Comparison of parameter types for the calibration of noninvasive continuous cardiac output monitoring of patients undergoing lumbar spinal surgery in the prone position
Abstract
BACKGROUND:
Cardiac output (CO) decreases on reversing the patient’s position to the prone position. Estimated continuous cardiac output (esCCO) systems can noninvasively and continuously monitor CO calibrated by patient information or transesophageal echocardiogram (TEE).
OBJECTIVE:
To compare the accuracy, precision, and trending ability of two calibration methods of CO estimation in patients in prone position.
METHODS:
The CO estimates calibrated by TEE (esT) and patient information (esP) of 26 participants were included. CO was collected at four time points. The accuracy and precision of agreement were evaluated using the Bland-Altman method. A four-quadrant plot was used for trending ability analysis.
RESULTS:
The bias between esP and TEE and between esT and TEE was 0.2594 L/min (95% limits of agreement (LoA):
CONCLUSION:
Despite limited accuracy and precision, esP showed acceptable trending ability. The trending ability of esCCO calibrated by the reference TEE value was comparable with that of TEE.
1.Introduction
An increase in age-related diseases, such as degenerative diseases of the lumbar spine [1], has been observed with the increase in the number of elderly individuals, resulting in an increase in the number of candidates requiring lumbar spine surgery. Nearly 180,000 general spine surgeries were performed in South Korea in 2020 [2]; lumbar spine surgery is one such surgery that is performed in the prone position. A significant decrease in cardiac output (CO) occurs on changing the position of the patient from the supine to the prone position due to a decrease in preload, resulting in decreased arterial pressure and tissue perfusion [3]. Perioperative cardiac complications are the main cause of mortality after noncardiac surgery, and 1.5% of patients undergoing noncardiac surgery die in the subsequent 30 days [4]. The overall incidence of cardiac complications ranges from 0.2–13% in lumbar spine surgery [5]. Therefore, the optimization of hemodynamic variables, including CO, is an integral part of perioperative care that reduces postoperative mortality and morbidity during lumbar spine and other surgeries that require patients to be placed in the prone position [6].
Many noninvasive hemodynamic monitoring techniques, such as impedance cardiography, arterial pressure-based CO (APCO), volume clamp method, and pulse wave transit time (PWTT) method, have been developed and studied recently [7, 8, 9]. Among these methods, the estimated continuous cardiac output (esCCO) system, a technique based on the PWTT method, can noninvasively and continuously monitor CO. The esCCO system determines a reference value for calibration by measuring and entering the basic vital sign parameters using electrocardiogram (ECG), pulse wave oximetry, and arterial blood pressure (ABP) and by entering patient information, such as age, gender, height, and weight [9]. Other cardiac output measurement devices, such as a transesophageal echocardiogram (TEE), can also be used for initial calibration [10].
Previous studies have demonstrated the accuracy, precision, and trending ability of esCCO, and several studies [9, 11, 12] have examined the use of esCCO calibrated using patient information and noninvasive basic vital sign parameters for the initial calibration. Terada et al. [9] studied the ability of the esCCO system to measure the trends during laparotomy without postural change and reported that esCCO has a reasonable trending ability. Magliocca et al. [11] compared esCCO with the thermodilution CO (TDCO) method in patients undergoing orthotopic liver transplantation and concluded that although esCCO has no interchangeability, it has an acceptable trending ability. Terada et al. [12] compared the ability of esCCO and APCO to detect the stroke volume index and demonstrated that the accuracy, precision, and dynamic trend of esCCO are better than those of APCO.
Some studies [13, 14] have used reference CO values for the initial calibration of esCCO and adopted the TDCO or APCO methods for reference CO measurement. The results of both studies indicated that esCCO has limited interchangeability in terms of accuracy and precision. Suzuki et al. [13] compared esCCO with APCO in patients undergoing cardiovascular surgery and showed that the trending ability of esCCO was almost acceptable. Tsutsui et al. [14] also assessed the trending ability of esCCO and reported that it is comparable with that of the TDCO method.
To the best of our knowledge, no study has measured CO using the PWTT method in patients undergoing surgery in the prone position. We aimed to compare the accuracy, precision, and trending ability of esCCO obtained using two different calibration methods with that of the TEE-measured CO values obtained from patients in the prone position during lumbar spine surgery.
2.Methods
This prospective study was conducted at the Dong-A University Hospital in accordance with the principles of the Declaration of Helsinki. We obtained approval from the Institutional Review Board of the Dong-A University Hospital. The study is registered with the Clinical Research Information Service of the Republic of Korea (no. KCT0006164; Available at: https://cris.nih.go.kr).
Twenty-six patients scheduled to undergo elective lumbar spinal surgery in the prone position who were between the ages of 30 and 80 years and with a physical status of 1, 2, or 3 according to the American Society of Anesthesiologists classification were enrolled in this study after obtaining written informed consent. Patients younger than 30 years or older than 80 years and patients with an ejection fraction less than 35% on preoperative echocardiography, severe valvular heart disease, preoperative hemodynamic instability, respiratory failure, persistent cardiac arrhythmia, permanent pacemaker, and/or an intra-aortic balloon pump were excluded. Patients with swallowing difficulties; any history of esophageal diseases, such as strictures, diverticula, and tumors; or recent gastroesophageal surgery were also excluded from the study.
Based on the PWTT method, esCCO can be calculated using ECG, ABP, and pulse wave oximetry according to the following formula:
The pulse wave transit time is the duration between the ECG R-wave peak to the rise point of the pulse wave oximeter. The rise point is defined as the time taken by the pulse wave to reach 30% of the maximum amplitude. The pulse wave transit time is correlated with SV, and K is calculated by the arterial pulse pressure. Experimental constants,
Two bedside monitors (BSM-6701K, Nihon Kohden, Tokyo, Japan) were connected to the patient to assess and monitor each esCCO. Two sets of electrodes were used to monitor the ECG (lead II), and two pulse oximeter probes were placed on the second and fourth fingertips of the same hand. The blood pressure was monitored noninvasively every 5 minutes until the insertion of the radial arterial catheter and every 15 minutes thereafter. The radial arterial and central venous catheters were placed under sevoflurane-induced (1.0–2.0%, end-tidal) general anesthesia, and a 2–7 MHz TEE transducer (Philips, X7-2t, MA, USA) was inserted to the depth of the lower esophagus. The position of the participant was then reversed from the supine to the prone position. The participants were positioned on a convex saddle frame, with their heads on the prone position foam headrest to prevent compression of the endotracheal tube and TEE. CO was evaluated through TEE, and the value was entered for the initial calibration of esCCO (esT). The reference CO value was calculated according to the modified Simpson’s method (biplane method of discs) using a portable ultrasound device (CX50, Philips, MA, USA) as it is the only recommended method that is known to fare well in the prediction of ventricular volume, CO, and ejection fraction even with abnormal ventricles [15]. The average of three sequential measurements of CO was used to improve precision and minimize the interventricular differences. The CO measurement with TEE was performed by an anesthesiologist who had performed this technique at least 200 times and had sufficient experience in monitoring cardiac function using TEE during cardiovascular surgery. Concurrently, another esCCO calibrated by patient information (esP) was also assessed. The CO measurements of esT, esP, and TEE were collected at four time points (T1: 10 minutes after reversing the patient to the prone position; T2: 10 minutes after skin incision; T3: mid-time of the surgery; and T4: at the time of placing the last suture).
Details regarding the demographic characteristics of the participants and types of surgery are shown in Table 1. The hemodynamic data of the participants at all time points (T1–T4) are presented in Table 2. The differences between esT, esP and TEE were evaluated at each of the four time points using one-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test or Kruskal-Wallis test with Dunn’s post-hoc test for multiple comparisons. Pearson correlation coefficients with 95% confidence intervals (Cis) for esT, esP, and TEE were calculated, and repeated measurements were conducted to correct the correlation coefficients [16]. The accuracy and precision of the agreement between esT and esP with TEE were evaluated using the Bland-Altman method [17]. The mean bias represents the systemic error between the two methods. When the estimated bias is small and near zero, and the 95% CI is narrow, it indicates that the agreement between the two measurements is accurate and precise. The results of the Bland-Altman analysis were presented as percentage error (PE) by dividing the limit of agreement (LoA) by mean CO. A PE cut-off of
Table 1
Patient and surgery characteristics (
| Characteristic | |
|---|---|
| Male, | 9 (35) |
| Age, y | 72 (52–80) |
| Height, cm | 157.8 |
| Weight, kg | 62.3 |
| BMI, kg/m | 25.1 |
| ASA score (I/II/III), | 2000/12/14 |
| Comorbidity | |
| Hypertension | 14 |
| Diabetes mellitus | 12 |
| Congestive heart failure | 3 |
| Angina | 2 |
| Chronic kidney failure | 5 |
| Cerebrovascular accident | 3 |
| Parkinson disease | 2 |
| Aortic stenosis | 1 |
| Asthma | 1 |
| Surgery time, min | 188 (176–229) |
| Diagnosis | |
| Spinal stenosis | 10 |
| Herniated nucleus pulposus | 1 |
| Spine fracture | 4 |
| Spine metastatic cancer | 1 |
| Post-operation complication | 4 |
| Spondylodiscitis | 3 |
| Spondylolisthesis | 3 |
| Fluid balance management, mL | |
| Input | 1800 (1500–2450) |
| Output including EBL | 525 (377–950) |
| Transfusion, RBC | |
| Patients, | 5 (19) |
| Phenylephrine, | 19 (61) |
| Ephedrine, | 7 (27) |
Age is presented as median (range), and the other values are presented as mean
Table 2
Hemodynamic values at four time points
| T1 | T2 | T3 | T4 | |||||
| sABP (mmHg) | 117 | 121 | 119 | 127 | ||||
| dABP (mmHg) | 61 | 63 | 64 | 63 | ||||
| mABP (mmHg) | 82 | 85 | 84 | 86 | ||||
| HR (beats/min) | 80 | 75 | 70 | 71 | ||||
| esCCO (esT, L/min) | 4.04 | 3.79 | 3.32 | 3.64 | ||||
| esCCO (esP, L/min) | 4.24 | 3.94 | 3.57 | 3.95 | ||||
| CO (TEE, L/min) | 4.09 | 3.66 | 3.43 | 3.49 | ||||
| CVP (cmH2O) | 11 (8–14) | 12 (7–15) | 10 (6–12) | 10 (6–13) | ||||
Data are presented as the mean
The sample size was calculated by estimating the mean bias and LoA of esCCO [17]. Based on accuracy, the expected mean bias of the CO value between esCCO and TEE was assumed to be 0.39 L/min. The expected precision between esCCO and TEE was
Statistical analyses were performed using SPSS v26.0 (IBM Corp., Armonk, NY, USA), MedCalc Statistical Software v20.009 (MedCalc Software Ltd., Ostend, Belgium) and R v4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
3.Results
Both esP and esT showed no significant differences compared with TEE at any time point during anesthesia and surgery (Fig. 1).
Figure 1.
TEE cardiac output (CO) measurements (circles, black line) esCCO calibrated by patient information (esP) (squares, red line), and esCCO calibrated by TEE (triangles, blue line). Data are presented as mean
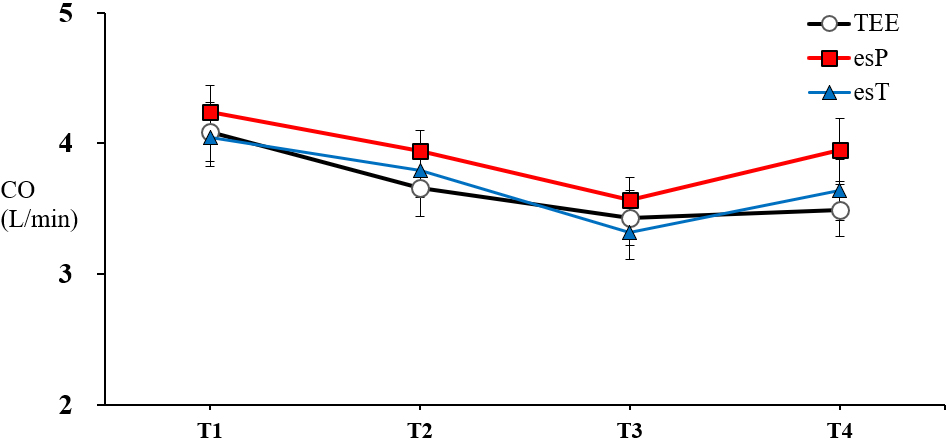
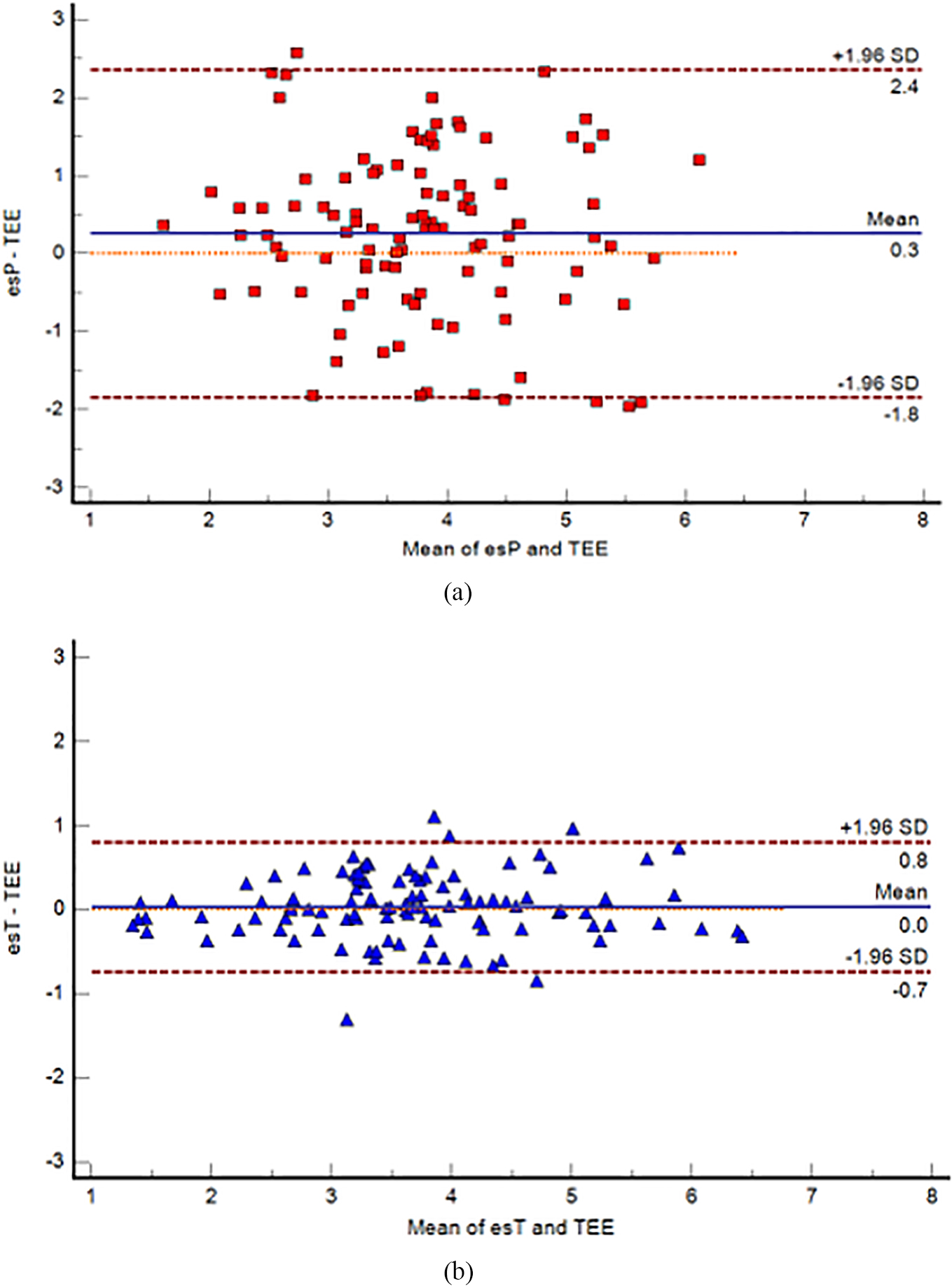
Figure 2.
Bland-Altman plots for the agreement analysis between esP and TEE (a), and esT and TEE (b). The blue continuous lines show the mean difference (bias), and the red dashed lines show the upper and lower 95% limits of agreement (LoA). TEE
Pearson correlation coefficient (r) with 95% confidence intervals (CIs) of esP, esT and TEE were calculated, and repeated measurements were conducted to correct the correlation coefficients. The correlation coefficients between esP and TEE and between esT and TEE were 0.713 (
The Bland-Altman analysis that was corrected for repeated measurements (26 measurements repeated four times) and included all time points (T1–T4) showed that the bias between esP and TEE was 0.2594 L/min (95% CI of LoA:
Table 3
Agreement analysis
| Method A | esP | esT |
|---|---|---|
| Method B | TEE | TEE |
| Mean | 0.2594 | 0.0337 |
| Lower limit | ||
| 95% CI | ||
| Upper limit | 2.3562 | 0.8055 |
| 95% CI | 1.8155 to 3.1536 | 0.6769 to 0.9831 |
| PE | 56% | 21% |
esP
Table 4
Four-quadrant analysis
|
| Concordant | Disconcordant | Concordance rate [%] | CCC (95% CI) | |
|---|---|---|---|---|---|
| esP | 37 | 34 | 3 | 91.9% | 0.700 (0.431–0.854) |
| esT | 34 | 33 | 1 | 97.1% | 0.794 (0.566–0.908) |
A strong correlation existed between
Figure 3.
The four-quadrant plot shows the changes in the cardiac output (CO),
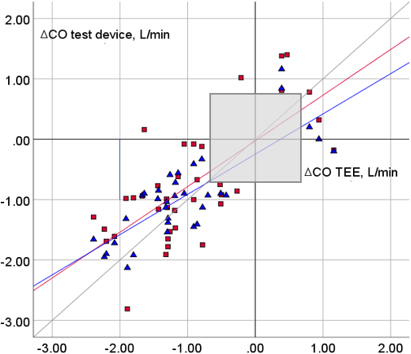
The trending abilities of esP and esT were expressed using the four-quadrant plot and CCC (Fig. 3). An exclusion zone of 0.75 L/min was applied based on previous literature [11].
4.Discussion
This clinical study is the first study that assesses the accuracy, precision, and trending ability of the initial calibration methods of patient information and reference TEE value for estimating CO using esCCO in patients undergoing prone position lumbar spine surgery. We hypothesized that regardless of the calibration method, esCCO might have a reliable trending ability. We also hypothesized that performing the initial calibration with the reference TEE values would help improve the accuracy and precision of esCCO. The correlation between esP and TEE was moderately strong, and the correlation between esT and TEE was also strong at all time points (T1–T4). In the Bland-Altman plot, the bias between esP and TEE and between esT and TEE was 0.2594 L/min and 0.0337 L/min, respectively. Compared with that of esT, esP reported higher CO measurement than TEE. The PE of esP was 56%, which was high, indicating that interchangeability was not acceptable. In contrast, the PE of esT was 21%, suggesting that the esCCO system that was calibrated using the reference CO value has interchangeability. CCC between
Previous studies enrolled patients undergoing procedures such as open-heart surgery and transplantation surgery. Vasoactive drugs are often likely to be required during these types of surgeries since a significant change in the systemic vascular resistance (SVR) might occur. The arterial elasticity affects PWTT; thus, esCCO may not provide reliable measurements when significant changes in SVR occur [22]. By enrolling patients undergoing lumbar spine surgery, we ensured that only less potent inotropes, such as phenylephrine and ephedrine, were required; this difference may influence the result. We can hypothesize that esCCO has interchangeability in lumbar spine surgery and can play a role in similar circumstances. The distance from the heart to the fingertip might differ between patients of similar age, height, and weight. Moreover, the arterial pulse wave velocity, which is influenced by SVR, can change from the point of initial calibration. Thus, the demographic data implied in esCCO cannot be generalized due to the presence of individual differences, which might further influence the accuracy and precision of esCCO when calibrated by patient information. However, it may be useful if a CO monitoring device has an acceptable trending ability, even if it fails to provide an absolute value of CO [19]. In addition to the comparable trending ability of esCCO with that of TEE, esCCO is noninvasive and easily accessible. A recent study revealed an excellent correlation between the ECG parameters in the supine and prone positions when detecting the QRS axis, PR, RR, QRS, and QT intervals with electrodes placed on the torso and dorsal aspect [23]. In addition, since the distance from the heart to the fingertip, where the upstroke of pulse oximetry is detected, does not change according to the patient’s position, the prone position does not influence the quality of PWTT and esCCO.
Judging from a previous study [23] and the principle of the esCCO system, the prone position itself may not interfere with the ability of esCCO to estimate CO. Further studies should determine the ability of esCCO under different circumstances as it may be useful and feasible for continuous monitoring CO in other noncardiac surgeries performed in the prone position.
Changing the position of the patient from supine to prone position under general anesthesia induces a significant decrease in CO (as high as 25%) caused by a decrease in preload [3]. Moreover, it increases the intrathoracic pressure and secondarily influences a decrease in systolic pulmonary venous flow, left ventricular compliance and venous return from the inferior vena cava [24]. Most patients scheduled for lumbar spine surgery are at an increased risk of developing cardiovascular disease including coronary artery disease, due to their advanced age and the presence of associated risk factors. Additionally, these patients cannot walk long distances due to pain and neurogenic claudication; they may be asymptomatic and have silent cardiovascular diseases [5]. There is a substantial risk of bleeding [25] and perioperative cardiac complications, including death [4] in many types of lumbar spine surgery. Therefore, it is essential to perform continuous monitoring of hemodynamic variables, including CO, using accurate and precise measurement tools to minimize these complications.
Transesophageal echocardiography, a well-established clinical tool for circulatory evaluation, is a feasible, rapid, safe, and semi-invasive monitoring device suitable for use in patients undergoing surgery under general anesthesia and mechanical ventilation, regardless of the position [26]. Dessap et al. [27] reported that the feasibility, tolerance, and image quality of the standard views of TEE were good in the prone and supine positions. Corresponding to this previous study, the image quality of the four-chamber long-axis view and two-chamber long-axis view for calculating the stroke volume in this study were comparable between the supine and prone positions when measuring the CO with TEE to obtain a reference value. TEE is used widely and routinely at our hospital to monitor cardiac performance, as the TDCO method is invasive and has the risk of severe complications [28]. The TEE method has been validated in comparison with the TDCO method in a previous study [10] and is now accepted as a reliable CO measurement method. The meta-analysis by Zhang et al. [10] concluded that there was no significant difference between the CO estimated using TEE and TDCO (random effects model: mean difference, 0.00; 95% CI,
The present study had some limitations. The small sample size cannot represent the hemodynamic variety of the population, and only patients undergoing lumbar spine surgery in the prone position were enrolled. The median age of the participants in our study was 72 years, and many of their diagnoses were age-related degenerative spine diseases. Further studies should be performed to determine the ability of esCCO to estimate CO in various noncardiac surgeries, circumstances, and age groups. It may also be suitable to measure esP and esT both before and after reversing the patient from supine to prone and vice versa and analyze the ability to detect the change in CO when the position of the patient changes as these two moments usually exhibit substantial hemodynamic changes. However, the patients were predominantly in the prone position during surgery. Situations, such as surgical incision, substantial bleeding, intraoperative hypotension and instituting vasoactive and inotropic support, which can happen often in lumbar spine surgery, were reflected in the results of our study.
5.Conclusion
esP, which noninvasively measures the estimated CO using basic vital signs and patient information, showed an acceptable trending ability, despite limited accuracy and precision. The accuracy and precision of esT calibrated by TEE were comparable with those of the reference CO values measured by TEE. The trending ability of esT was also good for tracking changes in CO in patients undergoing prone position lumbar spine surgery. Therefore, we propose that esCCO, a completely noninvasive and continuous CO monitoring system, may be a suitable alternative to other monitoring techniques.
Funding
No funding was received to assist with the preparation of this manuscript.
Data sharing availability
Due to the nature of this research, the participants did not agree to share their data publicly; hence, supporting data is not available. However, data are available from the authors upon reasonable request and with the permission of Dong-A University.
Acknowledgments
This study was supported by Dong-A University.
Conflict of interest
The authors declare that they have no conflict of interest.
References
[1] | Cloyd JM, Acosta FL, Ames CP. Complications and outcomes of lumbar spine surgery in elderly people: A review of the literature. J Am Geriatr Soc. (2008) ; 56: (7): 1318-1327. doi: 10.1111/j.1532-5415.2008.01771.x. |
[2] | Kang JH, Lee CJ. Major surgery statistical yearbook, 2020 [homepage on the internet]. National Health Insurance. (2021) [updated 15 December 2021; cited 18 June 2022]. Available from: www.nhis.or.kr/nhis/together/wbhaec06800m01.do?mode=view&articleNo=10813594. |
[3] | Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth. (2008) ; 100: (2): 165-183. doi: 10.1093/bja/aem380. |
[4] | Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. (2015) ; 373: (23): 258-269. doi: 10.1056/NEJMra1502824. |
[5] | Lee DY, Lee SH, Jang JS. Risk factors for perioperative cardiac complications after lumbar fusion surgery. Neurol Med Chir (Tokyo). (2007) ; 47: (11): 495-500. doi: 10.2176/nmc.47.495. |
[6] | Gurgel ST, do Nascimento P Jr. Maintaining tissue perfusion in high-risk surgical patients: A systematic review of randomized clinical trials. Anesth Analg. (2011) ; 112: (6): 1384-1391. doi: 10.1213/ane.0b013e3182055384. |
[7] | Heijne1 A, Krijtenburg P, Bremers A, Scheffer GJ, Malagon I, Slagt C. Four different methods of measuring cardiac index during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Korean J Anesthesiol. (2021) ; 74: (2): 120-133. doi: 10.4097/kja.20202. |
[8] | Stepanov R, Podtaev S, Dumler A, Chugainov S. Assessment of cardiac time intervals by wavelet transform of the impedance cardiogram. Technol Health Care. (2016) ; 24: : S803-S809. doi: 10.3233/THC-161213. |
[9] | Terada T, Kessoku S, Suzuki A, Kurosawa A, Nakagomi S, Oiwa A, et al. Comparison of the pulse wave transit time method and an arterial pressure-based cardiac output system for measuring cardiac output trends during laparotomy without postural change. Asian J Anesthesiol. (2019) ; 30: : 621-627. doi: 10.1007/s10877-015-9772-x. |
[10] | Zhang Y, Wang Y, Shi J, Hua Z, Xu J. Cardiac output measurements via echocardiography versus thermodilution: A systematic review and meta-analysis. PLoS One. (2019) ; 14: (10): e0222105. doi: 10.1371/journal.pone.0222105. |
[11] | Magliocca A, Rezoagli E, Anderson TA, Burns SM, Ichinose F, Chitilian HV. Cardiac output measurements based on the pulse wave transit time and thoracic impedance exhibit limited agreement with thermodilution method during orthotopic liver transplantation. Anesth Analg. (2018) ; 126: (1): 85-92. doi: 10.1213/ane.0000000000002171. |
[12] | Terada T, Ochiai R. Comparison of the ability of two continuous cardiac output monitors to detect stroke volume index: Estimated continuous cardiac output estimated by modified pulse wave transit time and measured by an arterial pulse contour-based cardiac output device. Technol Health Care. (2021) ; 29: (3): 499-504. doi: 10.3233/THC-202332. |
[13] | Suzuki T, Suzuki Y, Okuda J, Minoshima R, Misonoo Y, Ueda T, et al. Cardiac output and stroke volume variation measured by the pulse wave transit time method: A comparison with an arterial pressure-based cardiac output system. J Clin Monit Comput. (2019) ; 33: (3): 385-392. doi: 10.1007/s10877-018-0171-y. |
[14] | Tsutsui M, Araki Y, Masui K, Kazama T, Sugo Y, Archer TL, et al. Pulse wave transit time measurements of cardiac output in patients undergoing partial hepatectomy: A comparison of the esCCO system with thermodilution. Anesth Analg. (2013) ; 117: (6): 1307-1312. doi: 10.1213/ane.0b013e3182a44c87. |
[15] | Chengode S. Left ventricular global systolic function assessment by echocardiography. Ann Card Anaesth. (2016) ; 19: (Suppl 1): S26-S34. doi: 10.4103/0971-9784.192617. |
[16] | Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1–Correlation within subjects. BMJ. (1995) ; 310: (6977): 446. doi: 10.1136/bmj.310.6977.446. |
[17] | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. (1986) ; 1: (8476): 307-310. doi: 10.1016/s0140-6736(86)90837-8. |
[18] | Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. (1999) ; 15: (2): 85-91. doi: 10.1023/a1009982611386: . |
[19] | Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. (2010) ; 111: (5): 1180-1192. doi: 10.1213/ane.0b013e3181f08a5b. |
[20] | Terada T, Oiwa A, Maemura Y, Robert S, Kessoku S, Ochiai R. Comparison of the ability of two continuous cardiac output monitors to measure trends in cardiac output: Estimated continuous cardiac output measured by modified pulse wave transit time and an arterial pulse contour-based cardiac output device. J Clin Monit Comput. (2016) ; 30: : 621-627. doi: 10.1007/s10877-015-9772-x. |
[21] | Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth. (2011) ; 25: (3): 536-546. doi: 10.1053/j.jvca.2011.01.003. |
[22] | Bataille B, Bertuit M, Mora M, Mazerolles M, Cocquet P, Masson B, et al. Comparison of esCCO and transthoracic echocardiography for non-invasive measurement of cardiac output intensive care. Br J Anaesth. (2012) ; 109: (6): 879-886. doi: 10.1093/bja/aes298. |
[23] | Roccia H, Argaud L, Le Goic M, Guérin C, Cour M. Electrocardiogram monitoring in the prone position in coronavirus disease 2019 acute respiratory distress syndrome. Eur J Cardiovasc Nurs. (2021) ; 20: (8): 792-796. doi: 10.1093/eurjcn/zvab094. |
[24] | Chui J, Craen RA. An update on the prone position: Continuing professional development. Can J Anesth. (2016) ; 63: (6): 737-767. doi: 10.1007/s12630-016-0634-x. |
[25] | Butler JS, Burke JP, Dolan RT, Fitzpatrick P, O’Byrne JM, McCormack D, et al. Risk analysis of blood transfusion requirements in emergency and elective spinal surgery. Eur Spine J. (2011) ; 20: (5): 753-758. doi: 10.1007/s00586-010-1500-0. |
[26] | Lele A, Krishnamoorthy V. Transesophageal echocardiography. In: Prabhakar H, editor. Essentials of neuroanesthesia. London: Academic Press; (2017) . pp. 277-284. |
[27] | Dessap AM, Proost O, Boissier F, Louis B, Campo FR, Brochard L. Transesophageal echocardiography in prone position during severe acute respiratory distress syndrome. Intensive Care Med. (2011) ; 37: (3): 430-434. doi: 10.1007/s00134-010-2114-z. |
[28] | Youssef N, Whitlock R. The routine use of PAC should be abandoned. Can J Cardiol. (2017) ; 33: (1): 135-141. doi: 10.1016/j.cjca.2016.10.005. |




