Investigation on the mechanism of Ginkgo Folium in the treatment of Non-alcoholic Fatty Liver Disease by strategy of network pharmacology and molecular docking
Abstract
BACKGROUND:
Ginkgo Folium has a favorable effect on non-alcoholic fatty live disease (NAFLD), but its mechanism remains unclear.
OBJECTIVE:
The aim of this study is to reveal the underlying mechanism of Ginkgo Folium in the treatment of NAFLD.
METHODS:
Ingredients of Ginkgo Folium and ingredients-related genes were collected from TCMSP database and SwissTargetPrediction website, respectively. Genecards database was used to obtain NAFLD-related genes. Next, the protein-protein interaction network and key ingredients-genes network were constructed via Cytoscape3.7.0. Based on the Metascape website, gene ontology function analysis and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis were carried out for key genes. Finally, molecular docking was performed to present the interaction between components and genes using AutoDock Vina 1.1.2.
RESULTS:
Eighteen active ingredients and 10 target genes were screened from Ginkgo Folium. AKT1, TNF, EGFR, PTGS2, MAPK8, PPA
CONCLUSION:
The study showed that multi-components in Ginkgo Folium interacted with AKT1 and regulated AKT-AMPK/HIF pathway to alleviate NAFLD. Our findings provided an essential role and basis for new anti-NAFLD drug discovery and further research on Ginkgo Folium.
1.Introduction
Non-alcoholic fatty liver disease (NAFLD) is represented by excessive fat accumulation in the liver, and it causes non-alcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma [1]. There are 1.8 billion people were attacked by NAFLD worldwide until 2015, and patients with NAFLD face a high risk of cardiovascular disease with a mortality up to 75% [2, 3]. Incidence of NAFLD keeps ever-rising rapidly, which leads to an enormous threat to human health. Unfortunately, there is presently no effective and satisfactory treatment for NAFLD [4]. In decades, traditional Chinese medicine (TCM) treating with major chronic diseases has attracted more and more attention due to its unique efficacy and relative safety [5, 6]. For example, previous studies revealed that Dachaihu decoction had a good effect in attenuating NAFLD [7].
Ginkgo Folium (GF), the dried leave of Ginkgo biloba L. (family Ginkgoaceae), has effects of promoting blood-circulation, removing blood-stasis and reducing lipid [8]. GF contains flavonoids, organic acids, lactones and phenols [9]. Previous studies have discovered that Ginkgo biloba extract 50 has hepatoprotective effects on NAFLD, and its mechanism concerns with the IRS-1, NF
The network pharmacology is built based on the interconnected molecular systems targeting multiple nodes, and it is in line with the mechanism of multiple compounds and multiple targets in TCM. Network pharmacology is now commonly used to reveal the complex mechanism of TCM though cheminformatics and bioinformatics technology [15]. In this study, network pharmacology and molecular docking are used to explore the mechanism of GF in the treatment of NAFLD. The current study is carried out to provide scientific support for the further research of GF and for new drug development in the treatment of NAFLD. The design of this research is shown in Fig. 1.
Figure 1.
The workflow of the study to investigate the mechanism of GF in the treatment of NAFLD.
2.Method
2.1Ingredients and target-genes of GF
Ingredients of GF were collected from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (http://tcmspw.com/tcmsp.php, version 2.3). Oral bioavailability (OB) and drug-likeness (DL) values were selected for evaluation of absorption, distribution, metabolism and excretion of compound [16]. With conditions of OB
2.2Potential genes of GF treating NAFLD
NAFLD-related genes were collected from GeneCards database (http://www.genecards.org/, version 4.14).
Based on GF and NAFLD associated gene datasets, Venn diagram was constructed to identify common genes of NAFLD and GF.
2.3Network construction and analysis
The common genes of NAFLD and GF were input into the Metascape [19], and protein-protein interaction (PPI) network and module analysis were carried out. A node with a higher degree value indicates that it is more important to the network. We considered genes as key genes if its degree value was greater than twice the median of all genes in PPI network [20]. Finally, key ingredients-genes network of GF treating NAFLD was established by Cytoscape 3.7.0 [21].
To cluster the biological functions of key genes, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were run through Metascape.
2.4Molecular docking
The 3D structure of key ingredients and protein were acquired from PubChem database and The Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB; https://www.rcsb.org, version 4.9), respectively [22]. The centroid of the co-crystalized inhibitor (inositol-1,3,4,5-Tetrakisphos-phate) in the crystal structures of protein complex was defined as the binding site. Molecular docking was performed on AutoDock Vina 1.1.2, which was a updated version of docking software with high accuracy and fast speed from the Molecular Graphics Lab [23].
Table 1
The detail information of active compounds in Ginkgo Folium
| Mol ID | Molecule name | Formula | CAS no. | MW | OB (%) | Caco-2 | BBB | DL | FASA- | HL |
|---|---|---|---|---|---|---|---|---|---|---|
| MOL000358 | C | 64997-52-0 | 414.79 | 36.91 | 1.32 | 0.99 | 0.75 | 0.23 | 5.36 | |
| MOL001490 | bis[(2S)-2-ethylhexyl] benzene-1,2-dicarboxylate | C | 117-81-7 | 390.62 | 43.59 | 0.98 | 0.68 | 0.35 | 0.28 | 3.02 |
| MOL005043 | campest-5-en-3beta-ol | C | 474-62-4 | 400.76 | 37.58 | 1.32 | 0.94 | 0.71 | 0.23 | 4.43 |
| MOL003044 | Chryseriol | C | 491-71-4 | 300.28 | 35.85 | 0.39 | 0.27 | 0.32 | 16.31 | |
| MOL002881 | Diosmetin | C | 520-34-3 | 300.28 | 31.14 | 0.46 | 0.27 | 0.34 | 16.34 | |
| MOL002883 | Ethyl oleate (NF) | C | 111-62-6 | 310.58 | 32.4 | 1.4 | 1.1 | 0.19 | 0.19 | 4.85 |
| MOL002680 | Flavoxanthin | C | 512-29-8 | 584.96 | 60.41 | 0.97 | 0.56 | 0.32 | 16.38 | |
| MOL005573 | Genkwanin | C | 437-64-9 | 284.28 | 37.13 | 0.63 | 0.24 | 0.32 | 16.1 | |
| MOL000354 | Isorhamnetin | C | 480-19-3 | 316.28 | 49.6 | 0.31 | 0.31 | 0.32 | 14.34 | |
| MOL000422 | Kaempferol | C | 520-18-3 | 286.25 | 41.88 | 0.26 | 0.24 | 0 | 14.74 | |
| MOL009278 | Laricitrin | C | 53472-37-0 | 332.28 | 35.38 | 0.34 | 0.34 | 14.22 | ||
| MOL007179 | Linolenic acid ethyl ester | C | 1191-41-9 | 306.54 | 46.1 | 1.48 | 1.09 | 0.2 | 0.24 | 5.8 |
| MOL000006 | Luteolin | C | 491-70-3 | 286.25 | 36.16 | 0.19 | 0.25 | 0.39 | 15.94 | |
| MOL011597 | Luteolin-4’-glucoside | C | 6920-38-3 | 448.41 | 41.97 | 0.79 | 0.34 | 16.19 | ||
| MOL001494 | Mandenol | C | 544-35-4 | 308.56 | 42 | 1.46 | 1.14 | 0.19 | 0.25 | 5.39 |
| MOL000098 | Quercetin | C | 117-39-5 | 302.25 | 46.43 | 0.05 | 0.28 | 0.38 | 14.4 | |
| MOL001558 | Sesamin | C | 607-80-7 | 354.38 | 56.55 | 0.75 | 0.83 | 0.31 | 13.44 | |
| MOL000449 | Stigmasterol | C | 83-48-7 | 412.77 | 43.83 | 1.44 | 1 | 0.76 | 0.22 | 5.57 |
| MOL011604 | Syringetin | C | 4423-37-4 | 346.31 | 36.82 | 0.05 | 0.37 | 0.32 | 14.74 |
3.Results
3.1Identification of active components of GF
In the TCMSP database, Ginkgo Folium was searched, and a total of 307 compounds were found. There were 19 compounds satisfied the condition of OB
3.2Identification of common genes of NAFLD and GF
338 GF-related genes and 980 NAFLD-related genes were retrieved from SwissTargetPrediction and Genecards database, respectively. After mapping Venn diagram based on GF and NAFLD associated gene dataset, 98 overlap genes of NAFLD and GF were extracted (Fig. 2).
3.3Network construction and analysis
98 overlapping genes were inputted into the Metascape for PPI network and MCODE-based module analysis. Because CA1 had no interaction with the other genes and was excluded, 97 nodes and 608 edges were in PPI network, as shown in Fig. 3a. According to the criteria that degree of major hub was more than twice the median of all nodes in PPI network, 10 key target-genes were procured. They were AKT1, TNF, EGFR, PTGS2, MAPK8, PPAR
Table 2
The detailed information of the target genes for GF in the treatment of NAFLD
| Gene code | Protein | Uniprot ID | Degree |
|---|---|---|---|
| AKT1 | RAC- | P31749 | 54 |
| TNF | Tumor necrosis factor | P01375 | 42 |
| EGFR | Epidermal growth factor receptor | P00533 | 34 |
| PTGS2 | Cyclooxygenase-2 | P35354 | 34 |
| MAPK8 | c-Jun N-terminal kinase 1 | P45983 | 32 |
| PPAR | Peroxisome proliferator-activated receptor | P37231 | 31 |
| APP | P05067 | 30 | |
| ESR1 | Estrogen receptor | P03372 | 26 |
| HIF1 | Hypoxia-inducible factor 1 | Q16665 | 23 |
| PPAR | Peroxisome proliferator-activated receptor | Q07869 | 23 |
Figure 2.
The Venn diagram of the common genes between GF and NAFLD (blue part marks the unique genes of GF, red part marks the unique genes of NAFLD, and the purple part is the common genes).
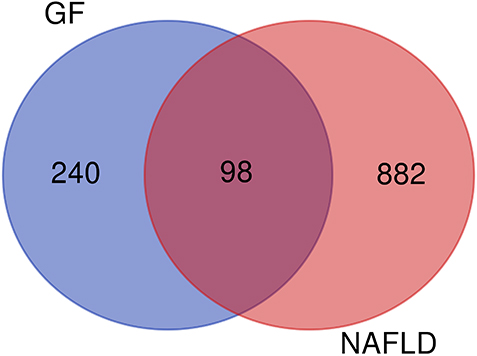
Figure 3.
PPI network of potential target-genes of GF in the treatment of NAFLD (a is PPI network, b is densely linked modules of PPI network; line represents the interaction relationship, nodes represent the target-genes; node size is proportional to its degree in the network, and red belongs to the module of response to reactive oxygen species, blue belongs to the module of G protein coupled receptor transport regulation, green belongs to the module of cell response to insulin stimulation, purple belongs to the module of protein modification regulation, orange belongs to the module of nuclear receptor activity).
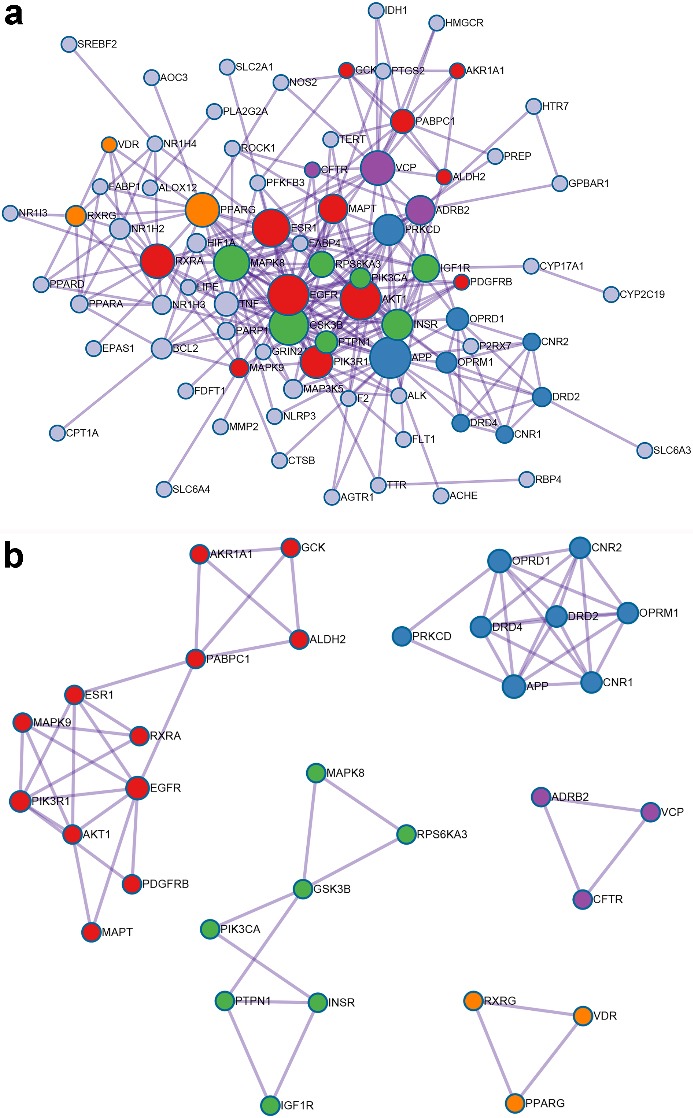
Figure 4.
The key bioactive ingredients-genes network for GF in the treatment of NAFLD (
Figure 5.
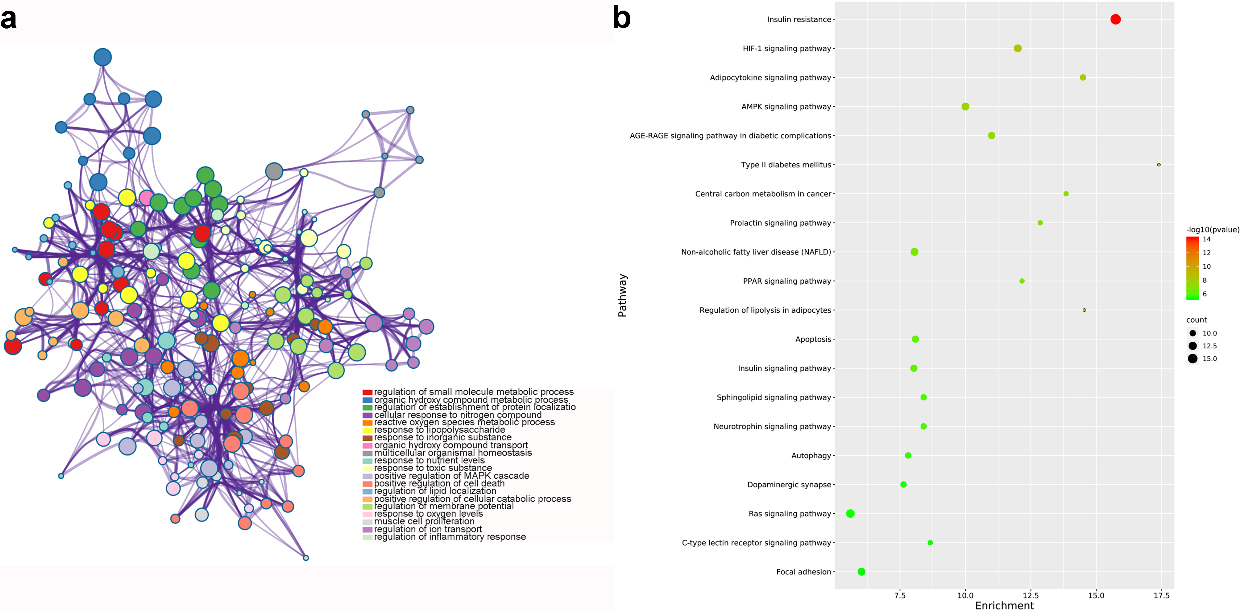
GO and KEGG analysis of common genes for GF in the treatment of NAFLD (nodes in a represent functions of common genes. node size is proportional to its counts, and node color is different with different function; b shows pathways of common genes. node color range from green to red represent -log
The PPI network was consisted with five densely linked modules, including response to reactive oxygen species, G protein coupled receptor transport regulation, cell response to insulin stimulation, protein modification regulation and nuclear receptor activity (Fig. 3b).
Flavoxanthin did not interact with any genes and was excluded. A total of 18 compounds in GF involved with 10 key target-genes. The data was entered into Cytoscape to construct a key ingredients-genes network, as shown in Fig. 4.
3.4GO and KEGG analysis
From GO analysis, results suggested that target genes were mainly enriched in regulation of small molecule metabolic process, nuclear receptor activity and RNA polymerase II transcription factor complex. Figure 5a highlighted the top 20 GO terms with smallest
3.5Molecular docking
In order to clarify mechanism of GF in the treatment of NAFLD, molecular docking was used to present interaction between key compound and protein. We selected AKT1 with the highest degree value (PDB ID: 1h10) and 18 compounds of GF for molecular docking. The docking results of 18 compounds of GF were compared with endogenous ligand (inositol-1,3,4,5-Tetrakisphosphate) of AKT1. The affinity value (reversely represented the degree of docking coincidence of molecules) and number of hydrogen bonds are listed in Table 3. Results showed that the affinity values of luteolin-4’-glucoside, sesamin, luteolin, chryseriol, isorhamnetin and laricitrin with AKT1 were lower than
Table 3
The docking results of 18 key bioactive compounds of GF to AKT1 protein, compared with endogenous ligand (inositol-1,3,4,5-Tetrakisphosphate) of AKT1
| Compound | Formula | CAS number | Binding energy (kcal/mol) | Binding-site number |
|---|---|---|---|---|
| Inositol-1,3,4,5-Tetrakisphosphate | C | 102850-29-3 | 21 | |
| Luteolin-4’-glucoside | C | 6920-38-3 | 9 | |
| Sesamin | C | 607-80-7 | 6 | |
| Quercetin | C | 117-39-5 | 5 | |
| Luteolin | C | 491-70-3 | 7 | |
| Chryseriol | C | 491-71-4 | 6 | |
| Isorhamnetin | C | 480-19-3 | 6 | |
| Diosmetin | C | 520-34-3 | 5 | |
| Laricitrin | C | 53472-37-0 | 7 | |
| Kaempferol | C | 520-18-3 | 4 | |
| Syringetin | C | 4423-37-4 | 6 | |
| Genkwanin | C | 437-64-9 | 4 | |
| Stigmasterol | C | 83-48-7 | 3 | |
| Campesterol | C | 474-62-4 | 3 | |
| C | 64997-52-0 | 2 | ||
| Bis (2-ethylhexyl) phthalate | C | 117-81-7 | 6 | |
| Linolenic acid ethyl ester | C | 1191-41-9 | 3 | |
| Ethyl linoleate | C | 544-35-4 | 2 | |
| Ethyl oleate | C | 111-62-6 | 5 |
Figure 6.
Docking model of endogenous ligand (inositol-1,3,4,5-tetraphosphate) (a) and luteolin-4’-glucoside (b) with AKT1 protein.
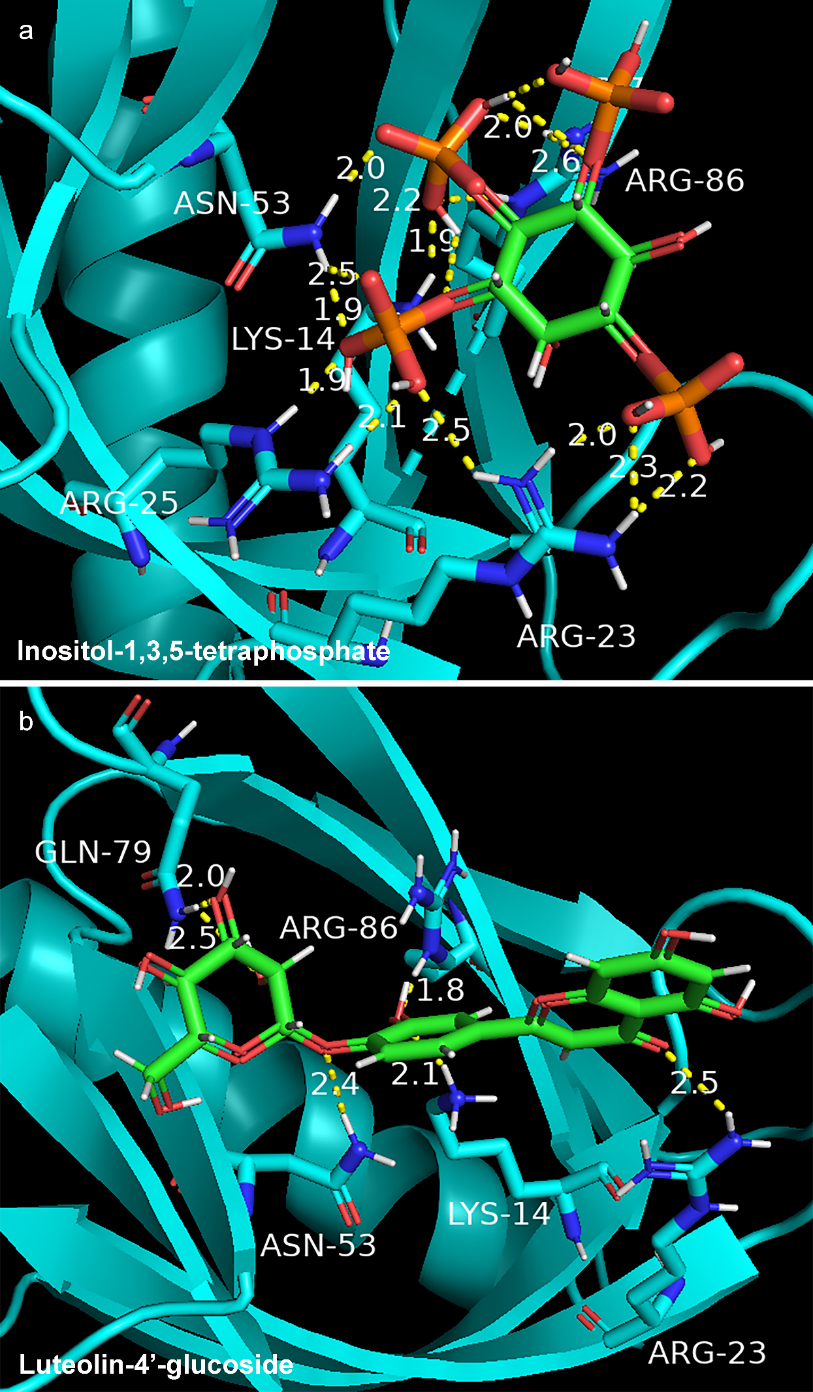
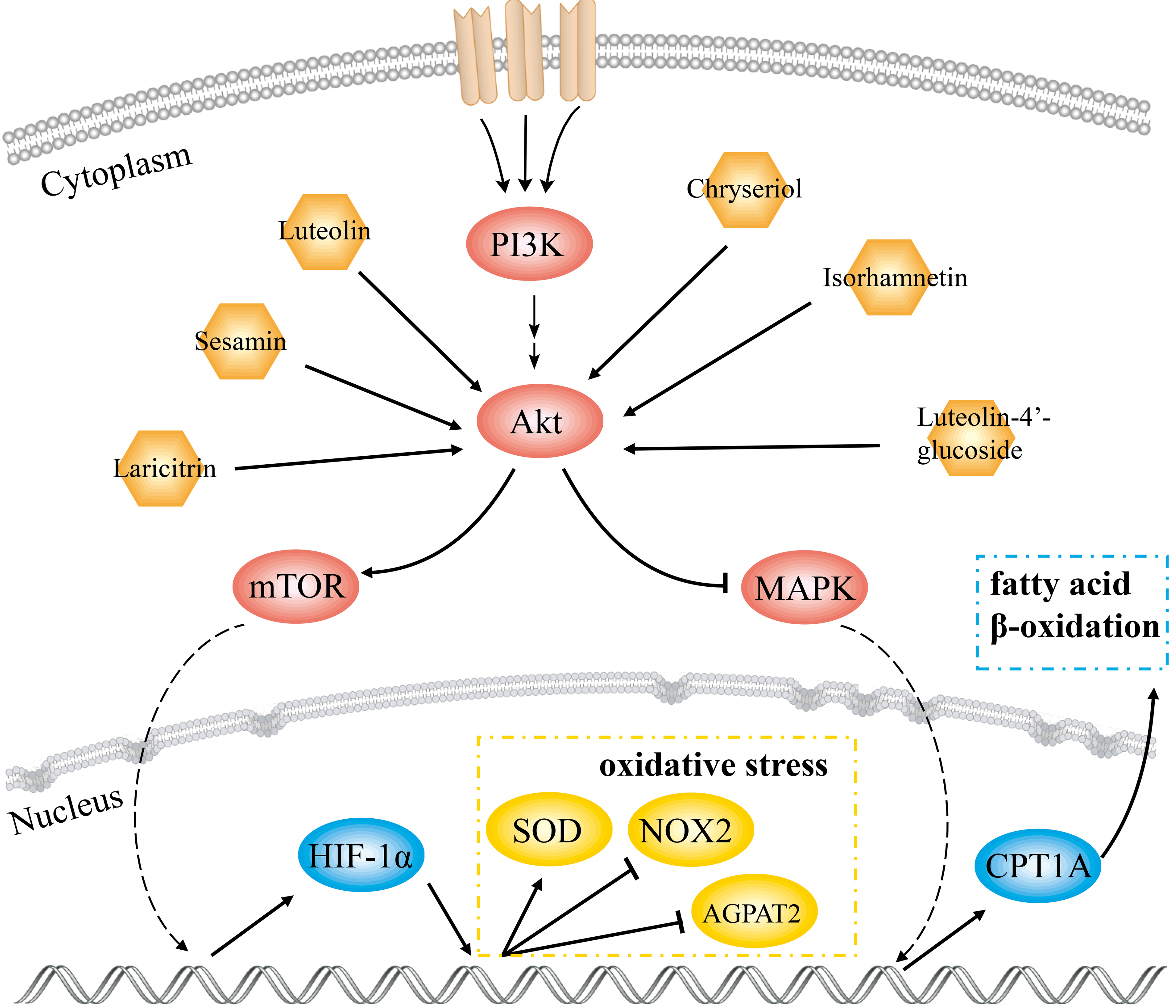
Figure 7.
Schematic diagram of the molecular mechanism of GF in the treatment of NAFLD.
4.Discussion
Globally, NAFLD is the most prevalent liver disease, which seriously threatens human health. Mechanisms for NAFLD progression and development are complex and multifactorial [24]. Studies have shown that NAFLD is influenced significantly by insulin resistance [25]. It increases production and secretion of adipokines and inflammatory cytokines [26]. Triglycerides accumulation in the liver causes lipo-toxicity and leads to oxidative stress and overloaded reactive oxygen species [27]. Additionally, altered gut flora causes increased fatty acid absorption and activated inflammatory [28]. Several factors contribute to the pathogenesis of NAFLD, including inflammation, lipo-toxicity and steatosis, as indicated by changes in serum biochemistry and histopathological features [29]. Previous studies indicated that GF has a favorable effect on regulating lipid metabolism [14], yet, its mechanism remains unclear.
In this study, 18 key compounds and 10 target genes of GF treating NAFLD were identified. AKT1 with highest degree value played a pivotal role in the PPI network. Results of GO and KEGG analysis showed that the mechanism of GF against NAFLD was associated with cell apoptosis, HIF-1, adipocytokine and AMPK signaling pathways. Molecular docking results displayed that luteolin-4’-glucoside, sesamin, luteolin, chryseriol, isorhamnetin and laricitrin had strong affinity with AKT1. This work indicated that these compounds were effective substances for GF in treatment of NAFLD.
Luteolin-4’-glucoside has an anti-dyslipidemia effect and inhibits expression of sterol regulatory element-binding protein-1 and
Overactivation of HIF-
5.Conclusion
The underlying mechanism of GF against NAFLD was investigated by network pharmacology combined with molecular docking. Our findings showed that GF alleviated NAFLD through interactions between effective ingredients and AKT1 and regulation of AKT-AMPK/HIF pathway. The present study revealed the molecular biological mechanism of GF against NAFLD and provided a basis to the clinical treatment of GF.
Acknowledgments
This research was supported by the Horizontal Research Project of Shanghai Jiao Tong University (Nos SA1700111, SA1700118), a project supported by the Department of Science and Technology of Guizhou (Grant no. [2018] 2831), and the Open Research Fund of NMPA Key Laboratory for Rapid Testing Technology of Drugs, Guangdong Institute for Drug Control (Nos KF2022002, KF2022006).
Conflict of interest
None to report.
References
[1] | Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med. (2017) ; 15: (1): 45. |
[2] | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) ; 64: (1): 73-84. |
[3] | Byrne CD, Targher G. NAFLD: A multisystem disease. J Hepatol. (2015) ; 62: (1 Suppl): S47-64. |
[4] | Antonucci L, Porcu C, Iannucci G, Balsano C, Barbaro B. Non-alcoholic fatty liver disease and nutritional implications: Special focus on copper. Nutrients. (2017) ; 9: (10): 1137. |
[5] | Xiong C, Li Y, Zhuang G, Zeng Y, Wei H, Li C, et al. Clinical efficacy and safety of Chinese herbal medicine versus placebo for the treatment of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Complement Ther Med. (2021) ; 59: : 102691. |
[6] | Dai X, Feng J, Chen Y, Huang S, Shi X, Liu X, et al. Traditional Chinese Medicine in nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Chin Med. (2021) ; 16: (1): 68. |
[7] | Yang JM, Sun Y, Wang M, Zhang XL, Zhang SJ, Gao YS, et al. Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J Gastroenterol. (2019) ; 25: (34): 5105-5119. |
[8] | Zhang PF, Liao LJ, Deng Z, Tan YP. Research progress of pharmacological effects and clinical application of Ginkgo biloba extract (in Chinese). Liaoning J Trad Chin Med. (2017) ; 44: (2): 426-429. |
[9] | Cheng WZ, Zhang PZ. Advances in pharmacology and clinical research of Ginkgo biloba extract (Part 1) (in Chinese). Chin J New Drugs and Clin Rem. (1999) ; 18: (5): 315-317. |
[10] | Li L, Yang L, Yang F, Zhao XL, Xue SJ, Gong FH. Ginkgo biloba Extract 50 (GBE50) Ameliorates Insulin Resistance, Hepatic Steatosis and Liver Injury in High Fat Diet-Fed Mice. J Inflamm Res. (2021) ; 14: : 1959-1971. |
[11] | Zhou ZY, Tang SQ, Zhou YM, Luo HS, Liu X. Antioxidant and hepatoprotective effects of extract of ginkgo biloba in rats of non-alcoholic steatohepatitis. Saudi Med J. (2010) ; 31: (10): 1114-1118. |
[12] | Tang JH, Ye XY, Liu J, Li P, Zhang Q, Hu JJ. Effects of Ginkgo biloba flavonoids on glucose and lipid metabolism and liver function in insulin resistant rats (in Chinese). J Shanghai Jiaotong U (Med Sci). (2009) ; 29: (2): 150-153. |
[13] | Xie ZQ. Study on the molecular mechanisms of Ginkgo biloba leaf extract in liver lipid metabolism regulation. Shanghai: Shanghai Jiaotong University; (2009) . |
[14] | Yan Z, Fan R, Yin S, Zhao X, Liu J, Li L, et al. Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int J Biol Macromol. (2015) ; 80: : 573-580. |
[15] | Luo TT, Lu Y, Yan SK, Xiao X, Rong XL, Guo J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin J Integr Med. (2020) ; 26: (1): 72-80. |
[16] | Gao L, Wang XD, Niu YY, Duan DD, Yang X, Hao J, et al. Molecular targets of Chinese herbs: A clinical study of hepatoma based on network pharmacology. Sci Rep. (2016) ; 6: : 24944. |
[17] | Kim S, Chen J, Cheng TJ, Gindulyte A, He J, He SQ, et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. (2019) ; 47: (D1): D1102-D1109. |
[18] | Daina A, Michielin O, Zoete V. Swiss Target Prediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. (2019) ; 47: (W1): W357-W364. |
[19] | Zhou YY, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. (2019) ; 10: : 1523. |
[20] | Zhang J, Jiang YY, Liu N, Shen T, Jung HW, Liu JX, et al. A Network-Based Method for Mechanistic Investigation and Neuroprotective Effect on Post-treatment of Senkyunolid-H Against Cerebral Ischemic Stroke in Mouse. Front Neurol. (2019) ; 10: : 14. |
[21] | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. (2003) ; 13: (11): 2498-504. |
[22] | Burley SK, Berman HM, Christie C, Duarte JM, Feng ZK, Westbrook J, et al. RCSB protein data bank: Sustaining a living digital data resource that enables breakthroughs in scientific research and biomedical education. Protein Sci. (2018) ; 27: (1): 316-330. |
[23] | Trott O, Olson AJ. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. (2010) ; 31: (2): 455-461. |
[24] | Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and Its Management. Nutr Clin Pract. (2020) ; 35: (1): 72-84. |
[25] | Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. (2019) ; 76: (1): 99-128. |
[26] | Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. (2008) ; 9: (5): 367-377. |
[27] | Yazıcı D, Sezer H. Insulin Resistance, Obesity and Lipotoxicity. Adv Exp Med Biol. (2017) ; 960: : 277-304. |
[28] | Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. (2015) ; 48: (13-14): 923-930. |
[29] | Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) – pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. (2017) ; 49: (2): 197-211. |
[30] | Sa C, Oliveira AR, Machado C, Azevedo M, Pereira-Wilson C. Effects on liver lipid metabolism of the naturally occurring dietary flavone luteolin-7-glucoside. Evid-Based Compl Alt. (2015) ; 2015: : 647832. |
[31] | Yin Y, Gao L, Lin HY, Wu Y, Han X, Zhu YX, et al. Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochem Bioph Res Co. (2017) ; 482: (4): 720-726. |
[32] | Zhu XH, Xiong T, Liu PY, Guo XP, Xiao L, Zhou F, et al. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem Toxicol. (2018) ; 114: : 52-60. |
[33] | Ganbold M, Owada Y, Ozawa Y, Shimamoto Y, Ferdousi F, Tominaga K, et al. Isorhamnetin alleviates steatosis and fibrosis in mice with nonalcoholic steatohepatitis. Sci Rep. (2019) ; 9: : 16210. |
[34] | Zheng FF, Guo XM, Zhong BL and Jiang XY. The protective effect of diosmetin on non-alcoholic fatty liver disease of young rats. Chin J Clin Anat. (2018) ; 36: (5): 520-526. |
[35] | Feng SM, Dai ZQ, Liu AB, Huang JB, Narsipur N, Guo G, et al. Intake of stigmasterol and beta-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. BBA-Mol Cell Biol L. (2018) ; 1863: (10): 1274-1284. |
[36] | Simonen P, Gylling H, Miettinen TA. The validity of serum squalene and non-cholesterol sterols as surrogate markers of cholesterol synthesis and absorption in type 2 diabetes. Atherosclerosis. (2008) ; 197: (2): 883-888. |
[37] | Pan ZX, Wang JW, Tang H, Li L, Lv J, Han CC, et al. Effects of linoleate on cell viability and lipid metabolic homeostasis in goose primary hepatocytes. Comp Biochem Phys A. (2011) ; 159: (2): 113-118. |
[38] | Murai A, Furuse M, Okumura J. Role of dietary gamma-linolenic acid in liver lipid metabolism in Japanese quail. Brit Poultry Sci. (1995) ; 36: (5): 821-827. |
[39] | Li J, Yuan YQ, Zhang L, Zhang H, Zhang SW, Zhang Y, et al. Exogenous hydrogen sulfide protects against high glucose-induced apoptosis and oxidative stress by inhibiting the STAT3/HIF-1 alpha pathway in H9c2 cardiomyocytes. Exp Therc Med. (2019) ; 18: (5): 3948-3958. |
[40] | Triantafyllou EA, Georgatsou E, Mylonis I, Simos G, Paraskeva E. Expression of AGPAT2, an enzyme involved in the glycerophospholipid/triacylglycerol biosynthesis pathway, is directly regulated by HIF-1 and promotes survival and etoposide resistance of cancer cells under hypoxia. BBA-Mol Cell Biol L. (2018) ; 1863: (9): 1142-1152. |
[41] | Guan RJ, Wang J, Li ZY, Ding MJ, Li DF, Xu GH, et al. Sodium tanshinone IIA sulfonate decreases cigarette smoke-induced inflammation and oxidative stress via blocking the activation of MAPK/HIF-1 alpha signaling pathway. Front Pharmacol. (2018) ; 9: : 263. |
[42] | Patsoukis N, Chatterjee P, Sari D, Petkova V, Li LQ, Boussiotis VA. PD-1 induces metabolic reprogramming of activated T cells from glycolysis to lipid oxidation. Blood. (2013) ; 122: (21): 187. |
[43] | Liang DD, Chen HJ, Zhao LP, Zhang WX, Hu J, Liu ZG, et al. Inhibition of EGFR attenuates fibrosis and stellate cell activation in diet-induced model of nonalcoholic fatty liver disease. BBA-Mol Basis of Dis. (2018) ; 1864: (1): 133-142. |
[44] | Zhang YY, Li C, Yao GF, Du LJ, Liu Y, Zheng XJ, et al. Deletion of macrophage mineralocorticoid receptor protects hepatic steatosis and insulin resistance through ER/HGF/Met pathway. Diabetes. (2017) ; 66: (6): 1535-1547. |
[45] | Jin Y, Tan YJ, Chen LP, Liu Y, Ren ZQ. Reactive oxygen species induces lipid droplet accumulation in HepG2 cells by increasing perilipin 2 expression. Int J Mol Sci. (2018) ; 19: (11): 3445. |




