The efficacy of probiotics on the prevention of pouchitis for patients after ileal pouch-anal anastomosis: A meta-analysis
Abstract
BACKGROUND:
To date, a few studies indicated that probiotics are beneficial to pouchitis, but no meta-analyses summarized the outcomes of probiotics in pouchitis in detail.
OBJECTIVE:
This meta-analysis discusses probiotics in the prevention of pouchitis for patients after ileal pouch-anal anastomosis (IPAA) and the relationship between probiotics preventive effect and the duration of therapy and history.
METHODS:
PubMed, EMBASE and Cochrane Library databases were searched from inception until February 2022. Risk ratio (RR), mean difference (MD) and their 95% confidence interval (CI) were analyzed by Review Manager 5.3. The subgroup analysis was also performed to explore the agent for influencing outcomes.
RESULTS:
A total of 8 studies were included in this meta-analysis. The incidence of pouchitis in probiotics was significantly lower than that in the control (RR
CONCLUSIONS:
Probiotics are beneficial in the prevention of pouchitis after IPAA, especially in the short-term.
1.Introduction
Pouchitis is a common complication after restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) seen in patients with ulcerative colitis (UC) and is a nonspecific inflammatory in the ileal pouch [1]. Over 50% of UC patients after IPAA experience pouchitis and preventive strategies are therefore of crucial importance. The pathogenesis of pouchitis is complicated. The dysbacteriosis of ileal pouch is one of the most important mechanisms [2]. An analysis of the microflora indicated that there is a great difference between pouchitis and non-pouchitis patients [3]. During pouchitis, the reduction of microflora diverse and the anaerobic to aerobic ratio are seen in pouchitis patients [4]. Some studies also indicated that sulfate-reducing bacteria, enterobacteriaceae are common bacteria associated with pouchitis [5, 6]. On the other hand, the anti-microbial treatment is an effective method for pouchitis and is superior to anti-inflammatory therapy in inducing remission in pouchitis patients [7, 8, 9]. Therefore, it is obvious that the microflora is closely related to pouchitis.
Probiotics are living microorganisms that are beneficial to host. They can regulate the tight junctions, properties of the mucus layer to maintain the intestinal homeostasis [10, 11, 12]. Laval et al. indicated that Lactobacillus rhamnosus CNCM I-3690 maintains the epithelial barrier through modulating occludin and E-cadherin in the murine model [12]. Probiotics also have an anti-microbial function to maintain intestinal balance [13]. Furthermore, some systematic reviews and meta-analyses indicated that probiotics are beneficial to the prevention and treatment of gastrointestinal disease, including the inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), antibiotic associated diarrhea (AAD) [14, 15, 16].
Some meta-analyses also mentioned that probiotics are beneficial to patients after IPAA, but they did not summarize the outcomes of probiotics for them in detail [14, 17, 18]. In this meta-analysis, we discuss the efficacy of probiotics in preventing pouchitis for patients after IPAA, the short-term and long-term preventive effects, and the pouch disease activity index (PDAI) after the administration. The agents that may influence the outcomes are also discussed.
2.Methods
2.1Search strategy
The MeSH terms “proctocolectomy, restorative”, “pouchitis”, “probiotics”, “escherichia coli”, “VSL3”, “streptococcus”, “saccharomyces”, “lactobacillus”, “bifidobacterium”, “enterococcus” and their entry terms were searched in PubMed, EMBASE and Cochrane Library databases from inception to February 2022. The study also gained from reference of relevant reviews.
2.2Study selection
We included studies that met the following criteria. Inclusion criteria: (1) All studies reported administration of probiotics for patients after restorative proctocolectomy with IPAA. (2) All patients were without pouchitis at the study entry (PDAI
The study selection was completed by two researchers. Any contradictions between the two researchers were solved by discussion or decided by a third reviewer.
2.3Data extraction
The following data were extracted: type of study; type of probiotics; the diagnostic criteria; the start time of probiotics administration; the population of the control and probiotics; the number of patients with pouchitis in different time periods; the population of pouchitis; the PDAI scores of pouchitis-free population after treatment. The data extraction was completed by two researchers. Any contradictions between the two researchers were solved by discussion or decided by a third reviewer.
2.4Assessing quality of included studies
The assessment quality was performed by the Cochrane Collaboration’s Tool for Assessing Risk of Bias. The quality was assessed according to the aspects as follows: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias). The assessing quality was completed by two researchers. Any contradictions between the two researchers were solved by discussion or decided by a third reviewer.
2.5Statistical analysis
All data were analyzed by Review Manager 5.3. The risk ratio (RR) and its 95% confidence interval (CI) were estimated by the Mantel-Haenszel analysis method. The mean difference (MD) and its 95% CI were estimated by the inverse variance analysis method. The heterogeneity was evaluated by Cochrane Q test and Quantity
3.Results
3.1Literature search results
The screening process and results are shown in Fig. 1. 1163 studies were searched from PubMed, EMBASE, Cochrane Library databases and other sources. 184 studies were removed due to duplication. 960 studies were removed according to the title and abstract. 11 studies were removed after screening through full-text based on the selection criteria. Finally, 8 studies were included in this meta-analysis [21, 22, 23, 24, 25, 26, 27, 28] (Fig. 1).
3.2Characteristics of included studies and patients
The characteristics of the studies are shown in Table 1. 3 studies recorded the patients with past history of recurrent or chronic pouchitis and 5 studies recorded the patients without past history. The bias of the included studies is shown in Fig. 2.
3.3The incidence of pouchitis after taking probiotics
The incidence in probiotics was significantly lower than the control (RR
3.4The short-term and long-term preventive effects of probiotics
The probiotic group was compared to the placebo group at two time periods: 0–6 months (Fig. 5A) and
Table 1
Characteristics of the included studies
| Year | Authors | Type of | Type of | Past history | Start time of | Type of | Diagnostic | Duration of | Episode/total | PDAI after treatment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| study | control | administration | probiotics (dose) | criteria | treatment | Control | Probiotics | Control | Probiotics | |||
| 2003 | Gionchetti et al. [21] | RCT: Placebo-controlled, Double-blind | Placebo | Without chronic or recurrent pouchitis | Within 1 week after ileostomy closure | VSL#3 (9 | Pouchitis: PDAI | 12 months | 8/20 | 2/20 | NA | NA |
| 2006 | Yasueda et al. [22] | RCT: Placebo-controlled, Double-blind | Placebo | Without chronic or recurrent pouchitis | At hospital discharge after IPAA completed. | Clostridium butyricum MIYAIRI (180 mg/day) | Pouchitis: mPDAI | 24 months | 4/8 | 1/7 | Clinical PDAI:1.63 | Clinical PDAI: 0.75 |
| 2004 | Brown et al. [23] | RCT: Placebo controlled Double-blind | Placebo | Without chronic or recurrent pouchitis | NA | Bifidobacterium longum BB-536 (NA) | Pouchitis: PDAI | 6 months | 2/5 | 1/7 | PDAI: 5.40 | PDAI: 1.83 |
| 2008 | Pronio et al. [24] | RCT: Open-label | No treatment | Without chronic or recurrent pouchitis | Probiotics: 97 | VSL#3 (9.0 | Pouchitis: PDAI | 12 months | 1/12 | /16 | NA | NA |
| 2004 | Gosselink et al. [25] | Cohort study | No treatment | Without chronic or recurrent pouchitis | Started immediately after IPAA completed | Lactobacillus rhamnosus GG (3.0 | Pouchitis: PDAI | 3 years | 27/78 | 3/39 | NA | NA |
|
Table 1, continued | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Authors | Type of | Type of | Past history | Start time of | Type of | Diagnostic | Duration of | Episode/total | PDAI after treatment | ||
| study | control | administration | probiotics (dose) | criteria | treatment | Control | Probiotics | Control | Probiotics | |||
| 2000 | Gionchetti et al. [26] | RCT: Placebo-controlled, Double-blind | Placebo | With chronic or recurrent pouchitis | Patients got remission after 1 month of antibiotic treatment | VSL#3 (1.8 | Relapse: an increase in the clinical PDAI score of | 9 months | 20/20 | 3/20 | PDAI: 12 (8–18) | Relapse ( |
|
Table 1, continued | ||||||||||||
| Year | Authors | Type of | Type of | Past history | Start time of | Type of | Diagnostic | Duration of | Episode/total | PDAI after treatment | ||
| study | control | administration | probiotics (dose) | criteria | treatment | Control | Probiotics | Control | Probiotics | |||
| 2004 | Mimura et al. [27] | RCT: Placebo-controlled, Double-blind | Placebo | With chronic or recurrent pouchitis | Patients got remission after 1 month of antibiotic treatment | VSL#3 (1.8 | Relapse: an increase in the clinical PDAI score of | 12 months | 15/16 | 3/20 | PDAI: 11 (6–14) | PDAI: 2 (0–12) |
| 2006 | Kühbacher et al. [28] | RCT: Placebo-controlled, Double-blind | Placebo | With chronic or recurrent pouchitis | Patients got remission after 1 month of antibiotic treatment | VSL#3 (1.8 | Remission: PDAI | 2month | 5/5 | /10 | NA | NA |
RCT: random-controlled trial; NA: Not available; IPAA: Ileal Pouch-Anal Anastomosis; PDAI: Pouchitis Disease Activity Index; mPDAI: modify Pouchitis Disease Activity Index; 1. Data represent mean
Figure 1.
Flowchart for study selection.
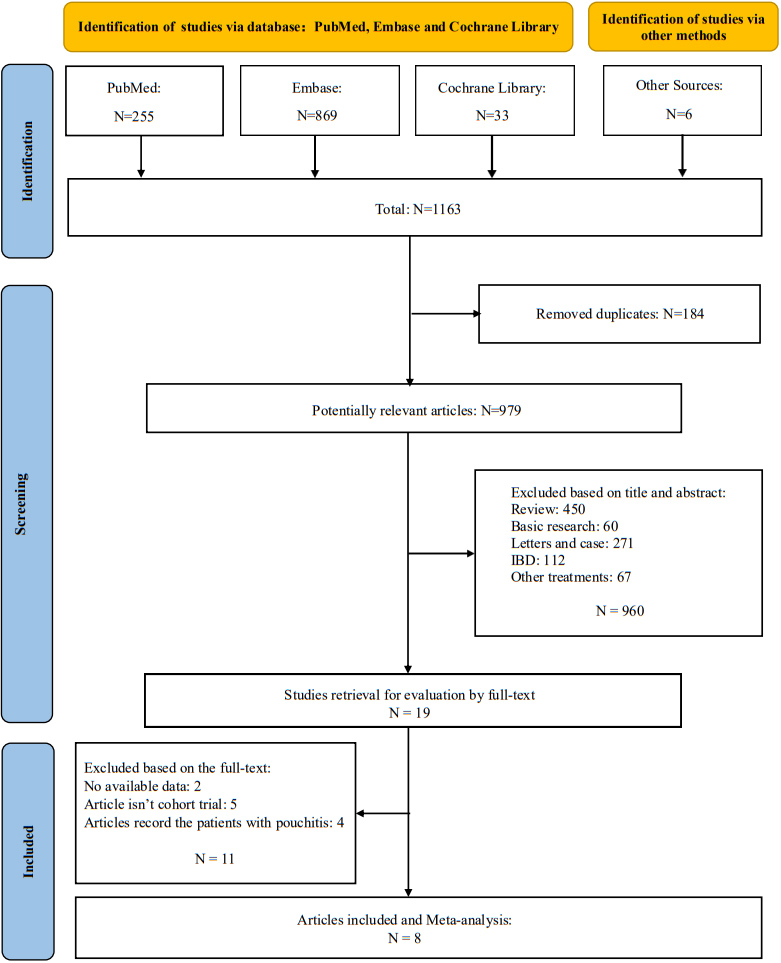
Figure 2.
Risk of bias in the included studies.

Figure 3.
Risk ratio (RR) for the pouchitis rate after administration of probiotics.
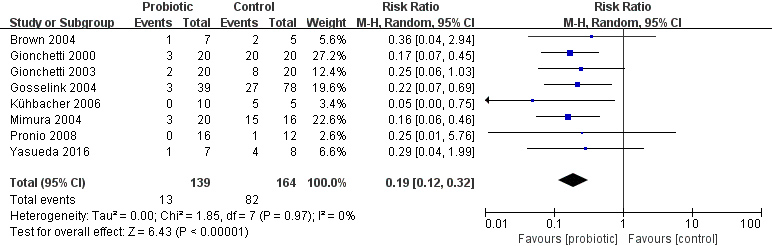
Figure 4.
Risk ratio (RR) for the time to onset of pouchitis in patients in the probiotic and placebo groups.
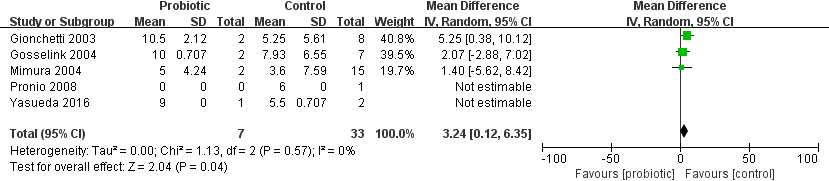
6–12 months (Fig. 5B). Due to the limitation of data, patients with pouchitis in different time periods were all diagnosed for the first time during the study period, and patients with recurrent or chronic pouchitis were not included in the number of patients in the next period. During 0–6 months, the probiotics group had a significant preventive effect on pouchitis compared with the placebo group, and the incidence of pouchitis was statistically significant between the two groups (RR
Figure 5.
(A) Risk ratio (RR) for the pouchitis rate after administration of probiotics between 0 and 6 months; (B) RR for the pouchitis rate after administration of probiotics between 6 and 12 months.
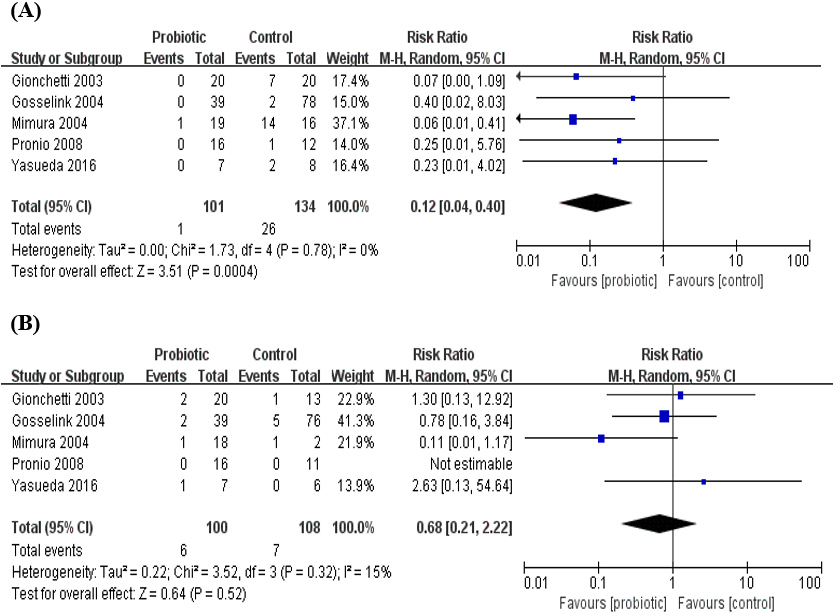
3.5The history of chronic or recurrent pouchitis and probiotics prevention effect
We performed a subgroup analysis based on past history (patient with or without chronic or recurrent pouchitis), duration of treatment, type of control (placebo or no treatment) and type of probiotics (Table 2). The results showed that the subgroup difference was significant after subgroup analysis according to the past history of chronic or recurrent pouchitis, but not significant based on the other agents. Subgroup analysis was performed on the number of patients with pouchitis in the probiotic group and the placebo group according to the presence or absence of previous history of chronic or recurrent pouchitis (Fig. 6). There was statistically significant difference between the probiotic group and placebo group regardless of prior history ((RR
Table 2
Subgroup analysis of outcomes for probiotics in the prevention of pouchitis
| Subgroup | RR, 95% CI | Heterogeneity |
| Test for subgroup differences: | |
|---|---|---|---|---|---|
| Past history | With chronic or recurrent pouchitis | 17.72 [4.67, 67.28] | |||
| Without chronic or recurrent pouchitis | 1.37 [1.18, 1.60] | ||||
| Duration of | 2 months | 11.45 [0.80, 163.26] | |||
| administration | 6 months | 1.43 [0.66, 3.11] | |||
| 9 months | 35.00 [2.25, 544.92] | ||||
| 12 months | 1.90 [0.70, 5.15] | ||||
| 24 months | 1.33 [0.58, 3.07] | ||||
| 36 months | 1.43 [1.15, 1.77] |
| |||
| Type of control | No treatment | 1.25 [0.94, 1.67] | |||
| Placebo | 3.12 [1.12, 8.70] | ||||
| Type of probiotics | VSL#3 | 4.14 [1.03, 16.57] | |||
| Lactobacillus rhamnosus GG | 1.43 [1.15, 1.77] |
| |||
| Clostridium butyricum MIYAIRI | 1.33 [0.58, 3.07] | ||||
| Bifidobacterium longum BB-536 | 1.43 [0.66, 3.11] |
RR: Risk ratio, CI: Confidence interval.
Figure 6.
Subgroup analysis of previous history of chronic or recurrent pouchitis.
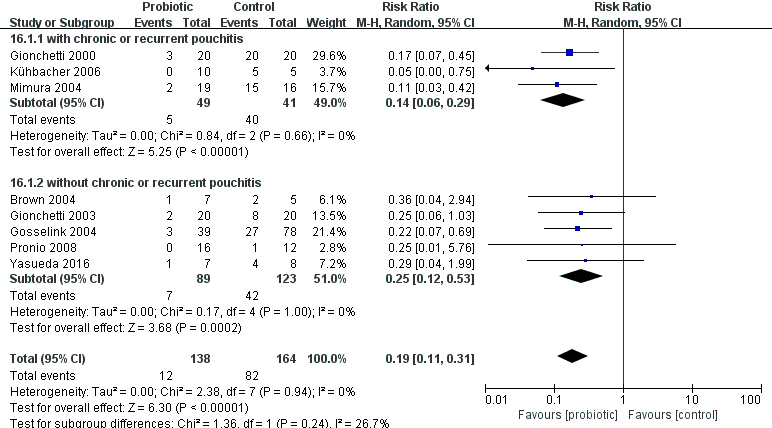
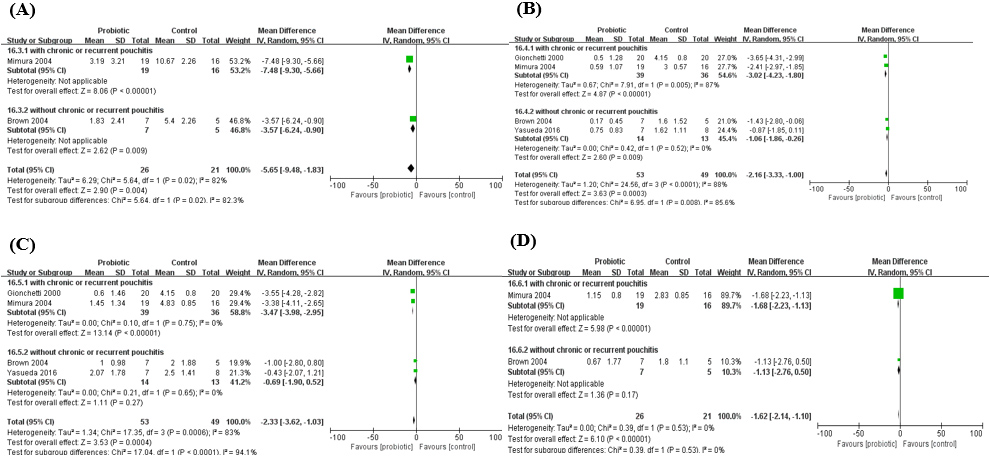
Figure 7.
Pouchitis disease activity index (PDAI) after the administration of probiotics. A. PDAI; B. Clinical PDAI; C. Endoscopic PDAI; D. Histological PDAI.
4.Discussion
Dysbiosis of the ileal pouch microbiota is a hypothesis about pathogenesis of pouchitis [2]. During pouchitis, the abundance of Enterobacteriaceae is increased and the abundance of Bacteroides and F. prausnitzii, which have an anti-inammatory effect, were decreased [4, 29]. There are a few meta-analyses regarding the efficacy of probiotics in administration of pouchitis. Elahi et al. indicated that probiotics are beneficial to management in pouchitis [17]. Shen et al. showed that VSL#3, a common production used in UC patients containing four strains of lactobacilli, three strains of bifidobacteria, and one strain of streptococcus, is beneficial to maintaining remission for patients with pouchitis [14]. VSL#3 has been shown to be effective in the prevention of pouchitis onset [36]. Singh et al. also showed that VSL#3 is beneficial to preventative therapy for patients after IPAA [18]. However, they did not discuss the agents which may influence outcome of probiotics for pouchitis. In this meta-analysis, we discussed the efficacy of probiotics in preventive therapy for patients after IPAA based on the number of pouchitis rate and PDAI score after administration of probiotics, and the short-term and long-term effects of probiotics was also discussed.
During the administration of probiotics, we found that patients after treatment are less likely to be attacked by pouchitis than the control group. The PDAI in probiotics group was also lower than the control. This indicates that probiotics prevent the episode of pouchitis for patients after IPAA, which is in line with previous meta-analyses. Then we performed the subgroup analysis based on type of probiotics, the duration of treatment, past history of chronic or recurrent pouchitis and type of control. We found that the past history of chronic or recurrent pouchitis was not a significant factor in the preventive effect of probiotics on pouchitis. However, we found that probiotics have a protective effect on pouchitis, but this prevention may differ in the short-term and long-term. Probiotics did not achieve the expected effect in the latter six months, but the reasons may be various. First, 6 months after surgery may be the peak period of pouchitis, and the incidence of pouchitis is higher than that after 6 months. However, the incidence of the two groups in the included study was not very high, leading to the possibility of bias error in the above data analysis results. Secondly, probiotics may be considered to have less effect in the long-term prevention of pouchitis. Long-term use of probiotics may reduce the effect on intestinal flora, or there is a possibility that long-term use may lead to intestinal adaptation to probiotics. Probiotics can promote the strengthening of the intestinal barrier, reduce inflammation, and improve intestinal barrier function by restoring mucus layer thickness, tight junction protein, and producing specific antimicrobial and bioactive lipids with anti-inflammatory properties [30]. It is not excluded that long-term use of probiotics may lead to a decrease in the effect of probiotics on the intestinal barrier. At present, the differences between the short-term and long-term effects of probiotics still need to be further discussed. However, it is undeniable that probiotics have preventive and therapeutic effects on pouchitis, and it is not certain whether the rebound phenomenon will occur after taking probiotics in the short-term, so whether patients should only take probiotics in a short period of time after IPAA has not been concluded.
Probiotics were also used in patients during pouchitis. However, we did not summarize these studies by meta-anlaysis because most studies on patients during pouchitis did not meet the criteria of meta-analyses. The efficacy of probiotics in patients during pouchitis was controversial. Gionchetti et al. indicated that VSL#3 effective for active pouchitis [31]. However, many studies indicated that patients cannot get clinical or endoscopic response after administration of probiotics [33, 34]. We think the successful colonization of probiotcs is a key to treatment. In Gionchetti’s study, S. thermophilus, lactobacilli, bifidobacteria was significantly increased in feces after administration of probiotics [31, 32]. Kuisma et al. indicated that the microbial flora did not have significant difference between before and after administration of probiotics, in which the patients did not have clinical response [34]. The oxidative stress often occurred in inflammatory response, which link to the dysbiosis in IBD [35, 36, 37]. Most probiotics belong to anaerobic bacteria. So inflammatory environment influences the colonization of probiotics, and the “warfare” between Reactive Oxygen Species (ROS) and probiotics may influence the efficacy of probiotics. In contrast, due to the less ROS produced in non-inflammatory pouch, colonization of probiotics in pouch is much easier. This provides a prerequisite for the good efficacy of probiotics in patients during no inflammation in pouch.
This meta-analysis is not without limitations. First, the number of included studies and patients was small, which limited further investigation into probiotics for pouch patients. Secondly, some continuous variables in the original studies did not represent in mean
5.Conclusion
Probiotics are beneficial to preventative therapy for patients after restorative proctocolectomy with ileal pouch-anal anastomosis. Long-term use of probiotics in the prevention of pouchitis is lower than short-term use, which may be difficult to achieve expectations, but there is no consensus on whether patients after IPAA should use probiotics only for the short-term.
Ethics statement
This study was exempt from ethics approval. Informed consent was obtained from all individual participants included in the study.
Availability of data and materials
The data used or analysed during the current study are available from the corresponding author on reasonable request.
Funding
None to report.
Author contributions
GL: Conceived and designed the study.
WX, XZ: Collected and analyzed the data and wrote the first draft of the paper.
CL, QH: Supervised the data collection process and assisted with writing the paper.
AH: Contributed to the revision of the paper and approved the final version.
Acknowledgments
None to report.
Conflict of interest
The authors have no competing interest to report.
References
[1] | Dalal RL, Shen B, Schwartz DA. Management of Pouchitis and Other Common Complications of the Pouch. Inflamm Bowel Dis. (2018) Apr 23; 24: (5): 989-996. doi: 10.1093/ibd/izy020. |
[2] | Schieffer KM, Williams ED, Yochum GS, Koltun WA. Review article: the pathogenesis of pouchitis. Aliment Pharmacol Ther. (2016) Oct; 44: (8): 817-35. doi: 10.1111/apt.13780. Epub 2016 Aug 24. |
[3] | Li KY, Wang JL, Wei JP, Gao SY, Zhang YY, Wang LT, Liu G. Fecal microbiota in pouchitis and ulcerative colitis. World J Gastroenterol. (2016) Oct 28; 22: (40): 8929-8939. doi: 10.3748/wjg.v22.i40.8929. |
[4] | McLaughlin SD, Walker AW, Churcher C, Clark SK, Tekkis PP, Johnson MW, Parkhill J, Ciclitira PJ, Dougan G, Nicholls RJ, Petrovska L. The bacteriology of pouchitis: a molecular phylogenetic analysis using 16S rRNA gene cloning and sequencing. Ann Surg. (2010) Jul; 252: (1): 90-8. doi: 10.1097/SLA.0b013e3181e3dc8b. |
[5] | Angriman I, Scarpa M, Castagliuolo I. Relationship between pouch microbiota and pouchitis following restorative proctocolectomy for ulcerative colitis. World J Gastroenterol. (2014) Aug 7; 20: (29): 9665-74. doi: 10.3748/wjg.v20.i29.9665. |
[6] | Reshef L, Kovacs A, Ofer A, Yahav L, Maharshak N, Keren N, Konikoff FM, Tulchinsky H, Gophna U, Dotan I. Pouch Inflammation Is Associated With a Decrease in Specific Bacterial Taxa. Gastroenterology. (2015) Sep; 149: (3): 718-27. doi: 10.1053/j.gastro.2015.05.041. Epub 2015 May 27. |
[7] | Nguyen N, Zhang B, Holubar SD, Pardi DS, Singh S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. (2019) May 28; 5: (5): CD001176. doi: 10.1002/14651858.CD001176.pub4. Update in: Cochrane Database Syst Rev. 2019 Nov 30; 11CD001176. |
[8] | Shen B, Fazio VW, Remzi FH, Bennett AE, Lopez R, Brzezinski A, Oikonomou I, Sherman KK, Lashner BA. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis Colon Rectum. (2007) Apr; 50: (4): 498-508. doi: 10.1007/s10350-006-0828-3. |
[9] | Steinhart AH, Ben-Bassat O. Pouchitis: a practical guide. Frontline Gastroenterol. (2013) Jul; 4: (3): 198-204. doi: 10.1136/flgastro-2012-100171. Epub 2013 Nov 12. |
[10] | Laval L, Martin R, Natividad JN, Chain F, Miquel S, Desclée de Maredsous C, Capronnier S, Sokol H, Verdu EF, van Hylckama Vlieg JE, Bermúdez-Humarán LG, Smokvina T, Langella P. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. (2015) ; 6: (1): 1-9. doi: 10.4161/19490976.2014.990784. Epub 2015 Jan 14. |
[11] | Orlando A, Linsalata M, Notarnicola M, Tutino V, Russo F. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: the role of cellular polyamines. BMC Microbiol. (2014) Jan 31; 14: : 19. doi: 10.1186/1471-2180-14-19. |
[12] | Hafez MM. Upregulation of Intestinal Mucin Expression by the Probiotic Bacterium E. coli Nissle 1917, Probiotics Antimicrob Proteins. 2012: Jun; 4: (2): 67-77. doi: 10.1007/s12602-012-9092-0. |
[13] | Sabia C, Anacarso I, Bergonzini A, Gargiulo R, Sarti M, Condò C, Messi P, de Niederhausern S, Iseppi R, Bondi M. Detection and partial characterization of a bacteriocin-like substance produced by Lactobacillus fermentum CS57 isolated from human vaginal secretions. Anaerobe. (2014) Apr; 26: : 41-5. doi: 10.1016/j.anaerobe.2014.01.004. Epub 2014 Jan 21. |
[14] | Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis. (2014) Jan; 20: (1): 21-35. doi: 10.1097/01.MIB.0000437495.30052.be. Erratum in: Inflamm Bowel Dis. 2014 Dec; 20: (12): 2526-8. |
[15] | Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. (2018) Nov; 48: (10): 1044-1060. doi: 10.1111/apt.15001. Epub 2018 Oct 8. |
[16] | Evans M, Salewski RP, Christman MC, Girard SA, Tompkins TA. Effectiveness of Lactobacillus helveticus and Lactobacillus rhamnosus for the management of antibiotic-associated diarrhoea in healthy adults: a randomised, double-blind, placebo-controlled trial. Br J Nutr. (2016) Jul; 116: (1): 94-103. doi: 10.1017/S0007114516001665. Epub 2016 May 12. |
[17] | Elahi B, Nikfar S, Derakhshani S, Vafaie M, Abdollahi M. On the benefit of probiotics in the management of pouchitis in patients underwent ileal pouch anal anastomosis: a meta-analysis of controlled clinical trials. Dig Dis Sci. (2008) May; 53: (5): 1278-84. doi: 10.1007/s10620-007-0006-z. Epub 2007 Oct 17. |
[18] | Singh S, Stroud AM, Holubar SD, Sandborn WJ, Pardi DS. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. (2015) Nov 23; (11): CD001176. doi: 10.1002/14651858.CD001176.pub3. Update in: Cochrane Database Syst Rev. 2019 May 28; 5CD001176. |
[19] | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) Dec 19; 14: : 135. doi: 10.1186/1471-2288-14-135. |
[20] | Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) Jun; 27: (6): 1785-1805. doi: 10.1177/0962280216669183. Epub 2016 Sep 27. |
[21] | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. (2003) May; 124: (5): 1202-9. doi: 10.1016/s0016-5085(03)00171-9. |
[22] | Yasueda A, Mizushima T, Nezu R, Sumi R, Tanaka M, Nishimura J, Kai Y, Hirota M, Osawa H, Nakajima K, Mori M, Ito T. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg Today. (2016) Aug; 46: (8): 939-49. doi: 10.1007/s00595-015-1261-9. Epub 2015 Oct 29. |
[23] | Brown SJ, Megan J, Smith S, et al. Bifidobacterium longum BB-536 and prevention of pouchitis. Gastroenterology. (2004) ; 126: : S465. |
[24] | Pronio A, Montesani C, Butteroni C, Vecchione S, Mumolo G, Vestri A, Vitolo D, Boirivant M. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells. Inflamm Bowel Dis. (2008) May; 14: (5): 662-8. doi: 10.1002/ibd.20369. |
[25] | Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden JG. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. (2004) Jun; 47: (6): 876-84. doi: 10.1007/s10350-004-0525-z. Epub 2004 Apr 19. |
[26] | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. (2000) Aug; 119: (2): 305-9. doi: 10.1053/gast.2000.9370. |
[27] | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. (2004) Jan; 53: (1): 108-14. doi: 10.1136/gut.53.1.108. |
[28] | Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Fölsch UR, Kamm MA, Schreiber S. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. (2006) Jun; 55: (6): 833-41. doi: 10.1136/gut.2005.078303. Epub 2006 Jan 9. |
[29] | Reshef L, Kovacs A, Ofer A, Yahav L, Maharshak N, Keren N, Konikoff FM, Tulchinsky H, Gophna U, Dotan I. Pouch Inflammation Is Associated With a Decrease in Specific Bacterial Taxa. Gastroenterology. (2015) Sep; 149: (3): 718-27. doi: 10.1053/j.gastro.2015.05.041. Epub 2015 May 27. |
[30] | Wieërs G, Belkhir L, Enaud R, Leclercq S, Philippart de Foy JM, Dequenne I, de Timary P, Cani PD. How Probiotics Affect the Microbiota. Front Cell Infect Microbiol. (2020) Jan 15; 9: : 454. doi: 10.3389/fcimb.2019.00454. |
[31] | Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, Brigidi P, Vitali B, Straforini G, Campieri M. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. (2007) Dec; 50: (12): 2075-82; discussion 2082-4. doi: 10.1007/s10350-007-9068-4. Epub 2007 Oct 13. |
[32] | Gionchetti P, Calafiore A, Riso D, Liguori G, Calabrese C, Vitali G, Laureti S, Poggioli G, Campieri M, Rizzello F. The role of antibiotics and probiotics in pouchitis. Ann Gastroenterol. (2012) ; 25: (2): 100-105. |
[33] | Bengtsson J, Adlerberth I, Östblom A, Saksena P, Öresland T, Börjesson L. Effect of probiotics (Lactobacillus plantarum 299 plus Bifidobacterium Cure21) in patients with poor ileal pouch function: a randomised controlled trial. Scand J Gastroenterol. (2016) Sep; 51: (9): 1087-92. doi: 10.3109/00365521.2016.1161067. Epub 2016 May 6. |
[34] | Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment Pharmacol Ther. (2003) Feb 15; 17: (4): 509-15. doi: 10.1046/j.1365-2036.2003.01465.x. |
[35] | Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. (2005) Aug 29; 579: (21): 4911-7. doi: 10.1016/j.febslet.2005.08.002. |
[36] | Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol. (2006) Feb; 290: (2): C433-43. doi: 10.1152/ajpcell.00135.2005. Epub 2005 Sep 14. |
[37] | Darnaud M, Dos Santos A, Gonzalez P, Augui S, Lacoste C, Desterke C, De Hertogh G, Valentino E, Braun E, Zheng J, Boisgard R, Neut C, Dubuquoy L, Chiappini F, Samuel D, Lepage P, Guerrieri F, Doré J, Bréchot C, Moniaux N, Faivre J. Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis. Gastroenterology. (2018) Mar; 154: (4): 1009-1023.e14. doi: 10.1053/j.gastro.2017.11.003. Epub 2017 Nov 11. |




