ||
| Yaw | 162304 | (441280) | 1160 | (3144) | 0.290 | ||
| Arm | Roll | 47477 | (88040) | 1008 | (2737) | 0.111 | |
| Pitch | 7807 | (13962) | 49 | (119) | 0.095 | ||
| Yaw | 11453 | (27548) | 23 | (47) | 0.199 | ||
PD, Parkinson’s disease; SWEDD, scans without evidence of dopaminergic deficit; SD, standard deviation; RMS, root mean square, CV, coefficient of variation.
Figure 3.
Comparison of quantitative analysis indices in the pitch direction between Parkinson’s disease (PD) and scans without evidence of dopaminergic deficit (SWEDD) patients (
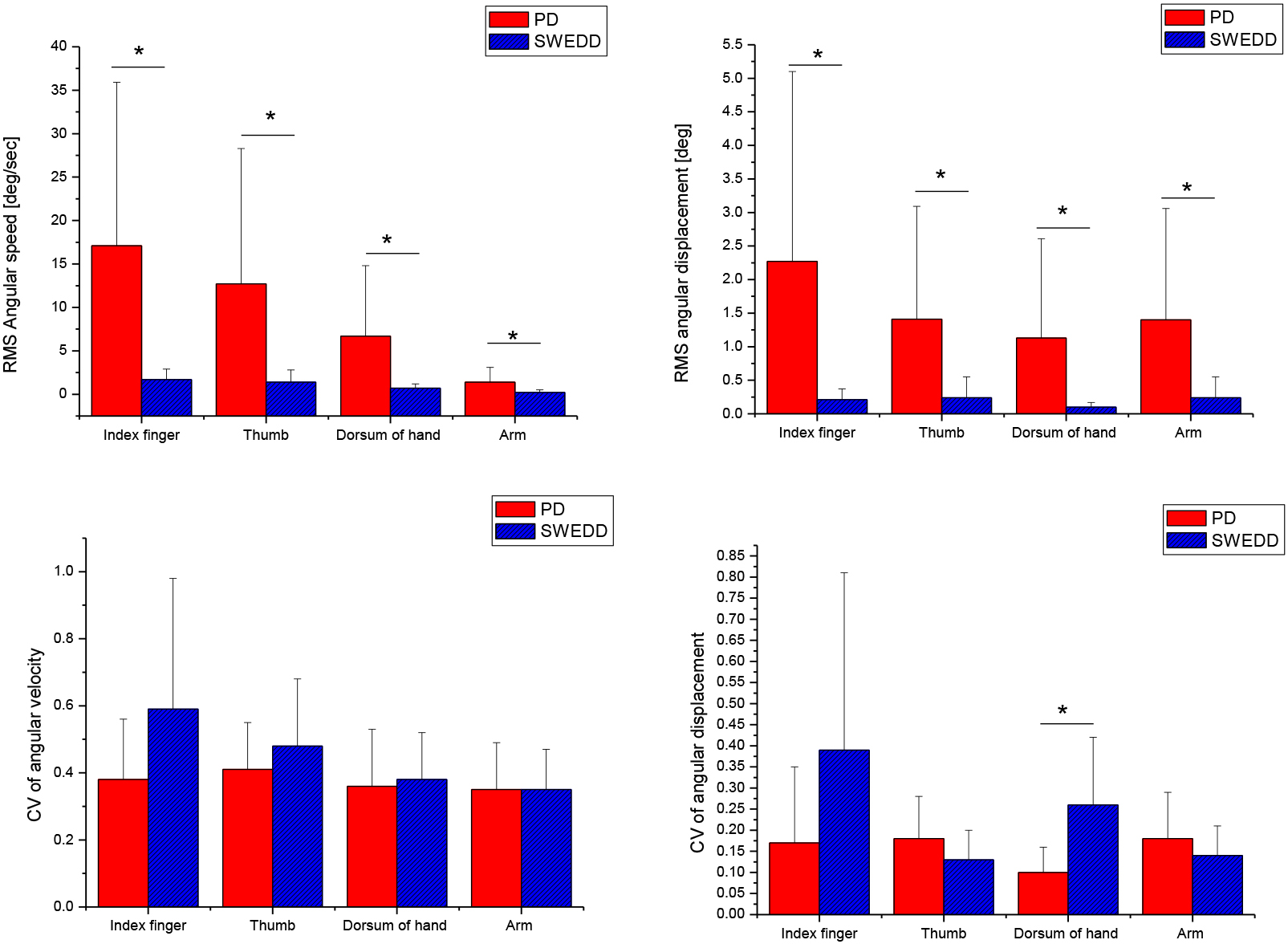
Table 3 shows the results of frequency analysis. PD patients tend to have higher frequency and greater peak power compared to SWDD patients. However, there were no significant differences between SWEDD and PD patients in all frequency domain variables (
4.Discussion
SWEDD patients present heterogeneous clinical manifestations due to various underlying etiologies [22], and their disease prognosis is different from that of PD patients [23]. Thus, differentiating SWEDD patients from PD patients is important to avoid inappropriate intervention. In the present study, quantitative measure of the upper limb during resting tremor task were performed and compared between SWEDD and PD patients. SWEDD patients showed significantly different tremor patterns (slower and smaller) than PD patients, particularly in the pitch direction at all joints. In addition, SWEDD patients showed more irregular amplitude at the dorsum of the hand than did PD patients.
In several studies, the clinical features were quantitatively measured and compared between SWEDD and PD patients. Kwon et al. quantitatively measured upper limb bradykinesia using a gyro sensor. The authors showed markedly different quantitative bradykinesia indices of forearm rotation between SWEDD and PD patients [17]. In a gait study, SWEDD patients had normal gait patterns in elbow posture, trunk, gait variability, and bilateral step phase coordination compared with PD patients [11]. Furthermore, SWEDD patients showed a shorter stance phase and a reduced double limb support period compared with PD patients [24]. In postural balance analysis, SWEDD patients presented a smaller center of pressure (COP) distance and a more frequent COP mainly in the medio-lateral direction and showed normal balance ability [25]. However, differences of resting tremor patterns have been investigated in only a few studies.
In the present study, SWEDD patients exhibited a slower average angular speed and lower amplitude in all sensor attachment positions compared with PD patients. This indicates that PD patients exhibited faster and greater tremulous patterns during the resting tremor task compared with SWEDD patients. In particular, this tendency was significantly pronounced in pitch direction (
The CV of average angular displacement in the pitch direction at the dorsum of the hand was greater in SWEDD patients (
In the present study, although comparable disease durations and Hoehn and Yahr stages were observed, some tremor patterns (average speed and amplitude of pitch direction at all joints and irregularity of average amplitude of pitch direction at the dorsum of the hand) in SWEDD patients differed from those in PD patients. This may be due to different deterioration rates between SWEDD and PD, which is supported by a report suggesting that the two are not likely to progress similarly. The results of the present study indicate that some quantitative indices obtained from the resting tremor task could be important biomarkers for identifying potential patients with SWEDD among patients diagnosed with PD.
SWEDD shows heterogeneous clinical manifestations with various underlying etiologies [22]. Our study demonstrated the feasibility of differentiating between PD and SWEDD using resting tremor patterns. However, evidence for clinical features of tremor in SWEDD patients is lacking. Therefore, further investigations are needed to differentiate SWEDD from PD by measuring various tremor types. Specifically, kinetic tremor as well as resting tremor should be investigated in more PD and SWEDD patients. In addition, tremor patterns of lower limb joint should also be investigated.
5.Conclusion
The results showed that quantitative resting tremor indices could be useful for characterizing SWEDD. Although disease duration and HY stages were comparable between PD and SWEDD patients, slower speed and smaller amplitude in pitch direction at all joints were observed in SWEDD patients. In addition, SWEDD patients had greater irregularity in amplitude in the pitch direction at the dorsum of the hand. These findings could help to identify potential SWEDD patients.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Nos 2018R1C1B6008083 and 2022R1I1A3065537).
Conflict of interest
None to report.
References
[1] | Varadi C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology (Basel). (2020) ; 9: . |
[2] | Zach H, Dirkx M, Bloem BR, Helmich RC. The Clinical Evaluation of Parkinson’s Tremor. J Parkinsons Dis. (2015) ; 5: : 471-474. |
[3] | Hurtado JM, Gray CM, Tamas LB, Sigvardt KA. Dynamics of tremor-related oscillations in the human globus pallidus: a single case study. Proc Natl Acad Sci USA. (1999) ; 961674-1679. |
[4] | Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. (2008) ; 79: : 368-376. |
[5] | Davidson MB, McGhee DJ, Counsell CE. Comparison of patient rated treatment response with measured improvement in Parkinson’s disease. J Neurol Neurosurg Psychiatry. (2012) ; 83: : 1001-1005. |
[6] | Kwon DY, Kwon YR, Choi YH, Eom GM, Ko J, Kim JW. Quantitative measures of postural tremor during clinical spiral drawing task using gyro sensors at the upper limb joints in patients with essential tremor. Technol Health Care. (2020) ; 28: : 499-507. |
[7] | Kwon YR, Eom GM, Ko J, Kim JW. Quantitative analysis of essiontial tremor measures of postural tremor at the upper limb joints in patients with essential tremor. JMMB. (2021) ; 21: : 2140050-2140062. |
[8] | Dai H, Zhang P, Lueth TC. Quantitative Assessment of Parkinsonian Tremor Based on an Inertial Measurement Unit. Sensors (Basel). (2015) ; 15: : 25055-25071. |
[9] | Jeon H, Lee W, Park H, Lee HJ, Kim SK, Kim HB, Jeon B, Park KS. Automatic Classification of Tremor Severity in Parkinson’s Disease Using a Wearable Device. Sensors (Basel). (2017) ; 17. |
[10] | Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, Lang AE, Rascol O, Ribeiro MJ, Remy P, Poewe WH, Hauser RA, Brooks DJ. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. (2003) ; 54: : 93-101. |
[11] | Mian OS, Schneider SA, Schwingenschuh P, Bhatia KP, Day BL. Gait in SWEDDs patients: comparison with Parkinson’s disease patients and healthy controls. Mov Disord. (2011) ; 26: : 1266-1273. |
[12] | Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, Quinn N, Bhatia KP. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord. (2007) ; 22: : 2210-2215. |
[13] | Fahn S, Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. (2005) ; 252: (Suppl 4): IV37-IV42. |
[14] | Marek K, Seibyl J, Eberly S, Oakes D, Shoulson I, Lang AE, Hyson C, Jennings D. Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology. (2014) ; 82: : 1791-1797. |
[15] | Nalls MA, McLean CY, Rick J, Eberly S, Hutten SJ, Gwinn K, Sutherland M, Martinez M, Heutink P, Williams NM, Hardy J, Gasser T, Brice A, Price TR, Nicolas A, Keller MF, Molony C, Gibbs JR, Chen-Plotkin A, Suh E, Letson C, Fiandaca MS, Mapstone M, Federoff HJ, Noyce AJ, Morris H, Van Deerlin VM, Weintraub D, Zabetian C, Hernandez DG, Lesage S, Mullins M, Conley ED, Northover CA, Frasier M, Marek K, Day-Williams AG, Stone DJ, Ioannidis JP, Singleton AB. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol. (2015) ; 14: : 1002-1009. |
[16] | Bajaj NP, Wang L, Gontu V, Grosset DG, Bain PG. Accuracy of subjective and objective handwriting assessment for differentiating Parkinson’s disease from tremulous subjects without evidence of dopaminergic deficits (SWEDDs): an FP-CIT-validated study. J Neurol. (2012) ; 259: : 2335-2340. |
[17] | Kwon DY, Kwon Y, Kim JW. Quantitative analysis of finger and forearm movements in patients with off state early stage Parkinson’s disease and scans without evidence of dopaminergic deficit (SWEDD). Parkinsonism Relat Disord. (2018) ; 57: : 33-38. |
[18] | Jennings DL, Seibyl JP, Oakes D, Eberly S, Murphy J, Marek K. (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol. (2004) ; 61: : 1224-1229. |
[19] | Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. (1992) ; 55: : 181-184. |
[20] | van de Wardt J, van der Stouwe AMM, Dirkx M, Elting JWJ, Post B, Tijssen MA, Helmich RC. Systematic clinical approach for diagnosing upper limb tremor. J Neurol Neurosurg Psychiatry. (2020) ; 91: : 822-830. |
[21] | Espay AJ, Giuffrida JP, Chen R, Payne M, Mazzella F, Dunn E, Vaughan JE, Duker AP, Sahay A, Kim SJ, Revilla FJ, Heldman DA. Differential response of speed, amplitude, and rhythm to dopaminergic medications in Parkinson’s disease. Mov Disord. (2011) ; 26: : 2504-2508. |
[22] | Cilia R, Reale C, Castagna A, Nasca A, Muzi-Falconi M, Barzaghi C, Marzegan A, Granata M, Marotta G, Sacilotto G, Vallauri D, Pezzoli G, Goldwurm S, Garavaglia B, Novel DYT11 gene mutation in patients without dopaminergic deficit (SWEDD) screened for dystonia. Neurology. (2014) ; 83: : 1155-1162. |
[23] | Schwingenschuh P, Ruge D, Edwards MJ, Terranova C, Katschnig P, Carrillo F, Silveira-Moriyama L, Schneider SA, Kagi G, Palomar FJ, Talelli P, Dickson J, Lees AJ, Quinn N, Mir P, Rothwell JC, Bhatia KP. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson’s disease: a clinical and electrophysiological study. Mov Disord. (2010) ; 25: : 560-569. |
[24] | Kwon DY, Choi YH, Kwon Y, Eom GM, Ko JH, Park MH, Kim JW. Comparison of spatio-temporal gait variables in patients with Parkinson’s disease and SWEDD. J Mech Med Biol. (2018) ; 18: : 1840023. |
[25] | Kwon DY, Choi YH, Kwon Y, Eom GM, Kim JW, Comparison of static postural balance in patients with SWEDDS and Parkinson’s disease. J Mech Med Biol. (2020) ; 9: : 2040013. |
[26] | Yuan H, Liu S, Liu J, Lin H, Yang C, Cai X, Zeng L, Li S. Detection and quantification of resting tremor in Parkinson’s disease using long-term acceleration data, Mathematical Problems in Engineering. (2021) , Article ID 5669932, 2021. |




