Network pharmacology and experimental studies for deciphering the molecular targets and mechanisms of Chaihu Shugan powder in the treatment of functional dyspepsia
Abstract
BACKGROUND:
Chaihu Shugan powder (CSP) is a prevalent prescription product used in the treatment functional dyspepsia (FD) in China. However, the underlying pharmacological mechanisms involved in the treatment of FD remain unclear.
OBJECTIVE:
To explore the key components of CSP and their molecular targets and mechanisms in the treatment of FD.
METHODS:
Active compounds for CSP were identified from the TCMSP and SymMap databases, and the relevant targets were predicted. FD-related targets were obtained from the GeneCards and CTD database. In addition, using the protein-protein interactions (PPI) analysis, the common targets were obtained. Furthermore, the compound-target networks were created with Cytoscape. Finally, molecular docking was performed to identify the core targets and validate them experimentally.
RESULTS:
In total, 78 active compounds and 671 related targets of CSP were obtained. PPI network analysis identified 15 key FD-related compound targets. Molecular docking revealed that sitosterol and hyndarin exhibited good binding activities with AKT1 and IL6, respectively. Animal experiments have shown that CSP effectively increased the protein levels of AKT1 and reduced the serum levels of IL-6 in FD rats.
CONCLUSION:
This study provides a theoretical evidence for the analysis of the molecular targets and mechanisms of the action of CSP in FD.
1.Introduction
Functional dyspepsia (FD) is a chronic recurrent disease with symptoms of dyspepsia, such as postprandial fullness, early satiety, epigastric pain, and burning sensation, and it cannot be explained by conventional clinical examinations [1]. The global prevalence of FD is 5%–10% and varies widely by country, from 10%–40% in Western countries to 5%–30% in the Asian countries [2, 3]. One study estimates the impact of FD on the U.S. economy at more than $1.8 billion per year [4]. The pathogenesis of FD includes gastrointestinal motor and sensory dysfunction, mucosal inflammation, abnormal brain – gut interactions, disruption of the intestinal flora, psychological factors, and genetics factors [3, 5]. The current treatment relieves the clinical symptoms of patients. However, most patients can easily develop tolerance to some of these drugs, particularly gastroprokinetic drugs and acid inhibitors. Antianxiety and antidepressants have more pronounced side effects. Therefore, more effective treatments are urgently required. In China, medicinal plants are widely used to treat various diseases owing to their remarkable curative effects. Many medicinal plants and their extracts offer a therapeutic efficacy in the treatment of digestive system disorders, such as stomach cancer, colon cancer, and ulcers [6, 7]. Therefore, medicinal plants could be effective, safe, and economical treatment options.
Chaihu Shugan powder (CSP) is a prevalent prescription used for a curative effect in FD in China, and its formula originates from an ancient book, “Jing Yue Quan Shu”. CSP is composed of seven ingredients: Chaihu (Radix bupleuri), Baishao (Radix Paeonia alba), Chenpi (Pericarpium citri reticulatae), Chuanxiong (Ligusticum chuanxiong Hort), Xiangfu (Rhizoma cyperi), Zhike (Fructus aurantia), and Gancao (licorice). In our previous studies, we found that an excessive autophagy and abnormal differentiation of the Interstitial cells of Cajal (ICCs) may be responsible for gastric motility disorders associated with FD [8]. Further, treatment of FD using CSP may be related to a reduced autophagic damage leading to protection of the ICCs, thus exerting a pro-gastric motility effect [9]. Nevertheless, the primary ingredients and molecular mechanisms of CSP have not been extensively studied in the treatment of FD.
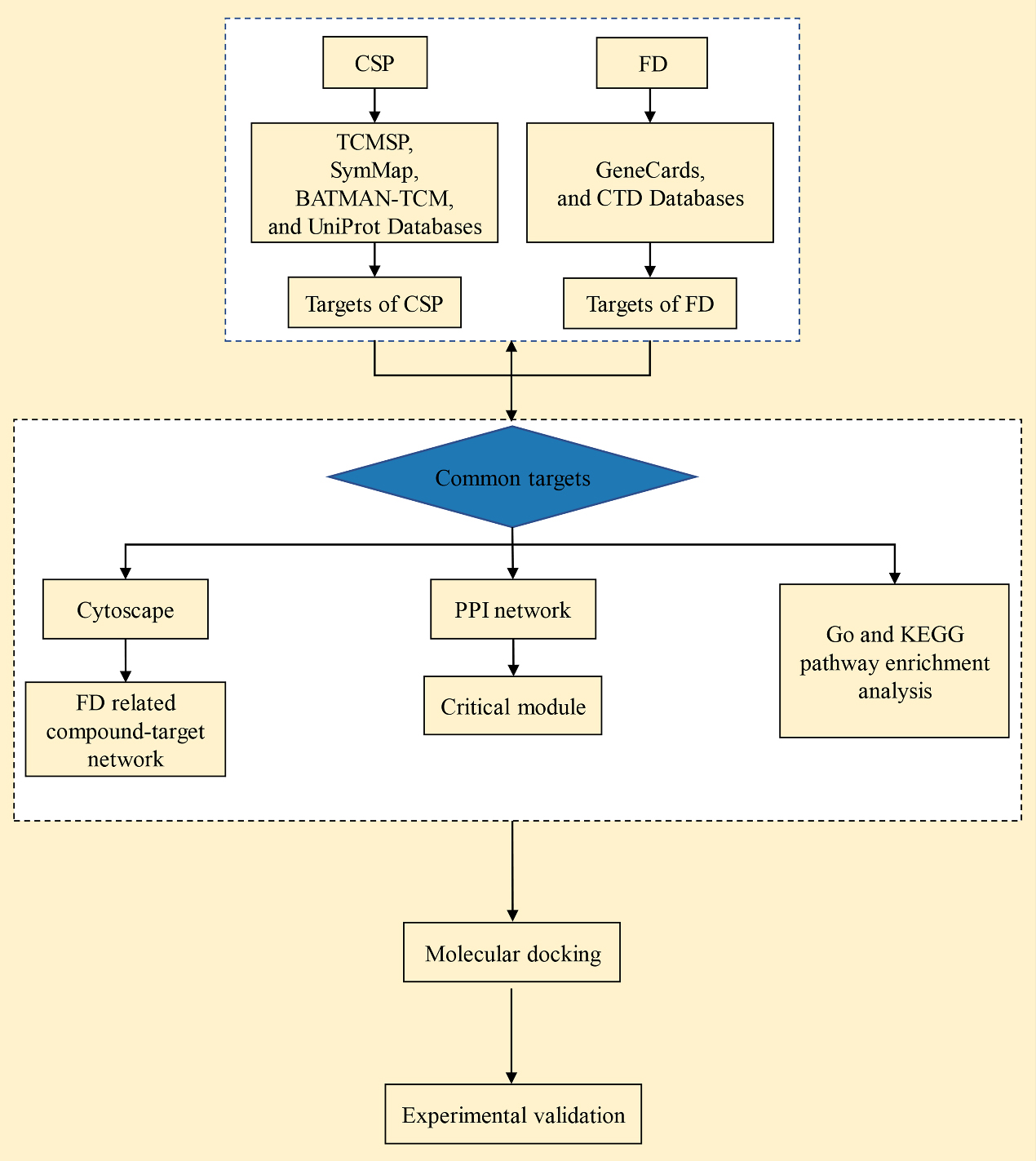
Chinese medicine formulas are characterized by multiple components, pathways, and targets. To ensure and improve their therapeutic effects, it is necessary to identify key targets in the mechanism of action of CSP for treating FD. Network pharmacology is a novel means to study the compounding of Chinese medicine that is based on the similarity of drugs in terms of their structure and efficacy, while combining the interactions between the target molecules and biological effector molecules in the organism using the construction of drug – drug and drug – target networks. This can effectively predict the efficacy of drugs and their mechanisms of action [10]. Therefore, the present study aimed to analyze the core targets, biological processes, and possible mechanisms of CSP in the treatment of FD using a network pharmacology approach. Our results can facilitate the screening of therapeutic drugs for FD, elucidate the mechanisms of action of CSP, and provide useful reference for the therapeutic mechanisms of other medicinal plants. The flowchart of the study is provided in Fig. 1.
Figure 1.
Flowchart of the study.
2.Method
2.1Materials
CSP was obtained from the herbal pharmacy of Shuguang Hospital, Shanghai, China. Domperidone was purchased from Xi’an Janssen Pharmaceutical Co., Ltd. IL-6 ELISA kit was purchased from Shanghai Hengyuan Biotechnology Co., Ltd. Akt1 antibody (7535), and rabbit IgG horseradish conjugate secondary antibody (7074) were purchased from Cell Signaling Technology, Inc., USA.
2.2Active compound screening
Related chemical compounds of CSP were acquired from the TCMSP (http://tcmspnw.com/) and the SymMap (http://www.symmap.org/) OB
2.3Target identification
Protein targets of the active compounds were queried in the BATMAN-TCM (http://bionet.ncpsb.org.cn/ batman-tcm/) and TCMSP database. The two generating datasets were merged to determine the active compound targets for further study. Before further study, the names of the targets were normalized to homo sapiens gene symbols using the UniProt Knowledgebase (UniProt) (https://www.uniprot.org/).
2.4Collection of FD disease target
The GeneCards database (https://www.genecards.org/) and the CTD database (http://ctdbase.org/) were utilized to predict the gene targets of FD. The keyword “functional dyspepsia” was used to screen the FD-related gene targets. Venn diagrams was utilized to obtain crossovers of the two generated datasets to identify disease targets for further study.
2.5Disease-related compound target identification and network construction
The intersection of the active compounds and FD-related targets was obtained to determine the FD-related compound target (CSP-FD targets). Then, the network of the CSP-FD targets and their corresponding compounds was constructed using Cytoscape software.
2.6GO and KEGG analyses
GO and KEGG analyses were employed that utilized the DAVID 6.8 (http://david.abcc.ncifcrf.gov/) to reveal the underlying mechanism through biological processes (BPs), molecular functions (MFs), cellular components (CCs), and key signaling pathways.
2.7Construction of the PPI network
In this study, STRING 11.0 (https://string-db.org/) was applied to conduct a PPI network on CSP-FD targets with a combined score
2.8Compounds-target molecular docking
The top 5 targets from the PPI network and the top 5 compounds from the active compound-target network were identified for the molecular docking analysis. The two-dimensional structure in SDF format were obtained for the compounds from the PubChem database. Docking of the receptor proteins with small molecule ligands of CSP active compounds was achieved using AutoDock 4.2.
2.9Animals
Sprague-Dawley male rats, weighting 180–200 g, were supplied by Shanghai Slaughter Laboratory Animal Co., Ltd. The animal protocol was approved by the Experimental Animal Welfare and Ethics Committee of Shanghai University of Traditional Chinese Medicine (PZSHUTCM210926008). The rats were randomly divided into the control group, the model group, the CSP group, and the domperidone group. Rats in all groups except the control group, were prepared by the tail clamping stimulation method for 30 min each time, twice a day for 4 weeks. CSP was given as aqueous decoction (9.7 g/kg) administered to the CSP group, and domperidone (3 mg/kg) was given to the domperidone group. The model and control groups received 2ml saline by oral gavage for 4 weeks.
2.10Western blotting
The gastric proteins were extracted from six rats in each group and measured by BCA protein assay. The proteins were loaded and separated by 5% and 10% SDS-PAGE, and then transferred to PVDF membranes. After being blocked with 5% skim milk powder for 2 h at room temperature, the membrane was washed with TBST for 15 min, incubated with anti-
2.11ELISA
The amount of IL-6 in rat serum was tested according to the manufacturer’s instructions on the ELISA kit.
2.12Statistics
SPSS 25.0 was utilized for statistical analysis. The measurement data is presented as the mean
3.Results
3.1The active compounds and potential targets of CSP
A total of 1807 compounds were obtained from CSP using the TCMSP database, and 159 of these satisfied the criteria of OB
Next, the BATMAN-TCM and TCMSP databases were employed to forecast the targets of the active compounds. By combining the results from these two databases, we obtained a dataset of 78 active compounds and 671 targets. The top ten active compounds were further identified based on the ranking of the number of active compounds corresponding to the target genes. Table 1 shows the general information of the top 10 active compounds in CSP.
Table 1
General information of the top 10 active compounds in CSP
| Molecule name | Gene counts | Source | 2D structure | PubChem CID |
|---|---|---|---|---|
| Gadelaidic acid | 183 | Gancao |

| 5460988 |
| Icos-5-enoic acid | 179 | Gancao |

| 3349565 |
| Hyndarin | 161 | Xiangfu |
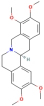
| 72301 |
| FA | 124 | Chuanxiong |
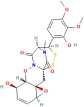
| 102059896 |
| Sitosterol | 96 | Baishao |
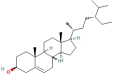
| 222284 |
| Stigmasterol | 74 | Chaihu |
| 5280794 |
| Mairin | 65 | Gancao |
| 64971 |
| Sugeonyl acetate | 46 | Xiangfu |
| 46173924 |
| Sainfuran | 38 | Chaihu |
| 185034 |
| Vestitol | 38 | Gancao |
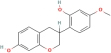
| 92503 |
3.2Disease related compound-target network construction
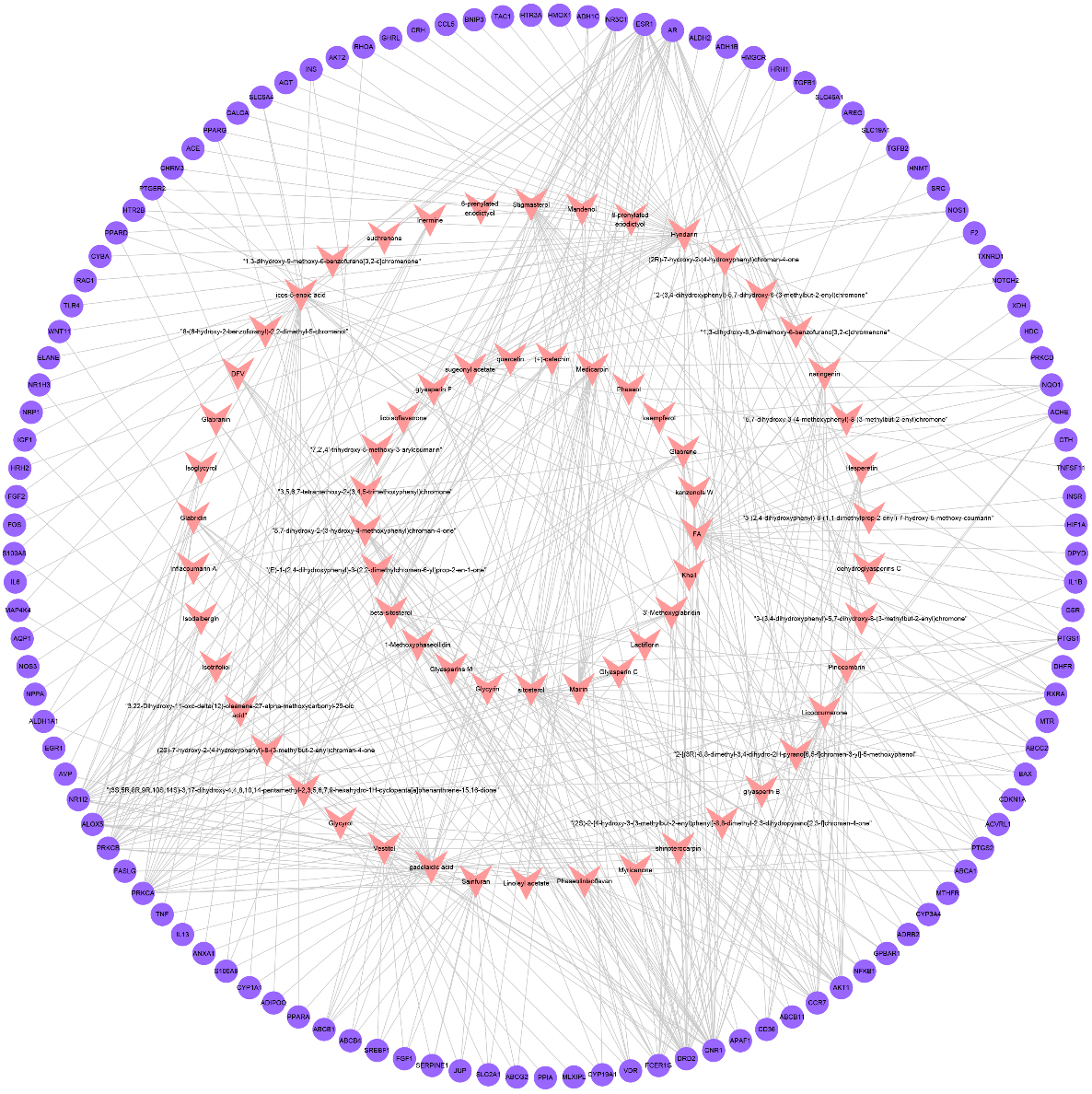
By taking the intersection of the databases of Genecards and CTD, 660 FD-related targets were acquired. Subsequently, an overlap of 660 FD-related targets and 671 active compound targets were obtained to identify the CSP-FD targets. In total,117 CSP-FD targets were generated. The CSP-FD targets were plotted using Venn diagram (Fig. 2). Cytoscape software was utilized to build the PPI network in Fig. 3. The network of CSP-FD targets and their corresponding compounds showed 450 compound-target interactions, 67 compounds and 117 CSP-FD targets.
Figure 2.
Venn diagram of the active components of CSP-FD targets.
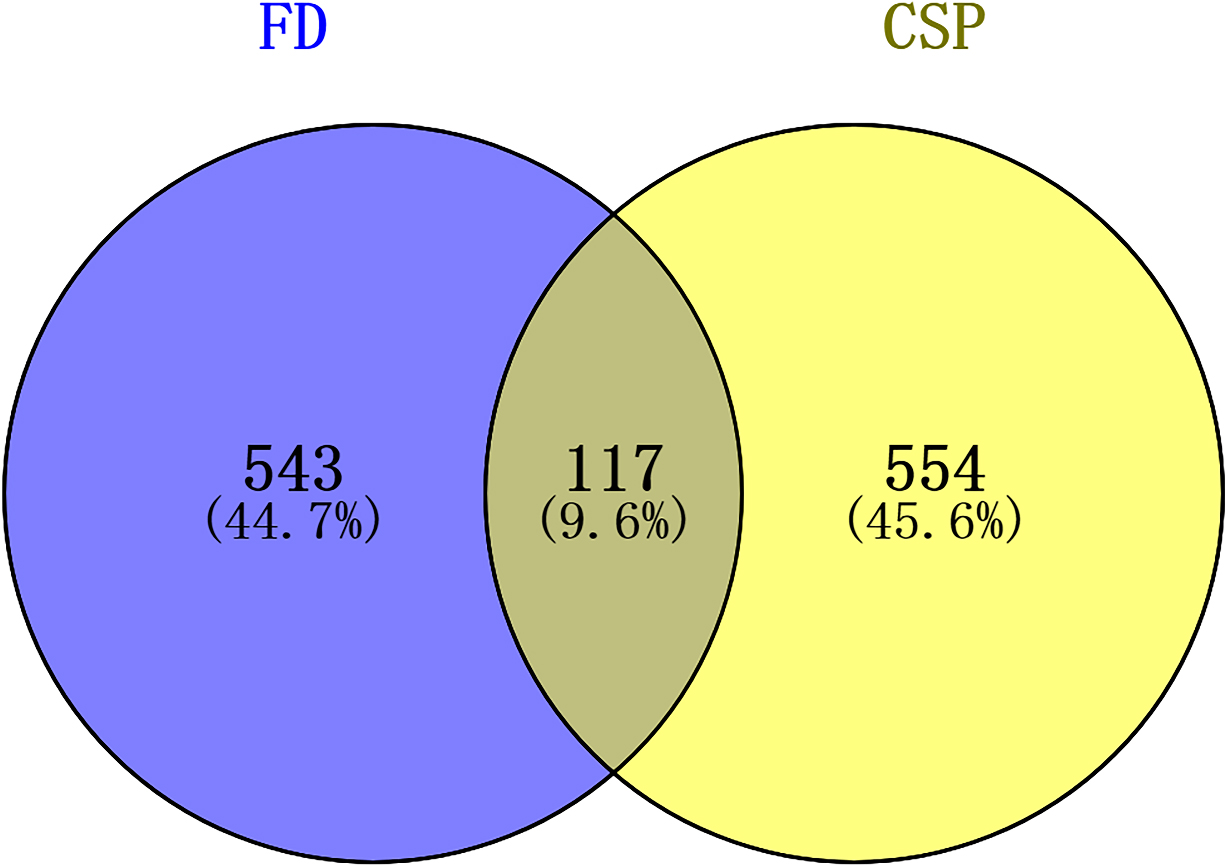
Figure 3.
Network of active compounds and CSP-FD targets (pink inverted triangle node denotes the active components of CSP; purple circular nodes denote the CSP-FD targets).
3.3Enrichment analysis
The 117 CSP-FD targets were introduced into the DAVID 6.8 for GO and KEGG analysis, and the results were selected using a statistical significance of
Figure 4.
GO and KEGG enrichment analysis of the 117 CSP-FD targets. (a) GO enrichment analysis: the top 10 BPs, MFs, and CCs. (b) Bubble chart of the top 20 KEGG pathways.
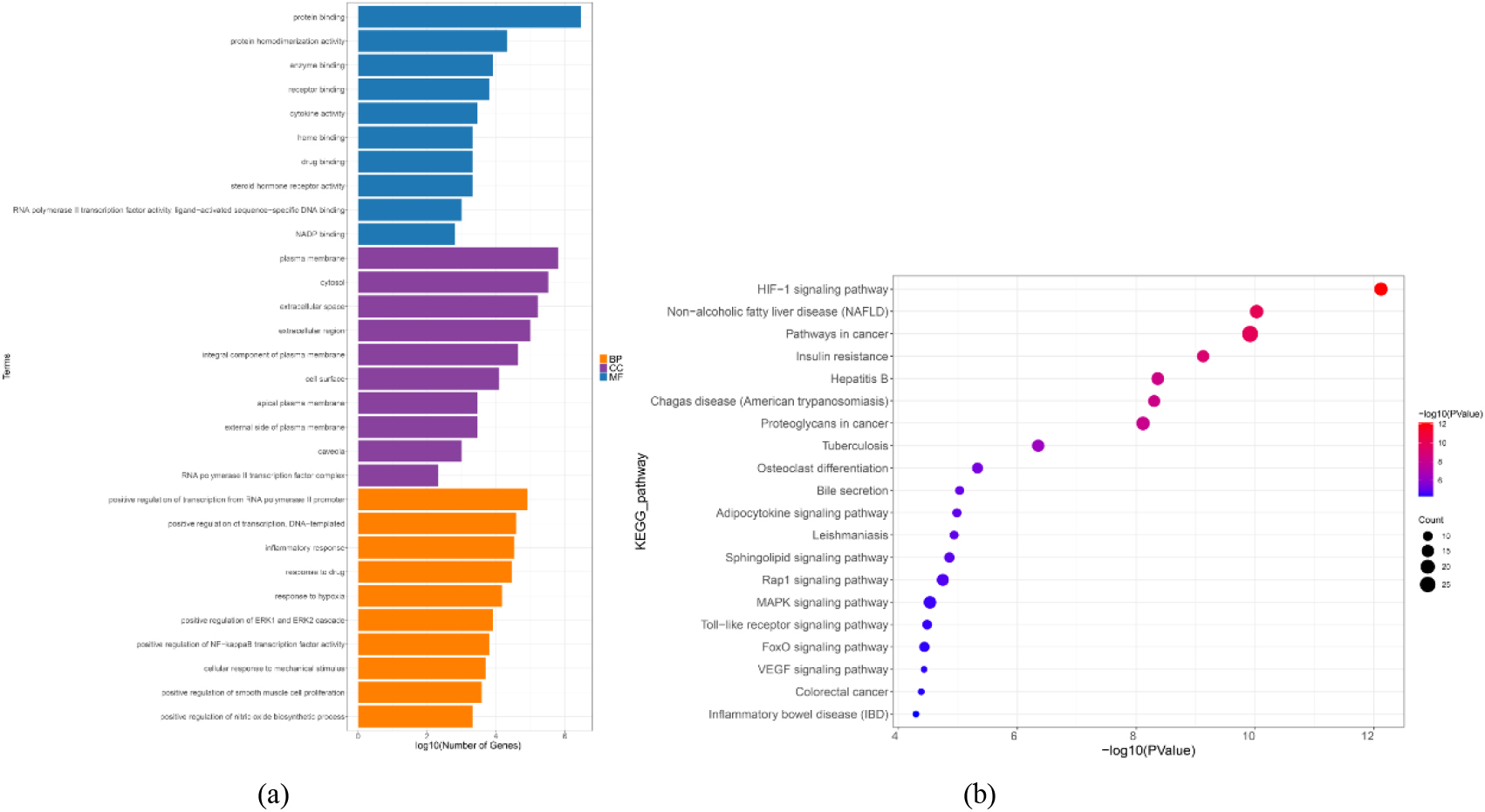
KEGG enrichment analysis revealed the presence of 99 signaling pathways. The visual analysis of the top 20 pathways revealed that the primary signaling pathways in CSP in the therapy of FD were focused on the HIF-1 signaling pathway, nonalcoholic fatty liver disease, pathways in cancer and Rap1 signaling pathway (Fig. 4b).
3.4PPI analysis
To acquire protein-protein interaction relationships, 117 CSP-FD targets were imported into the STRING database, and the outcomes were introduced into Cytoscape to build a PPI network. In total, there were 117 targets and 1207 PPIs in the PPI network (Fig. 5a).
Figure 5.
The PPI network of the CSP-FD targets. (a) The PPI network from STRING was analyzed using Cytoscape software. The MCODE was used to analyze critical modules in the PPI network in (b) Module 1 and (c) Module 2.
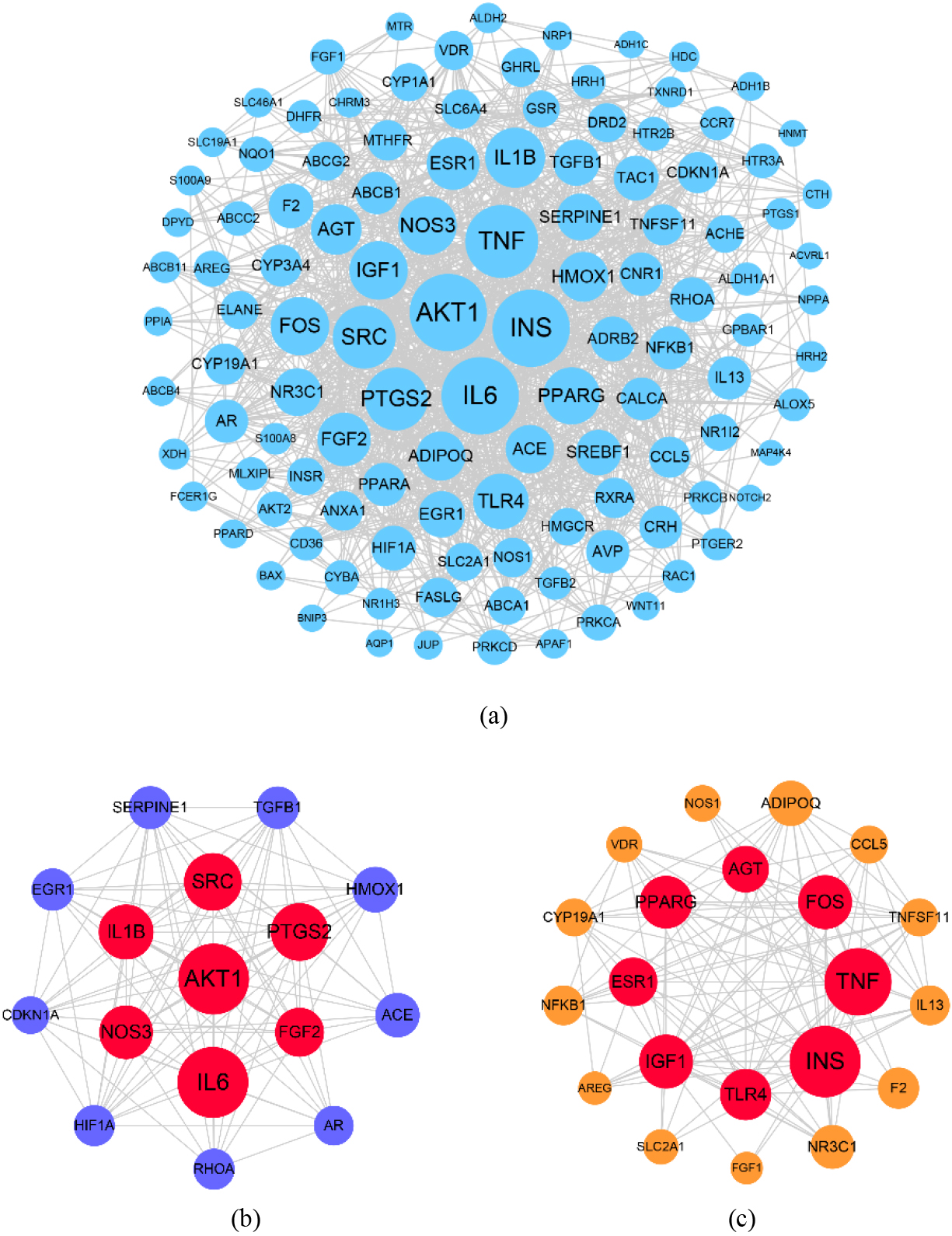
The CytoNCA module was employed to analyze the key targets in the PPI network. Four parameters were used to assess the significance of the target, including degree centrality (DC), betweenness centrality (BC), closeness centrality (CC), and eigenvector centrality (EC). The higher the score of those four parameters, the higher was importance of the target in the network. By setting the score of DC to greater than twice the median and the scores of BC, CC, and EC greater than the median, we obtained 15 key targets, including AKT1, IL6, INS, TNF, PTGS2, SRC, IL1B, NOS3, IGF1, FGF2, FOS, PPARG, TLR4, ESR1, and AGT.
The MCODE was employed to analyze the critical modules in the PPI network. Six modules were generated using MCODE, and two critical modules were screened from the six modules by setting a score greater than 10. Module 1 contained 16 targets and 102 PPIs with a score of 13.6. There were seven key targets in module 1, including AKT1, IL6, PTGS2, SRC, IL1B, NOS3, and FGF2 (Fig. 5b). Module 2 contained 21 targets and 115 PPIs with a score of 11.5. There were eight key targets in module 2, including INS, TNF, IGF1, FOS, PPARG, TLR4, ESR1, and AGT (Fig. 5c). By aggregating the key targets of the two modules, we obtained 15 targets. This was identical to the key targets obtained using the CytoNCA plug-in analysis, further demonstrating the importance of these 15 targets in the treatment FD by CSP.
3.5Molecular docking
Molecular docking enables the use of software to analyze the binding sites, the binding of the active pockets of small molecule compounds with certain target proteins. Molecular docking showed that the binding energy after docking was below
Table 2
Binding energy of molecular docking
| Docking pair | Docking score (kcal/mol) |
|---|---|
| Sitosterol – AKT1 | |
| Hyndarin – IL6 | |
| Gadelaidic aicid – PTGS2 | |
| Gadelaidic aicid – FGF2 | |
| Gadelaidic aicid – TNF |
Figure 6.
Molecular docking 2D-structure diagrams. (a) sitosterol-AKT1, (b) Hyndarin-IL6, (c) gadelaidic-FGF2, (d) gadelaidic-PTGS2, (e) gadelaidic-TNF.

3.6CSP increased the expression of AKT1 in stomach tissue
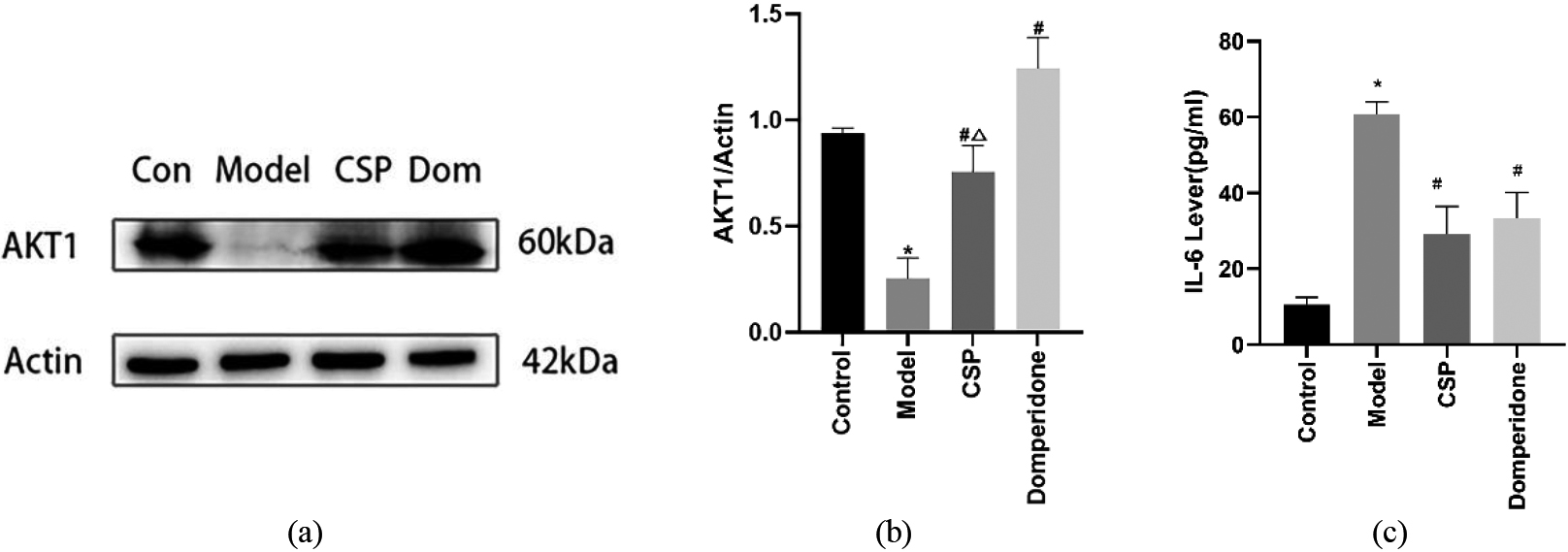
AKT1 is a key gene in the regulation of apoptosis and apoptosis is known to be involved in the development of FD [13]. The results of western blotting revealed that compared with the control group, the expression of AKT1 in the model group was downregulated, while CSP increased the expression of AKT1. In addition, the expression of AKT1 was remarkably upregulated in the domperidone group (Fig. 7a–b).
Figure 7.
CSP upregulated the expression of AKT1 and reduced the level of IL-6. (a–b) Representative western blotting pictures and quantitative analysis of AKT1,
3.7CSP inhibited the serum level of IL-6
IL-6 is an important inflammatory factor in the body, which is closely related to the development of FD. The results of ELISA revealed that, compared with the control group, the level of serum IL-6 in the model group increased significantly, suggesting an inflammatory response in FD. CSP and domperidone reduced the IL-6 levels. (Fig. 7c).
4.Discussion
In the present study, the chemical components of CSP for treating FD were investigated using the ADME and topological analysis to anticipate the main compounds involved in the treatment of FD. The active compounds were found to be organic acid, alcohols, and alkaloids. These include gadelaidic acid, icos-5-enoic acid, hyndarin (rotundine, levo-tetrahydropalmatine), FA, and sitosterol. Hyndarin is also known as rotundine. Rotundine was the first neurological drug to be extracted and developed from the medicine plant Corydalis yanhusuo in China. It also has sedative and analgesic effects [14]. This was similar to the efficacy of medicinal plant extracts studied earlier [15]. Rotundine has been reported to be effective in relieving pain symptoms in patients with FD, especially in those with epigastric pain syndrome [16]. It works by blocking the dopamine receptors in the brain, inhibiting the upstream activation system of the brainstem reticular nerve conduction, and decreasing the excitability of the central nervous system, thereby reducing the visceral sensitivity. In addition, rotundine showed significant analgesic effects in a mouse model of chronic inflammatory and neuropathic pain without producing motor impairment, the mechanism of which may be the activation of dopamine D1 receptors and spinal sigma-1 receptor [17, 18]. Oxidative stress is a known causative factor of FD [19]. A previous study showed that sitosterol could inhibit an increase in angiotensin II-induced reactive oxygen species in A7r5 cells [20]. Moreover, sitosterol significantly reduced the expression of inflammatory factors like TNF
Genes with high degree scores were chosen from the top two modules in the PPI network for molecular docking analysis. Of these, FGF2, IL6, AKT1, PTGS2, and TNF were the top five genes. These genes may participate in the development of FD. FGF2 is involved in promoting neuritogenesis, survival, and injury repair [23]. It is a novel neurotrophic factor with anxiolytic and antidepressant-like effects [24]. FGF2 is known to exert anxiolytic effects via a compensatory increase in the acetylation of the oxytocin receptor promoter to reduce oxytocin levels [25]. Moreover, a previous study demonstrated that FGF2 expression was reduced in a depressed mouse model, FGF2 infusion significantly improved the depressive behavior in mice, and the FGF2/FGF receptor signaling was involved in mediating its antidepressant effects [26, 27]. COX-2(PTGS2) is not only an important inducing enzyme in the inflammatory process, but also plays an important role in pain [28, 29]. Previous reports have shown that COX-2 expression was increased in FD rats [30, 31], and by suppressing COX-2 expression, the symptoms of dyspepsia in FD rats was significantly improved. Recently, a low-grade inflammation of the mucosa has gained attention in the pathogenesis of FD [5]. The core targets in this study were also screened for multiple inflammation-related targets, consistent with the literature. Among them, IL-6 and TNF are the inflammatory factors, which are correlated with gastrointestinal motility in addition to reflecting the inflammatory condition of the gastrointestinal tract. Recent research revealed that suppressing IL-6 and TNF improves the gastrointestinal dysmotility in FD [32]. The Akt kinase family has three members, of which Akt1 is involved in regulating cell survival pathway by inhibiting the apoptosis process [33]. Apoptosis is closely related to gastrointestinal motility. Gastric motility was also improved in FD rats when ICCs apoptosis was inhibited [13]. These genes are correlated with inflammation, apoptosis, anxiety, and depression, which are highly related to the development of FD. In this study, we verified two main targets (AKT1 and IL6) predicted by molecular docking using western blotting and ELISA. The results indicated that CSP increased the expression of AKT1 and decreased IL-6 levels compared to the control group. This suggests that CSP exerts its anti-FD effects by inhibiting apoptosis and reducing the inflammatory response. This finding provides an additional scientific basis for the clinical practice of CSP in the treatment of FD.
The above results were further supported by the GO and KEGG enrichment analyses. GO-BP data suggested that CSP could exert an anti-FD role by regulating the inflammatory response, NF-kappaB transcription factor activity, and smooth muscle cell proliferation. KEGG results revealed that other diseases were also among them, since some of the molecular targets also participated in the pathogenesis of those diseases. This also proves that herbal medicine has a multi-target mode of action. Recent studies have shown that inflammatory factors such as VEGF, TNF-
5.Conclusion
The present study identified 10 major compounds in CSP and multiple potential genes targeted in FD, and found that the AKT1 and IL-6 were the core roles in the treatment of FD by CSP. Furthermore, the pathways for treating FD by CSP may be related to the HIF-1, MAPK, and VEGF signaling pathways. This multi-target and multi-pathway mode of action of medicinal plants provides insights into the development of new herbal anti-FD drugs.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 81973774,82174 309).
Conflict of interest
None to report.
References
[1] | Talley NJ, Ford AC. Functional Dyspepsia. New England Journal of Medicine. (2015) ; 373: (19): 1853-63. doi: 10.1056/NEJMra1501505. |
[2] | Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. (2021) ; 160: (1): 99-114. doi: 10.1053/j.gastro.2020.04.014. |
[3] | Mahadeva S, Ford AC. Clinical and epidemiological differences in functional dyspepsia between the East and the West Neurogastroenterology. and Motility. (2016) ; 28: (2): 167-74. doi: 10.1111/nmo.12657. |
[4] | Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Alimentary Pharmacology & Therapeutics. (2013) ; 38: (2): 170-7. doi: 10.1111/apt.12355. |
[5] | Ford AC, Mahadeva S, Carbone MF, Lacy BE, Talley NJ. Functional Dyspepsia. Lancet. (2020) ; 396: (10263): 1689-1702. doi: 10.1056/NEJMra1501505. |
[6] | Ağagündüz D, Şahin TO, Yılmaz B, Ekenci KD, Özer SD, Capasso R. Cruciferous Vegetables and Their Bioactive Metabolites: from Prevention to Novel Therapies of Colorectal Cancer. Evidence Based Complementary and Alternative Medicine. (2022) ; 2022: (11): 1534083. doi: 10.1155/2022/1534083. |
[7] | Chakraborty AJ, Mitra M, Tallei TE, Tareq AM, Nainu F, Cicia D, et al. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life (Basel). (2021) ; 11: (4): 317. doi: 10.3390/life11040317. |
[8] | Zhang LM, Zeng LJ, Deng J, Zhang YQ, Wang YJ, Xie TY, et al. Investigation of autophagy and differentiation of myenteric interstitial cells of Cajal in the pathogenesis of gastric motility disorders in rats with functional dyspepsia. Biotechnology and Applied Biochemistry. (2018) ; 65: (4): 533-539. doi: 10.1002/bab.1635. |
[9] | Tang RQ, Zhang Z, Ju J, Ling JH. Effect of Chaihu Shugan Powder-Contained Serum on Glutamate-Induced Autophagy of Interstitial Cells of Cajal in the Rat Gastric Antrum. Evidence Based Complementary and Alternative Medicine. (2019) ; 29: : 7318616. doi: 10.1155/2019/7318616. |
[10] | Li S. Exploring traditional chinese medicine by a novel therapeutic concept of network target. Chinese Journal of Integrative Medicine. (2016) ; 22: (9): 647-52. doi: 10.1007/s11655-016-2499-9. |
[11] | Xu X, Zhang WX, Huang C, Li Y, Yu H, Wang YH, et al. A novel chemometric method for the prediction of human oral bioavailability. International Journal of Molecular Sciences. (2012) ; 13: (6): 6964-6982. doi: 10.1002/0471250953.bi0814s24. |
[12] | Morris GM, Huey R, Olson AJ. Using AutoDock for ligand-receptor docking. Bioinformatics. (2008) ; Chapter 8: Unit 8.14. doi: 10.1002/0471250953.bi0814s24. |
[13] | Zhang XY, Wang SY, Jin YJ, Wang JY, Wang RX, Yang XH, et al. Wei-Tong-Xin ameliorated cisplatin-induced mitophagy and apoptosis in gastric antral mucosa by activating the Nrf2/HO-1 pathway. Journal of Ethnopharmacology. (2023) ; 17: : 116253. doi: 10.1016/j.jep.2023.116253. |
[14] | Wang JB, Mantsch JR. l-tetrahydropalamatine: a potential new medication for the treatment of cocaine addiction. Future Medicinal Chemistry. (2012) ; 4: (2): 177-86. doi: 10.4155/fmc.11.166. |
[15] | Rahman MM, Islam F, Parvez A, Azad MA, Ashraf GM, Ullah MF, et al. Citrus limon L. (lemon) seed extract shows neuro-modulatory activity in an in vivo thiopental-sodium sleep model by reducing the sleep onset and enhancing the sleep duration. Journal of Integrative Neuroscience. (2022) ; 21: (1): 42. doi: 10.31083/j.jin2101042. |
[16] | Deng ZC. Clinical efficacy of rotundine in the treatment of functional dyspepsia in the community. Contemporary Medicine. (2011) ; 17: (10): 147-148. |
[17] | Zhou HH, Wu DL, Gao LY, Fang Y, Ge WH. L-Tetrahydropalmatine alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Neuroreport. (2016) ; 27: (7): 476-80. doi: 10.1097/WNR.0000000000000560. |
[18] | Kang DW, Moon JY, Choi JG, Kang SY, Ryu Y, Park JB, et al. Antinociceptive Profile of Levo-tetrahydropalmatine in Acute and Chronic Pain Mice Models: Role of spinal sigma-1 receptor. Scientific Reports. (2016) ; 2: (6): 37850. doi: 10.1038/srep37850. |
[19] | Joung JY, Choi SH, Son CG. Interstitial Cells of Cajal: Potential Targets for Functional Dyspepsia Treatment Using Medicinal Natural Products. Evidence Based Complementary and Alternative Medicine. (2021) ; 2021: (30): 9952691. doi: 10.1155/2021/9952691. |
[20] | Li CM, Liu Y, Xie Z, Lu Q, Luo SH. Stigmasterol protects against Ang II-induced proliferation of the A7r5 aortic smooth muscle cell-line. Food & Function. (2015) ; 6: (7): 2266-72. doi: 10.1039/c5fo00031a. |
[21] | Yuan LL, Zhang F, Shen MY, Jia S, Xie JH. Phytosterols Suppress Phagocytosis and Inhibit Inflammatory Mediators via ERK Pathway on LPS-Triggered Inflammatory Responses in RAW2647. Macrophages and the Correlation with Their Structure. Foods. (2019) ; 8: (11): 582. doi: 10.3390/foods8110582. |
[22] | Walker CIB, Oliveira SM, Tonello R, Rossato MF, Brum EDS, Ferreira J, et al. Anti-nociceptive effect of stigmasterol in mouse models of acute and chronic pain. Naunyn-Schmiedebergs Archives of Pharmacology. (2017) ; 390: (11): 1163-1172. doi: 10.1007/s00210-017-1416-x. |
[23] | Turner CA, Watson SJ, Akil H. The fibroblast growth factor family: neuromodulation of affective behavior. Neuron. (2012) ; 76: (1): 160-74. doi: 10.1016/j.neuron.2012.08.037. |
[24] | Lebowitz ER, Orbach M, Marin CE, Salmaso N, Vaccarino FM, Silverman WK. Fibroblast Growth Factor 2 Implicated in Childhood Anxiety and Depression Symptoms. Journal of Affective Disorders. (2021) ; 1: (282); 611-616. doi: 10.1016/j.jad.2020.12.055. |
[25] | Litvin Y, Turner CA, Rios MB, Maras PM, Chaudhury S, Baker MR, et al. Fibroblast growth factor 2 alters the oxytocin receptor in a developmental model of anxiety-like behavior in male rat pups. Hormones and Behavior. (2016) ; 84: : 64-70. doi: 10.1016/j.yhbeh.2016.09.009. |
[26] | Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, et al. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. (2007) ; 144: (4): 1219-28. doi: 10.1016/j.neuroscience.2006.11.026. |
[27] | Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biological Psychiatry. (2012) ; 72: (4): 258-65. doi: 10.1016/j.biopsych.2012.03.003. |
[28] | Zhu LY, Zhang YZ, Guo ZY, Wang M. Cardiovascular Biology of Prostanoids and Drug Discovery. Arteriosclerosis Thrombosis and Vascular Biology. (2020) ; 40: (6): 1454-1463. doi: 10.1161/ATVBAHA.119.313234. |
[29] | Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacological Reviews. (2007) ; 59: (3): 207-24. doi: 10.1124/pr.59.3.1. |
[30] | He YX, Yang C, Wang P, Yang L, Wu H, Liu H, et al. Child compound Endothelium corneum attenuates gastrointestinal dysmotility through regulating the homeostasis of brain-gut-microbiota axis in functional dyspepsia rats. Journal of Ethnopharmacology. (2019) ; 240: : 111953. doi: 10.1016/j.jep.2019.111953. |
[31] | Zou X, Wang Y, Wang YH, Yang JT, Guo HS, Cai ZX. Paeoniflorin Alleviates Abnormalities in Rats with Functional Dyspepsia by Stimulating the Release of Acetylcholine. Drug Design Development and Therapy. (2020) ; 14: : 5623-5632. doi: 10.2147/DDDT.S260703. |
[32] | Lin YL, Yuan HZ, Tan RQ, Ju J, Xie TY, Wang YJ, et al. Effect of Zhishi on IL-6, SCF and MTL in rats with functional dyspepsia. Shanxi Journal of Traditional Chinese Medicine. (2020) ; 36: (1): 52-53. |
[33] | Hamzehzadeh L, Atkin SL, Majeed M, Butler AE, Sahebkar A. The versatile role of curcumin in cancer prevention and treatment: A focus on PI3K/AKT pathway. Journal of Cellular Physiology. (2018) ; 233: (10): 6530-6537. doi: 10.1002/jcp.26620. |
[34] | Xiao LF, Wang ZH, Lu N, Hu YN, Qiao LM, Sheng XH, et al. GPER mediates the IL6/JAK2/STAT3 pathway involved in VEGF expression in swine ovary GCs. Journal of Molecular Endocrinology. (2021) ; 68: (1): 23-33. doi: 10.1530/JME-21-0125. |
[35] | Chen QF, Wang W, Huang Z, Huang DL. Hypoxia-inducible factor-1α attenuates myocardial inflammatory injury in rats induced by coronary microembolization. Anais Da Academia Brasileira De Ciencias. (2020) ; 92: (1): e20190658. doi: 10.1590/0001-3765202020190658. |
[36] | Li L, Candelario KM, Thomas K, Wang R, Wright K, Messier A, et al. Hypoxia inducible factor-1α (HIF-α) is required for neural stem cell maintenance and vascular stability in the adult mouse SVZ. Journal of Neuroscience. (2014) ; 34: (50): 16713-9. doi: 10.1523/JNEUROSCI.4590-13.2014. |
[37] | Udo H, Hamasu K, Furuse M, Sugiyama H. VEGF-induced antidepressant effects involve modulation of norepinephrine and serotonin systems. Behavioural Brain Research. (2014) ; 15: (275): 107-13. doi: 10.1016/j.bbr.2014.09.005. |
[38] | Preez AD, Onorato D, Eiben I, Musaelyan K, Egeland M, Zunszain PA, et al. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behavior and Immunity. (2021) ; 91: : 24-47. doi: 10.1016/j.bbi.2020.07.015. |
[39] | Kotan Z, Sarandöl E, Kırhan E, Ozkaya G, Kırlı S. Serum brain-derived neurotrophic factor, vascular endothelial growth factor and leptin levels in patients with a diagnosis of severe major depressive disorder with melancholic features. Therapeutic Advances in Psychopharmacology. (2012) ; 2: (2): 65-74. doi: 10.1177/2045125312436572. |
[40] | Hagiwara SI, Kaushal E, Paruthiyil S, Pasricha JP, Hasdemir B, Bhargava A. Gastric corticotropin-releasing factor influences mast cell infiltration in a rat model of functional dyspepsia. Plos One. (2018) ; 13: (9): e0203704. doi: 10.1371/journal.pone.0203704. |




