Frequency-following response effect according to gender using a 10-Hz binaural beat stimulation
Abstract
BACKGROUND:
Several studies have continuously investigated FFRs using binaural beat (BB) stimulations and their related effects. However, only a few studies have investigated the differences in BB stimulation effects according to basic demographic characteristics, such as gender and age.
OBJECTIVE:
This study aimed to determine the alpha wave activity after a 10-Hz BB stimulation and subsequently identify differences according to gender across all brain areas (frontal, central, parietal, temporal, and occipital areas).
METHODS:
A total of 23 healthy adults (11 male and 12 female), aged 20–29, participated in the study. For the 10-Hz BB stimulation, pure tone auditory stimuli of 250 and 260 Hz were given to the left and right ear, respectively. Through a power spectrum analysis of the phase-excluding BBs (non-BBs) and phase-including 10-Hz BBs (
RESULTS:
With the exception of the temporal area, all other brain areas showed a significant increase in alpha power for
CONCLUSION:
The results indicated the lack of gender effects in alpha wave generation through a 10-Hz BB stimulation.
1.Introduction
Frequency-following response (FFR) refers to the brain nerve activity synchronization phenomenon under frequency stimuli. In particular, binaural beats (BBs), a type of FFR, refer to the sounds that control the brain wave based on differences in the auditory stimuli frequency. BBs consist of a baseline and baseline
Numerous studies have reported FFRs using BB stimulations. Jirakittayakorn and Wongsawat [6] showed that BBs with a 6-Hz difference frequency could increase the theta wave. Furthermore, Puzi et al. [7] confirmed an increase in the alpha wave after BBs were stimulated with a 10-Hz difference frequency. In addition, Park et al. [8] reported an increase in the beta wave after BBs were stimulated with a 20-Hz difference frequency.
The generation of a specific brain wave through BB stimulation may also induce several emotional effects. BBs that led to delta or theta wave generation could induce physical and psychological comfort, thus enhancing the quality of sleep [9]. David et al. [10] reported an improvement in tinnitus through an 8-Hz BB stimulation. Furthermore, Beauchene et al. [11] revealed that a 15-Hz BB stimulation led to beta wave generation, which helped in enhancing short- and long-term memory and cognitive ability.
Accordingly, studies have continuously investigated FFRs using BB stimulations and their related effects. However, only a few studies have investigated the differences in BB stimulation effects according to basic demographic characteristics, such as gender and age. Norhazman et al. [12] found a higher level of increase in alpha waves in males than in females with a 9-Hz BB stimulation after induced stress, indicating that the effect of stress reduction was greater in males. However, their study was limited in that only the frontal brain area was examined. Although some attempts have been made to identify the effects of BB stimulations according to gender, it is essential to further verify whether gender has a significant effect on brainwave synchronization via BB stimulation across all brain areas under various emotional and cognitive perspectives. Furthermore, such studies are needed to perform an in-depth analysis of the effects and standards of BB stimulations.
Therefore, this study aimed to determine the effects of BB stimulation according to gender by first monitoring alpha wave generation with a 10-Hz BB stimulation. Subsequently, the differences in alpha waves according to gender across all brain areas (i.e., the frontal, central, parietal, temporal, and occipital areas) were examined.
2.Method
2.1Subjects
In this study, 23 healthy male (11) and female (12) adults, aged 20–29, voluntarily participated after being recruited through an advertisement. The subjects filled out the consent form to participate in the experiment. Table 1 presents the age characteristics of the study participants. The subjects did not have a medical history related to auditory function (hearing impairment or loss) and were instructed to take an adequate sleep of at least 7 h on the night before the day of the experiment. The subjects were also prohibited from engaging in activities that may affect the electroencephalogram (EEG) results, such as the intake of caffeinated drinks, drugs, and alcohol and smoking on the day of the experiment. In addition, this study was conducted with the approval of the Konkuk university institutional review board (7001355-202105-HR-439).
2.2Binaural Beats (BBs)
The BB was set to 10 Hz, which is the median value of the alpha spectrum (8–13 Hz) [13, 14]. An auditory stimulator (Q product, G company, South Korea) was used to induce 250 Hz and 260 Hz stimuli to the left and right ears, respectively, through a pair of earphones (sound pressure: about 60 dB). The experiment comprised a 5-min phase each without (non_BBs) and with BB stimulation (
Table 1
Age characteristics of the study participants
| Male | Female | Total | |
|---|---|---|---|
| Number of subjects | 11 | 12 | 23 |
| Mean age (SD) | 25.9 ( | 22.9 ( | 24.4 ( |
Figure 1.
Experimental design.
2.3EEG measurement and analysis
Using Enobio20 (NeuroElectrics, Spain), brainwaves were measured at a 500-Hz sampling rate. Based on the 10–20 system, the electrodes were attached to a total of 19 areas in the frontal (Fp1, Fp2, F3, F4, F7, F8, Fz), central (C3, C4, Cz), parietal (P3, P4, P7, P8, Pz), temporal (T7, T8), and occipital (O1, O2) areas. The reference and ground electrodes were attached to the right mastoid and right ear lobe, respectively. The impedance was maintained below 5 k
The resulting brainwave data were analyzed using MATLAB 2017 (MathWorks, USA). First, a bandpass filtering of 0.5–50 Hz was performed to remove different noises, including the power noise, electromagnetic waves, blinking, and other movements. To minimize possible noise at the start and end of the experiment, the initial and final 30 s of each measurement were excluded, and the brainwave signals were obtained through epoching for the non- and
The collected data were converted to power values using frequency-based fast Fourier transform (FFT), and the mean absolute power of the alpha frequency range (8–13 Hz) was produced via power spectrum analysis [6, 15, 16].
To compare the BB stimulation effects in terms of state and gender, the mean alpha power was obtained for each brain area, and a mixed-design ANOVA was performed (SPSS25, IBM, USA).
3.Results
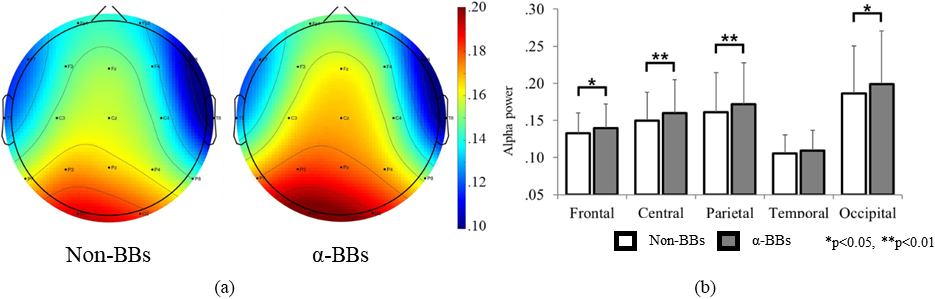
Figure 2a and b illustrate the changes in mean alpha wave power in accordance with the BB stimulation for the entire brain and for each different brain area, respectively. With the exception of the temporal area (
Table 2
Mixed-design ANOVA results
| Brain area | Effect | MS | df |
|
|
|
|---|---|---|---|---|---|---|
| Frontal | State | 0.001 | 1 | 5.255 | 0.032 | 0.200 |
| Gender | 0.004 | 1 | 2.247 | 0.149 | 0.097 | |
| State | 0.000 | 1 | 0.093 | 0.764 | 0.004 | |
| Central | State | 0.001 | 1 | 8.267 | 0.009 | 0.282 |
| Gender | 0.001 | 1 | 0.201 | 0.659 | 0.009 | |
| State | 0.000 | 1 | 0.943 | 0.343 | 0.043 | |
| Parietal | State | 0.001 | 1 | 9.278 | 0.006 | 0.306 |
| Gender | 0.001 | 1 | 0.234 | 0.634 | 0.011 | |
| State | 0.000 | 1 | 3.235 | 0.086 | 0.134 | |
| Temporal | State | 0.000 | 1 | 3.451 | 0.077 | 0.141 |
| Gender | 0.001 | 1 | 0.398 | 0.535 | 0.019 | |
| State | 0.000 | 1 | 0.926 | 0.347 | 0.042 | |
| Occipital | State | 0.002 | 1 | 5.540 | 0.028 | 0.209 |
| Gender | 0.004 | 1 | 0.493 | 0.490 | 0.023 | |
| State | 0.000 | 1 | 1.141 | 0.298 | 0.052 |
Figure 2.
Alpha wave comparison according to the BB stimulation state. (a) Brain topography for 19 channels. (b) Alpha power at each brain area.
4.Discussion
This study examined whether a 10-Hz BB stimulation led to changes in alpha waves across all brain areas (i.e., the frontal, central, parietal, temporal, and occipital areas) and whether there was any effect according to gender on the FFR. The magnitude of the effect was analyzed for the main BB stimulation state effect, differences according to gender, and interaction effect (state
The main effect (BB stimulation state) analysis indicated that all brain areas except the temporal area had an increase in alpha waves with a relatively high effect magnitude, which may be interpreted as the influence of the BB stimulation on alpha wave generation. This is consistent with previous studies that monitored alpha wave generation through BB stimulation [21, 22]. Conversely, the increasing trend in alpha waves for the temporal lobe was not statistically significant. The temporal lobe is the area that handles auditory information, and it is presumed that the actual auditory recognition of the baseline frequency stimulation (a fall in alpha wave) offsets the effect of the difference frequency (a rise in alpha wave).
The analysis of the other main variable (gender) revealed that it was not a significant variable because the magnitude of the effect in terms of alpha waves was substantially low.
The interaction effect was also analyzed to verify that gender did not influence the FFR after a BB stimulation across all brain areas. Both male and female participants in this study exhibited an increase in alpha waves after the BB stimulation, and the rate of increase was approximately 8% and 4%, respectively. This appear to be in agreement with a previous study reporting a higher level of brainwave synchronization after a BB stimulation in male subjects. However, as the difference in the rate of increase between genders and the magnitude of the effect were considerably low, it is difficult to conjecture that there was any significant effect according to gender on brainwave synchronization after a BB stimulation. From an anatomical and physiological perspective, the cochlea in females is shorter than in males; therefore, the delay time for auditory information processing is shorter in females, while males were able to better detect auditory information in complex masking tasks. This was a feature related to complex auditory stimuli detection, including linguistic sounds that indicated a difference according to gender [23]. In contrast, no difference according to gender was found in another study that applied pure tone stimuli [24]. Although further studies are necessary, it is plausible that differences regarding pure tones as relatively simple auditory stimuli are minimal across genders, despite differences in the processing of information containing complex linguistic factors. Therefore, no differences according to gender were found in this study, where the BB stimulation was applied by 10-Hz pure tone stimuli.
5.Conclusion
The results of this study indicated that a 10-Hz BB stimulation could cause alpha wave generation across all brain areas except the temporal area, and gender had no effect on the FFR process. This suggests that BB stimulations may produce effective outcomes regardless of gender. In addition, for the temporal lobe, where the auditory information is processed, the baseline frequency is likely to offset the difference frequency to render the BB stimulation without a significant effect. Based on these results, this study effectively addressed the limitations of previous studies that focused solely on a specific brain area.
Nevertheless, there still remains many variables that need to be analyzed for a more reliable determination of the effectiveness of BB stimulations. The effects of BB stimulations under various emotional/cognitive states and according to age as the most representative demographic variable will be investigated in a future study.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C2009136). And This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2023.
Conflict of interest
The authors declare that they have no conflict of interest.
References
[1] | Norhazman H, Zaini NM, Taib MN, Omar HA, Jailani R, Lias S, et al. Behaviour of EEG Alpha Asymmetry when stress is induced and binaural beat is applied. In 2012 International Symposium on Computer Applications and Industrial Electronics (ISCAIE). (2012) . pp. 297-301. doi: 10.1109/ISCAIE.2012.6482116. |
[2] | Schwarz DW, Taylor P. Human auditory steady state responses to binaural and monaural beats. Clin Neurophysiol. (2005) ; 116: (3): 658-668. doi: 10.1016/j.clinph.2004.09.014. |
[3] | Colzato LS, Barone H, Sellaro R, Hommel B. More attentional focusing through binaural beats: Evidence from the global-local task. Psychol Res. (2017) ; 81: (1): 271-277. doi: 10.1007/s00426-015-0727-0. |
[4] | Shamsi E, Ahmadi-Pajouh MA, Ala TS. Higuchi fractal dimension: An efficient approach to detection of brain entrainment to theta binaural beats. Biomedical Signal Processing and Control. (2021) ; 68: : 102580. doi: 10.1016/j.bspc.2021.102580. |
[5] | Hautus MJ, Shepherd D, Giang E, Landon J. Can binaural beats facilitate autonomic recovery following exposure to an acute stressor? Complement Ther Clin Pract. (2021) ; 45: : 101485. doi: 10.1016/j.ctcp.2021.101485. |
[6] | Jirakittayakorn N, Wongsawat Y. Brain responses to a 6-Hz binaural beat: effects on general theta rhythm and frontal midline theta activity. Front Neurosci. (2017) ; 11: : 365. doi: 10.3389/fnins.2017.00365. |
[7] | Puzi NM, Jailani R, Norhazman H, Zaini NM. Alpha and Beta brainwave characteristics to binaural beat treatment. In 2013 IEEE 9th International Colloquium on Signal Processing and its Applications. (2013) March. pp. 344-348. doi: 10.1109/CSPA.2013.6530069. |
[8] | Park J, Kwon H, Kang S, Lee Y. The effect of binaural beat-based audiovisual stimulation on brain waves and concentration. In 2018 International Conference on Information and Communication Technology Convergence (ICTC). (2018) October. pp. 420-423. doi: 10.1109/ICTC.2018.8539512. |
[9] | Abeln V, Kleinert J, Strüder HK, Schneider S. Brainwave entrainment for better sleep and post-sleep state of young elite soccer players – A pilot study. Eur J Sport Sci. (2014) ; 14: (5): 393-402. doi: 10.1080/17461391.2013.819384. |
[10] | David JB, Naftali A, Katz A. Tinntrain: A multifactorial treatment for tinnitus using binaural beats. Hearing Journal. (2010) ; 63: (11): 25-26. doi: 10.1097/01.HJ.0000390818.17619.65. |
[11] | Beauchene C, Abaid N, Moran R, Diana RA, Leonessa A. The effect of binaural beats on visuospatial working memory and cortical connectivity. PLoS One. (2016) ; 11: (11): e0166630. doi: 10.1371/journal.pone.0166630. |
[12] | Norhazman H, Mohamad Zaini N, Taib MN, Othman KA, Sani M, Jailani R, Omar HA. The Effect of Alpha Binaural Beat on Frontal ESD Alpha Asymmetry on Different Gender. (2006) . |
[13] | Beauchene C, Abaid N, Moran R, Diana RA, Leonessa A. The effect of binaural beats on verbal working memory and cortical connectivity. J Neural Eng. (2017) ; 14: (2): 026014. doi: 10.1088/1741-2552/aa5d67. |
[14] | Shekar L, Suryavanshi CA, Nayak KR. Effect of alpha and gamma binaural beats on reaction time and short-term memory. Natl J Physiol Pharm Pharmacol. (2018) ; 8: (6): 829-833. doi: 10.5455/njppp.2018.8.1246506022018. |
[15] | Guruprasath G, Gnanavel S. Effect of continuous and short burst binaural beats on EEG signals. In 2015 International Conference on Innovations in Information, Embedded and Communication Systems (ICIIECS). (2015) March. pp. 1-4. doi: 10.1109/ICIIECS.2015.7193197. |
[16] | Engelbregt H, Barmentlo M, Keeser D, Pogarell O, Deijen JB. Effects of binaural and monaural beat stimulation on attention and EEG. Exp Brain Res. (2021) ; 239: (9): 2781-2791. doi: 10.1007/s00221-021-06155-z. |
[17] | Nahm FS. Understanding effect sizes. Hanyang Med Rev. (2015) ; 35: (1): 40-43. doi: 10.7599/hmr.2015.35.1.40. |
[18] | Mihladiz G, Duran M, Dogan A. Examining primary school students’ attitudes towards science in terms of gender, class level and income level. Procedia-Social and Behavioral Sciences. (2011) ; 15: : 2582-2588. doi: 10.1016/j.sbspro.2011.04.150. |
[19] | Clifton RT, Gill DL. Gender differences in self-confidence on a feminine-typed task. J Sport Exerc Psychol. (1994) ; 16: (2): 150-162. |
[20] | Ali MS, Mohsin MN, Sherawan A. Gender differences in terms of test anxiety and attitude towards science. Int J Environment, Ecology, Family and Urban Studies (IJEEFUS). (2013) ; 3: : 133-8. |
[21] | McMurray JC. Binaural beats enhance alpha wave activity, memory, and* attention in healthy-aging seniors. University of Nevada, Las Vegas. (2006) . doi: 10.25669/ottz-gbnn. |
[22] | Solca M, Mottaz A, Guggisberg AG. Binaural beats increase interhemispheric alpha-band coherence between auditory cortices. Hear Res. (2016) ; 332: : 233-237. doi: 10.1016/j.heares.2015.09.011. |
[23] | McFadden D. Sex differences in the auditory system. DevNeuropsychol. (1998) ; 14: (2-3): 261-298. doi: 10.1080/87565649809540712. |
[24] | Ahadi M, Pourbakht A, Jafari AH, Shirjian Z, Jafarpisheh AS. Gender disparity in subcortical encoding of binaurally presented speech stimuli: An auditory evoked potentials study. Auris Nasus Larynx. (2014) ; 41: (3): 239-243. doi: 10.1016/j.anl.2013.10.010. |




