Effects of Prunus Tomentosa Thumb Total Flavones on adjuvant arthritis in rats and regulation of autophagy
Abstract
BACKGROUND:
Rheumatoid arthritis (RA) is a slow in taking effect systemic autoimmune disease. Prunus Tomentosa Thumb Total Flavones (PTTTF) has anti-inflammatory and antioxidant properties.
OBJECTIVE:
The purpose of this study is to the PTTTF on adjuvant arthritis (AA) in rats and to explore the mechanism of autophagy.
METHODS:
Adjuvant arthritis model was established in rats. The cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2), interleukin-1
RESULTS:
PTTTF (50, 100, 200 mg/kg) significantly inhibited inflammation in rats (
CONCLUSION:
PTTTF can inhibit adjuvant arthritis in rats and can inhibit the expression of autophagy-related proteins ATG5, ATG7, ATG12, Beclin1, Lc3II and Bcl-2.
1.Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by joint disease. Its main clinical manifestations are joint swelling and pain caused by synovium of facet joint, followed by cartilage destruction, joint space narrowing, joint stiffness, deformity, late severe bone destruction and dysfunction caused by absorption. The disease is easy to recur, poor prognosis with and high disability rate. Rheumatoid arthritis is primarily treated with disease-modifying antirheumatic drugs (DMARDs), which are pain relievers used to help relieve pain. There are two main categories of DMARDS: synthetic compounds and biologics [1], which require subcutaneous or intravenous administration, and are toxic and accompanied by many side effects of. There is so far no ideal treatment method [2].
Prunus Tomentosa Thumb can be found in Changbai Mountains, they are the fruits of wild shrubs and are a type of cherry. Prunus Tomentosa is abundant in number in China. However, little has been explored about it, and only few academic documents are found–most of the relative readings for Prunus Tomentosa are unofficial popular articles, saying “Cherry-soaked wine, folk Chinese used it to treat frostbite and rheumatism”.
Our research group has been long focusing on the anti-frostbite use of Prunus Tomentosa, because it is a very possible breakthrough for the cure for Rheumatoid arthritis (RA). The total flavonoids in Prunus Tomentosa has shown clear effects in anti-frostbite cases and anti-inflammatory cases. The analgesic and anti-inflammatory characteristics of Prunus Tomentosa may be in connection with the anti-oxidative stress of flavonoids and the inhibition of COX-1. COX-2 generation [3, 4]. In recent years, many studies done by worldwide scientists have confirmed that flavonoids have significant anti-inflammatory effects [5], which is of great medicinal development value.
In this paper, to establish the rat arthritis model induced by Freund’s adjuvant. The anti-inflammatory action of PTTTF on adjuvant arthritis in rats was observed. The changes of body weight, thymus index, spleen index, and cytokines in synovial membrane were determined. The effect of total flavonoids of Prunus Tomentosa Thumb on autophagy during anti-inflammatory process provides a theoretical basis for the exploitation of anti-inflammatory medicine to Prunus Tomentosa Thumb.
2.Materials and methods
2.1Experiment animals
SPF grade Wistar rats, 60 body weight 160–180 g, male, bought from Changchun City Yisi Experimental Animal Technology Co., Ltd. Experimental Animal Center, under the SPF experimental conditions of the Experimental Research Center of the College of Pharmacy, Beihua University.
2.2Instruments and reagents
Prunus Tomentosa Thumb Total Flavones are provided by the Laboratory of Medicinal Chemistry, School of Pharmacy, Beihua University. Freund’s complete adjuvant (Beijing Dingguo Changsheng Biotechnology Co., Ltd.), primers IL-6 ,TNF-
The Bio-Rad 680 microplate reader was purchased from Bio-Rad. The PCR instrument (Mastercycler gradient 5331) was purchased from Eppendorf. The gel imaging analysis system (Tocan 240 type) was purchased from Shanghai Lingcheng Biotechnology Co., Ltd. The UVP chemiluminescence imaging system was purchased from analytikjena. The Model 5810R Multi-Function Desktop Refrigeration Centrifuge was purchased from Eppendorf. The SW-CJ-2 ultra-clean workbench was purchased from Suzhou Purification Equipment Co., Ltd. PV-200 toe volume measuring instrument (Chengdu Qinmeng Technology Co., Ltd.)
2.3Animal grouping and administration
60 male Wistar rats were stochastically separated into 6 groups based on their body weight. There are normal group, model group, model with dexamethasone group (0.125 mg/kg), and the model with cherry extract group. The model with cherry extract group was further divided into low, medium and high doses (50, 100, 200 mg/kg) groups. There are 10 rats in each group, and after 3 days of adaptive feeding, the treated group was prevented from being administered for 7 days, referring to the modeling method [6]. Except for the normal group, rats in the other groups were injected hypodermic injection of 0.2% of Freund’s complete adjuvant in the right hind paw of the rats, and the normal rats were injected subcutaneously with the equal amount of physiological saline. During a modeling period, the joint redness, subcutaneous nodules, and erythema of the rats were observed and recorded, and the body mass and the degree of swelling of the rats were measured once every 2 days. After the inflammation, except for the model group, the treatment groups were started to be administered, and the normal group was given the same amount of distilled water for 28 days.
2.4Measuring rat paw swelling
The instrumental measures of the swelling of rats’ left and right paws were done using a PV-200 Toe Volume Measuring Instrument.
Applied Formula: Paw swelling extent
2.5Organ index
After 24 hours after the last administration, anesthesia was performed by intraperitoneal injection of 5% chloral hydrate. Then the spleen, thymus and joints were separated, rinsed with iced physiological saline, blotted with filter paper, weighed, and calculated the organ index.
2.6Pathological observation of joints (HE staining) [7]
The pathological changes of joints in each group were watched and took pictures under the microscope.
2.7PCR detection of inflammatory cytokine expression in the synovium tissue of the tested joint
The synovium tissue of the joint was taken, and total RNA was extracted according to the kit instructions, and reverse transcribed into cDNA by a PCR instrument. The resulting cDNA was amplified by a PCR machine according to the kit operation. The primer sequences of RT-PCR are referred to Table 1.
Table 1
The primer sequences of RT-PCR
| Primer | Forward primer (5 | Reverse primer (5 |
|---|---|---|
| COX-1 | GCC AGT ATT AGC AGC AGC AGG TATC | GCC GAA GAA TCA GAA TAG GTGT |
| COX-2 | CCC GCC CCA TCT AAC ATC TC | CCC CAC ATG GAG GAA TAGGC |
| IL-1 | GGA TGA TGA CGA CCT GCT AGT | CAC TTG TTG GCT TAT GTT CTGTC |
| IL-6 | CCA ACT TCC AAT GCT CTC TCC TAAT | CGA GTA GAC CTC ATA GTG ACCTT |
| IL-17 | CGC CGA GGC CAA ATA ACT TTC | GGT TGA GGT AGT CTG AGGGC |
| TNF- | ATG TGG AAC TGG CAG AGGAG | AGT AGA CAG AAG AGC GTGTG |
| GAPDH | GGC AAG TTC AAC GGC ACAG | GCC AGT AGA CTC CAC GACAT |
2.8Western blotting detection of the expression of autophagic factors in the synovium tissue of the joint
The synovium tissue of the joint was taken, and the total protein was extracted according to the kit operation. The same amount of protein was subjected to SDS-PAGE electrophoresis, and the protein was shifted to PVDF membrane. After blocking with 5% nonfat dried milk, it was incubated with anti-Beclin1, Lc3II, ATG5, ATG7, ATG12, Bcl-2, anti-
2.9Statistical analysis
The data obtained are expressed as mean
3.Results
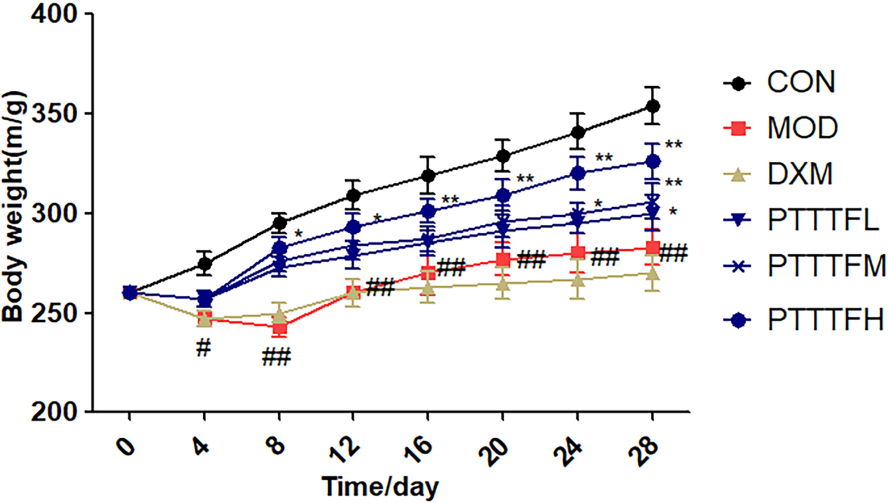
3.1The influence of prunus tomentosa thumb total flavones on body weight of rats
Compared with the blank group, there was a significant difference in the model group on the 4th day (
Figure 1.
The effect of PTTTF on weight in rats with adjuvant arthritis. The number of samples in each group was 10. All data represent the mean
3.2The effect of prunus tomentosa thumb total flavones on paw swelling of rat
An increase in paw swelling after one week of inflammation may be a secondary inflammatory reaction caused by inflammation [8]. PTTTF (50, 100, 200 mg/kg) significantly inhibited the swelling of the foot in rats with adjuvant arthritis. PTTTF (200 mg/kg) remarkably restrained the swelling of the foot in rats with adjuvant arthritis (
Table 2
The influence of PTTTF on paw swelling in rats with adjuvant arthritis
| Groups |
| 2 days | 7 days | 14 days | 21 days | 28 days | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | 10 | 0.02 | 0.01 | 0.01 | 0.03 | 0.03 | |||||
| Model group | 10 | 0.72 | 0.84 | 0.77 | 0.76 | 0.73 | |||||
| Positive group | 10 | 0.30 | 0.59 | 0.39 | 0.18 | 0.14 | |||||
| Low dose group | 10 | 0.59 | 0.69 | 0.54 | 0.53 | 0.49 | |||||
| Medium dose group | 10 | 0.54 | 0.63 | 0.54 | 0.48 | 0.47 | |||||
| High dose group | 10 | 0.48 | 0.53 | 0.49 | 0.46 | 0.36 | |||||
The number of samples in each group was 10. All data represent the mean
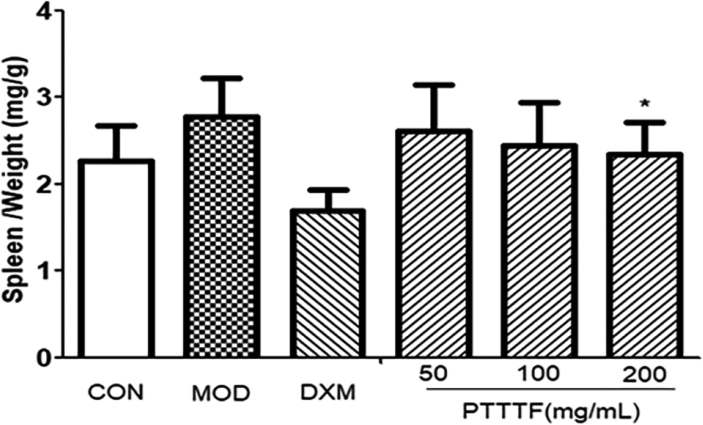
3.3The influence of prunus tomentosa thumb total flavones on immune organ index
The thymus and splenic organ are the main immune organs of human body, and changes in their relative quality play a significant role in immune evaluation. They have been widely used to evaluate the immune level of the body. The effect of drugs on the quality of thymus and spleen of animals can be used as an immunological pharmacological mechanism. Preliminary indicator [9]. PTTTF (50, 100, 200mg/kg) had a certain inhibitory effect on the spleen. PTTTF (200 mg/kg) significantly inhibited the growth of spleen (
Figure 2.
The influence of PTTTF on thymus index in rats with adjuvant arthritis. The number of samples in each group was 10. All data stand for the mean
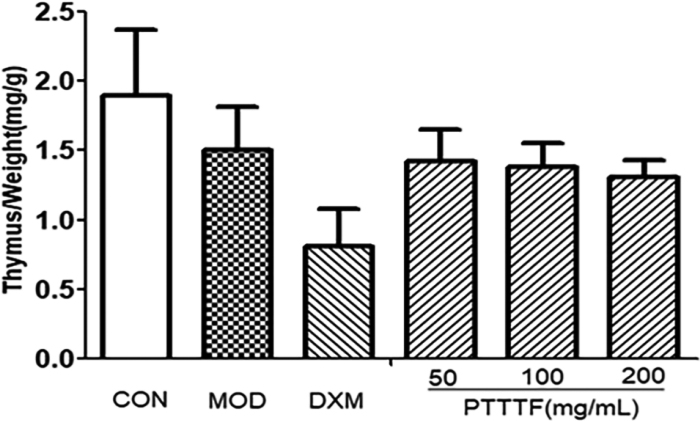
Figure 3.
The effect of PTTTF on spleen index in rats with adjuvant arthritis. The number of samples in each group was 10. All data stand for the mean
3.4The effect of prunus tomentosa thumb total flavones on pathological changes of joints
Pathological changes [10] are an important feature of RA, often manifested as joint effusion, synovial inflammation such as lining cell layer thickening, massive cell infiltration in the interstitial layer, microvascular neovascularization, synovial cell surface expression activation of antigen, taking shape of angiospasm and destruction of cartilage tissue. Pathological sections preserved pathological changes in the pathogenesis of RA in the form of sections. Compared with the pathological sections of the synovial cartilage in the treatment group, the blank group and the treatment group can objectively understand the progression of RA and the changes of histopathological structure before and after treatment.
In the model group, the synovial membrane of the synovium of the model group was thickened and hypertrophied, tissue vasodilation, fibrosis, and a large number of chronic inflammatory cells. PTTTF (50, 100, 200 mg/kg) can alleviate synovial cell and tissue proliferation of knee joints in AA rats and reduce inflammatory cell infiltration. The results are shown in Fig. 4.
Figure 4.
The influence of PTTTF on knee joint histopathology in rats with adjuvant arthritis(HE stain,
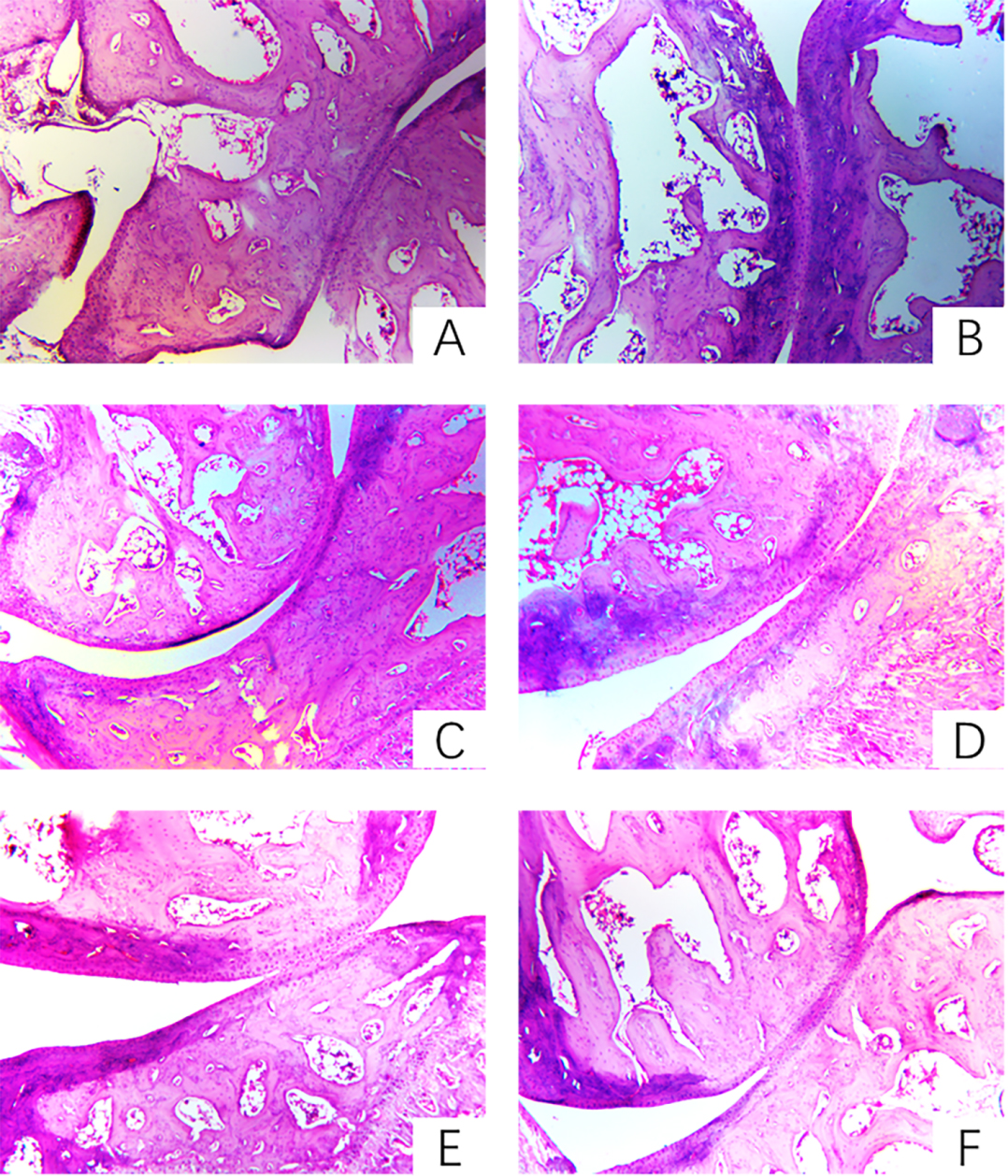
Figure 5.
The influence of PTTTF on the expression of COX-1 in synovial tissue of rats with adjuvant arthritis. The number of samples in each group was 10. All data stand for the mean
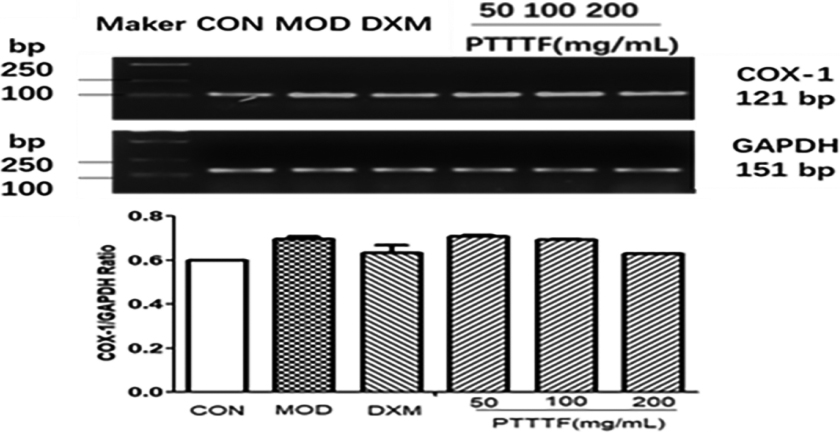
Figure 6.
The influence of PTTTF on COX-2 expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
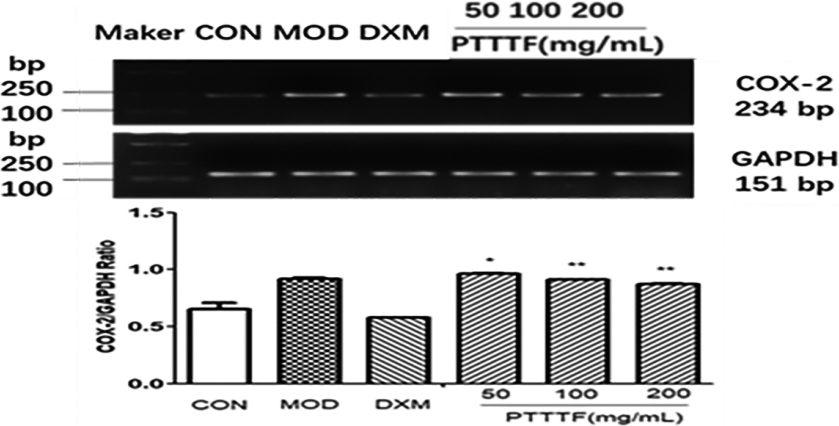
Figure 7.
The influence of PTTTF on IL-1
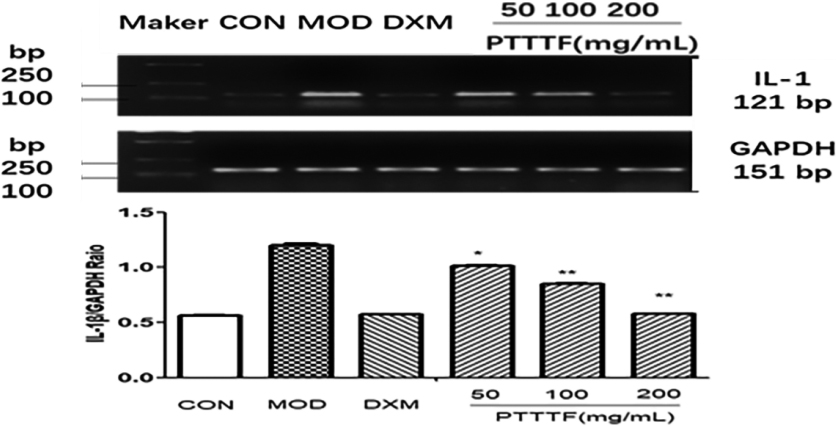
3.5The effect of prunus tomentosa thumb total flavones on synovial tissue inflammatory factors
PTTTF (50, 100, 200 mg/kg) remarkably decreased the expression of inflammatory factors, and PTTTF (50 mg/kg) significantly decreased IL-1
Figure 8.
The influence of PTTTF on IL-6 expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
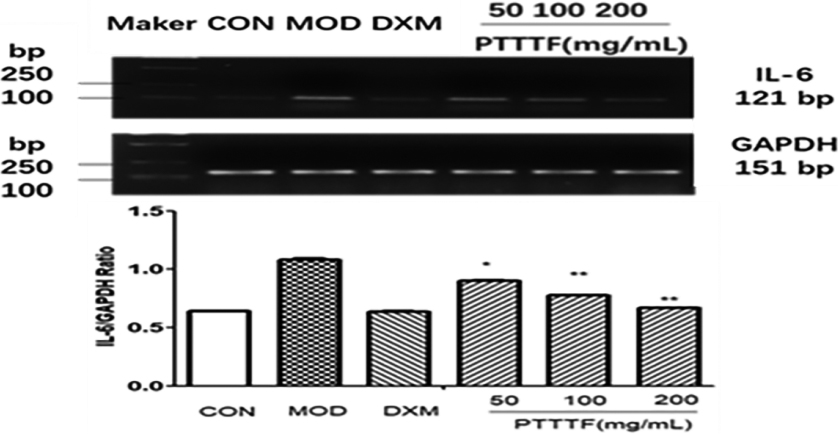
Figure 9.
The influence of PTTTF on IL-17 expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
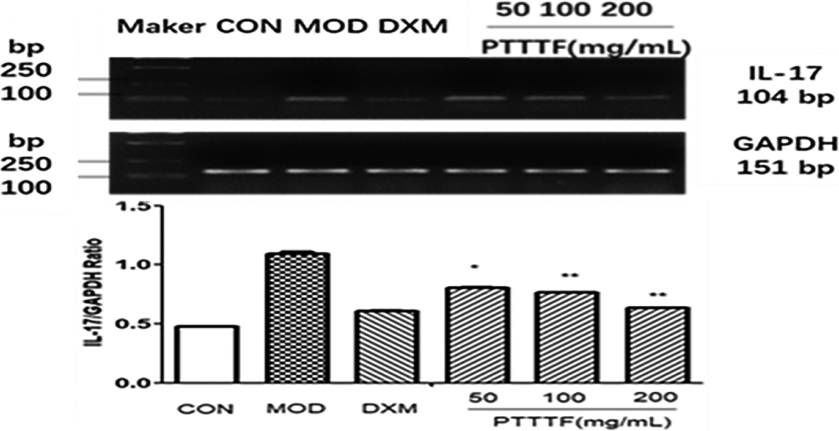
Figure 10.
The influence of PTTTF on TNF-
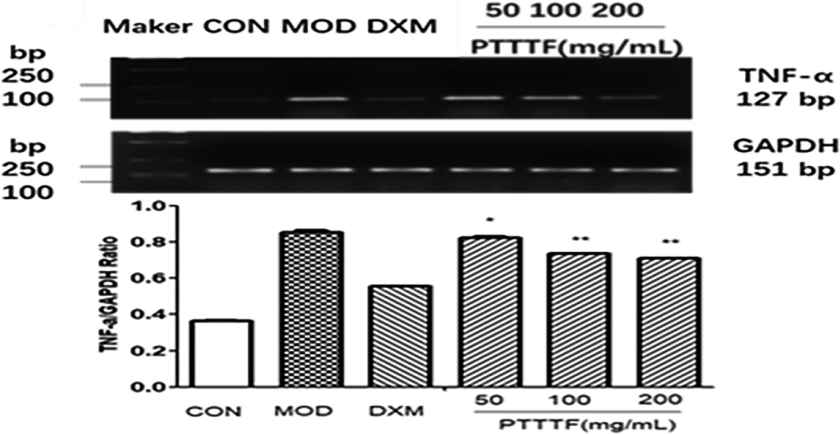
Figure 11.
The influence of PTTTF on ATG5 expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
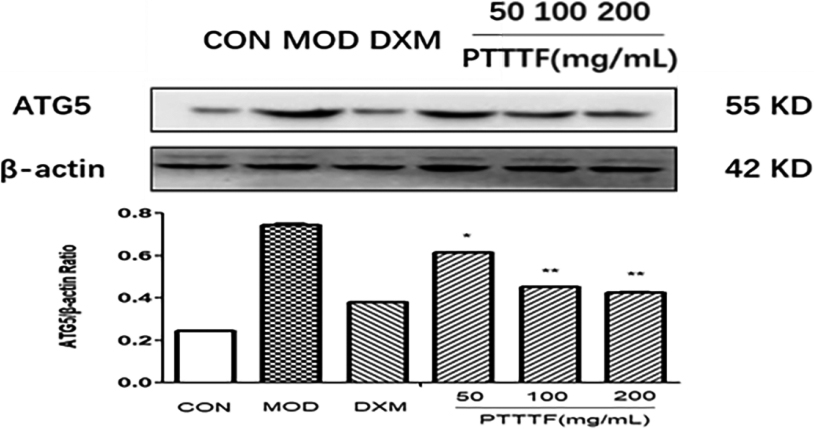
Figure 12.
The influence of PTTTF on ATG7 i expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
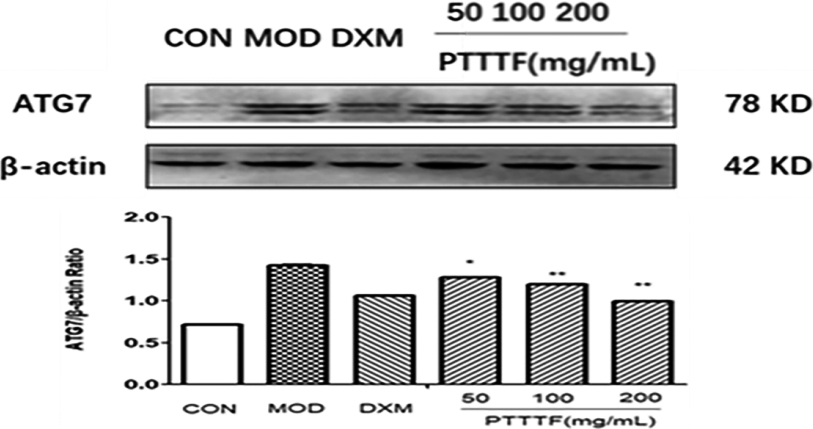
Figure 13.
The influence of PTTTF on ATG12 expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
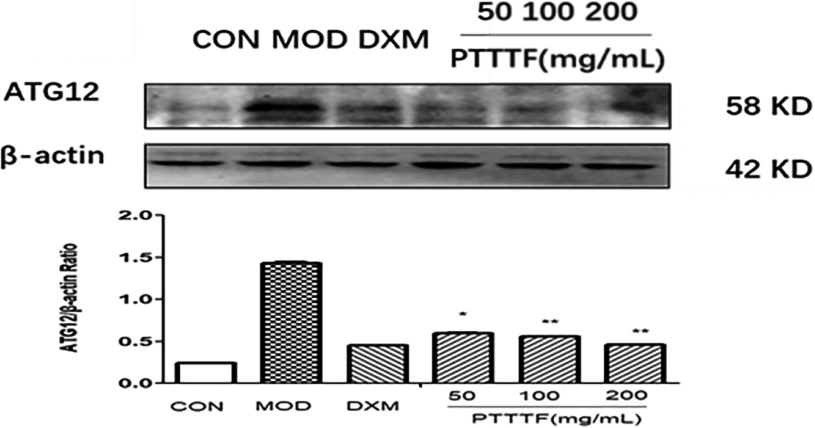
Figure 14.
The influence of PTTTF on Becinl1 expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
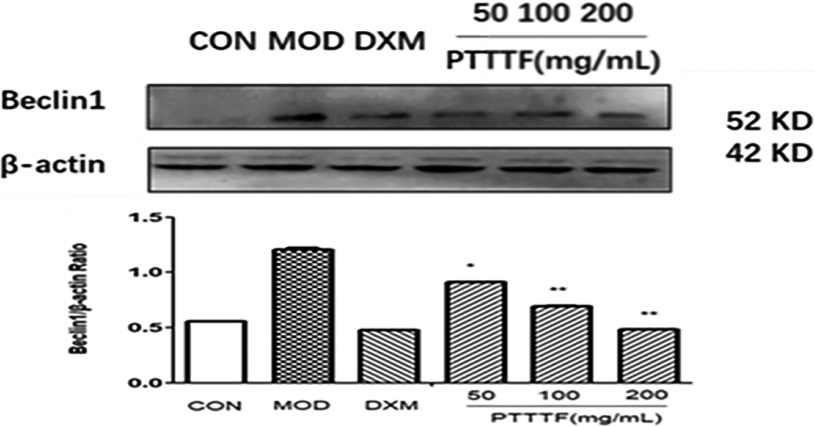
Figure 15.
The influence of PTTTF on LC3II expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
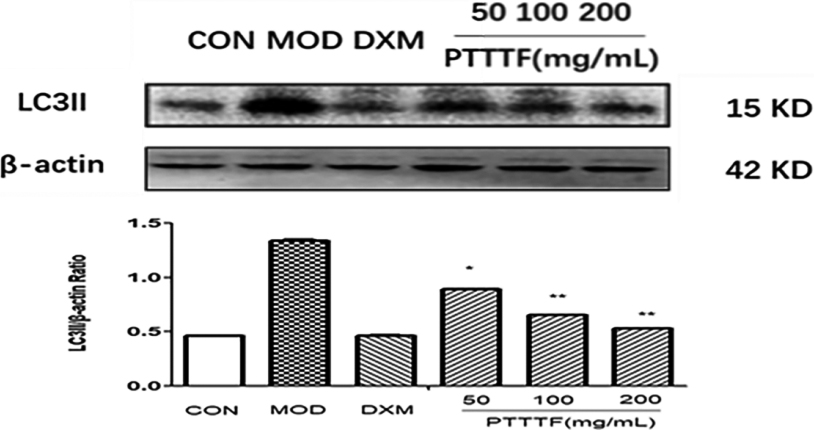
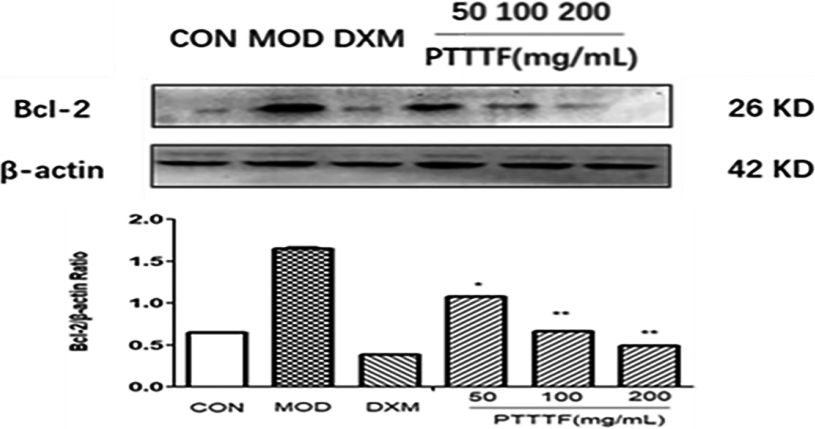
Figure 16.
The Effect of PTTTF on Bcl-2 expression in synovium of adjuvant arthritis rats. The number of samples in each group was 10. All data stand for the mean
3.6The effect of prunus tomentosa thumb total flavones on autophagy related factors in synovial tissue
PTTTF (50, 100, 200 mg/kg) significantly decreased the expression of autophagy-related proteins, PTTTF (50 mg/kg) significantly decreased ATG5 (
4.Discussion
AA rat is one of the widely used rheumatoid arthritis models. It is a commonly used animal model for rheumatoid arthritis, and its pathogenesis is basically consistent with rheumatoid arthritis. The model is characterized by multiple arthritis. The primary lesions are mainly local acute inflammation, and secondary lesions appear one week later, and immune dysfunction mainly due to cellular immune changes [12].
Autophagy is a transversion of phagocytizing its own cytoplasmic protein or organelle and coating it into vesicles, and fuses with lysosomes to form autophagosomes, which degrade the contents of the cells, thereby realizing the metabolism of the cells themselves. And the renewal of certain organelles, but this process is also used to degrade pathogenic microorganisms (such as viruses, bacteria and protozoa) [13, 14]. According to the way cell functions and the way cytoplasmic transports to lysosomes, it can be divided into three distinct modalities of autophagy: macroautophagy, microautophagy and molecular chaperone-mediated autophagy, respectively [15].
Under physiological conditions, moderate autophagy can clean up damaged organelles and intracellular macromolecules and recycle them, thereby maintaining intracellular homeostasis. Under pathological conditions, excessive cellular autophagy can cause damage to cells and tissues.
Beclin1 is an autophagy marker gene that adjusts the orientation of Atg protein in autophagy precursor structure and strengthens autophagy activity. Atg5 and Atg7 are important molecules in BeKlin1 affected by Beclin1 in the precursor structure, which is decisive for the activity of autophagy [16]. The autophagy-accelerating function of Beclin1 is effected by the anti-apoptotic protein Bcl-2; as a matter of fact, when Beclin1 binds to Bcl-2, autophagy is limited; by contrast; Bcl-2 disaggregation makes Beclin-1 and PI3K-III compound interactions and activation of autophagy [17]. Atg12-Atg5-Atg16L and microtubule-associated protein 1 light chain 3 (LC3)-phosphatidylethanolamine (PE) mediate autophagy and autophagosome expansion and closure [18]. LC3 is splitted by the cysteine protease atg4 to produce a cytosolic form of LC3-I. After being activated by Atg7, it is transferred to ATG3 and changed in a form coupled with PE, named LC3-II. Therefore, LC3-II is the most common marker of detecting autophagy activity, followed by the only protein associated with autophagosome stability during steps to ripeness [19].
Autophagy is a cellular catabolic process, which is extensively deemed as a reaction to various excitment, for instance, starvation, hypoxia and abnormal protein accumulation. More and more testimony shows that autophagy is involved in the pathological process of rheumatic diseases, ankylosing spondylitis, Crohn’s disease, and systemic lupus erythematosus [20]. As the whole world incidence rate of RA is 0.5–1.0%, and the mortality rate is increasing, the gravity of RA has aroused great concern [21]. Rheumatoid elements can lead to joint destruction, vasculitis and rheumatoid pleurisy, all of the above that will cause a serious economic and social burden. Autophagy is an important regulator of persistent inflammation in joints [22], but the link between autophagy and inflammation in RA is not clear. Studies have shown that LC3-II protein expression (an indicator of autophagosome formation) in RA patients is elevated, indicating an increase in autophagy activity in RA patients, suggesting that autophagy activation may participate in the pathogenesis of RA. Autophagy and inflammation interact in autoimmune diseases, and future studies should investigate the regulation of autophagy in inflammatory activity in RA [23]. The results of this study showed that the total flavonoids of cherries inhibited the expression of autophagy-related proteins ATG5, ATG7, ATG12, Beclin1, and LC3II. The therapeutic effect of total flavonoids in cherries may be at least partially concerned with the down-regulation of autophagy expression. Studying the pathogenic role of autophagy in RA may open up new ways for the exploitation of new drugs in the next. The relationship between autophagy and inflammation is intricate, and autophagy is participated in the induction and inhibition of inflammation [24]. Proinflammatory cytokines such as tumor necrosis factor (TNF-
Recent research shows that autophagy regulates metabolism is involved in the pathogenesis of RA, synovial fibroblasts, osteoclasts, chondrocytes, T lymphocytes and B lymphocytes, citrullin peptide presentation and proinflammatory cytokine release [27]. Our results showed that in the synovial tissue, the patient’s autophagy level was significantly reduced, while the expression level of inflammatory factors was significantly reduced. These results indicate a link between autophagy and RA-associated inflammation, which is consistent with other studies [28]. Resveratrol (an antioxidant) inhibits inflammation by reducing autophagy expression in AA rats [29]. Studies have found that levels of inflammatory factors in synovial tissue are positively correlated with autophagosome levels. Proinflammatory cytokines, for instance IL-1 and TNF-
5.Conclusion
This study illustrates that the total flavonoid in cherries inhibited the inflammation of adjuvant arthritis, reduced the expression of inflammatory factors and autophagy in adjuvant arthritis synovial tissue, and its anti-inflammatory and inhibitory effects reduced the expression of autophagy. No significant side effects were found in the treatment of adjuvant arthritis. Inflammation of synovial tissue, inhibition of autophagy may be benefit the curing of RA. This study provides a theoretical basis for studying the anti-inflammatory mechanism of total flavonoids in cherries, and the study also lays the theoretical foundation for the exploitation of new anti-inflammatory drugs before clinical development. Nevertheless, thinks to time and funding constraints, we have only explored how PTTTF regulates cell proliferation, apoptosis and autophagy in AA rats by inhibiting the expression of autophagy related proteins ATG5, ATG7, ATG12, Beclin1, Lc3II and Bcl-2.Further studies focusing on other signaling pathways are required to further corroborate our findings.
Acknowledgments
This research was supported by the Science and Technology Department of Jilin Province Science and Technology Research Project (grant number: 20190304048YY), Jilin, China; Jilin Province Health Science and Technology Capacity Improvement Project (grant number: 2021JC085), Jilin, China; Science and Technology Plan Project of Jilin City (grant number: 201820847), Jilin, China; and Jilin Province Health Science and Technology Capacity Improvement Project (grant number: 2019J046), Jilin, China.
Conflict of interest
None to report.
References
[1] | Lin YJ, Anzaghe M, Schülke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. (2020) ; 9: (4): 880. |
[2] | Zhao J, Jiang P, Guo S, Schrodi SJ, He D. Apoptosis, Autophagy, NETosis, Necroptosis, and Pyroptosis Mediated Programmed Cell Death as Targets for Innovative Therapy in Rheumatoid Arthritis. Front Immunol. (2021) ; 12: : 809806. |
[3] | Zhang DS, Liu YY, Chen X. Preparation and Pharmacodynamic Study of Anti-freeze Coating Agent for Prunus Tomentosa Thunb. Journal of Chinese Medicinal Materials. (2018) ; 41: (8): 1950-1953. |
[4] | Zhang CY, Shao SH, Shi YL. Anti-Frostbite Effects of PrunusTomentosa Thunb Total Flavone. European Journal of Inflammation. (2011) ; 65-71. |
[5] | Yan XT, Chen HG, Zhou X. Research progress of rheumatoid arthritis effect and mechanism of flavonoids. Nat Prod Res Dev. (2019) ; 31: (6): 1101-1108. |
[6] | Li X, Wu Z, He B, Zhong W. Tetrandrine alleviates symptoms of rheumatoid arthritis in rats by regulating the expression of cyclooxygenase-2 and inflammatory factors. Exp Ther Med. (2018) ; 16: (3): 2670-2676. |
[7] | Du CC, Tan YQ, Shen JY. Effect and mechanism of dihydroartemisinin on two models of rheumatoid arthritis. Chinese Journal of Experimental Formulology. (2019) ; 25: (10): 48-56. |
[8] | Li JY, Zhao F, Lu QJ. Effect of Angelica Nianhua soup on key factors in the apoptotic pathway inside and outside the synovial tissues in adjuvant arthritis rats. Chinese Journal of Experimental Formulology. (2021) ; 27: (4): 1-7. |
[9] | Qu DW, Wang XR, Feng B. Mechanism of anti-inflammatory effect of mother soup on rats with adjuvant arthritis. TCM Pharmacology and Clinical. (2020) ; 36: (3): 56-61. |
[10] | Miao Y, Yang J, Yun Y, Sun J, Wang X. Synthesis and anti-rheumatoid arthritis activities of 3-(4-aminophenyl)-coumarin derivatives. J Enzyme Inhib Med Chem. (2021) ; 36: (1): 450-461. |
[11] | Liu YY, Zhao DS, Zhuang WY. Study on the anti-inflammatory effects of total hairy cherry flavonoids on LPS-induced RAW2647 cells. J Chinese Journal of Cell Biology. (2018) ; 40: (2): 210-214. |
[12] | Liu P, Wang J, Wen W, Pan T, Chen H, Fu Y, et al. Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int Immunopharmacol. (2020) ; 84: : 106570. |
[13] | Deretic V, Levine B. Autophagy balances inflammation in innate immunity. Autophagy. (2018) ; 14: (2): 243-251. |
[14] | Yin HC, Shao SL, Jiang XJ, Xie PY, Sun WS, Yu TF. Interactions between Autophagy and DNA Viruses. Viruses. (2019) ; 11: (9): 776. |
[15] | Levine B, Kroemer G. Autophagy in the Pathogenesis of Disease. J Cell. (2008) ; 132: (1): 0-42. |
[16] | Zhang Q, Lai S, Hou X, Cao W, Zhang Y, Zhang Z. Protective effects of PI3K/Akt signal pathway induced cell autophagy in rat knee joint cartilage injury. Am J Transl Res. (2018) ; 10: (3): 762-770. |
[17] | Matsunaga T, Kawabata S, Yanagihara Y, Kezuka C, Kato M, Morikawa Y, et al. Pathophysiological roles of autophagy and aldo-keto reductases in development of doxorubicin resistance in gastrointestinal cancer cells. Chem Biol Interact. (2019) ; 314: : 108839. |
[18] | Li Z, Wang C, Wang Z, Zhu C, Li J, Sha T, et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds. Nature. (2019) ; 575: (7781): 203-209. |
[19] | Ueno T, Komatsu M. Monitoring Autophagy Flux and Activity: Principles and Applications. Bioessays. (2020) ; 42: (11): e2000122. |
[20] | Feng Y, Li B, Li XY, Wu ZB. The Role of Autophagy in Rheumatic Disease. Curr Drug Targets. (2018) ; 19: (9): 1009-1017. |
[21] | Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. (2016) ; 388: (10055): 2023-38. |
[22] | Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. miRNA-335-5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci. (2019) ; 226: : 164-172. |
[23] | Chadha S, Behl T, Bungau S, Kumar A, Kaur R, Venkatachalam T, et al. Focus on the Multimodal Role of Autophagy in Rheumatoid Arthritis. Inflammation. (2021) ; 44: (1): 1-12. |
[24] | Qian M, Fang X, Wang X. Autophagy and inflammation. Clin Transl Med. (2017) ; 6: (1): 24. |
[25] | Prokesch A, Blaschitz A, Bauer T, Moser G, Hiden U, Zadora J, et al. Placental DAPK1 and autophagy marker LC3B-II are dysregulated by TNF-α in a gestational age-dependent manner. Histochem Cell Biol. (2017) ; 147: (6): 695-705. |
[26] | Yang R, Zhang Y, Wang L, Hu J, Wen J, Xue L, et al. Increased autophagy in fibroblast-like synoviocytes leads to immune enhancement potential in rheumatoid arthritis. J Oncotarget. (2017) ; 8: (9). |
[27] | Du H, Wang Y, Zeng Y, Huang X, Liu D, Ye L, et al. Tanshinone IIA Suppresses Proliferation and Inflammatory Cytokine Production of Synovial Fibroblasts from Rheumatoid Arthritis Patients Induced by TNF-α and Attenuates the Inflammatory Response in AIA Mice. Front Pharmacol. (2020) ; 11: : 568. |
[28] | Li Z, Huaizhou W, Yu W, He Z, Qin Y, Shen Q. The Autophagy Level Is Increased in the Synovial Tissues of Patients with Active Rheumatoid Arthritis and Is Correlated with Disease Severity. J Mediators of Inflammation. (2017) ; 2017: : 1-9. |
[29] | Zhang J, Song X, Cao W, Lu J, Wang X, Wang G et al. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. J Scientific Reports. (2016) ; 6: : 32928. |
[30] | Ahmad Khan M, Sarwar AHMG, Rahat R, Ahmed RS, Umar S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int Immunopharmacol. (2020) ; 85: : 106642. |
[31] | Li S, Li F, Chen WJ, Tian J, Deng C, Wang J, et al. Autophagy inhibitor regulates apoptosis and proliferation of synovial fibroblasts through the inhibition of pi3k/akt pathway in collagen-induced arthritis rat model. American Journal of Translational Research. (2017) ; 9: (5): 2065. |
[32] | Chang L, Feng X, Gao W. Proliferation of rheumatoid arthritis fibroblast-like synoviocytes is enhanced by IL-17-mediated autophagy through STAT3 activation. Connective Tissue Research. (2018) . |




