Assessment of the potential value of combining western medicine therapies with traditional chinese medicine in the treatment of COVID-19: Mechanistic perspectives
Abstract
BACKGROUND:
The pandemic caused by the novel coronavirus disease (COVID-19) since early 2020 is one of the most significant global health issues in history. Although there is currently no specific treatment for COVID-19, researchers have provided a whole array of potential treatments, both from the Western medicine approach, which is molecular target and pathogenesis based, and from the traditional Chinese medicine (TCM) approach, which is based on the exposure to toxins/pathogens and the balance of the body to combat them for recovery.
OBJECTIVE:
The aim of this research is to find combinations of Western medicine and TCM that may offer better therapeutic efficacy synergystically with a better adverse events profile. The findings of the research may provide a new insight in the development of the treatment of COVID-19.
METHODS:
From the Western medicine perspective, drugs target the mechanisms of viral infection, including the stages of viral entry (Arbidol, Camostat Mesylate, Convalescent Plasma therapy) and viral replication (Lopinavir/Ritonavir, Redemsivir, Ribavirin). Additional therapies target host defenses, preventing cytokine storms (Tocilizumab) and stimulating the immune system (Interferons). On the other hand, TCM also proposed a number of treatment methods for COVID-19 with new scientific approaches identifying their antiviral and immunomodulatory activities. The novel combination of Western medicine and TCM can be proposed by analyzing their respective molecular targets.
RESULTS:
Although TCM is not generally accepted in the Western community because of the general lack of knowledge on their detailed mechanisms, studies and clinical trials suggest that TCM could be beneficial in combating COVID-19.
CONCLUSION:
Based on the principle of combining TCM and Western medicine, two combinations are tested effective in clinical trials, and three possible combinations that might be effective are proposed in the paper.
1.Introduction
1.1COVID-19
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It demonstrates symptoms similar to pneumonia, including cough, fever, and fatigue [1, 2]. According to Johns Hopkins University & Medicine, since the outbreak of COVID-19 from early 2020, there have been over 600 million confirmed cases and over 6.5 million deaths as of October 2022. After eighteen months of vigorous scientific research, the scientific community has gained significant knowledge on virology, pathogenesis, development of diagnostics and vaccines at an unprecedent speed. However, there is yet no specific cure for COVID-19 patients, and doctors treat patients by merely ameliorating the symptoms with limited options.
Figure 1.
The process of SARS-CoV-2 entering the cell, producing viral particles, and exiting the cell (created with BioRender.com).
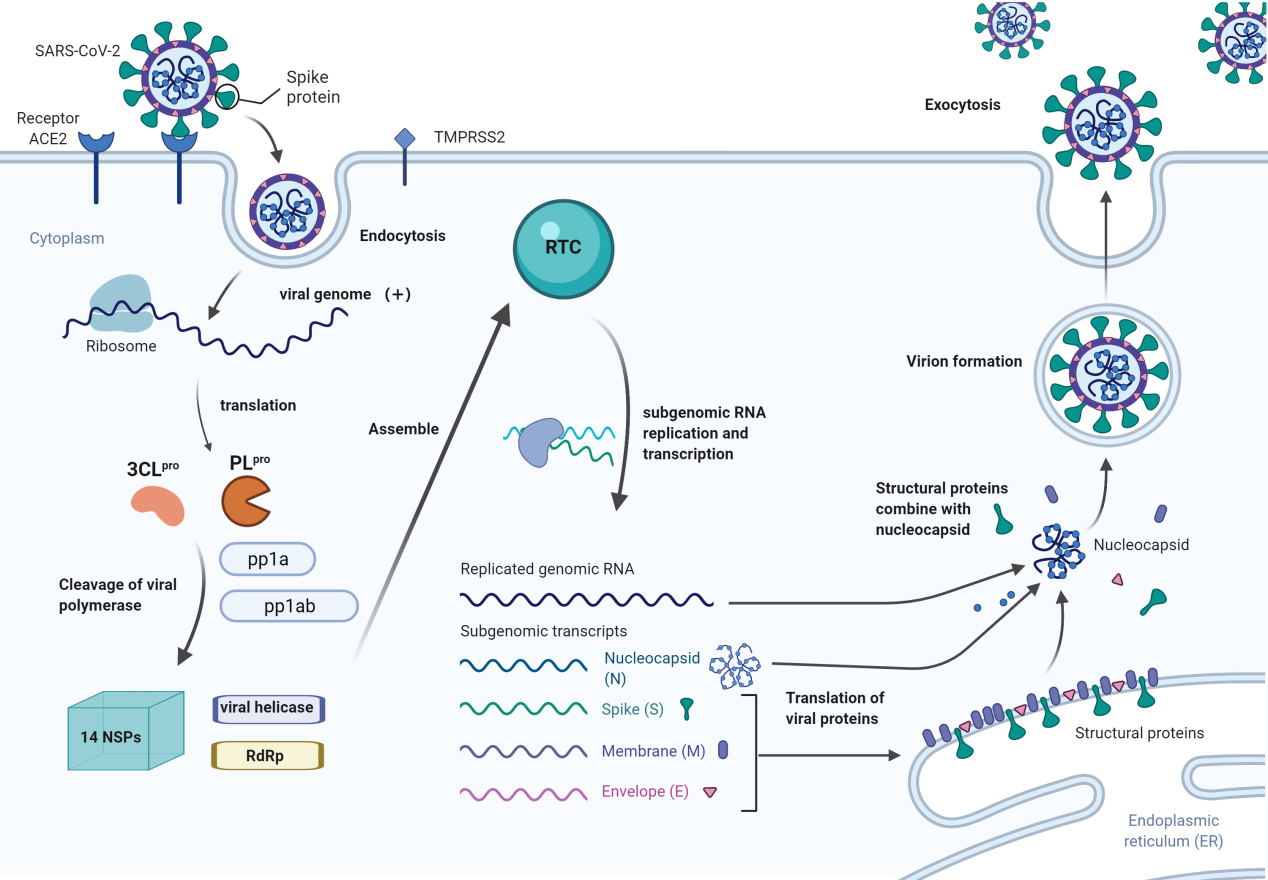
1.2Biological characteristics of SARS-CoV-2
SARS-CoV-2 consists of an enveloped, positive-sense, single-stranded RNA genome that encodes four structural proteins: the spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins [3]. As shown in Fig. 1, SARS-CoV-2 enters the cell by binding its spike protein to the receptor angiotensin-converting enzyme 2 (ACE2) on the cell surface, which causes the activation of the transmembrane protease serine protease 2 (TMPRSS2), which cleaves the spike protein and facilitates entry of the virus into the host cell [4]. After entering the cell, the viral RNA genome is translated into two viral proteases: papain-like protease (PL
Although many drug candidates targeting different aspects of SARS-CoV-2 infection and human response to the virus have been used to attempt to heal patients, none of them individually show a high cure rate. In China, some researchers are evaluating the potential of TCM for the treatment of COVID-19. TCM is not recognized by the Western world due to its unknown efficacy, and thus it has not yet gathered much scientific support. However, in combination with other Western therapies, TCM can show synergistic activities in either direct antiviral effect or for symptomatic relief. This paper will introduce the major therapies for COVID-19 patients using Western medicine and TCM. With the known information available for Western medicine, the potential combination of both Western medicine and TCM for treatment of COVID-19 are discussed and new possible combinations are proposed in the paper.
2.General therapies for COVID-19
2.1Therapies targeting mechanisms of SARS-CoV-2 infection
One of the possible treatment methods for COVID-19 is to target the mechanisms of viral attachment, entry, and replication. In general, SARS-CoV-2 mainly attacks lung epithelial cells, where the greatest concentration of ACE2 receptors is found [6]. The virus enters the cell through Spike protein-ACE2 (S-ACE2) interaction leading to membrane fusion. Then the virus synthesizes its viral proteins by using protease to splice polyprotein into smaller proteins, including RdRp. Therapies that target these mechanisms will be reviewed in the following sections.
2.1.1Blocking virus attachment to the cell
2.1.1.1 Arbidol
Arbidol, also known as Umifenovir, is a Russian-made antiviral drug mainly used to treat influenza in Russia and China. It has been shown to interact with lipids and proteins to alter intracellular viral trafficking and viral maturation in endosomal compartments in the hepatitis C virus model (another RNA virus) [7]. Arbidol has been shown to block S-ACE2 interaction to prevent the fusion between the viral envelope and cell membrane. Thus, the virus cannot enter the cell [8].
An in vitro experiment showed that Arbidol could efficiently inhibit SARS-CoV-2 infections [9]. In a clinical study including 111 patients, Arbidol was shown to promote viral clearance and reduce disease progression in terms of a reduced incidence of severe diseases [10]. However, using Arbidol as treatment is still in doubt since some other clinical studies have shown that Arbidol might not improve symptoms or speed up viral clearance in mild or moderate COVID-19 cases and its long-term side effects remain uncertain [7, 11, 12].
2.1.1.2 Camostat Mesylate
TMPRSS2 is a type of transmembrane serine protease that facilitates SARS-CoV-2 viral infection by priming the S protein thus promoting virus-cell membrane fusion through ACE2.
Camostat Mesylate is a serine protease inhibitor. It has been shown to be effective in blocking SARS-CoV-2 from entering human lung epithelial cells [13]. Other animal studies have reported that Camostat Mesylate is effective at protecting mice from contracting SARS-CoV infections, with a survival rate of 60% from SARS-CoV infection compared to that of lower than 10% using other protease inhibitors or no treatment at all [14]. Although there are not enough clinical studies to support its efficacy, Camostat Mesylate is believed to be a potential option to combat SARS-CoV-2 infection.
2.1.2Blocking membrane fusion
2.1.2.1 Chloroquine/Hydroxychloroquine
Chloroquine and its derivative hydroxychloroquine have been used as antimalarial drugs since the 1940s and play a role in treating autoimmune diseases. Chloroquine has been shown to increase endosomal pH that can have an inhibitory effect on virus-cell fusion by interfering with the glycosylation of the host cellular receptors [15, 16].
A recent study on the antiviral effect of chloroquine against SARS-CoV-2 in Vero E6 cells shows that chloroquine can inhibit viral replication at a low concentration with acceptable cytotoxicity [16]. However, opposite to the outcome of the in vitro study, many recent clinical studies involving hospitalized COVID-19 patients showed no benefits in speeding up viral clearance or reducing mortality of using chloroquine and hydroxychloroquine treatment [17]. Therefore, more treatment methods that combine chloroquine or hydroxychloroquine with other drugs need to be discovered since it is shown to be less effective when working alone.
2.1.3Inhibiting viral protease
2.1.3.1 Lopinavir/Ritonavir
Lopinavir is an HIV-1 protease inhibitor that has been shown to inhibit the viral protease 3CL
Although, Lopinavir/Ritonavir has been shown to be useful in treating SARS-CoV-2 in vitro, clinical trials show a different outcome. Lopinavir/Ritonavir treatment and standard care (treatment based on symptoms) were compared in a clinical study in Wuhan in early 2020 involving 199 confirmed COVID-19 patients. The results showed no difference in clinical improvement or viral load or mortality within 28 days [19]. Another study compared 111 patients treated only with Ribavirin and 41 patients treated with Lopinavir/Ritonavir and Ribavirin. The result showed that patients treated with the combined therapy had a lower risk of death [20]. Similar to the discussion above, the antiviral effect of lopinavir/ritonavir may only be revealed when used in combination with other antivirals.
2.1.4Inhibiting RNA dependent RNA polymerase (RdRp)
2.1.4.1 Remdesivir
Remdesivir is a broad-spectrum antiviral drug that was first developed against the Ebola virus [21]. Remdesivir ameliorates COVID-19 by inhibiting viral RdRp and thus preventing viral replication. Animal studies reported that Remdesivir can significantly reduce the viral load, enhance lung functioning, and minimize pathological detriment in the MERS-CoV infected mice lung [22]. Remdesivir inhibits SARS-CoV-2 replication at low concentrations and acceptable cytotoxicity in vitro [16].
However, for severe COVID-19 patients, clinical studies in China and the USA show inconsistent results. Clinical trials in China showed that there is no significant improvement using Remdesivir [23], despite the fact that it has been shown to reduce the number of hospital stays in severe COVID-19 cases that need ventilation machines to assist breathing in the United States [24]. Subsequently, Wang et al. analyzed over ten thousand SARS-CoV-2 viral genomes from different populations and found that the frequency of potential functional variations of the viral protein, including p.P4715L (which encodes the viral RdRp), is much lower in SARS-CoV-2 patients in China (11.2%) compared to the USA (63.0%). Researchers postulate that such innate genetic variation among the populations might account for the difference in the observed clinical benefits since RdRp, the target of Remdesivir, is produced by the pp1ab [25]. Though this hypothesis remains to be tested further in both in vitro and clinical studies, so far,
2.1.4.2 Ribavirin
Ribavirin is a guanine analog known for antiviral activities and has been found effective in treating hepatitis C virus and respiratory syncytial virus [26]. Ribavirin inhibits viral protein synthesis by interfering with the transcriptional initiation and elongation of RNA fragments [27].
A study evaluated clinical outcomes of Ribavirin involving 132 suspected SARS patients. The results showed that more than half of the patients treated with Ribavirin improved, as shown by multiple clinical indicators [28]. However, there is yet no sufficient evidence to show that Ribavirin is applicable used for therapeutic purposes. In addition, an in vitro study indicated that the high effective dose of Ribavirin needed for COVID-19 patients might cause side effects such as transaminase elevations and hemolytic anemia [28]. Thus, using Ribavirin alone as treatment is not recommended.
2.2Therapies targeting response to the virus
The immune response is critical in the host’s defense against the viral infection. Some of the antibodies can neutralize the virus from infecting more cells. When SARS-CoV-2 attacks the human body, viral antigens are presented to T cells and B cells by the major compatibility complex (MHC) to stimulate innate and adaptive immunity [29]. Inflammatory cytokines, mainly interleukin (IL-1, IL-2, IL-6), interferon (IFN
However, in some patients, when the viral infection is not under control and the immune system is overreacted, cytokines will release uncontrollably, inducing a cytokine storm. Cytokine storms may cause severe collateral tissue damage and acute respiratory distress syndrome (ARDS), a type of respiratory failure. Such damage can further cause lung injury and multiple organ failure, which will lead to death [29, 30]. Therefore, treatments that target cytokine storms are especially essential for severe COVID-19 patients to recover back to the mild stage of viral infection.
2.2.1Target on neutralizing cytokine storm
2.2.1.1 Tocilizumab
One of the cytokines released during the infection of SARS-CoV-2 is interleukin-6 (IL-6). IL-6 protects the host by inducing fever and acute inflammation and recruits other parts of the immune system to assist in the elimination of the viral infected cells [4]. In severe cases, overproduction of IL-6 may lead to impaired diffusion of oxygen in the lungs [31]. Therefore, tackling IL-6 before it is overproduced can be one way to treat COVID-19 patients.
Tocilizumab is a monoclonal immunoglobulin G1 (IgG1) antibody targeting the IL-6 receptor, mitigating the cytokine storms by making IL-6 unable to overproduce [32]. A study including 21 patients with COVID-19 who received Tocilizumab showed that 19 of them had clinical improvement as measured by improved respiratory function and CT imaging. What is more, the study showed no adverse effect from Tocilizumab treatment [33]. Studies on a larger scale are needed to confirm that Tocilizumab could be an option in treating COVID-19 patients.
2.2.1.2 Interferon (IFN)
Interferon (IFN) is a group of cytokines that interfere with every step of virus replication [34]. IFNs can be classified into Type I (IFN-
As for SARS-CoV-2, an in vitro study using IFN
2.2.2Immunoglobulin therapy
2.2.2.1 Convalescent Plasma therapy
Convalescent Plasma therapy (CP therapy) works by collecting antibody-rich plasma from recovered patients and transfusing the plasma into other patients. The antibodies in the plasma derived from the recovered patients can block viral entry and potentially mediate the host immune cells to remove the virus [40].
Sun et al. reviewed 37 studies about the efficacy of CP therapy in a variety of viral diseases. The authors concluded that early CP therapy if used early in the disease can significantly reduce mortality and viral load, and shorten the length of a hospital stay [40]. CP therapy can be an option when treating severe cases, but more studies are needed to ensure the method’s safety when being used in less severe cases.
3.TCM therapies
3.1Introduction to TCM therapies
TCM, Traditional Chinese Medicine, has been widely used in China for more than 2,000 years. TCM uses three approaches to curing disease: formula/prescriptions (mixture of herbs), acupuncture (needle therapy), and moxibustion (burnt-leaf induced vacuum therapy) [41].
Different from the Western medicine system that aims at reducing the effect of the disease, the TCM system focuses on maintaining the balance of Yin (essence, body fluids, blood) and Yang (qi, or vital energy, and spirit) within an individual [42]. According to the TCM system, when the balance of Yin and Yang is broken and the body is unable to regulate itself, disease occurs; and when the balance is back to normal, patients will recover from the disease and the symptoms are ameliorated.
Although there are major differences in treating patients between TCM and Western medicine, in both cases their therapeutic effects come from the compounds that constitute the medicine. Now I will examine the active compounds in TCM that may induce therapeutic benefits.
3.2Acceptance and non-acceptance of TCM in Western medicine
Although TCM has been applied in China for more than two thousand years and has achieved remarkable therapeutic effects in multiple diseases, the Western world still casts doubt on TCM. The major concern is the fundamental approach to the treatment of diseases. Western medicine focuses on the molecular target of the diseases, whereas TCM focuses on maintaining the balance of the body to enhance healing based on the body’s defense and repair mechanisms. The second concern is the purity of the products and the potential contamination with toxic minerals. The third concern is the overuse of animal tissues in many TCM formulas. Statistics showed that there are about 13% of TCM is derived from animals, the use of animal products would be criticized by ethical and legal issue by the Western world [43].
For the first concern, the concept of a holistic approach to the treatment of disease is getting traction in Western medicine. An example aiming to create a better balance in the body’s immune system in not hurting the host is using Tocilizumab to prevent IL-6 from overproduction in Western medicine treatment. On the other hand, modern researches on TCM mainly examine its general effects as related to the chemical compounds in its ingredients. Such analysis is similar to Western medicine, and the results can be accepted by most Western researchers. The second concern about possible toxic materials can be addressed by increased communication between the two systems. Today, herbs are mostly grown under controlled conditions in nontoxic soil. Toxic or mineral substances do not appear in the herbs, and safety issues should not be a concern. As for the doubt on animal tissue used in prescriptions, it could be solved by encouraging TCM doctors to substitute these animal tissues with herbs with similar compounds.
Although there are still many criticisms in the Western world towards using TCM herbal remedies, TCM should be gaining more acceptance gradually when most of the concerns are solved with approaches mentioned above. Currently, the European Union funded a project “Good practice in traditional Chinese medicine” (GP-TCM), preparing for the future use of TCM with more scientific support [44]. The World Health Organization also adopted TCM into the 11th version of the organization’s global compendium known as the International Statistical Classification of Diseases and Related Health Problems (ICD). This is a signal of TCM gaining increased attention, though more scientific support on safety and efficacy is needed for its popularization in the Western world [45].
3.3TCM therapies for COVID-19
3.3.1Pathogenesis of COVID-19 in a TCM perspective
In the TCM theoretical system, COVID-19 is classified as a plague caused by the special epidemic toxin SARS-CoV-2 [46]. As COVID-19 is featured as pneumonia which is “wetness of the lung” from the TCM perspective, the TCM classified this causative agent to be a “dampness” pathogen. Other features including shortness of breath and fever are resulted from the imbalance between qi and body fluid, and the imbalance of internal heat, respectively [47]. Currently, the pathogenesis of COVID-19 in a TCM perspective is considered as the interactions of toxin, dampness, heat (excessive internal heat), and stasis (slowing blood flow) [41]. Therefore, the objectives for TCM in the treatment of COVID-19 is to eliminate heat and dampness, or detoxification.
3.3.2Application of TCM to COVID-19: “Three prescriptions and three medicines”
In TCM, prescription is a list of herbs that work together to alleviate specific symptoms. Herbs in a prescription are usually boiled together into the form of decoction (medicinal soup). On the other hand, medicine is a combination of herbs prepared together in capsules or granules.
Three prescriptions and three medicines are being widely used among the 15 Chinese medicines recommended by the Guidelines for the Diagnosis and Treatment of COVID-19 [48]. The three prescriptions include Qingfei Paidu Decoction, Xuanfei Baidu Decoction, and Huashi Baidu Decoction; and the three medicines are Jinhua Qinggan Granule, Lianhua Qingwen Capsule, and Xuebijing Injection. The detailed information of “Three prescriptions and three medicines” are included in Table 1.
Table 1
Detailed information of “three prescriptions and three medicines”
| Prescription/ medicine | Active constituents [49] | Pharmalogical effects | Effectiveness for COVID-19 patients | References |
|---|---|---|---|---|
| Qingfei Paidu Decoction (QFPD) | Kaempferol, 18 | Inhibit virus entry, regulate cytokines to inhibit inflammatory response, reduce lung injury. | Large-scale clinical study showed effective when used at the mild stage of COVID-19. | [5, 49-52] |
| Xuanfei Baidu Decoction (XFBD) | – | Regulate cytokines to suppress cytokine storms. | More efficient when combined with other medicine, may work as monotherapy. | [53-54] |
| Huashi Baidu Decoction (HSBD) | Quercetin, Luteolin, Kaempferol, Baicalein. | Inhibit cytokines and signaling pathways to prevent inflammation response and inhibit viral replication. | Improved symptoms with no adverse reaction. | [55-56] |
| Jinhua Qinggan Granule (JHQG) | Kaempferol, Baicalein, Oroxylin A. | Regulate the virus signaling pathway. | May be effective but larger investigation is needed. | [57-58] |
| Lianhua Qingwen Capsule (LHQW) | Flavonoids, Glycyrrhizic acid, Anthraquinones, Amygdalin. | Inhibit the replication of SARS-CoV-2 and regulate cytokines. | Effective for mild and moderate COVID-19 cases, no significant help for severe cases. | [59-61] |
| Xuebijing Injection (XBJ) | Saffor yellow A, Ligustrazine, Ferulic acid, Salvianic acid A | Remove blood stasis and toxin to combat inflammatory response. | Especially beneficial for severe cases, also works for mild COVID-19 cases. | [62-64] |
3.3.3TCM treatment in different stages of viral infection
In vitro studies show that some active compounds of TCM work by targeting the process of viral infection. This section introduces possible medicines, herbs, and their active compounds that are shown to be effective in blocking virus entry, inhibiting viral replication, and neutralizing cytokine storms.
3.3.3.1 Block viral entry
There are mainly two ways to block the virus from entering the cell: blocking S-ACE2 interaction directly, and inhibiting TMPRSS2 to interfere with S-ACE2 interaction.
Emodin is an anthraquinone compound with three cyclic rings extracted from the herb Da Huang (Radix et Rhizoma Rhei). Da Huang is an active ingredient in many TCM prescriptions that shows antibacterial and anti-inflammatory effects (inhibits the pro-inflammatory transcription factor NF-
18
3.3.3.2 Inhibit viral replication
After the virus enters the cell, the replication process starts. Viral replication can also be blocked by inhibiting 3CL
Glycyrrhizin acid, another active constituent found inside the dry root of the licorice plant (Glycyrrhiza uralensis Fisch), has shown a wide range of antiviral effects by inhibiting the viral replication process and preventing the virus from accumulating inside the host. An in vitro study showed that glycyrrhizin and its derivative glycyrrhizic acid can effectively inhibit viral replication of SARS-CoV by inhibiting the early steps of the replicative cycle, including viral entry by inhibiting the host receptor ACE2 [70, 71]. Another recent in vitro study showed that glycyrrhizic acid could effectively reduce the activity of 3CL
Resveratrol, extracted from the Chinese herbal medicine Hu Zhang (Reynoutria japonica), has general functions in targeting the pathogenesis of COVID-19, including expelling dampness, clearing heat, removing toxicity, and resolving blood stasis. Multiple studies have also shown the compound to be beneficial in treating lung diseases such as chronic obstructive pulmonary disease by inhibiting oxidative stress and inflammation, suggesting that the beneficial effect is more for the organ rather than having negative effect on the virus [64, 74]. Resveratrol has antiviral effects against the influenza (H1N1) virus by increasing the expression of toll-like receptor 9, which activates interferon regulatory factors that lead to the expression of IFN
3.3.3.3 Target on neutralizing the cytokine storm
As discussed above, after the virus has infected the body, an overreaction of the inflammatory response may trigger a cytokine storm and cause death. A few TCM drugs can help patients by regulating the production of cytokines and strengthening the host immune response in COVID-19 patients, which is essential for viral clearance.
Flavonoids, including Quercetin and Kaempferol, are found in plants and fruits. Quercetin and Kaempferol are beneficial in inhibiting synthesis of cytokines (COX-2, 5-LOX, IL-6) because there is no C-3-hydroxyl group, which favors cytokine production, in their chemical structure [77, 78]. The compound is accepted by both Chinese and Western clinicians as a medicine for anti-inflammatory purposes. It is also the major constituent responsible for the anti-inflammatory ability of multiple approved drugs for COVID-19 in the Chinese Guidelines for the Diagnosis and Treatment of COVID-19, including QFPD, HSBD, and JHQG.
3.3.3.4 Multiple targets
Most TCM herbs or prescriptions are made up of multiple plant extracts or compounds and these compounds often work together and have different molecular targets.
Licorice (Gancao), is a herb that mainly contains glycyrrhizin acid, licorice polysaccharide, and 1
As a medicine considered most effective among “three prescriptions and three medicines”, in vitro experiments show that LHQW can effectively inhibit the replication of SARS-CoV-2 thanks to its active compound glycyrrhizic acid. The capsule also contains kaempferol that can modulate the production of cytokines in the infected host [59, 61]. Thus, LHQW used as clinical treatment showed effective in reducing viral load and the amount of cytokines.
4.TCM as a complement to Western medicine for COVID-19 treatment
Even though there are significant differences in therapeutic strategy between TCM and Western medicine, possible ways to combine the two to achieve a greater therapeutic effect can be established based on the TCM principle of “Jun-Chen-Zuo-Shi”. The principle notes there are four elements in a prescription necessary for it to have more desirable treatment outcomes. The four elements include:
(a) the denominator (directly targets the pathological factor),
(b) the enhancer (enhances the function of denominator),
(c) the corrector (prevents adverse effect), and
(d) the messenger (brings denominator to the function site).
When combining TCM and Western medicine, Western medicine often works as the denominator while TCM plays the role of an assistant [79]. Based on this principle, there are two possible ways to combine TCM with Western medicine in either trying to have the combination hitting the same molecular target or hitting different molecular targets. However, drugs used in combination may have detrimental effects on humans that neither of them have when working alone. Therefore, clinical trials are necessary to ensure that the combination can be implemented as effective treatment with trivial negative effects.
4.1Combination hitting the same molecular target
Targeting the same stage of a viral infection can ensure that the virus could be eliminated at one specific stage of the virus life cycle, such as viral replication or viral entry.
The Shufeng Jiedu Capsule (SFJD) contains licorice (Glycyrrhiza uralensis Fisch) that can inhibit ACE2 and TMPRSS2 to block virus entry and inhibit viral replication. A study divided 200 COVID-19 patients into two groups, each treated for a span of two weeks. One group was treated with Arbidol hydrochloride and SFJD, and the other was treated with Arbidol hydrochloride alone. Patients in the experimental group with combined therapy had lower body temperature, higher white blood cell count, and improved CT images than the control group treated only with Arbidol [80]. In addition to the clinical result, adverse effects including diarrhea, nausea, dizziness, and elevated serum transaminase, often seen with Arbidol monotherapy, were not shown in the combined therapy group [81]. SFJD combined with Arbidol would be an option for combined therapy as they strengthen the ability of cell blocking virus entry.
4.2Combination hitting different molecular targets
Targeting multiple molecular targets can provide synergistic inhibitory effects against the viral cycle or assist in the establishment of a better balance for suppressing the viral cycle and at the same time, assist the host to combat the infection and recovery.
LHQW is shown to have its antiviral effect by inhibiting the replication of SARS-CoV-2 and regulating cytokines, as mentioned above. Ribavirin can interfere with the process of viral replication by inhibiting RdRp. Research showed that the combination of Ribavirin and LHQW can significantly improve the therapeutic efficacy of upper respiratory tract infections [82]. Thus, the combination could possibly be effective for COVID-19 patients. In this case, LHQW works as an enhancer in the therapy by inhibiting viral replication along with Ribavirin, and reduces cytokine additionally. Another study, including 151 COVID-19 patients, found that combination therapy of Ribavirin, lopinavir/ritonavir, Arbidol, and LHQW is most effective among any other combinations of these four drugs [83]. It was mentioned in previous sections that Arbidol can prevent the virus from entering the cell by blocking S-ACE2 interaction; Lopinavir/Ritonavir work together can possibly inhibit the viral protease 3CL
4.3Other possible combinations
There are other combinations of TCM and Western medicine which could theoretically improve efficacy of COVID-19 treatment. Such combinations are mainly based on the theories mentioned above: having a same molecular target or addressing multiple targets. However, the combinations proposed are options that require evidence from in vitro experiments or clinical studies to support their actual efficacy as treatment.
TCM that contains Licorice (active compound: 18
TCM that contains Hu Zhang (active compounds include Resveratrol and Emodin) as major constituents, such as SFJD, inhibits both viral attachment and replication, while Remdesivir interferes with viral replication. Collectively they would interfere with the virus entering the cell and inhibit both nucleocapsid protein translation and RdRp to enhance this effect. Also, Resveratrol and Emodin are compounds found in TCM that have been shown to be effective in treating Chinese patients. The combined therapy might solve the problem of Remdesivir being ineffective for severe cases in China due to genetic variations. Additionally, SFJD can alleviate side effects including fever, vomiting, and dizziness that Remdesivir might cause.
Both Flavanoid and Tocilizumab can regulate cytokines to prevent cytokine storms from causing damage to the host body. The flaw of Tocilizumab is that it only regulates IL-6. Therapy integrating Flavanoids with Tocilizumab can target more types of cytokines such as COX2 that might cause cytokine storms. In addition, HSBD is also able to regulate signaling pathways such as IL-17 and NF-
5.Conclusion
This paper introduced major therapies for COVID-19 using Western medicine and TCM, focusing mainly on targeting viral entry, viral replication, and immune response. When analyzing the therapies, their former uses, micro-scale effects, and representative studies are highlighted. From the data in the literature, QFPD and LHQW may have some promise as monotherapy for the treatment of early COVID-19. Early studies also revealed the potential combination therapy including Arbidol combined with SFJD, and Ribavirin combined with LHQW in providing better therapeutic efficacy and reduced adverse effects in the treatment of COVID-19.
TCM is currently gaining increasing acceptance in the Western world since using modern technology can address many doubts that previously existed. However, many Western experts are still concerned about the safety and scientific explanation of efficacy of herbal remedies. Viewing the human body from a holistic perspective, the concept of integration of TCM and Western medicine emerged as an option for COVID-19 for a greater therapeutic effect than monotherapy of either.
Based on the mechanisms of different drugs, three possible combinations are suggested in the paper that may be worth considering for further clinical studies:
(a) QFPD and Camostat Mesylate combination for potential synergistic antiviral effects in inhibiting viral entry;
(b) SFJD and Remdesivir combination for potential synergistic antiviral effects in inhibiting viral replication; and
(c) HSBD and Tocilizumab for potential synergistic effects in preventing cytokine storm.
These proposed combinations might be more effective than conventional therapies and may attenuate adverse effects theoretically, although more clinical studies are needed to prove their actual efficacy in treating COVID-19 patients. If these combinations are shown to be more effective than conventional therapies, they may pave the way for a key milestone for merging the TCM concept together with the Western medicine principles. The success in combination may also provide a possible way of developing specific treatment for COVID-19.
Acknowledgments
The author would like to thank Professor Carole Gibson, Professor of Biology, Wake Forest University, and Professor Johnson Yiu-Nam Lau, MBBS, MD, FRCO, Professor of Biology and Chinese Medicine, Hong Kong Baptist University, for their advice in the preparation of this manuscript.
Conflict of interest
None to report.
References
[1] | Mohammadi M, Khoddamipour Z, Bagheri N. COVID-19 Possible Medical Treatments. Nanomedicine Research Journal. (2021) ; 6: (1): 1-10. doi: 10.22034/nmrj.2021.01.001. |
[2] | Duan C, Xia W, Zheng C, Sun G, Li Z, Li Q, Li P, Zhang H, Yang F, Zhang B, Liu Q. Clinical observation of Jinhua Qinggan granule in treating novel coronavirus infection pneumonia. J Tradit Chin Med. (2020) ; 1-5. |
[3] | Magrone T, Magrone M, Jirillo E. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin-Converting Enzyme 2 as a Potential Drug Target – A Perspective. Endocrine, Metabolic & Immune Disorders – Drug Targets. Endocr Metab Immune Disord Drug Targets. (2020) ; 20: (6): 807-811. |
[4] | Hussain A, Klaer J, Dubey AK. Emerging Pharmaceutical Treatments of Novel COVID-19: A Review. Cureus. (2020) May 24; 12. doi: 10.7759/cureus.8260. |
[5] | Choudhry N, Zhao X, Xu D, Zanin M, Chen WS, Yang ZF, Chen JX. Chinese Therapeutic Strategy for Fighting COVID-19 and Potential Small-Molecule Inhibitors against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Journal of Medicinal Chemistry. (2020) ; 63: : 13205-13227. doi: 10.1021/acs.jmedchem.0c00626. |
[6] | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. (2004) Jun; 203: (2): 631-7. doi: 10.1002/path.1570. |
[7] | Blaising J, Polyak SJ, Pécheur EI. Arbidol as a broad-spectrum antiviral: An update. Antiviral Res. (2014) Jul; 107: : 84-94. doi: 10.1016/j.antiviral.2014.04.006. |
[8] | Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. (2020) April; (323): 1824-1836. |
[9] | Wang X, Cao R, Zhang H, Liu J, Xu M, et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. (2020) ; 6: (28). doi: 10.1038/s414. |
[10] | Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W, Li Y, Ni Q, Zhou R, Li X, et al. Clinical Efficacy of Arbidol in Patients with 2019 Novel Coronavirus-Infected Pneumonia: A Retrospective Cohort Study. (2020) Feb 12. doi: 10.2139/ssrn.3542148. |
[11] | Li YP, Xie ZW, Lin WY, Cai WP, Wen CY, Guan YJ, et al. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: An exploratory randomized controlled trial. Med. (2020) Dec 18; 1: (1): 105-113. doi: 10.1016/j.medj.2020.04.001. |
[12] | Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: A retrospective study. Clin. Microbiol. Infect. (2020) Jul; 26: : 917-921. |
[13] | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. (2020) Apr 16; 181: (2): 271-280. doi: 10.1016/j.cell.2020.02.052. |
[14] | Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R, Nunneley JW, Barnard D, Pohlmann S, McKerrow JH, Renslo AR, Simmons G. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. (2015) ; 116: : 76-84. |
[15] | Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. (2005) ; 2: : 69. doi: 10.1186/1743-422X-2-69. |
[16] | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) ; 30: : 269-271. doi: 10.1038/s41422-020-0282-0. |
[17] | Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ. (2020) ; 369. doi: 10.1136/bmj.m1849. |
[18] | Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Ther Clin Risk Manag. (2008) Oct; 4: (5): 1023-33. doi: 10.2147/tcrm.s3285. |
[19] | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. (2020) May 7; 382: (19): 1787-1799. doi: 10.1056/NEJMoa2001282. |
[20] | Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. (2004) Mar; 59: (3): 252-256. doi: 10.1136/thorax.2003.012658. |
[21] | Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med Infect Dis. (2020) May–Jun; 35: : 101647. doi: 10.1016/j.tmaid.2020.101647. |
[22] | Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. (2020) Jan 10; 11: (1): 222. doi: 10.1038/s41467-019-13940-6. |
[23] | Wang Y, Zhang D, Du G. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2020) ; 395: : 1569-1578. |
[24] | US National Institutes of Health (NIH). Interim COVID-19 treatment guidelines. |
[25] | Wang LY, Cui JJ, Ouyang QY, Zhan Y, Guo CX, Yin JY. Remdesivir and COVID-19. Lancet. (2020) ; 396: : 953-954. doi: 10.1016/S0140-6736(20)32019-5. |
[26] | McCreary EK, Pogue JM. Coronavirus Disease 2019 Treatment: A Review of Early and Emerging Options. Open Forum Infect Dis. (2020) Mar 23; 7: (4). doi: 10.1093/ofid/ofaa105. |
[27] | Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. (2020) May; 92: (5): 479-490. doi: 10.1002/jmv.25707. |
[28] | Sung JJ, Wu A, Joynt GM, Yuen KY, Lee N, Chan PK, et al. Severe acute respiratory syndrome: Report of treatment and outcome after a major outbreak. Thorax. (2004) ; 59: : 414-20. doi: 10.1136/thx.2003.014076. |
[29] | Owji H, Negahdaripour M, Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. International Immunopharmacology. (2020) ; 88. doi: 10.1016/J.INTIMP.2020.106924.. |
[30] | Mangalmurti N, Hunter CA. Cytokine Storms: Understanding COVID-19. Immunity. (2020) ; 53: (1): 19-25. doi: 10.1016/J.IMMUNI.2020.06.017. |
[31] | Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H, 2020b. Aberrant Pathogenic GM-CSF T Cells and Inflammatory CD14 CD16 Monocytes in Severe Pulmonary Syndrome Patients of a New Coronavirus. bioRxiv. |
[32] | Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, Woo P, Nishimoto N, Yoshizaki K, Kishimoto T. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. (2005) Mar; 52: (3): 818-25. doi: 10.1002/art.20944. |
[33] | Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. (2020) May 19; 117: (20): 10970-10975. doi: 10.1073/pnas.2005615117. |
[34] | Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) Mar; 19: (3): 141-154. doi: 10.1038/s41579-020-00459-7. |
[35] | Chibber P, Haq SA, Ahmed I, Andrabi NI, Singh G. Advances in the possible treatment of COVID-19: A review. Eur J Pharmacol. (2020) Sep 15; 883: : 173372. doi: 10.1016/j.ejphar. |
[36] | Hensley LE, Fritz LE, Jahrling PB, Karp CL, Huggins JW, Geisbert TW. Interferon-beta 1a and SARS coronavirus replication. Emerg Infect Dis. (2004) Feb; 10: (2): 317-9. doi: 10.3201/eid. |
[37] | Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, Jones SM, Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-alpha. J Infect Dis. (2004) Apr 1; 189: (7): 1164-7. doi: 10.1086/382597. |
[38] | Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, Rajsbaum R, Menachery VD. Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV. J Virol. (2020) Nov 9; 94: (23): e01410-20. doi: 10.1128/JVI.01410-20. |
[39] | Alavi Darazam I, Shokouhi S, Pourhoseingholi MA, Naghibi Irvani SS, Mokhtari M, Shabani M, Amirdosara M, Torabinavid P, Golmohammadi M, Hashemi S, et al. Role of interferon therapy in severe COVID-19: The COVIFERON randomized controlled trial. Sci Rep. (2021) Apr 13; 11: (1): 8059. doi: 10.1038/s41598-021-86859-y. |
[40] | Sun M, Xu Y, He H, Zhang L, Wang X, Qiu Q, Sun C, Guo Y, Qiu S, Ma K. A potentially effective treatment for COVID-19: A systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease. Int J Infect Dis. (2020) Sep; 98: : 334-346. doi: 10.1016/j.ijid.2020.06.107. |
[41] | Luo H, Gao Y, Zou J, Zhang S, Chen H, Liu Q, Tan D, Han Y, Zhao Y, Wang S. Reflections on treatment of COVID-19 with traditional Chinese medicine. Chin Med. (2020) Sep 3; 15: : 94. doi: 10.1186/s13020-020-00375-1. |
[42] | Zhao JN. Moderation-integrated-balance presupposition of Chinese medicine compound and pharmacological problems in traditional Chinese drug research. Zhongguo Zhong Yao Za Zhi. (2017) Mar; 42: (5): 836-843. Chinese. doi: 10.19540/j.cnki.cjcmm. |
[43] | Still J. Use of animal products in traditional Chinese medicine: Environmental impact and health hazards. Complement Ther Med. (2003) Jun; 11: (2): 118-22. doi: 10.1016/s0965-2299(03)00055-4. |
[44] | Chan K, Shaw D, Simmonds MS, Leon CJ, Xu Q, Lu A, Sutherland I, Ignatova S, Zhu YP, Verpoorte R, Williamson EM, Duez P. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J Ethnopharmacol. (2012) Apr 10; 140: (3): 469-75. doi: 10.1016/j.jep.2012.01.038. |
[45] | Cyranoski D. Why Chinese medicine is heading for clinics around the world. Nature. (2018) Sep; 561: (7724): 448-450. doi: 10.1038/d41586-018-06782-7. |
[46] | Chen Y. Study on the compatible proportion and mechanism of Armeniacae Semen and Radix Platycodi in relieving cough, relieving asthma and expectorant. Psychologies. (2020) ; 15: : 190. |
[47] | Qi J, Qi X, Wang X. Clinical efficacy of different doses of jinhuaqinggan granule on influenza and serum levels of cytokines. Mod Med J. (2016) ; 44: : 1664-1669. |
[48] | Commision NH. Diagnosis and treatment Protocol for novel coronavirus pneumonia (trial version 7). Chinese Med J. (2020) ; 133: : 1087-1095. |
[49] | Ren W, Liang P, Ma Y, Sun Q, Pu Q, Dong L, Luo G, Mazhar M, Liu J, Wang R, Yang S. Research progress of traditional Chinese medicine against COVID-19. Biomed Pharmacother. (2021) May; 137: : 111310. doi: 10.1016/j.biopha.2021.111310. |
[50] | Wu H, Wang J, Yang Y, Li T, Cao Y, Qu Y, Jin Y, Zhang C, Sun Y. Preliminary exploration of the mechanism of Qingfei Paidu decoction against novel coronavirus pneumonia based on network pharmacology and molecular docking technology. Acta Pharm Sin. (2020) ; 55: : 374-83. |
[51] | Li C, Su Y, Liu Y, Xue X, Gong H, Li T, Niu S. Discussion on TCM theory and modern pharmacological mechanism of Qingfei Paidu decoction in the treatment of COVID-19. J Tradit Chin Med. (2020) ; 1-4. |
[52] | Zhao J, Tian S, Yang J, Liu J, Zhang W. Investigating mechanism of QingFei PaiDu Tang for treatment of COVID-19 by network pharmacology. Chin Tradit Herb Drugs. (2020) ; 51: : 829-35. |
[53] | Wang Y, Li X, Zhang J, Xue R, Qian J, Zhang X, Zhang H, Liu Q, Fan X, Zhang B. Mechanism of Xuanfei Baidu Tang in treatment of novel coronavirus pneumonia based on network pharmacology. China J Chin Materia Med. (2020) ; 1-9. |
[54] | Xiong WZ, Wang G, Du J, Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: A pilot randomized clinical trial. Integr Med Res. (2020) Sep; 9: (3): 100489. doi: 10.1016/j.imr.2020.100489. |
[55] | Dai YJ, Wan SY, Gong SS, Liu JC, Li F, Kou JP. Recent advances of traditional Chinese medicine on the prevention and treatment of COVID-19. Chin J Nat Med. 2020 Dec; 18(12): Disease. 2020; 35: 101647. doi: 10.1016/S1875-5364(20)60031-0. |
[56] | Luo H, Yang M, Tang QL, Hu XY, Willcox ML, Liu JP. Characteristics of registered clinical trials on traditional Chinese medicine for coronavirus disease 2019 (COVID-19): A scoping review. Eur J Integr Med. (2021) Jan; 41: : 101251. doi: 10.1016/j.eujim.2020.101251. |
[57] | Gong P, Guo Y, Li X, Wang N, Gu J. Exploring active compounds of Jinhua Qinggan granules for prevention of COVID-19 based on network pharmacology and molecular docking. Chin Tradit Herb Drugs. (2020) ; 51: : 1685-93. |
[58] | Liu Z, Li X, Gou C, Li L, Luo X, Zhang C, Wang X. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. Journal of Traditional Chinese Medicine. (2020) ; 40: : 467-472. |
[59] | Li RF, Hou YL, Huang JC, Pan WQ, Ma QH, Shi YX, Li CF, Zhao J, Jia ZH, Jiang HM, Zheng K, Huang SX, Dai J, Li XB, Hou XT, Wang L, Zhong NS, Yang ZF. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. (2020) Jun; 156: : 104761. doi: 10.1016/j.phrs. |
[60] | Hu K, Guan WJ, Bi Y, Zhang W, Li L, Zhang B, Liu Q, Song Y, Li X, Duan Z, Zheng Q, Yang Z, Liang J, Han M, Ruan L, Wu C, Zhang Y, Jia ZH, Zhong NS. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. (2021) May; 85: : 153242. doi: 10.1016/j.phymed. |
[61] | Jia W, Wang C, Wang Y, Pan G, Jiang M, Li Z, Zhu Y. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. ScientificWorldJournal. (2015) ; 2015: : 731765. doi: 10.1155/2015/731765. |
[62] | Zhao W, Li K, Zhang S, Fu Y, Shi X. The effect of Xuebijing injection on immune regulation in SIRS patients. J Sichuan Univ. (2014) ; 45: : 863-5. |
[63] | Guo H, Zheng J, Huang G, Xiang Y, Lang C, Li B, Huang D, Sun Q, Luo Y, Zhang Y, Huang L, Fang W, Zheng Y, Wan S. Xuebijing injection in the treatment of COVID-19: A retrospective case-control study. Ann Palliat Med. (2020) Sep; 9: (5): 3235-3248. doi: 10.21037/apm-20-1478. |
[64] | Ma BN, Li XJ. Resveratrol extracted from Chinese herbal medicines: A novel therapeutic strategy for lung diseases. Chin Herb Med. (2020) Oct; 12: (4): 349-358. doi: 10.1016/j.chmed. |
[65] | Koyama M, Kelly TR, Watanabe KA. Novel type of potential anticancer agents derived from chrysophanol and emodin. Some structure-activity relationship studies. J Med Chem. (1988) Feb; 31: (2): 283-4. |
[66] | Kumar A, Dhawan S, Aggarwal BB. Emodin (3-methyl-1, 6, 8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. (1998) Aug 20; 17: (7): 913-8. |
[67] | Dong X, Fu J, Yin X, Cao S, Li X, Lin L, Huyiligeqi, Ni J. Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. (2016) Aug; 30: (8): 1207-18. doi: 10.1002/ptr.5631. |
[68] | Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. (2007) May; 74: (2): 92-101. doi: 10.1016/j.antiviral.2006.04.014. |
[69] | Sun Y, Jiang M, Park PH, Song K. Transcriptional suppression of androgen receptor by 18β-glycyrrhetinic acid in LNCaP human prostate cancer cells. Arch Pharm Res. (2020) Apr; 43: (4): 433-448. doi: 10.1007/s12272-020-01228-z. |
[70] | Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. (2003) Jun 14; 361: (9374): 2045-6. doi: 10.1016/s0140-6736(03)13615-x. |
[71] | Hoever G, Baltina L, Michaelis M, Kondratenko R, Baltina L, Tolstikov GA, Doerr HW, Cinatl J Jr. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. (2005) Feb 24; 48: (4): 1256-9. doi: 10.1021/jm0493008. |
[72] | Van de Sand L, Bormann M, Alt M, Schipper L, Heilingloh CS, Steinmann E, Todt D, Dittmer U, Elsner C, Witzke O, Krawczyk A. Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses. (2021) Apr 2; 13: (4): 609. doi: 10.3390/v13040609. |
[73] | Sinha SK, Prasad SK, Islam MA, Chaudhary SK, Singh S, Shakya A. Potential Leads from Liquorice Against SARS-CoV-2 Main Protease using Molecular Docking Simulation Studies. Comb Chem High Throughput Screen. (2021) ; 24: (4): 591-597. doi: 10.2174/1386207323999200817103148. |
[74] | Zhang H, Li C, Kwok ST, Zhang QW, Chan SW. A Review of the Pharmacological Effects of the Dried Root of Polygonum cuspidatum (Hu Zhang) and Its Constituents. Evid Based Complement Alternat Med. (2013) ; 2013: : 208349. doi: 10.1155/2013/208349. |
[75] | Lin CJ, Lin HJ, Chen TH, Hsu YA, Liu CS, Hwang GY, Wan L. Polygonum cuspidatum and its active components inhibit replication of the influenza virus through toll-like receptor 9-induced interferon beta expression. PLoS One. (2015) ; 10: (2): e0117602. |
[76] | Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. (2017) Feb 13; 17: (1): 144. doi: 10.1186/s12879-01. |
[77] | Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. (2004) Nov; 96: (3): 229-45. doi: 10.1254/jphs.crj04003x. |
[78] | Huang YF, Bai C, He F, Xie Y, Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacol. Res. (2020) ; 158. |
[79] | Cao C, Brown B. Understanding chinese medicine and western medicine to reach the maximum treatment benefit. J Transl Sci. (2019) ; 5: : 1-2. |
[80] | Xiao Q, Jiang YJ, Wu SS, Wang Y, An J, Xu WP, Wu JJ. The combined therapy of Zhongyao Shufeng Jiedu capsule and Arbidol Hydrochloride Tablets in treating COVID-19 patients. J. Emerg. Tradit. Chin. Med. (2020) ; 1-3. |
[81] | Proskurnina EV, Izmailov DY, Sozarukova MM, Zhuravleva TA, Leneva IA, Poromov AA. Antioxidant potential of antiviral drug umifenovir. Molecules. (2020) Mar 30; 25: (7): 1577. doi: 10.3390/molecules25071577. |
[82] | Cheng S. Clinical observation of Lianhua Qingwen Capsule combined with ribavirin injection in treating viral upper respiratory tract infection. Chin Commun Doctors. (2011) ; 13: : 170. |
[83] | Li X, Yang Y, Liu L, Yang X, Zhao X, Li Y, Ge Y, Shi Y, Lv P, Zhang J, Bai T, Zhou H, Luo P, Huang S. Effect of combination antiviral therapy on hematological profiles in 151 adults hospitalized with severe coronavirus disease 2019. Pharmacol Res. (2020) Oct; 160: : 105036. doi: 10.1016/j.phrs.2020.105036. |




