Association of COVID-19 patient’s condition with fasting blood glucose and body mass index: A retrospective study
Abstract
BACKGROUND:
The COVID-19 pandemic broke out in 2019 and rapidly spread across the globe. Most of the severe and dead cases are middle-aged and elderly patients with chronic systemic diseases.
OBJECTIVE:
This study aimed to assess the association between fasting blood glucose (FPG) and body mass index (BMI) levels in patients with coronavirus disease 2019 (COVID-19) under different conditions.
METHODS:
Experimental-related information (age, gender, BMI, and FPG on the second day of admission) from 86 COVID-19 cases (47 males and 39 females) with an average age of (39
RESULTS:
1. Experimental group: 21 patients with asymptomatic or and mild symptoms (group A), 45 patients with common non-progression (group B), and 20 patients with common progression and severe symptoms (group C). 2. The age differences among the three groups were statistically significant and elderly patients had a higher risk of severe disease (
CONCLUSION:
The levels of FPG and BMI were significantly increased in the population with common progressive and severe COVID-19. FPG and age are independent risk factors for the progression of COVID-19.
1.Introduction
The new coronavirus disease broke out in 2019 and rapidly spread around the globe. The World Health Organization (WHO) defined the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as coronavirus disease 2019 (COVID-19) on 11 February 2020 [1]. As of April 16, 2022, there are 503,628,875 COVID-19 infected cases, 6,219,927 deaths, and 11.45 billion vaccinated cases that have been reported worldwide [2]. COVID-19 is the seventh type of coronavirus discovered so far that can infect humans. It belongs to the
By December 22, 2020, COVID-19 cases affected all seven continents, and the number of confirmed cases and deaths is still increasing [5]. Based on a reported analysis of 72,314 cases in China, mild cases of COVID-19 are about 81%, whereas severe cases are 14% and critically-ill patients are 5%. The case fatality rate (CFR) is 2.6% [6]. The initial CFR of the European COVID-19 outbreak is between 4% and 4.5% [7]. A systematic review and meta-analysis including patients in 2020 showed that the overall estimated pooled case fatality rate for COVID-19 was 10.0%. Composite CRF is only 1.0% in the general population, compared with 29% in ICU inpatients and 15% in inpatients [8]. The current pandemic is severe and occurred with an increased number of confirmed and dead patients as well as more infectious COVID-19 mutant strains being discovered such as the Omicron [9] and Delta variant [10]. There are increasing omicron COVID-19 cases and this strain is much more infectious than other strains [11]. Given that COVID-19 has dramatically spread worldwide and limited research has investigated the impact of COVID-19 pneumonia on FPG and BMI levels, we aim to assess the association of FPG and BMI with COVID-19 patients under different conditions.
2.Materials and methods
2.1Participants
Eighty-six COVID-19 patients treated by Hulunbuir People’s Hospital in April 2020 (58 cases) and November 2020 (28 cases) were collected in this study. Approval was obtained from the Hulunbuir People’s Hospital ethics committee. 47 were males (54.6%) and 39 were females (45.4%) with an average age of (39
2.2Data collection and study design
Ninety-two patients were initially included in the study. The age, gender, body mass index (BMI), and fasting plasma glucose (FPG) levels on the second day of admission were collected from all 92 cases. The suspected cases must meet one of the following etiological or serological evidence: 1. Real-time fluorescent RT-PCR positive detection of novel coronavirus nucleic acid; 2. Virus gene sequencing is highly homologous to known novel coronaviruses; 3. The novel coronavirus-specific IgG antibody changes from negative to positive or the IgG antibody titer in the recovery phase is 4 or more times higher than in the acute phase [12]. Six patients were subsequently excluded due to the following exclusion criteria: 4 patients tested COVID-19 negative for throat and nasal swabs and two patients were of Russian ethnicity instead of Chinese. Routine physical examinations and inquiries about family history and relevant information were collected followed by documenting the height and weight of all participants at admission. 3–5 ml of venous blood samples were collected (German Roche cobase 601) after an overnight fast lasting at least 8 hours from the patients in the early morning of the second day of admission. The serum samples free of hemolysis, lipemia, jaundice, and other influencing factors were tested for FPG levels according to the manufacturer’s instructions (Roche). 86 cases were divided into three groups according to the new coronavirus diagnosis and treatment plan (8
2.3Types of COVID-19 cases and diagnostic criteria for obesity and diabetes
According to the clinical classification based on the Guideline of Novel Coronavirus Pneumonia (8
Severe/critical early warning indicators were alerted to the deterioration of the disease: 1. Progressive aggravation of hypoxemia or respiratory distress; 2. Deterioration of tissue oxygenation indicators or progressive increase of lactate; 3. Peripheral Progressive decrease in blood lymphocyte count or progressive increase in peripheral blood inflammatory markers such as IL-6, CRP, and ferritin; 4. D-dimer and other coagulation-related indexes were significantly increased; 5. Chest imaging showed lung lesions progressed significantly [12].
BMI standard for adults was defined according to the Guideline for the Prevention and Treatment of Type 2 Diabetes Mellitus in China (2020 edition): less than 18.5 kg/m
1999 WHO diagnostic criteria for diabetes: diabetes symptoms (such as polyuria, polydipsia, and unexplained weight loss in type I diabetes patients); a random venous plasma glucose concentration
2.4Statistical analysis
Statistical analysis was carried out using SPSS 25.0 software and data was expressed as mean
3.Results
3.1Classification and determination of cases
According to the most severe classification of each case determined by the Chinese expert group: 21 patients were asymptomatic and mild cases; 60 patients were ordinary cases, and five patients were severe cases. Twenty-one asymptomatic infections and mild patients were included in group A and five severe patients were included in group C. Fifteen out of those 60 ordinary cases had different degrees of severe/critical early warning indicators including progressive aggravation of hypoxemia or respiratory distress, deterioration of tissue oxygenation indicators or progressively increased of lactate, progressively decreased peripheral blood lymphocytes or progressively increased peripheral blood inflammatory markers (IL-6, CRP, ferritin), increased D-dimer and other coagulation function-related indexes, progression of pulmonary lesions showed in chest imaging [12]. Fifteen cases were finally classified as common progressive cases in group C (Fig. 1).
Figure 1.
Comparison of general clinical data of three groups of cases
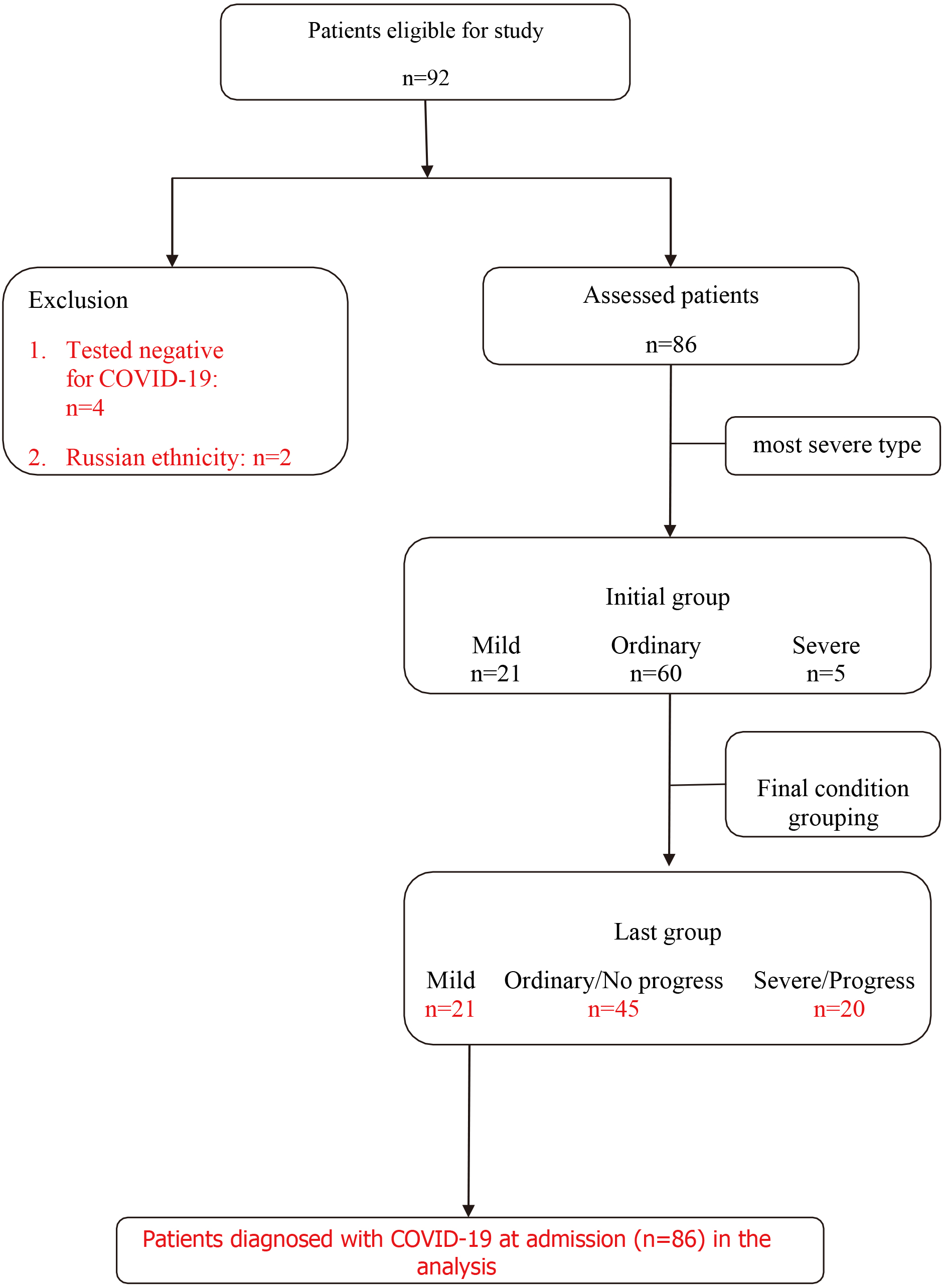
3.2Comparison of general clinical data of three groups of cases
Age was compared among the three groups and the differences were statistically significant (
Table 1
Comparison of general clinical data of three groups of cases (
| Different types of COVID-19 | Cases | Age | Male/female | Height (cm) | Weight (kg) |
|---|---|---|---|---|---|
| Group A | 21 | 24 | 14/07 | 168 | 63 |
| Group B | 45 | 40 | 25/20 | 167 | 63 |
| Group C | 20 | 53 | 06/14 | 157 | 70 |
Group A: asymptomatic infection and mild group; group B: common type without progression group; group C: common type progressive and severe group.
3.3Comparison of FPG levels in three groups of cases
The FPG level in group C was higher than in groups A (
Table 2
Comparison of FPG levels in three groups of cases (
| Group | Normal blood sugar | Diabetes or IFG | FPG (mmol/l) |
|---|---|---|---|
| Group A | 21 | 0 | 4.80 |
| Group B | 43 | 2 | 5.11 |
|
| 0.963 | 1.7341 | |
|
| 0.327 | 0.0877 | |
| Group B | 43 | 2 | 5.11 |
| Group C | 15 | 5 | 5.57 |
|
| 6.088 | 2.0212 | |
|
| 0.014 | 0.0475 | |
| Group A | 21 | 0 | 4.80 |
| Group C | 15 | 5 | 5.57 |
|
| 5.979 | 3.1655 | |
|
| 0.014 | 0.0030 |
Group A: asymptomatic infection and mild group; Group B: normal type without progression group; C group: normal type progressive and severe group; IFG: impaired fasting blood glucose regulation;
3.4Comparison of BMI levels of three groups of patients
The BMI in group C was significantly higher than in groups A (
Table 3
Comparison of BMI levels of three groups of patients (
| Group | Normal weight | Overweight or obese | BMI (kg/m |
|---|---|---|---|
| Group A | 15 | 6 | 21.72 |
| Group B | 31 | 14 | 22.50 |
|
| 0.044 | 1.0764 | |
|
| 0.834 | 0.2858 | |
| Group B | 31 | 14 | 22.50 |
| Group C | 5 | 15 | 25.79 |
|
| 10.794 | 3.8188 | |
|
| 0.001 | 0.0003 | |
| A | 15 | 6 | 21.72 |
| C | 5 | 15 | 25.79 |
|
| 8.838 | 3.8839 | |
|
| 0.003 | 0.0004 |
Group A: asymptomatic infection and mild group; group B: common type without progression group; C group: common type progressive and severe group;
3.5Multivariate linear regression analysis on the impact of COVID-19 conditions
The previously mentioned classification of COVID-19 patients was used as the dependent variable. Age, gender, BMI, and FPG were used as independent variables. Multivariate linear regression analysis showed that age (
Table 4
Multivariate linear regression models affecting COVID-19
| IV | B | SE |
|
|
|
|---|---|---|---|---|---|
| Age | 0.020 | 0.005 | 0.380 | 3.713 | 0.000 |
| Gender | 0.264 | 0.122 | 0.191 | 2.156 | 0.034 |
| FPG | 0.157 | 0.077 | 0.186 | 2.035 | 0.045 |
| BMI | 0.037 | 0.020 | 0.182 | 1.850 | 0.068 |
4.Discussion
In the 21
Previous COVID-19 data shows that the main risk factors in determining the severity of COVID-19 include age, gender, type 2 diabetes, obesity, smoking, and hypertension [22, 23, 24]. A meta-analysis showed that patients with diabetes had a higher COVID-19 infection rate and risk of developing to severe disease [25]. People with cardiovascular disease and diabetes have a higher chance of developing severe COVID-19 [26]. Diabetes is one of the most prevalent chronic diseases in the world and is closely associated with poor prognosis in COVID-19 [27]. Related studies have shown that diabetes increases mortality in patients with COVID-19 [28], and patients with diabetes who are infected by COVID-19 have a 2.95-fold higher risk of death compared with COVID-19 patients without diabetes [29]. Diabetes is associated with an increased risk of multiple infections such as viral infections [30]. The possible mechanism of diabetes-induced exacerbation of COVID-19 is the increased expression of the novel coronavirus angiotensin-converting enzyme 2 (ACE2) receptor in the lungs and other tissues of patients with type 2 diabetes [31], which is associated with chronic inflammation, insulin resistance, aggravated inflammatory responses and dysfunction of alveolar-capillary diffusion [32]. COVID-19 leads to insulin resistance and even direct damage to
Obesity is a serious public health problem with approximately 2 billion people are overweight [40, 41]. The obesity rate was 6.2% in China, 27.8% in the UK, 19.9% in Italy, 3.9% in India, 4.7% in South Korea, and 22.1% in Brazil according to a WHO report in 2016 [42]. Obesity is associated with different systemic diseases such as hypertension, angina pectoris, diabetes, and arthritis [43]. Overweight and obesity are the fifth highest risk of death in the world [44]. The COVID-19 pandemic has a dramatic impact on human health leading to changes in lifestyle through social distancing and home isolation accompanied by social and economic consequences [45, 46]. A meta-analysis showed that mortality, acute respiratory distress syndrome (ARDS), invasive mechanical ventilation (IMV), and increased visceral fat appears to be associated with severe adverse COVID-19 [47, 48]. The risk of COVID-19 exacerbation and death increases linearly with increased BMI [49]. Obesity may affect COVID-19 outcomes through multiple potential mechanisms [50]. Early detection and aggressive treatment of obesity and COVID-19 patients are imperative [51]. Clinicians should recognize that individuals with more severe obesity have higher risk of exacerbated COVID-19 [52]. Our data suggest that BMI levels of patients with severe and common progression groups are significantly higher than asymptomatic infection and mild and common non-progressive groups, which may ultimately affect the prognosis of COVID-19. In this study, three patients who met the Chinese obesity diagnostic criteria had a BMI
An earlier study of 79,394 confirmed COVID-19 patients (aged 30–59 years) clearly showed that older patients have a higher probability of more severe disease [53]. Patients over the age of 59 were 5.1 (4.2–6.1) times more likely to die after developing symptoms [54, 55]. The above studies are consistent with our findings that the age of the patients in the severe and common progression group of COVID-19 was significantly higher than that in the asymptomatic infection group. The elderly people needed more attention from society during the COVID-19 pandemic and vaccination is essential for the COVID-19 prevention. Regarding the effect of gender on the condition of COVID-19, most studies show that men have a higher risk of infection, severe disease, and mortality than females [22, 24]. Elderly males and coexisting with COVID-19 can lead to acute lung injury due to increased expression of ACE2 [56], and estrogen can participate in the regulation of the renin-angiotensin-aldosterone system (RAAS) including ACE2 [57]. Another study showed that nurses have a higher frequency of hospital visits made themselves more susceptible to infection [58]. Females with high FPG levels and low eGFR, age
This study demonstrates the impact of different conditions of COVID-19 on FPG and BMI levels. However, some limitations exist in this study: the retrospective data prevented us from assessing longitudinal changes and correlation analyses of biochemical and clinical parameters during the disease progression; the relatively limited number of patients. Therefore, larger-scale studies in the future may help to elucidate better the action of FPG and BMI on the impact of COVID-19. FPG and BMI might even serve as predictors of COVID-19 progression [61, 62]. Even though our study assessed the association of COVID-19 with BMI that is one of the measurements of obesity and is widely recognized as a predictor of chronic diseases [63, 64], it should be noted that the definition of overweight and obesity in China differs from that in the United States and Europe [65]. Therefore, it is necessary to consider the potential impact of this difference when applying the conclusions of this study to others. In addition, COVID-19 leads to a significant increased glycated hemoglobin, fasting blood glucose, and BMI levels in patients with type 2 diabetes [66]. It is documented that random blood glucose and BMI on admission are associated with mortality in patients with COVID-19 [67], and hyperglycemia is an independent predictor of high inflammation levels and severe COVID-19 [68]. Some studies suggest that COVID-19 patients with FPG
5.Conclusion
In our retrospective study, FPG and BMI were significantly increased in the severe and common disease progression group. Also, patients’ age in the severe and common disease progression group was significantly higher than that in the other two groups. FPG and age are independent risk factors for COVID-19 disease progression and it is speculated that FPG, BMI, and advanced age are predictors of COVID-19.
Author contributions
LGS and SRB conceived the study and collected patients; LGS conducted the experiments; LGS and DHH analyzed the data; LGS, DHH and LS wrote the manuscript; LS and LPD revised the manuscript. All authors read and approved the final version of the manuscript.
Data availability
All data generated and analyzed during this study are included in this article.
Acknowledgments
The authors thank LL Yang, K Shi and the participants in this study.
Conflict of interest
The authors have no conflicts of interest to report.
References
[1] | WHO. Novel Coronavirus (2019-nCoV) Situation Report – 22[EB/OL]. (2020-02-11); [2020-02-11]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. |
[2] | Hannah Ritchie, Edouard Mathieu, Lucas Rodés-Guirao, Cameron Appel, Charlie Giattino, Esteban Ortiz-Ospina, Joe Hasell, Bobbie Macdonald, Diana Beltekian and Max Roser ((2020) )– “Coronavirus Pandemic (COVID-19)”, Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/coronavirus [Online Resource] |
[3] | Chen ZM, Fu JF, Shu Q, Chen YH, Hua CZ, Li FB, Lin R, Tang LF, Wang TL, Wang W, Wang YS, Xu WZ, Yang ZH, Ye S, Yuan TM, Zhang CM, Zhang YY. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. (2020) Jun; 16: (3): 240-246. doi: 10.1007/s12519-020-00345-5. |
[4] | Kumar A, Prasoon P, Kumari C, Pareek V, Faiq MA, Narayan RK, Kulandhasamy M, Kant K. SARS-CoV-2-specific virulence factors in COVID-19. J Med Virol. (2021) Mar; 93: (3): 1343-1350. doi: 10.1002/jmv.26615. |
[5] | BBC. (22 December 2020). Coronavirus spreads to Antarctic research station. https://www.bbc.com/news/world-latin-America-55410065. Accessed 17 January 2021. |
[6] | Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. (2020) Apr 7; 323: (13): 1239-1242. doi: 10.1001/jama.2020.2648. |
[7] | Karadag E. Increase in COVID-19 cases and case-fatality and case-recovery rates in Europe: A cross-temporal meta-analysis. J Med Virol. (2020) Sep; 92: (9): 1511-1517. doi: 10.1002/jmv.26035. |
[8] | Alimohamadi Y, Tola HH, Abbasi-Ghahramanloo A, Janani M, Sepandi M. Case fatality rate of COVID-19: A systematic review and meta-analysis. J Prev Med Hyg. (2021) ; 62: (2): E311-E320. Published 2021 Jul 30. doi: 10.15167/2421-4248/jpmh2021.62.2.1627. |
[9] | Mehta S, Singh Gambhir R, Singh B, Goel R, Singh Ghuman K, Aggarwal A. Covid-19 update: Omicron variant – a new emerging threat. Rocz Panstw Zakl Hig. (2022) ; 73: (1): 13-16. doi: 10.32394/rpzh.2022.0198. |
[10] | Liu Y, Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. (2021) Oct 11; 28: (7): taab124. doi: 10.1093/jtm/taab124. |
[11] | Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: Global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. (2021) Dec; 25: (24): 8012-8018. doi: 10.26355/eurrev_202112_27652. |
[12] | The Diagnosis and Treatment Plan for the Novel Coronavirus Disease 8th ed. (2020), available online at: http://wwwnhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml (accessed October 15, 2020). |
[13] | Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellitus. (2021) ; 13: (4): 315-409. doi: 10.3760/cma.j.cn 115791-20210221-00095. |
[14] | World Health Organization: Definition, diagnosis, and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus. Geneva, World Health Organization, (1999) . |
[15] | Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, Khan ST. COVID-19: A global challenge with old history, epidemiology, and progress so far. Molecules. (2020) Dec 23; 26: (1): 39. doi: 10.3390/molecules26010039. |
[16] | Chan JWM, Ng CK, Chan YH, Mok TYW, Lee S, Chu SYY, Law WL, Lee MP, Li PCK. Short term outcome and risk factors for adverse clinical outcomes in adults with the severe acute respiratory syndrome (SARS). Thorax. (2003) ; 58: : 686-689. doi: 10.1136/thorax.58.8.686. |
[17] | Alanazi KH, Abedi GR, Midgley CM, Alkhamis A, Alsaqer T, Almoaddi A, Algwizani A, Ghazal SS, Assiri AM, Jokhdar H, Gerber SI, Alabdely H, Watson JT. Diabetes Mellitus, Hypertension, and Death among 32 Patients with MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis. (2020) Jan; 26: (1): 166-168. doi: 10.3201/eid2601.190952. |
[18] | Huang R, Zhu L, Xue L, Liu L, Yan X, Wang J, Zhang B, Xu T, Ji F, Zhao Y, Cheng J, Wang Y, Shao H, Hong S, Cao Q, Li C, Zhao XA, Zou L, Sang D, Zhao H, Guan X, Chen X, Shan C, Xia J, Chen Y, Yan X, Wei J, Zhu C, Wu C. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multicenter study. PLoS Negl Trop Dis. (2020) May 8; 14: (5): e0008280. doi: 10.1371/journal.and.0008280. |
[19] | Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. (2020) Aug 25; 324: (8): 782-793. doi: 10.1001/jama.2020.12839. |
[20] | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) Jan; 183: : 109119. doi: 10.1016/j.diabres.2021.109119. |
[21] | Mukona DM, Mathilda Z. Self-management of diabetes mellitus during the Covid-19 pandemic: Recommendations for a resource-limited setting. Diabetes & metabolic syndrome. (2020) ; 14: (6): 1575-1578. doi: 10.1016/j.dsx.2020.08.022. |
[22] | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. (2020) . |
[23] | Garibaldi BT, Fiksel J, Muschelli J, Robinson ML, Rouhizadeh M, Perin J, Schumock G, Nagy P, Gray JH, Malapati H, Ghobadi-Krueger M, Niessen TM, Kim BS, Hill PM, Ahmed MS, Dobkin ED, Blanding R, Abele J, Woods B, Harkness K, Thiemann DR, Bowring MG, Shah AB, Wang MC, Bandeen-Roche K, Rosen A, Zeger SL, Gupta A. Patient Trajectories Among Persons Hospitalized for COVID-19: A Cohort Study. Ann Intern Med. (2020) Sep 22; M20-3905. |
[24] | Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. (2020) . doi: 10.1016/j.ijid.2020.03.017. |
[25] | Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. (2020) . doi: 10.1007/s00392-020-01626-9. |
[26] | Papadokostaki E, Tentolouris N, Liberopoulos E. COVID-19, and diabetes: What does the clinician need to know? Prim Care Diabetes. (2020) ; 14: (5): 558-563. doi: 10.1016/j.pcd.2020.06.010. |
[27] | Pranata R, Henri J, Raffaello WM, Lawrence S, Huang I. Diabetes and COVID-19: The past, the present, and the future. Metabolism. (2021) Aug; 121: : 154814. doi: 10.1016/j.metabol.2021.154814. |
[28] | Wu ZH, Tang Y, Cheng Q. Diabetes increases the mortality of patients with COVID-19: A meta-analysis. Acta Diabetol. (2021) Feb; 58: (2): 139-144. doi: 10.1007/s00592-020-01546-0. |
[29] | Wu J, Zhang J, Sun X, Wang L, Xu Y, Zhang Y, Liu X, Dong C. Influence of diabetes mellitus on the severity and fatality of SARS-CoV-2 (COVID-19) infection. Diabetes Obes Metab. (2020) Oct; 22: (10): 1907-1914. doi: 10.1111/dom.14105. |
[30] | Abu-Ashour W, Twells L, Valcour J, Randell A, Donnan J, Howse P, Gamble JM. The association between diabetes mellitus and incident infections: a systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res. Care. (2017) ; 5. doi: 10.1136/bmjdrc-2016-000336. e000336. |
[31] | Rajpal A, Rahimi L, Ismail-Beigi F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J Diabetes. (2020) Dec; 12: (12): 895-908. doi: 10.1111/1753-0407.13085. |
[32] | Hayden MR. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J Int Med Res. (2020) ; 48: (7): 300060520939746. |
[33] | Govender N, Khaliq OP, Moodley J, Naicker T. Insulin resistance in COVID-19 and diabetes. Prim Care Diabetes. (2021) Aug; 15: (4): 629-634. doi: 10.1016/j.pcd.2021.04.004. |
[34] | Giustina A, Marazuela M, Reincke M, Yildiz BO, Puig-Domingo M. One year of the pandemic – how European endocrinologists responded to the crisis: a statement from the European Society of Endocrinology. Eur J Endocrinol. |
[35] | Kazakzakou P, Lambadiari V, Ikonomidis I, Kountouri A, Panagopoulos G, Athanasopoulos S, Korompoki E, Kalomenidis I, Dimopoulos MA, Mitrakou A. Diabetes and COVID-19; A Bidirectional Interplay. Front Endocrinol (Lausanne). (2022) Feb 17; 13: : 780663. doi: 10.3389/fendo.2022.780663. |
[36] | Yang P, Wang N, Wang J, Luo A, Gao F, Tu Y. Admission fasting plasma glucose is an independent risk factor for 28-day mortality in patients with COVID-19. J Med Virol. (2021) Apr; 93: (4): 2168-2176. doi: 10.1002/jmv.26608. |
[37] | Huang Y, Guo H, Zhou Y, Guo J, Wang T, Zhao X, Li H, Sun Y, Bian X, Fang C. The associations between fasting plasma glucose levels and mortality of COVID-19 in patients without diabetes. Diabetes Res Clin Pract. (2020) Nov; 169: : 108448. doi: 10.1016/j.diabres.2020.108448. |
[38] | Robert AA, Al Saeed A, Al Dawish MA. COVID-19 among people with diabetes mellitus in Saudi Arabia: Current situation and new perspectives. Diabetes Metab Syndr. (2021) Sep-Oct; 15: (5): 102231. doi: 10.1016/j.dsx.2021.102231. |
[39] | Giorgino F, Bhana S, Czupryniak L, Dagdelen S, Galstyan GR, Janež A, Lalić N, Nouri N, Rahelić D, Stoian AP, Raz I. Management of patients with diabetes and obesity in the COVID-19 era: Experiences and learnings from South and East Europe, the Middle East, and Africa. Diabetes Res Clin Pract. (2021) Feb; 172: : 108617. doi: 10.1016/j.diabres.2020.108617. |
[40] | Klingelhöfer D, Braun M, Quarcoo D, Brüggmann D, Groneberg DA. Epidemiological influences and requirements of global childhood obesity research. Obes Facts. (2021) ; 14: (4): 382-396. doi: 10.1159/000516777. |
[41] | Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. (2015) ; 66: (Suppl 2): 7-12. doi: 10.1159/000375143. |
[42] | World Health Organization Prevalence of obesity among adults, BMI |
[43] | Shukla A, Kumar K, Singh A. Association between obesity and selected morbidities: A study of BRICS countries. PLoS One. (2014) ; 9: (4): e94433. Published 2014 Apr 9. doi: 10.1371/journal.pone.0094433. |
[44] | WHO ((2013) )Obesity and Overweight, Geneva: World Health Organisation. http://www.who.int/mediacentre/factsheets/fs311/en |
[45] | Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attinà A, Cinelli G, Leggeri C, Caparello G, Barrera L, Scerbo F, Esposito E, De Lorenzo A. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J Transl Med. (2020) Jun 8; 18: (1): 229. doi: 10.1186/s12967-020-02399-5. |
[46] | Flanagan EW, Beyl RA, Fearnbach SN, Altazan AD, Martin CK, Redman LM. The Impact of COVID-19 Stay-At-Home Orders on Health Behaviors in Adults. Obesity (Silver Spring). (2021) Feb; 29: (2): 438-445. doi: 10.1002/oby.23066. |
[47] | Soetoroeroto AY, Soetedjo NN, Purwiga A, Santoso P, Kulsum ID, Suryadinata H, Ferdian F. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: A systematic review and meta-analysis. Diabetes Metab Syndr. (2020) Nov-Dec; 14: (6): 1897-1904. doi: 10.1016/j.dsx.2020.09.029. |
[48] | Huang Y, Lu Y, Huang YM, Wang M, Ling W, Sui Y, Zhao HL. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism. (2020) Dec; 113: : 154378. doi: 10.1016/j.metabol.2020.154378. |
[49] | Du Y, Lv Y, Zha W, Zhou N, Hong X. Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: A dose-response meta-analysis. Metabolism. (2021) Apr; 117: : 154373. doi: 10.1016/j.metabol.2020.154373. |
[50] | Kwok S, Adam S, Ho JH, Iqbal Z, Turkington P, Razvi S, Le Roux CW, Soran H, Syed AA. Obesity: A critical risk factor in the COVID-19 pandemic. Clin Obes. (2020) Dec; 10: (6): e12403. doi: 10.1111/cob.12403. |
[51] | Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, Mylonakis E. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring). (2020) Jul; 28: (7): 1200-1204. doi: 10.1002/oby.22859. |
[52] | Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin Proc. (2020) ; 95: (7): 1445-1453. doi: 10.1016/j.mayocp.2020.05.006. |
[53] | Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W, Liang HL, Cao YY, Yan Q, Cao C, Gao HY, Brüggen MC, van de Veen W, Sokolowska M, Akdis M, Akdis CA. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. (2021) Feb; 76: (2): 428-455. doi: 10.1111/all.14657. |
[54] | Mughal MS, Kaur IP, Jeffery AR, Dalmacion DL, Wang C, Koyoda S, Kramer VE, Patton CD, Weiner S, Eng MH, Granet KM. COVID-19 patients in a tertiary US hospital: Assessment of clinical course and predictors of the disease severity. Respir Med. (2020) Oct; 172: : 106130. doi: 10.1016/j.rmed.2020.106130. |
[55] | Wu JT, Leung K, Bushman M, Kishore N, Niehus R, de Salazar PM, Cowling BJ, Lipsitch M, Leung GM. Addendum: Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. (2020) Jul; 26: (7): 1149-1150. doi: 10.1038/s41591-020-0920-6. |
[56] | AlGhatrif M, Cingolani O, Lakatta EG. The Dilemma of Coronavirus Disease 2019, Aging, and Cardiovascular Disease: Insights From Cardiovascular Aging Science. JAMA Cardiol. (2020) ; 5: (7): 747-748. |
[57] | Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. (2020) May 25; 11: (1): 29. doi: 10.1186/s13293-020-00304-9. |
[58] | Liao JY, Yang ZX, Zhou T. The care role and susceptibility among female COVID-19 cases. Dian Zi Ke Ji Da Xue Bao. (2020) ; 49: : 425--430. |
[59] | Huh K, Lee R, Ji W, Kang M, Hwang IC, Lee DH, Jung J. Impact of obesity, fasting plasma glucose level, blood pressure, and renal function on the severity of COVID-19: A matter of sexual dimorphism? Diabetes Res Clin Pract. (2020) Dec; 170: : 108515. doi: 10.1016/j.diabres.2020.108515. |
[60] | Malik VS, Ravindra K, Attri SV, Bhadada SK, Singh M. Higher body mass index is an important risk factor in COVID-19 patients: A systematic review and meta-analysis. Environ Sci Pollut Res Int. (2020) Nov; 27: (33): 42115-42123. doi: 10.1007/s11356-020-10132-4. |
[61] | Deng M, Qi Y, Deng L, Wang H, Xu Y, Li Z, Meng Z, Tang J, Dai Z. Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity (Silver Spring). (2020) Oct; 28: (10): 1815-1825. doi: 10.1002/oby.22943. |
[62] | Liu SP, Zhang Q, Wang W, Zhang M, Liu C, Xiao X, Liu Z, Hu WM, Jin P. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res Clin Pract. (2020) Sep; 167: : 108338. doi: 10.1016/j.diabres.2020.108338. |
[63] | Khanna D, Peltzer C, Kahar P, Parmar MS. Body Mass Index (BMI): A Screening Tool Analysis. Cureus. (2022) ; 14: (2): e22119. Published 2022 Feb 11. doi: 10.7759/cureus.22119. |
[64] | Association between sex and body mass index as mediated by temperament in a nonclinical adult sample. Oniszczenko W, Stanisławiak E. Eat Weight Disord. (2019) ; 24: : 291-298. |
[65] | Goeller M, Achenbach S, Marwan M, Doris MK, Cadet S, Commandeur F, Chen X, Slomka PJ, Gransar H, Cao JJ, Wong ND, Albrecht MH, Rozanski A, Tamarappoo BK, Berman DS, Dey D. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. (2018) Jan-Feb; 12: (1): 67-73. doi: 10.1016/j.jcct.2017.11.007. |
[66] | Ojo O, Wang XH, Ojo OO, Orjih E, Pavithran N, Adegboye ARA, Feng QQ, McCrone P. The effects of COVID-19 lockdown on glycaemic control and lipid profile in patients with type 2 diabetes: A systematic review and meta-analysis. Int J Environ Res Public Health. (2022) Jan 19; 19: (3): 1095. doi: 10.3390/ijerph19031095. |
[67] | Permana H, Huang I, Susandi E, Wisaksana R. The association of admission random blood glucose concentration and body-mass index with mortality in COVID-19 patients. Eur Rev Med Pharmacol Sci. (2021) Nov; 25: (22): 7144-7150. doi: 10.26355/eurrev_202111_27268. |
[68] | Zhang W, Li C, Xu Y, He B, Hu M, Cao G, Li L, Wu S, Wang X, Zhang C, Zhao J, Xie J, Xu Z, Li Q, Wang G. Hyperglycemia and correlated high levels of inflammation have a positive relationship with the severity of coronavirus disease 2019. Mediators Inflamm. (2021) Mar 18; 2021: : 8812304. doi: 10.1155/2021/8812304. |
[69] | Zhao Y, Xing H. Influence of fasting plasma glucose level on admission of COVID-19 patients: A retrospective study. J Diabetes Res. (2022) ; 2022: : 7424748. Published 2022 Jan 6. doi: 10.1155/2022/7424748. |
[70] | Biamonte E, Pegoraro F, Carrone F, Facchi I, Favacchio G, Lania AG, Mazziotti G, Mirani M. Weight change and glycemic control in type 2; diabetes patients during COVID-19 pandemic: The lockdown effect. Endocrine. (2021) ; 72: : 604-610. doi: 10.1007/s12020-021-02739-5. |
[71] | Badnjevic A, Gurbeta L, Custovic E. An expert diagnostic system to automatically identify asthma and chronic obstructive pulmonary disease in clinical settings. Sci Rep. (2018) Aug 3; 8: (1): 11645. doi: 10.1038/s41598-018-30116-2. |
[72] | Gurbeta L, Badnjevic A, Maksimovic M, Omanovic-Miklicanin E, Sejdic E. A telehealth system for automated diagnosis of asthma and chronic obstructive pulmonary disease. J Am Med Inform Assoc. (2018) Sep 1; 25: (9): 1213-1217. doi: 10.1093/jamia/ocy055. |




