Comparison of chronic gastrointestinal and genitourinary toxicities between brachytherapy and external beam radiotherapy for patients with prostate cancer: A systematic review and meta-analysis
Abstract
BACKGROUND:
OBJECTIVE:
To define the GI and GU toxicities in prostate cancer to prevent adverse events after treatment.
METHODS:
We searched published studies in PubMed, Cochrane, and Embase databases up to December 31, 2022. The endpoints were the RRs of GI and GU toxicities. Pooled data were assessed using a random-effects model.
RESULTS:
Fifteen eligible studies were included into this analysis. LDR-BT had significantly lower RRs than LDR-BT
CONCLUSION:
The results implied that BT with and without EBRT can result in both GI and GU toxicities in patients with prostate cancer, with LDR-BT leading to a poorer urinary function than EBRT.
1.Introduction
Prostate cancer is malignant tumor worldwide that threatens the health of older men [1]. Currently, transperineal interstitial permanent (Iodine)
BT is a recommended monotherapy for low-malignant prostate cancer and boost for intermediate- and high-risk prostate cancer [5, 6, 7, 8]. The incidence of gastrointestinal (GI) and genitourinary (GU) toxic adverse events (AEs) varies with the choice of treatment. Given that the outcomes of BT, RP, and EBRT are similar, the prognosis accuracy, selection of a treatment regimen, and risks of GI and GU toxicities during and after radiotherapy (RT) are important. GI and GU toxicities from RT options should be considered because of their impact on patients’ health [9, 10]. The concern with BT is its toxicity to nearby organs and tissues, particularly with respect to GI and GU health [11].
An optimal therapeutic outcome would include maximal survival benefits with low GI and GU toxicities. Several clinical studies have indicated that BT, EBRT, and their combined therapy may be associated with a high prevalence of different GI and GU toxicities. However, there is no meta-analysis of recent clinical studies. Therefore, we assessed the risks of GI and GU toxicities from BT, EBRT, and combined-treatment use in men with prostate cancer.
2.Materials and methods
2.1Literature search
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and was registered at PROSPERO (Number: CRD42021249602) [12].
The literature was searched in Cochrane Library, PubMed and Embase databases up to December 31, 2022. The following Mesh keywords were used during the search: “prostatic neoplasms,” “prostatic intraepithelial neoplasia,” “brachytherapy,” “Iodine-125,” “I-125,” and “125I.”
2.2Inclusion and exclusion criteria
The inclusion criteria for clinical studies were as follows: only cohort studies in adult men with localized prostate cancer; no lymph node involvement; no distant metastases; comparisons between
2.3Data extraction
Studies were extracted by two reviewers independently to identify eligible studies. A third reviewer arbitrated any disagreements. These data included year of publication, first author, country of origin, study design, enrollment period, type of the patients, sample sizes, type of interventions, follow-up time, and details of toxic outcomes. The endpoints of GI and GU toxic complications were defined as AEs based on the CTCAE or RTOG. Only events greater than grade 2 were considered.
2.4Quality assessment
The Newcastle-Ottawa scale (NOS) to evaluate the quality of the clinic studies. Quality assessment included measurement of exposure factors, among-group similarity, and patient selection. Any disagreement was resolved by consensus. Studies with NOS scores
2.5Statistical analysis
A pooled estimate of the differences in risk for the single studies was calculated using a random-effect model base on the Mantel-Haenszel method; the results of studies are illustrated using forest plots. Dichotomous parameters were expressed as risk ratios (RRs). All results of studies were presented with a 95% confidence interval (95% CI). Homogeneity within the data of each study was assessed using a chi-square test, setting the degrees of freedom to the number of analyzed studies minus one. Heterogeneity was observed with the Cochran Q test and I
3.Results
3.1Study selection
We identified 16 213 potentially relevant studies. After removing 4407 duplicate studies, the title and abstract of the remaining 11 806 studies were assessed. Of these, 11 745 studies removed without inclusion criteria. Finally, 15 studies were included in the meta-analysis (Fig. 1): four prospective cohort studies and 11 retrospective cohort studies [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30].
3.2Study characteristics
Of the 12 773 patients in the included studies, 9405 were treated with LDR-BT, 2468 were treated with LDR-BT
3.3Meta-analysis of GI toxicities between LDR-BT and LDR-BT +
3.3.1Acute and late GI toxicity
A meta-analysis of six studies [20, 22, 23, 24, 26, 29] revealed that the RR of acute GI toxic complications from LDR-BT was significantly lower than that from LDR-BT
A meta-analysis of 11 studies [16, 19, 20, 21, 22, 23, 24, 25, 26, 27, 29] revealed that the RR of late GI toxic complications from LDR-BT was significantly lower than that from LDR-BT
Table 1
Studies characteristics
| Study/year | Country | Study design | Time | NCCN risk groups ( | Treatment group | Sample size | Prescribed dose | ||
|---|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | |||||||
| Taniguchi et al. [16], 2020 | Japan | RCS | 2004–2016 | 132 | 140 | 26 | LDR-BT | 120 | 104Gy |
| LDR-BT | 178 | 145Gy | |||||||
| Moll et al. [17], 2020 | Austria | RCS | 2000–2019 | 477 | 0 | 0 | LDR-BT | 264 | 145Gy |
| 3D-CRT/VMAT | 213 | NA | |||||||
| Tanaka et al. [18], 2019 | Japan | PCS | 2005–2010 | NA | NA | NA | LDR-BT | 547 | NA |
| LDR-BT | 1792 | NA | |||||||
| Tomoki et al. [19], 2018 | Japan | RCS | 2003–2013 | 781 | 1175 | 260 | LDR-BT | 955 | 100Gy |
| LDR-BT | 1261 | 145Gy/160Gy | |||||||
| Yamazaki et al. [20], 2018 | Japan | RCS | 2005–2013 | 194 | 250 | 42 | LDR-BT | 68 | 110Gy |
| LDR-BT | 418 | 145Gy | |||||||
| HDR-BT | 352 | 49–54Gy/7–9fr/4–7days | |||||||
| Maki et al. [21], 2017 | Japan | RCS | 2005–2011 | 132 | 147 | 21 | LDR-BT | 29 | 110Gy |
| LDR-BT | 271 | 145Gy | |||||||
| Katayama et al. [22], 2016 | Japan | PCS | 2005–2010 | NA | NA | NA | LDR-BT | 547 | 100–110Gy |
| LDR-BT | 1791 | 144Gy | |||||||
| Mukai et al. [23], 2018 | Japan | RCS | 2014–2017 | 4 | 60 | 41 | LDR-BT | 60 | 110Gy |
| LDR-BT | 45 | 160Gy | |||||||
| Ohashi et al. [24], 2015 | Japan | PCS | 2005–2007 | 2057 | 1836 | 158 | LDR-BT | 547 | 100–110Gy |
| LDR-BT | 1792 | 144Gy | |||||||
| Sutani et al. [25], 2015 | Japan | RCS | 2006–2013 | 364 | 389 | 331 | LDR-BT | 379 | 160Gy |
| 2D, 3D-CRT/IMRT | 410 | 60–76Gy/76–78Gy | |||||||
| LDR-BT | 295 | 110Gy | |||||||
| Tanaka et al. [26], 2013 | Japan | PCS | 2004–2008 | NA | NA | NA | LDR-BT | 63 | 110Gy |
| LDR-BT | 155 | 145Gy/160Gy | |||||||
| Kalakota et al. [27], 2010 | USA | RCS | 1997–2006 | 82 | 23 | 5 | LDR-BT | 48 | 110Gy |
| LDR-BT | 62 | 144Gy | |||||||
| Morimoto et al. [28], 2014 | Japan | RCS | 2008–2010 | 55 | 24 | 54 | LDR-BT | 37 | 145Gy |
| 3D-CRT/IMRT | 92 | 70Gy/70, 74Gy | |||||||
| HDR-BT | 27 | 45.5Gy/7fr/4days | |||||||
| Zelefsky et al. [29], 2008 | USA | RCS | 2002–2005 | 226 | 107 | 10 | LDR-BT | 127 | 110Gy |
| LDR-BT | 216 | 144Gy | |||||||
| Eade et al. [30], 2008 | USA | RCS | 1998–2004 | NA | NA | NA | LDR-BT | 158 | 145Gy |
| IMRT | 216 | 74–78Gy | |||||||
|
Table 1, continued | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study/year | RV 100 (ml) | RV 150 (ml) | U 200 (ml) | U 150 (ml) | PSA (ng/ml) | HT | Follow-up | Toxicity evaluation | End-points |
| Taniguchi et al. [16], 2020 | 0.37 (0.09–0.8) | 0.005 (0–0.07) | NA | NA | 7.9 (5.7–11.1) | 106 | 7.6 (5.1–10.2) years | CTCAE | GI toxicity |
| 0.32 (0.08–0.9) | 0 (0–0.07) | NA | NA | 5.9 (4.9–7.1) | 140 | 6.4 (4.8–8.5) years | |||
| Moll et al. [17], 2020 | NA | NA | NA | NA | 6.4 (0.6–10) | 33 | 68 (3–181) months | RTOG | GI/GU toxicity |
| NA | NA | NA | NA | 6.4 (0.3–10) | 77 | 71 (3–192) months | |||
| Tanaka et al. [18], 2019 | 0.4 | 0.0 | 0.0 | NA | 10.6 | 154 | 60 months | CTCAE | GU toxicity |
| 0.5 | 0.1 | 0.1 | NA | 7.2 | 1101 | 60 months | |||
| Tomoki et al. [19], 2018 | NA | NA | NA | NA | NA | 422 | 7.4 (4.9–9.8) year | CTCAE | GI toxicity |
| NA | NA | NA | NA | NA | 438 | 6.5 (4.5–9.1) year | |||
| Yamazaki et al. [20], 2018 | NA | NA | NA | NA | 7.0 (1.4–46) | NA | 90 (12–151) months | CTCAE | GI/GU toxicity |
| NA | NA | NA | NA | 7.0 (1.4–46) | NA | 90 (12–151) months | |||
| NA | NA | NA | NA | 7.0 (1.4–46) | NA | 84 (19–216) months | |||
| Maki et al. [21], 2017 | 0.35 (0 | 0.02 (0 | NA | NA | 7.0 (2.6–25) | NA | 53 (5–99) months | CTCAE | GI toxicity |
| 0.28 (0 | 0.01 (0 | NA | NA | 7.0 (2.6–25) | NA | 53 (5–99) months | |||
| Katayama et al. [22], 2016 | 0.43 | 0.04 | 0.01 | NA | 10.6 (1.9–42) | 390 | 7 years | CTCAE | GI toxicity |
| 0.50 | 0.05 | 0.07 | NA | 7.2 (1.6–42) | 764 | 7 years | |||
| Mukai et al. [23], 2018 | 0.08 | NA | NA | 0 | 9.0 (4–54.6) | 26 | 28 months | CTCAE | GI/GU toxicity |
| 0.2 | NA | NA | 0 | 7.5 (4–20.6) | 19 | 28months | |||
| Ohashi et al. [24], 2015 | 0.3 (0.0–3.7) | 0.0 (0.0–1.2) | 0.0 (0.0–0.6) | NA | 9.3 (1.9–42) | NA | 36 months | CTCAE | GI/GU toxicity |
| 0.3 (0.0–4.8) | 0.0 (0.0–1.5) | 0.0 (0.0–92.9) | NA | 9.3 (1.9–42) | NA | 36 months | |||
| Sutani et al. [25], 2015 | NA | NA | NA | NA | NA | 103 | 43 (25–61) months | RTOG | GI/GU toxicity |
| NA | NA | NA | NA | NA | 208 | 43 (27–61) months | |||
| NA | NA | NA | NA | NA | 108 | 45 (27–62) months | |||
| Tanaka et al. [26], 2013 | 0.11 | NA | NA | NA | 12.5 | 26 | 38.3 | CTCAE | GI/GU toxicity |
| 0.08 | NA | NA | NA | 7.2 | 43 | 44.2 | |||
| Kalakota et al. [27], 2010 | 0.09 (0–1.39) | NA | NA | NA | 6.0 (1.0–41) | 16 | 56 (4–100) months | RTOG | GI toxicity |
| 0.02 (0–1.31) | NA | NA | NA | 6.0 (2.0–26) | 2 | 34 (4–87) months | |||
| Morimoto et al. [28], 2014 | NA | NA | NA | NA | 8.7 (2.0–337) | NA | 19 (5–36) months | CTCAE | GI/GU toxicity |
| NA | NA | NA | NA | 8.7 (2.0–337) | NA | 19 (5–36) months | |||
| NA | NA | NA | NA | 8.7 (2.0–337) | NA | 19 (5–36) months | |||
| Zelefsky et al. [29], 2008 | NA | NA | NA | NA | NA | 43 | 30 months | CTCAE | GI/GU toxicity |
| NA | NA | NA | NA | NA | 2 | 30 months | |||
| Eade et al. [30], 2008 | NA | NA | NA | NA | 5.2 (0.5–9.8) | NA | 48 months | RTOG | GI/GU toxicity |
| NA | NA | NA | NA | 5.2 (0.4–9.6) | NA | 43 months | |||
RCS, Retrospective Cohort Study; PCS, Prospective Cohort Study; LDR-BT, Low-dose-rate brachytherapy; HDR-BT, High-dose-rate brachytherapy; 2D-CRT, 2-dimensional conformal radiation therapy; 3D-CRT, 3-dimensional conformal radiation therapy; IMRT, Intensity-modulated radiation therapy; VMAT, Volume-modulated arc therapy; CTCAE, Common Terminology Criteria for Adverse Events; RTOG, Radiation Therapy Oncology Group grading; GI toxicity, Gastrointestinal toxicity; GU toxicity, Genitourinary toxicity; NA, Not Available.
Table 2
Details of study quality evaluation via the Newcastle-Ottawa Scale
| Study/year | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age | Control for other confounding factors | Assessment of outcome | Enough long follow-up duration | Adequacy of follow-up of cohorts | Total |
| Taniguchi et al. [16], 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Moll et al. [17], 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Tanaka et al. [18], 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Tomoki et al. [19], 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Yamazaki et al. [20], 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Maki et al. [21], 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Katayama et al. [22], 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Mukai et al. [23], 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Ohashi et al. [24], 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Sutani et al. [25], 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Tanaka et al. [26], 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Kalakota et al. [27], 2010 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Morimoto et al. [28], 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Zelefsky et al. [29], 2008 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Eade et al. [30], 2008 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
Figure 1.
Study selection. Flow diagram summarising selection of studies that meet inclusion criteria.
Figure 2.
(a) Forest plot of RR for acute GI toxicity following LDR-BT
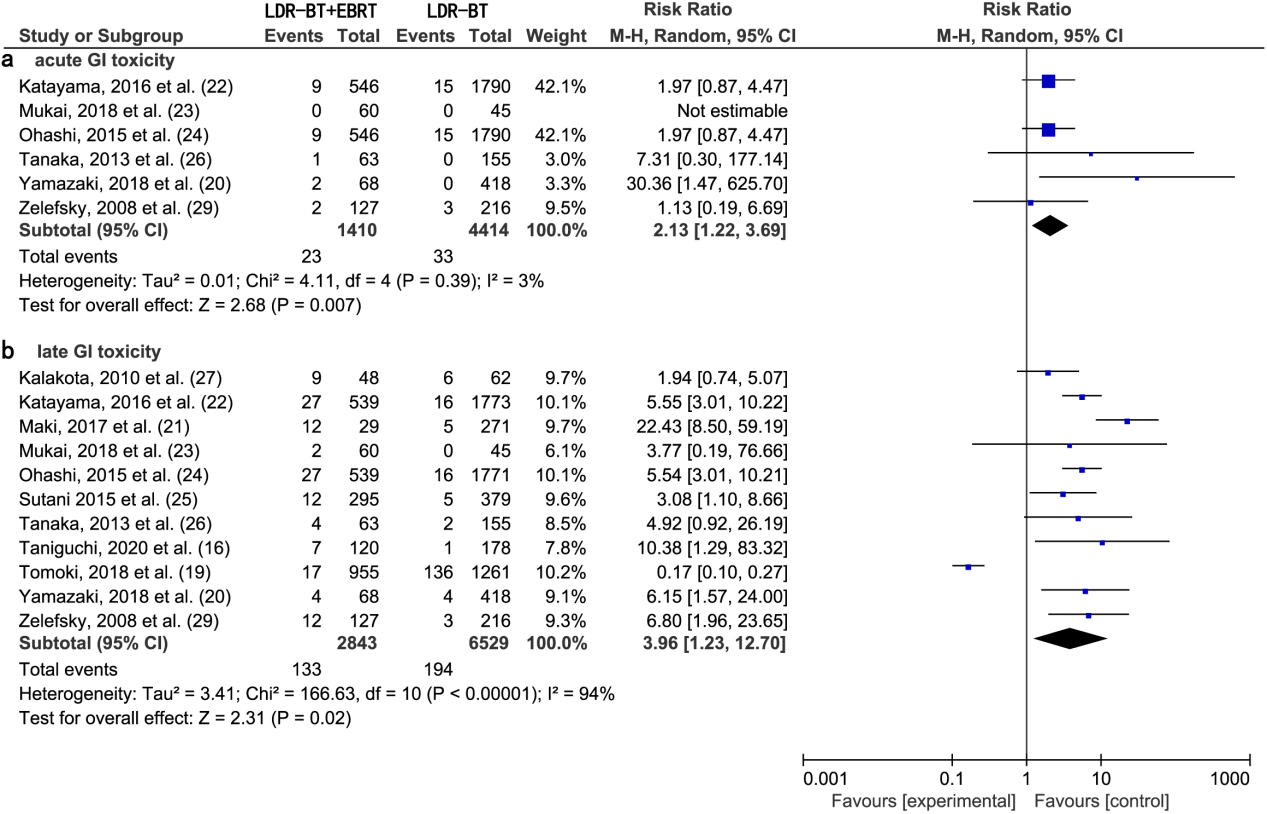
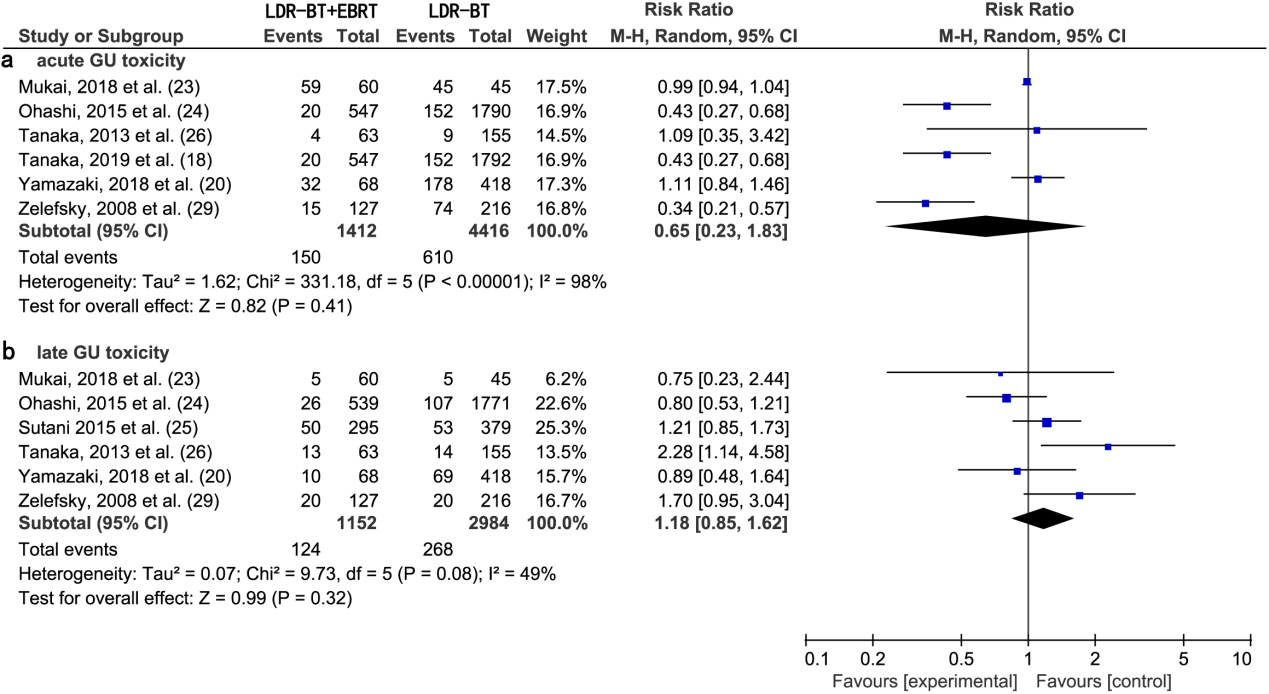
Figure 3.
(a) Forest plot of RR for acute GU toxicity following LDR-BT
3.4Meta-analysis of GU toxicities between LDR-BT and LDR-BT +
3.4.1Acute and late GU toxicity
A meta-analysis of six studies [18, 20, 23, 24, 26, 29] revealed that there was no significant difference in the RR of acute GU toxic complications between LDR-BT and LDR-BT
A meta-analysis of six studies [20, 23, 24, 25, 26, 29] revealed that there was no significant difference in the RR of late GU toxic complications between LDR-BT and LDR-BT
3.5Heterogeneity analysis of LDR-BT and LDR-BT +
Six studies reporting acute GI toxicity did not present heterogeneity (
Table 3
Sensitivity analysis (acute GI toxicity)
| RR | 95% CI | p | Q (p) | I | |
|---|---|---|---|---|---|
| All studies | 2.13 | 1.22–3.69 | 0.007 | 0.39 | 3% |
| Selected study omitted | |||||
| Yamazaki et al. [20], 2018 | 1.94 | 1.13–3.34 | 0.02 | 0.8 | 0% |
| Katayama et al. [22], 2016 | 2.58 | 0.92–7.27 | 0.07 | 0.25 | 26% |
| Mukai et al. [23], 2018 | 2.13 | 1.22–3.69 | 0.007 | 0.39 | 3% |
| Ohashi et al. [24], 2015 | 2.58 | 0.92–7.27 | 0.07 | 0.25 | 26% |
| Tanaka et al. [26], 2013 | 2.07 | 1.11–3.86 | 0.02 | 0.32 | 14% |
| Zelefsky et al. [29], 2008 | 2.38 | 1.21–4.68 | 0.01 | 0.31 | 17% |
Table 4
Sensitivity analysis (late GI toxicity)
| RR | 95% CI | p | Q (p) | I | |
|---|---|---|---|---|---|
| All studies | 3.96 | 1.23–12.7 | 0.02 | 94% | |
| Selected study omitted | |||||
| Taniguchi et al. [16], 2020 | 3.65 | 1.07–12.41 | 0.04 | 95% | |
| Tomoki et al. [19], 2018 | 5.60 | 3.63–8.65 | 0.11 | 38% | |
| Yamazaki et al. [20], 2018 | 3.79 | 1.10–13.13 | 0.04 | 94% | |
| Maki et al. [21], 2017 | 3.27 | 1.01–10.59 | 0.05 | 93% | |
| Katayama et al. [22], 2016 | 3.83 | 1.03–14.23 | 0.03 | 94% | |
| Mukai et al. [23], 2018 | 3.97 | 1.18–13.35 | 0.03 | 95% | |
| Ohashi et al. [24], 2015 | 3.83 | 1.03-14.23 | 0.04 | 94% | |
| Sutani et al. [25], 2015 | 4.08 | 1.14–14.64 | 0.03 | 95% | |
| Tanaka et al. [26], 2013 | 3.88 | 1.13–13.38 | 0.03 | 95% | |
| Kalakota et al. [27], 2010 | 4.29 | 1.18–15.57 | 0.03 | 95% | |
| Zelefsky et al. [29], 2008 | 3.75 | 1.08–13.08 | 0.04 | 94% |
3.6Meta-analysis of GI and GU toxicities between LDR-BT and EBRT
3.6.1Acute and late GI toxicity
A meta-analysis of two studies [28, 30] revealed that there was no significant difference in the RR of acute GI toxic complications between LDR-BT and EBRT alone (0.75; 95% CI, 0.21–2.74;
Figure 4.
(a) Forest plot of RR for acute GI toxicity following LDR-BT and EBRT. (b) Forest plot of RR for late GI toxicity following LDR-BT and EBRT.
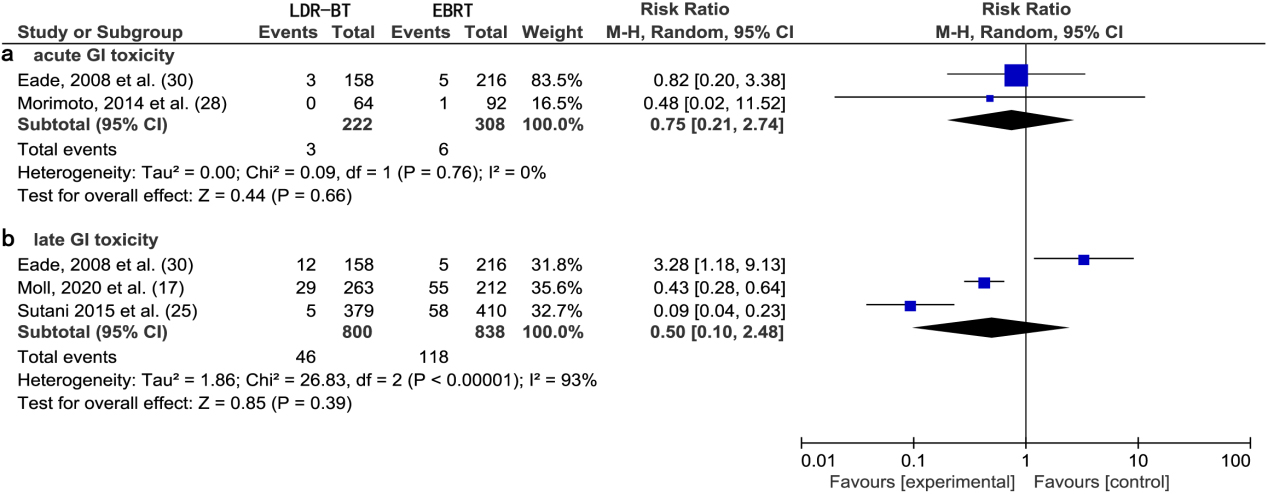
Figure 5.
(a) Forest plot of RR for acute GU toxicity following LDR-BT and EBRT. (b) Forest plot of RR for late GU toxicity following LDR-BT and EBRT.
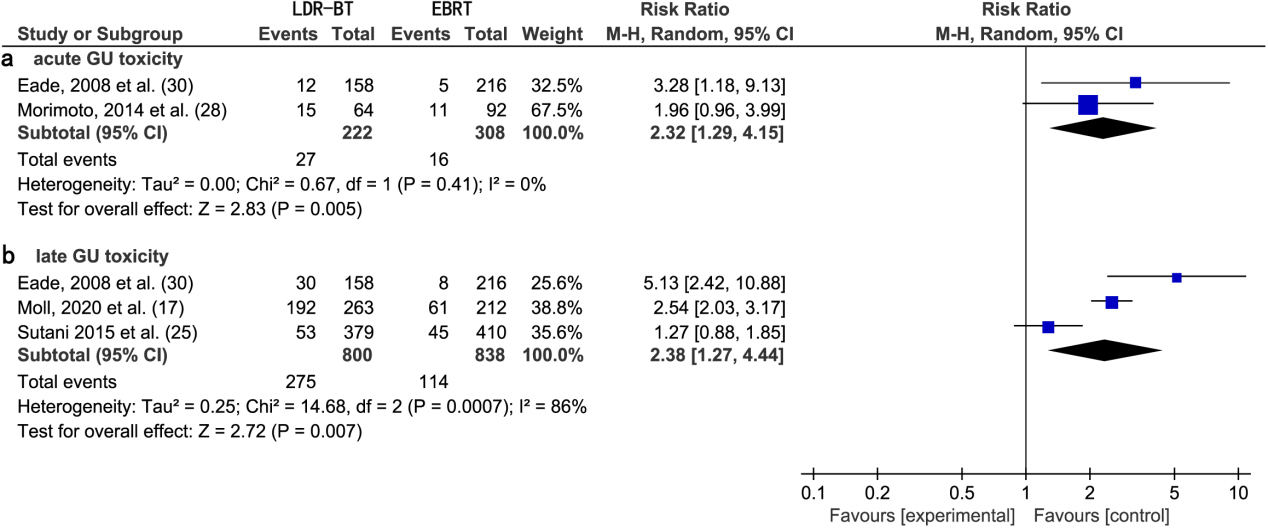
3.6.2Acute and late GU toxicity
A meta-analysis of two studies [28, 30] revealed that the RR of acute GU toxic complications from EBRT was significantly lower than that from LDR-BT alone (2.32; 95% CI, 1.29–4.15;
3.7Meta-analysis of GI and GU toxicities between HDR-BT and LDR-BT
3.7.1Acute GI toxicity and GU toxicity
A meta-analysis of two studies [20, 28] revealed no significant difference in the RR of acute GI toxic complications between HDR-BT and LDR-BT alone (4.14; 95% CI, 0.84–20.40;
Figure 6.
(a) Forest plot of RR for acute GI toxicity following HDR-BT and LDR-BT. (b) Forest plot of RR for acute GU toxicity following HDR-BT and LDR-B.
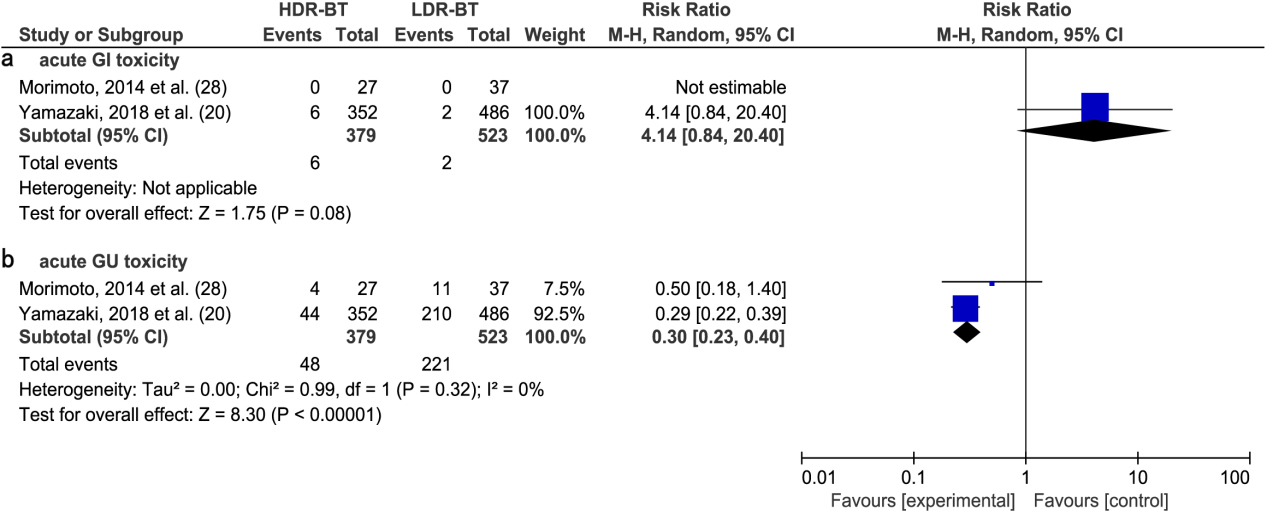
4.Discussion
With the results of this meta-analysis, we evaluated the significance of RRs of GI toxicities and GU toxicities in studies by comparing the following therapies: (A) LDR-BT and LDR-BT
The varied RT doses in studies must be considered when comparing the RRs of GI and GU toxicities between different therapies [31]. BT
Testosterone levels may decline as a result of hormone therapy (HT) on urinary toxicity [42, 43]. HT itself may affect urinary symptoms: this must be considered when comparing both the tumor outcome and toxicity of BT and BT
Large, population-based cancer patient cohort studies show that BT
Previous studies reported a 15 years disease-free survival rate of 80.4% after treatment of men with localized prostate cancer with BT [57, 58]. Another studies reported a 5 years and 10 years OS rate of 94% and 84%, respectively, among similar patients [59]. These findings confirm the long-term efficacy of BT treatment for localized prostate cancer. Kee et al. [60]. Published a meta-analysis of clinical randomized control trials, which revealed a significantly higher 5 years biochemical progression-free survival with
The literature search and screen of this meta-analysis were strict, the included studies were comprehensive, and outcomes were highly credible. However, our analysis has several limitations: (A) the total number of included studies was small (
5.Conclusions
Our findings implied that BT with and without EBRT can result in both acute and late GI toxic and GU toxic complications in men with localized prostate cancer, with LDR-BT leading to a poorer urinary function than EBRT. The results of this study reveal the need to prevent GI and GU toxic complications after multiple forms of radiotherapy in the future. Prospective clinical studies are needed to verify and expand on our results. When making decisions for treating local prostate cancer, clinicians should balance the effectiveness of different radiotherapies with their safety depends on the actual clinical characteristics.
Acknowledgments
This research was supported by the Natural Science Foundation of Fujian Province (2022J011393).
Conflict of interest
None to report.
References
[1] | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) ; 136: (5): 359-386. doi: 10.1002/ijc.29210. |
[2] | Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys. (2017) ; 98: (2): 275-285. doi: 10.1016/j.ijrobp.2016.11.026. |
[3] | Citrin DE. Recent developments in radiotherapy. N Engl J Med. (2017) ; 377: (11): 1065-1075. doi: 10.1056/NEJMra1608986. |
[4] | Lee DJ, Barocas DA, Zhao Z, Huang LC, Resnick MJ, Koyoma T, et al. Comparison of patient-reported outcomes after external beam radiation therapy and combined external beam with low-dose-rate brachytherapy boost in men with localized prostate cancer. Int J Radiat Oncol Biol Phys. (2018) ; 102: (1): 116-126. doi: 10.1016/j.ijrobp.2018.05.043. |
[5] | Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2019) ; 17: (5): 479-505. doi: 10.6004/jnccn.2019.0023. |
[6] | Tanaka N, Asakawa I, Nakai Y, Miyake M, Anai S, Fujii T, et al. Comparison of PSA value at last follow-up of patients who underwent low-dose rate brachytherapy and intensity-modulated radiation therapy for prostate cancer. BMC Cancer. (2017) ; 17: (1): 573-581. doi: 10.1186/s12885-017-3565-1. |
[7] | Goy BW, Burchette R. Ten year treatment outcomes of radical prostatectomy vs external beam radiation therapy vs brachytherapy for 1,503 patients with intermediate risk prostate cancer. Brachytherapy. (2021) ; 20: (6): 1083-1089. doi: 10.1016/j.brachy.2021.04.004. |
[8] | Chin J, Rumble RB, Kollmeier M, Heath E, Efstathiou J, Dorff T, et al. Brachytherapy for patients with prostate cancer: American society of clinical oncology/cancer care ontario joint guideline update. J Clin Oncol. (2017) ; 35: (15): 1737-1743. doi: 10.1200/jco.2016.72.0466. |
[9] | Chao M, Joon DL, Khoo V, Spencer S, Ho H, Guerrieri M, et al. Combined low dose rate brachytherapy and external beam radiation therapy for intermediate-risk prostate cancer. J Med Imaging Radiat Sci. (2019) ; 50: (1): 82-86. doi: 10.1016/j.jmir.2018.09.010. |
[10] | Taira AV, Merrick GS, Butler WM, Galbreath RW, Lief J, Adamovich E, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. (2011) ; 79: (5): 1336-1342. doi: 10.1016/j.ijrobp.2010.01.005. |
[11] | Goy BW, Soper MS, Chang T, Slezak JM, Cosmatos HA, Tome M. Treatment results of brachytherapy vs. external beam radiation therapy for intermediate-risk prostate cancer with 10-year followup. Brachytherapy. (2016) ; 15: (6): 687-694. doi: 10.1016/j.brachy.2016.06.015. |
[12] | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Rev Esp Nutr Hum Diet. (2009) ; 6: (7): e1000100. doi: 10.1371/journal.pmed.1000100. |
[13] | Zhang S, Liang F, Tannock I. Use and misuse of common terminology criteria for adverse events in cancer clinical trials. BMC Cancer. (2016) ; 16: (1): 392-398. doi: 10.1186/s12885-016-2408-9. |
[14] | Yoshida K, Yamazaki H, Nakamara S, Masui K, Kotsuma T, Akiyama H, et al. Comparison of common terminology criteria for adverse events v3.0. and radiation therapy oncology group toxicity score system after high-dose-rate interstitial brachytherapy as monotherapy for prostate cancer. Anticancer Res. (2014) ; 34: (4): 2015-2018. PMID: 24692740. |
[15] | Cox JD, Stetz JA, Pajak TFJIJROBP. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. (1995) ; 31: (5): 1341-1346. doi: 10.1016/0360-3016(95)00060-c. |
[16] | Taniguchi T, Iinuma K, Kato D, Takai M, Maekawa YM, Nakane K, et al. Predictive factors of rectal hemorrhage in patients with localized prostate cancer who underwent low-dose-rate brachytherapy. Int J Clin Oncol. (2020) ; 25: (9): 1711-1717. doi: 10.1007/s10147-020-01713-x. |
[17] | Moll M, Paschen C, Zaharie A, Berndl F, Goldner G. Treatment of low-risk prostate cancer: A retrospective study with 477 patients comparing external beam radiotherapy and I-125 seeds brachytherapy in terms of biochemical control and late side effects. Strahlenther Onkol. (2020) ; 197: (2): 118-123. doi: 10.1007/s00066-020-01657-1. |
[18] | Tanaka N, Yorozu A, Kikuchi T, Higashide S, Kojima S, Ohashi T, et al. Genitourinary toxicity after permanent iodine-125 seed implantation: The nationwide Japanese prostate cancer outcome study of permanent iodine-125 seed implantation (J-POPS). Brachytherapy. (2019) ; 18: (4): 484-492. doi: 10.1016/j.brachy.2019.03.007. |
[19] | Tanaka T, Yorozu A, Sutani S, Yagi Y, Nishiyama T, Shiraishi Y, et al. Predictive factors of long-term rectal toxicity following permanent iodine-125 prostate brachytherapy with or without supplemental external beam radiation therapy in 2216 patients. Brachytherapy. (2018) ; 17: (5): 799-807. doi: 10.1016/j.brachy.2018.05.008. |
[20] | Yamazaki H, Masui K, Suzuki G, Nakamura S, Yamada K, Okihara K, et al. High-dose-rate brachytherapy monotherapy versus low-dose-rate brachytherapy with or without external beam radiotherapy for clinically localized prostate cancer. Radiother Oncol. (2018) ; 132: (1): 162-170. doi: 10.1016/j.radonc.2018.10.020. |
[21] | Maki S, Itoh Y, Kubota S, Okada T, Nakahara R, Ito J, et al. Clinical outcomes of 125I brachytherapy with and without external-beam radiation therapy for localized prostate cancer: Results from 300 patients at a single institution in Japan. J Radiat Res. (2017) ; 58: (6): 870-880. doi: 10.1093/jrr/rrx051. |
[22] | Katayama N, Yorozu A, Maruo S, Kojima S, Ohashi T, Tanaka N, et al. Predictive factors of rectal toxicity after permanent iodine-125 seed implantation: Prospective cohort study in 2339 patients. Brachytherapy. (2016) ; 15: (6): 736-745. doi: 10.1016/j.brachy.2016.09.001. |
[23] | Mukai Y, Hayashi N, Koike I, Kaizu H, Takano S, Sugiura M, et al. Acute and late toxicities in localized prostate cancer patients treated with low-dose I brachytherapy (110 Gy) in combination with external beam radiation therapy versus brachytherapy alone (160 Gy). J Contemp Brachytherapy. (2018) ; 10: (5): 397-404. doi: 10.5114/jcb.2018.79379. |
[24] | Ohashi T, Yorozu A, Saito S, Tanaka N, Katayama N, Kojima S, et al. Urinary and Rectal Toxicity Profiles After Permanent Iodine-125 Implant Brachytherapy in Japanese Men: Nationwide J-POPS Multi-institutional Prospective Cohort Study. Int J Radiat Oncol Biol Phys. (2015) ; 93: (1): 141-149. doi: 10.1016/j.ijrobp.2015.05.014. |
[25] | Sutani S, Ohashi T, Sakayori M, Kaneda T, Yamashita S, Momma T, et al. Comparison of genitourinary and gastrointestinal toxicity among four radiotherapy modalities for prostate cancer: Conventional radiotherapy, intensity-modulated radiotherapy, and permanent iodine-125 implantation with or without external beam radiotherapy. Radiother Oncol. (2015) ; 117: (2): 270-276. doi: 10.1016/j.radonc.2015.08.019. |
[26] | Tanaka N, Asakawa I, Anai S, Hirayama A, Hasegawa M, Konishi N, et al. Periodical assessment of genitourinary and gastrointestinal toxicity in patients who underwent prostate low-dose-rate brachytherapy. Radiat Oncol. (2013) ; 8: (1): 25-32. doi: 10.1186/1748-717X-8-25. |
[27] | Kalakota K, Rakhno E, Pelizzari CA, Liauw SL. Late rectal toxicity after prostate brachytherapy: Influence of supplemental external beam radiation on dose-volume histogram analysis. Brachytherapy. (2010) ; 9: (2): 131-136. doi: 10.1016/j.brachy.2009.08.012. |
[28] | Morimoto M, Yoshioka Y, Konishi K, Isohashi F, Takahashi Y, Ogata T, et al. Comparison of acute and subacute genitourinary and gastrointestinal adverse events of radiotherapy for prostate cancer using intensity-modulated radiation therapy, three-dimensional conformal radiation therapy, permanent implant brachytherapy and high-dose-rate brachytherapy. Tumori. (2014) ; 100: (3): 265-271. doi: 10.1700/1578.17198. |
[29] | Zelefsky MJ, Nedelka MA, Arican Z-L, Yamada Y, Cohen GN, Shippy AM, et al. Combined brachytherapy with external beam radiotherapy for localized prostate cancer: Reduced morbidity with an intraoperative brachytherapy planning technique and supplemental intensity-modulated radiation therapy. Brachytherapy. (2008) ; 7: : 1-6. doi: 10.1016/j.brachy.2007.12.002. |
[30] | Eade TN, Horwitz EM, Ruth K, Buyyounouski MK, D’Ambrosio DJ, Feigenberg SJ, et al. A comparison of acute and chronic toxicity for men with low-risk prostate cancer treated with intensity-modulated radiation therapy or (125)I permanent implant. Int J Radiat Oncol Biol Phys. (2008) ; 71: (2): 338-345. doi: 10.1016/j.ijrobp.2007.10.019. |
[31] | Koga H, Naito S, Ishiyama H, Yorozu A, Saito S, Kojima S, et al. Patient-reported health-related quality of life up to three years after the treatment with permanent brachytherapy: Outcome of the large-scale, prospective longitudinal study in Japanese-Prostate Cancer Outcome Study by Permanent I-125 Seed Implantation (J-POPS). Brachytherapy. (2019) ; 18: (6): 806-813. doi: 10.1016/j.brachy.2019.06.006. |
[32] | Freiberger C, Berneking V, Vögeli T-A, Hermanns RK, Eble MJ 1, Pinkawa M. Quality of life up to 10 years after external beam radiotherapy and/or brachytherapy for prostate cancer. Brachytherapy. (2018) ; 17: (3): 517-523. doi: 10.1016/j.brachy.2018.01.008. |
[33] | Henderson A, Laing RW, Langley SEM. Quality of life following treatment for early prostate cancer: Does low dose rate (LDR) brachytherapy offer a better outcome? A review. Eur Urol. (2004) ; 45: (2): 134-141. doi: 10.1016/j.eururo.2003.09.015. |
[34] | Tsumura H, Tanaka N, Oguchi T, Prante O, Keller M, Maschauer S. Comparative effectiveness of low-dose-rate brachytherapy with or without external beam radiotherapy in favorable and unfavorable intermediate-risk prostate cancer. Sci Rep. (2022) ; 12: (1): 11023-11031. doi: 10.1038/s41598-022-15028-6. |
[35] | Zuber S, Weiß S, Baaske D, Schöpe M, Stevens S, Bodis S, et al. Iodine-125 seed brachytherapy for early stage prostate cancer: A single-institution review. Radiat Oncol. (2015) ; 10: (1): 49-58. doi: 10.1186/s13014-015-0349-0. |
[36] | Horwitz EM, Society AB. ABS brachytherapy consensus guidelines. Brachytherapy. (2012) ; 11: (1): 4-5. doi: 10.1016/j.brachy.2011.12.006. |
[37] | Eapen L, Kayser C, Deshaies Y, Perry G, Morash C, Cygler JE, et al. Correlating the degree of needle trauma during prostate brachytherapy and the development of acute urinary toxicity. Int J Radiat Oncol Biol Phys. (2004) ; 59: (5): 1392-1394. doi: 10.1016/j.ijrobp.2004.01.041. |
[38] | Yamazaki H, Masui K, Suzuki G, Aibe N, Shimizu D, Kimoto T, et al. Comparison of toxicities between ultrahypofractionated radiotherapy versus brachytherapy with or without external beam radiotherapy for clinically localized prostate cancer. Sci Rep. (2022) ; 12: (1): 5055-5065. doi: 10.1038/s41598-022-09120-0. |
[39] | Lee N, Wuu CS, Brody R, Katz AE, Bagiella E, Ennis RD. Factors predicting for postimplantation urinary retention after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. (2000) ; 48: (5): 1457-1460. doi: 10.1016/s0360-3016(00)00784-7. |
[40] | Yamazaki H, Masui K, Suzuki G, Aibe N, Shimizu D, Kimoto T, et al. High-dose-rate brachytherapy with external beam radiotherapy versus low-dose-rate brachytherapy with or without external beam radiotherapy for clinically localized prostate cancer. Sci Rep. (2021) ; 11: (1): 6165-6175. doi: 10.1038/s41598-021-85682-9. |
[41] | Wust P, von Borczyskowski DW, Henkel T, Rosner C, Graf R, Tilly W, et al. Clinical and physical determinants for toxicity of 125-I seed prostate brachytherapy. Radiother Oncol. (2004) ; 73: (1): 39-48. doi: 10.1016/j.radonc.2004.08.003. |
[42] | Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, Comeglio P, et al. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol. (2012) ; 214: (1): 31-43. doi: 10.1530/JOE-12-0142. |
[43] | Jia Y-l, Liu X, Yan J-y, Chong LM, Li L, Ma AC, et al. The alteration of inflammatory markers and apoptosis on chronic prostatitis induced by estrogen and androgen. Int Urol Nephrol. (2015) ; 47: (1): 39-46. doi: 10.1007/s11255-014-0845-4. |
[44] | Pinkawa M, Fischedick K, Gagel B, Piroth MD, Borchers H, Jakse G, et al. Association of neoadjuvant hormonal therapy with adverse health-related quality of life after permanent iodine-125 brachytherapy for localized prostate cancer. Urology. (2006) ; 68: (1): 104-109. doi: 10.1016/j.urology.2006.01.063. |
[45] | Merrick GS, Butler WM, Wallner KE, Allen ZA, Lief JH, Hinerman-Mulroy A, et al. The impact of prostate volume and neoadjuvant androgen-deprivation therapy on urinary function following prostate brachytherapy. Cancer J. (2004) ; 10: (3): 181-189. doi: 10.1097/00130404-200405000-00008. |
[46] | Kishan AU, Steigler A, Denham JW, Zapatero A, Guerrero A, Joseph D, et al. Interplay between duration of androgen deprivation therapy and external beam radiotherapy with or without a brachytherapy boost for optimal treatment of high-risk prostate cancer: A patient-level data analysis of 3 cohorts. JAMA Oncol. (2022) ; 8: (3): 6871-6880. doi: 10.1001/jamaoncol.2021.6871. |
[47] | Zhou X, Jiao D, Dou M, Chen J, Han B, Li Z, et al. Brachytherapy combined with or without hormone therapy for localized prostate cancer: A meta-analysis and systematic review. Front Oncol. (2020) ; 10: (1): 169-178. doi: 10.3389/fonc.2020.00169. |
[48] | Valicenti RK, Winter K, Cox JD, Sandler HM, Bosch W, Vijayakumar S, et al. RTOG 94-06: Is the addition of neoadjuvant hormonal therapy to dose-escalated 3D conformal radiation therapy for prostate cancer associated with treatment toxicity? Int J Radiat Oncol Biol Phys. (2003) ; 57: (3): 614-620. doi: 10.1016/s0360-3016(03)00640-0. |
[49] | Tamihardja J, Lawrenz I, Lutyj P, Weick S, Guckenberger M, Polat B, et al. Propensity score-matched analysis comparing dose-escalated intensity-modulated radiation therapy versus external beam radiation therapy plus high-dose-rate brachytherapy for localized prostate cancer. Strahlenther Onkol. (2022) ; 198: (8): 735-743. doi: 10.1007/s00066-022-01953-y. |
[50] | Wang Z, Ni Y, Chen J, Sun G, Zhang X, Zhao J, et al. The efficacy and safety of radical prostatectomy and radiotherapy in high-risk prostate cancer: A systematic review and meta-analysis. World J Surg Oncol. (2020) ; 18: (1): 42-54. doi: 10.1186/s12957-020-01824-9. |
[51] | Yin M, Zhao J, Monk P, Martin D, Folefac E, Joshi M, et al. Comparative effectiveness of surgery versus external beam radiation with/without brachytherapy in high-risk localized prostate cancer. Cancer Med. (2020) ; 9: (1): 27-34. doi: 10.1002/cam4.2605. |
[52] | Song P, Shu M, Yang L, Di X, Liu P, Liu Z, et al. The prognosis of radical prostatectomy, external beam radiotherapy plus brachytherapy, and external beam radiotherapy alone for patients above 70 years with very high-risk prostate cancer: A population-matched study. Urol Int. (2022) ; 106: (1): 11-19. doi: 10.1159/000518113. |
[53] | Kishan AU, Cook RR, Ciezki JP, Ross AE, Pomerantz MM, Nguyen PL, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9–10 prostate cancer. Jama. (2018) ; 319: (9): 896-905. doi: 10.1001/jama.2018.0587. |
[54] | Rodda S, Tyldesley S, Morris WJ, Keyes M, Halperin R, Pai H, et al. ASCENDE-RT: An analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2017) ; 98: (2): 286-295. doi: 10.1016/j.ijrobp.2017.01.008. |
[55] | Koontz BF, Chino J, Lee WR, Hahn CA, Buckley N, Huang S, et al. Morbidity and prostate-specific antigen control of external beam radiation therapy plus low-dose-rate brachytherapy boost for low, intermediate, and high-risk prostate cancer. Brachytherapy. (2009) ; 8: (2): 191-196. doi: 10.1016/j.brachy.2009.01.002. |
[56] | Pasalic D, Barocas DA, Huang L-C, Zhao Z, Koyama T, Tang C, et al. Five-year outcomes from a prospective comparative effectiveness study evaluating external-beam radiotherapy with or without low-dose-rate brachytherapy boost for localized prostate cancer. Cancer. (2021) ; 127: (11): 1912-1925. doi: 10.1002/cncr.33388. |
[57] | Sylvester JE, Grimm PD, Wong J, Galbreath RW, Merrick G, Blasko JC. Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I(125) prostate brachytherapy in clinically localized prostate cancer: Seattle experience. Int J Radiat Oncol Biol Phys. (2011) ; 81: (2): 376-381. doi: 10.1016/j.ijrobp.2010.05.042. |
[58] | Moll M, Renner A, Kirisits C, Paschen C, Zaharie A, Goldner G. Comparison of EBRT and I-125 seed brachytherapy concerning outcome in intermediate-risk prostate cancer. Strahlenther Onkol. (2021) ; 197: (11): 986-992. doi: 10.1007/s00066-021-01815-z. |
[59] | Martinez E, Daidone A, Gutierrez C, Pera J, Boladeras A, Ferrer F, et al. Permanent seed brachytherapy for clinically localized prostate cancer: Long-term outcomes in a 700 patient cohort. Brachytherapy. (2015) ; 14: (2): 166-172. doi: 10.1016/j.brachy.2014.11.015. |
[60] | Kee DLC, Gal J, Falk AT, Schiappa R, Chand ME, Gautier M, et al. Brachytherapy versus external beam radiotherapy boost for prostate cancer: Systematic review with meta-analysis of randomized trials. Cancer Treat Rev. (2018) ; 70: (1): 265-271. doi: 10.1016/j.ctrv.2018.10.004. |
[61] | Grills IS, Martinez AA, Hollander M, Huang R, Goldman K, Chen PY, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. The Journal of Urology. (2004) ; 117: (3): 1098-1104. doi: 10.1097/01.ju.0000113299.34404.22. |
[62] | Parry MG, Nossiter J, Sujenthiran A, Cowling TE, Patel RN, Morris M, et al. Impact of high-dose-rate and low-dose-rate brachytherapy boost on toxicity, functional and cancer outcomes in patients receiving external beam radiation therapy for prostate cancer: A national population-based study. Int J Radiat Oncol Biol Phys. (2021) ; 109: (5): 1219-1229. doi: 10.1016/j.ijrobp.2020.11.023. |
[63] | Major T, Polgár C, Jorgo K, Stelczer G, Ágoston P. Dosimetric comparison between treatment plans of patients treated with low-dose-rate vs. high-dose-rate interstitial prostate brachytherapy as monotherapy: Initial findings of a randomized clinical trial. Brachytherapy. (2017) ; 16: (3): 608-615. doi: 10.1016/j.brachy.2017.02.003. |
[64] | Strouthos I, Karagiannis E, Zamboglou N, Ferentinos K. High-dose-rate brachytherapy for prostate cancer: Rationale, current applications, and clinical outcome. Cancer Rep (Hoboken). (2022) ; 5: (1): 1450-1465. doi: 10.1002/cnr2.1450. |




